Abstract
Individuals treated for multidrug-resistant tuberculosis (MDR-TB) with aminoglycosides (AGs) in resource-limited settings often experience permanent hearing loss. However, AG ototoxicity has never been conceptually integrated or causally linked to MDR-TB patients’ pre-treatment health condition. We sought develop a framework that examines the relationships between pre-treatment conditions and AG-induced hearing loss among MDR-TB-infected individuals in sub-Saharan Africa.
The adverse outcome pathway (AOP) approach was used to develop a framework linking key events (KEs) within a biological pathway that result in adverse outcomes (AO), which are associated with chemical perturbation of a molecular initiating event (MIE). This AOP describes pathways initiating from AG accumulation in hair cells, sound transducers of the inner ear immediately after AG administration. After administration the drug catalyzes cellular oxidative stress due to overproduction of reactive oxygen species. Since oxidative stress inhibits mitochondrial protein synthesis, hair cells undergo apoptotic cell death—resulting in irreversible hearing loss (AO). We identified the following pre-treatment conditions that worsen the causal linkage between MIE and AO: HIV, malnutrition, aging, noise, smoking, and alcohol use. The KEs are: (1) nephrotoxicity, pre-existing hearing loss, and hypoalbuminemia that catalyzes AG accumulation; (2) immunodeficiency and antioxidant deficiency that trigger oxidative stress pathways; and (3) co-administration of mitochondrial toxic drugs that hinder mitochondrial protein synthesis, causing apoptosis.
This AOP clearly warrants the development of personalized interventions for patients undergoing MDR-TB treatment. Such interventions (i.e., choosing less ototoxic drugs, scheduling frequent monitoring, modifying nutritional status, avoiding poly-pharmacy) will be required to limit the burden of AG ototoxicity.
Keywords: aminoglycoside, ototoxicity, sensorineural hearing loss, tuberculosis
INTRODUCTION
Despite decades of effort to eradicate Mycobacterium tuberculosis (M.tb.), ~2.7 million people have been diagnosed with tuberculosis (TB) in sub-Saharan Africa—26% of the total global incidence of TB in 2015 (WHO 2016a). Particularly, multidrug-resistant TB (MDR-TB) has emerged as a global epidemic and results in significant mortality (WHO 2016a). Because MDR-TB is resistant to the powerful first-line regimens (i.e., rifampicin and isoniazid), second-line antimicrobials are used to treat this infection. Up to now, second-line regimens for MDR-TB have consisted of one injectable drug along with four or more oral anti-TB drugs. The most widely used injectable drug is an aminoglycoside (AG) given during the first phase of treatment (at least 4 months) (WHO 2016b). While the U.S. Food and Drug Administration approved AGs are gentamicin, tobramycin, amikacin, kanamycin, capreomycin, streptomycin, neomycin, and paromomycin for the treatment of serious infections caused by aerobic gram-negative bacilli, amikacin and kanamycin are the most frequently prescribed AGs globally for MDR-TB treatment recommended by the World Health Organization (WHO) (WHO 2016b).
One of the most debilitating adverse outcomes from long-term use of AGs is ototoxicity. Up to 69% of individuals with MDR-TB infection in sub-Saharan Africa experience hearing loss (Hong et al. 2018). This high incidence is almost 2-3 times higher than in high-resource countries, such as the U.S. (13%) (Marks et al. 2014), the Netherlands (18%) (de Jager and van Altena 2002), and the U.K. (28%) (Sturdy et al. 2011). AG ototoxicity appears to significantly contribute to hair cell injury, damaging both the cochlear and vestibular apparatus of the inner ear (Huth et al. 2011) (Figure 1). Typical manifestations of cochleotoxicity consist of tinnitus and/or hearing loss, which begins with high-frequency hearing loss, which may or may not be clinically apparent, and often progresses to more severe hearing loss even after discontinuation of AGs; while those of vestibulotoxicity include disequilibrium and dizziness with occasional nausea and vomiting (Ariano et al. 2008; Huth et al. 2011; Jiang et al. 2017). Vestibulocochlear impairment, moreover, can be permanent. While hearing loss is one of the most common and debilitating adverse outcomes of AGs from MDR-TB treatment, strategies to reduce the risk such as selection of less ototoxic antibiotics or systematic monitoring of hearing loss are limited in many TB programs or clinical settings in sub-Saharan Africa due to cost and constraints on human resources for health care. Compared to less-toxic antibiotics, AGs are extremely inexpensive relative to the high potency they offer (Gonzalez and Spencer 1998; Huth et al. 2011; Krause et al. 2016). Financial considerations may, in part, explain the higher incidence of AG-induced hearing loss in resource-limited countries compared to high-resource countries. However, HIV co-infection, which is substantially more common in many low resource settings may also play a major role (Hong et al. 2018). Presently, there are no practical and cost-effective tools to identify those at highest risk for developing hearing loss from AG treatment. To avoid the unnecessary occurrence of this adverse outcome and to guide clinical decision-making, it is critical to assess individuals’ potential risk for ototoxicity before initiation of MDR-TB regimen.
Figure 1.
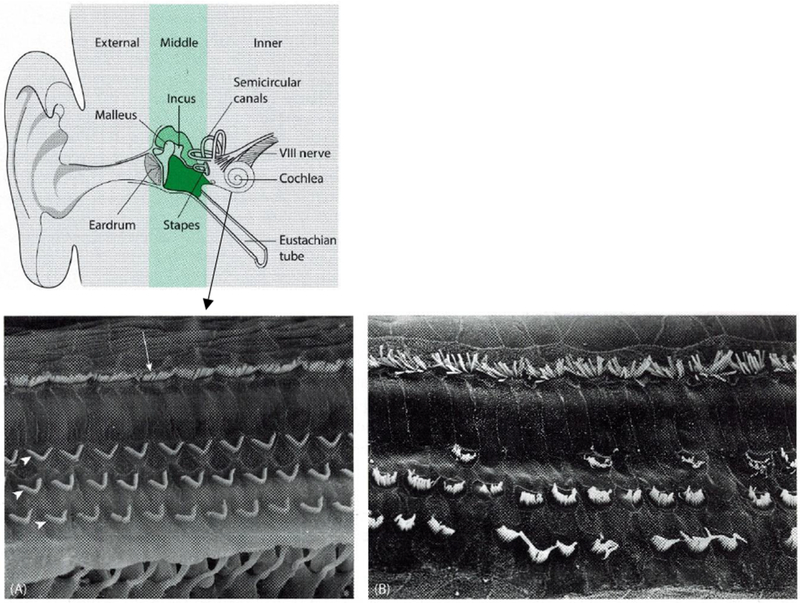
Anatomy of inner ear and hair cells
(A) Electron micrograph of normal outer (arrowheads) and inner (arrow) cochlear hair cells; (B) Electron micrograph of damaged cochlear hair cells. This illustration and image were adapted with permission from Taylor & Francis Group, LLC. (Tysome JR 2016)
The Adverse Outcome Pathway (AOP) is a conceptual framework representing a set of plausible connections from an initiating event to an adverse outcome considered relevant in risk assessment in predictive toxicology (Ankley et al. 2010; OECD 2012). The framework consists of conceptual constructs and depicts existing knowledge concerning the predictive and/or causal linkages between drug initiation, physiological and molecular responses, and organ and organism-level responses (OECD 2012). Since the AOP includes integrated sequential pathways, it is often used to develop integrated tools for predictive toxicology, regulatory toxicity testing, and risk assessment (OECD 2012; Vinken 2013). Despite examination of the mechanisms of AG-induced hearing loss at a cellular level, AG-induced hearing loss has never been conceptually integrated or causally linked to MDR-TB patients’ pre-treatment health condition, which may play a pivotal role in aggravating the ototoxicity pathway. Therefore, this study aimed to develop a framework that examines the relationships between pre-treatment conditions and AG ototoxicity among MDR-TB-infected individuals in sub-Saharan Africa.
MATERIAL AND METHODS
AOP Development
The conceptual framework development process was guided by the Organization for Economic Co-operation and Development’s (OECD) Guidance on Developing and Assessing the Completeness of Adverse Outcomes Pathways (OECD 2012; OECD 2013). AOP methodology shares a common schematic representation consisting of a molecular initiating event (MIE), intermediate key events (KE), and an adverse outcome (OECD 2012). MIE is defined as a chemical interaction with a biological target (OECD 2012). In this study, the MIE refers to AG molecular accumulation in the interstitium of hair cells in the inner ear that initiates the toxicity pathway. The MIE is associated with a set of potential apical hazard endpoints (OECD 2012), but the AG-induced sensorineural hearing loss (SNHL) is the apical adverse outcome of interest in this pathway. The MIE and adverse outcomes are causally linked with a series of KEs that are direct chemical effects or responses initiated from or prior to the target sites through the cellular or higher levels of biological organization, scientifically proven by in vitro and/or ex vivo studies (OECD 2012). In this pathway, three key events were identified: (1) KE1, defined as prerequisite events that directly impact MIE highlighted in orange arrows; (2) KE 2, defined as prerequisite events that impact initial cellular responses highlighted in green arrows; and (3) KE 3, defined as prerequisite events that impact latter cellular responses highlighted in a purple arrow in the proposed AOP framework (Figure 3). To evaluate whether scientific qualitative and quantitative data precisely support a causal relationship between observed outcomes and a given chemical, Weight-of-Evidence supporting the AOP was assessed by modified Bradford Hill Criteria per OECD guidelines (Hill 1965; OECD 2012). Institutional Review Board approval was not required for this study as human subjects were not involved in the research.
RESULTS
MDR-TB treatment.
Second-line injectable AGs that have been recommended by the WHO for MDR-TB treatment include amikacin, kanamycin and streptomycin (WHO 2016b); however, streptomycin is no longer considered a second-line agent because it was previously widely used for TB retreatment, and MDR-TB strains are more likely to be resistant to streptomycin than the other aminoglycosides (WHO 2016b). The selection of amikacin versus kanamycin for providers and organizations is determined by the likelihood of effectiveness, availability, and cost (WHO 2016b).
AG – Mechanism of Action.
AGs are highly potent and broad-spectrum bactericidal agents used for the treatment of serious gram-negative bacteria or mycobacteria including M.tb. (Mingeot-Leclercq et al. 1999). Their primary site of action is the 30S ribosomal subunit (Avent et al. 2011; Mingeot-Leclercq et al. 1999). To reach the site, molecules cross the bacterial cell wall through active transport into the cell cytosol; thereby they inhibit bacterial protein synthesis which results from misreading of the genetic code (Avent et al. 2011; Keene et al. 1982; Mingeot-Leclercq et al. 1999; Pagkalis et al. 2011). AGs have very poor oral bioavailability because they are highly polar cations. Only 0.3–1.5% of an orally or rectally administered dose of aminoglycoside reaches the systemic circulation and then appears in the urine (Pagkalis et al. 2011). Thus the route of AG administration is intravenous (IV), intramuscular (IM), intraosseous (IH), topical (cream/ointment), and ophthalmic. AGs are water-soluble and freely filtered across the glomerulus; almost all of the drug is then excreted (Avent et al. 2011; Blot et al. 2014).
MIE – Molecular interactions.
Although AGs preferentially target the bacterial ribosome, the inner ear and kidney are known to receive collateral damage (Huth et al. 2011). The mechanisms of AG uptake into sensory hair cells and renal epithelial cells increase the susceptibility to both ototoxicity and nephrotoxicity, which can be explained by the physiological similarities between the cochlea and kidney in terms of active transport of fluid and electrolytes to achieve iso-osmotic balance (Jiang et al. 2017). The accumulation of AGs appears to be dose- and duration-dependent, and uptake into the inner ear occurs rapidly and exposures persist for longer than other organs. In animal studies, AGs enter the cochlea within a few minutes and hair cells within 3 hours after systemic administration (Dai et al. 2006; Imamura and Adams 2003; Wang and Steyger 2009). AG concentrations in the inner ear are higher than plasma concentrations because the half-life of AGs in perilymph fluid are 10 to 15 times longer than in serum (Mörike et al. 1997). The receptor-mediated endocytosis at the apical surface of hair cells in the cochlea plays a role in AG uptake—AG molecules are found in vesicles beneath the hair cells (Hashino and Shero 1995). AGs are also taken up into the renal epithelial cell line via an endocytotic process, which explains the nephrotoxicity after glomerular filtration of the agent (Hashino and Shero 1995). Along with endocytosis, the presence of several ion channels at the hair cells, such as the mechanoelectrical transducer (MET) cation channel quickens AG accumulation. The MET channel increases the potential differences between extracellular fluid and cytoplasm and functions like a one-way valve, promoting the likelihood of cellular uptake and accumulation of cationic AGs in the cytoplasm in the hair cells and renal cells (Cernada et al. 2014; Farris et al. 2004; Marcotti et al. 2005). Consequently, AG molecules accumulate rapidly and are eliminated slowly from the inner ear; thus, hair cells are more susceptible to AG-related processes than other cell types.
Cellular and Organ Responses.
Reactive oxygen species (ROS) are byproducts of normal mitochondrial metabolism; ROS contribute to organ homeostasis by controlling normal cell growth, differentiation, development, and death (Benhar et al. 2002; Zorov et al. 2014). TB infection induces ROS production through activation of phagocytes—a part of host defense mechanism against M.tb. (Rajopadhye et al. 2017). Further, the AG molecules that enter hair cells readily bind to cytosolic proteins, specifically calreticulin, which plays a role in Ca2+ homeostasis (Karasawa et al. 2010). AG binding to calreticulin dysregulates cytosolic Ca2+ concentration (Krause and Michalak 1997), which in turn induces mitochondrial Ca2+ overload, producing cytoplasmic ROS and causing mitochondrial oxidation (Esterberg et al. 2016). In addition, since AGs act as iron chelators, the formation of redox-active iron-AG complexes catalyzes oxygen-derived free radicals (Priuska and Schacht 1995; Sha and Schacht 1999b). Thus, ROS overproduction with exhaustion of the capacity of the intrinsic protective and repair system results in oxidative stress (Murphy 2013; Zorov et al. 2014). Moreover, the Transient Receptor Potential (TRP) cation channels—particularly the subfamily TRPA1 containing pore helices—are located in the outer hair cells. The TRPA1 channels function as inflammatory, irritant, and oxidative stress sensors (Henderson et al. 2006; Lesniak et al. 2005). Activation of TRPA1 channels resulting from oxidative stress or noise-exposure, enlarges the pore diameter to dimensions that are larger than AG molecules, thereby facilitating AG uptake into the hair cell (Stepanyan et al. 2011). Oxidative stress contributes to mitochondrial depolarization and dysfunction, and mitochondrial protein synthesis inhibition, which in turn activates programmed cell death-signaling pathways, such as mitogen-activated protein kinases (MAPK) (Abi-Hachem et al. 2010; Hyde and Rubel 1995; Murphy 2013; Op de Beeck et al. 2011; Shokolenko et al. 2009). Consequently, hair cells along with ancillary sensory cells and neurons—mainly the cochlear portion of the auditory nerve—undergo apoptotic cell death, resulting in irreversible SNHL (Clerici et al. 1996; Hirose et al. 1997; Priuska and Schacht 1995; Sha and Schacht 1999b).
Key Event 1: Prerequisite events that directly impact MIE
Nephrotoxicity
Individuals with renal impairment may experience decreased AG clearance and increased AG accumulation, as AGs are mostly eliminated by glomerular filtration. As a result, sustained and excessive peak serum concentrations are considered a risk factor for hearing loss. AGs are also nephrotoxic; renal function at treatment initiation directly influences the level of AG accumulation in hair cells. Thus ototoxicity can be caused by AG toxic levels and/or renal impairment, which leads to reduced AG clearance and more drug accumulation (Huth et al. 2011). Comorbid conditions that influence renal function directly or indirectly through chronic use of nephrotoxic drugs would induce AG ototoxicity. A common example in sub-Saharan Africa is HIV co-infection. Renal complications of HIV infection are common and include proteinuria, interstitial nephritis, renal tubular damage, and nephrolithiasis; HIV-associated nephropathy, coupled with use of nephrotoxic antiretroviral drugs such as Nucleoside Reverse Transcriptase Inhibitors (NRTIs) in particular, leading to excessive AG accumulation (Calza et al. 2011; Jin et al. 2015; Kenyon et al. 2011; Kohler et al. 2009; Scherzer et al. 2012).
Pre-existing hearing loss
Pre-existing hearing loss at MDR-TB diagnosis commonly originates from previous exposure to ototoxic drugs, noise exposure, advanced age, or idiopathic SNHL (Tysome JR 2016). Particularly, because acoustic stimuli increase permeability of cation channels such as MET and TRP, the noise exposure also increases the AG uptake and directly accelerates intracellular accumulation of AGs within hair cells (Hayashida et al. 1985; Park et al. 2018; Ricci et al. 2005). Age-related hearing loss, or presbycusis, caused by the degeneration of cochlear cells is also a major cause of pre-existing hearing loss (Hu et al. 2018). As tissue ages, the hair cells also undergo progressive oxidative mitochondrial DNA damage modified by excessive ROS generation and chronic inflammatory damage due to immunosenescence (Cui et al. 2012; Iwai et al. 2008; Kalinec et al. 2017; Lee 2013; Saitoh et al. 1994). This results in auditory sensory cell degeneration. Further, HIV can cause pre-existing hearing loss directly and indirectly. It has been found that a primary HIV infection in either the central nervous system or peripheral auditory nerve causes SNHL, although the exact mechanism of nervous destruction is still unclear (Bankaitis and Keith 1995). A human study did not find histopathologic changes using electron microscopy supporting that HIV directly damages the cochlear end organs (Chandrasekhar et al. 1992). However, a recent observational study found that HIV infected adults had significantly poorer hearing threshold in both low and high frequencies than HIV uninfected adults (Torre et al. 2015a). Opportunistic infections are one of the common indirect causes of pre-existing hearing loss. The most frequent otologic opportunistic infections found in HIV infected individuals include seborrheic dermatitis of the external ear, otitis externa with otomycosis, and serous otitis media (Prasad et al. 2006; Rzewnicki et al. 2012). Because these infections are mostly caused by community-acquired organisms, such as Pseudomonas aeruginosa, Aspergillus, fumigatus, Candida albicans in outer ear and Streptococcus pneumoniae, Hemophilus influenzae and Moraxella catarrhalis in middle ear (Prasad et al. 2006; Rzewnicki et al. 2012), frequently recurrent acute or chronic ear infections lead to conductive hearing loss before or during AG treatment (Ivanov et al. 2017). Due to lack of trained healthcare providers or devices, it is difficult to confirm SNHL by differentiating it from conductive hearing loss by comprehensive audiological assessment, including otoscopy, tympanometry, and air-bone conduction audiometry in most countries in sub-Saharan Africa (Hong et al. 2018). Thus, underdiagnosed ear infections may masquerade as AG-induced hearing loss, and undertreated ear infections aggravate oxidative stress from altered metabolic pathways (Ivanov et al. 2017). While otosyphilis is a rare complication of syphilis, it is not an uncommon cause of inner ear infection in people living with HIV. A clinical manifestation of acute syphilis with cochleovestibular involvement includes sudden SNHL (Weder et al. 2013); otosyphilis amplifies oxidative stress but thereby may magnify symptoms of AG-induced hearing loss. Otosyphilis seems to lead to endolymphatic hydrops in the cochlea and/or atrophy of the organ of Corti, spiral ganglion, and stria vascularis (Miller et al. 2010) (Figure 2), which may reduce endocochlear potential, resulting in cochlear sensitivity to sound. Also, because HIV drugs, particularly NRTIs—including zidovudine, didanosine, stavudine, lamivudine—have ototoxic potential via their effect of reducing mitochondrial DNA content, use of NRTIs prior to MDR-TB treatment may potentiate the ototoxic effect of AGs (Simdon et al. 2001a; Torre et al. 2015b). This association has been specified in key event 3 (Figure 3).
Figure 2.
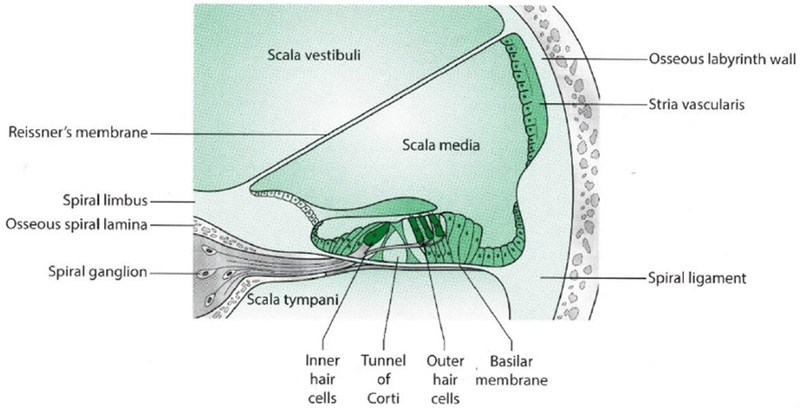
Cross-sectional view of the cochlear duct
Diagram not to relative scale. This illustration was adapted with permission from Taylor & Francis Group, LLC. (Tysome JR 2016)
Figure 3.
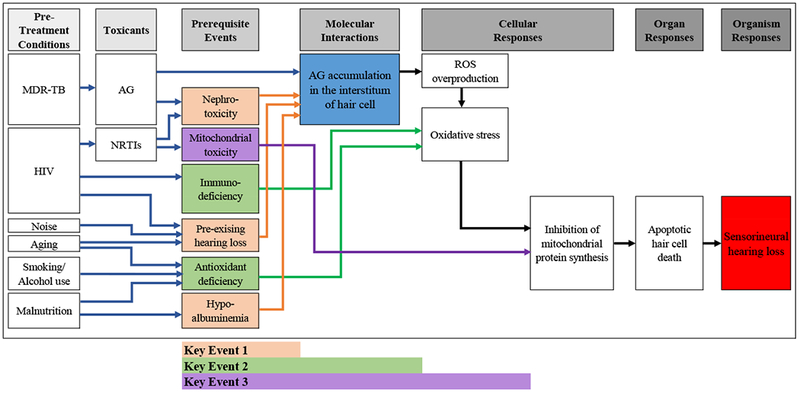
Conceptual framework of Adverse Outcome Pathway on AG ototoxicity in MDR-TB treatment
Abbreviations: AG= aminoglycoside; AO= adverse outcome; HIV= human immunodeficiency virus; KE= key event; MDR-TB= multidrug-resistant tuberculosis; MIE= molecular initiating event; mt= mitochondrial; NRTI= Nucleoside Reverse Transcriptase Inhibitor; ROS= reactive oxygen species; SNHL= sensorineural hearing loss
Hypoalbuminemia
Malnutrition—an insufficiency or unbalance of nutrition (Kelly et al. 1999)—is a significant health issue in people living with MDR-TB with or without HIV (Ivers et al. 2009; WHO 2003b) and is more prominent in resource-limited environments due to food insecurity (HealthyPeople.gov ; WHO 2012). Malnutrition is a result of a deficiency of both macronutrients (nutrients that provide calories or energy, including carbohydrates, proteins, and fat) and micronutrients (i.e., vitamins and minerals), vital dietary components necessary for physical and mental development, disease prevention, and well-being (de Pee and Semba 2010; WHO 2003a). Most individuals with active TB are in a catabolic state and experience weight loss and signs of vitamin and mineral deficiencies (Mohamed-Hussein et al. 2016). Protein-energy malnutrition (PEM) caused by insufficient intake of protein and calories is more prominent among TB and HIV co-infected patients and is worsened by TB-induced muscle wasting (Anema et al. 2009; de Pee and Semba 2010; Hood 2013; Koethe et al. 2009; van Lettow et al. 2003). In the case of PEM, albumin synthesis is impaired, leading to low serum albumin concentration (i.e., hypoalbuminemia) (Bisaso et al. 2014; van Lettow et al. 2003). Since albumin plays a pivotal role in maintaining colloid oncotic pressure, hypoalbuminemia results in abnormal increase of inner ear fluid volume by diminishing the osmotic gradient (Kim et al. 2017; Kim et al. 2011), accelerating AG accumulation because AG is water-soluble (Blot et al. 2014).
Key Event 2: Prerequisite events that impact initial cellular responses
Immunodeficiency
HIV infection weakens the human immune system by killing T-helper cells, macrophages, and dendritic cells, thus causing immunodeficiency (Freed and Martin 2007). HIV infection leads to chronic activation of nuclear factor (NF)-κB—a master regulator of pro-inflammatory genes, which produces pro-inflammatory cytokines, such as interleukin 1 (IL-1), IL-6, and tumor necrosis factor-alpha (TNF-α) (Fiume et al. 2012). Soon after secretion of pro-inflammatory cytokines, neutrophils and other immune cells migrate to the infection site in various cell types depending on opportunistic infections including TB, where they ingest bacterium and kill them by releasing ROS, which causes oxidative stress and mitochondrial DNA damage (Rajopadhye et al. 2017). Further, HIV-infected individuals with advanced disease have increased levels of oxidative DNA damage biomarkers (i.e., 7,8-dihydro-8-oxoguanine) in CD4+ T cells and show declines in DNA glycosylase activity for the repair of oxidative base lesions in these cells (Aukrust et al. 2005). In addition, the number of CD4+ cells is positively associated with the levels of intracellular concentration of antioxidants, especially glutathione (Staal et al. 1992; Wanchu et al. 2009). In particular, people living with HIV who are not taking ART may have increased risk of AG ototoxicity since ART restores the numbers of CD4+ T-cells while it augments the imbalanced redox status (Elias et al. 2013).
Antioxidant deficiency
Antioxidant deficiency causes hair cells to be more vulnerable to oxidative stress, which contributes to apoptotic hair cell death. For example, the presence of glutathione—an endogenous antioxidant resulting in detoxification of xenobiotics and protection against ROS (Marí et al. 2009)—protects the hair cells against oxidative stress (Garetz et al. 1994; Nishida and Takumida 1996). Several studies also found that dietary nutrient-based antioxidant supplementation, including vitamin A, β-carotene (one of the provitamin A carotenoids), vitamin C, and vitamin E, significantly attenuated outer hair cell damage, as they have anti-inflammatory properties (Aladag et al. 2016; Clerici et al. 1996; Le Prell et al. 2014). Albumin also has antioxidant properties through its multiple binding sites and capacity to trap free radicals (Roche et al. 2008). Thus, hypoalbuminemia also worsens antioxidant deficiency. Antioxidant deficiency is caused not only by poor intake of dietary sources but also by smoking, alcohol consumption, and aging which inhibit synthesis of antioxidant enzymes and reduce antioxidant concentrations (Albano 2006; de Grey 1999; Donohue 2006; Isik et al. 2007; Khare et al. 2014; Lemasters 2005; Sitar et al. 2013).
Key Event 3: Prerequisite events that impact latter cellular responses
Mitochondrial toxicity
Use of mitochondrial toxic drugs may potentially worsen AG ototoxicity. NRTIs can inhibit human DNA polymerases, including gamma polymerase—important to mtDNA replication—that may damage mtDNA; key event 3 may also directly activate the MAPK pathway (Marra et al. 1997; McNaghten et al. 2001; Simdon et al. 2001b). In particular, Tenofovir Disoproxil Fumarate (TDF) is one of the most common choices of NRTI; however, it targets the mitochondria of both hair cells and renal proximal tubules, increasing the risk of ototoxicity, as does key event 1. Since the combination of two NRTIs constitutes the backbone of ART regimens (Bartlett et al. 2012), individuals on both NRTIs and AGs are at higher risk of apoptotic hair cell death.
Assessment and Confidence Testing
Bradford Hill Criteria consist of 6 items, evaluating the concordance, strength, consistency, and specificity of associations between conceptual constructs within AOP, as well as the biological plausibility and coherence of experimental evidence (OECD 2012). To achieve confidence in the proposed AOP, 5 items were addressed to evaluate the mechanistic understanding of biological systems (OECD 2012).
Assessment of the AOP According to the Bradford Hill Criteria (Hill 1965)
Concordance of dose-response relationships
AG-induced ototoxicity occurs in basal outer hair cells and then extends to inner hair cells and further apical outer hair cells with increasing cumulative AG dose (Jiang et al. 2017). Many classic laboratory animal studies have revealed that AG-induced vestibulocochlear toxicity ranges over duration and levels of exposure (Aran and Darrouzet 1975; Lenoir and Puel 1987; Michaels 2012; Wersall and Hawkins 1962). Cochleotoxicity was tested in response to a range of amikacin doses in adult rats for 5 consecutive days (Lenoir and Puel 1987). They found that hair-cell stereocilia degeneration occurred in the high-dose group (i.e., 600-1000 mg/day),while the low-dose group did not develop cochlear abnormalities (i.e., 200 mg/day) (Lenoir and Puel 1987). In addition, the pattern of hair cell degeneration—most severe in the basal regions of the cochlea with decreasing gradient towards the apex—was dependent on the administered dose of amikacin (Lenoir and Puel 1987). Streptomycin also causes vestibulotoxicity in a dose-response manner. Vestibular disturbance was observed in cats 12-19 days after receiving a low-dose of streptomycin (100-200 mg/kg daily); however, the cats that received 400 mg/kg became ataxic shortly after administration of the first dose, which persisted for almost 24 hours (Wersall and Hawkins 1962).
Temporal concordance among the key events and adverse outcome
The temporal relationship among the three key events are dependent on the pre-treatment conditions that are present. Each pre-treatment factor may influence multiple key events that occurs in sequential order. Prerequisite events are mediated by presence of pre-treatment factors with or without exposure to toxicants, which preceded AG accumulation in the interstitium of hair cell (MIE). Since AG accumulation is an essential prerequisite for apoptosis of hair cells, the temporal sequence from pre-treatment conditions through AG induced SNHL is well supported.
Strength, consistency, and specificity of association of adverse outcome and initiating event
We explained that AG-induced SNHL is caused by the oxidative stress that results from excessive AG accumulation in the hair cells (MIE). The causality of this pathway can be inversely proven by the following experimental and clinical studies that tested protective effects by targeting various steps of the ototoxic cascades:
-
(1)
Reducing AG uptake: Evidence for the molecular identity of the MET channel strongly supports the potential modification of MET channel permeability, reducing AG uptake (Corns et al. 2017; Farris et al. 2004; Kirkwood et al. 2017).
-
(2)
Iron chelators and antioxidants as ROS scavengers: Attenuated hair cell apoptosis capacity have been confirmed by administration of iron chelators or antioxidative agents, such as salicylates (Lecain et al. 2007; Sha and Schacht 1999a), deferoxamine (Mostafa et al. 2007), N-Acetylcysteine (Aladag et al. 2016; Garcia-Alcantara et al. 2017), D-Methionine (Campbell et al. 2016; Fox et al. 2016), α-lipoic acid (Wang et al. 2012), ascorbic acid (vitamin C) (Le Prell et al. 2014; Wu et al. 2015), α-tocopherol (vitamin E) (Fetoni et al. 2004; Le Prell et al. 2014), magnesium (Le Prell et al. 2014), and misoprostol (Dogan et al. 2017).
-
(3)
Inhibiting the MAPK pathway: Inhibition of the MAPK pathway by application of D-JNKI-1 (Wang et al. 2003), CEP 11004 (Bodmer et al. 2002), and estradiol (Nakamagoe et al. 2010) prior to AG administration resulted in significant protection from hair cell death in vitro and hearing loss in vivo.
Biological plausibility, coherence, and consistency of the experimental evidence
The biological plausibility, coherence, consistency, and strength of the experimental evidence that supports the proposed AOP is detailed in Table 1.
Table 1.
Summary of Information on the Key Events of the AOP
| Key Events | Description of Events | Experimental Support and References |
|---|---|---|
| MIE: AG accumulation in the inner ear hair cell | The receptor-mediated endocytosis and presence of MET cation channel lead to rapid accumulation and slow elimination of AG in the inner ear | (1) KM was taken up into sensory hair cells via receptor-mediated endocytosis at their apical surfaces because AG molecules are found in vesicles beneath the hair cells from White Leghorn chicks, confirmed by immuno-gold electron microscopy (Hashino and Shero 1995; Richardson et al. 1997) |
| (2) MET channels on hair cell functions as open transducer channels that is the main route for aminoglycoside entry. AGs functioned as voltage-dependent MET channel blockers that also rapidly permeate through MET channels into hair cells, which was found in bullfrog model (Steyger et al. 2003), turtle model (Farris et al. 2004), and mouse model (Marcotti et al. 2005). The AG molecules enter the channel and block the ion-conducting pathway, thus such blockage increases voltage. Increased AG entry through the channel pore into the hair cell due to the large electrical driving force also increases the affinity for the blocker. This boosts both the entry of AG into the channel and the channel’s affinity for the drug (Farris et al. 2004; Marcotti et al. 2005; Steyger et al. 2003). | ||
| KE 1: Prerequisite events impacting MIE | Nephrotoxicity (KE1-1), hypoalbuminemia (KE1-2), and pre-existing hearing loss (KE1-3) accelerate AG accumulation in the interstitum of hair cell | (1) TDF is mitochondrial toxic, increasing number of abnormal mitochondria including irregular mitochondrial shape, and sparse, fragmented cristae. Abnormal proximal tubule functioning and decreased GFR occurred in patients who had been taking TDF in multiple studies (Calza et al., 2011; Jin et al., 2015; Kenyon et al., 2011; Kohler et al., 2009; Scherzer et al., 2012). |
| (2) The albumin-like proteins, including albumin, IgG, IgA, transferrin, antitrypsin, and haptoglobin were the major protein compositions of luminal fluid in inner ear (Kim et al. 2017; Kim et al. 2011). Among patients (n=11) with enlarged vestibular aqueducts, patients with recent hearing loss and increased volume of luminal fluid showed a significantly decreased proportion of the albumin-like proteins in the interstitial space (Kim et al. 2011). | ||
| (3) Pre-existing hearing loss includes mainly noise-induced and age-related hearing loss. Histological evaluation using mice (n=22), received repetitive exposure the acoustic stimuli (~4.5 kHz with 120.5 dB sound pressure level), showed significant structural hair cell damage (Park et al., 2018). It has been found that atrophy of the stria vascularis of cochlear duct was observed in older mice (older than 12 months at least), and that lipofuscin (aging associated pigment granules) accumulation in inner and outer hair cells of the mice were also found from the same age (Saitoh et al. 1994). This structural change is accelerated by the age-related dysfunctions of the systemic immune system accelerated, worsening presbycusis (Cui et al. 2012; Iwai et al. 2008; Kalinec et al. 2017; Lee 2013; Saitoh et al. 1994). | ||
| KE 2: Prerequisite events impacting initial cellular responses | Immunodeficiency (KE2-1) and antioxidant deficiency (KE2-2) trigger cellular oxidative stress | (1) In comparison between 8 HIV-infected patients (mean CD4+ T-cells count = 280 × 106/L), 7 AIDS patients (mean CD4+ T-cells count = 45 × 106/L), AIDS patients had increased levels of 7,8-dihydro-8-oxoguanine in CD4+ T cells and marked declines in DNA glycosylase activity for the repair of oxidative base lesions in these cells (Aukrust et al. 2005). People living with HIV showed elevated pro-inflammatory cytokines, including interleukin 2 (IL-2), IL-6, and tumor necrosis factor-alpha (TNF-α) and biomarkers associated with inflammation and coagulation, including C-reactive protein (CRP) and D-dimer due to chronic inflammation and immune activation (Abrams et al. 2009; Baker et al. 2010; Deeks et al. 2013; Neuhaus et al. 2010). In cross-sectional human study, the level of lipid peroxidation (LPO) and glutathione were used as a means of determining oxidative stress. The mean LPO levels were significantly higher in HIV-infected patients (n=100; mean= 0.7 ± 0.1 μmol/ml) as compared to healthy controls (n=30; mean= 0.3 ± 0.1 μmol/ml). The mean glutathione level in HIV-infected patients (0.06 ± 0.01 μmol/ml) was significantly lower in compared to healthy controls (0.09 ± 0.01 μmol/ml) (Staal et al. 1992; Wanchu et al. 2009). |
| (2) A human study found that compare to younger control subjects, elderly subjects had significantly lower level of glutathione (2.08 ± 0.12 vs. 1.12 ± 0.18 mmol/L RBCs; P < 0.05); glutathione synthesis rates (1.73 ± 0.16 vs. 0.55 ± 0.12 mmol/L RBCs per day; P < 0.01); and higher plasma oxidative stress (304 ± 16 vs. 346 ± 20 Carratelli units; P < 0.05) simultaneously (Sekhar et al. 2011). This indicates that glutathione deficiency in elderly humans resulted from a marked reduction in synthesis, related to oxidative stress. | ||
| KE 3: Prerequisite events impacting latter cellular responses | Mitochondrial toxicity (KE3) worsens inhibition of mitochondrial protein synthesis of hair cells | Since reductions in mitochondrial DNA content induced by NRTIs, significantly more HIV-infected patients had or developed persistent hearing loss with/without tinnitus during follow-up (Marra et al. 1997; McNaghten et al. 2001; Simdon et al. 2001a). After short-term exposure to AZT, d4T, ddC, ddI, and FLT (6-72 hours), mtDNA copy numbers were markedly decreased because the NRTIs inhibit mtDNA replication (Smith et al. 2017). Upregulation of glutathione S-transferase 4 expression were significantly increased, which suggests that ROS defense mechanisms likely to be induced by NRTI administration due to mtDNA intoxication (Smith et al. 2017). |
| AO: SNHL | When programmed cell death-signaling pathways has been activated, hair cells, ancillary sensory cells, and neurons undergo apoptotic cell death, resulting in irreversible SNHL | ROS formation through ototoxicants, including gentamicin and kanamycin, in cochlear tissues of was directly observed in guinea pig by electron paramagnetic resonance spectrometry (Clerici et al. 1996) and in chick by using dichlorofluorescin (Hirose et al. 1997). When chicks and mouse cochlear and vestibular hair cells were exposed to gentamicin, the incorporation of methionine-free medium over 24 hours was reduced by 30–60% compared to control conditions observed by fluorescence microscopy (Francis et al. 2013). This indicates gentamicin inhibited the medium uptake into hair cells by inhibiting protein synthesis in hair cells and activate a c-Jun N-terminal kinase (JNK) pathway as JNKs activate apoptotic signaling (Francis et al. 2013). |
Abbreviations: MIE= molecular initiating event; KE= key event; AO= adverse outcome; HIV= human immunodeficiency virus; CD4= cluster of differentiation 4; SNHL= sensorineural hearing loss; MET= mechanoelectrical transducer; NRTI= Nucleoside Reverse Transcriptase Inhibitor; KM= kanamycin; TDF= Tenofovir disoproxil Fumarate; ROS= reactive oxygen species; AZT= zidovudine (3’-Azido-3’-deoxythymidine); d4T= stavudine (2’,3’-didehydro-2’,3’-deoxythymidine); ddC= zalcitabine (2’,3’-dideoxycytidine); ddI= didanosine (2’,3’-dideoxyinosine); FLT= alovudine (3’-deoxy-3’-fluorothymidine)
Alternative mechanism(s) that logically present themselves and the extent to which they may distract from the postulated AOP
The mechanism of AG-induced ototoxicity with hearing loss is less understood. However, one potential alternative hypothesis is the presence of N-methyl-D-aspartate (NMDA) at the synapse between cochlear hair cells and spiral ganglion neural afferents (Basile et al. 1996; Puel et al. 1991). At NMDA receptors, AG mimics the positive modulation of polyamines, potentially leading to excitotoxic damage at the hair cell-afferent nerve synapses (Choi 1992). Since hair cell apoptosis resulted from ROS overproduction is a significant modifiable pathogenesis of AG ototoxicity, our AOP did not include mechanisms of NMDA receptors, and thereby separate AOP could depict such alternative mechanism.
Uncertainties, inconsistencies and data gaps
Assessments of human tissue from patients with MDR-TB, with or without HIV co-infection, for evidence of AG-related pathophysiology have not been conducted for obvious ethical reasons. Although AG ototoxicity has been comprehensively studied, the major events within the proposed AOP have been causally explained by healthy preclinical animal models, while AG has mostly been administered to those with severe infections in clinical settings. However, a recent animal study has induced systemic host-mediated inflammatory conditions by injecting lipopolysaccharide (LPS), an important component of bacterial endotoxin to experimental mice (Hirose et al. 2014; Koo et al. 2015). While LPS alone did not affect hearing, mice that received LPS prior to ototoxic agents had worse hearing loss than those that did not receive LPS pretreatment resulted from accelerated AG uptake (Hirose et al. 2014; Koo et al. 2015). Such animal studies are unable to fill the gap entirely, but evidence from preclinical work supports the hypothesis that persistent inflammation contributes to AG ototoxicity.
Confidence in the AOP
How well-characterized is the AOP?
AG-induced ototoxicity is a well-understood phenomenon. We adapted the Mitochondrial Free Radical Theory of Aging to explain the relationship between AG molecules and active free radicals, which are generally produced in the organism, at the cellular level (de Grey 1999). Such relationship is supported by experimental data, as specified in Table 1.
How well are the initiating and other key events causally linked to the outcome?
Multiple experiments demonstrated that AGs are causally linked to SNHL in a dose-dependent way in both animals and humans. Evidence is strong to support a causal relationship between each key event and SNHL.
What are the limitations in the evidence in support of the AOP?
There are unmeasurable variables that may confound the relationship outlined in the AOP, such as known and unknown genetic mutations or additional confounders we may not have thought of. Specifically, mtDNA mutation is a risk factor that may be considered as one of the pre-treatment conditions as several genetic mutations also increase the susceptibility to ototoxicity. The mitochondrial rRNA mutation, particularly in the 12S rRNA gene, such as A1555G (most common), C1494T, T1095C, T1291C, 961delT+C(n), and A827G, among others, increase the structural similarity of human mitochondrial ribosomal RNA (rRNA) to bacterial 16S rRNA (Hamasaki and Rando 1997; Hobbie et al. 2008; Prezant et al. 1993). As a result, mutated mitochondrial ribosomes in the cochlea become target-binding sites for AGs (Cox et al. 1964; Davies et al. 1965), and AGs lead to misreading of the genetic code along with perturbation of ribosomal translation (Hamasaki and Rando 1997; Hobbie et al. 2008). This causes mitochondrial ribosomal damage and further cytotoxicity as it directly activates the MAPK pathway with apoptosis (Benhar et al. 2001; Owens et al. 2007; Son et al. 2013; Wang et al. 2003). The most common type of mitochondrial A1555G gene mutation is most prevalent in Europeans (0.19%) (Bitner-Glindzicz et al. 2009; Vandebona et al. 2009) but not in Sub-Saharan Africans, where the prevalence of the mutation is extremely low (0% to 0.09%) (Bosch et al. 2014; Kabahuma et al. 2011; Lasisi et al. 2014; Wonkam et al. 2015). As a result, mtDNA mutation was not addressed and generalizability is limited because this model targets evidence obtained within the Sub-Saharan African MDR-TB populations and in resource-limited settings. To date, numerous experimental studies in this area are ongoing, so new evidence may change this AOP.
Is the AOP specific to certain tissues, life stages/age classes?
Advanced age may increase the risk for AG ototoxicity. Presbycusis is difficult to characterize because of genetic and environmental influences, and because of its complexity of structural changes confounded by various medical, psychological, and pharmacologic factors (Patel and McKinnon 2018). However, presbycusis is also caused by apoptotic hair cell death resulting from excessive oxidative cellular stress, which in turn stimulates the MAPK pathway. Age-dependent renal function is also closely related to this pathway because AG elimination is mostly completed through renal clearance. Age-related reduction in creatinine clearance among elderly populations increase the risk of ototoxicity (Fraisse et al. 2014; Weinstein and Anderson 2010). The glomerular filtration rate is low at birth, reaches about adult levels by the end of the second year of life, and declines after the fourth decade (Weinstein and Anderson 2010). Thus, infants, young children, and the elderly are more susceptible to AG-induced SNHL, but this AOP is developed targeting adult populations.
Are the initiating and key events expected to be conserved across taxa?
Experimental studies in multiple types of animals across species, including zebrafishes (Kim et al. 2018; Wang and Steyger 2009), bullfrogs (Dai et al. 2006), chicks (Dai et al. 2006; Hashino and Shero 1995), mice (Bächinger et al. 2018; Corns et al. 2017; Dai et al. 2006; Hirose et al. 2014; Koo et al. 2015; Yu et al. 2018), rats (Garcia-Alcantara et al. 2017; Ladrech et al. 2017; Lenoir and Puel 1987), turtles (Farris et al. 2004), cats (Wersall and Hawkins 1962), and guinea pigs (Aran and Darrouzet 1975; Bareggi et al. 1990; Campbell et al. 2016; Dai et al. 2006; Garetz et al. 1994; Hayashida et al. 1985; Imamura and Adams 2003; Tunstall et al. 1995)—all show evidence in support of this pathway. Human autopsies have also shown this relationship (Keene et al. 1982; Nordstrom et al. 1990).
DISCUSSION
Overall, AG ototoxicity caused by apoptotic hair cell death is a complex process, although our understanding of it has increased in recent years. Based on the modified Bradford Hill Criteria, we believe this AOP provides critical, evidence-based insights into AG-induced hearing loss. AG-induced hearing loss prevention in TB programs is a real challenge due to complicated clinical conditions, and the causal relationship between treatment and adverse outcomes is often difficult or impossible to determine definitively. Although maintaining therapeutic, but not supra-therapeutic, AG concentration aids in hearing loss prevention and cure of MDR-TB, frequent therapeutic drug monitoring (TDM) is impractical in most resource-limited settings. While the causative genomic variants have been studied to determine the phenotype-genotype correlations with AG-induced hearing loss (Alford et al. 2014), genetic services are not available in many clinical settings as a screening tool. As there are no practical screening tools to aid in the prevention of ototoxicity, knowing the mechanism of AG ototoxicity and its linkage with pre-treatment physical conditions associated with MDR-TB is critical for designing strategies to prevent AG-induced irreversible SNHL.
This is the first attempt to develop an AOP framework that outlines the apoptotic cascade in AG toxicity. This AOP framework will broaden our understanding of the complexity of AG-induced hearing loss and interactive health conditions in individuals before and after AG exposure. Such schematic representations can be used as a tool for healthcare providers to make clinical decisions, particularly in developing personalized interventions, such as choosing less ototoxic drugs or scheduling more frequent toxicity monitoring. The proposed AOP can be favorably applied not only in clinical practice but also widely in public health research as it is helpful in hypothesizing the relationships between different covariates associated with drug-induced adverse outcomes. Examples of clinical implications and recommendations based on the key elements and contributors to hearing loss are summarized in Table 2.
Table 2.
AOP implications and recommendations in MDR-TB treatment
| Pre-treatment conditions or prerequisite events | Recommendations |
|---|---|
| Untreated HIV and/or NRTI use | • Monitor CD4+ T-cell count and viral load. • Consider NRTI-sparing antiretroviral regimen. • Monitor oto- and nephro-toxicity more closely. |
| Renal insufficiency | • Monitor renal function more closely including BUN, creatinine (serum or urine), and creatinine clearance, etc. • If HIV-infected, consider NRTI-sparing antiretroviral regimen and avoid other nephrotoxic agents |
| Antioxidant deficiency Hypoalbuminemia |
• Provide dietary counseling. • Consider macro- and micronutrient supplementation. • Monitor serum albumin level more closely. |
| Pre-existing SNHL | • Conduct comprehensive audiologic evaluations including occupational/recreational noise exposure, family history of ototoxicity or hearing loss, audiometry, tympanometry, and otoscopy prior to AG initiation. • If moderate to severe hearing loss screened consider AG-sparing MDR-TB regimen or more frequent systematic audiologic evaluations should be followed. |
| Substance abuse | • Provide alcoholism and smoking cessation counseling and rehabilitation |
Abbreviations: AG= aminoglycoside; CD4= cluster of differentiation 4; HIV= human immunodeficiency virus; SNHL= sensorineural hearing loss; BUN= blood urea nitrogen; MDR-TB= multidrug-resistant tuberculosis; NRTI= Nucleoside Reverse Transcriptase Inhibitor
Since AG ototoxicity is concentration-dependent, AG dose and use should be tightly regulated in inpatient settings, with serial measurement of creatinine and estimation of creatinine clearance coupled with TDM, which is a measurement of aminoglycoside peaks and troughs, and adjustment of dosing to remain in the targeted therapeutic ranges (Avent et al. 2011). In outpatient settings or home-visiting programs; however, optimizing AG dosing is considerably challenging because TDM is unavailable in real time. As a result, detection of ototoxicity could be delayed because cochlear damage is initially asymptomatic. Thus, future research should consider to develop a surrogate measure of AG concentration without laboratory testing and examine its practical feasibility in resource limited environment.
Although the proposed AOP is theoretically and practically useful, application is limited to MDR-TB treatment in resource-limited settings particularly in sub-Saharan Africa because this study does not take account for genetic variance. Furthermore, we acknowledge that the proposed AOP oversimplifies the complex pharmacopathological and pharmacotoxicological process, which did not capture all potential mechanisms. Since this AOP was developed based on currently available scientific evidence, it must be considered an open and flexible framework that requires continuous refinement. There is a need for well-designed and adequately powered observational studies to identify the risk factors for AG ototoxicity that are present at MDR-TB treatment initiation and during treatment, through thorough history taking and frequent hearing screening. Since polypharmacy is common among people with MDR-TB and HIV (Alomar 2014), future studies may be helpful in elucidating drug-drug interactions and drug-gene interactions with AG and would be a good scientific addition to understanding and prevention of AG-induced hearing loss. Continuous attention to the prevention of AG-induced hearing loss during MDR-TB treatment is critical not only in resource-limited settings but also as global policy.
ACKNOWLEDGEMENTS
Research reported in this manuscript was funded by the National Institute of Allergy and Infectious Disease (R01 AI104488-01A1 to J. Farley), the National Institute of Nursing Research (F31 NR016910-01A1 to H. Hong) of the National Institutes of Health, Sigma Theta Tau International Global Nursing Research Grant, Sigma Theta Tau International/Association of Nurses in AIDS Care Grant, Global Korean Nursing Foundation Scientific Award, Dr. Scholl Foundation Dissertation Scholarship, the Johns Hopkins Center for Global Health Established Field Placements Grant. We would like to express our appreciation to Martin Blair for his editorial support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the aforementioned organizations/institutions. The authors declare that they have no conflict of interest.
References
- Abi-Hachem RN, Zine A, Van De Water TR (2010) The injured cochlea as a target for inflammatory processes, initiation of cell death pathways and application of related otoprotectives strategies. Recent patents on CNS drug discovery 5(2):147–63 [DOI] [PubMed] [Google Scholar]
- Aladag I, Guven M, Songu M (2016) Prevention of gentamicin ototoxicity with N-acetylcysteine and vitamin A. The Journal of laryngology and otology 130(5):440–6 doi: 10.1017/s0022215116000992 [DOI] [PubMed] [Google Scholar]
- Albano E (2006) Alcohol, oxidative stress and free radical damage. The Proceedings of the Nutrition Society 65(3):278–90 [DOI] [PubMed] [Google Scholar]
- Alford RL, Arnos KS, Fox M, et al. (2014) American college of medical genetics and genomics guideline for the clinical evaluation and etiologic diagnosis of hearing loss. Genetics in Medicine 16(4):347–355 doi: 10.1038/gim.2014.2 [DOI] [PubMed] [Google Scholar]
- Alomar MJ (2014) Factors affecting the development of adverse drug reactions (Review article). Saudi pharmaceutical journal : SPJ : the official publication of the Saudi Pharmaceutical Society 22(2):83–94 doi: 10.1016/j.jsps.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anema A, Vogenthaler N, Frongillo EA, Kadiyala S, Weiser SD (2009) Food insecurity and HIV/AIDS: current knowledge, gaps, and research priorities. Current HIV/AIDS reports 6(4):224–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankley GT, Bennett RS, Erickson RJ, et al. (2010) Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environmental toxicology and chemistry 29(3):730–41 doi: 10.1002/etc.34 [DOI] [PubMed] [Google Scholar]
- Aran JM, Darrouzet J (1975) Observation of click-evoked compound VIII nerve responses before, during, and over seven months after kanamycin treatment in the guinea pig. Acta oto-laryngologica 79(1-2):24–32 [DOI] [PubMed] [Google Scholar]
- Ariano RE, Zelenitsky SA, Kassum DA (2008) Aminoglycoside-induced vestibular injury: maintaining a sense of balance. The Annals of pharmacotherapy 42(9):1282–9 doi: 10.1345/aph.1L001 [DOI] [PubMed] [Google Scholar]
- Aukrust P, Luna L, Ueland T, et al. (2005) Impaired base excision repair and accumulation of oxidative base lesions in CD4+ T cells of HIV-infected patients. Blood 105(12):4730–5 doi: 10.1182/blood-2004-11-4272 [DOI] [PubMed] [Google Scholar]
- Avent ML, Rogers BA, Cheng AC, Paterson DL (2011) Current use of aminoglycosides: indications, pharmacokinetics and monitoring for toxicity. Internal medicine journal 41(6):441–9 doi: 10.1111/j.1445-5994.2011.02452.x [DOI] [PubMed] [Google Scholar]
- Bächinger D, Horvath L, Eckhard A, et al. (2018) Neuronal erythropoietin overexpression is protective against kanamycin-induced hearing loss in mice. Toxicology Letters 291:121–128 doi: 10.1016/j.toxlet.2018.04.007 [DOI] [PubMed] [Google Scholar]
- Bankaitis AE, Keith RW (1995) Audiological changes associated with HIV infection. Ear, nose, & throat journal 74(5):353–9 [PubMed] [Google Scholar]
- Bareggi R, Grill V, Narducci P, Zweyer M, Tesei L, Russolo M (1990) Gentamicin ototoxicity: histological and ultrastructural alterations after transtympanic administration. Pharmacological research 22(5):635–44 [DOI] [PubMed] [Google Scholar]
- Bartlett J, Gallant J, Pham P (2012) Medical Management of HIV infection. Knowledge Source Solutions, LLC., Durham, NC [Google Scholar]
- Basile AS, Huang JM, Xie C, Webster D, Berlin C, Skolnick P (1996) N-methyl-D-aspartate antagonists limit aminoglycoside antibiotic-induced hearing loss. Nature medicine 2(12):1338–43 [DOI] [PubMed] [Google Scholar]
- Benhar M, Dalyot I, Engelberg D, Levitzki A (2001) Enhanced ROS production in oncogenically transformed cells potentiates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase activation and sensitization to genotoxic stress. Molecular and cellular biology 21(20):6913–26 doi: 10.1128/mcb.21.20.6913-6926.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhar M, Engelberg D, Levitzki A (2002) ROS, stress-activated kinases and stress signaling in cancer. EMBO reports 3(5):420–5 doi: 10.1093/embo-reports/kvf094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaso KR, Owen JS, Ojara FW, et al. (2014) Characterizing plasma albumin concentration changes in TB/HIV patients on anti retroviral and anti –tuberculosis therapy. In Silico Pharmacology 2:3 doi: 10.1186/s40203-014-0003-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitner-Glindzicz M, Pembrey M, Duncan A, et al. (2009) Prevalence of mitochondrial 1555A-->G mutation in European children. The New England journal of medicine 360(6):640–2 doi: 10.1056/NEJMc0806396 [DOI] [PubMed] [Google Scholar]
- Blot SI, Pea F, Lipman J (2014) The effect of pathophysiology on pharmacokinetics in the critically ill patient--concepts appraised by the example of antimicrobial agents. Advanced drug delivery reviews 77:3–11 doi: 10.1016/j.addr.2014.07.006 [DOI] [PubMed] [Google Scholar]
- Bodmer D, Brors D, Bodmer M, Ryan AF (2002) Rescue of auditory hair cells from ototoxicity by CEP-11 004, an inhibitor of the JNK signaling pathway. Laryngo- rhino- otologie 81(12):853–6 doi: 10.1055/s-2002-36100 [DOI] [PubMed] [Google Scholar]
- Bosch J, Lebeko K, Nziale JJ, Dandara C, Makubalo N, Wonkam A (2014) In search of genetic markers for nonsyndromic deafness in Africa: a study in Cameroonians and Black South Africans with the GJB6 and GJA1 candidate genes. Omics : a journal of integrative biology 18(7):481–5 doi: 10.1089/omi.2013.0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calza L, Trapani F, Tedeschi S, et al. (2011) Tenofovir-induced renal toxicity in 324 HIV-infected, antiretroviral-naive patients. Scandinavian journal of infectious diseases 43(8):656–60 doi: 10.3109/00365548.2011.572906 [DOI] [PubMed] [Google Scholar]
- Campbell KC, Martin SM, Meech RP, Hargrove TL, Verhulst SJ, Fox DJ (2016) D-methionine (D-met) significantly reduces kanamycin-induced ototoxicity in pigmented guinea pigs. Int J Audiol 55(5):273–8 doi: 10.3109/14992027.2016.1143980 [DOI] [PubMed] [Google Scholar]
- Cernada M, Pérez-Aytes A, Vento M, Millán JM (2014) The Genetics of Aminoglycoside-Related Deafness. NeoReviews 15(10):e449–e457 doi: 10.1542/neo.15-10-e449 [DOI] [Google Scholar]
- Chandrasekhar SS, Siverls V, Sekhar HK (1992) Histopathologic and ultrastructural changes in the temporal bones of HIV-infected human adults. The American journal of otology 13(3):207–14 [PubMed] [Google Scholar]
- Choi DW (1992) Excitotoxic cell death. Journal of neurobiology 23(9):1261–76 doi: 10.1002/neu.480230915 [DOI] [PubMed] [Google Scholar]
- Clerici WJ, Hensley K, DiMartino DL, Butterfield DA (1996) Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hearing research 98(1-2):116–24 [DOI] [PubMed] [Google Scholar]
- Corns LF, Jeng JY, Richardson GP, Kros CJ, Marcotti W (2017) TMC2 Modifies Permeation Properties of the Mechanoelectrical Transducer Channel in Early Postnatal Mouse Cochlear Outer Hair Cells. Frontiers in molecular neuroscience 10:326 doi: 10.3389/fnmol.2017.00326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox EC, White JR, Flaks JG (1964) STREPTOMYCIN ACTION AND THE RIBOSOME. Proceedings of the National Academy of Sciences of the United States of America 51:703–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Kong Y, Zhang H (2012) Oxidative stress, mitochondrial dysfunction, and aging. Journal of signal transduction 2012:646354 doi: 10.1155/2012/646354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai CF, Mangiardi D, Cotanche DA, Steyger PS (2006) Uptake of fluorescent gentamicin by vertebrate sensory cells in vivo. Hearing research 213(1-2):64–78 doi: 10.1016/j.heares.2005.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J, Anderson P, Davis BD (1965) Inhibition of protein synthesis by spectinomycin. Science (New York, NY) 149(3688):1096–8 [DOI] [PubMed] [Google Scholar]
- de Grey ADNJ (1999) The Mitochondrial Free Radical Theory of Aging. R.G. Landes Company, Austin, Texas, U.S.A. [Google Scholar]
- de Jager P, van Altena R (2002) Hearing loss and nephrotoxicity in long-term aminoglycoside treatment in patients with tuberculosis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease 6(7):622–7 [PubMed] [Google Scholar]
- de Pee S, Semba RD (2010) Role of nutrition in HIV infection: review of evidence for more effective programming in resource-limited settings. Food and nutrition bulletin 31(4):S313–44 [PubMed] [Google Scholar]
- Dogan M, Polat H, Yasar M, et al. (2017) Protective role of misoprostol in prevention of gentamicin ototoxicity. International journal of pediatric otorhinolaryngology 96:140–144 doi: 10.1016/j.ijporl.2017.03.023 [DOI] [PubMed] [Google Scholar]
- Donohue JF (2006) Ageing, smoking and oxidative stress. Thorax 61(6):461–462 doi: 10.1136/thx.2005.053058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias A, Nelson B, Oputiri D, Geoffrey OBP (2013) Antiretroviral toxicity and oxidative stress. American Journal of Pharmacology and Toxicology 8(4):187–196 doi: 10.3844/ajptsp.2013.187.196 [DOI] [Google Scholar]
- Esterberg R, Linbo T, Pickett SB, et al. (2016) Mitochondrial calcium uptake underlies ROS generation during aminoglycoside-induced hair cell death. The Journal of clinical investigation 126(9):3556–66 doi: 10.1172/jci84939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris HE, LeBlanc CL, Goswami J, Ricci AJ (2004) Probing the pore of the auditory hair cell mechanotransducer channel in turtle. The Journal of physiology 558(Pt 3):769–92 doi: 10.1113/jphysiol.2004.061267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetoni AR, Sergi B, Ferraresi A, Paludetti G, Troiani D (2004) alpha-Tocopherol protective effects on gentamicin ototoxicity: an experimental study. Int J Audiol 43(3):166–71 [DOI] [PubMed] [Google Scholar]
- Fiume G, Vecchio E, De Laurentiis A, et al. (2012) Human immunodeficiency virus-1 Tat activates NF-kappaB via physical interaction with IkappaB-alpha and p65. Nucleic acids research 40(8):3548–62 doi: 10.1093/nar/gkr1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox DJ, Cooper MD, Speil CA, et al. (2016) d-Methionine reduces tobramycin-induced ototoxicity without antimicrobial interference in animal models. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society 15(4):518–30 doi: 10.1016/j.jcf.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraisse T, Gras aygon C, Paccalin M, et al. (2014) Aminoglycosides use in patients over 75 years old. Age and Ageing 43(5):676–681 doi: 10.1093/ageing/afu023 [DOI] [PubMed] [Google Scholar]
- Freed EO, Martin MA (2007) HIVs and their replication. Lippincott, Williams & Wilkins, Philadelphia, PA, USA [Google Scholar]
- Garcia-Alcantara F, Murillo-Cuesta S, Pulido S, et al. (2017) The expression of oxidative stress response genes is modulated by a combination of resveratrol and N-acetylcysteine to ameliorate ototoxicity in the rat cochlea. Hearing research 358:10–21 doi: 10.1016/j.heares.2017.12.004 [DOI] [PubMed] [Google Scholar]
- Garetz SL, Altschuler RA, Schacht J (1994) Attenuation of gentamicin ototoxicity by glutathione in the guinea pig in vivo. Hearing research 77(1-2):81–7 [DOI] [PubMed] [Google Scholar]
- Gonzalez LS 3rd, Spencer JP (1998) Aminoglycosides: a practical review. American family physician 58(8):1811–20 [PubMed] [Google Scholar]
- Hamasaki K, Rando RR (1997) Specific binding of aminoglycosides to a human rRNA construct based on a DNA polymorphism which causes aminoglycoside-induced deafness. Biochemistry 36(40):12323–8 doi: 10.1021/bi970962r [DOI] [PubMed] [Google Scholar]
- Hashino E, Shero M (1995) Endocytosis of aminoglycoside antibiotics in sensory hair cells. Brain research 704(1):135–40 [DOI] [PubMed] [Google Scholar]
- Hayashida T, Nomura Y, Iwamori M, Nagai Y, Kurata T (1985) Distribution of gentamicin by immunofluorescence in the guinea pig inner ear. Archives of oto-rhino-laryngology 242(3):257–64 [DOI] [PubMed] [Google Scholar]
- HealthyPeople.gov Social Determinants of Health. In. https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health
- Henderson D, Bielefeld EC, Harris KC, Hu BH (2006) The role of oxidative stress in noise-induced hearing loss. Ear and hearing 27(1):1–19 doi: 10.1097/01.aud.0000191942.36672.f3 [DOI] [PubMed] [Google Scholar]
- Hill AB (1965) The Environment and Disease: Association or Causation? Proceedings of the Royal Society of Medicine 58(5):295–300 [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Hockenbery DM, Rubel EW (1997) Reactive oxygen species in chick hair cells after gentamicin exposure in vitro. Hearing research 104(1–2):1–14 [DOI] [PubMed] [Google Scholar]
- Hirose K, Li S-Z, Ohlemiller KK, Ransohoff RM (2014) Systemic Lipopolysaccharide Induces Cochlear Inflammation and Exacerbates the Synergistic Ototoxicity of Kanamycin and Furosemide. JARO: Journal of the Association for Research in Otolaryngology 15(4):555–570 doi: 10.1007/s10162-014-0458-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie SN, Akshay S, Kalapala SK, Bruell CM, Shcherbakov D, Bottger EC (2008) Genetic analysis of interactions with eukaryotic rRNA identify the mitoribosome as target in aminoglycoside ototoxicity. Proceedings of the National Academy of Sciences of the United States of America 105(52):20888–93 doi: 10.1073/pnas.0811258106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Budhathoki C, Farley JE (2018) Increased risk of aminoglycoside-induced hearing loss in MDR-TB patients with HIV coinfection. The International Journal of Tuberculosis and Lung Disease 22(6):667–674 doi: 10.5588/ijtld.17.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood ML (2013) A narrative review of recent progress in understanding the relationship between tuberculosis and protein energy malnutrition. European journal of clinical nutrition 67(11):1122–8 doi: 10.1038/ejcn.2013.143 [DOI] [PubMed] [Google Scholar]
- Hu W, Wu J, Jiang W, Tang J (2018) MicroRNAs and Presbycusis. Aging and disease 9(1):133–142 doi: 10.14336/ad.2017.0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huth ME, Ricci AJ, Cheng AG (2011) Mechanisms of aminoglycoside ototoxicity and targets of hair cell protection. International journal of otolaryngology:937861 doi: 10.1155/2011/937861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde GE, Rubel EW (1995) Mitochondrial role in hair cell survival after injury. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 113(5):530–40 doi: 10.1177/019459989511300503 [DOI] [PubMed] [Google Scholar]
- Imamura S, Adams JC (2003) Distribution of gentamicin in the guinea pig inner ear after local or systemic application. Journal of the Association for Research in Otolaryngology : JARO 4(2):176–95 doi: 10.1007/s10162-002-2036-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isik B, Ceylan A, Isik R (2007) Oxidative stress in smokers and non-smokers. Inhalation toxicology 19(9):767–9 doi: 10.1080/08958370701401418 [DOI] [PubMed] [Google Scholar]
- Ivanov AV, Bartosch B, Isaguliants MG (2017) Oxidative Stress in Infection and Consequent Disease. Oxidative Medicine and Cellular Longevity 2017:3496043 doi: 10.1155/2017/3496043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivers LC, Cullen KA, Freedberg KA, Block S, Coates J, Webb P (2009) HIV/AIDS, Undernutrition and Food Insecurity. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 49(7):1096–1102 doi: 10.1086/605573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai H, Baba S, Omae M, Lee S, Yamashita T, Ikehara S (2008) Maintenance of systemic immune functions prevents accelerated presbycusis. Brain research 1208:8–16 doi: 10.1016/j.brainres.2008.02.069 [DOI] [PubMed] [Google Scholar]
- Jiang M, Karasawa T, Steyger PS (2017) Aminoglycoside-Induced Cochleotoxicity: A Review. Frontiers in cellular neuroscience 11:308 doi: 10.3389/fncel.2017.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Kim MH, Park JH, et al. (2015) The Incidence and Clinical Characteristics of Acute Serum Creatinine Elevation more than 1.5 mg/dL among the Patients Treated with Tenofovir/Emtricitabine-containing HAART Regimens. Infection & chemotherapy 47(4):239–46 doi: 10.3947/ic.2015.47.4.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabahuma RI, Ouyang X, Du LL, et al. (2011) Absence of GJB2 gene mutations, the GJB6 deletion (GJB6-D13S1830) and four common mitochondrial mutations in nonsyndromic genetic hearing loss in a South African population. International journal of pediatric otorhinolaryngology 75(5):611–7 doi: 10.1016/j.ijporl.2011.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinec GM, Lomberk G, Urrutia RA, Kalinec F (2017) Resolution of Cochlear Inflammation: Novel Target for Preventing or Ameliorating Drug-, Noise- and Age-related Hearing Loss. Frontiers in cellular neuroscience 11:192 doi: 10.3389/fncel.2017.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa T, Wang Q, David LL, Steyger PS (2010) CLIMP-63 is a gentamicin-binding protein that is involved in drug-induced cytotoxicity. Cell Death & Disease 1(11):e102 doi: 10.1038/cddis.2010.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene M, Hawke M, Barber HO, Farkashidy J (1982) Histopathological findings in clinical gentamicin ototoxicity. Archives of otolaryngology (Chicago, Ill : 1960) 108(2):65–70 [DOI] [PubMed] [Google Scholar]
- Kelly P, Musonda R, Kafwembe E, Kaetano L, Keane E, Farthing M (1999) Micronutrient supplementation in the AIDS diarrhoea-wasting syndrome in Zambia: a randomized controlled trial. AIDS (London, England) 13(4):495–500 [DOI] [PubMed] [Google Scholar]
- Kenyon C, Wearne N, Burton R, Meintjes G (2011) The Risks of Concurrent Treatment with Tenofovir and Aminoglycosides in Patients with HIV-Associated Tuberculosis Southern African journal of HIV medicine 12(1):43–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare M, Mohanty C, Das BK, Jyoti A, Mukhopadhyay B, Mishra SP (2014) Free radicals and antioxidant status in protein energy malnutrition. International journal of pediatrics 2014:254396 doi: 10.1155/2014/254396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CW, Han JH, Wu L, Choi JY (2018) microRNA-183 is Essential for Hair Cell Regeneration after Neomycin Injury in Zebrafish. Yonsei medical journal 59(1):141–147 doi: 10.3349/ymj.2018.59.1.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, McClave SA, Martindale RG, Miller KR, Hurt RT (2017) Hypoalbuminemia and Clinical Outcomes: What is the Mechanism behind the Relationship? The American surgeon 83(11):1220–1227 [DOI] [PubMed] [Google Scholar]
- Kim SH, Kim UK, Lee WS, et al. (2011) Albumin-like protein is the major protein constituent of luminal fluid in the human endolymphatic sac. PloS one 6(6):e21656 doi: 10.1371/journal.pone.0021656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood NK, O’Reilly M, Derudas M, et al. (2017) d-Tubocurarine and Berbamine: Alkaloids That Are Permeant Blockers of the Hair Cell’s Mechano-Electrical Transducer Channel and Protect from Aminoglycoside Toxicity. Frontiers in cellular neuroscience 11:262 doi: 10.3389/fncel.2017.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koethe JR, Chi BH, Megazzini KM, Heimburger DC, Stringer JS (2009) Macronutrient supplementation for malnourished HIV-infected adults: a review of the evidence in resource-adequate and resource-constrained settings. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 49(5):787–98 doi: 10.1086/605285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler JJ, Hosseini SH, Hoying-Brandt A, et al. (2009) Tenofovir renal toxicity targets mitochondria of renal proximal tubules. Laboratory investigation; a journal of technical methods and pathology 89(5):513–9 doi: 10.1038/labinvest.2009.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Quintanilla-Dieck L, Jiang M, et al. (2015) Endotoxemia-mediated inflammation potentiates aminoglycoside-induced ototoxicity. Science translational medicine 7(298):298ra118 doi: 10.1126/scitranslmed.aac5546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause KH, Michalak M (1997) Calreticulin. Cell 88(4):439–443 doi: 10.1016/S0092-8674(00)81884-X [DOI] [PubMed] [Google Scholar]
- Krause KM, Serio AW, Kane TR, Connolly LE (2016) Aminoglycosides: An Overview. Cold Spring Harbor Perspectives in Medicine 6(6):a027029 doi: 10.1101/cshperspect.a027029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladrech S, Eybalin M, Puel JL, Lenoir M (2017) Epithelial-mesenchymal transition, and collective and individual cell migration regulate epithelial changes in the amikacin-damaged organ of Corti. Histochemistry and cell biology 148(2):129–142 doi: 10.1007/s00418-017-1548-6 [DOI] [PubMed] [Google Scholar]
- Lasisi AO, Bademci G, Foster J 2nd, Blanton S, Tekin M (2014) Common genes for non-syndromic deafness are uncommon in sub-Saharan Africa: a report from Nigeria. International journal of pediatric otorhinolaryngology 78(11):1870–3 doi: 10.1016/j.ijporl.2014.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Ojano-Dirain C, Rudnick EW, et al. (2014) Assessment of nutrient supplement to reduce gentamicin-induced ototoxicity. Journal of the Association for Research in Otolaryngology : JARO 15(3):375–93 doi: 10.1007/s10162-014-0448-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecain E, Omri B, Behar-Cohen F, Tran Ba Huy P, Crisanti P (2007) The role of PKCzeta in amikacin-induced apoptosis in the cochlea: prevention by aspirin. Apoptosis : an international journal on programmed cell death 12(2):333–42 doi: 10.1007/s10495-006-0580-0 [DOI] [PubMed] [Google Scholar]
- Lee K-Y (2013) Pathophysiology of Age-Related Hearing Loss (Peripheral and Central). Korean Journal of Audiology 17(2):45–49 doi: 10.7874/kja.2013.17.2.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasters JJ (2005) Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Research 8(1):3–5 [DOI] [PubMed] [Google Scholar]
- Lenoir M, Puel JL (1987) Dose-dependent changes in the rat cochlea following aminoglycoside intoxication. II. Histological study. Hearing research 26(2):199–209 [DOI] [PubMed] [Google Scholar]
- Lesniak W, Pecoraro VL, Schacht J (2005) Ternary complexes of gentamicin with iron and lipid catalyze formation of reactive oxygen species. Chemical research in toxicology 18(2):357–64 doi: 10.1021/tx0496946 [DOI] [PubMed] [Google Scholar]
- Marcotti W, van Netten SM, Kros CJ (2005) The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. The Journal of physiology 567(Pt 2):505–521 doi: 10.1113/jphysiol.2005.085951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marí M, Morales A, Colell A, García-Ruiz C, Fernández-Checa JC (2009) Mitochondrial Glutathione, a Key Survival Antioxidant. Antioxidants & Redox Signaling 11(11):2685–2700 doi: 10.1089/ars.2009.2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks SM, Flood J, Seaworth B, et al. (2014) Treatment practices, outcomes, and costs of multidrug-resistant and extensively drug-resistant tuberculosis, United States, 2005–2007. Emerging infectious diseases 20(5):812–21 doi: 10.3201/eid2005.131037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra CM, Wechkin HA, Longstreth WT Jr., Rees TS, Syapin CL, Gates GA (1997) Hearing loss and antiretroviral therapy in patients infected with HIV-1. Archives of neurology 54(4):407–10 [DOI] [PubMed] [Google Scholar]
- McNaghten AD, Wan PC, Dworkin MS (2001) Prevalence of hearing loss in a cohort of HIV-infected patients. Archives of otolaryngology--head & neck surgery 127(12):1516–8 [PubMed] [Google Scholar]
- Michaels L (2012) Ear, Nose and Throat Histopathology In Ototoxic Damage to the Inner Ear. Springer-Verlag, Berlin, Heidelberg [Google Scholar]
- Miller ME, Makary C, Lopez IA, Ishiyama A (2010) Endolymphatic hydrops in otologic syphilis: a temporal bone study. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 31(4):681–6 doi: 10.1097/MAO.0b013e3181dbb7e4 [DOI] [PubMed] [Google Scholar]
- Mingeot-Leclercq MP, Glupczynski Y, Tulkens PM (1999) Aminoglycosides: activity and resistance. Antimicrobial agents and chemotherapy 43(4):727–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed-Hussein A, Salama S, Khalil M, Eid S (2016) Malnutrition in tuberculosis: value of fat-free mass and creatinine-height index. Egyptian Journal of Bronchology 10(1):58–63 doi: 10.4103/1687-8426.176790 [DOI] [Google Scholar]
- Mörike K, Schwab M, Klotz U (1997) Use of Aminoglycosides in Elderly Patients. Drugs & aging 10(4):259–277 doi: 10.2165/00002512-199710040-00003 [DOI] [PubMed] [Google Scholar]
- Mostafa BE, Tawfik S, Hefnawi NG, Hassan MA, Ismail FA (2007) The role of deferoxamine in the prevention of gentamicin ototoxicity: a histological and audiological study in guinea pigs. Acta oto-laryngologica 127(3):234–9 doi: 10.1080/00016480600794495 [DOI] [PubMed] [Google Scholar]
- Murphy MP (2013) Mitochondrial dysfunction indirectly elevates ROS production by the endoplasmic reticulum. Cell Metab 18(2):145–6 doi: 10.1016/j.cmet.2013.07.006 [DOI] [PubMed] [Google Scholar]
- Nakamagoe M, Tabuchi K, Uemaetomari I, Nishimura B, Hara A (2010) Estradiol protects the cochlea against gentamicin ototoxicity through inhibition of the JNK pathway. Hearing research 261(1–2):67–74 doi: 10.1016/j.heares.2010.01.004 [DOI] [PubMed] [Google Scholar]
- Nishida I, Takumida M (1996) Attenuation of aminoglycoside ototoxicity by glutathione. ORL; journal for oto-rhino-laryngology and its related specialties 58(2):68–73 [DOI] [PubMed] [Google Scholar]
- Nordstrom L, Ringberg H, Cronberg S, Tjernstrom O, Walder M (1990) Does administration of an aminoglycoside in a single daily dose affect its efficacy and toxicity? The Journal of antimicrobial chemotherapy 25(1):159–73 [DOI] [PubMed] [Google Scholar]
- OECD (2012) Proposal for a Template and Guidance on Developing and Assessing the Completeness of Adverse Outcome Pathways. In: Organisation for Economic Co-operation and Development; http://www.oecd.org/chemicalsafety/testing/49963554.pdf Accessed 1/17/2018 [Google Scholar]
- OECD (2013) Guidance document on developing and assessing adverse outcome pathways, Organisation for Economic Co-operation and Development. vol 184 Organisation for Economic Co-operations and Development, Paris, France [Google Scholar]
- Op de Beeck K, Schacht J, Van Camp G (2011) Apoptosis in acquired and genetic hearing impairment: the programmed death of the hair cell. Hearing research 281(1–2):18–27 doi: 10.1016/j.heares.2011.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens KN, Cunningham DE, MacDonald G, Rubel EW, Raible DW, Pujol R (2007) Ultrastructural analysis of aminoglycoside-induced hair cell death in the zebrafish lateral line reveals an early mitochondrial response. The Journal of comparative neurology 502(4):522–43 doi: 10.1002/cne.21345 [DOI] [PubMed] [Google Scholar]
- Pagkalis S, Mantadakis E, Mavros MN, Ammari C, Falagas ME (2011) Pharmacological considerations for the proper clinical use of aminoglycosides. Drugs 71(17):2277–94 doi: 10.2165/11597020-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Park YH, Chung J, Lee MY, Lee DY, Kim YH (2018) Cochlear Damage Caused by the Striking Noise of Titanium Head Golf Driver. Clinical and experimental otorhinolaryngology doi: 10.21053/ceo.2017.01669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, McKinnon BJ (2018) Hearing Loss in the Elderly. Clinics in geriatric medicine 34(2):163–174 doi: 10.1016/j.cger.2018.01.001 [DOI] [PubMed] [Google Scholar]
- Prasad HKC, Bhojwani KM, Shenoy V, Prasad SC (2006) HIV manifestations in otolaryngology. American Journal of Otolaryngology 27(3):179–185 doi: 10.1016/j.amjoto.2005.09.011 [DOI] [PubMed] [Google Scholar]
- Prezant TR, Agapian JV, Bohlman MC, et al. (1993) Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nature genetics 4(3):289–94 doi: 10.1038/ng0793-289 [DOI] [PubMed] [Google Scholar]
- Priuska EM, Schacht J (1995) Formation of free radicals by gentamicin and iron and evidence for an iron/gentamicin complex. Biochemical pharmacology 50(11):1749–52 [DOI] [PubMed] [Google Scholar]
- Puel JL, Ladrech S, Chabert R, Pujol R, Eybalin M (1991) Electrophysiological evidence for the presence of NMDA receptors in the guinea pig cochlea. Hearing research 51(2):255–64 [DOI] [PubMed] [Google Scholar]
- Rajopadhye SH, Mukherjee SR, Chowdhary AS, Dandekar SP (2017) Oxidative Stress Markers in Tuberculosis and HIV/TB Co-Infection. Journal of clinical and diagnostic research : JCDR 11(8):Bc24–bc28 doi: 10.7860/jcdr/2017/28478.10473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Kennedy HJ, Crawford AC, Fettiplace R (2005) The transduction channel filter in auditory hair cells. The Journal of neuroscience : the official journal of the Society for Neuroscience 25(34):7831–9 doi: 10.1523/jneurosci.1127-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E (2008) The antioxidant properties of serum albumin. FEBS letters 582(13):1783–7 doi: 10.1016/j.febslet.2008.04.057 [DOI] [PubMed] [Google Scholar]
- Rzewnicki I, Olszewska E, Rogowska-Szadkowska D (2012) HIV infections in otolaryngology. Medical Science Monitor : International Medical Journal of Experimental and Clinical Research 18(3):RA17–RA21 doi: 10.12659/MSM.882505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh Y, Hosokawa M, Shimada A, et al. (1994) Age-related hearing impairment in senescence-accelerated mouse (SAM). Hearing research 75(1-2):27–37 [DOI] [PubMed] [Google Scholar]
- Scherzer R, Estrella M, Li Y, et al. (2012) Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS (London, England) 26(7):867–75 doi: 10.1097/QAD.0b013e328351f68f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SH, Schacht J (1999a) Salicylate attenuates gentamicin-induced ototoxicity. Laboratory investigation; a journal of technical methods and pathology 79(7):807–13 [PubMed] [Google Scholar]
- Sha SH, Schacht J (1999b) Stimulation of free radical formation by aminoglycoside antibiotics. Hearing research 128(1-2):112–8 [DOI] [PubMed] [Google Scholar]
- Shokolenko I, Venediktova N, Bochkareva A, Wilson GL, Alexeyev MF (2009) Oxidative stress induces degradation of mitochondrial DNA. Nucleic acids research 37(8):2539–48 doi: 10.1093/nar/gkp100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simdon J, Watters D, Bartlett S, Connick E (2001a) Ototoxicity Associated with Use of Nucleoside Analog Reverse Transcriptase Inhibitors: A Report of 3 Possible Cases and Review of the Literature. Clinical Infectious Diseases 32(11):1623–1627 doi: 10.1086/320522 [DOI] [PubMed] [Google Scholar]
- Simdon J, Watters D, Bartlett S, Connick E (2001b) Ototoxicity associated with use of nucleoside analog reverse transcriptase inhibitors: a report of 3 possible cases and review of the literature. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 32(11):1623–7 doi: 10.1086/320522 [DOI] [PubMed] [Google Scholar]
- Sitar ME, Aydin S, Cakatay U (2013) Human serum albumin and its relation with oxidative stress. Clinical laboratory 59(9-10):945–52 [PubMed] [Google Scholar]
- Son Y, Kim S, Chung H-T, Pae H-O (2013) Chapter Two - Reactive Oxygen Species in the Activation of MAP Kinases In: Cadenas E, Packer L (eds) Methods in enzymology. vol 528 Academic Press, p 27–48 [DOI] [PubMed] [Google Scholar]
- Staal FJ, Ela SW, Roederer M, Anderson MT, Herzenberg LA, Herzenberg LA (1992) Glutathione deficiency and human immunodeficiency virus infection. Lancet (London, England) 339(8798):909–12 [DOI] [PubMed] [Google Scholar]
- Stepanyan RS, Indzhykulian AA, Velez-Ortega AC, et al. (2011) TRPA1-mediated accumulation of aminoglycosides in mouse cochlear outer hair cells. Journal of the Association for Research in Otolaryngology : JARO 12(6):729–40 doi: 10.1007/s10162-011-0288-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturdy A, Goodman A, Jose RJ, et al. (2011) Multidrug-resistant tuberculosis (MDR-TB) treatment in the UK: a study of injectable use and toxicity in practice. The Journal of antimicrobial chemotherapy 66(8):1815–20 doi: 10.1093/jac/dkr221 [DOI] [PubMed] [Google Scholar]
- Torre P 3rd, Hoffman HJ, Springer G, et al. (2015a) Hearing loss among HIV-seropositive and HIV-seronegative men and women. JAMA otolaryngology-- head & neck surgery 141(3):202–10 doi: 10.1001/jamaoto.2014.3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre P Iii, Hoffman HJ, Springer G, et al. (2015b) Hearing loss among hiv-seropositive and hiv-seronegative men and women. JAMA Otolaryngology–Head & Neck Surgery 141(3):202–210 doi: 10.1001/jamaoto.2014.3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall MJ, Gale JE, Ashmore JF (1995) Action of salicylate on membrane capacitance of outer hair cells from the guinea-pig cochlea. The Journal of physiology 485(Pt 3):739–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tysome JRKR (2016) Hearing: An Introduction & Practical Guide. CRC Press: Taylor & Francis Group, LLC, Baca Raton, FL [Google Scholar]
- van Lettow M, Fawzi WW, Semba RD (2003) Triple trouble: the role of malnutrition in tuberculosis and human immunodeficiency virus co-infection. Nutrition reviews 61(3):81–90 [DOI] [PubMed] [Google Scholar]
- Vandebona H, Mitchell P, Manwaring N, et al. (2009) Prevalence of mitochondrial 1555A-->G mutation in adults of European descent. The New England journal of medicine 360(6):642–4 doi: 10.1056/NEJMc0806397 [DOI] [PubMed] [Google Scholar]
- Vinken M (2013) The adverse outcome pathway concept: a pragmatic tool in toxicology. Toxicology 312:158–65 doi: 10.1016/j.tox.2013.08.011 [DOI] [PubMed] [Google Scholar]
- Wanchu A, Rana SV, Pallikkuth S, Sachdeva RK (2009) Short communication: oxidative stress in HIV-infected individuals: a cross-sectional study. AIDS research and human retroviruses 25(12):1307–11 doi: 10.1089/aid.2009.0062 [DOI] [PubMed] [Google Scholar]
- Wang A, Hou N, Bao D, Liu S, Xu T (2012) Mechanism of alpha-lipoic acid in attenuating kanamycin-induced ototoxicity. Neural regeneration research 7(35):2793–800 doi: 10.3969/j.issn.1673-5374.2012.35.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Van De Water TR, Bonny C, de Ribaupierre F, Puel JL, Zine A (2003) A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. The Journal of neuroscience : the official journal of the Society for Neuroscience 23(24):8596–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Steyger PS (2009) Trafficking of systemic fluorescent gentamicin into the cochlea and hair cells. Journal of the Association for Research in Otolaryngology : JARO 10(2):205–19 doi: 10.1007/s10162-009-0160-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weder S, Senn P, Caversaccio M, Vibert D (2013) Cochleovestibular Deficit as First Manifestation of Syphilis in a HIV-Infected Patient. Case Reports in Neurology 5(1):62–67 doi: 10.1159/000350574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JR, Anderson S (2010) THE AGING KIDNEY: PHYSIOLOGICAL CHANGES. Advances in chronic kidney disease 17(4):302–307 doi: 10.1053/j.ackd.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersall J, Hawkins JE Jr. (1962) The vestibular sensory epithelia in the cat labyrinth and their reactions in chronic streptomycin intoxication. Acta oto-laryngologica 54:1–23 [DOI] [PubMed] [Google Scholar]
- WHO (2003a) Nutrient requirements for people living with HIV/AIDS : report of a technical consultation. In: World Health Organization; https://apps.who.int/iris/bitstream/handle/10665/42853/9241591196.pdf?ua=1 Accessed 3/2/2018 [Google Scholar]
- WHO (2003b) Nutrient Requirements for People living with HIV/AIDS: Report of a technical consultation. World Health Organization; In: World Health Organization; http://www.who.int/nutrition/topics/hivaids/en/ [Google Scholar]
- WHO (2012) Social determinants of health: What are social determinants of health? World Health Organization; In. http://www.who.int/social_determinants/thecommission/finalreport/key_concepts/en/ [Google Scholar]
- WHO (2016a) Global tuberculosis report 2016. World Health Organization. World Health Organization, Geneva, Switzerland [Google Scholar]
- WHO (2016b) WHO treatment guidelines for drug-resistant tuberculosis, 2016 updates. World Health Organization. World Health Organization, Geneva, Switzerland [Google Scholar]
- Wonkam A, Bosch J, Noubiap JJ, Lebeko K, Makubalo N, Dandara C (2015) No evidence for clinical utility in investigating the connexin genes GJB2, GJB6 and GJA1 in non-syndromic hearing loss in black Africans. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde 105(1):23–6 [DOI] [PubMed] [Google Scholar]
- Wu CY, Lee HJ, Liu CF, Korivi M, Chen HH, Chan MH (2015) Protective role of L-ascorbic acid, N-acetylcysteine and apocynin on neomycin-induced hair cell loss in zebrafish. Journal of applied toxicology : JAT 35(3):273–9 doi: 10.1002/jat.3043 [DOI] [PubMed] [Google Scholar]
- Yu X, Fan Z, Han Y, et al. (2018) Paeoniflorin reduces neomycin-induced ototoxicity in hair cells by suppression of reactive oxygen species generation and extracellularly regulated kinase signalization. Toxicol Lett 285:9–19 doi: 10.1016/j.toxlet.2017.12.026 [DOI] [PubMed] [Google Scholar]
- Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiological reviews 94(3):909–50 doi: 10.1152/physrev.00026.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]


