Abstract
Previous studies have shown that prefrontal cortex (PFC) neurons carry task-related activity; however, it is largely unknown how this selectivity is implemented in PFC microcircuitry. Here, we exploited known differences in extracellular action potential waveforms, and antidromic identification, to classify PFC neurons as putative pyramidal or interneurons, and investigate their relative contributions to task-selectivity. We recorded the activity of prefrontal neurons while monkeys performed a blocked pro/antisaccade task in which they were required to look either toward or away from a peripheral visual stimulus. We found systematic differences in activity between neuron classes. Putative pyramidal neurons had higher stimulus-related activity on antisaccade trials, whereas putative interneurons exhibited greater activity for prosaccades. These findings suggest that task-selectivity in the PFC may be shaped by interactions between these neuronal classes. They are also consistent with the robust deficits in antisaccade performance frequently observed in disease states associated with PFC dysfunction.
Introduction
The ability to perform varied actions in response to identical sensory inputs is the hallmark of voluntary, goal-directed behavior. This ability is thought to depend on the prefrontal cortex (PFC), a neocortical area that receives input from all sensory systems and projects to motor-related cortical and subcortical areas (Fuster, 1991; Miller and Cohen, 2001). PFC lesions impair functions generally dependent on the implementation of behavioral rules, such as learning of arbitrary stimulus-response associations, and task switching (Parker and Gaffan, 1998; Bussey et al., 2001; Rushworth et al., 2005; Mansouri et al., 2007). In nonhuman primates, single PFC neurons have been shown to exhibit activity consistent with the instantiation of task rules. Responses to identical stimuli vary depending on the task and response requirements (Sakagami and Niki, 1994; Rainer et al., 1998; White and Wise, 1999; Asaad et al., 2000; Wallis et al., 2001; Everling et al., 2002; Johnston and Everling, 2006a). Although a large body of literature has demonstrated task-related activity in single PFC neurons, it is unknown how this activity is implemented within PFC microcircuitry.
The two major classes of cortical neurons are glutamatergic pyramidal neurons and GABAergic interneurons (Creutzfeldt, 1993; Markram et al., 2004; Wonders and Anderson, 2006). The dynamic interaction between these neuron types is thought to be responsible for many of the tuning properties of pyramidal neurons, and thus cortical function (Sillito et al., 1985; Wilson et al., 1994; Rao et al., 2000). Indeed, dysfunction of cortical interneurons has been implicated in disease states such as schizophrenia (Lewis et al., 2005). Electrophysiological studies have exploited known differences between the duration of the extracellularly recorded action potential waveforms of inter and pyramidal neurons to separate these two classes (Mountcastle et al., 1969; Connors and Gutnick, 1990; Markram et al., 2004). In the PFC, such studies in monkeys have implicated interneurons in tuning of spatial memory fields (Wilson et al., 1994; Rao et al., 1999, 2000; Constantinidis and Goldman-Rakic, 2002; Wang et al., 2004) and abstract numerical categories (Diester and Nieder, 2008). Such an approach has also been successful in somatosensory (Mountcastle et al., 1969; Simons, 1978; McCormick et al., 1985), motor (Merchant et al., 2008), and visual cortex (Gur et al., 1999; Mitchell et al., 2007).
Recently, we reported that PFC neurons showed task selectivity in a paradigm in which monkeys were required to alternate between blocks of prosaccade trials, in which they were required to generate a saccade to a flashed visual stimulus, and antisaccade trials (Hallett, 1978; Munoz and Everling, 2004), in which they were required to generate a saccade to the mirror location (Everling and DeSouza, 2005; Johnston and Everling, 2006b; Johnston et al., 2007). Here, we sought to investigate and quantify differences in task selectivity between cortical interneurons and pyramidal neurons. We separated putative inhibitory interneurons from putative pyramidal neurons based on their extracellular action potential durations. Furthermore, we directly identified a subset of broad spiking neurons as pyramidal using antidromic identification (Johnston and Everling, 2006b). Putative interneurons were more active on prosaccade trials whereas putative pyramidal neurons preferred antisaccade trials. This finding suggests that PFC task selectivity is shaped at least in part, by the interaction between these neuron classes.
Materials and Methods
All methods described (DeSouza and Everling, 2004) were performed in accordance with the guidelines of the Canadian Council of Animal Care Policy on the Use of Laboratory Animals and a protocol approved by the Animal Use Subcommittee of the University of Western Ontario Council on Animal Care. Four male rhesus monkeys (Macaca mulatta), weighing between 4 and 9 kg, were subjects in the present experiment. Animals were prepared for chronic experiments by undergoing a surgery in which a head restraint and recording chambers were stereotaxically implanted. All four animals were implanted with recording chambers over the lateral PFC (left hemisphere in two animals, right hemisphere in one, bilateral in one; anterior–posterior: 31 mm, medio–lateral: 18 mm) such that the principal sulcus and the surrounding cortex were readily accessible. Two of the animals were also implanted with a second recording chamber, which was centered on the midline and tilted 38° posterior of vertical to allow recordings from neurons in the superior colliculus (SC). Animals received analgesics and antibiotics postoperatively and were closely monitored by the university veterinarian.
Behavioral task
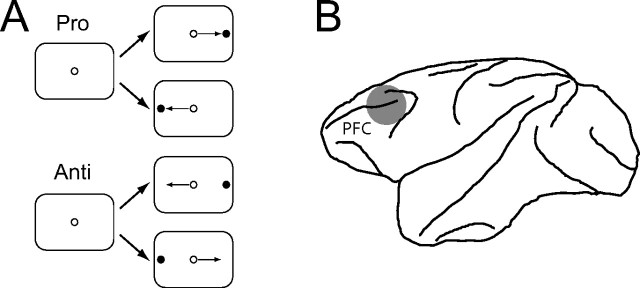
Each trial began with the presentation of a small fixation point at the center of the screen (Fig. 1A). Monkeys were required to fixate it within a 0.5° × 0.5° window for a random period of 1100–1400 ms. A visual stimulus (0.15°) was then presented pseudorandomly with equal probability 8° to the left or 8° to the right of the fixation spot. The central fixation point remained illuminated throughout the trial (overlap task). To receive a reward, monkeys had to generate a saccade within 500 ms to the stimulus on prosaccade trials or away from it to its mirror location on antisaccade trials (5° × 5°). After 30 correct responses, the task switched (e.g., from prosaccades to antisaccades) without any explicit signal to the monkeys [see Everling and DeSouza (2005) for details]. Eye movements were recorded at 1000 Hz using a magnetic search coil technique (David Northmore) in two of the animals, and a video eye tracker (ISCAN) at 120 Hz in the other two subjects. Monkeys performed between 4 and 20 task-switches (median 12) of alternating blocks of prosaccade and antisaccade trials.
Figure 1.
Task and recording area. A, Schematic diagram of the behavioral task. Monkeys were required to fixate a white fixation dot at the center of the screen for 1100–1400 ms. A peripheral stimulus then appeared to the left or right of fixation. Animals received a liquid reward if they looked toward the stimulus on prosaccade trials and to the mirror location on antisaccade trials. B, Location of the PFC.
Recording method
We recorded extracellular single neuron activity from the lateral prefrontal cortex (Fig. 1B). The locations of the implanted recording chambers were visualized in situ by MRI. Arrays of 2–8 dura-puncturing microelectrodes (FHC) were driven individually within a recording chamber by either custom-designed screw mini-microdrives that were attached to a delrin grid inside the recording chamber or a computer-controlled multi-microelectrode drive (NAN, Plexon). To ensure a relatively unbiased sampling of neural activity, neurons were not prescreened for task-related responses. Instead, we advanced the electrodes until the activity of one or more neurons was well isolated, after which data collection commenced. Waveforms were digitized, stored and sorted off-line using principal component analysis in two and three dimensions (Plexon). Waveforms were sampled at 40 kHz (25 μs/sample), and an 800–1000 μs trace of each waveform was stored.
In some sessions, we used antidromic activation to identify PFC pyramidal neurons sending direct projections to the superior colliculus. Details of this procedure have been described previously (Johnston and Everling, 2006b, 2008). Briefly, arrays of three to four microelectrodes were chronically implanted in the intermediate layers of the superior colliculus ipsilateral to the PFC recording hemisphere for a period of 4–6 weeks. The activity of isolated PFC neurons was monitored while single biphasic current pulses (0.15–0.3 ms per phase) were delivered to the SC through one of the implanted electrodes and an indifferent tungsten rod in the recording chamber. Neurons were required to meet several criteria including fixed latency, frequency, and collision testing, to be classified as antidromic (Lipski, 1981).
Data analysis
Data analysis was performed using custom-designed software running in Matlab (Mathworks). Trials associated with incorrect responses, broken, incorrect, or inaccurate fixation, or failure to generate a saccade within 500 ms were excluded from analyses of neural activity.
Waveform analysis.
For each neuron, we calculated its mean extracellular waveform duration using the identical procedure to that used by Mitchell et al. (2007). For each recorded neuron, all action potentials were aligned by their troughs and averaged. This average waveform was then normalized and spline interpolated to give a resolution of 1 μs. Waveform duration was determined by measuring the time from the trough (negative deflection) to the peak (positive deflection). All classifications of neurons as narrow-spiking (NS) or broad-spiking (BS) were based on these values.
ANOVA.
Task selective neurons were defined based on a two by two ANOVA on neural activity in the interval 100–200 ms after stimulus onset, evaluated at p < 0.05. The factors were Task (prosaccades or antisaccades) and Stimulus Location (ipsilateral or contralateral to recorded hemisphere). Because task selectivity could be superimposed on any stimulus location preference shown by PFC neurons, neurons showing a significant main effect of Task, main effects of both Task and Stimulus Location, or a Task × Stimulus Location interaction were classified as task selective. An interaction between Task and Stimulus location would indicate that effects of stimulus location differed depending on the task being performed.
Task preference.
As a measure of task preference (prosaccade vs antisaccade) in the preferred location, we computed a task preference index (TPI), as follows: TPI = (P − A)/(P + A), where P is activity on prosaccade trials at the preferred location in the interval 100–200 ms after stimulus onset, and A is activity on antisaccade trials at the preferred location in the same interval. Positive values of this index indicate that the neuron preferred prosaccades, whereas negative values indicate the neuron preferred antisaccades.
Time course of task selectivity.
To determine the time course of task selectivity for BS and NS neurons after stimulus presentation, we performed a sliding receiver operating characteristic (ROC) analysis. Starting from the time of peripheral stimulus presentation, the ROC value was calculated for a 50 ms epoch (centered around the time point). This analysis was repeated in 1 ms increments until 200 ms after stimulus presentation. An ROC time course was calculated for each neuron and then averaged separately across all BS and NS neurons with task selectivity. Values >0.5 indicate that the population was more active for prosaccades than antisaccades during the epoch, whereas values smaller than 0.5 indicate the population was more active for antisaccades. To test whether the ROC values were significant at any time points for the populations of NS and BS neurons, we conducted bootstrap analyses. To this end, the following procedure was repeated 10,000 times: For each neuron, a random decision was made to either exchange the two task conditions (pro and anti) (50% probability) or leave them unchanged (50% probability). Each of the 10,000 repetitions of the analysis, performed on all NS and BS neurons with task selectivity, yielded a single average time course. The distribution of the 10,000 average ROC values at each point in time was then used to calculate the 95th and 5th percentile values. Both were plotted together with the average ROC time course of the nonrandomized data. The 95th and 5th percentile indicate the 5% significance criterion.
Spike density function.
To evaluate the relationship between neural activity and stimulus onset, continuous spike density functions were constructed. The activation waveform was obtained by convolving each spike with an asymmetric function that resembled a postsynaptic potential (Hanes and Schall, 1996; Thompson et al., 1996; Everling et al., 1999). The advantage of this function over a standard Gaussian function (Richmond and Optican, 1987) is that a spike only exerts an effect forward and not backward in time.
Results
We recorded the extracellular waveforms of a total of 217 neurons in the lateral prefrontal cortex of four rhesus monkeys (33 in monkey K, 62 in monkey P, 80 in monkey R, 42 in monkey W) while they performed alternating blocks of prosaccades and antisaccades. Of these neurons, 170 were randomly sampled, i.e., we recorded from every neuron that we could isolate (monkeys K, P, R). Waveform durations as defined as the time from the initial trough to peak ranged from 133 to 473 μs. Consistent with previous reports (Constantinidis and Goldman-Rakic, 2002; Mitchell et al., 2007; Diester and Nieder, 2008), we found two populations of waveforms. A histogram of waveform durations with a binwidth of 20 ms showed a clear bimodality (Fig, 2A, top) which was confirmed by a Hartigan's Dip test (p < 0.05) (Hartigan and Hartigan, 1985).
Figure 2.
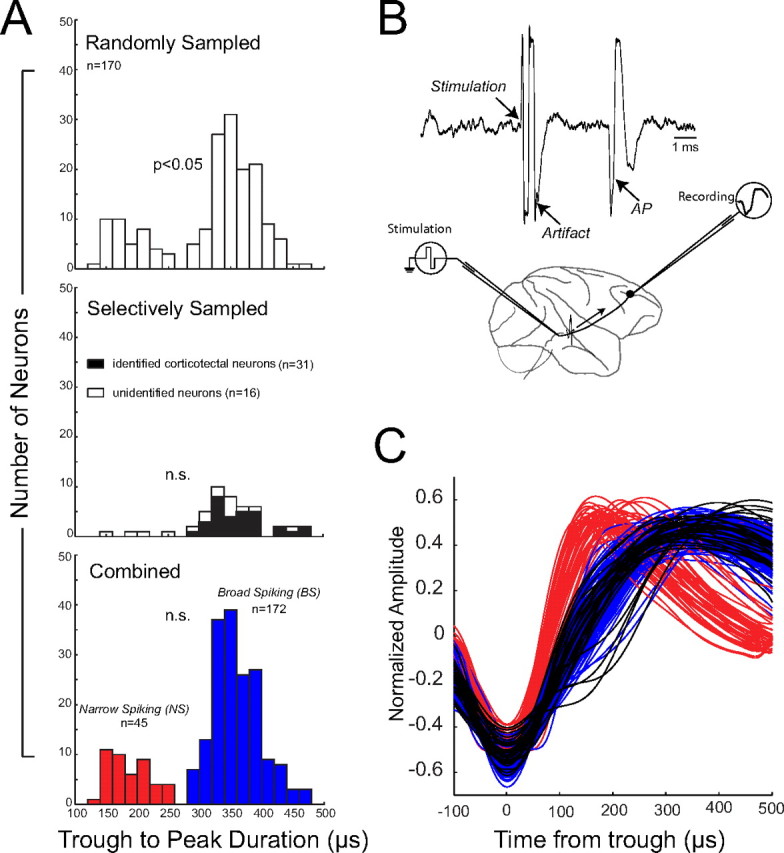
Classification of BS and NS neurons. A, Distribution of waveform durations of randomly sampled neurons (top), selectively sampled neurons (middle), and combined recordings (bottom). Waveform duration was defined a time from trough to peak. B, Left, Schematic representation of experimental method for antidromic activation of corticotectal neurons in the PFC. Right, Activation waveform showing activity recorded in the PFC. C, Normalized average waveforms of 217 neurons aligned by their trough. Red and blue correspond to narrow- and broad-spiking neurons, respectively. Waveforms of identified corticotectal neurons are plotted in black. Note that these waveforms overlap completely with other BS waveforms.
Previous studies have classified neurons with narrow (shorter duration) action potentials as interneurons and those with broad (longer duration) action potentials as pyramidal neurons (Constantinidis and Goldman-Rakic, 2002; Mitchell et al., 2007; Diester and Nieder, 2008). This classification was based on the known differences in waveform duration of recorded action potentials from morphologically identified interneurons and pyramidal neurons (McCormick et al., 1985; Connors and Gutnick, 1990; Nowak et al., 2003; González-Burgos et al., 2005). Such an approach is not feasible for recordings in the awake primate. As a further validation for a classification scheme based on waveform duration, we used antidromic identification techniques to identify PFC neurons sending direct projections to the SC, a midbrain oculomotor structure (Fig. 2B). These projection neurons are known to be layer V pyramidal neurons (Leichnetz et al., 1981; Creutzfeldt, 1993). We recorded a total of 47 neurons from monkeys R and W while we searched for corticotectal neurons using antidromic stimulation (Fig. 2A, middle). Of these 47 neurons, 31 were identified as corticotectal neurons. These identified pyramidal cells had broad extracellular waveform durations, ranging from 287 to 464 μs (median 355 μs). The 16 unidentified neurons that were recorded in the same sessions had waveform durations from 180 to 453 μs (median 343 μs). The waveforms that were recorded during these selective recording sessions did not show a significant bimodality [Hartigan's Dip test (p > 0.70)]. For the remainder of the analysis, we pooled the neurons that were randomly and selectively sampled (Fig. 2A, bottom). The distribution of all waveforms combined showed a clear separation between NS and BS waveform durations, with no waveforms falling between 260 and 280 μs. However, a test of bimodality failed to reach significance [Hartigan's Dip test (p = 0.13)]. We suspect that this may be because of an oversampling of pyramidal neurons, a result of the fact these neurons are easier to record because of their larger size, and because we searched selectively for this neuronal class in the sessions in which we used antidromic identification.
Based on the finding that the shortest waveform duration of identified pyramidal neurons was 287 μs, we classified neurons with waveform durations shorter than this value as NS and those with waveform durations of 270 μs or longer as BS (Fig. 2A, bottom, C).
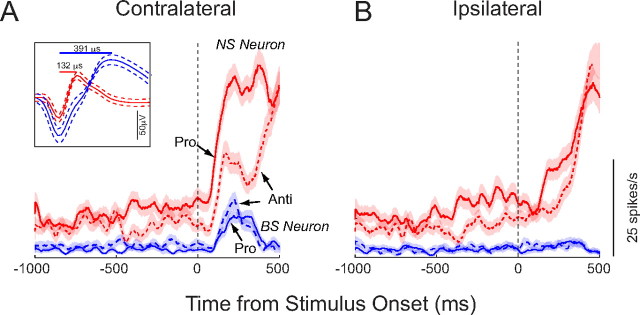
Figure 3 shows an example of a single BS (blue) and NS neuron (red) that were recorded simultaneously from the tip of the same microelectrode while monkey K performed alternating blocks of prosaccades (solid lines) and antisaccades (dashed lines). Both neurons had some tonic activity before the stimulus was presented, and both neurons showed a clear spatial preference. In this case, the neurons were more active for stimuli presented on the contralateral side (Fig. 3A) compared with the ispilateral side (Fig. 3B). For stimulus presentations at this preferred location, both neurons displayed a phasic increase in discharge on prosaccade and antisaccade trials. Despite these similarities in activity, the BS and NS neurons also showed a number of clear differences in their activity pattern. First, the NS neuron had higher levels of both tonic and phasic activity than the BS neuron. Higher activity levels for putative interneurons than putative pyramidal neurons have been previously reported by extracellular recording studies in nonhuman primates (Mitchell et al., 2007; Diester and Nieder, 2008). In fact, the higher discharge rates of NS neurons have been used as an additional classification tool (Constantinidis and Goldman-Rakic, 2002). Second, the NS neuron also exhibited an increase in discharge for the ipsilateral location. The BS neuron did not show any increase in activity at the ipsilateral location. Third, the discharge rate of the NS neuron in response to presentation of the peripheral stimulus was higher on prosaccade trials, whereas the BS neuron was more active on antisaccade trials.
Figure 3.
Activity of a single narrow- (red) and broad-spiking (blue) neuron recorded simultaneously at the tip of the same microelectrode. A, Activity on trials on which the stimulus was presented contralateral to the recording hemisphere. Solid and dashed lines indicate mean spike-density functions on prosaccade and antisaccade trials, respectively. Envelopes indicate SEM. The inset shows the extracellular waveforms of the two neurons. Colors match those of the spike-density functions, envelopes represent SD. B, Same as A, but for trials on which the stimulus appeared on the ipsilateral side.
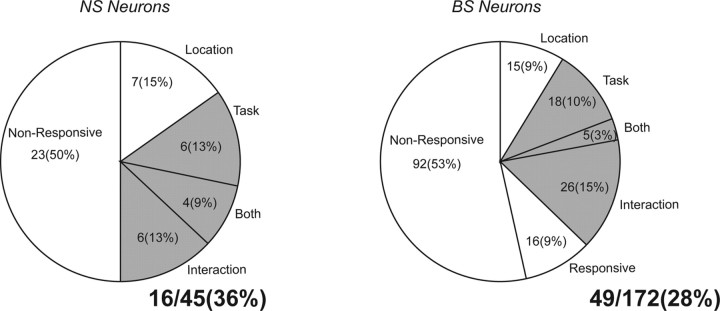
To identify NS and BS neurons that showed task-related responses, we performed ANOVAs on the poststimulus activity of each neuron (see Materials and Methods). We found that similar proportions of NS and BS neurons exhibited task-related activity (Fig. 4). In the sample of 45 NS neurons, we found that 16 neurons (36%) showed task selective activity. In the sample of 172 BS neurons, 49 neurons (28%) exhibited task selective activity. In addition to neurons that exhibited some form of task selectivity, we also found NS and BS neurons that were location-selective but not task selective (15% of NS neurons and 9% of BS neurons). Some of the BS neurons were responsive, i.e., they changed their activity in the interval from 100 to 200 ms after stimulus presentation compared with the 200 ms period immediately before stimulus onset (tested with paired t tests), but they were not task- or location-selective (9% of BS).
Figure 4.
Results of two-way ANOVAs on the activity in the epoch from 100 to 200 ms after stimulus onset for BS and NS neurons. The factors were stimulus location (left/right) and task (pro/anti). Responsive neurons were nonsignificant in the ANOVA but showed significant difference between the stimulus epoch and the fixation epoch. Shaded area indicates neurons with task selectivity subjected to further analysis.
To compare task-related activity between NS and BS neurons, we computed task preference indexes for all task selective NS and BS neurons (see Materials and Methods). The distribution of task preference indices is plotted in Figure 5. The mean (±SEM) task preference index of BS neurons was −0.11 ± 0.05, indicating that the population of BS neurons was more active on antisaccade than on prosaccade trials. In contrast, NS neurons were more active on prosaccade than on antisaccade trials (mean task preference index = 0.19 ± 0.08). Task preferences differed significantly between BS and NS neurons (p < 0.01, Mann–Whitney U test).
Figure 5.
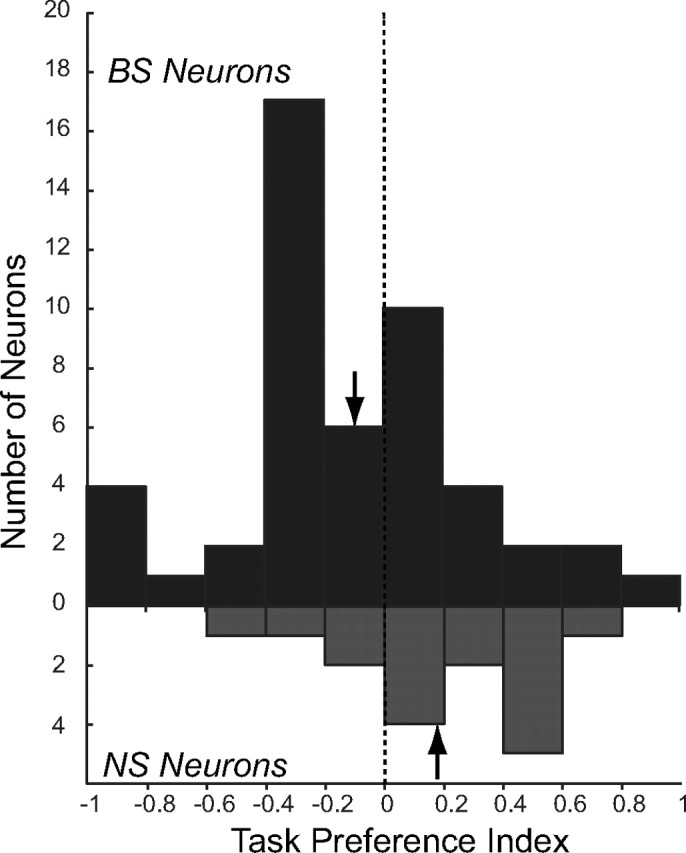
Values of task preference index for narrow- and broad-spiking neurons showing significant task effects in ANOVA. Negative values indicate higher activity on antisaccade trials, positive values, and prosaccade trials. Arrows indicate mean values of the index for each population of neurons.
Next, we compared the activity of BS and NS neurons with task selectivity on prosaccade and antisaccade trials for trials on which the stimulus was presented on the side contralateral to the recorded hemisphere, and those on which it was presented on the ipsilateral side. We found recently that electrical microstimulation has clear contralateral effects on task performance on prosaccade and antisaccade trials (Wegener et al., 2008). Figure 6A shows the activity of the population of BS (red lines) and NS (blue lines) neurons for prosaccade (solid lines) and antisaccade trials (dashed lines) at the contralateral location. NS neurons exhibited a stimulus-related response on prosaccade trials that was attenuated on antisaccade trials. BS neurons had a larger stimulus-related response on antisaccade than prosaccade trials. We compared the activity of NS and BS neurons between prosaccade and antisaccade trials in an early stimulus period (100–150 ms after stimulus onset) and a late stimulus period (150–200 ms after stimulus onset) as a coarse measure of the relative timing of selectivity between these neuronal classes. The activity of the population of NS neurons was significantly different between prosaccade and antisaccade trials in the early, but not late period (Fig. 6B), whereas BS neurons exhibited significant differences in both early and late periods (Fig. 6C). For trials on which the stimulus was presented on the ipsilateral side (Fig. 6D), NS neurons exhibited a marked increase in stimulus-related activity that was significant during the early stimulus period (Fig. 6E). BS neurons showed only a very small increase in discharge rate and no differences between prosaccade and antisaccade trials (Fig. 6F). These findings demonstrate that both NS and BS neurons display task selectivity but that there are clear differences in the nature of selectivity across these neuron classes. First, NS neurons were more active on prosaccade trials, whereas BS neurons were more active on antisaccade trials. Second, differences in laterality of task selectivity between NS and BS neurons were observed, such that the preference of NS neurons for prosaccade trials was present for both contralateral and ipsilateral stimulus presentations, whereas the preference of BS neurons for antisaccade trials was significant only for contralateral stimulus presentations. Third, we observed coarse differences in the timing of task selectivity between classes. Selectivity appeared in the early, but not late, epoch in the population of NS neurons, and within both early and late epochs in the population of BS neurons.
Figure 6.
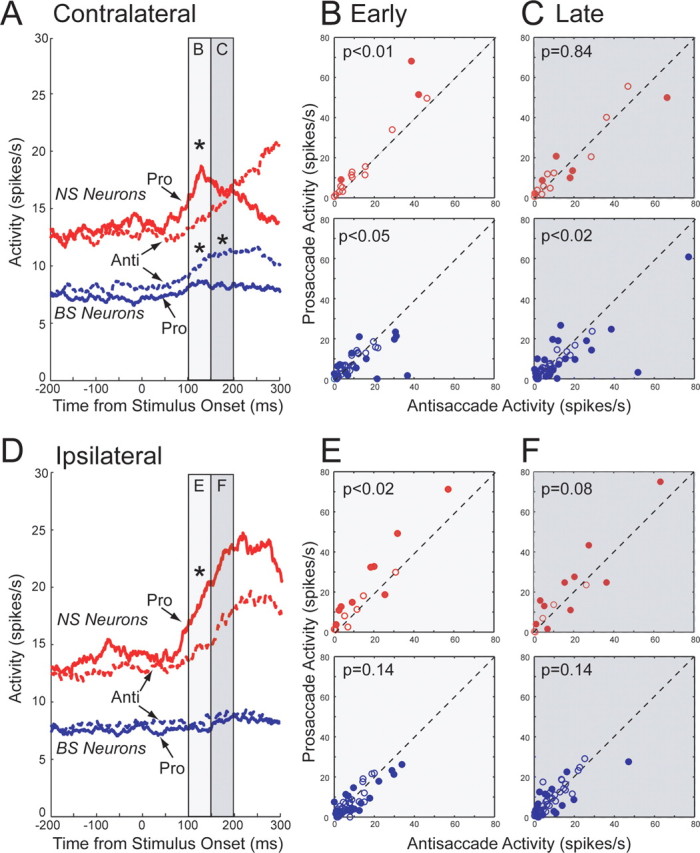
Activity in the selected neuron samples for prosaccade and antisaccade trials. A, Mean spike density of narrow-spiking (red) and broad-spiking (blue) neurons on prosaccade (solid lines) and antisaccade trials (dashed lines) for trials on which the stimulus appeared contralateral to the recording hemisphere. B, Activities of individual narrow-spiking (red) and broad-spiking (blue) PFC neurons are plotted for prosaccade trials (y-axis) versus antisaccade trials (x-axis). Plotted values are mean spike counts in the epoch from 100 to 150 ms after stimulus onset. Filled circles indicate neurons with significant differences (p < 0.05, Wilcoxon signed rank test) between prosaccade and antisaccade trials. C, Same as B, but for activities in the epoch from 150 to 200 ms after stimulus onset. D–F, Same as A–C, but for trials on which the stimulus appeared ipsilateral to the recording hemisphere.
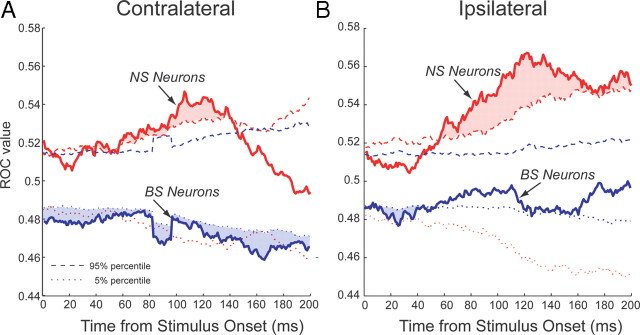
To perform a more principled comparison of the relative time courses of task selectivity after stimulus onset for NS and BS neurons, we performed an ROC analysis. To test whether these ROC values were significantly different from chance, we also conducted bootstrap analyses (see Materials and Methods). For contralateral stimulus presentations, BS neurons exhibited task selectivity earlier than NS neurons (Fig. 7A). For ipsilateral stimulus presentations, BS neurons showed some early task selectivity, whereas NS neurons displayed later and more robust task selective responses (Fig. 7B). Examination of Figure 7 also shows that NS neurons were more active on prosaccade trials (ROC values >0.5), whereas BS neurons were more active on antisaccade trials (ROC values <0.5). This finding verifies that task preferences were opposite between neuron classes, and indicates that BS neurons displayed task selectivity earlier than NS neurons.
Figure 7.
Time course of task selectivity of narrow- and broad-spiking neurons. A, Time course of average population ROC values (solid lines) of NS (red) and BS (blue) PFC neurons on trials on which the stimulus appeared contralateral to the recording hemisphere. Dashed and dotted lines indicate 95th and 5th percentile of randomized data, respectively. Red lines represent percentile values for narrow-spiking neurons, blue, percentile values for broad-spiking neurons. Periods in which the solid lines lay above the dashed line or below the dotted line (red and blue shaded regions) indicate periods with significant task selectivity (p < 0.05). B, Same as A, but for trials on which the stimulus appeared on the ipsilateral side.
Discussion
Task selective responses are a ubiquitous property of PFC neurons, but little is known about how such selectivity is implemented in PFC microcircuitry. Here, we used known differences in the extracellular waveforms of cortical pyramidal and interneurons as a classification tool (Constantinidis and Goldman-Rakic, 2002; González-Burgos et al., 2005; Mitchell et al., 2007; Diester and Nieder, 2008), and compared the response properties of narrow-spiking putative interneurons (NS), and broad-spiking putative pyramidal neurons (BS) in a blocked pro/antisaccade task. We found systematic differences in the response properties of these two neuronal types suggestive of different functional roles in performance of the task. First, NS neurons exhibited a higher level of activity on prosaccade than antisaccade trials, whereas BS neurons showed the opposite pattern of selectivity. Second, task selectivity of BS neurons was lateralized, whereas that of NS neurons was present for both contralateral and ipsilateral stimulus presentations. Third, task selectivity evolved earlier in BS than NS neurons. Together, these data suggest that task selectivity of prefrontal pyramidal neurons may be shaped, in part, by the activity of inhibitory interneurons.
The broad-spiking class as pyramidal neurons
Consistent with several previous studies (Constantinidis and Goldman-Rakic, 2002; González-Burgos et al., 2005; Mitchell et al., 2007; Diester and Nieder, 2008), we found that the distribution of extracellular waveform durations exhibited a clear bimodality. NS neurons also had higher levels of activity than BS neurons. Previous studies have argued that NS waveforms originate from the spiking of interneurons, whereas BS waveforms have been attributed to pyramidal neurons. The main argument in support of this classification has been the temporal profile of intracellular recorded activity of interneurons and pyramidal neurons and the differences in ion-channel kinetics between these types of neurons (Martina et al., 1998; González-Burgos et al., 2004; Bean, 2007). These differences have been confirmed by direct comparisons between in vitro intracellular and extracellular recordings of action potentials in the monkey dorsolateral PFC (González-Burgos et al., 2005).
Here, we used antidromic stimulation to directly identify layer V pyramidal neurons that project to the superior colliculus. We found the waveform durations of PFC neurons recorded here fell into two distinct and clearly separated populations, and that all identified output neurons had trough to peak durations longer than 270 μs, placing them within the BS class. Although this result is based on a fairly small sample size (31 neurons), this finding provides further support for the assumption that BS neurons are mainly pyramidal neurons whereas NS neurons are interneurons. Our finding is also consistent with a previous report that identified corticotectal neurons in the primate frontal eye field as BS neurons (Shin and Sommer, 2006).
Task-related differences in prefrontal cortex
Neural correlates of behavioral rules and categories have been found in many studies of PFC function (Rainer et al., 1998; White and Wise, 1999). Miller and Cohen (2001) proposed a model in which PFC neurons participate in cognitive control by actively maintaining patterns of activity representing task requirements and sending outputs that modulate the activity of other brain areas in the service of behavioral goals. The need for such control is especially high in situations when strong direct stimulus-response mappings must be overridden in favor of weaker, purposive ones (Miller, 1999; Miller and Cohen, 2001). The antisaccade task (Hallett, 1978) represents a clear example of a task with such requirements. Subjects are instructed not to look toward a flashed visual stimulus but instead to look to the opposite side. As such, the antisaccade task has become a popular tool for the study the flexible behavioral control (Everling and Fischer, 1998; Munoz and Everling, 2004). Patients with prefrontal damage (Guitton et al., 1985; Pierrot-Deseilligny et al., 1991), and psychiatric disorders such as schizophrenia (Fukushima et al., 1988) reliably make performance errors in this task consistent with an inability to inhibit reflexive saccades toward the visual stimulus.
Task-related differences between prosaccade and antisaccades have been found in PFC neurons (Funahashi et al., 1993; Everling and DeSouza, 2005; Johnston and Everling, 2006a; Johnston et al., 2007), as well as a number of other cortical and subcortical areas including the frontal eye field (FEF) (Everling and Munoz, 2000), anterior cingulate cortex (ACC) (Johnston et al., 2007), supplementary eye field (SEF) (Schlag-Rey et al., 1997; Amador et al., 2004), lateral intraparietal area (LIP) (Gottlieb and Goldberg, 1999; Zhang and Barash, 2000), globus pallidus (Yoshida and Tanaka, 2008), and SC (Everling et al., 1998, 1999). Based mainly on differences in activity of SC neurons during prosaccade and antisaccade tasks, Munoz and Everling proposed an accumulator model in which correct antisaccade performance depends on top-down control of SC saccade neurons by frontal cortical areas (Munoz and Everling, 2004). Direct support for this hypothesis is provided by the finding that corticotectal PFC neurons are more active on antisaccade than prosaccade trials (Johnston and Everling, 2006b). The current study indicates that not only output neurons but also interneurons in the PFC carry task-related activity.
Implications for representation of task selectivity in PFC
Previous models of the interactions between pyramidal neurons and inhibitory interneurons (Compte et al., 2000; Wang et al., 2004; Fusi et al., 2007) have made basic assumptions that are in partial agreement with our data. Such models assume that the activity of inhibitory interneurons is driven by pyramidal cell input, is broadly tuned, and is not task selective. The first two assumptions are consistent with the earlier onset of task selectivity we observed in BS than NS neurons, and the broad tuning we observed in NS neurons. Here, however, we observed robust task selective responses in NS neurons. This, in combination with the numerosity-selective responses recently observed in NS neurons (Diester and Nieder, 2008), suggests that inhibitory interneurons may play a more active role in the implementation of top-down control than previously thought.
Our finding that task selectivity appeared earlier in the population of BS than NS neurons is contrary to the latency differences between these neuron classes in some previous studies. Both Wilson et al. (1994) and Diester and Nieder (2008) found that putative interneurons had shorter visual response latencies than putative pyramidal neurons. This discrepancy may be the result of differences between the tasks they used and the pro/antisaccade task used here. Differences in preparatory activity between prosaccade and antisaccade trials has been observed in many areas, and is thought to reflect a presetting of the oculomotor circuitry in advance of stimulus presentation to facilitate suppression of reflexive saccades (Munoz and Everling, 2004). We have previously reported that preparatory activity of corticotectal PFC neurons is greater on antisaccade than prosaccade trials (Johnston and Everling, 2006b). We speculate that the early task selectivity we observed in BS neurons could reflect changes in preparatory set that were not present in the saccade and delayed match to sample tasks used in other studies.
We have previously reported that lateralized increases of the activity of corticotectal PFC neurons on antisaccade trials represents a suppression signal that acts to decrease the activity of SC saccade neurons and thereby prevent the saccade signal from reaching threshold (Munoz and Everling, 2004; Johnston and Everling, 2006b). This could be mediated via an enhancement of the activity of either fixation or inhibitory interneurons in the SC. A schematic depiction of this model is presented in Figure 8. For example, on an antisaccade trial on which the visual stimulus appears to the left of fixation (Fig. 8A), output (BS) neurons in the right PFC would show an enhancement of activity. Since cortical outputs are excitatory (Creutzfeldt, 1993), this leads to increased activity of either fixation or interneurons in the right SC, and a concomitant reduction in the activity of SC saccade neurons, suppressing a reflexive saccade toward the stimulus. On prosaccade trials (Fig. 8B), this enhanced signal is absent, and visual inputs are allowed to drive SC saccade neurons. This would facilitate performance on prosaccade trials, since the visual stimulus location and saccade direction are the same. By such a mechanism, cortical output selective for the antisaccade “rule” could act to modulate the downstream SC to facilitate the antisaccade “goal.” Here, we found that NS neurons have a reduced stimulus-related response on antisaccade trials. We hypothesize that as a consequence, pyramidal neurons receive less inhibition during the stimulus period on antisaccade trials and therefore increase their response to contralateral stimulus presentations. Although the limitations of extracellular recording in the awake animal preclude us from making direct empirical statements about connectivity between the NS and BS neurons recorded here, our results, combined with the demonstrated anatomical connectivity between interneurons and pyramidal neurons, suggest that interaction between task selective responses of these neuronal types could be one mechanism that acts to regulate cortical output and thus performance in the antisaccade task.
Figure 8.
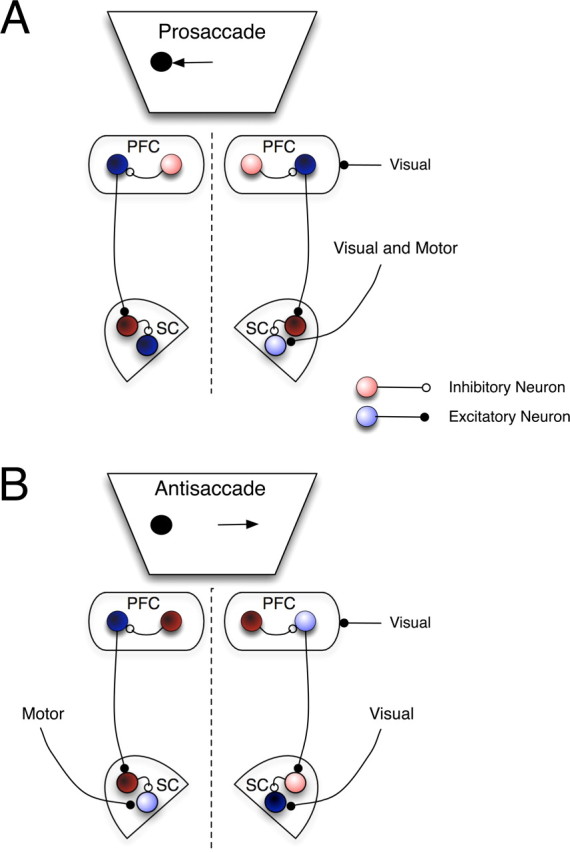
Schematic representation of putative mechanism underlying contribution of BS and NS neurons to antisaccade and prosaccade task performance. Blue circles represent excitatory neurons, red circles, inhibitory neurons. Color saturation represents activity level, lighter: higher activity, darker: lower activity. A, Prosaccade trial on which visual stimulus appears in left hemifield and correct response is a leftward saccade. Inhibitory NS neurons in PFC are more active on prosaccade trials and reduce activity of BS PFC output neurons to SC. This leads to reduced activity of SC fixation or inhibitory interneurons (dark red circles) and reduced inhibition of SC saccade neurons. Visual input from stimulus in left hemifield and motor command for leftward saccade can combine to drive activity of saccade neuron and generate correct leftward prosaccade. Enhanced activity of inhibitory NS neurons could, therefore, attenuate outputs of BS neurons and allow reflexive prosaccade to proceed. B, Antisaccade trial on which visual stimulus appears in left hemifield, and a rightward saccade (arrow) is the correct response. Inhibitory interneurons in PFC (dark red circles) are less active on antisaccade trials and thus allow the following chain of events to proceed. Visual stimulus evokes responses in excitatory, BS output neurons (light blue circle) of the right PFC and saccade neuron (dark blue circle) of the right SC. Excitatory input of BS neuron in right PFC increases activity of inhibitory interneuron or fixation neuron in SC (pink circle), which attenuates stimulus-driven visual activity of SC saccade neuron and prevents it from reaching threshold for generating a saccade. Thus, reflexive saccades toward the visual stimulus are inhibited. Voluntary motor input then drives saccade neuron in left SC (light blue circle) to generate correct rightward saccade. Decreased activity of inhibitory NS neurons in PFC on antisaccade trials could therefore act to allow outputs of BS neurons to regulate SC activity in support of antisaccade performance.
Our results suggest that the decrease of activity of interneurons in the PFC immediately after stimulus presentation might be a crucial step in correct antisaccade performance. Dysfunction of some classes of cortical inhibitory interneurons has been demonstrated in schizophrenic patients (Lewis et al., 2005). Hence, a disruption in the normal function activity of PFC interneurons could contribute to the well established performance deficits of schizophrenic patients in the antisaccade task (Fukushima et al., 1988; Broerse et al., 2001; Hutton and Ettinger, 2006; Gooding and Basso, 2008).
Footnotes
This work was supported by grants from the Canadian Institutes of Health Research.
References
- Amador N, Schlag-Rey M, Schlag J. Primate antisaccade. II. Supplementary eye field neuronal activity predicts correct performance. J Neurophysiol. 2004;91:1672–1689. doi: 10.1152/jn.00138.2003. [DOI] [PubMed] [Google Scholar]
- Asaad WF, Rainer G, Miller EK. Task-specific neural activity in the primate prefrontal cortex. J Neurophysiol. 2000;84:451–459. doi: 10.1152/jn.2000.84.1.451. [DOI] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- Broerse A, Crawford TJ, den Boer JA. Parsing cognition in schizophrenia using saccadic eye movements: a selective overview. Neuropsychologia. 2001;39:742–756. doi: 10.1016/s0028-3932(00)00155-x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Wise SP, Murray EA. The role of ventral and orbital prefrontal cortex in conditional visuomotor learning and strategy use in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2001;115:971–982. doi: 10.1037//0735-7044.115.5.971. [DOI] [PubMed] [Google Scholar]
- Compte A, Brunel N, Goldman-Rakic PS, Wang XJ. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex. 2000;10:910–923. doi: 10.1093/cercor/10.9.910. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Goldman-Rakic PS. Correlated discharges among putative pyramidal neurons and interneurons in the primate prefrontal cortex. J Neurophysiol. 2002;88:3487–3497. doi: 10.1152/jn.00188.2002. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt OD. Cortex cerebri. Goettingen: Springer; 1993. [Google Scholar]
- DeSouza JF, Everling S. Focused attention modulates visual responses in the primate prefrontal cortex. J Neurophysiol. 2004;91:855–862. doi: 10.1152/jn.00273.2003. [DOI] [PubMed] [Google Scholar]
- Diester I, Nieder A. Complementary contributions of prefrontal neuron classes in abstract numerical categorization. J Neurosci. 2008;28:7737–7747. doi: 10.1523/JNEUROSCI.1347-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, DeSouza JF. Rule-dependent activity for prosaccades and antisaccades in the primate prefrontal cortex. J Cogn Neurosci. 2005;17:1483–1496. doi: 10.1162/0898929054985455. [DOI] [PubMed] [Google Scholar]
- Everling S, Fischer B. The antisaccade: a review of basic research and clinical studies. Neuropsychologia. 1998;36:885–899. doi: 10.1016/s0028-3932(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Dorris MC, Munoz DP. Reflex suppression in the anti-saccade task is dependent on prestimulus neural processes. J Neurophysiol. 1998;80:1584–1589. doi: 10.1152/jn.1998.80.3.1584. [DOI] [PubMed] [Google Scholar]
- Everling S, Dorris MC, Klein RM, Munoz DP. Role of primate superior colliculus in preparation and execution of anti-saccades and pro-saccades. J Neurosci. 1999;19:2740–2754. doi: 10.1523/JNEUROSCI.19-07-02740.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Tinsley CJ, Gaffan D, Duncan J. Filtering of neural signals by focused attention in the monkey prefrontal cortex. Nat Neurosci. 2002;5:671–676. doi: 10.1038/nn874. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Fukushima K, Chiba T, Tanaka S, Yamashita I, Kato M. Disturbances of voluntary control of saccadic eye movements in schizophrenic patients. Biol Psychiatry. 1988;23:670–677. doi: 10.1016/0006-3223(88)90050-9. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365:753–756. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- Fusi S, Asaad WF, Miller EK, Wang XJ. A neural circuit model of flexible sensorimotor mapping: learning and forgetting on multiple timescales. Neuron. 2007;54:319–333. doi: 10.1016/j.neuron.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex: anatomy, physiology, and neuropsychology of the frontal lobe. New York: Raven; 1991. [Google Scholar]
- González-Burgos G, Krimer LS, Urban NN, Barrionuevo G, Lewis DA. Synaptic efficacy during repetitive activation of excitatory inputs in primate dorsolateral prefrontal cortex. Cereb Cortex. 2004;14:530–542. doi: 10.1093/cercor/bhh015. [DOI] [PubMed] [Google Scholar]
- González-Burgos G, Krimer LS, Povysheva NV, Barrionuevo G, Lewis DA. Functional properties of fast spiking interneurons and their synaptic connections with pyramidal cells in primate dorsolateral prefrontal cortex. J Neurophysiol. 2005;93:942–953. doi: 10.1152/jn.00787.2004. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Basso MA. The tell-tale tasks: a review of saccadic research in psychiatric patient populations. Brain Cogn. 2008;68:371–390. doi: 10.1016/j.bandc.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb J, Goldberg ME. Activity of neurons in the lateral intraparietal area of the monkey during an antisaccade task. Nat Neurosci. 1999;2:906–912. doi: 10.1038/13209. [DOI] [PubMed] [Google Scholar]
- Guitton D, Buchtel HA, Douglas RM. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp Brain Res. 1985;58:455–472. doi: 10.1007/BF00235863. [DOI] [PubMed] [Google Scholar]
- Gur M, Beylin A, Snodderly DM. Physiological properties of macaque V1 neurons are correlated with extracellular spike amplitude, duration, and polarity. J Neurophysiol. 1999;82:1451–1464. doi: 10.1152/jn.1999.82.3.1451. [DOI] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- Hartigan JA, Hartigan PM. The dip test of unimodality. Ann Statistics. 1985;13:70–84. [Google Scholar]
- Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology. 2006;43:302–313. doi: 10.1111/j.1469-8986.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- Johnston K, Everling S. Neural activity in monkey prefrontal cortex is modulated by task context and behavioral instruction during delayed-match-to-sample and conditional prosaccade-antisaccade tasks. J Cogn Neurosci. 2006a;18:749–765. doi: 10.1162/jocn.2006.18.5.749. [DOI] [PubMed] [Google Scholar]
- Johnston K, Everling S. Monkey dorsolateral prefrontal cortex sends task-selective signals directly to the superior colliculus. J Neurosci. 2006b;26:12471–12478. doi: 10.1523/JNEUROSCI.4101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K, Everling S. Task-relevant output signals are sent from monkey dorsolateral prefrontal cortex to the superior colliculus during a visuospatial working memory task. J Cogn Neurosci. 2008;21:1023–1038. doi: 10.1162/jocn.2009.21067. [DOI] [PubMed] [Google Scholar]
- Johnston K, Levin HM, Koval MJ, Everling S. Top-down control-signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron. 2007;53:453–462. doi: 10.1016/j.neuron.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR, Spencer RF, Hardy SG, Astruc J. The prefrontal corticotectal projection in the monkey; an anterograde and retrograde horseradish peroxidase study. Neuroscience. 1981;6:1023–1041. doi: 10.1016/0306-4522(81)90068-3. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lipski J. Antidromic activation of neurones as an analytic tool in the study of the central nervous system. J Neurosci Methods. 1981;4:1–32. doi: 10.1016/0165-0270(81)90015-7. [DOI] [PubMed] [Google Scholar]
- Mansouri FA, Buckley MJ, Tanaka K. Mnemonic function of the dorsolateral prefrontal cortex in conflict-induced behavioral adjustment. Science. 2007;318:987–990. doi: 10.1126/science.1146384. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Martina M, Schultz JH, Ehmke H, Monyer H, Jonas P. Functional and molecular differences between voltage-gated K+ channels of fast-spiking interneurons and pyramidal neurons of rat hippocampus. J Neurosci. 1998;18:8111–8125. doi: 10.1523/JNEUROSCI.18-20-08111.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- Merchant H, Naselaris T, Georgopoulos AP. Dynamic sculpting of directional tuning in the primate motor cortex during three-dimensional reaching. J Neurosci. 2008;28:9164–9172. doi: 10.1523/JNEUROSCI.1898-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK. Neurobiology. Straight from the top. Nature. 1999;401:650–651. doi: 10.1038/44291. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron. 2007;55:131–141. doi: 10.1016/j.neuron.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Talbot WH, Sakata H, Hyvärinen J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol. 1969;32:452–484. doi: 10.1152/jn.1969.32.3.452. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Nowak LG, Azouz R, Sanchez-Vives MV, Gray CM, McCormick DA. Electrophysiological classes of cat primary visual cortical neurons in vivo as revealed by quantitative analyses. J Neurophysiol. 2003;89:1541–1566. doi: 10.1152/jn.00580.2002. [DOI] [PubMed] [Google Scholar]
- Parker A, Gaffan D. Memory after frontal/temporal disconnection in monkeys: conditional and non-conditional tasks, unilateral and bilateral frontal lesions. Neuropsychologia. 1998;36:259–271. doi: 10.1016/s0028-3932(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Rivaud S, Gaymard B, Agid Y. Cortical control of reflexive visually guided saccades. Brain. 1991;114:1473–1485. doi: 10.1093/brain/114.3.1473. [DOI] [PubMed] [Google Scholar]
- Rainer G, Asaad WF, Miller EK. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature. 1998;393:577–579. doi: 10.1038/31235. [DOI] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J Neurophysiol. 1999;81:1903–1916. doi: 10.1152/jn.1999.81.4.1903. [DOI] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABAA blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond BJ, Optican LM. Temporal encoding of two-dimensional patterns by single units in primate inferior temporal cortex. II. Quantification of response waveform. J Neurophysiol. 1987;57:147–161. doi: 10.1152/jn.1987.57.1.147. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Buckley MJ, Gough PM, Alexander IH, Kyriazis D, McDonald KR, Passingham RE. Attentional selection and action selection in the ventral and orbital prefrontal cortex. J Neurosci. 2005;25:11628–11636. doi: 10.1523/JNEUROSCI.2765-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami M, Niki H. Encoding of behavioral significance of visual stimuli by primate prefrontal neurons: relation to relevant task conditions. Exp Brain Res. 1994;97:423–436. doi: 10.1007/BF00241536. [DOI] [PubMed] [Google Scholar]
- Schlag-Rey M, Amador N, Sanchez H, Schlag J. Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature. 1997;390:398–401. doi: 10.1038/37114. [DOI] [PubMed] [Google Scholar]
- Shin SY, Sommer MA. Frontal eye field input neurons have higher spontaneous firing rates and narrower action potentials than output neurons. Soc Neurosci Abstr. 2006;32:138–11. [Google Scholar]
- Sillito AM, Salt TE, Kemp JA. Modulatory and inhibitory processes in the visual cortex. Vision Res. 25:375–381. doi: 10.1016/0042-6989(85)90062-8. [DOI] [PubMed] [Google Scholar]
- Simons DJ. Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol. 1978;41:798–820. doi: 10.1152/jn.1978.41.3.798. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Hanes DP, Bichot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol. 1996;76:4040–4055. doi: 10.1152/jn.1996.76.6.4040. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Anderson KC, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature. 2001;411:953–956. doi: 10.1038/35082081. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Tegnér J, Constantinidis C, Goldman-Rakic PS. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci U S A. 2004;101:1368. doi: 10.1073/pnas.0305337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener SP, Johnston K, Everling S. Microstimulation of monkey dorsolateral prefrontal cortex impairs antisaccade performance. Exp Brain Res. 2008;190:463–473. doi: 10.1007/s00221-008-1488-4. [DOI] [PubMed] [Google Scholar]
- White IM, Wise SP. Rule-dependent neuronal activity in the prefrontal cortex. Exp Brain Res. 1999;126:315–335. doi: 10.1007/s002210050740. [DOI] [PubMed] [Google Scholar]
- Wilson FA, O'Scalaidhe SP, Goldman-Rakic PS. Functional synergism between putative gamma-aminobutyrate-containing neurons and pyramidal neurons in prefrontal cortex. Proc Natl Acad Sci U S A. 1994;91:4009–4013. doi: 10.1073/pnas.91.9.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Tanaka M. Enhanced modulation of neuronal activity during antisaccades in the primate globus pallidus. Cereb Cortex. 2008;19:206–217. doi: 10.1093/cercor/bhn069. [DOI] [PubMed] [Google Scholar]
- Zhang M, Barash S. Neuronal switching of sensorimotor transformations for antisaccades. Nature. 2000;408:971–975. doi: 10.1038/35050097. [DOI] [PubMed] [Google Scholar]