Abstract
Background: Autism spectrum disorders (ASD) have been found to occur more frequently in individuals with Tourette syndrome (TS) than in the general population. Similarities exist between ASD and TS clinically, which suggests a potential relationship between the two conditions.
Purpose: The purpose of this study was to explore the occurrence of autism-related features in ASD and TS, focusing on areas of overlap and difference.
Patients and methods: This study examined the nature and extent of autistic traits as measured by the Social Communication Questionnaire (SCQ) in a sample with a diagnosis of TS, a sample diagnosed to have ASD, and a normative general population sample.
Results: The TS sample had significantly higher mean SCQ scores than the general population, but generally lower scores than the ASD sample. The group differences in mean SCQ scores between the TS and ASD sample were significant except in the domain of restricted repetitive behaviours (RRB).
Conclusion: This suggests that ASD traits occur commonly in the TS population, with a significant overlap in certain clinical features. This was especially the case for complex movements or repetitive behaviours, which may represent either: i) a shared phenotype which is subclinical, ii) a phenocopy where some clinical symptoms mimic each other, or iii) a co-morbidity. Awareness of this association can be useful in identifying these symptoms as part of the comprehensive assessment of TS and addressing these to improve the overall clinical outcomes in these patients.
Keywords: Autism spectrum disorder, Tourette syndrome, clinical features, co-occurrence
Introduction
Tourette syndrome (TS) is a neurodevelopmental disorder characterized by motor and vocal tics, typically involving a pre-pubertal age of onset, a waxing and waning course, and symptomatic improvement in adulthood.1,2 Approximately 1% of school-aged children will be diagnosed with TS,3 with the burden of the disorder often exacerbated by frequently co-occurring psychiatric disorders,1 particularly obsessive compulsive disorder (OCD; 50% of individuals with TS affected), Attention Deficit Hyperactivity Disorder (ADHD; 54% affected) and mood disorders (30% affected). Autism Spectrum Disorders (ASDs) and Learning Disorder4 are neurodevelopmental disorders that co-occur less frequently with TS, however, there is emerging evidence to suggest substantial convergence between the disorders at the clinical, epidemiological, genetic, and neurocognitive levels.5 ASDs are characterized by variable, persistent deficits in social interaction, verbal and non-verbal communication, and repetitive, restrictive and stereotypic (RRS) behaviors.6
TS and ASD share patterns of onset, with symptoms becoming apparent in childhood and most commonly affecting males at an estimated male:female ratio of between 4:1 and 5:1.7 Symptoms such as obsessions, compulsive behaviors, excessive sniffing and smelling, and poor control of speech including echolalia, palilalia and echopraxia, are also common in both conditions.6 More recently, visual disturbances linked to visual stress has been found to exist in children diagnosed with ASD and in children with TS.8 Perhaps the clearest point of partial symptomatic convergence is in the stereotypic behaviors which are typical of both disorders; tics are experienced by those with TS, while RRS behaviors are central to ASD. Tics and stereotypic movements can be difficult to differentiate, especially when discriminating between complex motor tics and stereotypic motor mannerisms, but there are some distinguishing features. Tics tend to be quick movements that last for very brief periods of time whilst stereotypic movements are more coordinated and unhurried. While tics are often associated with awareness and a premonitory urge, or sense of inner tension requiring release, stereotypic behaviors tend to be unconscious. Tics and RRS movements also affect different areas of the body, with tics affecting mostly the face, shoulders, arms and whole body whilst RRS movements affect the whole body, hands or trunk.9,10
Several studies have looked at the co-occurrence of TS and ASD; or, prior to 2013, the co-occurrence of TS with ASD, Pervasive Developmental Disorder (PDD), or Asperger’s Disorder, all of which are included under the umbrella of ASD in the most recent iteration of the Diagnostic and Statistical Manual of Mental Disorders.6 The most recent of these undertaken by Burd, Li, Kerbeshian, Klug, Freeman11 indicates that PDD was diagnosed in 4.6% of individuals with TS. Another study conducted by Freeman and colleagues used thorough diagnostic criteria and obtained a similar prevalence rate of 4.5%.12 Similarly, Kadesjo, Gillberg13 found 5% of individuals with TS also had previously been diagnosed with Asperger’s Disorder. However, considerably higher rates have also been found, with a recent study reporting 18% of participants with TS meeting cut-off criteria for a probable diagnosis of ASD.14 Additionally, it has been noted that autistic features occur in other neuropsychiatric disorders such as OCD and ADHD, which are often associated with TS.15 On the other hand, a study by Baron-Cohen and colleagues found the prevalence of TS in ASD to be 6.5% in institutional settings16 while Canitano, Vivanti10 reported 11% of their ASD sample as having comorbid TS. Thus it seems that the rate of TS in ASD populations is between 6–11%, while the rate of ASD in TS is 4.5–18%.
As ASDs occur on a spectrum and are highly variable in the severity and combination of symptoms experienced, individuals may possess autistic features that do not fulfill the criteria for a formal diagnosis of ASD. There is evidence that difficulties with empathy can occur at above population normative levels in people with tic disorders.13 Specifically, around two-thirds of children and adolescents with TS, who did not have a co-morbid ASD, scored above the clinical cut-off on the autism screening test used, and were also rated by their teachers as having major social interaction difficulties, while around one third were also rated as having empathy difficulties (ie they were reported to lack the ability to adjust to expectations or demands made by peers and/or they did not notice implicit negative reactions on the part of peers).13 Previous studies have found that individuals with TS have poorer expressive and receptive language compared with matched controls, and that abnormal speech patterns are sometimes present.17 There is also a growing body of evidence of a link between atypical motor function and deficits in social cognition, such as understanding another’s nonverbal communication of feelings, desires, or intentions.18 This link is more strongly evidenced in ASD than in TS populations due to a scarcity of studies focusing on TS,18 and while no clear direction of causality has been established, if the same link were to be reliably found in both populations it could hold further keys regarding potential shared mechanisms underlying features of ASD and TS.
The strong heritability of both ASD and TS implies a genetic etiology,19 while the symptomatic convergence suggests that some genetic variants may be common to both disorders, supported by recent genetic epidemiology studies.20 Molecular studies have implicated mutations of synaptic genes in several neurodevelopmental disorders, with the identification of NLGN4X mutations and the independent findings of rare variants in neurodevelopmental genes such as NRXN1, SHANK3, and SHANK2 having drawn attention to the neuroligin-neurexin complex and related molecules in the post synaptic density with consequent impact on circuitry formation.21 This would suggest a shared genetic etiology to general psychiatric impairment through overlap in involvement of neuronal circuitry and related neurotransmitters and in this regard a recent study observed a shared genetic background between TS and OCD in terms of social impairment which in turn was attributed to result in a potential overlap with ASD.14 However another study examining the shared genetic etiology through analysis of heritability among brain phenotypes found common genetic variation across a number of neurological and psychiatric disorders but failed to find a specific relationship between TS and ASD.22
Although the genetic underpinnings of the disorders may remain unclear, the presence of features in TS that also appear in symptom lists for ASD is important for researchers and clinicians to address. Greater awareness of these features when diagnosing and managing TS will facilitate treatments which address the specific symptom profile and any overlap it shares with autism.
This study therefore seeks to explore the nature and occurrence of autism-related features as assessed by the Social and Communication Questionnaire (SCQ) in a cohort of TS patients as compared to a cohort of patients diagnosed with ASD and to a general population cohort, and in so doing, to stimulate debate and enquiry about how our understanding of the etiology and management of these neurodevelopmental disorders may be sharpened by a better understanding of their shared features. Specifically, we aim to determine whether features related to autism as measured by the subscales and total scores of the SCQ differ for TS patients both in relation to ASD patients and to the general population. Secondly, we aim to explore whether any differences between the three groups are simply quantitative (that is, based just on severity or on number of endorsed features) or if such differences can be considered as being qualitative (that is, based on a distinguishable pattern of reported features that is different between groups).
Materials and methods
Participants
This study incorporated data from three groups: a sample diagnosed with TS, a sample diagnosed with ASD, and a general population or non-clinical sample (data from Mulligan, Richardson, Anney, Gill23). In total, data were analyzed for N=203 participants: n=44 with TS, n=26 with ASD, n=133 general sample. No exclusion criteria were used. The characteristics of the three cohorts are summarized in Table 1.
Table 1.
Summary of sample characteristics
| Sample | Location | Age (years) | Gender | Version of SCQ |
|---|---|---|---|---|
| Tourette Syndrome cohort (n=44) | Sydney, Australia | Min: 4 Max: 61 Mean: 18.17 |
Male: 75% Female: 25% |
Lifetime |
| Autistic Spectrum Disorder cohort (n=26) | Sydney, Australia | Min: 3 Max: 6 Mean: 4.67 |
Male: 80.77% Female: 19.23% |
Current |
| General cohort (n=133) | Dublin, Ireland | Min: 4 Max: 12 Mean: 8.25 |
Male: 48.87% Female: 51.13% |
Lifetime |
Tourette syndrome cohort
Using newsletter announcement to all of the past and present members of the Tourette Syndrome Association of Australia (n=767), an invitation was extended to participate in a research study involving completing some questionnaires without announcing the actual aim or the details of the study. Those who responded indicating an interest in participating in the research (n=101) were then provided a detailed information sheet and consent form and invited to complete the study questionnaires. This approach was taken in order to obtain as large a sample as possible for a population that is relatively small within Australia to enable meaningful analyses. Whilst this introduced a wider age range into the study, as the TS sample did include both adults and children, the use of the lifetime version of the Social and Communication Questionnaire24 has allowed the ascertainment of symptoms across children and adults in a consistent manner. Ethical approval for collection of data for the Tourette Syndrome cohort was granted by the University of New South Wales Human Research Ethics Committee.
Of the 44 individuals with complete data (6% uptake), around 46% had completed the questionnaires themselves, while the remainder had been completed by a parent or guardian.
The mean age of participants in the TS sample was 18.17 years (SD=12.65), with a range of 4–61 years. Three of the participants were non-verbal and of these, one reported a diagnosis of ASD. The sample consisted of 11 females (25.00%) and 33 males (75.00%), and the sample predominantly identified themselves as white Australian (89.00%). There were high levels of comorbid disorders in the cohort, with many reported diagnoses of anxiety (38.64%; n=17), OCD (29.55%; n=13), depression (22.73%; n=10), learning disabilities (25.00%; n=11) and ADHD (25.00%; n=11), with a comorbid diagnosis of ASD reported by five (11.36%) of the group. The average number of TS symptoms reported by each participant was 4.34 (SD=1.98).
Autism spectrum disorder cohort
A sample of individuals with ASD was recruited from a day care center for children with ASD located in Sydney, Australia. Parents completed consent forms for all participating children. Although 32 SCQ Current Forms were distributed to parents, only 26 were ultimately usable due to excessive missing data or withdrawal from the study (81.25% uptake). Thus the final sample consisted of 26 children all of whom were formally diagnosed as having an ASD by a clinician in the community. Ethical approval for collection of data for the Autism cohort was granted by the University of New South Wales Human Research Ethics Committee.
This sample comprised 21 males (80.77%) and five females (19.23%), aged between 3.34 years and 5.62 years with a mean age of 4.67 years (SD =0.61). It was found that around 53.85% of these children were also diagnosed with mild-to-severe developmental delay, and eight (30.77%) were non-verbal.
General sample
The general non-clinical sample recruited by Mulligan et al (2009) consisted of 153 individuals enrolled in a primary school serving both suburban and rural areas in the vicinity of Dublin, Ireland. The questionnaire was distributed to the pupils by their teachers, accompanied by an information letter about the study and the possibility of publication of the results. Parents were requested to complete the questionnaire anonymously and return the questionnaire blank if they did not consent to participate. The questionnaire data was incomplete for 20 members of the original cohort. These individuals were excluded from the analysis, leaving a non-clinical sample of 133 individuals between the ages of four and 12 years, with an average age of 8.25 years (SD =2.40). The group comprised 68 females (51.13%) and 65 males (48.87%), with one male participant reported to be non-verbal. Ethical approval for collection of the general sample was granted by the Child and Adolescent Mental Health Services Ethics Board.
Measures
Social communication questionnaire (SCQ)
All participants completed the Social Communication Questionnaire (SCQ). In the case of the ASD cohort the Current form was used, and in the case of the TS and general cohorts the Lifetime form was used. Both forms contain the same questions and are answered with reference to symptoms and features that are/were present in early childhood. The Current form asks respondents to reflect on the present and the recent past (the last three months), and the Lifetime form asks respondents to partly reflect on the childhood as a whole, and, for more focused items, on the year when the child was aged 4–5. The SCQ assesses autistic features as they relate to the three domains of impairment in ASDs; communication, reciprocal social interaction and restricted, repetitive and stereotyped (RRS) behaviors. It is a 40 question survey completed by parents or primary caregivers based on the Autism Diagnostic Interview Revised,24 with the first of the items not contributing to any total score calculation but indexing whether the individual is verbal or non-verbal.
The communication portion examines deficits in verbal and non-verbal communication, whilst the social section assesses deficits in social skills, facial expression and impaired imagination. RRS behaviors include: verbal rituals, stereotypic movements, and unusual preoccupations.24,25 For each item, if the abnormal feature is present it is given a score of one. The total score flags whether an individual screens positive for ASD (total score ≥15). The domain scores can also be calculated to determine if an individual has deficits in their social or communication skills, or exhibits RRS behaviors.24
The SCQ is a reliable first level instrument to screen for ASDs. The questionnaire has been used to screen both the general population and probable (at-risk) groups and has been found to have a strong specificity and sensitivity. Chandler et al.’s26 validation study of the SCQ found the survey had a strong ability to discriminate between ASD and non-ASD with a sensitivity of 0.88 and a specificity of 0.72.
Consistent with previous work, the SCQ is scored differently for non-verbal and verbal individuals, as six of the items administered to verbal participants are not applicable to non-verbal participants.27–29 Hence, the SCQ scores of non-verbal participants were converted to a ratio out of the non-verbal maximum score, then multiplied by the maximum score attainable by verbal participants to facilitate comparison between groups. For example, if a non-verbal child had a total score of 22, their adjusted total score would be calculated as: (22/33) × 39=26. The same process was used for calculation of subscale scores.
National hospital interview schedule - brief
The TS cohort completed a brief version of the National Hospital Interview Schedule.30 The sections selected for completion included items on demographics and on specific tic characteristics.
Data analysis
Group differences between the three groups’ SCQ total and domain scores (social, communication and RRS behaviors) were investigated using four sets of three planned pairwise contrasts (not assuming equal variances) based on a One-Way ANOVA variance structure, assessed at α=0.017 in order to keep family-wise Type I error rates to a maximum of 0.05. Note that according to Levene’s tests none of the SCQ scales was measured with equal variance in the three groups (all p<0.05), but that non-parametric tests returned the same pattern of significant and non-significant results as the ANOVA-based analysis, so the latter is presented for ease of interpretation. Additionally, prior to this analysis age was regressed on all SCQ scales to determine whether it was a significant covariate requiring inclusion in the models, and in all cases this resulted in a non-significant (p>0.05) model. Age was therefore not controlled for statistically as it was determined to have not affected results.
In order to determine whether SCQ item responses could be used to predict membership to each of the three groups, discriminant analysis was performed. A stepwise entry method was used, allowing all items of the SCQ for which full data was available (that is, excluding items 1 through 7 as these are left blank for non-verbal participants) to be considered within the model and the items meeting prediction thresholds to be identified. This resulted in ten items being identified, which meant the final discriminant analysis model presented is based on ten variables – well under the sample size of the smallest group (n=26).
All data were analyzed using SPSS v.23.
Results
Comparison of SCQ domain and total scores
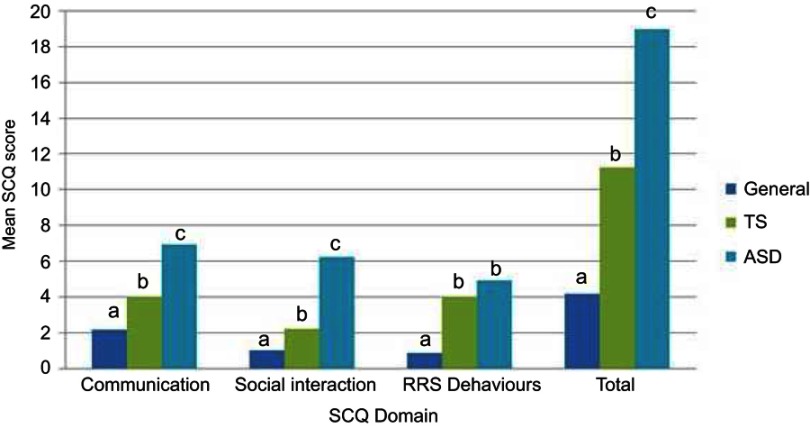
When comparing groups on total and domain scores for the SCQ, it was found that in general the ASD cohort had the most elevated scores, followed by the TS cohort, and lastly the General cohort (see Table 2). In fact, all pairwise differences between groups within domains were found to be significant (p<0.017) except for the difference between the ASD and TS groups in the domain of RRS behaviors (t(43.32)= −1.56, p=0.13). Figure 1 provides a graphical representation of the planned contrast results.
Table 2.
Means and SDs for total and domain SCQ scores by cohort
| Mean (SD) scores by SCQ domain | ||||
|---|---|---|---|---|
| Group | Communication | Social | RRS | Total SCQ |
| General (n=133) | 2.17 (1.75) | 1.02 (1.23) | 0.88 (1.24) | 4.19 (3.19) |
| TS cohort (n=44) | 4.05 (2.04) | 2.23 (2.62) | 4.04 (1.95) | 11.27 (5.26) |
| ASD cohort (n=26) | 6.93 (3.11) | 6.27 (3.40) | 4.92 (2.48) | 18.98 (7.68) |
Figure 1.
Mean differences between TS (n=44), ASD (n=26), and general (n=133) groups on the domains of the Social Communication Questionnaire (SCQ) using pairwise contrasts (within each domain, columns with the same letter label (a, b, or c) did not differ significantly from one another at p=0.017).
Discriminant analysis
All of SCQ items 8 through to 40 were entered into a stepwise discriminant analysis model based on the three cohorts represented in this study: General, TS, and ASD. This allowed all 203 participants to be included rather than just the participants who were verbal, given that the early part of the SCQ contains questions that are only answered for verbal individuals. The final model retained ten items, which are listed in the bottom panel of Table 3, using partial F values to determine threshold levels for entry and removal of items. Wilks’ λ =0.16, indicating that combination of the ten items explained around 84% of the variance.
Table 3.
Standardized coefficients and functions at group centroids for the discriminant analysis
| Functions evaluated at group centroids | ||
|---|---|---|
| Group | Function 1 | Function 2 |
| General cohort | −0.96 | 0.23 |
| TS cohort | 1.29 | −1.79 |
| ASD cohort | 2.73 | 1.84 |
| Standardized canonical discriminant function coefficients | ||
| SCQ item | Function 1 | Function 2 |
| 8. Having particular rituals | 0.22 | −0.23 |
| 10. Using other’s hand as tool | 0.10 | 0.36 |
| 11. Having special interests | 0.24 | 0.17 |
| 15. Having hand mannerisms or movements | 0.43 | −0.04 |
| 16. Having complicated whole body movements | 0.19 | −0.30 |
| 17. Engaging in self-injury | 0.22 | −0.53 |
| 19. Not having close friends | 0.33 | 0.39 |
| 21. Not copying others’ behavior | 0.21 | −0.33 |
| 24. Not nodding to indicate yes | 0.08 | 0.28 |
| 39. Not engaging in imaginative play | 0.36 | 0.25 |
Notes: All items are expressed in terms of what is indicated by a score of 1 on that item, which indicates the presence of a clinically significant symptom/feature (some have been reverse scored from how they appear in the questionnaire form)
The top panel of Table 3 illustrates that the first function separates the clinical groups from the non-clinical group, with higher values tending to belong to the ASD group rather than the TS group, and the second function more clearly separates the two clinical groups from one another. Thus, in the bottom panel of Table 3, the standardized coefficients for the first function are all positive – that is, the presence of any of these items versus its absence contributes to separating the clinical and non-clinical groups. However, particular weight is put on whether the individual exhibits hand mannerisms, does not engage in imaginative play, and does not have close friends. To a lesser extent, having special interests, engaging in self-injury, having particular rituals, and not copying other people’s behavior are also important for separating out the two clinical groups from the non-clinical group. The second function operates to distinguish the TS and ASD groups from one another. Here, participants who do not engage in self-injury, do not have close friends, who copy others’ behavior, use others’ hands as a tool, and who do not exhibit complicated whole body movements are classified as belonging to the ASD group and those who have the converse pattern are classified to the TS group.
The classification results from the discriminant analysis revealed that the model correctly allocated 91.13% of the 203 participants into their correct groups. The model operated particularly well for the general cohort, of whom 131 (98.50%) were correctly assigned with one person being misallocated to each of the TS and ASD groups. The ASD group was correctly assigned for 22 individuals (84.62%), with the remaining four members of that group all misallocated to the general group. The TS group showed the highest rate of misallocation, with two people being allocated to the ASD group, 10 being allocated to the general group, and the remaining 32 (72.73%) being allocated correctly as belonging to the TS group. By and large, the 14 individuals from the clinical cohorts who were misallocated to the general group had low scores on the SCQ (13 participants scored below 15, which is the nominated cut-point for a clinical flag on the SCQ, and 10 participants had a total SCQ of 8 or less). Similarly, of the three non-ASD cohort members assigned to that group, two had total SCQ scores above 20. Only one person was misallocated to the TS group. They came from the general cohort and had a total SCQ score above 15.
Discussion
Three distinct cohorts of individuals, in completing the SCQ, showed clear differences in their responses to the instrument. Not only were the general and ASD cohorts easily distinguishable, as would be expected for an instrument designed to screen between ASD and non-clinical populations, but the TS cohort was distinguishable from both the general and ASD cohorts. This indicates that the content of the SCQ is to an extent differentially relevant to individuals diagnosed with TS.
Results indicated that shared features between ASD and TS that maximally differentiate populations with these diagnoses from a non-clinical group include having hand mannerisms, a lack of friends, and a lack of imaginative play. Put another way, these features are highlighted in current results as being to some extent shared between ASD and TS presentations in a way that stands out in comparison to non-clinical individuals. Having special interests, engaging in self-injury, having particular rituals, and not copying other people’s behavior were also implicated as distinguishing features of the two clinical groups, however, these features together with using other peoples’ hands as tool and exhibiting complicated whole body movements were identified as the key discriminators between the ASD and TS cohorts as well. That is, participants diagnosed with TS were more likely to have reports of injuring themselves and having complicated whole body movements than those diagnosed with ASD, but they were also more likely to have friends, not copy other people’s behavior, and not attempt to use other people’s hands as tools when compared to the ASD cohort. The domain level results show another angle on this, in that the ASD and TS cohorts were differentiated from each other on all subscales except for the RRS domain, on which they could not be distinguished. The RRS domain includes some of the features from the above lists, such as hand or body movements, special interests, and having particular rituals – this is a key area where the two clinical groups stand clearly apart from a non-clinical group but not from one another.
The overlap in the RRS subscale and associated items is unsurprising, as it rests quite specifically on the known common features of ASD and TS in that both presentations involve abnormal patterns of movement and physical behavior. If we assume the “common variant common disease hypothesis” as the predominant paradigm in the field of neurodevelopmental disorders including TS and ASD,31–33 the expectation would be that any given common allele would carry moderate effects and is likely to be neither necessary nor sufficient to lead to the clinical phenotype. Instead a combination of risk alleles would be likely to contribute to the emergence of pathological traits falling at the extremes of a population distribution. However, another possibility exists in that certain traits or symptoms, whilst shared between two disorder profiles, may be duplicated due to environmental or other factors rather than due to common alleles. That is, the points of similarity could be due to phenocopy rather than phenotypic overlap. In this instance, with similarities largely contained to the RRS domain rather then spread across RRS and social or communication domains, the evidence is more consistent with an explanation based on the phenocopy hypothesis. Further research is indicated, however, to further explore this possibility.
Several other aspects of the current results show points of connection with existing knowledge. For example, having few or no friends has been reported as a common experience amongst school-aged individuals with TS,34,35 whilst deficits in the area of social interaction along with a paucity of relationships are hallmark diagnostic features of ASD.6 However, this surface level similarity glosses over the very different mechanisms that might be at work to create these outcomes. With respect to ASD, challenges in building or maintaining social connections and friendships are linked to a cluster of symptoms involving deficits in nonverbal communication, both expressive and receptive, and an abnormal approach to interaction and social reciprocity,6 whilst in TS a reduction in satisfying relationships and increasing loneliness has been posited to be linked to high rates of peer victimization or bullying.36 In other words, the ASD group might find it difficult to make friends because of their own deficits in being able to interact positively, whilst people with TS might find the same outcome because of the reticence of others to engage positively with them. The two disorders, then, might share some similar outcomes without these arising from similar mechanisms.
Thus it is possible that there are different explanations for the overlap in symptoms; 1) for a subgroup, the symptoms may represent a true overlap through a shared phenotype; 2) for some the overlap may be a phenocopy, where the clinical symptoms may suggest an overlap – for example, complex tics and tic related obsessive compulsive symptoms and non-obscene socially inappropriate behaviors characteristic of TS may mimic ASD symptoms and vice versa where the stereotypic and repetitive behaviors characteristic of ASD may mimic TS; 3) the conditions are co-morbid with different underlying mechanisms. Regardless of the reasons for the shared clinical features, it is important in clinical settings to recognize these symptoms and address them accordingly. For example, in ASD, social deficits may occur in part because the patient does not understand the social nuances while for the TS patient, they may know to some extent that the behavior is not conforming to the social norm but are not able to voluntarily modulate it. For the latter group, the age at which the symptoms start may provide an important clue in that the ASD symptoms may have an onset in the second of year of life (SCQ specifically probes for the onset of symptoms before four years), while the Tourette symptoms may arise at a later age typically around 6 years. As detailed in Table 3, the type of symptoms may also offer important ways of distinguishing the two conditions as some symptoms may be more characteristic and useful in delineating the conditions.
Conversely, some areas of overlap between TS and ASD may exist not just at the level of outcome but at the level of the underlying mechanism. One obvious place for this is in the co-occurrence of atypical features in social cognition and motor function existing in both disorders, and in the potential for a shared mechanism being at least somewhat responsible for this link.18 Whilst the current study on the domain level found a close concordance between the two disorders on RRS and less on social or communication scores, it would be of great interest to study this aspect of overlap and whether task-based and neuroimaging studies showed similarities in ASD and TS populations with respect to links between social cognition and motor ability. With regard to neurocognitive deficits, research has reported a TS cohort experiencing increased intrusions during recall on a word list, suggesting an inhibitory dysfunction during learning task, with this inhibitory dysfunction coupled with increased attention to irrelevant stimuli potentially at the core of the neuro-cognitive impairment found in pure TS without any comorbidities.37 Of course, with respect to ASD, selective attention to detail and a piece-meal rather than coherent or contextual perception of information is a key feature. There is potential here for similar underlying processes to be driving the outcome in both groups. Both ASD and TS occur more frequently in those with Attention Deficit Hyperactivity Disorder than in those without ADHD, suggesting that there may be one underlying syndrome driving the emergence of ADHD, ASD, and TS. In addition, a recent case-study has shown the thalamic deep brain stimulation was able to modulate motor tics and improve some psychiatric symptoms, including anxiety and depression, in an adult patient with TS.38 Further research is needed to better understand any similarities that might exist on a causal level.
Clinically it is important to recognize the potential co-occurrence of TS and ASD as this has implications for management. In the current study, five individuals self-reported that they held diagnoses for both ASD and TS, however the variability of results on the SCQ suggests that many more may have held a primary diagnosis of one of the disorders as well as features of the other disorder. The presence of autistic features alongside TS may lead to deficits in an individual’s communication and social skills, which can negatively affect an individual’s quality of life regardless of tic symptoms.4 Similarly, identifying tic symptoms in ASD subjects would allow appropriate management and treatment of this alongside other strategies to alleviate the impairment caused by the primary autistic features. Thus appropriate clinical intervention is dependent on a symptom-level understanding of an individual presentation, something with which an instrument like the SCQ can be used to assist.
Several limitations apply to the current study. Foremost among these is that the datasets used were recruited by different means in different cohorts, leading to potential confounding factors pertaining between the three groups. Whilst this was deemed a reasonable approach in the bid to obtain a large enough sample size to permit meaningful analyses, it did introduce a range of other potential problems to the study. These included selection bias (the TS group had a very low response rate, possibly due to the mail-out approach used for recruitment, whilst the other two groups covered the majority of individuals approached for recruitment), differing age ranges in the groups (partially offset by the use of the Current form of the SCQ for preschoolers and the Lifetime SCQ for all others), and different social contexts (Ireland and Australia, and within a school/preschool versus by membership of an association). The study also used parent-report responses for children and self-report responses for independent adults, rather than a mixture of parent/teacher reports and direct clinical assessment. In addition, whilst both SCQ forms contain the same questions, the Lifetime SCQ form refers either to childhood in general or to the period when the child was 4–5 years old, whilst the Current SCQ form is used in young children to refer to their current period of childhood. Future replications should be designed to remove these concerns through consistent data collection and sampling processes. Studies utilizing epidemiological longitudinal samples to further investigate and clarify the nature of overlap between ASD and TS, and the potential for causal pathways from one to the other, would also be of benefit
Conclusion
This study was able to demonstrate that the SCQ, designed for use in screening between ASD and general population groups, can in fact effectively differentiate between ASD, TS, and general groups. Future research should focus not only on replicating the current results with larger samples and more consistent sampling processes, but particularly on teasing out the extent to which the utility of this instrument in cohorts with ASD and TS respectively reflects similar mechanisms of deficit and dysfunction between the two disorders or, alternatively, similar outcomes born of different underlying mechanisms.
Acknowledgments
We would like to acknowledge the International Multicentre ADHD Genetics Project (IMAGE), a multisite international effort supported by NIH Grant R01MH62873 to SV Faraone, which assisted in the electronic storage of the data collected at the Irish site.
Disclosure
Professor Aisling Mulligan reports personal fees from SHIRE, during the conduct of the study and personal fees from SHIRE and from “Point of Care”, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Eapen V, Črnčec R. Tourette syndrome in children and adolescents: special considerations. J Psychosom Res. 2009;67(6):525–532. doi: 10.1016/j.jpsychores.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 2.Robertson MM, Cavanna AE, Eapen V. Gilles de la tourette syndrome and disruptive behavior disorders: prevalence, associations, and explanation of the relationships. J Neuropsychiatry Clin Neurosci. 201. 5;27(1):33–41. [DOI] [PubMed] [Google Scholar]
- 3.Robertson MM. A personal 35 year perspective on Gilles de la tourette syndrome: prevalence, phenomenology, comorbidities, and coexistent psychopathologies. Lancet Psychiatry. 2015;2(1):68–87. doi: 10.1016/S2215-0366(14)00132-1 [DOI] [PubMed] [Google Scholar]
- 4.Eapen V, Črnčec R, McPherson S, Snedden C. Tic disorders and learning disability: clinical characteristics, cognitive performance and comorbidity. Australas J Spec Educ. 2013;37(2):162–172. doi: 10.1017/jse.2013.2 [DOI] [Google Scholar]
- 5.Robertson MM, Eapen V, Singer HS, et al. Gilles de la tourette syndrome. Nat Rev Dis Primers. 2017;3:16097. doi: 10.1038/nrdp.2016.97 [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders - Fifth Edition (DSM-5). Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 7.Lai MC, Lerch JP, Floris DL, et al. Imaging sex/gender and autism in the brain: etiological implications. J Neurosci Res. 2017;95:380–397. doi: 10.1002/jnr.23948 [DOI] [PubMed] [Google Scholar]
- 8.Ludlow A, Wilkins A. Atypical sensory behaviours in children with tourette’s syndrome and in children with autism spectrum disorder. Res Dev Disabil. 2016;56:108–116. doi: 10.1016/j.ridd.2016.05.019 [DOI] [PubMed] [Google Scholar]
- 9.Stern JS, Robertson MM. Tics associated with autistic and pervasive developmental disorders. Neurol Clin. 1997;15(2):345–355. [DOI] [PubMed] [Google Scholar]
- 10.Canitano R, Vivanti G. Tics and tourette syndrome in autism spectrum disorders. Autism. 2007;11(1):19–28. doi: 10.1177/1362361307070992 [DOI] [PubMed] [Google Scholar]
- 11.Burd L, Li Q, Kerbeshian J, Klug MG, Freeman RD. Tourette syndrome and comorbid pervasive developmental disorders. J Child Neurol. 2009;24(2):170–175. doi: 10.1177/0883073808322666 [DOI] [PubMed] [Google Scholar]
- 12.Freeman RD, Fast DK, Burd L, Kerbeshian J, Robertson MM, Sandor P. An international perspective on tourette syndrome: selected findings from 3500 individuals in 22 countries. Dev Med Child Neurol. 2000;42(7):436–447. doi: 10.1017/S0012162200000839 [DOI] [PubMed] [Google Scholar]
- 13.Kadesjo B, Gillberg C. Tourette’s disorder: epidemiology and comorbidity in primary school children. J Am Acad Child Adolesc Psychiatry. 2000;39(5):548–555. doi: 10.1097/00004583-200005000-00007 [DOI] [PubMed] [Google Scholar]
- 14.Darrow SM, Grados M, Sandor P, et al. Autism spectrum symptoms in a tourette’s disorder sample. J Am Acad Child Adolesc Psychiatry. 2017;56(7):610–617. doi: 10.1016/j.jaac.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivarsson T, Melin K. Autism spectrum traits in children and adolescents with obsessive-compulsive disorder (OCD). J Anxiety Disord. 2008;22(6):969–978. doi: 10.1016/j.janxdis.2007.10.003 [DOI] [PubMed] [Google Scholar]
- 16.Baron-Cohen S, Scahill VL, Izaguirre J, Hornsey H, Robertson MM. The prevalence of Gilles de la tourette syndrome in children and adolescents with autism: a large scale study. Psychol Med. 1999;29(05):1151–1159. [DOI] [PubMed] [Google Scholar]
- 17.Burd L, Christensen T, Kerbeshian J. Speech, language, and communication in tourette’s syndrome. Annu Rev Appl Linguist. 2008;28:170–190. doi: 10.1017/S026719050808001X [DOI] [Google Scholar]
- 18.Eddy CM, Cook JL. Emotions in action: the relationship between motor function and social cognition across multiple clinical populations. Prog Neuro-Psychopharmacol Biol Psychiatry. 2018;86:229–244. doi: 10.1016/j.pnpbp.2018.05.021 [DOI] [PubMed] [Google Scholar]
- 19.O’Rourke JA, Scharf JM, Yu D, Pauls DL. The genetics of tourette syndrome: a review. J Psychosom Res. 2009;67(6):533–545. doi: 10.1016/j.jpsychores.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.State MW. The genetics of child psychiatric disorders: focus on autism and tourette syndrome. Neuron. 2010;68(2):254–269. doi: 10.1016/j.neuron.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke RA, Eapen V. Balance within the neurexin trans-synaptic connexus stabilizes behavioral control. Front Hum Neurosci. 2014;8:52. doi: 10.3389/fnhum.2014.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anttila V, Bulik-Sullivan B, Finucane H, et al. Analysis of shared heritability in common disorders of the brain. bioRxiv. 2016. doi: 10.1101/048991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulligan A, Richardson T, Anney RJL, Gill M. The social communication questionnaire in a sample of the general population of school-going children. Ir J Med Sci. 2009;178(2):193–199. doi: 10.1007/s11845-008-0184-5 [DOI] [PubMed] [Google Scholar]
- 24.Rutter M, Bailey A, Lord C. The Social Communication Questionnaire: Manual. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- 25.Goin-Kochel RP, Cohen R. Screening cases within a statewide autism registry: a comparison of parental reports using DSM-IV-TR criteria versus the SCQ. Focus Autism Other Dev Disabl. 2008;23(3):148–154. doi: 10.1177/1088357608316270 [DOI] [Google Scholar]
- 26.Chandler S, Charman T, Baird G, et al. Validation of the social communication questionnaire in a population cohort of children with autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(10):1324–1332. doi: 10.1097/chi.0b013e31812f7d8d [DOI] [PubMed] [Google Scholar]
- 27.Eapen V, Grove R, Aylward E, et al. Transition from early intervention program to primary school in children with autism spectrum disorder. World J Clin Pediatr. 2017;6(4):169–175. doi: 10.5409/wjcp.v6.i4.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fulton E, Eapen V, Crnčec R, Walter A, Rogers S. Reducing maladaptive behaviors in preschool-aged children with autism spectrum disorder using the early Start Denver model. Front Pediatr. 2014;2:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eapen V, Crnčec R, Walter A. Clinical outcomes of an early intervention program for preschool children with autism spectrum disorder in a community group setting. BMC Pediatr. 2013;13:1. doi: 10.1186/1471-2431-13-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson MM, Eapen V. The national hospital interview schedule for the assessment of Gilles de la tourette syndrome. Int J Methods Psychiatr Res. 1996;6:203–226. doi: [DOI] [Google Scholar]
- 31.Reich DE, Lander ES. On the allelic spectrum of human disease. Trends Genet. 2001;17(9):502–510. [DOI] [PubMed] [Google Scholar]
- 32.Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science. 1996;273(5281):1516–1517. doi: 10.1126/science.273.5281.1516 [DOI] [PubMed] [Google Scholar]
- 33.Chakravarti A. Population genetics—making sense out of sequence. Nat Genet. 1999;21:56–60. doi: 10.1038/4482 [DOI] [PubMed] [Google Scholar]
- 34.Sukhodolsky DG, Scahill L, Zhang H, Peterson BS, King RA, Lambroso PJ. Disruptive behavior in children with tourette’s syndrome: association with ADHD comorbidity, tic severity, and functional impairment. J Am Acad Child Adolesc Psychiatry. 2003;42:98–105. doi: 10.1097/00004583-200301000-00016 [DOI] [PubMed] [Google Scholar]
- 35.Kadesio B, Gillberg C. Tourette’s disorder: epidemiology and comorbidity in primary school children. J Am Acad Child Adolesc Psychiatry. 2000;39:548–555. doi: 10.1097/00004583-200005000-00007 [DOI] [PubMed] [Google Scholar]
- 36.Hanks CE, Lewin AB, Mutch PJ, Storch EA, Murphy TK. Social deficits and autism spectrum disorders in tourette’s syndrome. Curr Dev Disord Rep. 2015;2:285–292. doi: 10.1007/s40474-015-0060-8 [DOI] [Google Scholar]
- 37.Eddy CM, Rizzo R, Cavanna AE. Neurological aspects of tourette syndrome: a review. J Psychosom Res. 2009;67(6):503–513. doi: 10.1016/j.jpsychores.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 38.Marano M, Migliore S, Squitieri F, Insola A, Scarnati E, Mazzone P. CM-Pf deep brain stimulation and the long term management of motor and psychiatric symptoms in a case of tourette syndrome. J Clin Neurosci. 2019;62:269–272. doi: 10.1016/j.jocn.2018.12.029 [DOI] [PubMed] [Google Scholar]