Abstract
One of the major evolutionary advances of human primates in the motor domain is their ability to use verbal instructions to guide their behavior. Despite this fundamental role of verbal information for our behavioral regulation, the functional and neural mechanisms underlying the transformation of verbal instructions into efficient behavior are still poorly understood. To gain deeper insights into the motor representation of verbal instructions, we investigated the neural circuits involved in overcoming interference from stimulus– response (S-R) mappings that are merely instructed and S-R mappings that are implemented. Implemented and instructed S-R mappings revealed a partly overlapping pattern of fronto-parietal brain activity when compared with a neutral condition. However, the direct contrast revealed a clear difference with stronger activation for the implemented condition in the ACC, bilateral inferior parietal cortex, the cerebellum and the precentral sulcus. This indicates that instructed S-R mappings share some properties with implemented S-R mappings but that they are lacking the motor-related properties of implemented mappings.
Keywords: verbal instruction, prefrontal cortex, conflict, fMRI, cognitive control, ACC, pre-SMA
Introduction
The ability to follow verbal instructions when learning new behavior distinguishes human primates from all other species. Humans are able to apply a verbally instructed behavior immediately and effortlessly, even when it is completely arbitrary and has never been implemented before. Due to this unique ability, humans do not have to acquire all behavior via effortful trial and error learning. Furthermore, understanding verbal instructions to guide behavior as well as using verbal self-instructions plays a crucial role in the ontogenetic development of behavioral self-regulation and behavioral control (Luria, 1980). Despite this fundamental role of verbal information for our behavioral regulation, surprisingly little is know of how verbal instructions are represented beyond the pure linguistic level.
One fundamental question regarding the representation of verbal instructions is how far verbal instructions “forge ahead” into the action system. Does the mere instruction of an action result in a representation that is comparable with a stimulus–response (S-R) link acquired via associative learning? Directly addressing this question experimentally has proven to be difficult, since an instructed S-R mapping can only be presented once before it becomes implemented. One solution to this problem is to investigate the influence of verbally instructed S-R mappings indirectly by presenting them on the irrelevant stimulus dimension. This indirect approach has recently been used in a number of behavioral studies (De Houwer et al., 2005; Wenke and Frensch, 2005; Cohen-Kdoshay and Meiran, 2007; Wenke et al., 2007; Waszak et al., 2008).
The present study seeks to investigate the functional level on which instructed S-R mappings are represented by exploring the neural mechanisms involved in overcoming interference from merely instructed compared with already implemented S-R mappings. There is an extensive literature on the neural basis of overcoming interference from implemented S-R associations. Functional brain imaging studies using interference paradigms revealed that areas in the frontal and parietal cortex are related to interference control and conflict detection (Bush et al., 1999; Cabeza and Nyberg, 2000; MacDonald et al., 2000; Brass and von Cramon, 2002, 2004). One brain region that has received much attention in this respect is the anterior cingulate cortex (ACC) that has been considered to be involved in detecting response conflict caused by habitual S-R associations (Botvinick et al., 2001; Ridderinkhof et al., 2004).
However, to our knowledge, no study has ever investigated which brain areas are involved in overcoming interference from a mere instructed S-R mapping. If instructed S-R mappings are represented on a sensori-motor level, interference from these mappings should result in activation that is very similar to the activation associated to overcoming interference from implemented S-R associations. However, if merely instructed S-R associations are represented on an abstract level, the pattern of brain activation should be very different for instructed and implemented S-R mappings.
Materials and Methods
Participants.
Twenty-one subjects (10 males, 11 females, mean age: 25.9, SD: 2.67) participated in the experiment. All had normal or corrected-to-normal vision. None of the subjects had a history of neurological, major medical, or psychiatric disorders. All were right handed, as assessed by the Edinburgh Inventory (Oldfield, 1971).
Design and stimuli.
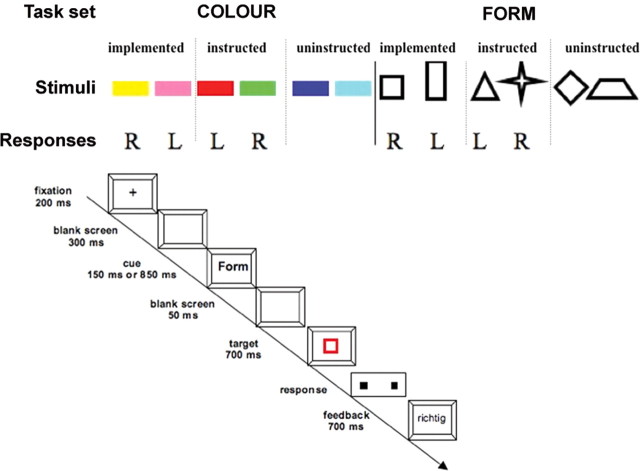
To investigate the brain areas involved in overcoming interference from instructed and implemented S-R mappings, we used a typical task switching paradigm (Meiran, 1996; Monsell, 2003) with a manipulation of stimulus valence (Meiran, 2000; Brass et al., 2003; Rubin and Meiran, 2005; Cohen-Kdoshay and Meiran, 2007; Waszak and Hommel, 2007). In this paradigm, which is based on a study by Waszak et al. (2008), participants had to carry out either a simple color or a form judgment on colored geometrical figures. Each experimental trial started with a task cue unequivocally indicating which task to perform next. After a cue-target interval (CTI) that was either long or short, the target stimulus appeared. There were six possible stimulus values for each dimension: six different colors (pink, red, green, yellow, dark blue, light blue) and six different shapes (triangle, rectangle, star, trapezoid, diamond, square). For four of the colors (yellow, pink, red, green) and four of the shapes (square, rectangle, triangle, star), a response mapping was verbally instructed before the experiment started (Fig. 1, top). The other two colors (light blue and dark blue) and shapes (diamond, trapezoid) received no task instruction. Two of the four instructed stimulus values could appear as targets on the relevant stimulus dimension (e.g., on the color dimension when color was the relevant task), as well as on the irrelevant stimulus dimension as distractors (e.g., on the form dimension when color was the relevant task). The other two instructed stimulus values for each task only appeared as distractors on the irrelevant stimulus dimension and, therefore, the corresponding instructed S-R mappings were never implemented (at least in the first two experimental blocks, see below). As a matter of course, the uninstructed stimulus values occurred on the irrelevant stimulus dimension only. Three types of experimental trials can be distinguished on the basis of the irrelevant stimulus dimension: trials in which the irrelevant stimulus dimension contained stimulus values that had previously appeared as a target on the relevant stimulus dimension (implemented condition); trials where the irrelevant stimulus dimension contained values that never appeared on the relevant stimulus dimension but which have been assigned to a motor response during the instructions (instructed condition); and finally trials where the irrelevant stimulus dimension contained values that never received a task instruction (uninstructed condition). After two experimental blocks (preimplementation phase), each instructed stimulus value (which, up to this point, occurred only as distractor on the irrelevant stimulus dimension), was presented six times as a target on the relevant stimulus dimension. Thus, in this implementation block participants actually responded to the instructed stimuli. Finally, another block identical to the first two blocks was presented (postimplementation phase). The aim of including the implementation block was to compare activations in the preimplementation phase with activation in the postimplementation phase. It allowed us to test whether implementing the task instruction for the instructed stimulus values a few times yields effects similar to the continued implementation of the instructions.
Figure 1.
Top, Stimulus values for the color and the form task. Implemented stimulus values appeared on the relevant and irrelevant dimension. Instructed stimulus values appeared only on the irrelevant dimension in block I and block II. Uninstructed stimulus values only appeared on the irrelevant stimulus dimension. Bottom, Exact stimulus timing of the experimental trial.
Each trial started with a variable jitter interval of 0, 500, 1000 or 1500 ms (Fig. 1, bottom). Then a fixation cross was presented in the middle of the screen for 200 ms which was followed by a blank screen for 300 ms. On each trial, a cue indicated which task to perform next. The cue consisted of the German word “Farbe” (color) or “Form” (form). The cue was presented in the middle of the screen for 150 ms. The interval between the cue and the target (CTI) was either long (850 ms) or short (50 ms). After the CTI, the target was presented for 700 ms. After a blank screen of 1800 ms, the feedback was presented for 300 ms. It consisted of the German words “Falsch” (wrong), “Richtig” (correct) and “Zu langsam” (too slow). Both the sequence of the tasks and the CTI were pseudo-randomized leading to task alternations and task repetitions. However, since we were not interested in switch-related effects, we did not split our data into switch and repeat trials.
In the first experimental block, 108 trials were presented in a pseudo-randomized order: 12 null events, 32 implemented trials, 32 instructed trials and 32 uninstructed trials. Block II was identical to block I. In the implementation block, each instructed stimulus value that, up to this point, did not appear on the relevant stimulus dimension, appeared six times on the relevant stimulus dimension (6 × 4 trials = 24 trials). Furthermore, each trial type (implemented, instructed, uninstructed) was presented 16 times, resulting in 72 trials. The postimplementation block was identical to block I and II.
Functional MRI methods.
The experiment was performed on a 3T scanner (Siemens, Trio). Twenty axial slices (19.2 cm FOV, 64 × 64 matrix, 4 mm thickness, 1 mm spacing), parallel to the anterior commissure–posterior commissure plane, and covering the whole brain, were acquired using a single-shot, gradient-recalled echoplanar imaging (EPI) sequence (TR 2000 ms, TE 30 ms, 90 flip angle). Before the functional runs, 20 corresponding anatomical modified driven equilibrium Fourier transformation slices and 20 EPI-T1 slices were acquired. Stimuli were presented using a head-mounted display with a resolution of 1024 × 768 and a refresh rate of 60 Hz.
The functional MRI (fMRI) data was analyzed using the software package LIPSIA (Leipzig Image Processing and Statistical Interference Algorithms) (Lohmann et al., 2001). First, the functional data was motion corrected with a three-dimensional motion correction using 6 degrees of freedom (3 translational and 3 rotational). To correct for the temporal offset between the slices acquired in one scan, a cubic spline interpolation algorithm was applied. The functional slices were then coregistered with the high-resolution full-brain scan by a rigid, affine linear transformation using 3 translational and 3 rotational parameters. Subsequently, these rotational and translational parameters were transformed to standard size (Talairach and Tournoux, 1988) by linear scaling. The transformation parameters obtained from this step were then applied to the functional data so that the functional slices were also registered into the stereotactic space by means of a trilinear interpolation. Voxel size was interpolated during the coregistration to a spatial resolution of 3 × 3 × 3 mm. A high-pass filter was applied (cutoff frequency: 1/80 Hz), and the data were filtered with a spatial Gaussian filter with 5.65 mm full-width at half-maximum (FWHM). The statistical evaluation was based on a least-squares estimation using the general linear model for serially autocorrelated observations (Friston et al., 1995; Worsley and Friston, 1995). An event-related design was used; that is, the hemodynamic response function was modeled with respect to the experimental conditions for each stimulus event. The trigger for the event-related analysis was the presentation time of the cue. The design matrix was generated using a synthetic hemodynamic response function (gamma function) (Glover, 1999) and a response delay of 6 s. The model equation, including the observation data, the design matrix, and the error term, was convolved with a Gaussian kernel of dispersion of 4 s FWHM to deal with the temporal autocorrelation (“precoloring”) (Worsley and Friston, 1995). Afterward, contrast images, that is, estimates of the raw score differences between specified conditions, were generated for each subject. The single-subject contrast images from the first-level were entered into a second-level random effects analysis, using a one-sample t test across the contrast images of all subjects to indicate whether observed differences between conditions were significantly different from zero. Subsequently, t values were transformed into Z scores. The tested model combined the preimplementation phase (block 1 and 2) and the CTIs (short, long), to reduce the number of relevant regressors. Included in the model were the crossed factors Instruction (implemented, instructed and uninstructed conditions) and Block (preimplementation and postimplementation blocks), as well as a regressor coding error trials.
To protect against false-positive activations, we used a double-threshold approach, that is, combining a voxel-based threshold with a minimum cluster size (Forman et al., 1995). This nonarbitrary voxel cluster size was determined by using the program AlphaSim (afni.nimh.nih.gov/afni/doc/manual/AlphaSim) (Ward, 2000). The multiple comparison correction used a combination of single voxel probability thresholding on the one hand, and cluster size and cluster z-value thresholding, however. The probability threshold was set to Z >2.4 (p < 0.008, uncorrected), the cluster size and cluster z-value threshold was computed by the program using Monte Carlo simulations. The program contains thresholds that specify features that a cluster must have to qualify as being significant: the first feature is cluster size (i.e., number of voxels in a cluster). The second feature is the maximum z-value in a cluster. Thus, a cluster may qualify as being significant if it is either large enough, or if it has a large maximum z-value, or both. On the basis of a Monte Carlo simulation (1000 iterations), with our brain volume and an individual voxel height threshold of Z >2.4, areas were determined which were significant at an overall imagewise false-positive rate of 5%. Activations exceeding this cluster-based threshold are, therefore, considered to be activated at an experiment-wise threshold of p < 0.05, corrected for multiple comparisons. Therefore, only activations exceeding this threshold are reported.
Additionally, a signal strength analysis was performed by taking the presupplementary motor area (pre-SMA) coordinates from the contrast of the instructed condition and the uninstructed condition (see below). Then we averaged the β values for the most activated voxel and the adjacent voxels.
Results
Performance data
For the performance analysis, we performed a three-factorial ANOVA with the factors Experimental block (block I, block II, postimplementation block), Instruction (implemented, instructed, uninstructed) and CTI (short, long) (Fig. 2). The ANOVA of reaction times yielded a main effect of Instruction (F(2,40) = 5.45, p < 0.01) with the implemented (729 ms) and instructed condition (728 ms) being slower than the uninstructed condition (712 ms). It also showed a main effect of CTI (F(1,20) = 355.15, p < 0.001). As in almost all task-cuing experiments, the long CTI (636 ms) yielded faster reaction times than the short CTI (810 ms). Furthermore, the two-way interaction of Block × Instruction was significant (F(4,80) = 3.15, p < 0.05). While the reaction times for the three instruction conditions were statistically not different in block I, the implemented condition was statistically slower than the uninstructed condition in block II (t(20) = 1.85, p < 0.05, one-tailed). In the postimplementation block, the implemented (t(20) = 3.2, p < 0.01) and instructed condition (t(20) = 2.2, p < 0.05) were statistically slower than the uninstructed condition. Finally, the Block × CTI interaction was significant (F(2,40) = 11.15, p < 0.001).
Figure 2.
Mean reaction time and percentage errors as a function of condition (implemented, instructed, uninstructed) and test block (block I, block II, postimplementation block).
The error analysis also revealed a main effect of Instruction (F(2,40) = 14.52, p < 0.001) and CTI (F(1,20) = 24.56, p < 0.001). The implemented condition showed a statistically higher error rate than the uninstructed (t(20) = 4.5, p < 0.001) and the instructed condition (t(20) = 4.1, p < 0.01). Furthermore, the error rate for the instructed condition was higher than for the uninstructed condition (t(20) = 1.8, p < 0.05, one-tailed. Crucially, in test block II, the error rate for the instructed condition was significantly higher than the uninstructed condition (t(20) = 2.1, p < 0.05, one tailed) (Fig. 2). In addition, the instruction × CTI interaction was significant (F(2,40) = 5.5, p < 0.001).
Finally, we calculated the error rate for instructed trials in the implementation block. The error rate was <5% suggesting that participants memorized the instructed S-R mappings over the first two blocks.
fMRI analysis
To reduce the number of regressors in the fMRI analysis, and because we had no specific hypothesis regarding the influence of CTI on brain activation, we pooled trials across CTIs. Furthermore, we aggregated the two blocks before the implementation block to a preimplementation phase.
First, we were interested in the question of whether overcoming interference from instructed and implemented S-R mappings yield different or similar brain activation. To address this question, we contrasted the implemented and instructed condition, respectively, with the uninstructed condition. This first analysis was restricted to preimplementation phase, in which the instructed trials had not been implemented yet. The contrast of the implemented and uninstructed condition revealed activation in an extended fronto-parietal network (Table 1). In the fronto-lateral cortex, activation was found in the middle frontal gyrus (MFG) and the dorsal premotor cortex. In the fronto-median cortex, the SMA/pre-SMA (x: −8, y: 1, z: 51) and the ACC (x: 1, y: 7, z: 33) (Fig. 3a, Table 1) was found to be activated. Parietal activations were located in the postcentral gyrus and the inferior parietal lobe. The contrast of the instructed and uninstructed condition also revealed activation in frontal and parietal areas including MFG and a fronto-median activation in the pre-SMA (x: −2, y: −2, z: 51). However, no activation was found in the ACC and the IPL (Fig. 3b, Table 2). The direct contrast of the implemented versus the instructed condition yielded activation in frontal and parietal brain areas including the lateral-frontal cortex, the inferior parietal cortex, and, most importantly, in the ACC (x: 10, y: 4, z: 36) (Fig. 3c, Table 3). These data suggest, thus, that the functional mechanisms involved in overcoming interference from implemented S-R mappings activates a more extended network of brain areas than overcoming interference from instructed S-R mappings. In particular, the ACC seems to be more strongly involved in overcoming interference from implemented compared with instructed S-R associations.
Table 1.
Brain activation for the contrast of implemented and uninstructed trials in the preimplementation phase
| Location | Brodmann area | z-max | Talairach coordinates (x, y, z) | Laterality |
|---|---|---|---|---|
| Pre-SMA (l.m.) | (BA 6) | 3.98 | −8, 1, 51 | Left |
| ACC | (BA 24/32) | 4.06 | 1, 7, 33 | Right |
| MFG | (BA 9) | 3.80 | −23, 37, 21 | Left |
| MFG | (BA 9) | 3.34 | 28, 46, 18 | Right |
| Pre-CS | (BA 6) | 3.56 | −53, 7, 6 | Left |
| Postcentral gyrus (l.m.) | (BA 2) | 3.84 | −38, −23, 45 | Left |
| iPL | (BA 40) | 4.20 | 46, −29, 21 | Right |
| STG | (BA 22) | 4.23 | −44, −32, 15 | Left |
| Pons | 3.81 | 10, −17, −24 | Right |
pre-CS, Precentral sulcus; iPL, inferior parietal lobe; l.m., local maxima; STG, superior temporal gyrus.
Figure 3.
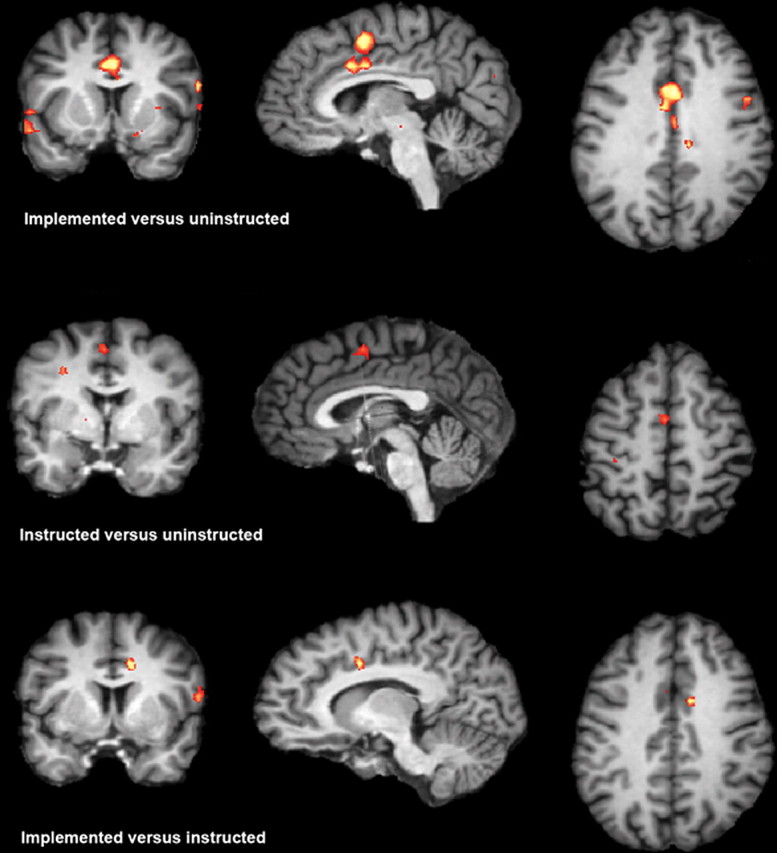
Top, Brain activation for the contrast of implemented and uninstructed trials in the preimplementation phase. Middle, Brain activation for the contrast of instructed versus uninstructed trials in the preimplementation phase. Bottom, Brain activation for the contrast of implemented versus instructed in preimplementation phase. The significance threshold is Z = 3.09 in all three contrasts.
Table 2.
Brain activation for the contrast of instructed and uninstructed trials in the preimplementation phase
| Location | Brodmann area | z-max | Talairach coordinates (x, y, z) | Laterality |
|---|---|---|---|---|
| Pre-SMA | (BA 6) | 3.44 | −2, −2, 51 | Left |
| MFG | (BA 10) | 4.63 | 31, 40, 24 | Right |
| Pre-CS | (BA 6) | 4.67 | −53, 10, 3 | Left |
| Postcentral gyrus | (BA 2) | 3.53 | −38, −23, 42 | Left |
| PC | (BA 7) | 3.68 | 13, −65, 45 | Right |
| Rectal gyrus | (BA 11) | 3.54 | 13, 16, −21 | Right |
| NC | 3.60 | 16, −8, 18 | Right | |
| Pons | 4.01 | 7, −17, −24 | Right |
PC, Precuneus; iFG, inferior frontal gyrus; iPL, inferior parietal lobe; NC, nucleus caudatus; pre-CS, precentral sulcus.
Table 3.
Brain activation for the contrast of implemented versus instructed trials in the preimplementation phase
| Location | Brodmann area | z-max | Talairach coordinates (x, y, z) | Laterality |
|---|---|---|---|---|
| ACC | (BA 24/32) | 3.98 | 10, 4, 36 | Right |
| Pre-CS | (BA 6) | 4.03 | 55, 7, 15 | Right |
| iPL | (BA 40) | 4.41 | 49, −29, 24 | Right |
| iPL | (BA 40) | 3.15 | −53, −23, 21 | Left |
| Cerebellum | 3.68 | 2, −68, 3 | Right | |
| Pons | 3.60 | 34, 13, −27 | Left |
iPL, Inferior parietal lobe; pre-CS, precentral sulcus.
To test how the implementation block affected brain activation, we computed the interaction of Instruction (implemented vs instructed) and Block (preimplementation vs postimplementation). This analysis yielded again activation in the ACC (x: 13, y: 3, z: 33, Z-max: 3.4, p < 0.001, uncorrected), corroborating the notion that the ACC is sensitive to the implementation of S-R mappings.
Furthermore, we were interested in the question as to whether the performance data correlated with brain activation in the ACC and the pre-SMA. When correlating the activation difference in the ACC (x: 1, y: 7, z: 32) for the implemented and instructed condition in the preimplementation phase with the reaction time difference of implemented and instructed trials, we found a significant correlation across participants (r = 0.51, p < 0.05). The activation difference in the pre-SMA (x: −8, y: 1, z: 51) was not significantly correlated with the reaction time difference (r = −0.04, p = 0.88). This correlation suggests that the performance difference between the implemented and instructed condition is indeed related to activation in the ACC.
Another crucial variable regarding the interference from implemented S-R mappings is the congruency of the responses. In some trials, both tasks require the same response (congruent condition), while in other trials, the relevant dimension indicates a response that is incongruent to the irrelevant dimension (incongruent response). We performed a whole-brain analysis in which we wanted to test whether response congruency has an influence on brain activation. The whole-brain analysis showed no significant activation difference for incongruent compared with congruent trials. Furthermore, congruency did not interact with Instruction or Block.
Discussion
To investigate the functional level of verbally instructed S-R mappings, we assessed brain activation involved in overcoming interference from instructed and implemented S-R associations.
Compared with the uninstructed condition, implemented and instructed mappings revealed an overlapping pattern of brain activation. Both conditions showed activation in the pre-SMA, the MFG, the postcentral gyrus, and the inferior precentral sulcus, suggesting that an instructed S-R mapping causes interference when presented on the irrelevant stimulus dimension, similar to implemented S-R mappings. However, stronger activation for the implemented than for the instructed condition was found in the ACC, bilateral inferior parietal cortex, the cerebellum and the precentral sulcus, indicating that instructed S-R mappings lack the implementation component that results in response-related interference. Moreover, the comparison of instruction conditions before and after the implementation block showed that the activation difference in the ACC between implemented and instructed mappings was diminished after the instructed mappings had been executed a few times. Finally, a correlation analysis of the reaction time difference between implemented and instructed trials in the preimplementation phase revealed a high correlation with activation in the ACC, suggesting that the ACC activation is linked to response related interference processes.
Overcoming interference from implemented S-R mappings
One central finding of the present study is the sensitivity of the ACC to interference from implemented S-R mappings. There is a debate on the role of the ACC in cognitive control (Ridderinkhof et al., 2004; Rushworth et al., 2004). The most dominant account assumes that the ACC is involved in conflict monitoring (Carter et al., 1998; Botvinick et al., 2004; Liston et al., 2006). Furthermore, it has been assumed that ACC is involved in error processing (Menon et al., 2001; Swick and Turken, 2002) or in relating actions to their consequences (Rushworth et al., 2004, 2005). Our findings are highly compatible with the conflict monitoring account. We assume that applying an S-R mapping compiles a direct association between stimulus and response (Logan, 1988). When presented as a distractor, the stimulus automatically activates the associated response. This, in turn, causes response conflict summoning activation in the ACC (Botvinick et al., 2001).
However, notice that, usually, experiments on conflict monitoring assess interference effects of implemented arbitrary S-R mappings or highly automatized mappings (e.g., Stroop interference) but not of merely instructed S-R mappings. The present study demonstrates for the first time that ACC is relatively insensitive to interference from instructed S-R mappings and that ACC activity depends on the S-R mappings being applied.
One might argue that our results are not surprising given that implemented S-R mappings are extensively practiced over the whole experiment. However, the data from the implementation block suggest that extended practice is not required to induce conflict-related ACC activation. Six implementation trials were sufficient to attenuate the activation difference in the ACC. The correlation analysis outlined above further supports the conflict interpretation: the automatic retrieval of implemented S-R mappings results in higher response conflict and, as a consequence, stronger activation of the ACC.
The fact that we did not find an effect of response congruency on ACC activation appears to be at odds with the idea that retrieval of direct S-R links are the reason for the activation in ACC we observed. However, notice that even congruent responses that are kinematically identical may be coded differently, depending on the task they serve to realize. This idea is consistent with recent behavioral findings. Koch and Allport (2006) and Waszak and Hommel (2007) compared effects of the retrieval of S-R associations across tasks separately for stimuli that map to the same response in both tasks (congruent) or to different responses (incongruent). They found that retrieval effects were not (much) larger for incongruent than for congruent stimuli. Notice also that, in the present study, we did not use highly over-learned stimulus–response associations. Thus, it is possible that context-specific S-R encoding may be predominant during early stages of compilation of S-R traces, whereas these traces may become more context-independent as the number of encoded traces increases.
To summarize, the most crucial point is that the activation pattern in the ACC is different for the instructed and the implemented condition indicating a functional dissociation of both types of S-R associations. This dissociation gains further support by the observation that in addition to the ACC we found activation in the premotor cortex, the inferior parietal cortex and the cerebellum, all areas that are tightly linked to motor execution. In particular, this motor-related activity might suggest that the activation we observe is not only related to overcoming interference from the irrelevant stimulus dimension but rather might also reflect to some degree activation of the irrelevant task and/or response sets.
Overcoming interference from instructed S-R mappings
Our second crucial finding is that verbally instructed S-R mappings cause interference even if they have never been executed. This finding is consistent with recent behavioral work (De Houwer et al., 2005; Wenke and Frensch, 2005; Cohen-Kdoshay and Meiran, 2007; Wenke et al., 2007; Waszak et al., 2008). On the neural level this interference effect is reflected in the partial overlap of brain circuits when overcoming interference from instructed and implemented trials. In particular, an activation overlap was observed in the middle frontal gyrus, the precentral sulcus, somato-sensory cortex and the pre-SMA. The extent of this overlap is surprising given the fact that the implemented and instructed conditions differ substantially regarding practice. Moreover, since uninstructed stimulus values occur as often on the irrelevant stimulus dimension as instructed stimulus values, this effect cannot be reduced to practice or mere exposure.
A dissociation in the fronto-median wall: ACC versus pre-SMA
As outlined above, overcoming interference from implemented S-R mappings activated the ACC and the pre-SMA, overcoming interference from instructed S-R mappings activated the pre-SMA but not the ACC. The pre-SMA can be distinguished from the ACC both on a neuroanatomical (Picard and Strick, 1996) and on a functional level (Ullsperger and von Cramon, 2001). The dissociation of pre-SMA activity from activity in the ACC we observed fits nicely to the fact that, in contrast to the ACC, the pre-SMA seems to be involved in more abstract cognitive control processes that are not directly related to the response. The pre-SMA has been shown to be involved in task-set related control (Brass and von Cramon, 2002; Rushworth et al., 2002; Crone et al., 2006). Furthermore, Milham and Banich (2005) associated an anterior part of the ACC with response conflict and a more posterior part of the ACC which overlaps with the pre-SMA with a more general form of conflict. In accordance with this proposal, Nachev et al. (2007) argued that the pre-SMA is involved in resolution of conflict between different condition-action associations rather than stimulus–response associations.
Another interpretation of the activation pattern in the instructed condition is that it reflects subvocal rehearsal of the instructed S-R mappings. In fact the supplementary motor speech area (Alario et al., 2006) has been proposed to be closely located to the pre-SMA activation we report here. Nevertheless, we consider this explanation to be unlikely. First, such a strategy would be extremely maladaptive given that merely instructed mappings appeared on the irrelevant stimulus dimension on only one third of the trials. Second, behavioral studies that blocked subvocal rehearsal found the automatic effect of merely instructed mappings to be completely independent of such manipulations (Cohen-Kdoshay and Meiran, 2007, Wenke et al., 2007). Hence, we propose that representations are compiled on a subverbal level.
A three-stage model of implementing verbally instructed behavior
We propose a three-stage model of implementing verbally instructed behavior. The linguistic stage consists of an explicit verbal representation of the relevant S-R rules. Recent research shows that this linguistic representation of behavioral rules is very crucial for learning new behavior (Luria, 1980; Kray et al., 2006) as well as for the control of behavior (Goschke, 2000; Miyake et al., 2004). However, this representation alone is not sufficient for behavioral implementation as witnessed by the dissociation of knowing and doing in prefrontal patients (Teuber, 1964). Our findings suggest that healthy adults can easily translate the linguistic information to a sublinguistic level. This sublinguistic representation seems to be a transition stage between a mere verbal representation and a direct S-R link. A sublinguistic representation differs from a verbal representation in that it does not involve explicit, verbally mediated rule translation. Instead, it is implicit and, thus, can automatically influence behavior. However, the sublinguistic stage is also distinguishable from the sensori-motor stage. It is only on this third stage that direct S-R links are formed. Our data suggest that building up direct S-R links is a very fast process. As shown by the effect of the implementation block of our experiment on activation in the ACC, executing an instructed mapping a few times seems sufficient for establishing such a link.
Evidently, further research is needed to specify the content of the sublinguistic stage. One possibility is that verbalizing specific S-R relations might trigger mental imagery or simulation processes that result in mental S-R images.
Footnotes
This work was supported by the Special Research Fund (Bijzonder Onderzoeksfond) of Ghent University.
References
- Alario FX, Chainay H, Lehericy S, Cohen L. The role of the supplementary motor area (SMA) in word production. Brain research. 2006;1076:129–143. doi: 10.1016/j.brainres.2005.11.104. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. The role of the frontal cortex in task preparation. Cereb Cortex. 2002;12:908–914. doi: 10.1093/cercor/12.9.908. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. Decomposing components of task preparation with functional magnetic resonance imaging. J Cogn Neurosci. 2004;16:609–620. doi: 10.1162/089892904323057335. [DOI] [PubMed] [Google Scholar]
- Brass M, Ruge H, Meiran N, Rubin O, Koch I, Zysset S, Prinz W, von Cramon DY. When the same response has different meanings: recoding the response meaning in the lateral prefrontal cortex. Neuroimage. 2003;20:1026–1031. doi: 10.1016/S1053-8119(03)00357-4. [DOI] [PubMed] [Google Scholar]
- Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen BR, Biederman J. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biol Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Cohen-Kdoshay O, Meiran N. The representation of instructions in working memory leads to autonomous response activation: evidence from the first trials in the flanker paradigm. Q J Exp Psychol (Colchester) 2007;60:1140–1154. doi: 10.1080/17470210600896674. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cereb Cortex. 2006;16:475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- De Houwer J, Beckers T, Vandorpe S, Custers R. Further evidence for the role of mode-independent short-term associations in spatial Simon effects. Percept Psychophys. 2005;67:659–666. doi: 10.3758/bf03193522. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage. 1995;2:166–172. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- Glover GH. Deconvolution of impulse response in event-related BOLD fMRI. Neuroimage. 1999;9:416–429. doi: 10.1006/nimg.1998.0419. [DOI] [PubMed] [Google Scholar]
- Goschke T. Intentional reconfiguration and voluntary persistence in task set switching. In: Monsell S, Driver J, editors. Control of cognitive processes: attention and performance XVIII. Cambridge, MA: MIT; 2000. pp. 331–355. [Google Scholar]
- Koch I, Allport A. Cue-based preparation and stimulus-based priming of tasks in task switching. Mem Cognit. 2006;34:433–444. doi: 10.3758/bf03193420. [DOI] [PubMed] [Google Scholar]
- Kray J, Eenshuistra R, Kerstner H, Weidema M, Hommel B. Language and action control: the acquisition of action goals in early childhood. Psychol Sci. 2006;17:737–741. doi: 10.1111/j.1467-9280.2006.01774.x. [DOI] [PubMed] [Google Scholar]
- Liston C, Matalon S, Hare TA, Davidson MC, Casey BJ. Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron. 2006;50:643–653. doi: 10.1016/j.neuron.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Logan GD. Toward an instance theory of automatization. Psychol Rev. 1988;95:492–527. [Google Scholar]
- Lohmann G, Muller K, Bosch V, Mentzel H, Hessler S, Chen L, Zysset S, von Cramon DY. LIPSIA—a new software system for the evaluation of functional magnetic resonance images of the human brain. Comput Med Imaging Graph. 2001;25:449–457. doi: 10.1016/s0895-6111(01)00008-8. [DOI] [PubMed] [Google Scholar]
- Luria AR, editor. Higher order functions in man. New York: Consultants Bureau; 1980. [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Meiran N. Reconfiguration of processing mode prior to task performance. J Exp Psychol Learn Mem Cogn. 1996;22:1423–1442. [Google Scholar]
- Meiran N. Reconfiguration of stimulus task sets and response task sets during task switching. In: Monsell S, Driver J, editors. Control of cognitive processes: attention and performance XVIII. Cambridge, MA: MIT; 2000. pp. 377–399. [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Banich MT. Anterior cingulate cortex: an fMRI analysis of conflict specificity and functional differentiation. Hum Brain Mapp. 2005;25:328–335. doi: 10.1002/hbm.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Emerson MJ, Padilla F, Ahn JC. Inner speech as a retrieval aid for task goals: the effects of cue type and articulatory suppression in the random task cuing paradigm. Acta psychologica. 2004;115:123–142. doi: 10.1016/j.actpsy.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends Cogn Sci. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Nachev P, Wydell H, O'Neill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. Neuroimage 36 Suppl. 2007;2:T155–T163. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rubin O, Meiran N. On the origins of the task mixing cost in the cuing task-switching paradigm. J Exp Psychol Learn Mem Cogn. 2005;31:1477–1491. doi: 10.1037/0278-7393.31.6.1477. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol. 2002;87:2577–2592. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Kennerley SW, Walton ME. Cognitive neuroscience: resolving conflict in and over the medial frontal cortex. Curr Biol. 2005;15:R54–R56. doi: 10.1016/j.cub.2004.12.054. [DOI] [PubMed] [Google Scholar]
- Swick D, Turken AU. Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proc Natl Acad Sci U S A. 2002;99:16354–16359. doi: 10.1073/pnas.252521499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach P, Tournoux J, editors. A stereotactic coplanar atlas of the human brain. Stuttgart, Germany: Thieme; 1988. [Google Scholar]
- Teuber HL. The riddle of frontal lobe function in man. In: Warren JM, Akert K, editors. The frontal granular cortex and behaviour. New York: McGraw-Hill; 1964. pp. 410–440. [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. 2000. Retrieved August 23, 2007, from http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim.
- Waszak F, Hommel B. The costs and benefits of cross-task priming. Mem Cognit. 2007;35:1175–1186. doi: 10.3758/bf03193487. [DOI] [PubMed] [Google Scholar]
- Waszak F, Wenke D, Brass M. Cross-talk of instructed and applied arbitrary visuomotor mappings. Acta Psychologica. 2008;127:30–35. doi: 10.1016/j.actpsy.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Wenke D, Frensch PA. The influence of task instruction on action coding: constraint setting or direct coding? J Exp Psychol Hum Percept Perform. 2005;31:803–819. doi: 10.1037/0096-1523.31.4.803. [DOI] [PubMed] [Google Scholar]
- Wenke D, Gaschler R, Nattkemper D. Instruction-induced feature binding. Psychol Res. 2007;71:92–106. doi: 10.1007/s00426-005-0038-y. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited—again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]