Alzheimer's disease (AD) is characterized by extracellular deposition of β-amyloid (Aβ) as senile plaques, as well as intracellular accumulation of hyperphosphorylated, aggregated tau as neurofibrillary tangles. Increased production and/or deposition of Aβ is thought to precede tangle formation, but evidence suggests that tau pathology is more closely related to neuronal death (reviewed by Hanger et al., 2009). The importance of tau in mediating Aβ toxicity has been demonstrated by the resistance of tau knock-out neurons to Aβ-induced neurotoxicity and the rescue of cognitive impairment in mutant amyloid precursor protein (APP) transgenic mice when crossed with a tau knock-out line (Roberson et al., 2007). However, the mechanistic link between Aβ, disease-associated changes in tau proteins, and cell death remains the subject of intensive research.
Several studies demonstrate that fragments generated by the proteolytic cleavage of tau by caspases and/or calpains play a role in neurodegeneration. Immunohistochemical studies have demonstrated that tau cleaved by caspase-3 is present in neuronal lesions of several tauopathies. Caspase-3-cleaved tau fragments show a higher propensity to aggregate than full-length tau, and generation of these fragments precedes alterations in tau conformation, phosphorylation, and aggregation, suggesting cleavage is a relatively early event in neurofibrillary tangle formation (Cotman et al., 2005). Mutant forms of tau that are resistant to caspase-3 cleavage prevent caspase-induced cell death, indicating a direct relationship between the generation of tau fragments and neuronal demise (Cotman et al., 2005). The calcium-activated cysteine protease, calpain, also digests tau, producing several fragments, including a 17 kDa N-terminal fragment comprising residues 44–228 of the longest CNS tau isoform that induces apoptosis when exogenously expressed in cell lines. Furthermore, Aβ activates calpain and increases tau proteolysis in primary neurons (Park and Ferreira., 2005). Thus, proteolytic cleavage of tau may be a key step in Aβ-induced cell death pathways.
In a recent issue of The Journal of Neuroscience, Nicholson and Ferreira (2009) extend their previous findings (Park and Ferreira, 2005) by showing that Aβ-mediated production of 17 kDa calpain-cleaved tau fragments changes increases with neuronal development. The authors cultured hippocampal neurons for 7–12 or 17–21 d in vitro, classing these as immature or mature cultures, respectively, based on differential expression of fetal and adult tau isoforms. Treatment with 10–20 μm preaggregated Aβ demonstrated that immature neurons were more resistant than mature neurons to Aβ-induced toxicity and that the apparent vulnerability of mature neurons was associated with calpain activation and production of 17 kDa tau species. The authors conclude that, since there is no difference in the susceptibility of fetal and human tau isoforms to cleavage by calpain in vitro, the differences between immature and mature neurons are attributable to the impact of heightened Aβ-induced calpain activation in mature neurons.
Membrane cholesterol content regulates the rate of calcium influx and calpain activation in neurons by increasing the activity of glutamatergic receptors and membrane-associated calcium transporters. Nicholson and Ferreira (2009) investigated whether the increased cholesterol content observed in mature neurons induces calpain over-activation in response to Aβ. Lowering membrane cholesterol in mature neurons with MBCD (methyl-β-cyclodextrin) resulted in a transient reversal of the Aβ-induced increases in intraneuronal calcium concentrations and the accompanying calpain activation, generation of 17 kDa tau fragments, and neuronal death. Conversely, increasing membrane cholesterol in immature neurons increased their sensitivity to Aβ. The authors conclude that maturation-dependent elevations in membrane cholesterol increase the susceptibility of neurons to Aβ-induced calpain activation and tau toxicity.
Nicholson and Ferreira (2009) are the first to suggest an association between membrane cholesterol levels and tau toxicity in the context of AD; however, some of their results raise additional questions, and some conclusions seem premature.
The first, and perhaps most important, issue that needs to be addressed, is the relevance of the 17 kDa tau fragment to AD. Although this fragment has been observed in primary neurons, it has yet to be found in human brain. Calpain-cleaved fragments of tau have been identified in human brain tissue (Mercken et al., 1995), but these are different (30 and 36 kDa) species to those described by Nicholson and Ferreira. In AD, tau forms insoluble paired helical filaments (PHF), and although calpain activity is increased in AD, it has been shown that PHF-tau is less susceptible to calpain degradation than tau from control brain (Mercken et al., 1995). It is possible that the 17 kDa tau species might be generated before PHF formation, but until this species is identified in human brain, it cannot be asserted that its production is an early event in AD.
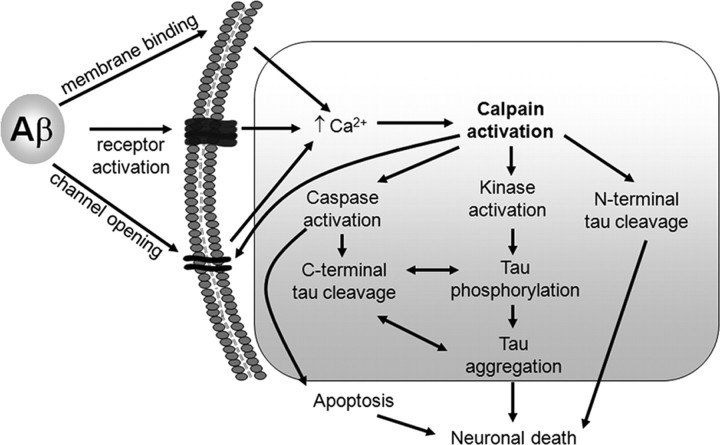
Second, it is not clear whether calpain cleavage of tau precedes or follows tau phosphorylation. Hyperphosphorylation of tau is a prominent feature in AD and recent evidence indicates a neurotoxic role of soluble species of phosphorylated tau (reviewed by Hanger et al., 2009). Aβ treatment activates several tau kinases, including cdk5 and GSK-3, both of which can be activated by calpain, and that phosphorylate many residues on PHF-tau (Hanger et al., 2009). Thus, although this is not reported, it is likely that some degree of tau phosphorylation occurs in the model used by Nicholson and Ferreira (2009). The generation of other tau fragments, such as caspase-3-cleaved tau, is thought to precede tau phosphorylation (Cotman et al., 2005), and so this issue is certainly worthy of further investigation. The pathogenic cascade proposed by Nicholson and Ferreira (2009), together with other Aβ-induced events, is shown in Figure 1.
Figure 1.
Involvement of multiple pathways in Aβ-induced neurodegeneration. Extracellular Aβ induces increased intraneuronal calcium through several different mechanisms, including those represented in this summary. Calpain activation is linked to both N- and C-terminal tau cleavage and the activation of tau kinases. Caspase-cleaved tau is more likely to become aggregated and highly phosphorylated, all or some of which may result in the induction of neuronal death pathways, including apoptosis-associated caspase activation.
Third, Nicholson and Ferreira (2009) refer to tau toxicity; however, a direct neurotoxic effect of the calpain-cleaved 17 kDa tau species has not been shown in this study. Although the neuronal death observed is associated with Aβ-induced calpain activation, calpain is involved in several different neurodegenerative pathways. Calpain induces aberrant processing of APP, activation of key protein kinases, processing of the synaptic vesicle recycling protein, dynamin-1, inactivation of NMDA receptors involved in learning and memory, such as NR2B, and disruption of plasma membrane ion channels. As an example of the latter, members of the plasma membrane calcium ATPase family remove excess calcium from neurons and thus maintain calcium homeostasis. Several groups have now shown that Aβ induces calpain-mediated cleavage of ion channels, causing their inactivation and resulting in excess intraneuronal calcium and subsequent excitotoxic cell death. In the cell culture system used by Nicholson and Ferreira (2009), there is likely to be significant cleavage of membrane calcium channels by calpain in the mature neurons, which, regardless of tau cleavage, will certainly contribute to the Aβ-induced neuronal death observed in this study.
A fourth confound that complicates interpretation of Nicholson and Ferreira's (2009) results is the effects of membrane cholesterol depletion on lipid rafts/detergent-resistant membranes. Cholesterol and sphingolipids are enriched in lipid rafts, which function as signal transduction platforms in neurons, and are a major site of Aβ production (Hung et al., 2008). Several laboratories have demonstrated that key neuronal proteins involved in calcium transport and signaling are also localized in these compartments. Depleting cholesterol from membranes alters the protein composition of lipid rafts, disturbing membrane trafficking, calcium transporter activity, and related functions. Thus, it is important to know whether the effects on tau described by Nicholson and Ferreira (2009) are mediated specifically by altered lipid raft function after reduction of membrane cholesterol. Interestingly, the human Prion Protein (PrPc) has recently been shown to be important in Aβ-mediated synaptic plasticity as it acts as a receptor for Aβ (Laurén et al., 2009). It would be intriguing to determine whether membrane cholesterol influences recruitment of PrPc to the cell membrane and whether the generation of 17 kDa tau is dependent on the Aβ-PrPc interaction, a question that could be addressed by replicating the current study in PrPc knock-out neuronal cultures.
Finally, Nicholson and Ferreira (2009) appear to have selected suboptimal conditions for treating neuronal cultures with Aβ. There is now a consensus in the field that soluble, oligomeric Aβ is more toxic than the fibrillar form used here (Glabe and Kayed, 2006), and the authors have themselves previously reported that soluble oligomeric, and not fibrillar, Aβ leads to a sustained calcium influx and calpain activation that is linked to synaptic loss and neurodegeneration (Kelly and Ferreira, 2006). Aggregated preparations of Aβ are likely to be heterogeneous in nature, and thus it would be worthwhile to characterize the precise nature of the aggregates used, for example, by size-exclusion chromatography and/or electron microscopy. This would allow determination of the subpopulation of Aβ species responsible for producing the observed effects and comparison with the results of other laboratories. Another interesting consideration in relation to this study is that soluble and aggregated Aβ have differing membrane-binding capabilities, with soluble, intermediate-sized species of Aβ binding strongly to lipid membranes, an event required for Aβ-induced neurotoxicity (Hung et al., 2008). Thus, the authors' observation of increased cholesterol in mature neurons might represent an increased binding surface for Aβ, contributing to the neuronal death observed only after maturation of neurons in culture.
In summary, the recent paper by Nicholson and Ferreira (2009) is an interesting study that describes a possible relationship between membrane cholesterol and Aβ-induced tau neurotoxicity. Although high cholesterol is viewed as a risk factor for AD and cholesterol-lowering agents are the focus of intensive research, the involvement of calpain-mediated tau cleavage in disease pathogenesis remains unclear. The development of transgenic animals expressing other fragments of tau derived from AD brain and cleaved huntingtin species has provided robust support for the involvement of specific protein fragments in AD and Huntington's disease, respectively, and a similar approach using calpain-cleaved tau fragments could provide substantial proof that these are also involved in disease pathogenesis. The development of an animal model expressing the 17 kDa tau fragment would allow the authors to more accurately examine the effects of aging, and dietary cholesterol could be modified to clarify the relationship between age, cholesterol content, and tau-induced neurodegeneration. Such studies will provide further insights into basic disease processes in AD and may also open the possibility of new avenues for therapeutic intervention.
Footnotes
Editor's Note: These short, critical reviews of recent papers in the Journal, written exclusively by graduate students or postdoctoral fellows, are intended to summarize the important findings of the paper and provide additional insight and commentary. For more information on the format and purpose of the Journal Club, please see http://www.jneurosci.org/misc/ifa_features.shtml.
We thank Dr. Diane Hanger and Dr. Patrick Lewis for helpful discussion and editorial comments on this manuscript.
References
- Cotman CW, Poon WW, Rissman RA, Blurton-Jones M. The role of caspase cleavage of tau in Alzheimer disease neuropathology. J Neuropathol Exp Neurol. 2005;64:104–112. doi: 10.1093/jnen/64.2.104. [DOI] [PubMed] [Google Scholar]
- Glabe CG, Kayed R. Common structure and toxic function of amyloid oligomers implies a common mechanism of pathogenesis. Neurology. 2006;66(2) Suppl 1:S74–S78. doi: 10.1212/01.wnl.0000192103.24796.42. [DOI] [PubMed] [Google Scholar]
- Hanger DP, Anderton BH, Noble W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol Med. 2009;15:112–119. doi: 10.1016/j.molmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Hung LW, Ciccotosto GD, Giannakis E, Tew DJ, Perez K, Masters CL, Cappai R, Wade JD, Barnham KJ. Amyloid-beta peptide (Abeta) neurotoxicity is modulated by the rate of peptide aggregation: Abeta dimers and trimers correlate with neurotoxicity. J Neurosci. 2008;28:11950–11958. doi: 10.1523/JNEUROSCI.3916-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BL, Ferreira A. beta-Amyloid-induced dynamin 1 degradation is mediated by N-methyl-D-aspartate receptors in hippocampal neurons. J Biol Chem. 2006;281:28079–28089. doi: 10.1074/jbc.M605081200. [DOI] [PubMed] [Google Scholar]
- Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken M, Grynspan F, Nixon RA. Differential sensitivity to proteolysis by brain calpain of adult human tau, fetal human tau and PHF-tau. FEBS Lett. 1995;368:10–14. doi: 10.1016/0014-5793(95)00590-6. [DOI] [PubMed] [Google Scholar]
- Nicholson AM, Ferreira A. Increased membrane cholesterol might render mature hippocampal neurons more susceptible to beta-amyloid-induced calpain activation and tau toxicity. J Neurosci. 2009;29:4640–4651. doi: 10.1523/JNEUROSCI.0862-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Ferreira A. The generation of a 17 kDa neurotoxic fragment: an alternative mechanism by which tau mediates beta-amyloid-induced neurodegeneration. J Neurosci. 2005;25:5365–5375. doi: 10.1523/JNEUROSCI.1125-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]