Abstract
Shigellosis is one of the most important acute enteric infections caused by different species of Shigella, such as Shigella flexneri. Despite the use of antibiotic therapy to reduce disease duration, this approach is becoming less effective due to the emergence of antibiotic resistance among Shigella spp. Bacteriophages have been introduced as an alternative for controlling shigellosis. However, the bacteriophages must be without any lysogenic or virulence factors, toxin coding, or antibiotic-resistant genes. In this study, the whole genome sequence of vB-SflS-ISF001, a virulent Siphoviridae bacteriophage specific for Shigella flexneri, was obtained, and a comparative genomic analysis was carried out to identify its properties and safety. vB-SflS-ISF001 genomic DNA was measured at 50,552 bp with 78 deduced open reading frames (ORFs), with 24 ORFs (30.77%) sharing similarities with proteins from the genomes of homologous phages that had been reported earlier. Genetic analysis classifies it under the genus T1virus of the subfamily Tunavirinae . Moreover, comparative genomic analysis revealed no undesirable genes in the genome of vB-SflS-ISF001, such as antibiotic resistance, virulence, lysogeny, or toxin-coding genes. The results of this investigation indicate that vB-SflS-ISF001 is a new species, and confirm its safety for the biocontrol of S. flexneri.
Keywords: Bacteriophage, Shigella flexneri, whole genome sequence, Siphoviridae, T1virus
1. Introduction
Shigella flexneri is a gram-negative, rod-shaped, invasive pathogen for humans and primates that causes inflammation in colonic mucosa (Jennison and Verma, 2004), a causative agent of diarrhea that is frequently bloody. It has been reported as the main cause of endemic shigellosis in developing countries and has resulted in the annual infection of more than 2 million individuals worldwide (Niu et al., 2017).
The first line of drugs to treat shigellosis is antibiotics, but due to the occurrence of antibiotic resistance among Shigella spp., it seems that these drugs are getting less effective over time (Ye et al., 2010) . To tackle such an important issue, it is very important to come up with effective new alternatives. Bacteriophage therapy is a promising approach. Bacteriophages are the most common biological entities in the world (Olszak et al., 2017); previous studies have indicated that lytic bacteriophages can control a bacterial population (Wommack and Colwell, 2000). On the other hand, phages that are known as temperate bacteriophages can transfer undesirable genes within a bacterial population, including adhesion and invasion, exotoxin production, and other types of virulence genes (Wagner and Waldor, 2002; Shahin et al., 2018).
Previous studies have reported a number of Shigella species and Escherichia coli strains susceptible to lysogenic phages (James et al., 2001). Additionally, antigen conversion by phage in S. flexneri has been reported (Gemski et al., 1975). S. flexneri harbors various bacteriophage-mediated virulence genes on its plasmids and chromosomes (Walker and Verma, 2002). Thus, to avoid transmission of such virulence genes to the bacterial host in a lytic bacteriophage product for the biocontrol of S. flexneri, analyzing the genome sequence for such genes is absolutely essential.
vB-SF1S-ISF001, a specific phage for S. flexneri, belongs to the Siphoviridae family. It has been isolated from wastewater; its biological characteristics such as host range, host range, absorption rate, burst size, lytic activity, pH, and thermal and saline stability were reported in our previous study (Shahin and Bouzari, 2018). In the current study, we aimed to sequence the entire genome of the S. flexneri vB-SflS-ISF001 phage and perform a comparative genomic analysis and phylogenic analysis. Additionally, we have evaluated the safety of vB-SflS-ISF001 phage for use as a biocontrol agent by looking for any undesirable genes such as antibiotic resistance, virulence factors, or lysogeny genes.
2. Materials and methods
2.1. Bacterial culture
S. flexneri [Persian Type Culture Collection (PTCC 1234)] was obtained from the Iranian Research Organization for Science and Technology (IROST), Tehran, Iran, and stored at −80 °C. An overnight culture was prepared by adding 50 μL of the thawed stock suspension of the bacterium to 5 mL of brain heart infusion (BHI) broth (Merck, Darmstadt, Germany), and then incubated at 37 °C for 18 h with constant shaking (220 rpm).
2.2. Bacteriophage propagation and concentration
Bacteriophage vB-SflS-ISF001 (Shahin and Bouzari, 2018) was used in this study at a primary titer of 1010 PFU/mL. vB-SflS-ISF001 was propagated using S. flexneri (PTCC 1234) as host according to the method of Sambrook and Russell (2001). One hundred milliliters of sterile BHI broth was inoculated with 1 mL of the overnight culture of the host bacterium and incubated at 37 °C with constant shaking (220 rpm). The biomass production of the host bacterium was routinely checked until it reached an earlylog phase (OD600nm ≈ 0.2), when it was supplemented with 200 μL of the bacteriophage suspension (1010 PFU/mL). The mixture was incubated again at 37 °C for 24 h with constant shaking at 100 rpm. The media was then centrifuged at 10,000 × g for 10 min at 4 °C, and the phagecontaining supernatant was filtered through 0.22 µm syringe filters (Sartorius, Bangalore, India). The phage titer was then determined using the double-layer agar method (Kropinski et al., 2009). A high-titer stock of the phage was prepared using ultracentrifugation in an ultracentrifuge at 105,000 × g, 3 h, and 4 °C (Beckman Optima L-80 XP, TYPE 45 Ti rotor; Beckman Coulter, Brea, CA, USA). The pellet was then resuspended in 1 mL of sterilized SM buffer (100 mM NaCl, 8 mM MgSO4, 2% gelatin, 50 mM Tris-HCl, pH 7.5). This high-titer phage suspension was stored at 4 °C until further use.
2.3. Phage genome extraction and the whole genome sequencing
The genomic DNA of the phage was extracted according to Sambrook and Russell (2001). To remove nonphagerelated DNA and RNA, 10 μg/mL DNase I and RNase I (Sigma, Hong Kong, China) were added to the high-titer phage suspension (750 μL) and incubated for 1 h at 37 °C. Then, 78 μL of 20% SDS and proteinase K (20 mg/mL) (Sigma, Hong Kong, China) were added to the mixture, followed by an overnight incubation at 56 °C. DNA was then precipitated by adding 150 μL of 5 M sodium chloride. Subsequently, an equal volume of phenol/ chloroform/isoamyl alcohol solution was added before centrifugation at 13,000 × g for 10 min. The aqueous phase was collected carefully and remixed with an equal volume of phenol/chloroform/isoamyl alcohol solution before centrifugation at 13,000 × g for 10 min. The aqueous phase was then transferred to a new sterile tube. The phage DNA was precipitated by adding 3 M sodium acetate (one-tenth volume of the aqueous phase) and cold pure ethanol (twice volume of the aqueous phase). The sample was mixed well and incubated overnight at –20 °C before centrifugation at 20,000 × g for 20 min. Finally, the DNA pellet was washed twice with ethanol (70%) and then resuspended in RNaseand DNase-free water (Takara, Shiga, Japan). The phage genome DNA was stored at –20 °C until sequencing. DNA libraries were prepared by DNA fragmentation, adapter ligation, and amplification, and then subjected to the whole-genome DNA sequencing with 2 × 300 bp pairedend reads, carried out by the TGS Company (Shenzhen, China) on an Illumina HiSeq. The sequencing data were assembled using default parameters with SOAPdenovo (v2.04), and the sequence was deposited in DDBJ/EMBL/ GenBank under accession number MG049919.
2.4. Bioinformatic analysis
Open reading frames (ORFs) were predicted with Prokaryotic GeneMark.hmm version 3.25 (http://opal. biology.gatech.edu/genemark/gmhmmp.cgi) (Besemer et al., 2001) , and then were checked manually using the NCBI ORF Finder to confirm the predictions (https://www.ncbi.nlm.nih.gov/orfinder/). Isoelectric pH and molecular weight of translated ORFs and tRNA sequences were predicted using the ExPASy compute pI/Mw tool (http://web.expasy.org/compute_pi/) (Gasteiger et al., 2005) and tRNAscan-SE (Schattner et al., 2005) , respectively. ORF regions were translated to protein sequences using online ExPASy translate tool (http://web.expasy.org/translate/). Basic Local Alignment Search Tool (BLASTp), (https://blast.ncbi.nlm.nih.gov/Blast.cgi), HHpred (https://toolkit.tuebingen.mpg.de/#/tools/hhpred), Pfam (http://pfam.xfam.org/search#tabview=tab1) (Finn et al., 2015) , and InterProScan (http://www.ebi.ac.uk/interpro/search/sequence-search) (Altschul et al., 1997) programs with various protein domain databases were used for comparative analyses of the putative functions and conserved domains of the translated products.
2.5. Comparative genomics
CoreGenes 3.5 (http://gateway.binf.gmu.edu:8080/CoreGenes3.5/) (Turner et al., 2013) was used to find the proteins of vB_SflS-ISF001 that are similar to those of related phages. Mauve was used for the whole genome comparison at a DNA level with other related phages (Darling et al., 2004).
2.6. Phage protein analysis
Phage proteins were analyzed using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) as previously described (Ghasemi et al., 2014). The high-titer phage suspension (prepared using ultracentrifugation as described above) was mixed with the loading buffer (YEASEN, China) and heated in a boiling water bath for 10 min. Phage suspension (25–30 μL) was introduced to 12% (w/v) SDS-PAGE gel (YEASEN, China), and the separated protein bands were visualized by staining the gel with Coomassie blue G-250. A PageRuler Prestained Protein Ladder (Thermo Scientific, Waltham, MA, USA) was used as the size standard (10 to 180 kDa).
2.7. Phylogenetic analysis
The amino acid sequences of 1 structural ORF (ORF29, the major tail protein) and 1 nonstructural ORF (ORF14, the DNA primase) were selected to construct the phylogenic tree of the vB-SflS-ISF001 phage. The gene sequences of other phages belonging to different genera of Siphoviridae were obtained from GenBank. All sequences were aligned in MEGA 7.0 using MUSCLE, and then the phylogenetic tree was generated using UPGMA (unweighted pair group method with arithmetic mean) with 2000 bootstrap replications (Kumar et al., 2016) . Salmonella phage vB-SPuM-SP116 (accession number: KP010413) was used as the outgroup for both analyses.
3. Results
3.1. Genome characterizations
The whole genome sequencing was performed with 12,290,282 total reads (184,354,300 total bases). The sequencing data assembled using default parameters with SOAPdenovo (v2.04) showed that the dsDNA genome of vB-SflS-ISF001 phage had a 50,552 bp size (coverage > 1000×), a G + C content of 45.58%, and included LTRs of 52 bp in both ends of the genome. Bioinformatic analysis revealed that phage vB-SflS-ISF001 genome contained 78 putative ORFs (19 on the forward strand and 59 on the reverse strand) which are fairly similar to other T1virus members (Table 1). ATG was identified as the only start codon for all ORFs (Table 2). According to BLASTP searches in the GenBank database, the function of 24 ORFs (30.77%) were predicted, and the remaining ORFs (54 ORFs, 69.23%) were considered as hypothetical proteins due to their shared similarities with uncharacterized database entries (Table 2). A different range of identified ORFs from 25% (Shfl1) to 31.8% (SH6) was reported in the phages belonging to the T1 virus genus (Table 1). Among the identified ORFs and detected conserved domains of the vB-SflSISF001 genome, no sequences related to undesirable genes including antibiotic resistance, virulence, lysogenic mediated, or toxin coding genes were found. In addition, no tRNA-encoding sequences were found in the genome (Figure 1 and Table 2). The predicted ORFs of phage vB-SflS-ISF001 were divided into 4 groups according to their function (Figure 1).
Table 1.
Comparison of the basic genomic properties of phage vB_SflS-ISF001 and other similar phages.
| Shigella phages | Escherichia phages | |||||||
|---|---|---|---|---|---|---|---|---|
| Properties | vB_SflS-ISF001 | SH6 | Shfl1 | pSf-2 | ADB-2 | JMPW2 | T1 | JMPW1 |
| % identity | - | 89 | 89 | 90 | 91 | 89 | 89 | 88 |
| GC-content | 45.58 | 45.83 | 45.41 | 45.44 | 45.55 | 45.38 | 45.55 | 45.56 |
| Total/identified ORF | 78/24 | 82/26 | 80/20 | 83/24 | 79/25 | 80/24 | 77/23 | 7823 |
| No. of tRNA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Isolation country | Iran | Canada | Brazil | South Korea | India | China | Canada | China |
| Accession no. | MG049919 | KX828710 | HM035024 | KP085586 | JX912252 | KU194205 | AY216660 | KU194206 |
Table 2.
Analysis of the predicted ORFs of vB_SflS-ISF001 and their putative functions.
| Best match (NCBI database) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ORFs | Strand | Left | Right | Start codon | Size (aa) | PI | Mw (Kda) | Predicted product [organism] | E value | Identity | Accession no |
| 1 | + | 209 | 616 | ATG | 135 | 9.2 | 16166.87 | Hypothetical protein T1p10 [Escherichia virus T1] | 7E-90 | 95% | YP_003935.1 |
| 2 | + | 806 | 1021 | ATG | 71 | 4.78 | 8176.19 | Hypothetical protein B508_00390 [Escherichia phage ADB-2] | 3E-35 | 83% | YP_007112743.1 |
| 3 | - | 1035 | 1436 | ATG | 133 | 8.89 | 13972.21 | Hypothetical protein JMPW1_065 [Escherichia phage JMPW1] | 1E-73 | 89% | ALT58269.1 |
| 4 | - | 1436 | 1924 | ATG | 162 | 9.35 | 18135.87 | Endolysin [Shigella phage SH6] | 2E-101 | 90% | APC44908.1 |
| 5 | - | 1924 | 2139 | ATG | 71 | 6.06 | 7645.94 | Putative holin [Escherichia virus T1] | 6E-36 | 90% | YP_003932.1 |
| 6 | - | 2498 | 3649 | ATG | 383 | 6.54 | 43031.97 | Hypothetical protein JMPW1_061 [Escherichia phage JMPW1] | 0 | 92% | ALT58265.1 |
| 7 | - | 3728 | 4015 | ATG | 95 | 7.84 | 10788.54 | Hypothetical protein B508_00365 [Escherichia phage ADB-2] | 4E-55 | 88% | YP_007112738.1 |
| 8 | - | 4210 | 4434 | ATG | 74 | 9.73 | 8432.87 | Hypothetical protein ISF001_007 [Shigella phage vB_SsoS-ISF002] | 9E-39 | 88% | ASD50891.1 |
| 9 | - | 4486 | 4734 | ATG | 82 | 4.67 | 9713.19 | Hypothetical protein ISF001_008 [Shigella phage vB_SsoS-ISF002] | 8E-45 | 90% | ASD50892.1 |
| 10 | - | 4742 | 5455 | ATG | 273 | 6.84 | 26966.43 | DNA adenine methyltransferase [Escherichia phage vB_EcoS_SH2] | 3E-147 | 87% | ARW57245.1 |
| 11 | - | 5523 | 5939 | ATG | 138 | 8.59 | 15797.96 | Hypothetical protein ISF001_0010 [Shigella phage vB_SsoS-ISF002] | 4E-98 | 100% | ASD50894.1 |
| 12 | - | 5936 | 7948 | ATG | 670 | 6.59 | 75636.28 | ATP-dependent helicase [Escherichia phage JMPW2] | 0 | 95% | ALT58178.2 |
| 13 | + | 8048 | 8500 | ATG | 150 | 10.47 | 16913.61 | Hypothetical protein Shfl1p58 [Shigella virus Shfl1] | 9E-93 | 90% | YP_004414874.1 |
| 14 | + | 8577 | 9497 | ATG | 306 | 6.04 | 34833.12 | DNA primase/helicase [Escherichia phage JMPW1] | 0 | 91% | ALT58257.1 |
| 15 | + | 9598 | 11382 | ATG | 594 | 4.8 | 64226.31 | Putative tail fiber [Shigella virus Shfl1] | 0 | 87% | YP_004414872.1 |
| 16 | - | 11411 | 11824 | ATG | 137 | 7.87 | 15667.35 | Single-stranded DNA-binding protein [Shigella phage SH6] | 1E-62 | 78% | APC44921.1 |
| 17 | - | 11870 | 12517 | ATG | 215 | 8.52 | 23707.2 | Putative recombination protein [Shigella phage vB_SsoS-ISF002] | 8E-128 | 91% | ASD50900.1 |
| 18 | - | 12592 | 13656 | ATG | 354 | 5.02 | 39954.22 | Exodeoxyribonuclease VIII [Shigella phage SH6] | 0 | 93% | APC44928.1 |
| 19 | + | 14184 | 14414 | ATG | 76 | 8.71 | 8399.77 | Phage lipoprotein [Shigella phage SH6] | 6E-38 | 84% | APC44941.1 |
| 20 | + | 14417 | 15373 | ATG | 318 | 8.09 | 34098.65 | Hypothetical protein pSf2_021 [Shigella phage pSf-2] | 0 | 94% | YP_009112959.1 |
| 21 | - | 15468 | 18851 | ATG | 1127 | 4.88 | 125022.94 | Tail fiber protein [Shigella phage SH6] | 0 | 94% | APC44985.1 |
| 22 | - | 18929 | 19528 | ATG | 199 | 9.1 | 20875.01 | Putative tail assembly protein [Escherichia phage ADB-2] | 9E-135 | 96% | YP_007112720.1 |
| 23 | - | 19525 | 20259 | ATG | 244 | 5.74 | 28258.09 | Putative minor tail protein [Escherichia virus T1] | 0 | 99% | YP_003910.1 |
| 24 | - | 20256 | 21038 | ATG | 260 | 8.52 | 28774.74 | Putative minor tail protein [Escherichia virus T1] | 2E-173 | 90% | YP_003909.1 |
| 25 | - | 21118 | 21471 | ATG | 117 | 4.64 | 13011.49 | Tail fiber protein [Escherichia phage JMPW1] | 6E-72 | 87% | ALT58245.1 |
| 26 | - | 21474 | 24347 | ATG | 957 | 6.05 | 103770.01 | Tail length tape measure protein [Escherichia phage JMPW2] | 0 | 94% | ALT58162.1 |
| 27 | - | 24387 | 24656 | ATG | 89 | 4.25 | 10131.71 | Hypothetical protein pSf2_028 [Shigella phage pSf-2] | 7E-49 | 89% | YP_009112966.1 |
| 28 | - | 24704 | 25021 | ATG | 105 | 6.72 | 11815.39 | Hypothetical protein pSf2_029 [ Shigella phage pSf-2] | 4E-53 | 88% | YP_009112967.1 |
| 29 | - | 25136 | 25804 | ATG | 222 | 5.07 | 24090.32 | Putative major tail protein [Shigella virus Shfl1] | 2E-140 | 88% | YP_004414858.1 |
| 30 | - | 25807 | 26205 | ATG | 132 | 9.07 | 15330.60 | Hypothetical protein pSf2_031 [Shigella phage pSf-2] | 3E-79 | 87% | YP_009112969.1 |
| 31 | - | 26195 | 26638 | ATG | 147 | 6.91 | 16523.76 | Hypothetical protein Shfl1p40 [Shigella virus Shfl1] | 9E-96 | 91% | YP_004414856.1 |
| 32 | - | 26631 | 27002 | ATG | 123 | 5.69 | 13904.7 | Hypothetical protein pSf2_033 [Shigella phage pSf-2] | 2E-76 | 92% | YP_009112971.1 |
| 33 | - | 26999 | 27412 | ATG | 137 | 9.25 | 15542.96 | Hypothetical protein JMPW2_033 [Escherichia phage JMPW2] | 8E-79 | 85% | ALT58155.2 |
| 34 | - | 27455 | 27745 | ATG | 96 | 4.76 | 10348.09 | Hypothetical protein T1p46 [Escherichia virus T1] | 2E-46 | 82% | YP_003899.1 |
| 35 | - | 27795 | 28754 | ATG | 319 | 6.61 | 35068.32 | Hypothetical protein Shfl1p36 [Shigella virus Shfl1] | 0 | 93% | YP_004414852.1 |
| 36 | - | 28847 | 29614 | ATG | 255 | 4.65 | 26691.97 | Hypothetical protein Shfl1p35 [Shigella virus Shfl1] | 1E-144 | 81% | YP_004414851.1 |
| 37 | - | 29674 | 30150 | ATG | 158 | 5.54 | 17268.48 | Hypothetical protein pSf2_038 [Shigella phage pSf-2] | 9E-89 | 83% | YP_009112976.1 |
| 38 | - | 30162 | 31274 | ATG | 370 | 5.33 | 40269.73 | Major head subunit precursor [Escherichia virus T1] | 0 | 92% | YP_003895.1 |
| 39 | - | 31277 | 32038 | ATG | 253 | 9.09 | 28826.61 | Minor capsid protein [Escherichia phage JMPW1] | 7E-163 | 90% | ALT58231.1 |
| 40 | - | 32028 | 33311 | ATG | 427 | 4.71 | 47760.74 | Putative portal protein [Shigella virus Shfl1] | 0 | 93% | YP_004414847.1 |
| 41 | - | 33368 | 34936 | ATG | 522 | 6.91 | 59967.82 | Putative terminase large subunit [Shigella virus Shfl1] | 0 | 94% | YP_004414846.1 |
| 42 | - | 34975 | 35499 | ATG | 174 | 4.93 | 19287.68 | Putative terminase small subunit [Escherichia virus T1] | 3E-114 | 93% | YP_003891.1 |
| 43 | - | 35584 | 35811 | ATG | 75 | 9.39 | 8557.22 | Hypothetical protein Shfl1p28 [Shigella virus Shfl1] | 4E-33 | 88% | YP_004414844.1 |
| 44 | - | 35813 | 35998 | ATG | 61 | 9.57 | 7039.22 | Hypothetical protein JMPW1_022 [Escherichia phage JMPW1] | 2E-25 | 80% | ALT58226.1 |
| 45 | - | 35979 | 36140 | ATG | 53 | 9.22 | 5894.76 | Hypothetical protein pSf2_046 [Shigella phage pSf-2] | 3E-20 | 83% | YP_009112984.1 |
| 46 | - | 36305 | 36508 | ATG | 67 | 5.07 | 7230.12 | Hypothetical protein B508_00150 [Escherichia phage ADB-2] | 4E-34 | 84% | YP_007112695.1 |
| 47 | - | 36508 | 36738 | ATG | 76 | 9.75 | 8737.25 | Hypothetical protein JMPW1_019 [Escherichia phage JMPW1] | 7E-39 | 88% | ALT58223.1 |
| 48 | - | 36738 | 37082 | ATG | 114 | 9.16 | 12972 | Hypothetical protein B508_00140 [Escherichia phage ADB-2] | 1E-65 | 87% | YP_007112693.1 |
| 49 | - | 37079 | 37288 | ATG | 69 | 4 | 8032.77 | Hypothetical protein B508_00135 [Escherichia phage ADB-2] | 1E-33 | 83% | YP_007112692.1 |
| 50 | - | 37361 | 37933 | ATG | 190 | 5.55 | 21575.59 | Hypothetical protein T1p62 [Escherichia virus T1] | 2E-123 | 91% | YP_003883.1 |
| 51 | - | 38042 | 38575 | ATG | 177 | 5.95 | 20038.7 | Putative morphogenetic protein [Escherichia phage ADB-2] | 6E-112 | 90% | YP_007112690.1 |
| 52 | - | 38659 | 39105 | ATG | 148 | 8.51 | 17383.97 | Hypothetical protein SH6_0017 [Shigella phage SH6] | 2E-81 | 95% | APC44930.1 |
| 53 | - | 39163 | 39381 | ATG | 72 | 4.75 | 7840.97 | Hypothetical protein T1p66 [Escherichia virus T1] | 5E-33 | 83% | YP_003878.1 |
| 54 | - | 39530 | 40168 | ATG | 212 | 9.38 | 23844.42 | Hypothetical protein pSf2_055 [Shigella phage pSf-2] | 9E-138 | 91% | YP_009112993.1 |
| 55 | - | 40173 | 40460 | ATG | 95 | 7.84 | 11139.68 | Hypothetical protein B508_00110 [Escherichia phage ADB-2] | 7E-50 | 81% | YP_007112687.1 |
| 56 | - | 40539 | 40688 | ATG | 49 | 7.82 | 5667.66 | Hypothetical protein [Escherichia phage vB_EcoS_SH2] | 5E-25 | 88% | ARW57197.1 |
| 57 | - | 40688 | 41194 | ATG | 168 | 6.96 | 18812.91 | Hypothetical protein pSf2_059 [Shigella phage pSf-2] | 3E-88 | 77% | YP_009112997.1 |
| 58 | - | 41266 | 41754 | ATG | 162 | 4.43 | 18232.68 | Hypothetical protein B508_00095 [Escherichia phage ADB-2] | 2E-98 | 89% | YP_007112684.1 |
| 59 | - | 41826 | 42017 | ATG | 63 | 4.05 | 7383.14 | Hypothetical protein JMPW2_006 [Escherichia phage JMPW2] | 3E-29 | 84% | ALT58128.1 |
| 60 | - | 42027 | 42200 | ATG | 57 | 6.52 | 6161.42 | Hypothetical protein ISF001_0059 [Shigella phage vB_SsoS-ISF002] | 5E-24 | 86% | ASD50943.1 |
| 61 | - | 42304 | 42531 | ATG | 75 | 10.07 | 8613.07 | Hypothetical protein JMPW2_004 [Escherichia phage JMPW2] | 2E-30 | 68% | ALT58126.1 |
| 62 | - | 42538 | 42768 | ATG | 76 | 6.54 | 8632.82 | Hypothetical protein Shfl1p05 [Shigella virus Shfl1] | 5E-39 | 88% | YP_004414824.1 |
| 63 | - | 42847 | 43317 | ATG | 156 | 5.51 | 17590.09 | Hypothetical protein B508_00070 [Escherichia phage ADB-2] | 4E-77 | 74% | YP_007112679.1 |
| 64 | - | 43320 | 43514 | ATG | 64 | 5.1 | 7308.31 | Hypothetical protein Shfl1p02 [Shigella virus Shfl1] | 2E-29 | 86% | YP_004414821.1 |
| 65 | - | 43586 | 43915 | ATG | 109 | 5.7 | 12370.27 | Hypothetical protein ISF001_0064 [Shigella phage vB_SsoS-ISF002] | 2E-60 | 87% | ASD50948.1 |
| 66 | - | 43928 | 44503 | ATG | 191 | 4.99 | 21342.21 | Hypothetical protein JMPW2_001 [Escherichia phage JMPW2] | 5E-104 | 81% | ALT58123.1 |
| 67 | + | 45206 | 45910 | ATG | 234 | 9.14 | 26394.97 | DNA methylase [Shigella phage SH6] | 6E-146 | 89% | APC44923.1 |
| 68 | + | 45971 | 46156 | ATG | 61 | 9.16 | 7030.22 | Hypothetical protein B508_00040 [Escherichia phage ADB-2] | 2E-24 | 79% | YP_007112675.1 |
| 69 | + | 46172 | 46357 | ATG | 61 | 6.14 | 6920.14 | Hypothetical protein ISF001_0068 [Shigella phage vB_SsoS-ISF002] | 9E-25 | 82% | ASD50952.1 |
| 70 | + | 46433 | 46813 | ATG | 126 | 4.49 | 14559.40 | Hypothetical protein ISF001_0069 [Shigella phage vB_SsoS-ISF002] | 2E-66 | 83% | ASD50953.1 |
| 71 | + | 46810 | 46983 | ATG | 57 | 8.01 | 6695.60 | Hypothetical protein ISF001_0070 [Shigella phage vB_SsoS-ISF002] | 4E-20 | 75% | ASD50954.1 |
| 72 | + | 47055 | 47426 | ATG | 123 | 4.51 | 13557.28 | Hypothetical protein JMPW1_074 [Escherichia phage JMPW1] | 4E-61 | 80% | ALT58278.1 |
| 73 | + | 47419 | 47619 | ATG | 66 | 6.18 | 7624.69 | Hypothetical protein T1p02 [Escherichia virus T1] | 2E-28 | 82% | YP_003943.1 |
| 74 | + | 47637 | 47957 | ATG | 106 | 9.71 | 12105.10 | Hypothetical protein pSf2_078 [Shigella phage pSf-2] | 9E-56 | 81% | YP_009113016.1 |
| 75 | + | 48174 | 48398 | ATG | 74 | 10.16 | 7966.25 | Hypothetical protein B508_00015 [Escherichia phage ADB-2] | 1 | 85% | YP_007112670.1 |
| 76 | + | 48402 | 48614 | ATG | 70 | 3.93 | 8105.68 | Hypothetical protein T1p06 [Escherichia virus T1] | 1E-25 | 71% | YP_003939.1 |
| 77 | + | 48695 | 49057 | ATG | 120 | 9.62 | 13975.26 | Hypothetical protein B508_00005 [Escherichia phage ADB-2] | 1E-70 | 90% | YP_007112668.1 |
| 78 | + | 49188 | 50009 | ATG | 273 | 5.89 | 30123.19 | Hypothetical protein pSf2_083 [Shigella phage pSf-2] | 0 | 98% | YP_009113021.1 |
Figure 1.
The linear genome map of Shigella flexneri bacteriophage vB-SflS-ISF001 drawn in a circularized format using DNAPlotter (Carver et al., 2009). The 4 circular tracks describe (from inner to outer layers): GC skew [(G – C) / (G + C)], G + C content, ORFs located in negative strand, and ORFs located in positive strand.
3.1.1. DNA replication, modification, regulation
In this group, ORF12 was the longest ORF (2013 bp, 670 aa), and its predicted protein product shared high similarity with the ATP-dependent helicase from Escherichia phage JMPW2 (95% identity). ORF10 product was predicted as DNA adenine methyltransferase due to 87% similarity (E value: 3E-147) to the DNA adenine methyltransferase of Escherichia phage vB-EcoS-SH2 (accession number: KY985004). ORF14 showed 91% identity to the DNA primase/helicase of Escherichia phage JMPW1. The deduced product of ORF16 displayed 78% similarity (E value: 1E-62) with the single-stranded DNA-binding protein from Shigella phage SH6. The proteins encoded by ORF17, ORF18, and ORF67 matched the putative recombination protein of Shigella phage vB-SsoS-ISF002 (accession number: MF093736), exodeoxyribonuclease VIII of Shigella phage SH6, and DNA methylase of Shigella phage SH6 with 91% (E value: 8E-128), 93%, and 89% (E value: 6E-146) similarity, respectively.
3.1.2. Structure, morphogenesis
ORF21, which was the largest ORF in this group (3384 bp, 1127 aa), encoded a protein similar to the tail fiber protein from Shigella phage SH6 (94%). The protein sequences of products of ORFs 15 and 25 also showed similarity to the tail fiber proteins of Shigella virus Shfl1 (accession number: HM035024) and Escherichia phage JMPW1, with 87% (E value: 0) and 87% (E value: 6E-72) identity, respectively. The predicted proteins of ORFs 23 and 24 showed 100% identity (E value: 2E-173) to the putative minor tail protein of Escherichia virus T1. Moreover, the major tail protein was found to be encoded by ORF29 with 88% identity (E value: 2E-140) to the major tail protein of Shigella virus Shfl1. The predicted proteins of ORFs 22 and 26 were identified as the putative tail assembly protein and tail length tape measure protein due to 96% (E value: 9E-135) and 94% similarity with the putative tail assembly protein of Escherichia phage ADB-2 and tail length tape measure protein of Escherichia phage JMPW2, respectively. ORF38 was predicted to encode the major head subunit precursor, with 92% sequence similarity to the major head subunit precursor of Escherichia virus T1. The predicted protein of ORF39 was identified as the minor capsid protein, displaying 90% similarity (E value: 7E-163) with the minor capsid protein from Escherichia phage JMPW1. The portal protein and morphogenetic protein were found to be encoded by ORFs 40 and 51, respectively. The product of ORF40 showed 93% similarity with the portal protein from Shigella virus Shfl1, and the protein sequence of ORF51 showed 90% similarity (E value: 6E-112) with the putative morphogenetic protein of Escherichia phage ADB-2. Furthermore, the product encoded by ORF19 had 84% similarity (E value: 6E-38) with the phage lipoprotein of Shigella phage SH6.
3.1.3. DNA packaging
Terminase complex is composed of 2 separate gene products of ORFs 41 and 42. The product of ORF41 showed 94% similarity to the putative terminase large subunit from Shigella virus Shfl1 and the protein sequence of ORF42 product shared 93% similarity (E value: 3E-114) to the putative terminase small subunit from Shigella virus Shfl1.
3.1.4. Bacterial cell wall lysis
The product of ORF5 showed 90% similarity (E value: 6E36) to the putative holin of Escherichia virus T1, and the predicted protein of ORF4 showed 90% similarity (E value: 2E-101) to endolysin from Shigella phage SH6.
3.2. Comparative genomics analysis
A MegaBLAST search of the phage genome indicated that vB-SflS-ISF001 had 88%–91% sequence similarity with Shigella and Escherichia phages (Table 1). CoreGene analysis demonstrated that vB-SflS-ISF001 shared similarity to 50 proteins of other related phages (score >70), including 22 known (2 bacterial cell wall lysis, 7 DNA replication, modification, regulation protein, 11 structural, and 2 DNA packaging proteins) and 38 hypothetical proteins (Table 3). These amino acid coding sequences were not restricted to any particular region or functional group of genes and were distributed over the phage genome. Moreover, comparison of the genome sequence of phage vB-SflS-ISF001 with other members of the T1virus genus demonstrated that vB-SflS-ISF001 genome sequence, organization, and ORF orientations were generally similar to other members of the genus T1virus (Figure 2).
Table 3.
Conserved proteins of vB_SflS-ISF001 phage shared with related phages (SH6, Shfl1, ADB-2, JMPW2) as determined by CoreGenes.
*Data presented in these columns are accession numbers for each individual protein of each phage.
Figure 2.

Alignment of the genome of S. flexneri bacteriophage vB-SflS-ISF001 with others of the genus T1virus using Mauve. Names of the bacteriophages are mentioned under their maps line. Colored blocks indicate corresponding regions of nucleotide similarity, while colorless blocks correspond to dissimilar regions.
3.3. Phylogenetic position of vB-SflS-ISF001
The constructed phylogenetic tree using the major tail protein and the DNA primase revealed that vB-SflSISF001 had homology to genus T1virus phages (Shigella phage SH6, Shigella phage Shfl1, Shigella phage pSf-2, Escherichia phage ADB-2, Escherichia phage JMPW2, Enterobacteria phage T1, and Escherichia phage JMPW1) (Figure 3). Based on the UPGMA dendrograms, vB-SflSISF001, a Shigella flexneri phage, can be classified as a new species in the genus T1virus of the subfamily Tunavirinae (Figure ).
Figure 3.
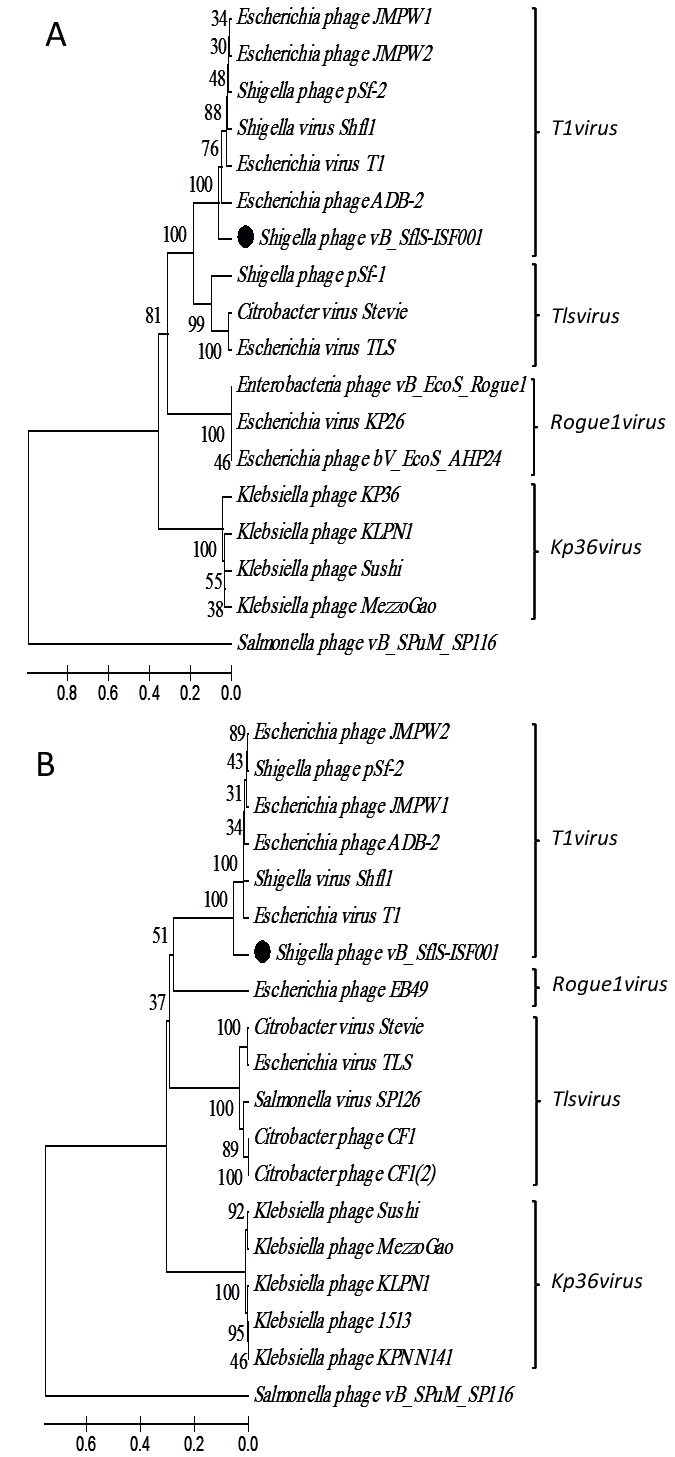
Phylogenetic relationship of S. flexneri bacteriophage vB-SflS-ISF001. Phylogenetic trees were constructed based on the amino acid sequence of the major tail (A) and the DNA primase (B) using the UPGMA method with 2000 bootstrap replications. The numbers on the lines show the supporting rates.
3.4. Analysis of vB-SflS-ISF001 structural proteins
To further characterize vB-SflS-ISF001, the high-titer phage suspension was subjected to 12% (w/v) SDS-PAGE gel. As shown in Figure 4, at least 11 individual protein bands with molecular masses ranging from 13 to 103.7 kDa were detected. In addition, each of the bands was attributed to one of the predicted structural proteins of phage vB-SflS-ISF001 based on their molecular weights (Figure 4).
Figure 4.
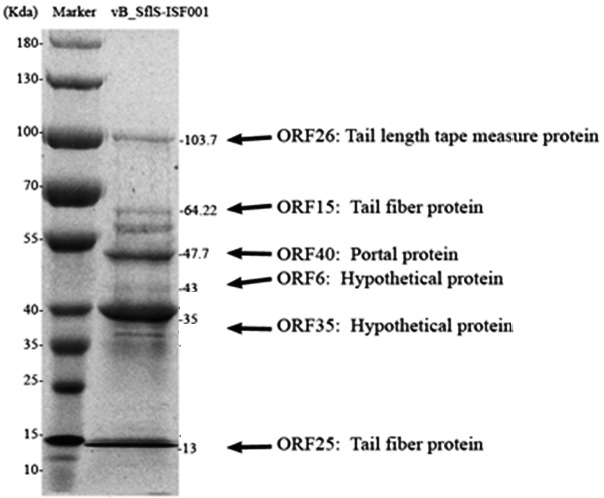
SDS-PAGE analysis of the S. flexneri bacteriophage vB-SflS-ISF001. Lane M, Page Ruler TM Prestained Protein Ladder 26616 (Thermo Scientific, Waltham, MA, USA). The predicted ORFs products related to each band are presented on the left side.
4. Discussion
Shigella is one of the most important groups of Enterobacteriaceae which cause enteric infections (Zhang et al., 2013) . With the emergence of resistant strains, phage therapy has been introduced as an alternative method and a new generation of antibacterial agents. A candidate phage must be analyzed thoroughly before its use in phage therapy (Shahin et al., 2018) . Therefore, the current study aimed to perform a comparative genomic analysis and phylogenic analysis, and look for any sequences related to antibiotic resistance, bacterial virulence factor, or phage lysogeny genes. According to whole genome sequencing and bioinformatic analysis, the most and the least similarity between the ORFs of vB-SflS-ISF001 and other T1virus phages were observed in SH6 and SH2, respectively. Six out of 24 ORFs (ORFs 4, 16, 18, 19, 21, and 67), and 1 out of 24 ORFs (ORF10) of vB-SflS-ISF001 had similarity to ORFs of SH6 and SH2, respectively. In the DNA replication, modification, and regulation group of genes, the function of 7 ORFs were predicted due to their similarity to JMPW2 (1 ORF), vB-EcoS-SH2 (1 ORF), JMPW1 (1 ORF), SH6 (3 ORF), and vB-SsoS-ISF002 (1 ORF). DNA primase/ helicase, which plays a regulatory role in the bacteriophage DNA replication process, is encoded by ORF 14 (Shen et al., 2016) . In the structure and morphogenesis group of genes, the function of 13 ORFs were predicted due to their similarity to JMPW2 (1 ORF), vB-EcoS-SH2 (1 ORF), JMPW1 (2 ORF), SH6 (1 ORF), T1 (3 ORF), Shfl1 (3 ORF), and ADB-2 (2 ORF). Terminases are phage-encoded endonuclease enzymes with ATPase activity that act in the headful DNA packaging process during phage assembly (Hamdi et al., 2017). This enzyme, which was classified in the DNA packaging group, is composed of 2 separate units: the small subunit (ORF41) and the large subunit (ORF42). Double-strand DNA (dsDNA) phages employ the holin–endolysin complex to destroy bacterial host cells. In the genome of vB-SflS-ISF001, ORFs 4 (endolysin) and 5 (holin) were predicted to encode this complex. Holins are hydrophobic proteins that produce holes in the bacterial cytoplasmic membrane by oligomerization and ease the access of endolysins to the cell wall (Fernandes and São‐ José, 2016) . In contrast, endolysins have a crucial role in cleaving the peptidoglycan (murein), the main part of the bacterial cell wall structure (Fernandes and São‐José, 2016). Furthermore, the position of predicted ORFs of the lysis group was similar with those of other Siphoviridae phages (Escherichia virus T1, Escherichia phage JMPW1, Shigella phage SH6, Escherichia phage ADB-2, Shigella phage pSf-2, and Shigella virus Shfl1), which were located at the right or left end of the genome (Roberts et al., 2004; Bhensdadia et al., 2013; Jun et al., 2016; Shen et al., 2016; Hamdi et al., 2017) . Among the identified ORFs and detected conserved domains of the vB-SflS-ISF001 genome, no sequences related to undesirable genes including antibiotic resistance, virulence, or lysogenic mediated or toxin-coding genes were found. Therefore, vB-SflS-ISF001 can be considered a safe agent for biocontrol applications. Additionally, as with other T1virus phages, no tRNA-encoding sequences were identified in the genome of vB-SflS-ISF001.
Genomic comparison showed that the organization, orientations, and distribution of the ORFs were generally similar to those of other members of the genus T1virus. Moreover, MegaBLAST analysis and UPGMA dendrograms revealed that vB-SflS-ISF001 can be classified as a new member of the genus T1virus, subfamily Tunavirinae.
In conclusion, in the current study, genomic characteristics of Shigella flexneri phage vB-SflS-ISF001 were comparatively analyzed. Phage vB-SflS-ISF001 genome is a dsDNA (50,552 bp) with 45.58% G + C content. Seventy-eight distinct ORFs and no tRNA were predicted in the vB-SflS-ISF001 genome. Comparative genomic analysis of vB-SflS-ISF001 demonstrated that this phage could be classified as a new species in the genus T1virus of the subfamily Tunavirinae. Moreover, no undesirable genes, e.g., antibiotic resistance, virulence, lysogenic mediated genes, or toxin-coding genes, were found in the vB-SflS-ISF001 genome sequence. Phylogenetic analysis (based on major tail and DNA primase) of vB-SflS-ISF001 showed a high similarity to other T1virus species, and was further validated through genome and comparative genomic analyses, which not only constitute a much more accurate classification approach, but also a powerful methodology to investigate and certify the safety of phages for potential application as biocontrol agents. Therefore, the data suggest that vB-SflS-ISF001 can be used as a safe agent for phage therapy.
Acknowledgments
This research was funded by an operating grant of the Dean of Research and Graduate Studies at the University of Isfahan (No: A/94/32650) and Jiangsu Agricultural Science and Technology Foundation (No. CX[16]1060).
References
- Altschul SF , Madden TL , Schäefr AA , Zhang J , Zhang Z , Miller W , Lipman DJ ( 1997. ). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs . Nucleic Acids Res 25 : 3389 - 3402 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besemer J , Lomsadze A , Borodovsky M ( 2001. ). GeneMarkS: a selftraining method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions . Nucleic Acids Res 29 : 2607 - 2618 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhensdadia D , Bhimani H , Rawal C , Kothari V , Raval V , Kothari C , Patel A , Bhatt V , Parmar N , Sajnani M ( 2013. ). Complete genome sequence of Escherichia phage ADB-2 isolated from a fecal sample of poultry . Genome Announc 1 : e00043 - 00013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver T , oThmson N , Bleasby A , Berriman M , Parkhill J ( 2009. ). DNAPlotter: circular and linear interactive genome visualization . Bioinformatics 25 : 119 - 120 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling AC , Mau B , Blattner FR , Perna NT ( 2004. ). Mauve: multiple alignment of conserved genomic sequence with rearrangements . Genome Res 14 : 1394 - 1403 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes S , São‐José C ( 2016. ). More than a hole: the holin lethal function may be required to fully sensitize bacteria to the lytic action of canonical endolysins . Mol Microbiol 102 : 92 - 106 . [DOI] [PubMed] [Google Scholar]
- Finn RD , Coggill P , Eberhardt RY , Eddy SR , Mistry J , Mitchell AL , Potter SC , Punta M , Qureshi M , Sangrador-Vegas A ( 2015. ). The Pfam protein families database: towards a more sustainable future . Nucleic Acids Res 44 : D279 - D285 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasteiger E , Hoogland C , Gattiker A , Duvaud Se , Wilkins MR , Appel RD , Bairoch A ( 2005. ). Protein identification and analysis tools on the ExPASy server . In: Walker JM, editor. The Proteomics Protocols Handbook. Totowa , NJ, USA: Humana Press. pp. 571 - 607 .
- Kumar S , Stecher G , Tamura K ( 2016. ). MEGA7: Molecular Evolutionary Genetics Analysis version 7 . 0 for bigger datasets . Mol Biol Evol 33 : 1870 - 1874 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu C , Yang J , Liu H , Cui Y , Xu H , Wang R , Liu X , Feng E , Wang D , Pan C ( 2017. ). Role of the virulence plasmid in acid resistance of Shigella flexneri . Sci Rep 7 : 46465 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszak T , Latka A , Roszniowski B , Valvano M , Drulis-Kawa Z ( 2017. ). Phage life cycles behind bacterial biodiversity . Curr Med Chem 24 : 3987 - 4001 . [DOI] [PubMed] [Google Scholar]
- Roberts MD , Martin NL , Kropinski AM ( 2004. ). The genome and proteome of coliphage T1 . Virology 318 : 245 - 266 . [DOI] [PubMed] [Google Scholar]
- Sambrook J , Russell DW ( 2001. ) Molecular Cloning: A Laboratory Manual . 2nd ed. Cold Spring Harbor, NY , USA: Cold Spring Harbor Laboratory Press.
- Schattner P , Brooks AN , Lowe TM ( 2005. ). The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs . Nucleic Acids Res 33 : W686 - W689 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahin K , Bouzari M ( 2018. ). Bacteriophage application for biocontrolling Shigella flexneri in contaminated foods . J Food Sci Technol 55 : 550 - 559 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahin K , Bouzari M , Wang R ( 2018. ). Isolation, characterization and genomic analysis of a novel lytic bacteriophage vB-SsoSISF002 infecting Shigella sonnei and Shigella flexneri . J Med Microbiol 67 : 376 - 386 . [DOI] [PubMed] [Google Scholar]
- Shen M , Zhu H , Lu S , Le S , Li G , Tan Y , Zhao X , Shen W , Hu F , Wang J ( 2016. ). Complete genome sequences of T1-like phages JMPW1 and JMPW2 . Genome Announc 4 : e00601 - 00616 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D , Reynolds D , Seto D , Mahadevan P ( 2013. ). CoreGenes3. . 5: a webserver for the determination of core genes from sets of viral and small bacterial genomes . BMC Res Notes 6 : 140 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner PL , Waldor MK ( 2002. ). Bacteriophage control of bacterial virulence . Infect Immun 70 : 3985 - 3993 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JC , Verma NK ( 2002. ). Identification of a putative pathogenicity island in Shigella flexneri using subtractive hybridisation of the S. flexneri and Escherichia coli genomes . FEMS Microbiol Lett 213 : 257 - 264 . [DOI] [PubMed] [Google Scholar]
- Wommack KE , Colwell RR ( 2000. ). Virioplankton: viruses in aquatic ecosystems . Microbiol Mol Biol Rev 64 : 69 - 114 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye C , Lan R , Xia S , Zhang J , Sun Q , Zhang S , Jing H , Wang L , Li Z , Zhou Z ( 2010. ). Emergence of a new multidrug-resistant serotype X variant in an epidemic clone of Shigella flexneri . J Clin Microbiol 48 : 419 - 426 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H , Wang R , Bao H ( 2013. ). Phage inactivation of foodborne Shigella on ready-to-eat spiced chicken . Poult Sci 92 : 211 - 217 . [DOI] [PubMed] [Google Scholar]
- Gemski P , Koeltzow D , Formal S ( 1975. ). Phage conversion of Shigella flexneri group antigens. Infect Immun 11 : 685 - 691 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi SM , Bouzari M , Baygloo NS , Chang H-I ( 2014). ). Insights into new bacteriophages of Lactococcus garvieae belonging to the family Podoviridae. Arch Virol 159 : 2909 - 2915 . [DOI] [PubMed] [Google Scholar]
- Hamdi S , Rousseau GM , Labrie SJ , Tremblay DM , Kourda RS , Slama KB , Moineau S ( 2017. ). Characterization of two polyvalent phages infecting Enterobacteriaceae. Sci Rep 7 : 40349 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- James CE , Stanley KN , Allison HE , Flint HJ , Stewart CS , Sharp RJ , Saunders JR , McCarthy AJ ( 2001. ). Lytic and lysogenic infection of diverse Escherichia coli and Shigella strains with a verocytotoxigenic bacteriophage. Appl Environ Microbiol 67 : 4335 - 4337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennison AV , Verma NK ( 2004. ). Shigella flexneri infection: pathogenesis and vaccine development. FEMS Microbiol Rev 28 : 43 - 58 . [DOI] [PubMed] [Google Scholar]
- Jun JW , Giri SS , Kim HJ , Yun SK , Chi C , Chai JY , Lee BC , Park SC , ( 2016. ). Bacteriophage application to control the contaminated water with Shigella. Sci Rep 6 : 22636 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropinski AM , Mazzocco A , Waddell TE , Lingohr E , Johnson RP ( 2009. ). Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol Biol 501 : 69 - 76 . [DOI] [PubMed] [Google Scholar]



