To the Editor:
Pomalidomide is an oral immunomodulatory drug with antitumoral efficacy against relapsed or refractory multiple myeloma. It binds moderately (16%−42%) to plasma proteins.1 It is mainly eliminated by cytochrome-mediated hydroxylation in the liver.2 Its high plasma free fraction and low molecular weight (273 g/mol) make it susceptible to glomerular filtration. Despite that, <4% of the dose is excreted unchanged in urine,3 suggesting that substantial tubular reabsorption may occur.4 Pomalidomide elimination does not seem to be substantially affected by moderately decreased kidney function,2 but data from patients with advanced kidney failure are limited and clinical trials regarding its disposition in this subpopulation are ongoing. In theory, pomalidomide could be significantly removed by hemodialysis because this technique replaces glomerular filtration without affecting further reabsorption. The FDA recently published updated pomalidomide dosage recommendations for patients requiring dialysis, advising a 25% dose reduction (ie, 3 mg/d). Per the manufacturer, there is an increase in pomalidomide’s AUC (of 38%) and the rate of severe adverse events (by 64%) in dialysis patients.1
Multiple myeloma is frequently associated with alterations in kidney function, and high-cutoff (HCO) dialysis is increasingly used in this condition to ensure blood purification and remove nephrotoxic light chains. However, data regarding pomalidomide disposition during HCO dialysis are lacking. HCO dialysis implies longer, more frequent sessions, using higher filter permeability for substances in the molecular weight range of 15 to 45 kDa, and requiring albumin substitution; it may therefore lead to higher drug elimination compared to traditional intermittent hemodialysis. We report the pharmacokinetics of pomalidomide during HCO dialysis in a patient with multiple myeloma and kidney failure.
A 59-year-old man with refractory IgGκ multiple myeloma presented with secondary kidney failure requiring dialysis (CLcr estimated at 10 mL/min). HCO dialysis (Theralite; Gambro Lundia AB) was initiated 3 to 4 d/wk, 8 hours per session, with albumin substitution of 7 g/h, and average blood and dialysate flows of 300 to 350 and 500 mL/min, respectively (ultrafiltration rate, 250 mL/h). Pomalidomide, administered orally just after dialysis, was introduced as recommended in cycles of 21 consecutive days separated by 7 off-treatment days, along with ongoing bortezomib and dexamethasone. The dose was started at 2 mg/d because of a concern of increased hematologic toxicity with the usually recommended dosage of 4 mg/d.1 More information about clinical presentation, kidney biopsy, previous multiple myeloma therapy, comedications, and clinical course is given in Item S1.
The patient’s written consent was obtained to collect a series of blood samples (schedule described in Item S1) to determine pomalidomide plasma levels and validate the given dosage during dialysis. Plasma and dialysate concentrations were determined by a validated ultra high-performance liquid chromatography method with fluorescence detection.5
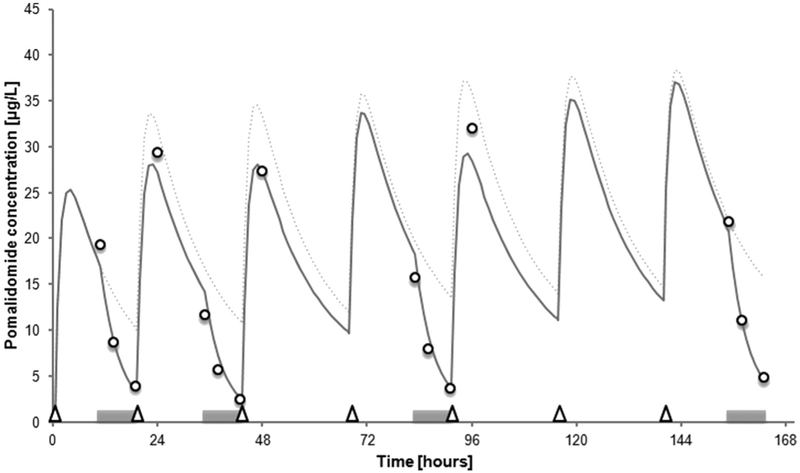
A 2-compartment pharmacokinetic model (detailed in Item S1) was used to predict plasma concentrations of pomalidomide during HCO dialysis, based on published pomalidomide pharmacokinetic parameters, the patient’s kidney and liver status on admission, and dialysis settings (Fig 1).6 Pharmacokinetic and dialysis elimination parameters are reported in Table 1.
Figure 1.
Predicted (continuous line) and measured (open circles) pomalidomide concentrations over time. HCO dialysis sessions are indicated by grey horizontal bars; pomalidomide doses (2 mg), by open triangles. Predicted concentrations (with 2 mg/d dosage) in the absence of dialysis denoted by dotted line.
Table 1.
Pharmacokinetic Parameters of Pomalidomide in the Reported Patient With HCO Dialysis
| Parameter | Our Patient | Reference Values | |
|---|---|---|---|
| Healthy Person | Multiple Myeloma | ||
| Dose, mg/d | 2a | 2 | 4 |
| Pharmacokinetic parameters | |||
| CLR, mL/min | 3 | 4 | 3 |
| CLNR, mL/min | 47 | 172 | 127 |
| CLTot/F, mL/min | 50 | 176b | 130 |
| Vss/F, L | 132 | — | 141 |
| t1/2 off dialysis, h | 14.3 | 8.9 | 7.5 |
| AUC24h, μg·h/L | 475 on dialysis; 574 off dialysis | 189 ± 52 | 513b |
| Hemodialysis elimination | |||
| E, % | 37–65 | — | — |
| CLDial predicted, mL/min | 155–181 | — | — |
| CLDial observed, mL/min | 187–663 | — | — |
| t1/2 on dialysis, h | 3.5 | — | — |
Abbreviations and definitions (further information in Item S1): AUC24h, area under the curve calculated by numerical integration over 24 h; CLDial observed, observed dialytic clearance, or difference between concentration entering the dialyzer vs that leaving it, multiplied by blood flow, divided by concentration entering the dialyzer; CLDial predicted, predicted dialytic clearance, or product of blood flow, fraction unbound, and blood-plasma concentration ratio; CLNR, nonrenal clearance or CLTot/F multiplied by hepatic extraction ratio; CLR, renal clearance; CLTot, total clearance; E, extraction coefficient, or observed dialytic clearance divided by blood flow; F, bioavailability; t1/2, half-life; VSS, sum of central and peripheral volumes of distribution.
At the end of HCO dialysis.
Based on the relation AUC:Dose/(CL/F).
According to our observations, pomalidomide’s dialytic extraction ratio is significant and consistent with its molecular features (low protein binding and small molecular size).
Our model-based prediction of AUC24h (574 μg ·h/L) for a dosage of 2 mg/d off dialysis was ~21% higher than the AUC24h calculated under HCO dialysis (475 μg·h/L). The estimated AUC24h in our patient is comparable to the prediction for a dosage of 4 mg/d in average patients with multiple myeloma (Table 1),6,7 which is compatible with particularly low total and intercompartmental pomalidomide clearances in this patient. This observation could be explained by effects of kidney failure on liver metabolism,8 individual variation of cytochrome CYP3A4 function, or organ dysfunction associated with advanced disease. An effect of disease has been observed on pomalidomide disposition, with faster drug distribution and deeper penetration into tissues in patients with multiple myeloma compared with healthy individuals.6
In conclusion, our model and observations suggest that HCO dialysis affects pomalidomide exposure to a definite extent. However, this effect does not override the decrease in systemic clearance reported in patients with kidney failure, which justifies the 25% dose reduction recently recommended by the manufacturer. Administering the dose just after dialysis sessions may limit the impact of dialytic clearance. Prospective studies recording clinical outcomes are still needed to confirm whether pomalidomide can be effectively and safely used at a 25% reduced dosage during HCO dialysis.
Supplementary Material
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Pomalyst (pomalidomide) capsule, label information. Labeling revision, updated June 30, 2016. http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/204026s012s014lbl.pdf. Accessed July 14, 2016.
- 2.Pomalyst (pomalidomide) clinical pharmacology and biopharmaceutics review(s). http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204026Orig1s000ClinPharmR.pdf. Accessed July 14, 2016.
- 3.Hoffmann M, Kasserra C, Reyes J, et al. Absorption, metabolism and excretion of [14C]pomalidomide in humans following oral administration. Cancer Chemother Pharmacol. 2013;71(2):489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowland M, Tozer TN. Elimination - tubular reabsorption In: Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications. 4th ed. Philadelphia, PA: Wolters Kluwer Health; 2011:132–137. [Google Scholar]
- 5.Shahbazi S, Peer CJ, Polizzotto MN, et al. A sensitive and robust HPLC assay with fluorescence detection for the quantification of pomalidomide in human plasma for pharmacokinetic analyses. J Pharm Biomed Anal. 2014;92:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Xu Y, Liu L, Wang X, Palmisano M, Zhou S. Population pharmacokinetics of pomalidomide. J Clin Pharmacol. 2015;55(5):563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gay F, Mina R, Troia R, Bringhen S. Pharmacokinetic evaluation of pomalidomide for the treatment of myeloma. Expert Opin Drug Metab Toxicol. 2013;9(11):1517–1527. [DOI] [PubMed] [Google Scholar]
- 8.Velenosi TJ, Urquhart BL. Pharmacokinetic considerations in chronic kidney disease and patients requiring dialysis. Expert Opin Drug Metab Toxicol. 2014;10(8):1131–1143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.