Abstract
X-linked hyper IgM Syndrome (XLHIGM), the most frequent form of the Hyper IgM syndromes is a primary immune deficiency resulting from a mutation in the CD40 ligand gene (CD40LG). We analyzed the clinical and laboratory features of ten patients with XLHIGM, who were diagnosed at a tertiary care hospital in North India. Most common infections were sinopulmonary infections (80%) and diarrhea (50%). Sclerosing cholangitis and necrotising fasciitis were noted in one patient each. Three novel mutations in CD40LG (c.429_429 delA, p. G144DfsX5; c.500 G > A, p.G167E and c.156 G > C, p.K52 N) were detected. In addition, we found one missense mutation, two splice site mutations and two large deletions, which have been previously reported. Four (4) patients had expired at the time of analysis. We report the first series of XLHIGM from North India where we have documented unique features such as pulmonary alveolar proteinosis and infections with Mycobacterium sp.
Keywords: X-linked hyper-IgM syndrome, CD40 ligand, Immunoglobulin class switching, Neutropenia, Mycobacterium sp., Pulmonary alveolar proteinosis
1. Introduction
Immunoglobulin (Ig) deficiency with “elevated” IgM (HIGM) is an inborn error of immunity characterized by markedly low or nearly absent serum immunoglobulins G, A and E with an elevated or normal immunoglobulin M. Affected individuals have an increased susceptibility to develop recurrent sino-pulmonary and opportunistic infections, auto-immune diseases, neutropenia and malignancy [1]. In 1993, five groups independently discovered that X-linked form of Hyper IgM syndrome (XLHIGM) is caused by mutations in the gene encoding the protein, CD40 ligand [2–7]. The CD40 ligand protein is expressed on the surface of activated CD4 + helper T lymphocytes and binds to CD40 expressed on antigen presenting cells and B lymphocytes [8]. This cognate interaction between CD40L and CD40 provides vital co-stimulatory signals for immunoglobulin class switching, somatic hypermutation, T cell priming, macrophage and dendritic cell activation. Most infants having CD40 ligand deficiency present with early onset of severe recurrent sino-pulmonary infections, diarrhea, and intermittent or persistent neutropenia. Pneumonia due to Pneumocystis jirovecii and diarrhea due to Cryptosporidium parvum are classically noted in XLHIGM syndrome [9]–[11].
In this study, we describe the clinical and molecular details of a North Indian cohort of patients with XLHIGM syndrome.
2. Materials and methods
2.1. Sample collection from patients and healthy controls
Patients included in this study were followed in the Primary Immunodeficiency Clinic at the Department of Pediatrics, Advanced Pediatrics Centre, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India, between 1995 and 2017. PGIMER is a tertiary referral care centre in North India. Our unit has also been sanctioned a centre for “Advanced Research in Primary Immunodeficiency Diseases” by the Indian Council of Medical Research, New Delhi. Inclusion criteria for enrolment included normal or elevated Immunoglobulin M, low or absent serum Immunoglobulin G and Immunoglobulin A (≤2 standard deviation [SD] below normal values for age) and early onset of severe sino-pulmonary infections. After obtaining an informed written consent, five to eight milliliters venous blood was collected from each patient in heparin and EDTA vacutainers. Blood samples of other family members and a healthy adult control were also obtained simultaneously, whenever possible.
2.2. PBMC isolation
PBMCs from the healthy controls (HCs) and patients were isolated using density gradient centrifugation. Briefly, 5 mL of heparinized blood was diluted with RPMI 1640 medium (Sigma Aldrich, USA) in 1:1 ratio and layered over Histopaque-1077 (Sigma Aldrich, USA) in a ratio of 3:1 followed by centrifugation at 1200 rpm for 40 min at 18–22 °C. The buffy layer containing PBMCs was obtained, washed and re-suspended in 1 mL RPMI-1640 medium supplemented with 2 mM L-glutamine and antibiotics cocktail (penicillin, streptomycin and amphotericin B, Sigma Aldrich, USA). Cell viability was checked by trypan-blue dye (Sigma Aldrich, USA) exclusion test (> 90% PBMCs were viable) and cells were counted on hematocytometer (Neubauer’s chamber, Marienfeld, Germany) under an upright light microscope (Olympus, Japan).
2.3. Detection of CD40L expression by flow cytometry
Briefly, 5 × 105 PBMCs in RPMI 1640 media supplemented with 10% FBS were stimulated for 4 h with 40 ng/mL phorbol-12-myristate 13-acetate (PMA, Sigma Aldrich, USA) and 1 μg/mL Ionomycin (Sigma Aldrich, USA) in humidified atmosphere at 5% CO2 in CO2 incubator (New Brunswick, Eppendorf, USA). Cells were washed with PBS, and cell surface staining was done with FITC labelled anti-CD3 antibody, (BD Biosciences, USA) and Allophycocyanin (APC) conjugated anti-CD154 antibody (anti-CD40L) (BD Biosciences, USA). Cells were washed, and flow cytometry studies were performed on BD FACS Aria III using BD FACS Diva software (BD Biosciences, USA). Expression of CD40L in the patients is always compared with the expression in the normal controls. The normal controls were age and sex-matched, and belong to North India.
2.4. Detection of mutation in CD40LG by polymerase chain reaction and sanger sequencing
Genomic DNA was extracted using QIAamp DNA Blood Mini Kit according to the manufacturer’s protocol (Qiagen, Hilden, Germany) from EDTA anticoagulated blood samples. All five exons and exon/intron junctions of the CD40LG were individually amplified using polymerase chain reaction and specific oligonucleotide primers which were obtained from Resource of Asian Primary Immunodeficiency Database (RAPID). The PCR products were qualitatively examined by 1.5% Agarose gel electrophoresis followed by purification and direct sequencing using the ABI Big Dye Terminator kit and ABI 3500 Gene Analyzer (Applied Biosystems). Sequencing results were analyzed using CodonCode Aligner software (CodonCode Corporation, Centerville, MA).
3. Results
Ten patients were diagnosed with X-linked Hyper IgM syndrome. CD40LG gene sequencing was performed in 9 patients. Parents of Pt.10 did not give consent for genetic analysis. Patients 1 and 2 were brothers. However, others were not related to each other. Median age at onset of clinical manifestations and diagnosis of Hyper IgM syndrome was 0.63 years (range: 0.25–1 years), and 3.5 years (range: 0.67–9 years) respectively. Median delay in the diagnosis of hyper IgM syndrome was 3.13 years (range: 0–8.75 years). Two children were managed initially as a case of congenital neutropenia before the diagnosis of hyper IgM syndrome was established [Table 1].
Table 1.
Clinical and immunological features of the North Indian cohort of patients with XLHIGM
| Patient No. | Age of onset (years) | Age at diagnosis (years) | Delay in diagnosis (years) | Predominant infections | Organisms isolated | IgG (mg/dl) | IgA (mg/dl) | IgM (mg/dl) | Treatment given | Outcome | Other manifestations | Neutropenia | GCSF | Follow up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt.l | 0.67 | 0.67 | 0 | Pneumonia, diarrhea, psoas abscess | C.difficile in stools, Klebsiella pneumoniae in urine culture | < 95 | 10 (15–70) | 63 (40–160) | IVIg (intermittent), cotrimoxazole, antimicrobials | Expired at 2.5 years of age (psoas abscess with sepsis) | None | Yes | Given (only at first month at diagnosis) | 2 years |
| Pt.2 | 0.67 | 0.67 | 0 | Otherwise asymptomatic | None | < 92 | < 17 | 31 (60–210) | Cotrimoxazole prophylaxis | Surviving | None | NA | Not given | 1 year |
| Pt.3 | 1 | 5 | 4 | Pneumonia, diarrhea | Klebsiella pneumoniae | 308 (490–1610) | 65 (40–200) | 314 (50–200) | IVIg, cotrimoxazole, antimicrobials | Well on IVIg | Pulmonary alveolar proteinosis, Recurrent episodes of anemia requiring blood transfusions | No | Not given | 8 years |
| Pt.4 | 0.58 | 1.5 | 1 | Recurrent pneumonia, pulmonary tuberculosis, diarrhea, recurrent oral ulcers | Mycobacterium tuberculosis | < 94 | < 17 | 60 (50–220) | IVIg, cotrimoxazole, antimicrobials | Expired at 3 years of age (bronchopneumonia) | None | Yes (ANC: 1328) | Not given | 1.5 years |
| Pt.5 | 0.5 | 4 | 3.5 | Cervical lymphadenitis, Necrotizing fasciitis of the right eyelid leading to thinning of cornea | None | 475 (490–1610) | < 17 | 676 (50–200) | IVIg 5 g monthly, cotrimoxazole, antimicrobials | Well on IVIg | None | Yes | Not given | 1 year |
| Pt.6 | 0.25 | 3 | 2.75 | Recurrent episodes of fever with cough, oral candidiasis | None | 500 (490–1610) | 60 (40–200) | 76 (50–200) | IVIg, GCSF, antimicrobials | Well on IVIg | None | Yes | Given | 1 year |
| Pt.7 | 1 | 5 | 4 | Pneumonia | None | < 75 | < 10 | 130 | IVIg, Cotrimoxazole prophylaxis | Lost to follow-up | Arthritis (left knee, right elbow, right ankle), anemia requiring recurrent blood transfusion | Yes | - | Lost to follow up |
| Pt.8 | 1.5 | 3 | 1.5 | Diarrhea, Otitis media, Pneumonia, Empyema | None | < 202 | < 17 | 197 (50–200) | Cotrimoxazole (therapeutic for 14 days followed by prophylaxis), IVIg, antimicrobials | Lost to follow-up | Right Femoral Vein thrombosis | No | Not given | Lost to follow-up |
| Pt.9 | 0.25 | 9 | 8.75 | Recurrent pneumonia, diarrhea, Otitis media, meningitis, Transfusion acquired hepatitis B | None | 16 (72–265) | 34 (61–227) | 410 (51–332) | IVIg, Cotrimoxazole, antimicrobials | Expired at 11 years (cause: Not known) | Arthritis | No | NA | 2 years |
| Pt.10 | 0.25 | 5 | 4.5 | Recurrent diarrhea, Pneumonia, Sclerosing cholangitis | None | < 95 | 6 | 125 | Not given | Expired at the time of diagnosis (5 years) (severe pneumonia) | - | No | Not given | 0.1 years |
IVIg – Intravenous Immunoglobulin, G-CSF – Granulocyte Colony Stimulating Factor.
3.1. Clinical profile
Clinical manifestations present at the onset of disease included sinopulmonary infections (n = 5), diarrhea (n = 2), cervical adenitis (n = 1), and oral ulcers (n = 1). Pt.2, younger brother of Pt.1 was clinically asymptomatic, and diagnosed at the age of 7 months during screening as he was suspected because of his affected elder brother. Recurrent sinopulmonary infections were noted in 8 children. Two children had typical clinical features of Pneumocystis jirovecii pneumonia- bilateral parahilar infiltrates with ground glass opacities, respiratory distress with minimal auscultatory signs, and hypoxemia, however, microbiological confirmation could not be done in these cases. Recurrent diarrheal illness was noted in 5 patients. Pt. 1 had persistent troublesome diarrhea which was unresponsive to anti-microbials, nitazoxanide, intravenous immunoglobulin (IVIg), and nutritional rehabilitation. Clostridium difficile toxin assay was positive in the stool of that patient. One patient (Pt. 5) had necrotising fasciitis of right side of face involving the eyelid that progressed to corneal thinning and prolapse of the anterior chamber and subsequent vision loss. One patient (Pt. 3) had features of chronic interstitial lung disease and the lung biopsy revealed features of pulmonary alveolar proteinosis. After initiation of monthly immunoglobulin replacement therapy, the pulmonary manifestations resolved over time. Arthritis was noted in 2 patients. The arthritis of Pt. 7 resolved with the use of non-steroidal anti-inflammatory medications and monthly IVIg replacement therapy. Recurrent episodes of anemia requiring blood transfusions were noted in 2 patients (Pt. 3 & 9) that resolved with monthly replacement IVIg.
3.2. Immunological features
IgG levels were decreased in 9 patients at the time of diagnosis. One patient had IgG levels within the normal range [9]. IgA levels were decreased in 8 patients, whereas, two patients had normal levels [12, 13]. Class-switched memory B cells were assessed in three children and were found to be low when compared to age and sex-matched pediatric healthy control for each patient. Neutropenia was noted in 5 children (50%). G-CSF therapy was used in 3 patients only at the time of diagnosis, and, none of the patients required long-term usage. Two patients (Pt. 5 and 6) had severe neutropenia (ANC- 0.42 × 109/L & 0.3 × 109/L, respectively) before the diagnosis and it showed progressive improvement following initiation of monthly replacement IVIg prophylaxis. ANC at last follow-up was 6.2 × 109/L and 2.1 × 109/L, respectively. Patient 1 who had a complete absence of CD40L protein expression by flow cytometry had severe neutropenia (ANC- 0.28 × 109/L) at presentation requiring 5 μg/kg of subcutaneous granulocyte colony stimulating factor (G-CSF) for 15 days that improved the ANC to 2.2 × 109/L. However, 2 years later, his ANC was 12.6 × 109/L and this spontaneous improvement was noted without use of G-CSF.
3.3. CD40 ligand expression by flow cytometry
CD40 ligand expression on activated T helper lymphocytes by flow cytometry was assessed in 7 children and was found to be low in all of them (Supplementary Table 1).
Median percentage expression and MFI in the cases were 0.9% (range: 0–6%), and 542 (range: 0–865), respectively. Median percentage expression and MFI in the controls were 50.2% (range: 37.4–51.5%), and 2462 (range: 1474–3976), respectively.
3.4. Follow-up and mortality
Pt. 7 was lost to follow-up after initial work-up. Median follow-up period of the remaining 9 patients was 1 year (Range: 0.1–8). IVIg replacement therapy was successfully continued in 6 children. Three (3) children (Pts 1, 2 and 10) received IVIg only during clinical presentation and the parents denied regular monthly IVIg replacement therapy. Four children had expired at the time of analysis. Two patients (Pt. 4& 9) succumbed to severe pneumonia despite adequate replacement IVIg therapy. Patient 1 expired at the age of 2.75 years due to psoas abscess, refractory diarrhea, and septicemia. Patient 10, succumbed to severe pneumonia at the time of initial evaluation.
3.5. CD40LG mutation analysis
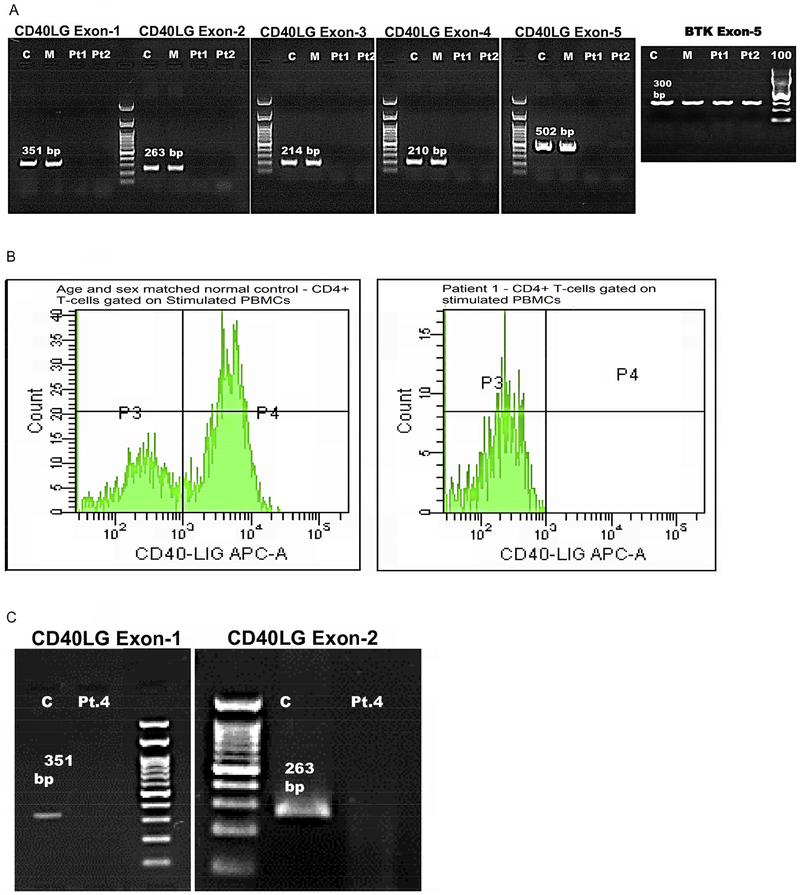
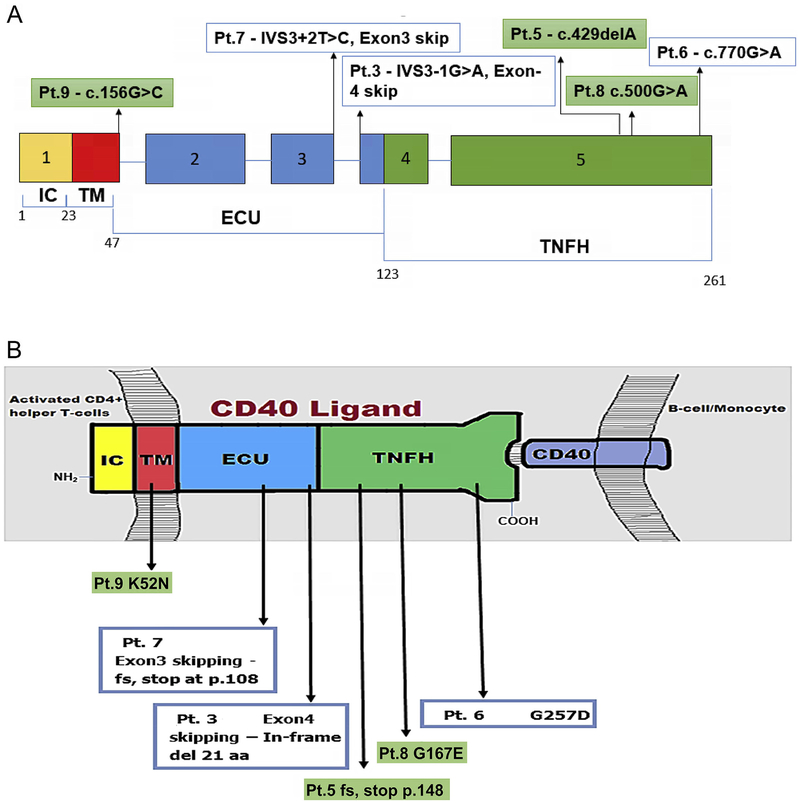
In our cohort of 10 patients, CD40LG sequencing was performed in 9 patients from 8 families. Eight variants: 5 previously reported and 3 novel variants were found [Supplementary Table 1]. The 3 novel variants have not been reported in the 1000 genomes database, Human Gene Mutation Database (HGMD), CD40Ligand gene database (CD40Lbase), ClinVar database and The Genome Aggregation Database (gnomAD), and were not detected in 50 normal healthy individuals who were screened for these variants. Novel variants included a single nucleotide deletion and two missense mutations. Single nucleotide deletion of A at c.429_429 of exon 5 resulting in a premature stop codon at p.148 lead to the formation of a truncated protein (Pt. 5) affecting the region encoding for part of the extracellular Tumor necrosis factor homology domain (TNFH). A missense mutation of c.G500A in Exon 5 (Pt. 8) resulted in the incorporation of polar, charged Glutamate instead of hydrophobic non-polar Glycine amino acid at codon 167 affecting the extracellular Tumor necrosis factor homology domain (TNFH). Another novel missense mutation of c.G156C in Exon-1 (Pt. 9) resulted in incorporation of polar, uncharged Asparagine instead of polar, positively charged basic amino acid Lysine at codon 52, affecting the N-terminal Intracellular domain (IC). Two brothers had gross gene deletion involving all the five coding exons of the CD40LG (Pt. 1& Pt. 2) as previously reported [Fig. 1a] [12]. Another patient, (Pt. 4) showed Exon 1–2 deletion [13]. Splice acceptor mutation IVS3–1 (G → A) affecting extracellular TNFH domain was detected in a patient (Pt. 3). Splice donor mutation IVS3 + 2 (T → C) was seen in another patient (Pt. 7).
Fig. 1.
(A) Agarose Gel (1.5%) electrophoresis of PCR products from amplification of the five exons of CD40LG gene and BTK Exon-5 (300 bp) of Pt.1, Pt.2, Mother and an age-matched healthy control. Fig. 1(B) CD40L expression by flow cytometry: Histogram depicting CD40L expression on CD4+ activated T cells; Left panel showing normal CD40L expression in control (50.3%); Right panel with no CD40L expression in Pt 1 (0%). Fig. 1(C) Agarose Gel (1.5%) electrophoresis of PCR products from amplification of Exon 1 and Exon 2 CD40LG of Pt.4 and an age matched healthy control. No amplification of exons 1 and 2 in Pt. 4.
4. Discussion
We describe a cohort of 10 patients with X-linked hyper IgM syndrome from North India. The previous series from India described 7 patients from the Western part of India (Mumbai) [14]. Median age of onset of symptoms in our cohort (7.2 months) is comparable to other cohorts. However, there was a significant delay in diagnosis of immunodeficiency with the median age at diagnosis of 3.5 years. Contributing factors are the lack of easy access to the immunological investigations and lack of sufficient awareness of primary immunodeficiency diseases among the general physicians and pediatricians [15]. Two children in our cohort were managed as cases of congenital neutropenia with G-CSF for several months before a correct diagnosis of XLHIGM syndrome could be made. This emphasizes the fact that serum immunoglobulin profile must be performed in cases of neutropenia especially in male children with recurrent infections.
Infection profile seen in our cohort is similar to that of previously published reports [Table 1]. Recurrent sinopulmonary infections and diarrhea were the most common infections in our cohort. Though we had 2 children with typical clinical features of Pneumocystis jirovecii pneumonia, we could not isolate the organism in any of our patients. Inability to perform invasive procedures such as bronchoalveolar lavage and lung biopsy in sick children and non-availability of molecular methods to identify the organism are the probable reasons. Two children had manifestations of neutropenia in the form of recurrent oral ulcers. Sclerosing cholangitis was seen in one of our patients which could be the result of persistent Cryptosporidium infection of the bile ductular epithelium. Pt.4 presented with pneumonia due to Mycobacterium sp. Infections with Mycobacterium sp. in XL hyper IgM syndrome has been documented in countries that are endemic for tuberculosis and where neonatal BCG vaccination is mandatory i.e., China, India, and Latin America [14, 16, 17]. It reiterates the fact that CD40-CD40L interaction among the macrophage and lymphocytes is required in the clearance of Mycobacterium sp.
Recurrent anemia requiring transfusion and arthritis have been documented in the report from USIDNET registry [18]. Pulmonary alveolar proteinosis (PAP) was seen in the lung biopsy of one of our patients with a splice site mutation in CD40L. To the best of our knowledge, only one case of PAP has been previously documented in XL hyper IgM syndrome [19]. PAP could be either primary or secondary due to infections in our patient. The proposed pathogenic mechanism for PAP is the defective macrophage function in CD40L defect leading to reduced catabolism of surfactant in the alveoli. After institution of regular monthly IVIg infusions, our patient had a clearance of lung infiltrates, indicating that the etiology be either post-infective or autoimmune.
Etiology of neutropenia is not known in XLHIGM. Previous studies have shown that CD40–40 L interactions play a role in myelopoiesis and this could be a possible reason for neutropenia [20]. In the present study, neutropenia was seen in five of our patients. Improvement in neutrophil counts over time noted in our cohort suggest either an autoimmune process or better control of infections that could result in secondary neutropenia. However, previous studies have not documented the presence of anti-neutrophil antibodies in XLHIGM [1]. An arrest of myelopoiesis at the promyelocyte or myelocyte stage has been documented on bone marrow examination in XLHIGM patients with neutropenia [10]. In contrast, serum granulocyte colony stimulating factor (G-CSF) levels in XLHIGM with neutropenia are normal or increased. However, treatment with recombinant G-CSF have been documented to result in increase in neutrophil counts and significantly reduction in infections in XLHIGM patients with neutropenia [21]. Presence of neutropenia probably did not influence the mortality outcomes in our cohort as only one child had uncorrected neutropenia and the remaining three patients were not noted to have neutropenia at the time of their demise. Functional defects in neutrophils have also been recently described in XLHIGM syndrome that could be partially reversed with recombinant human interferon gamma (rhIFNγ) [22].
We have documented normal IgA levels in 2 patients and normal IgG levels in 1 patient with XL hyper IgM syndrome. IgG levels can be normal in some cases as documented in previous studies [9, 12]. Also, it is also important to note that only 50% of patients have increased levels of IgM and some patients also have elevated IgA levels [13]. CD40 ligand expression performed in 7 of our patients showed reduced expression compared to the normal control. A complete absence of the protein expression (0%) was noted in patients (1&2) who had a deletion of all 5 exons of CD40LG [Fig. 1a]. A normal CD40L expression in the activated lymphocytes with defective CD40-Ig binding has also been documented in patients with CD40L defect, especially in in-frame insertions/deletions and missense mutations [13]. However, we have not documented normal expression in our cohort.
CD40LG comprises five exons encoding the various domains of the 39-kDa protein composed of 261 amino acids [23]. CD40L is a type-II surface glycoprotein which belongs to the family of Tumor Necrosis Factor receptor proteins. It is a monomer consisting of 4 structural domains: An N-terminal intracellular domain (IC), a trans-membrane domain (TM), extracellular unique domain (EU) and extracellular Tumor Necrosis Factor Homology domain (TNFH) at the C-terminal [Fig. 2b] [24]. Most of the previously reported CD40LG variants are single nucleotide substitutions in Exon-5 encoding for the TNFH domain which binds the CD40 [13, 14, 18]. Previous studies have also reported mutations in the CD40LG promoter region [24]. The Human Gene Mutation Database (HGMD) has recorded 180 mutations in the CD40LG till date, missense and nonsense mutations are the most common. Previous studies in XLHIGM have shown a similar spectrum of mutations in CD40LG seen in our cohort i.e., deletions are the commonest among the mutations followed by missense, nonsense and splice site mutations [Supplementary Table 2] [[12]–[14, 16]–[18, 25–28].
Fig. 2.
(A)Schematic diagram showing exons of CD40L gene and corresponding domains of CD40L protein with position of single nucleotide substitutions (n = 5) and single nucleotide deletion (n = 1) in 6 pts. Pts 1&2 with deletions of Exons1–5 and Pt.4 with deletion of Exons 1–2 not depicted. Fig. 2(B) Cartoon depicting CD40L protein domains with predicted effects of the six mutations annotated in Fig. 2A. [IC- Intracellular Domain, TM- Transmembrane domain, ECU – Extracellular Unique Domain, TNFH–Tumor Necrosis Factor Homology Domain].
In our cohort, we encountered three novel mutations. All the three patients showed clinical phenotypes suggestive of XLHIGM characterized by early onset of sino-pulmonary infections and reduced CD40L expression. Pt. 8 had novel missense mutation of c.G500A in Exon-5. The PolyPhen-2 scored this mutation as 1.000, which is a probably damaging mutation. This mutation was predicted to be disease-causing by Mutation Taster, with a score of 0.999963. Another online pathogenicity prediction tool, Combined Annotation Dependent Depletion (CADD) scored 27.5, which is pathogenic. Another novel missense mutation- c.G156C in Exon-1 in Pt. 9 was predicted to be probably damaging with a PolyPhen-2 score of 0.999. This mutation was predicted to be disease-causing by Mutation Taster, with a score of 0.74961. CADD score for this mutation was 25.8, which is pathogenic. We were able to do carrier screening for only four patients namely Pt. 5, Pt. 6, Pt. 7 and Pt. 8. All the mothers were carriers for the same mutation found in their respective children.
Four (4) children had expired at the time of analysis. The mortality rate is disproportionately high (40%) compared to other cohorts [Supplementary Table 2]. Contributing factors for high mortality are the late clinical diagnosis and lack of readily available hematopoietic stem cell transplant (HSCT) services. Two children expired despite replacement IVIg therapy and co-trimoxazole prophylaxis, emphasizing the need of early hematopoietic stem cell transplant (HSCT) in XL hyper IgM syndrome. Survival rates in XLHIGM following HSCT, have proven to be superior compared to patients who are not transplanted in a study from Japan [12]. However, a recent multicentre data found no difference in long-term survival rates between HSCT and non-HSCT groups in XLHIGM. The study has also shown that children who were diagnosed and transplanted before 5 years of age had better survival rates than that of children who are transplanted > 5 years of age [29].
5. Conclusion
Amidst the significant lack of awareness of primary immunodeficiency disorders (PIDs) among physicians and delayed referrals and diagnosis, we could identify 10 patients with XLHIGM syndrome in the last 22 years at our Centre. We envisage that the numbers would go up with the increase in awareness of PIDs and prompt referral for appropriate investigations. Notable clinical phenotypes of XLHIGM in our cohort include pulmonary alveolar proteinosis and infection with Mycobacterium sp. Neutropenia, though, observed in 5 of our patients, was not long-lasting and improved with age. Among the molecular features, we observed deletions and missense mutations in CD40LG to be prevalent. Three novel variants in CD40LG were also observed.
Supplementary Material
Acknowledgements
The authors thankfully acknowledge India Council of Medical Research, New Delhi, India and Department of Health Research, Ministry of Health and Family Welfare, Govt of India, New Delhi, India for funding vide Grant No GIA/48/2014-DHR and The Foundation for Primary Immunodeficiencies (FPID), USA. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
This study was approved by the Departmental Review Board of Pediatrics at the Advanced Pediatrics Centre, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
We deeply thank the patients and their parents for their cooperation.
Funding
This work was supported by the grant from Indian Council of Medical Research, New Delhi and Department of Health Research, Ministry of Health and Family Welfare, Govt of India, New Delhi. [Grant No GIA/48/2014-DHR to one of the authors (SS)].
Abbreviations:
- XLHIGM
X-linked hyper IgM syndrome
- IVIg
Intravenous immunoglobulin
- EDTA
Ethylene diamine tetraacetate
- G-CSF
Granculocyte Colony Stimulating factor
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clim.2018.07.013.
Conflict of interest
The authors declare no conflict of interest.
References
- [1].Notarangelo LD, Duse M, Ugazio AG, Immunodeficiency with hyper-IgM (HIM), Immunodefic. Rev 3 (1992) 101–121. [PubMed] [Google Scholar]
- [2].Fuleihan R, et al. , Defective expression of the CD40 ligand in X chromosome-linked immunoglobulin deficiency with normal or elevated IgM, Proc. Natl. Acad. Sci. U. S. A 90 (1993) 2170–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Allen RC, et al. , CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome, Science 259 (1993) 990–993. [DOI] [PubMed] [Google Scholar]
- [4].Aruffo A, et al. , The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome, Cell 72 (1993) 291–300. [DOI] [PubMed] [Google Scholar]
- [5].Disanto JP, Bonnefoy JY, Gauchat JF, Fischer A, de Saint Basile G, CD40 ligand mutations in x-linked immunodeficiency with hyper-IgM, Nature 361 (1993) 541–543. [DOI] [PubMed] [Google Scholar]
- [6].Korthäuer U, et al. , Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM, Nature 361 (1992) 539–541. [DOI] [PubMed] [Google Scholar]
- [7].Ramesh N, et al. , Deletions in the ligand for CD40 in X-linked immunoglobulin deficiency with normal or elevated IgM (HIGMX-1), Int. Immunol 5 (1993) 769–773. [DOI] [PubMed] [Google Scholar]
- [8].Fuleihan R, et al. , Cyclosporin A inhibits CD40 ligand expression in T lymphocytes, J. Clin. Invest 93 (1994) 1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].de la Morena MT, Clinical phenotypes of hyper-IgM syndromes, J Allergy Clin Immunol Pract 4 (2016) 1023–1036. [DOI] [PubMed] [Google Scholar]
- [10].Levy J, et al. , Clinical spectrum of X-linked hyper-IgM syndrome, J. Pediatr 131 (1) (1997) 47–54. [DOI] [PubMed] [Google Scholar]
- [11].Aghamohammadi A, et al. , Clinical and laboratory findings in hyper-IgM syndrome with novel CD40L and AICDA mutations, J. Clin. Immunol 29 (2009) 769–776. [DOI] [PubMed] [Google Scholar]
- [12].Mitsui-Sekinaka K, et al. , Clinical features and hematopoietic stem cell transplantations for CD40 ligand deficiency in Japan, J. Allergy Clin. Immunol 136 (2015) 1018–1024. [DOI] [PubMed] [Google Scholar]
- [13].Lee W-I, Torgerson TR, Schumacher MJ, Yel L, Zhu Q, Ochs HD, Molecular analysis of a large cohort of patients with the hyper immunoglobulin M (IgM) syndrome, Blood 105 (2005) (1881–1890). [DOI] [PubMed] [Google Scholar]
- [14].Madkaikar M, et al. , X-linked hyper IgM syndrome: clinical, immunological and molecular features in patients from India, Blood Cells Mol. Dis 53 (2014) 99–104. [DOI] [PubMed] [Google Scholar]
- [15].Gupta S, Madkaikar M, Singh S, Sehgal S, Primary immunodeficiencies in India: a perspective, Ann. N. Y. Acad. Sci 1250 (2012) 73–79. [DOI] [PubMed] [Google Scholar]
- [16].Wang L-L, et al. , Clinical features and genetic analysis of 20 Chinese patients with X-linked hyper-IgM syndrome, J Immunol Res 683160 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cabral-Marques O, et al. , First report of the Hyper-IgM syndrome Registry of the Latin American Society for Immunodeficiencies: novel mutations, unique infections, and outcomes, J. Clin. Immunol 34 (2014) 146–156. [DOI] [PubMed] [Google Scholar]
- [18].Leven EA, et al. , Hyper IgM syndrome: a report from the USIDNET registry, J. Clin. Immunol 36 (2016) 490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gallagher J, et al. , X-linked hyper IgM syndrome presenting as pulmonary alveolar Proteinosis, J. Clin. Immunol 36 (2016) 564–570. [DOI] [PubMed] [Google Scholar]
- [20].Mavroudi I, Papadaki HA, The role of CD40/CD40 ligand interactions in bone marrow Granulopoiesis, Sci. World J 11 (2011) 2011–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang WC, Cordoba J, Infante AJ, Conley ME, Successful treatment of neutropenia in the hyper-immunoglobulin M syndrome with granulocyte colony-stimulating factor, Am. J. Pediatr. Hematol. Oncol 16 (1994) 160–163. [PubMed] [Google Scholar]
- [22].Cabral-Marques O, et al. , CD40 ligand deficiency causes functional defects of peripheral neutrophils that are improved by exogenous IFN-γ, J. Allergy Clin. Immunol (2018. March 5) [E pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Thusberg J, Vihinen M, The structural basis of hyper IgM deficiency - CD40L mutations, Protein Eng. Des. Sel. PEDS 20 (2007) 133–141. [DOI] [PubMed] [Google Scholar]
- [24].Van Hoeyveld E, Zhang P-X, De Boeck K, Fuleihan R, Bossuyt X, Hyper-immunoglobulin M syndrome caused by a mutation in the promotor for CD40L, Immunology 120 (2007) 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Allewelt H, Martin PL, Szabolcs P, Chao N, Buckley R, Parikh S, Hematopoietic stem cell transplantation for CD40 ligand deficiency: single institution experience, Pediatr. Blood Cancer 62 (2015) 2216–2222. [DOI] [PubMed] [Google Scholar]
- [26].Vargas-Hernández A, et al. , Clinical and genetic analysis of patients with X-linked hyper-IgM syndrome, Clin. Genet 83 (2013) 585–587. [DOI] [PubMed] [Google Scholar]
- [27].Gilmour KC, et al. , Immunological and genetic analysis of 65 patients with a clinical suspicion of X linked hyper-IgM, Mol. Pathol. MP 56 (2003) 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lee W-I, et al. , Clinical features and genetic analysis of Taiwanese patients with the hyper IgM syndrome phenotype, Pediatr. Infect. Dis. J 32 (2013) 1010–1016. [DOI] [PubMed] [Google Scholar]
- [29].de la Morena MT, et al. , Long-term outcomes of 176 patients with X-linked hyper-IgM syndrome treated with or without hematopoietic cell transplantation, J. Allergy Clin. Immunol 139 (2017) 1282–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.