Abstract
Activation of cannabinoid receptors (CB1, CB2 and GPR55) produces analgesic effects in several experimental pain models, including visceral pain arising from the gastrointestinal tract. We assessed the role of CB1, CB2, and GPR55 receptors and the endogenous cannabinoid system on basal pain responses and acute mechanical hyperalgesia during colorectal distension (CRD) in rodents. The effects of cannabinoid receptor agonists and antagonists on pain-related responses to CRD were assessed in rats and in wild-type and CB1 receptor knock-out mice. The dual CB1/2 agonist, WIN55,212-2, and the peripherally acting CB1-selective agonist, SAB-378, inhibited pain-related responses to repetitive noxious CRD (80 mmHg) in a dose-related manner in rats. The analgesic effects of WIN55,212-2 and SAB-378 were blocked by the selective CB1 antagonist SR141716, but were not affected by the selective CB2 antagonist SR144528. SR141716, per se, increased the responses to repetitive noxious CRD, indicative of hyperalgesia, and induced pain-related responses during non-noxious CRD (20 mmHg), indicative of allodynia. The cannabinoid receptor agonists anandamide, virodhamine and O-1602 had no effect. At analgesic doses, WIN55,212-2 did not affect colonic compliance. In accordance to the rat data, WIN55,212-2 produced analgesia, whereas SR141716 induced hyperalgesia, during noxious CRD (55 mmHg) in wild-type but not in CB1-knock-out mice. These data indicate that peripheral CB1 receptors mediate the analgesic effects of cannabinoids on visceral pain from the gastrointestinal tract. The allodynic and hyperalgesic responses induced by SR141716 suggest the existence of an endogenous cannabinoid tone and the activation of CB1 receptors during noxious CRD.
Keywords: cannabinoid receptors; endocannabinoids; hyperalgesia; SR141716; visceral pain; WIN55,212-2
Introduction
Biological effects of cannabinoids are mediated primarily through specific G-protein-coupled cannabinoid receptors. Two cannabinoid receptor subtypes (CB1 and CB2) and a putative third cannabinoid receptor (referred to as GPR55) have been identified (Howlett et al., 2002; Brown, 2007; Ryberg et al., 2007). All three cannabinoid receptors are activated by endogenous lipid ligands known as endocannabinoids, with anandamide and 2-arachinodonyl glycerol being the most abundant endocannabinoids (for review, see Bisogno, 2008; Di Marzo, 2008). CB1 receptors (CB1Rs) are mostly expressed in the nervous system at both central and peripheral sites, including the enteric nervous system (Matsuda et al., 1990, 1992; Ahluwalia et al., 2000; Coutts et al., 2002; Bridges et al., 2003; MacNaughton et al., 2004). In addition, CB1R expression has also been localized on normal colonic epithelium and smooth muscle (Casu et al., 2003; Ligresti et al., 2003). However, CB2 receptors (CB2Rs) are predominantly present in immune cells (Munro et al., 1993), whereas GPR55 has a more promiscuous distribution with high expression levels in the adrenal glands, small intestine and CNS (Ryberg et al., 2007).
Among the roles of the endocannabinoid system, analgesia at central and peripheral levels, particularly associated to somatic pain, is largely documented in both clinical and preclinical studies (Ashton and Milligan, 2008; Hosking and Zajicek, 2008). With respect to visceral pain, the information related to the effects of cannabinoids is still limited and somewhat controversial. For instance, the endocannabinoid system has been involved in both proalgesic (Dinis et al., 2004) and analgesic responses in the urinary tract (Farquhar-Smith et al., 2002). A limited number of studies have implicated both CB1Rs and CB2Rs in the modulation of gastrointestinal functions, including sensitivity (for review, see Hornby and Prouty, 2004; Di Marzo and Izzo, 2006; Storr and Sharkey, 2007; Storr et al., 2008; Wright et al., 2008). So far, only two independent studies have evaluated the role of CB1Rs and CB2Rs in viscerosensitivity during colorectal distension in rats (Sanson et al., 2006; Kikuchi et al., 2008). According to these studies, both CB1Rs and CB2Rs elicited analgesic responses under basal conditions (Sanson et al., 2006) and during states of inflammation-induced hyperalgesia (Sanson et al., 2006; Kikuchi et al., 2008). Interestingly, the only data available in humans showed that the CB1/CB2 nonselective agonist, dronabinol, relaxed the colon but increased sensation ratings to distension in healthy volunteers (Esfandyari et al., 2007). This was interpreted as an interaction between the colonic motor effects and the central action of cannabinoids modulating perception (increasing awareness).
The aim of the present study was to further assess the role of cannabinoid receptors and the endogenous cannabinoid system on basal pain responses and acute mechanical hyperalgesia during colorectal distension (CRD)-elicited visceral pain in rodents. For this, the effects of different cannabinoids (agonists and antagonists) with various selectivity for CB1Rs and CB2Rs were assessed on visceral pain-related responses during CRD in rats and in wild-type and CB1-knock-out mice.
Materials and Methods
Animals
Adult Sprague Dawley female rats (250–350 g; Harlan Netherlands) were used. In addition, adult female mice with a C57BL/6JOlaHsd background, normal (CB1+/+, wild-type mice) or deficient for the gene encoding for the CB1R [CB1−/−, knock-out mice; originally created by Zimmer et al. (1999)] were used in some of the experiments. Mice were bred in house (Astra-Zeneca R&D Mölndal) and genotyped by PCR on DNA isolated from tail biopsy samples. Homozygous mutant mice grew and reproduced normally, with no obvious phenotypical differences when compared with age- and gender-matched wild-type control mice. Animals were housed in group cages (2–5 animals per cage) in an enriched environment with free access to food (Standard pellets, R3, Lactamin) and tap water on a 12:12 h light-dark cycle and controlled conditions of temperature (21°C) and humidity (50%). Before starting any experimental procedure, animals were allowed to acclimatize to the animal facility for at least 1 week after arrival. The phase of the oestrus cycle was not taken into account. All experiments were approved by the local animal ethics review committee in Göteborg, Sweden.
Colorectal distension
For CRD in rats, a 3 cm polyethylene balloon with a connecting catheter (made in-house) was inserted into the distal colon, 2 cm from the base of the balloon to the anus, during light isoflurane anesthesia (Forene, Abbott Scandinavia). The catheter was fixed to the base of the tail with tape. At the same time, an intravenous catheter (Neoflon, Becton Dickinson) was inserted in a tail vein for compounds administration. Similar procedures were followed in mice. In that case, a 2 cm polyethylene balloon with a connecting catheter (in-house made) was inserted in the distal colon, 0.5 cm proximal to the anus, during light isoflurane anesthesia (Forene), and fixed to the tail with tape to avoid any displacement. For compound administration, when needed, a polyethylene catheter (PE25) was placed subcutaneously in the neck region. Thereafter, animals were placed in Bollmann cages and allowed to recover from sedation for at least 15 min before starting the experiments.
During the CRD procedure, the balloons, through the connecting catheter, were connected to pressure transducers (P-602, CFM-k33, 100 mmHg; Bronkhorst Hi-Tec). A customized barostat (Astra-Zeneca) was used to control the air inflation and intraballoon pressure. A customized computer software (PharmLab on-line 4.0.1) running on a standard PC was used to control the barostat and to perform data collection and storage. The distension paradigms generated by the barostat were achieved by generating pulse patterns on an analog output channel. When assessing pain relates responses in rats, two CRD paradigms were used: (1) repeated noxious phasic distensions, 12 times at 80 mmHg, with a pulse duration of 30 s at 5 min intervals; and (2) repeated non-noxious phasic distensions, 10 times at 20 mmHg, with a pulse duration of 30 s at 5 min intervals. Distensions at 20 mmHg are considered non-noxious because they are below the estimated threshold pressure for pain in this model and do not evoke a significant visceromotor response (Tammpere et al., 2005; Martínez el al., 2007). In mice, the CRD paradigm used consisted on repeated noxious phasic distensions, 12 times at 55 mmHg, with a pulse duration of 10 s at 5 min intervals. Similar CRD paradigms have been used previously to assess pain-related responses in mice and rats (Tammpere et al., 2005; Arvidsson et al., 2006; Martinez and Melgar, 2008).
Responses to CRD were assessed by recording and quantitation of phasic changes in intraballoon pressure during the distending pulses, as previously described (Tammpere et al., 2005; Arvidsson et al., 2006). Pressure oscillations during the isobaric inflation of the intracolonic balloon reflect abdominal muscle contractions associated to the distension procedure and, therefore, are considered a valid assessment of the visceromotor response associated to the presence of pain of visceral origin (Tammpere et al., 2005; Arvidsson et al., 2006).
For compliance measurements, ascending phasic isobaric distensions from 2 to 20 mmHg, with a pulse duration of 1 min at 5 min intervals, were used. In this case, pressure dependent changes in volume during isobaric CRD were taken as a measure of colorectal compliance (Käll et al., 2007; Martínez et al., 2007). When assessing compliance, low distension pressures (within a range considered non-noxious) were chosen to avoid pain-related visceromotor responses that might interfere with the measurements of the intraballoon volume. In this case, the barostat system was used to inflate the balloon to a predetermined pressure and to simultaneously measure pressure and volume changes.
Data collection and analysis
The analog input channels were sampled with individual sampling rates and digital filtering was performed on the signals. The balloons were connected to pressure transducers (P-602, CFM-k33, 100 mmHg, Bronkhorst Hi-Tec) and the pressure signals sampled at 50 Hz. A highpass filter at 1 Hz was used to separate the contraction-induced pressure changes from the slow varying pressure generated by the barostat. A resistance in the airflow between the pressure generator and the pressure transducer further enhanced the pressure variations induced by the abdominal contractions of the animal. In addition, a band-stop filtered at 49–51 Hz was used to remove line frequency interference. A customized computer software (PharmLab off-line 4.0.1) was used to quantify the phasic changes of the balloon pressure signals. Changes in the highpass filtered balloon pressure signals were determined for the 30 s (rat) or 5 s (mouse) period before each pulse (baseline) and for each pulse. When assessing CRD in mice, and to minimize variability due to movement-related artifacts, responses to CRD were determined for each 3 consecutive pulses as a group, as previously described (Arvidsson et al., 2006; Martinez and Melgar, 2008).
Drugs
WIN55,212-2 [[(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de)-1,4-benzoxazin-6-yl]-1-napthalenylmethanone], mesylate form; Tocris Bioscience] (D'Ambra et al., 1992) was dissolved in 5% ethanol:5% Solutol HS 15:90% saline (v:v:v). Anandamide (arachidonoylethanolamide, N-(2-hydroxyethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide; Cayman Chemical) was dissolved in 5% (v:v) N,N-dimethylacetamide (DMA) in water. SAB-378 [naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone (Astra-Zeneca R&D Mölndal) was administered as a nanosuspension in 5% DMA. O-1602 (5-methyl-4-[(1R,6R)-3-methyl-6-(1-cyclohexen-1-yl]-1,3-benzenediol, Tocris Cookson) was administered as a nanosuspension in 10% DMA. SR141716 [5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide; rimonabant; Astra-Zeneca R&D, Mölndal] (Rinaldi-Carmona et al., 1995) and SR144528 [N-[(1S)-endo-1,3,3-trimethyl bicycle [2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide; Astra-Zeneca R&D Mölndal) (Rinaldi-Carmona et al., 1998) were administered as nanosuspensions in 3% DMA. The corresponding vehicles were used as controls. Doses were selected based on preliminary experiments and published data showing efficacy in different pain models (Dziadulewicz et al., 2007). See Table 1 for biological activity and relative receptor affinity of compounds used in this study.
Table 1.
Biological activity and relative receptor affinity of cannabinoids and cannabinoid-related compounds
| Compound | Activity | CB1 | CB2 | GPR55 | Reference |
|---|---|---|---|---|---|
| Anandamide | Agonist | 31 ± 6a | 27 ± 6a | 18 ± 3a | Ryberg et al., 2007 |
| WIN55,212-2 | Agonist | 18 ± 3a | 1 ± 0.2a | >30,000a | Ryberg et al., 2007 |
| SAB-378 | Agonist | 15 ± 5b | 98 ± 7.6c | Dziadulewicz et al., 2007 | |
| O-1602 | Agonist | >30,000a | >30,000a | 13 ± 2a | Ryberg et al., 2007 |
| Virodhamine | Agonist | 2920 ± 325a | 381 ± 34a | 12 ± 3a | Ryberg et al., 2007 |
| SR-141716 | Antagonist/Inverse agonist | 12d | 790d | Silvestri et al., 2008 | |
| SR-144528 | Antagonist/Inverse agonist | >2820d | 5.4d | Silvestri et al., 2008 |
aEC50 (nm) for the stimulation of GTPγS binding on membranes from human embryonic kidney-HEK293s cells transiently transfected with human CB1 or CB2 receptors.
bEC50 (nm) inhibiting specific binding of [3H]CP55,940 in cell membranes from HEK293s cells transfected with the human CB1 receptor.
cEC50 (nm) inhibiting specific binding of [3H]CP55,940 in cell membranes from CHO-K1 cells transfected with the human CB2 receptor.
dKi (nm) values for the inhibition of [3H]CP55,940 binding in membranes from HEK cells transfected with human CB1 or CB2 receptors.
Experimental protocols
Before starting the experiments, to minimize the effects of restraint and to avoid movement-related artifacts, animals were habituated to Bollmann cages [Plexi-glass tubes; 18 cm length × 6 cm internal diameter (rat) or 10 cm length × 2.6 cm internal diameter (mouse); Astra-Zeneca Mölndal] 30 min per day, for at least 3 d. Animals were used in multiple occasions, with at least a 5 d interval between consecutive experiments.
Effects of cannabinoid receptor agonists on pain-related responses to CRD in rats.
During the noxious, repetitive CRD paradigm, after the third distension, either anandamide (0.1 or 3 μmol/kg; corresponding to ∼0.3 and 9 mg/kg, respectively), WIN55,212-2 (30, 50 or 100 nmol/kg; corresponding to ∼16, 26 and 52 μg/kg, respectively), O-1602 (20 μmol/kg, corresponding to ∼5 mg/kg), SAB-378 (0.27, 1.4, 2.7 or 5.4 μmol/kg; equivalent to ∼0.1, 0.5, 1 and 2 mg/kg, respectively) or their respective vehicles (1 ml/kg) was administered as a slow intravenous bolus injections over a 1 min period. Effects on the pain-related responses to CRD were assessed during distensions 4–12. In a separate experiment, the effects of the repeated administration of WIN55,212-2 (100 nmol/kg), given intravenously (1 ml/kg) after pulses 3 and 7 (total dose of 200 nmol/kg), were also tested. Virodhamine (3 μmol/kg; equivalent to ∼1 mg/kg) or its vehicle (1 ml/kg) was administered intraperitoneally 10 min before starting the noxious, repetitive CRD protocol.
In a separate experiment after 3 phasic distensions at 80 mmHg, SR141716 (300 nmol/kg, corresponding to ∼0.14 mg/kg), SR144528 (300 nmol/kg, corresponding to ∼0.14 mg/kg) or their vehicle (1 ml/kg) was administered as an slow intravenous bolus injection, over a 1 min period, immediately before the administration of WIN55,212-2 (100 nmol/kg, i.v.); thereafter the responses to CRD were assessed during the remaining 9 phasic distensions.
Based on the former experiments, the effects of SR141716 on SAB-378 effects on CRD-induced pain-related responses were also investigated. For these experiments, SR141716 (300 nmol/kg) or vehicle (1 ml/kg) was administered intravenously after the third distension during the repetitive noxious CRD paradigm. Thereafter, after the seventh distension of the CRD protocol, either SAB-378 (2.7 μmol/kg) or its vehicle (1 ml/kg) were administered intravenously and the response to CRD during distensions 7–12 assessed.
Effects of the cannabinoid receptor antagonist SR141716 on pain-related responses to CRD in rats.
During the noxious CRD paradigm, after three phasic distensions at 80 mmHg, SR141716 (300 or 1000 nmol/kg) or vehicle (1 ml/kg) was administered as a slow intravenous bolus injection over a 1 min period and the responses to CRD assessed for nine additional pulses at 80 mmHg.
In a separate experiment, after five consecutive non-noxious distensions (20 mmHg), SR141716 (300 nmol/kg) or its vehicle (1 ml/kg) was administered intravenously and the responses to five additional distensions at 20 mmHg assessed.
Effects of WIN55,212-2 on colonic compliance in rats.
When assessing compliance, vehicle (1 ml/kg) or WIN55,212-2 (100 nmol/kg) was administered intravenously at the beginning of the ascending phasic CRD (2–20 mmHg) paradigm. In a set of experiments, treatments were repeated after the CRD at 10 mmHg (total dose of WIN55,212-2 administered: 200 nmol/kg). The maximal intracolonic volume achieved during each distension was determined and pressure-volume curves were constructed as a measure of colonic compliance (Käll et al., 2007; Martínez et al., 2007; Ravnefjord et al., 2008).
Pain-related responses to CRD in wild-type and CB1-knock-out mice and effects of WIN55,212-2 and SR141716.
Normal pain responses to mechanical stimulation of the colon were assessed in wild-type and CB1-knock-out mice during the repetitive phasic (12 pulses × 55 mmHg) CRD protocol.
In separate experiments, the effects of WIN55,212-2 and SR141716 on pain responses to CRD were also assessed. In wild-type and CB1-knock-out mice, WIN55,212-2 (200 nmol/kg), SR141716 (2000 nmol/kg), or the appropriate vehicles (5 ml/kg) was administered subcutaneously, as a slow bolus injection, after the third distension at 55 mmHg and the responses to CRD evaluated for the following nine distensions at 55 mmHg.
Statistical analysis
Data are expressed as mean ± SEM. In some cases data represent the cumulative response during the part of the CRD procedure after treatments (area under the curve, AUC). Differences between two groups were determined using paired or unpaired Student′s t test, as appropriate. Differences between multiple groups were assessed by ANOVA followed, when necessary, by a Student-Newman–Keuls multiple comparisons test. Within-group differences in the responses to repeated distensions were assessed by repeated measures ANOVA followed, when necessary, by a Student-Newman–Keuls multiple comparisons test. ED50 values were determined by nonlinear regression to a sigmoidal equation with variable slope (GraphPad Prism, version 4.0.3, GraphPad Software). Data were considered statistically significant when p was ≤0.05.
Results
Effects of cannabinoid receptor agonists on pain-related responses to CRD in rats
Under basal conditions, activity of the abdominal musculature was low (mean basal activity: 0.06 ± 0.01) and stable over the experimental time. Phasic CRD at 80 mmHg induced abdominal contractions, as revealed by the oscillations in intraballoon pressure during the distension time (0.19 ± 0.04; p < 0.05 vs basal) (Fig. 1A). In addition, during repetitive distensions at 80 mmHg in vehicle-treated rats (n = 8), the response to distension increased over time by 113 ± 38% (first distension: 0.16 ± 0.03; 12th distension: 0.30 ± 0.05 mmHg; p < 0.05) (Fig. 1A).
Figure 1.
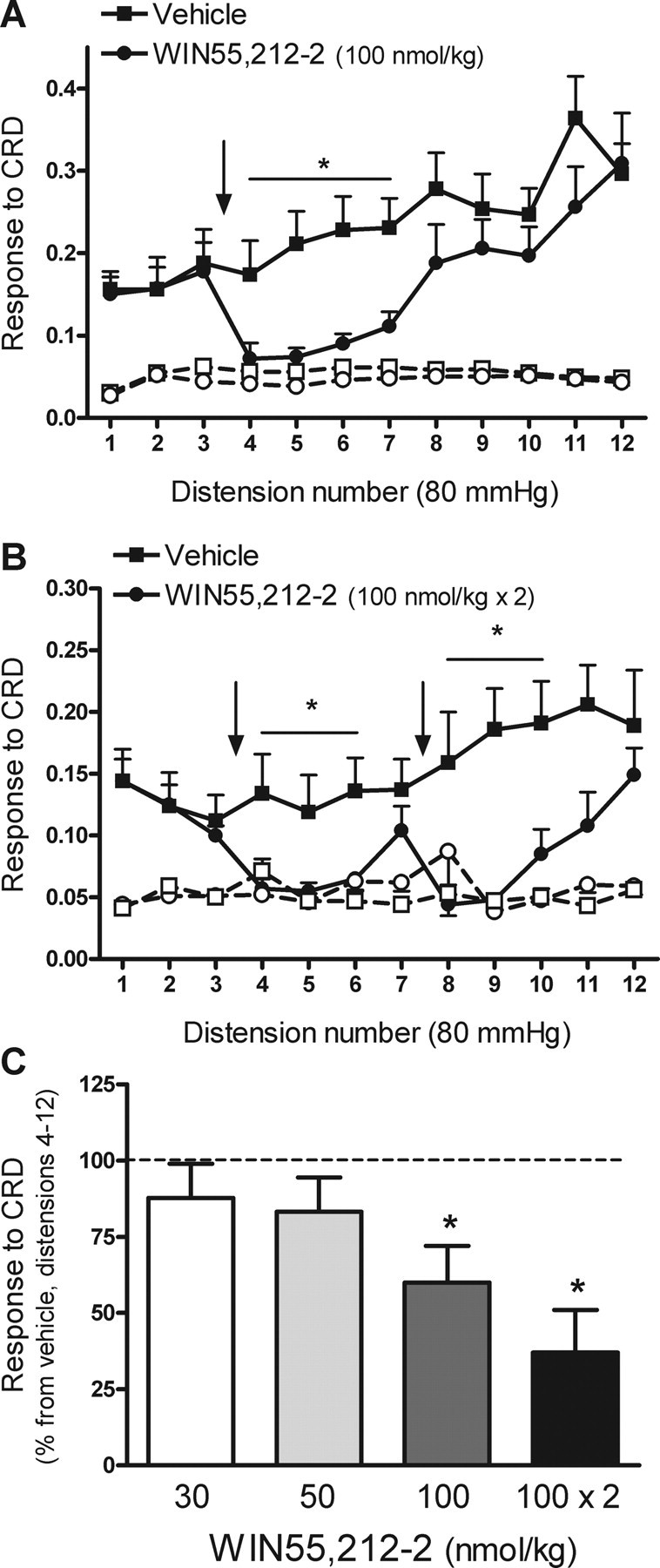
Effects of WIN55,212-2 on repetitive noxious CRD-evoked pain related responses in rats. A, Effects of WIN55,212-2 (100 nmol/kg) or vehicle (1 ml/kg), administered intravenously between distensions 3 and 4, on CRD-evoked pain related responses. Graphs with open symbols and broken lines correspond to the basal activity of the abdominal musculature between distensions. The arrow indicates the point of WIN55,212-2 or vehicle administration. Data are mean ± SEM of eight animals per group. *p < 0.05 versus corresponding response in the vehicle-treated group. B, Effects on pain-related responses to CRD of repeated administration of WIN55,212-2 (100 nmol/kg) between distensions 3 and 4 and 7 and 8 (as indicated by the arrows). Graphs with open symbols and broken lines correspond to the basal activity of the abdominal musculature between distensions. Data are mean ± SEM of seven animals per group. *p < 0.05 versus corresponding response in the vehicle-treated group. C, Overall effects of WIN55,212-2 (% inhibition vs the response in the vehicle group, set up at 100%; broken line) on pain-related responses during distensions 4–12 of the noxious CRD protocol. Data are mean ± SEM of 4–8 animals per group. *p < 0.05 versus vehicle.
WIN55,212-2 (100 nmol/kg, n = 8), administered intravenously between distensions 3 and 4, inhibited the overall response to CRD (distensions 4–12) by 40 ± 9.5% (p < 0.05 vs vehicle) (Fig. 1A,C). The effects of WIN55,212-2 were transitory and present between distension 4 and 7 (∼15 min), reaching values similar to those observed for the basal activity of the abdominal musculature. Thereafter, responses to CRD recovered and reached values similar to those observed in the vehicle-treated group (Fig. 1A). Based on these observations, the effects of two consecutive doses of WIN55,212-2 were also determined. Two doses of WIN55,212-2 (100 nmol/kg each, n = 7), administered after distensions 3 and 7, respectively, resulted in an enhanced inhibitory effect of CRD responses, with an overall inhibition of 63 ± 14% of the response to CRD (distensions 4–12) (Fig. 1B,C). Lower doses of WIN55,212-2 (30 or 50 nmol/kg) had no significant effects on the responses to CRD (Fig. 1C).
SAB-378 inhibited the responses to repetitive noxious CRD in a dose-related manner. At doses of 1.4 (n = 4), 2.7 (n = 8) and 5.4 μmol/kg (n = 4), SAB-378 inhibited the response to CRD (distension 4–12) by 82 ± 3%, 98 ± 6% and 90 ± 2%, respectively (all p < 0.05 vs vehicle) (Fig. 2). A lower dose, 0.27 μmol/kg (n = 4) inhibited the responses to CRD by 28 ± 15%, without reaching statistical significance. According to these data, the calculated ED50 was 0.48 μmol/kg (95% confidence interval: 0.33–0.67 μmol/kg; r2 = 0.9085). At effective doses, the effects of SAB-378 were long-lasting (distensions 4–12) and lead to a complete inhibition of the response to distension with values comparable to the basal activity of the abdominal musculature and no signs of recovery at the end of the CRD protocol (∼45 min posttreatment) (Fig. 2).
Figure 2.
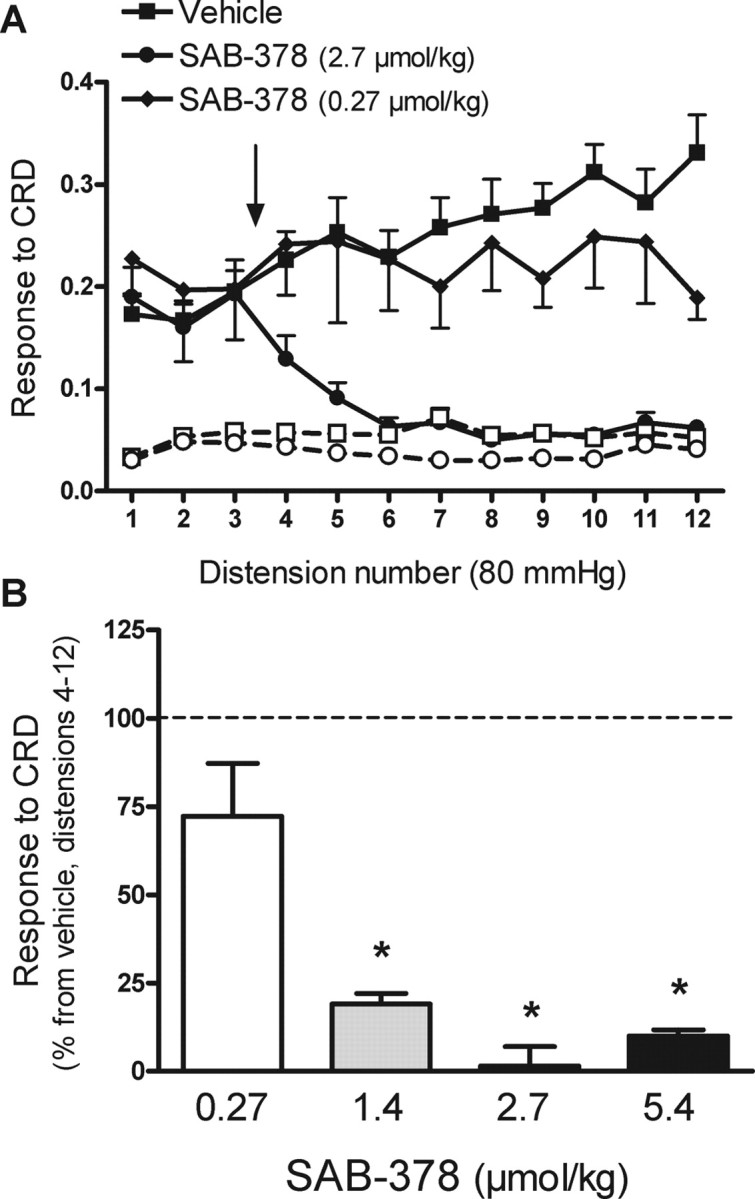
Effects of SAB-378 on repetitive noxious CRD-evoked pain related responses in rats. A, Effects of SAB-378 (0.27 or 2.7 μmol/kg) or vehicle (1 ml/kg), administered intravenously between distensions 3 and 4, on CRD-evoked pain-related responses. Graphs with open symbols and broken lines correspond to the basal activity of the abdominal musculature between distensions. The arrow indicates the point of SAB-378 or vehicle administration. Data are mean ± SEM of 4–8 animals per group. B, Overall effects of SAB-378 (% inhibition vs the response in the vehicle group, set up at 100%; broken line) on pain-related responses during distensions 4–12 of the noxious CRD protocol. Data are mean ± SEM of 4–8 animals per group. *p < 0.05 versus vehicle.
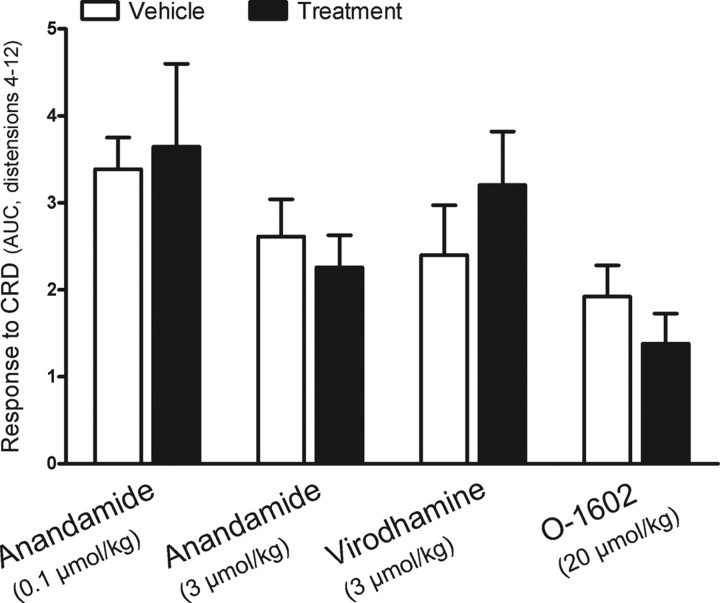
Neither anandamide (0.1 or 3 μmol/kg; n = 3 and 8, respectively) nor virodhamine (3 μmol/kg, n = 4) nor O-l602 (20 μmol/kg, n = 4) affected the response to CRD (Fig. 3).
Figure 3.
Effects of the cannabinoid agonists anandamide, virodhamine, and O-1602 on repetitive noxious CRD-evoked pain-related responses in rats. Compounds, or their respective vehicles, were administered intravenously between distensions 3 and 4 and the data represent the overall response (AUC) for distensions 4–12. In the case of virodhamine, treatments were applied before starting the CDR protocol (see Materials and Methods for details) and data represent the overall response (AUC) for the complete protocol (distensions 1–12). Data are mean ± SEM of 3–8 animals per group.
At the doses tested, no gross side-effects were observed for none of the compounds used.
Effects of the cannabinoid receptor antagonists SR141716 and SR144528 on pain-related responses to CRD and on WIN55,212-2 and SAB-378 effects in rats
In vehicle + WIN55,212-2 (100 nmol/kg, i.v.)-treated rats (n = 6), the response to CRD was inhibited between distensions 4 and 8. Responses to distension during this period were similar in magnitude to the basal activity of the abdominal musculature (Fig. 4A), consistent with the experiments described above. SR141716 (300 nmol/kg, i.v.), administered immediately before WIN55,212-2 (100 nmol/kg, i.v.) (n = 6), completely prevented the inhibitory effects of WIN55,212-2 on the pain responses to CRD (Fig. 4A). In contrast, pretreatment with SR144528 (0.3 μmol/kg, n = 8) did not affect the inhibitory effects of WIN55,212-2 on CRD responses (Fig. 4B).
Figure 4.
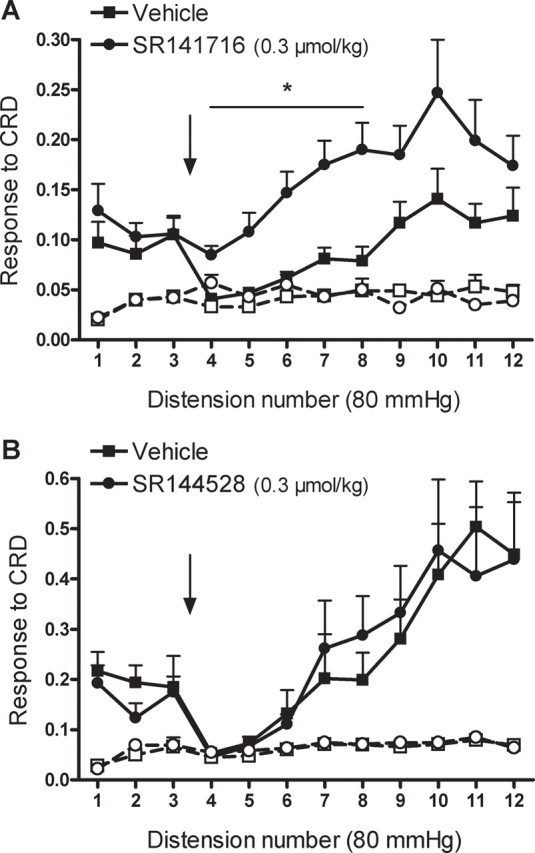
A, B, Effects of the selective CB1 antagonist, SR141716 (A), and the selective CB2 antagonist, SR141528 (B), on WIN55,212-2-induced analgesic responses during repetitive noxious CRD ni rats. SR141716 (0.3 μmol/kg), SR144528 (0.3 μmol/kg), or vehicle (1 ml/kg) was administered intravenously between distensions 3 and 4 and immediately before the intravenous administration of WIN55,212-2 (100 nmol/kg) (indicated by the arrow). Graphs with open symbols and broken lines correspond to the basal activity of the abdominal musculature between distensions. Data are mean ± SEM of 6–8 animals per group. *p < 0.05 versus corresponding response in the vehicle-treated group.
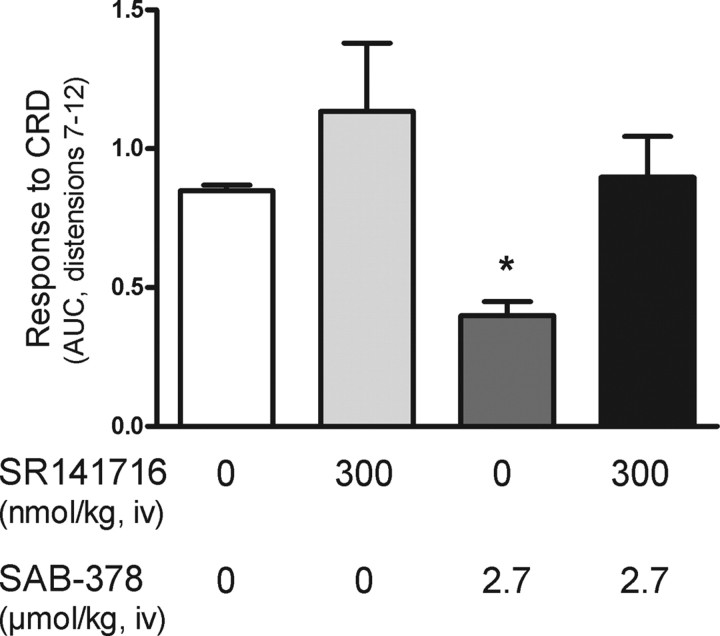
Similarly, pretreatment with SR141716 (0.3 μmol/kg, i.v.) completely prevented SAB-378 (2.7 μmol/kg, i.v.)-induced inhibition of pain responses during noxious CRD (Fig. 5).
Figure 5.
Blocking effects of the selective CB1 antagonist, SR141716, on SAB-378-indcued analgesia during noxious CRD in rats. Data represent the overall response to CRD (AUC of distensions 7–12) in animals pretreated with either vehicle or SR141716 (300 nmol/kg) and receiving thereafter either vehicle or SAB-378 (2.7 μmol/kg). Data are mean ± SEM of 4–5 animals per group. *p < 0.05 vs other experimental groups.
Based on these observations, the effects of SR141716, per se, on visceromotor responses to noxious and non-noxious CRD were assessed in separate experiments. SR141716 (0.3 or 1 μmol/kg, i.v.) administered between distensions 3 and 4 of the repeated noxious CRD protocol resulted in an increase in the response to CRD compared with vehicle-treated rats. Overall, SR141716 increased the response to CRD by 47 ± 27% and 57 ± 29% at 0.3 and 1 μmol/kg, respectively (n = 8 for each, both p < 0.05 vs vehicle) (Fig. 6).
Figure 6.
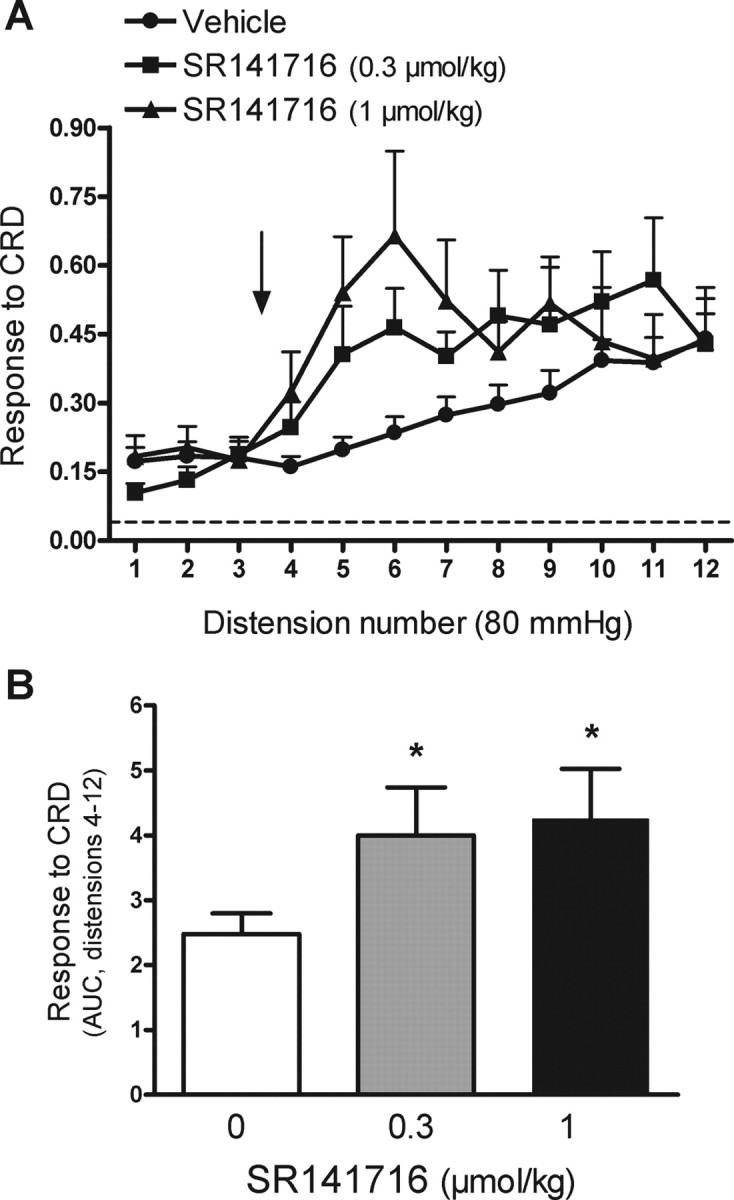
Effects of the selective CB1 antagonist, SR141716 on visceral pain-related responses during repetitive noxious CRD ni rats. A, SR141716 (0.3 or 1 μmol/kg) or vehicle (1 ml/kg) was administered intravenously between distensions 3 and 4 of the noxious CRD protocol (arrow). The broken line represents the mean basal activity of the abdominal musculature between distensions. B, Overall response to CRD (area under the curve, AUC, for distensions 4–12) for the different experimental groups. Data are mean ± SEM of 8 animals per group. *p < 0.05 versus the vehicle group.
Repetitive distensions at 20 mmHg did not elicit any increase in the activity of the abdominal musculature over basal values (mean basal: 0.05 ± 0.01; mean response to five repeated distensions at 20 mmHg: 0.06 ± 0.01; pooled data from all animals corresponding to the five consecutive distensions before treatments, n = 24). After five consecutive distensions at 20 mmHg, administration of SR141716 (0.3 μmol/kg, i.v., n = 12) resulted in an increase in the response to subsequent distensions (Fig. 7A). Peak response to distension reached values (0.13 ± 0.02) similar to those observed during distensions at 80 mmHg in previous control experiments (0.19 ± 0.04). Overall, during the experimental time after SR141716 administration (distension 6–10) the response to CRD increased by 100 ± 33% and 110 ± 49% over the basal response before treatment or the response in vehicle-treated animals, respectively (both p < 0.05) (Fig. 7B).
Figure 7.
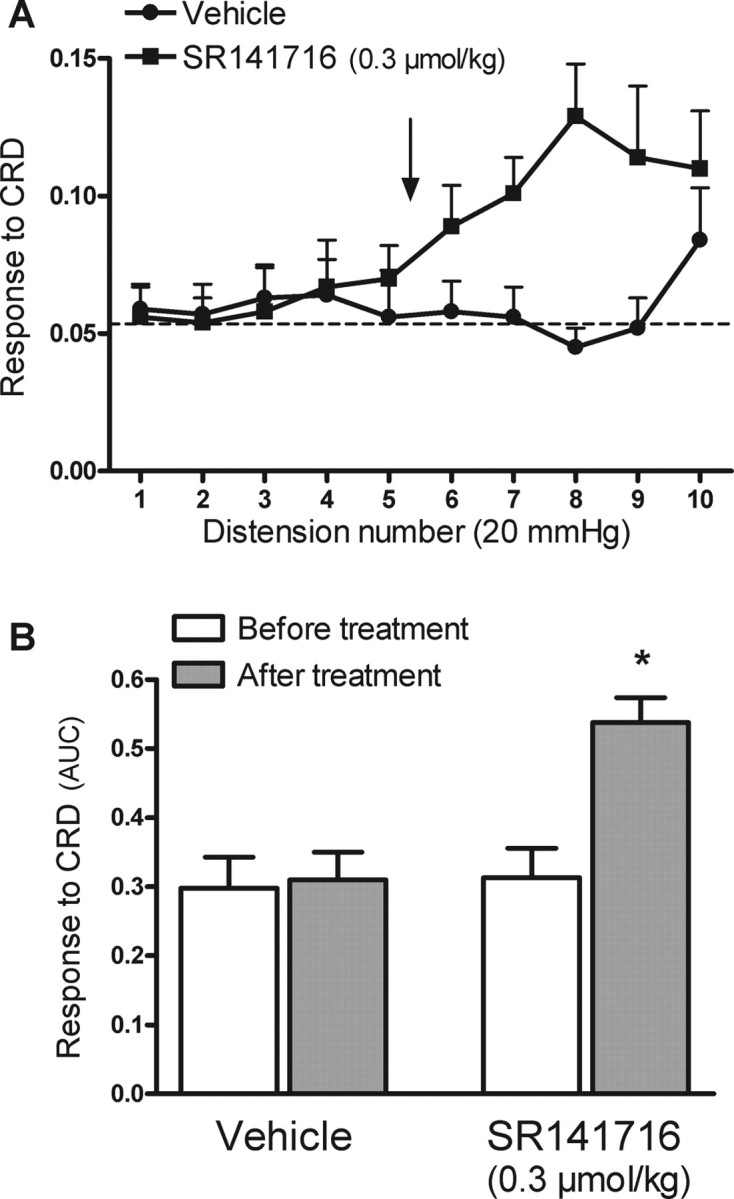
Effects of the selective CB1 antagonist, SR141716 on visceral pain-related responses during repetitive non-noxious CRD ni rats (10 distensions at 20 mmHg). A, SR141716 (0.3 μmol/kg) or vehicle (1 ml/kg) was administered intravenously between distensions 5 and 6 of the non-noxious CRD protocol (arrow). The broken line represents the mean basal activity of the abdominal musculature between distensions. B, Overall response to CRD (area under the curve, AUC, before and after treatments, distensions 1–5 and 6–10, respectively) for the different experimental groups. Data are mean ± SEM of 12 animals per group. *p < 0.05 versus the response to CRD before treatment.
Effects of WIN55,212-2 on pressure-volume responses during CRD in rats
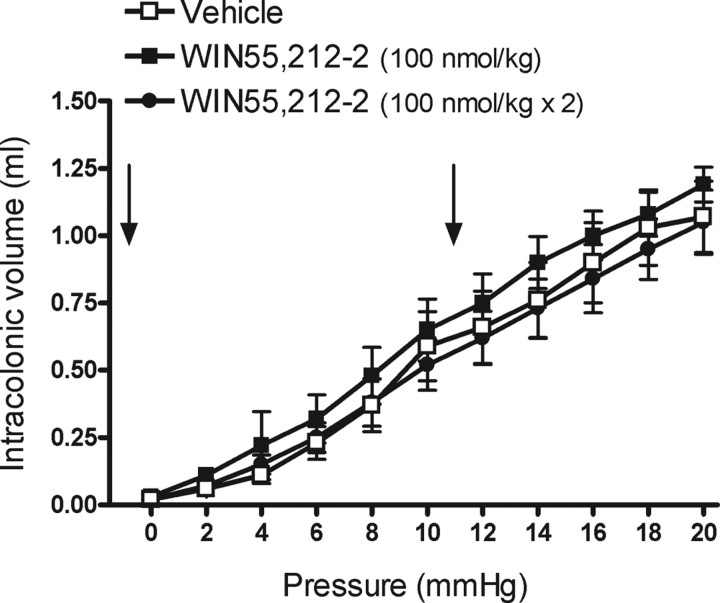
In vehicle-treated animals (n = 8), during phasic CRD at 2–20 mmHg, intracolonic volume increased in a pressure-related manner (Fig. 8). WIN55,212-2 (100 nmol/kg or 100 nmol/kg × 2; n = 8 for each) did not affect the volume-pressure relationship during CRD (Fig. 8).
Figure 8.
Effects of WIN55,212-2 on the pressure–volume relationship during CRD in rats. Vehicle (1 ml/kg × 2) or WIN55,212-2 (100 nmol/kg, a single dose or two doses) was administered intravenously immediately before starting the CRD procedure (single dose) and after the 5fh distension (repeated treatment), as indicated by the arrows. Data are mean ± SEM of eight animals per group.
Pain-related response to CRD and effects of WIN55,212-2 and SR141716 in wild-type and CB1-knock-out mice
Basal activity of the abdominal musculature was similar in wild-type (0.020 ± 0.002, n = 8) and CB1-knock-out mice (0.016 ± 0.001, n = 5; p > 0.05 vs wild type). CRD at 55 mmHg elicited a distension-related increase in the activity of the abdominal musculature, with similar magnitude in both groups of animals (overall response to distension, wild type: 0.26 ± 0.02; CB1-knock-out: 0.31 ± 0.08; p > 0.05).
In wild-type mice, WIN55,212-2 (200 nmol/kg, n = 7), administered subcutaneously after the third distension, reduced the responses to CRD compared with vehicle-treated animals. Inhibition of CRD responses was observed between distensions 4 and 9; thereafter the response to CRD was similar to that observed in vehicle-treated wild-type mice (Fig. 9A). Overall, WIN55,212-2 inhibited the response to CRD by 30 ± 7% for distensions 4–12 (p < 0.05 vs vehicle) (Fig. 8C). Under the same experimental conditions, WIN55,212-2 (200 nmol/kg, n = 7) did not affect the responses to CRD in CB1-knock-out mice (Fig. 9B,C).
Figure 9.
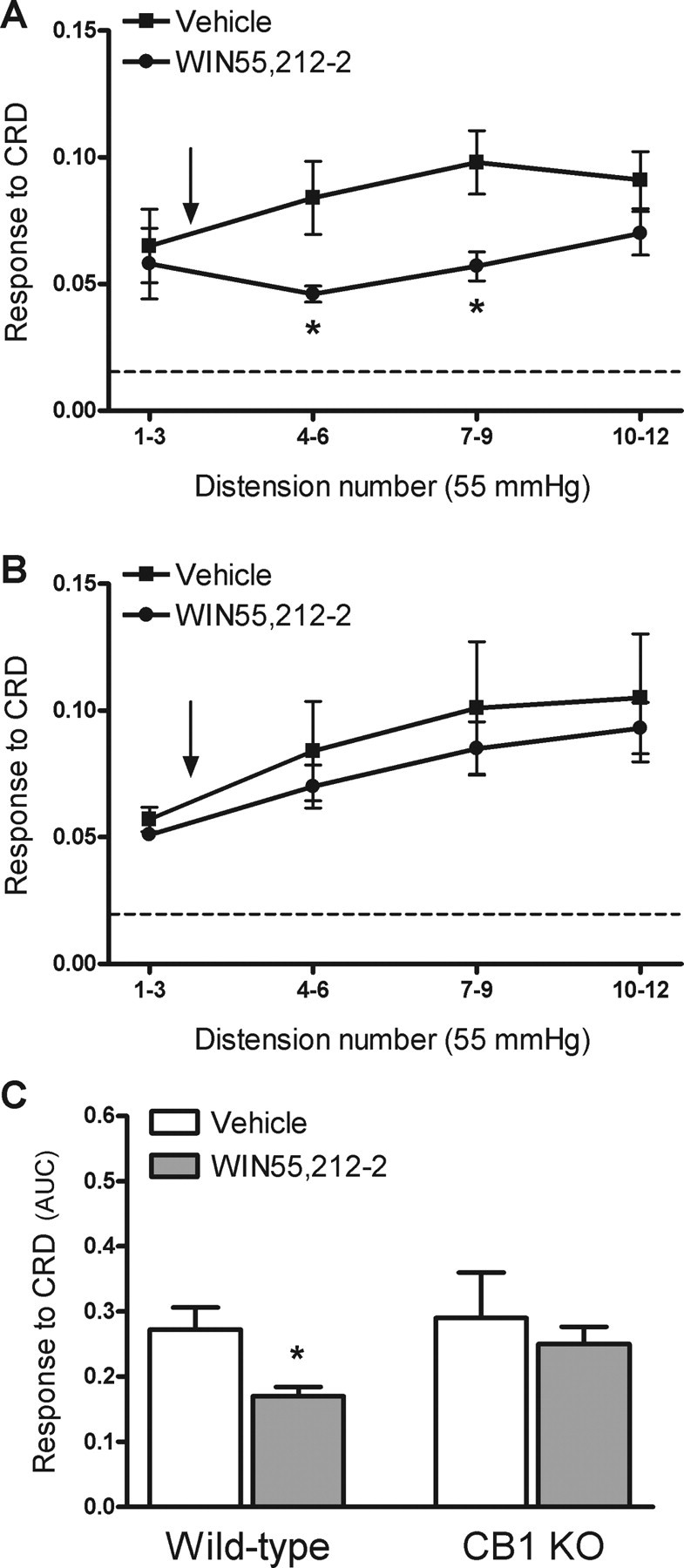
Effects of WIN55,212-2 on visceral pain-related responses to CRD in wild-type and CB1-knock-out (KO) mice. WIN55,212-2 (200 nmol/kg) or vehicle (5 ml/kg) was administered subcutaneously after the third distension at 55 mmHg (arrow). A, Responses to CRD in wild-type mice. The broken line represents the mean basal activity of the abdominal musculature between distensions. B, Responses to CRD in CB1-knock-out mice. The broken line represents the mean basal activity of the abdominal musculature between distensions. C, Overall response to CRD (area under the curve, AUC, for distensions 4–12) in wild-type and CB1-knock-out mice. Data are mean ± SEM of 7–8 animals per group. *p < 0.05 versus response in the vehicle-treated group.
In wild-type mice, SR141716 (2000 nmol/kg, n = 7), administered subcutaneously after the third distension, increased the overall response to CRD by 93 ± 24% over the response to distension observed in vehicle-treated wild-type animals (Fig. 10A,C). In CB1-knock-out mice, administration of SR141716 (2000 nmol/kg, n = 7), did not affect the responses to CRD when compared with the response in vehicle-treated animals (Fig. 10B,C).
Figure 10.
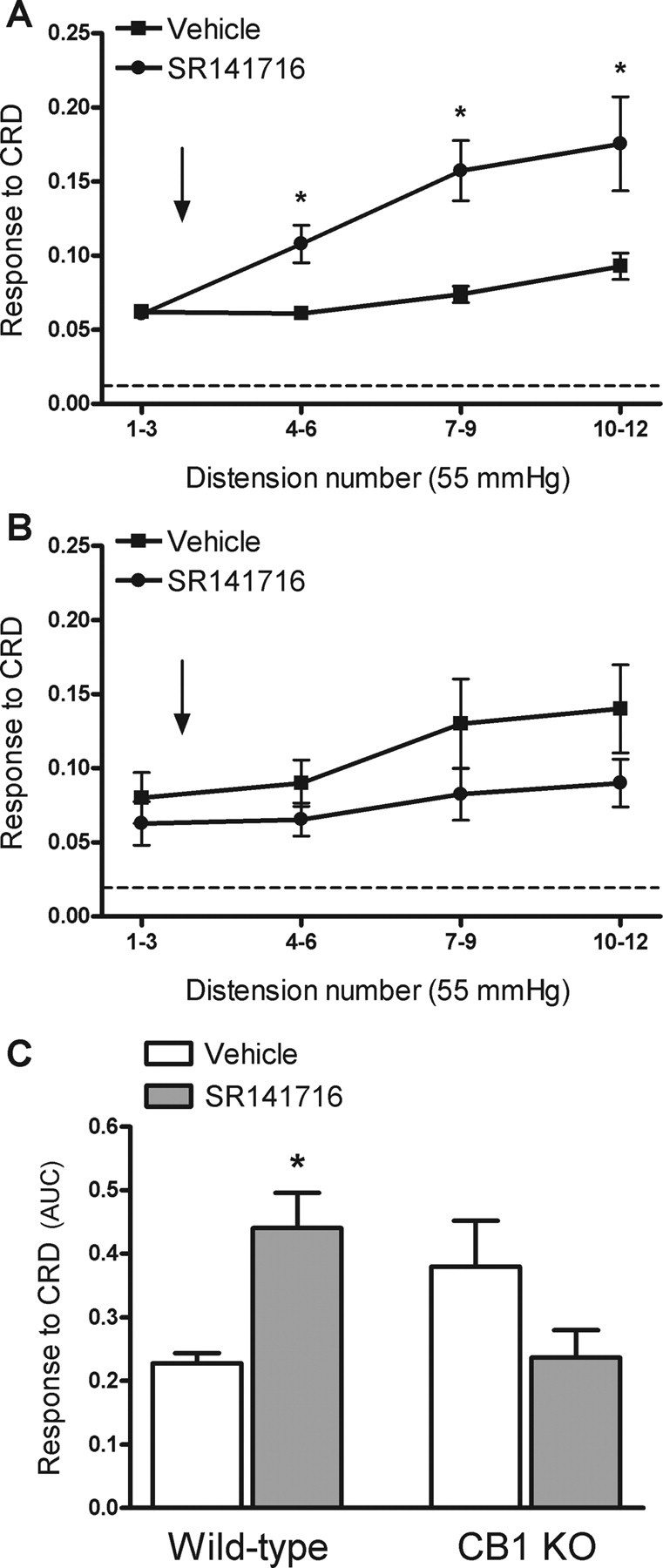
Effects of the selective CB1 antagonist, SR141716 on visceral pain-related responses to CRD in wild-type and CB1-knock-out (KO) mice. SR141716 (2 μmol/kg) or vehicle (5 ml/kg) was administered subcutaneously after the third distension at 55 mmHg (arrow). A, Responses to CRD in wild-type mice. The broken line represents the mean basal activity of the abdominal musculature between distensions. B, Responses to CRD in CB1-knock-out mice. The broken line represents the mean basal activity of the abdominal musculature between distensions. C, Overall response to CRD (area under the curve, AUC, for distensions 4–12) in wild-type and CB1-knock-out mice. Data are mean ± SEM of 4–7 animals per group. *p < 0.05 versus response in the vehicle-treated group.
Discussion
The present data indicates that CB1Rs, but not CB2Rs or the putative cannabinoid receptor, GPR55, are involved in the modulation of basal visceral sensitivity in a model of visceral pain in rodents. Enhancement of visceral pain-related responses, resulting in a hyperalgesic state, after treatment with the CB1 antagonist/inverse agonist, SR141716, suggests the endogenous release of endocannabinoids during the stimulation of pain-related pathways. Moreover, the presence of allodynic-like responses after treatment with SR141716 suggests the existence of an endogenous cannabinoid tone, likely modulating (containing) pain-related responses.
Cannabinoids are well known analgesics, particularly as it relates to somatic pain (Rice at el., 2002; Fox and Bevan, 2005). Recent studies in several species, including humans, support a role for endocannabinoids modulating visceral pain (Jaggar et al., 1998; Farquhar-Smith et al., 2002; Sanson et al., 2006; Esfandyari et al., 2007; Rousseaux et al., 2007; Kikuchi et al., 2008). Sanson et al. (2006) implicated CB1/2Rs in the modulation of basal viscerosensitivity in rats. However, according to the present results, CB1Rs account for the full analgesic effects of cannabinoids on basal CRD-associated pain responses. Several observations support a predominant role of CB1Rs mediating the visceral analgesic effects of cannabinoids. First, the distribution of CB1Rs is consistent with a role in mediating pain responses. CB1Rs are present in enteric primary sensory neurons, considered the primary nociceptive pathway within the gut (Ahluwalia et al., 2000). Moreover, CB1Rs are present in the dorsal horn of the spinal cord and in brain nuclei involved in the integration of pain-related responses (Farquhar-Smith et al., 2000; Salio et al., 2002a,b; Cristino et al., 2006). Pharmacological data also support a role for CB1Rs because the effects of WIN55,212-2 were blocked by SR141716, but not by the CB2 antagonist/inverse agonist, SR144528. Moreover, the CB1-preferring agonist, SAB-378, also inhibited the responses to CRD in a SR141716-sensitive manner. These observations in rats might be considered biased from having chosen a noninactive per se dose of SR141716. However, a clear-cut evidence for the role of CB1Rs is provided by the use of CB1-knock-out mice. Indeed, effects of WIN55,212-2 and SR141716 were absent in CB1-knock-out mice, but present in wild-type animals. For the first time, a potential role for the putative CB3 receptor, GPR55, in viscerosensitivity was investigated. None of the GPR55-preferring agonists tested, virrodhamine or O-1602 (Table 1), affected CRD-induced pain responses. All together, these observations strongly indicate that analgesic effects of cannabinoids on visceral pain, in basal conditions, are mediated through the activation of CB1Rs. This contrasts with reports indicating that CB2Rs might be involved in normal somatic anti-nociception (Malan et al., 2001; Nackley et al., 2004), suggesting differences between somatic and visceral pain in cannabinoid-dependent analgesia. In addition, direct and/or indirect effects of cannabionids on TRPV1 channels, also implicated in pain responses, cannot be excluded as contributing mechanisms to the effects observed (De Petrocellis et al., 2001; Dinis et al., 2004; Jeske et al., 2006; Patwardhan et al., 2006; Fioravanti et al., 2008).
Pharmacokinetic properties of the cannabinoid agonists tested may explain the differences in the effects observed. The relatively short duration of action of WIN55,212-2 (15–20 min in rats and ∼30 min in mice) might be explained by its short active half life in vivo (Croxford and Miller, 2003). Similarly, low metabolic stability (Khanolkar and Makriyannis, 1999) most likely accounts for the lack of effects of anandamide. Indeed, anandamide inhibited the response to CRD when its half-life was prolonged by inhibiting its endogenous metabolism (data not shown) (Pinto et al., 2002; Capasso et al., 2005; Palmer et al., 2008). Likewise, the long-lasting analgesic effects of SAB-378 probably reflect its long half life in vivo after intravenous administration (Dziadulewicz et al., 2007).
CRD-induced pain responses were similar in CB1-knock-out and wild-type mice. This agrees with data showing that CB1-knock-out mice are normoalgesic in the tail flick test while being hypoalgesic in the hot plate or the formalin tests (Zimmer et al., 1999), and suggests heterogeneity in the involvement of CB1Rs in basal pain responses, depending on the nociceptive stimuli and the sensory pathway stimulated. Alternatively, compensatory changes might account for the normalization in visceral pain responses in CB1-knock-out mice. Nevertheless, the lack of effects of WIN55,212-2 and SR141716 in CB1-knock-out mice strongly supports a role for CB1Rs mediating the analgesic effects of cannabinoids on visceral pain. In general, effective doses were higher in mice than in rats, likely due to the different routes of administration used and/or species-related pharmacodynamic differences.
Interestingly, treatment with SR141716 led to the development of both hyperalgesia and allodynia. SR141716 increased pain-related responses to noxious CRD in rats and wild-type mice, but not in CB1-knock-out mice, indicating the development of visceral hyperalgesia in response to CB1R-blockade. This suggests that endogenous cannabinoids are released during the CRD procedure, probably due to the activation of pain-related pathways. Indeed, endocannabinoids and other pain-modulatory mediators, such as GABA, seem to be released during the activation of pain mechanisms (Petrosino et al., 2007; Brusberg et al., 2009; Hong et al., 2009) or colitis (D'Argenio et al., 2006). This might be interpreted as an endogenous mechanism containing the activation of pain pathways and thus maintaining the overall response to pain within a physiologically manageable range. Supporting this view, tonic release of endocannabinoids has been described in several systems, including the gastrointestinal tract (Izzo et al., 1999; Pinto et al., 2002; Hentges et al., 2005; Narushima et al., 2007), and has also been postulated from results obtained after the pharmacological blockade (Cippitelli et al., 2008; Moreira et al., 2008) or the genetic deletion of the endocannabinoids catabolic system (Cravatt et al., 2001; Moreira et al., 2008). In vitro studies showed that SR141716 behaves as an inverse agonist on the CB1R (Landsman et al., 1997; Shire et al., 1999) and that CB1Rs are constitutively active (Leterrier et al., 2006; Canals and Milligan, 2008). Therefore, although constitutive activity is difficult to demonstrate in vivo, the effects observed here could be explained by an inverse agonistic activity of SR141716 on constitutively active CB1Rs. In these conditions, an apparent antagonistic effect will be observed in the absence of any endogenous ligand. Functionally, a tonic constitutive activity of cannabinoid receptors might lead to a sustained tone of analgesia without any tonic endocannabinoid release. Overall, our observations contrast with previous results showing that SR141716 did not affect normal pain responses to CRD in rats (Sanson et al., 2006). All together, these observations warrant further studies assessing the tonic release of endocannabinoids at pain-relevant sites as well as the activity of CB1Rs in normal and pathophysiological conditions (such as during inflammation).
Altered colonic tone/accommodation to distension have been suggested as contributing mechanisms to altered colonic sensitivity in functional gastrointestinal disorders (Delgado-Aros and Camilleri, 2005). Nevertheless, there is not a clear correlation between compliance and pain perception (Käll et al., 2007; Lindström et al., 2008; Ravnefjord et al., 2008). Experimental data in humans showed that the nonselective cannabinoid dronabinol reduced colonic tone and increased compliance while increasing sensation ratings to distension, probably through the central modulation of perception (Esfandyari et al., 2007). In the present experiments, WIN55,212-2, at analgesic doses, did not modify the pressure-volume relationship during distension indicating a lack of effect modulating colonic compliance. These results argue for a lack of direct relationship between analgesia and compliance and suggest differences between humans and rodents in cannabinoid-dependent modulation of colonic tone/compliance.
Analgesic effect of cannabinoids may be exerted at multiple central and peripheral locations. Although the site of action was not directly assessed in the present studies, several observations suggest a peripheral effect. First, as mentioned, CB1Rs are expressed within the gastrointestinal tract and in sensory endings on primary sensory afferents (Kulkarni-Narla and Brown, 2000; Coutts et al., 2002; Bridges et al., 2003; Casu et al., 2003; MacNaughton et al., 2004), allowing a peripheral effect modulating sensory afferent information. Second, SAB-378 is a peripherally acting compound with limited brain access, showing CNS-related effects only at doses ∼170-fold greater than that required to produce analgesia (Dziadulewicz et al., 2007). Finally, none of the compounds tested evoked visible CNS-related side effects at the doses used, which would be expected if stimulation of central cannabinoid receptors occurred, although this was not assessed systematically. However, a potential contribution of central CB1Rs in the analgesic responses observed cannot be totally excluded. Nevertheless, the present observations suggest that the peripheral blockade of CB1Rs is sufficient to elicit visceral analgesia. Therefore, highly peripheralized CB1 agonists may be a valid therapeutic option for the treatment of visceral pain arising from the gut.
In summary, cannabinoids, acting through a CB1-dependent mechanism, have analgesic effects in a rodent model of mechanically induced visceral pain, likely acting at peripheral sites. Observations derived from the use of SR141716 indicate that, through a tonic release of endocannabinoids and/or the constitutive activity of CB1Rs, the endogenous cannabinoid system might be tonically active, likely constraining the activity of pain pathways. These observations suggest that peripherally active CB1 agonists have a potential interest for the treatment of visceral pain-related disorders, such as irritable bowel syndrome, avoiding the undesirable profile associated to the use of centrally acting cannabinoids.
References
- Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- Arvidsson S, Larsson M, Larsson H, Lindström E, Martinez V. Assessment of visceral pain-related pseudo-affective responses to colorectal distension in mice by intracolonic manometric recordings. J Pain. 2006;7:108–118. doi: 10.1016/j.jpain.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Ashton JC, Milligan ED. Cannabinoids for the treatment of neuropathic pain: clinical evidence. Curr Opin Investig Drugs. 2008;9:65–75. [PubMed] [Google Scholar]
- Bisogno T. Endogenous cannabinoids: structure and metabolism. J Neuroendocrinol. 2008;20(Suppl 1):1–9. doi: 10.1111/j.1365-2826.2008.01676.x. [DOI] [PubMed] [Google Scholar]
- Bridges D, Rice AS, Egertová M, Elphick MR, Winter J, Michael GJ. Localisation of cannabinoid receptor 1 in rat dorsal root ganglion using in situ hybridisation and immunohistochemistry. Neuroscience. 2003;119:803–812. doi: 10.1016/s0306-4522(03)00200-8. [DOI] [PubMed] [Google Scholar]
- Brown AJ. Novel cannabinoid receptors. Br J Pharmacol. 2007;152:567–575. doi: 10.1038/sj.bjp.0707481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusberg M, Ravnefjord A, Martinsson R, Larsson H, Martinez V, Lindström E. The GABAb receptor agonist, baclofen, and the positive allosteric modulator, CGP7930, inhibit visceral pain-related responses to colorectal distension in rats. Neuropharmacology. 2009;56:362–367. doi: 10.1016/j.neuropharm.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Canals M, Milligan G. Constitutive activity of the cannabinoid CB1 receptor regulates the function of co-expressed Mu opioid receptors. J Biol Chem. 2008;283:11424–11434. doi: 10.1074/jbc.M710300200. [DOI] [PubMed] [Google Scholar]
- Capasso R, Matias I, Lutz B, Borrelli F, Capasso F, Marsicano G, Mascolo N, Petrosino S, Monory K, Valenti M, Di Marzo V, Izzo AA. Fatty acid amide hydrolase controls mouse intestinal motility in vivo. Gastroenterology. 2005;129:941–951. doi: 10.1053/j.gastro.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Casu MA, Porcella A, Ruiu S, Saba P, Marchese G, Carai MA, Reali R, Gessa GL, Pani L. Differential distribution of functional cannabinoid CB1 receptors in the mouse gastroenteric tract. Eur J Pharmacol. 2003;459:97–105. doi: 10.1016/s0014-2999(02)02830-3. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A, Defonseca FR, Piomelli D, Ciccocioppo R. Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology. 2008;198:449–460. doi: 10.1007/s00213-008-1104-0. [DOI] [PubMed] [Google Scholar]
- Coutts AA, Irving AJ, Mackie K, Pertwee RG, Anavi-Goffer S. Localisation of cannabinoid CB(1) receptor immunoreactivity in the guinea pig and rat myenteric plexus. J Comp Neurol. 2002;448:410–422. doi: 10.1002/cne.10270. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Croxford JL, Miller SD. Immunoregulation of a viral model of multiple sclerosis using the synthetic cannabinoid R+WIN55,212. J Clin Invest. 2003;111:1231–1240. doi: 10.1172/JCI17652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambra TE, Estep KG, Bell MR, Eissenstat MA, Josef KA, Ward SJ, Haycock DA, Baizman ER, Casiano FM, Beglin NC, et al. Conformationally restrained analogues of pravadoline: nanomolar potent, enantioselective (aminoalkyl)indole agonists of the cannabinoid receptor. J Med Chem. 1992;35:124–135. doi: 10.1021/jm00079a016. [DOI] [PubMed] [Google Scholar]
- D'Argenio G, Calvani M, Casamassimi A, Petillo O, Margarucci S, Rienzo M, Peluso I, Calvani R, Ciccodicola A, Caporaso N, Peluso G. Experimental colitis: decreased Octn2 and Atb0+ expression in rat colonocytes induces carnitine depletion that is reversible by carnitine-loaded liposomes. FASEB J. 2006;20:2544–2546. doi: 10.1096/fj.06-5950fje. [DOI] [PubMed] [Google Scholar]
- Delgado-Aros S, Camilleri M. Visceral hypersensitivity. J Clin Gastroenterol. 2005;39(Suppl 3):S194–S203. doi: 10.1097/01.mcg.0000156114.22598.1b. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Bisogno T, Maccarrone M, Davis JB, Finazzi-Agro A, Di Marzo V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J Biol Chem. 2001;276:12856–12863. doi: 10.1074/jbc.M008555200. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Endocannabinoids: synthesis and degradation. Rev Physiol Biochem Pharmacol. 2008;160:1–24. doi: 10.1007/112_0505. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Izzo AA. Endocannabinoid overactivity and intestinal inflammation. Gut. 2006;55:1373–1376. doi: 10.1136/gut.2005.090472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinis P, Charrua A, Avelino A, Yaqoob M, Bevan S, Nagy I, Cruz F. Anandamide-evoked activation of vanilloid receptor 1 contributes to the development of bladder hyperreflexia and nociceptive transmission to spinal dorsal horn neurons in cystitis. J Neurosci. 2004;24:11253–11263. doi: 10.1523/JNEUROSCI.2657-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziadulewicz EK, Bevan SJ, Brain CT, Coote PR, Culshaw AJ, Davis AJ, Edwards LJ, Fisher AJ, Fox AJ, Gentry C, Groarke A, Hart TW, Huber W, James IF, Kesingland A, La Vecchia L, Loong Y, Lyothier I, McNair K, O'Farrell C, et al. Naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone: a potent, orally bioavailable human CB1/CB2 dual agonist with antihyperalgesic properties and restricted central nervous system penetration. J Med Chem. 2007;50:3851–3856. doi: 10.1021/jm070317a. [DOI] [PubMed] [Google Scholar]
- Esfandyari T, Camilleri M, Busciglio I, Burton D, Baxter K, Zinsmeister AR. Effects of a cannabinoid receptor agonist on colonic motor and sensory functions in humans: a randomized, placebo-controlled study. Am J Physiol Gastrointest Liver Physiol. 2007;293:G137–G145. doi: 10.1152/ajpgi.00565.2006. [DOI] [PubMed] [Google Scholar]
- Farquhar-Smith WP, Egertová M, Bradbury EJ, McMahon SB, Rice AS, Elphick MR. Cannabinoid CB(1) receptor expression in rat spinal cord. Mol Cell Neurosci. 2000;15:510–521. doi: 10.1006/mcne.2000.0844. [DOI] [PubMed] [Google Scholar]
- Farquhar-Smith WP, Jaggar SI, Rice AS. Attenuation of nerve growth factor-induced visceral hyperalgesia via cannabinoid CB(1) and CB(2)-like receptors. Pain. 2002;97:11–21. doi: 10.1016/s0304-3959(01)00419-5. [DOI] [PubMed] [Google Scholar]
- Fioravanti B, De Felice M, Stucky CL, Medler KA, Luo MC, Gardell LR, Ibrahim M, Malan TP, Jr, Yamamura HI, Ossipov MH, King T, Lai J, Porreca F, Vanderah TW. Constitutive activity at the cannabinoid CB1 receptor is required for behavioral response to noxious chemical stimulation of TRPV1: antinociceptive actions of CB1 inverse agonists. J Neurosci. 2008;28:11593–11602. doi: 10.1523/JNEUROSCI.3322-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A, Bevan S. Therapeutic potential of cannabinoid receptor agonists as analgesic agents. Expert Opin Investig Drugs. 2005;14:695–703. doi: 10.1517/13543784.14.6.695. [DOI] [PubMed] [Google Scholar]
- Hentges ST, Low MJ, Williams JT. Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J Neurosci. 2005;25:9746–9751. doi: 10.1523/JNEUROSCI.2769-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Fan J, Kemmerer ES, Evans S, Li Y, Wiley JW. Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat. Gut. 2009;58:202–210. doi: 10.1136/gut.2008.157594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornby PJ, Prouty SM. Involvement of cannabinoid receptors in gut motility and visceral perception. Br J Pharmacol. 2004;141:1335–1345. doi: 10.1038/sj.bjp.0705783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking RD, Zajicek JP. Therapeutic potential of cannabis in pain medicine. Br J Anaesth. 2008;101:59–68. doi: 10.1093/bja/aen119. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Mascolo N, Pinto L, Capasso R, Capasso F. The role of cannabinoid receptors in intestinal motility, defaecation and diarrhoea in rats. Eur J Pharmacol. 1999;384:37–42. doi: 10.1016/s0014-2999(99)00673-1. [DOI] [PubMed] [Google Scholar]
- Jaggar SI, Hasnie FS, Sellaturay S, Rice AS. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain. 1998;76:189–199. doi: 10.1016/s0304-3959(98)00041-4. [DOI] [PubMed] [Google Scholar]
- Jeske NA, Patwardhan AM, Gamper N, Price TJ, Akopian AN, Hargreaves KM. Cannabinoid WIN 55,212-2 regulates TRPV1 phosphorylation in sensory neurons. J Biol Chem. 2006;281:32879–32890. doi: 10.1074/jbc.M603220200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käll E, Lindström E, Martinez V. The serotonin reuptake inhibitor citalopram does not affect colonic sensitivity or compliance in rats. Eur J Pharmacol. 2007;570:203–211. doi: 10.1016/j.ejphar.2007.05.032. [DOI] [PubMed] [Google Scholar]
- Khanolkar AD, Makriyannis A. Structure-activity relationships of anandamide, an endogenous cannabinoid ligand. Life Sci. 1999;65:607–616. doi: 10.1016/s0024-3205(99)00283-0. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Ohashi K, Sugie Y, Sugimoto H, Omura H. Pharmacological evaluation of a novel cannabinoid 2 (CB2) ligand, PF-03550096, in vitro and in vivo by using a rat model of visceral hypersensitivity. J Pharmacol Sci. 2008;106:219–224. doi: 10.1254/jphs.fp0071599. [DOI] [PubMed] [Google Scholar]
- Kulkarni-Narla A, Brown DR. Localization of CB1-cannabinoid receptor immunoreactivity in the porcine enteric nervous system. Cell Tissue Res. 2000;302:73–80. doi: 10.1007/s004410000261. [DOI] [PubMed] [Google Scholar]
- Landsman RS, Burkey TH, Consroe P, Roeske WR, Yamamura HI. SR141716A is an inverse agonist at the human cannabinoid CB1 receptor. Eur J Pharmacol. 1997;334:R1–R2. doi: 10.1016/s0014-2999(97)01160-6. [DOI] [PubMed] [Google Scholar]
- Leterrier C, Lainé J, Darmon M, Boudin H, Rossier J, Lenkei Z. Constitutive activation drives compartment-selective endocytosis and axonal targeting of type 1 cannabinoid receptors. J Neurosci. 2006;26:3141–3153. doi: 10.1523/JNEUROSCI.5437-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligresti A, Bisogno T, Matias I, De Petrocellis L, Cascio MG, Cosenza V, D'argenio G, Scaglione G, Bifulco M, Sorrentini I, Di Marzo V. Possible endocannabinoid control of colorectal cancer growth. Gastroenterology. 2003;125:677–687. doi: 10.1016/s0016-5085(03)00881-3. [DOI] [PubMed] [Google Scholar]
- Lindström E, Brusberg M, Hughes PA, Martin CM, Brierley SM, Phillis BD, Martinsson R, Abrahamsson C, Larsson H, Martinez V, Blackshaw LA. Involvement of metabotropic glutamate 5 receptor in visceral pain. Pain. 2008;137:295–305. doi: 10.1016/j.pain.2007.09.008. [DOI] [PubMed] [Google Scholar]
- MacNaughton WK, Van Sickle MD, Keenan CM, Cushing K, Mackie K, Sharkey KA. Distribution and function of the cannabinoid-1 receptor in the modulation of ion transport in the guinea pig ileum: relationship to capsaicin-sensitive nerves. Am J Physiol Gastrointest Liver Physiol. 2004;286:G863–G871. doi: 10.1152/ajpgi.00482.2003. [DOI] [PubMed] [Google Scholar]
- Malan TP, Jr, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, Porreca F, Makriyannis A. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- Martinez V, Melgar S. Lack of colonic inflammation-induced acute visceral hypersensitivity to colorectal distension in Na. (v) 1.9 knockout mice. Eur J Pain. 2008;12:934–944. doi: 10.1016/j.ejpain.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Martínez V, Ryttinger M, Kjerling M, Astin-Nielsen M. Characterisation of colonic accommodation in Wistar Kyoto rats with impaired gastric accommodation. Naunyn Schmiedebergs Arch Pharmacol. 2007;376:205–216. doi: 10.1007/s00210-007-0195-1. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ. Cannabinoid receptors: which cells, where, how, and why? NIDA Res Monogr. 1992;126:48–56. [PubMed] [Google Scholar]
- Moreira FA, Kaiser N, Monory K, Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54:141–150. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Zvonok AM, Makriyannis A, Hohmann AG. Activation of cannabinoid CB2 receptors suppresses C-fiber responses and windup in spinal wide dynamic range neurons in the absence and presence of inflammation. J Neurophysiol. 2004;92:3562–3574. doi: 10.1152/jn.00886.2003. [DOI] [PubMed] [Google Scholar]
- Narushima M, Uchigashima M, Fukaya M, Matsui M, Manabe T, Hashimoto K, Watanabe M, Kano M. Tonic enhancement of endocannabinoid-mediated retrograde suppression of inhibition by cholinergic interneuron activity in the striatum. J Neurosci. 2007;27:496–506. doi: 10.1523/JNEUROSCI.4644-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JA, Higuera ES, Chang L, Chaplan SR. Fatty acid amide hydrolase inhibition enhances the anti-allodynic actions of endocannabinoids in a model of acute pain adapted for the mouse. Neuroscience. 2008;154:1554–1561. doi: 10.1016/j.neuroscience.2008.04.047. [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Jeske NA, Price TJ, Gamper N, Akopian AN, Hargreaves KM. The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proc Natl Acad Sci U S A. 2006;103:11393–11398. doi: 10.1073/pnas.0603861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosino S, Palazzo E, de Novellis V, Bisogno T, Rossi F, Maione S, Di Marzo V. Changes in spinal and supraspinal endocannabinoid levels in neuropathic rats. Neuropharmacology. 2007;52:415–422. doi: 10.1016/j.neuropharm.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Pinto L, Izzo AA, Cascio MG, Bisogno T, Hospodar-Scott K, Brown DR, Mascolo N, Di Marzo V, Capasso F. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology. 2002;123:227–234. doi: 10.1053/gast.2002.34242. [DOI] [PubMed] [Google Scholar]
- Ravnefjord A, Brusberg M, Larsson H, Lindström E, Martínez V. Effects of pregabalin on visceral pain responses and colonic compliance in rats. Br J Pharmacol. 2008;155:407–416. doi: 10.1038/bjp.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice AS, Farquhar-Smith WP, Nagy I. Endocannabinoids and pain: spinal and peripheral analgesia in inflammation and neuropathy. Prostaglandins Leukot Essent Fatty Acids. 2002;66:243–256. doi: 10.1054/plef.2001.0362. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Héaulme M, Alonso R, Shire D, Congy C, Soubrié P, Brelière JC, Le Fur G. Biochemical and pharmacological characterisation of SR141716A, the first potent and selective brain cannabinoid receptor antagonist. Life Sci. 1995;56:1941–1947. doi: 10.1016/0024-3205(95)00174-5. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, Oustric D, Sarran M, Bouaboula M, Calandra B, Portier M, Shire D, Brelière JC, Le Fur GL. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M, Ouwehand A, Leyer G, Carcano D, Colombel JF, Ardid D, Desreumaux P. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13:35–37. doi: 10.1038/nm1521. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salio C, Doly S, Fischer J, Franzoni MF, Conrath M. Neuronal and astrocytic localization of the cannabinoid receptor-1 in the dorsal horn of the rat spinal cord. Neurosci Lett. 2002a;329:13–16. doi: 10.1016/s0304-3940(02)00549-9. [DOI] [PubMed] [Google Scholar]
- Salio C, Fischer J, Franzoni MF, Conrath M. Pre- and postsynaptic localizations of the CB1 cannabinoid receptor in the dorsal horn of the rat spinal cord. Neuroscience. 2002b;110:755–764. doi: 10.1016/s0306-4522(01)00584-x. [DOI] [PubMed] [Google Scholar]
- Sanson M, Bueno L, Fioramonti J. Involvement of cannabinoid receptors in inflammatory hypersensitivity to colonic distension in rats. Neurogastroenterol Motil. 2006;18:949–956. doi: 10.1111/j.1365-2982.2006.00819.x. [DOI] [PubMed] [Google Scholar]
- Shire D, Calandra B, Bouaboula M, Barth F, Rinaldi-Carmona M, Casellas P, Ferrara P. Cannabinoid receptor interactions with the antagonists SR 141716A and SR 144528. Life Sci. 1999;65:627–635. doi: 10.1016/s0024-3205(99)00285-4. [DOI] [PubMed] [Google Scholar]
- Silvestri R, Cascio MG, La Regina G, Piscitelli F, Lavecchia A, Brizzi A, Pasquini S, Botta M, Novellino E, Di Marzo V, Corelli F. Synthesis, cannabinoid receptor affinity, and molecular modeling studies of substituted 1-aryl-5-(1H-pyrrol-1-yl)-1H-pyrazole-3-carboxamides. J Med Chem. 2008;51:1560–1576. doi: 10.1021/jm070566z. [DOI] [PubMed] [Google Scholar]
- Storr MA, Sharkey KA. The endocannabinoid system and gut-brain signalling. Curr Opin Pharmacol. 2007;7:575–582. doi: 10.1016/j.coph.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Storr MA, Yüce B, Andrews CN, Sharkey KA. The role of the endocannabinoid system in the pathophysiology and treatment of irritable bowel syndrome. Neurogastroenterol Motil. 2008;20:857–868. doi: 10.1111/j.1365-2982.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- Tammpere A, Brusberg M, Axenborg J, Hirsch I, Larsson H, Lindström E. Evaluation of pseudo-affective responses to noxious colorectal distension in rats by manometric recordings. Pain. 2005;116:220–226. doi: 10.1016/j.pain.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Wright KL, Duncan M, Sharkey KA. Cannabinoid CB2 receptors in the gastrointestinal tract: a regulatory system in states of inflammation. Br J Pharmacol. 2008;153:263–270. doi: 10.1038/sj.bjp.0707486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]