Abstract
Neuronal orientation selectivity has been shown in animal models to require corticocortical network cooperation and to be dependent on the presence of GABAergic inhibition. However, it is not known whether variability in these fundamental neurophysiological parameters leads to variability in behavioral performance. Here, using a combination of magnetic resonance spectroscopy, magnetoencephalography, and visual psychophysics, we show that individual performance on a visual orientation discrimination task is correlated with both the resting concentration of GABA and the frequency of stimulus-induced gamma oscillations in human visual cortex. Behaviorally, a strong oblique effect was found, with the mean angular threshold for oblique discrimination being five times higher than that for vertically oriented stimuli. Similarly, we found an oblique effect for the dependency of performance on neurophysiological parameters. Orientation detection thresholds were significantly negatively correlated with visual cortex GABA concentration for obliquely oriented patterns (r = −0.65, p < 0.015) but did not reach significance for vertically oriented stimuli (r = −0.39, p = 0.2). Similarly, thresholds for obliquely oriented stimuli were negatively correlated with gamma oscillation frequency (r = −0.65, p < 0.017), but thresholds for vertical orientations were not (r = −0.02, p = 0.9). Gamma oscillation frequency was positively correlated with GABA concentration in primary visual cortex (r = 0.67, p < 0.013). These results confirm the importance of GABAergic inhibition in orientation selectivity and demonstrate, for the first time, that interindividual performance on a simple visual task is linked to neurotransmitter concentration. The results also suggest a key role for GABAergic gamma oscillations in visual discrimination tasks.
Introduction
Within visual cortex, individual neural receptive fields are strongly tuned for stimulus orientation (Hubel and Wiesel, 1962), allowing the detection and analysis of contours within the visual scene and also providing the neural substrate for our ability to discriminate differences in pattern orientation. It has also been shown that GABAergic inhibition appears to play a key role in determining the orientation profile of these neurons, although this is the subject of much debate (Allison and Bonds, 1994; Ferster et al., 1996; Ferster and Miller, 2000; Shapley et al., 2003). The application of a GABA antagonist, such as bicuculline, can reversibly reduce orientation selectivity (Sillito, 1975; Tsumoto et al., 1979; Sillito et al., 1980; Wolf et al., 1986), while cells can become more sharply tuned when GABA is applied (Li et al., 2008).
Inhibitory mechanisms are also important in determining the properties of cortical oscillations (Traub et al., 1996; Wang and Buzsáki, 1996; Whittington et al., 2000; Bartos et al., 2007), especially in the 30–80 Hz gamma range. Stimulus-induced gamma oscillations arise in coupled populations of GABAergic inhibitory interneurons and excitatory pyramidal cells, and have been recorded from the primary visual cortex of cat (Gray and Singer, 1989), monkey (Friedman-Hill et al., 2000; Henrie and Shapley, 2005), and human (Adjamian et al., 2004; Muthukumaraswamy et al., 2009). Both modeling and neurophysiological recordings have demonstrated that the properties of gamma oscillations are controlled by the relative contributions of cortical excitation and inhibition (Whittington et al., 2000; Brunel and Wang, 2003) and a recent human study shows that gamma oscillation frequency is predicted by the concentration of resting GABA in visual cortex (Muthukumaraswamy et al., 2009). Recordings in monkey have also shown that visual gamma oscillations are modulated by orientation (Friedman-Hill et al., 2000) and show sharper orientation tuning than lower frequency oscillations (Frien et al., 2000). Given that behavioral orientation discrimination thresholds are better than might be predicted from individual neuronal receptive fields, it has been proposed that visual gamma oscillations may also provide a mechanism for synchronizing neurons into a cooperative neural assembly that then enhances orientation discrimination performance (Samonds et al., 2004; Samonds and Bonds, 2005).
The above evidence suggests that the properties of both GABAergic inhibition and gamma oscillations in primary visual cortex may be important in determining orientation selectivity at the neuronal level. What is it not known, however, is whether variability in these measures leads to variability in human behavioral performance. The aim of this work was therefore to investigate whether individual variability in orientation discrimination performance is related both to the level of GABA within an individual's cortex, measured using magnetic resonance spectroscopy (MRS), and the properties of stimulus-driven gamma oscillations in visual cortex, measured using magnetoencephalography (MEG).
Materials and Methods
Participants.
Fifteen healthy right-handed male volunteers with normal or corrected-to-normal vision were recruited. However, one volunteer could not be scanned with MEG and one participant was unable to successfully perform the oblique discrimination task (threshold = 4.6°, 4.3 SD from the mean). The final cohort therefore consisted of 13 volunteers (mean age 33.3; range 23.5–42.9) who participated after giving informed consent. Seven of these were scanned using MEG and MRS for a previous study (Muthukumaraswamy et al., 2009). MEG, MRS, and behavioral data were collected on separate days. All procedures were approved by the local Ethics Committee.
Psychophysics.
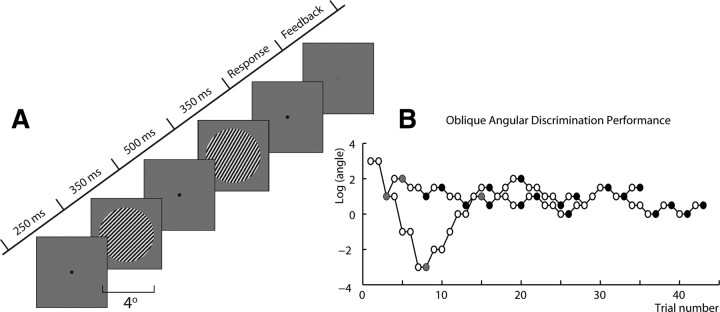
Orientation discrimination thresholds were measured using a two-alternative forced choice procedure shown in Figure 1 A. The display was a Sony Trinitron G400 CRT monitor controlled by an ATI RADEON X1600 Pro graphics card. Participants were seated 57 cm from the monitor, using a chin rest to stabilize the head. The room was completely dark, and a circular aperture was placed over the monitor to remove all external orientation clues, such as those from the edges of the screen. On each trial, two circular gratings (diameter 4°; spatial frequency 3 cycles/degree; contrast 80%; mean luminance 44.5 cd/m2) were presented sequentially, each for 350 ms, interpresentation time chosen randomly from 400–600 ms. The orientation difference between the gratings was adjusted logarithmically, using two interleaved one-up two-down staircases that converged on 71% correct performance. The mean orientation for the two gratings was held fixed at 0° in the “vertical” condition and 45° in the “oblique” condition. Participants were asked to judge whether the second grating was rotated clockwise or counterclockwise compared to the first. Responses were made using the buttons on a computer mouse and auditory tone feedback was given on each trial. Each run consisted of a block of either vertical or oblique trials, with the block order counterbalanced across participants. The run continued until both staircases completed 12 reversals, typically lasting ∼4 min. The first two reversals of each staircase were discarded before computing the threshold in degrees by taking the mean over the last 10 and then averaging the thresholds from the two staircases. Participants performed three runs each of the vertical and oblique conditions. To control for training effects, only the last two thresholds per condition were averaged to provide final threshold estimates for each participant.
Figure 1.
A, Schematic of the orientation discrimination task used in the experiment. B, Example data from the orientation discrimination task showing the two interleaved staircases—in this case for obliquely oriented stimuli. Filled dots represent turning points in the staircases.
Magnetoencephalography.
MEG recordings were made using a CTF-Omega 275-channel system sampled at 1200 Hz (0–300 Hz bandpass) in third-order gradiometer mode. Three of the 275 channels were turned off due to excessive sensor noise. To achieve MRI/MEG coregistration, fiduciary markers were placed at fixed distances from anatomical landmarks identifiable in the participant's anatomical MRIs (tragus, eye center).
Visual stimuli consisted of vertical, stationary, maximum-contrast, 3 cycles/degree, square-wave gratings presented on a mean luminance background. Stimuli were presented in the lower left visual field and subtended 4° both horizontally and vertically, with the upper right corner of the stimulus located 0.5° horizontally and vertically from a small red fixation point. Participants were instructed to maintain fixation for the entire experiment and to press a response key as fast as possible at the termination of each stimulation period. The duration of each stimulus was 1.5–2 s followed by 2 s of fixation cross only. Two hundred stimuli were presented in a session, and participants responded to the first 100 stimuli with either the right or left hand and to the second 100 trials with the opposite hand. A Mitsubishi Diamond Pro 2070 monitor controlled by Presentation software (Neurobehavioral Systems) was used to present all stimuli at 1024 × 768 resolution at 100 Hz.
Synthetic aperture magnetometry (SAM) (Vrba and Robinson, 2001) was used to create three-dimensional differential images of source power (pseudo-t statistics) for 1.5 s of baseline (−1.5 to 0 s) compared to 1.5 s of visual stimulation (0–1.5 s) at an isotropic resolution of 4 mm. To localize visual gamma responses, volumetric SAM images were initially constructed within four frequency bands, 0–20 Hz, 20–40 Hz, 40–60 Hz, and 60–80 Hz, based on our previous work (Muthukumaraswamy and Singh, 2009). The peak locations of gamma activity in each primary visual cortex were located in the volumetric images and SAM virtual electrodes reconstructions were generated for these locations using covariance matrices bandpass filtered between 0 and 100 Hz. Time–frequency analysis was then performed using the Hilbert transform between 1 and 100 Hz in 0.5 Hz frequency steps. From these time–frequency spectra, peak gamma band frequency and amplitudes, expressed as percentage change from baseline, were obtained. In four of the participants, peak gamma oscillation frequency was measured on another five occasions, each separated by 1 week, to estimate intersession variability in this parameter.
Magnetic resonance imaging.
MR data were acquired on a 3 tesla General Electric Signa HDx scanner with an eight-channel receive-only head RF coil (Medical Devices). For each participant we obtained a 3D FSPGR scan with 1 mm isotropic voxel resolution for use with the MEG analyses.
GABA-edited MR spectra were acquired from a 3 × 3 × 3 cm3 volume positioned medially in the occipital lobe using the MEGA-PRESS method (Mescher et al., 1998; Edden and Barker, 2007). The lower face of the voxel was aligned with the cerebellar tentorium and positioned so as to avoid including the sagittal sinus and to ensure the volume remained inside the occipital lobe. The following experimental parameters were used: TE = 68 ms; TR = 1800 ms; 512 transients of 2048 data points were acquired in 15 min experiment time; a 20 ms Gaussian editing pulse was applied at 1.9 ppm in alternate scans. Four hertz exponential line broadening and a high-pass water filter were applied, and the MEGA-PRESS difference spectrum was produced. The edited GABA signal at 3 ppm and the unsuppressed PRESS water signal were integrated. A concentration measurement in institutional units was derived by accounting for the editing efficiency and the T1 and T2 relaxation times of water and GABA. The integral of the GABA peak was calculated automatically using a linear fit of the baseline and a Gaussian fit to the peak itself (Marshall et al., 2000). Two 15 min measurements were made, and the mean concentration measurement was calculated for each participant.
Cortical mesh models were constructed for each participant from their anatomical FSPGR scan using FreeSurfer (Dale et al., 1999). Freesurfer was also used to obtain measures of cortical volume and cortical thickness in the occipital lobe, within the pericalcarine, lingual, and cuneal gyri.
The robustness of the significant Pearson correlation coefficients was estimated using bootstrapping with replacement, with 10,000 iterations used to provide 95% confidence intervals. Linear regression analysis was also performed in SPSS16, to investigate partial correlations between variables of interest.
Results
Figure 1 B shows example behavioral data from one participant, demonstrating convergence of the two staircases for the oblique orientation task. There was a clear, significant, difference between thresholds for the oblique and vertical discrimination tasks (t (12) = 6.8, p < 0.0001). For vertical, the final discrimination threshold was 0.5 ± 0.1°, while for oblique it was 1.8 ± 0.2°. The threshold for oblique discrimination is clearly higher than that for vertical by a factor of 5.0 ± 1.0. There was also a significant effect of training for the oblique task, but not the vertical. For the oblique task, mean thresholds fell from 2.3° to 1.8° over the three measurement sessions, while for the vertical task, thresholds were essentially unchanged (0.55–0.54°).
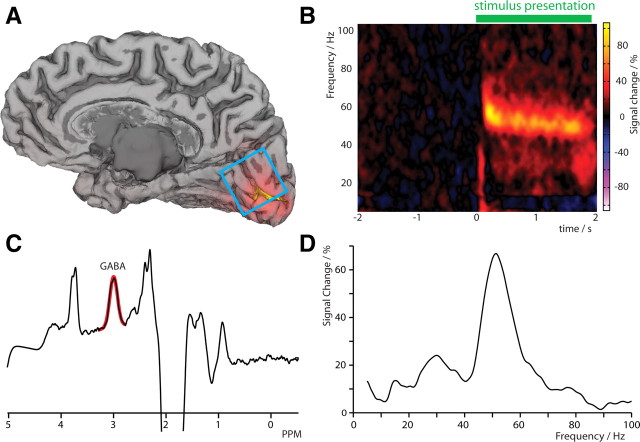
In the MEG recording session, all participants demonstrated a sustained stimulus-induced gamma oscillation in primary visual cortex (Fig. 2 A). Virtual electrode analysis was performed at the cortical location showing the peak gamma amplitude for each participant (Fig. 2 B). Figure 2 D shows a representative gamma spectrum for one participant, integrated over the latency range 0.5–1.5 s, during stimulus presentation. The amplitude of the gamma oscillation was expressed as the percentage change in amplitude from the prestimulus baseline and, across participants, ranged from 4.0 to 57.0% (mean = 19.0 ± 4.1%). The frequency of the gamma oscillation ranged from 43.5 to 58.0 Hz (mean: 50.9 ± 1.3 Hz).
Figure 2.
A, Cortical mesh model from a representative participant showing the location of the MRS voxel (blue box) and the source localization of induced gamma band (40–60 Hz) activity (orange) in the calcarine sulcus. B, Time–frequency representation of the peak location of gamma band activity in primary visual cortex showing a clear sustained oscillation that is present for the entire duration of the stimulus and is shown as an integrated spectrum in D. C, Edited MR spectra for the same participant (black) demonstrating a clearly resolved peak for GABA at 3 ppm, with the fitted GABA model indicated in red.
In the MRS session, all participants' spectra showed a clearly resolved GABA peak at 3 ppm, which was well modeled by a Gaussian fit to the spectra. GABA concentration varied across the participants from 1.07 to 1.41 IU (mean: 1.22 ± 0.03). Figure 2 C shows the spectrum from a representative participant.
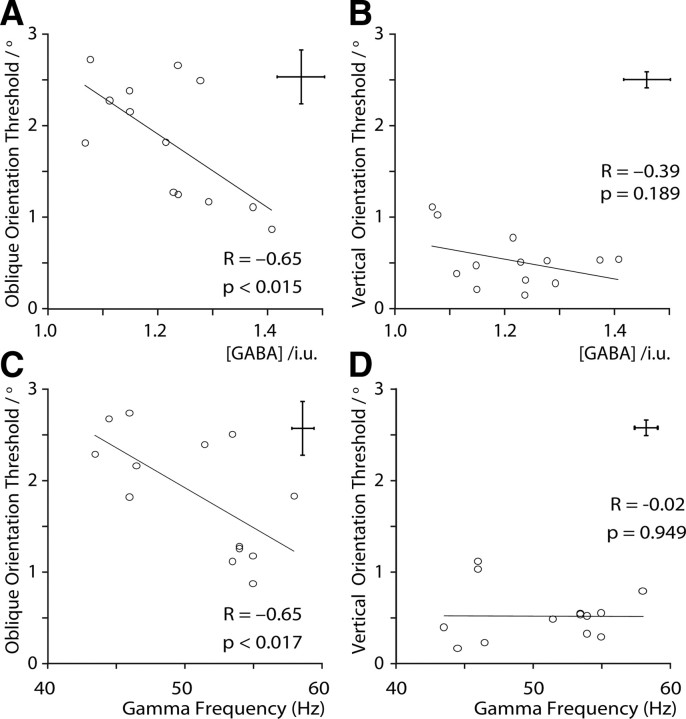
As shown in Figure 3, the angular threshold for the oblique orientation discrimination task was inversely correlated with GABA concentration (r = −0.65, p < 0.015), whereas the correlation for vertical stimuli was weaker and not statistically significant (r = −0.39, p = 0.2). Similarly, the orientation discrimination threshold was negatively correlated with an individual's gamma frequency for oblique stimuli (r = −0.65, p < 0.017) but not for vertical stimuli (r = −0.02, p = 0.9). No significant correlations were found between orientation performance and gamma amplitude, for either oblique or vertical stimuli.
Figure 3.
Vertical and oblique angular orientation discrimination thresholds as a function of both GABA concentration (A, B) and gamma frequency (C, D). The crosshairs in the top right show estimates of uncertainty for each measure, averaged across participants. For gamma frequency this is the mean SEM obtained for the four participants with repeat data (see Materials and Methods) and was calculated to be 0.8 ± 0.2 Hz. For GABA the crosshairs depict the mean within-session difference between the two GABA concentration estimates, which was 0.043 ± 0.006 IU. For the behavioral thresholds, the crosshairs depict the SEM of the last 10 staircase reversals, averaged over both interleaved staircases. For oblique discrimination, this was 0.29 ± 0.03° and for vertical discrimination, it was 0.09 ± 0.01°.
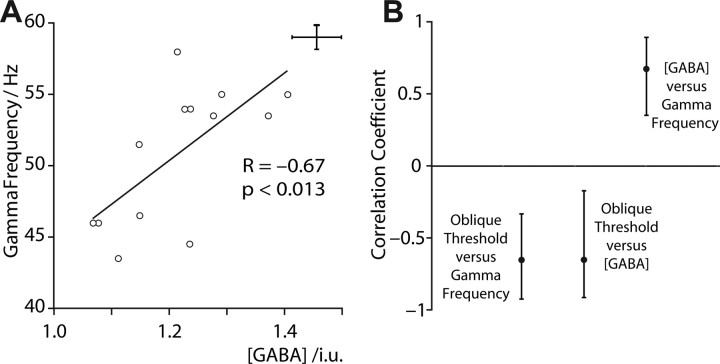
As we previously reported (Muthukumaraswamy et al., 2009), gamma frequency was strongly correlated with GABA concentration (r = 0.67, p < 0.013) (Fig. 4 A) again suggesting a key role for inhibitory GABAergic mechanisms in controlling the parameters of gamma oscillations. Note that seven of the current participants were in our previous study. However, if only the six new participants are included we still find a significant correlation between GABA and gamma frequency (r = 0.82, p < 0.048).
Figure 4.
A, Gamma frequency is plotted as a function of GABA concentration. B, Stability of the observed significant correlations assessed using bootstrapping. The crosshairs depict the 95% confidence intervals.
The three highly significant correlations that we found between GABA concentration, gamma frequency, and oblique orientation discrimination threshold were tested against a bootstrapped distribution, to provide 95% confidence intervals (CIs), and the results are shown in Figure 4 B. In all three cases, the correlation coefficient appears robust, with CIs that do not intersect the axis.
Performance on the discrimination tasks was not correlated with the age of our participants for either the oblique task (r = 0.36, p > 0.2) or the vertical task (r = −0.17, p > 0.6). Neither the oblique or vertical discrimination threshold showed a significant correlation with either gray-matter thickness or volume in the occipital lobe. Similarly, none of GABA concentration, gamma amplitude, or gamma frequency were significantly correlated with age, occipital gray matter thickness or occipital gray matter volume. The total volume of occipital gray matter did show a significant age-related decline (r = −0.58, p < 0.04).
Linear regression analysis using GABA, gamma frequency, and age as covariates to explain variance in oblique discrimination was performed. When controlled for gamma frequency, GABA did not show a significant correlation with threshold (r = −0.39, p = 0.21). Similarly, when controlled for GABA, gamma frequency did not show a significant correlation with threshold (r = −0.38, p = 0.23). Interestingly, when the correlations due to gamma frequency and GABA are accounted for, there was a trend to significance for age versus oblique orientation threshold (r = 0.534, p = 0.09), suggesting that some of the variance across participants may be explained by a general decline in performance with age, independent of the effects of GABA and gamma frequency. In support of this, the correlation between GABA and oblique orientation threshold increased when age was controlled for (−0.67 to −0.75, p < 0.005). Similarly, the correlation between gamma frequency and behavioral threshold also increased slightly (−0.65 to −0.66).
Discussion
In this work we have demonstrated that individual variability of performance on an orientation discrimination task is correlated with the resting GABA concentration in an individual's cortex. These findings confirm a central role for GABAergic inhibition in orientation discrimination and provide a direct link between previous animal neurophysiology studies and human behavioral measures. The strong correlations we find are particularly remarkable given that resting GABA concentration is assessed over such a large volume of occipital cortex. In addition, the MRS concentration presumably reflects the total baseline amount of GABA that is available, both intracellular and extracellular, and so there is no guarantee that this measure should reveal anything about the active GABAergic current at the synapses. The fact such strong behavioral and electrophysiological relationships to GABA exist suggests that MRS measures of bulk resting GABA do indeed tell us something about the functional action of GABA, but caution must be maintained when interpreting what are only correlational findings.
Neuronal inhibition, mediated by GABAergic interneurons, may influence performance of orientation discrimination tasks by (at least) two possible mechanisms, at either a neuronal or network level. First, it is thought that inhibition plays a direct role in sharpening orientation tuning across a wide range of stimulus contrasts, although the precise contribution and mechanisms are the subject of much debate (Ferster et al., 1996; Ferster and Miller, 2000; Shapley et al., 2003). Second, it has been proposed that the coordinated action of several neurons is needed to explain why human behavioral performance appears better than that of a single neuron (Samonds et al., 2004). The mechanism for binding orientation representation across neural assemblies is unknown, but some have proposed a role for gamma oscillations in helping to synchronize neural firing within this assembly (Samonds and Bonds, 2005). Thus, GABA may also influence orientation discrimination performance through modulation of the properties of visual gamma oscillations.
In support of this, we also found that orientation discrimination performance was correlated with gamma oscillation frequency in primary visual cortex. Note that we did not measure gamma oscillations while people performed the orientation discrimination task, rather we use the frequency of response to the same grating stimulus as a trait measure of the excitation/inhibition balance in cortex. Again, this correlation may be observed for two reasons, associative and causative. First, it may simply be, as shown in Figure 4 A and in previous studies (Muthukumaraswamy et al., 2009), that gamma frequency is positively correlated with orientation performance simply through a mutual dependency on GABA, and plays no real role itself in the task.
Alternatively, a more rapid gamma oscillation may confer a direct advantage in terms of task performance. On initial presentation of a visual stimulus, a high-frequency transient gamma response is generated from the retina through the LGN to primary visual cortex (Castelo-Branco et al., 1998) where it synchronizes the neural assembly needed to perform extraction of salient stimulus features such as orientation (Samonds and Bonds, 2005). This transient synchrony tends to decay, but it has been shown that when sustained gamma oscillations are present, neural synchrony within these neural subgroups tends to be preserved (Fries et al., 2001; Samonds and Bonds, 2005). This is important because synchronization of neural firing has been shown to enhance stable transmission of information through the cortex (Diesmann et al., 1999). We therefore suggest that within the cortex of individuals demonstrating a higher gamma frequency trait, neural synchrony is maintained more efficiently, enhancing the stability and accuracy of perceptual grouping and thereby contributing to better performance on orientation tasks. A direct test of this hypothesis would be to measure gamma oscillation frequency during performance of the orientation discrimination task and assess both intersubject and trial-by-trial intrasubject correlations with behavioral performance.
As expected, we observed the “oblique effect,” in which performance is much poorer for obliquely oriented stimuli than for the cardinals (Appelle, 1972). This has been observed many times, but the fundamental mechanism is poorly understood (McMahon and MacLeod, 2003). Some studies show that there are fewer cells optimally tuned for oblique orientations (Mansfield, 1974; Li et al., 2003) and less cortical area devoted to oblique representations (Coppola et al., 1998; Wang et al., 2003). Macroscopic investigations, such as evoked potentials in cat (Bonds, 1982) and human (Maffei and Campbell, 1970) and functional magnetic resonance imaging (Furmanski and Engel, 2000) also provide support for a cortical origin of this oblique effect. However, these differences in cortical representation and activation tend to be rather modest compared to the large differences in thresholds that we and others observe, especially for stimuli that are well above detection thresholds.
One possibility is that the difference between cardinal and oblique performance may not arise from primary visual cortex itself, but may result from top-down modulation from higher-visual areas. A recent study in cat primary visual area 17 showed that the increased areal representation of the cardinal axes, compared to the obliques, was greatly increased by glutamate excitation of cat visual area 21a and decreased by the deactivation of the same area by GABA (Liang et al., 2007). These studies suggest that the oblique effect may be at least partly due to feedback from higher-level areas and we could speculate that those individuals with higher GABA within specific visual areas may have increased inhibitory control of these feedback processes. This would then explain why we found a correlation between oblique discrimination performance and GABA concentration, but not for vertical stimuli. Given the large extent of our MRS voxel, we cannot speak to this issue. However, future studies could investigate this hypothesis directly by the targeted placement of multiple smaller MRS voxels, at the expense of increased acquisition time, to see whether behavioral performance correlates most strongly with GABA concentration in the ventral visual areas thought to contain the human homolog of 21a.
Whatever the neurophysiological substrate underpinning the oblique effect, our failure to observe a correlation between GABA concentration and vertical discrimination performance may also be a reflection of a “ceiling” effect in which performance is so good on the vertical task that intersubject variability is too small to detect the correlation with intrinsic GABA levels, at least in our moderately sized cohort. In support of this, we note that in Figure 3 B there does appear to be an apparent negative correlation between vertical orientation discrimination and GABA, which does not reach significance (r = −0.37, p < 0.02). However, this is not the case for the correlation between vertical performance and gamma frequency (Fig. 3 D). It is worth noting that a previous study also found that migraineurs were significantly impaired on orientation discrimination compared to controls, but only for oblique orientations (Tibber et al., 2006).
In conclusion, we believe that this study underlines the importance of studying interparticipant variability in neuroimaging, neurophysiological, and behavioral experiments, and queries the wisdom of studying small numbers of observers or averaging across groups. The results we present here show that variability in an individual's perceptual performance is, at least partly, explained by variability in neurophysiological traits of that individual. The current experiment provides a strong example of how the study of these variances can potentially provide useful information on the mechanisms underlying perceptual and cognitive performance.
Footnotes
This work was supported by the Schools of Psychology, Biosciences, and Chemistry at Cardiff University and the Wales Institute of Cognitive Neuroscience. The Cardiff University Brain Research Imaging Centre was established with support from the UK Department of Trade and Industry, Cardiff University, and the Welsh Assembly government. R.A.E.E. holds a Research Councils UK fellowship and acknowledges pulse programming advice from Gareth Barker and Dikoma Shungu.
References
- Adjamian P, Holliday IE, Barnes GR, Hillebrand A, Hadjipapas A, Singh KD. Induced visual illusions and gamma oscillations in human primary visual cortex. Eur J Neurosci. 2004;20:587–592. doi: 10.1111/j.1460-9568.2004.03495.x. [DOI] [PubMed] [Google Scholar]
- Allison JD, Bonds AB. Inactivation of the infragranular striate cortex broadens orientation tuning of supragranular visual neurons in the cat. Exp Brain Res. 1994;101:415–426. doi: 10.1007/BF00227335. [DOI] [PubMed] [Google Scholar]
- Appelle S. Perception and discrimination as a function of stimulus orientation: the “oblique effect” in man and animals. Psychol Bull. 1972;78:266–278. doi: 10.1037/h0033117. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Bonds AB. An “oblique effect” in the visual evoked potential of the cat. Exp Brain Res. 1982;46:151–154. doi: 10.1007/BF00238110. [DOI] [PubMed] [Google Scholar]
- Brunel N, Wang X-J. What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation-inhibition balance. J Neurophysiol. 2003;90:415–430. doi: 10.1152/jn.01095.2002. [DOI] [PubMed] [Google Scholar]
- Castelo-Branco M, Neuenschwander S, Singer W. Synchronization of visual responses between the cortex, lateral geniculate nucleus, and retina in the anesthetized cat. J Neurosci. 1998;18:6395–6410. doi: 10.1523/JNEUROSCI.18-16-06395.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola DM, White LE, Fitzpatrick D, Purves D. Unequal representation of cardinal and oblique contours in ferret visual cortex. Proc Natl Acad Sci U S A. 1998;95:2621–2623. doi: 10.1073/pnas.95.5.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Diesmann M, Gewaltig MO, Aertsen A. Stable propagation of synchronous spiking in cortical neural networks. Nature. 1999;402:529–533. doi: 10.1038/990101. [DOI] [PubMed] [Google Scholar]
- Edden RAE, Barker PB. Spatial effects in the detection of gamma-aminobutyric acid: improved sensitivity at high fields using inner volume saturation. Magn Reson Med. 2007;58:1276–1282. doi: 10.1002/mrm.21383. [DOI] [PubMed] [Google Scholar]
- Ferster D, Miller KD. Neural mechanisms of orientation selectivity in the visual cortex. Annu Rev Neurosci. 2000;23:441–471. doi: 10.1146/annurev.neuro.23.1.441. [DOI] [PubMed] [Google Scholar]
- Ferster D, Chung S, Wheat H. Orientation selectivity of thalamic input to simple cells of cat visual cortex. Nature. 1996;380:249–252. doi: 10.1038/380249a0. [DOI] [PubMed] [Google Scholar]
- Friedman-Hill S, Maldonado PE, Gray CM. Dynamics of striate cortical activity in the alert macaque: I. Incidence and stimulus-dependence of gamma-band neuronal oscillations. Cereb Cortex. 2000;10:1105–1116. doi: 10.1093/cercor/10.11.1105. [DOI] [PubMed] [Google Scholar]
- Frien A, Eckhorn R, Bauer R, Woelbern T, Gabriel A. Fast oscillations display sharper orientation tuning than slower components of the same recordings in striate cortex of the awake monkey. Eur J Neurosci. 2000;12:1453–1465. doi: 10.1046/j.1460-9568.2000.00025.x. [DOI] [PubMed] [Google Scholar]
- Fries P, Neuenschwander S, Engel AK, Goebel R, Singer W. Rapid feature selective neuronal synchronization through correlated latency shifting. Nat Neurosci. 2001;4:194–200. doi: 10.1038/84032. [DOI] [PubMed] [Google Scholar]
- Furmanski CS, Engel SA. An oblique effect in human primary visual cortex. Nat Neurosci. 2000;3:535–536. doi: 10.1038/75702. [DOI] [PubMed] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci U S A. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrie JA, Shapley R. LFP power spectra in V1 cortex: the graded effect of stimulus contrast. J Neurophysiol. 2005;94:479–490. doi: 10.1152/jn.00919.2004. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Peterson MR, Freeman RD. Oblique effect: a neural basis in the visual cortex. J Neurophysiol. 2003;90:204–217. doi: 10.1152/jn.00954.2002. [DOI] [PubMed] [Google Scholar]
- Li G, Yang Y, Liang Z, Xia J, Yang Y, Zhou Y. GABA-mediated inhibition correlates with orientation selectivity in primary visual cortex of cat. Neuroscience. 2008;155:914–922. doi: 10.1016/j.neuroscience.2008.06.032. [DOI] [PubMed] [Google Scholar]
- Liang Z, Shen W, Shou T. Enhancement of oblique effect in the cat's primary visual cortex via orientation preference shifting induced by excitatory feedback from higher-order cortical area 21a. Neuroscience. 2007;145:377–383. doi: 10.1016/j.neuroscience.2006.11.051. [DOI] [PubMed] [Google Scholar]
- Maffei L, Campbell FW. Neurophysiological localization of the vertical and horizontal visual coordinates in man. Science. 1970;167:386–387. doi: 10.1126/science.167.3917.386. [DOI] [PubMed] [Google Scholar]
- Mansfield RJ. Neural basis of orientation perception in primate vision. Science. 1974;186:1133–1135. doi: 10.1126/science.186.4169.1133. [DOI] [PubMed] [Google Scholar]
- Marshall I, Bruce SD, Higinbotham J, MacLullich A, Wardlaw JM, Ferguson KJ, Seckl J. Choice of spectroscopic lineshape model affects metabolite peak areas and area ratios. Magn Reson Med. 2000;44:646–649. doi: 10.1002/1522-2594(200010)44:4<646::aid-mrm20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- McMahon MJ, MacLeod DIA. The origin of the oblique effect examined with pattern adaptation and masking. J Vis. 2003;3:230–239. doi: 10.1167/3.3.4. [DOI] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci U S A. 2009;106:8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samonds JM, Bonds AB. Gamma oscillation maintains stimulus structure-dependent synchronization in cat visual cortex. J Neurophysiol. 2005;93:223–236. doi: 10.1152/jn.00548.2004. [DOI] [PubMed] [Google Scholar]
- Samonds JM, Allison JD, Brown HA, Bonds AB. Cooperative synchronized assemblies enhance orientation discrimination. Proc Natl Acad Sci U S A. 2004;101:6722–6727. doi: 10.1073/pnas.0401661101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley R, Hawken M, Ringach DL. Dynamics of orientation selectivity in the primary visual cortex and the importance of cortical inhibition. Neuron. 2003;38:689–699. doi: 10.1016/s0896-6273(03)00332-5. [DOI] [PubMed] [Google Scholar]
- Sillito AM. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J Physiol. 1975;250:305–329. doi: 10.1113/jphysiol.1975.sp011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito AM, Kemp JA, Milson JA, Berardi N. A re-evaluation of the mechanisms underlying simple cell orientation selectivity. Brain Res. 1980;194:517–520. doi: 10.1016/0006-8993(80)91234-2. [DOI] [PubMed] [Google Scholar]
- Tibber MS, Guedes A, Shepherd AJ. Orientation discrimination and contrast detection thresholds in migraine for cardinal and oblique angles. Invest Ophthalmol Vis Sci. 2006;47:5599–5604. doi: 10.1167/iovs.06-0640. [DOI] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Colling SB, Buzsáki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol. 1996;493:471–484. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumoto T, Eckart W, Creutzfeldt OD. Modification of orientation sensitivity of cat visual cortex neurons by removal of GABA-mediated inhibition. Exp Brain Res. 1979;34:351–363. doi: 10.1007/BF00235678. [DOI] [PubMed] [Google Scholar]
- Vrba J, Robinson SE. Signal processing in magnetoencephalography. Methods. 2001;25:249–271. doi: 10.1006/meth.2001.1238. [DOI] [PubMed] [Google Scholar]
- Wang G, Ding S, Yunokuchi K. Difference in the representation of cardinal and oblique contours in cat visual cortex. Neurosci Lett. 2003;338:77–81. doi: 10.1016/s0304-3940(02)01355-1. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Buzsáki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci. 1996;16:6402–6413. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol. 2000;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Wolf W, Hicks TP, Albus K. The contribution of GABA-mediated inhibitory mechanisms to visual response properties of neurons in the kitten's striate cortex. J Neurosci. 1986;6:2779–2795. doi: 10.1523/JNEUROSCI.06-10-02779.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]