Abstract
Background: In cancer biology, metastasizing is one of the most poorly studied processes. Pancreatic ductal adenocarcinoma (PDAC) is characterized by early metastasis, which is the leading cause of death. The PDX1 protein is crucial for the development of cancer, and its low levels are characteristic of the most aggressive PDAC tumors. The PDX1 is a mediator of initiation and progression of PDAC. However, further studies are needed to elucidate the role of PDX1 in the cancer metastasis.
Purpose: To confirm the hypothesis that PDX1 in PDAC plays suppressor role of epithelial–mesenchymal transition (EMT), and to study its possible ability to inhibit metastasis.
Methods: A PDX1-overexpressing PDAC cell line was obtained by lentiviral transduction of PANC-1 cells. PDX1 overexpression was confirmed by RT-PCR and Western blotting. Effects of PDX1 ectopic expression on cell proliferation and motility were determined in PANC-1 cells using MTS, cell cycle analysis, transwell and wound-healing assay. EMT genes expression was analyzed in PDX1-overexpressing and Control PANC-1. Finally, the migration potential of pancreatic cancer cells expressing PDX1 was evaluated using a zebrafish embryo model.
Results: The motility of human PDAC cells PANC-1 considerably decreased at ectopic expression of PDX1. The decreased expression of ZEB1, the key factor of EMT, and almost unchanged expression of the genes that characterize the epithelial state suggest a decrease in the EMT ability. Suppression of PDX1 expression by siRNA knockdown restored the PANC1 motility.
Conclusion: The results obtained suggest a possible therapeutic use of PDX1 delivery into PDAC patients with a reduced or absent expression of PDX1 in the most aggressive tumors.
Keywords: metastasis, PDAC, PDX1, epithelial–mesenchymal transition, Danio rerio
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is on the way from the fourth to the second top position in global cancer mortality by 2030. The frequency of emergence and mortality of PDAC is almost the same due to the combination of usually late diagnostics, aggressive clinical course, and poor reaction to chemo- and radiotherapy.1 Early resection of primary tumor without detectable metastases prevents further growth of tumor and increases the survival rate. Unfortunately, even radical surgery does not prevent the development of metastases from previously disseminated cancer cells.2 The genetic heterogeneity of PDAC and different oncogenic susceptibility of different compartments within the pancreas make this disease opposite to what clinicians call “a chameleon,” that is one disease with many faces. Instead, PDAC may represent numerous diseases with the same appearance.3 In addition to the genetic heterogeneity, the results of some studies indicate possible multifocal neoplasia in the pancreas.4,5
In PDAC, as well as in other malignant tumors, metastasis is the main cause of death of cancer patients and often occurs at early stages of cancer development. In cancer biology, metastasizing is one of the most poorly studied phenomena.6,7 It is a complex process; the majority of cancers have specific features of metastasis, so that the exact ways of dissemination of cancer cells from their primary sites, their migration, and subsequent invasion into distant parts of the organism remain unknown. In addition, it is currently evident that metastasizing is not an autonomous program of cancer cells, but a complex chain of events affected by intracellular mutations, multiple interactions between malignant and stromal cells, and signals of the extracellular matrix. Steps of successful metastasis include angiogenesis/lymphangiogenesis, epithelial–mesenchymal transition (EMT), invasion to surrounding tissues and migration, formation of a premetastatic niche, and growth at the metastatic site,6 with its own regulatory factors involved. Previous reviews6,8–10 mention also the role of the richness of genomic aberrations and a strong link between cancer stem cells and metastatic events in PDAC.9 Recently, microRNAs and exosomes have also been suggested to play a regulatory role in the metastatic behavior of many tumors, including PDAC.9 The development of metastases may be correlated to a more mesenchymal transcriptomic subtype.10
Another key determinant in cancer development and metastasis is the tumor microenvironment (for a recent review, see11). In particular, cancer-associated fibroblasts (CAFs) are often found in the vicinity of or in direct contact with neoplastic cells and can co-travel in the blood with circulating murine metastatic lung carcinoma cancer cells, probably supporting the cancer cell viability and growth advantage at the metastatic site. It is quite possible that other factors implicated in the formation of PDAC metastasis remain largely unknown and undefined.9
It is usually accepted that metastasis is linked to the EMT of cancer cells12, although recently it was called in question.13,14 It was supposed that there are EMT-dependent and EMT-independent mechanisms of metastasis, although the mechanisms of these processes remain unclear and are possibly different for different types of cancer. In its turn, the process of EMT depends on a complex network of cytokines, transcription factors, growth factors, signaling pathways, and the tumor microenvironment. The transition of cancer cells to a mesenchymal state increases their migratory and invasive properties, increasing metastasis probability.
Over the last two decades, therapy of malignant tumors has made considerable progress due to a breakthrough in cancer immunotherapy. However, therapy of PDAC cancer developed slowly, and the success of immunotherapy did not have any effect on it. Generally, PDAC remains “A Riddle Wrapped in a Mystery inside an Enigma.”15 Therefore, the problem of PDAC therapy is extremely urgent. Recently, the attention of researchers was attracted by PDX1, the key regulator of the pancreas development. Yu et al16 developed different methods of inhibiting Pdx1 expression, which led to a prolonged survival in mouse PDAC models. The authors argued that strategies directed at the PDX1 therapy might allow to cure PDAC.16 However, these data were criticized by another group of researchers,12 who showed that PDX1 was a context-dependent mediator of initiation and progression of PDAC. According to their data, the definition of PDX1 as a fully pro- or anticancer factor is false, and it would be premature to treat it as a therapeutic target without further understanding of its various functions, as proposed by a previous study.16 The PDX1 protein is crucial for cancer development, but blocking it may lead to more aggressive tumors. Further studies are needed to examine how PDX1 interacts with various co-regulators and modulates critical aspects of cancer development.12
In the present study, using Danio rerio (zebrafish) as a model organism , we report that the motility of human PDAC cells PANC-1 considerably decreased at ectopic expression of a key factor of pancreas embryogenesis PDX1. The decreased expression of ZEB1, the key factor of epithelial-mesenchymal transition (EMT), and almost unchanged expression of the genes that characterize the epithelial state suggest a decrease in the EMT ability. Suppression of PDX1 expression by siRNA restores the motility. On the assumption that EMT is a prerequisite of metastasis, the decreased motility may indicate a decline in metastatic potential. These results suggest a possibility of gene therapeutic use of PDX1 delivery into PDAC cells with the reduced or absent expression of PDX1, which are known to be the most aggressive.
Materials and methods
Cell cultures
PANC-1 (ATCC® CRL-1469) and 293T (ATCC® CRL-3216™) cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in DMEM supplemented with 10,000 U/mL penicillin, 10 mg/mL streptomycin, and 10% FBS. Media and supplements were purchased from Gibco (Carlsbad, CA, USA). Cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C.
Lentivirus vector design and production
Three lentivirus vectors were designed to express green fluorescent protein (GFP) under the control of the CMV promoter, to express the PDX1 gene under control of the PCNA promoter and the puromycin-resistance gene under control of the PGK promoter, and to express solely the puromycin-resistance gene under the control of the PGK promoter. To express GFP under the control of the CMV promoter, lentiviral expression vector pLVT (kindly provided by Prof. V.V. Belousov, Institute of Bioorganic Chemistry, Russian Academy of Sciences, Moscow) was created as previously described.17 The promoter of the human PCNA gene18 was amplified from a fragment of human genomic DNA at –241 to +148 from the transcription start site. The PCNA promoter was amplified by PCR with the primers PCNA-LS 5’-TCTCCACATATGCCCGGACT-3’ and PCNA-R 5’-GCAACAACGCCGCTACAG-3’. The amplification products were cloned into a pAL-TA vector (Evrogen, Moscow, Russia) and then subcloned into a pLVPGm.1 vector. The PDX1 (Pancreatic and duodenal homeobox 1) human gene was derived from a pCMV6 plasmid (OriGene Technologies, Rockville, MD, USA, cat. no. RC222354) and inserted under the control of the PCNA promoter.18,19 The negative control vector contained only a puromycin-resistance cassette. Following DNA sequencing confirmation, the successfully constructed vectors containing PDX1 and control were transfected with Lipofectamine® 2000 (Invitrogen Life Technologies,Carlsbad, CA, USA) into 80% confluent 293T cells for 48 hrs at 37°C. The cell culture medium containing the lentivirus was collected and stored at −70°C.
Lentiviral transduction of cancer cells
PANC-1 cells were transduced with lentiviral particles containing the PCNA promoter to express PDX1 and those containing the control vector. Transduction was performed in 6-well plates seeded with 3×105 PANC-1 cells/well followed by puromycin selection (4 μg/mL) for 10 days. The cell lines stably expressing PDX1 and control cells were maintained in puromycin (2 μg/mL). The generated stable cell lines were named PANC-1PDX1 and PANC-1Control, respectively.
Next, PANC-1PDX1 and PANC-1Control cells were transduced with lentiviral particles containing Turbo-GFP under the control of the CMV promoter. The transduction was performed as described previously. GFP-positive (GFP+) transduced cells were selected using a fluorescence-activated cell sorter FACSAria III (BD Bioscience, San Jose, CA, USA).
Quantitative polymerase chain reaction (qPCR)
Total RNA was extracted using Extract RNA reagent (Evrogen) and reverse-transcribed into cDNA using Mint Reverse Transcriptase (Evrogen). qPCR was performed to determine the gene expression levels in the PANC-1PDX1and PANC-1 Control cells on a LightCycler480 Real-Time PCR platform (Roche Applied Science, Mannheim, Germany). qPCRmix-HSSYBR was used to determine the relative RNA expression. Primer sequences are shown in Table 1. The 18SRNA and EEF1a genes were used as an internal control. The PCR reaction conditions were as follows: 1 cycle at 90°C for 5 mins; 40 cycles at 95°C for 20 s, 60°C for 20 s. and 72°C for 35 sec; and 1 cycle at 95°C for 5 s, 55°C for 60 s, and 97°C for 15 sec. The experiments were performed in triplicate for each sample. A relative expression ratio of PDX1 was normalized by using geometric means of the 18SRNA and EEF1a expression levels. Calculations were performed according to Ganger et al20 for the relative expression ratio.
Table 1.
Real-time PCR primers used in gene expression analysis
| Gene | Primer sequence (5’ → 3’) | PCR product (bp) | |
|---|---|---|---|
| 18S | Fw | CGCGGTTCTATTTTGTTGGT | 501 |
| Rv | ATGCCAGAGTCTCGTTCGTT | ||
| EEF1a | Fw | GACACGTAGATTCGGGCAAG | 173 |
| Rv | GATACCACGTTCACGCTCA | ||
| PDX1 | Fw | GTCCTGGAGGAGCCCAAC | 272 |
| Rv | CGGCGGTTTTGGAACCAGAT | ||
| CDH1 | Fw | AGTGCCTGCTTTTGATGATG | 338 |
| Rv | AGCTTGAACTGCCGAAAAATC | ||
| KRT8 | Fw | ATGTTGTCCATGTTGCTTCG | 125 |
| Rv | ACCCTCAACAAGTTTGCC | ||
| MUC1 | Fw | CTGGTCTGTGTTCTGGTTGC | 250 |
| Rv | CCACTGCTGGGTTTGTGTAAG | ||
| KLF5 | Fw | ACAAATCAGACAGCAGCAATGGACA | 312 |
| Rv | GGTGGTGGGTAAATTTGGATTGTGA | ||
| VIM | Fw | GCAGAAGAATGGTACAAATCCA | 144 |
| Rv | TTTAAGGGCATCCACTTCACA | ||
| SNAIL | Fw | CCAATCGGAAGCCTAACTAC | 124 |
| Rv | GCGGTGGGGTTGAGGATCTC | ||
| SLUG | Fw | AGAAGGTTTTGGAGCAGTTTTTG | 160 |
| Rv | TGGTTGCTTCAAGGACACAT | ||
| ZEB1 | Fw | GAACAGTGTTCCATGCTTAAGAGCG | 217 |
| Rv | GGGCGGTGTAGAATCAGAGTCATTC | ||
Abbreviations: Fw, forward primer; Rw, reverse primer; bp, base pair.
Western blotting
The lentivirus-transduced cells were lysed in SDS sample buffer containing 1% SDS, 2% 2-mercaptoethanol, and 62 mM Tris–HCl, pH 6.8, subjected to SDS electrophoresis on 10–15% polyacrylamide gels and then electrotransferred to a Polyvinylidene difluoride (PVDF) Immobilon-P membrane (Millipore, Burlington, MA, USA) using a Bio-Rad Trans-Blot SD cell (Bio-Rad Laboratories, Hercules, CA, USA). The membranes were blocked with 5% skimmed milk in PBS-T (PBS containing 0.1% Tween 20) for 1 hr at room temperature, incubated in PBS-T containing 5% skimmed milk and the relevant primary antibody overnight at 4°C, and finally washed three times with PBS-T. The membranes were then incubated with indicated primary antibodies rabbit anti-PDX1 (1:1000; Cat. no. 5679; Cell Signaling Technology, Danvers, MA, USA) and mouse anti-GAPDH (1:60,000; Cat. no. 10494–1-AP; Santa Cruz Biotechnology Inc., Dallas, TX, USA) at 4°C overnight, followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5000; Cat. no. sc-2054; Santa Cruz Biotechnology Inc.) at room temperature for 1 hr. Mouse monoclonal anti-GAPDH antibody was used as a loading control. After washing, the membranes were incubated in PBS-T containing 5% skimmed milk and goat anti-mouse or anti-rabbit antibody HRP conjugates (Santa Cruz, 1:5.000) for 1 hr at room temperature. The membranes were finally washed with PBS-T, and specific signals were visualized using a Clarity Western ECL (Bio-Rad Laboratories) with a VersaDoc Imaging System (Bio-Rad Laboratories).
RNA interference
A siRNA sequence targeting human PDX1 (Si-135: 5′-GCCACGCAGCTTTACAAG-3’, Si-464: 5’-TCCCATGGATGAAGTCTAC-3’, Si-599 5’-AGTTCCTATTCAACAAGTA-3’) was designed and synthesized (Syntol, Moscow, Russia). Silencer™ Negative Control No. 1 siRNA (Ambion, Waltham, MA, USA, Cat #AM4635) was used as a negative control for siRNA.
Transfections were carried out using the Lipofectamine RNAiMAX reagent (Invitrogen), following the manufacturer’s instructions. To achieve the best depletion of PDX1, transfection was conducted twice: 24 hrs after seeding the cells and 48 hrs after the first transfection. siRNA-treated cells were injected into zebrafish embryos 24 hrs after the second transfection.
Cell proliferation assay
Cell proliferaion assay was performed using CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) (Promega, Madison, WI, USA). Following the lentivirus transduction, the PANC-1PDX1 and PANC-1Control (2000 cells/well) cells were seeded each into 96-well plates. MTS solution (5 mg/mL; 20 µL) was added into each well. The MTS solution was aspirated off following incubation for 1 hr at 37°C. The absorbance of each plate was measured at 595 nm using a Benchmark Plus microplate reader (Bio-Rad Laboratories).
Cell cycle analysis
Cells were collected in the logarithmic phase, plated onto 6-well plates at 1×105 cells per well, and incubated for 24 hrs. After that, the cells were digested with 0.25% trypsin, centrifuged at 1000 rpm for 5 mins, and then the supernatant was discarded. Remainders were washed with PBS twice. One milliliter of 70% ethanol was added, and after that the samples were held at 4°C for 24 hrs. Later, the samples were washed and centrifuged again. Staining buffer, propidium iodide (PI) staining solution (20×), and RNase A (50×) were added to the samples, which were then incubated for 30 mins at room temperature under darkroom conditions. PI fluorescence was detected using a Cytomics FC500 (Beckman Coulter, Bray, CA, USA) flow cytometry system and analyzed using a MultiCycle (Beckman Coulter).
Migration — cell wound closure assay
The PANC-1PDX1 and PANC-1 Control cells (4×105 cells/well) were plated onto 6-well plates for 24 hrs to a confluence of about 80%, then wounded by scratching with a p200 pipette tip. The debris was removed, and the cells were washed once with 1 mL of growth medium to assure that the edges of the scratch were smoothed by washing. We took utmost care to make wounds of the same dimension, both for the experimental and control cells, to minimize any possible variety resulting from a difference in the scratch width. GFP-labeled PANC-1PDX1 and PANC-1Control cell migration was assessed by a monolayer gap closure migration assay, using the free ImageJ software (version 1.50i, National Institute of Health, Bethesda, MD, USA). The area of the initial wound was measured, followed by gap area measurements after 12 and 24 hrs.
Transwell assay
The migration assay was performed on transwell plates. For cell migration assay, 2×105 cells were seeded on a polycarbonate membrane transwell inserts containing 8 µm pores (Corning, Corning, NY, USA) and cultured in DMEM without serum. DMEM containing 10% FBS was added to the lower chamber. After incubation for 24 hrs at 37°C in a CO2 incubator, the insert was washed with PBS, and cells on the top surface of the insert were removed using a cotton swab. GFP-labeled PANC-1PDX1 and PANC-1Control cells that migrated to the bottom surface of the insert were counted in five random fields.
Statistical analysis
Data were expressed as the mean (SD) or SEM. The significance of differences for the data obtained was estimated using the Wilcoxon–Mann–Whitney U test and the STATISTICA software package (Stat-Soft, Dell Software Company, Round Rock, TX, USA).
Zebrafish husbandry
Experiments on animals were conducted in strict accordance with the ethics principles prescribed by the European Convention for the Protection of Vertebrates and the bioethics norms (https://rm.coe.int/168007a67b).
The fish were kept in a flow-through aquarium system (Aqua Schwarz GmbH, Göttingen, Germany) at 28 °C. The light conditions corresponding to the international standards were maintained in a light/dark proportion of 14 hrs/10 hrs. The fish were fed once a day with Artemia salina nauplia (Barrom, Barnaul, Russia) and dry Sera Vipan food (Sera GmbH, Heinsberg, Germany). The wild-type Danio rerio AB line was used in the work.
Preparation of cancer cells for xenotransplantation
To prepare cell samples, cells were treated with 0.25% trypsin in a Hanks solution (PanEco, Moscow, Russia) and washed with a PBS solution (PanEco) to obtain cellular suspension at the concentration of 106 cells per 100 µL of PBS.
Xenotransplantation of cancer cells into Danio rerio embryo
To perform xenotransplantation, embryos at the age of 48 hpf (hours post fertilization), previously dechorionized and anesthetized with a 0.006% tricaine solution (Sigma-Aldrich, St. Louis, MO, USA), were used.
The embryos were placed onto an agarose support, and cells were injected into the yolk sac using a PicoPump PV820 pneumatic microinjector (World Precision Instruments, Inc., Sarasota, FL, USA) with the pressure parameters set at 20 psi and the sample submission time of 100 ms; an M-152 micromanipulator (Narashige Group, Tokyo, Japan); and an Olympus CKX41 microscope (Olympus, Japan). Glass capillaries (cat.# BF100-50-10 Sutter Instrument, Novato, CA, USA), with the outer diameter of 40 μm, produced by a Model P-97 device (Sutter Instrument), were employed.
The postinjection embryos were held in water at +28°C for 2 hrs, and the temperature was raised to +34 °C.
Five hours after the injections, bioimaging of the injected embryos was carried out. Individuals that showed 100–200 cells at the injection area and with no cells observed in blood vessels were selected for future analysis.
Bioimaging
Bioimaging of the embryos after xenotransplantation was performed using a Leica ICC50 HD direct fluorescence microscope (Leica Microsystems CMS GmbH, Wetzlar, Germany) equipped with a filter for GFP reporter. Evaluation of tumor cell migration in the embryo body was done using the injected embryos’ digitized images. The bioimaging of injected embryos was performed 2 days after transplantation. The percentage of the embryos with migration of transplanted cells from the injection site was estimated.
Results
Construction of PANC-1 cell line overexpressing PDX1 and PDX1 knockdown with anti-PDX1 siRNA
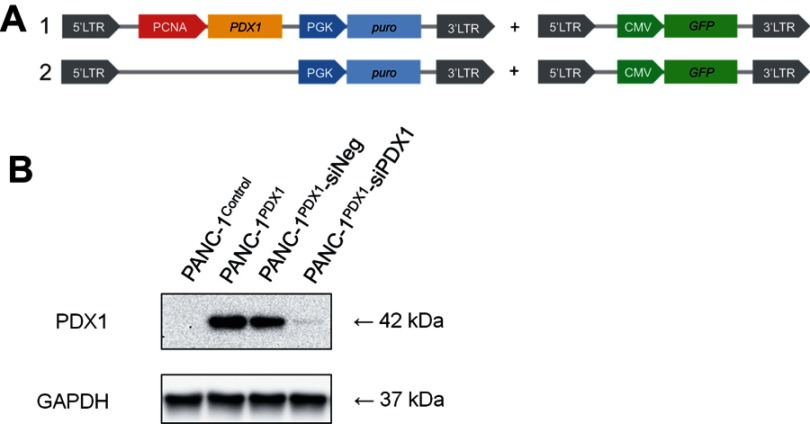
Figure 1A shows the scheme of the lentiviral construct used to analyze the effects of PDX1 overexpression on gene expression in PANC-1 cells. We used the PCNA promoter, a constitutive cellular promoter described by us earlier18 for gene expression. This promoter provides some benefits for constitutive expression of transgenes in human cells. The PCNA promoter is highly active compared to other known human promoters in a wide spectrum of normal and tumor cells of human and murine origin. Due to its smaller size, the promoter is convenient for cloning into many genetically engineered vectors sensitive to the size of inserts and allows to use longer transgene inserts.
Figure 1.
Construction of PDX1 overexpression in PANC-1 cells. (A) Scheme of the lentiviral construct used to obtain GFP-labeled PANC-1PDX1 (1) and PANC-1Control (2) cells. (B) Western blot analysis of the expression of the PDX1 protein in PANC-1PDX1 cells and in the PANC-1Control group, and the expression of PDX1 in PANC-1PDX1 after treatment with anti-PDX1 siRNA and negative siRNA. Abbreviations: PCNA - proliferating cell nuclear antigen gene promoter; PDX1 -Pancreatic And Duodenal Homeobox 1 gene; PGK - promoter of 3-phosphoglycerate kinase gene; puro - puromycin-resistance gene encoding N-acetyl-transferase; CMV - cytomegalovirus promoter; GFP - green fluorescent protein gene, 5'-LTR, 3'- LTR - 5' and 3' long terminal repeat; GAPDH - Glyceraldehyde 3-phosphate dehydrogenase; kDa - kilodalton.
According to Western blot data (Figure 1B), the expression of PDX1 protein in PANC-1PDX1 cells was conspicuously higher than in PANC-1Control cells. The expression of PDX1 protein in PANC-1PDX1 cells treated with siPDX1 was lower than in those treated with siNeg. Real-time PCR data confirmed that the relative level of PDX1 transcript in PANC-1PDX1 cells 100-fold exceeded that in PANC-1Control cells (data not shown). All the results above confirmed the successful construction of PDX1 overexpression in PANC-1 cells and PDX1 downregulation in PANC-1PDX1 cells.
PDX1 impact on PANC-1 cell proliferation
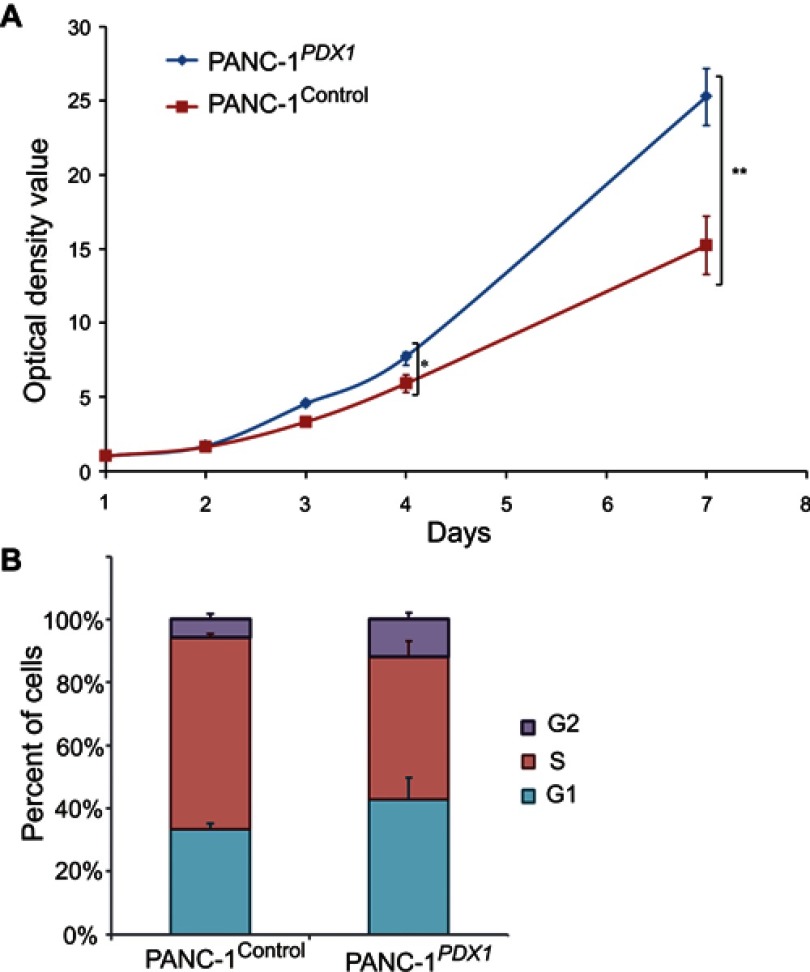
Cell proliferation rates of PANC-1Control and PANC-1PDX1 cells were analyzed by MTS assay. The results of the MTS assay showed that the growth of PANC-1Control optical density of cells in the control group was lower than that of PANC-1PDX1 cells (P<0.05, P<0.01) (Figure 2A).
Figure 2.
Effects of PDX1 overexpression on PANC-1 cell proliferation. (A) Growth curves of cells were plotted after transfection with indicated vectors by MTT assays. The OD value of cells in the control group was lower than that of PANC-1PDX1+ cells. *P<0.05, **P<0.01 compared with control group (B) Flow cytometry results showed that the number of cells in the S stage of PANC-1PDX1+ was significantly lower than of PANC1Control, whereas the number of cells in the G1 and G2 stages of PANC-1PDX1+ cells was higher than that of PANC1.Control
According to the flow cytometry results, the percentage of PANC-1Control and PANC-1PDX1 cells in the S stage was 62% and 45%, respectively. The results revealed that the number of cells in the S stage of PANC-1PDX1 cells was smaller compared with that in the control group, whereas the number of cells in the G1 and G2 stages of PANC-1PDX1 cells was higher than that of PANC-1Control cells (Figure 2B).
Suppression of migration and invasion of the PANC-1 cells due to overexpression of PDX1
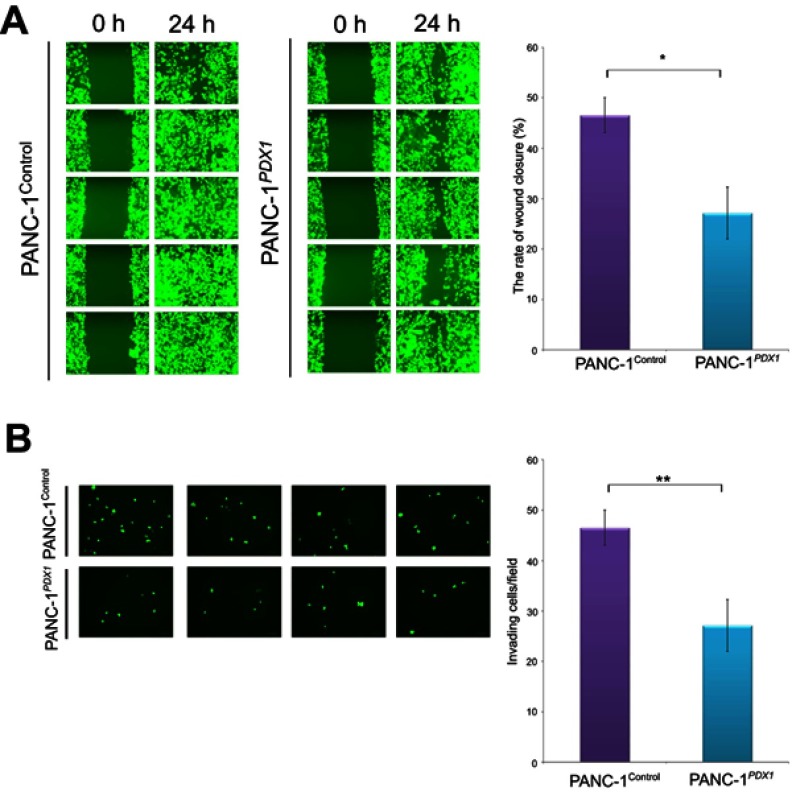
To test the effect of PDX1 expression on cell motility, a wound healing assay was employed. Compared with PANC-1Control cells, wound repair of PANC-1PDX1 cells was slowed down (Figure 3A). In addition, transwell migration assay results showed that the number of migrated cells expressing PDX1 was smaller than that of the control cells after 24 hrs (Figure 3B). As a whole, the expression of PDX1 significantly reduced both the migration and the invasion of human pancreatic cancer cells.
Figure 3.
Expression of PDX1 significantly reduces both migration and invasion of human pancreatic cancer cells. (А) Effect of PDX1 overexpression on pancreatic cancer cell wound recovery. Nearly confluent cell monolayers of PANC-1Control and PANC-1PDX1 cells were scratched, and wound recovery was monitored after 24 hrs. The rate of wound closure ± SEM is shown. The experiment was performed in triplicate with a similar trend. (B) The transwell cell invasion assay of PANC-1Control and PANC-1PDX1 cells. Representative fields of invasive cells on a membrane are captured. The average number of invasive cells per field from three independent experiments ± SEM is shown. *P<0.05; **P<0.01.
PDX1 expression affects the expression of pro-epithelial and pro-mesenchymal genes
The expression of the main pro-epithelial and pro-mesenchymal genes in PANC-1 cell line that expresses PDX1 was estimated. The results obtained are presented in Figure 4.
Figure 4.
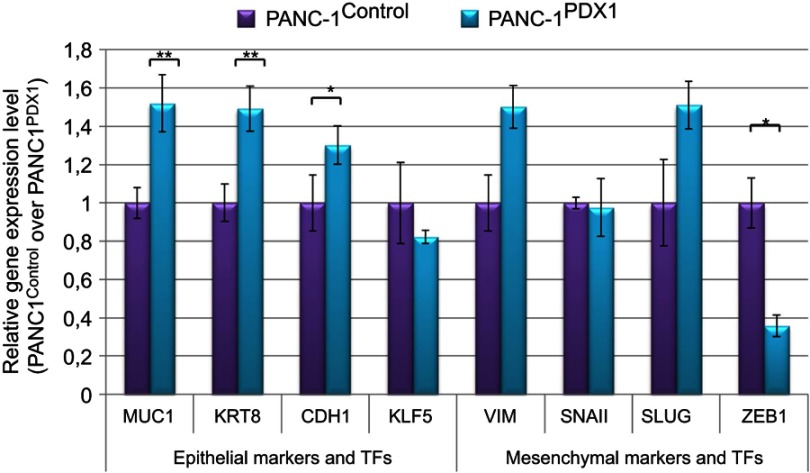
Relative expression of pro-epithelial and pro-mesenchymal genes in PANC1Control and PANC-1PDX1 cells. TFs are the transcription factors. Data are presented as the mean ± SEM for three to five independent experiments; *P<0.05, **P<0.01 compared with the control group.
The MUC1, KRT8, and CDH1 genes whose expression is characteristic of the epithelial cell type and KLF5 that maintains epithelial characteristics of cells were used as pro-epithelial markers.21 VIM, which is actively expressed in the mesenchymal cells, and SNAIL, SLUG, and ZEB1, which are transcriptional repressors of E-cadherin transcription and expressed in mesenchymal cell types, were used as pro-mesenchymal markers.
It can be seen that the expression levels of epithelial genes, such as MUC1, KRT8, and CDH1, were slightly increased in PANC-1PDX1 cells, but the expression level of the mesenchymal transcription factor gene ZEB1 was decreased, although the expression of the mesenchymal genes VIM and SLUG was slightly increased.
Assessment of the migration potential of pancreatic cancer cells expressing PDX1 in zebrafish
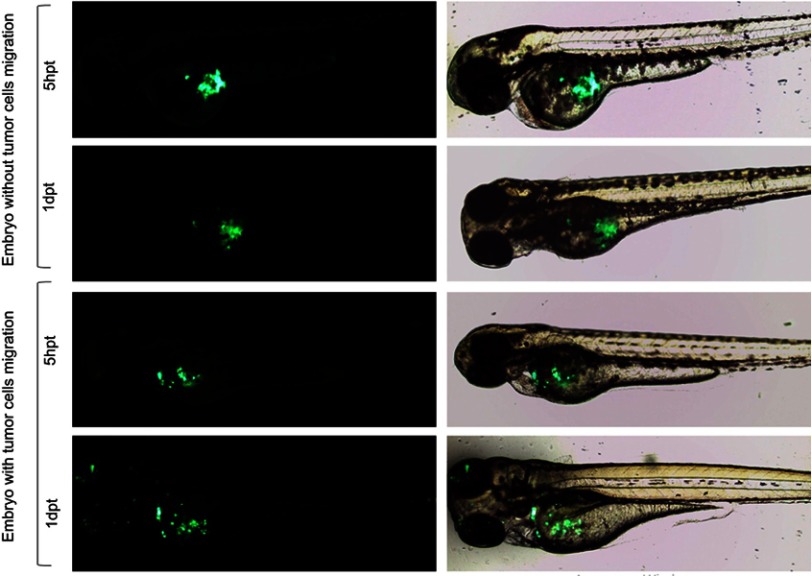
Green fluorescent-labeled PANC-1Control and PANC-1PDX1 cells were injected into the yolk sac of zebrafish embryos at 48 hpf. In all, over 500 embryos were analyzed in 8 independent experiments. As shown in Table 2, PANC-1PDX1 cells microinjected into zebrafish embryos at 2 dpt (days post-transplantation) exhibited a lower level (average 12.5%) of migration as compared with PANC-1Control cells (average 50%). Figure 5 demonstrates representative images of the migration of PANC-1PDX1/PANC-1Control cells within the injected embryos.
Table 2.
Quantitation of embryos with migrating cellsa
| Cells | PANC-1Control | PANC-1PDX1 | ||||
|---|---|---|---|---|---|---|
| siRNA | No siRNA | siPDX1 | siNeg | No siRNA | siPDX1 | siNeg |
| % of embryos with migration | 50% | 30% | 23% | 12,5% | 40% | 6% |
Note: aResults of 8 independent experiments are summarized in the table. The variation between the experiments was 10%.
Abbreviation: PDX1, pancreatic and duodenal homeobox 1.
Figure 5.
Representative images of migration of PANC-1PDX1/PANC-1Control cells within injected embryos. Fluorescent (left) and bright/fluorescent fields (right) images are presented 20× magnification. hpt is hour post-transplantation, dpt is days post-transplantation.
Further, we examined the effects of knockdown of PDX1 expressed in PANC-1PDX1 cells. The cells were transfected with siRNA prior to their injection into the yolk sac of the fish. The efficient knockdown of PDX1 in PANC-1PDX1 -siPDX1 was confirmed by Western blot analysis (Figure 1B). The effect of the PDX1 knockdown on PANC-1PDX1 cells was then evaluated morphologically in vivo. The PDX1-knockdowned and control cells were injected into the yolk sac of zebrafish embryos at 48 hpf. As shown in Table 2, PANC-1PDX1 -siPDX1 cells microinjected into zebrafish embryos at 2 dpt exhibited considerably higher levels of migration (average 40%) as compared with PANC-1PDX1 -siNeg cells (average 6%).
These findings suggested that, first, zebrafish embryo models could be used to monitor the migration of pancreatic cancer cells in a living animal, and, second, expression of PDX1 caused a significant reduction in pancreatic cancer cell migration in vivo.
Discussion
Yu et al16 tried to apply Pdx1 to achieve a therapeutic effect by suppressing Pdx1 expression. However, as pointed out by Roy et al, this was in disagreement with the fact that the most aggressive types of cancer are characterized by the lowest levels of PDX1 and the worst results were in patients whose tumors lacked PDX1.12 This observation raises the question, if gene therapeutic delivery of PDX1 into tumors with low levels of PDX1 could have a therapeutic value and, in particular, if it could not be that PDX1, which, as Roy assumed, was suppressing EMT, is an antimetastatic agent. To check this hypothesis, Danio rerio is an ideal model. It allowed to evaluate the inherent activity of PDX1, separating it from multiple factors of the PDAC microenvironment, although one cannot fully exclude that in the organism of this fish PDX1 still has partner(s) to inhibit the motility of human cells.
The results obtained in our work allow suggesting that PDX1 has indeed potential EMT suppressor activity, which is fully in keeping with the data of Roy et al12 regarding the role of PDX1 as a suppressor of the EMT at the late stages of PDAC development. This activity decreases the motility of cells probably due to inhibiting their epithelial-mesenchymal transition.
This conclusion is based on three groups of observations.
Cell motility in a wound healing assay was slowed down in PANC-1PDX1 cells as compared to original PANC-1Control cells (Figure 3A).
Transwell migration assay results showed that the number of migrated cells expressing PDX1 was smaller than that of control cells (Figure 3B).
The data of Table 2 demonstrate a significant decrease in the migration rate of PANC-1PDX1 cells as compared to original PANC-1 cells. This effect is most likely in a causal relationship with the expression of PDX1, since the suppression of this expression with the help of siRNA causes a synchronous increase in cell motility.
The mechanism of this inhibition of cancer cell spread and its relation to metastasis remains to be determined. As mentioned above, the development of metastases may be correlated to a more mesenchymal transcriptomic subtype.10 We evaluated the expression of the main pro-epithelial and pro-mesenchymal genes in PANC-1 cell line that expresses PDX1. The results obtained are presented in Figure 5 concerning pro-mesenchymal genes, expression of VIM and SLUG is slightly enhanced, while that of ZEB1 is markedly decreased. Recently, it has been claimed that EMT is dispensable for metastasis13,14 because genetic depletion of the Snail or Twist1 EMT activators had no effect on tumor initiation, invasion, or metastasis in a mouse model of PDAC. Later, Chen et al22 confirmed the existence of EMT-dependent and EMT-independent metastasis in an independent model. However, Krebs et al23 demonstrated that “In contrast to SNAIL and TWIST1, depletion of ZEB1 strongly affected the formation of precursor lesions, tumor grading, invasion and notably metastasis during PDAC progression.” The authors concluded “that EMT is important for metastasis, but there is considerable variability and tissue specificity (and not redundancy) in the role and function of different EMT-transcription factors.”
Based on the data of Krebs et al,23 we suppose that in our case a ZEB1-dependent EMT program is being carried out. A significantly reduced level of ZEB1 expression, together with the almost unchanged expression of SNAIL and SLUG may lead to a diminished EMT ability of PANC-1 cells and their reduced motility and metastatic activity.
On the other hand, factors maintaining the epithelial character of cells act in the same direction. The content of these factors (eg, PDX1: KRT8, MUC1, and CDH1) in cells expressing PDX1 is increased, although the content of KLF5 is not changed.
Conclusion
In conclusion, we believe that our data confirmed the PDX1 role as an EMT suppressor and thus, possibly, metastasis inhibitor. Further experiments on a more close-to-human model, such as a mouse, will allow to investigate this problem in more detail and to evaluate the possibility of using PDX1 as an antimetastatic agent in medicine.
Acknowledgments
The authors are thankful to Dr. B.O. Glotov for his help in preparing the paper and to Dr. K.N. Kashkin for siRNA design. The study was supported by the Russian Science Foundation (project no. 19-15-00317). The Danio rerio model development was supported by the Russian Foundation of Basic Research (project no.17-00-00194(К)). In vivo experiments were carried out on the equipment of the Center for Collective Use of the Institute of Molecular Genetics of the Russian Academy of Sciences.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dugnani E, Sordi V, Pellegrini S, et al. Gene expression analysis of embryonic pancreas development master regulators and terminal cell fate markers in resected pancreatic cancer: A correlation with clinical outcome. Pancreatology. 2018;18:945–953. doi: 10.1016/j.pan.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 2.Ansari D, Friess H, Bauden M, Samnegard J, Andersson R. Pancreatic cancer: disease dynamics, tumor biology and the role of the microenvironment. Oncotarget. 2018;9(5):6644–6651. doi: 10.18632/oncotarget.24019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reichert M, Blume K, Kleger A, Hartmann D, von Figura G. Developmental pathways direct pancreatic cancer initiation from its cellular origin. Stem Cells Int. 2016;2016:1–8. doi: 10.1155/2016/9298535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer CG, Wood LD. From somatic mutation to early detection: insights from molecular characterization of pancreatic cancer precursor lesions. J Pathol. 2018;246(4):395–404 doi: 10.1002/path.5154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mostafa ME, Erbarut-Seven I, Pehlivanoglu B, Adsay V. Pathologic classification of “pancreatic cancers”: current concepts and challenges. Chin Clin Oncol. 2017;6(6):59. doi: 10.21037/cco.2017.12.01 [DOI] [PubMed] [Google Scholar]
- 6.Ren B, Cui M, Yang G, et al. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer. 2018;17(1):108. doi: 10.1186/s12943-018-0858-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chitty JL, Filipe EC, Lucas MC, Herrmann D, Cox TR, Timpson P. Recent advances in understanding the complexities of metastasis. F1000Res. 2018;7:1169. doi: 10.12688/f1000research.15064.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S, Huang S, Sun YL. Epithelial-mesenchymal transition in pancreatic cancer: a review. Biomed Res Int. 2017;2017:1–10. doi: 10.1155/2017/2646148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giovannetti E, van der Borden CL, Frampton AE, Ali A, Firuzi O, Peters GJ. Never let it go: stopping key mechanisms underlying metastasis to fight pancreatic cancer. Semin Cancer Biol. 2017;44:43–59. doi: 10.1016/j.semcancer.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 10.Le Large TYS, Bijlsma MF, Kazemier G, van Laarhoven HWM, Giovannetti E, Jimenez CR. Key biological processes driving metastatic spread of pancreatic cancer as identified by multi-omics studies. Semin Cancer Biol. 2017;44:153–169. doi: 10.1016/j.semcancer.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 11.Sverdlov E. Missed druggable cancer hallmark: cancer-stroma symbiotic crosstalk as paradigm and hypothesis for cancer therapy. Bioessays. 2018;40(11):1800079. doi: 10.1002/bies.201800079 [DOI] [PubMed] [Google Scholar]
- 12.Roy N, Takeuchi KK, Ruggeri JM, et al. PDX1 dynamically regulates pancreatic ductal adenocarcinoma initiation and maintenance. Genes Dev. 2016;30(24):2669–2683. doi: 10.1101/gad.291021.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer KR, Durrans A, Lee S, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527(7579):472–476. doi: 10.1038/nature15748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng X, Carstens JL, Kim J, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527(7579):525–530. doi: 10.1038/nature16064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borazanci E, Dang CV, Robey RW, Bates SE, Chabot JA, Von Hoff DD. Pancreatic cancer: “a riddle wrapped in a mystery inside an enigma”. Clin Cancer Res. 2017;23(7):1629–1637. doi: 10.1158/1078-0432.CCR-16-2070 [DOI] [PubMed] [Google Scholar]
- 16.Yu J, Liu SH, Sanchez R, Nemunaitis J, Rozengurt E, Brunicardi FC. PDX1 associated therapy in translational medicine. Ann Transl Med. 2016;4(11):214. doi: 10.21037/atm.2016.03.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashkin KN, Chernov IP, Didych DA, Sverdlov ED. Construction of a combinatorial library of chimeric tumor-specific promoters. BioTechniques. 2017;63(3). doi: 10.2144/000114586 [DOI] [PubMed] [Google Scholar]
- 18.Kondratyeva LG, Kashkin KN, Chernov IP, et al. PCNA: a constitutive human promoter for gene expression for functional studies and therapeutic applications. Mol Genet Microbiol Virol. 2018;32(3):137–140. doi: 10.3103/S089141681703003X [DOI] [Google Scholar]
- 19.Kondratyeva LG, Didych DA, Chernov IP, et al. Dependence of expression of regulatory master genes of embryonic development in pancreatic cancer cells on the intracellular concentration of the master regulator PDX1. Doklady Biochem Biophys. 2017;475(1):259–263. doi: 10.1134/S1607672917040056 [DOI] [PubMed] [Google Scholar]
- 20.Ganger MT, Dietz GD, Ewing SJ. A common base method for analysis of qPCR data and the application of simple blocking in qPCR experiments. BMC Bioinformatics. 2017;18(1). doi: 10.1186/s12859-017-1949-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abjalimov IR, Zinovyeva MV, Nikolaev LG, Kopantzeva MR, Kopantzev EP, Sverdlov ED. Expression of transcription factor genes in cell lines corresponding to different stages of pancreatic cancer progression. Doklady Biochem Biophys. 2017;475(1):267–270. doi: 10.1134/S160767291704007X [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, LeBleu VS, Carstens JL, et al. Dual reporter genetic mouse models of pancreatic cancer identify an epithelial-to-mesenchymal transition-independent metastasis program. EMBO Mol Med. 2018;10(10):e9085. doi: 10.15252/emmm.201809085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krebs AM, Mitschke J, Lasierra Losada M, et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol. 2017;19(5):518–529. doi: 10.1038/ncb3513 [DOI] [PubMed] [Google Scholar]