Abstract
This study was designed to determine the effects of deuteration in pyruvate on exchange reactions in alanine aminotransferase (ALT), lactate dehydrogenase (LDH) and flux through pyruvate dehydrogenase (PDH). Although deuteration of a 13C enriched substrate is commonly used to increase the lifetime of a probe for hyperpolarization experiments, the potential impact of kinetic isotope effects on such substitutions has not been studied in detail. Metabolism of deuterated pyruvate was investigated in isolated rat hearts. Hearts were perfused with a 1:1 mixture of [U-13C3]pyruvate and [2-13C1]pyruvate or a 1:1 mixture of [U-13C3]pyruvate plus [2-13C1, U-2H3]pyruvate for 30 min before being freeze clamped. Another set of hearts received [2-13C1, U-2H3]pyruvate and was freeze-clamped at 3 min or 6 min. Tissue extracts were analyzed by 1H and 13C{1H} NMR spectroscopy. The chemical shift isotope effect of 2H was monitored in the 13C NMR spectra of the C2 resonance of lactate and alanine plus the C5 of glutamate. There was little kinetic isotope effect of 2H in pyruvate on flux through PDH, LDH or ALT as detected by the distribution of 13C, but the distribution of 2H differed markedly between alanine and lactate. At steady-state, alanine was a mixture of deuterated species, while lactate was largely perdeuterated. Consistent with results at steady-state, hearts freeze-clamped at 3 min or 6 min showed rapid removal of deuterium in alanine but not in lactate. Metabolism of hyperpolarized [l-13C1]pyruvate was compared to [l-13C1,U-2H3]pyruvate in isolated hearts. Consistent with the results from tissue extracts, there was little effect of deuteration on the kinetics of appearance of lactate, alanine or bicarbonate, but there was a small, time-dependent upfield chemical shift in the HP[l-13C1]alanine signal reflecting exchange of methyl deuterons with water protons. Together, these results demonstrate that (1) the kinetics of pyruvate metabolism in hearts detected by 13C NMR are not affected by replacement of the pyruvate methyl protons with deuterons and (2) that the loss of deuterium from the methyl position occurs rapidly during the conversion of pyruvate to alanine. The majority of the deuterium atoms are lost on the time-scale of a hyperpolarization experiment.
Keywords: Isolated hearts, Perfusions, Hyperpolarization, Isotopomer analysis, Deuteration, Kinetic isotope effects
1. Introduction
An analysis of intermediary metabolism in vivo using stable isotope tracers and 13C NMR spectroscopy is made possible by the information encoded in the chemical shift and multiplet patterns due to 13C-13C spin-spin couplings in common metabolites [1]. The inherent poor sensitivity limitation of 13C NMR may be overcome by using hyperpolarization (HP) methods [2,3]·However, for practical purposes, the sensitivity advantage of HP is limited by the Τ1 relaxation of 13C nuclei. In many cases, the Τ1 relaxation of 13C may be prolonged by replacing all protons, especially directly bonded C-H protons, with deuterium [4,5]. Protons have been replaced by deuterium atoms for a variety of different metabolites and the results show longer 13C T1’s both in vitro and in vivo [4–7]. Conclusions about flux through a biochemical pathway, based on observations of 13C-labelled metabolic products, would be most reliable if it could be shown that deuteration does not significantly influence reaction kinetics. The effects of deuteration are enzyme-specific but, in general, kinetic isotope effects have not been widely studied in intact tissues [8].
The 13C NMR spectrum of deuterated products is sensitive to multiple chemical processes. The 2H nucleus is quadrupolar, so it increases the complexity of 13C NMR spectra for carbon atoms with one or more directly attached deuterium atoms. The mass differences between 1H and 2H also induces a chemical shift in the 13C NMR spectrum, typically, a one-bond chemical shift of 0.25 ppm per 2H, and a two-bond shift of about 0.05 ppm per 2H is observed [9]. A third factor is exchange of 2H labels in a metabolite with 1H in tissue water in vivo. For example, formation of a Schiff base intermediate in the alanine transaminase reaction results in 2H-1H exchange in the methyl protons of pyruvate and alanine [10,11]. These factors were previously examined during metabolism of [U-13C6, U-2H7]glucose in isolated hearts. One surprising finding was the dramatic difference in deuterium distribution in alanine compared to lactate, which can only arise if pyruvate ↔ alanine exchange and pyruvate ↔ lactate exchange occur in distinctly different cellular compartments [9]. Although no kinetic isotope effects were observed in the conversion of glucose to lactate, a kinetic difference was observed in the conversion of pyruvate → alanine (catalyzed by ALT) and conversion of pyruvate → glutamate (via the TCA cycle).
Given the importance of glycolysis in most tissues, the ability to image flux through this pathway using hyperpolarized glucose would have a major impact in cancer metabolism but unfortunately the Ti’s of the carbon atoms even in the fully deuterated analog, [U-2H7, U-13C6]glucose, are too short for practical imaging of glycolysis in most tissues. For this reason, hyperpolarized [l-13C1]pyruvate remains the most widely-used agent for HP studies even though it only reflects the last step of the glycolytic pathway [12]. Perdeuteration of pyruvate prolongs the Τ1 of the C3 carbon and the C2 to a lesser extent [4,5] so for some metabolic experiments, these deuterated analogs remain of interest. However, the consequences of perdeuteration in pyruvate on flux measurements has not been evaluated.
The aim of this study was to investigate the kinetic isotope effects that could arise when using deuterated pyruvate in an HP experiment (Fig. la). Conventional 13C NMR of tissue extracts as well as direct kinetic measurements of conversion of HP-[1-13C1, U-2H3]pyruvate versus HP-[l-13C1]pyruvate to alanine, lactate and bicarbonate were used to probe these effects. A dramatic difference in 2H labeling in lactate versus alanine was evident in hearts provided with deuterated pyruvate, consistent with compartment-specific metabolism of pyruvate. Based on analysis of 13C isotopomer data in both hyperpolarized and conventional spectra, there was little effect on the kinetics of deuterated pyruvate exchange with lactate or alanine, or oxidation via pyruvate dehydrogenase. However, exchange of 2H in alanine (but not lactate) with water protons is rapid and can be detected as a small chemical shift in the alanine Cl resonance during conversion of HP-pyruvate to HP-alanine in isolated, perfused hearts.
Fig. 1.
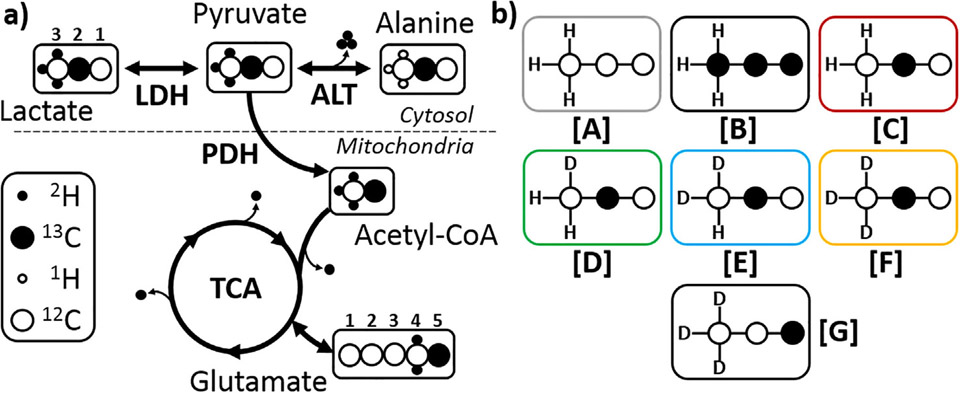
Illustrations of (a) where possible 2H (●) and 1H (o) exchange reactions could occur when tissue is presented with [2-13C1 U-2H3]pyruvate and (b) the resulting isotopomer species labelled [A] though [F]. Species [G] was used in the hyperpolarization experiments.
2. Methods & materials
2.1. Materials
[U-13C3]pyruvate, [2-13C1]pyruvate, [2-13C1,U-2H3]pyruvate and [l-13C1,U-2H3]pyruvic acid were purchased from Sigma-Aldrich Isotec (Miamisburg, OH) and were used without further purification.
2.2. Selection of 2H and 13C labeling patterns
To limit the effects of multiplicity arising from the quadrupolar 2H nucleus coupling to the 13C labels, [2-13C1,U-2H3]pyruvate was chosen because there is only a chemical shift isotope effect in C2, allowing detection of all deuterium isotopomers, but there is no direct coupling with 2H. Potential kinetic isotope effects on metabolism of perdeuterated pyruvate in the isolated heart were studied by using a 1:1 mixture of [U-13C3]pyruvate (Species [B], Fig. lb) with [2-13C1,U-2H3]pyruvate ([F]). The relative rates of [2-13C1, U-2H3]pyruvate can be directly compared to [U-13C3]pyruvate by analysis of the C2 resonance of both lactate and alanine and in the C5 resonance of glutamate (Fig. la). Exchange of deuterium with solvent protons introduces mixtures of protonated/deuterated isotopomers of [2-13C1,U-2H3]pyruvate (Fig. lb, Species [C] through [E]).
2.3. Heart perfusions and hyperpolarization
The study was performed under a protocol approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center. Sprague-Dawley rats (about 250−300 g), purchased from Charles River (Cambridge, MA), were fed ad libitum. Three different conditions were used for the perfused rat heart experiments. In all experiments, the rats were anesthetized with isoflurane (1.5−2%); the depth of anesthesia was monitored by respiratory rate, paw pinch reflex and palpebral reflex of the animal. Under deep general anesthesia, hearts were excised and arrested in ice-cold perfusion medium. All hearts were Langendorff perfused at a pressure of 100 cm H20 using a standard Krebs-Henseleit buffered medium containing 25 mM NaHC03, 118 mM NaCl, 4.7 mM KC1, 1.2 mM MgS04, 1.2 mM KH2P04, and 1.25 mM CaCl2. The medium was continuously bubbled with a 95/5 mixture of 02/C02 to maintain a pH of 7.4 and maintained at 37 °C by use of circulating warm water.
Hearts in group 1 were perfused with a 1:1 mixture of [U-13C1] pyruvate and [2-13C1,U-2H3]pyruvate (1 mM each) for 30 min to ensure metabolic and isotopic steady-state followed by rapid freeze clamping (n = 5). Hearts perfused with [U-13C3]pyruvate and [2-13Ci]pyruvate (n = 3) for the same period were used as controls. Hearts in group 2 were perfused for shorter periods to mimic non-steady-state isotopic conditions as typically done in an HP experiment. Here, hearts were perfused with unlabeled pyruvate (2 mM) for 25 min before the perfusate was quickly switched to a medium containing [2-13C1,U-2H3]pyruvate (2 mM) for either 3 min (n = 2), or 6 min (n = 2) before rapid freeze clamping. Hearts in group 3 were perfused with unlabeled pyruvate (2 mM) for 25 min followed by addition of either hyperpolarized [l-13C1, U-2H3]pyruvate or [l-13C1]pyruvate as control (2 mM each) directly into the perfusate (n = 3 each). After collecting HP-13C NMR spectra for over 3 min, hearts were immediately freeze-clamped. Pyruvate was polarized using standard methods [2] using a HyperSense DNP Polariser (Oxford Instruments) together with 0X063 (15 mM) and Gd3+ (2 mM). The frequency of irradiation was 94.112 GHz. After polarizing for 90 min, the samples were rapidly dissolved in hot PBS (5 ml), mixed in 15 ml of substrate free KH and then mixed with perfusate directly flowing into the aorta. In all cases, the frozen tissue was extracted using the perchloric acid procedure, then dissolved in 5% D20, 0.5 mM DSS-d3 and 1 mM EDTA in H20 at pH 7 and analyzed by 1H and 13C NMR spectroscopy.
2.4. 1Η and 13C NMR spectroscopy
All high-resolution NMR spectra of tissue extracts were collected using a Bruker Avance HDIII 14.1 T spectrometer equipped with a 5 mm DCH cryoprobe. The 1H NMR spectra were obtained via a ID NOESY sequence that allows presaturation during relaxation (1 s) and mixing time (0.1 s) with an acquisition time of 4 s and 128 scans. The tissue extracts 13C NMR spectra were run with 1H decoupling, a 30 deg flip angle, a repetition time of 2 sec and acquisition time of 1 sec for 8000−12,000 scans. The spectra were referenced and the peak areas were fitted using a combination of Gaussian/Lorentzian lineshapes using TOPSPIN 3.5 (Bruker, Germany). Relative peak areas of unlabeled and 13C-enriched products were obtained from the spectrum. The analysis of the 13C NMR spectra followed procedures reported previously [1,13,14]. The HP experiment was performed on a 9.4 T Varian (Agilent) VNMRS Direct Drive system using a 20 mm broadband probe (DOTY Scientific). The 13C NMR were obtained every 2 s, with a 10 deg flip angle, for about 2–3 min after injection of the hyperpolarized substrate or until the signal had fully decayed. All 13C NMR spectra were acquired with complete proton decoupling.
2.5. Isotopomer analysis of lactate, alanine and glutamate
The distribution of species [A] through [F] (Fig. lb) were calculated using methods reported previously [9]. Here, the analysis is performed using the ratio of the quartet area (representing [U-13C3]pyruvate) versus the area of the individual singlet species [C] though [F] in the C2 resonance of lactate and alanine in the 13C NMR spectra [9]. The contribution of the unlabeled species, [A], is measured from the C3 resonance in the Ή spectrum of lactate (1.33 ppm) and alanine (1.47 ppm) versus the resonances produced by [B]. The C5 glutamate resonance reported this same ratio (singlet from [2-13C1]pyruvate and doublet from [U-13C3]pyruvate). However, the chemical shift isotope effect is the opposite direction for a carbonyl group with neighboring 2H nuclei. In addition, only a maximum of two 2H nuclei is possible at C4 of glutamate. The contribution of [A] to glutamate was estimated from the C4 and C3 ratio as reported previously [15].
2.6. Error analysis
A statistical error analysis was performed on the data obtained from the 1H and 13C NMR spectra of the individual hearts. Table 2, ESI Table 2 and 3 show the estimated errors for all experiments. The error for the data in Table 1 was calculated from the experimental error of the hearts perfused with [2-13C1,U-2H3]pyruvate (n = 5) or [2-13C1]pyruvate (n = 3). The error in the ratios was estimated through the standard deviation from the individual ratios of the hearts. The error in the calculation in [A] is often larger than for the other isotopomers due to the data being obtained from the crowded 1H spectrum instead of the 13C NMR spectrum.
Table 2.
Isotopomer distribution in lactate, alanine and glutamate at three different perfusion time points. Data were derived from 13C NMR spectra of tissue extracts. The population of each species is reported as a percentage of all species.
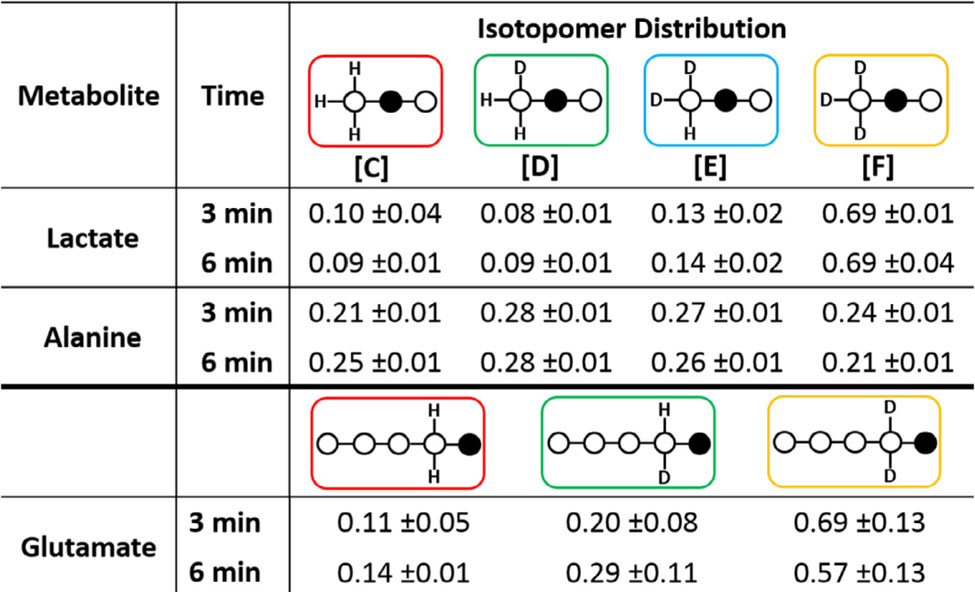
|
Table 1.
The relative concentrations of species [A] through [F] as reported by 13C and 1H NMR spectra of each metabolite in tissue extracts. The glutamate values were determined by analysis of glutamate C4 and C5 resonances. A full table of all values with error values is provided in ESI Table S3.
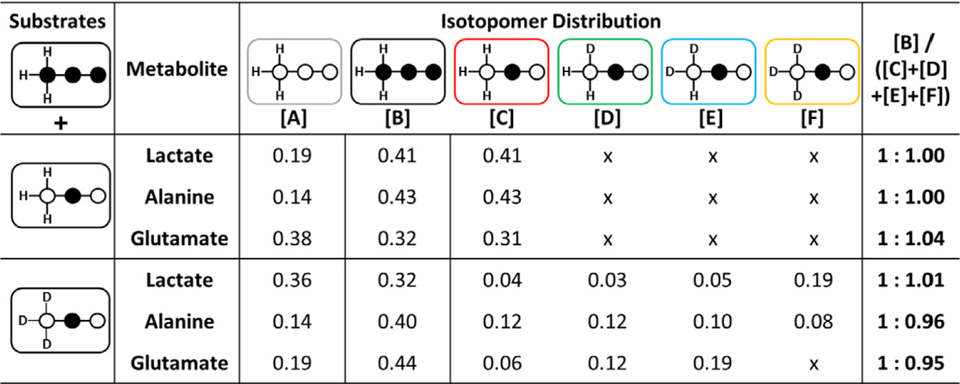
|
3. Results
3.1. Exchange of deuterium from [2-13C1U-2H3]pyruvate with solvent protons during conversion of pyruvate to lactate and alanine
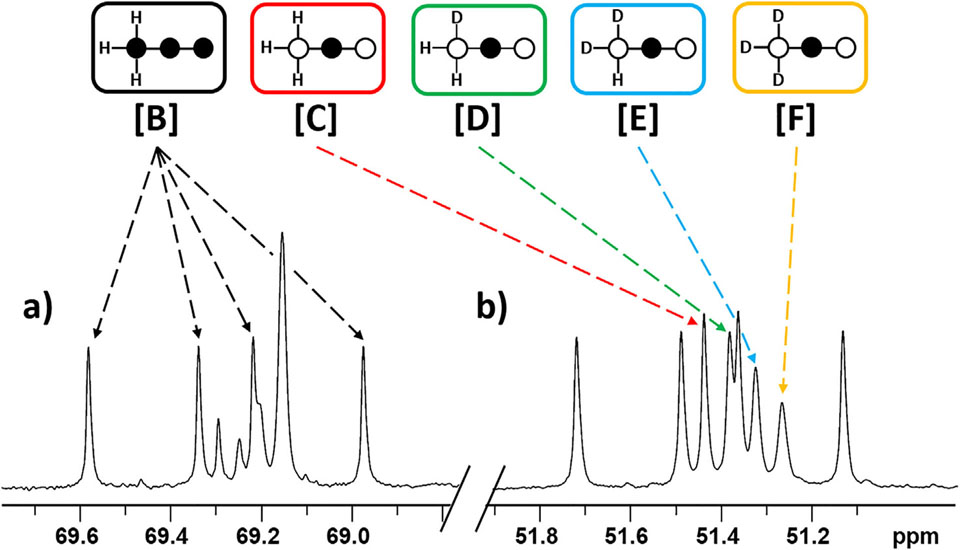
The deuterated species ([C] through [F]) generated during metabolism of [2-13C1,U-2H3]pyruvate are easily discriminated by the chemical shift of the C2 resonance of each species. The chemical shift of the perdeuterated species [F] is lies furthest upfield while [D] is only shifted by about 0.05 ppm away from the non-deuterated species [C] (see ESI Table SI for full chemical shift table). Uniformly 13C labelled pyruvate, used as an internal control, appears as a doublet of doublets or quartet in the C2 resonance of lactate and alanine with little overlap between all other resonances (Fig. 2). Any deviation of the ratio of species [B] over the sum of all other 13C species ([C] + [D] + [E] + [F]) would reflect a kinetic isotope effect. As seen in Table 1, the relative rates between the internal control and the deuterated species were 1:1.01 ±0.10 in lactate, 1:0.96 ±0.01 in alanine and 1:0.95 ±0.05 in glutamate. The control experiments performed with [2-13C1]pyruvate gave the exact expected ratio of 1:1.00 ±0.02 of [B]/[C]. These results indicate that there is no effect on the exchange process between lactate and pyruvate. However, the results show that there is a minor slowing for alanine and glutamate. These minor effects could be attributed to the different labeling patterns of 13C and 2H labels and their effect on the starting metabolite.
Fig. 2.
13C{1H} NMR spectrum of tissue extracts of hearts perfused with [U-13C3]pyruvate and [2-13C1,U-2H3]pyruvate highlighting the C2 resonances of (a) lactate and (b) alanine. Species [B] through [F] are all present. The shift pattern of lactate and alanine are the same, so not all resonances are highlighted for clarity.
3.2. Relative fluxes of non-deuterated versus perdeuterated pyruvate through PDH
The 13C NMR spectrum of the tissue extracts also shows labeling of 13C and 2H in the glutamate C5 resonance. [U-13C3]pyruvate yields [l,2-13C2]acetyl-CoA via PDH and ultimately [4,5-13C2]gluta-mate via the TCA cycle whereas [2-13C1,U-2H3]pyruvate yields [l-13C1,U-2H3]acetyl-CoA via PDH and ultimately appears as a singlet glutamate C5 that is influenced by any 2H remaining in C4 (see Fig. la). One of the potential three 2H labels in acetyl-CoA is lost upon reacting with oxaloacetate. Interestingly, the chemical shift isotope effect observed in glutamate C5 is the opposite direction, downfield, compared to the effects of methyl deuteration in lactate and alanine (ESI Fig. SI), likely due to bond-angle constraints of the C5 carboxyl group of glutamate [16]. Similar to the lactate and alanine analysis, the singlet in glutamate C5 reflects loss of all deuterium labels along this pathway while the other singlets appearing on the downfield side of the central singlet reflect the mono-deuterated and di-deuterated species. A ratio of the C5 doublet area relative to the sum of the singlet areas should equal 1.0 if there are no kinetic isotope effects along the pathway from pyruvate to glutamate. As shown in Table 1, this ratio was 1:0.95 ± 0.02, similar to that found for alanine. The control experiment also showed the expected 1:1.00 ± 0.01 ratio.
3.3. Exchange of solvent protons with 2H in alanine transaminase
The perdeuterated species [F] can lose 2H during the conversion of pyruvate to alanine in the reaction catalyzed by alanine transaminase [11]. This enzyme uses pyridoxal-5’- phosphate as a co-factor which forms a Schiff base during this interconversion. Hydrolysis of the Schiff base intermediate provides a mechanism to replace one or more of the deuterium atoms originally on pyruvate with solvent protons. One interesting question that arises then is does this deuterium exchange reaction occur slower or faster that the conversion of HP-pyruvate to HP-alanine as measured by 13C NMR? To investigate the rate of deuterium exchange on the same timescale of an HP experiment, additional heart perfusions were performed for 3 or 6 min using non-polarized [2-13C1, U-2H3] pyruvate.
Table 2 summarizes the isotopomer distribution in lactate, alanine and glutamate at the two different time points after exposure of hearts to [2-13C1,U-2H3]pyruvate alone. These data show that deuterium/proton exchange is rapid and near maximal at 3 min or before. This near-behavior is reflected in all three metabolites. For example, the amount of triply-deuterated lactate (species [F]) is near 70% by 3–6 min but only 20−25% in alanine. Similarly, the amount of fully deuterated C4 glutamate also dominates (near 60%) all possible isotopomers, similar to lactate. This indicates that the exogenous [2-13C1,U-2H3]pyruvate must be compartmentalized in cells where one pool rapidly exchanges with alanine via ALT (pyruvateALA), another pool exchanges with lactate via LDH (pyruvateLAC), and perhaps a third pool that feeds directly into the TCA cycle (pyruvateTcA) Given that the LDH reaction is not known to catalyze deuterium/proton exchange it is not surprising to find that ~70% of the lactate pool at near-equilibrium is largely the fully deuterated form. If [2-13C1,U-2H3]pyruvate was the only pyruvate isotopomer in exchange with lactate, then the population of the fully deuterated isotopomer would have been close to 100%. However, if one allows mixing of some of pyruvateALA with pyruvateLAC, then other observed lactate isotopomers would indeed be generated. The numbers suggest that if ~10% of the pyruvateALA pool, which appears to reach a near equilibrium population of 25% of each deuterated species very rapidly, mixes with the pyruvateLAC pool, then one would obtain close to the observed lactate isotopomer distribution shown in Table 2. One can do a similar estimate for glutamate. In this case, there is an added possibility of losing a deuterium in the citrate synthase reaction. In this case, if [2-13C1,U-2H3]pyruvate was the only form contributing to pyruvateTcA, then the only isotopomer that should have been found in glutamate would be [E]. As one sees from Table 2, this was the predominant isotopomer found in glutamate but its population was not 100% but rather only 60−70%. This means that either pyruvateALA or pyruvateLAc contribute about 30−40% to the pool of pyruvate entering the TCA cycle (pyruvateTcA)·If pymvateALA contributed 30−40%, then the population of [C] and [D] should have been equal. This was not observed. However, if pyruvateLAc contributed 30−40%, then one would predict that [D] > [C] as observed. This certainly does not preclude the possibility of pyruvateALA contributing a small fraction to pyruvateTcA but it does suggest that pyruvateLAc is the dominant contributor.
3.4. HP of [1-13C1U-2H3]pyruvic acid
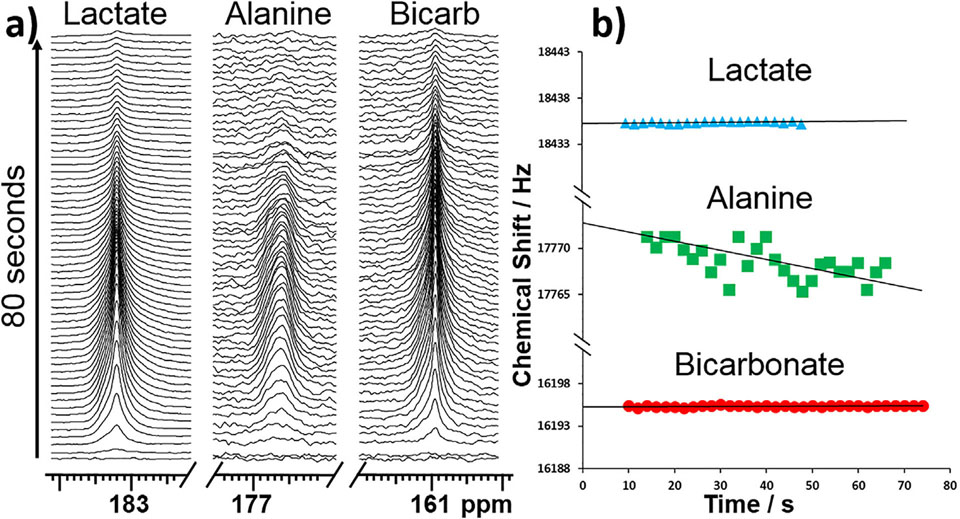
Studies using hyperpolarized [l-13C1,U-2H3]pyruvate were performed in isolated hearts to assess the impact of deuteration on the resulting hyperpolarized products. The 13C label in position Cl is 3 bonds away from the 2H atoms so the chemical shift isotope effects are comparatively small (0.02 ppm per 2H nucleus) but, nonetheless, the alanine resonance is in fact much broader than lactate and bicarbonate resonances. The shape of the resonance also becomes more asymmetric over time, indicating a change in the distribution of isotopomers, likely from [F] to [C] as shown in Fig. 3a. This is also manifested in an upheld shift of the alanine Cl signal (Fig. 3b). The shift behavior here is similar to that of glutamate C5 (confirmed by [U-13C3,U-2H3]pyruvate in ESI Fig. SI). The changing shape of the HP-alanine resonance was the only significant difference observed between the spectra recorded from HP-[1 −13C1,U-2H3]pyruvate versus HP-[l-13Ci]pyruvate. This feature agrees with the observations from NMR spectra of alanine in tissue extracts. Exchange of deuterium with protons occurs rapidly in the pyruvateALA pool on the time-scale of the HP experiment while deuterium/proton exchange is not detected by either the HP-lactate signal or the HP-bicarbonate signal.
Fig. 3.
Stack of (a) 13C spectra acquired after presenting hearts with HP-[1-13C1,U-2H3]pyruvate (scan every 2 s); lactate (left), alanine (middle) and bicarbonate (right), (b) chemical shift of bicarbonate (top), alanine (middle), lactate (bottom) are shown as a function of time.
When comparing the ratio of the lactate and bicarbonate resonances between hearts perfused with [l-13C1,2H3]pyruvate versus just [l-13C1]pyruvate, one finds essentially no difference (2H/1H:1.04 ± 0.40). This indicates a kinetic isotope effect is not an important factor in the rate of entry of pyruvate into the TCA cycle via PDH. This is in agreement with the steady-state measurements.
4. Discussion
4.1. Deuteration of pyruvate has little effect on metabolism detected by 13C NMR
A fundamental limitation of hyperpolarization technology, the short observation time imposed by Τ1, can be improved to some extent by deuteration of the reporter molecule. However, kinetic isotope effects due to deuterium are well-known in isolated enzyme systems [6,7]. Somewhat surprisingly, in intact hearts supplied with perdeuterated glucose, there was little effect of deuteration on pyruvate exchange with lactate and a small effect with alanine [9]. Traditionally, a kinetic isotope effect of deuterium is interpreted as evidence that breaking a carbon-hydrogen bond is rate limiting in a reaction mechanism. In the current study with perdeuterated pyruvate other factors appear to be more important in controlling rates of these metabolic steps involving pyruvate. For practical purposes, the presence of deuterium does not modify the information about pyruvate metabolism, at least as measured by conventional or hyperpolarized 13C NMR methods. Since the Τ1 of 13C in pyruvate C2 is prolonged slightly by deuteration, studies with [2-13C1,U-2H3]pyruvate maybe useful. Exploration of deuteration in other informative molecules such as lactate and alanine is also warranted.
4.2. Functional compartmentation of pyruvate is detected by 2H distribution
Isotopic exchange of water protons with lactate [17–19] and alanine [11] protons have previously been reported in studies of erythrocytes and isolated enzymes. The current results indicate that the process is rapid in the case of alanine, but not for lactate, and occurs on a time scale relevant to hyperpolarization experiments. However, the relative rate constants have been reported to be an order of magnitude faster for the exchange between pyruvate and lactate than for pyruvate and alanine [20]. This would indicate that alanine is exchanging on and off the ALT much faster than net conversion to pyruvate as the proton/deuterium exchange process is far more complete for alanine than lactate. Although the processes are readily detected by high-resolution analysis of tissue extracts, aside from the line broadening in the alanine resonance due to the rapid exchange, there is a minimal effect detectable in an HP experiment.
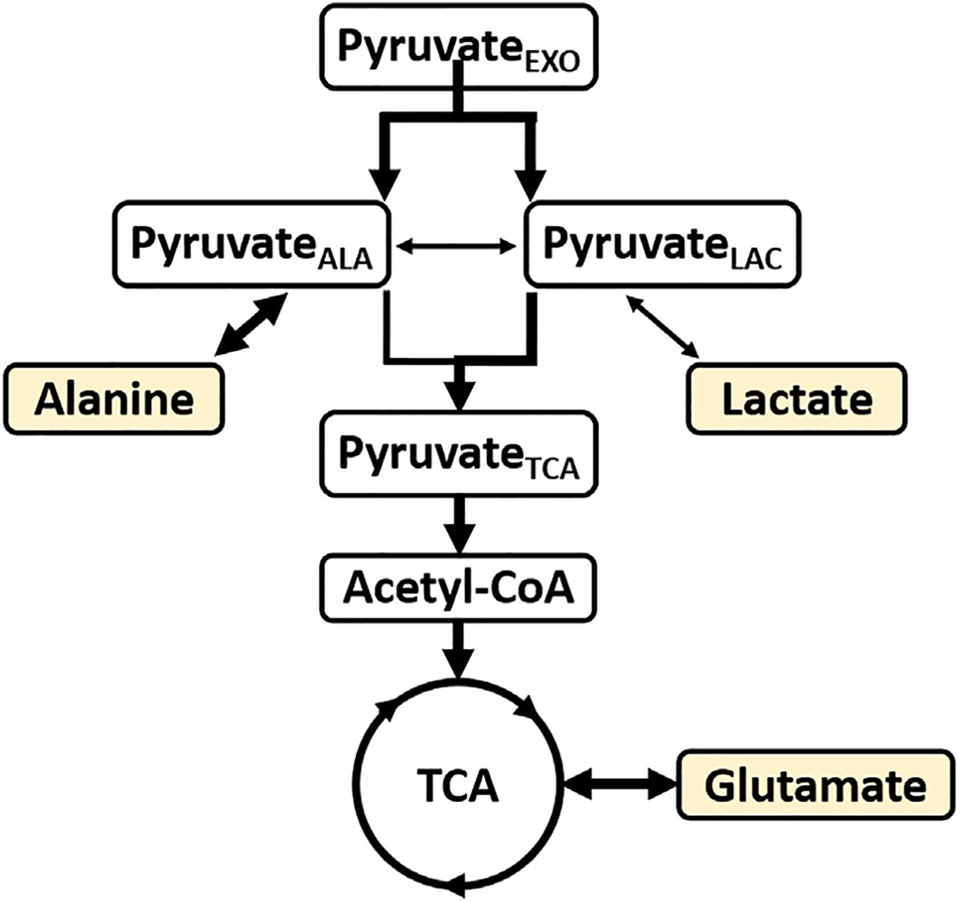
Given the high activity of both alanine aminotransferase and lactate dehydrogenase, investigators generally assume that distribution of a tracer in alanine or lactate in the heart reflects labeling in pyruvate. However, the current results are not consistent with that assumption. Compartmentation of intracellular pyruvate has been described in multiple studies and may have important implications for understanding results of experiments with hyperpolarized pyruvate. In hearts supplied with [l-14C]pyruvate, specific radioactivities of tissue alanine and lactate differed substantially. Based on studies at tracer concentrations of pyruvate, these results were most consistent with two functional pools of pyruvate in the cytosol of the heart, one in communication with pyruvate from glycolysis and the other in communication with extracellular pyruvate [21]. In the presence of [2-14C]lactate, not all pyruvate in the heart exchanges with lactate [22]. 13C and 1H NMR methods found differing enrichments in lactate and alanine when the tracer originated in glucose [23]. A separate 13C NMR study of [1 −13C1]glucose found functional compartmentation of glycolytic and glycogenolytic processes. In that analysis, the 13C labeling in pyruvate feeding the TCA cycle was from the pyruvateLAc pool [24]. Our earlier study of perdeuterated 13C glucose [9] found substantial de-deuteration of alanine, similar to the current results, and that the 2H labeling patterns in glutamate followed pattern of the pyruvateALA pool. These results are consistent with the earlier report [21] that pyruvateALA is the source of pyruvate entering the TCA cycle via PDH.
However, when the 2H label originates in exogenous pyruvate rather than glucose, the pyruvateLAc pool seems to contribute the majority of the glutamate pool (pyruvateTcA, Fig. 4), with only a small contribution from the pyruvateALA pool. The current results indicate that when pyruvate is present in high concentration or as a bolus, functional compartmentation is detected by the 2H distribution of products derived from pyruvate. These effects were easily detected in heart tissue. In other organs, such as the liver, the relative activity of the mitochondrial isoform of ALT is much higher and different results may be observed [25,26].
Fig. 4.
Pyruvate and lactate compartmentation based on 13C and 2H distribution in lactate, alanine and glutamate. The 13C isotopomers indicate a complete equilibration in the alanine and lactate pools. However, the 2H isotopomers show that exchange between pyruvate and alanine is rapid, indicated by the thicker arrow. Based on 2H distribution in glutamate, pyruvate exchanging with lactate preferentially supplies the acetyl-CoA pool entering the TCA cycle. The readout metabolites are highlighted.
5. Conclusions
We sought to investigate the effect of deuteration on metabolism of pyruvate in the intact heart. There were no substantial effects of deuteration on the kinetics of metabolism as investigated by 13C and hyperpolarization methods. However, flux through alanine aminotransferase induced a dramatic difference in deuteration of alanine compared to lactate. These results indicate that the pool of pyruvate feeding the TCA cycle is identical to the pool in exchange with lactate. These results are relevant to interpretation of conventional HP studies in the heart and indicate that deuterated pyruvate provides additional information about compartmentation of pyruvate metabolism.
Supplementary Material
Acknowledgements
The authors like to acknowledge funding by NIH grants P41EB015908 (CRM) and R37HL034557 (ADS).
Footnotes
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmr.2019.03.003.
References
- [1].Jeffrey FM, Rajagopal A, Malloy CR, Sherry AD, 13C-NMR: a simple yet comprehensive method for analysis of intermediary metabolism, Trends Biochem. Sei 16 (1991) 5–10. [DOI] [PubMed] [Google Scholar]
- [2].Keshari KR, Wilson DM, Chemistry and biochemistry of 13C hyperpolarized magnetic resonance using dynamic nuclear polarization, Chem. Soc. Rev 43 (2014) 1627–1659, 10.1039/C3CS60124B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ardenkjær-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K, Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR, Proc. Natl. Acad. Sei 100 (2003) 10158–10163, 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vuichoud B, Milani J, Bornet A, Melzi R, Jannin S, Bodenhausen G, Hyperpolarization of deuterated metabolites via remote cross-polarization and dissolution dynamic nuclear polarization, J. Phys. Chem. B 118 (2014) 1411–1415, 10.1021/jp4118776. [DOI] [PubMed] [Google Scholar]
- [5].Taglang C, Korenchan DE, von Morze C, Yu J, Najac C, Wang S, Blecha JE, Subramaniam S, Bok R, VanBrocklin HF, Vigneron DB, Ronen SM, Sriram R, Kurhanewicz J, Wilson DM, Flavell RR, Late-stage deuteration of 13 C-enriched substrates for T 1 prolongation in hyperpolarized 13 C MRI, Chem. Commun 54 (2018) 5233–5236, 10.1039/C8CC02246A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Meier S, Karlsson M, Jensen PR, Lerche MH, Duus JØ, Metabolic pathway visualization in living yeast by DNP-NMR, Mol. BioSyst 7 (2011) 2834–2836, 10.1039/ClMB05202K. [DOI] [PubMed] [Google Scholar]
- [7].Harris T, Degani H, Frydman L, Hyperpolarized 13C NMR studies of glucose metabolism in living breast cancer cell cultures, NMR Biomed 26 (2013) 1831–1843, 10.1002/nbm.3024. [DOI] [PubMed] [Google Scholar]
- [8].Cooper AJ, Proton magnetic resonance studies of glutamate-alanine transaminase-catalyzed deuterium exchange. Evidence for proton conservation during prototropic transfer from the alpha carbon of L-alanine to the C4-position of pyridoxal 5’-phosphate, J. Biol. Chem 251 (1976) 1088–1096. [PubMed] [Google Scholar]
- [9].Funk AM, Anderson BL, Wen X, Hever T, Khemtong C, Kovacs Z, Sherry AD, Malloy CR, The rate of lactate production from glucose in hearts is not altered by per-deuteration of glucose, J. Magn. Reson 284 (2017) 86–93, 10.1016/j.jmr.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tonelli M, Singarapu KK, Makino S, Sahu SC, Matsubara Y, Endo Y, Kainosho M, Markley JL, Hydrogen exchange during cell-free incorporation of deuterated amino acids and an approach to its inhibition, J. Biomol. NMR 51 (2011) 467–476, 10.1007/sl0858-011-9575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Barb AW, Hekmatyar SK, Glushka JN, Prestegard JH, Probing alanine transaminase catalysis with hyperpolarized 13CD3-pyruvate, J. Magn. Reson 228 (2013) 59–65, 10.1016/j.jmr.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cunningham CH, Lau JYC, Chen AP, Geraghty BJ, Perks WJ, Roifman I, Wright GA, Connelly KA, Hyperpolarized 13C metabolic MRI of the human heart: initial experience, Circ. Res 119 (2016) 1177–1182, 10.1161/CIRCRESAHA.116.309769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Malloy CR, Sherry AD, Jeffrey FM, Evaluation of carbon flux and substrate selection through alternate pathways involving the citric acid cycle of the heart by 13C NMR spectroscopy, J. Biol. Chem 263 (1988) 6964–6971. [PubMed] [Google Scholar]
- [14].Malloy CR, Sherry AD, Jeffrey FM, Analysis of tricarboxylic acid cycle of the heart using 13C isotope isomers, Am. J.Physiol.- Heart Circ. Physiol 259 (1990) H987–H995. [DOI] [PubMed] [Google Scholar]
- [15].Burgess SC, Carvalho RA, Merritt ME, Jones JG, Malloy CR, Sherry AD, 13C Isotopomer analysis of glutamate by J-resolved heteronuclear single quantum coherence spectroscopy, Anal. Biochem 289 (2001) 187–195, 10.1006/abio.2000.4930. [DOI] [PubMed] [Google Scholar]
- [16].Hansen PE, Isotope effects on nuclear shielding, in: Webb GA (Ed.), Annu. Rep. NMR Spectrosc, Academic Press, 1984, pp. 105–234, 10.1016/S0066-4103(08)60208-2. [DOI] [Google Scholar]
- [17].Brindle KM, Brown FF, Campbell ID, Foxall DL, Simpson RJ, A 1H n.m.r. study of isotope exchange catalysed by glycolytic enzymes in the human erythrocyte, Biochem. J 202 (1982) 589–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Simpson RJ, Brindle KM, Brown FF, Campbell ID, Foxall DL, A p.m.r. isotope-exchange method for studying the kinetic properties of dehydrogenases in intact cells, Biochem. J 202 (1982) 573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Simpson RJ, Brindle KM, Brown FF, Campbell ID, Foxall DL, Studies of pyruvate-water isotope exchange catalysed by erythrocytes and proteins, Biochem. J 193 (1981) 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mariotti E, Orton MR, Eerbeek O, Ashruf JF, Zuurbier CJ, Southworth R, Eykyn TR, Modeling non-linear kinetics of hyperpolarized [1–13C] pyruvate in the crystalloid-perfused rat heart, Nmr Biomed 29 (2016) 377–386, 10.1002/nbm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Peuhkurinen KJ, Hiltunen JK, Hassinen IE, Metabolic compartmentation of pyruvate in the isolated perfused rat heart, Biochem. J 210 (1983) 193–198, 10.1042/bj2100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mowbray J, Ottaway JH, The effect of insulin and growth hormone on the flux of tracer from labelled lactate in perfused rat heart, Eur. J. Biochem 36 (1973) 369–379. [DOI] [PubMed] [Google Scholar]
- [23].Chatham JC, Forder JR, Metabolic compartmentation of lactate in the glucose-perfused rat heart, Am. J. Physiol. - Heart Circ. Physiol 270 (1996) H224–H229. [DOI] [PubMed] [Google Scholar]
- [24].Anousis N, Carvalho RA, Zhao P, Malloy CR, Sherry AD, Compartmentation of glycolysis and glycogenolysis in the perfused rat heart, NMR Biomed 17 (2004) 51–59, 10.1002/nbm.860. [DOI] [PubMed] [Google Scholar]
- [25].DeRosa G, Swick RW, Metabolic implications of the distribution of the alanine aminotransferase isoenzymes, J. Biol. Chem 250 (1975) 7961–7967. [PubMed] [Google Scholar]
- [26].Taegtmeyer H, Peterson MB, Ragavan VV, Ferguson AG, Lesch M, De novo alanine synthesis in isolated oxygen-deprived rabbit myocardium, J. Biol. Chem 252 (1977) 5010–5018. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.