Abstract
A history of prior fracture is the most reliable indicator of prospective fracture risk. Increased fracture risk is not confined to the region of the prior fracture, but is operant at all skeletal sites, providing strong evidence of systemic bone loss after fracture. Animal and human studies suggest that systemic bone loss begins shortly after fracture and persists for several years in humans. In fact, bone quantity and bone quality may never fully return to their pre-fracture levels, especially in older subjects, demonstrating a need for improved understanding of the mechanisms leading to systemic bone loss after fracture in order to reduce subsequent fracture risk. Although the process remains incompletely understood, mechanical unloading (disuse), systemic inflammation, and hormones that control calcium homeostasis may all contribute to systemic bone loss. Additionally, individual factors can potentially affect the magnitude and time course of systemic bone loss and recovery. The magnitude of systemic bone loss correlates positively with injury severity and age. Men may also experience greater bone loss or less recovery than women after fracture. This review details the current understanding of systemic bone loss following fracture, including possible underlying mechanisms and individual factors that may affect this injury response.
Keywords: Fracture healing, Osteoporosis, Bone Loss, Fracture Risk
Introduction
Osteoporosis-related fractures are a significant clinical concern for the aging population, with two million osteoporotic fractures in the United States on a yearly basis accounting for approximately 19 billion dollars in medical costs [1]. Despite the clinical significance of bone fragility, factors underlying age-related bone loss and fracture risk remain incompletely understood. The development of osteoporosis depends on changes in the balance between bone formation and bone resorption throughout life [2–6]. Therefore, it is important to identify and better understand the mechanisms that modulate the relative balance of these processes and lead to decreases in bone mass and strength. One underappreciated risk factor for osteoporotic fracture is systemic bone loss after a prior fracture. This review first summarizes evidence for systemic bone loss after fracture, then discusses potential mechanisms driving this phenomenon, and finally examines individual factors affecting the magnitude of systemic bone loss after fracture.
Evidence for Systemic Bone Loss After Fracture
Increased Fracture Risk Following an Initial Fracture
Increase of prospective fracture risk after an initial fracture is consistent with fracture causing systemic changes in bone quantity and bone quality. While many risk factors for fracture have been identified, a prior fracture is the most reliable predictor of future fracture risk at any skeletal site [2,3]. Even minor fractures increase the risk of subsequent major fractures [4]. Individuals with a prior fracture have a 2–10 times higher risk of sustaining a future fracture than individuals without a prior history of fracture, and subsequent fractures further increase prospective fracture risk [5–9]. These changes in fracture risk are sustained for many years. Women that experienced fracture between the ages of 20–50 years had an increased risk into their 70s, and men with a history of fracture before the age of 18 years had a significantly higher risk of fracture after the age of 35 years [10,11]. Prior fracture remains a significant predictor of future risk even after controlling for bone mineral density (BMD), indicating that this phenomenon is not solely a correlate of individuals with lower BMD being more prone to fracture [11,12].
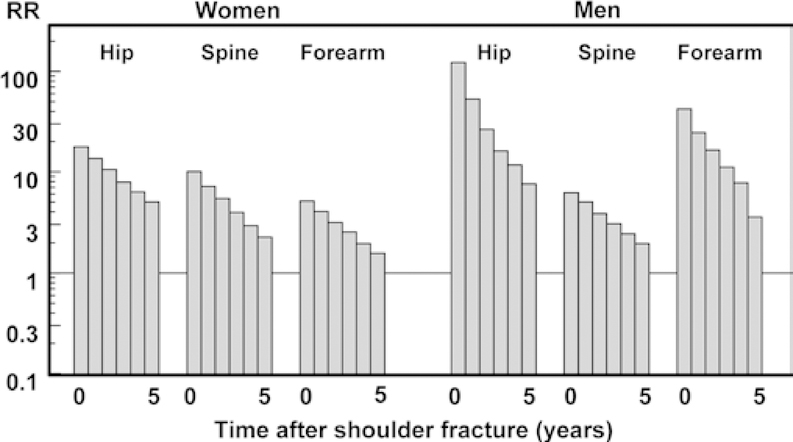
The risk of subsequent fracture decreases with time after injury, but remains elevated above that of individuals without a history of fracture in the long term [3,5,13–15]. For example, the relative risk for hip, spine, and wrist fractures declined in the 5 years after a shoulder fracture in 60 year-old patients, but remained elevated relative to the general population (Figure 1) [3]. This suggests that after an acute elevation in fracture risk, partial recovery occurs. Therefore, the heightened risk of subsequent fracture, while most acute for the first few years after an index fracture, may persist for several decades.
Fig. 1.
Relative Risk (RR) of hip, spine, and forearm fracture for 5 years after a shoulder fracture in men and women aged 60 years [3]
The etiology of increased fracture risk following an initial fracture is unclear. However, over two decades ago, Silman proposed several potential mechanisms by which an initial fracture could predict future fracture risk [16]:
“Risk factors for the development of one fracture are still operative to increase susceptibility to a second and subsequent event”
“There may be mechanical influences caused by having had one fracture, and it may be these mechanical effects that increase this subsequent risk”
“The occurrence of a fracture, particularly in the limbs, is followed by bone loss, not completely reversible, which could lead to an increased risk of subsequent fracture”
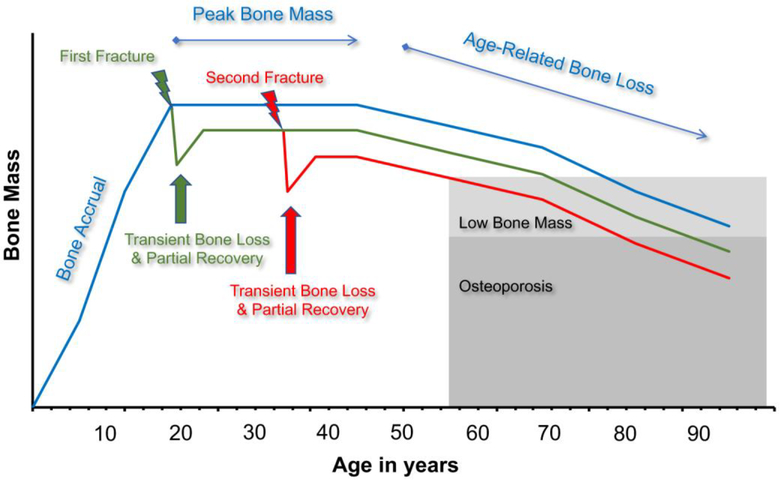
With regard to the first point, previous studies have shown that preexisting poor physical function, higher risk of falls, or other individual cofactors do contribute to higher fracture risk [17–20]. Index fracture may also negatively impair physical function, through derangement of joint mobility and discoordination, supporting the second mechanism [21,22]. However, these two mechanisms may not entirely explain elevated risk of subsequent fracture. It is also likely that fracture causes systemic bone loss and reductions in bone quality, which decrease skeletal strength, and individuals may not completely recover from this for many years, if ever. Posttraumatic bone loss may interact with natural age-related skeletal degeneration, placing individuals on a higher risk trajectory for the development of osteoporosis and future fractures (Figure 2) [16].
Fig. 2.
Proposed lifetime trajectory of bone mass. Fractures may cause a transient loss of bone that is not fully recovered, resulting in an altered bone mass trajectory and an increased risk of future fractures
Acceleration of Bone Remodeling Following Fracture
Although studies of bone remodeling following fracture have not typically investigated systemic bone loss, accelerated remodeling after fracture supports the hypothesis that fracture leads to long-term systemic decreases in bone mass. After fracture, bone remodeling increases both in the skeletal regions close to the fracture site and systemically. Frost named the increase in bone turnover near the fracture the Regional Acceleratory Phenomenon (RAP) [23,24]. RAP peaks within 1–2 months after injury, with reported increases anywhere between 2–20 times above normal levels, and this increased remodeling rate persists for 6–24 months [24,25]. While the RAP increases both bone formation by osteoblasts and bone resorption by osteoclasts, the rate of resorption typically outpaces the rate of formation [26,27]. Since the 19th century, it has been recognized that greater resorption than formation after fracture leads to regional bone loss in the fractured element or injured limb, commonly referred to as either post-traumatic osteoporosis or osteopenia [28,29]. Importantly, a Systemic Acceleratory Phenomenon (SAP) has also been documented after skeletal damage. Tibial osteotomy increased bone formation rate and mineralizing surface in mice and rats 3–3.5 fold at the contralateral tibia and lumbar vertebrae [30–32]. Other histomorphometry studies detected increases in osteoclast activity in lumbar vertebrae following femur osteotomy or fracture in mice [33,34]. Thus, as occurs during the RAP, the balance of increased remodeling during the SAP may skew towards bone resorption, resulting in a net decrease in bone mass.
Clinical studies support an SAP in humans, during which resorption exceeds bone formation. These studies typically measured bone resorption markers in serum or urine following fracture, so it was not possible to assess differences in remodeling at individual skeletal sites. Nevertheless, following fracture, increased osteoclast activity preceded elevation of osteoblast activity. Markers of resorption increased within two weeks after fracture, whereas markers of bone formation did not increase until 24 weeks [35]. Bone formation and bone resorption rates can remain imbalanced for many years after fracture [35–40]. For instance, women who sustained a fracture in the past six years still had decreased bone formation but unchanged levels of bone resorption compared to controls [36]. Another study found marginally elevated markers of both bone formation and resorption in women who had suffered a fracture at any point in their life [37]. This pattern became stronger and statistically significant when only women who had sustained fractures in the two years prior were examined. Thus, the timeline of bone remodeling after fracture closely follows that of prospective fracture risk: most acute in the first few years after injury, but remaining elevated compared to non-fractured individuals in the long term, strongly suggesting that systemic bone loss is a mechanism for increased risk of prospective fracture [3]. Additional research is required better quantify the duration of increased osteoclast and osteoblast activity following fracture and identify time points at which the greatest imbalance exists between systemic resorption and formation.
Animal Studies of Systemic Bone Loss After Fracture
A better understanding of the magnitude and duration of post-fracture systemic bone loss requires direct measurement of changes in bone quantity and architecture. Animal models provide the best means for determining the mechanisms and time course of bone loss and recovery following fracture, as they allow for evaluation at a level of detail not easily achieved in clinical studies. Animal studies either create fracture using a controlled impact, or they simulate a fracture using osteotomy [30,33,41,42]. Animals are then examined at various time points to assess short- and long-term skeletal adaptation. Most studies of fracture healing have focused exclusively on the affected limb; only a few studies have considered systemic changes in bone quantity after fracture as a primary variable. Two recent studies reported systemic bone loss following femur osteotomy in mice [33,43]. Fischer et al. studied the effect of fracture on posttraumatic bone loss in mice with different amounts of dietary calcium [33]. Three weeks after osteotomy, lumbar vertebrae trabecular bone volume fraction (BV/TV) decreased and osteoclast activity increased in fractured mice compared to controls. However, decreases in BV/TV only reached statistical significance in mice fed a mineral deficient diet, whereas the group on a control diet exhibited a non-significant decrease. Haffner-Luntzer et al. examined systemic calcium mobilization after femur osteotomy in wild type and Cckbr−/− mice, a model for calcium malabsorption due to gastric hypochlorhydria [43]. While this study did not compare fracture to non-fractured animals, osteoclast activity in the lumbar vertebrae was higher in fractured Cckbr−/− mice, which also exhibited reduced bending strength of the uninjured femur compared to fractured wild type mice. These two studies strongly suggest that increased remodeling after fracture leads to systemic bone loss in uninjured elements. However, they did not extensively examine the magnitude and time course of systemic bone loss outside the context of mineral deficiency.
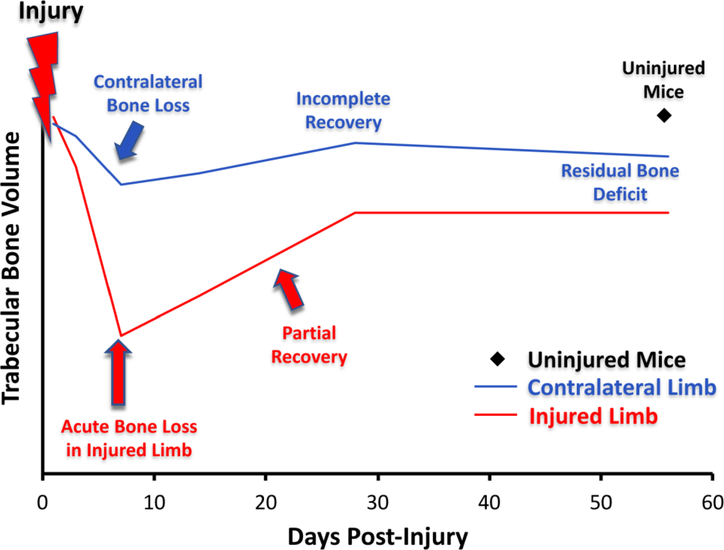
A recent study from our lab explicitly investigated the time course and mechanisms of systemic bone loss and recovery in mice following femur fracture [34]. Analysis of whole-body BMD and bone mineral content (BMC) with dual-energy x-ray absorptiometry (DXA) revealed loss of bone 2 weeks after fracture. At later time points, bone was recovered in young (3 month old) but not middle-aged (12 month old) mice. Similarly, vertebral trabecular bone volume fraction (BV/TV) was decreased 12–18% by 2 weeks post-fracture in both young and middle-aged mice. By 6 weeks post-fracture, BV/TV had recovered to pre-fracture levels in young mice, but BV/TV did not change from 2–6 weeks in middle-aged fractured mice. These results are similar to the time course of trabecular bone loss we have observed in the injured and contralateral knees following ACL rupture in mice (Figure 3) [44,45]. In this injury model, rapid bone loss occurred for 1–2 weeks post-injury, followed by a partial recovery by 4 weeks. It remains unclear if the skeleton ever returns to pre-fracture levels of bone quantity and strength.
Fig. 3.
Trabecular bone loss post-injury. Injury causes acute bone loss followed by an incomplete recovery in both the injured and contralateral limb
Obviously, it is difficult to translate the magnitude and time course of fracture repair in mice to that of humans, but the general injury response has important clinical implications. Fracture immediately initiates a period of bone loss, and, while most pronounced in the damaged region, this process occurs throughout the skeleton. Subsequently, partial recovery occurs, but bone mass may not return to pre-fracture levels. Again, this trajectory parallels the time course of future fracture risk following an initial fracture, with especially high fracture risk in the period immediately following fracture and decreased fracture risk at later time points, though still slightly elevated compared to non-fractured individuals.
Clinical Evidence for Systemic Bone Loss After Fracture
Clinical studies measuring changes in bone during follow-up after fracture provide evidence of systemic bone loss by comparing fractured individuals with controls matched for sex, age, and hormonal status. Reported decreases in BMD within the injured limb following fracture range from 3–31% relative to baseline values measured close to the time of fracture [35,38,46,47]. After proximal femur fracture, men exhibited 9–13% lower forearm BMC and lumbar spine BMD than age-matched controls [48,49]. However, given a lack of pre-fracture baseline, these finding may reflect either systemic bone loss or that men with lower BMC and BMD had an inherently higher fracture risk.
Importantly, longitudinal studies have shed light on the time course of systemic changes in humans [50–53]. Six months after tibial shaft fracture, contralateral proximal femur BMC decreased by an average of 2–3 percent [51]. Another longitudinal study of the contralateral radius after Colles’ fracture showed that BMC decreased by 10% in the first 4 months [52]. This study is particularly interesting, because bone loss in the injured limb was greatest after 4 months and recovered thereafter, suggesting that regional and systemic bone loss follow similar time courses. Another study modeling ultra-distal tibia stiffness and failure load following surgery on the tibia showed that trabecular thickness, as well as overall stiffness and failure load of both the injured and contralateral limb decreased within 6 weeks of the procedure [54]. Material properties of either limb did not return to baseline levels by 4 months after surgery.
A recent study from our lab further investigated systemic bone loss by examining changes in total hip BMD in women enrolled in the Study of Osteoporotic Fractures (SOF) who had an incident fracture during a 4-year period compared to those that did not fracture [2]. We found that women with an incident fracture during this period experienced a greater decline in total hip BMD than women without fracture. Bone loss was particularly pronounced during intervals that included an incident fracture of the vertebrae, elbow, humerus, clavicle, hip, and pelvis. Accelerated bone loss was isolated to the 2-year period that included the fracture; this accelerated bone loss was not observed during the 2 years before or 2 years after the fracture interval. This finding is in agreement with studies that found accelerated losses in BMD at the contralateral hip, proximal femur, and lumbar vertebrae in the year following hip fracture [55–58]. After one year, fractured individuals lost 5–10% BMD, compared to non-fractured controls who lost between 0.5 to 1%.
Altogether, these longitudinal clinical studies suggest a trajectory of systemic bone changes similar to that seen in the mouse model of systemic bone loss used in our lab, though on a much longer timeline. Systemic bone loss begins within weeks of a fracture, and lasts between 6–24 months. Bone loss in the first 1–2 years after fracture also correlates with the period of increased fracture risk observed in the years immediately after injury, suggesting that acute systemic bone loss following a fracture may be a primary mechanism underlying the increased risk.
Unfortunately, no longitudinal study has evaluated systemic changes at later time points, so the extent and time course of subsequent recovery remains unclear. Decreases in fracture risk in the years after fracture indicate that some recovery towards pre-fracture levels of bone strength does occur [2,34,44]. However, particularly in older people, fracture risk may never return to the level of non-fractured individuals, suggesting that the new steady state for bone mass and strength falls below pre-injury levels. Increased awareness of systemic bone loss can guide the development of clinical strategies for maintaining bone health. As post-traumatic bone loss likely occurs within two years of the injury, maintaining bone mass and bone strength may require early interventions. Further improving understanding of systemic bone loss requires consideration of the mechanisms driving bone loss and the individual factors that affect this response.
Mechanisms of Bone Loss After Fracture
The etiology of systemic bone loss after fracture remains poorly understood. Animal and clinical studies have implicated unloading (disuse), inflammation, and hormones that regulate calcium homeostasis in post-traumatic bone loss, but the relative contribution of these factors and how they alter the activity of bone cells has not been sufficiently explored.
Bone Cells Involved in Bone Loss Following Fracture
Bone resorption and formation initiated by fracture results from changes in the activity of bone cells: osteoblasts, osteoclasts, and osteocytes. Osteoblasts deposit bone matrix and regulate increases in the number and activity of osteoclasts, bone resorbing cells, during the fracture repair process via Macrophage-Colony Stimulating Factor (M-CSF) and Receptor Activator of Nuclear kappa B Ligand (RANKL) [59–62]. Osteocytes derive from osteoblasts that become embedded in lacunae within bone matrix and their processes extend through canaliculi. These cells regulate osteoclast and osteoblast activity, and can also directly remove bone matrix through perilacunar remodeling [59,63–66]. Changes in the activity of osteoblasts, osteoclasts, and osteocytes arise due to changes in mechanical loading and circulating signaling factors following fracture.
Mechanical Unloading (Disuse) Following Injury
Fracture has predominantly been associated with disuse of the injured region, but generalized reduction in activity could also contribute to systemic changes. A relationship between total or partial disuse and bone loss has long been recognized based on the skeleton’s catabolic response to bed rest, immobilization, and space flight [27,29,67]. Similarly, following fracture, a patient’s overall activity levels are typically reduced. In our study of systemic bone loss following fracture in mice, we found that overall activity level of mice was decreased 4 days post-fracture, with no significant differences at later time points [34]. This suggests that reductions in mechanical loading might contribute to the observed systemic bone loss in mice, but the brief duration of changes makes it unlikely that reduced mechanical loading fully accounts for all systemic bone loss. Unloading may actually play a bigger role in humans, in which fracture typically leads to a greater degree of disuse for a longer period of time. In humans, the ability to recover bone lost due to a 4 month period of disuse appears variable, with some regions of the body showing minimal or no recovery [68]. While disuse following fracture is not typically full unloading, this finding demonstrates that generalized disuse can contribute to systemic loss of bone tissue that may not be fully reversible.
Systemic Chemical Messengers Produced by Disuse
In addition to the direct effects of unloading on the activity of bone cells, disuse may also release signaling factors that increase bone resorption systemically. As a result of unloading, increased RANKL production by osteoblasts and osteocytes upregulates osteoclastic bone resorption, and increased production of sclerostin by osteocytes reduces osteoblastic bone formation [69–74]. Circulation of these factors beyond the fracture region could lead to net loss of bone systemically. A year-long clinical study found that sclerostin serum levels increased in patients with long bone fractures for 8 weeks after fracture [75]. They then decreased gradually for the rest of the year but remained elevated relative to controls. Consistent with this being due to disuse, a bed rest study found that serum sclerostin peaked at 8 weeks and decreased thereafter [76].
Altogether, unloading may contribute to systemic bone loss, either due to direct effects on the activity of bone cells or due to the release of signaling factors that systemically decrease bone formation and increase bone resorption. Thus, disuse is likely one of several relevant factors contributing to systemic bone loss after fracture. Importantly, studies of unloading have primarily dealt with total unloading rather than partial disuse, and no study has specifically evaluated the effect of unloading following fracture on systemic bone loss. Therefore, future studies on animal fracture models could evaluate if different degrees of disuse change the amount of systemic bone loss post-fracture, and serum levels of RANKL, sclerostin, and other circulating factors associated with bone formation and resorption.
Inflammation
Fracture, like any traumatic injury, produces an inflammatory response that plays an essential role in initiating and regulating the repair process [77]. A link between chronic inflammation and bone loss has been well established. Patients with auto-immune diseases or other chronic inflammatory conditions such as rheumatoid arthritis, inflammatory bowel disease, chronic obstructive pulmonary disease, and ankylosing spondylitis exhibit systemic bone loss and an increased risk of osteopenia or osteoporosis [78–85]. However, the effect of acute inflammation following fracture on bone has not been well established.
Effect of Pro-Inflammatory Cytokines on Bone Cells
During the inflammatory response, immune cells and osteoblasts release pro-inflammatory cytokines, notably TNF-α, IL-1, and IL-6, which have catabolic effects on bone [60,86–91]. These cytokines induce RANKL production in osteoblasts and osteocytes, increasing osteoclast differentiation; they may also directly activate osteoclasts [60,91,92]. Pro-inflammatory cytokines also reduce the deposition of bone matrix by osteoblasts either through direct interaction or by increasing osteocyte production of sclerostin and Dickkopf-related protein 1 (DKK1), which decrease osteoblast activity by inhibiting the Wnt/ β-catenin pathway [60,78,85,88,93,94].
Evidence for Bone Loss Due to Post-Fracture Inflammation
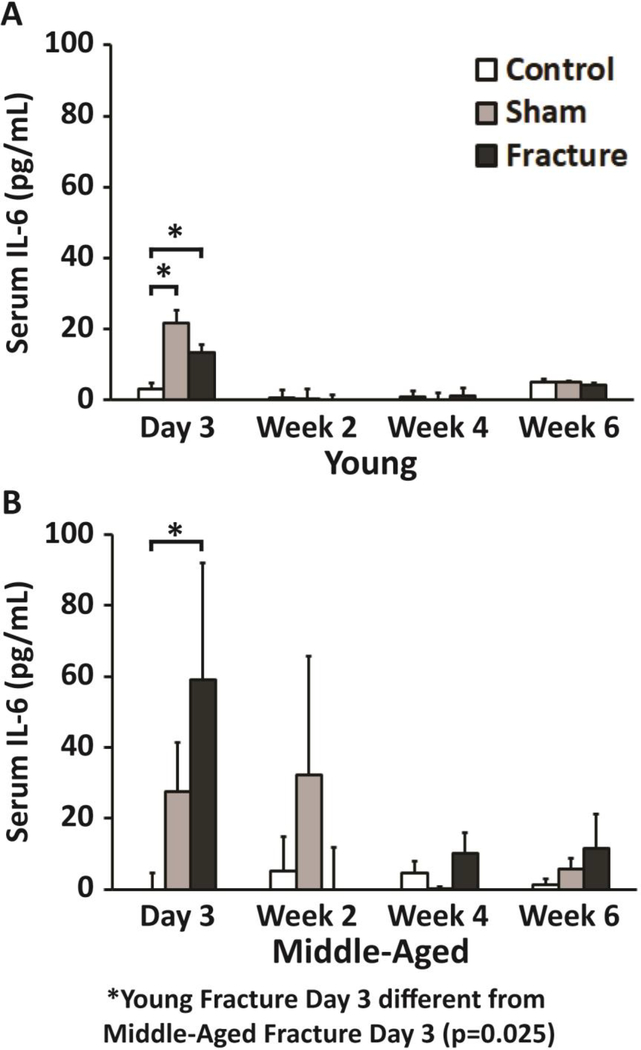
Evidence that increases in pro-inflammatory cytokine concentrations after fracture directly cause systemic bone loss is limited, but compelling. In our study of systemic bone loss following fracture in mice, we observed elevated IL-6 concentration in serum compared to controls 3 days post-fracture in both young and middle-aged mice (Figure 4) [34]. At the same time point, osteoclast number and resorbing surface area was increased in the lumbar vertebrae. This correlation suggests that increased pro-inflammatory cytokine concentrations may initiate increases in systemic resorption. Another study of mice detected a similar pattern at the fracture site, with IL-1 and TNF-α expression in the fracture callus peaking in the first 24 hours after fracture, then subsequently decreasing before rising again in the third week after fracture [95]. In rats, expression of IL-6 in blood vessels and muscles reaches a maximum within the first day following femur fracture, both at the fracture site and in the contralateral limb [96]. Clinical study of humans found that IL-6 levels in plasma reached a maxima 48 hours post-fracture; a similar pattern was seen following thoracoabdominal surgery [97,98]. These early elevations in pro-inflammatory cytokine concentration may trigger post-traumatic bone loss, though this remains to be shown mechanistically.
Fig. 4.
Enzyme-linked immunosorbent assay (ELISA) of serum interleukin-6 levels in (A) young and (B) middle-aged mice after fracture. At day 3, young Fractured mice had a 3.5 fold increase in serum IL-6, while middle-aged Fractured mice had a 21.9 fold (p<0.001) increase in serum IL-6 compared to age-matched Control mice. Error bars represent standard deviation. * denotes p≤0.05 [34]
Further work is required to conclusively demonstrate that post-fracture inflammation causes systemic bone loss. Also, which pro-inflammatory cytokines have the greatest effect on bone remodeling remains unclear. The majority of studies track IL-6 concentrations because it is the most abundant inflammatory cytokine in circulation, and it moderates the effect of other cytokines [60,88]. Future animal and clinical studies could better characterize how cytokine concentrations change during fracture healing and examine how these changes correlate with rates of bone formation and resorption. Also, if post-fracture inflammation is a primary cause of systemic bone loss, then anti-inflammatory treatments should be able to reduce bone loss after fracture [60].
Systemic Bone Loss Addresses Mineral Need During Fracture Repair
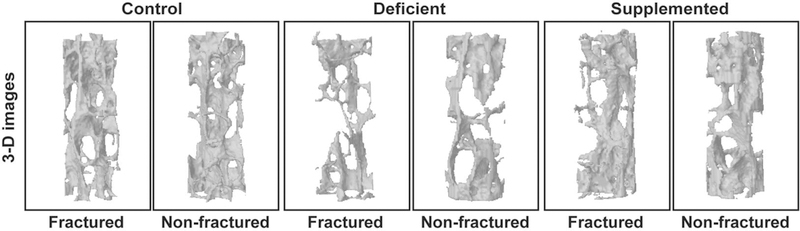
It has been proposed that systemic increases in remodeling play an important role in local bone repair. The SAP may increase bone remodeling to make available mineral stored throughout the body to address an acute local need (fracture healing) [34]. Consequently, the availability of dietary calcium could modulate the utilization of mineral stores in the skeleton [43], and individuals with diets deficient in mineral may experience greater systemic bone loss after fracture. Consistent with this hypothesis, Fischer et al. found that fractured mice fed a mineral deficient diet exhibited greater loss of bone at the lumbar vertebrae after femur osteotomy than mice on a normal diet (Figure 5) [33]. Also, Haffner-Luntzer et al. found that calcium supplementation after femur osteotomy in mice decreased osteoclast activity and increased BV/TV and BMD in the lumbar vertebrae relative to fractured mice on a control diet, suggesting that post-fracture systemic bone resorption addresses mineral need [43]. A clinical examination of bone loss in the lumbar spine and contralateral femoral neck after hip fracture similarly found that decrease in BMD was greater in the year after fracture in individuals with lower dietary calcium intake and serum levels of Vitamin D [58]. In another study, individuals with either an upper or lower limb fracture that received calcium and Vitamin D supplements demonstrated an increase in lumbar BMD in the year after fracture, whereas this variable decreased in fractured individuals who did not receive supplements [99]. These findings suggest that systemic bone loss following fracture provides mineral for callus formation and fracture repair, since the extent of mineral need affects the magnitude of systemic changes in bone quantity. The efficacy of mineral supplementation for preventing post-traumatic bone loss certainly merits further study.
Fig. 5.
μCT images of lumbar vertebrae from non-fractured and fractured ovariectomized mice fed a control, Calcium/ Vitamin D-deficient, or Calcium/ Vitamin D- supplemented diet. Fractured Calcium/ Vitamin D-deficient mice exhibited the greatest bone loss post-fracture compared to non-fractured controls from the same dietary group [33]
PTH as a Regulator of Systemic Bone Loss
If the SAP provides mineral for fracture healing, then hormones that regulate release of calcium and Vitamin D from the skeleton may play an important regulatory role in systemic bone loss [100,101]; parathyroid hormone (PTH) may be particularly important. While intermittent low doses of PTH are anabolic, continuous exposure to PTH stimulates bone formation [101]. PTH indirectly stimulates bone resorption by upregulating RANKL and IL-6 production in osteoblasts and osteocytes [101,102]. In addition, PTH may increase direct bone resorption by osteocytes [103–105].
The regulation of bone remodeling after fracture by PTH has been the subject of several investigations [106]. For example, PTH levels increased following leg fractures in dogs from the time of injury to the formation of the bony callus, suggesting that PTH mediates systemic resorption of bone for callus formation [107]. A study of mice directly assessed the relationship between systemic bone loss, PTH levels, and mineral need after fracture. Comparisons of mice with a normal and calcium/Vitamin D deficient diet showed that PTH concentration and osteoclast surface per bone surface of the lumbar vertebrae increased in both groups after osteotomy. However, the increases were significantly greater in the deficient diet group [33]. Conversely, elevated PTH was not seen in fractured mice with mineral supplemented diet, and osteoclast activity was lower than in the other two groups. Mineral deficient mice also showed significant decreases in BV/TV compared to non-fractured mice on the same diet, whereas mice with a control or mineral supplemented diet did not. Increased PTH, osteoclast activity, and bone loss when the diet lacks mineral strongly suggest that PTH increases systemic bone resorption to address mineral need at the fracture site.
Several clinical studies have also found that PTH level increases in the first year after fracture; corresponding to the period during which bone loss may occur [2,3,40,52,108]. For example, one study showed that individuals recovering from fracture and receiving calcium and Vitamin D had lower PTH levels than fractured individuals not receiving mineral supplementation. After one year, only non-supplemented individuals showed decreases in lumbar BMD [99]. Thus, systemic bone loss may address a mineral need at the fracture site, and PTH may act as a key regulator of systemic bone resorption. Subsequently, calcium and Vitamin D supplementation may have the potential to reduce circulating levels of PTH and reduce systemic bone loss following fracture. Further animal and clinical studies could also evaluate if long-term mineral supplementation improves recovery from systemic bone loss.
Importantly, PTH may have opposing effects on fracture healing and systemic bone loss. Animal models have found that PTH improves localized bone healing. Administration of exogenous PTH in rats improved callus size, strength, BMD, and BMC [106,109–112]. Therefore, it is likely that improvements in callus volume and mineralization associated with PTH treatment may come at the expense of increased systemic resorption. Given clinical interest in PTH as a method for improving fracture healing, future studies should investigate the potentially contrasting effects of PTH on localized and systemic bone adaptation to fracture [106].
Variation in Systemic Bone Loss after Fracture
Current research has established that post-traumatic bone loss throughout the skeleton is not uniform. The amount of bone lost may correlate positively with injury severity, older individuals may not recover bone lost after fracture to the same extent as younger individuals, and men may experience greater systemic bone loss than women.
Severity of Injury
The severity and number of fractures likely affects the magnitude of systemic bone loss. Our previous study examining loss of hip BMD in women found greater bone loss in women that had incident fractures of larger skeletal sites (vertebra, hip, humerus, elbow, clavicle), while fractures of smaller skeletal sites (hand, knee, foot, toe) were not associated with increased loss of hip BMD [2]. Similarly, individuals that experienced multiple incident fractures during the study period lost considerably more hip BMD than those with a single fracture.
The correlation between injury severity and bone loss may relate to the extent of inflammation. Serum levels of IL-6 positively correlate with clinical injury severity scores [113,114]. Mice with both soft tissue damage and bone fragment injections exhibited higher circulating IL-6 than individuals with only soft tissue damage or injected bone fragments [115]. Similarly, a rat trauma model compared the inflammatory response to different combinations of injuries (blunt chest trauma, skull trauma, femur fracture) [116]. Rats subjected to all three injuries showed higher serum pro-inflammatory cytokine levels than rats with only one or two injuries. These results are consistent with more severe injuries producing a greater inflammatory response, and this may increase bone resorption systemically. However, no study has directly demonstrated that increased cytokine concentrations after a larger fracture or in the context of multiple fractures produces greater bone loss throughout the skeleton. Additionally, more severe injuries may result in greater disuse or even complete immobilization.
The specific relationship between fracture severity and the magnitude of bone loss has not been investigated. Systemic bone loss may increase linearly with the number or severity of fractures, or alternatively, one or more thresholds of injury severity may exist above which greater systemic bone loss occurs. Future animal studies can test this possibility, and can better quantify the relationship between the severity and number of fractures and the magnitude of systemic inflammation, mineral mobilization from the skeleton, and disuse.
Age
The magnitude of systemic bone loss following fracture may increase with age, and the ability to recover from this acute bone loss may decrease. Our study investigating systemic bone loss and recovery following femur fracture in mice found significant age-related differences in the trajectory of systemic bone loss and recovery after fracture [34]. Two weeks after fracture, whole-body BMD and BMC decreased in both young (3 month old) and middle-aged (12 month old) mice. By 6 weeks post-fracture, young mice fully recovered from this bone loss, whereas whole-body BMD did not recover in middle-aged mice. These data suggest that younger people may have the ability to fully recover from transient bone lost after fracture, while older individuals may be left with a longer-term or even permanent deficit.
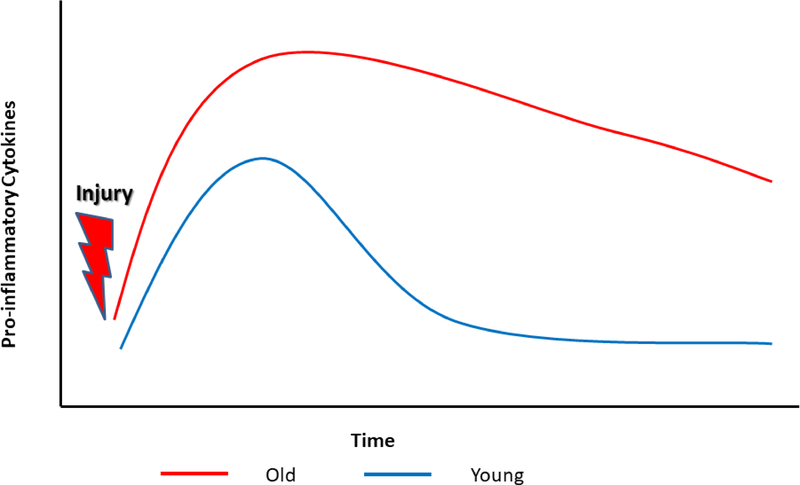
Age-related changes in post-fracture inflammation may be an underlying mechanism driving greater systemic bone loss and poorer recovery following fracture in older individuals. Dysregulation of the immune system in the elderly chronically increases inflammatory status [117,118]. Humans also show age-related differences in the magnitude and duration of inflammatory response to injury. For example, proximal femur fracture increased serum concentrations of IL-6 more in patients older than 70 years than in patients younger than 50 years [119]. Furthermore, after surgery, IL-6 concentrations increase more rapidly and remain elevated longer in elderly than middle aged people [120,121]. Based on these observations, Hazeldine et al. proposed a model of age-differences in the inflammatory response to injury (Figure 6) [121]. Aged individuals have higher circulating baseline levels of pro-inflammatory cytokines than young individuals. After injury, they also produce more pro-inflammatory cytokines, and the immune response lasts longer [119,121–123]. In the context of fracture, dysregulation of the immune system may increase osteoclast activity or prolong the period of bone resorption. As pro-inflammatory cytokines also decrease osteoblast activity, elderly people may also exhibit reduced bone formation due to higher baseline levels of inflammation. Our study of mice supports the hypothesis that greater inflammation in older animals causes poorer recovery of bone quantity. Three days after fracture, middle aged mice exhibit 4-fold greater concentrations of IL-6 than young mice [34].
Fig. 6.
Changes in pro-inflammatory cytokine concentrations post-fracture in young and old individuals. Prior to fracture, old people have higher baseline pro-inflammatory cytokine level. After fracture cytokine levels reach a higher peak in old people and take longer to decrease [121]
Age-related decrease in estrogen and testosterone levels may explain increased pro-inflammatory cytokine production in older individuals [102,123,124]. Sex hormones suppress pro-inflammatory cytokine mediated bone resorption. Experiments on ovarectomized mice and in vitro mouse osteoblast lines demonstrate that estradiol suppresses osteoblast production of IL-6 and osteoclastogenesis [122,125]. Similarly, a clinical study found that after oophorectomy, women exhibited increases in IL-1, IL-6, TNF-α, and bone resorption [126]. Estrogen supplementation decreased concentrations of both pro-inflammatory cytokines and markers of bone resorption. Decrease in testosterone levels also increases osteoclastogenesis, and treatment with IL-6 antibody counteracts this, demonstrating the ability of testosterone to reduce proinflammatory cytokine mediated bone resorption [127,128]. As would be expected, individuals with low sex hormone levels have greater fracture risk and decreased BMD [129–132]. Thus, greater post-fracture bone loss in older people may be partially due to lower sex hormone concentrations.
Diminished ability to recover bone after fracture in aged individuals may also reflect a reduced anabolic response to mechanical loading compared to younger individuals [133,134]. As a result, return to normal activity levels may not be sufficient to fully recover lost bone in aged individuals. Thus, increased inflammation, reduction of sex hormones, and reduced anabolic response to loading may explain greater systemic bone loss and diminished recovery of bone in older individuals. From a clinical perspective, greater systemic bone loss post-fracture in older individuals suggests that interventions to preserve bone mass after fracture, potentially through decreasing the inflammatory response or increasing bone deposition during recovery, may be especially important for older patients. Future animal studies can evaluate systemic bone loss throughout the life course, and ovariectomized and orchidectomized animals can be used to explore the role of sex hormones in reducing bone loss after fracture.
Sex
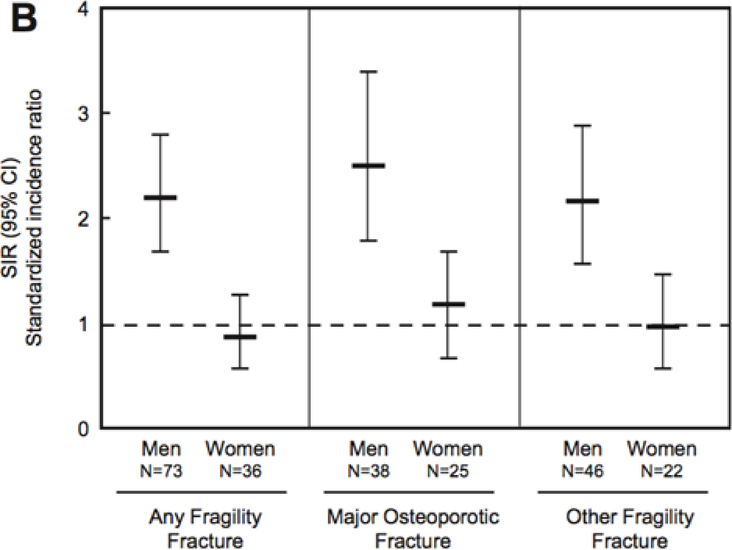
Sex-differences in the magnitude of bone loss following fracture have never been directly demonstrated. However, studies of fracture risk following an initial fracture suggest possible differences in either the magnitude of systemic bone loss or the extent of recovery. Amin et al. found that fractures before the age of 18 years were associated with a higher risk of future fracture in men but not in women (Figure 7) [10]. A meta-analysis of five studies determined that men older than fifty years with history of Colles’ fracture had a higher risk of subsequent hip fracture than women [7]. Another study found that 5 years after fracture, men had a considerably higher risk of hip and forearm fracture than women [3]. Thus, not only does risk of fracture appear greater in males, but the period of elevated risk may be longer as well. Although other factors such as behavioral differences cannot be ruled out, higher risk of subsequent fracture in men could result from greater systemic bone loss. The underlying mechanisms of sex-based differences in post-fracture bone loss and subsequent fracture risk remain unclear, but differences in growth and the inflammatory response are likely candidates.
Fig. 7.
Standardized incidence ratio (SIR) for the risk of fracture at age ≥50 years for men and women following a distal forearm fracture in childhood (age ≤18 years) from 1935–1992 [10]
Effect of Adolescent Growth on Sex-Differences in Systemic Bone Loss
Due to sexual differences in adolescent growth, a fracture during adolescence may create a lasting systemic bone deficit in men more than in women. During the growth spurt, adolescents exhibit a transient increase in bone porosity, fragility, and the incidence of fracture [10,135]. This may reflect the skeletal system addressing the mineral need created by rapid increase in stature, and a fracture would create a competing mineral demand [136]. As the male growth spurt is longer and achieves a greater velocity, this creates a greater mineral need, and adolescent males exhibit higher rates of bone turnover and a more marked increase in cortical porosity [137–139]. Consequently, males may not be able to divert as much mineral to recovering post-fracture bone loss as females. Adolescent males will therefore have a greater risk than females of achieving lower peak bone mass by the end of the growth period, potentially increasing fracture risk throughout adulthood.
Sex Differences in Systemic Bone Loss Due to Inflammation
While growth differences may increase systemic bone loss in adolescent males, variation in the inflammatory response may contribute to sex-differences in systemic bone loss throughout life. Males exhibit higher serum concentrations of IL-6 and TNF-α after injury than females [124,140–142]. This may partly reflect the differential effect of sex hormones on post-fracture inflammation. Although both estrogen and testosterone reduce the expression of IL-6 and other pro-inflammatory cytokines, estrogen may inhibit pro-inflammatory cytokine production more effectively than testosterone [126,127,143]. An in vitro study demonstrated that, compared to estradiol, two-fold greater androgen concentrations were required to inhibit production of IL-6 by human bone cells as well as osteoblast cell lines from rats [128]. Studies of fracture risk also indicate that estrogen may reduce bone resorption after fracture more than testosterone. In males low estrogen levels are better predictors of prospective fracture than testosterone [129,131]. In addition to direct inhibition of pro-inflammatory cytokine production, estrogen may also decrease monocyte levels by inducing mitotic arrest and apoptosis [144]. This is supported by an in vitro study of inflammatory response to lipopolysaccharide stimulation, which showed that normalizing TNF-α levels to monocyte counts removed the difference between men and women in cytokine concentrations [145]. Furthermore, higher monocyte levels in men may increase bone resorption, because monocytes can differentiate into osteoclasts in the presence of RANKL [60,146].
Overall, males may experience greater bone loss following fracture and reduced recovery compared to females, though this remains to be shown experimentally. This may partially due to differences in growth rate during adolescence, the protective effects of estrogen, or differences in immune system function. As with studies of age-differences, animal studies can provide a better understanding of sex differences in systemic bone loss and the extent to which this is mediated by differences in inflammation. Additional clinical studies could also be performed to evaluate sex-differences in systemic bone loss after fracture [2].
Summary
Based on current data, we can postulate that fracture initiates a period of systemic bone loss that may increase the risk of a future fracture at any skeletal site, and this increased risk may persist for several years if not longer. In the long term, incomplete recovery of bone quantity and quality increases the risk of osteoporotic fractures. In humans, bone loss begins almost immediately after fracture, and it may continue for up to two years. Subsequently, recovery in bone quantity occurs, but bone quantity and strength may not return to their pre-fracture levels, especially in older people. Disuse, inflammation, and circulating hormones that regulate calcium homeostasis are all likely mechanisms that contribute to systemic bone loss after fracture. Clinical and animal studies suggest that the magnitude of bone loss is greater in more severe injuries, in older individuals, and in males. To some degree, this likely reflects increased inflammatory response. Other factors such as greater mineral need and disuse following more severe fracture may also play important roles.
Although post-fracture bone loss likely increases fracture risk and accelerates the development of osteoporosis, understanding of this phenomenon remains incomplete. Both animal models and clinical studies can improve understanding of systemic bone loss after fracture. Specifically, future animal and clinical studies can better characterize the chronology of systemic bone loss and extent of recovery, as well as how this varies due to sex and age. Furthermore, research should explore the effect of specific signaling molecules such as pro-inflammatory cytokines, sclerostin, PTH, and sex hormones on systemic bone remodeling and skeletal strength following fracture. Ultimately, this knowledge could lead to treatments that can be applied soon after a fracture that will ameliorate post-traumatic bone loss and reduce future fracture risk for these patients.
Acknowledgments
The authors are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), under award number R01 AR071459.
Compliance with Ethical Standards
The authors, Benjamin Osipov, Armaun John Emami, and Blaine A. Christiansen, declare that they have no conflict of interest. The authors are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), under award number R01 AR071459. In animal studies cited involving the authors, all applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Works Cited
- 1.Learn What Osteoporosis Is and What It’s Caused by [Internet]. Natl. Osteoporos. Found. [cited 2018 Jul 27]. Available from: https://www.nof.org/patients/what-is-osteoporosis/
- 2.Christiansen BA, Harrison SL, Fink HA, Lane NE, Study of Osteoporotic Fractures Research Group. Incident fracture is associated with a period of accelerated loss of hip BMD: the study of osteoporotic fractures. Osteoporos Int. 2018;1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnell O, Kanis JA, Odén A, Sernbo I, Redlund-Johnell I, Petterson C, et al. Fracture risk following an osteoporotic fracture. Osteoporos Int. 2004;15:175–9. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed LA, Center JR, Bjørnerem Å, Bluic D, Joakimsen RM, Jørgensen L, et al. Progressively increasing fracture risk with advancing age after initial incident fragility fracture: the Tromsø study. J Bone Miner Res. 2013;28:2214–21. [DOI] [PubMed] [Google Scholar]
- 5.Clinton J, Franta A, Polissar NL, Neradilek B, Mounce D, Fink HA, et al. Proximal humeral fracture as a risk factor for subsequent hip fractures. J Bone Joint Surg Am. 2009;91:503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson CM, Royds M, Abraham A, Mcqueen MM, Court-Brown CM, Christie J. Refractures in patients at least forty-five years old: a prospective analysis of twenty-two thousand and sixty patients. J Bone Jt Surg-Am Vol. 2002;84:1528–33. [DOI] [PubMed] [Google Scholar]
- 7.Haentjens P, Autier P, Collins J, Velkeniers B, Vanderschueren D, Boonen S. Colles fracture, spine fracture, and subsequent risk of hip fracture in men and women: a meta-analysis. J Bone Jt Surg-Am Vol. 2003;85:1936–43. [DOI] [PubMed] [Google Scholar]
- 8.Lauritzen JB, Schwarz P, McNair P, Lund B, Transbøl I. Radial and humeral fractures as predictors of subsequent hip, radial or humeral fractures in women, and their seasonal variation. Osteoporos Int. 1993;3:133–7. [DOI] [PubMed] [Google Scholar]
- 9.Honkanen R, Tuppurainen M, Kroger H, Alhava E, Puntila E. Associations of early premenopausal fractures with subsequent fractures vary by sites and mechanisms of fractures. Calcif Tissue Int. 1997;60:327–31. [DOI] [PubMed] [Google Scholar]
- 10.Amin S, Melton LJ, Achenbach SJ, Atkinson EJ, Dekutoski MB, Kirmani S, et al. A distal forearm fracture in childhood is associated with an increased risk for future fragility fractures in adult men, but not women. J Bone Miner Res Off J Am Soc Bone Miner Res. 2013;28:1751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu F, Mason B, Horne A, Ames R, Clearwater J, Liu M, et al. Fractures between the ages of 20 and 50 years increase women’s risk of subsequent fractures. Arch Intern Med. 2002;162:33. [DOI] [PubMed] [Google Scholar]
- 12.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–39. [DOI] [PubMed] [Google Scholar]
- 13.Johnell O, Oden A, Caulin F, Kanis JA. Acute and long-term increase in fracture risk after hospitalization for vertebral fracture. Osteoporos Int. 2001;12:207–14. [DOI] [PubMed] [Google Scholar]
- 14.Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320–3. [DOI] [PubMed] [Google Scholar]
- 15.Schousboe JT, Fink HA, Lui L-Y, Taylor BC, Ensrud KE. Association between prior non-spine non-hip fractures or prevalent radiographic vertebral deformities known to be at least 10 years old and incident hip fracture. J Bone Miner Res. 2006;21:1557–64. [DOI] [PubMed] [Google Scholar]
- 16.Silman AJ. The patient with fracture: the risk of subsequent fractures. Am J Med. 1995;98:12S–16S. [DOI] [PubMed] [Google Scholar]
- 17.Szulc P, Feyt C, Chapurlat R. High risk of fall, poor physical function, and low grip strength in men with fracture—the STRAMBO study. J Cachexia Sarcopenia Muscle. 2016;7:299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whooley MA, Kip KE, Cauley JA, Ensrud KE, Nevitt MC, Browner WS. Depression, falls, and risk of fracture in older women. Arch Intern Med. 1999;159:484–90. [DOI] [PubMed] [Google Scholar]
- 19.Ensrud KE, Ewing SK, Taylor BC, Fink HA, Stone KL, Cauley JA, et al. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62:744–51. [DOI] [PubMed] [Google Scholar]
- 20.Bliuc D, Alarkawi D, Nguyen TV, Eisman JA, Center JR. Risk of subsequent fractures and mortality in elderly women and men with fragility fractures with and without osteoporotic bone density: the Dubbo osteoporosis epidemiology study. J Bone Miner Res. 2015;30:637–46. [DOI] [PubMed] [Google Scholar]
- 21.Karlsson MK, Hasserius R, Obrant KJ. The ankle fracture as an index of future fracture risk: a 25–40 year follow-up of 1063 cases. Acta Orthop Scand. 1993;64:482–4. [DOI] [PubMed] [Google Scholar]
- 22.Myers TA, Briffa NK. Secondary and tertiary prevention in the management of low-trauma fracture. Aust J Physiother. 2003;49:25–9. [DOI] [PubMed] [Google Scholar]
- 23.Frost HM. The regional acceleratory phenomenon: a review. Henry Ford Hosp Med J. 1983;31:3–9. [PubMed] [Google Scholar]
- 24.Frost HM. The biology of fracture healing: an overview for clinicians. Part 1. Clin Orthop. 1989;248:283–93. [PubMed] [Google Scholar]
- 25.Schnitzler CM, Solomon L. Histomorphometric analysis of a calcaneal stress fracture: a possible complication of fluoride therapy for osteoporosis. Bone. 1986;7:193–8. [DOI] [PubMed] [Google Scholar]
- 26.Obrant KJ, Nilsson BE. Histomorphologic changes in the tibial epiphysis after diaphyseal fracture. Clin Orthop. 1984;270–5. [PubMed] [Google Scholar]
- 27.Wendeberg B Mineral metabolism of fractures of the tibia in man studied with external counting of Sr 85. Acta Orthop Scand. 1961;32:3–81. [PubMed] [Google Scholar]
- 28.Nilsson BER. Post-traumatic osteopenia: a quantitative study of the bone mineral mass in the femur following fracture of the tibia in man using americium-241 as a photon source. Acta Orthop Scand. 1966;37:1–55. [DOI] [PubMed] [Google Scholar]
- 29.Finsen V, Haave O, Benum P. Fracture interaction in the extremities: the possible relevance of posttraumatic osteopenia. Clin Orthop. 1989;240:244–9. [PubMed] [Google Scholar]
- 30.Mueller M, Schilling T, Minne HW, Ziegler R. A systemic acceleratory phenomenon (SAP) accompanies the regional acceleratory phenomenon (RAP) during healing of a bone defect in the rat. J Bone Miner Res. 1991;6:401–10. [DOI] [PubMed] [Google Scholar]
- 31.Schilling T, Müller M, Minne HW, Ziegler R. Influence of inflammation-mediated osteopenia on the regional acceleratory phenomenon and the systemic acceleratory phenomenon during healing of a bone defect in the rat. Calcif Tissue Int. 1998;63:160–6. [DOI] [PubMed] [Google Scholar]
- 32.Einhorn TA, Simon G, Devlin VJ, Warman J, Sidhu SP, Vigorita VJ. The osteogenic response to distant skeletal injury. J Bone Jt Surg. 1990;72:1374–8. [PubMed] [Google Scholar]
- 33.Fischer V, Haffner-Luntzer M, Prystaz K, vom Scheidt A, Busse B, Schinke T, et al. Calcium and vitamin-D deficiency marginally impairs fracture healing but aggravates posttraumatic bone loss in osteoporotic mice. Sci Rep. 2017;7:7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emami AJ, Toupadakis CA, Telek SM, Fyhrie DP, Yellowley CE, Christiansen BA. Age dependence of systemic bone loss and recovery following femur fracture in mice. J Bone Miner Res. 2018;33:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veitch SW, Findlay SC, Hamer AJ, Blumsohn A, Eastell R, Ingle BM. Changes in bone mass and bone turnover following tibial shaft fracture. Osteoporos Int. 2006;17:364–72. [DOI] [PubMed] [Google Scholar]
- 36.Åkesson K, Ljunghall S, Jonsson B, Sernbo I, Johnell O, Gärdsell P, et al. Assessment of biochemical markers of bone metabolism in relation to the occurrence of fracture: a retrospective and prospective population-based study of women. J Bone Miner Res. 1995;10:1823–9. [DOI] [PubMed] [Google Scholar]
- 37.Obrant KJ, Ivaska KK, Gerdhem P, Alatalo SL, Pettersson K, Väänänen HK. Biochemical markers of bone turnover are influenced by recently sustained fracture. Bone. 2005;36:786–92. [DOI] [PubMed] [Google Scholar]
- 38.Ingle BM, Hay SM, Bottjer HM, Eastell R. Changes in bone mass and bone turnover following distal forearm fracture. Osteoporos Int. 1999;10:399–407. [DOI] [PubMed] [Google Scholar]
- 39.Ivaska KK, Gerdhem P, Åkesson K, Garnero P, Obrant KJ. Effect of fracture on bone turnover markers: a longitudinal study comparing marker levels before and after injury in 113 elderly women. J Bone Miner Res. 22:1155–64. [DOI] [PubMed] [Google Scholar]
- 40.Yu‐Yahiro JA, Michael RH, Dubin NH, Fox KM, Sachs M, Hawkes WG, et al. Serum and urine markers of bone metabolism during the year after hip fracture. J Am Geriatr Soc. 2001;49:877–83. [DOI] [PubMed] [Google Scholar]
- 41.Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2:97–101. [DOI] [PubMed] [Google Scholar]
- 42.Lindsey BA, Clovis NB, Smith ES, Salihu S, Hubbard DF. An animal model for open femur fracture and osteomyelitis—Part II: Immunomodulation with systemic IL-12. J Orthop Res. 2010;28:43–7. [DOI] [PubMed] [Google Scholar]
- 43.Haffner-Luntzer M, Heilmann A, Heidler V, Liedert A, Schinke T, Amling M, et al. Hypochlorhydria-induced calcium malabsorption does not affect fracture healing but increases post-traumatic bone loss in the intact skeleton: calcium prevents secondary hyperparathyroidism after fracture. J Orthop Res. 2016;34:1914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christiansen BA, Anderson MJ, Lee CA, Williams JC, Yik JHN, Haudenschild DR. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2012;20:773–82. [DOI] [PubMed] [Google Scholar]
- 45.Christiansen BA, Emami AJ, Fyhrie DP, Satkunananthan PB, Hardisty MR. Trabecular bone loss at a distant skeletal site following noninvasive knee injury in mice. J Biomech Eng. 2015;137:0110051–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ceroni D, Martin X, Delhumeau C, Rizzoli R, Kaelin A, Farpour-Lambert N. Effects of cast-mediated immobilization on bone mineral mass at various sites in adolescents with lower-extremity fracture. J Bone Jt Surg-Am Vol. 2012;94:208–16. [DOI] [PubMed] [Google Scholar]
- 47.Findlay SC, Eastell R, Ingle BM. Measurement of bone adjacent to tibial shaft fracture. Osteoporos Int. 2002;13:980–9. [DOI] [PubMed] [Google Scholar]
- 48.Johnell O, Nilsson BE. Bone mineral content in men with fractures of the upper end of the femur. Int Orthop. 1984;7:229–31. [DOI] [PubMed] [Google Scholar]
- 49.Kannus P, Järvinen M, Sievänen H, Järvinen TAH, Oja P, Vuori I. Reduced bone mineral density in men with a previous femur fracture. J Bone Miner Res. 1994;9:1729–36. [DOI] [PubMed] [Google Scholar]
- 50.van der Poest Clement E, van der Wiel H, Patka P, Roos JC, Lips P. Long-term consequences of fracture of the lower leg: cross-sectional study and long-term longitudinal follow-up of bone mineral density in the hip after fracture of lower leg. Bone. 1999;24:131–4. [DOI] [PubMed] [Google Scholar]
- 51.Petersen MM, Gehrchen PM, Nielsen PK, Lund B. Loss of bone mineral of the hip assessed by DEXA following tibial shaft fractures. Bone. 1997;20:491–5. [DOI] [PubMed] [Google Scholar]
- 52.Westlin NE. Loss of bone mineral after Colles’ fracture. Clin Orthop. 1974;194–9. [DOI] [PubMed] [Google Scholar]
- 53.Härmä M, Karjalainen P. Trabecular osteopenia in Colles’ fracture. Acta Orthop Scand. 1986;57:38–40. [DOI] [PubMed] [Google Scholar]
- 54.Kazakia GJ, Tjong W, Nirody JA, Burghardt AJ, Carballido-Gamio J, Patsch JM, et al. The influence of disuse on bone microstructure and mechanics assessed by HR-pQCT. Bone. 2014;63:132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magaziner J, Wehren L, Hawkes WG, Orwig D, Hebel JR, Fredman L, et al. Women with hip fracture have a greater rate of decline in bone mineral density than expected: another significant consequence of a common geriatric problem. Osteoporos Int. 2006;17:971–7. [DOI] [PubMed] [Google Scholar]
- 56.Karlsson M, Nilsson JÅ, Sernbo I, Redlund-Johnell I, Johnell O, Obrant KJ. Changes of bone mineral mass and soft tissue composition after hip fracture. Bone. 1996;18:19–22. [DOI] [PubMed] [Google Scholar]
- 57.Rathbun AM, Magaziner J, Shardell MD, Yerges-Armstrong LM, Orwig D, Hicks GE, et al. Older men who sustain a hip fracture experience greater declines in bone mineral density at the contralateral hip than non-fractured comparators. Osteoporos Int. 2018;29:365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dirschl DR, Henderson RC, Oakley WC. Accelerated bone mineral loss following a hip fracture: a prospective longitudinal study. Bone. 1997;21:79–82. [DOI] [PubMed] [Google Scholar]
- 59.Capulli M, Paone R, Rucci N. Osteoblast and osteocyte: games without frontiers. Arch Biochem Biophys. 2014;561:3–12. [DOI] [PubMed] [Google Scholar]
- 60.Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 2012;11:234–50. [DOI] [PubMed] [Google Scholar]
- 61.Lacey DL, Timms E, Tan H-L, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. [DOI] [PubMed] [Google Scholar]
- 62.Boyce BF, Xing L. The RANKL/RANK/OPG pathway. Curr Osteoporos Rep. 2007;5:98–104. [DOI] [PubMed] [Google Scholar]
- 63.Prideaux M, Findlay DM, Atkins GJ. Osteocytes: The master cells in bone remodelling. Curr Opin Pharmacol. 2016;28:24–30. [DOI] [PubMed] [Google Scholar]
- 64.Schaffler MB, Cheung W-Y, Majeska R, Kennedy O. Osteocytes: master orchestrators of bone. Calcif Tissue Int. 2014;94:5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Brien CA. The variety of osteocyte function Osteoimmunology. San Diego: Academic Press; 2016. p. 83–102. [Google Scholar]
- 66.Chen H, Senda T, Kubo K. The osteocyte plays multiple roles in bone remodeling and mineral homeostasis. Med Mol Morphol. 2015;48:61–8. [DOI] [PubMed] [Google Scholar]
- 67.Finsen V, Benum P. Osteopenia after ankle fractures. The influence of early weight bearing and muscle activity. Clin Orthop. 1989;261–8. [PubMed] [Google Scholar]
- 68.Leblanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM. Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res. 1990;5:843–50. [DOI] [PubMed] [Google Scholar]
- 69.Moriishi T, Fukuyama R, Ito M, Miyazaki T, Maeno T, Kawai Y, et al. Osteocyte network: a negative regulatory system for bone mass augmented by the induction of RANKL in osteoblasts and SOST in osteocytes at unloading. PLOS ONE. 2012;7:e40143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Komori T Functions of the osteocyte network in the regulation of bone mass. Cell Tissue Res. 2013;352:191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pathak JL, Bravenboer N, Luyten FP, Verschueren P, Lems WF, Klein-Nulend J, et al. Mechanical loading reduces inflammation-induced human osteocyte-to-osteoclast communication. Calcif Tissue Int. 2015;97:169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Komori T Animal models for osteoporosis. Eur J Pharmacol. 2015;759:287–94. [DOI] [PubMed] [Google Scholar]
- 74.Gaudio A, Pennisi P, Bratengeier C, Torrisi V, Lindner B, Mangiafico RA, et al. Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J Clin Endocrinol Metab. 2010;95:2248–53. [DOI] [PubMed] [Google Scholar]
- 75.Sarahrudi K, Thomas A, Albrecht C, Aharinejad S. Strongly enhanced levels of sclerostin during human fracture healing. J Orthop Res. 2012;30:1549–55. [DOI] [PubMed] [Google Scholar]
- 76.Spatz JM, Fields EE, Yu EW, Divieti Pajevic P, Bouxsein ML, Sibonga JD, et al. Serum sclerostin increases in healthy adult men during bed rest. J Clin Endocrinol Metab. 2012;97:E1736–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury. 2007;38:1336–45. [DOI] [PubMed] [Google Scholar]
- 78.Walsh NC, Gravallese EM. Bone remodeling in rheumatic disease: a question of balance. Immunol Rev. 2010;233:301–12. [DOI] [PubMed] [Google Scholar]
- 79.Joffe I, Epstein S. Osteoporosis associated with rheumatoid arthritis: pathogenesis and management. Semin Arthritis Rheum. 1991;20:256–72. [DOI] [PubMed] [Google Scholar]
- 80.Peel NF, Eastell R, Russell RG. Osteoporosis in rheumatoid arthritis--the laboratory perspective. Br J Rheumatol. 1991;30:84–5. [DOI] [PubMed] [Google Scholar]
- 81.Lombardi F, Franzese A, Iafusco D, del Puente A, Esposito A, Prisco F, et al. Bone involvement in clusters of autoimmune diseases: just a complication? Bone. 2010;46:551–5. [DOI] [PubMed] [Google Scholar]
- 82.Agrawal M, Arora S, Li J, Rahmani R, Sun L, Steinlauf AF, et al. Bone, inflammation, and inflammatory bowel disease. Curr Osteoporos Rep. 2011;9:251–7. [DOI] [PubMed] [Google Scholar]
- 83.Montalcini T, Romeo S, Ferro Y, Migliaccio V, Gazzaruso C, Pujia A. Osteoporosis in chronic inflammatory disease: the role of malnutrition. Endocrine. 2013;43:59–64. [DOI] [PubMed] [Google Scholar]
- 84.Braun T, Schett G. Pathways for bone loss in inflammatory disease. Curr Osteoporos Rep. 2012;10:101–8. [DOI] [PubMed] [Google Scholar]
- 85.Baker-LePain JC, Nakamura MC, Lane NE. Effects of inflammation on bone: an update: Curr Opin Rheumatol. 2011;23:389–95. [DOI] [PubMed] [Google Scholar]
- 86.Loi F, Córdova LA, Pajarinen J, Lin T, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone. 2016;86:119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsiridis E, Upadhyay N, Giannoudis P. Molecular aspects of fracture healing: which are the important molecules? Injury. 2007;38:S11–25. [DOI] [PubMed] [Google Scholar]
- 88.Steeve KT, Marc P, Sandrine T, Dominique H, Yannick F. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15:49–60. [DOI] [PubMed] [Google Scholar]
- 89.Charles JF, Nakamura MC. Bone and the innate immune system. Curr Osteoporos Rep. 2014;12:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Einhorn TA, Majeska RJ, Rush EB, Levine PM, Horowitz MC. The expression of cytokine activity by fracture callus. J Bone Miner Res. 1995;10:1272–81. [DOI] [PubMed] [Google Scholar]
- 91.Souza PPC, Lerner UH. The role of cytokines in inflammatory bone loss. Immunol Invest. 2013;42:555–622. [DOI] [PubMed] [Google Scholar]
- 92.Wu Q, Zhou X, Huang D, Ji Y, Kang F. IL-6 enhances osteocyte-mediated osteoclastogenesis by promoting JAK2 and RANKL Activity in vitro. Cell Physiol Biochem. 2017;41:1360–9. [DOI] [PubMed] [Google Scholar]
- 93.Ke HZ, Richards WG, Li X, Ominsky MS. Sclerostin and dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev. 2012;33:747–83. [DOI] [PubMed] [Google Scholar]
- 94.Chang JC, Christiansen BA, Murugesh DK, Sebastian A, Hum NR, Collette NM, et al. SOST/sclerostin improves post traumatic osteoarthritis and inhibits MMP2/3 expression after injury. J Bone Miner Res. 2018;33:1105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kon T, Cho T-J, Aizawa T, Yamazaki M, Nooh N, Graves D, et al. Expression of osteoprotegerin, receptor activator of NF-κB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res. 16:1004–14. [DOI] [PubMed] [Google Scholar]
- 96.Currie HN, Loos MS, Vrana JA, Dragan K, Boyd JW. Spatial cytokine distribution following traumatic injury. Cytokine. 2014;66:112–8. [DOI] [PubMed] [Google Scholar]
- 97.Hauser CJ, Zhou X, Joshi P, Cuchens MA, Kregor P, Devidas M, et al. The immune microenvironment of human fracture/soft-tissue hematomas and its relationship to systemic immunity. J Trauma Acute Care Surg. 1997;42:895–904. [DOI] [PubMed] [Google Scholar]
- 98.Hisano S, Sakamoto K, Ishiko T, Kamohara H, Ogawa M. IL-6 And soluble IL-6 receptor levels change differently after surgery both in the blood and in the operative field. Cytokine. 1997;9:447–52. [DOI] [PubMed] [Google Scholar]
- 99.Hitz MF, Jensen J-EB, Eskildsen PC. Bone mineral density and bone markers in patients with a recent low-energy fracture: effect of 1 y of treatment with calcium and vitamin D. Am J Clin Nutr. 2007;86:251–9. [DOI] [PubMed] [Google Scholar]
- 100.Dedic C, Hung TS, Shipley AM, Maeda A, Gardella T, Miller AL, et al. Calcium fluxes at the bone/plasma interface: acute effects of parathyroid hormone (PTH) and targeted deletion of PTH/PTH-related peptide (PTHrP) receptor in the osteocytes. Bone. 2018;116:135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Silva BC, Bilezikian JP. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol. 2015;22:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jilka RL. Cytokines, bone remodeling, and estrogen deficiency: a 1998 update. Bone. 1998;23:75–81. [DOI] [PubMed] [Google Scholar]
- 103.Qing H, Ardeshirpour L, Pajevic PD, Dusevich V, Jähn K, Kato S, et al. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res Off J Am Soc Bone Miner Res. 2012;27:1018–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saftig P, Hunziker E, Wehmeyer O, Jones S, Boyde A, Rommerskirch W, et al. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci. 1998;95:13453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hayman AR. Tartrate-resistant acid phosphatase (TRAP) and the osteoclast/immune cell dichotomy. Autoimmunity. 2008;41:218–23. [DOI] [PubMed] [Google Scholar]
- 106.Della Rocca GJ, Crist BD, Murtha YM. Parathyroid hormone: is there a role in fracture healing? J Orthop Trauma. 2010;24:S31–5. [DOI] [PubMed] [Google Scholar]
- 107.Meller Y, Kestenbaum RS, Mozes M, Mozes G, Yagil R, Shany S. Mineral and endocrine metabolism during fracture healing in dogs. Clin Orthop. 1984;289–95. [PubMed] [Google Scholar]
- 108.Cappola AR, Hawkes WG, Blocher N, Yu-Yahiro J, Orwig D, Fredman L, et al. The hormonal profile of hip fracture female patients differs from community-dwelling peers over a 1-year follow-up period. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2011;22:339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ellegaard M, Kringelbach T, Syberg S, Petersen S, Beck Jensen J-E, Brüel A, et al. The effect of PTH(134) on fracture healing during different loading conditions. J Bone Miner Res. 2013;28:2145–55. [DOI] [PubMed] [Google Scholar]
- 110.Holzer G, Majeska RJ, Lundy MW, Hartke JR, Einhorn TA. Parathyroid hormone enhances fracture healing: a preliminary report. Clin Orthop. 1999;366:258–63. [DOI] [PubMed] [Google Scholar]
- 111.Barnes GL, Kakar S, Vora S, Morgan EF, Gerstenfeld LC, Einhorn TA. Stimulation of fracture-healing with systemic intermittent parathyroid hormone treatment. J Bone Jt Surg-Am Vol. 2008;90:120–7. [DOI] [PubMed] [Google Scholar]
- 112.Zhang D, Potty A, Vyas P, Lane J. The role of recombinant PTH in human fracture healing: a systematic review. J Orthop Trauma. 2014;28:57–62. [DOI] [PubMed] [Google Scholar]
- 113.Okeny PK, Ongom P, Kituuka O. Serum interleukin-6 level as an early marker of injury severity in trauma patients in an urban low-income setting: a cross-sectional study. BMC Emerg Med. 2015;15:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stensballe J, Christiansen M, Tønnesen E, Espersen K, Lippert FK, Rasmussen LS. The early IL-6 and IL-10 response in trauma is correlated with injury severity and mortality. Acta Anaesthesiol Scand. 2009;53:515–21. [DOI] [PubMed] [Google Scholar]
- 115.Pfeifer R, Darwiche S, Kohut L, Billiar TR, Pape H-C. Cumulative effects of bone and soft tissue injury on systemic inflammation: a pilot study. Clin Orthop. 2013;471:2815–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Weckbach S, Perl M, Heiland T, Braumüller S, Stahel PF, Flierl MA, et al. A new experimental polytrauma model in rats: molecular characterization of the early inflammatory response. Mediators Inflamm [Internet]. 2012; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3317068/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ginaldi L, Di Benedetto MC, De Martinis M. Osteoporosis, inflammation and ageing. Immun Ageing A. 2005;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vester H, Huber-Lang MS, Kida Q, Scola A, van Griensven M, Gebhard F, et al. The immune response after fracture trauma is different in old compared to young patients. Immun Ageing. 2014;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thaeter M, Knobe M, Kerckhove MV, Böhle F, Herold J, Verhaven E, et al. Perioperative inflammatory response in major fracture: do geriatric patients behave differently? Eur J Trauma Emerg Surg. 2016;42:547–51. [DOI] [PubMed] [Google Scholar]
- 121.Hazeldine J, Lord JM, Hampson P. Immunesenescence and inflammaging: a contributory factor in the poor outcome of the geriatric trauma patient. Ageing Res Rev. 2015;24:349–57. [DOI] [PubMed] [Google Scholar]
- 122.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–70. [DOI] [PubMed] [Google Scholar]
- 123.Starr ME, Evers BM, Saito H. Age-associated increase in cytokine production during systemic inflammation: adipose tissue as a major source of IL-6. J Gerontol A Biol Sci Med Sci. 2009;64A:723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pacifici R Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res. 1996;11:1043–51. [DOI] [PubMed] [Google Scholar]
- 125.Jilka RL, Hangoc G, Girasole G, Passeri G, Williams DC, Abrams JS, et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257:88–91. [DOI] [PubMed] [Google Scholar]
- 126.Pacifici R, Brown C, Puscheck E, Friedrich E, Slatopolsky E, Maggio D, et al. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci U S A. 1991;88:5134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bellido T, Jilka RL, Boyce BF, Girasole G, Broxmeyer H, Dalrymple SA, et al. Regulation of interleukin-6, osteoclastogenesis, and bone mass by androgens. The role of the androgen receptor. J Clin Invest. 1995;95:2886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Girasole G, Jilka RL, Passeri G, Boswell S, Boder G, Williams DC, et al. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest. 1992;89:883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mellström D, Vandenput L, Mallmin H, Holmberg AH, Lorentzon M, Odén A, et al. Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J Bone Miner Res. 2008;23:1552–60. [DOI] [PubMed] [Google Scholar]
- 130.Mellström D, Johnell O, Ljunggren Ö, Eriksson A-L, Lorentzon M, Mallmin H, et al. Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res. 2006;21:529–35. [DOI] [PubMed] [Google Scholar]
- 131.LeBlanc ES, Nielson CM, Marshall LM, Lapidus JA, Barrett-Connor E, Ensrud KE, et al. The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. J Clin Endocrinol Metab. 2009;94:3337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Orwoll ES, Lapidus J, Wang PY, Vandenput L, Hoffman AR, Fink HA, et al. The limited clinical utility of testosterone, estradiol and sex hormone binding globulin measurements in the prediction of fracture risk and bone loss in older men. J Bone Miner Res Off J Am Soc Bone Miner Res. 2017;32:633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ruff CB, Walker A, Trinkaus E. Postcranial robusticity in Homo. III: ontogeny. Am J Phys Anthropol. 1994;93:35–54. [DOI] [PubMed] [Google Scholar]
- 134.Lieberman DE, Devlin MJ, Pearson OM. Articular area responses to mechanical loading: effects of exercise, age, and skeletal location. Am J Phys Anthropol. 2001;116:266–277. [DOI] [PubMed] [Google Scholar]
- 135.Cooper C, Dennison EM, Leufkens HG, Bishop N, van Staa TP. Epidemiology of childhood fractures in Britain: a study using the general practice research database. J Bone Miner Res. 2004;19:1976–81. [DOI] [PubMed] [Google Scholar]
- 136.Parfitt AM. The two faces of growth: benefits and risks to bone integrity. Osteoporos Int. 1994;4:382–98. [DOI] [PubMed] [Google Scholar]
- 137.Gabel L, Macdonald HM, McKay HA. Sex differences and growth-related adaptations in bone microarchitecture, geometry, density, and strength from childhood to early adulthood: a mixed longitudinal HR-pQCT study. J Bone Miner Res. 2017;32:250–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Largo RH, Gasser T, Prader A, Stuetzle W, Huber PJ. Analysis of the adolescent growth spurt using smoothing spline functions. Ann Hum Biol. 1978;5:421–34. [DOI] [PubMed] [Google Scholar]
- 139.Tanner JM, Whitehouse RH, Marubini E, Resele LF. The adolescent growth spurt of boys and girls of the Harpenden growth study. Ann Hum Biol. 1976;3:109–26. [DOI] [PubMed] [Google Scholar]
- 140.Schroder J, Kahlke V, Staubach K-H, Zabel P. Gender differences in human sepsis. Arch Surg. 1998;133:1200–5. [DOI] [PubMed] [Google Scholar]
- 141.Diodato MD, Knöferl MW, Schwacha MG, Bland KI, Chaudry IH. Gender differences in the inflammatory response and survival following haemorrhage and subsequent sepsis. Cytokine. 2001;14:162–9. [DOI] [PubMed] [Google Scholar]
- 142.Kovacs EJ, Plackett TP, Witte PL. Estrogen replacement, aging, and cell-mediated immunity after injury. J Leukoc Biol. 2004;76:36–41. [DOI] [PubMed] [Google Scholar]
- 143.Bruunsgaard, Pedersen Schroll, Skinhøj Pedersen. Impaired production of proinflammatory cytokines in response to lipopolysaccharide (LPS) stimulation in elderly humans. Clin Exp Immunol. 1999;118:235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11:411–23. [DOI] [PubMed] [Google Scholar]
- 145.Lefèvre N, Corazza F, Duchateau J, Desir J, Casimir G. Sex differences in inflammatory cytokines and CD99 expression following in vitro lipopolysaccharide stimulation. Shock. 2012;38:37–42. [DOI] [PubMed] [Google Scholar]
- 146.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–49. [DOI] [PubMed] [Google Scholar]