Abstract
Disease processes that frequently require emergency care constitute approximately 50% of the total disease burden in low-income and middle-income countries (LMICs). Many LMICs continue to deal with emergencies caused by communicable disease states such as pneumonia, diarrhoea, malaria and meningitis, while also experiencing a marked increase in non-communicable diseases, such as cardiovascular diseases, diabetes mellitus and trauma. For many of these states, emergency care interventions have been developed through research in high-income countries (HICs) and advances in care have been achieved. However, in LMICs, clinical research, especially interventional trials, in emergency care are rare. Furthermore, there exists minimal research on the emergency management of diseases, which are rarely encountered in HICs but impact the majority of LMIC populations. This paper explores challenges in conducting clinical research in patients with emergency conditions in LMICs, identifies examples of successful clinical research and highlights the system, individual and study design characteristics that made such research possible in LMICs. Derived from the available literature, a focused list of high impact research considerations are put forth.
Keywords: public health, study design, diseases, disorders, injuries
Summary box.
There is a paucity of high-quality research on the emergency management of diseases, which are rarely encountered in high-income countries (HICs) but impact the majority of populations in low-income and middle-income countries (LMICs).
Emergency care clinical research has substantial potential to impact overall morbidity and mortality in LMICs.
Addressable constraints to conducting clinical emergency care research in LMICs include: (1) lack of relevant and reproducible measures; (2) resource and capacity barriers; (3) ethical constraints and (4) disconnect in recognised emergency care dimensions.
The following aspects should be considered to conduct high-quality and relevant emergency care clinical research in and for LMICs: (1) utilisation of standardised, relevant and clinically credible measures; (2) focus on local capacity building, use of technology and collaboration; (3) increased financial and non-financial support must be prioritised; (4) studies must focus on setting specific emergency disease burdens and (5) translation of research findings into practice and policy through education and engagement of local stakeholders is required.
Background
The burden of critical illness and injury in low-income and middle-income countries (LMICs) is larger than in the higher-resource settings.1 2 Research focused on improving outcomes early in the presentation of acute, potentially life-threatening or therapeutically responsive disease processes could substantially reduce LMIC morbidity and mortality. Emergency care clinical research has a significant role in such care transformations. The goal of the Collaborative for Enhancing Emergency Care Research in LMICs (CLEER) project was to identify barriers to clinical research in emergency care in LMICs, propose solutions and recommend high impact clinical research priorities that have the potential to impact global public health.
Emergency department (ED) mortality in LMICs is high, with mortality occurring in 1.8% (IQR 0.2%–5.1%) of overall cases and 4.8% (IQR 2.3%–8.4%) of paediatric cases.3 Injuries and infectious diseases4 contribute the highest morbidity burden. Injury alone accounts for ~5 million deaths annually; a magnitude 1.7 times greater than the number of fatalities from HIV, tuberculosis and malaria combined.5 Sepsis, a common final pathway of infectious diseases in life-threatening presentations, warrants special consideration in LMICs which account for the majority of the >30 million global cases annually.6 Additionally, initial presentations and complications of non-communicable diseases such as diabetes mellitus, hypertension and cerebrovascular disease are rising and will continue to increase health burdens in LMICs.7 8 Continued and broadened research in the resuscitation and management of injury, the early recognition and treatment of infectious diseases and complications of non-communicable diseases has potential to improve health outcomes in LMICs.
There are several constraints and barriers to this effort. These include inadequate resources, lack of staff support and underdeveloped research infrastructure for scientific endeavours. Applying care guidelines from high-income countries (HICs) to LMICs is constrained by insufficient data and heterogeneity of resources and practice. Furthermore, LMIC patient populations are relatively young, more often critically ill, and have higher age-matched mortality than HIC emergency patients.3 The differences in resources, organisation, epidemiology, and practice all suggest that investment in research in LMICs will make emergency care more effective, responsive, and appropriate for LMIC populations. Through improvements in data collection and implementation of high-quality clinical studies, researchers in LMICs may develop cost-effective, disease specific and locally relevant interventions. The focus of this paper is to identify challenges and propose strategies for conducting clinical research in LMICs, while highlighting key areas and implementation considerations for future clinical research.
Emergency care research in LMICs: challenges and strategies
Research metrics
Consistently applied, relevant and reproducible measures are required for emergency care research in LMICs to demonstrate high impact and to improve practice both locally and more broadly. The table 1 provides an overview of key data items with examples and issues germane to LMIC research and emergency care. Additionally, to help overcome barriers to high-quality systematised data being gathered and maintained in LMICs factors inclusive of partnerships between academic institutions and healthcare systems, material support for data collection and linkage and incentives to overcome local opportunity costs would be beneficial.9 Specific to clinical interventional and observational studies, documented metrics and measures should include structural and process measures that complement clinical and patient level outcome data.
Table 1.
Overview of metrics and considerations for use
| Data Items | Examples | Issues |
| Setting | ||
| Facility | Prehospital Facility (rural vs urban) |
Different levels of data will be appropriate based on facility and the research capacity |
| Personnel | Level of training Proportion of providers across each levels of training |
Large variability and must be thoroughly reported to ensure understanding and generalisability of findings |
| Equipment | CT scan, sterile equipment, basic disposable items, for example, catheters, fluids, medications | Supply chain limitations and sustainability of access |
| Demographics | ||
| Country demographics | Age and sex distinctions with generally younger populations with larger burdens of patients living with minimal resources | Demographic and risk transitions are poorly understood in LMICs and need to be well documented |
| Comorbidities | Concurrence of infectious disease with non-infectious ones (eg, burdens of anaemia in injured patients) Chronic diseases often unrecognised in LMIC |
Difficulty to assess and categorise for existence and overlap |
| Processes | ||
| Prehospital care ED Admission as inpatient Need for ICU Discharge |
ED length of stay ED disposition Inpatient length of stay ICU availability and usage |
Lack of clear definitions across physical structures and within single facilities |
| Laboratory testing Point of care testing |
Blood counts TuberculosiB or HIV tests ECG Ultrasound |
Availability of tests and types Documentation of results |
| Interventions for treatments | Antimicrobials Haemodynamic and respiratory support |
Availability of equipment Implementation costs |
| Implementation Quality assurance |
Uptake of and compliance with care algorithms | Difficult to maintain sustainability without resource Need for simple evaluation points |
| Outcomes | ||
| Mortality Cause of death |
ED based (initial treatment <24 hours) In-hospital mortality and 30/60/90-day mortality |
ED based outcomes not commonly collected Posthospital follow-up difficult and resource intensive |
| Post-discharge function and morbidity | Quality of life and functionality assessments | Difficult to collect Cultural appropriateness |
| Provider acceptability | Date from surveys, interviews, focus groups. | Poor uptake resulting in lack of representativeness and inaccuracy Uncommonly done in LMICs |
| Costing | Fees incurred, lengths of stay, ICU usage, treatments | Variability in costs across settings |
ED, emergency department; ICU, intensive care unit; LMICs, low-income and middle-income countries.
Clinical outcomes
Clinical outcome measures vary according to study design and intervention type. However, in-hospital mortality is commonly collected as an objective, patient-centred outcome measure. In addition to the initial ED resuscitation, in-hospital mortality may be affected by timing of pre-ED interventions, post-ED care like surgical and critical care treatments. Due to the potential contribution of these factors, research using in-hospital mortality must take into account characteristics prior to ED presentation as well as post-ED care delivery so that studies are able to capture factors impacting outcomes across the continuum of treatment. Furthermore, understanding ED-specific mortality pertinent to the initial treatment period, especially through the first 24 hours of treatment, is an important and rarely reported outcome that is greatly needed to inform and advance ED care provision. A common mortality metric in research is 90-day mortality, but due to resource limitations and barriers in many LMICs related to communication mechanisms, geographic distance and cultural practices, this commonly used outcome may not be feasible and more proximate outcomes should be used by LMIC researchers.10
In certain pathologies, mortality may be uncommon and not be the most relevant research measure for assessing emergency interventions.11 Thus, morbidity metrics can be key outcome measures for emergency care studies. Disability assessments and pragmatic evaluation of pre-illness versus post-illness functionality, along with abilities to perform social and vocational roles, should be considered by researchers in LMICs where there is limited information on such outcomes. Additionally, clinical process measures could serve as surrogate outcomes of importance, by providing a dimension of understanding on resource availability and delivery.12 13 These clinical process measures should be considered at the outset of studies by LMIC emergency care researchers. For instance, the ordering and actual administration of intravenous fluid and blood products may be used as clinical process measures in acute resuscitation research.
Emergency care research in LMICs should collect demographic, physiologic, laboratory and process measures that are reproducible. To ensure that key data are collected consistently and reliably, a parsimonious minimum required data set should be ubiquitously used with additional data gathered as needed, based on the specific research question. These required minimum data must include information regarding reasons for presentation for emergency care and ED outcomes.12 Standardised clinical documentation forms, with uniform sets of minimum variables, would help to facilitate data consistency and acquisition. A smaller amount of data collected with completeness and accuracy is more valuable than larger inaccurate and incomplete data collections. Establishing a standard reporting of these aspects across LMIC ED research endeavours will facilitate pooling of data and subsequently be a powerful repository to be able to better understand patient characteristics, disease burdens and outcomes within and across LMIC ED settings.
Box 1. Injury Care Research (Example of outcome metrics).
Injuries affect approximately 1 billion people per year, leading to over 5 million deaths and 138 million disability-adjusted life-years annually. Over 90% of this burdenfalls on those living in LMICs, where emergency andtrauma care resources are limited.5 35 36 Despite the highmorbidity and mortality, funding for, and execution of, emergency care clinical research in LMICs has beenminimal. However, an exemplary body of work in injuryscience derived from the group implementing the CRASH trials does exist. This group runs large international randomised controlled trials that use pragmatic monitoring and data collection designs to evaluate large numbers of injured patients with a deliberate inclusion of LMIC settings. The Corticosteroid Randomisation After Significant Head Injury (CRASH) 1 trial randomised>10 000 patients to corticosteroid or placebo after significant head injury and the Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage (CRASH) 2 trial randomised >20 000 patients to tranexamic acid or placebo after injury, both evaluating short-term mortality outcomes.37 38 This body of work highlights the role that appropriate and realistic outcome metrics serve inperforming emergency care clinical trials in LMICs andhow impactful results are attainable when comprehensiveresearch platforms are utilised and integrated intothe provision of emergency care.
Cost-effectiveness
Metrics on cost-effectiveness need to be collected in research endeavours globally,13 especially in LMICs. In relation to HIV and TB in LMICs, there exists data on cost-effectiveness14; however, emergency care data is sparse. Research on acute myocardial infarctions from Latin America employing telemedicine referral pathways to more well-resourced institutions has been shown to be cost-effective and represents a collaborative methodology that could be used in other LMICs.15 Also previously studied in emergency care research in Uganda is task shifting from more highly trained practitioners to other providers with less training and this has been found to have beneficial clinical impacts.16 Task shifting has also been put forth as cost-effective in community programmes for non-emergent health issues and study in emergency care settings is warranted.17 Although cost measurements are often difficult to attribute unless there is a fee-for-service system of care, researchers should be mindful of incorporating costing data into their work. Until comprehensive data systems are widely in place, a pragmatic approach in LMICs would be to use estimates of resource usage, such as length of stay across care venues, as well as medication administration, which in aggregate will provide a functional approximation of cost burden.
Human resource and infrastructure
Challenges in LMIC emergency care clinical research are amplified by human resource and logistical and infrastructural challenges.18 There are fewer trained researchers per capita in LMICs versus HICs despite the disproportionate disease burden. Additionally, academic groups and researchers in LMICs often have less guidance from institutions, fewer mentorship resources and less access to training to enhance research skills.19 20 In LMICs, there are high rates of turnover and migration of trained professionals, which inhibits longitudinal development at instructional and national levels.21 Moreover, emergency care providers often treat patients with high levels of severity and insufficient resources, further inhibiting their abilities to focus time on designing and performing clinical research.3
Capacity building in LMICs may be enhanced by training of emergency care clinicians and researchers in methods and logistical implementation appropriate for their settings. Mentored research training that takes into account cross-disciplinary and transgenerational personnel interactions is a key component to building research capacity in LMICs. Also important are developing a centralised institutional research agenda and establishing leadership at institutions in LMICs that can promote work at both system and individual levels and contribute to focused scientific goals. As not all LMICs can currently support such actions, collaboration either regionally or globally with institutions able to support education and mentoring of LMICs researches will be beneficial. An example of a successful longitudinal initiative exists in Pakistan, where a collaborative research and training programme with institutional buy-in for injury prevention and treatment work has been developed and produces not only high-quality emergency care research but also an enhanced pool of local researchers and scientific outputs.22 23
Research ethics
Research ethics is of key importance in all medical research and especially in LMIC emergency care research where there is potentially limited protection for patients due to less regulatory infrastructure and limited guidance and oversight. This is further compounded by the paucity personnel formally trained in ethical frameworks in many LMICs to support clinical trials and other research activities.24 The ethical complexities are exacerbated by barriers to patient follow-up and the high prevalence of vulnerable patients. Consequently, there is a need for development of ethical review boards with understanding of emergency care research in LMICs. Such development will require local institutional investment to foster individual well-trained researchers and more broadly research cultures, actions which could be bolstered through partnerships between institutions with existing systems to bridge the development of a strong foundation in research ethics. Partnerships of this nature would support promotion of emergency care research training as highlighted above and provide protection to the vulnerable patients needing acute emergency treatments.
Technology
Technology is frequently not utilised to its maximum benefit to perform high=quality clinical emergency care research in LMICs.13 Specifically, there tends to be less comfort with and use of digital research data collection in LMICs. In addition, health systems often have less access to online resources and training programmes either due to financial or technological constraints. The provision of technological materials in LMIC settings to enhance awareness, usability and implementation via support from HIC partners and donors would assist in overcoming these barriers.
Although these barriers exist, leveraging technology represents a promising opportunity to improve emergency care research in LMICs. Tools such as tablet-based data collection, text-based messaging for rapid follow-up and telemedicine can assist with patient screening, consent and enrolment and help streamline and standardise data collection and analysis leading to more efficient manuscript production and dissemination. Use of technology in training LMIC emergency care researchers, including distance learning and non-traditional media, is a minimally studied but potentially powerful area for development of human resources, as research educators and institutions from HICs can be strategically and efficiently utilised to train their counterparts in LMICs.25 Furthermore. partnering approaches could have far-reaching magnitude as they could be replicated in multiple settings using the initially established processes in an economically efficient manner.
Additionally, use of innovation in emergency care research in LMICs could be better fostered through engagement of diverse personnel and social media, as has been demonstrated in medical hack-a-thons.26 Such events in India, Pakistan and Uganda have generated innovative ideas that have been used as launch pads for internally and externally funded research projects.27 These platforms are a novel model to support implementation of research that intersects with traditional and non-traditional clinical research personnel and designs.
Financial support
Funding for emergency care research in LMICs is insufficient. Across major research funders globally between 2012 and 2016, there were only 115 supported projects for emergency care research in Africa, four in South America and 13 in Asia. This investment is compared with 1411 projects in North American countries funded during the same time period.28 The minimal funding in emergency care clinical research is mirrored in deficient publication outputs from LMICs. As illustrated in figure 1, although there is an increasing frequency in the global trend in emergency care clinical research, the proportion occurring in LMICs is a small minority and has only comprised 2%–5% of published reports annually over the preceding decade (Average=3.2%, ±SD: 0.9%), with an even smaller proportion represented by clinical trials.
Figure 1.
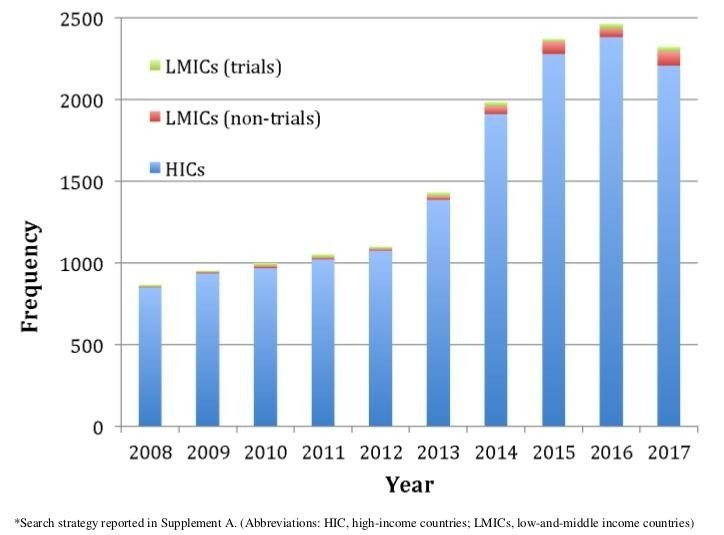
Publications in emergency care clinical research by income strata over time.
The disconnect between emergency health burdens and support for clinical research and scholarly outputs in LMICs illustrates the need for increased funding.29 Emergency medicine is a new specialty in most LMICs; hence, it is difficult to identify local champions to engage in grant funding applications, further lowering the possibilities of securing research funding. To overcome these challenges, partnerships with researchers that are successful in securing funding should be developed with emergency care researchers in LMICs. Such partnerships could be with local, regional or international researchers who work in research both within and external to emergency care provision, as the practice of emergency care allows for study of diverse disease processes. Funding for partnered research should include sources from conventional mechanisms and as well non-traditional sources such as governmental organisations, non-governmental organisations, device manufacturers, pharmaceuticals and philanthropists. The dearth of funding must be addressed in order to improve outcomes among some of the most vulnerable and understudied populations globally.
Key research focus areas
As outlined in table 2, emergency care research can be characterised by the considerations of time, location and priority syndromes and diseases. Emergency care in LMICs differs from that in HICs in each of these dimensions, as a result of infrastructure, density of comprehensive care facilities and differing burden of disease (including superimposed burden of communicable and non-communicable diseases resulting from the risk transition, eg, HIV infection complicating recovery in a patient with severe injuries). Relative to higher resource settings, initial illness presentation in LMICs tends to occur later in the disease course and at clinics with fewer resources than hospitals. Also, preclinical transport is more frequently ad hoc without formalised medical providers and support services, and care systems tend to be fragmented both horizontally and vertically.30 31 Emergency care system development and capacity also tend to be relatively constrained, often mirroring health system development and capacity overall.
Table 2.
Dimensions of emergency care by resource setting
| Emergency care dimension | Low resource setting |
Middle resource setting |
High resource setting |
| Temporal | Relatively long time from illness onset to presentation for care; distribution skewed to right | Variable time from illness onset to presentation for care; distribution with long tails | Shorter time from illness onset to presentation for care; distribution skewed to left |
| Spatial | Supermajority of initial illness presentation to local acute intake areas of available health facilities with middle-level health providers. | Variable presentations across health system, from local clinics to district hospitals | Supermajority of initial illness presentations to hospital-based emergency departments with physician staff |
| Health burdens and priorities | Substantial burden of disease related to acute infectious disease, injuries, high burden of paediatric illness; certain settings may have unusually or uniquely high prevalence of certain exposures or conditions (eg, Ebola, extreme heat) | Variable range of threats across settings; larger overall burden of disease associated with non-communicable and communicable disease related to risk transition | Substantial proportion of disease related to acute exacerbations of chronic disease |
| System Capacity (clinical care and research) |
Lower average levels of training among care providers, lower per capita provider rates, lower research capacity | Variable skill and capacity, typically concentrated in urban areas; variable research capacity | Higher per capita rates of physician coverage, relatively high research capacity |
These considerations all factor into prioritisation for clinical emergency care research in LMIC settings. Identifying priorities is context specific and depends on disease burden (certain disease processes may be more prevalent in specific areas such as HIV or Ebola Virus Disease), resource availability and system structure, among others. There is wide variability, even within specific LMICs, and this can further increase the complexity of specific research prioritisation efforts. As a result, in many LMIC settings, identifying and evaluating innovative ways to improve the systems and quality of care are a crucial part of a broader research agenda. Similarly, reducing barriers and constraints to accessing care, which can be assessed using metrics such as time between illness development and presentation for care, is important, particularly in rural areas with disproportionately limited capacity. Overlaying assessment of access and epidemiology of priority health threats in terms of disease burden and amenability to intervention can help guide infrastructure and research investment. In many cases, emergency care research in LMICs has focused on translation and implementation of practices deemed efficacious in HICs to LMIC settings without taking into account the appropriate systems and disease factors that are present, as has been demonstrated in sepsis care in Africa.32 33
Box 2. Sepsis Research in Africa (Example of key focus area).
The emergency diagnosis and management of sepsis is an area of LMIC research that has dimensions with unique characteristics as compared with that in HICs and that could serve as a model for research with crucial importance.39 Mortality due to sepsis is more common in LMICs, and patients with sepsis often present to health centreswith limited resources in advanced shock states. Additionally, controlled trial data from Africa assessing the application of sepsis treatments based on HIC protocols hasdemonstrated worse outcomes. In a trial by Maitland etal, higher mortality in paediatric patients with sepsis wasassociated with intravenous fluid bolus therapy. Similarly, trial data among adult patients with sepsis from Zambia found that protocol-based resuscitation with administration of intravenous fluids and vasopressors were associated with a higher in-hospital mortality compared withusual care.32 33 As such, research aimed at identifying, triaging and treating patients with sepsis early and appropriatelyis of high value. Such research could entail usingvital signs or simple clinical assessments such as the QuickSepsis-Related Organ Failure Assessment score and integrating these with accessible low-cost technology, suchas oxygen saturation monitoring, to determine setting appropriate risk stratification algorithms.40 41 Subsequently, these pathways could be linked to context-appropriate bundles of care which could be studied as an area of investigation pertinent to patient-centred outcomes.Studies to identify patients with infections who requireadmission versus those who can be managed as outpatientsmay also ease the burden in the lower-resourcedED. These questions could be approached using iterativestudy designs of simple cohort studies followed by trialslooking at outcomes in LMIC settings.
In the majority of settings, emergency care of the undifferentiated patient is driven by chief complaint and syndromic presentations rather than pre-existing diagnoses. In LMIC settings, this approach has often resulted in a focus on identifying syndromic presentations, which de-emphasises the need for expensive and scarce laboratory and imaging services and promotes care directed by common final pathological states (eg, immune dysregulation and shock), as these are responsible for the largest burdens of morbidity and mortality in LMIC emergency care settings.6 Moreover, these final common states may lend themselves to treatment with simple and inexpensive bundled interventions, which can be taught to and delivered by providers with limited but focused skills and training.34 Priorities for choosing intervention bundles are context specific and frequently chosen based on pragmatic criteria for reducing morbidity and mortality burdens and interaction with cost-effectiveness considerations (ie, interventions with a low number-needed-to-treat and an appropriate cost-effectiveness estimate under the constraints of the specific LMICs’ ability to support the intervention). Accordingly, LMIC researchers and funders of such research should adopt a framing paradigm, which focuses on the impact and cost-effectiveness in the development, funding and execution of emergency care studies.
There is a paucity of implementation research, especially in emergency care from LMICs. Addressing this data void will be fundamental to ensure the appropriateness and success of interventions moving forward. For implementation studies, acceptability to practitioners and patients should be a central focus along with feasibility based on existing resources in the specific LMIC settings of interest. For acceptability evaluations, varying approaches have been used including focus groups, electronic surveys and interviews. As such work is carried out, cultural issues implicit in each approach should be taken into consideration and leveraged to ensure the best possible data are attained.
Conclusions
Emergency care clinical research has substantial potential to impact overall morbidity and mortality in LMICs. There are, however, addressable constraints to conducting clinical research in LMICs including: (1) lack of agreed on, relevant and reproducible measures; (2) barriers in funding, resources, training and capacity; (3) ethical constraints and (4) disconnect in recognised emergency care dimensions.
In light of these challenges, the following approaches should be considered when developing and conducting high-quality emergency care clinical research in LMICs. Utilisation of standardised outcome measures such as mortality and the consistent collection of parsimonious minimum data sets. Systematic incorporation of structural and human capacity development and the leveraging of technology and international collaborations to catalyse development and improve equity in emergency care research. Advocacy for funding from traditional and non-traditional sources using collaborative partnership should be strengthened. Research foci based on disease burdens and syndromic presentations responsible for the largest contributions to morbidity and mortality in emergency care settings must be prioritised. Research findings from LMICs should be translated into practice and policy through education and engagement of local stakeholders which will support knowledge dissemination and integration into care delivery.
Acknowledgments
The authors would like to thank Dr Judd Walson, School of Public Health, University of Washington.
Footnotes
Handling editor: Seye Abimbola
Contributors: All authors took part in drafting and revising the manuscript and approve of the submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There is no original data associated with this manuscript.
References
- 1. Adhikari NKJ, Fowler RA, Bhagwanjee S, et al. . Critical care and the global burden of critical illness in adults. The Lancet 2010;376:1339–46. 10.1016/S0140-6736(10)60446-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stewart B, Khanduri P, McCord C, et al. . Global disease burden of conditions requiring emergency surgery. Br J Surg 2014;101:e9–22. 10.1002/bjs.9329 [DOI] [PubMed] [Google Scholar]
- 3. Obermeyer Z, Abujaber S, Makar M, et al. . Emergency care in 59 low- and middle-income countries: a systematic review. Bull World Health Organ 2015;93:577–86. 10.2471/BLT.14.148338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vos T, Barber RM, Bell B, et al. . Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the global burden of Disease Study 2013. The Lancet 2015;386:743–800. 10.1016/S0140-6736(15)60692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Injuries and violence: the facts 2014. Geneva:: World Health Organization.; 2014.. WHO Department for Management of Noncommunicable Disease, Disability, Violence and Injury Prevention. [Google Scholar]
- 6. Fleischmann C, Scherag A, Adhikari NK, et al. . Assessment of global incidence and mortality of Hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016;193:259–72. 10.1164/rccm.201504-0781OC [DOI] [PubMed] [Google Scholar]
- 7. GBD Lifetime risk of stroke Collaborators. global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J of Med 2016;2018:2429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. GBD Disease and injury incidence and prevalence Collaborators. global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet 2017;2018:1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Panhuis WG, Paul P, Emerson C, et al. . A systematic review of barriers to data sharing in public health. BMC Public Health 2014;14 10.1186/1471-2458-14-1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nel E, Rich E, Morojele N, et al. . Data collection challenges experienced while conducting the International alcohol control study (IAC) in Tshwane, South Africa. Drugs: Education, Prevention and Policy 2017;24:376–83. 10.1080/09687637.2016.1226774 [DOI] [Google Scholar]
- 11. Sege RD, Kharasch S, Perron C, et al. . Pediatric violence-related injuries in Boston: results of a city- wide emergency department surveillance program. Arch Pediatr Adolesc Med 2002;156:73–6. [DOI] [PubMed] [Google Scholar]
- 12. Asplin BR, Yealy DM. Key requirements for a new era of emergency department Operations research. Ann Emerg Med 2011;57:101–3. 10.1016/j.annemergmed.2010.07.026 [DOI] [PubMed] [Google Scholar]
- 13. Kelly WN, Randolph MA. Careers in clinical research: obstacles and opportunities. Washington, DC: National Academy Press, 1994. [PubMed] [Google Scholar]
- 14. Laxminarayan R, Mills AJ, Breman JG, et al. . Advancement of global health: key messages from the disease control priorities project. The Lancet 2006;367:1193–208. 10.1016/S0140-6736(06)68440-7 [DOI] [PubMed] [Google Scholar]
- 15. Mehta S, Botelho R, Fernandez F, et al. . TCT-851 providing cost-effective, population based AMI care with the use of telemedicine. J Am Coll Cardiol 2016;68 10.1016/j.jacc.2016.09.881 [DOI] [Google Scholar]
- 16. Chamberlain S, Stolz U, Dreifuss B, et al. . Mortality related to acute illness and injury in rural Uganda: task shifting to improve outcomes. PLoS One 2015;10:e0122559 10.1371/journal.pone.0122559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lewin S, Lavis JN, Oxman AD, et al. . Supporting the delivery of cost-effective interventions in primary health-care systems in low-income and middle-income countries: an overview of systematic reviews. Lancet 2008;372:928–39. 10.1016/S0140-6736(08)61403-8 [DOI] [PubMed] [Google Scholar]
- 18. Porter ME. What is the value in healthcare: crating value-based competition on results. Boston, MA: Harvard Business Review Press, 2006. [Google Scholar]
- 19. Ijsselmuiden C, Marais DL, Becerra-Posada F, et al. . Africa's neglected area of human resources for health research—the way forward. S Afr Med J 2012;102:228–33. [PubMed] [Google Scholar]
- 20. Beran D, Byass P, Gbakima A, et al. . Research capacity building—obligations for global health Partners. Lancet Glob Health 2017;5:e567–8. 10.1016/S2214-109X(17)30180-8 [DOI] [PubMed] [Google Scholar]
- 21. Duvivier RJ, Burch VC, Boulet JR. A comparison of physician emigration from Africa to the United States of America between 2005 and 2015. Hum Resour Health 2017;15 10.1186/s12960-017-0217-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mir MU, Bachani AM, Khawaja H, et al. . The Pakistan national emergency department surveillance study (Pak-NEDS): introducing a pilot surveillance. BMC Emerg Med 2015;15 10.1186/1471-227X-15-S2-S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Puvanachandra P, Razzak JA, Hyder AA. Establishing a national emergency department surveillance: an innovative study from Pakistan. BMC Emerg Med 2015;15 Suppl 2 10.1186/1471-227X-15-S2-I1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. European Commission Clinical trials – regulation EU No. 536/2014, 2014. Available: https://ec.europa.eu/health/human-use/clinical-trials/regulation_en [Accessed Mar 2018].
- 25. Sutherland S, Jalali A. Social media as an open-learning resource in medical education: current perspectives. Adv Med Educ Pract 2017;8:369–75. 10.2147/AMEP.S112594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farooqi W, Subhani F, Mian A. Paediatric innovation in Pakistan: our experience and a call to action. Arch Dis Child 2017;102:963–7. 10.1136/archdischild-2016-312123 [DOI] [PubMed] [Google Scholar]
- 27. Madhani S, Farooqi WH, Mian AI. Stimulating innovation through the hackathon concept in paediatrics: our experience at the Aga Khan university. Arch Dis Child 2017;102 10.1136/archdischild-2017-313648 [DOI] [PubMed] [Google Scholar]
- 28. RePORT W. National Institutes of Health (NIH) Report. Available: https://worldreport.nih.gov [Accessed 12 June 2018].
- 29. Vu A, Duber HC, Sasser SM, et al. . Emergency care research funding in the global health context: trends, priorities, and future directions. Acad Emerg Med 2013;20:1259–63. 10.1111/acem.12267 [DOI] [PubMed] [Google Scholar]
- 30. Jones CH, Ward A, Hodkinson PW, et al. . Caregivers' experiences of pathways to care for seriously ill children in Cape Town, South Africa: a qualitative investigation. PLoS One 2016;11:e0151606 10.1371/journal.pone.0151606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hodkinson P, Argent A, Wallis L, et al. . Pathways to care for critically ill or injured children: a cohort study from first presentation to healthcare services through to admission to intensive care or death. PLoS One 2016;11:e0145473 10.1371/journal.pone.0145473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maitland K, Kiguli S, Opoka RO, et al. . Mortality after fluid bolus in African children with severe infection. N Engl J Med 2011;364:2483–95. 10.1056/NEJMoa1101549 [DOI] [PubMed] [Google Scholar]
- 33. Andrews B, Semler MW, Muchemwa L, et al. . Effect of an early resuscitation protocol on in-hospital mortality among adults with sepsis and hypotension: a randomized clinical trial. JAMA 2017;318:1233–40. 10.1001/jama.2017.10913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kissoon N, Carapetis J. Pediatric sepsis in the developing world. Journal of Infection 2015;71(Suppl 1):S21–S26. 10.1016/j.jinf.2015.04.016 [DOI] [PubMed] [Google Scholar]
- 35. Haagsma JA, Graetz N, Bolliger I, et al. . The global burden of injury: incidence, mortality, disability-adjusted life years and time trends from the global burden of Disease Study 2013. Inj Prev 2016;22:3–18. 10.1136/injuryprev-2015-041616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lozano R, Naghavi M, Foreman K, et al. . Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of Disease Study 2010. The Lancet 2012;380:2095–128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roberts I, Yates D, Sandercock P, et al. . Effect of intravenous corticosteroids on death within 14 days in 10 008 adults with clinically significant head injury (MRC crash trial): randomised placebo-controlled trial. Lancet 2004;364:1321–8. [DOI] [PubMed] [Google Scholar]
- 38. CRASH-2 trial collaborators, Shakur H, Roberts I, et al. . Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376:23–32. 10.1016/S0140-6736(10)60835-5 [DOI] [PubMed] [Google Scholar]
- 39. Reinhart K, Daniels R, Kissoon N, et al. . Recognizing Sepsis as a Global Health Priority - A WHO Resolution. N Engl J Med 2017;377:414–7. 10.1056/NEJMp1707170 [DOI] [PubMed] [Google Scholar]
- 40. Rudd KE, Seymour CW, Aluisio AR, et al. . Association of the quick sequential (sepsis-related) organ failure assessment (qSOFA) score with excess hospital mortality in adults with suspected infection in low- and middle-income countries. JAMA 2018;319:2202–11. 10.1001/jama.2018.6229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aluisio AR, Garbern S, Wiskel T, et al. . Mortality outcomes based on ED qSOFA score and HIV status in a developing low income country. Am J Emerg Med 2018;36:2010–9. 10.1016/j.ajem.2018.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]


