Abstract
Objective
We sought to determine whether the early luteal serum progesterone (P4) level predicts the success of IVF treatment with oral dydrogesterone for luteal support.
Method
This retrospective monocentric cohort study included 242 women who underwent IVF treatment with fresh embryo transfer (ET) between July 2017 and June 2018. The population was unselected, and women were treated according to our unit’s usual stimulation protocols. For the luteal phase support (LPS), all women were supplemented with a 10 mg three-times-daily dose of oral dydrogesterone beginning on the day of oocyte pick-up (OPU). Blood sampling was performed on the day of ET (Day 2–3 after OPU) to determine the early luteal serum progesterone level.
Results
ROC curve analysis allowed us to determine two thresholds for the prediction of live birth using the early P4 level. Women who had early luteal P4 levels greater than 252 nmol/l had a significantly higher live birth rate (27.1%) than women with early luteal P4 between 115 and 252 nmol/l (17.2%) and women with early luteal P4 below 115 nmol/l (6.0%; p = 0.011). After a multiple regression analysis, an early luteal P4 level greater than 252 nmol/l was still associated with a higher chance of a live birth than a P4 between 115 and 252 nmol/l (OR = 0.40 [0.18–0.91]; p = 0.028) or a P4 below 115 nmol/l (OR = 0.10 [0.01–0.52]; p = 0.006).
Conclusions
Our study suggests a positive association between early P4 levels and reproductive outcomes in IVF using oral dydrogesterone for luteal support. The inconsistencies between our results and those of other studies suggest that extrapolation is impractical. Further larger prospective cohort studies should be conducted to determine reliable thresholds that could be used to personalize luteal phase support.
Introduction
Over the past decades, increasing efforts have been made to identify best practices for controlled ovarian stimulation (COS) and embryo culture for In Vitro Fertilization (IVF) treatment [1]. In contrast, the luteal phase subsequent to the COS and oocyte retrieval have received less interest for a long time [2]. By modifying gene expression, progesterone guides the endometrial secretory transformation and therefore plays a fundamental role in implantation and early embryologic development [3,4]. After COS, the endogenous secretion of progesterone by the granulosa of the corpus luteum is insufficient [5]. The luteolytic effect of the GnRH analogs or GnRH antagonists used for the COS, inhibition of the hypothalamic-pituitary axis by the supra-physiological secretion of steroids, and aspiration of the granulosa cells during oocyte retrieval are the main hypotheses for this inadequate secretion [6]. Luteal phase support (LPS) compensates for this deficiency by supplementing with either progesterone or an hCG or GnRH agonist [7].
In recent years, clinical investigators have shown a renewed interest in the luteal phase subsequent to ART treatments. Evidence is accumulating regarding the existence of an optimal range of P4 levels on the day of embryo transfer (ET) in frozen embryo transfer cycles with hormone replacement therapy [8–10]. In 2018, Thomsen et al. published the results of a large prospective cohort study of 602 women who underwent IVF treatment and had serum P4 levels measured on the day of ET [11]. The authors determined that very low P4 levels (< 60 nmol/l) on the day of fresh ET (Day 2–3 after oocyte pick-up (OPU)) were associated with decreased chance of success of the IVF treatment. This result allows us to consider a possible value of early luteal P4 monitoring and luteal rescue by increasing the P4 supplementation. Thomsen et al. also reported that high P4 levels (> 400 nmol/l) were associated with a decreased chance of live birth after IVF treatment. If confirmed by other studies, these thresholds could encourage the cancellation of ET for women with high early luteal P4 levels.
However, concerns have been raised regarding the statistical analysis of the aforementioned study [12], and further studies are needed to assess the reproducibility of the suggested thresholds before they can be used in common practice, in particular with different COS and LPS protocols.
A recent large phase III RCT demonstrated the noninferiority of oral dydrogesterone compared with micronized vaginal progesterone for LPS in terms of pregnancy rates and tolerability [13]. Thus, dydrogesterone was recently approved for LPS in IVF [14]. Furthermore, given the assumed preference for the oral route over vaginal route, dydrogesterone is presumed to become the new standard for LPS in IVF treatment according to some authors [15]. The early luteal phase P4 levels have never been studied in the context of the use of dydrogesterone for LPS [16].
Throughout this retrospective data analysis, we aimed to determine if early luteal P4 levels (on the day of fresh ET) could allow us to predict the success of IVF on an unselected population treated with oral dydrogesterone for LPS.
Materials and methods
Study design
We conducted a monocentric retrospective cohort study. Data were retrospectively and anonymously collected. Before the study began, it was approved by the ethics committee of Aix Marseille University. All patients were informed of the anonymous and retrospective use of their data and could refuse to participate by simple notification.
Study population
We enrolled couples who underwent IVF or ICSI treatments in our ART unit between July 2017 and June 2018 and who had early luteal phase serum P4 levels measured on the day of fresh ET (Day 2–3 after OPU). Women were eligible for inclusion regardless of the reason for the infertility or the number of previous unsuccessful IVF or ICSI cycles.
Ovarian stimulation protocols
The choices of COS protocols and gonadotrophin doses were made in accordance with the standards of our ART unit based on age, BMI, ovarian reserve, outcomes of previous IVF or ICSI cycles, and other comorbidities such as endometriosis or polycystic ovary syndrome (PCOS). GnRH antagonist protocols were generally used as the first choice, especially for patients with an expected low ovarian response and for women with PCOS; in contrast, GnRH long agonist protocols were used for patients with endometriosis or as a second choice for patients with an inadequate response to the previous GnRH antagonist protocol. Short GnRH agonist protocols were used for low responders who had already experienced several failures after previous stimulations.
Patients who were treated in a long GnRH-agonist protocol were downregulated using a unique IM injection of 3 mg triptorelin (Decapeptyl, Ipsen Pharma, France) on the 22nd day of the preceding cycle. Ovarian stimulation started after 14 days of downregulation after checking that the endometrial thickness was less than 4 mm. Final follicular maturation was induced by a single injection of 250 μg of choriogonadotropin alpha (Ovitrelle, Merck Serono Europe Limited, United Kingdom) when two or more leading follicles reached a mean diameter of 17 mm.
If the GnRH antagonist protocol was used, ovarian stimulation started on cycle Day 2. Daily GnRH antagonist cotreatment was added from cycle Day 6. Final maturation could be induced by a single injection of 250 μg of choriogonadotropin alpha (Ovitrelle, Merck Serono Europe Limited, United Kingdom) or by a dual trigger (250 μg of choriogonadotropin alpha plus 0.3 mg of Triptorelin) when two or more leading follicles reached a mean diameter of 17 mm.
Ovarian stimulation was performed using either r-FSH, r-FSH/r-LH or hMG. Dose adjustments were performed according to ovarian response, as monitored by transvaginal ultrasound during treatment. OPU was performed 36 h after the ovulation trigger. IVF or ICSI was performed according to normal clinical practice.
Luteal phase support
All patients received a 10 mg three-times-daily dose of oral dydrogesterone (Duphaston, Mylan Medical, France) starting on the day of OPU [14,15]. We systematically checked with the patients’ compliance with this treatment on the day of ET. No additional treatment (neither hCG nor GnRH agonist bolus) was ever used during the luteal phase. Oral dydrogesterone administration continued from the day of OPU until the day of pregnancy testing or until the seventh gestational week (in the case of pregnancy).
Hormone analysis
On day 2–3 after OPU, immediately before ET, blood samples were collected for P4 and estradiol determination. We used an automated Cobas e411 instrument (Roche Diagnostics, Mannheim, Germany) and the same assays for all hormone measurements during the entire study. Samples were tested by an electrochemiluminescence immunoassay for Progesterone III (Cobas 07092539 190). The intra- and inter-assay variation coefficients for the P determinations were 3.3% and 5.2%, respectively, and sensitivity was 0.2 ng/ml.
Endpoints
hCG serum level was measured 14 days after ET and considered positive if hCG > 10 UI/ml. Clinical pregnancy was defined as the presence of a live fetus within an intrauterine gestational sac upon ultrasound examination at gestational weeks 6–7. Early pregnancy loss was defined by positive hCG testing at Day 14 and the absence of a live fetus within an intrauterine gestational sac upon ultrasound examination at gestational weeks 6–7. Thus, this definition includes patients with decreasing hCG after the first test, patients with no intrauterine gestational sac during the first ultrasound, patients with ectopic pregnancy and patients with a visible embryo without cardiac activity. Clinical pregnancy loss was defined as the loss of a viable intrauterine pregnancy between the first ultrasound up to and including gestational weeks 24 + 0. A live birth was defined as the delivery of a live infant after gestational week 24 + 0. Clinical gestational dating was performed using the day of OPU as gestational week 2 + 0 [11].
Statistical analysis
Data are presented as percentages for categorical variables, as means and standard deviations for continuous parametric variables and as medians and ranges for continuous nonparametric variables.
First, a receiver operating characteristic (ROC) curve was used to evaluate the ability of early luteal P4 levels to predict live birth and to determine thresholds that could discriminate patients while maximizing sensitivity (Se) and specificity (Sp) with a good Youden index (Se + Sp– 1). Three P4 groups were thus formed using the optimal thresholds found (< 115 nmol/l, [115–252] nmol/l and > 252 nmol/l). ANOVA and χ2 tests were used, respectively, to compare continuous and categorical characteristics among the three P4 groups.
Second, the multiple logistic regression model used by Thomsen et al. was applied to assess the differences in terms of reproductive outcomes among the aforementioned P4 groups [11]. The model included the independent variables maternal age, maternal BMI, final follicle count on the day of trigger (>14 mm) and late follicular P4 level (>4.77 nmol/l) to estimate positive hCG levels, clinical pregnancy and live birth. For estimates of early pregnancy loss, adjustments were made for maternal age, maternal BMI, smoking, final follicle count and peak estradiol level on the day of trigger.
Third, to compare our results to those of the Thomsen et al. study, we conducted a multiple logistic regression by retaining only patients who responded to the same inclusion criteria (age < 41, BMI < 35, and excluding the flare up protocols). We compared the same P4 groups (< 60 nmol/l; [60–100 nmol/l]; [100–400 nmol/l]; > 400 nmol/l) to determine the differences in terms of reproductive outcomes.
All statistical analyses were two-tailed, and results were considered significant when p-values < 0.05 were obtained. These analyses were performed using IBM SPSS Statistics 20.0 (IBM Inc., New York, USA).
Results
Study population and reproductive outcomes
During the selected time period, 242 women who were undergoing IVF treatment at our ART unit had a blood sample on the day of ET and were included in the study. Baseline characteristics of participants are provided in Table 1, and cycle characteristics with crude reproductive outcomes are presented in Table 2. The mean age of patients enrolled was 34.9 ± 4.7 years, and the mean BMI was 24.8 ± 5.3 kg/m2. After IVF treatment, 69 (28.5%) patients had a positive hCG level and 22 (9.1%) patients had early pregnancy loss, leaving 47 (19.4%) patients with clinical pregnancy. Overall, 43 (17.8%) patients gave birth to live children.
Table 1. Baseline characteristics.
| Progesterone (nmol/l) | N | All | < 115 | 115–252 | > 252 | P |
|---|---|---|---|---|---|---|
| Number of patients, n | 242 | 50 | 122 | 70 | ||
| Maternal age (years) | 242 | 34.9 ± 4.8 | 37.4 ± 3.9 | 34.5 ± 4.6 | 33.7 ± 5.0 | <0.01** |
| Maternal BMI (kg/m2) | 242 | 24.8 ± 5.3 | 27.5 ± 6.8 | 23.8 ± 4.15 | 24.5 ± 5.2 | <0.01** |
| Maternal smoking (%) | 242 | 21 | 20 | 25 | 14 | 0.235 |
| Basal FSH (IU) | 236 | 6.8 (1.7–16.3) | 7.5 (3.6–13.9) | 7.2 (1.7–16.3) | 6.2 (2.4–13.4) | 0.001** |
| Basal LH (IU) | 234 | 5.0 (0.1–35.0) | 4.5 (1.6–17.0) | 5.1 (1.4–35.0) | 4.9 (0.1–23.0) | 0.549 |
| Basal AMH (IU) | 242 | 1.9 (0.1–17.0) | 1.1 (0.1–11.5) | 1.9 (0.1–17.0) | 2.9 (0.5–9.8) | <0.01** |
| Antral follicle count, n | 217 | 13 (2–55) | 11 (2–55) | 13 (4–38) | 16 (4–42) | <0.01** |
| Primary diagnosis | 242 | |||||
| Unexplained (%) | 23 | 16 | 33 | 10 | <0.01** | |
| Tubal (%) | 27 | 32 | 25 | 27 | 0.608 | |
| Endometriosis (%) | 13 | 14 | 14 | 11 | 0.871 | |
| PCO/PCOS (%) | 12 | 8 | 7 | 23 | <0.01** | |
| Male (%) | 25 | 30 | 21 | 29 | 0.377 |
Descriptive data are presented as the mean ± SD for continuous parametric data and as the median (range) for continuous nonparametric data. Categorical data are presented as percentages (%).
SI conversion factor for P4: nmol/l = 3.18 ng/ml.
**p< 0.01
Table 2. Descriptive data for controlled ovarian stimulation, oocytes, embryo transfer, and reproductive outcomes.
| Progesterone (nmol/l) | N | All | < 115 | 115–252 | > 252 | P |
|---|---|---|---|---|---|---|
| Number of patients, n (%) | 242 | 50 (21%) | 122 (50%) | 70 (29%) | ||
| Protocol | 242 | |||||
| Antagonist (%) | 64 | 60 | 60 | 76 | 0.066 | |
| Long GnRH agonist (%) | 28 | 22 | 33 | 21 | 0.449 | |
| Flare up (%) | 8 | 18 | 7 | 3 | 0.011* | |
| Total FSH dose (IU) | 242 | 2925 (1000–7200) | 3450 (1125–6750) | 2850 (1125–7200) | 2475 (1000–6750) | <0.001** |
| Stim duration (days) | 242 | 10.5 ± 1.7 | 10.3 ± 1.9 | 10.4 ± 1.7 | 10.7 ± 1.6 | 0.399 |
| Final follicle count >14 mm on trigger day | 240 | 8 (2–24) | 5 (2–17) | 8 (3–16) | 10 (2–24) | <0.001** |
| Mode of triggering for final oocyte maturation | 242 | 0.353 | ||||
| hCG (%) | 69 | 62 | 70 | 75 | ||
| Dual trigger (%) | 31 | 38 | 30 | 25 | ||
| Number of mature oocytes retrieved (n) | 242 | 8 (1–25) | 4 (1–14) | 8 (1–20) | 12 (2–25) | <0.001** |
| Number of fertilized oocytes (n) | 242 | 5 (1–19) | 3 (1–8) | 5 (1–17) | 6 (1–19) | <0.001** |
| Single embryo transfer (%) | 242 | 26 | 40 | 24 | 21 | 0.048* |
| Double embryo transfer (%) | 242 | 74 | 60 | 76 | 79 | |
| At least one top quality embryo for transfer (%) | 242 | 25 | 26 | 26 | 21 | 0.741 |
| Positive hCG, n (%) | 242 | 69 (28.5%) | 9 (18.0%) | 33 (27.0%) | 27 (38.6%) | 0.043* |
| Early pregnancy loss, n (%) | 242 | 22/69 (31.9%) | 5/9 (55.6%) | 11/33 (33.3%) | 6/27 (22.2%) | 0.182 |
| Clinical pregnancy, n (%) | 242 | 47 (19.4%) | 4 (8.0%) | 22 (18,0%) | 21 (30.0%) | 0.009** |
| Live birth, n (%) | 242 | 43 (17.8%) | 3 (6.0%) | 21 (17.2%) | 19 (27.1%) | 0.011* |
Descriptive data are presented as the mean ± SD for continuous parametric data and as the median (range) for continuous nonparametric data. Categorical data are presented as percentages (%).
SI conversion factor for P4: nmol/l = 3.18 ng/ml.
*p<0.05
**p<0.01
Threshold and P4 groups
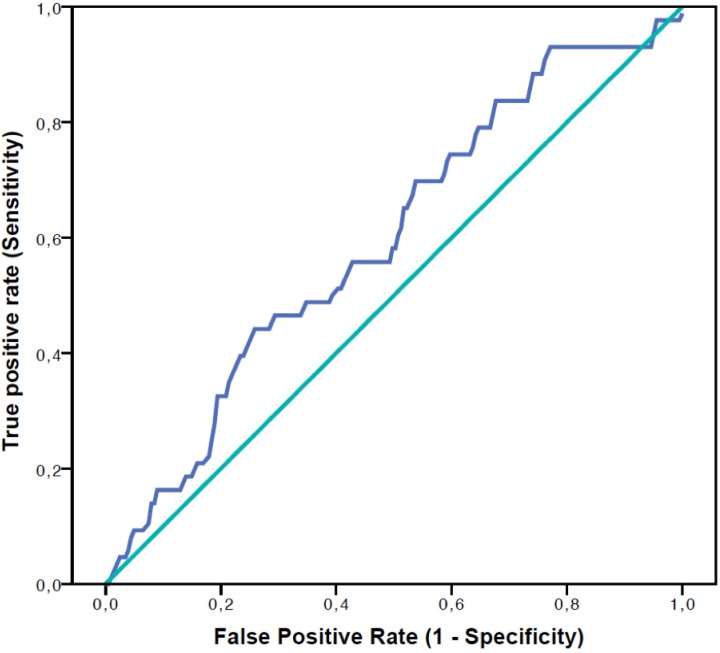
The ROC curve for prediction of live birth based on early luteal P4 levels (Fig 1) allowed us to find two optimal thresholds and to identify three P4 groups: patients (n = 50) who had early luteal P4 < 115 nmol/l had a significantly lower live birth rate (6.0%) than patients (n = 122) with P4 levels [115–252 nmol/l] (17.2%) and patients (n = 70) with P4 levels > 252 nmol/l (27.1%; p = 0.011) (Table 2). Similarly, the rates of positive hCG (p = 0.043) and clinical pregnancies (p = 0.009) significantly differed across the three groups. The rates of early pregnancy loss were 5/9 (55.6%), 11/33 (33.3%) and 6/27 (22.2%) respectively for the P4 groups < 115 nmol/l, [115–252 nmol/l], > 252 nmol/l. The low number of observations did not allow us to find any significant difference across groups for this reproductive outcome (p = 0.182).
Fig 1. Receiving operative characteristic (ROC) curve for the prediction of live birth based on early luteal progesterone levels during IVF.
Area under ROC curve = 0.599 (0.507–0.691), p = 0.042.
Multiple regression analysis
Due to missing data, the multiple regression analysis using the maternal age, the maternal BMI, the number of follicles > 14 mm and the late follicular P4 levels > 4.77 nmol/l was only conducted on 231 patients. The results of this analysis are presented in Table 3. Patients who had early luteal P4 levels < 115 nmol/l had the poorest chance of giving live birth (adjusted OR = 0.10 [0.02–0.52]) compared with patients with early luteal P4 > 252 nmol/l. Patients with early luteal P4 [115–252 nmol/l] also had a significantly lower chance of giving live birth than patients with P4 > 252 nmol/l (adjusted OR = 0.40 [0.18–0.91]). The same significative differences were obtained for the positive hCG and the clinical pregnancies but not for the early pregnancy losses (Table 3).
Table 3. Reproductive outcomes in the different luteal progesterone groups.
| OR for positive hCG | OR for clinical pregnancy | OR for live birth | OR for early pregnancy loss | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Crude OR [95% CI] |
N | Adjusted OR [95% CI] |
N | Crude OR [95% CI] |
N | Adjusted OR [95% CI] |
N | Crude OR [95% CI] |
N | Adjusted OR [95% CI] |
N | Crude OR [95% CI] |
N | Adjusted OR [95% CI] |
|
| N | 69/242 | 242* | 63/231 | 231** | 47/242 | 242* | 43/231 | 231** | 43/242 | 242* | 39/231 | 231** | 22/69 | 69 | 20/65 | 65 |
| P4 < 115 nmol/l | 9/50 | 0.35 [0.15–0.83] | 8/48 | 0.26 [0.09–0.76] | 4/50 | 0.20 [0.06–0.64] | 3/48 | 0.13 [0.03–0.53] | 3/50 | 0.17 [0.05–0.617] | 2/48 | 0.10 [0.01–0.52] | 5/9 | 4.37 [0.89–21.61] | 5/9 | 10.42 [0.84–129.16] |
| P4 115–252 nmol/l | 33/122 | 0.59 [0.32–1.10] | 30/117 | 0.47 [0.24–0.99] | 22/122 | 0.51 [0.26–1.02] | 20/117 | 0.36 [0.16–0.80] | 21/122 | 0.56 [0.27–1.13] | 19/117 | 0.40 [0.18–0.91] | 11/33 | 1.750 [0.55–5.59] | 10/31 | 3.64 [0.72–18.23] |
| P4 > 252 nmol/l | 27/70 | 1 | 25/66 | 1 | 21/70 | 1 | 20/66 | 1 | 19/70 | 1 | 18/66 | 1 | 6/27 | 1 | 5/25 | 1 |
*In the crude OR estimates, all 242 patients with embryo transfer are included.
**Due to missing data for the covariate late follicular phase in 7 patients, the final follicle count > 14 mm on trigger day for 2 patients, and the BMI for 2 patients, the final adjusted regression model included 231 patients.
Discussion
To our knowledge, our study is the first to study the effect of the early luteal phase during IVF with the use of oral dydrogesterone for the LPS. Lotus I, a large double-blinded RCT recently demonstrated the noninferiority of oral dydrogesterone compared with micronized vaginal progesterone for LPS in terms of pregnancy rates and tolerability [13]. Several other prospective studies have shown the effectiveness and safety of oral dydrogesterone for LPS during IVF or ICSI cycles and higher satisfaction rates for women compared with micronized vaginal progesterone [7,17–21]. Thus, according to some authors, dydrogesterone may become the new standard for LPS during fresh ET IVF cycles [15].
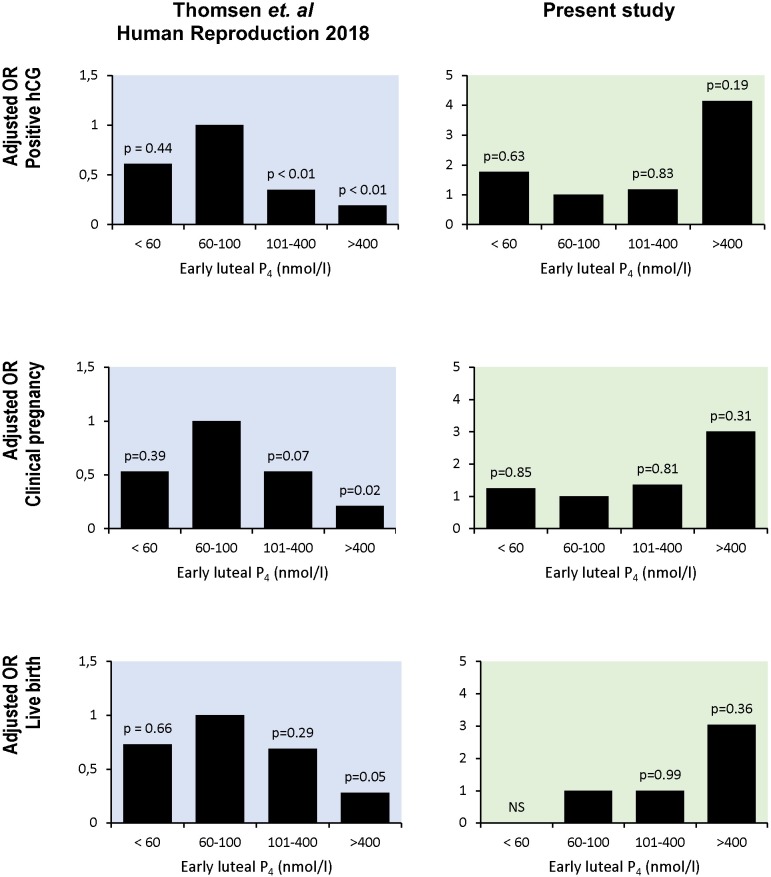
Our results suggest a positive association between early luteal P4 levels and reproductive outcomes in IVF treatment with oral dydrogesterone for the luteal support. Therefore, these results are not only inconsistent with the conclusion of Thomsen et al. but the complete opposite [11]. After applying the same exclusion criteria as in the Thomsen et al. study (age < 41, BMI < 35, and excluding the flare up protocols), only 179 patients were divided into the same P4 groups <60 nmol/l; [60–100 nmol/l]; [100–400 nmol/l]; and > 400 nmol/l (respectively n = 5; n = 12; n = 154; n = 8). Our results are compared to those of the Thomsen et al. study in Fig 2. Although the low number of subjects in three of the four groups in this analysis did not allow us to find any significant differences among groups, the results of the two cohorts of patients are clearly inconsistent. The P4 group [60–100 nmol/l] that was associated with the best clinical pregnancy rate in the Thomsen et al. study had the poorest chance in our study. Similarly, the P4 group > 400 nmol/l that was associated with the poorest live birth rate in the aforementioned study was associated with the best live birth rate in our study.
Fig 2.
Comparison of the reproductive outcomes of our retrospective cohort (in green, right section, 179 patients) with the Thomsen et al. prospective cohort (in blue, left section, 389 patients).
The most plausible hypothesis for these discrepancies is that dydrogesterone interferes with the progesterone secretion by the corpus lutei. Indeed, we measured P4 levels only and do not report the dydrogesterone levels, which would have required the use of an instrumental chromatographic method [16,22]. Although the influence of dydrogesterone on the secretion of progesterone during the luteal phase has not been fully elucidated, its administration lowers the P4 levels [23]. Two studies have also shown the efficacy of dydrogesterone administration to prevent premature LH surges in the context of frozen-thawed ET, suggesting negative feedback on the pituitary gland [23,24]. Although it is clearly impossible with the present study to estimate the respective weights of influence of exogenous dydrogesterone and endogenous progesterone on the outcomes of IVF treatment, our study clearly illustrates that the thresholds suggested by Thomsen et al. are not transposable when dydrogesterone is used for the LPS.
The present study has several limitations. The small sample size forced us to conduct the statistical analysis on early luteal P4 cut-off values that were determined in a data-dependent way. This method of analysis is known to bear the risk of finding an effect in the sample while no real difference exists in the population [12,25]. Although we noticed the same trends when the data were analyzed in an objective way–i.e. percentile groups–due to sample size, we were unable to show significant differences using this analysis. Comparability of our P4 groups could also be criticized, as there are significant differences between groups. To overcome this issue, we chose to use the exact same variables as Thomsen et al. for the multiple regression analysis [11]. They used a Directed Acyclic Graph to identify a minimum set of covariates to adjust for in the statistical analysis. However, in the present study, the sample size was too small to show significant differences between groups by using a classical selection process for the multiple regression. Therefore, we cannot formally exclude a confusion bias. Overall, in addition to the retrospective nature of the study, the lack of statistical power does not allow us to present an irreproachable conclusion and further larger prospective cohort studies must be conducted. Another obvious limitation of the present study is the absence of early luteal phase dydrogesterone level measurements. This missing information prevents us from fully concluding on the pathophysiological mechanisms involved in the relationship between P4 levels and reproductive outcomes. Nevertheless, we successfully identified early luteal P4 thresholds that–if confirmed by prospective studies–may be used in clinical practice. Indeed, we determined that 21% of our patients who had P4 levels below 115 nmol/l had a dramatically poor live-birth rate (6.0%). If confirmed, this finding would encourage physicians to cancel the ET for these patients. The effect of increasing the supplementation is more intangible because the dydrogesterone might induce negative feedback over the progesterone secretion of the corpus lutei [23]. Furthermore, increasing progesterone or dydrogesterone supplementation does not proportionally increase the serum levels, and uterine progesterone levels do not correlate well with serum levels [26–29]. These pharmacokinetic data add to the uncertainty regarding strategies to increase the LPS in the case of low early luteal P4 levels.
Thus, our results should be interpreted with utmost precautions, and we only conclude that early luteal P4 levels and the reproductive outcomes in IVF with oral dydrogesterone for luteal support appear to be positively associated. The current state of knowledge on early luteal P4 monitoring is still too weak to allow for consideration of interventional studies or clinical applications. Further larger prospective cohort studies must be conducted to determine reliable thresholds that could be used to personalize LPS.
Data Availability
The underlying data set has been publicly released via the following DOI: 10.6084/m9.figshare.8187413 (doi.org/10.6084/m9.figshare.8187413).
Funding Statement
The authors received no specific funding for this work.
References
- 1.Kushnir VA, Barad DH, Albertini DF, Darmon SK, Gleicher N. Systematic review of worldwide trends in assisted reproductive technology 2004–2013. Reprod Biol Endocrinol. 2017;15: 6 10.1186/s12958-016-0225-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yding Andersen C, Vilbour Andersen K. Improving the luteal phase after ovarian stimulation: reviewing new options. Reproductive BioMedicine Online. 2014;28: 552–559. 10.1016/j.rbmo.2014.01.012 [DOI] [PubMed] [Google Scholar]
- 3.Carson DD. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Molecular Human Reproduction. 2002;8: 871–879. 10.1093/molehr/8.9.871 [DOI] [PubMed] [Google Scholar]
- 4.Wetendorf M, DeMayo FJ. The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol Cell Endocrinol. 2012;357: 108–118. 10.1016/j.mce.2011.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fauser BC, de Jong D, Olivennes F, Wramsby H, Tay C, Itskovitz-Eldor J, et al. Endocrine profiles after triggering of final oocyte maturation with GnRH agonist after cotreatment with the GnRH antagonist ganirelix during ovarian hyperstimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87: 709–715. 10.1210/jcem.87.2.8197 [DOI] [PubMed] [Google Scholar]
- 6.Child T, Leonard SA, Evans JS, Lass A. Systematic review of the clinical efficacy of vaginal progesterone for luteal phase support in assisted reproductive technology cycles. Reprod Biomed Online. 2018;36: 630–645. 10.1016/j.rbmo.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 7.van der Linden M, Buckingham K, Farquhar C, Kremer JAM, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. 2015; CD009154. 10.1002/14651858.CD009154.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labarta E, Mariani G, Holtmann N, Celada P, Remohí J, Bosch E. Low serum progesterone on the day of embryo transfer is associated with a diminished ongoing pregnancy rate in oocyte donation cycles after artificial endometrial preparation: a prospective study. Hum Reprod. 2017;32: 2437–2442. 10.1093/humrep/dex316 [DOI] [PubMed] [Google Scholar]
- 9.Yovich JL, Conceicao JL, Stanger JD, Hinchliffe PM, Keane KN. Mid-luteal serum progesterone concentrations govern implantation rates for cryopreserved embryo transfers conducted under hormone replacement. Reprod Biomed Online. 2015;31: 180–191. 10.1016/j.rbmo.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 10.Alsbjerg B, Thomsen L, Elbaek HO, Laursen R, Povlsen BB, Haahr T, et al. Progesterone levels on pregnancy test day after hormone replacement therapy-cryopreserved embryo transfer cycles and related reproductive outcomes. Reprod Biomed Online. 2018;37: 641–647. 10.1016/j.rbmo.2018.08.022 [DOI] [PubMed] [Google Scholar]
- 11.Thomsen LH, Kesmodel US, Erb K, Bungum L, Pedersen D, Hauge B, et al. The impact of luteal serum progesterone levels on live birth rates-a prospective study of 602 IVF/ICSI cycles. Hum Reprod. 2018; 10.1093/humrep/dey226 [DOI] [PubMed] [Google Scholar]
- 12.Venetis CA, Mol BW, Kolibianakis EM. Low as well as high serum P4 levels in the early and mid-luteal phase reduce the chance of a live birth following IVF treatment with fresh embryo transfer: where is the evidence? Hum Reprod. 2018;33: 2312–2313. 10.1093/humrep/dey311 [DOI] [PubMed] [Google Scholar]
- 13.Tournaye H, Sukhikh GT, Kahler E, Griesinger G. A Phase III randomized controlled trial comparing the efficacy, safety and tolerability of oral dydrogesterone versus micronized vaginal progesterone for luteal support in in vitro fertilization. Hum Reprod. 2017;32: 1019–1027. 10.1093/humrep/dex023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbott B.V. film-coated tablets 10 mg Summary of Product Characteristics. 2017. [Google Scholar]
- 15.Griesinger G, Blockeel C, Tournaye H. Oral dydrogesterone for luteal phase support in fresh in vitro fertilization cycles: a new standard? Fertil Steril. 2018;109: 756–762. 10.1016/j.fertnstert.2018.03.034 [DOI] [PubMed] [Google Scholar]
- 16.Griesinger G, Tournaye H, Macklon N, Petraglia F, Arck P, Blockeel C, et al. Dydrogesterone: pharmacological profile and mechanism of action as luteal phase support in assisted reproduction. Reprod Biomed Online. 2019;38: 249–259. 10.1016/j.rbmo.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 17.Chakravarty BN, Shirazee HH, Dam P, Goswami SK, Chatterjee R, Ghosh S. Oral dydrogesterone versus intravaginal micronised progesterone as luteal phase support in assisted reproductive technology (ART) cycles: results of a randomised study. J Steroid Biochem Mol Biol. 2005;97: 416–420. 10.1016/j.jsbmb.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 18.Patki A, Pawar VC. Modulating fertility outcome in assisted reproductive technologies by the use of dydrogesterone. Gynecol Endocrinol. 2007;23 Suppl 1: 68–72. 10.1080/09513590701584857 [DOI] [PubMed] [Google Scholar]
- 19.Ganesh A, Chakravorty N, Mukherjee R, Goswami S, Chaudhury K, Chakravarty B. Comparison of oral dydrogestrone with progesterone gel and micronized progesterone for luteal support in 1,373 women undergoing in vitro fertilization: a randomized clinical study. Fertil Steril. 2011;95: 1961–1965. 10.1016/j.fertnstert.2011.01.148 [DOI] [PubMed] [Google Scholar]
- 20.Tomic V, Tomic J, Klaic DZ, Kasum M, Kuna K. Oral dydrogesterone versus vaginal progesterone gel in the luteal phase support: randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2015;186: 49–53. 10.1016/j.ejogrb.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 21.Saharkhiz N, Zamaniyan M, Salehpour S, Zadehmodarres S, Hoseini S, Cheraghi L, et al. A comparative study of dydrogesterone and micronized progesterone for luteal phase support during in vitro fertilization (IVF) cycles. Gynecol Endocrinol. 2016;32: 213–217. 10.3109/09513590.2015.1110136 [DOI] [PubMed] [Google Scholar]
- 22.Abdel-Hamid ME, Sharaf LH, Kombian SB, Diejomaoh FME. Determination of Dydrogesterone in Human Plasma by Tandem Mass Spectrometry: Application to Therapeutic Drug Monitoring of Dydrogesterone in Gynecological Disorders. Chroma. 2006;64: 287–292. 10.1365/s10337-006-0035-3 [DOI] [Google Scholar]
- 23.Zhu X, Ye H, Fu Y. Duphaston and human menopausal gonadotropin protocol in normally ovulatory women undergoing controlled ovarian hyperstimulation during in vitro fertilization/intracytoplasmic sperm injection treatments in combination with embryo cryopreservation. Fertil Steril. 2017;108: 505–512.e2. 10.1016/j.fertnstert.2017.06.017 [DOI] [PubMed] [Google Scholar]
- 24.Yu S, Long H, Chang HY-N, Liu Y, Gao H, Zhu J, et al. New application of dydrogesterone as a part of a progestin-primed ovarian stimulation protocol for IVF: a randomized controlled trial including 516 first IVF/ICSI cycles. Hum Reprod. 2018;33: 229–237. 10.1093/humrep/dex367 [DOI] [PubMed] [Google Scholar]
- 25.Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst. 1994;86: 829–835. 10.1093/jnci/86.11.829 [DOI] [PubMed] [Google Scholar]
- 26.Mol BW, Bossuyt PM, Sunkara SK, Garcia Velasco JA, Venetis C, Sakkas D, et al. Personalized ovarian stimulation for assisted reproductive technology: study design considerations to move from hype to added value for patients. Fertil Steril. 2018;109: 968–979. 10.1016/j.fertnstert.2018.04.037 [DOI] [PubMed] [Google Scholar]
- 27.Paulson RJ, Collins MG, Yankov VI. Progesterone pharmacokinetics and pharmacodynamics with 3 dosages and 2 regimens of an effervescent micronized progesterone vaginal insert. J Clin Endocrinol Metab. 2014;99: 4241–4249. 10.1210/jc.2013-3937 [DOI] [PubMed] [Google Scholar]
- 28.Cicinelli E, de Ziegler D, Bulletti C, Matteo MG, Schonauer LM, Galantino P. Direct transport of progesterone from vagina to uterus. Obstet Gynecol. 2000;95: 403–406. [DOI] [PubMed] [Google Scholar]
- 29.Gibbons WE, Toner JP, Hamacher P, Kolm P. Experience with a novel vaginal progesterone preparation in a donor oocyte program. Fertil Steril. 1998;69: 96–101. 10.1016/s0015-0282(97)00457-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The underlying data set has been publicly released via the following DOI: 10.6084/m9.figshare.8187413 (doi.org/10.6084/m9.figshare.8187413).