SUMMARY
The V1V2 region of the HIV-1 envelope is the target of several broadly neutralizing antibodies (bNAbs). Antibodies to V1V2 elicited in the RV144 clinical trial correlated with a reduced risk of HIV infection, but these antibodies were without broad neutralizing activity. Antibodies targeting V1V2 also correlated with a reduced viral load in immunized macaques challenged with simian immunodeficiency virus (SIV) or simian/human immunodeficiency virus (SHIV). To focus immune responses on V1V2, we engrafted the native, glycosylated V1V2 domain onto five different multimeric scaffold proteins and conducted comparative immunogenicity studies in macaques. Vaccinated macaques developed high titers of plasma and mucosal antibodies that targeted structurally distinct V1V2 epitopes. Plasma antibodies displayed limited neutralizing activity but were functionally active for ADCC and phagocytosis, which was detectable 1–2 years after immunizations ended. This study demonstrates that multivalent, glycosylated V1V2-scaffold protein immunogens focus the antibody response on V1V2 and are differentially effective at inducing poly-functional antibodies with characteristics associated with protection.
In Brief
The V1V2 region of HIV Env is an important vaccine target. In comparative immunogenicity studies in macaques, Hessell et al. reveal that a cocktail of multiple V1V2-scaffold immunogens readily induces durable, polyfunctional V1V2-specific antibodies with antiviral activities that are associated with a reduced risk of acquisition of infection and viral control.
Graphical Abstract
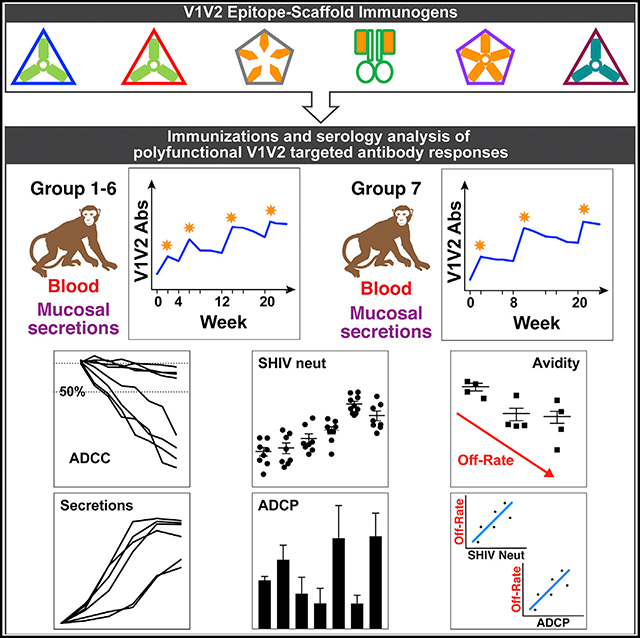
INTRODUCTION
The HIV-1 envelope protein (Env) is a heterotrimeric glycoprotein that is the sole target of neutralizing antibodies (NAbs). Despite being the most diverse in both sequence and length (Pan et al., 2015; Zolla-Pazner and Cardozo, 2010), the first and second variable region (V1V2) of the Env glycoprotein is recognized as a strategic target for vaccine development. Human monoclonal antibodies (mAbs) specific for V1V2 that are potent neutralizers of HIV-1 often require extreme rates of somatic mutation and a lengthy process of affinity maturation, hindering efforts to elicit similar antibodies with vaccine candidates (Moore and Williamson, 2016). In contrast, human mAbs directed to other V1V2 epitopes require far less somatic mutation and are inducible by vaccines (Li et al., 2015; Zolla-Pazner et al., 2016).
Recent data have shown that V1V2 forms a unique five-stranded beta-barrel structure with strands A, B, C, C’, and D and has a relatively constrained structure within the stabilized BG505 SOSIP.664 trimer (Gorman et al., 2016; Pan et al., 2015; Pancera et al., 2014). In the trimer context, the V1V2 domain is located at the apex, and all three V1V2 loops in the trimer join together at the trimer association domain (Mao et al., 2012) to form the top layer of the Env complex (Julien et al., 2013; Lee et al., 2016; Lyumkis et al., 2013; Pancera et al., 2014). This layer shields the co-receptor binding site and partially occludes the third variable loop region (V3). However, upon binding of CD4, V1V2 relocates to expose the co-receptor binding sites and V3 loop (Munro et al., 2014; Ozorowski et al., 2017; Pancera et al., 2014; Spurrier et al., 2011; Wang et al., 2018).
Several cross-clade-reactive human mAbs targeting the V1V2 region on the Env spike have been isolated and extensively characterized (Haynes and Mascola, 2017; Li et al., 2015). For example, the V2p epitope family is defined by human mAbs CH58 and CH59, isolated from an RV144 vaccinee (Liao et al., 2013), and CAP228–16H, isolated from an HIV+ individual (van Eeden et al., 2018; Wibmer et al., 2018). These mAbs react with V2 peptides, which are structurally unconstrained and preferentially assume a helical or helical coil structure (Liao et al., 2013). Antibodies in this family are not neutralizing (Aiyegbo et al., 2017; Liao et al., 2013) but are able to block α4β7 binding to gp120 (Chand et al., 2017; C. Cicala et al., 2018, Keystone Symposium, conference; Lertjuthaporn et al., 2018).
The V2i epitope family is comprised of discontinuous residues in V1V2, including the α4β7 integrin binding motif at residues 179–181 in V2 (Gorny et al., 2012; Mayr et al., 2013; Pan et al., 2015; Spurrier et al., 2014). The V2i epitopes are defined by a panel of human mAbs, including 830A, 697, 1393, and 2158 (Gorny et al., 1994, 2012; Mayr et al., 2013; Nyambi et al., 2000; Pinter et al., 2004; Spurrier et al., 2014). Extensive immunological, mutagenesis, and structural data have shown that mAbs that recognize the V2i epitopes are generally weakly neutralizing but mediate antibody (Ab)-dependent cellular cytotoxicity (ADCC) and Ab-dependent cellular phagocytosis (ADCP) (Chung et al., 2014a; Gorny et al., 2012; Musich et al., 2017a; Pollara et al., 2014).
The V2q epitope family is defined by a group of broadly Nabs (bNAbs), including PG9 and PG16 (Walker et al., 2009), and is preferentially expressed on the quaternary Env trimer (Doria-Rose et al., 2015), although PG9 and PG16 can bind to selected gp120 monomers (McLellan et al., 2011). Structural data of PG9 and PG16 in complex with engineered V1V2 protein scaffolds or SOSIP have shown that V2q mAbs recognize a region in the C strand of V1V2 that assumes a β strand (rather than an α-helical) configuration and also bind to two N-linked glycans (McLellan et al., 2011; Pancera et al., 2013, 2014; Wang et al., 2017).
The V2qt epitope family is defined by trimer-specific bNAbs, such as PGT145 and PGDM1400, that have long and straight complementarity determining region (CDR) H3 regions (Sok et al., 2014; Walker et al., 2011). These bNAbs bind at the axial center of the Env trimer, with the hairpin tip of the long CDR H3 domain reaching into the central opening of the trimer apex (Lee et al., 2017; Liu et al., 2017; Sok et al., 2014).
The crystallographic, cryo-EM and immunologic studies cited above indicate that the V1V2 domain transitions in position and conformation on the Env trimer and displays a rich set of diverse but conserved epitopes, resulting in the ability of many V1V2 mAbs to display extensive cross-reactivity (Braibant et al., 2013; Gorny et al., 2012). Therefore, this Env domain provides a unique opportunity for the design and construction of immunogens that target the immune response to V1V2 epitopes. Data from active immunization studies in primates have provided support for the hypothesis that vaccine-induced antibodies specific for V1V2 are correlated with control of viremia and/or reduce the risk of infection, establishing a baseline for further vaccine improvement (Barouch et al., 2012; Gordon et al., 2014; Julg et al., 2017; Roederer et al., 2014; Vaccari et al., 2016a). Moreover, a robust Ab response to V1V2 correlated with a reduced risk of HIV infection in the RV144 HIV vaccine clinical trial, and we have recently shown that a single passively transferred V2i mAb reduced seeding of the virus in primates and reduced plasma and peripheral blood mononuclear cell (PBMC) virus levels (Hessell et al., 2018). Taken together, these data suggest that V1V2-specific antibodies are at least one of the major components that contribute to protective immune responses.
“Reverse vaccinology” is an approach to vaccine design that uses epitopes recognized by mAbs to design vaccines and has been used successfully in the development of vaccines against various human pathogens (Rinaudo et al., 2009; Wang et al., 2010a, 2010b). We used this approach to develop several immunogens by engrafting the V1V2 region from HIV isolates of clades A, B, C, and E into scaffold proteins in which the V1V2 domain can adopt different conformations. We reported the design and production of several protein scaffold immunogens engrafting the V1V2 sequences of HIV-1 isolates from clades A, B, and C (Jiang et al., 2016) and showed that nine of these V1V2-scaffold proteins focused the antibody response on V1V2 and induced cross-reactive and biologically active V2-specific antibodies in rabbits (Zolla-Pazner et al., 2016). The results of the rabbit experiments informed our selection of V1V2-scaffold immunogens to test in macaques. Guided by previous experience, we used immunization regimens that combined HIV-1 env plasmid DNA with protein immunogens (Hessell et al., 2016; Jalah et al., 2014; Malherbe et al., 2014; Singh et al., 2018). Here we show that these V1V2-scaffold protein immunogens, in conjunction with DNA immunization, programmed a targeted immune response in macaques toward V1V2. Used either alone or in combination, these immunogens induced (1) high titers of antibodies in macaques with cross-clade antigen binding, (2) antibodies that mediated phagocytosis and ADCC activity, and (3) long-lived antibody activity demonstrable for more than a year after the last immunization. These experiments show that poly-functional antibodies can be induced differentially by individual and combinations of V1V2-scaffold immunogens, demonstrating that it is possible to design and develop immunogens that effectively tune the humoral immune response and elicit antibodies with anti-viral function.
RESULTS
Immunogenicity of Multivalent V1V2-Scaffold Proteins in Macaques
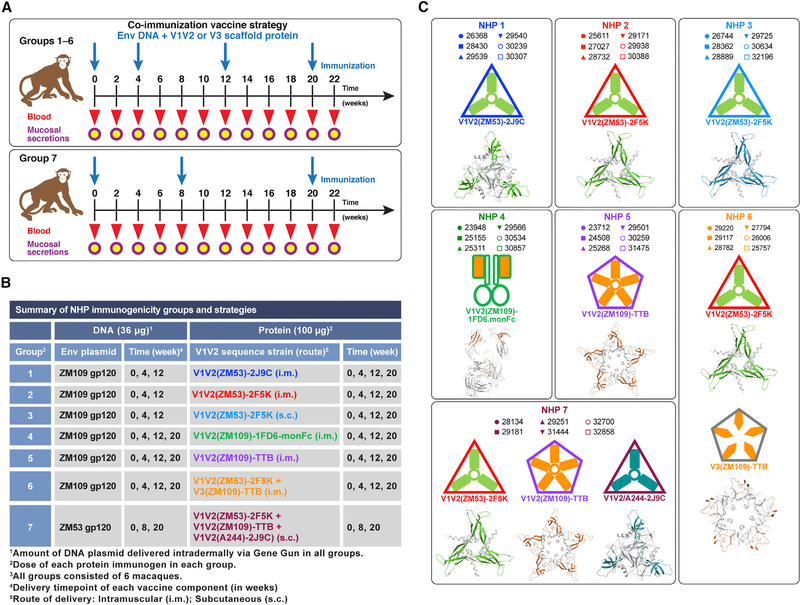
A total of seven vaccine groups, each comprised of six adult rhesus macaques, were immunized according to the schedules shown in Figure 1A using single or multiple V1V2-scaffold proteins formulated in Adjuplex adjuvant. Simultaneously, all animals received either clade C gp120(ZM109) or gp120(ZM53) plasmid DNA intradermally via gene gun, a procedure used previously for optimal immunogenicity in animal models (Hessell et al., 2016; Jaworski et al., 2012; Krebs et al., 2014; Malherbe et al., 2014, 2018; Pissani et al., 2014). The immunization regimens, summarized in Figure 1B, varied in the choice and number of V1V2 scaffolds to examine (1) DNA and protein co-immunization priming followed by a protein-only boost (groups 1–3), (2) subcutaneous (s.c.) versus intramuscular (i.m.) delivery of the protein immunogen (Pauthner et al., 2017) (group 2 versus group 3), (3) DNA and protein co-immunization at all vaccine time points (groups 4–6), and (4) effects of extending the interval of time between the first and second co-immunization of DNA and protein (Pauthner et al., 2017) (group 7).
Figure 1. Immunogenicity of Multivalent V1V2-Scaffold Proteins in Macaques.
(A) Immunization protocols and NHP groups with timelines and regimens.
(B) Individual group strategies for immunogenicity studies using DNA and protein co-immunizations.
(C) Animal IDs and structural models of V1V2- and V3-scaffold immunogens, with icons representing protein immunogens. Scaffold structural models are shown in gray, and engrafted V1V2 or V3 regions are colored. TTB, typhoid toxin B subunit; monFc, monkey IgG Fc.
A model structure of each V1V2-scaffold protein used in this study and described in detail by Jiang et al. (2016) is provided in Figure 1C, along with an icon representing each immunogen and the ID number of each immunized animal. The icons represent the V1V2 scaffolds used in each experiment. Immunogens V1V2(ZM53)-2F5K, V1V2(ZM53)-2J9C, and V1V2(A244)-2J9C (nonhuman primate [NHP] groups 1–3, 6, and 7; Figure 1C) include the V1V2 domains engrafted onto trimeric scaffolds. We have also previously identified other scaffolds for presenting V1V2 epitopes, including 1FD6-monkey immunoglobulin G (IgG) Fc (NHP group 4; Figure 1C), which was designed to increase the interaction with macaque Fcγ receptors on immune cells. Fusing HIV proteins to IgG Fc has been tested in mice, resulting in improved antibody responses (Chen et al., 2007; Qi et al., 2010; Zaharatos et al., 2011), including increased potency and breadth of neutralizing and ADCC activities in macaques (Shubin et al., 2017). Binding of immune complexes to Fcγ receptors on dendritic cells has also been shown to enhance antigen presentation (Akiyama et al., 2003; Regnault et al., 1999; Sallusto and Lanzavecchia, 1994; Song et al., 1999) and promote somatic hypermutation and affinity maturation (Allen and Cyster, 2008; Aydar et al., 2005; Wu et al., 2008). The self-assembling pentameric typhoid toxin B (TTB) subunit possesses a structural site suitable for the engraftment of the V1V2 domain, providing an antigenically and immunologically accessible configuration. The V1V2-TTB scaffold protein presents five copies of the V1V2 domain, binds to cell surface gangliosides, and induces mucosal immunity (NHP groups 5 and 7; Figure 1C; Jiang et al., 2016; Totrov et al., 2010).
Epitope-Specific mAbs Define the Antigenicity of the V1V2 and V3 Scaffolds
We evaluated the antigenicity of each of the V1V2-scaffold proteins using a panel of V1V2- and V3-specific mAbs (Figure 2) to assess whether the immunogens display epitopes that are properly folded, glycosylated, and presented on scaffolds that maintain the integrity of the V1V2 and V3 epitopes. Binding experiments by ELISA reveal that the V2q mAb PG9 epitope is presented on all multimeric V1V2 scaffolds tested, and the 50% half-maximum binding response (EC50) values determined by ELISA are comparable with those reported with BG505 SOSIP.664 gp140 (Figure 2A; Sanders et al., 2013). Differences in epitope recognition among V2p mAb binding to the various V1V2-scaffold proteins can be seen by the variation in EC50 values (Figure 2H). Notably, V2p mAb CH59 binds poorly to V1V2(ZM53)-2F5K compared with CH58, and mAb CH58 does not bind to V1V2(ZM109)-1FD6.monFc or V1V2(ZM109)-TTB (Figures 2E and 2F), reflecting the differences in the epitopes recognized by these two V2p mAbs (Liao et al., 2013). Similarly, there are differences in the binding of the three V2i mAbs tested (Figures 2B–2D), with V1V2(ZM109)-TTB again being the least reactive of the multimeric V1V2-scaffold proteins. V2i mAb 697D, in particular, detected differences between the five multimeric molecules, displaying strong, moderate, and very weak binding. In contrast, strong binding by anti-V3 mAbs 447–52D and 2219 to the V3(ZM109)-TTB scaffold denotes the structural and antigenic integrity in the V3 loop engrafted in this V3-scaffold immunogen. The reduced binding by V2q, V2i, and V2p mAbs to V1V2(ZM109)-TTB suggests that this molecule may present V1V2 in an unusual configuration.
Figure 2. Epitope-Specific mAbs Define the Antigenicity of V1V2 and V3 Scaffolds.
V1V2-specific mAbs were serially diluted and assayed by ELISA in duplicate at a starting concentration of 5 μg/mL to evaluate the antigenicity of each immunogen. Curves are mean ± SEM.
(A-F). Each mAb and specificity is shown: PG9 V2q (A), 830A V2i (B), 2158 V2i (C), 697D V2i (D), CH58 V2p (E), CH59 V2p (F). Unique symbols identify each V1V2-scaffold protein.
(G) Binding to the V3(ZM109)-TTB scaffold with V3-specific mAbs 447–52D and 2219 and control V2 and CD4bs mAbs.
(H) EC50 values derived from ELISA curves in (A)–(G).
Data are representative of assays repeated at least twice with comparable results.
V1V2-Scaffold Immunogens Induce Primarily V1V2-Focused Antibody Responses that Transudate to Vaginal Mucosal Tissues
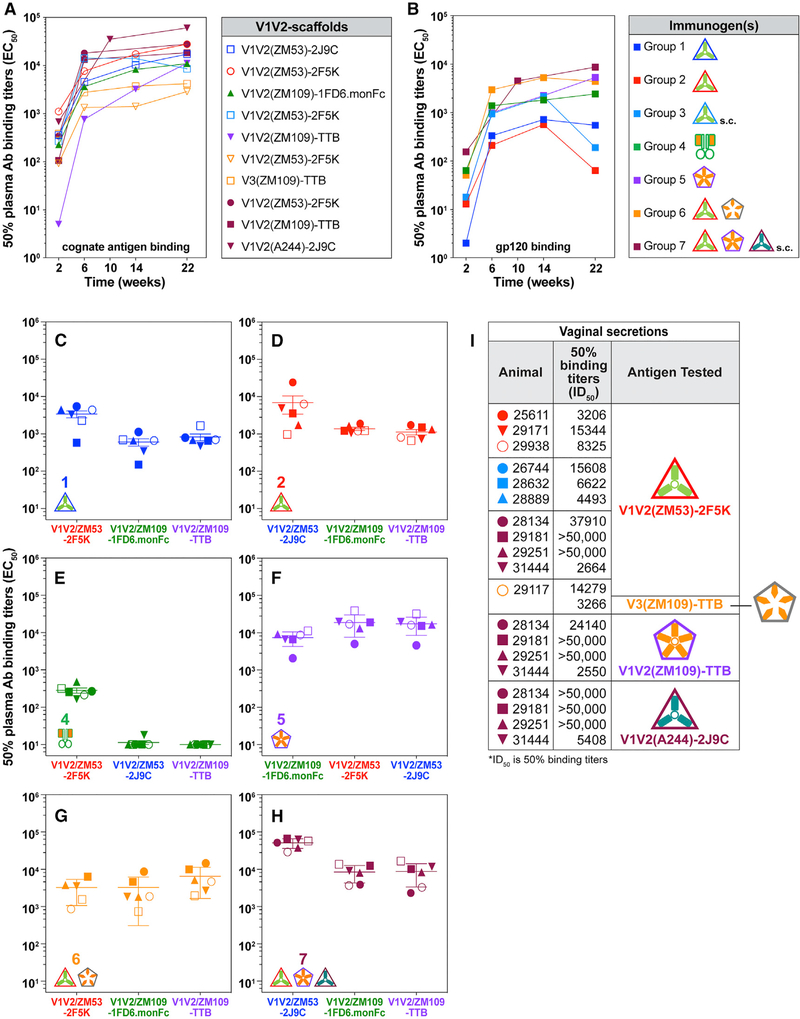
As a first comparison among immunized NHP groups, we assessed plasma antibody binding titers by ELISA to each of the cognate V1V2-scaffold proteins (Figure 3A) and to gp120(ZM109) (Figure 3B). Mean midpoint binding plasma antibody titers (EC50) for each group are shown at 2 weeks after each immunization. Notably, antibody titers in each group are evident after a single immunization (week 2) and increase by 1–2 orders of magnitude with the second immunization in all groups. A group-wise comparison reveals that the immunization strategy used in group 7 induced higher plasma antibody titers than all other groups (Table S1). Titers to the cognate V1V2-scaffold immunogens were approximately 1 order of magnitude higher than the titers against gp120 in all groups except groups 5 and 6 and accounted for a significant difference in reactivity (compare Figures 3A and 3B; Table S1). Increasing the concentration of gp120 on the ELISA plates to reach equimolar equivalency between V1V2-scaffolds and gp120 with respect to V1V2 did not result in any significant change in EC50, confirming that the V1V2-directed antibody response to this domain as presented on gp120 was lower than that directed to V1V2 on the scaffold proteins (data not shown). This suggests that macaque antibody responses were focused primarily on the V1V2 epitopes, with little reactivity to other gp120 epitopes (see also Figure 4).
Figure 3. V1V2-Scaffold Immunogens Induce Primarily V1V2-Focused Antibody Responses that Transudate to Vaginal Mucosal Tissues.
(A and B) Serially diluted plasma samples from vaccinated macaques were tested in duplicate by ELISA. Each curve is the mean EC50 from each group (n = 6), graphed to show reactivity to (A) cognate V1V2-scaffold protein immunogens and (B) gp120 ZM109 protein.
(C–H) Duplicates of plasma samples from each group were assayed for binding against heterologous scaffolds. Group 1 (C), group 2 (D), group 4 (E), group 5 (F), group 6 (G), and group 7 (H). Data are EC50 values for individual macaques, and mean ± SEM values for each group (n = 6) are indicated.
(I) Mean EC50 binding titers from week 22 duplicate vaginal secretions collected from female animals in each group, assessed in an ELISA for IgG binding to cognate epitope-scaffold proteins (indicated as antigen tested). Animal symbols match those in Figure 1C. See also titration curves for vaginal secretions in Figure S1.
Statistical analysis of the data in (A)–(H) is provided in Table S1. Data are representative of assays that were repeated at least twice with comparable results.
Figure 4. V1V2-Scaffold Immunogens Induce Antibodies that Are Cross-Clade Reactive and Differentially Target V1V2 When Presented in the Context of Recombinant V1V2-Scaffold Proteins.
A Luminex multiplex assay for binding of immune NHP plasma is shown as a heatmap of mean fluorescence intensity (MFI). Binding of week 22 plasma was tested in duplicate samples and, diluted 1:200, assayed against a set of 15 Env antigens and BSA (see antigen key). Negative controls were BSA-coated beads, pooled pre-bleed plasma and PBS with 0.02% Tween and 0.01% bovine serum albumin (PBS-TB*Avg). The positive control was mAb pool*Avg: a cocktail of mAbs 697 (anti-V2i), 830A (anti-V2i), 1393A (anti-V2i), CH58 (anti-V2p), 3869 (anti-V3), 670 (anti-C5), and 1331A (anti-C5). The intensity of the reactivity of each sample with each antigen is designated by color, corresponding to the MFI spectrum shown. Statistical analysis is shown in Table S2.
Groups 1–3 received only the V1V2-scaffold protein in the fourth immunization, without the gp120 DNA immunogen (see Figure 1B). Group 1 plasma antibody binding to monomeric gp120 did not increase after the third immunization, and binding was markedly decreased in groups 2 and 3 after the fourth immunization (Figure 3B). This decrease was not seen in groups 4–7, where all four immunizations included both gp120 DNA and V1V2-scaffold proteins (Figure 1B), suggesting a contribution by the DNA component that is not maintained with a protein-only boost, as we have reported previously (Pissani et al., 2014).
We also tested plasma antibodies from each group for binding in ELISA to homologous and heterologous V1V2 domains and for binding to V1V2 presented on heterologous scaffolds to evaluate cross-reactivity and the presence or absence of scaffold-specific responses. The V1V2(ZM53) domain was engrafted onto both the 2J9C and 2F5K scaffold proteins and was used to immunize macaques in groups 1, 2, and 3. Group 1 and 2 scaffold proteins were immunized via the i.m. route. For a direct comparison of how well group 1 and 2 vaccine-induced antibodies recognized the homologous V1V2(ZM53) on heterologous scaffolds, we tested plasma from each group for cross-binding to each V1V2 scaffold and found no difference in either group recognizing the homologous ZM53 domain (p = 1.0; Figures 3C and 3D; Table S1). Groups 1 and 2 also equally recognized the heterologous V1V2(ZM109) as presented by the V1V2(ZM109)-1FD6.monFc and the V1V2(ZM109)-TTB scaffolds (p = 0.4567 and p = 1.0, respectively; Figures 3C and 3D; Table S1). Antibody titers against V1V2(ZM019)-TTB in groups 1 and 2 were also comparable with the titers of group 6 against the same scaffold (p = 0.1662 and p = 0.7608, respectively; Figures 3C, 3D, and 3G; Table S1). These various comparisons suggest that the vaccine-induced antibodies were targeting homologous and heterologous V1V2 domains similarly, whether presented on homologous or heterologous scaffolds.
V1V2 in the context of the 1FD6 scaffold is strongly reactive with V2i but not V2p mAbs (Figures 2B–2E). In contrast, V2i mAbs bind much more weakly to V1V2(ZM109)-TTB, and V2p mAb CH58 binds neither. Group 4 animals, immunized with the V1V2(ZM109)-1FD6.monFc protein, generated the weakest responses despite the presence of the Fc fragment in the protein immunogen, which was included theoretically to enhance antigen processing (Figure 3E). However, despite its relatively poor antigenicity, the group 5 immunogen, V1V2(ZM109)-TTB, generated the strongest antibody responses to homologous and heterologous V1V2 segments displayed on heterologous scaffolds with higher 50% inhibitory dilution (ID50) values than all groups, except group 7, where it was combined with two other scaffold immunogens (Figure 3F; Table S1).
Group 6 animals were immunized with a combination of V1V2(ZM53)-2F5K and V3(ZM109)-TTB to determine whether induction of antibodies to V1V2 and V3 would be advantageous. Figure 3G shows that induction of V1V2 antibodies was not improved, and further studies of antibody binding (see below) with a more extensive antigen panel suggest that V3 immunogenicity may have impaired the V1V2 response. Animals in group 7 were immunized with three different V1V2-scaffold protein immunogens. Except for group 5, mean antibody titers in group 7 animals were higher than for all other groups against heterologous V1V2 scaffolds (Figure 3; Table S1).
The strong cross-reactivity with V1V2 domains from different strains by vaccine-elicited antibodies from animals immunized with V1V2-scaffold immunogens demonstrates that shared epitopes in this highly sequence-variable region of Env are readily recognized by polyclonal immune plasma antibodies, and the variability in the strength of the responses shows that the immunogenicity of V1V2 is dramatically influenced by the context in which it is presented. Taken together, this supports the concept that there may be multiple ways to target specific highly valued HIV-1 Env determinants.
The immunization regimen was systemic, with intradermal (i.d.) delivery of gp120 DNA and i.m. or s.c. protein delivery. To determine whether an antibody was transudated to mucosal sites, we collected vaginal secretions from each female animal and assayed non-blood-contaminated samples for the presence of V1V2 antibodies. Binding reactivity against cognate V1V2-scaffold immunogens was tested with specimens taken 2 weeks after the fourth immunization (week 22) using a starting dilution of 1:300 of secretions collected on Wek Cel swabs. The strongest responders were in group 7, where mucosal secretions from three of four females showed very strong binding signals in ELISA, demonstrating the presence of mucosal V1V2-specific IgG at dilutions of more than 1:50,000 against all three V1V2 scaffolds tested (Figure S1). When analyzed, 20 of the 43 secretion samples tested (47%) had 50% binding titers (ID50) of more than 1:2,000 against cognate V1V2 scaffolds (Figure 3I). Responses varied among the groups, indicating that the immunogens differentially induced mucosal IgG responses.
V1V2-Scaffold Immunogens Induce Antibodies that Are Cross-Clade Reactive and Differentially Target V1V2 When Presented in the Context of Recombinant V1V2-Scaffold Proteins
Luminex multiplex binding assays were used to produce data simultaneously against a panel of 16 antigens, with individual plasma samples from all animals in each group drawn 2 weeks after the last immunization (week 22). Data from a representative experiment are shown as a heatmap (Figure 4), where binding responses are represented as the mean fluorescence intensity (MFI) of replicates of plasma samples normalized to the reactivity of the antigen tested with a mAb pool. Antigens tested included (1) gp120s from clades C (ZM53), B (YU2), and A (MG505); (2) structurally unconstrained peptides representing the linear V3 clade C consensus sequence, the linear C5 sequence from clade C isolate ZM109, and the cyclic V2 sequence from clade E isolate 92TH023 (Karasavvas et al., 2012); (3) an unconstrained V1V2 sequence presented in the context of C-terminal his and avi tags (V1V2(1086) tags) (Liao et al., 2013); (4) a truncated form of gp70 from the murine leukemia virus spliced at its C terminus to clade B V1V2(CaseA2) (Pinter et al., 1998), (V1V2(CaseA2)-WT), or V1V2(CaseA2) carrying three mutations (K169V, V172E, and H173Y) to make it more similar to the V1V2 of clade E (V1V2(CaseA2)-mut 3-gp70) (Liao et al., 2013); (5) monomeric 1FD6 engrafted with clade C V1V2(ZM109), clade B V1V2(YU2), or clade A V1V2(MG505) (McLellan et al., 2011); (6) V1V2-scaffold proteins in which the V1V2 domain was grafted at a site identified using the Internal Coordinate Mechanics (ICM) molecular modeling environment (Abagyan et al., 1994) and used as immunogens in this study; and (7) BSA used as a negative control protein. Pools of pre-bleeds from animals in each group served as negative controls.
Luminex binding data from each group were analyzed by comparing the MFI of each group generated against antigens across each category shown in Figure 4. Plasma from animals in groups 1–3 that received V1V2-scaffold proteins only in the final immunization showed little reactivity with gp120 molecules (Figure 4, columns 1–3) compared with groups 4–7, which were co-immunized with gp120 DNA and V1V2-scaffold proteins for all four immunizations. Thus, it is likely that the gp120 DNA in the fourth immunization accounts for the gp120 and V3 plasma antibodies in animals from groups 4–7. Groups 1, 2, and 3 also had the poorest reactivity with V3 and C5 peptides (Figure 4, columns 4 and 5, respectively). Group 6 received the V3(ZM109)-TTB immunogen along with V1V2(ZM109)-TTB, resulting in the strongest gp120 and V3 peptide binding.
Except for group 4, nearly every animal developed antibodies to V1V2 antigens, indicated by the high MFI values in columns 7–15, in contrast with the lower MFI values for gp120, V3, and C5 (columns 1–6), indicating that the V1V2-scaffold immunogens primarily focused the antibody response on epitopes within V1V2. Moreover, the anti-V1V2 responses were cross-reactive, as shown by positive responses to reagents from clades A, B, C, and E. The varying patterns of reactivity between groups 1–6 suggest that each immunogen and immunization regimen induced antibodies differentially recognizing various V1V2 epitopes. Strikingly, the combination of trimeric and pentameric scaffold immunogens used in group 7 resulted in broad reactivity with all V1V2-scaffold antigens that was significantly greater than for all groups except group 5 (Figure 4, columns 10–15; Table S2).
All of the V1V2-scaffold immunogens induced antibodies reactive with V1V2 antigens presenting the V1V2 domain in slightly different configurations, as indicated by the antigenic characterizations (Figure 2). The strong binding of the immune plasma to V1V2 domains on different scaffolds diminishes the likelihood that these signals are due to or influenced by anti-scaffold responses. This is shown by (1) essentially identical reactivity by plasma from groups 1 and 2, where V1V2(ZM53) is presented on two different scaffolds (Figure 4, columns 13 and 14); (b) strong binding by group 5 plasma to heterologous V1V2(ZM53) on both the 2F5K and 2J9C scaffolds (Figure 4, columns 13 and 14); and (C) reactivity of group 4 plasma to homologous V1V2(ZM109) on the heterologous TTB scaffold (Figure 4, column 15).
Maturation of the Polyclonal Antibody Response in Immunized Macaques
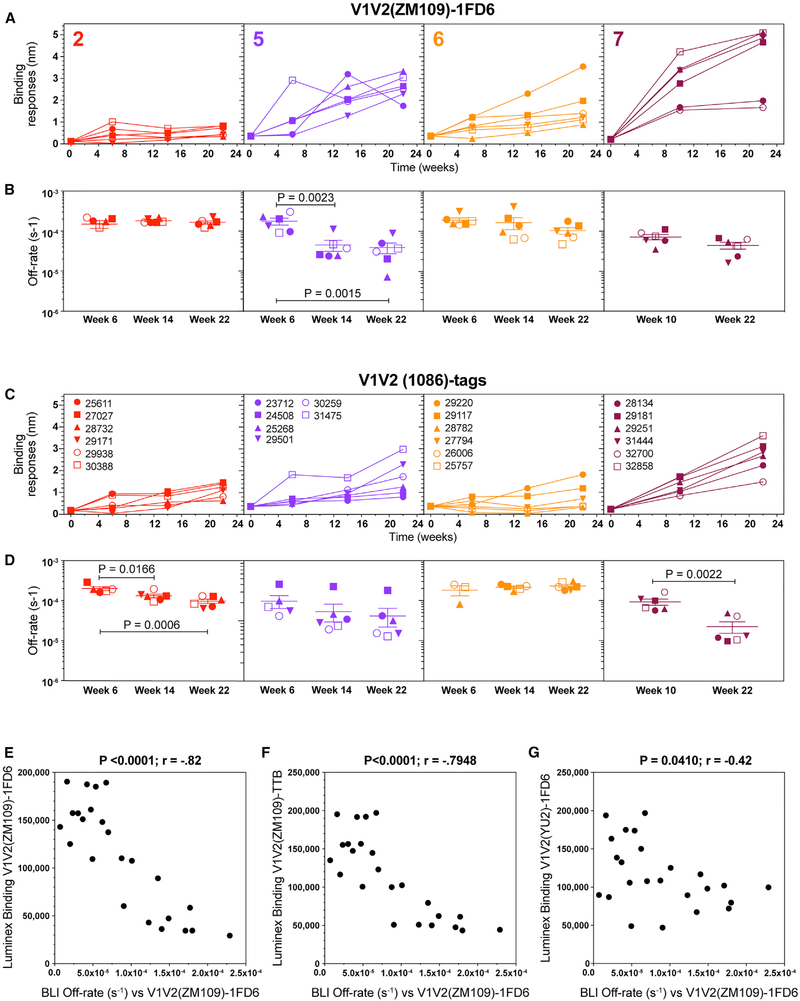
We have shown that the DNA + protein co-immunization strategy leads to a polyclonal plasma antibody response with increasing affinity during vaccination (Hessell et al., 2016). As shown in Figure 3, binding antibody titers in plasma increased rapidly, reaching peak or near-peak levels after only two immunizations. Here we used longitudinal biolayer interferometry (BLI) to assess increases in binding responses and affinity maturation in plasma drawn from animals in groups 2, 5, 6, and 7 at 2 weeks after each immunization. Using area under the curve (AUC) group-wise longitudinal comparisons of binding responses to V1V2(ZM109)-1FD6, group 7 binding responses continued to increase over the course of the immunizations and were significantly greater than those of group 2 and group 6 (p < 0.0001 and p = 0.0009, respectively; Table S3). Group 5 responses were comparable with group 7 (p = 0.0546; Table S3). Similarly, binding responses to V1V2(1086) tags developed continuously in all group 7 animals and were comparable to group 5 and significantly greater than those of groups 2 and 6 (p = 0.0004 and p = 0.0008, respectively; Figure 5C; Table S3). Group 2 binding responses were minimal and did not increase during the immunizations against either V1V2 antigens (Figures 5A and 5C).
Figure 5. Maturation of the Polyclonal Antibody Response in Immunized Macaques.
Real-time interactions between polyclonal macaque plasma antibodies and V1V2(ZM109)-1FD6 and V1V2(1086)-tags were monitored by biolayer interferometry (BLI).
(A and C) Real-time interactions between polyclonal macaque plasma antibodies were monitored by BioLayer Interferometry (BLI). Binding response: means of duplicate samples over the course of the immunizations reported in wavelengths of light (nanometers, nm) against V1V2(ZM109)-1FD6 (A) and V1V2(1086)-tags (C).
(B and D) Off-rates, based on an average dissociation constant (s−1) (more fully described in STAR Methods), are shown as group (n = 6) means (± SEM) for V1V2(ZM109)-1FD6 (B) and V1V2(1086)-tags (D).
(E-G) Statistically significant correlations are shown between decreasing off-rates (equivalent to increasing antibody affinity) and Luminex binding for V1V2(ZM109)-1FD6 (E), V1V2(ZM109)-TTB (F), and V1V2(YU2)-1FD6 (G).
Spearman correlation analyses were performed in GraphPad Prism 7.0 for Mac.
See Table S3 for statistical analysis of group comparisons.
To determine affinity maturation, we used BLI to measure the binding of the polyclonal antibodies as a function of immunization. The concentration of binding antibodies is required for accurate measurement of on-rates, which is difficult to determine in plasma. Here we determined off rates because, by definition, off-rates are independent of antibody concentration and can be used as a surrogate for affinity measurements in plasma samples, values that reflect antibody affinity maturation (Kamat and Rafique, 2017). Thus, decreasing off-rates in samples from groups 5, 6, and 7 indicate increasing binding affinities to V1V2(ZM109)-1FD6 as the immunization regimen progressed (Figure 5B), although this only reached statistical significance in group 5 between immunizations 2 (week 6) and 3 (week 14) (p=0.0023) and between immunizations 2 (week 6) and 4 (week 22) (p = 0.0015). Groups 5 and 7 were comparable regarding affinity increase during the immunizations, but group 7 surpassed group 6 (p = 0.0120; Table S3), despite fewer immunizations in the regimen.
Affinity for V1V2(1086) tags by group 7 surpassed that of groups 5 and 6 (p = 0.024 and p = 0.0044, respectively; Figure 5D; Table S3). Despite overall low binding responses in group 2 for this antigen, significant affinity increases were measured between the second and third immunizations (p = 0.0166) and between the first and last immunizations (p = 0.0006). Antibody affinity in group 7 with the strongest and most consistent response significantly increased after the second and third immunizations (p = 0.0022).
When the data from all 24 animals in groups 2, 5, 6, and 7 were analyzed together, correlations were found between decreasing off-rates for V1V2(ZM109)-1FD6 measured in BLI and the binding responses measured in Luminex to V1V2(ZM109)-1FD6 (p < 0.0001; Figure 5E), V1V2(ZM109)-TTB (p < 0.0001; Figure 5F), and V1V2(YU2)-1FD6 (p < 0.0410; Figure 5G). These data are equivalent to a positive correlation between antibody affinity and binding and indicate that a robust polyclonal antibody response was accompanied by affinity maturation.
V1V2 Scaffolds Induce Neutralizing Antibody Titers that Correlate with Increasing Antibody Affinity
Plasma samples collected 2 weeks after each immunization were tested in the TZM-bl assay for neutralization of a panel of HIV tier 1A and 1B pseudoviruses (Figure 6A). We also assayed tier 1B clade C ZM197 and tier 2 clade C ZM53, but the resulting neutralization titers were low and sporadic against these isolates (data not shown). Neutralization titers against the tier 1A clade C virus MW965 and clade B virus SF162 were seen in all groups. The higher titers against these isolates by group 6 are likely due to the inclusion of the V3-scaffold immunogen in the regimen. Notably, although neutralizing antibody titers against tier 1B viruses were low and infrequent in groups 1–6, all group 7 macaques neutralized tier 1B clade C ZM109 after three immunizations.
Figure 6. V1V2-Scaffold Immunogens Induce Neutralizing Antibody Titers that Correlate with Increasing Antibody Affinity.
Neutralization data are expressed as the ID50; i.e., the plasma dilution that neutralized 50% of the infecting virus in the TZM-bl assay.
(A) Individual ID50 values from plasma samples collected 2 weeks after each immunization, tested in duplicate for neutralization of tier 1B ZM109 and tier 1A MW965 and SF162 pseudoviruses.
(B) ID50 values for each animal in each group and the mean ID50 +/− SEM for each group (n = 6) using week 22 plasma against tier 1 clade B SHIVBaL and tier 1 clade C SHIV1157-ipEL.
(C) Group (n = 6) mean (± SEM) SHIV ID50 neutralizing titers in plasma. Data are representative of assays that were repeated at least twice with comparable results.
(D–F) Correlations between HIV(ZM109) neutralization (ID50) and Luminex binding (MFI) for V1V2(YU2)-1FD6 (D), V1V2(ZM109)-1FD6 (E), and V1V2(1086) (F) tags.
(G) Correlation between SHIV1157.ipEL neutralization (ID50) and BLI off-rates (s−1) for V1V2(1086) tags.
Spearman correlation tests were performed in GraphPad Prism 7.0 for Mac.
Plasma samples collected 2 weeks after the final immunization were also tested for neutralization of tier 1 simian/human immunodeficiency virus, strain BaL (SHIVBaL) (clade B) and SHIV1157-ipEL (clade C). The mean ID50 for each animal in each group against both SHIVs are shown in Figure 6B, with the ID50 values for each group against each SHIV shown in Figure 6C. The Envs of these viruses are heterologous to all of the immunogens used, and, with the exception of plasma from group 2, these SHIVs were neutralized by plasma from the majority of animals. Higher titers against both SHIVs were induced in groups 4–7, which were co-immunized with DNA and protein immunogens in each dose, whereas groups 1–3, which received only a protein immunogen for the last immunization, induced lower mean neutralizing titers against these SHIVs.
We further analyzed neutralization, binding, and affinity data for statistical correlations. Neutralization of tier 1B clade C ZM109 correlated with binding responses in Luminex to V1V2(ZM109)-1FD6 (p = 0.0088; Figure 6D), V1V2(YU2)-1FD6 (p = 0.0492; Figure 6E), and V1V2(1086),tags (p = 0.0003; Figure 6F). Neutralization of clade C SHIV1157.ipEL correlated with affinity increases (equivalent to off-rate decreases) against V1V2(1086) tags (p = 0.0309; Figure 6G). These correlations demonstrate that gp120 DNA + V1V2 scaffold co-immunization regimens induced antibodies that targeted the V1V2 region and that these antibodies increased in magnitude, affinity, and neutralizing capacity over the course of the immunizations.
Vaccine-Induced V1V2-Specific Antibodies Mediate Fc-Dependent Functional Responses that Correlate with Binding Responses and Increasing Antibody Affinity during Immunization
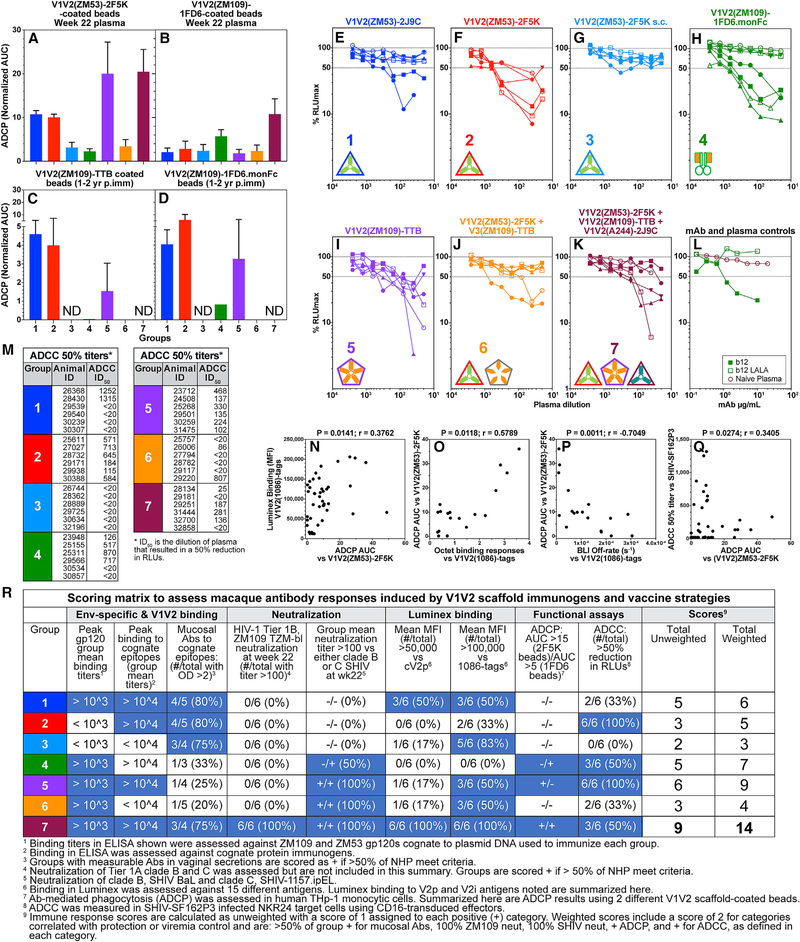
There is continuing evidence of the contribution of vaccine-induced functional antibody responses correlating with protection in vivo (Barouch et al., 2015, 2018; Chung et al., 2015). In vitro, we previously demonstrated (1) ADCP activity by human V2i-specific mAbs (Musich et al., 2017b), (2) Fc-mediated inhibitory activity by V2i mAbs against HIV replication in monocyte-derived macrophages and immature dendritic cells (Holl et al., 2006), (3) ADCP by serum antibodies from rabbits immunized with gp120 DNA and V1V2-scaffold immunogens (Zolla-Pazner et al., 2016), and (4) a significant association of Env IgG3 and V1V2 antibodies with ADCC levels in RV144 (Yates et al., 2014). We now show that durable antibody responses that mediate ADCP can be induced in macaques using different V1V2-scaffold immunogens when vaccine strategies contain single, double, or triple immunogens. We tested plasma for ADCP from all animals 2 weeks after the final immunization (week 22) (Figures 7A and 7B) and from animals that were still available for blood draws 13 and 27 months after the last immunization (Figures 7C and 7D). Phagocytosis was measured using THP-1 cells and beads coated with V1V2(ZM53)-2F5K, V1V2(ZM109)-1FD6, or V1V2(ZM109)-TTB. Group 7 ADCP activity was greater than that of groups 3 (p = 0.0114), 4 (p = 0.0067), and 6 (p = 0.0134) against the V1V2(ZM53)-2F5K antigen and was greater than that of all groups, except group 4, against V1V2(ZM109)-1FD6 (p = 0.0039, p = 0.0076, p = 0.0051, p = 0.0030, and p = 0.0049; Figure 7A; Table S4). The strength and consistency of the ADCP activity generated by group 7 plasma against both antigens may indicate an advantage of immunizations with V1V2 from both ZM53 and ZM109. Interestingly, binding antibodies in group 5 samples generated by the V1V2(ZM109)-TTB scaffold were consistently cross-reactive with V1V2(ZM53) scaffolds in ELISA and Luminex. The strong ADCP against the heterologous V1V2(ZM53)-2F5K antigen by group 5, immunized with only V1V2(ZM109)-TTB, further suggests strong immunogenicity generated by the TTB scaffold immunogen.
Figure 7. Vaccine-Induced V1V2-Specific Antibodies Mediate Fc-Dependent Functional Responses that Correlate with Binding Responses and Increasing Antibody Affinity during Immunization.
(A and B) ADCP is expressed as normalized area under the curve values (AUC; + SEM) using plasma samples tested from each group (n = 6), collected at week 22 against beads coated with V1V2(ZM53)-2F5K (A) and V1V2(ZM109)-1FD6 (B).
(C and D) Mean AUC values for samples available for testing 1–2 years after the last immunization against beads coated with V1V2(ZM109)-TTB (C) and V1V2(ZM109)-1FD6-monFc (D). Samples tested and months after final immunization: group 1 (n = 2) at 27 months; group 2 (n = 2) after 26 months; group 3, not done, no animals available; group 4 (n = 1) after 13 months; group 5 (n = 2) after 13 months; group 6 (n = 1), zero value; group 7, not done, no animals available. All samples were tested in duplicate. See Table S4 for statistical analysis.
(E-K) ADCC activity in NHP plasma tested in triplicate against target cells infected with SHIVSF162P3: group 1 (E), group 2 (F), group 3 (G), group 4 (H), group 5 (I), group 6 (J), group 7 (K), control mAbs, and naïve plasma (L). See Table S5 for statistical analysis.
(L) Positive and negative controls for ADCC. Data shown are representative of assays that were repeated at least twice with comparable results.
(M) Dilutions of plasma causing a 50% reduction in RLUs, indicating 50% SHIVSF162P3-infected cell killing.
(N–Q) Correlations of binding, off-rates, and functional immune responses of plasma Abs: ADCP AUC of V1V2(ZM53)-2F5K coated beads versus binding in Luminex against V1V2(1086)-tags, P=0.0141 (N), ADCP AUC of V1V2(ZM53)-2F5K coated beads versus BLI binding responses to V1V2(1086)-tags, P=0.0118 (O), ADCP AUC of V1V2(ZM53)-2F5K coated beads versus BLI off-rates (s−1) for V1V2(1086)-tags, P=0.0011 (P), and ADCP AUC of V1V2(ZM53)-2F5K coated beads versus ADCC vs SHIVSF162P3, P=0.0274 (Q). Spearman correlations were performed in GraphPad Prism 7.0 for Mac.
(R) Scoring matrix comparing antibody responses across groups and summarized with total unweighted and weighted scores for each group as described in the text.
We also measured ADCP activity in the plasma of animals that were available 13 and 27 months after the last immunization (Figures 7C and 7D). Two animals from group 1 and two from group 2 were available for blood sampling at 26 and 27 months, respectively, after the last immunization time point (week 22). Additional animals from groups 4 (n = 1), 5 (n = 2), and 6 (n = 1) were sampled 13 months after the final immunization. Remarkably, specimens from all but one of these animals retained phagocytic activity in the plasma 13 or 27 months post-immunization. ADCP activity in macaque plasma against V1V2(ZM53)-2F5K from a tier 2 clade C isolate correlated with binding to V1V2(1086) tags in Luminex (p = 0.0141; Figure 7N) with BLI binding responses (p = 0.0118; Figure 7O) and, inversely, with BLI off-rates, which is equivalent to a positive correlation with antibody affinity (p = 0.0011; Figure 7P).
Vaccine-Induced Macaque Antibodies Mediate ADCC Activity against SHIVSF162P3-Infected Target Cells
The importance of ADCC in the context of natural infection as well as vaccine pre-clinical and clinical trials is well-documented (Lewis, 2014). To evaluate plasma ADCC in vaccinated macaques, we assayed samples from all animals in each group at week 22 against SHIVSF162P3-infected cells (Figures 7E–7L). We found that, with the exception of group 3, immunized with DNA gp120 and V1V2(ZM53)-2F5K (Figure 7G), which showed no ADCC activity, plasma from several animals in each of the other groups was able to mediate more than 50% of ADCC activity (defined as the plasma dilution giving a 50% or more reduction in relative lights units (RLUs). Overall, except for group 3, ADCC activity among all other groups was comparable (statistical analyses are shown in Table S5). The macaque ADCC activity against tier 2 SHIVSF162P3-infected cells correlated with ADCP measured using V1V2(ZM53)-2F5K-coated beads (p = 0.0274; Figure 7Q). Importantly, antibodies induced by V1V2-scaffold immunogens derived from clade C viruses killed cells infected with the heterologous clade B tier 2 SHIVSF162P3, although there was no neutralization against this virus (data not shown).
Vaccine-Induced Responses with V1V2 Scaffold Immunogens Can Be Assessed in a Scoring Matrix
The outcomes of all vaccine-induced antibody responses tested are summarized in a matrix that compares each group and calculates an overall vaccine score (Figure 7R). To compose this table and evaluate the overall results of each group, we assigned a score of 1 for each parameter that met or exceeded a “success criterion,” and the sum of these scores created an “unweighted score” for each group. For parameters that were previously correlated with protection or viral control, the score for that parameter was doubled, resulting in a “weighted score.” Group 7 (immunized with DNA gp120 and three V1V2-scaffold proteins), which is positive for each of the nine criteria, garnered the highest rating in both unweighted and weighted scores, whereas group 5 (immunized with DNA gp120 + V1V2-TTB) had the next-highest score, suggesting that the V1V2-TTB tested alone in group 5 and included in the trivalent antigen cocktail used in group 7 likely played an important role in the immunogenicity of the group 7 regimen. Inclusion of the V3-TTB pentamer with V1V2(ZM53)-2F5K in group 6 did not enhance the immunogenicity of the V1V2(ZM109)-2F5K antigen used in group 2 other than increasing the neutralization of tier 1A and SHIV viruses, likely because of V3 targeting. Vaccine-induced antibodies in Group 7 macaques were the most potent in mucosal secretions (Figure 3I; Figure S1); had the broadest reactivity with V1V2 antigens from clades A, B, and C (Figure 4); developed the strongest increase in affinity (Figures 5A–5D); neutralized the tier 1B clade C virus ZM109 (Figure 6A); and showed functional antigen-specific ADCP and ADCC against tier 2 SHIV-infected cells (Figures 7A–7M).
DISCUSSION
Thirty-eight years into the HIV epidemic, there is still a critical need for an effective AIDS vaccine that protects against or controls HIV-1 infection (Fauci et al., 2008). Four large human vaccine efficacy trials have been completed, with only one partially successful outcome, the RV144 Thai trial, in which vaccine efficacy at 1 year was 60% (Robb et al., 2012) and at 3.5 years was 31.2% (Rerks-Ngarm et al., 2009), a level that is insufficient as a public health standard (Baden and Dolin, 2012). The RV144 trial revealed only a single independent biomarker of vaccine efficacy among more than 150 variables tested: an inverse correlation between reduced risk of HIV infection and a robust antibody response specific for the V1V2 region of gp120 as presented by a V1V2-gp70 fusion protein (Haynes et al., 2012; Zolla-Pazner et al., 2013, 2014). Subsequent meta-analyses indicated that protection was also correlated with antibodies reactive with V2 peptides (Gottardo et al., 2013), and characterization of the RV144 vaccinees’ break-through viruses also supported the role of V1V2 antibodies (Rolland et al., 2012). Moreover, although neutralizing antibody activity was not a correlate of reduced infection risk, the RV144 HIV-1 vaccine trial induced antibodies that mediated ADCC (Bonsignori et al., 2012; Chung et al., 2014b; Gordon et al., 2014; Julg et al., 2017; Roederer et al., 2014) and complement activation (Perez et al., 2017), both of which may have played a role in the partial protection observed.
Other active immunization studies subsequent to RV144 in macaques have identified V1V2 antibodies as playing a role in reduced risk of acquisition of simian immunodeficiency virus (SIV) (Barouch et al., 2012; Vaccari et al., 2016a, 2016b). Systems serology analysis of animal vaccine data also support the importance of Fc-mediated anti-viral effects (Ackerman et al., 2017; Bradley et al., 2017). Finally, passive immunization of macaques with a single V2i mAb followed by mucosal challenge with SHIVBaL has been shown to decrease viral DNA significantly in PBMCs and lymphoid tissues and reduce plasma virus levels (Hessell et al., 2018). Together, the results of these human and NHP vaccine experiments suggest that V1V2 antibodies and Fc-dependent activities can participate in control of SHIV and SIV infection and are correlated with a reduced risk of SIV, SHIV, and HIV infection.
Vaccines that did not induce bNAbs but reduced the risk of acquisition following repeated SHIV and SIV challenge in rhesus macaques have been reported to correlate with Env-specific binding and functional antibody responses, including ADCP (Barouch et al., 2012, 2013; Roederer et al., 2014). More recently, a human vaccine trial using mosaic Ad26 and a high-dose gp140 protein boost induced strong Env-specific binding antibody responses in all subjects and ADCC responses in 80%. Importantly, this study included a parallel vaccine-challenge study in rhesus macaques that resulted in 67% efficacy against SHIV challenge with reported correlates that included Env-specific antibodies (Barouch et al., 2018).
We initiated comparative vaccine studies using co-immunization with gp120 DNA and five different V1V2-scaffold immunogens, individually and in combinations, using varying immunization routes and time courses, with the primary goal of focusing the antibody response and inducing antibodies with anti-viral activies. The scaffolds were designed to present the V1V2 region as dimeric, trimeric, or pentameric glycosylated immunogens. Antigenicity studies using human mAbs revealed that these scaffolds had related but distinguishable properties. For example, none of the current versions of V1V2 scaffolds studied here displayed the V2qt epitope, but all displayed V2q and V2i epitopes (as determined by ELISA with mAb PG9 and V2i mAbs), and only the trimeric versions reacted with V2p mAbs. Several of these V1V2-scaffold protein immunogens induced V1V2-focused antibody responses in rabbits (Jiang et al., 2016; Zolla-Pazner et al., 2016), and the selection of V1V2-scaffolds immunogens for testing in macaques here was based on the rabbit data. In addition to the specific goal of targeting epitopes within the V1V2 domain, we used vaccine regimens known to elicit durable, high-avidity systemic and mucosal antibodies with multiple anti-viral functions. Macaque groups were immunized with different V1V2-scaffold immunogens or immunogen combinations or using different vaccine regimens, resulting in antibodies focused on V1V2 but nuanced in the fine specificity patterns induced. Mucosal antibodies were detected at high titers in most vaginal samples tested, and there was evidence of increasing affinity against V1V2 antigens in groups 2, 5, and 7. Only modest neutralizing activity developed against heterologous tier 1A and 1B HIV isolates as well as heterologous SHIVs.
Presentation of V1V2 in the context of the Fc fragment of monkey IgG was tested in group 4 to enhance ADCC activity. In this group, three of six animals developed strong ADCC activity that was durable for at least 1 year in the one macaque available for testing. However, the overall antibody response of group 4 was moderate and not as strong or cross-reactive as the response of animals in group 7, which were not immunized with the V1V2–1FD6.monFc immunogen. Thus, multiple scaffolds can be effective, and the TTB pentamer and the 2F5K trimer also generated antibodies with ADCC activity when delivered i.m. but not s.c.
Taken together, the data suggest that a cocktail of three V1V2-scaffold immunogens presenting V1V2 from different viruses and clades in the context of different scaffolds is the best regimen tested for induction of humoral vaccine antiviral responses in macaques in terms of binding, mucosal targeting, neutralization, and effector functions while increasing affinity with each immunization. The scoring matrix comparing all groups shows that the immune response induced by the regimen used in group 7 (immunization with gp120 DNA and three V1V2-scaffold proteins) resulted in the most potent, broad, and functional antibodies. Group 7 antibodies also displayed the highest affinity (lowest off-rate) 2 weeks after the last immunization. The improved antibody responses in group 7 may be due to the increased amount of protein immunogens delivered (three times as much as received by animals in groups 1–5), the modified timing of immunization, or the s.c. delivery of the scaffold proteins. It is also possible that the increased immunogenicity is the result of including V1V2(A244)-2J9C in the protein immunogen cocktail, given that the A244 Env appears to be unusual in its ability to induce V1V2 antibodies. This was the only V1V2 scaffold not previously tested as a single immunogen, and, therefore, its efficacy when used alone remains to be studied. The potency of the cocktail used in group 7 may also be due to the inclusion of V1V2(ZM109)-TTB (used alone in group 5), which is an exceptionally immunogenic fusion protein.
These immunogenicity studies were not designed or powered to include SHIV challenge experiments, and because there was no viral challenge, the effectiveness of these responses in protection from infection is unknown and should be assessed. One important addition to our vaccine in future experiments will be the inclusion of additional antigens to broaden T cell responses.
Despite progress, further development of scaffolds that can present the V2qt epitope is warranted to improve the neutralization breadth and potency of vaccine-induced antibodies. Our focus on immunogens that can induce antibodies directed to the V1V2 region is based on human and NHP active and passive immunization experiments that suggest an important but not exclusive role for these antibodies. As a result, we posit that, in a setting where the induction of bNAbs by vaccination is limited (Pauthner et al., 2019), the development of immunogens like those described in this study that readily induce durable, polyfunctional V1V2 antibodies will constitute a modality providing reduced risk of acquisition and better viral control, which would be a major step forward, especially in areas of the world where the incidence of HIV infection is high.
STAR★METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by Dr. Susan Zolla-Pazner (Susan.Zolla-Pazner@mssm.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
A total of forty-two (n = 42) male and female outbred Indian rhesus macaques (Macaca mulatta) between 2 and 10 years of age were sourced and housed at the Oregon National Primate Research Center (ONPRC) in Beaverton, OR. Assignments were made to create immunogenicity groups comprised of six (n = 6) animals in each group. All animals were free of Cercopithicine herpesvirus 1, D-type simian retrovirus, simian T-lymphotrophic virus type 1 and SIV infection at the start of the study. All procedures were performed according to rules and protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Oregon Health & Science University. At the end of the study, animals were not euthanized, but instead all animals were released from assignment and returned to the colony at ONPRC.
Cell lines
HEK293 GnTI−/− cells are used for downstream large-scale transient transfection of epitope-scaffold proteins using 293S(GnTI−/−, restricts N-linked glycans to a homogeneous Man5-GlcNac2 structure) or 6E (EBNA cells) cell lines. Cells are cultured in suspension with Freestyle 293 Expression medium in polycarbonate Erlenmeyer flasks (with ventilation membrane caps) using 15%−25% of the nominal volume. They are incubated in an upright CO2 incubator under standard humidified conditions (37°C and 5% CO2) and rotated at 110–130 rpm in an orbital shaker. Cells are passaged to 1 × 106 per ml on the day of transfection. The transfection reagents are prepared by mixing high-quality plasmid DNA and PEI in fresh serum-free culture medium and incubating approximately 10 minutes while the polymer associates with the DNA. The DNA/PEI solution is added to the passaged cells and incubated for approximately 4–5 days before harvesting. Typically, we use 1 μg of expression plasmid for each ml of transfection volume. Cell supernatants are harvested by pelleting the cells and filtering through a 0.22 μm membrane. The filtered supernatants are then loaded onto affinity columns for protein purification.
TZM-bl cells are used for HIV-1 pseudovirus neutralization assays and are obtained from the NIHARRP, Cat. No. 8129. Complete growth medium (GM) consists of D-MEM supplemented with 10% fetal bovine serum (FBS, heat-inactivated), 25 mM HEPES and 50 μg/ml gentamicin. TZM-bl is an adherent cell line that can be disrupted and removed by treatment with trypsin/EDTA at confluency for routine maintenance and for assay preparation. Cultures are passaged up to a maximum of 20 times every 2–3 days with approximately 106 cells in 15ml of GM. Cultures are incubated at 37°C in a 5% CO2/95% air environment.
HEK293 cells (NIHARRP, Cat. No. 103) are used to produce pseudovirions that are harvested in media, frozen, and titered before use in neutralization assays. The original frozen vial is thawed and maintained individually during initial tissue culture to prevent cross contamination. Cells are expanded until sufficient cell numbers are obtained to viably freeze. A bank of 10–20 vials containing between 5–10×106 cells per vial are stored in liquid nitrogen. Careful monitoring of passages is recorded and when needed, a new vial is thawed, viability assessed, expanded, and frozen as above to obtain a secondary bank. Cells from banks >2 years old are discarded and replaced as new banks are created. 293F cells (FreeStyle™, Life Technologies/Thermo Fisher) are used for transient transfection to produce human monoclonal antibodies. The integrity of the stock is maintained as described above for the HEK293 cells. Cultures are incubated at 37°C in a 5% CO2/95% air environment.
THP-1 cells (ATCC, Cat. No. TIB-202, RRID:CVCL_0006) are cultured in a base medium of RPMI-1640 Medium supplemented with 2-mercaptoethanol to a final concentration of 0.05 mM and fetal bovine serum to a final concentration of 10% to make the complete growth medium. Cultures are maintained by the addition or replacement of fresh medium every 2 to 3 days or when cell concentrations reach 8×105 cells/mL. Cell concentrations are not allowed to exceed 1 × 106 cells/mL.
NKR24 luciferase-reporter cell line was derived from CEM.NKR.CCR5 CD4+ T cells (Howell et al., 1985; Trkola et al., 1999) and obtained from the AIDS Research and Reference Reagent Program.
KHYG-1 rhCD16 effector cells were derived from the CD16-negative human NK cell line KHYG-1 (Japan Health Sciences Foundation) (Yagita et al., 2000).
METHOD DETAILS
Macaque immunizations
Groups of adult rhesus macaques (Macaca mulatta) were assigned to the study and balanced for age and gender of the animals. For each group (n = 6) of male and female macaques, co-immunizations with HIV-1 ZM109 Env gp120 gene expression plasmids and one or more soluble V1V2 protein scaffolds were administered. Six groups were co-immunized at weeks 0, 4, 12 and 20. One group was co-immunized at weeks 0, 8, and 20. Three groups (1–3) received only protein at the final boost at week 20 (details are provided in Figure 1B and the text). At each co-immunization, a total of 36 μg HIV-1 ZM109 or ZM53 gp120 plasmid DNA was delivered epidermally with a Particle Mediated Epidermal Delivery (PMED) device (gene gun, XR-1 research model, PowderMed, Oxford, UK), by administering 2 μg of the DNA vaccines coated onto 1 μm-size gold particles into each of 18 sites along the shaved abdomen and upper thighs, as previously described (Hessell et al., 2016). Simultaneously, 50 μg or 100 μg of one or more of the recombinant V1V2-scaffold protein immunogens were delivered intramuscularly, formulated with not more than 20% Adjuplex (Sigma) adjuvant (Hessell et al., 2016). Regular blood sampling and vaginal secretions were obtained every two weeks to monitor immune responses in blood and at the mucosae.
Recombinant V1V2-scaffold proteins
Codon-optimized gp120 DNA expressing env from HIV clade C primary isolate ZM109F (Li et al., 2006) or ZM53 was prepared in the pJW4303 vector with a tissue plasminogen activator (tPA) leader sequence, as described previously (Wang et al., 2006; Zolla-Pazner et al., 2008). The genes of the following proteins were used to prepare the scaffolds of the V1V2-scaffold immunogens: PDB accession number 1FD6 (Ross et al., 2001), typhoid toxin subunit B (TTB) (Song et al., 2013), and PDB accession numbers 2J9C (Yildiz et al., 2007) and 2F5K (Zhang et al., 2006). The V1V2 sequences of the full-length V1V2 regions used as inserts into the scaffolds were previously provided in Jiang et al. (2016). Genes of the V1V2 and V3 scaffolds were chemically synthesized and cloned into the pVRC8400 plasmid, followed by expression in HEK293 GnTr−/− cells with protein purification performed by affinity chromatography (Jiang et al., 2016; Reeves et al., 2002). Details of the design and construction of the immunogens are described separately (Jiang et al., 2016; McLellan et al., 2011). Scaffolds were designed by engrafting the V1V2 or V3 domains into trimeric or pentameric scaffolds. The designations of 2F5K, 2J9C, and 1FD6 are PDB accession numbers, and V1V2 and V3 sequences were derived from HIV-1 Clade C, ZM53 or ZM109 isolates. V1V2-scaffold immunogens were generated from codon-optimized gene constructs expressed in mammalian cells. See Jiang et al. (2016) for details.
Recombinant proteins and peptides used as antigens
The HIV-1 BaL gp120, ZM53 gp120, and ZM109 gp120 were purchased from Immune Tech (New York, NY), and gp120 A244 was provided by Global Solutions for Infectious Diseases (South San Francisco, CA). V1V2-tags protein of strain 1086 (V1V2/1086-tags), and V1V2/CaseA2-mut 3 were provided by Dr. H-X. Liao (Duke University), and V1V2/CaseA2-gp70 was provided by Dr. A. Pinter. A clade C V3 consensus linear, non-biotinylated 23-mer peptide (NNTRKS IRIGPGQTFYATGDIIG) and the cyclic V2/92TH023 (cV2 E, clade E) biotinylated peptide were purchased from BioPeptide (San Diego, CA). A C5 linear non-biotinylated 22-mer peptide (from clade C ZM109, amino acids 495 to 516 of gp120, KIEPLGVAPWKAKRRVVQREKR) was purchased from PolyPeptide Group (Torrance, CA).
DNA immunogens
Codon-optimized gp120 DNA expressing env from HIV clade C primary isolate ZM109F (Li et al., 2006) was prepared in the pJW4303 vector with a tissue plasminogen activator (tPA) leader sequence, as described previously (Wang et al., 2006; Zolla-Pazner et al., 2008). The V1V2 sequences of the full-length V1V2 regions of ZM109 and ZM53 used as inserts into the scaffolds were previously shown (Jiang et al., 2016).
ELISA Binding Assays
The binding IgG antibody responses in macaque plasma were measured by ELISA. Briefly, Costar half-well assay plates (Corning) were coated with either cognate immunogen proteins at 2 μg/ml or recombinant gp120 proteins at 1 μg/ml. IgG responses in vaginal secretions were measured against cognate immunogens coated on plates at 2 μg/ml. Antigens were coated at 50 μl per well in 0.2M sodium carbonate/bicarbonate buffer, pH 9.4 overnight at 4°C. Plates were washed in dilution buffer, 1X PBS plus 0.1% Triton X-100, followed by a blocking step with 150 μl per well of 1X PBS, 1% normal goat serum, 5% BSA for 1 hr at room temperature. Plasma samples were heat-inactivated at 56° for 30 min. Serially diluted samples were transferred to the assay plate and incubated for 1 hr at room temperature. Plates were washed three times and bound antibodies were detected with Peroxidase AffiniPure Goat Anti-Human IgG, Fcγ Fragment Specific (Jackson Immuno Research) diluted 1:5000 and then incubated for 1 hr at room temperature. After washing, 50 μl per well of TMB One Component Substrate (Southern Biotech) was added and incubated for 10 min. The reaction was stopped with the addition of 50 μl per well of 1N sulfuric acid. The plates were read at two wavelengths, 450 nm and 650 nm, in a SpectraMax190 plate reader (Molecular Devices, Sunnyvale, CA). Assays are standardized with positive and negative control mAbs and naive plasma which are included with each assay. The dilution at which 50% maximum binding is detected is calculated in Prism 7.0 and reported as 50% plasma antibody binding titers.
Pseudovirus Neutralization Assays
Serum and purified IgG samples were tested for their ability to neutralize HIV-1 and SHIV pseudoviruses using the single-cycle TZM-bl neutralization assay as described previously (Montefiori, 2009; Wei et al., 2003). As a negative control, a pool of preimmune plasma was used at the lowest dilution tested (1:20). Neutralization dose-response curves were fitted by nonlinear regression and a final titer is reported as the reciprocal of the dilution of serum necessary to achieve 50% neutralization.
Biolayer interferometry (BLI) binding assay
Real-time interactions between polyclonal macaque antibodies and V1V2 antigens were monitored on an Octet RED96 instrument (Pall FortéBio, Fremont, CA) at 30°C. Avi-tagged recombinant V1V2(ZM109)-1FD6 and V1V2(1086)-tags were biotinylated with the BirA biotinylation kit (Avidity), according to the manufacturer’s instructions. Biotinylated V1V2 antigens were diluted in kinetics buffer (0.1% [w/v] bovine serum albumin [BSA], 0.02% [v/v] Tween-20 in phosphate-buffered saline; Pall FortéBio) and immobilized on streptavidin (SA) biosensors (Pall FortéBio) at ~50% of the sensor maximum binding capacity. Loaded biosensors were dipped into wells containing 2-fold serial dilutions (from 1:10 to 1:160) of plasma in kinetics buffer for 900 s, followed by an 1800 s dissociation step in buffer. A buffer-only reference was subtracted from all curves, and preimmune plasmas were used as a negative control. Indicated binding responses were measured at the end of the 900 s association step for the 1:10 plasma dilution. Off-rates were obtained by fitting curves to a 1:1 binding model using the Data analysis software 9.0 (Pall FortéBio). For each plasma sample, an average dissociation constant (s−1) was derived from dilutions that generated binding responses above background (measured from a preimmune pool for each group) and well-fitted curves (χ2 < 3, R2 > 0.95).
Luminex Binding Assay
HIV-1 antigens included recombinant gp120 molecules (ZM53, YU2 and MG505) from Immune Tech (New York, NY). To ensure sufficient coupling of peptides to the beads, all peptides used bore an N-terminal 6x Lys-Gly (KG)-linker. The clade C consensus V3 linear peptide (NNTRKSIRIGPGQTFYATGDIIG) and the clade E cyclic V2 (92TH023) (cV2 E) peptide (CSFNMTTELRDKKQKVHALFYKLDIV PIEDNTSSSEYRLINC) were purchased from GenScript (Piscataway, NJ) and the C5 (ZM109) linear peptide (VEIKPLGIAPTEA KRRVVQREKR) was purchased from BioPeptide (San Diego, CA). V1V2-scaffold proteins bearing V1V2 Env inserts from a variety of HIV-1 strains and clades included V1V2–1FD6, V1V2–2J9C, V1V2–2F5K and V1V2-TTB synthesized by X. Jiang and X-P. Kong (NYU School of Medicine), V1V2-tags and V1V2VRC-gp70 reagents provided by B. Haynes and J. Peacock (Duke University) and V1V2-gp70 (CaseA2) provided by A. Pinter (Rutgers University). Antigens were covalently coupled individually to magnetic beads using a two-step carbodiimide reaction with the xMAP Ab Coupling (AbC) Kit according to manufacturers’ instructions (Luminex, Austin, TX). Carboxylated xMAP beads were coupled to 0.5 μg protein/million beads (V1V2/ZM109-TTB and V1V2/ZM109–1FD6 and V1V2/YU2–1FD6) or 1 μg protein/million beads (all peptides and V1V2/MG505–1FD6) or 4 μg protein/million beads (all gp120s, V1V2/ZM53–2F5K, all V1V2-tags, -gp70, and BSA), as determined by titration of Ags against a mAb pool and a control serum sample. The coupled beads were counted, diluted to a concentration of 500,000 beads/mL and stored at 4°C for up to 1 month prior to use. Each antigen was bound to a bead with a different fluorescent profile. Fluorescence was measured using a Luminex FlexMAP3D device with xPONENT 4.2 software. Samples were tested in duplicates and results are shown as mean fluorescent intensity (MFI). Beads coupled to BSA and serum from pre-bleeds served as negative controls. A cocktail of mAbs composed of multiple V2 (697, 830A, 1393A and CH58), V3 (3869) and C5 (670, 1331A) mAbs was used in each experiment for inter-experimental standardization. Heatmaps were generated in GraphPad Prism 7.03.
Antibody-dependent cellular phagocytosis (ADCP) assay
Using the assay developed by Ackerman et al. (2017) (109), 5 μg of V1V2(ZM53)-2F5K, V1V2(ZM109)-1FD6, or V1V2(ZM109)-TTB were biotinylated using EZ-Link Sulfo-NHS-LC-LC-Biotin (Thermo Scientific) and then conjugated to fluorescent neutravidin beads (Thermo) according to manufacturer’s instructions. Conjugated beads were washed and resuspended in 0.1% BSA-PBS to a working dilution of 1:100. Nine hundred thousand (9 × 105) beads were aliquoted per well in round bottom 96-well plates. Four-fold dilutions of plasma obtained at week 22 or at late time points after immunization of the NHPs were titrated, added to beads, incubated for 2 hr at 37°C, and washed. Twenty-five thousand (2.5 × 104) THP-1 cells (ATCC, Cat. No. TIB-202, RRID:CVCL_0006) were added to each well and were incubated overnight. Phagocytosis was measured by flow cytometry. Antibody-dependent cellular phagocytosis (ADCP) scores were calculated as (% bead-positive cells × mean fluorescence intensity [MFI] of bead-positive cells)/106. Area under the curve (AUC) was calculated from the titration curves using GraphPad Prism.
Antibody-dependent cellular cytotoxicity (ADCC) assay
Using the assay developed by Alpert et al. (2012), ADCC measurements were made with SHIVSF162P3-infected target cells. Briefly, NKR24 cells were used as targets which were derived from CEM.NKR.CCR5 CD4+ T cells (Howell et al., 1985; Trkola et al., 1999) and obtained from the AIDS Research and Reference Reagent Program. The KHYG-1 rhCD16 effector cells were derived from the CD16-negative human NK cell line KHYG-1 (Japan Health Sciences Foundation) (Yagita et al., 2000). Briefly, the NKR24 luciferase-reporter cell line is infected by spinoculation and incubated for 3–4 days before starting the assay to achieve an infection titer that is at least five-fold over background (RLU readout of mock infected cells). SHIV-infected NKR24 target cells are washed and combined with KHYG-1 rhCD16 cells at a ratio of effectors to targets (E:T) of 10:1 and added to the assay plate along with serially diluted V2i mAbs or positive and negative mAb controls. Controls that define 100% RLU and 0% RLU are included on the plate. The assay is incubated for 8 hours after which the luciferase substrate reagent, Bright-Glo (Promega), is used to read activity. Data are reported as both 50% ADCC titers.
QUANTIFICATION AND STATISTICAL ANALYSIS
The following statistical analyses were performed with SAS V9.4 software packages. Figures 3A, 3B, and 5: The polynomial function for each animal was first determined using regression. Area under the curve (AUC) was calculated for each polynomial function f (x), using integration method. That is, . Then, two-way ANOVA with an interaction model was used compare difference in AUC between groups (n = 6) and vaccine type. Within group affinity increase comparisons were done using one-way ANOVA and Mann-Whitney tests in GraphPad Prism, Version 7.0 for Mac. Statistical analysis for Figures 3C–3H: Log10 transformed EC50 values were compared using mixed effect model. Figures 4 and 7E–7K: Log10 transformed antibodies were compared using one-way ANOVA. Figure 7A: ADCP normalized values between groups (n = 6) were compared using one-way ANOVA. Log10 transformed ID50 values were compared using a mixed effect model followed by pairwise comparisons. Tukey multiple comparison correction was used to control overall type I error rate at 0.05 for all analyses. Spearman correlations in Figures 5, 6, and 7 were performed in GraphPad Prism, Version 7.0 for Mac. For each analysis: Figures 5E–5G, (n = 24); Figure 6D, (n = 42); Figure 6E–6, (n = 24); Figures 7N and 7Q, (n = 42); Figures 7O and 7P, (n = 24).
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Peroxidase-conjugated AffiniPure Goat Anti-Human IgG, Fcγ Fragment Specific | Jackson ImmunoResearch | Cat. No. 109–035-098; RRID:AB_2337586 |
| ZyMax Goat anti-Human IgG (H+L) FITC Conjugate | Invitrogen | Cat. No. 817111 |
| Monoclonal anti-HIV-1 Env2158 | Dr. Susan Zolla-Pazner (PMID:PMC5126359) | N/A |
| Monoclonal anti-HIV-1 Env2219 | NIH AIDS Reagents Reference Program | Cat. No. 11683 |
| Monoclonal anti-HIV-1 Env 447–52D | NIH AIDS Reagents Program | Cat# BS4030 |
| Monoclonal anti-HIV-1 Env 697–30D | NIH AIDS Reagents Program | Cat. No. 7371 |
| Monoclonal anti-HIV-1 Env 830A IgG1 | Dr. Susan Zolla-Pazner (PMID:PMC5126359) | N/A |
| Monoclonal anti-HIV-1 Env CH58 | NIH AIDS Reagents Program | Cat. No. 12550 |
| Monoclonal anti-HIV-1 Env IgG1 b12 | NIH AIDS Reagents Program | Cat. No. 2640 |
| Monoclonal anti-HIV-1 Env CH59 | NIH AIDS Reagents Program | Cat. No. 12551 |
| Monoclonal anti-HIV-1 EnvPG16 | NIH AIDS Reagents Program | Cat. No. 12150 |
| Monoclonal anti-HIV-1 Env PG9 | NIH AIDS Reagents Program | Cat. No. 12149 |
| Monoclonal anti-HIV-1 Env1393A | Dr. Susan Zolla-Pazner (PMID:PMC5126359) | N/A |
| Monoclonal anti-HIV-1 Env 3869 | Dr. Susan Zolla-Pazner (PMC5126359) | N/A |
| Monoclonal anti-HIV-1 Env 670 | Dr. Susan Zolla-Pazner (PMID:PMC5126359) | N/A |
| Monoclonal anti-HIV-1 Env1331A | Dr. Susan Zolla-Pazner (PMID: PMC5126359) | N/A |
| Bacterial and Virus Strains | ||
| pSVIII-93MW965.26 | NIH AIDS Reagents Program | Cat. No. 3094 |
| SHIV BaL.P4 (replicating virus) | Dr. Sampa Santra (PMID:PMC4523205) | N/A |
| SHIV BaL (pseudovirus) | Dr. Nancy Haigwood (PMID:PMC5952134) | N/A |
| SHIV NL-LucR.1157ipEL | Dr. Ruth Ruprecht (PMID:PMC2908149) | N/A |
| ZM109F.PB4, SVPC13 | NIH AIDS Reagents Program | Cat. No. 11314 |
| ZM197M.PB7, SVPC6 | NIH AIDS Reagents Program | Cat No. 11309 |
| ZM53M.PB12, SVPC11 | NIH AIDS Reagents Program | Cat. No. 11313 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| HRP-Protein A | Life Technologies | Cat. No. 101023 Cat# ABIN101023 |
| Adjuplex | Emperion LLC (Columbus, OH) | N/A |
| Calcium Chloride Solution, 1M | Fluka Analytical | Cat. No 21115 |
| jetPEI | Polyplus Transfection | Cat. No 101–10N |
| Protease Inhibitor Cocktail | Sigma | Cat. No P8340–1ML |
| Polyvinylpyrrolidone, 0.5gm | Bio-Rad | Cat. No S 22 |
| Spermidine | Sigma | Cat. No S0266–1G |
| V1V2(ZM53)-2F5K | Dr. Xiang-Peng Kong (PMID: PMC5126360) | N/A |
| V1V2(ZM53)-2J9C | Dr. Xiang-Peng Kong (PMID: PMC5126360) | N/A |
| V1V2(ZM109)-1FD6.monFc | Dr. Xiang-Peng Kong (PMID: PMC5126360) | N/A |
| V1V2(ZM109)-TTB | Dr. Xiang-Peng Kong (PMID: PMC5126360) | N/A |
| V3(ZM109)-TTB | Dr. Xiang-Peng Kong (PMID: PMC5126360) | N/A |
| V1V2(A244)-2J9C | This paper | N/A |
| HIV-1 BaL gp120 | Immune Tech (NY) | Cat. No. |
| HIV-1 ZM53 gp120 | Immune Tech (NY) | Cat. No. |
| HIV-1 ZM109 gp120 | Immune Tech (NY) | Cat. No. |
| HIV-1 A244 gp120 | Global Solutions for Infectious Diseases (S.San Francisco, CA) | Cat. No. |
| V1V2/1086-tags | Drs. Barton Haynes and J. Peacock (Duke Univ.) (PMID: 3371689) | N/A |
| V1V2/CaseA2-mut3 | Drs. Barton Hayens and H-X Liao (Duke Univ) (PMID: 3371689) | N/A |
| V1V2/CaseA2-gp70 | Dr. Barton Haynes (PMID: 3371689) | N/A |
| HIV-1 clade C, V3 peptide | GenScript (Piscataway, NJ) | N/A |
| HIV-1 clade E, 92TH023 cyclic V2 peptide | GenScript (Piscataway, NJ) | N/A |
| HIV-1 clade C, ZM109 linear C5 peptide | BioPeptide (San Diego, CA) | N/A |
| Critical Commercial Assays | ||
| EZ-Link™ Sulfo-NHS-LC-LC-Biotin | Thermo-Fisher Scientific | Cat. No 21338 |
| Experimental Models: Cell Lines | ||
| Human: HeLA-derived TZM-bl cells | NIH AIDS Reagents Program | Cat. No. 8129 |
| CEM.NKR CCR5+Luc+ (NKR24) | NIH AIDS Reagents Program | Cat. No. 5198 |
| KHG1 rhCD16 | Japan Health Sciences Foundation | N/A |
| HEK293 GnTI−/− cells | ATCC | ATCC CRL3022; RRID:CVCL_A785 |
| Cos7 cells | ATCC | ATCC CRL-1651; RRID:CVCL_0224 |
| Human HEK293T | NIH AIDS Reagent Program | Cat. No. 103 |
| THP-1 | ATCC | Cat.No. TIB-202; RRID:CVCL_0006 |
| Experimental Models: Organisms/Strains | ||
| Indian-origin rhesus macaques (outbred) | Oregon National Primate Research Center | N/A |
| Recombinant DNA | ||
| HIV-1 SG3 ΔEnv Non-infectous Molecular Clone (pSG3DEnv) | NIH AIDS Reagent Program | Cat. No. 11051 |
| pEMC* | Dr. Nancy Haigwood (PMID: PMC4797725) | N/A |
| pJW4303 | Dr. Xiang-Peng Kong (PMID: PMC5126360) | N/A |
| pVRC8400 | Dr. Xiang-Peng Kong (PMID: PMC5126360) | N/A |
| ZM109 gp120 DNA | Dr. Xiang-Peng Kong (PMID: PMC5126360) | N/A |
| ZM53 gp120 DNA | Dr. Xiang-Peng Kong (PMID: PMC5126360) | N/A |
| Software and Algorithms | ||
| GraphPad Prism 7.0 | GraphPad Prism | http://www.graphpad.com/scientific-software/prism/ |
| SAS V9.4 Software | SAS Institute, Cary, NC | SAS/STAT 9.4m5; https://www.sas.com/en_us/home.html |
| Data analysis software for Octet RED96 assay | Pall ForteBio, Fremont, CA | https://www.moleculardevices.com/products/biologics/octet-systems-software#gref |
| xPONENT 4.2 Software for analysis of Luminex data | Luminex (Austin, TX) | https://www.luminexcorp.com/xponent/ |
| Other | ||
| Particle Mediated Epidermal Delivery (PMED) device (gene gun, XR-1 research model; PowderMed, Oxford, UK | Dr. Deborah Fuller, Univ. of Washington (PMID: PMC4797725) | N/A |
| Helios Gene Gun Prep Station | Bio-Rad | 165–2418 |
| TUBE,ETFE.125”0DX.093”ID 50 FT Length (Gene Gun Tubing) | Saint-Gobain | TSTZ83–0125-016–50 |
| 1.0mm Gold Microcarriers, 0.25 g | Bio-Rad | Cat. No 1652263 |
| xMAP Ab Coupling (AbC) Kit | Luminex (Austin, TX) | 40–50016 |
Highlights.
Macaque immunogenicity studies with glycosylated V1V2 multimeric scaffold proteins
V1V2-scaffold immunogens induce V1V2-specific plasma and mucosal antibodies
V1V2 antibodies are functionally active and durable with increasing affinity
V1V2-scaffold immunogen cocktails made from multiple viruses improve humoral responses
ACKNOWLEDGMENTS
We wish to thank William F. Sutton, Letzibeth Mendez-Riveria, Philip Barnette, Heidi Henderson, and Rebecca Lewinsohn for excellent technical assistance. We thank ONPRC veterinarians and animal technicians for animal procedures and sample collection. We thank Catarina Hioe and Mirek Gorny for valuable discussions throughout the study. This work was supported by P01 AI100151 and P51 OD011092. The Icahn School of Medicine at Mount Sinai provided partial support for S.Z.-P. This work was also supported by cooperative agreements W81XWH-07-2-0067 and W81XWH-11-2-0174 between The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (DOD). The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army or Department of Defense.
Footnotes
DECLARATION OF INTERESTS
M.T., X.J., S.Z.-P., and X.-P.K. have filed for a patent (USPTO_29527.1901) covering the design of the V1V2-scaffold protein immunogens
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2019.06.074.
REFERENCES
- Abagyan R, Totrov M, and Kuznetsov D (1994). ICM-A new method for protein modeling and design: Applications to docking and structure prediction from the distorted native conformation. J. Comput. Chem 15, 488–506. [Google Scholar]
- Ackerman ME, Barouch DH, and Alter G (2017). Systems serology for evaluation of HIV vaccine trials. Immunol. Rev 275, 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiyegbo MS, Shmelkov E, Dominguez L, Goger M, Battacharya S, deCamp AC, Gilbert PB, Berman PW, and Cardozo T (2017). Peptide Targeted by Human Antibodies Associated with HIV Vaccine-Associated Protection Assumes a Dynamic α-Helical Structure. PLoS ONE 12, e0170530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama K, Ebihara S, Yada A, Matsumura K, Aiba S, Nukiwa T, and Takai T (2003). Targeting apoptotic tumor cells to Fc gamma R provides efficient and versatile vaccination against tumors by dendritic cells. J. Immunol 170, 1641–1648. [DOI] [PubMed] [Google Scholar]
- Allen CD, and Cyster JG (2008). Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin. Immunol 20, 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert MD, Harvey JD, Lauer WA, Reeves RK, Piatak M Jr., Carville A, Mansfield KG, Lifson JD, Li W, Desrosiers RC, et al. (2012). ADCC develops over time during persistent infection with live-attenuated SIV and is associated with complete protection against SIV(mac)251 challenge. PLoS Pathog. 8, e1002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydar Y, Sukumar S, Szakal AK, and Tew JG (2005). The influence of immune complex-bearing follicular dendritic cells on the IgM response, Ig class switching, and production of high affinity IgG. J. Immunol 174, 5358–5366. [DOI] [PubMed] [Google Scholar]
- Baden LR, and Dolin R (2012). The road to an effective HIV vaccine. N. Engl. J. Med 366, 1343–1344. [DOI] [PubMed] [Google Scholar]
- Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, et al. (2012). Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482, 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, Stephenson KE, Borducchi EN, Smith K, Stanley K, McNally AG, Liu J, Abbink P, Maxfield LF, Seaman MS, et al. (2013). Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell 155, 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, Alter G, Broge T, Linde C, Ackerman ME, Brown EP, Borducchi EN, Smith KM, Nkolola JP, Liu J, et al. (2015). Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science 349, 320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, Tomaka FL, Wegmann F, Stieh DJ, Alter G, Robb ML, Michael NL, Peter L, Nkolola JP, Borducchi EN, et al. (2018). Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13–19). Lancet 392, 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang KK, Gilbert PB, Huang Y, Gurley TC, Kozink DM, et al. (2012). Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J. Virol 86, 11521–11532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley T, Pollara J, Santra S, Vandergrift N, Pittala S, Bailey-Kellogg C, Shen X, Parks R, Goodman D, Eaton A, et al. (2017). Pentavalent HIV-1 vaccine protects against simian-human immunodeficiency virus challenge. Nat. Commun 8, 15711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braibant M, Gong EY, Plantier JC, Moreau T, Alessandri E, Simon F, and Barin F (2013). Cross-group neutralization of HIV-1 and evidence for conservation of the PG9/PG16 epitopes within divergent groups. AIDS 27, 1239–1244. [DOI] [PubMed] [Google Scholar]
- Chand S, Messina EL, AlSalmi W, Ananthaswamy N, Gao G, Uritskiy G, Padilla-Sanchez V, Mahalingam M, Peachman KK, Robb ML, et al. (2017). Glycosylation and oligomeric state of envelope protein might influence HIV-1 virion capture by α4β7 integrin. Virology 508, 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Xu X, and Jones IM (2007). Immunogenicity of the outer domain of a HIV-1 clade C gp120. Retrovirology 4, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AW, Crispin M, Pritchard L, Robinson H, Gorny MK, Yu X, Bailey-Kellogg C, Ackerman ME, Scanlan C, Zolla-Pazner S, and Alter G (2014a). Identification of antibody glycosylation structures that predict monoclonal antibody Fc-effector function. AIDS 28, 2523–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, Dugast AS, Schoen MK, Rolland M, Suscovich TJ, et al. (2014b). Poly-functional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci. Transl. Med 6, 228ra38. [DOI] [PubMed] [Google Scholar]
- Chung AW, Kumar MP, Arnold KB, Yu WH, Schoen MK, Dunphy LJ, Suscovich TJ, Frahm N, Linde C, Mahan AE, et al. (2015). Dissecting Polyclonal Vaccine-Induced Humoral Immunity against HIV Using Systems Serology. Cell 163, 988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria-Rose NA, Bhiman JN, Roark RS, Schramm CA, Gorman J, Chuang GY, Pancera M, Cale EM, Ernandes MJ, Louder MK, et al. (2015). New Member of the V1V2-Directed CAP256-VRC26 Lineage That Shows Increased Breadth and Exceptional Potency. J. Virol 90, 76–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci AS, Johnston MI, Dieffenbach CW, Burton DR, Hammer SM, Hoxie JA, Martin M, Overbaugh J, Watkins DI, Mahmoud A, and Greene WC (2008). HIV vaccine research: the way forward. Science 321, 530–532. [DOI] [PubMed] [Google Scholar]
- Gordon SN, Doster MN, Kines RC, Keele BF, Brocca-Cofano E, Guan Y, Pegu P, Liyanage NP, Vaccari M, Cuburu N, et al. (2014). Antibody to the gp120 V1/V2 loops and CD4+ and CD8+ T cell responses in protection from SIVmac251 vaginal acquisition and persistent viremia. J. Immunol 193, 6172–6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman J, Soto C, Yang MM, Davenport TM, Guttman M, Bailer RT, Chambers M, Chuang GY, DeKosky BJ, Doria-Rose NA, et al. ; NISC Comparative Sequencing Program (2016). Structures of HIV-1 Env V1V2 with broadly neutralizing antibodies reveal commonalities that enable vaccine design. Nat. Struct. Mol. Biol 23, 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny MK, Moore JP, Conley AJ, Karwowska S, Sodroski J, Williams C, Burda S, Boots LJ, and Zolla-Pazner S (1994). Human anti-V2 monoclonal antibody that neutralizes primary but not laboratory isolates of human immunodeficiency virus type 1. J. Virol 68, 8312–8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny MK, Pan R, Williams C, Wang XH, Volsky B, O’Neal T, Spurrier B, Sampson JM, Li L, Seaman MS, et al. (2012). Functional and immunochemical cross-reactivity of V2-specific monoclonal antibodies from HIV-1-infected individuals. Virology 427, 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardo R, Bailer RT, Korber BT, Gnanakaran S, Phillips J, Shen X, Tomaras GD, Turk E, Imholte G, Eckler L, et al. (2013). Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS ONE 8, e75665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, and Mascola JR (2017). The quest for an antibody-based HIV vaccine. Immunol. Rev 275, 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, et al. (2012). Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med 366, 1275–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Malherbe DC, Pissani F, McBurney S, Krebs SJ, Gomes M, Pandey S, Sutton WF, Burwitz BJ, Gray M, et al. (2016). Achieving Potent Autologous Neutralizing Antibody Responses against Tier 2 HIV-1 Viruses by Strategic Selection of Envelope Immunogens. J. Immunol 196, 3064–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Shapiro MB, Powell R, Malherbe DC, McBurney SP, Pandey S, Cheever T, Sutton WF, Kahl C, Park B, et al. (2018). Reduced cell-associated DNA and improved viral control in macaques following passive transfer of a single anti-V2 monoclonal antibody and repeated SHIV challenges. J. Virol 92, e02198–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holl V, Peressin M, Decoville T, Schmidt S, Zolla-Pazner S, Aubertin AM, and Moog C (2006). Nonneutralizing antibodies are able to inhibit human immunodeficiency virus type 1 replication in macrophages and immature dendritic cells. J. Virol 80, 6177–6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DN, Andreotti PE, Dawson JR, and Cresswell P (1985). Natural killing target antigens as inducers of interferon: studies with an immunoselected, natural killing-resistant human T lymphoblastoid cell line. J. Immunol 134, 971–976. [PubMed] [Google Scholar]
- Jalah R, Kulkarni V, Patel V, Rosati M, Alicea C, Bear J, Yu L, Guan Y, Shen X, Tomaras GD, et al. (2014). DNA and protein co-immunization improves the magnitude and longevity of humoral immune responses in macaques. PLoS ONE 9, e91550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski JP, Krebs SJ, Trovato M, Kovarik DN, Brower Z, Sutton WF, Waagmeester G, Sartorius R, D’Apice L, Caivano A, et al. (2012). Co-immunization with multimeric scaffolds and DNA rapidly induces potent autologous HIV-1 neutralizing antibodies and CD8+ T cells. PLoS ONE 7, e31464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Totrov M, Li W, Sampson JM, Williams C, Lu H, Wu X, Lu S, Wang S, Zolla-Pazner S, and Kong XP (2016). Rationally Designed Immunogens Targeting HIV-1 gp120 V1V2 Induce Distinct Conformation-Specific Antibody Responses in Rabbits. J. Virol 90, 11007–11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julg B, Tartaglia LJ, Keele BF, Wagh K, Pegu A, Sok D, Abbink P, Schmidt SD,Wang K, Chen X, et al. (2017). Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer protection against a clade C SHIV challenge. Sci. Transl. Med 9, eaal1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien JP, Cupo A, Sok D, Stanfield RL, Lyumkis D, Deller MC, Klasse PJ, Burton DR, Sanders RW, Moore JP, et al. (2013). Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science 342, 1477–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat V, and Rafique A (2017). Designing binding kinetic assay on the bio-layer interferometry (BLI) biosensor to characterize antibody-antigen interactions. Anal. Biochem 536, 16–31. [DOI] [PubMed] [Google Scholar]
- Karasavvas N, Billings E, Rao M, Williams C, Zolla-Pazner S, Bailer RT, Koup RA, Madnote S, Arworn D, Shen X, et al. ; MOPH TAVEG Collaboration (2012). The Thai Phase III HIV Type 1 Vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res. Hum. Retroviruses 28, 1444–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs SJ, McBurney SP, Kovarik DN, Waddell CD, Jaworski JP, Sutton WF, Gomes MM, Trovato M, Waagmeester G, Barnett SJ, et al. (2014). Multimeric scaffolds displaying the HIV-1 envelope MPER induce MPER-specific antibodies and cross-neutralizing antibodies when co-immunized with gp160 DNA. PLoS ONE 9, e113463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Ozorowski G, and Ward AB (2016). Cryo-EM structure of a native, fully glycosylated, cleaved HIV-1 envelope trimer. Science 351, 1043–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Andrabi R, Su CY, Yasmeen A, Julien JP, Kong L, Wu NC, McBride R, Sok D, Pauthner M, et al. (2017). A Broadly Neutralizing Antibody Targets the Dynamic HIV Envelope Trimer Apex via a Long, Rigidified, and Anionic β-Hairpin Structure. Immunity 46, 690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lertjuthaporn S, Cicala C, Van Ryk D, Liu M, Yolitz J, Wei D, Nawaz F, Doyle A, Horowitch B, Park C, et al. (2018). Select gp120 V2 domain specific antibodies derived from HIV and SIV infection and vaccination inhibit gp120 binding to α4β7. PLoS Pathog. 14, e1007278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GK (2014). Role of Fc-mediated antibody function in protective immunity against HIV-1. Immunology 142, 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Salazar-Gonzalez JF, Derdeyn CA, Morris L, Williamson C, Robinson JE, Decker JM, Li Y, Salazar MG, Polonis VR, et al. (2006). Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J. Virol 80, 11776–11790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang XH, Williams C, Volsky B, Steczko O, Seaman MS, Luthra K, Nyambi P, Nadas A, Giudicelli V, et al. (2015). A broad range of mutations in HIV-1 neutralizing human monoclonal antibodies specific for V2, V3, and the CD4 binding site. Mol. Immunol 66, 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, et al. (2013). Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 38, 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Acharya P, Dolan MA, Zhang P, Guzzo C, Lu J, Kwon A, Gururani D, Miao H, Bylund T, et al. (2017). Quaternary contact in the initial interaction of CD4 with the HIV-1 envelope trimer. Nat. Struct. Mol. Biol 24, 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyumkis D, Julien JP, de Val N, Cupo A, Potter CS, Klasse PJ, Burton DR, Sanders RW, Moore JP, Carragher B, et al. (2013). Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science 342, 1484–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malherbe DC, Pissani F, Sather DN, Guo B, Pandey S, Sutton WF, Stuart AB, Robins H, Park B, Krebs SJ, et al. (2014). Envelope variants circulating as initial neutralization breadth developed in two HIV-infected subjects stimulate multiclade neutralizing antibodies in rabbits. J. Virol 88, 12949–12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malherbe DC, Mendy J, Vang L, Barnette PT, Reed J, Lakhashe SK, Owuor J, Gach JS, Legasse AW, Axthelm MK, et al. (2018). Combination Adenovirus and Protein Vaccines Prevent Infection or Reduce Viral Burden after Heterologous Clade C Simian-Human Immunodeficiency Virus Mucosal Challenge. J. Virol 92, e01092–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Wang L, Gu C, Herschhorn A, Xiang SH, Haim H, Yang X, and Sodroski J (2012). Subunit organization of the membrane-bound HIV-1 envelope glycoprotein trimer. Nat. Struct. Mol. Biol 19, 893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr LM, Cohen S, Spurrier B, Kong XP, and Zolla-Pazner S (2013). Epitope mapping of conformational V2-specific anti-HIV human monoclonal antibodies reveals an immunodominant site in V2. PLoS ONE 8, e70859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, et al. (2011). Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 480, 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori DC (2009). Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol. Biol 485, 395–405. [DOI] [PubMed] [Google Scholar]
- Moore PL, and Williamson C (2016). Approaches to the induction of HIV broadly neutralizing antibodies. Curr. Opin. HIV AIDS 11, 569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro JB, Gorman J, Ma X, Zhou Z, Arthos J, Burton DR, Koff WC, Courter JR, Smith AB 3rd, Kwong PD, et al. (2014). Conformational dynamics of single HIV-1 envelope trimers on the surface of native virions. Science 346, 759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musich T, Li L, Liu L, Zolla-Pazner S, Robert-Guroff M, and Gorny MK (2017a). Monoclonal Antibodies Specific for the V2, V3, CD4-Binding Site, and gp41 of HIV-1 Mediate Phagocytosis in a Dose-Dependent Manner. J. Virol 91, e02325–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musich T, Li L, Liu L, Zolla-Pazner S, Robert-Guroff M, and Gorny MK (2017b). Monoclonal Antibodies to V2, V3, the CD4-binding site and gp41 HIV-1 Mediate Phagocytosis in a Dose-dependent Manner. J Virol 91, e02325–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyambi PN, Mbah HA, Burda S, Williams C, Gorny MK, Nádas A, and Zolla-Pazner S (2000). Conserved and exposed epitopes on intact, native, primary human immunodeficiency virus type 1 virions of group M. J. Virol 74, 7096–7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozorowski G, Pallesen J, de Val N, Lyumkis D, Cottrell CA, Torres JL, Copps J, Stanfield RL, Cupo A, Pugach P, et al. (2017). Open and closed structures reveal allostery and pliability in the HIV-1 envelope spike. Nature 547, 360–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan R, Gorny MK, Zolla-Pazner S, and Kong XP (2015). The V1V2 Region of HIV-1 gp120 Forms a Five-Stranded Beta Barrel. J. Virol 89, 8003–8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancera M, Shahzad-Ul-Hussan S, Doria-Rose NA, McLellan JS, Bailer RT, Dai K, Loesgen S, Louder MK, Staupe RP, Yang Y, et al. (2013). Structural basis for diverse N-glycan recognition by HIV-1-neutralizing V1-V2-directed antibody PG16. Nat. Struct. Mol. Biol 20, 804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancera M, Zhou T, Druz A, Georgiev IS, Soto C, Gorman J, Huang J, Acharya P, Chuang GY, Ofek G, et al. (2014). Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature 514, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauthner M, Havenar-Daughton C, Sok D, Nkolola JP, Bastidas R, Boopathy AV, Carnathan DG, Chandrashekar A, Cirelli KM, Cottrell CA, et al. (2017). Elicitation of Robust Tier 2 Neutralizing Antibody Responses in Nonhuman Primates by HIV Envelope Trimer Immunization Using Optimized Approaches. Immunity 46, 1073–1088.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauthner MG, Nkolola JP, Havenar-Daughton C, Murrell B, Reiss SM, Bastidas R, Prevost J, Nedellec R, von Bredow B, Abbink P, et al. (2019). Vaccine-Induced Protection from Homologous Tier 2 SHIV Challenge in Nonhuman Primates Depends on Serum-Neutralizing Antibody Titers. Immunity 50, 241–252.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez LG, Martinez DR, deCamp AC, Pinter A, Berman PW, Francis D, Sinangil F, Lee C, Greene K, Gao H, et al. (2017). V1V2-specific complement activating serum IgG as a correlate of reduced HIV-1 infection risk in RV144. PLoS ONE 12, e0180720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter A, Honnen WJ, Kayman SC, Trochev O, and Wu Z (1998). Potent neutralization of primary HIV-1 isolates by antibodies directed against epitopes present in the V1/V2 domain of HIV-1 gp120. Vaccine 16, 1803–1811. [DOI] [PubMed] [Google Scholar]
- Pinter A, Honnen WJ, He Y, Gorny MK, Zolla-Pazner S, and Kayman SC (2004). The V1/V2 domain of gp120 is a global regulator of the sensitivity of primary human immunodeficiency virus type 1 isolates to neutralization by antibodies commonly induced upon infection. J. Virol 78, 5205–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissani F, Malherbe DC, Schuman JT, Robins H, Park BS, Krebs SJ, Barnett SW, and Haigwood NL (2014). Improvement of antibody responses by HIV envelope DNA and protein co-immunization. Vaccine 32, 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollara J, Bonsignori M, Moody MA, Liu P,Alam SM, Hwang KK, Gurley TC, Kozink DM, Armand LC, Marshall DJ, et al. (2014). HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. J. Virol 88, 7715–7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Pan C, Lu H, Shui Y, Li L, Li X, Xu X, Liu S, and Jiang S (2010). A recombinant mimetics of the HIV-1 gp41 prehairpin fusion intermediate fused with human IgG Fc fragment elicits neutralizing antibody response in the vaccinated mice. Biochem. Biophys. Res. Commun. 398, 506–512. [DOI] [PubMed] [Google Scholar]
- Reeves PJ, Callewaert N, Contreras R, and Khorana HG (2002). Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc. Natl. Acad. Sci. USA 99, 13419–13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault A, Lankar D, Lacabanne V, Rodriguez A, Théry C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, and Amigorena S (1999). Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med 189, 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al. ; MOPH-TAVEG Investigators (2009). Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med 361, 2209–2220. [DOI] [PubMed] [Google Scholar]
- Rinaudo CD, Telford JL, Rappuoli R, and Seib KL (2009). Vaccinology in the genome era. J. Clin. Invest 119, 2515–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb ML, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Kunasol P, Khamboonruang C, Thongcharoen P, Morgan P, Benenson M, et al. (2012). Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: a post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect. Dis 12, 531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roederer M, Keele BF, Schmidt SD, Mason RD, Welles HC, Fischer W, Labranche C, Foulds KE, Louder MK, Yang ZY, et al. (2014). Immunological and virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature 505, 502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, deCamp AC, Carrico C, Menis S, Magaret CA, et al. (2012). Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature 490, 417–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SA, Sarisky CA, Su A, and Mayo SL (2001). Designed protein G core variants fold to native-like structures: sequence selection by ORBIT tolerates variation in backbone specification. Protein Sci. 10, 450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, and Lanzavecchia A (1994). Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med 179, 1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders RW, Derking R, Cupo A, Julien JP, Yasmeen A, de Val N, Kim HJ, Blattner C, de la Peña AT, Korzun J, et al. (2013). A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog. 9, e1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubin Z, Li W, Poonia B, Ferrari G, LaBranche C, Montefiori D, Zhu X, and Pauza CD (2017). An HIV Envelope gp120-Fc Fusion Protein Elicits Effector Antibody Responses in Rhesus Macaques. Clin. Vaccine Immunol 24, e00028–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Ramírez-Salazar EG, Doueiri R, Valentin A, Rosati M, Hu X, Keele BF, Shen X, Tomaras GD, Ferrari G, et al. (2018). Control of Heterologous Simian Immunodeficiency Virus SIVsmE660 Infection by DNA and Protein Coimmunization Regimens Combined with Different Toll-Like-Receptor-4-Based Adjuvants in Macaques. J. Virol 92, e00281–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sok D, van Gils MJ, Pauthner M, Julien JP, Saye-Francisco KL, Hsueh J, Briney B, Lee JH, Le KM, Lee PS, et al. (2014). Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proc. Natl. Acad. Sci. USA 111, 17624–17629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Nie X, Basu S, Singh M, and Cerny J (1999). Regulation of VH gene repertoire and somatic mutation in germinal centre B cells by passively administered antibody. Immunology 98, 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Gao X, and Galán JE (2013). Structure and function of the Salmonella Typhi chimaeric A(2)B(5) typhoid toxin. Nature 499, 350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurrier B, Sampson JM, Totrov M, Li H, O’Neal T, Williams C, Robinson J, Gorny MK, Zolla-Pazner S, and Kong XP (2011). Structural analysis of human and macaque mAbs 2909 and 2.5B: implications for the configuration of the quaternary neutralizing epitope of HIV-1 gp120. Structure 19, 691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurrier B, Sampson J, Gorny MK, Zolla-Pazner S, and Kong XP (2014). Functional implications of the binding mode of a human conformation-dependent V2 monoclonal antibody against HIV. J. Virol 88, 4100–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totrov M, Jiang X, Kong XP, Cohen S, Krachmarov C, Salomon A, Williams C, Seaman MS, Abagyan R, Cardozo T, et al. (2010). Structure-guided design and immunological characterization of immunogens presenting the HIV-1 gp120 V3 loop on a CTB scaffold. Virology 405, 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trkola A, Matthews J, Gordon C, Ketas T, and Moore JP (1999). A cell line-based neutralization assay for primary human immunodeficiency virus type 1 isolates that use either the CCR5 or the CXCR4 coreceptor. J. Virol 73, 8966–8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari M, Gordon SN, Fourati S, Schifanella L, Liyanage NP, Cameron M, Keele BF, Shen X, Tomaras GD, Billings E, et al. (2016a). Adjuvant-dependent innate and adaptive immune signatures of risk of SIVmac251 acquisition. Nat. Med 22, 762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari M, Gordon SN, Fourati S, Schifanella L, Liyanage NP, Cameron M, Keele BF, Shen X, Tomaras GD, Billings E, et al. (2016b). Corrigendum: Adjuvant-dependent innate and adaptive immune signatures of risk of SIVmac251 acquisition. Nat. Med 22, 1192. [DOI] [PubMed] [Google Scholar]
- van Eeden C, Wibmer CK, Scheepers C, Richardson SI, Nonyane M, Lambson B, Mkhize NN, Vijayakumar B, Sheng Z, Stanfield-Oakley S, et al. (2018). V2-Directed Vaccine-like Antibodies from HIV-1 Infection Identify an Additional K169-Binding Light Chain Motif with Broad ADCC Activity. Cell Rep. 25, 3123–3135.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, et al. ; Protocol G Principal Investigators (2009). Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326, 285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, et al. ; Protocol G Principal Investigators (2011). Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477, 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Farfan-Arribas DJ, Shen S, Chou TH, Hirsch A, He F, and Lu S (2006). Relative contributions of codon usage, promoter efficiency and leader sequence to the antigen expression and immunogenicity of HIV-1 Env DNA vaccine. Vaccine 24, 4531–4540. [DOI] [PubMed] [Google Scholar]
- Wang TT, Tan GS, Hai R, Pica N, Ngai L, Ekiert DC, Wilson IA, Garcia-Sastre A, Moran TM, and Palese P (2010a). Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc. Natl. Acad. Sci. USA 107, 18979–18984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT, Tan GS, Hai R, Pica N, Petersen E, Moran TM, and Palese P (2010b). Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog. 6, e1000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Gristick HB, Scharf L, West AP, Galimidi RP, Seaman MS, Freund NT, Nussenzweig MC, and Bjorkman PJ (2017). Asymmetric recognition of HIV-1 Envelope trimer by V1V2 loop-targeting antibodies. eLife 6, e27389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Barnes CO, Yang Z, Nussenzweig MC, and Bjorkman PJ (2018). Partially Open HIV-1 Envelope Structures Exhibit Conformational Changes Relevant for Coreceptor Binding and Fusion. Cell Host Microbe 24, 579–592.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, et al. (2003). Antibody neutralization and escape by HIV-1. Nature 422, 307–312. [DOI] [PubMed] [Google Scholar]
- Wibmer CK, Richardson SI, Yolitz J, Cicala C, Arthos J, Moore PL, and Morris L (2018). Common helical V1V2 conformations of HIV-1 Envelope expose the α4β7 binding site on intact virions. Nat. Commun 9, 4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Sukumar S, El Shikh ME, Best AM, Szakal AK, and Tew JG (2008). Immune complex-bearing follicular dendritic cells deliver a late antigenic signal that promotes somatic hypermutation. J. Immunol 180, 281–290. [DOI] [PubMed] [Google Scholar]
- Yagita M, Huang CL, Umehara H, Matsuo Y, Tabata R, Miyake M, Konaka Y, and Takatsuki K (2000). A novel natural killer cell line (KHYG-1) from a patient with aggressive natural killer cell leukemia carrying a p53 point mutation. Leukemia 14, 922–930. [DOI] [PubMed] [Google Scholar]
- Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, Alam SM, Ferrari G, Yang ZY, Seaton KE, et al. (2014). Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci. Transl. Med 6, 228ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz O, Kalthoff C, Raunser S, and Kühlbrandt W (2007). Structure of GlnK1 with bound effectors indicates regulatory mechanism for ammonia uptake. EMBO J. 26, 589–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharatos GJ, Yu J, Pace C, Song Y, Vasan S, Ho DD, and Huang Y (2011). HIV-1 and influenza antigens synthetically linked to IgG2a Fc elicit superior humoral responses compared to unmodified antigens in mice. Vaccine 30, 42–50. [DOI] [PubMed] [Google Scholar]
- Zhang P, Du J, Sun B, Dong X, Xu G, Zhou J, Huang Q, Liu Q, Hao Q, and Ding J (2006). Structure of human MRG15 chromo domain and its binding to Lys36-methylated histone H3. Nucleic Acids Res. 34, 6621–6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla-Pazner S, and Cardozo T (2010). Structure-function relationships of HIV-1 envelope sequence-variable regions refocus vaccine design. Nat. Rev. Immunol 10, 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla-Pazner S, Cohen SS, Krachmarov C, Wang S, Pinter A, and Lu S (2008). Focusing the immune response on the V3 loop, a neutralizing epitope of the HIV-1 gp120 envelope. Virology 372, 233–246. [DOI] [PubMed] [Google Scholar]
- Zolla-Pazner S, deCamp AC, Cardozo T, Karasavvas N, Gottardo R, Williams C, Morris DE, Tomaras G, Rao M, Billings E, et al. (2013). Analysis of V2 antibody responses induced in vaccinees in the ALVAC/AIDSVAX HIV-1 vaccine efficacy trial. PLoS ONE 8, e53629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla-Pazner S, deCamp A, Gilbert PB, Williams C, Yates NL, Williams WT, Howington R, Fong Y, Morris DE, Soderberg KA, et al. (2014). Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS ONE 9, e87572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla-Pazner S, Powell R, Yahyaei S, Williams C, Jiang X, Li W, Lu S, Wang S, Upadhyay C, Hioe CE, et al. (2016). Rationally Designed Vaccines Targeting the V2 Region of HIV-1 gp120 Induce a Focused, Cross-Clade-Reactive, Biologically Functional Antibody Response. J. Virol 90, 10993–11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.