Abstract
Amyloid β (Aβ) immunotherapy is emerging as a promising disease-modifying therapy for Alzheimer's disease, although the precise mechanisms whereby anti-Aβ antibodies act against amyloid deposition and cognitive deficits remain elusive. To test the “peripheral sink” theory, which postulates that the effects of anti-Aβ antibodies in the systemic circulation are to promote the Aβ efflux from brain to blood, we studied the clearance of 125I-Aβ1-40 microinjected into mouse brains after intraperitoneal administration of an anti-Aβ monoclonal antibody 266. 125I-Aβ1-40 was rapidly eliminated from brains with a half-life of ∼30 min in control mice, whereas 266 significantly retarded the elimination of Aβ, presumably due to formation of Aβ-antibody complex in brains. Administration of 266 to APP transgenic mice increased the levels of monomer Aβ species in an antibody-bound form, without affecting that of total Aβ. We propose a novel mechanism of Aβ immunotherapy by the class of anti-Aβ antibodies that preferentially bind soluble Aβ, i.e., intracerebral, rather than peripheral, sequestration of soluble, monomer form of Aβ, thereby preventing the accumulation of multimeric toxic Aβ species in brains.
Introduction
Immunization with amyloid β peptide (Aβ), the pathogenic protein in Alzheimer's disease (AD), or passive transfer of anti-Aβ antibodies, have been shown to be effective at reducing the amyloid burden and reversing the memory deficiency phenotype in APP transgenic (Tg) mice (Schenk et al., 1999; Janus et al., 2000; Morgan et al., 2000). These observations bolstered the clinical development of Aβ immunotherapy for treatment of AD, although a long-term follow-up study of Aβ immunization in patients with AD showed that clearance of Aβ deposits did not necessarily prevent progressive neurodegeneration (Holmes et al., 2008). Anti-Aβ antibodies are presumed to be the effector molecules in Aβ immunotherapy, although the precise mechanisms, as well as the site of action of anti-Aβ antibodies in immunotherapy remain elusive. A class of anti-Aβ antibodies directed to the Aβ N terminus have been shown to act within the CNS by binding to Aβ aggregates in plaques, triggering microglial phagocytotic clearance of amyloid plaques through an Fc receptor-mediated mechanism (Bard et al., 2000), or inhibiting aggregation or neurotoxicity of Aβ (McLaurin et al., 2002; Mamikonyan et al., 2007). Non-Fc-mediated mechanisms may be involved in clearance of Aβ plaques because F(ab′)2 fragments that lack the Fc region of the antibody also are effective (Bacskai et al., 2002). Another proposed mechanism of Aβ clearance is that the site of antibody action is in the periphery, where the anti-Aβ antibodies would sequestrate soluble forms of Aβ in the peripheral circulation and drive an efflux of Aβ from the brain to the blood plasma, providing a “peripheral sink” for Aβ clearance (DeMattos et al., 2001, 2002a,b; Lemere et al., 2003). This hypothesis was proposed based on data on passive transfer of an anti-Aβ monoclonal antibody (mAb) 266, which recognizes the midportion of Aβ and has a high affinity to soluble Aβ but not to aggregated β-amyloid (Seubert et al., 1992; DeMattos et al., 2001), in PDAPP Tg mice. Intraperitoneal administration of 266 resulted in a rapid and robust increase in the level of plasma Aβ, and chronic treatment with 266 reduced Aβ deposition in the brains of PDAPP mice, leading to the notion that 266 might have reduced brain Aβ by changing the equilibrium of Aβ levels between brain interstitial fluids and blood, thereby accelerating the Aβ efflux and causing an acute improvement of learning and memory (DeMattos et al., 2001; Dodart et al., 2002). Other studies have not observed cerebral amyloid reduction with 266 therapy, and attributed the increased plasma Aβ levels to a reduced clearance rate of Aβ complexed to antibody (Seubert et al., 2008). Moreover, direct evidence for whether anti-Aβ antibodies in the blood acutely accelerate the efflux of Aβ from the brain, has not been shown.
In this study, we conducted further examination of the mechanism of action of mAb 266 in vivo, using brain injection of radiolabeled Aβ and biochemical analysis of Aβ in the brains of APP transgenic mice, and showed that mAb 266 acts within the brain parenchyma by binding to and stabilizing the soluble, monomeric form of Aβ. Our observation argues against the peripheral sink theory and proposes a novel mechanism whereby Aβ antibodies act in Aβ immunotherapy, i.e., sequestration of soluble Aβ within the CNS, not in the peripheral blood stream.
Materials and Methods
Animals.
Seven- to nine-week-old male C57BL/6J mice were purchased from Charles River Laboratories. A7 are transgenic mice that overexpress human APP695 harboring K670N, M671L, and T714I FAD mutations in neurons under the control of Thy1.2 promoter. The expression level of the transgenic human mutant APP is ∼1.4-fold that of endogenous murine APP. These mice develop progressive amyloid deposition in cerebral cortices at the age of ∼9- to 12-month-old in an age-dependent manner (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). All animals were maintained on food and water with a 12 h light/dark cycle. All experiments were approved by the Institutional Animal Care and Use Committee of the Graduate School of Pharmaceutical Sciences, The University of Tokyo.
Reagents.
Radioiodinated human [125I]-Aβ1-40 was purchased from Perkin-Elmer Life Sciences as described previously (Yamada et al., 2008). [14C]-Inulin was obtained from American Radiolabeled Chemicals. 10D5 (against residues 3–7 of Aβ) and 266 (against residues 16–24 of Aβ) used in the passive immunization experiments are anti-Aβ mouse mAbs (IgG1) as described previously (Seubert et al., 1992; Bard et al., 2000). LB509, an anti-α-synuclein mouse mAb (IgG1), was used as a control antibody (Baba et al., 1998). Anti-Aβ antibody 82E1, which recognizes the N terminus of human Aβ, was purchased from IBL.
Brain microinjection of 125I-Aβ1-40 and assay of efflux from brain.
10D5, 266, and LB509 were administrated by intraperitoneal injection at a dose of 300 μg. One, twenty-four, or one hundred twenty hours after antibody administration, 125I-Aβ1-40 was injected into the cerebral cortices, and its clearance was monitored as previously described (Kakee et al., 1996). Briefly, mice were anesthetized with an intraperitoneal injection of pentobarbital (100 mg/kg) and placed on the heating pad to maintain the body temperature. A burr hole was made right above the parietal cortex area 2 (Par2) region, and a unilateral injection of the applied solution (0.30 μl) containing [125I]-Aβ1-40 (0.007–0.02 μCi) and [14C]-inulin (0.20 μCi) in an extracellular fluid (ECF) buffer (122 mm NaCl, 25 mm NaHCO3, 3 mm KCl, 1.4 mm CaCl2, 1.2 mm MgSO4, 0.4 mm K2HPO4, 10 mm d-glucose, and 10 mm HEPES, pH 7.4) was made using a Hamilton syringe over a period of 30 s. The target coordinate (Par2) was 0.0 mm anterior/posterior and 3.9 mm lateral to bregma, and 2.5 mm ventral to the surface of the skull, determined by using a stereotaxic frame (SR-6; Narishige). The precise location of the target region was fixed according to Franklin and Paxinos (2007). Par2 region was selected because the transfer of the molecule to CSF or contralateral hemisphere is reported to be minimal upon injection into this region (Kakee et al., 1996). The concentration of 125I-Aβ in the solution was ∼10–30 nm. After microinjection, the microsyringe was kept in place for an additional 4 min before gentle withdrawal to minimize backflow. At designated times, the injected side of the brain was excised and dissolved in 2 ml of 2 m NaOH at 60°C for 1 h. The [125I] radioactivity of the samples was measured in a gamma counter (ART300, Aloka). The samples were then mixed with 14 ml of Hionic-fluor (Perkin-Elmer), and the [14C] radioactivity was measured in a liquid scintillation counter (Perkin-Elmer). The brain efflux index (BEI), which represents the percentage of 125I-Aβ effluxed from the brain, was defined by Equation 1, and the percentage of 125I-Aβ remaining in the ipsilateral cerebrum (100 − BEI) was determined using Equation 2:
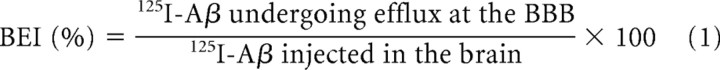 |
 |
In the brain coinjection study, 125I-Aβ was mixed with 600 ng/ml antibodies (10D5, 266, or LB509) and was injected into the Par2 as above. The apparent elimination rate constant (kapp,el) was determined from the slope given by fitting a semilogarithmic plot of (100 − BEI) versus time (Shiiki et al., 2004).
The 125I-Aβ level in blood plasma at 60 min after brain microinjection in control IgG- and 266-treated mice were 8.65 ± 0.53% and 7.34 ± 0.69% (n = 4, each), respectively, which were considerably lower than the predicted levels of 125I-Aβ to be detected outside the brain (∼85% in control IgG- and ∼55% in 266-treated, respectively, based on the BEI results), presumably due to the rapid uptake and degradation by peripheral organs.
Determination of binding of antibodies to 125I-Aβ.
The binding of anti-Aβ antibodies to Aβ was determined by immunoprecipitation of 125I-Aβ1-40 in vitro. We have confirmed that 125I-Aβ1-40 in solution exists in a monomeric state (Yamada et al., 2008). 125I-Aβ1-40 was mixed in PBS, pH 7.6 with 0, 0.01, 0.1, 1, or 10 μg/ml antibodies (10D5, 266, or LB509) and protein G agarose beads (Invitrogen), and incubated for 1 h at 4°C. After incubation, the beads were washed with radioimmunoprecipitation assay (RIPA) buffer (50 mm Tris-HCl, pH = 7.4, 150 mm NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) and counted by a gamma counter.
Determination of binding of antibodies to fibrillized Aβ.
The binding of anti-Aβ antibodies to fibrillized Aβ was determined by ELISA. Microtiter wells were coated with fibrillized Aβ1-42 at 4°C as previously described (Osada et al., 2005). Unoccupied sites were blocked with Block Ace (Snow Brand), and washed with PBS containing 0.05% Tween 20. Wells were then incubated with anti-Aβ antibodies (10D5 or 266) or LB509 for 3 h. After incubation with a horseradish peroxidase-tagged secondary antibody (GE Healthcare Life Sciences), the levels of antibodies bound to fibrillized Aβ were quantitated by development using TMB microwell system (KPL).
Brain extraction and guanidine HCl denaturation.
A7 transgenic mice (4 months old) were killed 120 h after intraperitoneal injection of 600 μg of antibodies (LB509 or 266). Mice were extensively perfused with PBS before brain excision. The brains were homogenized in RIPA buffer containing Complete protease inhibitor cocktail (Roche). Homogenates were centrifuged at 75,000 × g for 20 min at 4°C, and the resultant supernatant was collected as brain RIPA extract. The RIPA extract was mixed with 8 m guanidine HCl (Gdn) to a final concentration of 4 m Gdn and incubated at room temperature for 30 min. These Gdn-denatured RIPA extracts of brains were diluted to 500 mm Gdn and Aβ level was analyzed by ELISA.
ELISA.
Levels of Aβ monomer in brain extracts were quantitated by BAN50/BA27 or BAN50/BC05 ELISAs, that specifically recognize monomeric form of Aβ, as previously described (Iwatsubo et al., 1994; Enya et al., 1999) (supplemental Fig. S2, available at www.jneurosci.org as supplemental material).
Immunoblot analysis.
SDS-PAGE was performed as described previously (Hashimoto et al., 2002) using an anti-Aβ antibody 82E1. The immunoblots were visualized using Immunostar reagents (Wako Pure Chemical) using LAS-1000plus (FUJIFILM) as described previously (Hashimoto et al., 2002).
Statistics.
Statistical analyses were performed using Student's t test or ANOVA.
Results
Peripheral administration of an anti-Aβmonoclonal antibody 266 retards the Aβ clearance from mouse brains
To examine whether passive immunization by peripheral administration of anti-Aβ antibodies results in a “sink effect,” i.e., acute promotion of the Aβ efflux from the brain, we microinjected 125I-Aβ1-40 into the cerebral neocortex of mice after intraperitoneal injection of anti-Aβ or control (LB509; anti-α-synuclein) murine mAbs, and analyzed the time course of labeled-Aβ clearance from the brain (Fig. 1A). The radioactivity derived from 125I-Aβ microinjected into the cerebrum of nontreated or control mAb-treated mice was rapidly eliminated from the brain with a half-life of ∼30 min (Fig. 1B), in agreement with the previous reports (Shibata et al., 2000; Shiiki et al., 2004; Ito et al., 2007). Mice treated with 10D5, which recognizes the N terminus and preferentially binds to fibrillized form of Aβ (supplemental Fig. S3, available at www.jneurosci.org as supplemental material), showed no change in the elimination of 125I radioactivity compared with the rate in control mice. In sharp contrast, administration of 266, an antibody that recognizes the midportion of Aβ and prefers the soluble form of Aβ, caused a marked retardation in the elimination of 125I radioactivity from the brain (Fig. 1B). The Aβ retention effects by 266 were similarly observed at shorter intervals of 1 or 24 h after intraperitoneal administration of antibodies (Fig. 1C). Altogether, these results indicate that passive immunization with the anti-Aβ antibodies does not cause an acute promotion of Aβ efflux from the brain, and that administration of 266 actually caused retention of Aβ in the brain.
Figure 1.

Brain injection and clearance of 125I-Aβ1-40 after peripheral administration of anti-Aβ antibodies in mice. A, Time course of intraperitoneal administration of antibodies and 125I-Aβ injection. 125I-Aβ was injected into the cortex of mice brains 1, 24, or 120 h after antibody administration. The radioactivities of Aβ remaining in the brain were calculated as 100 − BEI (%) at 15, 30, 60, or 120 min after injection of 125I-Aβ. B, Time course of elimination of 125I-Aβ from brains 120 h after intraperitoneal administration of 10D5, 266, or LB509 as a control IgG. The means ± SEMs in three to eight independent assays are shown. **p < 0.01, ANOVA. C, The remaining 125I-Aβ at 60 min after microinjection of 125I-Aβ at 1, 24, or 120 h after intraperitoneal administration of 266 or LB509. The means ± SEMs in three to eight independent assays are shown. *p < 0.05, **p < 0.01, by Student's t test.
Anti-Aβ monoclonal antibody 266 enters the brain parenchyma and binds to Aβ
To examine whether peripherally administrated 266 entered the brain parenchyma and formed a complex with injected 125I-Aβ, we captured the Ig moiety of the Aβ-antibody complex in the RIPA buffer lysates of brains by protein G after an extensive perfusion. Approximately 45% of the 125I-Aβ brain-injected radioactivity was retrieved from the protein G precipitates of the RIPA-extracts of brains of 266-treated mice, suggesting that 266 formed a complex with 125I-Aβ in the brain parenchyma (Fig. 2A). In contrast, only ∼10% of 125I radioactivity was precipitated in 10D5-treated mice, a level comparable to controls. To further examine whether the formation of Aβ/266 complex delayed Aβ efflux, we coinjected anti-Aβ antibodies with 125I-Aβ into the cortices of C57BL/6J mice. Coinjection of 266 with 125I-Aβ significantly reduced the elimination rate constant (kapp,el) of 125I-Aβ efflux from the brain, by ∼70% compared with those by administration of 10D5 or a control mAb (Fig. 2B). Together, the data strongly suggest that a fraction of peripherally administered 266 entered the brain and captured 125I-Aβ, thereby inhibiting the brain clearance of Aβ.
Figure 2.
Formation of 125I-Aβ/antibody complex in mouse brains. A, Peripheral administration of antibodies 10D5, 266, or LB509 followed by intracerebral injection of 125I-Aβ and protein G precipitation of antibodies from brain extracts. 125I-Aβ was injected 5 d after intraperitoneal administration of antibodies. After extensive transcardiac perfusion with PBS, brains were removed and extracted with RIPA buffer and the resulting supernatants were precipitated by protein G agarose. The radioactivity derived from 125I-Aβ bound to the protein G-precipitated antibody as a percentage of total injected 125I-Aβ is shown. The means ± SEMs in three independent assays are shown. ***p < 0.001, ANOVA. B, Coinjection of 125I-Aβ with antibodies 10D5, 266, or LB509 (1 mg/ml) into brains. Apparent elimination rate constant (kapp,el %) of 125I-Aβ efflux is indicated as a percentage of that with LB509 (control antibody) as 100%. The means ± SEMs in three independent assays are shown. **p < 0.01, ANOVA.
Anti-Aβ antibody 266 increases the level of the monomeric, antibody-bound form of Aβ, without affecting that of the total soluble Aβ, in the brain
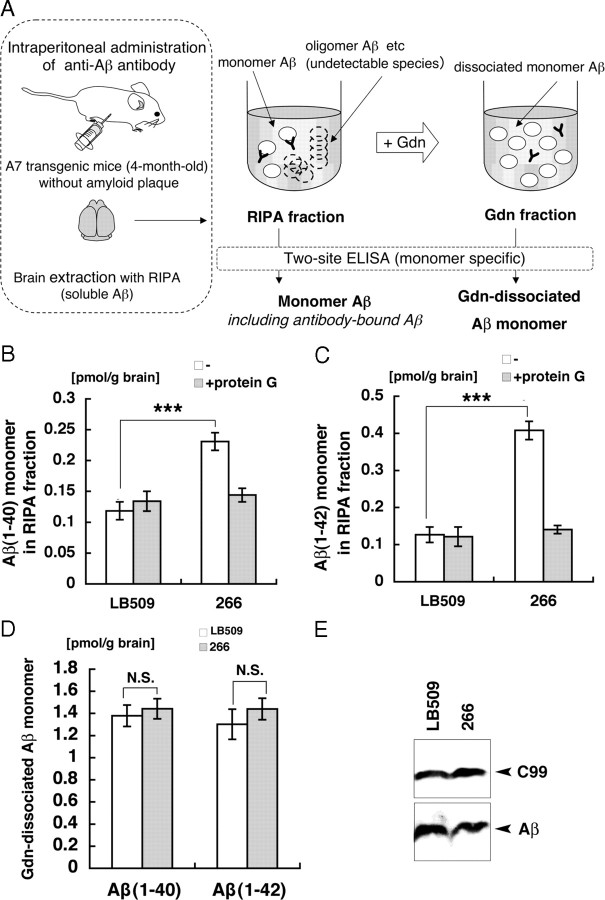
We further analyzed the effects of peripheral administration of 266 on the relatively soluble Aβ species in the brains of young (4-month-old) Tg mice (A7) that overexpress human APP harboring Swedish/Austrian mutations and have not developed amyloid deposits yet. These mice showed an increased level of plasma Aβ upon 266 treatment (supplemental Fig. S4, available at www.jneurosci.org as supplemental material). We quantitated the levels of the monomer form of Aβ in two sequentially prepared fractions, using two-site ELISAs that have been shown to specifically recognize monomer Aβ, but not multimers including dimers (Enya et al., 1999) (supplemental Fig. S3, available at www.jneurosci.org as supplemental material). We first extracted brains of A7 Tg mice with RIPA buffer (RIPA fraction), and further denatured the RIPA fraction with 4 m Guanidine HCl (Gdn fraction) (Fig. 3A). Thus, Aβ detected in the RIPA fraction represents Aβ species that were present in monomeric state (monomer Aβ) in the brain; it may either be in antibody-bound or unbound forms, because the ELISAs detected 266-bound monomer Aβ at a comparable sensitivity to unbound form (supplemental Fig. S5, available at www.jneurosci.org as supplemental material). In contrast, Aβ detected in the Gdn fraction by the monomer-specific ELISAs should include Aβ that was present in the RIPA-extractable fraction in multimeric state or whose epitope has been masked before Gdn denaturation (Gdn-dissociated monomer Aβ).
Figure 3.
Differential extraction of brains of APP transgenic mice following peripheral administration of anti-Aβ antibodies. A, Procedures of ELISA quantitation of Aβ in two sequentially prepared fractions from brains of APP transgenic mice. The level of “monomer Aβ” is defined as the concentration of Aβ in RIPA fraction detected by monomer-specific two-site ELISA, and “Gdn-dissociated soluble Aβ” as that of Aβ in the Gdn fraction. The levels of monomer Aβ1-40 (B) and Aβ1-42 (C) in the RIPA fractions of 4-month-old A7 mice 120 h after intraperitoneal administration of 266 or LB509 were quantitated by ELISA with (shaded) or without (blank) immunodepletion by protein G. The means ± SEMs in four independent assays are shown. ***p < 0.001, ANOVA. D, The levels of Gdn-dissociated monomer Aβ in the Gdn-fraction of the brains of 4-month-old A7 mice 120 h after intraperitoneal administration of 266 (shaded) or LB509 (blank) were quantitated by ELISA. The means ± SEMs in four independent assays are shown. E, The total Aβ and its precursor C99 in the RIPA fractions were analyzed by immunoblotting with 82E1 after immunoprecipitation with the same antibody.
The levels of monomer Aβ in the RIPA fraction of A7 APP Tg mice were significantly elevated by 266 treatment by approximately twofold for Aβ1-40 and approximately threefold for Aβ1-42 (Fig. 3B,C). These increases in the levels of monomer Aβ1-40 and Aβ1-42 were not observed after protein G immunodepletion of the RIPA fraction, suggesting that the increased Aβ was complexed with 266 in the brain parenchyma (Fig. 3B,C), whereas the levels of Gdn-dissociated monomer Aβ were not altered (Fig. 3D). The total level of Aβ in the RIPA fraction detected by immunoprecipitation by an anti-Aβ mAb 82E1, whose binding is not competed by 266 (supplemental Fig. S4C, available at www.jneurosci.org as supplemental material), also was not altered by 266 treatment (Fig. 3E). Together, we concluded that peripherally administered anti-Aβ mAb 266 entered the brain and selectively bound and stabilized the soluble, monomer form of Aβ, without affecting the total level of Aβ in the soluble fraction of brains of APP Tg mice.
Discussion
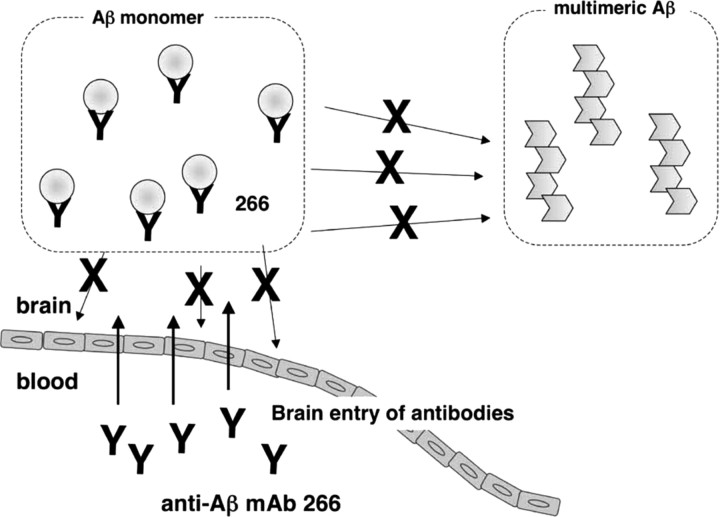
Previous studies have been mixed as to whether peripheral administration of a high-affinity capture anti-Aβ antibody can reduce central amyloidosis in a transgenic mouse model of AD (DeMattos et al., 2001; Seubert et al., 2008). Furthermore, administration of anti-Aβ antibodies to APP Tg mice did not alter the levels of RIPA-soluble Aβ, despite a dramatic increase in plasma Aβ (Levites et al., 2006), questioning the ability of peripheral anti-Aβ antibodies to reduce the levels of soluble Aβ within the brain. Specifically, it has not been directly addressed whether anti-Aβ antibodies in the periphery accelerate the efflux of Aβ from brains. Combining 125I-Aβ microinjection with passive immunization of anti-Aβ mAbs, we showed that peripheral administration of mAb 266 actually retards the efflux transport of Aβ from brain to blood stream. We further asked what types of Aβ species in multimerization state or solubility are targeted by anti-Aβ mAbs in the brains of APP tg mice using different denaturation techniques and detection by monomer Aβ-specific ELISA, and showed that 266 caused an increase in the soluble, monomer form of Aβ in brain (Fig. 3B,C). Immunodepletion by protein G significantly decreased the levels of Aβ monomer in 266-treated mice, suggesting that the increased monomer was complexed with mAb 266. In contrast, the levels of Aβ in the RIPA fraction detected after Gdn denaturation, that represent a larger pool of soluble Aβ in the brain, were not altered by administration of 266 (Fig. 3D,E). These data suggest that 266 binds to and sequesters monomer Aβ in the brain parenchyma, resulting in the reduction in the level of antibody-free Aβ, potentially influencing the ability of free Aβ to form into oligomers and amyloid fibrils that may be neurotoxic and causative to functional deterioration (Legleiter et al., 2004) (Fig. 4). The antibody-free Aβ should comprise a subfraction of the protein G-insensitive Aβ, although the total levels of the latter fraction were similar regardless of immunodepletion by protein G (Fig. 3B,C). Further studies to detect the Aβ species decreased in the antibody-free fraction, the latter being a mixture of Aβ in different multimerization and interaction states, would be crucial to the understanding of the mechanism of action of 266. It would also be interesting to see whether 266 may have different effects upon administration in older animals after amyloid deposition is established.
Figure 4.
Possible mechanism of action of anti-Aβ mAb 266 in passive Aβ immunotherapy. 266 enters the brain parenchyma and sequesters Aβ chiefly in a monomeric state, thereby inhibiting further multimerization of Aβ and neurotoxicity.
In addition to the potential mechanisms of action for immunotherapy including binding to and promoting clearance of insoluble plaques in the brain (Bard et al., 2000) and the perturbation of efflux from the brain by binding to plasma forms of Aβ (DeMattos et al., 2001, 2002a,b), we must also consider the potential for anti-Aβ antibodies to directly bind soluble Aβ within the CNS. That this binding may have biological importance is supported by acute behavioral improvements noted in APP transgenic models following immunotherapy (Dodart et al., 2002; Kotilinek et al., 2002). Other possible mechanisms may include perturbation of APP processing by binding of 266 (Tampellini et al., 2007). Our findings modify the current understanding of the mechanism of Aβ immunotherapy, and suggest an important contribution of antibody interactions with soluble Aβ within the CNS.
Footnotes
This work was supported by Core Research for Evolutional Science and Technology of Japan Science and Technology Agency, and by grants-in-aid from the Japan Society for the Promotion of Science. We thank Drs. D. M. Holtzman, R. B. DeMattos, Y. Nagae, and T. Wakabayashi for helpful discussions, D. M. A. Mann and T. Tomita for critical reading of the manuscript, and S. Ito for kind instructions on the 125I-Aβ microinjection experiments.
References
- Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T. Aggregation of α-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- Bacskai BJ, Kajdasz ST, McLellan ME, Games D, Seubert P, Schenk D, Hyman BT. Non-Fc-mediated mechanisms are involved in clearance of amyloid-β in vivo by immunotherapy. J Neurosci. 2002;22:7873–7878. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-Aβ antibody alters CNS and plasma Aβ clearance and decreases brain Aβ burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-β efflux: a measure of brain amyloid burden in a mouse model of Alzheimer's disease. Science. 2002a;295:2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Parsadanian M, O'Dell MA, Foss EM, Paul SM, Holtzman DM. Plaque-associated disruption of CSF and plasma amyloid-β (Aβ) equilibrium in a mouse model of Alzheimer's disease. J Neurochem. 2002b;81:229–236. doi: 10.1046/j.1471-4159.2002.00889.x. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reverses memory deficits without reducing brain Aβ burden in Alzheimer's disease model. Nat Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- Enya M, Morishima-Kawashima M, Yoshimura M, Shinkai Y, Kusui K, Khan K, Games D, Schenk D, Sugihara S, Yamaguchi H, Ihara Y. Appearance of sodium dodecyl sulfate-stable amyloid β-protein (Aβ) dimer in the cortex during aging. Am J Pathol. 1999;154:271–279. doi: 10.1016/s0002-9440(10)65273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. The mouse brain in stereotaxic coordinates. London: Academic; 2007. [Google Scholar]
- Hashimoto T, Wakabayashi T, Watanabe A, Kowa H, Hosoda R, Nakamura A, Kanazawa I, Arai T, Takio K, Mann DMA, Iwatsubo T. CLAC: a novel Alzheimer amyloid plaque component derived from a transmembrane precursor, CLAC-P/collagen type XXV. EMBO J. 2002;21:1524–1534. doi: 10.1093/emboj/21.7.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA. Long-term effects of Aβ42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Ito S, Ohtsuki S, Kamiie J, Nezu Y, Terasaki T. Cerebral clearance of human amyloid-β peptide (1-40) across the blood-brain barrier is reduced by self-aggregation and formation of low-density lipoprotein receptor-related protein-1 ligand complexes. J Neurochem. 2007;103:2482–2490. doi: 10.1111/j.1471-4159.2007.04938.x. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of Aβ42(43) and Aβ40 in senile plaques with end-specific Aβ monoclonals: evidence that an initially deposited species is Aβ42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HTJ, Nixon RA, Mercken M, Bergeron C, Fraser PE, St George-Hyslop P, Westaway D. Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- Kakee A, Terasaki T, Sugiyama Y. Brain efflux index as a novel method of analyzing efflux transport at the blood-brain barrier. J Pharmacol Exp Ther. 1996;277:1550–1559. [PubMed] [Google Scholar]
- Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, Younkin S, Ashe KH. Reversible memory loss in a mouse transgenic model of Alzheimer's disease. J Neurosci. 2002;22:6331–6335. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legleiter J, Czilli DL, Gitter B, DeMattos RB, Holtzman DM, Kowalewski T. Effect of different anti-Aβ antibodies on Aβ fibrillogenesis as assessed by atomic force microscopy. J Mol Biol. 2004;335:997–1006. doi: 10.1016/j.jmb.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Spooner ET, LaFrancois J, Malester B, Mori C, Leverone JF, Matsuoka Y, Taylor JW, DeMattos RB, Holtzman DM, Clements JD, Selkoe DJ, Duff KE. Evidence for peripheral clearance of cerebral Aβ protein following chronic, active Aβ immunization in PSAPP mice. Neurobiol Dis. 2003;14:10–18. doi: 10.1016/s0969-9961(03)00044-5. [DOI] [PubMed] [Google Scholar]
- Levites Y, Smithson LA, Price RW, Dakin RS, Yuan B, Sierks MR, Kim J, McGowan E, Reed DK, Rosenberry TL, Das P, Golde TE. Insights into the mechanisms of action of anti-Aβ antibodies in Alzheimer's disease mouse models. FASEB J. 2006;20:2576–2578. doi: 10.1096/fj.06-6463fje. [DOI] [PubMed] [Google Scholar]
- Mamikonyan G, Necula M, Mkrtichyan M, Ghochikyan A, Petrushina I, Movsesyan N, Mina E, Kiyatkin A, Glabe CG, Cribbs DH, Agadjanyan MG. Anti-Aβ 1–11 antibody binds to different β-amyloid species, inhibits fibril formation, and disaggregates preformed fibrils but not the most toxic oligomers. J Biol Chem. 2007;282:22376–22386. doi: 10.1074/jbc.M700088200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin J, Cecal R, Kierstead ME, Tian X, Phinney AL, Manea M, French JE, Lambermon MH, Darabie AA, Brown ME, Janus C, Chishti MA, Horne P, Westaway D, Fraser PE, Mount HT, Przybylski M, St George-Hyslop P. Therapeutically effective antibodies against amyloid-β peptide target amyloid-β residues 4–10 and inhibit cytotoxicity and fibrillogenesis. Nat Med. 2002;8:1263–1269. doi: 10.1038/nm790. [DOI] [PubMed] [Google Scholar]
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- Osada Y, Hashimoto T, Nishimura A, Matsuo Y, Wakabayashi T, Iwatsubo T. CLAC binds to amyloid β peptides through the positively charged amino acid cluster within the collagenous domain 1 and inhibits formation of amyloid fibrils. J Biol Chem. 2005;280:8596–8605. doi: 10.1074/jbc.M413340200. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, McCormack R, Wolfert R, Selkoe D, Lieberburg I, Schenk D. Isolation and quantification of soluble Alzheimer's β-peptide from biological fluids. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Seubert P, Barbour R, Khan K, Motter R, Tang P, Kholodenko D, Kling K, Schenk D, Johnson-Wood K, Schroeter S, Gill D, Jacobsen JS, Pangalos M, Basi G, Games D. Antibody capture of soluble Aβ does not reduce cortical Aβ amyloidosis in the PDAPP mouse. Neurodegener Dis. 2008;5:65–71. doi: 10.1159/000112834. [DOI] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer's amyloid-β(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiiki T, Ohtsuki S, Kurihara A, Naganuma H, Nishimura K, Tachikawa M, Hosoya K, Terasaki T. Brain insulin impairs amyloid-β(1-40) clearance from the brain. J Neurosci. 2004;24:9632–9637. doi: 10.1523/JNEUROSCI.2236-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampellini D, Magrané J, Takahashi RH, Li F, Lin MT, Almeida CG, Gouras GK. Internalized antibodies to the Abeta domain of APP reduce neuronal Abeta and protect against synaptic alterations. J Biol Chem. 2007;282:18895–18906. doi: 10.1074/jbc.M700373200. [DOI] [PubMed] [Google Scholar]
- Yamada K, Hashimoto T, Yabuki C, Nagae Y, Tachikawa M, Strickland DK, Liu Q, Bu G, Basak JM, Holtzman DM, Ohtsuki S, Terasaki T, Iwatsubo T. The low density lipoprotein receptor-related protein 1 mediates uptake of amyloid β peptides in an in vitro model of the blood-brain barrier cells. J Biol Chem. 2008;283:34554–34562. doi: 10.1074/jbc.M801487200. [DOI] [PMC free article] [PubMed] [Google Scholar]