Abstract
Repeated pairs of timed presynaptic and postsynaptic potentials cause lasting changes in efficacy of transmission at many synapses. The corticospinal tract is the major pathway controlling voluntary movement in humans, and corticospinal neurons have monosynaptic connections to motoneurons of many muscles. We hypothesized that corticospinal transmission in humans could be altered by delivering, to the corticospinal–motoneuronal synapses, timed pairs of presynaptic volleys (produced by cortical stimulation) and antidromic postsynaptic volleys (by peripheral nerve stimulation). To test corticospinal transmission, electrical cervicomedullary stimuli evoked motor responses [cervicomedullary motor-evoked potentials (CMEPs)] in biceps brachii before and for 1 h after conditioning with 50 paired cortical and peripheral nerve stimuli. Seven interstimulus intervals (ISIs) of conditioning stimulus pairs were tested on different days. With one ISI (+3 ms; cortical before peripheral nerve stimulation), CMEPs were significantly increased in size by 33 ± 30% (mean ± SD; n = 7) from 4 until 32 min after conditioning. With two other ISIs (−13 ms, +22 ms), CMEPs were decreased from ∼30 until 60 min after conditioning (by 25 ± 23% and 27 ± 32%; n = 8). The remaining ISIs produced no changes. In a second study, subjects performed weak bilateral voluntary elbow flexion contractions before and after conditioning of the right elbow flexors. Conditioning ISIs that increased or decreased CMEPs similarly increased or decreased voluntary force and EMG on the right. Thus, depending on their timing, repeated paired stimuli can potentiate or depress corticospinal transmission, and these changes are functionally relevant. We suggest that bidirectional spike-timing-dependent plasticity can be induced at corticospinal–motoneuronal synapses and can influence voluntary motor output.
Introduction
Many synapses in the CNS show lasting activity-dependent changes in efficacy of transmission. Both increases and decreases in synaptic strength can be brought about by repeated paired presynaptic and postsynaptic action potentials that are precisely timed with respect to each other (Levy and Steward, 1983; Markram et al., 1997; Bi and Poo, 1998, 2001; Dan and Poo, 2004). These changes are known as spike-timing-dependent plasticity (STDP) and can last for tens of minutes to hours. Depolarization of the postsynaptic membrane by backpropagation of the somatic action potential into the dendrites may be an important mechanism for feedback to synapses on the coincidence of the presynaptic and postsynaptic events and thus be important for STDP (Markram et al., 1997; Debanne et al., 1998; Stuart and Häusser, 2001; Sjüstrüm et al., 2008). If antidromic stimulation of a postsynaptic cell is paired with timed orthodromic stimulation of a presynaptic cell, bidirectional STDP can be induced (Markram et al., 1997; Bi and Poo, 1998). The majority of STDP studies have been performed in slices or culture, but in vivo studies include demonstration of changes in the human motor and somatosensory cortex induced by timed paired stimuli (Wolters et al., 2003, 2005).
Little is known about the potential for synaptic plasticity in the spinal cord in the descending pathways to the motoneurons. For limb muscles, plastic changes have been postulated in association with exercise and motor skill training (Carroll et al., 2002; Adkins et al., 2006), but acutely induced changes have not been investigated. In humans, the corticospinal tract is the major descending pathway for the control of voluntary movement (Lemon et al., 2004). Corticospinal neurons have monosynaptic inputs to motoneurons innervating many muscles (Palmer and Ashby, 1992; de Noordhout et al., 1999) and provide an opportunity for in vivo pairing of presynaptic input with antidromic postsynaptic action potentials. Corticospinal neurons can be activated at a cortical level by transcranial magnetic stimulation (TMS), whereas antidromic action potentials can be elicited in the motoneurons by peripheral nerve stimulation. Backpropagation of action potentials into the dendrites of motoneurons of rats is similar to that in neocortical and hippocampal pyramidal neurons (Larkum et al., 1996; Stuart et al., 1997), which suggests that antidromic activation of axons of motoneurons should produce postsynaptic depolarization in the dendrites.
Here, we aimed to alter corticospinal–motoneuronal synapses by delivering repeated paired transcranial magnetic stimuli and peripheral nerve stimuli at different interstimulus intervals (ISIs) to human subjects. We tested the efficacy of transmission through the corticospinal pathway with electrical transmastoid (cervicomedullary) stimulation, which activates corticospinal axons at a subcortical level (Ugawa et al., 1991; Taylor et al., 2002). At particular ISIs, we found persistent increases or decreases in responses to corticospinal stimulation. Furthermore, when subjects made voluntary contractions after repeated paired stimuli at appropriate ISIs, their EMG and force output was increased or decreased. Thus, a protocol designed to produce STDP at the corticospinal–motoneuronal synapse can produce increases and decreases in corticospinal transmission that alter normal physiological use of the pathway.
Materials and Methods
Subjects
Fifteen healthy subjects (five females; 27 ± 10 years, mean age ± SD) participated in two sets of studies. The first set of studies had seven conditions that were tested on different days. Initially, four of these conditions were tested on the same eight subjects. An additional three conditions were later added and tested on seven subjects. Five subjects completed all seven conditions. The second set of studies had three conditions that were tested on separate days. The same 10 subjects completed each part of this second study. All subjects gave their written informed consent to participate. The procedures were approved by the ethics committee of the University of New South Wales, and the study was conducted according to the Declaration of Helsinki.
Experimental setup
For study 1, subjects were seated with their arms relaxed and placed on a pillow across their lap. EMG was recorded from right biceps brachii through surface electrodes secured to the skin over the belly and tendon of the muscle (Ag–AgCl, 10 mm diameter). For study 2, subjects sat with both arms strapped in isometric myographs with the forearms upright and the elbows and shoulders flexed to 90°. Elbow flexion forces could be measured and stored on a computer. EMG was recorded from biceps brachii and brachioradialis in both arms by surface electrodes secured to the skin over each muscle. The signals were amplified, filtered (16–1000 Hz), and collected at 2 kHz for off-line analysis using customized software (CED 1401 with Signal software; Cambridge Electronic Design).
Conditioning stimulation
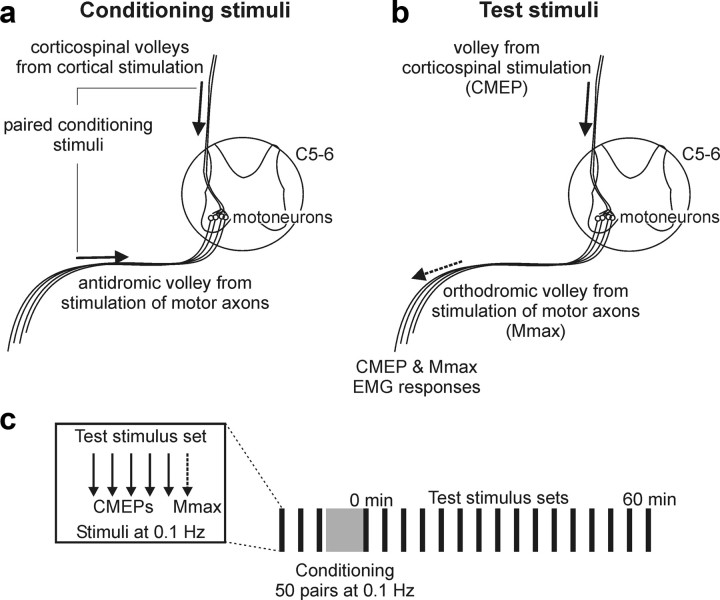
Conditioning involved 50 pairs of stimuli delivered at 0.1 Hz. Pairs comprised transcranial magnetic stimuli and peripheral nerve stimuli delivered at different ISIs. TMS activated corticospinal neurons and produced presynaptic action potentials at the corticospinal–motoneuronal synapses of biceps, whereas antidromic activation of the peripheral nerve produced depolarization of the postsynaptic membrane in biceps motoneurons (Fig. 1a).
Figure 1.
Conditioning stimuli used to alter transmission in the corticospinal pathway, stimuli used to test for these changes, and the study protocol. a, Corticospinal–motoneuronal synapses were conditioned with pairs of stimuli delivered to the motor cortex with TMS and to the peripheral nerve at the brachial plexus. TMS evoked orthodromic volleys in corticospinal neurons and provided presynaptic action potentials at the synapses, whereas brachial plexus stimulation caused antidromic activation of motoneurons (see arrows) to depolarize the postsynaptic membrane. Conditioning stimuli were delivered in pairs with different ISIs on different days. b, Changes in transmission at the corticospinal–motoneuronal synapses were tested with stimulation of corticospinal axons at the cervicomedullary junction. The single descending volley (solid arrow) activated motoneurons, and a response (CMEP) was recorded in biceps brachii EMG. The maximal M-wave (Mmax) evoked by stimulation of motor axons at the brachial plexus (dashed arrow) was also recorded in biceps brachii EMG to monitor any changes in the muscle fiber action potential. c, Protocol for study 1. Test stimulus sets were delivered each 4 min before and after conditioning with 50 paired stimuli over 8 min. Each test stimulus set consisted of five stimuli to the corticospinal axons to evoke CMEPs and a single stimulus delivered over the brachial plexus to evoke Mmax (shown in box).
Peripheral nerve stimulation.
Single electrical stimuli were delivered to the peripheral nerves innervating right elbow flexor muscles with a cathode in the supraclavicular fossa and an anode on the acromion (100 μs; DS7AH constant-current stimulator; Digitimer). To provide an index of the size of the antidromic activation of biceps motoneurons, EMG responses were recorded from biceps of the right arm (Fig. 2a). The stimulus intensity (mean stimulus intensity ± SD: study 1, 75 ± 23 mA; study 2, 82 ± 27 mA) was set to evoke a maximal compound muscle action potential (Mmax; typical response shown in Fig. 2b).
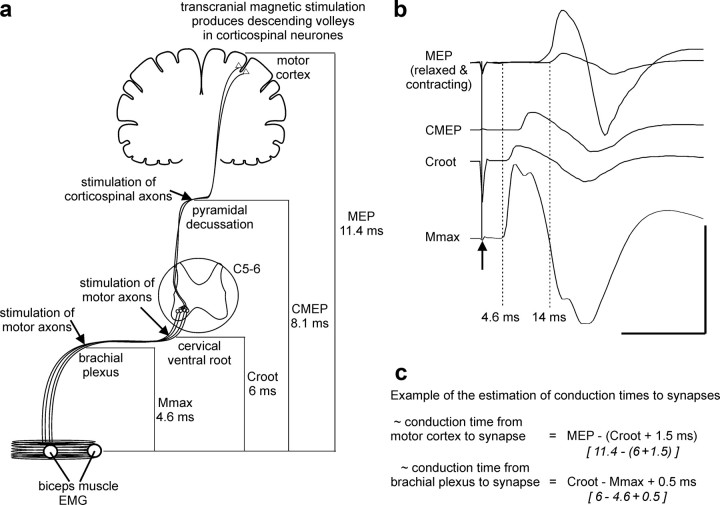
Figure 2.
Sites of stimulation, typical evoked motor responses, and calculation of central delays. a, Stimulation at various points in the motor pathway resulted in motor responses that were measured in the EMG of biceps brachii muscle. The relative latencies of these responses were used for calculation of conduction times to the corticospinal–motoneuronal synapses at the biceps brachii motoneuron pool, which is at the C5–C6 cervical spinal level. Typical latencies for each response are given. TMS was delivered to the motor cortex during a voluntary contraction. TMS evokes descending volleys in corticospinal axons and results in activation of motoneurons and a MEP in the muscle. Stimulation of corticospinal axons at the pyramidal decussation (or cervicomedullary junction) results in a CMEP. Motor axons were stimulated at the cervical root (CRoot) and at the brachial plexus to evoke a maximal compound muscle action potential (Mmax). b, Data for a single subject showing typical sizes and latencies of EMG responses in biceps to each type of stimulation. MEPs recorded from biceps brachii at rest and during a voluntary contraction are overlaid. The arrow and solid line mark the stimulation. Dotted lines indicate the onset of Mmax and the MEP in resting muscle. Calibration: horizontal, 20 ms; vertical, 8 mV (Mmax) and 4 mV (all other potentials). c, Example calculation for one subject of conduction time for volleys from the cortex to the corticospinal–motoneuronal synapse and for antidromic volleys from the brachial plexus to the synapse.
TMS.
A circular coil (13.5 cm outside diameter) was positioned over the vertex and oriented to preferentially activate the left motor cortex (Magstim 200; Magstim). To provide an index of the size of corticospinal volleys reaching the corticospinal–motoneuronal synapses, EMG responses were recorded from biceps of the right arm (Fig. 2a). The stimulus intensity (mean ± SD; study 1, 66 ± 13% of stimulator output; study 2, 70 ± 14% of stimulator output) was set to elicit a small motor-evoked potential (MEP; typical response shown in Fig. 2b; mean area ± SD; study 1, 2.1 ± 1.4% Mmax; study 2, 4.4 ± 3.5% Mmax) and remained the same for each subject across all ISIs tested.
ISIs between TMS and peripheral nerve stimulation.
Seven different ISIs were used. Two ISIs, in which peripheral nerve stimulation preceded TMS by 13 and 3 ms, were designed for the antidromic volley in the motoneurons to reach the dendrites ∼15 and ∼5 ms before the initial volley evoked by TMS reached the presynaptic terminals of the corticospinal neurons. These ISIs are referred to throughout as the −13 and −3 ms ISIs. At five other ISIs, in which TMS preceded peripheral nerve stimulation (by 3, 6, 9, 12, and 22 ms), the initial TMS volley was timed to reach the synapse ∼1, 4, 7, 10, or 20 ms before the antidromic postsynaptic volley. These are referred to as the +3, +6, +9, +12, and +22 ms ISIs, respectively.
ISIs for the delivery of conditioning stimuli were estimated from the relative latencies of EMG responses to stimulation at different levels of the motor pathway in pilot subjects (Fig. 2). These responses included the MEP, Mmax, and a response evoked by magnetic stimulation of the cervical nerve roots (CRoot). The relative latencies of these responses were then used to establish the likely conduction time to the corticospinal–motoneuronal synapses of biceps for volleys evoked by the two conditioning stimuli. The conduction time to the presynaptic terminals of corticospinal neurons for the first volley evoked by TMS was calculated using the following equation (Fig. 2c): MEP − (CRoot + 1.5 ms), where 1.5 ms estimated the time of synaptic transmission plus conduction to the nerve root at the vertebral foramina. The MEP latency was established during a weak voluntary contraction of biceps. TMS evokes multiple descending volleys in corticospinal neurons, and the latency during contraction is likely to indicate the arrival of the initial descending volley at the motoneurons. The influence of subsequent volleys on this estimation of timing is considered in the Discussion. The conduction time to the dendrites for the antidromic volley in motoneurons produced by peripheral nerve stimulation was calculated using the following equation: CRoot − Mmax + 0.5 ms, where 0.5 ms estimated antidromic conduction time from the vertebral foramina to the dendrites. Differences in the estimates of time to the synapse were then used to establish seven different ISIs used for conditioning in all subjects.
MEPs during contraction, Mmax, and CRoot potentials were recorded for all subjects at each experiment (see Table 1 for mean latencies) and were subsequently used to calculate the estimated arrival of volleys at the synapse for individual subjects. Data in Figure 4 are plotted relative to these individual estimates, and the means are given in Table 2.
Table 1.
Response latencies and conduction times (mean ± SD; 10 subjects)
| Response latencies (ms) | |
| CMEP | 8.1 ± 0.40 |
| MEP (contracting) | 11.1 ± 0.50 |
| CRoot | 5.5 ± 0.56 |
| Mmax | 4.4 ± 0.39 |
| Calculated conduction time (ms) | |
| Cortex to synapse [MEP − (CRoot + 1.5)] | 4.1 ± 0.41 |
| Brachial plexus to synapse [CRoot − Mmax + 0.5] | 1.6 ± 0.44 |
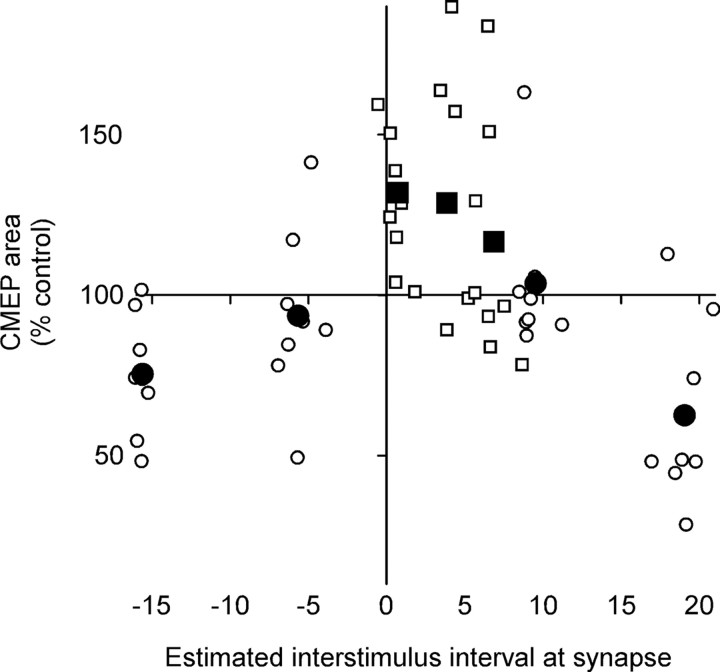
Figure 4.
Changes in muscle responses to corticospinal stimulation plotted against the estimated arrival of the conditioning volleys at the corticospinal–motoneuronal synapses of biceps. Each small point represents the average area of CMEPs after conditioning as a percentage of control in an individual subject (with the group mean for each ISI shown with larger symbols). Because depression and facilitation of the CMEPs followed different time courses, CMEPs were averaged from 32 to 60 min after conditioning for ISIs (−13, −3, +12, +22 ms; ○) collected in the initial series of experiments, but from 0 to 28 min after conditioning for ISIs (+3, +6, +9 ms; □) collected in the second series. The ISIs between TMS and peripheral nerve stimulation were designed so that the antidromic volley in the motoneurons produced by peripheral nerve stimulation (postsynaptic potential) would reach the dendrites before or after the initial descending volley evoked by TMS reached the presynaptic terminals of the corticospinal neurons. On the x-axis, each point is plotted as the difference in estimated time of arrival of the volleys at the synapses (presynaptic potential before postsynaptic potential shown as positive intervals).
Table 2.
Estimated difference in arrival time of presynaptic and postsynaptic volleys at the corticospinal–motoneuronal synapses calculated from evoked responses in individual subjects (mean ± SD; 8 subjects for ISIs of −13, −3, +12, and +22 ms; 7 subjects for other intervals)
| ISI | Planned interval at synapse | Calculated interval at synapse (ms) |
|---|---|---|
| −13 ms | −15 ms | −15.7 ± 0.28 |
| −3 ms | −5 ms | −5.6 ± 0.97 |
| +3 ms | 1 ms | 0.6 ± 0.78 |
| +6 ms | 4 ms | 4.2 ± 1.00 |
| +9 ms | 7 ms | 6.5 ± 1.16 |
| +12 ms | 10 ms | 9.3 ± 0.95 |
| +22 ms | 20 ms | 19.0 ± 1.22 |
Positive intervals indicate presynaptic volley before postsynaptic volley.
Test stimulation
The efficacy of the corticospinal pathway was tested before and after conditioning by stimulating descending corticospinal axons and recording motor responses from biceps muscles. Because there is a one-to-one correspondence between firing of a motoneuron and generation of action potentials in the muscle fibers that it innervates, muscle responses are a surrogate measure of motoneuron responses. There is graded recruitment of motoneurons of increasing size by increasing synaptic input (Clamann et al., 1974; Henneman et al., 1974). Furthermore, corticospinal synapses onto motoneurons lack classical presynaptic inhibition (Nielsen and Petersen, 1994; Jackson et al., 2006). Thus, changes in the size of EMG responses evoked through corticospinal stimulation and measured at the muscle mainly reflect changes at the corticospinal–motoneuronal synapses or the motoneurons (Fig. 1b). Changes in the muscle fiber action potential can also affect these responses but can be ruled out if the compound muscle action potential evoked by peripheral stimulation (Mmax; see above) is unchanged (Fig. 1b).
Corticospinal axons were stimulated by passing an electrical current (100 μs duration; Digitimer DS7AH) between electrodes placed over the mastoids (1–2 cm posterior and superior to the tip of the mastoid processes) (Ugawa et al., 1991; Gandevia et al., 1999; Taylor and Gandevia, 2004). Activation occurs at the cervicomedullary junction and evokes large short-latency responses [cervicomedullary motor-evoked potential (CMEPs)] in arm muscles (Fig. 2a,b). The stimulus activates many of the same axons as motor cortical stimulation because the single volley evoked by transmastoid stimulation can occlude a large part of the response to cortical stimulation when the ISI is appropriate (Taylor et al., 2002). A significant proportion of the motoneuronal response to cervicomedullary stimulation is monosynaptic for biceps (Petersen et al., 2002). The latency of responses was monitored to ensure that high stimulation intensities did not activate the motor axons at or near the ventral roots. A jump in latency of ∼2 ms occurs when the site of stimulation spreads from descending tracts to the ventral roots (Taylor and Gandevia, 2004). The stimulus intensity (246 ± 35 mA, mean stimulus intensity ± SD) was set to evoke responses ∼5% Mmax (5.5 ± 2.1% Mmax, mean area ± SD).
Protocol
Study 1.
In the first study, changes in the efficacy of transmission through the corticospinal pathway produced by conditioning pairs of stimuli were tested by assessing muscle responses to stimulation of corticospinal axons. CMEPs were evoked at rest before and after a set of conditioning stimuli (Fig. 1c). Before conditioning, 15 CMEPs were collected in three sets of five (delivered at 0.1 Hz) with 4 min between sets. After conditioning, sets of CMEPs were collected each 4 min for 1 h. Mmax was recorded after each set of CMEPs to rule out any changes in the compound muscle action potential such as have been reported with activity and with prolonged rest (Cupido et al., 1996; Crone et al., 1999). Conditioning consisted of 50 pairs of peripheral nerve stimulation and TMS (delivered at 0.1 Hz) at different ISIs. Initially, four different ISIs (−13, −3, +12, and +22 ms) were investigated (n = 8). Because none of these ISIs produced facilitation of the CMEP, an additional three ISIs (+3, +6, and +9 ms) of the conditioning pairs were tested (n = 7). Each ISI was tested on a different day, separated by at least 3 d in individual subjects.
Study 2.
A second study investigated whether changes in corticospinal transmission observed with the CMEP after conditioning at some ISIs could also influence the voluntary motor output to perform muscle contractions. With their arms strapped into isometric myographs, subjects made weak bilateral contractions of the elbow flexors. On a light-emitting diode display, they received visual feedback of the force produced with the left arm but were not told whether this feedback derived from the left or right side (Petersen et al., 2003). This feedback allowed subjects to generate reliable voluntary output with the left arm throughout the experiment, whereas output of the right arm relied on subjects' internal calibration of drive to the two arms. Subjects (n = 10) were instructed to contract both arms simultaneously to reach a target (10% of maximal voluntary contraction) within 1 s after an auditory signal delivered at 5 s intervals. They were instructed not to correct contractions once initiated. These weak, brief efforts were designed to minimize fatigue and to minimize any correction of force based on afferent input. After practice, subjects performed three sets of 20 control contractions separated by 8 min intervals. Conditioning at one of three different ISIs was then delivered to the right arm. The conditioning ISIs used in study 2 were those that produced the most consistent changes in CMEPs in study 1 (−13, +3, and +22 ms). Three additional sets of contractions were then performed, separated by 8 min intervals. Analysis of CMEPs in study 1 indicated that changes in the efficacy of transmission in the corticospinal pathway were delayed after conditioning, and this delay was different for different ISIs. Hence, contractions did not commence immediately after conditioning but were delayed by 8 min (after conditioning at the +3 ms ISI) or 28 min (after the −13 or +22 ms ISI conditioning). Force and EMG activity in the left and right arms were compared before and after conditioning of the right arm.
Statistics
In study 1, the area of each CMEP was measured. For each subject, the mean size of the five CMEPs in each postconditioning set was calculated and normalized to the mean of the 15 CMEPs before conditioning. The area of Mmax was measured similarly and normalized to the mean of three controls for each subject. One-way repeated-measures ANOVAs and the Bonferroni test were then used to compare the size of potentials before and at different time points after conditioning. In study 2, the force produced in each arm was calculated over a 500 ms window coinciding with the plateau in force production. Biceps root mean square (RMS) EMG and brachioradialis RMS EMG were assessed for the same window. Ratios of the forces between the right and left arms and of biceps EMG and brachioradialis EMG produced in the right and left arms were then calculated for each subject. The mean ratios for each set of 20 contractions were calculated for each subject. Initial analyses using one-way repeated-measures ANOVAs revealed no differences in any of these variables between the three sets performed before conditioning (see Results). Hence, the postcontraction ratios were normalized to the mean ratios of the 60 control contractions for each subject. One-way repeated-measures ANOVAs were then used to compare these variables before and at different time points after conditioning. Statistical significance was set at p < 0.05. Group data are presented as mean ± SD in the text and mean ± SEM in the figures.
Results
Experiment 1: single synchronized descending volley
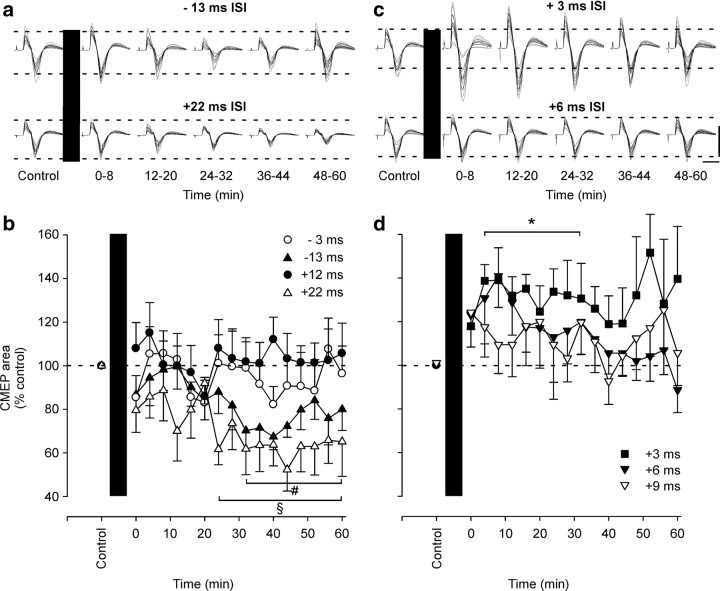
The size of CMEPs elicited in the biceps brachii muscle was altered by conditioning with pairs of TMS and peripheral nerve stimulation at some ISIs. Initially, four different ISIs were tested. After conditioning with stimulus pairs in which peripheral nerve stimulation was given 13 ms before TMS (−13 ms ISI), a significant decrease in the CMEP developed at 32 min after the end of conditioning and lasted for 60 min (one-way repeated-measures ANOVA and post hoc comparison to control; main effect of time: F(7,16) = 3.324; p < 0.001) (Fig. 3a,b, top traces and ▲). During this period, the CMEP was 75 ± 23% of its control area. Similarly, when TMS was given 22 ms before peripheral nerve stimulation (+22 ms ISI), CMEPs were significantly depressed to 63 ± 32% of control size from 24 min to the end of testing at 60 min after conditioning (main effect of time: F(7,16) = 2.350; p = 0.005) (Fig. 3a,b, bottom traces and △). CMEPs were not altered when TMS was given 3 ms after or 12 ms before peripheral nerve stimulation (−3 ms ISI: F(7,16) = 0.714, p = 0.775; +12 ms ISI: F(7,16) = 0.542, p = 0.919) (Fig. 3b). Because none of the four tested ISIs induced facilitation of the CMEP, an additional three ISIs of the conditioning pairs (+3, +6, and +9 ms ISIs) were tested. Conditioning with one of the pairs (TMS 3 ms before peripheral nerve stimulation, +3 ms ISI) produced consistent facilitation of the CMEP to 133 ± 30% of control area from 4 to 32 min after conditioning (+3 ms ISI: F(6,16) = 1.959; p = 0.047) (Fig. 3c,d, top traces and ■). No consistent changes were seen for the other two ISIs (+6 ms ISI: F(6,16) = 1.663, p = 0.067; +9 ms ISI: F(6,16) = 0.765, p = 0.720) (Fig. 3c,d). To exclude the possibility that changes in CMEPs resulted from activity-induced changes in the muscle fiber action potential, Mmax evoked in biceps was measured throughout all the protocols and showed no significant changes in area.
Figure 3.
Changes in the efficacy of transmission through the corticospinal pathway assessed by muscle responses to stimulation of corticospinal axons. EMG responses to stimulation of corticospinal axons (CMEP) for a single subject (a, c) and for the group (b, d) before and after 50 pairs of conditioning stimuli. Conditioning (black bar) comprised TMS and peripheral nerve stimulation delivered at different ISIs in different experiments. a, b, In the first series of experiments (n = 8), TMS was delivered 13 ms (−13 ms, top traces and ▲) and 3 ms (−3 ms, ○) after or 12 ms (+12 ms, •) and 22 ms (+22 ms, bottom traces and △) before peripheral nerve stimulation. CMEPs were significantly reduced by conditioning at +22 ms (§p = 0.005) or −13 ms (#p < 0.001). c, d, In the second series of experiments (n = 7), TMS was delivered 3 ms (+3 ms, top traces and ■), 6 ms (+6 ms, bottom traces and ▾), and 9 ms (+9 ms, ▿) before peripheral nerve stimulation. CMEPs were significantly facilitated by conditioning at +3 ms (*p = 0.047). In a and c, all CMEPs evoked before conditioning are overlaid, as are all CMEPs evoked over the indicated time periods after conditioning. Calibration: vertical, 1 mV; horizontal, 20 ms. In b and d, CMEPs in each postconditioning set were normalized to the preconditioned CMEPs. Error bars indicate SEM.
Data for all subjects are shown in Figure 4 to illustrate the pattern of changes in the CMEP after conditioning with stimulus pairs with different ISIs. Each small point represents the average area of CMEPs as a percentage of control in an individual subject (with the group mean for each ISI shown with larger symbols). However, because depression and facilitation of the CMEPs followed different time courses (Fig. 3b,d), CMEPs were averaged from 32 to 60 min after conditioning for ISIs (−13, −3, +12, +22 ms) collected in the initial series of experiments but from 0 to 28 min after conditioning for ISIs (+3, +6, +9 ms) collected in the second series. On the x-axis, each point is plotted as the difference in estimated time of arrival of the presynaptic and postsynaptic potentials at the dendrites of the motoneurons (presynaptic before postsynaptic shown as positive intervals).
Experiment 2: voluntary contraction
Subjects made brief bilateral elbow flexion contractions before and after conditioning with paired TMS and peripheral nerve stimulation of the right brachial plexus. Before conditioning, subjects made very similar contractions with the two arms (Fig. 5), and the ratio of forces between the right and left arms (mean, 0.93 ± 0.18) was consistent over three sets of contractions (one-way repeated-measures ANOVAs; main effect of set for −13 ms ISI: F(2,9) = 1.810, p = 0.192; +3 ms ISI: F(2,9) = 0.0184, p = 0.982; +22 ms ISI: F(2,9) = 2.339, p = 0.125). Elbow flexion force produced by the control left arm did not change after conditioning, nor did the EMG produced in the left biceps or brachioradialis.
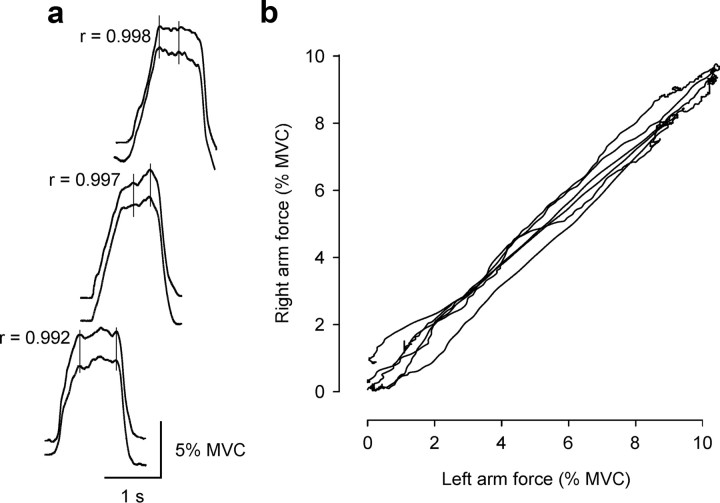
Figure 5.
Typical matching forces produced in consecutive “identical” contractions of the left and right sides in one subject before conditioning. a, Force record for the left side [10% maximal voluntary contraction (MVC)] as the top trace and for the right side as the bottom trace in each pair. Traces are offset for illustrative purposes. Pearson correlation coefficients are shown for each contraction pair. These correlations compare the absolute force produced in the left arm versus the right arm across all of the individual data points illustrated for each contraction pair. Vertical lines mark some force increments occurring bilaterally. Calibration: vertical, 5% MVC; horizontal, 1 s. b, Corresponding x–y plot of the forces produced on the two sides from the contractions shown in a.
After conditioning with paired stimuli that produced depression of CMEPs, force produced by the conditioned right arm was decreased relative to the control arm by 9 ± 12% (−13 ms ISI: F(3,9) = 4.616, p = 0.01) (Figs. 6a, 7, ▲) and 11 ± 14% (+22 ms ISI: F(3,9) = 5.409, p = 0.005) (Fig. 7, △). After conditioning with the +22 ms ISI, all three sets of contractions showed decreased force (post hoc comparisons vs control: 28 min, p = 0.023; 36 min, p = 0.031; 44 min, p = 0.002). After conditioning with the −13 ms ISI, force was not significantly decreased in the set of contractions at 28 min (p = 0.214) but was in the later sets at 36 and 44 min (p = 0.021 and p = 0.006). After conditioning with paired stimuli that produced facilitation of the CMEP, force produced by the right conditioned arm was increased by 15 ± 20% compared with the control left arm (main effect of time: F(3,9) = 3.651; p = 0.025). This increase was not significant for the set of contractions at 8 min (post hoc comparison versus control: p = 0.064) but was for sets at 16 and 24 min (p = 0.035 and p = 0.023) (Figs. 6b, 7, ■). Combined analysis of EMG recorded from two elbow flexor muscles, biceps and brachioradialis, showed changes in their muscle activity, consistent with the changes in force (two-way repeated-measures ANOVA time × muscle, main effect of time: −13 ms ISI: F(3,9) = 3.911, p = 0.019; +22 ms ISI: F(3,9) = 5.798, p = 0.003; +3 ms ISI: F(3,9) = 4.180, p = 0.015). Separate analysis of EMG from the two muscles showed more robust changes in biceps, with similar trends but more intersubject variability in brachioradialis (Fig. 7).
Figure 6.
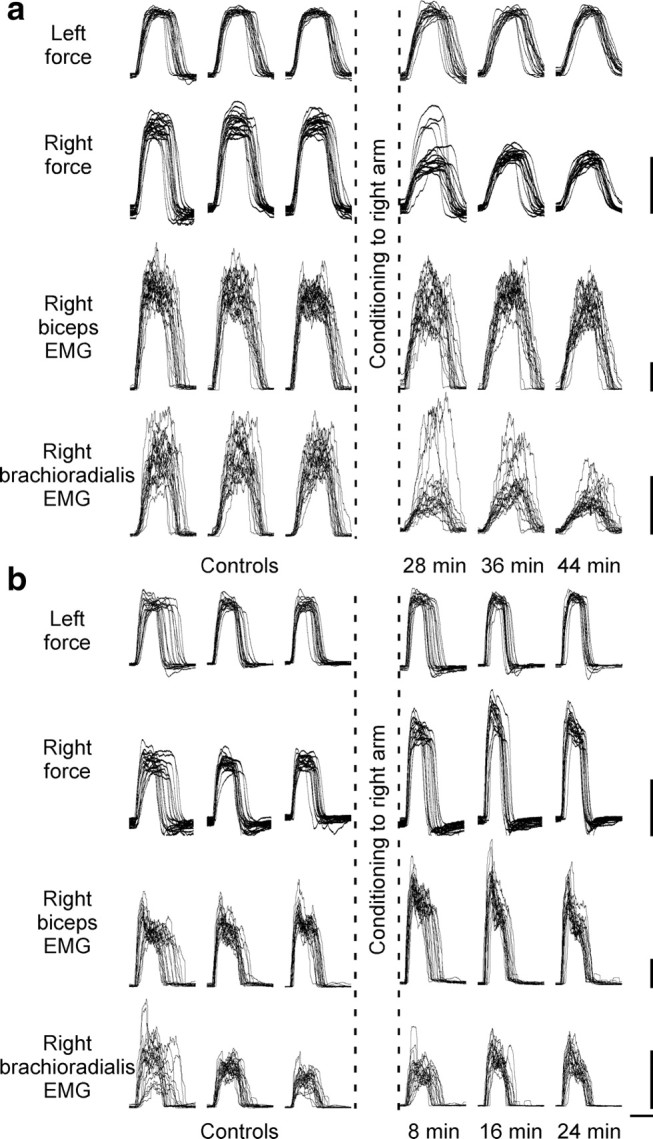
Changes in voluntary motor output produced by conditioning pairs of stimuli for a single subject. To demonstrate altered motor output after conditioning stimulation, subjects made weak bilateral contractions of the elbow flexors before and after 50 pairs of conditioning stimuli designed to alter corticospinal transmission to the right biceps. Subjects were provided with feedback of left-arm force. Three sets of contractions were performed before and after conditioning. The 20 contractions performed within each set are overlaid. In each panel, left-arm force (control arm), right-arm force, right biceps RMS EMG, and right brachioradialis RMS EMG are illustrated. For the ISIs chosen for conditioning, voluntary motor output was altered in a similar way to the responses to stimulation of the corticospinal axons. a, When peripheral nerve stimulation was delivered 13 ms before TMS (−13 ms ISI), there was a significant decrease in the force produced by the right arm. This change is most evident for contractions performed in the final two sets after conditioning. For this subject, biceps EMG remained relatively stable across all sets, whereas brachioradialis EMG was reduced after conditioning. b, When TMS was delivered 3 ms before peripheral nerve stimulation (+3 ms ISI), there was a significant increase in the force and biceps EMG of the right arm. Calibration: vertical, 4 Nm (force traces) and 0.05 mV (EMG traces); horizontal, 1 s.
Figure 7.
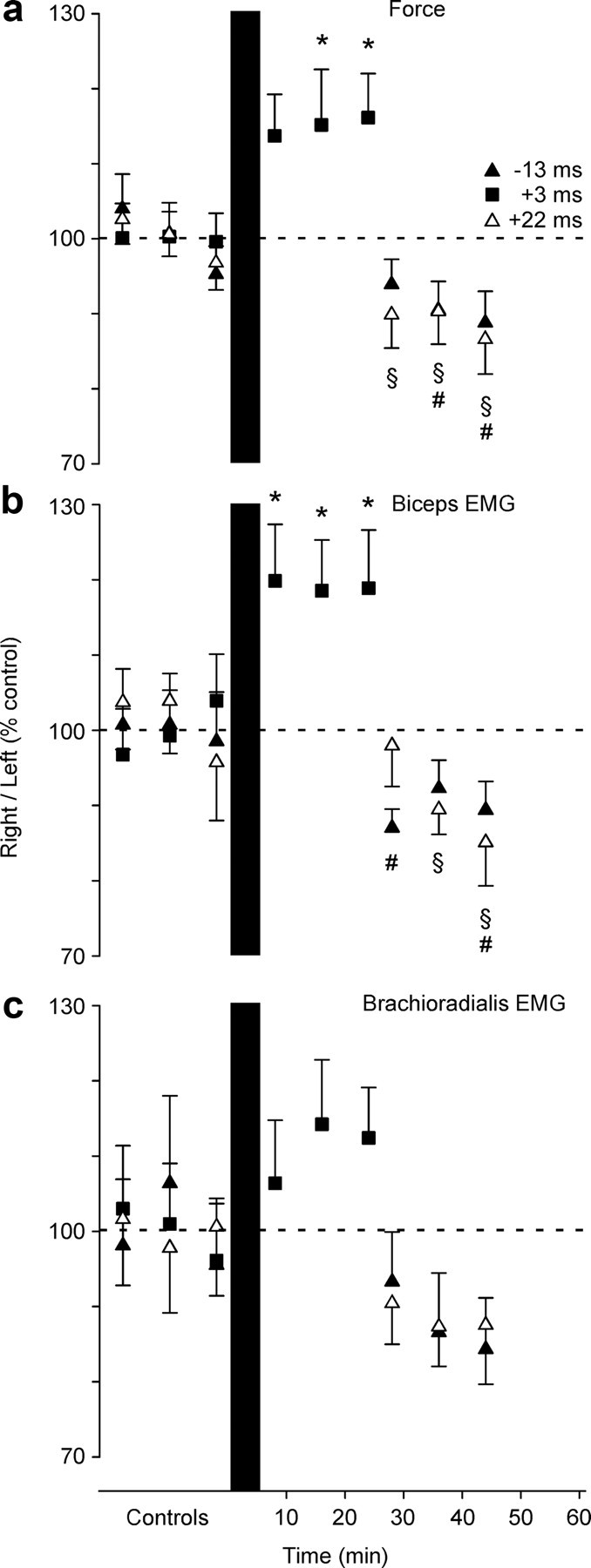
Changes in voluntary motor output produced by conditioning pairs of stimuli for the group of subjects. Grouped data (mean ± SEM; n = 10) are shown for the contractions before and after 50 pairs of conditioning stimuli in which TMS was delivered 3 ms (+3 ms; ■) and 22 ms (+22 ms; △) before or 13 ms (−13 ms; ▲) after peripheral nerve stimulation of the right brachial plexus. Force (a), biceps (b), and brachioradialis (c) EMG activity is plotted as a ratio between the right and left (control) side. The values were normalized to the mean for the 60 control contractions before the conditioning. a, Changes in the ratio of right-arm force to left-arm force. The force produced in the right arm compared with the left arm was depressed after −13 ms (p = 0.01) and +22 ms (p = 0.005) conditioning but was facilitated after +3 ms conditioning (p = 0.025). b, The ratio of right-arm biceps EMG to left-arm biceps EMG. c, The ratio of right-arm brachioradialis EMG to left-arm brachioradialis EMG. Changes in biceps and brachioradialis EMG mostly mirrored changes in force, but were more robust for biceps. * and ■, Different from controls; § and △, different from controls; # and ▲, different from controls (p < 0.05).
Discussion
Our findings show that 50 pairs of stimuli designed to produce STDP at the corticospinal–motoneuronal synapses of the biceps brachii motoneuron pool in humans can alter transmission both of a single synchronized descending volley and of normal physiological cortical output. Transmission can be either facilitated or depressed by conditioning with stimulus pairs with different ISIs. Conditioning that facilitates responses to a single descending volley also facilitates voluntary activity, and conditioning that depresses responses to a volley depresses voluntary activity. Thus, functionally relevant changes in corticospinal transmission that persist for at least 30 min can be induced acutely by controlled activity. This is the first evidence for acutely induced plasticity at a spinal level in the corticospinal pathway.
Because these studies are performed on intact human subjects, there can be argument about exactly what neurons are activated by the different stimuli, exactly what pathways are involved in transmission of responses, and the exact timing of interactions. Therefore, the precise mechanism(s) of the changes described here is uncertain. However, the findings clearly demonstrate plasticity at a spinal level, and we argue that the most plausible site for this plasticity is the corticospinal–motoneuronal synapses. Although the methods involved stimulation and recording remote to the synapses under investigation, we chose the corticospinal–motoneuronal synapse because it can be activated antidromically and orthodromically using electrophysiological techniques and can be tested relatively directly in awake human subjects. Cervicomedullary stimulation preferentially excites corticospinal axons at the level of the cervicomedullary junction with responses recorded in surface EMG (Ugawa et al., 1991; Taylor and Gandevia, 2004). The response from biceps motoneurons has a strong monosynaptic component (Petersen et al., 2002). Moreover, corticospinal terminals are not subject to afferent presynaptic inhibition (Nielsen and Petersen, 1994; Jackson et al., 2006). There is one-to-one correspondence between firing of a motoneuron and generation of action potentials in the muscle fibers that it innervates. Hence, muscle responses are a surrogate measure of the generation of action potentials in motoneurons. These factors limit the possible explanations for changes in the size of the CMEP. The most likely mechanisms are changes at the corticospinal–motoneuronal synapse or in the excitability of the motoneurons. Changes in the muscle fiber action potential can be excluded because the peripherally evoked Mmax was unchanged by conditioning.
Although the excitability of motoneurons can be altered through repetitive activation (Spielmann et al., 1993; Gandevia et al., 1999) or through ongoing changes in afferent input (Martin et al., 2008), neither of these mechanisms is likely to produce the differential effects observed here, as both afferents and motoneurons experienced the same total extra activity in each conditioning protocol. Furthermore, this extra activity was very little compared with normal motoneuron firing in voluntary contractions (>15 Hz) (Bellemare et al., 1983). This leaves synapses onto the motoneurons as the most likely site of the depression and facilitation of the CMEPs. However, in addition to strong monosynaptic inputs to the motoneurons, there is likely to be a nonmonosynaptic component to the CMEP, and so altered interneuronal transmission may also contribute to plasticity.
A number of features of the changes we observed are consistent with STDP as seen in simpler models. Millisecond changes in the relative timing of the paired TMS and peripheral nerve stimulation altered whether transmission of corticospinal input was increased, decreased, or unchanged (Bi and Poo, 2001; Dan and Poo, 2004). Significant changes in transmission occurred after only 50 pairs of stimuli presented at 10 s intervals (Debanne et al., 1998; Feldman, 2000; Sjöström et al., 2001). Changes developed over time after the end of conditioning and lasted for tens of minutes (Sjöström et al., 2001; Letzkus et al., 2006). In contrast, the direction of change at different interstimulus timings was not completely consistent with that most common for STDP, although the limited number of ISIs tested may not have revealed the full time course. Previous studies predict depression of transmission when postsynaptic potentials precede the presynaptic potentials by approximately ≤20 ms in conditioning pairs, and facilitation when the order is reversed (Bi and Poo, 1998; Feldman, 2000; Dan and Poo, 2004; Caporale and Dan, 2008). In the current study, responses were significantly facilitated when the presynaptic potential arrived ∼1 ms before the postsynaptic, with variable facilitation in individual subjects with timings out to ∼7 ms. Responses were depressed with one postsynaptic before presynaptic timing (∼15 ms), but not with a shorter ISI, and were also depressed with the presynaptic potential ∼20 ms before the postsynaptic. Different patterns of STDP have been described at different synapses (Nishiyama et al., 2000; Letzkus et al., 2006; Caporale and Dan, 2008; Sjöström et al., 2008), and a narrow window for potentiation may be beneficial in this pathway where output neurons mostly have no resting discharge and firing must be tightly controlled for successful motor performance. Furthermore, a recent study of STDP in neurons in the primary auditory cortex shows that the addition of conductances to mimic intact network behavior can shift the usual pattern of changes closer to that seen here (Delgado and Desai, 2008).
TMS as a conditioning stimulus may have promoted variability between subjects, particularly where it narrowly preceded the peripheral stimulus (+3, +6, and +9 ms ISIs). TMS produces multiple descending volleys over several milliseconds with some corticospinal neurons firing more than once (Edgley et al., 1990; Rothwell et al., 1991). Our timing was based on estimates of the arrival of the first descending volley at the motoneuron, but clearly timing of later volleys is also likely to be important. Some corticospinal synapses could be potentiated, but others depressed at a specific conditioning ISI. Thus, the overall change in transmission represented by a change in the size of the CMEP may well depend on the number and size of specific volleys in individual subjects.
Whereas the CMEP confined testing to the corticospinal pathway, the characteristics of the conditioning stimuli determined where changes might occur. TMS evokes volleys in corticospinal neurons and so has monosynaptic and nonmonosynaptic inputs to the motoneurons (Palmer and Ashby, 1992). The peripheral stimulus was designed to activate all motor axons innervating biceps brachii and thus result in backpropagating action potentials in the dendrites of the biceps motoneurons with the aim of influencing synapses onto these neurons. At the same time, it activated motor axons innervating many other muscles in the arm. In addition, it would also activate many large-diameter sensory axons from the arm including the muscle spindle Ia afferents, which have strong monosynaptic connections to motoneurons (Mendell and Henneman, 1968; Miller et al., 1995). These afferent inputs might contribute to the observed changes in the CMEP by adding to the timed depolarization of motoneurons, or might alter other synapses or neurons in the corticospinal pathway. The afferent terminals onto motoneurons or interneurons might themselves also undergo changes. However, any effect at synapses onto the motoneurons (from afferents or interneurons conveying signals from afferents) should be constant across the protocols because the peripheral nerve stimulus provided large time-locked presynaptic and postsynaptic volleys.
Changes in voluntary activity were demonstrated by asking subjects to make simultaneous brief bilateral efforts. In such contractions, the strongly correlated force profiles produced by the two arms suggest common drive. After conditioning with paired stimuli, the common drive was transmitted differently to the two arms. Increases and decreases in motor output in the conditioned arm were consistent with facilitation and depression of CMEPs by the same conditioning. Changes in elbow flexion force were matched by underlying changes in EMG, and this represents altered activation of motoneurons by descending voluntary drive. Changes were more robust in biceps than brachioradialis. As both muscles are innervated from the C5–C6 segmental level, the effective ISIs for the conditioning pairs should be the same. However, stimuli were optimized for biceps. In addition, the wrist and finger extensors can contribute to surface EMG recorded over brachioradialis without altering elbow flexion force.
While the size of CMEPs is only influenced by subcortical mechanisms, voluntary activity could also be influenced by cortical events, and repeated paired TMS and afferent stimulation can alter excitability of the motor cortex (Stefan et al., 2000; Wolters et al., 2003). Our stimulus pairs may have altered cortical excitability, but their timing is such that our facilitatory conditioning (+3 ms) should result in depression in the cortex (sensory input arrives at the cortex ∼9 ms after TMS), and the −13 ms ISI protocol, which shows depression, should result in cortical facilitation (Wolters et al., 2003). Thus, it is most probable that the changes in voluntary output are produced through the same subcortical mechanisms as the changes in CMEPs. We suggest that altered efficacy at corticospinal–motoneuronal synapses alters the synaptic current provided to the motoneurons by descending drive. More motoneurons will be recruited and/or will fire faster if synapses are potentiated and the reverse if synapses are depressed. As the function of motoneurons is well understood and changes in motoneuron firing are easily measured, it is possible to see clear behavioral consequences of induced plasticity in the corticospinal pathway.
In addition to providing a unique model for investigation of the functional consequences of synaptic plasticity, our findings suggest an enhanced role for the spinal cord in motor learning. Furthermore, induction of persistent changes in the efficacy of corticospinal input to the motoneurons may also prove useful clinically. In particular, if longer-duration conditioning or repeated applications lead to more enduring facilitation (Debanne et al., 1998; Dan and Poo, 2004), STDP may provide a mechanism for strengthening residual descending connections to enhance muscle function after stroke or incomplete spinal cord injury.
Footnotes
This work was supported by the National Health and Medical Research Council of Australia. We are grateful to Drs. James Brock and Jane Butler for comments on this manuscript.
References
- Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol. 2006;101:1776–1782. doi: 10.1152/japplphysiol.00515.2006. [DOI] [PubMed] [Google Scholar]
- Bellemare F, Woods JJ, Johansson R, Bigland-Ritchie B. Motor-unit discharge rates in maximal voluntary contractions of three human muscles. J Neurophysiol. 1983;50:1380–1392. doi: 10.1152/jn.1983.50.6.1380. [DOI] [PubMed] [Google Scholar]
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Riek S, Carson RG. The sites of neural adaptation induced by resistance training in humans. J Physiol. 2002;544:641–652. doi: 10.1113/jphysiol.2002.024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamann HP, Gillies JD, Skinner RD, Henneman E. Quantitative measures of output of a motoneuron pool during monosynaptic reflexes. J Neurophysiol. 1974;37:1328–1337. doi: 10.1152/jn.1974.37.6.1328. [DOI] [PubMed] [Google Scholar]
- Crone C, Johnsen LL, Hultborn H, Orsnes GB. Amplitude of the maximum motor response (Mmax) in human muscles typically decreases during the course of an experiment. Exp Brain Res. 1999;124:265–270. doi: 10.1007/s002210050621. [DOI] [PubMed] [Google Scholar]
- Cupido CM, Galea V, McComas AJ. Potentiation and depression of the M wave in human biceps brachii. J Physiol. 1996;491:541–550. doi: 10.1113/jphysiol.1996.sp021238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Debanne D, Gahwiler BH, Thompson SM. Long-term synaptic plasticity between pairs of individual CA3 pyramidal cells in rat hippocampal slice cultures. J Physiol. 1998;507:237–247. doi: 10.1111/j.1469-7793.1998.237bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado JY, Desai NS. In vivo-like conductances limit spike-timing dependent plasticity. Soc Neurosci Abstr. 2008;34:40–43. [Google Scholar]
- de Noordhout AM, Rapisarda G, Bogacz D, Gerard P, De Pasqua V, Pennisi G, Delwaide PJ. Corticomotoneuronal synaptic connections in normal man: an electrophysiological study. Brain. 1999;122:1327–1340. doi: 10.1093/brain/122.7.1327. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Eyre JA, Lemon RN, Miller S. Excitation of the corticospinal tract by electromagnetic and electrical stimulation of the scalp in the macaque monkey. J Physiol. 1990;425:301–320. doi: 10.1113/jphysiol.1990.sp018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. Timing-based LTP and LTD at vertical inputs to layer II/III pyramidal cells in rat barrel cortex. Neuron. 2000;27:45–56. doi: 10.1016/s0896-6273(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Petersen N, Butler JE, Taylor JL. Impaired response of human motoneurones to corticospinal stimulation after voluntary exercise. J Physiol. 1999;521:749–759. doi: 10.1111/j.1469-7793.1999.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E, Clamann HP, Gillies JD, Skinner RD. Rank order of motoneurons within a pool: law of combination. J. Neurophysiol. 1974;37:1338–1349. doi: 10.1152/jn.1974.37.6.1338. [DOI] [PubMed] [Google Scholar]
- Jackson A, Baker SN, Fetz EE. Tests for presynaptic modulation of corticospinal terminals from peripheral afferents and pyramidal tract in the macaque. J Physiol. 2006;573:107–120. doi: 10.1113/jphysiol.2005.100537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Rioult MG, L̈uscher HR. Propagation of action potentials in the dendrites of neurons from rat spinal cord slice cultures. J Neurophysiol. 1996;75:154–170. doi: 10.1152/jn.1996.75.1.154. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Kirkwood PA, Maier MA, Nakajima K, Nathan P. Direct and indirect pathways for corticospinal control of upper limb motoneurons in the primate. Prog Brain Res. 2004;143:263–279. doi: 10.1016/S0079-6123(03)43026-4. [DOI] [PubMed] [Google Scholar]
- Letzkus JJ, Kampa BM, Stuart GJ. Learning rules for spike timing-dependent plasticity depend on dendritic synapse location. J Neurosci. 2006;26:10420–10429. doi: 10.1523/JNEUROSCI.2650-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy WB, Steward O. Temporal contiguity requirements for long-term associative potentiation/depression in the hippocampus. Neuroscience. 1983;8:791–797. doi: 10.1016/0306-4522(83)90010-6. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Martin PG, Weerakkody N, Gandevia SC, Taylor JL. Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J Physiol. 2008;586:1277–1289. doi: 10.1113/jphysiol.2007.140426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell LM, Henneman E. Terminals of single Ia fibers: distribution within a pool of 300 homonymous motor neurons. Science. 1968;160:96–98. doi: 10.1126/science.160.3823.96. [DOI] [PubMed] [Google Scholar]
- Miller TA, Mogyoros I, Burke D. Homonymous and heteronymous monosynaptic reflexes in biceps brachii. Muscle Nerve. 1995;18:585–592. doi: 10.1002/mus.880180604. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Petersen N. Is presynaptic inhibition distributed to corticospinal fibres in man? J Physiol. 1994;477:47–58. doi: 10.1113/jphysiol.1994.sp020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama M, Hong K, Mikoshiba K, Poo MM, Kato K. Calcium stores regulate the polarity and input specificity of synaptic modification. Nature. 2000;408:584–588. doi: 10.1038/35046067. [DOI] [PubMed] [Google Scholar]
- Palmer E, Ashby P. Corticospinal projections to upper limb motoneurones in humans. J Physiol. 1992;448:397–412. doi: 10.1113/jphysiol.1992.sp019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen NT, Taylor JL, Gandevia SC. The effect of electrical stimulation of the corticospinal tract on motor units of the human biceps brachii. J Physiol. 2002;544:277–284. doi: 10.1113/jphysiol.2002.024539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen NT, Taylor JL, Butler JE, Gandevia SC. Depression of activity in the corticospinal pathway during human motor behavior after strong voluntary contractions. J Neurosci. 2003;23:7974–7980. doi: 10.1523/JNEUROSCI.23-22-07974.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. Stimulation of the human motor cortex through the scalp. Exp Physiol. 1991;76:159–200. doi: 10.1113/expphysiol.1991.sp003485. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32:1149–1164. doi: 10.1016/s0896-6273(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Rancz EA, Roth A, Hausser M. Dendritic excitability and synaptic plasticity. Physiol Rev. 2008;88:769–840. doi: 10.1152/physrev.00016.2007. [DOI] [PubMed] [Google Scholar]
- Spielmann JM, Laouris Y, Nordstrom MA, Robinson GA, Reinking RM, Stuart DG. Adaptation of cat motoneurons to sustained and intermittent extracellular activation. J Physiol. 1993;464:75–120. doi: 10.1113/jphysiol.1993.sp019625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Stuart G, Spruston N, Sakmann B, Häusser M. Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci. 1997;20:125–131. doi: 10.1016/s0166-2236(96)10075-8. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Häusser M. Dendritic coincidence detection of EPSPs and action potentials. Nat Neurosci. 2001;4:63–71. doi: 10.1038/82910. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Gandevia SC. Noninvasive stimulation of the human corticospinal tract. J Appl Physiol. 2004;96:1496–1503. doi: 10.1152/japplphysiol.01116.2003. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Petersen NT, Butler JE, Gandevia SC. Interaction of transcranial magnetic stimulation and electrical transmastoid stimulation in human subjects. J Physiol. 2002;541:949–958. doi: 10.1113/jphysiol.2002.016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa Y, Rothwell JC, Day BL, Thompson PD, Marsden CD. Percutaneous electrical stimulation of corticospinal pathways at the level of the pyramidal decussation in humans. Ann Neurol. 1991;29:418–427. doi: 10.1002/ana.410290413. [DOI] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Wolters A, Schmidt A, Schramm A, Zeller D, Naumann M, Kunesch E, Benecke R, Reiners K, Classen J. Timing-dependent plasticity in human primary somatosensory cortex. J Physiol. 2005;565:1039–1052. doi: 10.1113/jphysiol.2005.084954. [DOI] [PMC free article] [PubMed] [Google Scholar]