Abstract
Dopamine improves learning and memory formation. The neurophysiological basis for these effects might be a focusing effect of dopamine on neuroplasticity: Accordingly, in humans l-dopa prolongs focal facilitatory plasticity, but turns nonfocal facilitatory plasticity into inhibition. Here we explore the impact of D1 receptors on plasticity. Nonfocal plasticity was induced by transcranial direct current stimulation (tDCS), and focal plasticity by paired associative stimulation (PAS). Subjects received sulpiride, a D2 antagonist, to increase the relative contribution of D1 receptors to dopaminergic activity, combined sulpiride and l-dopa, to increase the relation of D1/D2 activity further, or placebo medication. Under placebo, anodal tDCS and excitatory PAS (ePAS) increased motor cortex excitability. Cathodal tDCS and inhibitory PAS (iPAS) reduced it. Sulpiride abolished iPAS-induced inhibition, but not ePAS-generated facilitation, underlining the importance of D1-receptor activity for focal facilitatory neuroplasticity. Combining sulpiride with l-dopa reestablished iPAS-induced inhibition, but did not affect ePAS-induced plasticity. tDCS-induced plasticity, which was abolished by sulpiride in a former study, also recovered. Thus enhancing D1 activity further relative to D2 activity is relevant for facilitatory and inhibitory plasticity. However, comparison with former results show that an appropriate balance of D1 and D2 activity seems necessary to (1) consolidate the respective excitability modifications and (2) to elicit a focusing effect.
Keywords: dopamine, human, motor cortex, neuroplasticity, paired associative stimulation, transcranial direct current stimulation
Introduction
Dopamine (DA) modulates learning and memory. Dopaminergic enhancement improves verbal learning in healthy subjects and motor learning in healthy subjects and stroke patients (Knecht et al., 2004; Flöel et al., 2005a; Floel et al., 2005b). Cognitive functions are impaired in diseases accompanied by dopaminergic dysfunction, like Parkinson's disease and schizophrenia (Grace et al., 1998).
The neurophysiological basis for these effects of DA, as primarily studied in animal and slice experiments, might be its prolonging effect on neuroplasticity, i.e., long-term potentiation (LTP) and depression (LTD) (Otani et al., 1998; Bailey et al., 2000). D1-receptor activation prolongs LTP and LTD (Chen et al., 1996; Otmakhova and Lisman, 1998; Bach et al., 1999; Bailey et al., 2000; Gurden et al., 2000; Huang et al., 2004). For D2 receptors, positive as well as negative effects on LTP and LTD are described (Frey et al., 1989; Chen et al., 1996; Otani et al., 1998; Gurden et al., 2000; Spencer and Murphy, 2000; Manahan-Vaughan and Kulla, 2003). Thus DA has no uniform excitatory or inhibitory function, but acts as neuromodulator. On cortical network level, this results in a focusing effect of DA on cortical activity and plasticity, i.e., an enhancement of the signal-to-noise ratio (Foote and Morrison, 1987; Seamans and Yang, 2004).
This focusing effect was confirmed in humans by use of neuroplasticity-inducing stimulation tools, namely transcranial direct current stimulation (tDCS) and paired associative stimulation (PAS), which elicit LTP- and LTD-like effects on cortical excitability in terms of time course [excitability changes lasting for ∼1 h (Stefan et al., 2000; Nitsche and Paulus, 2001; Nitsche et al., 2003a; Wolters et al., 2003), and NMDA receptor dependence (Stefan et al., 2002; Nitsche et al., 2003b)]. PAS induces focal plasticity by modification of the strength of somatosensory-motor cortical connections (Stefan et al., 2000). tDCS induces nonfocal plasticity within the motor cortex not restricted to synaptic subgroups (Purpura and McMurtry, 1965; Nitsche et al., 2003c, 2008). Since l-dopa prolongs the focal excitability enhancement induced by excitatory PAS (ePAS), turns anodal tDCS-generated nonfocal excitability enhancement into inhibition and prolongs cathodal tDCS-induced excitability diminution (Kuo et al., 2008), it has a focusing effect on excitatory plasticity. With regard to dopaminergic subreceptors, D2-antagonism eliminates tDCS-induced plasticity, whereas D2-agonism prolongs the inhibitory effect of cathodal tDCS (Nitsche et al., 2006). Thus D2-receptor activity is essential for stabilizing nonfocal inhibitory plasticity and might also contribute to nonfocal excitatory plasticity.
We aimed to shed light on the relevance of D1-receptor activation for plasticity in humans. Since no selective D1 agonist or antagonist is available for use in humans, we chose an indirect approach. In the first experiment, the balance of D1/D2 receptor activation was shifted toward the D1 receptor by blocking D2 receptors with sulpiride. We explored the impact of this drug solely on focal, PAS-induced plasticity, because its effect on nonfocal, tDCS-induced plasticity was already tested (Nitsche et al., 2006). In the second experiment, D1 activation was enhanced by combining l-dopa with sulpiride. The effects of this drug combination on plasticity were explored for PAS- and tDCS-induced plasticity. The primary motor cortex was taken as a model system.
Materials and Methods
Subjects.
Ten to 12 healthy subjects without acute or chronic CNS-affecting medication participated in each experiment (experiment 1 (sulpiride/placebo) 10 subjects, 5 female, mean age 27.1 years ± 6.10 SD; experiment 2 (sulpiride-l-dopa/placebo 12 subjects, 7 female, mean age 30.67 ± 10.53 SD). All gave written informed consent. The study was approved by the ethics committee of the University of Göttingen, and we conform to the Declaration of Helsinki.
tDCS of the motor cortex.
tDCS was applied to induce nonfocal neuroplasticity. It generates a modulation of cortical network plasticity by application of weak direct currents through the surface of the scalp. Depending on stimulation duration anodal tDCS enhances and cathodal tDCS diminishes cortical excitability for about an hour after the end of stimulation (Nitsche and Paulus, 2001; Nitsche et al., 2003a). The primary mechanism is a modulation of resting membrane potential, and the resulting polarity-specific excitability and changes in cortical activity subsequently induce changes in synaptic strength; which are however not restricted to specific synaptic subgroups, since excitability and activity of a broad range of cortical neurons is modulated by tDCS, as shown in animal experiments (Purpura and McMurtry, 1965; Nitsche et al., 2003b). The after-effects of tDCS are NMDA receptor-dependent (Nitsche et al., 2003c).
Direct currents were transferred via a pair of saline-soaked surface sponge electrodes (35 cm2) fixed to the scalp and delivered by a specially developed battery-driven constant current stimulator (Schneider Electronic) with a maximum output of 2 mA. The motor-cortical electrode was placed over the representational field of the right abductor digiti minimi (ADM) as identified by transcranial magnetic stimulation (TMS), and the other electrode was located contralaterally above the right orbit. The currents flowed continuously for 9 (cathodal tDCS) and 13 (anodal tDCS) min with an intensity of 1.0 mA. These stimulation durations have been shown to elicit the intended excitability shift durations in former experiments (Nitsche and Paulus, 2001; Nitsche et al., 2003a).
PAS of the motor cortex.
For PAS, repetitive peripheral nerve stimulation is paired with TMS of the human motor cortex (Stefan et al., 2000). It is postulated that PAS-induced excitability changes specifically facilitate somatosensory-motor cortical connections. Recently, it has moreover been demonstrated that the effects of PAS in the human motor cortex are restricted to the motor cortical representations affected by the stimulation protocol, but do not spread to neighbored ones (Weise et al., 2006). The efficacy of PAS to induce motor cortical excitability alterations specifically depend on the interstimulus interval. Thus, PAS shares some critical features of associative synaptic LTP and LTD (Stefan et al., 2000, 2002; Wolters et al., 2003). PAS-induced excitability changes have been demonstrated to be NMDA receptor-dependent (Stefan et al., 2002).
Peripheral nerve stimulation was applied to the right ulnar nerve at the level of the wrist. Single-pulse TMS was delivered over the representing area of the right ADM and preceded by an ulnar nerve stimulus with an interval of 25 ms (ePAS) or 10 ms [inhibitory PAS (iPAS)] with stimulation intensity of 300% of the perceptual threshold. With an interstimulus interval of 25 ms, the somatosensory afferent stimulus elicited by peripheral stimulation reaches the motor cortex nearly simultaneously with the TMS stimulus delivered directly to the motor cortex. The resulting simultaneous activation of a postsynaptic neuron delivered by conjoint afferent inputs has been shown to induce LTP in animals (Baranyi et al., 1991; Hess and Donoghue, 1994). In the case of the 10 ms interstimulus interval, however, the somatosensory afferent stimulus reaches the respective motor cortical neurons relevantly later than the TMS stimulus. This a-synchronous activation has been shown to induce LTD in animals (Zhang et al., 1998). Both effects might be dependent on the resulting calcium concentration of the postsynaptic neurons. Simultaneous activation might induce a strong enhancement of intraneuronal calcium sufficient for the induction of LTP, while asynchronous activation might enhance calcium to a lower degree, thus resulting in LTD (Lisman, 2001). Ninety pairs were applied at 0.05 Hz >30 min, which has been shown to induce a long-lasting excitability modification of the motor cortex (Stefan et al., 2000).
Pharmacological interventions.
Four-hundred milligrams of sulpiride, a combination of 400 mg of sulpiride and 100 mg of l-dopa (combined with 20 mg of domperidone to avoid side-effects, e.g., vomiting, of the medication) or equivalent placebo (PLC) drugs were taken by the subjects 1 h before the start of the experimental session. By this means, the verum drugs induce stable plasma levels and produce prominent effects in the CNS (Flöel et al., 2005a; Nitsche et al., 2006; Kuo et al., 2008). Each experimental session was performed in a randomized order and was separated by at least 1 week to avoid cumulative drug effects. Subjects were blinded to the drugs received in the specific sessions.
Measurement of motor cortical excitability.
TMS-elicited muscle-evoked potentials (MEPs) were recorded to measure excitability changes of the representational motor cortical area of the right ADM. Single-pulse TMS was conducted by a Magstim 200 magnetic stimulator (Magstim Company) with a figure-of-eight magnetic coil (diameter of one winding = 70 mm, peak magnetic field = 2.2 Tesla). The coil was held tangentially to the skull, with the handle pointing backwards and laterally at an angle of 45° from midline. The optimal position was defined as the site where stimulation resulted consistently in the largest MEPs. Surface EMG was recorded from the right ADM with Ag-AgCl electrodes in a belly-tendon montage. The signals were amplified and filtered with a time constant of 10 ms and a low-pass filter of 2.5 kHz, then digitized at an analog-to-digital rate of 5 kHz and further relayed into a laboratory computer using the Signal software and CED 1401 hardware (Cambridge Electronic Design). The intensity was adjusted to elicit baseline MEPs of, on average, 1 mV peak-to-peak amplitude and was kept constant for the poststimulation assessment unless adjusted (see below).
Experimental procedures.
The experiments were conducted in a repeated measurement design. First the optimal position of the magnetic coil for eliciting MEPs in the resting ADM was assessed over the left motor cortex and 20 MEPs were recorded for the first baseline. One hour after intake of the medication, a second baseline was determined to control for a possible influence of the drug on cortical excitability and adjusted if necessary. Subjects were not aware about and could not distinguish between the specific stimulation protocols used nor were they informed about the medication administered in a specific session.
In the experiments with tDCS, one of the DC electrodes, to which in the following the terms cathodal or anodal tDCS refer, was fixed at the cortical representational area of ADM as defined during the first baseline recording, and the other one was fixed at the contralateral forehead area above the right orbit. Direct currents were applied on 12 subjects for 9 (cathodal) or 13 min (anodal). After cessation of tDCS, 20 MEPs were recorded at 0.25 Hz every 5 min for half an hour, and then every 30 min until 2 h after the end of DC stimulation, since tDCS-induced after-effects without medication will not last longer than this period of time (Nitsche and Paulus, 2000, 2001; Nitsche et al., 2003a). Because of the relatively diurnal stability of corticospinal excitability, we did not expect further excitability changes after MEPs returned to baseline level again. For the medication conditions, TMS recordings were performed also at the evening of the stimulation day [same day evening (SE)], and the day after plasticity-inducing intervention [next morning (nm), next noon (nn), and next evening (ne)] in experiment 2.
In the experiments with PAS, the interventional PAS protocols as described above were used. TMS recording procedures were the same as described above. Each experimental session was performed in a randomized order and was separated by at least 1 week to avoid cumulative drug or stimulation effects.
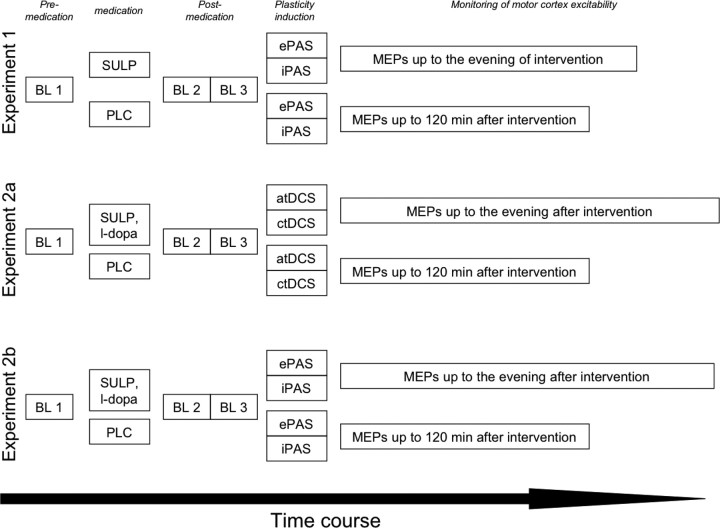
A synopsis of the experimental procedures is shown in Figure 1. Experiment 1, which tested the impact of sulpiride only on plasticity, was only conducted for PAS, and not for tDCS, because the latter experiment was already performed in a former study of our group (Nitsche et al., 2006). Experiment 2, i.e., the sulpiride/l-dopa drug combination, was performed for tDCS (experiment 2a), and PAS (experiment 2b).
Figure 1.
Synopsis of experimental procedures. For each experiment, before drug intake a first baseline MEP measure (BL1) was performed (determination of TMS intensity resulting in an MEP amplitude of 1 mV). Immediately afterward, drug intake followed. In experiment 1, the subjects received sulpiride (SULP) or PLC medication, in experiment 2, they received a combination of SULP and l-dopa or PLC. One hour after drug intake, a second baseline (BL2) was recorded with the TMS intensity of BL1. If MEP amplitudes were different from the first baseline, TMS intensity was adjusted such that a MEP amplitude of 1 mV (BL3) resulted. The TMS intensity of BL3 was kept constant during the remaining experiment. Afterward, the plasticity induction procedures (only PAS in experiment 1, PAS and tDCS in experiment 2) were performed. Post-interventionally MEPs were recorded for 30 min every fifth minute, then every 30th min up to 120 min for each condition. For PLC medication, MEP recording was terminated afterward, because it is known that baseline excitability is reached after approximately 1 h after tDCS and PAS without pharmacological intervention. For the drug conditions, MEPs were recorded once more at the evening of the day under SULP and until the evening of the following day under SULP/l-dopa to cover also prolonged excitability changes. atDCS, Anodal tDCS; ctDCS, cathodal tDCS.
Data analysis and statistics.
MEP amplitude means were calculated first individually, then interindividually for each time bin including the baseline values (before medication, 1 h after medication, TMS intensity not adjusted and 1 h after medication, TMS intensity adjusted), to detect medication-induced baseline modifications. The postintervention MEPs were normalized and are given as ratios of the third baseline.
For the tDCS and PAS experiments, repeated measure ANOVAs for the time bins up to 120 min after stimulation were calculated with the within-subject factors time course, current stimulation (anodal and cathodal tDCS, ePAS, and iPAS), drug condition [sulpiride (experiment 1), sulpiride/l-dopa (experiment 2) vs placebo], and the dependent variable MEP amplitude. If appropriate, post hoc Student's t tests (paired samples, two-tailed, p < 0.05, not adjusted) were performed to determine whether the MEP amplitudes before and after the interventional brain stimulations differed in each intervention condition, and if those differences depended on the drug conditions. Additional post hoc tests (Student's t tests, p < 0.05) were performed to explore whether sulpiride or the combination of sulpiride and l-dopa modified baseline MEPs.
Results
Beyond a slight itching sensation under the tDCS electrodes and a discrete dizziness under medication in 3 subjects under the sulpiride/l-dopa combination, no side-effects of stimulation or pharmacological intervention were reported by the subjects.
Effect of sulpiride on focal plasticity induced by PAS (experiment 1)
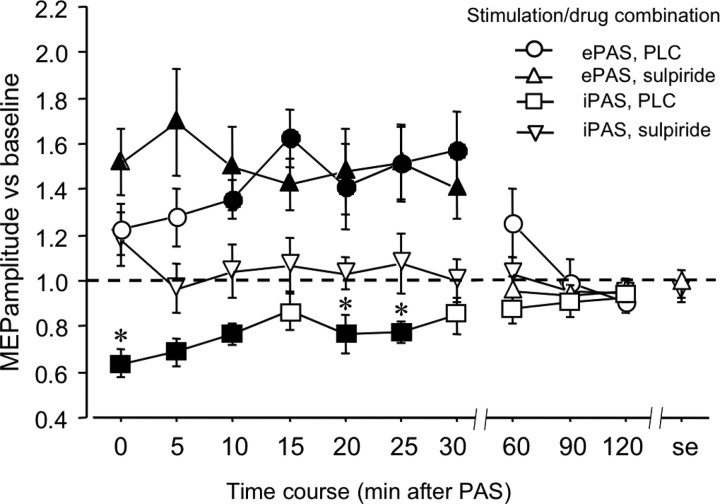
The ANOVA shows significant main effects of time and PAS. Moreover, the interactions time × drug and time × PAS were significant (Table 1). The excitability enhancement accomplished by ePAS was significant up to 30 min after stimulation independent of the drug condition. During the first minutes after stimulation, a nonsignificant trend for an enlarged excitability enhancement under sulpiride, compared with PLC medication, can be noticed. For iPAS, however, a significant reduction of the MEP amplitude was apparent for 25 min under placebo medication only. Sulpiride abolished this effect of iPAS (Fig. 2).
Table 1.
Results of the ANOVAs conducted for experiment 1 (sulpiride) and experiment 2 (sulpiride and l-dopa)
| Experiment | df | F | p |
|---|---|---|---|
| Sulpiride or placebo medication, PAS | |||
| Time | 10 | 6.625 | <0.001* |
| Drug | 1 | 4.763 | 0.057 |
| PAS | 1 | 33.172 | <0.001* |
| Time × drug | 10 | 3.075 | 0.002* |
| Time × PAS | 10 | 6.434 | <0.001* |
| Drug × PAS | 1 | 1.984 | 0.193 |
| Time × drug × PAS | 10 | 0.875 | 0.559 |
| Sulpiride/l-dopa or placebo medication, tDCS | |||
| Time | 10 | 1.568 | 0.126 |
| Drug | 1 | 1.034 | 0.331 |
| tDCS | 1 | 58.192 | <0.001* |
| Time × drug | 10 | 0.831 | 0.600 |
| Time × tDCS | 10 | 13,323 | <0.001* |
| Drug × tDCS | 1 | 0.414 | 0.533 |
| Time × drug × tDCS | 10 | 0.931 | 0.508 |
| Sulpiride/l-dopa or placebo medication, PAS | |||
| Time | 10 | 2.428 | 0.012* |
| Drug | 1 | 2.089 | 0.176 |
| PAS | 1 | 21.658 | 0.001* |
| Time × drug | 10 | 1.860 | 0.059 |
| Time × PAS | 10 | 11.223 | <0.001* |
| Drug × PAS | 1 | 0.016 | 0.903 |
| Time × drug × PAS | 10 | 1.251 | 0.267 |
Note that for both experiments, the ANOVA encompasses the time course up to 120 min after tDCS, because the remaining time points were only measured for the medication conditions. Asterisks indicate significant results (p < 0.05).
Figure 2.
Impact of D2 block on focal plasticity induced by ePAS and iPAS (experiment 1). Depicted are the baseline-standardized MEP amplitudes up to the evening after ePAS or iPAS under placebo medication or sulpiride. Under placebo medication, ePAS enhances while iPAS diminishes excitability significantly for up to 30 min after stimulation. Under sulpiride, the ePAS-generated excitability enhancement remains largely unaffected, whereas sulpiride abolishes the iPAS-induced excitability reduction. Filled symbols indicate significant deviations of the post-PAS MEP amplitudes from baseline, asterisks indicate significant deviations of the drug vs PLC conditions with regard to identical time points (Student's t test, two-tailed, paired samples, p < 0.05).
Effect of l-dopa combined with sulpiride on tDCS-induced plasticity (experiment 2a)
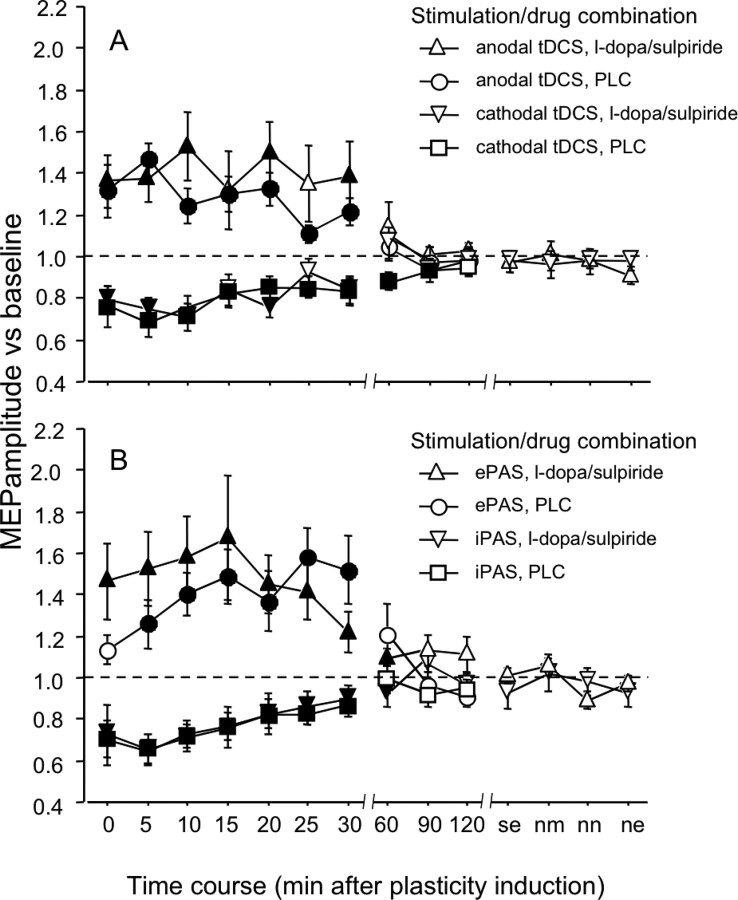
The ANOVA resulted in a significant main effect of tDCS and in a significant interaction of tDCS × time (Table 1). The excitability enhancement accomplished by anodal tDCS was stable for ∼30 min after the end of stimulation, independent from real or placebo medication. However, a nonsignificant trend for an enlarged excitability enhancement under sulpiride and l-dopa, compared with PLC medication, can be noticed. In the cathodal tDCS condition, under placebo medication MEP amplitudes were significantly reduced for up to 90 min after tDCS, while for only 30 min after tDCS under l-dopa and sulpiride (Fig. 3a).
Figure 3.
Impact of combined D1 activation and D2 block on plasticity induced by ePAS/and anodal or cathodal tDCS/iPAS (experiment 2). A, B, Shown are baseline-standardized MEP amplitudes after plasticity induction by anodal/cathodal tDCS (A) or ePAS/iPAS (B) under placebo medication or sulpiride and l-dopa up to the evening of the poststimulation day. In the placebo medication condition, anodal tDCS enhances excitability significantly for 30 min while cathodal tDCS results in an excitability reduction for 90 min. Whereas the anodal tDCS-induced excitability enhancement is not affected by sulpiride and l-dopa, these drugs reduce duration of the cathodal tDCS-generated excitability diminution. The iPAS- and ePAS-induced excitability modification was largely identical under placebo and real medication. Filled symbols indicate significant deviations of the post-tDCS MEP amplitudes from baseline (Students t test, two-tailed, paired samples, p < 0.05).
Effect of l-dopa combined with sulpiride on PAS-induced plasticity (experiment 2b)
Here the ANOVA resulted in significant main effects of PAS and time. The interaction PAS × time was also significant (Table 1). Medication status had no significant influence on the excitability diminution or enhancement accomplished, which was prominent for ∼30 min in both, the drug and placebo condition. Again a nonsignificant trend for an enlarged excitability enhancement under sulpiride and l-dopa, compared with PLC medication, can be noticed. (Fig. 3b).
Drug-induced baseline MEP modifications
In experiment 1, sulpiride alone did not modify corticospinal excitability. MEP amplitudes were identical before (1.022 ± 0.092 SD) and 1 h after medication (1.042 ± 0.204 SD, t = 0.353, p = 0.730). With regard to experiment 2, however, the combination of l-dopa and sulpiride diminished MEP amplitudes slightly, but significantly [1.010 ± 0.084 SD before medication (baseline 1), 0.811 ± 0.219 after medication (baseline 2), t = 4.5, p < 0.001]. After adjustment, baseline MEP values were identical for each plasticity-inducing protocol immediately before the plasticity-inducing procedure (baseline 3).
Discussion
The results of our study show a nonlinear contribution of D1 receptor activity to focal and nonfocal plasticity in the human motor cortex. Modification of the relative activation of D1 and D2 receptors by blocking the latter resulted in an abolition of iPAS-induced focal inhibitory plasticity. Further enhancement of relative D1 activation by l-dopa under D2 block reestablished focal and nonfocal inhibitory as well as excitatory plasticity. These results are in favor for a dose-dependent effect of D1 activation on plasticity and deliver indirect evidence for the importance of balanced dopamine receptor activation for achieving a focusing dopaminergic effect on plasticity.
The effect of relative D-receptor enhancement by D2 block on plasticity
As shown by the results of experiment 1, blocking D2 receptors, and thus shifting the relation of activity of D1/D2 receptors to the direction of D1 receptors, but without enhancing absolute D1-receptor activity, abolishes focal inhibitory plasticity, as induced by iPAS. Similarly, in a former experiment nonfocal inhibitory plasticity, induced by cathodal tDCS, was abolished by D2-receptor block via sulpiride (Nitsche et al., 2006). This pattern of results is in accordance with an important influence of the D2 receptor on inhibitory plasticity, as already shown for LTD in animals (Otani et al., 1998; Spencer and Murphy, 2000). Here, physiological D1 activity without D2 activity does not suffice to induce inhibitory plasticity.
In contrast, sulpiride did not extinguish, but slightly enhance the magnitude, of focal excitatory plasticity induced by ePAS. Interestingly, in another experiment sulpiride abolished the induction of nonfocal excitatory plasticity induced by anodal tDCS (Nitsche et al., 2006). Thus it seems that with regard to facilitatory plasticity, block of D2 receptors has different effects dependent on the focality of plasticity induction. For the induction of focal plasticity, D2 receptor involvement seems to be of no major importance. This result is in principal accordance with some animal experiments, showing a missing impact of D2 receptors on LTP (Gurden et al., 2000) but an important function of D1 receptors (Otmakhova and Lisman, 1996; Chen et al., 1996; Bach et al., 1999; Bailey et al., 2000; Gurden et al., 2000; Huang et al., 2004). However, the slight, nonsignificant enhancement of the magnitude of the MEP amplitude increase under sulpiride, which might have become significant with larger subject numbers, might hint for a reducing effect of D2 activity on ePAS-generated LTP-like plasticity. For nonfocal excitatory plasticity, as induced by anodal tDCS, however, under physiological D1 activation D2 activity seems to be essential for achieving the respective excitability enhancement. Since in another animal experiment weak, but not strong LTP was prevented by D2 antagonism (Manahan-Vaughan and Kulla, 2003), this might indicate that the importance of the D2 receptor differs with regard to the specific kind of excitatory plasticity induced.
Since sulpiride alone did not affect single pulse MEPs and baseline MEP amplitudes were identical between the respective experimental conditions, an impact of these factors on the results can be ruled out.
D1-receptor activation under D2-receptor block reestablishes plasticity
Adding l-dopa to sulpiride, and thus activating D1 receptors absolutely under D2 block, resulted in clearly different effects. Here, inhibitory plasticity induced by focal iPAS was reestablished, while facilitatory focal plasticity was preserved. Similarly, the effects of nonfocal (anodal/cathodal tDCS) stimulation protocols were reestablished, compared with the plasticity-abolishing effects of sulpiride in a foregoing experiment (Nitsche et al., 2006). Moreover, for both excitability-enhancing plasticity-inducing protocols, a slight, nonsignificant increase of MEP-amplitudes under the medication condition compared with PLC can be noticed, which might have become significant with larger subject numbers studied. On first sight, it might be argued that the l-dopa medication could have removed the D2-receptor block and thus these effects are due to an activation of the whole dopaminergic system. However, l-dopa alone was demonstrated in a former study to affect plasticity, as induced by tDCS and PAS, in a different way: It turned the anodal tDCS-generated nonfocal excitability enhancement into inhibition, but prolonged nonfocal and focal inhibitory plasticity and focal excitatory plasticity (Kuo et al., 2008). This focusing effect is clearly not apparent in the present experiment. Moreover, the prolonging effect of l-dopa on plasticity without D2 block has vanished, whereas a slight enlargement of the excitability-enhancing stimulation protocols occurs. This qualitatively different pattern of results is in favor for an effective D1 activation and a still relevant D2-receptor block accomplished by our medication. However, strictly speaking this is indirect evidence for an effective selective D1 activation by this experimental protocol. Studies at the receptor level are needed to validate this design more directly.
Since baseline MEP amplitudes were identical between the respective experimental conditions, an impact of these factors on the results can be ruled out.
The inhibitory effect of D1-receptor activity on acute motor cortex excitability is compatible with former animal experiments (Gorelova et al., 2002; Rosenkranz and Johnston, 2006; Kröner et al., 2007).
General remarks
The results of the current experiments in connection with those of former experiments of our group (Nitsche et al., 2006; Kuo et al., 2008) shed light on the interaction of dopaminergic D1 and D2 receptors in producing a focusing effect on neuroplasticity.
Under physiological conditions, D1-receptor activity does suffice for the induction of focal excitability-enhancing plasticity by brain stimulation, but additional D2 activity is needed for the induction of nonfocal excitatory as well as inhibitory plasticity. This importance of D2 receptors for the induction of plasticity can be overruled by increased D1 receptor activation: enhancing D1 receptor activity not only relative to D2 activity, but by an absolute degree via l-dopa administration under D2 receptor block reestablishes focal as well as nonfocal inhibitory and excitatory plasticity.
Interestingly, our experiments deliver indirect evidence for an importance of concomitant D1 and D2 activation on certain further aspects of plasticity. (1) D1 activation alone does not exert a specific focusing effect on excitatory plasticity, as achieved by l-dopa medication in a former study (Kuo et al., 2008). Thus additional D2 activity is needed to suppress the nonfocally induced excitability enhancement generated by anodal tDCS. At first sight, this is in opposition to the fact that D2 block by sulpiride suppressed the anodal tDCS-induced excitability enhancement, but it fits to the fact that the combined D1/D2-, but predominant D2-agonist pergolide slightly diminished the ability of anodal tDCS to enhance excitability (Nitsche et al., 2006). (2) Contrary to l-dopa alone, l-dopa combined with sulpiride did not prolong neuroplasticity. Under l-dopa, the effects of all plasticity protocols studied were relevantly stabilized in a former experiment (Kuo et al., 2008). In the current study, only a minor prolongation of the after-effects of facilitatory PAS was accomplished. Thus for consolidating neuroplasticity, a balanced D1/D2 activity seems necessary.
The results of the current study are in favor for an important impact of D1 receptors on learning and memory formation via its supportive role with regard to the induction of neuroplastic network alterations. Accordingly, it was shown in animal experiments that D1 receptor activation improves long-term memory (Seamans et al., 1998). Indirect evidence for a cognition-enhancing effect of D1 activity is also available for humans. Application of a combined D1/D2 agonist and relative strengthening of D1 receptor activity by D2 receptor block enhanced performance in overlearned working memory tasks, while a selective D2 agonist was without effect (Müller et al., 1998; Mehta et al., 2004). In contrast, the D1/D2 antagonist haloperidol impaired performance in a procedural learning task, but the selective D2 antagonist sulpiride did not affect it (Paquet et al., 2004; Mehta et al., 2005). This, however, does not mean that D2 activity is not important for or without effect on cognition in general. Indeed, the beneficial effect of dopaminergic subreceptors on cognition might be task-specific: D1 activity might improve performance, when stable reactions, and thus respective plastic network alterations are needed. D2 receptors might be beneficial for tasks that demand flexibility, where stable network modifications would be dysfunctional (Seamans and Yang 2004). Indeed, it has been shown by Mehta et al. (2004) that the D2 antagonist sulpiride impaired performance in a working memory task requiring such flexible information processing. These different patterns of D1 and D2 effects on network function might also improve our understanding of dopaminergic dysfunction in neuropsychiatric diseases. In schizophrenia, it is suggested that distractibility, tangential and intrusive thought patterns are caused by an overactivity of D2 receptors, and thus pathological enhanced “flexibility” of information processing (Seamans and Yang 2004). Consequently, blocking D2 receptors and thus enhancing stability of information processing by relative enhancement of D1 activation is the major therapeutic activity of antipsychotic medication.
Together, the present experiments reveal distinct features of the D1 receptor with regard to focal and nonfocal plasticity. Neuroplasticity, as tested here, is thought to be the neurophysiological basis of learning and memory formation. Since pharmacological intervention with different dopaminergic substances has been shown to result in specific modifications of plasticity, it might be worth to explore the property of specific dopaminergic drugs to enhance cognition in detail. Hereby, due to its unique focusing effect, it might come out that simultaneous D1 and D2 stimulation is better suited than stimulation of single subreceptors to enhance cognition. If so, this might have implications for the future treatment of patients with a dopaminergic dysfunction, accompanied by cognitive disturbances, e.g., patients with schizophrenia, Parkinson's, or Lewy body disease.
Footnotes
This work was supported by the German Ministry for Education and Research, Bernstein Center for Computational Neuroscience, Göttingen. M.-F.K. is supported by European Graduiertenkolleg 632, Neuroplasticity: From Molecules to Systems, which is supported by the German Research Foundation (Deutsche Forschungsgemeinschaft Grant NI 683/4-1).
References
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci U S A. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Giustetto M, Huang YY, Hawkins RD, Kandel ER. Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory? Nat Rev Neurosci. 2000;1:11–20. doi: 10.1038/35036191. [DOI] [PubMed] [Google Scholar]
- Baranyi A, Szente MB, Woody CD. Properties of associative long-lasting potentiation induced by cellular conditioning in the motor cortex of conscious cats. Neuroscience. 1991;42:321–334. doi: 10.1016/0306-4522(91)90378-2. [DOI] [PubMed] [Google Scholar]
- Chen Z, Ito K, Fujii S, Miura M, Furuse H, Sasaki H, Kaneko K, Kato H, Miyakawa H. Roles of dopamine receptors in long-term depression: enhancement via D1 receptors and inhibition via D2 receptors. Receptors Channels. 1996;4:1–8. [PubMed] [Google Scholar]
- Flöel A, Breitenstein C, Hummel F, Celnik P, Gingert C, Sawaki L, Knecht S, Cohen LG. Dopaminergic influences on formation of a motor memory. Ann Neurol. 2005a;58:121–130. doi: 10.1002/ana.20536. [DOI] [PubMed] [Google Scholar]
- Floel A, Hummel F, Breitenstein C, Knecht S, Cohen LG. Dopaminergic effects on encoding of a motor memory in chronic stroke. Neurology. 2005b;65:472–474. doi: 10.1212/01.wnl.0000172340.56307.5e. [DOI] [PubMed] [Google Scholar]
- Foote SL, Morrison JH. Extrathalamic modulation of cortical function. Annu Rev Neurosci. 1987;10:67–95. doi: 10.1146/annurev.ne.10.030187.000435. [DOI] [PubMed] [Google Scholar]
- Frey U, Hartmann S, Matthies H. Domperidone, an inhibitor of the D2-receptor, blocks a late phase of an electrically induced long-term potentiation in the CA1-region in rats. Biomed Biochim Acta. 1989;48:473–476. [PubMed] [Google Scholar]
- Gorelova N, Seamans JK, Yang CR. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol. 2002;88:3150–3166. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- Grace AA, Gerfen CR, Aston-Jones G. Catecholamines in the central nervous system. Overview. Adv Pharmacol. 1998;42:655–670. doi: 10.1016/s1054-3589(08)60836-4. [DOI] [PubMed] [Google Scholar]
- Gurden H, Takita M, Jay TM. Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. J Neurosci. 2000;20:RC106. doi: 10.1523/JNEUROSCI.20-22-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G, Donoghue JP. Long-term potentiation of horizontal connections provides a mechanism to reorganize cortical motor maps. J Neurophysiol. 1994;71:2543–2547. doi: 10.1152/jn.1994.71.6.2543. [DOI] [PubMed] [Google Scholar]
- Huang YY, Simpson E, Kellendonk C, Kandel ER. Genetic evidence for the bidirectional modulation of synaptic plasticity in the prefrontal cortex by D1 receptors. Proc Natl Acad Sci U S A. 2004;101:3236–32341. doi: 10.1073/pnas.0308280101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Breitenstein C, Bushuven S, Wailke S, Kamping S, Flöel A, Zwitserlood P, Ringelstein EB. Levodopa: faster and better word learning in normal humans. Ann Neurol. 2004;56:20–26. doi: 10.1002/ana.20125. [DOI] [PubMed] [Google Scholar]
- Kröner S, Krimer LS, Lewis DA, Barrionuevo G. Dopamine increases inhibition in the monkey dorsolateral prefrontal cortex through cell type-specific modulation of interneurons. Cereb Cortex. 2007;17:1020–1032. doi: 10.1093/cercor/bhl012. [DOI] [PubMed] [Google Scholar]
- Kuo MF, Paulus W, Nitsche MA. Boosting focally-induced brain plasticity by dopamine. Cereb Cortex. 2008;18:648–651. doi: 10.1093/cercor/bhm098. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Three Ca2+ levels affect plasticity differently: the LTP zone, the LTD zone and no man′s land. J Physiol. 2001;532:285. doi: 10.1111/j.1469-7793.2001.0285f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Kulla A. Regulation of depotentiation and long-term potentiation in the dentate gyrus of freely moving rats by dopamine D2-like receptors. Cereb Cortex. 2003;13:123–135. doi: 10.1093/cercor/13.2.123. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Manes FF, Magnolfi G, Sahakian BJ, Robbins TW. Impaired set-shifting and dissociable effects on tests of spatial working memory following the dopamine D2 receptor antagonist sulpiride in human volunteers. Psychopharmacology. 2004;176:331–342. doi: 10.1007/s00213-004-1899-2. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Hinton EC, Montgomery AJ, Bantick RA, Grasby PM. Sulpiride and mnemonic function: effects of a dopamine D2 receptor antagonist on working memory, emotional memory and long-term memory in healthy volunteers. J Psychopharmacol. 2005;19:29–38. doi: 10.1177/0269881105048889. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. 2003a;114:600–604. doi: 10.1016/s1388-2457(02)00412-1. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003b;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W. Modulation of cortical excitability by weak direct current stimulation – technical, safety and functional aspects. In: Transcranial magnetic and transcranial direct current stimulation. Clin Neurophysiol Suppl. 2003c;56:255–276. doi: 10.1016/s1567-424x(09)70230-2. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Lampe C, Antal A, Liebetanz D, Lang N, Tergau F, Paulus W. Dopaminergic modulation of long-lasting direct current-induced cortical excitability changes in the human motor cortex. Eur J Neurosci. 2006;23:1651–1657. doi: 10.1111/j.1460-9568.2006.04676.x. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A. Transcranial direct current stimulation: state of the art 2008. Brain Stim. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Otani S, Blond O, Desce JM, Crépel F. Dopamine facilitates long-term depression of glutamatergic transmission in rat prefrontal cortex. Neuroscience. 1998;85:669–676. doi: 10.1016/s0306-4522(97)00677-5. [DOI] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. D1/D5 receptors inhibit depotentiation at CA1-synapses via camp-dependent mechanism. J Neurosci. 1998;18:1270–1279. doi: 10.1523/JNEUROSCI.18-04-01270.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet F, Soucy JP, Stip E, Lévesque M, Elie A, Bédard MA. Comparison between olanzapine and haloperidol on procedural learning and the relationship with striatal D2 receptor occupancy in schizophrenia. J Neuropsychiatry Clin Neurosci. 2004;16:47–56. doi: 10.1176/jnp.16.1.47. [DOI] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Johnston D. Dopaminergic regulation of neuronal excitability through modulation of Ih in layer V entorhinal cortex. J Neurosci. 2006;26:3229–3244. doi: 10.1523/JNEUROSCI.4333-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal–prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Spencer JP, Murphy KP. Bi-directional changes in synaptic plasticity induced at corticostriatal synapses in vitro. Exp Brain Res. 2000;135:497–503. doi: 10.1007/s002210000523. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise D, Schramm A, Stefan K, Wolters A, Reiners K, Naumann M, Classen J. The two sides of associative plasticity in writer's cramp. Brain. 2006;129:2709–2721. doi: 10.1093/brain/awl221. [DOI] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Tao HW, Holt CE, Harris WA, Poo M. A critical window for cooperation and competition among developing retinotectal synapses. Nature. 1998;395:37–44. doi: 10.1038/25665. [DOI] [PubMed] [Google Scholar]