Abstract
Pregnancy is associated with changes in mood and anxiety level as well as with marked hormonal fluctuations. Increases in the brain concentrations of neuroactive steroids during pregnancy in rats are accompanied by changes in expression of subunits of the GABA type A receptor (GABAA-R) in the brain. Granule cells of the dentate gyrus (DGGCs) exhibit two components of inhibitory GABAergic transmission: a phasic component mediated by synaptic GABAA-Rs, and a tonic component mediated by extrasynaptic GABAA-Rs. Recordings of GABAergic currents were obtained from hippocampal slices prepared from rats in estrus, at pregnancy day 15 (P15) or P19, or at 2 d after delivery. Exogenous GABA or 3α,5α-THP induced an increase in tonic current in DGGCs that was significantly greater at P19 than in estrus. Neither tonic nor phasic currents were affected by pregnancy in CA1 pyramidal cells. Immunohistochemical analysis revealed a marked increase in the abundance of the δ subunit of the GABAA-R and a concomitant decrease in that of the γ2 subunit in the hippocampus at P19. Expression of the α4 subunit did not change during pregnancy but was increased 2 d after delivery. Treatment of rats from P12 to P18 with the 5α-reductase inhibitor finasteride prevented the changes in tonic current and in δ and γ2 subunit expression normally apparent at P19. These data suggest that the number of extrasynaptic GABAA-Rs is increased in DGGCs during late pregnancy as a consequence of the associated marked fluctuations in the brain levels of neuroactive steroids.
Keywords: GABAA receptor δ, subunit, pregnancy, delivery, 3α-hydroxy-5α-pregnan-20-one, dentate gyrus granule cells, tonic current
Introduction
Fast inhibitory synaptic transmission in the CNS is mediated predominantly by GABA type A receptors (GABAA-Rs). These ligand-gated Cl− channels are characterized by a high level of structural (subunit composition) and functional (agonist affinity, channel kinetics) heterogeneity (Barnard et al., 1998; Whiting et al., 1999; Sieghart and Sperk, 2002; Jacob et al., 2008) and are also the principal target of endogenous neuroactive steroids (Belelli and Lambert, 2005; Mitchell et al., 2008) as well as of anxiolytic, hypnotic, myorelaxant, anticonvulsant, and anesthetic drugs (Sieghart, 1995; Barnard et al., 1998).
Whereas synaptic GABAA-Rs mediate a phasic inhibitory current, extrasynaptic GABAA-Rs with a high affinity for GABA and nondesensitizing properties are activated by the low concentrations of GABA normally present in the extracellular space and mediate a tonic inhibitory current (Brickley et al., 1996; Semyanov et al., 2004; Farrant and Nusser, 2005). Synaptic GABAA-Rs are pentamers consisting of combinations of αn, βn, and γ2 subunits, whereas extrasynaptic GABAA-Rs are pentamers characterized by the presence of the δ subunit together either with the α4 subunit in granule cells of the dentate gyrus (Pirker et al., 2000; Nusser and Mody, 2002; Wei et al., 2003) and in thalamic neurons (Sur et al., 1999), with the α6 subunit in cerebellar granule cells (Jones et al., 1997; Nusser et al., 1998; Pirker et al., 2000), or with the α1 subunit in a small population of dentate gyrus interneurons (Glykys et al., 2007). Additionally, extrasynaptic α5-containing GABAA-Rs mediate tonic current in CA1/CA3 pyramidal cells (Pirker et al., 2000; Caraiscos et al., 2004), and ∼30% of the total tonic current in the dentate gyrus (Glykys et al., 2008).
Neurosteroids are steroid derivatives that are synthesized both locally in neurons and glia as well as peripherally in the gonads and adrenals (Hu et al., 1987; Mathur et al., 1993; Mellon and Griffin, 2002; Sanna et al., 2004). Among these compounds, the progesterone metabolite 3α-hydroxy-5α-pregnan-20-one (3α,5α-THP or allopregnanolone) exerts a selective and potent modulatory action at GABAA-Rs and exhibits an especially high affinity and efficacy at extrasynaptic GABAA-Rs (Majewska et al., 1986; Belelli and Lambert, 2005). Prolonged physiological or pharmacologically induced fluctuations in brain and plasma concentrations of 3α,5α-THP play an important role in regulation of GABAA-R plasticity and function (Brussaard et al., 1997; Smith et al., 1998a,b; Biggio et al.,. 2006; Mostallino et al., 2006) as well as in development of the GABAergic system in the prefrontal cortex (Grobin et al., 2003). Fluctuations in neurosteroid levels during pregnancy (Brussaard et al., 1997; Concas et al., 1998) and the ovarian cycle (Maguire et al., 2005; Maguire and Mody, 2007) thus result in selective changes in the expression and function of GABAA-Rs in various regions of the rat and mouse brain, effects that are mediated by a direct regulatory action of neurosteroids at GABAA-Rs. We have now shown that the expression and function of extrasynaptic GABAA-Rs in the rat hippocampus are markedly modified during pregnancy and after delivery.
Materials and Methods
Animals.
Adult (60–90 d old; body mass, 200–250 g) female Sprague Dawley CD rats (Charles River Laboratories) were studied. They were housed under an artificial 12 h light/dark cycle (lights on from 8:00 A.M. to 8:00 P.M.) and at a constant temperature of 23 ± 2°C and relative humidity of 65%. Standard laboratory food and water were freely available at all times.
The specific stage of the estrous cycle (diestrus, proestrus, or estrus) was determined from analysis of daily vaginal smears collected between 9:00 A.M. and 10:00 A.M. for 2–4 weeks. The estrous cycle was monitored for two full cycles before animals were mated by placing them individually in a cage with a proven male on the evening of proestrus. The male was removed from the breeding cage after the appearance of a vaginal plug in the female; the day the plug was detected was designated day 0 of pregnancy (P0). Animals were killed between 9:00 A.M. and 10:00 A.M. when in estrus (control group) or at various stages of pregnancy or after delivery.
Animal care and handling throughout the experimental procedures were in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC). The experimental protocols were also approved by the Animal Ethics Committee of the University of Cagliari.
Pharmacological treatments.
In one series of experiments, pregnant rats were treated with finasteride (25 mg per kilogram of body mass) once a day from P12 to P18 (Concas et al., 1998). Finasteride was extracted and purified from commercial tablets as described previously (Trapani et al., 2002), suspended in a mixture of ethanol (20%) and corn oil (80%), and injected subcutaneously at the nape of the neck in a volume of 3 ml/kg. In another series of experiments, clomiphene citrate (Bruno Farmaceutici) was dissolved in physiological saline and administered intragastrically at a dose of 5 mg/kg once a day from P12 to P18. Control pregnant rats received the respective vehicle according to the same schedule as that for the two drugs.
Immunohistochemistry.
Rats were administered a lethal dose (0.3 ml/g) of Equithesin (1 g of sodium pentobarbital, 4.251 g of choral hydrate, 2.125 g of MgSO4, 12 ml of ethanol, and 43.6 ml of propylene glycol, adjusted to a total volume of 100 ml with distilled water) and were then perfused through the ascending aorta with 100 ml of PBS followed by 250 ml of 4% paraformaldehyde in PBS. The brain was removed, exposed to the same fixative for 2 h, and then transferred to 20% (w/v) sucrose in PBS. Sagittal sections (thickness, 50 μm) were cut with a Vibratome 1000 Plus instrument (Vibratome) and stored in antifreeze solution (30% ethylene glycol, 20% glycerol, 50% 50 mm phosphate buffer) at −20°C.
Indirect immunohistochemistry for subunits of the GABAA-R was performed with free-floating sections. The sections were washed with PBS, incubated for 30 min at room temperature with 0.1% phenylhydrazine to block endogenous peroxidase activity, permeabilized for 1 h with 0.2% Triton X-100 in PBS (PBS-T), and incubated for 1 h with 10% normal donkey serum (Jackson ImmunoResearch) in PBS-T. They were then incubated at 4°C for 4 d with goat polyclonal antibodies to the α4 or δ subunits (Santa Cruz Biotechnology) or with rabbit polyclonal antibodies to the γ2 subunit (Alomone) at dilutions of 1:500, 1:100, or 1:250, respectively, in PBS-T containing 10% normal donkey serum. After several washes, the sections were incubated at room temperature first for 2 h with appropriate biotinylated donkey antibodies to IgG (Jackson ImmunoResearch) diluted 1:200 in PBS-T and then for 30 min with avidin-peroxidase solution (Vectastain Elite Kit; Vector Laboratories), with the sections being washed three times with PBS-T after each incubation. The reaction product was visualized by exposure of the sections to 0.4 mm 3,3′- diaminobenzidine (Sigma) and 0.01% H2O2. After several washes with PBS, the sections were mounted on gelatin-coated slides, air-dried, dehydrated in ethanol, and cleared in xylene, and a coverslip was then applied in the presence of Eukitt mounting medium (O. Kindler). Control sections either incubated with primary antibodies in the presence of the corresponding peptide antigen or not exposed to primary antibodies did not yield positive staining. Sections were examined with a BX-41 microscope (Olympus) and photographed with an F-View charge-coupled device camera. Representative images obtained with a Plan 2× objective (numerical aperture, 0.05) are shown.
Semiquantitative analysis of images was performed with AnalySIS 3.2 software (Soft Imaging System). In each image, the different areas of the hippocampus, based on plates 48–50 of the Paxinos and Watson (1986) atlas, were selected by drawing a line surrounding the region of interest (ROI). The intensity of immunostaining in each ROI was determined as an integral from the intensity value for each pixel and the total number of pixels and was corrected for the background obtained in negative control sections. The intensity values, which represent the abundance of the corresponding GABAA-R subunit, were represented by a gray scale and expressed as percentage change relative to the control rats in estrus.
The antibodies to the δ subunit were generated in response to a peptide corresponding to the C terminus of the human protein; the amino acid sequence of the peptide differs from that of the corresponding region of the rat protein at two positions. Immunoblot analysis of a crude membrane fraction of the rat hippocampus with these antibodies yielded a single immunoreactive band at a position corresponding to a molecular size of ∼54 kDa (Follesa et al., 2005). The specificity of the antibody preparation was also confirmed by immunocytochemical analysis (Follesa et al., 2005; Serra et al., 2006). The antibodies to the α4 subunit were generated in response to a peptide corresponding to the N-terminal region of the rat protein. Immunoblot analysis showed that these antibodies recognized a single protein of ∼70 kDa in a crude membrane fraction prepared from rat hippocampal neurons (Sanna et al., 2003). The specificity of this antibody preparation was also confirmed by immunocytochemistry (Sanna et al., 2003; Serra et al., 2006). The antibodies to the γ2 subunit were generated in response to a synthetic peptide corresponding to residues 39–53 of the rat protein. Immunoblot analysis of a crude membrane fraction of rat hippocampus with these antibodies yielded a single immunoreactive band of ∼45–47 kDa; this band was not detected when the blot was incubated with the antibodies in the presence of the peptide antigen, demonstrating antibody specificity (data not shown).
Electrophysiology.
Rats were anesthetized in a chamber saturated with chloroform and then decapitated. The brain was removed rapidly and placed in an ice-cold solution containing 220 mm sucrose, 2 mm KCl, 1.3 mm NaH2PO4, 12 mm MgSO4, 0.2 mm CaCl2, 10 mm glucose, 2.6 mm NaHCO3 (pH 7.3, equilibrated with 95% O2 and 5% CO2), and 3 mm kynurenic acid. Coronal hippocampal slices (thickness, 200–250 μm) were prepared with a Vibratome 1000 Plus and then incubated first for 40 min at 34°C and then for 30 min at room temperature in artificial CSF (ACSF), consisting of 126 mm NaCl, 3 mm KCl, 1.25 mm NaH2PO4, 1 mm MgSO4, 2 mm CaCl2, 10 mm glucose, and 26 mm NaHCO3 (pH 7.3, equilibrated with 95% O2 and 5% CO2).
Slices were transferred to a recording chamber perfused with ACSF at a rate of ∼2 ml/min and at room temperature. Whole-cell patch-clamp electrophysiological recordings were performed with an Axopatch 200-B amplifier (Axon Instruments) and using an infrared-differential interference contrast microscope. Patch microelectrodes (borosilicate capillaries with a filament and an outer diameter of 1.5 μm; Sutter Instruments) were prepared with a two-step vertical puller (Sutter Instruments) and had a resistance of 4–6 MΩ. Spontaneous IPSCs (sIPSCs) as well as tonic GABAergic currents were recorded at a holding potential of −65 mV with an internal solution containing 140 mm CsCl, 2 mm MgCl2, 1 mm CaCl2, 10 mm EGTA, 10 mm HEPES-CsOH, pH 7.3, 2 mm ATP (disodium salt), and 5 mm QX-314 (lidocaine N-ethyl bromide). Access resistance was between 20 and 30 MΩ; if it changed by >20% during the recording, the recording was discarded.
All GABAergic currents were recorded in the presence of kynurenic acid (3 mm) in the external solution. For the recording of miniature IPSCs (mIPSCs), lidocaine (500 μm) was added in the external solution. Currents through the patch-clamp amplifier were filtered at 2 kHz and digitized at 5.5 kHz with commercial software (pClamp 8.2, Axon Instruments). Analysis of tonic GABAergic currents was performed with parameters described previously (Carta et al., 2004). In brief, we selected epochs of 3 s every 30 s of recording and visually excluded sIPSCs from the analysis. For the analysis of sIPSCs or mIPSCs, we visually selected all events, which are readily distinguishable from the background noise on the basis of their characteristic fast rise times and slower decay times. All-point histograms were constructed from these epochs and mean values were determined from Gaussian fits. (GABA, bicuculline methiodide, 3α,5α-THP, and Ro15-4513 were from Sigma. Lorazepam was a kind gift from Wyeth).
Statistical analysis.
Electrophysiological data are presented as means ± SEM. Statistical comparisons of pooled data were performed by ANOVA followed by Scheffe's post hoc test. Immunohistochemical data are expressed as percentage change in gray-scale values relative to the estrus group and are means ± SEM of values from the indicated numbers of animals. Significance of differences was assessed by one-way ANOVA followed by Scheffe's test. For all statistical analysis, a p value of <0.05 was considered statistically significant.
Results
Expression of the δ subunit during pregnancy and after delivery
The expression of the α4, δ, and γ2 subunits of the GABAA-R in the hippocampus of rats during pregnancy and after delivery was examined by immunohistochemistry with specific antibodies generated in response to extracellular epitopes of these proteins. In all instances, specific immunostaining was blocked by previous incubation of the antibodies with the respective peptide antigen (data not shown). Staining intensity for the individual subunits depended on the properties of the corresponding antibodies. We therefore used different antibody dilutions to obtain similar intensities of staining. Differences in staining intensity obtained with the subunit-specific antibodies thus do not necessarily reflect real differences in expression level among the respective proteins.
In control (estrus) rats, moderate levels of immunoreactivity for the δ subunit of the GABAA-R were apparent throughout most regions of the hippocampal formation (Fig. 1; supplemental Fig. S1, available at www.jneurosci.org as supplemental material). The abundance of the δ subunit appeared greatest in the granule cell layer of the dentate gyrus and in the pyramidal cell layer of regions CA1 (Fig. 1A,B). Expression of the δ subunit was also prominent in the molecular layer of the dentate gyrus. Measurement of δ subunit immunoreactivity in rats on P15, P19, and P21 revealed that specific staining increased progressively in the dentate gyrus and the CA1 region compared with that in control rats (Fig. 1A–D). A significant increase was already evident at P15 in the dentate gyrus and the pyramidal cell layer of CA1, with maximal upregulation in all regions being apparent at P19 or P21. In contrast, the amount of δ subunit immunoreactivity had decreased to values significantly less than those for control rats by 2 d after delivery (pp2) (Fig. 1A–D). This downregulation of δ subunit expression was still apparent 7 d postpartum (pp7) in the granular and molecular layers of the dentate gyrus (Fig. 1A,C) and in the stratum oriens of CA1 (Fig. 1B,D).
Figure 1.
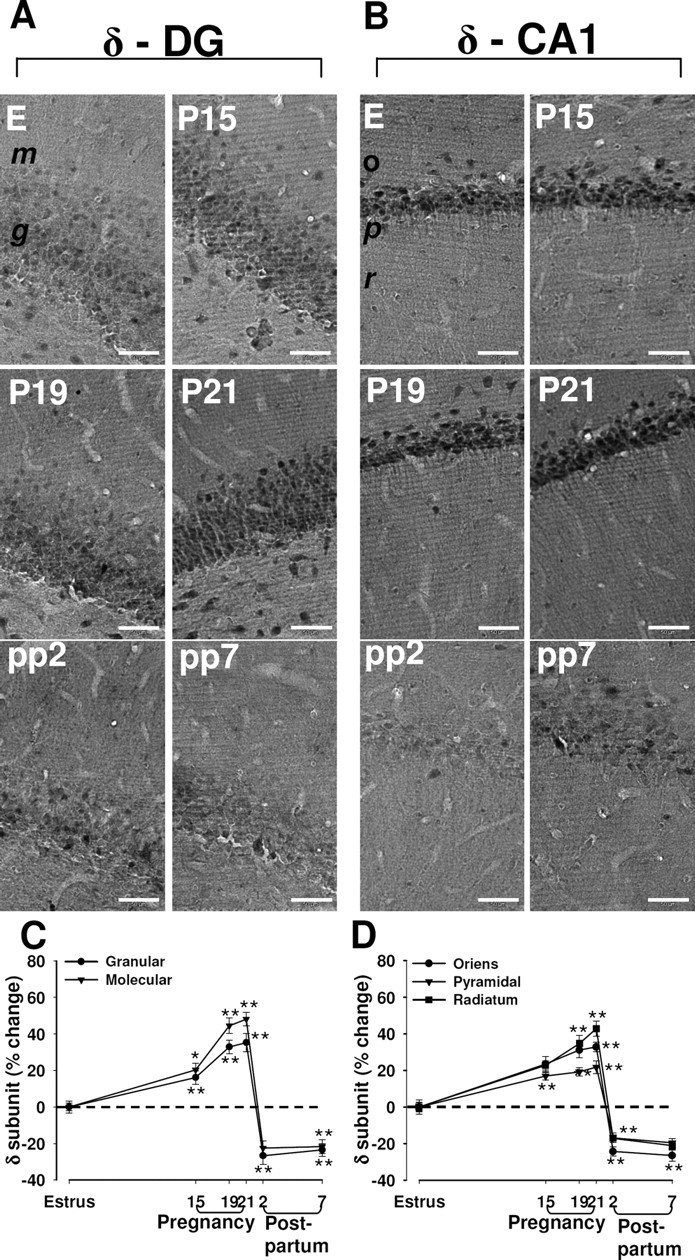
Changes in immunoreactivity for the δ subunit of the GABAA-R in the rat hippocampus during pregnancy and after delivery. A, B, Representative immunohistochemical images of the distribution of the δ subunit in the dentate gyrus (A) and in the CA1 region (B) in hippocampal sections from rats in estrus (E), at P15, P19, or P21, or at pp2 or pp7. m, Molecular layer of the dentate gyrus; g, granule layer of the dentate gyrus; o, stratum oriens of the CA1 region; p, pyramidal of the CA1 region; r, radians of the CA1 region. Scale bar, 50 μm. C, D, Images similar to those in A and B were subjected to semiquantitative measurement of δ subunit immunoreactivity in the granular and molecular cell layers of the dentate gyrus (C), and in the stratum oriens, pyramidal cell layer, and stratum radiatum of CA1 (D). Data are expressed as the percentage change in gray-scale values relative to the corresponding value for rats in estrus (control) and are means ± SEM from six to eight animals at each time point. *p < 0.05, **p < 0.01 versus estrus (one-way ANOVA followed by Scheffe's test).
Expression of the α4 subunit during pregnancy and after delivery
Immunoreactivity for the α4 subunit of the GABAA-R appeared diffuse throughout the hippocampal formation of control rats, being most abundant in the granule cell layer of the dentate gyrus and in the pyramidal cell layer of CA1 (Fig. 2; supplemental Fig. S2, available at www.jneurosci.org as supplemental material). Staining was also prominent in the molecular layer of the dentate gyrus. The amount of the α4 subunit did not change significantly during pregnancy, but it was increased markedly both in the dentate gyrus and the CA1 region at 2 d after delivery (Fig. 2A–D). This upregulation of α4 subunit expression was no longer significant at 7 d after delivery (Fig. 2A–D).
Figure 2.
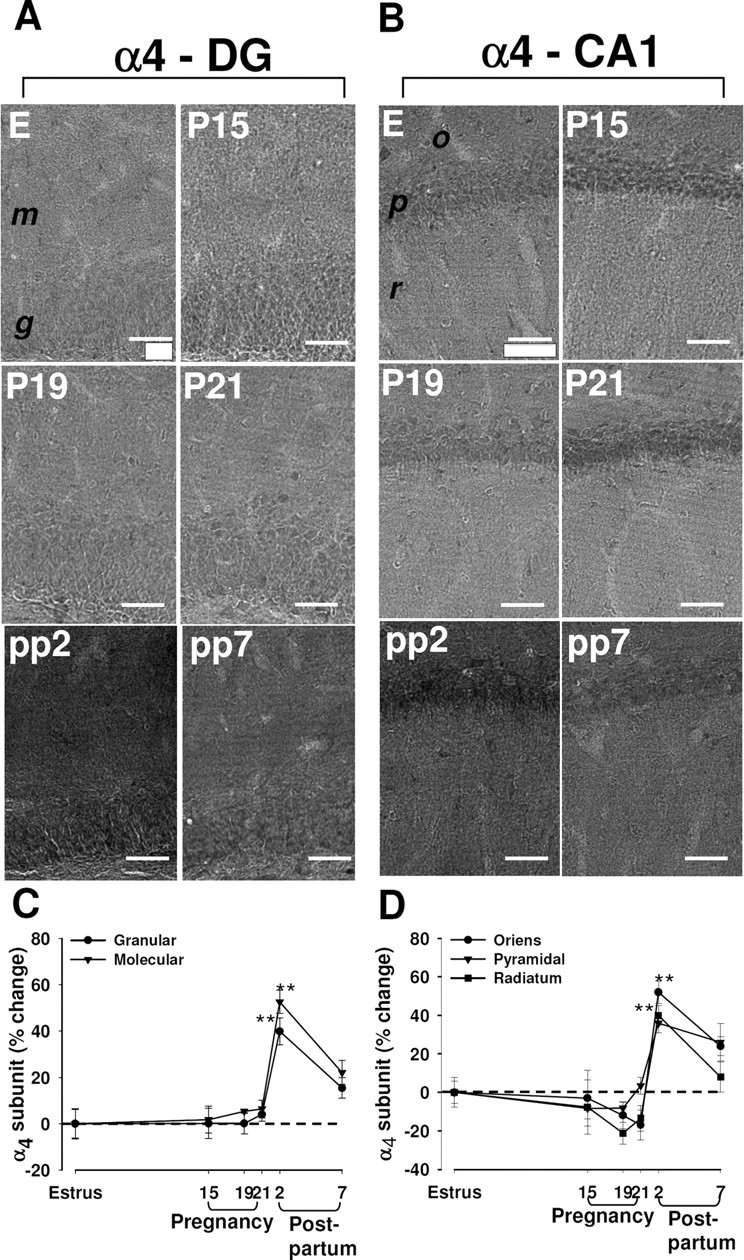
Changes in immunoreactivity for the α4 subunit of the GABAA-R in the rat hippocampus during pregnancy and after delivery. A, B, Representative immunohistochemical images of the distribution of the α4 subunit in the dentate gyrus (A) and in the CA1 region (B) in hippocampal sections from rats in estrus (E), at P15, P19, or P21, or at pp2 or pp7. m, Molecular layer of the dentate gyrus; g, granule layer of the dentate gyrus; o, stratum oriens of the CA1 region; p, pyramidal of the CA1 region; r, radians of the CA1 region. Scale bar, 50 μm. C, D, Images similar to those in A and B were subjected to semiquantitative measurement of α4 subunit immunoreactivity in the granular and molecular cell layers of the dentate gyrus (C), and in the stratum oriens, pyramidal cell layer, and stratum radiatum of CA1 (D). Data are expressed as the percentage change in gray-scale values relative to the corresponding value for rats in estrus (control) and are means ± SEM from six to eight animals at each time point. **p < 0.01 versus estrus (one-way ANOVA followed by Scheffe's test).
Expression of the γ2 subunit during pregnancy and after delivery
In control rats, γ2 subunit immunoreactivity was concentrated in the strata oriens and radiatum of CA1 and CA3, in the stratum lacunosum-moleculare of CA1, and in granule cells of the dentate gyrus (Fig. 3; supplemental Fig. S3, available at www.jneurosci.org as supplemental material). The level of γ2 subunit immunoreactivity was decreased at P15 and P19 in the dentate gyrus and the CA1 region (Fig. 3A–D). It then increased immediately before delivery at P21, remained increased at 2 d after delivery, and returned to control values by 7 d after delivery (Fig. 3A–D).
Figure 3.
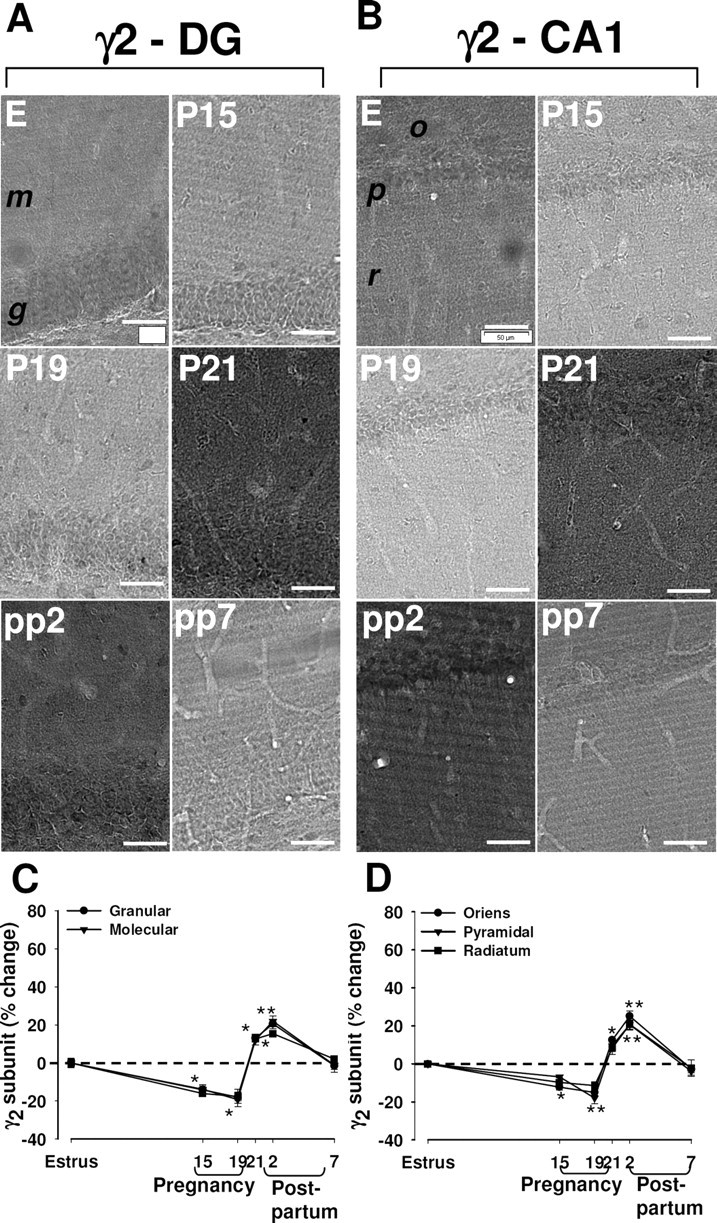
Changes in immunoreactivity for the γ2 subunit of the GABAA-R in the rat hippocampus during pregnancy and after delivery. A, B, Representative immunohistochemical images of the distribution of the γ2 subunit in the dentate gyrus (A) and in the CA1 region (B) in hippocampal sections from rats in estrus (E), at P15, P19, or P21, or at pp2 or pp7. m, Molecular layer of the dentate gyrus; g, granule layer of the dentate gyrus; o, stratum oriens of the CA1 region; p, pyramidal of the CA1 region; r, radians of the CA1 region. Scale bar, 50 μm. C, D, Images similar to those in A and B were subjected to semiquantitative measurement of γ2 subunit immunoreactivity in the granular and molecular cell layers of the dentate gyrus (C), and in the stratum oriens, pyramidal cell layer, and stratum radiatum of CA1 (D). Data are expressed as the percentage change in gray-scale values relative to the corresponding value for rats in estrus (control) and are means ± SEM from six to eight animals at each time point. *p < 0.05, **p < 0.01 versus estrus (one-way ANOVA followed by Scheffe's test).
GABAergic tonic current in granule cells during pregnancy and after delivery
We next examined GABAergic transmission in granule cells of the dentate gyrus using hippocampal slices obtained from rats at P15 and P19 as well as at 2 d after delivery and during estrus (control). Granule cell bodies are densely packed and are readily recognizable on the basis of their rounded shape and small size. In adult rats, granule cells of the dentate gyrus are characterized by a prominent GABAergic tonic current mediated by extrasynaptic GABAA-Rs containing mainly α4, βn, and δ subunits (Nusser and Mody, 2002) and, in a lower proportion, by extrasynaptic receptors containing the α5 subunit (Glykys et al., 2008). There is also a phasic component to inhibition of these neurons that is attributable to the activation of synaptic GABAA-Rs with an αnβnγ2 subunit composition. We recorded from granule cells in the voltage-clamp mode (holding potential, −65 mV) with patch pipettes containing a solution with a high Cl− concentration that gives rise to a Cl− equilibrium potential of ∼0 mV. Under these recording conditions, activation of GABAA-Rs generates inward currents that reflect an outflow of Cl−. GABAergic currents were pharmacologically isolated by the addition to ACSF of kynurenic acid (3 mm), a broad-spectrum antagonist of ionotropic glutamate receptors.
After a baseline period of ∼5 min, slices were exposed to exogenous GABA (5 μm) for 5 min to activate high-affinity extrasynaptic GABAA-Rs (Maguire et al., 2005). Exposure of granule cells from control rats to GABA increased current noise variance and shifted the holding current in the negative direction with respect to baseline (Fig. 4), effects attributable to the activation of extrasynaptic GABAA-Rs. Bath application of bicuculline (30 μm), a competitive antagonist of GABAA-Rs, blocked all GABA-induced currents, confirming that they were mediated by GABAA-Rs, as well as reduced the noise variance and the holding current compared with those observed during the baseline period (Fig. 4A). The bath application of GABA resulted in marked increases in noise variance and in a shift of the holding current in slices obtained from rats at P15, P19, or 2 d after delivery (Fig. 4A–C). Statistical analysis revealed a significant difference between rats at P19 and control animals for both noise variance and holding current. Tonic current parameters were also increased at P15 relative to control values, but the changes were not significant, and they had returned to control values by 2 d after delivery (Fig. 4B,C).
Figure 4.
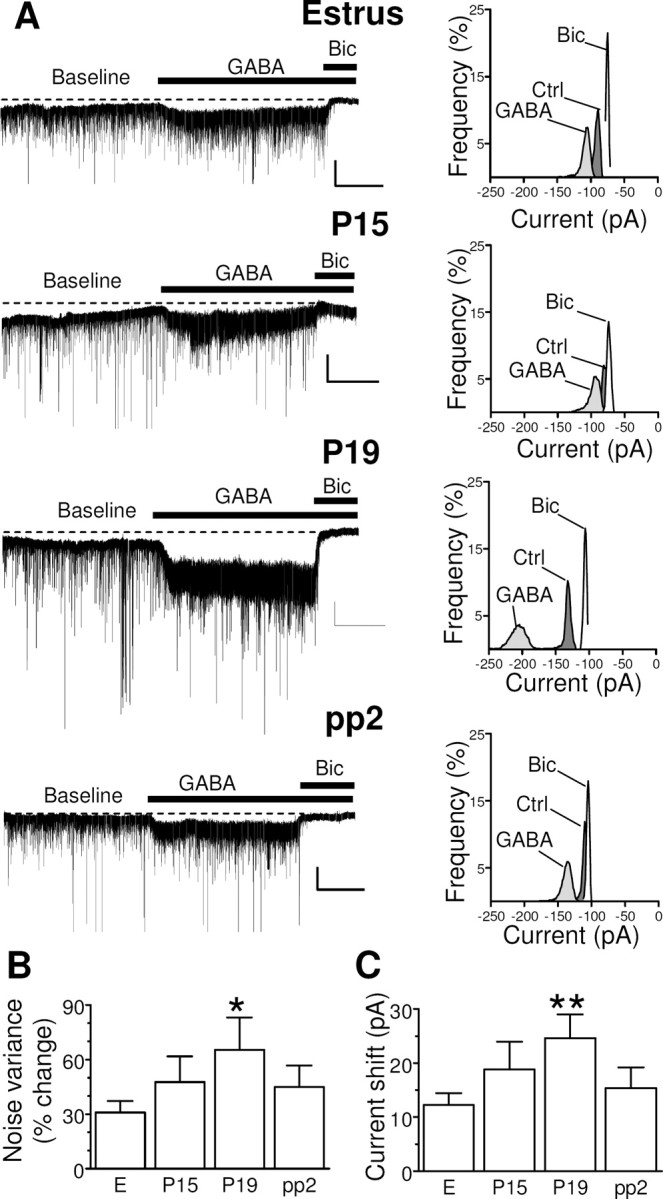
Changes in tonic GABAergic current in granule cells of the dentate gyrus during pregnancy and after delivery. A, Representative traces of GABAergic currents recorded in the whole-cell mode (holding potential, −65 mV) from granule cells of the dentate gyrus are shown (left panels) for hippocampal slices isolated from female rats in estrus (E), at day P15 or P19, or at pp2. All recordings were performed in the presence of kynurenic acid (3 mm) to block glutamatergic currents. After a baseline period of 5 min, application of GABA (5 μm) induced an increase in noise variance and a shift in the holding current. All GABAergic currents were blocked by the application of bicuculline (Bic) at 30 μm. Calibration: 50 pA, 2 min. An all-point histogram for 2 min periods before (Ctrl) or during the application of GABA alone or with bicuculline is also shown for each trace (right panels). B, C, Bar graphs summarizing the changes in noise variance (B) and holding current (C) induced by the application of exogenous GABA in experiments similar to those shown in A. Data are means ± SEM from 32 to 67 neurons. *p < 0.05, **p < 0.01 versus estrus (ANOVA followed by Scheffe's test).
GABAergic phasic inhibition in granule cells during pregnancy and after delivery
The high frequency of sIPSCs observed in the electrophysiological traces in Figure 4A showed that the granule cells were also subjected to pronounced phasic inhibition mediated by synaptic GABAA receptors. These currents were readily distinguishable from the tonic current on the basis of their fast rise time and slower decay time. Analysis of the kinetic properties of these sIPSCs revealed no significant differences among rats of the different experimental groups (Table 1). Because sIPSCs may be affected by alterations in spontaneous action potential firing levels of interneurons, we also recorded mIPSCs in dentate gyrus granule cells. Quantitative analysis of basal mIPSC kinetic characteristics did not indicate any statistically significant difference among animals of the various experimental groups, although amplitude, decay time constant, and area of mIPSCs were slightly decreased and increased in P19 and 2 d after delivery rats, respectively, compared with animals in estrus (supplemental Table S1, available at www.jneurosci.org as supplemental material).
Table 1.
Kinetic characteristics of GABAA-R-mediated sIPSCs recorded from granule cells of the dentate gyrus during pregnancy and after delivery
| Group (n) | Amplitude (pA) | Decay time (ms) | Area (pA × ms) | Frequency (Hz) |
|---|---|---|---|---|
| E (68) | 50.8 ± 3.5 | 16.5 ± 0.6 | 1617 ± 104 | 1.5 ± 0.1 |
| P15 (38) | 47.4 ± 2.2 | 13.7 ± 0.3 | 1555 ± 113 | 2.0 ± 0.2 |
| P19 (55) | 46.6 ± 2.1 | 15.3 ± 0.5 | 1457 ± 121 | 1.2 ± 0.1 |
| pp2 (38) | 55.1 ± 3.7 | 15.5 ± 0.6 | 1726 ± 243 | 1.6 ± 0.1 |
Data are means ± SEM for the indicated numbers (n) of neurons. The sIPSCs were recorded in the presence of 3 mm kynurenic acid (external) from voltage-clamped (−65 mV) cells in hippocampal slices isolated from female rats in estrus (E), at P15 or P19, or at pp2.
Sensitivity of GABAA-Rs to 3α,5α-THP during pregnancy
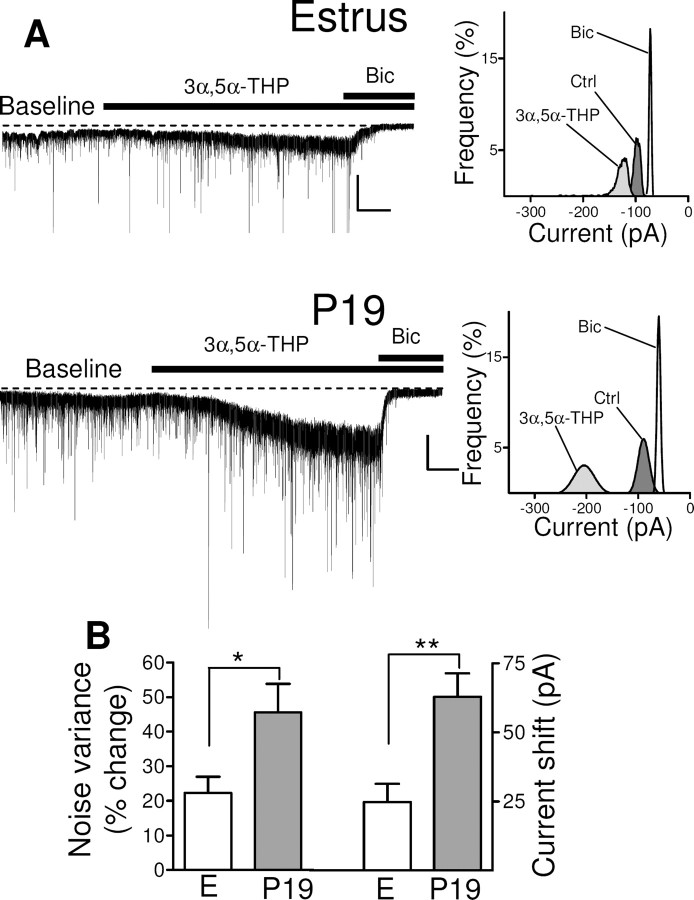
Neurosteroids are among the most potent endogenous positive modulators of GABAA-Rs, but their activity is dependent on the receptor subunit composition (Belelli and Lambert, 2005). They thus manifest a higher efficacy at GABAA-Rs that contain the δ subunit than at those that contain the γ2 subunit (Adkins et al., 2001; Brown et al., 2002; Wohlfarth et al., 2002). Extrasynaptic GABAA-Rs are therefore more sensitive (nanomolar affinity) to this class of modulators than are synaptic receptors (micromolar affinity) (Stell et al., 2003). We next tested the effects of the neurosteroid 3α,5α-THP on both tonic and phasic GABAergic transmission in rats at P19 in comparison with those in estrus.
Hippocampal slices were exposed to 3α,5α-THP (1 μm) for 7 min after a stable baseline had been established for 4 min. As expected, 3α,5α-THP induced a marked increase in the tonic current (Fig. 5A). However, because this effect of 3α,5α-THP was slower in onset and increased more gradually with time compared with that of GABA (Fig. 5A), we selected only the last 2 min period of 3α,5α-THP application before the addition of bicuculline (corresponding to the maximal increase in tonic current) for our analysis. 3α,5α-THP induced an increase in noise variance and holding current in slices from rats in estrus or at P19 (Fig. 5B,C). The differences in both noise variance and holding current between estrus and P19 were statistically significant. At a lower concentration (0.5 μm), 3α,5α-THP induced a small but nonsignificant increase in the tonic current in both groups of animals.
Figure 5.
Modulatory action of 3α,5α-THP on tonic GABAergic current in granule cells of the dentate gyrus during pregnancy. A, Representative traces of GABAergic currents recorded from granule cells in hippocampal slices from rats in estrus or at P19 (left panels). Recordings were obtained before (Baseline) and during bath application of 1 μm 3α,5α-THP either alone or in the presence of 30 μm bicuculline. Calibration: 100 pA, 1 min. An all-point histogram for 2 min periods at the baseline (Ctrl) or during the application of 3α,5α-THP alone (final 2 min before the addition of bicuculline) or with bicuculline is shown for each trace (right panels). B, Bar graph summarizing the percentage changes in noise variance (left panel) and holding current (right panel) induced by 3α,5α-THP in experiments similar to those shown in A. Data are means ± SEM from 13 to 23 neurons. *p < 0.05, **p < 0.01 versus estrus (ANOVA followed by Scheffe's test).
We also examined the effect of 3α,5α-THP on sIPSCs in granule cells. As expected, 3α,5α-THP (1 μm) induced marked increases in the decay time constant, amplitude, and area of sIPSCs both in estrus and at P19, but the extent of these increases did not differ between rats of the two groups (supplemental Fig. S4, available at www.jneurosci.org as supplemental material). In contrast, 3α,5α-THP substantially reduced the frequency of sIPSCs in both groups (supplemental Fig. S4, available at www.jneurosci.org as supplemental material), an effect that was likely attributable to the masking of phasic events of smaller amplitude by the marked enhancement of tonic conductance.
Effect of lorazepam on GABAA-R function during pregnancy
To examine further the functional consequences of the pregnancy-induced changes in GABAA-R subunit expression, we examined the effect of the benzodiazepine lorazepam on GABAergic currents in granule cells of the dentate gyrus at P19. As expected (Nusser and Mody, 2002), bath application of lorazepam (3 μm) for 5 min had no effect on tonic current in rats either in estrus or at P19 (data not shown). In contrast, lorazepam markedly increased the amplitude, area and decay time constant of sIPSCs by similar extents for rats in estrus or at P19 (supplemental Table S2, available at www.jneurosci.org as supplemental material). The effect of lorazepam on sIPSC kinetic parameters was however slightly reduced at P19 compared with rats in estrus but this effect did not reach statistical significance.
Effect of Ro15-4513 on GABAA-R-mediated sIPSCs during pregnancy and after delivery
To evaluate the functional relevance of the increased expression of the α4 and γ2 subunits of the GABAA-R in the hippocampus 2 d after delivery, we tested the modulatory effect of Ro15-4513 on GABAA-R-mediated sIPSCs in granule cells of the dentate gyrus. Ro15-4513 is an inverse agonist at the benzodiazepine-binding site of GABAA-Rs containing either the α1, α2, α3, or α5 subunit together with the γ2 subunit (Barnard et al., 1998), but it is able to bind and positively modulate receptors comprising α4, β, and γ2 subunits (Knoflach et al., 1996; Wafford et al., 1996). In granule cells of the dentate gyrus of rats in estrus and at day 19 of pregnancy, bath application of 3 μm Ro15-4513 reduced (24 and 19%, respectively) the time constant of sIPSC decay. In contrast, in granule cells of the dentate gyrus from pp2 rats, Ro15-4513 increased by 28% the time constant of sIPSC decay (Table 2). The frequency and amplitude of sIPSCs were not significantly altered by Ro15-4513.
Table 2.
Effect of Ro15–4513 on GABAA-R-mediated sIPSCs in granule cells of the dentate gyrus during pregnancy and after delivery
| Group (n) | Amplitude (% change) | Decay time (% change) | Area (% change) | Frequency (% change) |
|---|---|---|---|---|
| E (5) | −5.5 ± 7.2 | −24.1 ± 4.1* | −31.2 ± 9.5* | −11.2 ± 7.3 |
| P19 (8) | −10.3 ± 6.8 | −19.5 ± 5.2* | −23.1 ± 10.3* | −3.6 ± 6.9 |
| pp2 (8) | 12.2 ± 10.2 | 28.6 ± 8.3* | 25 ± 11.5 | −5.8 ± 7.7 |
Data are expressed as the percentage of change in current parameters induced by Ro15-4513 (3 μm) and are means ± SEM for the indicated numbers (n) of neurons in hippocampal slices obtained from rats in estrus, at day 19 of pregnancy, or at 2 d after delivery.
*p < 0.05 versus estrus (one-way ANOVA followed by Scheffe's test).
GABAergic inhibitory transmission in CA1 pyramidal neurons during pregnancy
Although tonic currents in the hippocampus have been characterized most extensively in granule cells of the dentate gyrus, this type of inhibitory current has also been detected in CA1 pyramidal neurons (Caraiscos et al., 2004). After a baseline period of 5 min, we exposed hippocampal slices to GABA (5 μm) and then blocked all currents with bicuculline (30 μm). However, we failed to detect a substantial tonic current in CA1 pyramidal neurons of rats in estrus or at P19 with this protocol (supplemental Fig. S5, available at www.jneurosci.org as supplemental material). We also characterized the kinetic parameters of sIPSCs (supplemental Table S3, available at www.jneurosci.org as supplemental material) as well as the effect of lorazepam on these currents (supplemental Table S4, available at www.jneurosci.org as supplemental material) in CA1 pyramidal neurons of rats of the various experimental groups. No significant effects of pregnancy or delivery were apparent.
Role of neurosteroids in the changes in GABAA-R expression and function during pregnancy
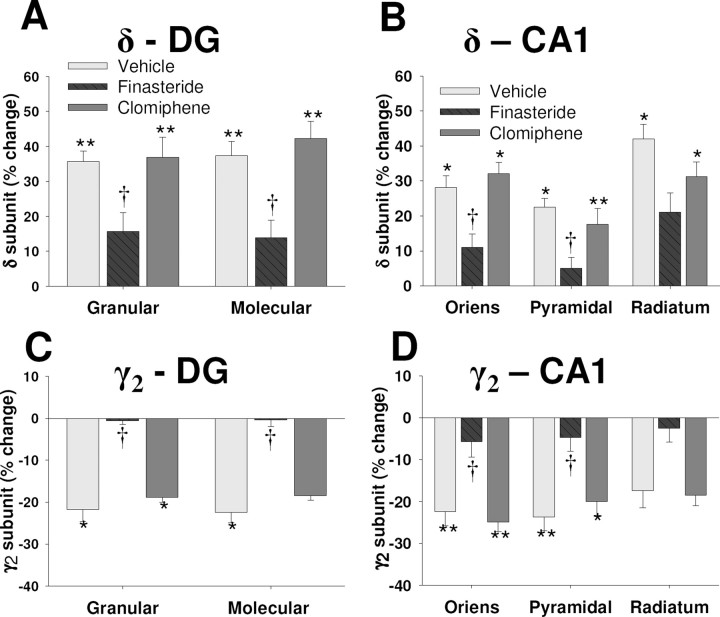
Given that the selective and opposite changes in the expression of δ and γ2 subunits of the GABAA-R as well as the enhancement of GABAergic tonic current in granule cells of the dentate gyrus observed in rats at P19 appeared temporally correlated with the previously demonstrated increases in the brain concentrations of progesterone and 3α,5α-THP (Concas et al., 1998) as well as of estrogen (Taya and Greenwald, 1981), we examined whether these events might be causally related. Finasteride, an inhibitor of the enzyme 5α-reductase, or clomiphene, an estrogen receptor antagonist, was administered to pregnant rats from P12 to P18 to inhibit the synthesis of 3α,5α-THP or the activation of estrogen receptors, respectively (Azzolina et al., 1997; Homburg, 2005; Finn et al., 2006). Finasteride treatment inhibited the upregulation of δ subunit expression (Fig. 6A,B) and the downregulation of γ2 subunit expression (Fig. 6C,D) observed in the dentate gyrus and the CA1 region at P19. In contrast, clomiphene administration had no significant effect on the expression level of the δ subunit (Fig. 6A,B) or the γ2 subunit (Fig. 6C,D) apparent at P19.
Figure 6.
Effect of treatment with finasteride or clomiphene on expression of the δ and γ2 subunits of the GABAA-R during pregnancy. Finasteride (25 mg/kg), clomiphene (5 mg/kg), or vehicle was administered daily from day 12 to day 18 of pregnancy. A–D, Rats were killed at day 19 of pregnancy and the hippocampus was subjected to immunohistochemistry for semiquantitative measurement of δ subunit (A, B) or γ2 subunit (C, D) immunoreactivity in the dentate gyrus and the CA1 region, respectively. Data are expressed as percentage of change relative to the corresponding value for rats in estrus and are means ± SEM from at least eight animals per group. Results from rats treated with the vehicle for finasteride or with that for clomiphene were pooled. *p < 0.05, **p < 0.01 versus rats in estrus; †p < 0.05 versus vehicle-treated rats at day 19 of pregnancy (one-way ANOVA followed by Scheffe's test).
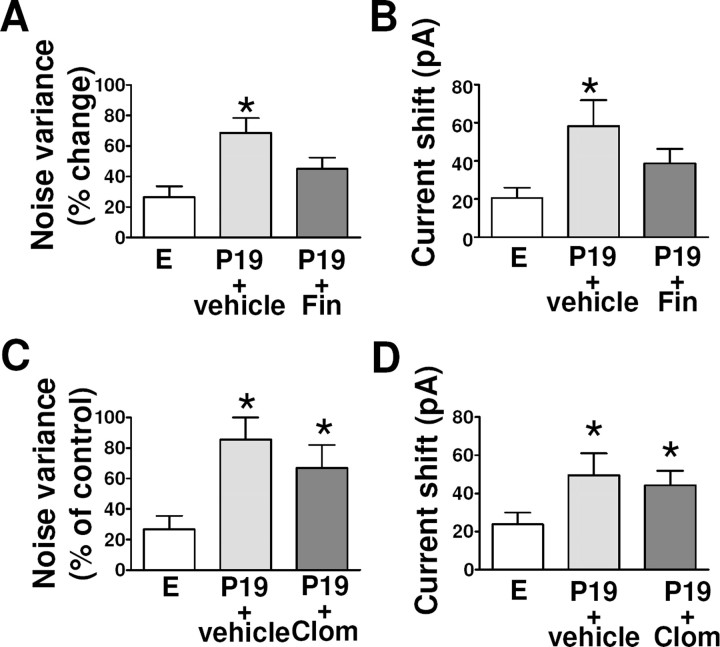
We also examined GABAA-R function in granule cells of the dentate gyrus in hippocampal slices prepared from rats in estrus or from those at P19 that had been injected with finasteride or vehicle. The differences in the effects of GABA on tonic current parameters (noise variance and holding current) between rats at P19 and those in estrus were reduced and rendered statistically insignificant by finasteride treatment (Fig. 7A,B). In contrast, finasteride treatment had no effect on the kinetic properties of GABAergic sIPSCs in granule cells of rats at P19 (data not shown). Finally, clomiphene treatment had no effect on tonic current parameters in granule cells of rats at P19 (Fig. 7C,D). Clomiphene treatment also had no significant effect on the kinetic parameters of GABAergic sIPSCs recorded from granule cells at P19 in the absence or presence of 3α,5α-THP (data not shown).
Figure 7.
Effect of finasteride or clomiphene treatment on the changes in GABAergic tonic current in granule cells of the dentate gyrus during pregnancy. A–D, The percentage of changes in noise variance (A, C) and holding current (B, D) induced by GABA were determined in hippocampal slices from rats in estrus as well as from those at day 19 of pregnancy that had been treated with finasteride (A, B) or clomiphene (C, D) as in Figure 6. *p < 0.05 versus estrus (ANOVA followed by Scheffe's test).
Discussion
Our present results provide further support to the notion that both the structure and function of GABAA-Rs in the brain change during pregnancy and immediately after delivery as a result of the marked associated fluctuations in the brain levels of neuroactive steroids. We have thus shown that the late stage of pregnancy in rats is associated with an increase in the expression of the δ subunit of the GABAA-R and a decrease in that of the γ2 subunit in various regions of the hippocampus. In contrast, the expression of the α4 subunit did not change during pregnancy but rather increased markedly after delivery. This selective switch in subunit composition of GABAA-Rs during pregnancy and after delivery was associated with a selective enhancement and reduction of tonic current mediated by extrasynaptic GABAA-Rs in granule cells of the dentate gyrus, respectively, with phasic sIPSCs mediated by synaptic receptors being unaffected. These effects of pregnancy on the subunit composition and function of GABAA-Rs were inhibited by treatment with finasteride, suggesting that they are mediated by the associated changes in the brain levels of 3α,5α-THP and other neuroactive steroids during late pregnancy (Concas et al., 1998). Such an increase in GABAergic tonic at day 19 of pregnancy may be important to further reduce the excitability and anxiety level usually associated with the end of pregnancy and immediately preceding delivery (Zuluaga et al., 2005; de Brito Faturi et al., 2006; Skouteris et al., 2009).
The brain levels of steroid hormones increase markedly during pregnancy in rats (Concas et al., 1998). Indeed the concentrations of progesterone, 3α,5α-THP, and 3α,5α-tetrahydrodeoxycorticosterone increase severalfold in both plasma and the brain (relative to estrus values) starting from day 15 and peaking at day 19 of pregnancy. However, the brain levels of these hormones fall suddenly to control levels at day 21 of pregnancy, immediately preceding delivery, and they remain at such levels for up to 2 d (Concas et al., 1998).
We have previously shown that the sustained increases in the brain levels of neuroactive steroids during pregnancy are responsible for the associated reduction in the expression of the α5 and γ2 subunits of the GABAA-R in both the cerebral cortex and hippocampus of rats (Concas et al., 1998; Follesa et al., 1998). Late pregnancy was also associated with an increase in the abundance of the α1 subunit mRNA in oxytocin neurons of the supraoptic nucleus of the hypothalamus (Fénelon and Herbison, 1996). Our present immunohistochemical data confirm our previous observation of the downregulation of γ2 subunit expression in the hippocampus of rats during pregnancy. The amount of γ2 subunit immunoreactivity in the hippocampus was decreased from P15 to P19, an effect that was well correlated temporally with the increase in the brain levels of neuroactive steroids and which was both largely abolished by finasteride treatment (Concas et al., 1998). Furthermore, this reduction was promptly reversed in response to the sudden fall in the brain concentrations of these steroid hormones at day 21 of pregnancy and during the first 2 d after delivery.
The abundance of immunoreactivity for the δ subunit showed changes opposite to those for γ2 subunit immunoreactivity during pregnancy and after delivery. In the hippocampus, the δ subunit is expressed predominantly in granule cells of the dentate gyrus as well as in CA1 and CA3 pyramidal neurons, where it coassembles mostly with the α4 subunit (Pirker et al., 2000; Nusser and Mody, 2002; Wei et al., 2003), although it also coassembles with the α1 subunit in interneurons in the molecular layer of the dentate gyrus (Glykys et al., 2007). Specific immunostaining for the δ subunit in the hippocampus increased progressively during pregnancy, reaching a peak at days 19–21, and then fell markedly, being below control levels at 2 and 7 d after delivery. Consistent with the higher sensitivity of GABAA-Rs containing the δ subunit to neuroactive steroids (Adkins et al., 2001; Brown et al., 2002; Wohlfarth et al., 2002), fluctuations in the plasma and brain content of these compounds associated with various pathophysiological or pharmacological conditions are accompanied by changes in the expression of the δ subunit together, in some instances, with changes in α4 subunit expression (Smith et al., 1998a,b; Sundstrom-Poromaa et al., 2002; Griffiths and Lovick, 2005; Lovick et al., 2005; Maguire et al., 2005; Shen et al., 2005).
We found that expression of the α4 subunit in the hippocampus did not change during pregnancy but rather increased markedly after delivery, likely as a consequence of the sudden decrease in the anxiolytic steroid concentrations. This finding under the physiological conditions of pregnancy and delivery is consistent with those of pharmacological studies of prolonged treatment with and subsequent withdrawal of neuroactive steroids (Smith et al., 1998a,b; Sundstrom-Poromaa et al., 2002; Biggio et al., 2006; Mostallino et al., 2006). Immediately after delivery, the marked reduction in the expression of the δ subunit is accompanied with an increase in the number of receptors containing the α4 and γ2 subunit, which, being endowed with a lower sensitivity to GABA, increased desensitization rate, and likely synaptic localization, may contribute to the reversal of the increase in tonic current level and to the development of an arousal state observed in the perinatal period. The idea of an enhanced density of α4β1–3γ2 receptors is further supported by the finding that Ro15-4513 increases (Knoflach et al., 1996; Wafford et al., 1996) the time constant for sIPSC decay in granule cells of the dentate gyrus from rats at 2 d after delivery whereas it reduced this parameter in animals in estrus or at day 19 of pregnancy. This pattern of changes in the expression of these subunits is similar to that described for the ovarian cycle, during which the abundance of the δ and γ2 subunits is regulated in opposite manners (Maguire et al., 2005).
Our results have revealed substantial and selective changes in GABA-mediated transmission in granule cells of the dentate gyrus during pregnancy and after delivery. In particular, we detected a marked increase in tonic current during the late stage of pregnancy relative to that in control rats in estrus, and this increase was no longer apparent 2 d after delivery. Application of exogenous GABA thus induced a larger percentage increase in noise variance and shift in the holding current in rats at P19 than in control animals. Given that tonic transmission in granule cells is mediated by receptors containing the δ subunit, our functional data are consistent with an increase in the density of these receptors in the extrasynaptic membrane.
Neuroactive steroids are among the most potent endogenous modulators of GABAA-Rs (Majewska et al., 1986; Belelli and Lambert, 2005). These compounds modulate more potently and effectively the tonic transmission mediated by δ subunit-containing receptors than the phasic transmission mediated by γ2 subunit-containing receptors (Stell et al., 2003). The finding that 3α,5α-THP potentiated to a greater extent the tonic GABAergic transmission in granule cells of the dentate gyrus at P19 than at 2 d after delivery or in estrus supports the idea that upregulation of the expression of GABAA-Rs containing the δ subunit plays an important role during late pregnancy. Similarly, changes in the plasma levels of neuroactive steroids, an increase in tonic transmission in granule cells of the dentate gyrus, and upregulation of expression of the δ subunit have been shown to be tightly associated during the estrous cycle in mice (Maguire et al., 2005; Maguire and Mody, 2007).
Such changes in tonic current appear to be limited to granule cells of the dentate gyrus, given that neither tonic nor phasic currents recorded from CA1 pyramidal neurons were affected by pregnancy or delivery. The tonic current in CA1 pyramidal neurons has previously been recorded by only a few laboratories and under particular experimental conditions and pharmacological manipulations (Caraiscos et al., 2004).
The kinetic properties of synaptic currents in granule cells of the dentate gyrus were not substantially altered during pregnancy despite a decrease in γ2 subunit expression. Downregulation of the expression of the γ2 subunit during the diestrus phase of the ovarian cycle in mice was also found not to be accompanied by changes in the kinetic parameters of synaptic currents (Maguire et al., 2005). Our results now suggest that the density of GABAA-Rs containing the δ subunit and the associated tonic current are selectively modified during the late stage of pregnancy and after delivery.
Our results show that the opposite changes in GABAergic tonic inhibition in the dentate gyrus during pregnancy and after delivery correlate with characteristic hormonal changes (Concas et al., 1998). Very recently, a similar study (Maguire and Mody, 2008) evaluated in mice the changes during pregnancy and post partum of GABAAR δ and γ2 subunit expression in the hippocampus and found, at variance with our work, a decreased expression of both subunits at P18 compared with virgin animals. Also in that work, both tonic and phasic inhibitory currents in DGGCs were markedly reduced at P18 compared with control. All of these changes rebounded 48 h after delivery. Apart from the species difference and other differences related to the experimental conditions (i.e., anti-δ antibodies used, higher temperature used in the patch-clamp recordings, and the continuous application of exogenous GABA to the slices to bust tonic current in Maguire's study), a more likely and most influential factor that could account for the difference among the two studies may be the animals that were used as controls. In fact, while in our study we used rats in the estrus phase of the ovary cycle, Maguire and Mody used female mice in the diestrus phase. This may be relevant because at diestrus δ expression (and tonic currents) is at its highest level (in association with the peak of neuroactive steroid levels) during the ovary cycle, whereas in the estrus phase both δ expression (and tonic current) reaches its lowest level (Maguire et al., 2005). Thus, it is conceivable that the use of such opposite reference points may have contributed to reach a different interpretation of the changes occurring during pregnancy. However, this may not completely account for the different pattern of changes observed during pregnancy and after delivery in these tow studies, and at the moment we do not find more convincing reasons that may justify these discrepancies.
In conclusion, the opposite regulation of extrasynaptic GABAA-R-mediated tonic current in response to fluctuations of brain concentrations of neuroactive steroids may be crucial for the changes in mood and for the anxiolytic effect associated with pregnancy (de Brito Faturi et al., 2006) as well as for the arousal state typical of period immediately preceding delivery and early postpartum.
Footnotes
This work was supported by grants from the National Research Council, the Istituto Superiore della Sanità, the Sardinian Department of Health and Welfare, and the Gioventù in Armonia (GIO I A) Foundation (Pisa, Italy).
References
- Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB. α4β3δ GABAA receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J Biol Chem. 2001;276:38934–38939. doi: 10.1074/jbc.M104318200. [DOI] [PubMed] [Google Scholar]
- Azzolina B, Ellsworth K, Andersson S, Geissler W, Bull HG, Harris GS. Inhibition of rat α-reductases by finasteride: evidence for isozyme differences in the mechanism of inhibition. J Steroid Biochem Mol Biol. 1997;61:55–64. doi: 10.1016/s0960-0760(97)00002-2. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Biggio F, Gorini G, Caria S, Murru L, Mostallino MC, Sanna E, Follesa P. Plastic neuronal changes in GABAA receptor gene expression induced by progesterone metabolites: in vitro molecular and functional studies. Pharmacol Biochem Behav. 2006;84:545–554. doi: 10.1016/j.pbb.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussaard AB, Kits KS, Baker RE, Willems WP, Leyting-Vermeulen JW, Voorn P, Smit AB, Bicknell RJ, Herbison AE. Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABAA receptor subunit expression. Neuron. 1997;19:1103–1114. doi: 10.1016/s0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of γ-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci U S A. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito Faturi C, Teixeira-Silva F, Leite JR. The anxiolytic effect of pregnancy in rats is reversed by finasteride. Pharmacol Biochem Behav. 2006;85:569–574. doi: 10.1016/j.pbb.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fénelon VS, Herbison AE. Plasticity in GABAA receptor subunit mRNA expression by hypothalamic magnocellular neurons in the adult rat. J Neurosci. 1996;16:4872–4880. doi: 10.1523/JNEUROSCI.16-16-04872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Beadles-Bohling AS, Beckley EH, Ford MM, Gililland KR, Gorin-Meyer RE, Wiren KM. A new look at the 5α-reductase inhibitor finasteride. CNS Drug Rev. 2006;12:53–76. doi: 10.1111/j.1527-3458.2006.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follesa P, Floris S, Tuligi G, Mostallino MC, Concas A, Biggio G. Molecular and functional adaptation of the GABAA receptor complex during pregnancy and after delivery in the rat brain. Eur J Neurosci. 1998;10:2905–2912. doi: 10.1111/j.1460-9568.1998.00300.x. [DOI] [PubMed] [Google Scholar]
- Follesa P, Mostallino MC, Biggio F, Gorini G, Caria S, Busonero F, Murru L, Mura ML, Sanna E, Biggio G. Distinct patterns of expression and regulation of GABA receptors containing the δ subunit in cerebellar granule and hippocampal neurons. J Neurochem. 2005;94:659–671. doi: 10.1111/j.1471-4159.2005.03303.x. [DOI] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths JL, Lovick TA. GABAergic neurones in the rat periaqueductal grey matter express α4, β1 and δ GABAA receptor subunits: plasticity of expression during the estrous cycle. Neuroscience. 2005;136:457–466. doi: 10.1016/j.neuroscience.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Heenan EJ, Lieberman JA, Morrow AL. Perinatal neurosteroid levels influence GABAergic interneuron localization in adult rat prefrontal cortex. J Neurosci. 2003;23:1832–1839. doi: 10.1523/JNEUROSCI.23-05-01832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homburg R. Clomiphene citrate—end of an era? A mini-review. Hum Reprod. 2005;20:2043–2051. doi: 10.1093/humrep/dei042. [DOI] [PubMed] [Google Scholar]
- Hu ZY, Bourreau E, Jung-Testas I, Robel P, Baulieu EE. Neurosteroids: oligodendrocyte mitochondria convert cholesterol to pregnenolone. Proc Natl Acad Sci U S A. 1987;84:8215–8219. doi: 10.1073/pnas.84.23.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Mäkelä R, Mellor JR, Pollard S, Bahn S, Stephenson FA, Randall AD, Sieghart W, Somogyi P, Smith AJ, Wisden W. Ligand-gated ion channel subunit partnerships: GABAA receptor α6 subunit gene inactivation inhibits δ subunit expression. J Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoflach F, Benke D, Wang Y, Scheurer L, Lüddens H, Hamilton BJ, Carter DB, Mohler H, Benson JA. Pharmacological modulation of the diazepam-insensitive recombinant gamma-aminobutyric acidA receptors alpha 4 beta 2 gamma 2 and alpha 6 beta 2 gamma 2. Mol Pharmacol. 1996;50:1253–1261. [PubMed] [Google Scholar]
- Lovick TA, Griffiths JL, Dunn SM, Martin IL. Changes in GABAA receptor subunit expression in the midbrain during the oestrous cycle in Wistar rats. Neuroscience. 2005;131:397–405. doi: 10.1016/j.neuroscience.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABAA receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. GABAAR plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Mathur C, Prasad VV, Raju VS, Welch M, Lieberman S. Steroids and their conjugates in the mammalian brain. Proc Natl Acad Sci U S A. 1993;90:85–88. doi: 10.1073/pnas.90.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- Mitchell EA, Herd MB, Gunn BG, Lambert JJ, Belelli D. Neurosteroid modulation of GABAA receptors: molecular determinants and significance in health and disease. Neurochem Int. 2008;52:588–595. doi: 10.1016/j.neuint.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Mostallino MC, Mura ML, Maciocco E, Murru L, Sanna E, Biggio G. Changes in expression of the δ subunit of the GABAA receptor and in receptor function induced by progesterone exposure and withdrawal. J Neurochem. 2006;99:321–332. doi: 10.1111/j.1471-4159.2006.04055.x. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Busonero F, Talani G, Tranquilli S, Mameli M, Spiga S, Follesa P, Biggio G. Changes in GABAA receptor gene expression associated with selective alterations in receptor function and pharmacology after ethanol withdrawal. J Neurosci. 2003;23:11711–11724. doi: 10.1523/JNEUROSCI.23-37-11711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Serra M, Mostallino MC, Talani G, Pisu MG, Carta M, Mura ML, Floris I, Maciocco E, Sanna E, Biggio G. Social isolation-induced increase in α and δ subunit gene expression is associated with a greater efficacy of ethanol on steroidogenesis and GABA receptor function. J Neurochem. 2006;98:122–133. doi: 10.1111/j.1471-4159.2006.03850.x. [DOI] [PubMed] [Google Scholar]
- Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases δ GABAA receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–586. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Skouteris H, Wertheim EH, Rallis S, Milgrom J, Paxton SJ. Depression and anxiety through pregnancy and the early postpartum: an examination of prospective relationships. J Affect Disord. 2009;113:303–308. doi: 10.1016/j.jad.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JM, Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998a;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. Withdrawal from 3α-OH-5α-pregnan-20-one using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor α4 subunit in association with increased anxiety. J Neurosci. 1998b;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of α4 and δ subunits of the γ-aminobutyric acidA receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- Taya K, Greenwald GS. In vivo and in vitro ovarian steroidogenesis in the pregnant rat. Biol Reprod. 1981;25:683–691. doi: 10.1095/biolreprod25.4.683. [DOI] [PubMed] [Google Scholar]
- Trapani G, Dazzi L, Pisu MG, Reho A, Seu E, Biggio G. A rapid method for obtaining finasteride, a 5alpha-reductase inhibitor, from commercial tablets. Brain Res Brain Res Protoc. 2002;9:130–134. doi: 10.1016/s1385-299x(02)00146-0. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human gamma-aminobutyric acidA receptors containing the alpha 4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ, Bonnert TP, McKernan RM, Farrar S, Le Bourdellès B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Thompson SA, Wafford KA. Molecular and functional diversity of the expanding GABAA receptor gene family. Ann N Y Acad Sci. 1999;868:645–653. doi: 10.1111/j.1749-6632.1999.tb11341.x. [DOI] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuluaga MJ, Agrati D, Pereira M, Uriarte N, Fernández-Guasti A, Ferreira A. Experimental anxiety in the black and white model in cycling, pregnant and lactating rats. Physiol Behav. 2005;84:279–286. doi: 10.1016/j.physbeh.2004.12.004. [DOI] [PubMed] [Google Scholar]