Abstract
Introduction:
Voluntary medical male circumcision (VMMC) among men who have sex with men (MSM) may protect against HIV acquisition. We conducted a series of analyses to assess if expanded VMMC might reduce HIV incidence among MSM effectively and economically.
Methods:
We used a deterministic compartmental model to project new HIV cases (2016–2026) under annual VMMC coverage rates (λ) ranging from 0.0001 to 0.15. The “number needed to avert (NNA)” that defined as the cumulative number of VMMC conducted up to that year divided by the cumulative number of HIV cases averted in that specific year.
Results:
Compared with the baseline circumcision coverage rate, we projected that new HIV cases would be reduced with increasing coverage. By 2026 (last year simulated), the model generated the lowest ratio (15.76) when the annual circumcision rate was the most optimistic (λ=0.15). The breakeven point was observed at the year of 2019 with the annual VMMC coverage rate of 0.001. The total cost saved by averting HIV cases would range from 2.5 to 811 million by the end of 2026 with different hypothetical coverage rates.
Discussion:
Our model suggests that acceleration in VMMC implementation among MSM could help stem the HIV/AIDS epidemic.
Keywords: Male Circumcision, HIV, Men who have Sex with Men, China, “number needed to avert” analysis
Introduction
Three randomized controlled trials (RCT) have shown voluntary medical male circumcision (VMMC) to be effective in reducing HIV acquisition among heterosexual men by over 50% (1–3). However, the role of VMMC in preventing HIV infection among men who have sex with men (MSM) is controversial and circumstantial (4–9). In a meta-analysis using published data through August 2016 assessing the efficacy of VMMC among MSM, 30 of 33 studies reported non-significant associations between VMMC and HIV although the meta-analysis suggested an overall weak, but significant protective efficacy (10). Two studies from India suggested that VMMC might reduce HIV risk significantly among MSM, even among males who primarily practiced receptive sex (11, 12). Among MSM in Beijing, a remarkably strong protection effect was noted, especially among MSM who predominately practice insertive sex (aOR=0.15, 95% CI=0.04, 0.65) vs. MSM who were uncircumcised and practicing receptive and/or versatile (both receptive and insertive) sexual positioning. These Asian studies found the strongest evidence to date that VMMC might be a useful biomedical tool for HIV risk reduction among MSM, especially for MSM with comparatively lower risk profiles, such as MSM practicing insertive anal intercourse in Asia (10). Circumcision is rare in Asia except among Muslims, and circumcised men have undergone the procedure in childhood or adolescence, typically, due to pre-existing medical conditions such as phimosis (13). Hence, the observational studies comparing circumcised and uncircumcised men are not likely to be biased, given the lack of association between phimosis and later sexual preference.
The willingness of VMMC uptake among Chinese males ranged from less than one-third among male miners in Guangxi (14), to more than two-thirds among general Chinese male population who received an intervention for circumcision uptake promotion (15, 16). The willingness for receiving VMMC among MSM was consistent with that among general population in China, among whom two-thirds of them expressed their willingness to accept the VMMC as a strategy to prevent HIV and other sexually transmitted infections (15, 17).Well-designed interventions may facilitate increased willingness of accepting VMMC among MSM in China (18). For instance, an intervention successfully increased the willingness to be circumcised from 8.1% to 35.1% among a group of bisexual MSM in the Southern China (18). Therefore, promoting VMMC may be beneficial for HIV prevention and is also feasible among MSM, particularly among some subgroups including youths and those who practice insertive anal sex.
To assess long-term population-level impacts of expanding VMMC among MSM, we should consider the balance between investment (e.g., costs for VMMC performed) and impact (e.g., numbers of HIV cases averted). The United States Agency for International Development (USAID) and the Joint United Nations Programme on HIV/AIDS (UNAIDS) have developed a modeling tool called “Decision Makers’ Program Planning Tool” (DMPPT) to facilitate decision makers’ estimates of the epidemiological impact and cost of alternative programmatic options for scaling up VMMC (19–21). However, these model-based studies only focused on heterosexual sexual men in specific settings (e.g., African nations) with generalized HIV epidemics (19, 21–23).
The DMPPT model assumes the HIV incidence rate to remain constant during the prediction period, resulting in potentially over- or underestimating the impact of VMMC (20). To date, no analogous modeling studies have specifically addressed the potential utility of circumcision programs targeting MSM. Given the lower prevalence of VMMC among Chinese MSM (5, 10, 24), it is conceivable that VMMC would have a greater impact in China than in areas of high circumcision prevalence (e.g., the United States). In order to assess context-specific impacts of VMMC among MSM in China(25), we conducted the current analysis to assess the extent to which expanding VMMC programs might reduce HIV incidence among MSM effectively and economically.
Methods:
Model Building Procedure
We employed a deterministic compartmental model to project the HIV epidemic for the period between 2016 and 2026 among MSM in Beijing. Susceptible men are HIV-uninfected, in contrast to HIV-infected men. The fixed estimate for the protective efficacy of VMMC is derived from the meta-analysis (10) and is set conservatively at 7%. It is plausible that efficacy for HIV prevention in MSM is much higher than this (26). The study population fell into four mutually exclusive subgroups: (1) circumcised susceptible MSM (Sc+); (2) circumcised infected MSM (Ic+); (3) uncircumcised susceptible MSM (Sc-); and (4) uncircumcised infected MSM (Ic-). With the HIV transmission rate of ρ-, uncircumcised susceptible MSM (Sc-) became uncircumcised infected MSM (Ic-). Similarly, with the HIV transmission rate of ρ+, circumcised susceptible MSM (Sc+) became circumcised infected MSM (Ic+). In this dynamic model, seronegative MSM entered the susceptible pool at a rate of σ. The rate of entering the circumcised susceptible compartment (Sc+) was σ+, while σ- represented the rate of entering the uncircumcised susceptible compartment (Sc-). Meanwhile, MSM were considered to have left the susceptible pool by aging beyond 65 years, emigrating out of Beijing, or dying of any causes. We used the parameter τ to indicate the rate of leaving the model among susceptible MSM, with τ+ representing the rate of leaving the circumcised susceptible pool (Sc+), and τ- representing the rate of leaving the uncircumcised susceptible pool (Sc-). In this model, susceptible MSM would change from being uncircumcised to being circumcised at a rate of λ.
As very few MSM are expected to be circumcised after HIV infection (27), we did not consider the number of infected MSM who transited from being uncircumcised to being circumcised. Both circumcised and uncircumcised MSM would leave the infectious compartments for one reason: 1) death due to HIV infection, or 2) having undetectable viral loads due to either being an immunologically elite controller (e.g., the small group of HIV-infected patients who can maintain high CD4+ cell counts and low viral loads without progressing immunologically towards AIDS over years in absence of ART (28) or an effective ART regime at a rate of for uncircumcised and for circumcised MSM (Figure 1). When possible, demographic and transmission parameters were estimated from the published literature (27, 29, 30) or from available unpublished data sources (personal communication); a few parameters were calculated within the model itself (Supplementary Table 1).
Figure 1.
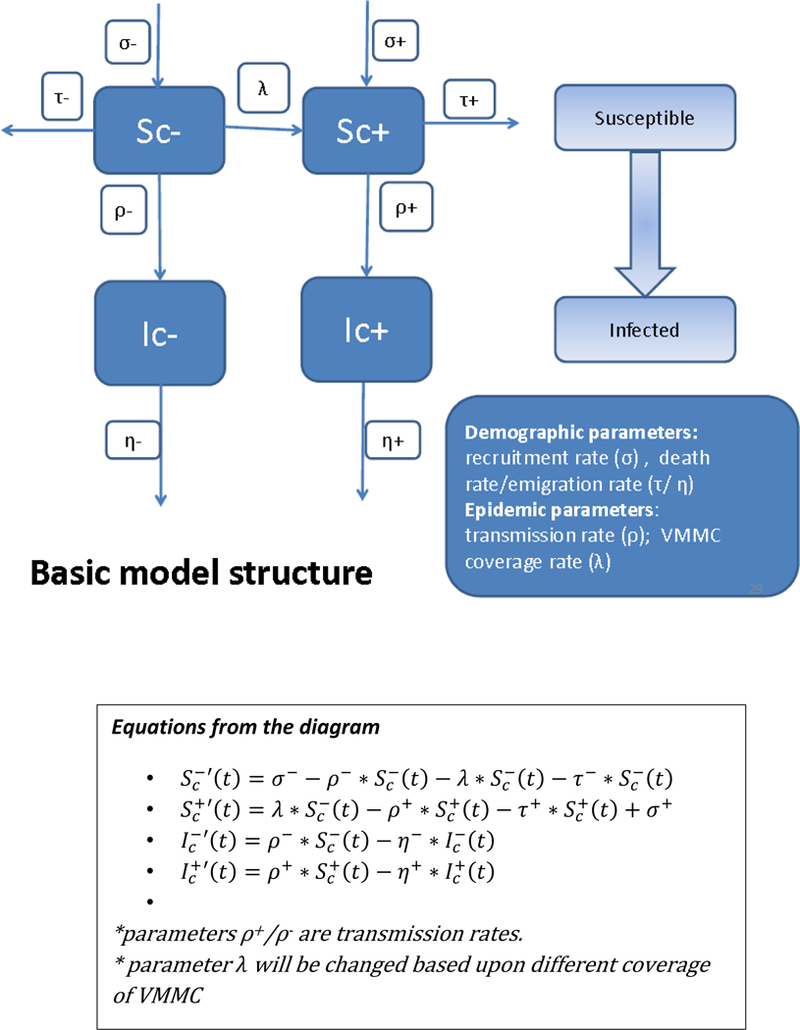
Schematic diagram for the HIV transmission model and voluntary medical male circumcision among men who have sex with men
In order to refine the model to provide the most valid predictions, we calibrated the model parameters to enable the model to predict historical surveillance data. When the prediction model overlapped with the surveillance data, using plausible and defensible parameter estimations, we considered the generated model to serve well for predictions forward in time. Additionally, through 50 repeated simulations, we generated the predicted transmission rates (e.g., ρ- and ρ+) for 2016–2025 among both uncircumcised and circumcised MSM. An assumption that the protection of VMMC is similar for MSM across all ages has been made during the model building. Although the life expectation protected by VMMC varied for MSM at different ages, we calculated the average benefits among MSM in this study.
Economic impact analysis
In order to assess the economic impact for VMMC scale-up among MSM, we conducted the current analysis from the perspectives of budget holders and health professionals (31). We employed the “numbers needed to avert” (NNA) that defined as “the cumulative number of VMMC conducted up to that year divided by the cumulative number of HIV case averted” as an indicator. NNA was calculated by dividing the cumulative number of HIV cases averted (compared with the baseline [λ=0.0001]) by the cumulative number of VMMC conducted at a given year. The lower the NNA, the more economically effective the VMMC campaign would be among MSM. We calculated the NNA under different annual VMMC coverage rates at each predicted year.
In addition, we further identified the breakeven point that balances the total cost of VMMC with the cost saved from averting new HIV cases. The breakeven point can be considered as the tipping point that it was economically desirable to proceed with the VMMC program among MSM in Beijing. We also calculated the total cost saved compared to the cost at baseline after expanding the VMMC program in each projected year. The cost at each scenario included the cost of VMMC and cost of HIV treatment. The cost of each individual VMMC and HIV treatment in each projected year (2016–2026) was discounted by 3% (32)that started from the baseline cost derived from existing studies (33–36)(Supplementary Table 2).
Sensitivity analyses
We conducted sensitivity analyses by setting the transmission rates for both circumcised and uncircumcised MSM at the same level as the incidence rate seen in 2015. If nothing changes from 2015 (i.e., very, very few VMMC), it represents a lower bound for the transmission rate. In addition, we assessed the economic impact in terms of the NNA and breakeven points by different protective efficacies (e.g., 7%, 17%, 27%, 37%, and 47%) ranging from being very conservative 7% (10) to being optimistic 47%(26) incrementally in the current study.
Results
Model predictions of the numbers of new HIV cases and averted HIV cases
We presented findings from the prediction model in Table 1, including the number of new VMMC, total number of new HIV cases, and numbers of HIV cases averted compared to baseline at each predicted year (2016–2026), varying the annual coverage rates for VMMC from λ=0.0001 to λ=0.15. With an increased VMMC coverage rate, the numbers of VMMC were increased proportionately. For example in the year 2026, under a coverage rate of 0.0001 there would be 42 new VMMC in Beijing, but under an annual coverage rate of 0.15 there would be 18,836 new VMMC. The number of susceptible MSM will have decreased over time in the latter case, but will have changed little in the former. In the model, when a hypothetical VMMC coverage rate was no greater than 0.01, the number of new VMMC increased linearly with each year due to a minimal change in the susceptible MSM. In contrast, when a hypothetical VMMC coverage rate reached 0.05 or more, numbers of new VMMC decreased over time due to the decline in susceptible uncircumcised MSM. For instance, under an annual VMMC coverage rate of 0.1, the number of new VMMC would be 33,652 in 2016, decreasing to 18,094 in 2026. As expected given the protective estimates of VMMC for HIV prevention in MSM, new HIV cases decreased as VMMC coverage increased. By contracting the number of new HIV cases at the baseline from the number of new HIV cases under different hypothetical coverage rates of VMMC, we calculated the potential number of HIV cases averted (Table 1).
Table 1.
Results from mathematical modeling of predicting HIV cases at different voluntary medical male circumcision coverage rates (2016–2026)
| Year | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | 2026 | Total number |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λ1=0.0001 (baseline) | ||||||||||||
| # of new VMMC per year | 37 | 38 | 39 | 39 | 40 | 40 | 41 | 41 | 42 | 42 | 42 | 441 |
| total # of new HIV cases (baseline) | 6344 | 9816 | 13581 | 17547 | 21688 | 26104 | 30694 | 35397 | 40242 | 45190 | 50301 | 296904 |
| λ1=0.0005 | ||||||||||||
| # of new VMMC per year | 185 | 189 | 193 | 196 | 199 | 202 | 204 | 206 | 207 | 208 | 209 | 2198 |
| # of new HIV cases | 6344 | 9816 | 13580 | 17546 | 21685 | 26099 | 30687 | 35386 | 40225 | 45167 | 50270 | 296805 |
| # HIV cases averted vs. baseline | 0 | 0 | 1 | 1 | 3 | 5 | 7 | 11 | 17 | 23 | 31 | 99 |
| λ1=0.001 | ||||||||||||
| # of new VMMC per year | 371 | 378 | 386 | 392 | 397 | 402 | 406 | 410 | 413 | 415 | 417 | 4387 |
| # of new HIV cases | 6344 | 9815 | 13580 | 17543 | 21679 | 26090 | 30673 | 35366 | 40199 | 45132 | 50224 | 296645 |
| # HIV cases averted vs. baseline | 0 | 0 | 1 | 4 | 9 | 14 | 21 | 31 | 43 | 58 | 77 | 259 |
| λ1=0.005 | ||||||||||||
| # of new VMMC per year | 1847 | 1878 | 1905 | 1929 | 1950 | 1968 | 1982 | 1994 | 2002 | 2007 | 2009 | 21471 |
| # of new HIV cases | 6343 | 9812 | 13570 | 17524 | 21644 | 26031 | 30582 | 35233 | 40013 | 44881 | 49897 | 295530 |
| # HIV cases averted vs. baseline | 1 | 4 | 11 | 24 | 44 | 73 | 113 | 164 | 229 | 308 | 404 | 1374 |
| λ1=0.01 | ||||||||||||
| # of new VMMC per year | 3675 | 3719 | 3756 | 3787 | 3813 | 3832 | 3845 | 3852 | 3854 | 3851 | 3842 | 41826 |
| # of new HIV cases | 6343 | 9809 | 13560 | 17501 | 21603 | 25961 | 30472 | 35073 | 39788 | 44577 | 49499 | 294186 |
| # HIV cases averted vs. baseline | 1 | 7 | 21 | 46 | 85 | 143 | 222 | 324 | 454 | 612 | 802 | 2718 |
| λ1=0.05 | ||||||||||||
| # of new VMMC per year | 17671 | 17221 | 16776 | 16340 | 15915 | 15496 | 15086 | 14689 | 14302 | 13927 | 13561 | 170984 |
| # of new HIV cases | 6337 | 9779 | 13476 | 17322 | 21279 | 25427 | 29656 | 33897 | 38170 | 42431 | 46724 | 284498 |
| # HIV cases averted vs. baseline | 7 | 37 | 105 | 225 | 409 | 677 | 1038 | 1500 | 2071 | 2759 | 3577 | 12406 |
| λ1=0.1 | ||||||||||||
| # of new VMMC per year | 33652 | 31295 | 29157 | 27226 | 25485 | 23909 | 22488 | 21211 | 20060 | 19026 | 18094 | 271603 |
| # of new HIV cases | 6330 | 9744 | 13380 | 17122 | 20923 | 24853 | 28800 | 32696 | 36553 | 40331 | 44074 | 274806 |
| # HIV cases averted vs. baseline | 13 | 72 | 201 | 425 | 765 | 1251 | 1894 | 2701 | 3689 | 4858 | 6227 | 22098 |
| λ1=0.15 | ||||||||||||
| # of new VMMC per year | 48067 | 42665 | 38042 | 34099 | 30740 | 27873 | 25434 | 23362 | 21601 | 20107 | 18836 | 330826 |
| # of new HIV cases | 6324 | 9711 | 13292 | 16943 | 20614 | 24368 | 28094 | 31726 | 35279 | 38716 | 42081 | 267148 |
| # HIV cases averted vs. baseline | 20 | 104 | 290 | 604 | 1074 | 1736 | 2600 | 3671 | 4963 | 6474 | 8220 | 29756 |
Notes: ρm [ρ-]: transmission rate among uncircumcised; ρp [ρ+]: transmission rate among circumcised; Scp0: number of circumcised susceptible MSM; Scm0: number of uncircumcised susceptible MSM; Ichp0: number of circumcised infected MSM; Ichm0: number of uncircumcised infected MSM;
Economic impact assessment
The NNA under different coverage rates were presented in Table 2. Our analyses revealed that the higher the VMMC coverage rate was, the fewer numbers of VMMC was needed for one HIV case averted. For instance, under the VMMC coverage rate of 0.0005, the cumulative number of VMMC conducted up to that year for averting one HIV case was 880.76 at 2026, and the number dropped to 345.60 when the coverage rate increased to 0.001, 78.50 under the coverage rate of 0.005, 47.82 under the coverage rate of 0.01, 22.06 under the coverage rate of 0.05, 17.76 under the coverage rate of 0.1, and 15.79 under the coverage rate of 0.15 (Table 2).
Table 2.
Numbers of voluntary medical male circumcision needed per HIV case averted among men who have sex with men under different coverage rates (2016–2026) *
| Year | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | 2026 | Overall |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λ1=0.0005 | n/a | n/a | 90965.94 | 41840.61 | 18061.80 | 9287.96 | 5146.19 | 3063.60 | 1933.97 | 1282.82 | 880.76 | 22.20 |
| λ1=0.001 | n/a | 127512.93 | 42568.75 | 13493.38 | 5795.49 | 2979.75 | 1734.52 | 1085.53 | 713.28 | 489.01 | 345.60 | 16.94 |
| λ1=0.005 | 130914.12 | 19325.26 | 5873.78 | 2395.22 | 1157.90 | 628.47 | 372.18 | 235.42 | 157.00 | 109.18 | 78.50 | 15.63 |
| λ1=0.01 | 67408.05 | 11224.19 | 3350.17 | 1360.71 | 663.64 | 364.12 | 217.77 | 139.25 | 93.76 | 65.83 | 47.82 | 15.39 |
| λ1=0.05 | 17759.12 | 3141.80 | 1037.66 | 457.23 | 238.59 | 138.54 | 86.98 | 58.02 | 40.57 | 29.47 | 22.06 | 13.78 |
| λ1=0.1 | 11553.96 | 2172.94 | 749.29 | 340.30 | 181.27 | 106.90 | 67.95 | 45.78 | 32.27 | 23.59 | 17.76 | 12.29 |
| λ1=0.15 | 9468.16 | 1848.99 | 647.02 | 296.50 | 158.97 | 94.15 | 60.02 | 40.52 | 28.60 | 20.94 | 15.79 | 11.12 |
Notes:
The ratio is the cumulative number of VMMC conducted up to that year divided by the cumulative number of infections averted
We further calculated the prevalence of VMMC over the projected period (2016–2026). If the annual circumcision coverage rate remained at the 2015 baseline (λ=0.0001), the prevalence of VMMC would remain stable over the projected years (1.89% in 2015 vs. 1.74% in 2026), while with a high annual coverage rate (λ=0.15) in the 2016–2026 period, the coverage would rise from 1.89% in 2015 to 58.8% in 2026 (Table 3).
Table 3.
Prevalence of voluntary medical male circumcision among men who have sex with men in China under different coverage rates over the projected period (2016–2026)
| Coverage rates/Year |
2015 (%) |
2016 (%) |
2017 (%) |
2018 (%) |
2019 (%) |
2020 (%) |
2021 (%) |
2022 (%) |
2023 (%) |
2024 (%) |
2025 (%) |
2026 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λ1=0.0001 | 1.89 | 1.87 | 1.85 | 1.83 | 1.82 | 1.80 | 1.79 | 1.78 | 1.77 | 1.76 | 1.75 | 1.74 |
| λ1=0.0005 | 1.89 | 1.90 | 1.92 | 1.93 | 1.94 | 1.95 | 1.95 | 1.96 | 1.97 | 1.97 | 1.98 | 1.98 |
| λ1=0.001 | 1.89 | 1.95 | 2.00 | 2.05 | 2.09 | 2.13 | 2.16 | 2.19 | 2.21 | 2.23 | 2.26 | 2.27 |
| λ1=0.005 | 1.89 | 2.31 | 2.69 | 3.02 | 3.30 | 3.56 | 3.79 | 3.99 | 4.17 | 4.33 | 4.47 | 4.60 |
| λ1=0.01 | 1.89 | 2.77 | 3.54 | 4.21 | 4.80 | 5.32 | 5.78 | 6.19 | 6.56 | 6.88 | 7.17 | 7.43 |
| λ1=0.05 | 1.89 | 6.32 | 10.06 | 13.25 | 15.98 | 18.33 | 20.37 | 22.14 | 23.68 | 25.03 | 26.20 | 27.23 |
| λ1=0.1 | 1.89 | 10.58 | 17.61 | 23.36 | 28.13 | 32.10 | 35.44 | 38.24 | 40.61 | 42.61 | 44.30 | 45.72 |
| λ1=0.15 | 1.89 | 14.64 | 24.52 | 32.29 | 38.49 | 43.49 | 47.53 | 50.81 | 53.47 | 55.63 | 57.37 | 58.76 |
The analytical result for the “breakeven point” was presented in Table 4. We compared the cost spent for expanding VMMC with the cost saved from averting HIV cases. At the year of 2019 with the annual VMMC coverage rate of 0.001, the cost after scaling up VMMC was approximately equal to the cost at the baseline (costed save was approximately equal to zero), which was considered as the breakeven point of the VMMC coverage rate. Therefore, it is economically desirable to proceed with the VMMC program forward among MSM in Beijing, China beyond the breakeven point in terms of the time (e.g.,2019) and the coverage rate (e.g., 0.001). The amount of cost saved got increased with a higher coverage rate. Compared to baseline, the saved cost ranged from $2534.2 (in 1,000 USD) under the coverage rate of 0.0005 to $811,092.2 (in 1,000 USD) under the coverage rate of 0.15.
Table 4.
Cost saved after expanding the voluntary medical male circumcision compared to the baseline under different coverage rates &, #
| Year | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | 2026 | Total cost |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cost at baseline* | 150964.0 | 240589.4 | 342848.3 | 456249.4 | 580836.7 | 720089.6 | 872089.2 | 1035904.8 | 1213026.9 | 1403026.6 | 1608538.4 | 8624163.3 |
| cost saved at 0.0005 | 34.2 | 35.9 | 12.5 | 13.6 | −39.0 | −94.5 | −153.9 | −275.1 | −464.3 | −664.1 | −939.5 | −2534.2 |
| cost saved at 0.001 | 77.2 | 56.4 | 59.8 | −15.0@ | −148.2 | −289.2 | −495.9 | −802.4 | −1187.8 | −1688.5 | −2346.1 | −6779.8 |
| cost saved at 0.005 | 394.3 | 339.9 | 179.5 | −121.7 | −681.8 | −1497.0 | −2646.4 | −4244.8 | −6330.4 | −9002.1 | −12309.3 | −35919.9 |
| cost saved at 0.01 | 816.6 | 704.5 | 380.5 | −251.6 | −1295.4 | −2928.4 | −5257.6 | −8399.5 | −12571.8 | −17885.3 | −24468.4 | −71156.3 |
| cost saved at 0.05 | 3906.9 | 3182.7 | 1449.9 | −1742.4 | −6825.9 | −14532.8 | −25339.2 | −39737.5 | −58292.4 | −81479.3 | −110194.4 | −329604.4 |
| cost saved at 0.1 | 7431.9 | 5674.5 | 2059.9 | −4199.3 | −13871.8 | −28111.9 | −47617.0 | −73032.5 | −105352.3 | −145143.2 | −193530.9 | −595692.5 |
| cost saved at 0.15 | 10619.0 | 7571.8 | 2015.2 | −7121.5 | −20780.8 | −40428.3 | −66862.7 | −100808.7 | −143304.5 | −194958.7 | −257033.0 | −811092.2 |
Notes:
in 1,000 US dollars;
if the cost at any given voluntary medical male circumcision coverage rate is higher than the baseline, the number is positive, which means not economically desirable; if the cost is lower than the baseline, the number is negative, which means economically desirable;
total cost at the baseline when λ1=0.0001
breakeven point for the economically desirable of voluntary medical male circumcision (when λ=0.001 at the year of 2019).
Results from sensitivity analyses
In the sensitivity analyses, the same pattern for HIV infections and susceptibility by circumcision status was observed when the transmission rates were set at the same incidence rate seen in 2015.The higher the VMMC coverage rate, the fewer numbers of VMMC needed per HIV case averted. Similarly, the trend of a lower NNA was observed for each given coverage rate over time (results not shown).
A series of sensitivity analyses with incrementally increased protective efficacies revealed that the higher the protective efficacy of VMMC, the lower coverage rate and less time was required to reach the breakeven point. For instance, at the protective efficacy of 17%,27% and 37%, the breakeven point was achieved in the year of 2018 with the coverage rate of 0.0005; while the breakeven point was achieved earlier (e.g., 2017) with the same coverage rate (e.g., λ=0.0005) when the protective efficacy increased to 47% (Supplementary Table 3 ). The amount of cost saved increased either with a higher protective efficacy for each given coverage rate, or with a higher coverage rate for each given protective efficacy (Supplementary Figure).
Discussion
We employed a deterministic, compartmental model to assess epidemiologic and economic impact of expanding VMMC on HIV prevention among MSM in China. Economic impact analysis was an essential part of a comprehensive assessment of a health intervention, playing an increasingly important role in decision-making for policy makers in the current study (31). We found that the higher the annual coverage rate, the lower numbers of VMMC needed per HIV case averted was over time. A lower NNA can be obtained by expanding VMMC campaign significantly among MSM, which in turn, requires a higher budget cost of scaling-up the VMMC. If we achieved the greatest impact with the NNA as low as 15.79 when the annual VMMC coverage rate was 0.15, a total of 330,826 VMMC would be conducted by the year of 2026. We observed that he NNA was very sensitive to the level of VMMC coverage. It could be due to the “herd immunity effect” which individuals were indirectly protected as the result of a large percentage of a population become immune to an infection (4,5,26). In this case, the great proportion of MSM who are immune from VMMC in Beijing, the smaller the probability that those who are not circumcised will contract with HIV. On the other hand, if we adopted the most frugal budget to provide a low annual coverage rate (e.g., 0.0005) of VMMC, the impact would be trivial even though this coverage is five times the very low baseline. Furthermore, the analyses for “breakeven points” and total cost saved compared to baseline can assist health authorities to visualize time period and coverage rate for the tipping point of the intervention. Therefore, preventive health and decision-makers can use findings from the current modeling study for resource and budget planning.
A study conducted among heterosexual men in high HIV prevalence settings indicated that one HIV infection can be averted by every five to 15 male circumcisions performed over a 10-year time-frame with a 80% VMMC coverage (37). Our study revealed that to avert one HIV case, 11 to 22 male circumcisions needed to be performed on average over a 10-year period with an eventual 58.7% VMMC coverage with our use of very conservative estimates of the efficacy of VMMC in protecting MSM (e.g., 7%) (10). With a higher protective efficacy, fewer numbers of VMMC would be needed for averting one HIV case. Although WHO/UNAIDS guidance has stated that the VMMC would exert its greatest potential public health impact in settings where HIV prevalence exceeds 15% (37, 38), the HIV prevalence in Chinese MSM approaches this prevalence in many cities(5, 10, 39–41). Hence, the argument for VMMC deployment among Chinese MSM rivals that for heterosexual men in Africa, even with a lower anticipated efficacy (e.g., 7%) (10), though this assumption may overly conservative (26).
Several strengths of our study are notable, particularly its unique contribution to the existing literature. Although the current study employed a deterministic compartmental model similar to the DMPPT of UNAIDS/UNAID (20), we simulated transmission rates based upon the National HIV Surveillance data in order to capture possible randomness properties (42). Compared to the arbitrary 15% variance strategy that employed by other published studies (19, 20), the HIV surveillance data can improve the validity of the model parameters and likely accuracy of context-specific predictions. In addition to the simulation, sensitivity analyses estimated new HIV cases at their lower bound when transmission rates remained the same as seen in 2015, suggesting our findings to be robust and plausible. Both parameter (e.g., uncertainty of transmission rates) and structural uncertainties (e.g., uncertainty of VMMC coverage rates) were assessed. The analyses based upon incrementally increased protective efficacies also comprehensively assessed the intervention impact of VMMC. Furthermore, as one of the first prediction models to evaluate VMMC from an economic perspective outside Africa (43), our model assists decision making and resource planning for other settings similar to China, especially for places where MSM increasingly dominated the HIV epidemic.
Limitations of other models also apply here. Our model in the current study has limited generalizability due to the context-specific parameters although we used a meta-analysis derived circumcision efficacy estimate which would presumably apply to many settings(10). Unlike the DMPPT model in which age-specific VMMC scaling up strategies were assessed (20), we only assessed the overall epidemic among all MSM with an extended age range from 18 to 65 years old. We did not examine the age pattern among MSM who would have VMMC as we calculated the average benefits of MSM across all ages in their life span. Due to the limits of predictability of the deterministic model, we cannot project the VMMC benefits for the lifelong period of MSM (27, 29, 30). However, the life-long protection from VMMC would definitely continue beyond our model prediction limits (e.g., 2026). In addition, we did not assess epidemiologic and economic impacts of VMMC with other concurrent behavioral (e.g., condom use), structural (e.g., microfinance for at-risk populations) or biomedical intervention strategies (e.g., pre-exposure prophylaxis) (5, 27, 44). The nature of these available tools depends on user-adherence, a potential disadvantage compared to VMMC (5, 27, 44–46). If acceptable, if offered, and if taken up by MSM, VMMC can confer potentially lifelong protective benefits once the one-time surgical procedure is performed (37). Future research can consider synergistic effects of other available prevention tools (27) as well as considering indirect protective effects toward women and children among bisexual men who get circumcised (20, 37), or potential modulation of benefit due to risk compensation after the VMMC procedure was conducted (37). Finally, due to the controversial definition of quality-adjusted life year, we did not conduct the cost-effectiveness analysis as other investigators have done (47–49). Nonetheless, these current analyses can provide health system and decision-makers estimates useful for deciding when and how to deploy a VMMC scale-up strategy among MSM in settings like China (31).
Our model serves as one of the first studies to provide economic data (e.g., breakeven analysis, NNA, total cost saved), suggesting VMMC to be a viable prevention option for control of HIV among MSM in China and similar venues. Policy-makers would do well to consider accelerating access to VMMC targeting MSM, a neglected component in the “prevention tool box” in addition to other strategies(50, 51), to stem the HIV/AIDS epidemic.
Supplementary Material
Acknowledgement
Thanks for the helps from Vanderbilt Institute of Global Health during this manuscript development.
References
- 1.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS medicine. 2005;2(11):e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey RC, Moses S, Parker CB, Agot K, Maclean I, Krieger JN, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369(9562):643–56. [DOI] [PubMed] [Google Scholar]
- 3.Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369(9562):657–66. [DOI] [PubMed] [Google Scholar]
- 4.Qian HZ, Ruan Y, Liu Y, Milam DF, HM LS, Yin L, et al. Lower HIV risk among circumcised men who have sex with men in China: Interaction with anal sex role in a cross-sectional study. Journal of acquired immune deficiency syndromes (1999). 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vermund SH, Qian HZ. Circumcision and HIV prevention among men who have sex with men: no final word. Jama. 2008;300(14):1698–700. [DOI] [PubMed] [Google Scholar]
- 6.Millett GA, Flores SA, Marks G, Reed JB, Herbst JH. Circumcision status and risk of HIV and sexually transmitted infections among men who have sex with men: a meta-analysis. Jama. 2008;300(14):1674–84. [DOI] [PubMed] [Google Scholar]
- 7.Smith AD, Tapsoba P, Peshu N, Sanders EJ, Jaffe HW. Men who have sex with men and HIV/AIDS in sub-Saharan Africa. Lancet. 2009;374(9687):416–22. [DOI] [PubMed] [Google Scholar]
- 8.Baral S, Sifakis F, Cleghorn F, Beyrer C. Elevated risk for HIV infection among men who have sex with men in low- and middle-income countries 2000–2006: a systematic review. PLoS medicine. 2007;4(12):e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grund JM, Chetty-Makkan CM, Ginindza S, Munyai R, Kisbey-Green H, Maraisane M, et al. Effectiveness of an “Exclusive Intervention Strategy” to increase medical male circumcision uptake among men aged 25–49 years in South Africa . BMC public health. 2018;18(1):868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C, Qian HZ, Liu Y, Vermund SH. Effect of circumcision on risk of HIV infection among men who have sex with men: a systematic review and meta-analysis. Journal of Acquired Immune Difficiency Syndrome. Under review. [Google Scholar]
- 11.Schneider JA, Michaels S, Gandham SR, McFadden R, Liao C, Yeldandi VV, et al. A protective effect of circumcision among receptive male sex partners of Indian men who have sex with men. AIDS and behavior. 2012;16(2):350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solomon SS, Mehta S, Srikrishnan AK, McFall A, Balakrishman P, Anand S, et al. , editors. Circumcision is associated with lower HIV prevalence among men who have sex with men in India. 2014 International Conference 2014; Melbourne, Australia. [Google Scholar]
- 13.Ben KL, Xu JC, Lu L, Lu NQ, Cheng Y, Tao J, et al. [Male circumcision is an effective “surgical vaccine” for HIV prevention and reproductive health]. Zhonghua nan ke xue = National journal of andrology. 2009;15(5):395–402. [PubMed] [Google Scholar]
- 14.Wei FM, Yang XB, Jiang JJ, Yuan XY, Chen YH, Lin ZS, et al. [Benefits of promoting male circumcision among the general population in the high HIV prevalence areas of Guangxi Province]. Zhonghua nan ke xue = National journal of andrology. 2012;18(5):391–6. [PubMed] [Google Scholar]
- 15.Luo H, Liang X, Chen J, Yang XB, Jiang JJ, Deng W, et al. [Acceptability of male circumcision among male miners in Baise of Guangxi]. Zhongguo yi xue ke xue yuan xue bao Acta Academiae Medicinae Sinicae. 2011;33(3):313–7. [DOI] [PubMed] [Google Scholar]
- 16.Ruan Y, Qian HZ, Li D, Shi W, Li Q, Liang H, et al. Willingness to be circumcised for preventing HIV among Chinese men who have sex with men. AIDS patient care and STDs. 2009;23(5):315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau JT, Zhang J, Yan H, Lin C, Choi KC, Wang Z, et al. Acceptability of circumcision as a means of HIV prevention among men who have sex with men in China. AIDS care. 2011;23(11):1472–82. [DOI] [PubMed] [Google Scholar]
- 18.Lau JT, Yan H, Lin C, Zhang J, Choi KC, Wang Z, et al. How willing are men who have sex with men in China to be circumcised for the sake of protecting his female sex partner? The journal of sexual medicine. 2012;9(7):1904–12. [DOI] [PubMed] [Google Scholar]
- 19.Kripke K, Njeuhmeli E. Assessing Progress, Impact, and Next Steps in Rolling Out Voluntary Medical Male Circumcision for HIV Prevention in 14 Priority Countries in Eastern and Southern Africa through 2014. PloS one. 2016;11(7):e0158767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kripke K, Opuni M, Schnure M, Sgaier S, Castor D, Reed J, et al. Age Targeting of Voluntary Medical Male Circumcision Programs Using the Decision Makers’ Program Planning Toolkit (DMPPT) 2.0. 2016;11(7):e0156909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Njeuhmeli E, Forsythe S, Reed J, Opuni M, Bollinger L, Heard N, et al. Voluntary medical male circumcision: modeling the impact and cost of expanding male circumcision for HIV prevention in eastern and southern Africa. PloS one. 2011;8(11):e1001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bollinger LA, Stover J, Musuka G, Fidzani B, Moeti T, Busang L. The cost and impact of male circumcision on HIV/AIDS in Botswana. Journal of the International AIDS Society. 2009;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stover J, Kripke K, Perales N, Lija J, Fimbo B, Mlanga E, et al. The Economic and Epidemiological Impact of Focusing Voluntary Medical Male Circumcision for HIV Prevention on Specific Age Groups and Regions in Tanzania. PloS one. 2016;11(7):e0153363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380(9839):367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan SW, Carpiano RM, Li D, Zhang Z, Schechter MT, Spittal PM, et al. Ethnicity and HIV vulnerabilities among men who have sex with men in China. AIDS care. 2018;30(8):1025–30. [DOI] [PubMed] [Google Scholar]
- 26.Qian HZ, Ruan Y, Liu Y, Milam DF, Spiegel HM, Yin L, et al. Lower HIV Risk Among Circumcised Men Who Have Sex With Men in China: Interaction With Anal Sex Role in a Cross-Sectional Study. Journal of acquired immune deficiency syndromes (1999). 2016;71(4):444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lou J, Blevins M, Ruan Y, Vermund SH, Tang S, Webb GF, et al. Modeling the impact on HIV incidence of combination prevention strategies among men who have sex with men in Beijing, China. PloS one. 2014;9(3):e90985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pantaleo G, Menzo S, Vaccarezza M, Graziosi C, Cohen OJ, Demarest JF, et al. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. The New England journal of medicine. 1995;332(4):209–16. [DOI] [PubMed] [Google Scholar]
- 29.Lou J, Smith RJ. Modelling the effects of adherence to the HIV fusion inhibitor enfuvirtide. Journal of theoretical biology. 2011;268(1):1–13. [DOI] [PubMed] [Google Scholar]
- 30.Lou J, Wu J, Chen L, Ruan Y, Shao Y. A sex-role-preference model for HIV transmission among men who have sex with men in China. BMC public health. 2009;9 Suppl 1:S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan SD, Mauskopf JA, Augustovski F, Jaime Caro J, Lee KM, Minchin M, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2014;17(1):5–14. [DOI] [PubMed] [Google Scholar]
- 32.Hunink M, Glasziou P, Siegel J, Weeks JC, Pliskin JS, Elstein AS, et al. Decision Making in Health and Medicine: Integrating Evidence and Values: Cambridge University Press; 2001. [Google Scholar]
- 33.Drabo EF, Hay JW, Vardavas R, Wagner ZR, Sood N. A Cost-effectiveness Analysis of Preexposure Prophylaxis for the Prevention of HIV Among Los Angeles County Men Who Have Sex With Men. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;63(11):1495–504. [DOI] [PubMed] [Google Scholar]
- 34.Farnham PG, Gopalappa C, Sansom SL, Hutchinson AB, Brooks JT, Weidle PJ, et al. Updates of lifetime costs of care and quality-of-life estimates for HIV-infected persons in the United States: late versus early diagnosis and entry into care. Journal of acquired immune deficiency syndromes (1999). 2013;64(2):183–9. [DOI] [PubMed] [Google Scholar]
- 35.Moon S, Van Leemput L, Durier N, Jambert E, Dahmane A, Jie Y, et al. Out-of-pocket costs of AIDS care in China: are free antiretroviral drugs enough? AIDS care. 2008;20(8):984–94. [DOI] [PubMed] [Google Scholar]
- 36.Schackman BR, Fleishman JA, Su AE, Berkowitz BK, Moore RD, Walensky RP, et al. The lifetime medical cost savings from preventing HIV in the United States. Medical care. 2015;53(4):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Group UWS. Male circumcision for HIV prevention in high HIV prevalence settings: what can mathematical modelling contribute to informed decision making? PLoS medicine. 2009;6(9):e1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO/UNAIDS. New Data on Male Circumcision and HIV Prevention: Policy and Programme Implications 2007. [cited 2016 October 26]. Available from: http://www.unaids.org/sites/default/files/media_asset/mc_recommendations_en_0.pdf.
- 39.Zeng Y, Zhang L, Li T, Lai W, Jia Y, Aliyu MH, et al. Risk Factors for HIV/Syphilis Infection and Male Circumcision Practices and Preferences among Men Who Have Sex with Men in China. BioMed research international. 2014;2014:498987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang C, Shephard BE, Lou J, Vermund SH, Qian HZ, Penson DF, et al. Predicting the impact of voluntary medical male circumcision on HIV incidence among men who have sex with men in Beijing, China. PloS one. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou C, Raymond HF, Ding X, Lu R, Xu J, Wu G, et al. Anal sex role, circumcision status, and HIV infection among men who have sex with men in Chongqing, China. Archives of sexual behavior. 2013;42(7):1275–83. [DOI] [PubMed] [Google Scholar]
- 42.Vynnycky E, White RG. An introductory book on infectious disease modelling and its applications New York: Oxford University Press; 2010. [Google Scholar]
- 43.Goodreau SM, Carnegie NB, Vittinghoff E, Lama JR, Fuchs JD, Sanchez J, et al. Can male circumcision have an impact on the HIV epidemic in men who have sex with men? PloS one. 2014;9(7):e102960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rotheram-Borus MJ, Swendeman D, Chovnick G. The past, present, and future of HIV prevention: integrating behavioral, biomedical, and structural intervention strategies for the next generation of HIV prevention. Annual review of clinical psychology. 2009;5:143–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang W, Du S, Xu J, Zhou J, Liang S, Yu F, et al. Acceptability of Condoms, Circumcision and PrEP among Young Black Men Who Have Sex with Men: A Descriptive Study Based on Effectiveness and Cost. BioMed research international. 2014;2(1):129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pretorius C, Stover J, Bollinger L, Bacaer N, Williams B. Evaluating the cost-effectiveness of pre-exposure prophylaxis (PrEP) and its impact on HIV-1 transmission in South Africa. PloS one. 2010;5(11):e13646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson J, Wilson D, Templeton DJ, Grulich A, Carter R, Kaldor J. Cost-effectiveness of adult circumcision in a resource-rich setting for HIV prevention among men who have sex with men. The Journal of infectious diseases. 2009;200(12):1803–12. [DOI] [PubMed] [Google Scholar]
- 48.Kahn JG, Marseille E, Auvert B. Cost-effectiveness of male circumcision for HIV prevention in a South African setting. PLoS medicine. 2006;3(12):e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sansom SL, Prabhu VS, Hutchinson AB, An Q, Hall HI, Shrestha RK, et al. Cost-effectiveness of newborn circumcision in reducing lifetime HIV risk among U.S. males. PloS one. 2010;5(1):e8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paparini S, Nutland W, Rhodes T, Nguyen VK, Anderson J. DIY HIV prevention: Formative qualitative research with men who have sex with men who source PrEP outside of clinical trials. PloS one. 2018;13(8):e0202830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reed JB, Patel RR, Baggaley R. Lessons from a decade of voluntary medical male circumcision implementation and their application to HIV pre-exposure prophylaxis scale up. International journal of STD & AIDS. 2018:956462418787896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


