Abstract
Extinction procedures are clinically relevant for reducing pathological fear, and the mechanisms of fear regulation are a subject of intense research. The amygdala, hippocampus, and prefrontal cortex (PFC) have all been suggested to be key brain areas in extinction of conditioned fear. GABA has particularly been implicated in extinction learning, and the 65 kDa isoform of glutamic acid decarboxylase (GAD65) may be important in elevating GABA levels in response to environmental signals. Extinction of conditioned fear was examined in Gad65−/− mice while recording local field potentials from the amygdala, hippocampus, and PFC simultaneously while monitoring behavior. Gad65−/− mice showed generalization of cued fear, as reported previously, and impaired extinction of cued fear, such that fear remained high across extinction training. This endurance in cued fear was associated with theta frequency synchronization between the amygdala and hippocampus. Extinction of contextual fear, however, was unaltered in Gad65−/− mice when compared with wild-type littermates. The data imply that GAD65 plays a critical role in regulating cued fear responses during extinction learning and that, during this process, GABAergic signaling is involved in modulating synchronized activity between the amygdala and hippocampus. In view of the more pronounced effect on cued versus contextual fear extinction, these influences may rely more on GABAergic mechanisms in the amygdala.
Introduction
Anxiety disorders are the most common emotional disorders, making it important to understand the methods aimed at alleviating anxiety and fear, such as extinction, which involves reexposure to fearful stimuli once coupled with an aversive outcome. Extinction is considered new learning rather than erasure of the fear memory (Myers and Davis, 2002), although some unlearning may also contribute (Myers et al., 2006; Kim et al., 2007). Precisely how extinction regulates fear is unclear, but it is firmly established that fear learning processes require the amygdala (LeDoux, 2000). Via projections to the brainstem and hypothalamus, the amygdala can regulate fear behavior, such as freezing in rodents (LeDoux, 2000). Hence, the amygdala is critical for integrating sensory information predicting danger with the motor output needed to avoid such danger. In fact, confrontation with fear-associated stimuli elicits synchronized theta activity within the amygdala (Paré and Collins, 2000) and hippocampus (Moita et al., 2003), as well as between these two areas (Seidenbecher et al., 2003; Narayanan et al., 2007a,b), supporting theta synchrony as a key mechanism underlying sensorimotor integration (Bland and Oddie, 2001).
The prefrontal cortex (PFC) modulates amygdala-dependent fear memories and has become a central component of current extinction models (Quirk and Mueller, 2008). The PFC might decrease fear expression via projections to GABAergic intercalated (ITC) cells in the amygdala, which inhibit amygdalar projection neurons (Paré et al., 2004; Likhtik et al., 2008). In fact, GABAergic transmission is critical for extinction learning (Myers and Davis, 2002). GABA is generated in the brain by the enzyme glutamic acid decarboxylase (GAD), which exists in two isoforms, GAD65 and GAD67. GAD67 is responsible for metabolic GABA synthesis, whereas GAD65 may be rapidly activated in times of high GABA demand (Martin and Rimvall, 1993). Cued fear conditioning transiently reduces Gad65 gene expression in the hippocampus and amygdala (Bergado-Acosta et al., 2008). Moreover, mice lacking GAD65 (Gad65−/−), with ∼50% decrease in GAD activity and GABA content, display elevated anxiety in the open-field test and elevated zero maze (Kash et al., 1999; Stork et al., 2000, 2003) and show less prepulse inhibition (Heldt et al., 2004). Otherwise, they have normal brain cytoarchitecture (Kash et al., 1997) and no apparent loss of neuronal populations (Kash et al., 1997). Compensatory GAD67 upregulation (Asada et al., 1996), postsynaptic changes in GABAA receptor density (Kash et al., 1999), or changes in pain threshold (Bergado-Acosta et al., 2008) have not be observed in these mutants. Importantly, cued fear conditioning in Gad65−/− mice results in generalization of fear to neutral stimuli: both neutral and conditioned stimuli elicit freezing and theta frequency synchronization between the amygdala and hippocampus (Bergado-Acosta et al., 2008).
We therefore hypothesized that the GAD65 null mutation might affect neural activities during fear memory extinction and examined local field potentials (LFPs) from the lateral amygdala (LA), the CA1 area of the hippocampus, and the infralimbic area of the PFC during this process in Gad65−/− mice. Our data imply that GAD65 plays an important role in regulating fear responses during extinction of cued but apparently not contextual fear.
Materials and Methods
Animals.
Eight- to 12-week-old homozygous (Gad65−/−) Gad65 mice mutants and their wild-type littermates (Gad65+/+) were obtained from Gad65+/− × Gad65+/− breeding on a C57BL/6 genetic background (>10 generations of backcross) (Asada et al., 1996). Animals were kept under a 12 h light/dark cycle (lights on at 7:00 A.M.) with food and water provided ad libitum. Genotypes were determined with allele-specific PCR at the time of weaning, and selected animals were transferred to individual housing 1 week before starting experiments. All procedures were performed under strict observance of the European Committees Council Directive (86/609/EEC) for experimentation on animals and were approved by the Bezirksregierung Münster (AZ 50.0835.1.0, G 53/2005).
Extinction of cued fear.
Mice were first adapted to the fear conditioning apparatus (light-blue acrylic glass, 36 cm length × 21 cm width × 21 cm height arena with a grid floor for electric shock delivery; TSE Systems) and six neutral tone presentations [neutral stimulus (CS−); 2.5 kHz, 85 dB, 10 s, 20s interstimulus interval (ISI)] twice (2–3 h apart). Fear training commenced the next day and consisted of three tones [conditioned stimulus (CS+); 10 kHz, 85 dB, 10 s, randomized 10–30 s ISI], which were each coterminated with a 1 s footshock [scrambled 0.2 mA (“weak training”) or 0.4 mA (“standard training”)]; this training was repeated on the following day. The two tones were counter-conditioned, and control mice received footshocks unpaired with the tones. In the unpaired paradigm, mice were first exposed to three presentations of the tone serving as the CS+ in the paired group (randomized 10–30 s ISI). After a 2 min pause, three footshocks were delivered (randomized 10–30 s ISI). We found this to be the optimal protocol to induce similar freezing levels for the two genotypes (compared with Bergado-Acosta et al., 2008). One day after fear conditioning, mice were placed in a neutral context (clear, plastic 19 cm length × 19 cm width × 13 cm height box, filled with sawdust bedding) for extinction training. Extinction training consisted of six retrieval sessions (R1–R6, 6 min each), each 30 min apart, in which both the CS− and CS+ were presented without footshocks (4 × 10 s CS− at 20 s ISI, 40 s silence, 4 × 10 s CS+ at 20 s ISI); mice remained in the neutral context between retrieval sessions. Retention of this extinction training was assessed on the following day in the extinction context (E; identical to the retrieval sessions). One hour later, reinstatement of fear was examined immediately after a tail pinch in the extinction context (RS; identical to the retrieval sessions). For mice that were implanted with recording electrodes, recordings were obtained during R1–R6, E, and RS sessions. At the beginning of each of the two recording days, all implanted mice were connected to the recording apparatus via a swivel commutator under light inhalational anesthesia (isoflurane) and given at least 30 min to recover from the anesthesia. Because of the high-anxiety phenotype, anesthesia was necessary.
Extinction of contextual fear.
Before training, mice were twice transferred to the neutral context for 10 min (2–3 h apart) to adapt them to handling. One day after handling, mice were placed in the fear conditioning apparatus (TSE Systems) for 3 min before one footshock was delivered (scrambled 0.7 mA, 2 s) and 30 s later were returned to their home cages. Extinction training occurred the next day and consisted of six reexposures to the context (R1–R6, 6 min each) 30 min apart; mice were returned to their home cages between reexposures. Retention of this extinction training was assessed the following day (E, 6 min context reexposure), immediately followed by a footshock (scrambled 0.7 mA, 2 s), and observations continued for an additional 6 min to assess reinstatement (RS). “No-shock” controls underwent the same procedure except that the footshock during fear training was omitted.
Behavioral analyses.
Training and extinction sessions were videotaped for offline behavioral analysis. Freezing, complete immobility with the exception of respiratory movements, is an innate defensive behavior and was taken as a behavioral measurement of fear (Seidenbecher et al., 2003). The total time spent freezing was quantified during the entire 10 s of each tone presentation separately for cued fear experiments and during the entire 6 min context reexposure for contextual fear experiments and was reported as percentage of time spent freezing. In Figure 2, risk assessment (a) refers to defensive, nonfreezing behaviors, such as overt watching or stretched attending, and watching (w) refers to nondefensive sitting quiet behavior.
Figure 2.
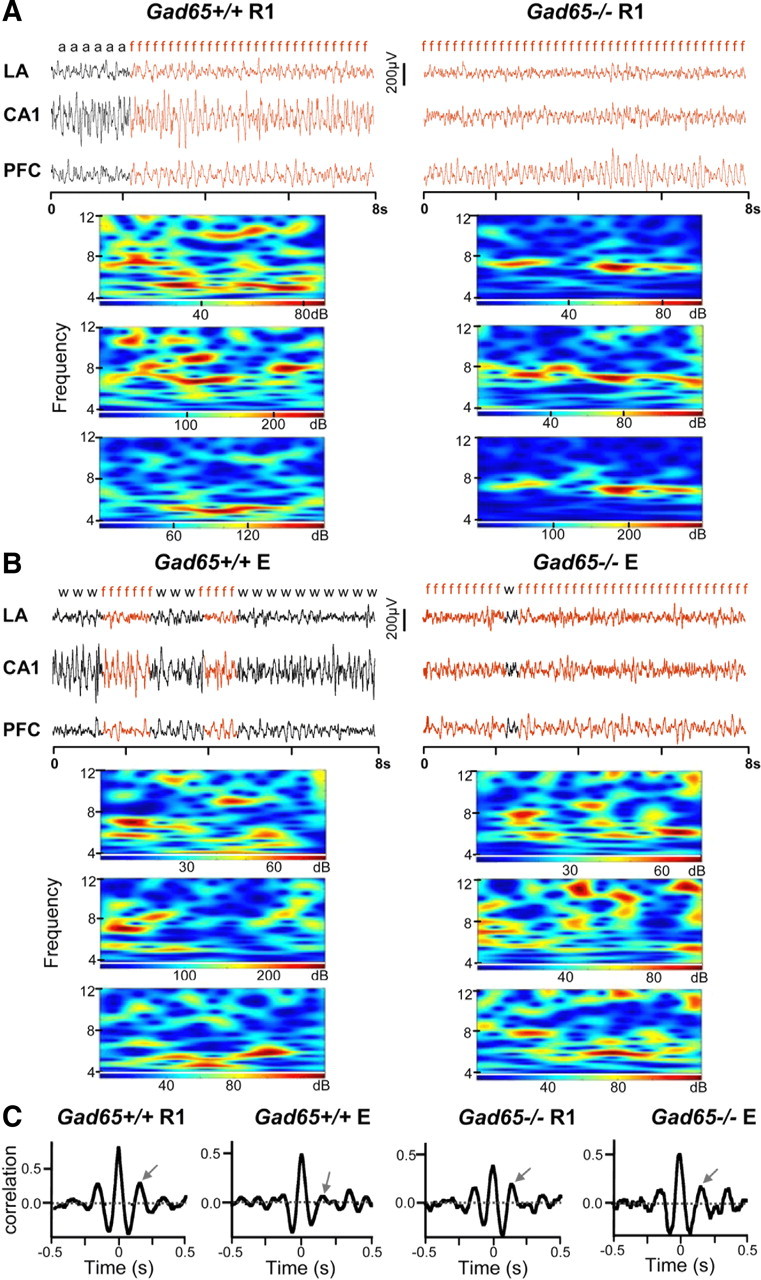
A, Theta synchronization in different stages of fear extinction. LFPs from CA1, LA, and PFC were simultaneously recorded during extinction training, extinction retention, and reinstatement while monitoring the animals' behavior. The figure shows representative LFP traces (8 s) for each recording area from R1 during the first CS+ for a Gad65+/+ and Gad65−/− mouse (left and right, respectively). The behavior of the mouse at that particular time is indicated above the traces (f, freezing; a, risk assessment; w, watching), and wavelet transforms of the 8 s LFP segments for each recording area are displayed underneath. Increased activity in the theta frequency band can be seen during bouts of freezing (highlighted in red) in all recording areas for both genotypes. B, Same as A but for session E (extinction retention). C, Representative cross-correlograms for LA–CA1 are shown. The y-value of the second positive peak (indicated by arrow) was taken to quantify theta phase correlation levels between two recording areas.
Surgery.
Stainless steel electrodes were implanted in the CA1 area of the hippocampus [anteroposterior (AP), 1.94 mm; mediolateral (ML), 1 mm; dorsoventral (DV), 1.25 mm], LA (AP, 2.06 mm; ML, 3.25 mm; DV, 3.5 mm), and infralimbic area of the PFC (AP, 1.78 mm; ML, 0.3 mm; DV, 2 mm) (Franklin and Paxinos, 1997), under deep pentobarbital anesthesia (Narkoren, 50 mg/kg, i.p.). Silver electrodes were implanted close to the midline over the nasal and cerebellar regions for reference and ground, respectively. The free ends of the electrodes were fed through a rubber socket that was fixed on the skull with dental cement. During recording, this ensemble was connected to a swivel commutator, which was connected to amplifiers for tracking and to a personal computer for data storage. All mice were given 3–5 d for surgical recovery before beginning any experiment. After all experiments, animals were killed with an overdose of isoflurane, and the brains were removed and preserved in 4% Formalin. Cryosections were stained with cresyl violet to verify the electrode positions.
Local field potential analyses.
LFP waveforms were fed through a differential amplifier (DPA-2F; Science Products), bandpass filtered from 1 to 30 Hz, transformed by an analog-to-digital interface (sampling rate, 1 kHz; CED Power 1401; Cambridge Electronic Design), and stored on a personal computer. LFPs from CA1, LA, and PFC were simultaneously recorded during the R1–R6, E, and RS sessions of extinction of cued fear (standard training) while monitoring behavior (see Fig. 2A,B). Time–frequency color representations (see Fig. 2A,B) were calculated via wavelet transformation of the time-domain data using 120 Morlet wavelets linearly spaced between 4 and 12 Hz using a customized Matlab routine (MathWorks). The color plots depict the modulus of the complex wavelet coefficients (i.e., oscillation amplitude) in units normalized to the time-domain amplitude. Cross-correlograms were calculated via the program Spike 2 (Cambridge Electronic Design), and the y-value of the second positive peak, corresponding to the theta frequency peak, was taken to quantify correlation levels between two recording areas (see Fig. 2C) (Seidenbecher et al., 2003; Bergado-Acosta et al., 2008). The reference channel was set to CA1 for LA–CA1 and CA1–PFC cross-correlograms and to PFC for LA–PFC cross-correlograms.
Statistical analyses.
Unless otherwise stated, data were analyzed with two-way repeated measures ANOVAs to detect significant interactions between group or genotype and session and main effects of session, genotype, or group. Post hoc Bonferroni's tests were performed when appropriate. Within-group differences across extinction training were analyzed via one-way repeated measures ANOVAs using the post hoc Tukey's multiple comparison test to detect significant differences between sessions, unless otherwise stated. p values <0.05 were considered statistically significant.
Results
Impaired extinction of cued fear in Gad65−/− mice
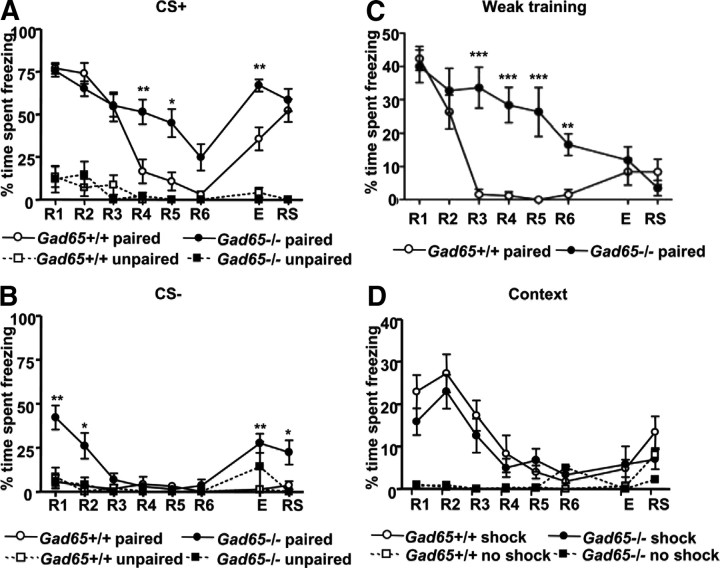
The percentage of time spent freezing during the first CS+ presentation across R1–R6, E, and RS was quantified and taken for analysis (Fig. 1A). A repeated measures two-way ANOVA revealed a significant interaction between genotype and session (F(7,217) = 4.59, p < 0.0001), as well as a main effect of both session (F(7,217) = 26.33, p < 0.0001) and genotype (F(1,217) = 12.15, p < 0.01). When compared with wild-type littermates, Gad65−/− exhibited significantly higher freezing at R4, R5, and E (post hoc Bonferroni's test, p < 0.001, p < 0.01, p < 0.01, respectively). Gad65+/+ (implanted; n = 14) mice showed a reduction in freezing from R1 to R6 and freezing remained relatively low during E, demonstrating successful extinction of fear (repeated measures one-way ANOVA, F(7,91) = 24.59, p < 0.0001; post hoc Tukey's test: R1 vs R4, p < 0.001; R1 vs R5, p < 0.001; R1 vs R6, p < 0.001; R1 vs E, p < 0.001). During reinstatement, freezing behavior increased during the CS+ presentation (R1 vs RS, p > 0.05) but not the CS− presentation (repeated measures one-way ANOVA, F(7,91) = 0.539, p > 0.05), demonstrating that the original fear association remained after extinction training. Gad65−/− mice (implanted; n = 19) displayed pronounced freezing across all sessions with the exception of R5 and R6 (repeated measures one-way ANOVA, F(7,126) = 7.557, p < 0.0001; post hoc Tukey's test: R1 vs R5, p < 0.01; R1 vs R6, p < 0.001). Thus, Gad65−/− mice are disrupted in their ability to extinguish fear responses elicited by a cue. A comparable lack of extinction learning was observed in unimplanted Gad65−/− mice (data not shown).
Figure 1.
A, Impaired extinction of cued fear. Freezing behavior during the CS+ across R1–R6, E, and RS (standard training). Freezing in Gad65+/+ mice declined from R1 to R6 and remained low during E. Gad65−/− mice displayed pronounced freezing across all sessions with the exception of R6. Very little freezing was observed in unpaired controls of either genotype. Asterisks indicate between-genotype differences. B, Generalization of fear. Freezing behavior during the CS− across R1–R6, E, and RS (standard training). Gad65+/+ mice showed very little freezing to the CS− across all sessions. Gad65−/− mice exhibited high freezing to the CS− early in extinction training (R1, R2) and during the extinction retention test (E). C, Weak training. Freezing behavior during the CS+ across R1–R6, E, and RS. Gad65−/− mice were similar to Gad65+/+ mice at R1 and E. However, extinction learning across R1–R6 remained impaired in Gad65−/− mice. D, Unaffected extinction of contextual fear. Both genotypes showed similar levels of freezing after conditioning (R1), whereas no-shock controls for both genotypes showed no freezing. No differences were seen between genotypes across extinction training. All data are expressed as mean ± SEM. Asterisks indicate between-genotype differences. *p < 0.05, **p < 0.01, ***p < 0.001.
Unpaired controls (implanted; Gad65+/+, n = 6; Gad65−/−, n = 6) (Fig. 1A) showed significantly lower freezing to the CS+ after training than genotype-matched paired mice, demonstrating that, in both genotypes, a specific pairing between the CS+ and the unconditioned stimulus (US) is required to develop a learned fear response to the CS+ (R1: both Gad65+/+ and Gad65−/−, paired vs unpaired, unpaired t tests, p < 0.0001).
Generalization of fear in Gad65−/− mice
Freezing was also measured during the first CS− presentation (neutral tone) across R1–R6, E, and RS to assess fear specificity to the cues (Fig. 1B). A repeated measures two-way ANOVA revealed a significant interaction between genotype and session (F(7,217) = 6.61, p < 0.0001), as well as a main effect of both session (F(7,217) = 8.05, p < 0.0001) and genotype (F(1,217) = 17.61, p < 0.001). Compared with Gad65+/+ mice, Gad65−/− mice (n = 19) exhibited significantly higher freezing to the CS− early in extinction training, during the extinction retention test, and after the reinstating stimulus (post hoc Bonferroni's test; R1, p < 0.001; R2, p < 0.01; E, p < 0.001; RS, p < 0.05), demonstrating that their freezing responses generalized to the neutral CS− as well. However, Gad65−/− mice (n = 19) showed lower freezing to the CS− compared with the CS+ (R1, paired t test, p < 0.0001), indicating that they were able to discriminate between the two tones. Gad65+/+ mice (n = 14) showed very little freezing to the CS− across all sessions, thus demonstrating that their freezing response was specific to the cue associated with the footshock (CS+). Last, there was no freezing to the neutral context in either genotype as measured during the first minute of each retrieval session before any tone presentations (data not shown).
Impaired extinction in Gad65−/− mice after weak training
The above data were obtained under standard training conditions using a 0.4 mA footshock intensity, which elicits a strong fear response during R1. Although freezing levels during R1 were similar for both genotypes, there is the possibility that fear conditioning was stronger in Gad65−/− mice that was not reflected in the freezing score because freezing was already quite high. Thus, a weaker training protocol in which the footshock intensity was reduced to 0.2 mA was used to address this issue (Fig. 1C). Under these conditions, freezing levels were again similar during R1, arguing against the idea that there is stronger fear conditioning in the knock-outs. Neither genotype exhibited any freezing during the CS− (data not shown). Extinction learning was again attenuated in Gad65−/− mice. A repeated measures two-way ANOVA revealed a significant interaction between genotype and session (F(7,105) = 9.17, p < 0.0001), as well as a main effect for both session (F(7,105) = 22.57, p < 0.0001) and genotype (F(1,105) = 9.1, p < 0.01). Compared with wild-type littermates, Gad65−/− mice displayed significantly higher freezing at R3, R4, R5, and R6 (post hoc Bonferroni's test, p < 0.001, p < 0.001, p < 0.001, p < 0.01, respectively). Gad65+/+ mice (unimplanted; n = 9) showed a significant reduction in freezing beginning at R2 (repeated measures one-way ANOVA, F(7,56) = 28.26, p < 0.0001; post hoc Tukey's test: R1 vs R2, p < 0.01; R1 vs R3, R4, R5, R6, E, and RS, p < 0.001). Gad65−/− mice (unimplanted; n = 8) did not show a significant reduction in freezing across extinction training until R6, which continued to remain low during E and RS (repeated measures one-way ANOVA, F(7,49) = 11.72, p < 0.0001; post hoc Tukey's test: R1 vs R6, E, and RS, p < 0.001). Because initial freezing levels were lower with weak training compared with standard training, extinction of the fear response was more complete at R6 in Gad65−/− mice (Fig. 1C). This allowed us to examine whether the poor extinction recall with standard training was attributable to the high level of fear still apparent in R6 (Fig. 1A). With weak training, freezing was comparably low for both Gad65+/+ and Gad65−/− mice during E, demonstrating that, when fear in the knock-outs is extinguished to a lower level, there is similar extinction recall. This suggests that GAD65-mediated GABAergic transmission is important for within-session extinction learning but perhaps not for recall of extinction.
Unimpaired extinction of contextual fear in Gad65−/− mice
We have shown previously that the generalization seen after fear conditioning in Gad65−/− mice is context independent (Bergado-Acosta et al., 2008). Moreover, extinction of contextual fear was assessed in the present study and found to be unimpaired in Gad65−/− mice (Fig. 1D). A repeated measures two-way ANOVA revealed no significant interaction between genotype and session (F(7,49) = 1.01, p > 0.05) and no main effect of genotype (F(1,49) = 2.94, p > 0.05) but a significant effect of session (F(7,49) = 13.15, p < 0.0001). Both genotypes (unimplanted; Gad65+/+, n = 5; Gad65−/−, n = 4) showed similar levels of freezing 1 d after fear training during R1, whereas no-shock controls for both genotypes (Gad65+/+, n = 4; Gad65−/−, n = 2) showed no freezing. Furthermore, no significant differences were found between genotypes at any additional time point.
Persistence of cued fear in Gad65−/− mice is associated with higher LA–CA1 theta frequency synchronization
Conditioned stimulus (CS+)
LFPs from CA1, LA, and PFC were simultaneously recorded during the R1–R6, E, and RS sessions of extinction of cued fear (standard training) while monitoring behavior (Fig. 2A,B). Because our previous results (Seidenbecher et al., 2003) (standard training) demonstrated a change in theta frequency synchronization between the LA and CA1 after fear conditioning during freezing behavior, freezing-related correlations were calculated (Fig. 2C) during the first CS+ presentation between the LA and CA1, CA1 and PFC, and LA and PFC (Fig. 3). R1, R4, E, and RS sessions were chosen for analysis because, (1) sufficient freezing behavior existed to obtain a cross-correlogram and (2) with respect to R4 and E, a clear difference in freezing behavior between genotypes was present. A two-way ANOVA of LA–CA1 correlation values revealed a significant interaction between genotype and session (F(3,81) = 3.20, p < 0.05), as well as a main effect of both session (F(3,81) = 6.5, p < 0.001) and genotype (F(1,81) = 4.26, p < 0.05). A post hoc Bonferroni's test showed that correlations in Gad65−/− mice were significantly higher than Gad65+/+ mice at E. LA–CA1 correlation values significantly decreased in Gad65+/+ mice during R4 and E when compared with R1 (one-way ANOVA, F(3,31) = 8.566, p = 0.0003; post hoc Tukey's test: R4 vs R1, p < 0.01; E vs R1, p < 0.01). Gad65−/− mice did not display this decrease, with the LA–CA1 correlation values remaining high across extinction training (one-way ANOVA, F(3,50) = 1.864, p > 0.05), thus paralleling the observed persistence in fear behavior. No significant interactions between genotype and session were detected in CA1–PFC or LA–PFC freezing-related correlations (two-way ANOVA, F(3,66) = 1.574, p > 0.05; F(3,65) = 4.097, p > 0.05, respectively). There were also no effects of genotype in CA1–PFC or LA–PFC correlations (F(1,66) = 0.07, p > 0.05; F(1,65) = 0.02, p > 0.05, respectively) and no effect of session between CA1–PFC (F(3,66) = 1.57, p > 0.05). There was, however, an effect of session between LA–PFC (F(3,65) = 4.1, p < 0.05). Freezing-related correlations between the LA–PFC for the Gad65−/− mice were found to significantly decrease from R1 to R4 and E (one-way ANOVA, F(3,37) = 4.2, p = 0.0118; post hoc Tukey's test: R1 vs R4, p < 0.05; R1 vs E, p < 0.05); however, these values were not significantly different from +/+ mice for the respective time points.
Figure 3.
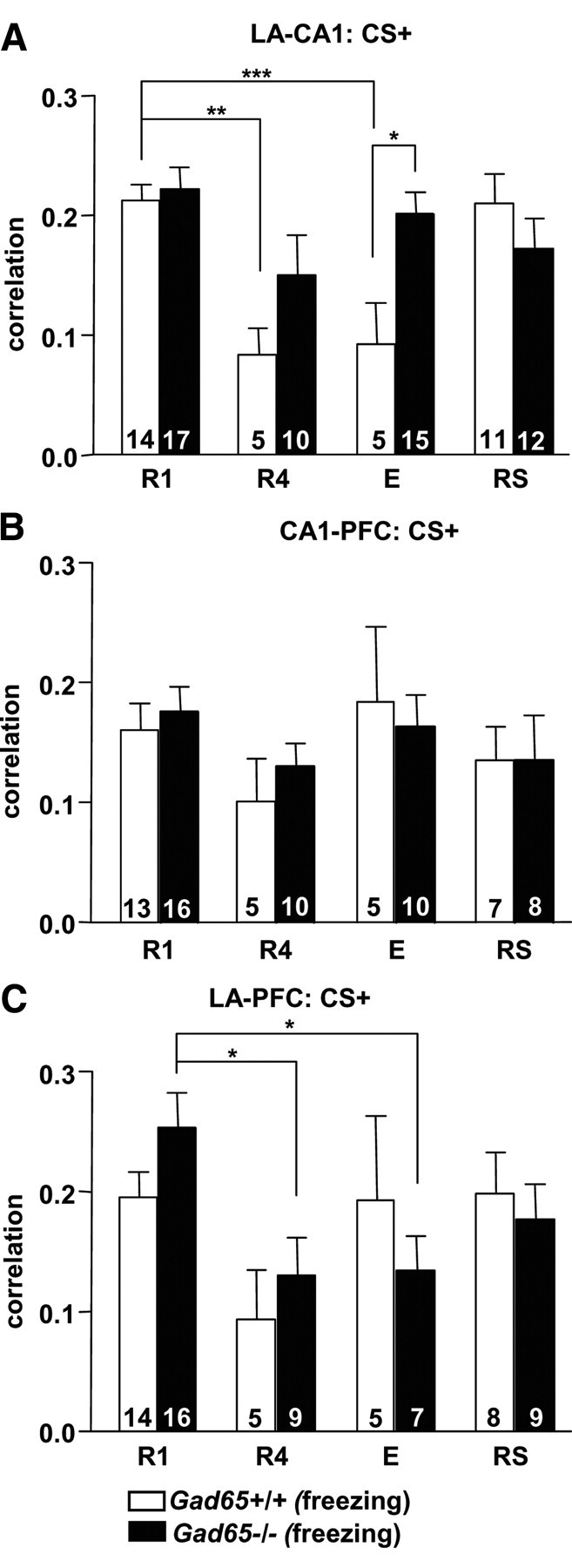
Theta synchronization deficits in Gad65−/− mice during fear extinction. Average cross-correlation values for the second positive peak during freezing during the CS+ between the LA and CA1 (A), CA1 and PFC (B), and LA and PFC (C). Gad65+/+ mice showed significantly decreased LA–CA1 correlation values during R4 and E when compared with R1. Gad65−/− mice did not display this decrease, with the correlation values remaining high across extinction training and being significantly higher than for Gad65+/+ littermates during E. LA–PFC correlation values significantly decreased in Gad65−/− mice during R4 and E when compared with R1 but were not significantly different than for Gad65+/+ littermates during any session. All data are expressed as mean ± SEM. Numbers within each bar indicates the number of observations. *p < 0.05, **p < 0.01, ***p < 0.001.
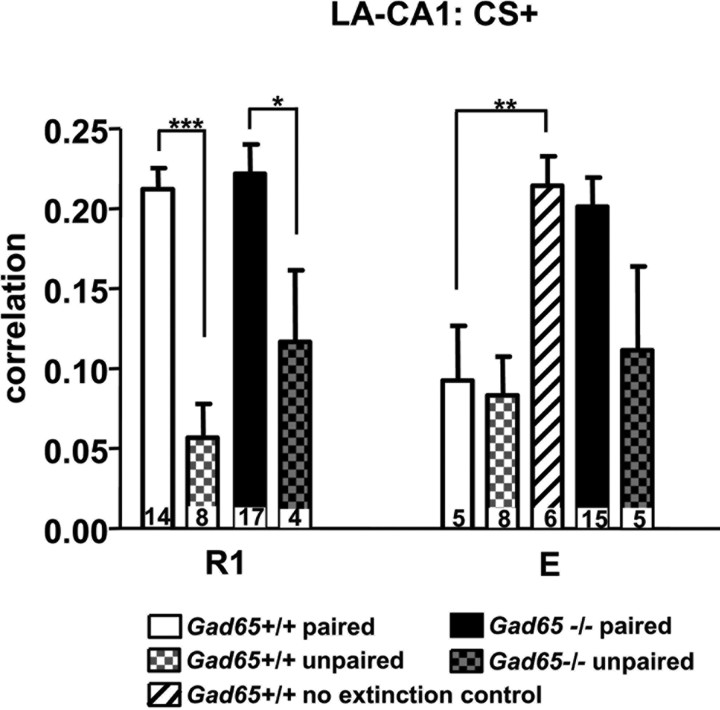
The changes in LA–CA1 freezing-related correlative activity were not seen in “no-extinction” controls (Fig. 4), i.e., Gad65+/+ mice (n = 6) that did not receive extinction training, but were still tested parallel to the E session. A one-way ANOVA showed that this group was significantly higher than both paired and unpaired Gad65+/+ mice at E (F(2,16) = 8.155, p < 0.01; post hoc Tukey's test: paired vs no extinction, p < 0.05; unpaired vs no extinction, p < 0.01). Furthermore, LA–CA1 correlative activity for no-extinction Gad65+/+ mice was not significantly different from R1 (unpaired t test, p > 0.05). This demonstrates that the decrease seen in the LA–CA1 correlative activity after extinction training was attributable to the extinction training and not to the passage of time. Learning the fear-associated cue resulted in increased LA–CA1 correlative activity, because Gad65+/+ (n = 8) and Gad65−/− (n = 4) unpaired controls (Fig. 4) showed significantly lower LA–CA1 correlative activity during R1 compared with genotype-matched paired mice (Gad65+/+ and Gad65−/−: unpaired t tests, p < 0.001).
Figure 4.
Unpaired and no-extinction controls. LA–CA1 correlation values for unpaired controls for both genotypes were significantly lower than for paired mice during R1. Values for no-extinction controls were significantly higher than in mice that received E training but not significantly different from R1. All data are expressed as mean ± SEM. Numbers within each bar indicates the number of observations. *p < 0.05, **p < 0.01, ***p < 0.001.
Neutral stimulus (CS−)
To compare synchronization patterns between Gad65+/+ mice, which did not show generalization, and Gad65−/− mice, which did, cross-correlograms were also produced for LFPs during the CS− presentation (Fig. 5). Those obtained for Gad65+/+ mice represented nonfreezing behavior because these mice did not freeze appreciably during the CS− in any given session [Fig. 5, Gad65+/+ (non-freezing)]. Gad65−/− did freeze significantly for some sessions (R1, E, and RS), and cross-correlograms were produced for LFPs during epochs in which only freezing occurred [Fig. 5, Gad65−/− (freezing)]. Two-way ANOVAs on LA–CA1, CA1–PFC, and LA–PFC correlation values showed no significant interactions between group and session (F(4,101) = 2.07, p > 0.05; F(4,95) = 0.53, p > 0.05; F(4,100) = 1.26, p > 0.05, respectively) and no effects of session (LA–CA1, F(2,101) = 1.75, p > 0.05; CA1–PFC, F(2,95) = 1.68, p > 0.05; LA–PFC, F(2,100) = 1.91, p > 0.05) but significant effects of group (LA–CA1, F(2,101) = 6.47, p < 0.01; CA1–PFC, F(2,95) = 4.54, p < 0.05; LA–PFC, F(2,100) = 2.75, p < 0.05). Post hoc Bonferroni's tests revealed significant differences between Gad65+/+ (nonfreezing) and Gad65−/− (freezing) at R1 for LA–CA1, CA1–PFC, and LA–PFC (p < 0.05 each).
Figure 5.
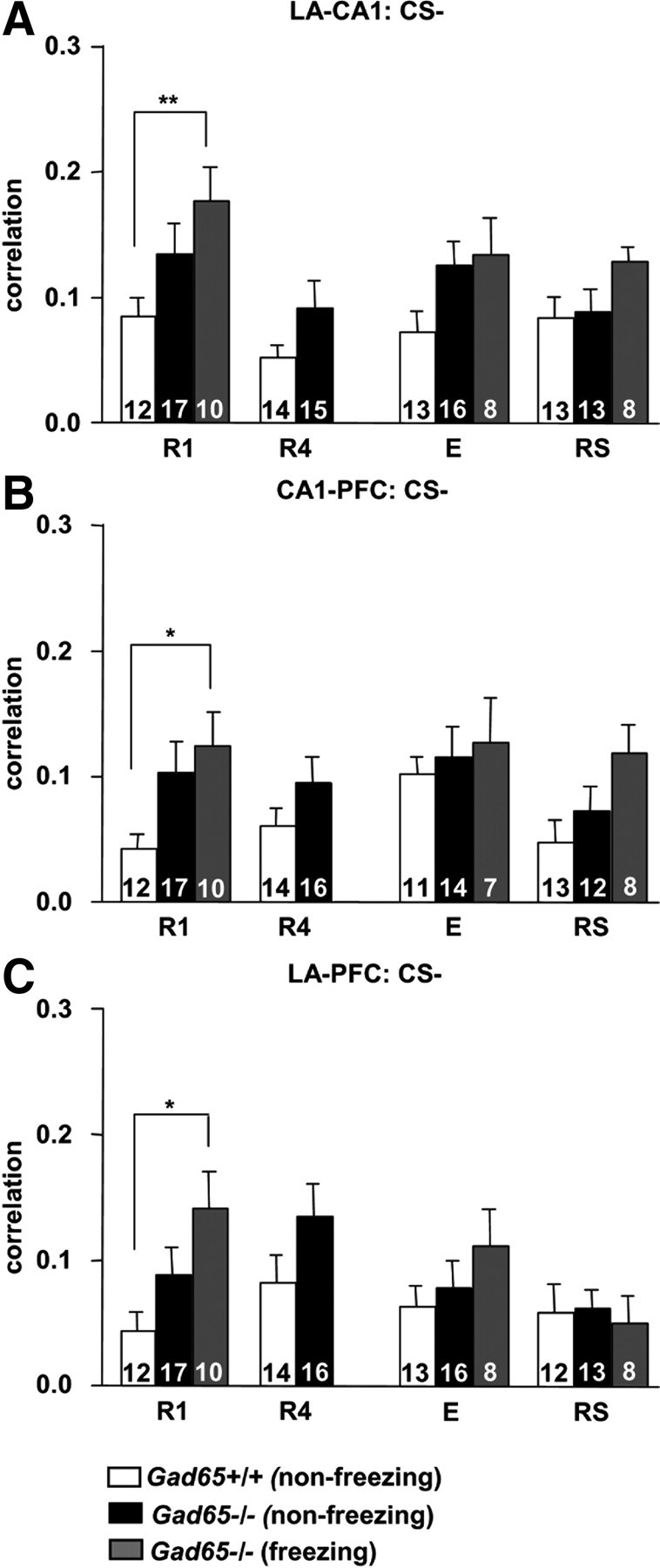
Theta synchronization in Gad65−/− mice during generalized fear. Average values for the second positive peak for cross-correlations between the LA and CA1 (A), CA1 and PFC (B), and LA and PFC (C), recorded during the CS−. Gad65+/+ mice did not freeze during the CS−, so all correlations in this genotype represent nonfreezing behavior. In contrast, generalized fear occurred in Gad65−/− mice during R1, E, and RS allowing cross-correlograms for both freezing and nonfreezing periods to be obtained. During R1, freezing-specific correlations between all recording areas in Gad65−/− mice were significantly higher than those obtained for Gad65+/+. All data are expressed as mean ± SEM. Numbers within each bar indicates the number of observations. *p < 0.05, **p < 0.01.
Furthermore, correlative activity for Gad65+/+ mice during the CS+ (freezing) was significantly higher than during the CS− (nonfreezing) between the LA–CA1, CA1–PFC, and LA–PFC (unpaired t tests, p < 0.0001, p < 0.001, p < 0.0001, respectively) (Figs. 3 vs 5). In Gad65−/− mice, only the LA–PFC correlations during CS− (freezing) were significantly lower than CS+ (freezing) (unpaired t test, p < 0.05). CS− (nonfreezing) correlations in Gad65−/− mice were significantly lower than CS+ (freezing) for all recording groups (unpaired t tests, LA–CA1, p < 0.01; CA1–PFC, p < 0.05; LA–PFC, p < 0.0001). Thus, generalized fear in Gad65−/− mice was also correlated with increased theta synchronization in the LA–CA1–PFC tripartite circuit.
Discussion
Gad65−/− mice, deficient in activity-dependent GABA synthesis, displayed persistence of cued fear during extinction learning (Fig. 1A), stimulus generalization (Fig. 1B), and enduring high LA–CA1 theta phase synchronization (Fig. 3). In contrast, extinction of contextual fear was unaffected (Fig. 1D), and extinction recall for cued fear appeared normal in the knock-outs under weak training conditions (Fig. 1C), suggesting an impairment in extinction acquisition for cued fear memories but no disturbance in the recall of extinction in the GAD65 null mutant mice.
Although Gad65−/− mice showed considerable freezing during the neutral tone (CS−), freezing levels for these mice were significantly lower to the CS− than to the CS+, indicating that they can discriminate between the two tones and recognize the CS+ as more predictive of danger. Moreover, freezing to the CS− was not influenced by high fear responses to the CS+ because, in each retrieval session, all CS− were presented before the first CS+. Our data confirm the reported lack in the preciseness of the cue memory in these mutants (Bergado-Acosta et al., 2008). A similar loss in precision of an auditory fear memory is seen after exogenously increasing cAMP response element-binding protein in the auditory thalamus, with no concomitant impairment in contextual fear memory (Han et al., 2008). The auditory thalamus projects both directly and indirectly to the LA. The thalamo-amygdala pathway is thought to process auditory information in a rapid but crude manner, whereas the thalamo-cortico-amygdala pathway processes more detailed representations of sound stimuli (LeDoux, 1995). Perhaps the more direct thalamo-amygdala pathway is potentiated in Gad65−/− mice as a result of less activity-dependent GABA release, leading to generalized fear to auditory cues that is more resistant to extinction. Although Han et al. (2008) did not investigate extinction processes, their results may provide an explanation for the more pronounced impairment in extinction to an auditory cue versus context seen in Gad65−/− mice.
Generalization of fear has been associated with a lack of presynaptic inhibition through GABAB(1a) receptors in the amygdala (Shaban et al., 2006). The generalization of fear observed in GABAB(1a) receptor-deficient mice was associated with a shift from the associative, NMDA receptor-dependent form of long-term potentiation (LTP) toward a non-associative, NMDA receptor-independent form at cortico-amygdala afferents (Shaban et al., 2006). Interestingly, the level of presynaptic GABAB receptor-mediated inhibition balancing the two forms of LTP was modulated by GABAergic activity of the local synaptic network (Shaban et al., 2006). A lack of activity-dependent GABA release in Gad65−/− mice may thus result in reduced GABAB receptor-mediated presynaptic inhibition, leading to non-associative plasticity in the LA and generalization of conditional fear. Additional evidence implicating reduced GABAergic transmission and concomitant lowered threshold for fear generalization comes from mice lacking the γ2 subunit of the GABAA receptor, which show a fear response to both a fully and partially conditioned stimulus (Crestani et al., 1999). Similar to our results, this study also demonstrated normal contextual fear learning.
The generalization seen in Gad65−/− mice is context independent (Bergado-Acosta et al., 2008), and Gad65−/− mice show normal performance in two hippocampus-dependent tasks: passive avoidance and the Morris water maze (Asada et al., 1996). Similarly, we observed that their ability to extinguish cued fear was impaired, without affecting the ability to extinguish contextual fear. The role of the amygdala has been traditionally viewed as important in fear responses to simpler, modality-specific stimuli, with the hippocampus playing a sensory relay role in fear conditioning (Phillips and LeDoux, 1992). The hippocampus has also been assigned an important role in extinction learning because it is sensitive to contextual information (Ji and Maren, 2007). Because the context is predicted to be influential during extinction of both contextual and cued fear, it was surprising that a greater deficit in the extinction of cued fear existed. To our knowledge, this is the first demonstration of impaired extinction of cued fear without a similar effect on the extinction of contextual fear in a mouse model. One reason for this may be that the synaptic mechanisms leading to generalization of fear in Gad65−/− mice also made the memory for cued fear more resistant to extinction. This is in agreement with the proposed role of reduced GABAergic inhibition in the amygdala producing generalization of fear.
Activity-dependent GABA release via GAD65 may also be more critical during the extinction of cued fear than contextual fear. Convergence of GAD65 deficiency and GABAergic dysfunction may lie in the intercalated (ITC) cell masses of the amygdala. ITC cell masses are clusters of GABAergic neurons forming a network surrounding the basolateral complex of the amygdala and provide feedforward inhibition to the basolateral and central amygdala (Marowsky et al., 2005). Lesioning these cells causes a deficit in the expression of fear extinction (Likhtik et al., 2008), whereas increasing their excitatory drive results in facilitation of extinction learning and recall (Jüngling et al., 2008). The ITC neurons thus provide a site at which a decrease in activity-dependent GABA release in GAD65 mutants would likely result in impaired extinction acquisition and/or consolidation. Fear-specific and extinction-specific neurons have been identified in the basal amygdala (Herry et al., 2008). Fear-specific neurons increase CS+-evoked spike firing during and after fear conditioning, and extinction training abolishes this increase, converting the response to a CS+-evoked inhibition. In addition, extinction-specific neurons were found that responded with increased CS+-evoked activity only after extinction training. Although Herry et al. (2008) did not locate extinction neurons in the LA, fear neurons in this area could still exist that decrease their responses after extinction training, and it is possible that this change in activity of fear neurons in response to extinction training may require GABAergic signaling.
GABAergic modulation may influence neuronal activity on a timescale consistent with the theta rhythm (Wallenstein and Hasselmo, 1997). The theta rhythm is seen in much of the temporal lobe during emotional arousal (Paré et al., 2002), and theta oscillations have been suggested as a signaling mechanism in amygdalo-hippocampal pathways for consolidation of emotional memories (Seidenbecher et al., 2003; Pelletier and Paré, 2004; Narayanan et al., 2007a,b). Indeed, in our study, high LA–CA1 theta correlations paralleled high conditional fear behavior in both genotypes. In Gad65+/+, the decline in conditional freezing responses during extinction learning and recall was accompanied by a decline in theta synchrony in LA–CA1. Gad65−/− mice displayed high freezing levels to the CS− early in extinction and to the CS+ across extinction and showed high LA–CA1 theta phase correlations throughout. These data support the notion that theta synchronization relates to fear memory in these pathways (Narayanan et al., 2007b).
A different scenario seems to hold for theta activity involving the PFC. Theta correlations in LA–PFC did decrease during extinction learning in Gad65−/− mice, although these values were not significantly different from wild-type littermates at any given session. In view of the role of the infralimbic area of the PFC in the recall and maintenance of extinction (Quirk and Mueller, 2008), these findings were unexpected. Although we did not attempt a systematic study of regionally specific differences in theta synchrony related to conditional fear behavior in the present study, a number of mechanisms may contribute. First, we may have not fully monitored theta patterns related to extinction in our study. Our recordings were restricted to the LA not necessarily including the population of GABAergic intercalated cells targeted by the PFC to decrease fear expression (Jüngling et al., 2008; Likhtik et al., 2008) nor the extinction-specific neurons in the BA (Herry et al., 2008). Second, given the theta decline during extinction in Gad65−/−, our data suggest that GABA synthesis through GAD65 activity may be important for sculpturing theta activity in these circuits. A modeling study has linked GABA-mediated conductances to rising and falling in rhythm with theta oscillations and has identified a critical role of GABAB receptor activation (Wallenstein and Hasselmo, 1997). Presynaptic and postsynaptic GABAB receptors in the LA typically require repetitive, high-frequency activity for activation (Szinyei et al., 2000; Klueva et al., 2003), and high-frequency burst activity typifies prefrontal cortical neurons during extinction recall (Burgos-Robles et al., 2007). Therefore, it is tempting to suggest that GABAB receptor activation is involved in extinction-related theta synchrony in PFC–LA circuits and that this function requires GAD65 activity. The conclusion of an interrelation of GAD65 and GABAB receptor activity is supported by the findings that both GABAB receptor (Shaban et al., 2006) and GAD65 deficiency (Bergado-Acosta et al., 2008) result in generalization of conditioned fear responses.
In conclusion, reduced GABA activity attributable to a deficiency in GAD65 results in both generalization of conditional fear and impaired extinction of cued fear but leaves extinction of contextual fear unaffected. These behavioral deficits seem to reflect altered GABAergic transmission affecting the timing of neuronal firing, the specificity of synaptic plasticity at afferent input systems to the amygdala, and the synchronization at theta frequency of functionally connected neuronal populations in the amygdala, hippocampus, and prefrontal cortex.
Footnotes
This work was supported by Deutsche Forschungsgemeinschaft Grants Pa 336/17-1 (H.-C.P.), SFB-TRR58 TPA02 (H.-C.P., T.S.), and SFB779 TPB5 (O.S.). S.S. is supported by the International Human Frontier Science Program Organization. We thank S. Ruppel, P. Berenbrock, and E. Boening for animal care and technical assistance and Dr. C. Kluge for Matlab assistance.
References
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding R, Ji FY, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem Biophys Res Commun. 1996;229:891–895. doi: 10.1006/bbrc.1996.1898. [DOI] [PubMed] [Google Scholar]
- Bergado-Acosta JR, Sangha S, Narayanan RT, Obata K, Pape HC, Stork O. Critical role of the 65-kDa isoform of glutamic acid decarboxylase in consolidation and generalization of Pavlovian fear memory. Learn Mem. 2008;15:163–171. doi: 10.1101/lm.705408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland BH, Oddie SD. Theta band oscillation and synchrony in the hippocampal formation and associated structures: the case for its role in sensorimotor integration. Behav Brain Res. 2001;127:119–136. doi: 10.1016/s0166-4328(01)00358-8. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, Belzung C, Fritschy JM, Lüscher B, Mohler H. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. San Diego: Academic; 1997. The mouse brain in stereotaxic coordinates. [Google Scholar]
- Han JH, Yiu AP, Cole CJ, Hsiang HL, Neve RL, Josselyn SA. Increasing CREB in the auditory thalamus enhances memory and generalization of auditory conditioned fear. Learn Mem. 2008;15:443–453. doi: 10.1101/lm.993608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Green A, Ressler KJ. Prepulse inhibition deficits in GAD65 knockout mice and the effect of antipsychotic treatment. Neuropsychopharmacology. 2004;29:1610–1619. doi: 10.1038/sj.npp.1300468. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17:749–758. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- Jüngling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, Okamura N, Duangdao DM, Xu YL, Reinscheid RK, Pape HC. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron. 2008;59:298–310. doi: 10.1016/j.neuron.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash SF, Johnson RS, Tecott LH, Noebels JL, Mayfield RD, Hanahan D, Baekkeskov S. Epilepsy in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1997;94:14060–14065. doi: 10.1073/pnas.94.25.14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash SF, Tecott LH, Hodge C, Baekkeskov S. Increased anxiety and altered responses to anxiolytics in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1999;96:1698–1703. doi: 10.1073/pnas.96.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee S, Park K, Hong I, Song B, Son G, Park H, Kim WR, Park E, Choe HK, Kim H, Lee C, Sun W, Kim K, Shin KS, Choi S. Amygdala depotentiation and fear extinction. Proc Natl Acad Sci U S A. 2007;104:20955–20960. doi: 10.1073/pnas.0710548105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klueva J, Munsch T, Albrecht D, Pape HC. Synaptic and non-synaptic mechanisms of amygdala recruitment into temporolimbic epileptiform activities. Eur J Neurosci. 2003;18:2779–2791. doi: 10.1111/j.1460-9568.2003.02984.x. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion: clues from the brain. Annu Rev Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdale function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Martin DL, Rimvall K. Regulation of γ-aminobutyric acid synthesis in the brain. J Neurochem. 1993;60:395–407. doi: 10.1111/j.1471-4159.1993.tb03165.x. [DOI] [PubMed] [Google Scholar]
- Moita MA, Rosis S, Zhou Y, LeDoux JE, Blair HT. Hippocampal place cells acquire location-specific responses to the conditioned stimulus during auditory fear conditioning. Neuron. 2003;37:485–497. doi: 10.1016/s0896-6273(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Myers KM, Ressler KJ, Davis M. Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learn Mem. 2006;13:216–223. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan RT, Seidenbecher T, Kluge C, Bergado J, Stork O, Pape HC. Dissociated theta phase synchronization in amygdalo-hippocampal circuits during various stages of fear memory. Eur J Neurosci. 2007a;25:1823–1831. doi: 10.1111/j.1460-9568.2007.05437.x. [DOI] [PubMed] [Google Scholar]
- Narayanan RT, Seidenbecher T, Sangha S, Stork O, Pape HC. Theta resynchronization during reconsolidation of remote contextual fear memory. Neuroreport. 2007b;18:1107–1111. doi: 10.1097/WNR.0b013e3282004992. [DOI] [PubMed] [Google Scholar]
- Paré D, Collins DR. Neuronal correlates of fear in the lateral amygdala: multiple extracellular recordings in conscious cats. J Neurosci. 2000;20:2701–2710. doi: 10.1523/JNEUROSCI.20-07-02701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Collins DR, Pelletier JG. Amygdala oscillations and the consolidation of emotional memories. Trends Cogn Sci. 2002;6:306–314. doi: 10.1016/s1364-6613(02)01924-1. [DOI] [PubMed] [Google Scholar]
- Paré D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Pelletier JG, Paré D. Role of amygdala oscillations in the consolidation of emotional memories. Biol Psychiatry. 2004;55:559–562. doi: 10.1016/j.biopsych.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- Shaban H, Humeau Y, Herry C, Cassasus G, Shigemoto R, Ciocchi S, Barbieri S, van der Putten H, Kaupmann K, Bettler B, Lüthi A. Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nat Neurosci. 2006;9:1028–1035. doi: 10.1038/nn1732. [DOI] [PubMed] [Google Scholar]
- Stork O, Ji FY, Kaneko K, Stork S, Yoshinobu Y, Moriya T, Shibata S, Obata K. Postnatal development of a GABA deficit and disturbance of neural functions in mice lacking GAD65. Brain Res. 2000;865:45–58. doi: 10.1016/s0006-8993(00)02206-x. [DOI] [PubMed] [Google Scholar]
- Stork O, Yamanaka H, Stork S, Kume N, Obata K. Altered conditioned fear behavior in glutamate decarboxylase 65 null mutant mice. Genes Brain Behav. 2003;2:65–70. doi: 10.1034/j.1601-183x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- Szinyei C, Heinbockel T, Montagne J, Pape HC. Putative cortical and thalamic inputs elicit convergent excitation in a population of GABAergic interneurons of the lateral amygdala. J Neurosci. 2000;20:8909–8915. doi: 10.1523/JNEUROSCI.20-23-08909.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein GV, Hasselmo ME. GABAergic modulation of hippocampal population activity: sequence learning, place field development, and the phase precession effect. J Neurophysiol. 1997;78:393–408. doi: 10.1152/jn.1997.78.1.393. [DOI] [PubMed] [Google Scholar]