Abstract
To investigate the role of human apolipoprotein E (apoE) on Aβ deposition in vivo, we crossed PDAPP mice lacking mouse Apoe to targeted replacement mice expressing human apoE (PDAPP/TRE2, PDAPP/TRE3, or PDAPP/TRE4). We then measured the levels of apoE protein and Aβ peptides in plasma, CSF, and brain homogenates in these mice at different ages. We also quantified the amount of brain Aβ and amyloid burden in 18-month-old mice. In young PDAPP/TRE4 mice that were analyzed at an age before brain Aβ deposition, we observed a significant decrease in the levels of apoE in CSF and brain when compared with age-matched mice expressing either human E2 or E3. The brain levels of Aβ42 in PDAPP/TRE4 mice were substantially elevated even at this very early time point. In older PDAPP/TRE4 mice, the levels of insoluble apoE protein increased in parallel to the dramatic rise in brain Aβ burden, and the majority of apoE was associated with Aβ. In TRE4 only mice, we also observed a significant decrease in the level of apoE in brain homogenates. Since the relative level of apoE mRNA was equivalent in PDAPP/TRE and TRE only mice, it appears that post-translational mechanisms influence the levels of apoE protein in brain (E4 < E3 ≪ E2), resulting in early and dramatic apoE isoform-dependent effects on brain Aβ levels (E4 ≫ E3 > E2) that increase with age. Therapeutic strategies aimed at increasing the soluble levels of apoE protein, regardless of isoform, may effectively prevent and (or) treat Alzheimer's disease.
Introduction
The ε4 allele of the apolipoprotein E gene is a well established risk factor for late-onset Alzheimer's disease (AD), even in populations where the ε4 allele is under-represented (Saunders et al., 1993; Farrer et al., 1997). Conversely, the less frequent ε2 allele appears to delay as well as reduce the relative risk for developing AD (Corder et al., 1994). Exactly how these APOE alleles influence disease risk is unclear, since individuals who are homozygous for ε4 do not invariably develop AD (Meyer et al., 1998). Neuropathological examination of brain tissue from ε4 patients suggests that apoE influences the amount of β-amyloid (Aβ) deposition in addition to the number of neurofibrillary tangles (Schmechel et al., 1993; Nagy et al., 1995).
In brain, apolipoprotein E (apoE) is synthesized primarily by glia and represents the most abundant apolipoprotein within the CNS (Boyles et al., 1985). ApoE appears to play a critical role in cholesterol homeostasis, synaptic plasticity, and neuronal repair (Mahley and Rall, 2000). Although apoE can be internalized by neurons, it is widely believed that de novo synthesis of apoE by neurons occurs rarely and perhaps only under conditions of injury and (or) disease (Boschert et al., 1999).
We have previously reported an important role for apoE in the development of brain amyloid burden in the PDAPP transgenic mouse model of AD (Bales et al., 1997). Surprisingly, when these same transgenic mice were crossed to mice expressing human apoE from astrocytes, a dramatic reduction in brain Aβ burden was observed (Holtzman et al., 1999). However, as these animals age, we observed greater Aβ deposition in the brains of mice expressing human E4 (Holtzman et al., 2000). Here, we extend those findings by characterizing PDAPP mice that express human apoE under control of the mouse regulatory elements (Sullivan et al., 1997). We demonstrate an apoE isoform-dependent difference in the level of apoE in brain and CSF with the lowest levels observed in mice expressing E4 (PDAPP/TRE4). Importantly, PDAPP/TRE4 mice manifest elevated levels of soluble brain Aβ when measured at an early age. In old PDAPP/TRE4 mice, brain Aβ/amyloid burden was also significantly greater, and nearly all of the apoE measured by immunohistochemistry was associated with deposited Aβ. To investigate whether or not Aβ was influencing the levels of apoE in PDAPP/TRE mice, we also measured apoE in TRE mice. The levels of soluble apoE in brain homogenates from TRE4 mice were also significantly reduced. The levels of apoE mRNA were identical between the various genotypes and, therefore, could not account for the reduced apoE protein that we observed in mice expressing human E4, suggesting that post-translational mechanisms strongly influence brain levels of apoE as well as brain Aβ/amyloid deposition. Thus, a persistent decrease in the level of soluble apoE protein in brain as observed in PDAPP/TRE4 mice results in dramatic effects on brain Aβ levels and deposition over a lifetime. Treatments that upregulate apoE expression (regardless of isoform) may, therefore, be beneficial for preventing or slowing the progression of AD.
Materials and Methods
Transgenic mice.
Homozygous PDAPP transgenic (APPV717F mice) without mouse Apoe derived from a heterogeneous background comprising the strains: DBA/2J, C57BL/6J, and Swiss Webster were crossed to mice that had been genetically engineered to express human apolipoprotein E by homologous recombination (Bales et al., 1997; Sullivan et al., 1997). Heterozygous mice were then intercrossed, and mice homozygous for the PDAPP transgene and human apolipoprotein E2, E3, or E4 (PDAPP/TRE2, PDAPP/TRE3, or PDAPP/TRE4) at various ages were used. Mice expressing human apolipoprotein E2, 3, or 4 (TRE2, 3, or 4; 3 months of age) only were purchased from Taconic. All experiments were performed under protocols that had been approved by the Internal Animal Care and Use Committee, Lilly Research Laboratories, Eli Lilly and Company.
Tissue preparation and immunohistochemistry.
Animals were anesthetized (Avertin 0.032 mg/ml), and CSF was collected from the cisterna magna. Whole blood was collected via cardiac puncture before transcardiac perfusion with heparinized saline. Each brain from mice that were 3 or 12 months of age was microdissected into hippocampus and cortex before storage at −80°C until use. For mice that were 18 months of age, the brain was divided along the sagittal plane, and one-half of the brain was microdissected into hippocampus and cortex. The remaining half was frozen in liquid nitrogen and stored at −80°C until sectioning. For quantification of brain Aβ and amyloid burden, five sagittal sections (10 μm) representing the extent of the hippocampus and cortex from each mouse per genotype were selected and processed as described previously (Bales et al., 1999). Quantification of brain Aβ burden was completed after immunostaining with the anti-Aβ antibody 3D6 which recognizes the N terminus of the human Aβ peptide (Johnson-Wood et al., 1997). The signal was visualized using a fluorescein conjugated secondary antibody (Invitrogen). For investigating apoE and Aβ colocalization in brain, hemisections from the 18-month-old PDAPP/TRE mice used above were double stained with anti-Aβ (as described above) and anti-apoE antibody that was biotinylated (Biodesign International) followed by detection with secondary antibodies that were labeled with rhodamine (anti-Aβ; red) or fluorescent-labeled streptavidin (anti-apoE, green; Invitrogen). The specificity of anti-apoE staining was verified by the lack of staining in an apoE knock-out mouse brain section as well as the lack of signal when the primary antibody was omitted (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Brain amyloid was quantified after thioflavine S histochemistry (Bales et al., 1997).
Quantification of amyloid/Aβ burden.
Aβ (immunoreactive Aβ deposits) and amyloid (thioflavine S-positive deposits) burden was quantified by capturing stained images using Image Pro plus software (Media Cybernetics), defining an area of interest within the hippocampus or cortex, and setting a threshold (in control mice) to discriminate nonspecific staining. The percentage of surface area covered by Aβ immunoreactivity (Aβ burden) or thioflavine S (amyloid burden) was determined for two areas: an area of parietal cortex comprising layers 1–V1 and an area of the hippocampal formation comprising layers oriens, pyramidal layer, stratum radiatum, and the dentate gyrus. Brain Aβ or thioflavine S burden was then represented as a percentage of total area quantified. For quantifying apoE and Aβ colocalization, regions of hemibrain (that included cortex and hippocampus) were captured and the relative area of fluorescence for each protein was quantified using Image Pro (v5.1; Media Cybernetics). Intensity thresholds were set based on visual review of the images and were used consistently for analysis of all images. A macro was computed in Image-Pro to facilitate data collection that included total amount of Aβ or apoE signal as well as the amount of apoE/Aβ signal that was colocalized. The amount of colocalized signal was then expressed as a percentage of the apoE signal.
Aβ ELISA measurements.
Previously microdissected brain regions (hippocampus or cortex) were frozen on dry ice and stored at −80°C until processing. A serial extraction method was used to determine the levels of Aβ in soluble (PBS) or insoluble (guanidine) pools (Johnson-Wood et al., 1997). Soluble levels of Aβ were measured from hippocampal or cortical regions that were rapidly homogenized in PBS (200 μl for hippocampus and 400 μl for cortex). After centrifugation (14,000 rpm, 4°C for 15 min), the supernatant was collected, and the pellet was re-extracted in the same volume of PBS as described above. The two PBS fractions were combined and represented the soluble pool. The remaining pellet was extracted in 5.0 m guanidine in 50 mm Tris-HCl, pH 8.0 (400 μl for hippocampus and 800 μl for cortex) by rotating the samples at room temperature for at least 4 h. The samples were then centrifuged at 14,000 rpm for 15 min and diluted with cold PBS-T (1% BSA, 0.05% Tween) before measurement of Aβ40 or Aβ42 as described previously (Bales et al., 1997). Aβ measured after guanidine extraction represented the insoluble pool. Levels of Aβ40 and Aβ42 in plasma and CSF were measured using the same sandwich ELISA as brain homogenates, except plasma samples were diluted 1:2 (in PBS-T) and CSF samples were diluted 1:10 (in PBS-T) before loading.
ApoE ELISA measurements.
ApoE levels were measured in plasma, CSF, and brain homogenates from PDAPP mice expressing one of the three human apoE alleles at various ages using a sandwich ELISA. Additionally, we measured apoE in brain homogenates from 3-month-old mice expressing the human apoE alleles only using this same ELISA that we had cross validated with plasma samples using a quantitative mass spectrometry method (our unpublished observation). Briefly, plasma, CSF, or brain homogenates (diluted 3000-, 100-, and 50-fold, respectively, in PBS-T and 0.4% glycine) were loaded into a 96-well plate that had been coated with an anti-apoE antibody (goat anti-apoE; 1 to 2000 dilution; Millipore Bioscience Research Reagents). After overnight incubation at 4°C, the samples were removed and the plate washed 3× with PBS-T before incubation with a biotinylated anti-apoE antibody (goat anti-apoE; 1:5000; Biodesign) for 1 h at room temperature. The plates were then washed again 3× with PBS-T and incubated with streptavidin–HRP for an additional 1 h at room temperature. The immunocomplex was reacted with TMB substrate and detected using a Spectra MAX 190 (Molecular Devices) plate reader. Levels of apoE were normalized to a standard curve generated using recombinant human apoE (Biodesign). Plasma and CSF apoE levels were determined in triplicate and expressed as the amount of apoE per milliliter. Levels of apoE in brain homogenates were determined in triplicate, normalized to protein content, and expressed as the amount of apoE per milligram of protein.
Amyloid precursor protein ELISA measurements.
Amyloid precursor protein (APP) holoprotein levels were measured in guanidine homogenates from the hippocampus and cortex in the same 12-month-old cohort used for Aβ and apoE brain measurements described above (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Briefly, measurement of APP holoprotein levels followed the same ELISA protocol for measuring Aβ levels, except the anti-APP antibody 8E5 was used as the capture antibody and an anti-APP C-terminal antibody was used as the reporter antibody (Johnson-Wood et al., 1997) (Invitrogen). Levels of APP were normalized to a standard curve generated using recombinant human APP. The levels of APP holoprotein in brain homogenates were measured in triplicate, normalized to protein, and expressed as the amount of APP per milligram of protein.
RNA analysis.
Total RNA was isolated from cortical samples collected from 12-month-old PDAPP mice expressing human apoE using a BioRobot M48, with the MagAttract RNA mini M48 kit (supplemental Fig. 3, available at www.jneurosci.org as supplemental material) (Qiagen). Total RNA (∼1–2 μg) was reverse transcribed using the High-Capacity cDNA Archive kit (Perkin Elmer Applied Biosystems), according to the manufacturers' recommendations. A dilution of the reverse transcribed cDNA (1/25) was used as the template for the PCR. An equivalent volume of template without reverse transcription served as a negative control. The PCR primers and probe for detecting human APOE or the 695 aa isoform of the amyloid precursor protein transgene were designed using Primer Express 1.0 software program (supplemental Methods, available at www.jneurosci.org as supplemental material). Primers corresponding to rodent glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Perkin Elmer Applied Biosystems. For PCR amplification, 2 μl of cDNA with 8 pmol of each primer and 2 pmol of the probe primer in a total volume of 10 μl was used. Cycling conditions were 2 min at 50°C, 10 min at 95°C for 40 cycles with the final cycle consisting of 95°C for 15 s, 60°C for 1 min in a PE Applied Biosystems 7900HT sequence detection system. Each sample was run in triplicate, and the relative level of APOE mRNA was calculated by the standard curve method and normalized to levels of GAPDH.
Results
Isoform-dependent differences in the level of apolipoprotein E protein in brain
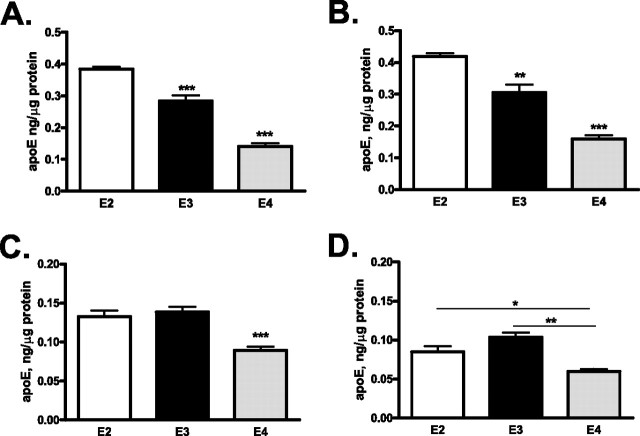
Using a sensitive ELISA, we determined the levels of apoE protein in plasma, CSF, and brain (hippocampus and cortex) in PDAPP/TRE2, 3, or 4 mice at various ages. The level of apoE in the plasma of PDAPP/TRE2 mice was significantly greater at 3 and 18 months of age than that measured in either PDAPP/TRE3 or PDAPP/TRE4 mice at the same age (Fig. 1 A). Similarly, the level of apoE in CSF was significantly greater in PDAPP/TRE2 mice when compared with PDAPP/TRE3 or PDAPP/TRE4 mice at either 3 or 18 months of age (Fig. 1 B). At 18 months of age, there was also an age-dependent increase in the level of apoE measured in the CSF of PDAPP/TRE3 or PDAPP/TRE4 mice that was not observed in PDAPP/TRE2 (Fig. 1 B). Importantly, the levels of apoE in CSF samples from PDAPP/TRE3 mice were significantly greater (∼50%) than those measured in PDAPP/TRE4 samples at either 3 or 18 months of age (Fig. 1 B).
Figure 1.
A–F, Levels of apoE in plasma, CSF, and brain homogenates in PDAPP mice expressing human apoE at various ages. A, B, ApoE levels in plasma (A) and CSF (B) were significantly greater in PDAPP/TRE2 mice, whereas the levels of apoE were the lowest in PDAPP/TRE4 mice. C, D, Soluble levels of apoE in hippocampal (C) or cortical (D) homogenates were significantly greater in PDAPP/TRE2 mice at all ages examined, whereas the levels of soluble apoE were significantly lower in PDAPP/TRE4 mice. E, F, Insoluble levels of apoE measured in the hippocampus (E) were significantly lower in PDAPP/TRE4 mice at a young age. There was an age-dependent increase in insoluble apoE levels in hippocampus (E) and cortex (F) of PDAPP mice expressing one of the human apoE alleles. A, ***p < 0.001 versus all other groups ANOVA, Tukey–Kramer post test, *p < 0.05 t test. B, ***p < 0.001 versus all other groups ANOVA, Tukey–Kramer post test, **p < 0.01 t test. C, ∧∧∧ p < 0.001 versus all other groups, ### p < 0.001 versus all other groups except # p < 0.05 versus E4 at 3 months ANOVA, Tukey–Kramer post test, *p < 0.05, **p < 0.01, ***p < 0.001 t test. D, ∧∧∧ p < 0.001 versus all other groups, ### p < 0.001 versus all other groups except E4 at 3 months, & p < 0.001 versus all other groups except E2 and E3 at 3 months ANOVA, Tukey–Kramer post test, **p < 0.05, ***p < 0.001 t test. E, & p < 0.001 versus all other groups except E4 at 18 months ∧ p < 0.05 versus E3 at 12 months, # p < 0.05 versus E4 at 18 months, ANOVA, Tukey–Kramer post test, *p < 0.05 t test. F, & p < 0.001 versus all other groups, # p < 0.05 versus al other groups, ANOVA, Tukey–Kramer post test.
We then measured the level of apoE in two brain regions: hippocampus and cerebral cortex, using a serial extraction method to quantify apoE in the soluble (PBS) and insoluble (guanidine) compartments. At 3 months of age, there was a significant isoform-dependent difference in the level of apoE protein measured in either the soluble or insoluble fractions from the hippocampus or cortex (Fig. 1). PDAPP/TRE4 mice had significantly reduced levels of apoE protein in both the cortex and hippocampus in both fractions analyzed. In contrast, young PDAPP/TRE2 mice had the highest levels of apoE in the soluble fraction after extraction of hippocampal or cortical tissue (Fig. 1 C,D). By 12 months of age, the levels of insoluble apoE measured in the hippocampus of PDAPP/TRE4 mice began to increase (Fig. 1 E). In very old mice, the level of insoluble apoE increased in an apoE-isoform dependent manner with 18-month-old PDAPP/TRE4 mice having significantly more apoE present in the insoluble cortical fraction when compared with age-matched PDAPP/TRE2 or PDAPP/TRE3 mice (Fig. 1 F). The age-dependent increase in insoluble apoE we observed in old PDAPP/TRE4 mice most likely reflects apoE that has codeposited with Aβ in brain parenchyma (Bales et al., 1999) (see Figs. 6, 7).
Figure 6.
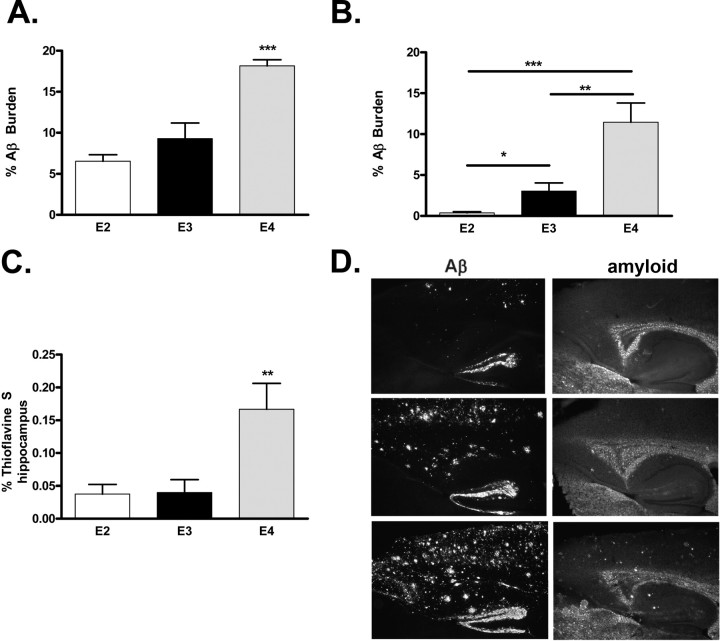
A–D, Brain Aβ and amyloid burden in PDAPP mice expressing human apolipoprotein E alleles at 18 months of age. Significantly more Aβ is deposited in the hippocampus (A, ***p < 0.001, ANOVA, Tukey–Kramer post test) or cortex (B, ***p < 0.001, **p < 0.01, *p < 0.05, t test) of PDAPP/TRE4 mice at 18 months of age. Significantly more amyloid (thioflavine S material) is present in the hippocampus of PDAPP/TRE4 mice at 18 months of age (C, **p < 0.01, ANOVA, Tukey–Kramer post test). D, Representative photomicrographs of Aβ immunoreactivity and amyloid (thioflavine S staining) in PDAPP mice expressing various human apoE alleles at 18 months of age.
Figure 7.
A, Representative images from 18-month-old PDAPP/TRE mice double stained with anti-apoE (apoE, green) or anti-Aβ (Aβ, red) and merged (yellow). B, Quantification of apoE and Aβ colocalization in PDAPP/TRE brain sections. The vast majority (∼90%) the apoE signal that is measured in the brain from PDAPP/TRE4 mice is associated with Aβ, whereas only ∼25% of apoE and Aβ are colocalized in very old PDAPP/TRE2 mice. ***p < 0.001 versus all other groups, ANOVA, Tukey–Kramer post test.
Since the expression of Aβ in PDAPP mice could affect the overall levels of apoE that we measured, we next measured the levels of both soluble (PBS extractable) and insoluble (guanidine extractable) apoE in brain (hippocampus and cortex) homogenates from mice expressing human apoE only (TRE mice) (Fig. 2). Similar to the results observed in PDAPP/TRE4 mice, TRE4 mice had the lowest levels of apoE regardless of extraction method or brain region (Fig. 2). In both PDAPP/TRE4 as well as TRE4 mice, the relative level of soluble apoE4 was very similar with PDAPP/TRE4 mice having ∼40% and TRE4 mice having ∼37% of the levels of apoE measured in PDAPP/TRE2 or TRE2 mice of a similar age (Figs. 1 C, 2 A). Additionally, the levels of insoluble apoE that we measured in hippocampal and cortical homogenates from TRE2 and TRE3 mice were virtually identical (Fig. 2 C,D).
Figure 2.
A–D, Levels of apoE in brain homogenates from mice expressing human apoE2, 3, or 4 (E2, E3, or E4; 3 months of age) only. A, B, Soluble levels of apoE in hippocampal (A) or cortical (B) homogenates were significantly greater in TRE2 mice, whereas the levels of soluble apoE were significantly lower in TRE4 mice. C, D, Insoluble levels of apoE measured in the hippocampus (C) or cortex (D) were significantly lower in TRE4 mice. A–C, ***p < 0.001, **p < 0.01 versus all other groups ANOVA, Tukey–Kramer post test. D, **p < 0.05, *p < 0.01 t test.
Isoform-dependent differences in the level of Aβ peptides
We next determined the levels of Aβ40 and Aβ42 in plasma, CSF, and brain (hippocampus and cortex) in the same cohort of PDAPP/TRE mice that we had used to determine the levels of apoE. The levels of plasma Aβ42 were not significantly different at any of the ages sampled, nor were there any apoE-isoform-dependent effects (Fig. 3 A). There was an age-dependent decrease in the levels of Aβ40, such that by 18 months of age, the level of Aβ40 in plasma was reduced to only ∼50% of that observed in younger 3-month-old mice (Fig. 3 B). The significant decrease in plasma Aβ40 levels occurred without regard to apoE-isoform, although the highest levels of plasma Aβ40 were measured in PDAPP/TRE3 mice. In CSF, the levels of Aβ42 and Aβ40 did not differ according to age or apoE allele status, except for a highly significant decrease in Aβ42 levels observed in CSF from 18-month-old PDAPP/TRE4 mice (Fig. 3 C,D).
Figure 3.
A–D, Levels of Aβ in plasma (A, B) or CSF (C, D) in PDAPP mice expressing one of the human apolipoprotein E alleles (E2, E3, or E4) at 3 or 18 months. B, C, ***p < 0.001 versus all groups at 18 months of age, **p < 0.01 versus E2 at 3 months of age, *p < 0.01 versus E4 at 3 months of age, ANOVA, Tukey–Kramer post test, ***p < 0.001, **p < 0.01 t test.
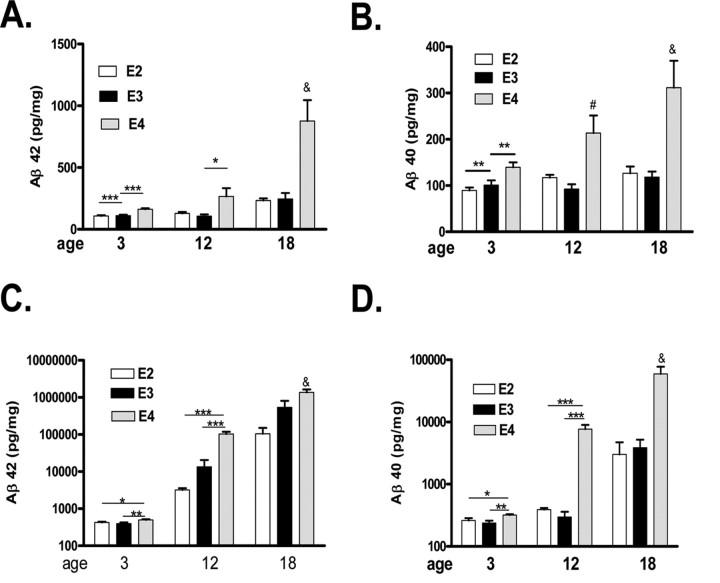
We then measured the levels of Aβ40 and Aβ42 in the hippocampus or cortex after extraction of soluble (PBS) or insoluble (guanidine) pools of Aβ (Figs. 4, 5). There was an age-dependent increase in both Aβ42 and Aβ40, regardless of apoE isoform or fraction (soluble or insoluble) analyzed, which was more pronounced in the PDAPP/TRE4 mice (Figs. 4, 5). Soluble levels of both Aβ40 as well as Aβ42 measured in the hippocampus of 3-month-old PDAPP/TRE4 exceeded or were equivalent to 12-month-old PDAPP/TRE3 or PDAPP/TRE2 mice (Fig. 4 A,B). In the hippocampus, insoluble Aβ40 and Aβ42 levels increased in both an age- and apoE isoform-dependent manner, with the greatest levels observed in PDAPP/TRE4 mice even when measured at a young age (Fig. 4 C,D). Soluble and insoluble levels of Aβ40 and Aβ42 measured in the cortex followed a pattern similar to that we observed in the hippocampus with the highest levels measured in PDAPP/TRE4 mice at all ages sampled (Fig. 5). In contrast to PDAPP/TRE4 mice, the levels of both Aβ40 and Aβ42 measured in any of the brain regions sampled were lowest in PDAPP/TRE2 mice (Figs. 4, 5).
Figure 4.
A–D, Levels of soluble (A, B) and insoluble (C, D) Aβ in hippocampal homogenates from PDAPP mice expressing human apolipoprotein E alleles at different ages. A, & p < 0.001 versus all groups, ### p < 0.001 versus E2 or E3 at 12 months; E4 at 3 months, # p < 0.01 versus E3 18 months, ANOVA, Tukey–Kramer post test, ***p < 0.001, t test. B, & p < 0.001 versus all groups except E4 at 12 months, # p < 0.01 versus E4 at 3 months and E3 at 12 months, ANOVA, Tukey–Kramer post test, ***p < 0.001 t test. C, & p < 0.01 versus all other groups expect E3 at 18 months; # p < 0.01 versus all other groups except E4 at 18 months; ∧p < 0.01 versus E2 at 3 and 12 months, ANOVA, Tukey–Kramer post test, **p < 0.01, ***p < 0.001, t test. D, & p < 0.001 versus all other groups, ANOVA, Tukey–Kramer post test, ***p < 0.001, **p < 0.05 t test.
Figure 5.
A–D, Levels of soluble (A, B) and insoluble Aβ (C, D) in cortical homogenates from PDAPP mice expressing human apolipoprotein E alleles at different ages. A, & p < 0.001 versus all other groups, ANOVA, Tukey–Kramer post test, *p < 0.05, ***p < 0.001 t test. B, & p < 0.001 versus all other groups except E4 at 12 months, # p < 0.05 versus E3 at 12 months ANOVA, Tukey–Kramer post test, **p < 0.01, t test. C, & p < 0.001 versus all other groups, ANOVA, Tukey–Kramer post test, *p < 0.05, **p < 0.01, ***p < 0.001, t test. D, & p < 0.001 versus all other groups, ANOVA, Tukey–Kramer post test, *p < 0.05, **p < 0.01, ***p < 0.001, t test.
ApoE isoform-dependent amyloid deposition and apoE/Aβ colocalization
We next determined brain Aβ and amyloid burden in very old PDAPP mice (18 months of age) that expressed apoE2, E3, or E4 (Fig. 6). There was an apoE-isoform-dependent difference in the amount of Aβ deposited in both the hippocampus and cortex with PDAPP/TRE4 mice, demonstrating significantly more brain Aβ burden than either PDAPP/TRE3 or PDAPP/TRE2 mice (Fig. 6). Although the level of brain Aβ burden that we measured in the hippocampus of PDAPP/TRE2 or PDAPP/TRE3 mice was roughly equivalent (6.5 vs 9.3%), the amount of Aβ in the cortex of PDAPP/TRE3 mice was ∼7.5× greater than in PDAPP/TRE2 mice (3.0 vs 0.4%) (Fig. 6 A,B). The amount of fibrillar amyloid (thioflavine S-positive material) was significantly greater in PDAPP/TRE4 mice compared with PDAPP/TRE2 or PDAPP/TRE3 mice, whereas the amount of amyloid quantified in the hippocampi of PDAPP/TRE2 or PDAPP/TRE3 mice was virtually identical (Fig. 6 C) (0.06 vs 0.04%).
To determine if the dramatic rise in insoluble apoE levels that we observed in old PDAPP/TRE4 mice was the result of apoE codepositing with Aβ, we quantified colocalized apoE and Aβ immunoreactivity in the brains of old PDAPP/TRE mice (Fig. 7). Nearly all (∼90%) of the apoE that we quantified in old PDAPP/TRE4 brain sections was associated with Aβ, whereas only ∼25% of the apoE in PDAPP/TRE2 mice was associated with Aβ (Fig. 7 B).
Significant correlation between soluble levels of apoE and Aβ42 in PDAPP/TRE2 mice
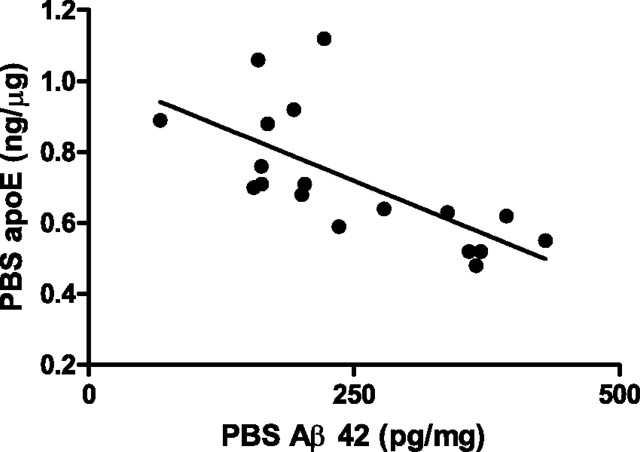
We next investigated the correlation between the levels of soluble apoE and Aβ42 in hippocampal homogenates from PDAPP/TRE mice. We observed a significant inverse correlation between the levels of soluble apoE and Aβ42 in hippocampal homogenates from PDAPP/TRE2 mice (Fig. 8). We did not observe a significant correlation between soluble apoE and Aβ42 levels in hippocampal extracts from either PDAPP/TRE3 or PDAPP/TRE4 mice (supplemental Fig. 4, available at www.jneurosci.org as supplemental material).
Figure 8.
Significant inverse correlation between soluble levels of apolipoprotein E2 and Aβ 42 extracted from the hippocampus of PDAPP mice expressing human E2 (r 2 = 0.46, p < 0.001).
APP protein levels in PDAPP/TRE mice
We next measured the level of APP holoprotein in 12-month-old PDAPP/TRE mice to ensure that an increase in APP substrate was not responsible for the dramatic increase in Aβ that we observed in PDAPP/TRE4 mice. There was a small but significant reduction in the level of APP holoprotein in hippocampal homogenates from PDAPP/TRE2 mice that was not apparent in the cortex (supplemental Fig. 2, available at www.jneurosci.org as supplemental material).
No change in APOE or APP mRNA
To ensure that the differences we measured in apoE or Aβ were not attributable to apoE isoform-dependent differences in the relative levels of apoE or APP transgene mRNA, we quantified mRNA levels for these two genes in brain samples from PDAPP/TRE2, PDAPP/TRE3, and PDAPP/TRE4 mice using quantitative PCR (supplemental Fig. 3A,B, available at www.jneurosci.org as supplemental material). Additionally, we quantified the level of mRNA in brain (cortex and hippocampus) samples from mice expressing apoE only (supplemental Fig. 3C,D, available at www.jneurosci.org as supplemental material). There was no significant difference in the level of APP mRNA regardless of APOE isoform (supplemental Fig. 3B, available at www.jneurosci.org as supplemental material) in PDAPP/TRE mice as well as no significant difference in the level of APOE in either PDAPP/TRE or TRE only mice (supplemental Fig. 3A,C,D, available at www.jneurosci.org as supplemental material).
Discussion
The ε4 allele of the APOE gene represents the most important genetic risk factor identified to date for the development of late onset AD, since ε4 carriers have an increased risk for and an earlier age of disease onset (Saunders et al., 1993). Additionally, ε4 carriers have an increased risk of dementia after acute head injury or stoke and an increased rate of mortality after intracerebral hemorrhage (Nicoll et al., 1996; Slooter et al., 1997; Teasdale et al., 1997). The ε4 allele also confers susceptibility to other neurological conditions such as Parkinson's disease, multiple sclerosis, and amyotrophic lateral sclerosis, as well as an increased risk of CNS-permissive viral infections such as HIV (human immunodeficiency virus) and HSV (herpes simplex virus) (Mahley et al., 2006; Burt et al., 2008; Miller and Federoff, 2008). Additionally, ε4 carriers have a more rapid decline in memory function associated with aging (Deary et al., 2002). In contrast, the e2 allele appears to be in some way “protective” even when other genetic risk factors such as mutations associated with familial forms of AD or the ε4 allele are present (Corder et al., 1994; Royston et al., 1994).
Although the exact mechanisms underlying the relationship between the different APOE alleles and increased susceptibility to or protection from AD remain to be defined, several possibilities exist. Each of the apoE isoforms differ from one another by a single amino acid at residue 112 or 158; however, divergence at these key residues has profound effects on the stability of the protein as well as how apoE interacts with its receptors (Weisgraber, 1994). Although little is known about the binding preference of the various apoE isoforms for lipoproteins in the CNS, in the periphery, apoE3 and apoE2 bind preferentially to smaller more phospholipid-enriched high-density lipoprotein (HDL), whereas apoE4 prefers very low-density lipoprotein particles (Weisgraber, 1994). In vitro studies with the various apoE isoforms have also documented an isoform-specific effect on neurite outgrowth with apoE2 ≫ apoE3 > apoE4 (Holtzman and Fagan, 1998).
An important role for apoE in determining brain Aβ burden in vivo has been established and numerous studies have documented an increase in brain Aβ burden in AD patients who are ε4 carriers (Bales et al., 1999; Walker et al., 2000; DeMattos, 2004). Previous studies from our laboratory used PDAPP mice that were crossed to transgenic mice expressing human apoE exclusively in astrocytes (Fagan et al., 2002). Additionally, we investigated the formation of cerebral amyloid angiopathy in APPswe mice crossed to TRE mice (Fryer et al., 2005). In both reports and similar to the findings reported here, the levels of Aβ were significantly increased in transgenic mice expressing human apoE4. Here, we present data in PDAPP mice expressing various human apoE isoforms in all relevant tissues, and, once again, PDAPP/TRE4 mice had the highest levels of brain Aβ/amyloid burden (Fig. 6). Nearly all (∼90%) of the apoE that we measured in the brains of old PDAPP/TRE4 mice was associated with Aβ, whereas only ∼25% of the apoE in PDAPP/TRE2 mice was colocalized with Aβ (Fig. 7). We also observed an increase in the levels of soluble Aβ42, the more profibrillogenic Aβ species, in brain homogenates from young PDAPP/TRE4 mice at an age when deposited Aβ is rarely detected by sensitive immunohistochemical methods (Fig. 4). We also observed a significant decrease in apoE levels in plasma, CSF, and brain homogenates in young PDAPP/TRE4 (Fig. 1). Several reports have documented a significant decrease in apoE levels in TRE4 mice, and we also observed a significant reduction (Fig. 2) (Ramaswamy et al., 2005; Riddell et al., 2008). An apoE isoform-dependent difference in the level of plasma apoE has been reported in healthy control subjects with ε2/ε2 individuals having the highest and ε4/ε4 individuals having the lowest levels (Utermann et al., 1980). Reports of isoform-dependent differences in apoE levels in CSF have been inconsistent, most probably because of methodological differences in sample collection and analysis. However, several reports have consistently documented lower apoE levels in the hippocampus of AD patients who are ε4 carriers (Beffert et al., 1999, Poirier, 2008). The cumulative effect of this significant decrease in apoE4 levels on brain Aβ burden over time appears to be quite dramatic, since the level of brain Aβ/amyloid burden was quite substantial in PDAPP/TRE4 mice (Fig. 6). Conversely, PDAPP/TRE2 mice had the highest levels of soluble apoE measured in any of the sampled compartments, along with the lowest levels of brain Aβ. In fact, the levels of soluble apoE2 and Aβ42 in hippocampal homogenates were significantly and inversely correlated in PDAPP/TRE2 mice only (Fig. 8).
Since several studies have reported an effect of apoE on APP processing, we measured the levels of APP in hippocampal and cortical homogenates from PDAPP/TRE mice (Irizarry et al., 2004; Ye et al., 2005). There was a small but significant decrease in APP protein levels in hippocampal homogenates from PDAPP/TRE2 mice, whereas the levels of cortical APP were equivalent (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Additional studies will be required to elucidate the exact mechanisms, whereby apoE might influence APP holoprotein levels in the hippocampus; however, a recent report suggests that γ-secretase-dependent cleavage of APP plays a central role in regulating cholesterol and apoE via lipoprotein receptor-related protein (Liu et al., 2007).
Since apoE mRNA levels are equivalent in PDAPP/TRE and TRE mice, post-translational mechanisms must be responsible for the differences in protein levels that we measured. Although apoE undergoes biochemical modifications, these changes do not appear to affect apoE's interaction with its receptors; however, these modifications may influence the association of apoE for particular HDL's, especially in brain parenchyma (Marmillot et al., 1999). Previously, several reports have characterized lipoproteins in CSF and apoE-containing particles that are secreted from immortalized cells in culture that express human apoE (LaDu et al., 1998; DeMattos et al., 2001; Koch et al., 2001). Although no apoE isoform-specific differences were found, critical differences in the ability of these particles to form a complex with Aβ under native conditions were reported (Morikawa et al., 2005).
Although little is known about the characteristics of apoE–HDL particles in brain, the lipidation status of apoE appears to be critical for the removal of brain Aβ (Wahrle et al., 2004, 2005; Hirsch-Reinshagen et al., 2005; Jiang et al., 2008). When ABCA1, a transmembrane transporter critical for lipidating apoE, is deleted, poorly lipidated brain apoE results in an increase in brain Aβ burden in PDAPP transgenic mice (Wahrle et al., 2005). Conversely, when ABCA1 is overexpressed, apoE appears to be maximally lipidated, and brain Aβ burden is significantly reduced (Wahrle et al., 2008). Unlike apoE–HDL particles isolated from CSF, the exact composition of brain “HDL-like” particles is unknown, and as Aβ is cleared from brain parenchyma via the periarterial instersitial drainage pathway, the protein composition of these particles is likely to change (Weller et al., 1998; Deane et al., 2008). Just as the presence of apoE is required for the formation of fibrillar amyloid, the lipidation status of apoE is also critical for amyloid formation, since poorly lipidated apoE results in more thioflavine S Aβ deposits (Bales et al., 1997; Wahrle et al., 2004). In PDAPP transgenic mice, the neuroanatomical distribution of brain Aβ is determined by ΑPOE genotype and Apoe deficiency, as well as apoE lipidation status, since mice expressing human apoE2, deficient in apoE or ABCA1, display an identical phenotype with no Aβ deposits evident in the molecular layer of the hippocampus (Fig. 6) (Bales et al., 1999; Fagan et al., 2002; Wahrle et al., 2004).
Although little is known about the cellular trafficking and metabolism of apoE in brain, several important observations about apoE secretion and internalization from macrophages are worth mentioning. Nearly one-third of the apoE that is secreted by macrophages is internalized and then re-released (Hasty et al., 2005). Similarly, apoE bound to cell surface proteoglycans may represent an important and “accessible” pool of apoE, suggesting that an important attribute of apoE in the periphery, and perhaps also a critical requirement for CNS-derived apoE, is to maintain a basal as well as a “readily accessible” pool of apoE (Rees et al., 1999). Similarly, newly synthesized apoE4, but not apoE3, is rapidly degraded intracellularly, thus minimizing potential “reserve” pools available for secretion (Riddell et al., 2008).
Regardless of the exact pathophysiological mechanisms by which the ε4 allele confers AD susceptibility, our results recapitulate in a transgenic mouse model an isoform-dependent increase in Aβ/amyloid burden that appears to be tightly coupled to soluble apoE levels. Our results presented here suggest that the most physiologically relevant pool of apoE is represented by the soluble fraction and reductions in this pool, which appear to occur post-translationally, may compromise normal Aβ clearance. This compromised apoE-dependent clearance capacity for brain-derived Aβ results in a very early and persistent increase in soluble Aβ42, as well as age-dependent increases in brain Aβ/amyloid burden. Brain penetrable compounds that can elevate apoE levels (regardless of isoform) may, therefore, provide novel therapeutic approaches for the treatment and prevention of late onset AD.
References
- Bales KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, Piccardo P, Ghetti B, Paul SM. Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nat Genet. 1997;17:263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- Bales KR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J, Fishman CE, DeLong CA, Piccardo P, Petegnief V, Ghetti B, Paul SM. Apolipoprotein E is essential for amyloid deposition in the APP V717F transgenic mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 1999;96:15233–15238. doi: 10.1073/pnas.96.26.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffert U, Cohn JS, Petit-Turcotte C, Tremblay M, Aumont N, Ramassamy C, Davignon J, Poirier J. Apolipoprotein E and beta-amyloid levels in the hippocampus and frontal cortex of Alzheimer's disease subjects are disease-related and apolipoprotein E genotype dependent. Brain Res. 1999;843:87–94. doi: 10.1016/s0006-8993(99)01894-6. [DOI] [PubMed] [Google Scholar]
- Boschert U, Merlo-Pich E, Higgins G, Roses AD, Catsicas S. Apolipoprotein E expression by neurons surviving excitotoxic stress. Neurobiol Dis. 1999;6:508–514. doi: 10.1006/nbdi.1999.0251. [DOI] [PubMed] [Google Scholar]
- Boyles JK, Pitas RE, Wilson E, Mahley RW, Taylor JM. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmylinating glia of the peripheral nervous system. J Clin Invest. 1985;76:1501–1513. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt TD, Agan BK, Marconi VC, He W, Kulkarni H, Mold JE, Cavrois M, Huang Y, Mahley RW, Dolan MJ, McCune JM, Ahuja SK. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE ε4/ε4 genotype accelerates HIV disease progression. Proc Natl Acad Sci U S A. 2008;105:8718–8723. doi: 10.1073/pnas.0803526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, Rimmler JB, Locke PA, Conneally PM, Schmader KE. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. ApoE isoform-specific disruption of amyloid β peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF, Carothers A, Whalley LJ. Cognitive change and APOE ε4 allele. Nature. 2002;418:932. doi: 10.1038/418932a. [DOI] [PubMed] [Google Scholar]
- DeMattos RB. Apolipoprotein E dose-dependent modulation of β-amyloid deposition in a transgenic mouse model of Alzheimer's disease. J Mol Neurosci. 2004;23:255–262. doi: 10.1385/JMN:23:3:255. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Brendza RP, Heuser JE, Kierson M, Cirrito JR, Fryer J, Sullivan PM, Fagan AM, Han X, Holtzman DM. Purification and characterization of astrocyte-secreted apolipoprotein E and J-containing lipoproteins from wild-type and human apoE transgenic mice. Neurochem Int. 2001;39:415–425. doi: 10.1016/s0197-0186(01)00049-3. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Watson M, Parsadanian M, Bales KR, Paul SM, Holtzman DM. Human and murine ApoE markedly alters Aβ metabolism before and after plaque formation in a mouse model of Alzheimer's disease. Neurobiol Dis. 2002;9:305–318. doi: 10.1006/nbdi.2002.0483. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Fryer JD, Simmons K, Parsadanian M, Bales KR, Paul SM, Sullivan PM, Holtzman DM. Human apolipoprotein E4 alters the amyloid-β40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J Neurosci. 2005;25:2803–2810. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty AH, Plummer MR, Weisgraber KH, Linton MF, Fazio S, Swift LL. The recycling of apolipoprotein E in macrophages: influence of HDL and apolipoprotein A-I. J Lipid Res. 2005;46:1433–1439. doi: 10.1194/jlr.M400418-JLR200. [DOI] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, Maia LF, Burgess BL, Blain JF, Naus KE, McIsaac SA, Parkinson PF, Chan JY, Tansley GH, Hayden MR, Poirier J, Van Nostrand W, Wellington CL. The absence of ABCA1 decreases soluble ApoE levels but does not diminish amyloid deposition in two murine models of Alzheimer disease. J Biol Chem. 2005;280:43243–43256. doi: 10.1074/jbc.M508781200. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Fagan AM. Potential role of apoE in structural plasticity in the nervous system: implications for diseases of the central nervous system. Trends Cardiovasc Med. 1998;6:250–255. doi: 10.1016/s1050-1738(98)00017-6. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Wu S, Bhat P, Parsadanian M, Fagan AM, Chang LK, Sun Y, Paul SM. Expression of human apolipoprotein E reduces amyloid-β deposition in a mouse model of Alzheimer's disease. J Clin Invest. 1999;103:R15–R21. doi: 10.1172/JCI6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry MC, Deng A, Lleo A, Berezovska O, Von Arnim CA, Martin-Rehrmann M, Manelli A, LaDu MJ, Hyman BT, Rebeck GW. Apolipoprotein E modulates γ-secretase cleavage of the amyloid precursor protein. J Neurochem. 2004;90:1132–1143. doi: 10.1111/j.1471-4159.2004.02581.x. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Lee CY, Mandrekar S, Wilkinson B, Cramer P, Zelcer N, Mann K, Lamb B, Willson TM, Collins JL, Richardson JC, Smith JD, Comery TA, Riddell D, Holtzman DM, Tontonoz P, Landreth GE. ApoE promotes the proteolytic degradation of Aβ. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Wood K, Lee M, Motter R, Hu K, Gordon G, Barbour R, Khan K, Gordon M, Tan H, Games D, Lieberburg I, Schenk D, Seubert P, McConlogue L. Amyloid precursor protein processing and Abeta42 deposition in a transgenic mouse model of Alzheimer disease. Proc Natl Acad Sci U S A. 1997;94:1550–1555. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Donarski N, Goetze K, Kreckel M, Stuerenburg HJ, Buhmann C, Beisiegel U. Characterization of four lipoprotein classes in human cerebrospinal fluid. J Lipid Res. 2001;42:1143–1151. [PubMed] [Google Scholar]
- LaDu MJ, Gilligan SM, Lukens JR, Cabana VG, Reardon CA, Van Eldik LJ, Holtzman DM. Nascent astrocyte particles differ from lipoproteins in CSF. J Neurochem. 1998;70:2070–2081. doi: 10.1046/j.1471-4159.1998.70052070.x. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zerbinatti CV, Zhang J, Hoe HS, Wang B, Cole SL, Herz J, Muglia L, Bu G. Amyloid precursor protein upregulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron. 2007;56:66–78. doi: 10.1016/j.neuron.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Rall SC., Jr Apolipoprotien E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc Natl Acad Sci U S A. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmillot P, Rao MN, Liu QH, Lakshman MR. Desialylation of human apolipoprotein E decreases its binding to human high-density lipoprotein and its ability to deliver esterified cholesterol to the liver. Metabolism. 1999;48:1184–1192. doi: 10.1016/s0026-0495(99)90136-1. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Tschanz JT, Norton MC, Welsh-Bohmer KA, Steffens DC, Wyse BW, Breitner JC. APOE genotype predicts when-not whether-one is predisposed to develop Alzheimer disease. Nat Genet. 1998;19:321–322. doi: 10.1038/1206. [DOI] [PubMed] [Google Scholar]
- Miller RM, Federoff HJ. Isoform-specific effects of ApoE on HSV immediate early gene expression and establishment of latency. Neurobiol Aging. 2008;29:71–77. doi: 10.1016/j.neurobiolaging.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Morikawa M, Fryer JD, Sullivan PM, Christopher EA, Wahrle SE, DeMattos RB, O'Dell MA, Fagan AM, Lashuel HA, Walz T, Asai K, Holtzman DM. Production and characterization of astrocyte-derived human apolipoprotein E isoforms from immortalized astrocytes and their interactions with amyloid-β. Neurobiol Dis. 2005;19:66–76. doi: 10.1016/j.nbd.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Esiri MM, Jobst KA, Johnston C, Litchfield S, Sim E, Smith AD. Influence of the apolipoprotein E genotype on amyloid deposition and neurofibrillary tangle formation in Alzheimer's disease. Neuroscience. 1995;69:757–761. doi: 10.1016/0306-4522(95)00331-c. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Roberts GW, Graham DI. Amyloid β-protein, APOE genotype and head injury. Ann N Y Acad Sci. 1996;777:271–275. doi: 10.1111/j.1749-6632.1996.tb34431.x. [DOI] [PubMed] [Google Scholar]
- Poirier J. Apolipoprotein E represents a potent gene-based therapeutic target for the treatment of sporadic Alzheimer's disease. Alzheimer's and Dementia. 2008;4:S91–S98. doi: 10.1016/j.jalz.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Ramaswamy G, Xu Q, Huang Y, Weisgraber KH. Effect of domain interaction on Apolipoprotein E levels in mouse brain. J Neurosci. 2005;25:10658–10663. doi: 10.1523/JNEUROSCI.1922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D, Sloane T, Jessup W, Dean RT, Kritharides L. Apolipoprotein A-I stimulates secretion of apolipoprotein E by foam macrophages. J Biol Chem. 1999;274:27925–27933. doi: 10.1074/jbc.274.39.27925. [DOI] [PubMed] [Google Scholar]
- Riddell DR, Zhou H, Atchison K, Warwick HK, Atkinson PJ, Jefferson J, Xu L, Aschmies S, Kirksey Y, Hu Y, Wagner E, Parratt A, Xu J, Li Z, Zaleska MM, Jacobsen JS, Pangalos MN, Reinhart PH. Impact of apolipoprotein E (ApoE) polymorphism on brain apoE levels. J Neurosci. 2008;28:11445–11453. doi: 10.1523/JNEUROSCI.1972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston MC, Mann D, Pickering-Brown S, Owen F, Perry R, Raghavan R, Khin-Nu C, Tyrer S, Day K, Crook R. Apolipoprotein E ε2 allele promotes longevity and protects patients with Down's syndrome from dementia. Neuroreport. 1994;5:2583–2585. doi: 10.1097/00001756-199412000-00044. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ. Association of apolipoprotein allele ε4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD. Increased amyloid β-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slooter AJ, Tang MX, van Duijn CM, Stern Y, Ott A, Bell K, Breteler MM, Van Broeckhoven C, Tatemichi TK, Tycko B, Hofman A, Mayeux R. Apolipoprotein E ε4 and the risk of dementia with stroke: a population-based investigation. JAMA. 1997;277:818–821. doi: 10.1001/jama.277.10.818. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia. J Biol Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- Teasdale GM, Nicoll JA, Murray G, Fiddes M. Association of apolipoprotein E polymorphism with outcome after head injury. Lancet. 1997;350:1069–1071. doi: 10.1016/S0140-6736(97)04318-3. [DOI] [PubMed] [Google Scholar]
- Utermann G, Langenbeck U, Beisiegel U, Weber W. Genetics of the apolipoprotein E system in man. Am J Hum Genet. 1980;32:339–347. [PMC free article] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Legleiter J, Han X, Fryer JD, Kowalewski T, Holtzman DM. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J Biol Chem. 2004;279:40987–40993. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Hartman RE, Bales KR, Paul SM, Holtzman DM. Deletion of Abca1 increases Aβ deposition in the PDAPP transgenic mouse model of Alzheimer disease. J Biol Chem. 2005;280:43236–43242. doi: 10.1074/jbc.M508780200. [DOI] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Kim J, Li A, Knoten A, Jain S, Hirsch-Reinshagen V, Wellington CL, Bales KR, Paul SM, Holtzman DM. Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J Clin Invest. 2008;118:671–682. doi: 10.1172/JCI33622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, Pahnke J, Madauss M, Vogelgesang S, Pahnke A, Herbst EW, Stausske D, Walther R, Kessler C, Warzok RW. Apolipoprotein E4 promotes the early deposition of Aβ42 and then Aβ40 in the elderly. Acta Neuropathol. 2000;100:36–42. doi: 10.1007/s004010051190. [DOI] [PubMed] [Google Scholar]
- Weisgraber KH. Apolipoprotein E: structure-function relationships. Adv Protein Chem. 1994;45:249–302. doi: 10.1016/s0065-3233(08)60642-7. [DOI] [PubMed] [Google Scholar]
- Weller RO, Massey A, Newman TA, Hutchings M, Kuo YM, Roher AE. Cerebral amyloid angiopathy: amyloid beta accumulation in putative interstitial fluid drainage pathways in Alzheimer's disease. Am J Pathol. 1998;153:725–733. doi: 10.1016/s0002-9440(10)65616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Huang Y, Müllendorff K, Dong L, Giedt G, Meng EC, Cohen FE, Kuntz ID, Weisgraber KH, Mahley RW. Apolipoprotein (apo) E4 enhances amyloid β peptide production in cultured neuronal cells: ApoE structure as a potential therapeutic target. Proc Natl Acad Sci U S A. 2005;102:18700–18705. doi: 10.1073/pnas.0508693102. [DOI] [PMC free article] [PubMed] [Google Scholar]