Abstract
Purpose
In solid tumors such as prostate cancer, novel paradigms are needed to assess therapeutic efficacy. We utilized a method estimating tumor growth and regression rate constants from serial PSA measurements, and assessed its potential in patients with metastatic castration resistant prostate cancer (mCRPC).
Experimental Design
Patients were enrolled in five phase II studies, including an experimental vaccine trial, representing the evolution of therapy in mCRPC. PSA measurements obtained prior to, and during, therapy were used. Data analysis using a two-phase mathematical equation yielded concomitant PSA growth and regression rate constants.
Results
Growth rate constants (g) can be estimated while patients receive therapy and in such patients g is superior to PSA-DT in predicting OS. Incremental reductions in growth rate constants were recorded in successive trials with a 10-fold slower g in the most recent combination therapy trial (log g, 10−3.17) relative to single-agent thalidomide (log g, 10−2.08) more than a decade earlier. Growth rate constants correlated with survival, except in patients receiving vaccine-based therapy where the evidence demonstrates prolonged survival presumably due to immunity developing subsequent to vaccine administration.
Conclusion
Incremental reductions in tumor growth rate constants suggest increased efficacy in successive chemotherapy trials. The derived growth rate constant correlates with survival, and may be used to assess efficacy. The PSA-TRICOM vaccine appears to have provided marked benefit not apparent during vaccination, but consistent with subsequent development of a beneficial immune response. If validated as a surrogate for survival, growth rate constants would offer an important new efficacy endpoint for clinical trials.
Keywords: Prostate cancer therapies, PSA, TRICOM, Cancer clinical trial assessment, bevacizumab
INTRODUCTION
In 2009 in the United States, approximately 562,340 people died of cancer, most from chemotherapy-refractory solid tumors [1]. Among these, almost 27,360 died from metastatic castration resistant prostate carcinoma (mCRPC) [1]. Traditionally treated with hormonal therapies and orchiectomy, the demonstration of docetaxel’s activity began a shift in outcomes for patients with mCRPC. However, because these therapies are not curative, alternative treatment strategies and novel decision paradigms must be developed [2, 3]. We explore whether tumor growth rates could furnish an efficacy endpoint to help decide between alternative therapies.
We previously described an equation that models tumor response to therapy as the sum of simultaneous regression (decay, d) and progression (growth, g), generating rate constants for each parameter [4, 5]. The rate constants are derived using equations based on first order kinetics fitted to data collected in clinical trials. In tumors that respond to therapy, regression dominates from the beginning of therapy until nadir, while growth dominates after nadir. In tumors that do not respond, the latter dominates throughout. In renal cell cancer the kinetics were based on CT measurements of tumor dimensions [4], while in prostate cancer, serum PSA was used [5]. The growth rate constant, g, was found to correlate with survival, while, surprisingly, the regression rate constant, d, did not. These results parallel studies in prostate cancer showing that PSA doubling time (PSA-DT) correlates with survival and can guide determination of therapeutic efficacy [6 – 13]. However, unlike PSA-DT, which cannot be determined while concomitant regression occurs during therapy, the growth rate constant of the treatment refractory fraction of a tumor can be discerned even as the nadir tumor amount is approached.
Here, we report the use of this equation in analysis of PSA data obtained in patients with mCRPC, enrolled in a series of phase II trials conducted at the National Institutes of Health over more than a decade. We illustrate the growth rate constant’s potential value in predicting survival, and in evaluating, comparing and providing insight into different therapies.
MATERIALS AND METHODS
Clinical Trial and Study Design
The Institutional Review Board of the NCI approved all the clinical studies. All patients provided written informed consent. The primary objective of all trials was to determine whether novel combinations of chemotherapy or a vaccine therapy produced sufficiently high clinical responses or benefit to warrant further investigation in patients with mCRPC. All patients had mCRPC and had disease progression following combined androgen blockade, as well as anti-androgen withdrawal. The therapies employed included thalidomide as a single agent [14], ketoconazole plus alendronate [15], thalidomide plus docetaxel [16, 17], bevacizumab (Avastin®), Thalidomide, docetaxel (Taxotere®) and Prednisone (ATTP) [18], and PSA-TRICOM, an experimental vaccine composed of recombinant poxviral vectors containing transgenes for PSA and the co-stimulatory molecules B7.1, ICAM-1, and LFA-3 [19 – 20]. In a separate randomized phase II study PSA-TRICOM has demonstrated an overall survival advantage compared to the control arm [21]. There were no detectable anti-PSA antibodies seen in either vaccine study which if present could have artificially altered PSA levels.
Overall survival (OS) was calculated from the on-study date until date of death. PSA values were obtained in some cases before the start of therapy, and in all cases at regular intervals after the start of therapy. Responses were scored according to PSA Working Group criteria [22, 23].
Mathematical, Data and Statistical Analyses
Mathematical analysis
The regression-growth equation
We have developed an equation based on the assumption that the change of a tumor’s quantity during therapy, here and throughout indicated by the change in the PSA measurement, results from two independent component processes: an exponential (first order kinetics) decrease/regression and an exponential re-growth of the tumor. (To allow ready adaptation of this method to other data sets, Supplemental Table 1 can be downloaded as an Excel spreadsheet and tumor measurements inserted to compute the appropriate rate constants. Alternatively, tumor quantity data can be uploaded into the web site at http://www.tumorgrowthanalyses.com and the rate constants extracted.
The equation is:
| Eq. (1) |
where exp is the base of the natural logarithm, e = 2.7182…, and f(t) is the tumor (or, here, PSA) measurement at time t in days, normalized to (divided by) the tumor measurement at day 0, the time at which treatment is commenced. Rate constant d (decay, in days−1) represents the exponential decrease/regression of the PSA signal during therapy. Rate constant g (growth, also in days−1) represents the exponential growth/re-growth of the tumor during treatment. These rate constants may be expressed in terms of half-lives and doubling times. Thus, d equals ln2 (0.693..) divided by the time it takes for the regressing part to shrink by half, while g equals ln2 divided by the time for the growing component to double.
Two earlier papers depict theoretical curves depicting the separate components of Eq. (1) and how these combine together to give the time dependence of the tumor size, f [4,5]. When the data showed a continuous decrease from the time of treatment start, so that only the regression parameter d was found to differ significantly from zero with p < 0.05, Eq. (1) was replaced by the following reduced form, with the growth rate constant eliminated:
| Eq. (2) |
When tumor measurements showed a continuous increase, so that only the growth parameter g was found to differ significantly from zero with p < 0.05, Eq. (1) was replaced by the following reduced form, with the decay constant eliminated:
| Eq. (3) |
Supplemental Figure 1 depicts a set of PSA measurements through which lines were fitted using this model.
Data analysis
We attempted to fit Eq. (1) to each data set for which more than one data point was available. Curve fitting was performed using Sigmaplot (Systat Software, Point Richmond, CA), or by using the Solver routine in an Excel spreadsheet as exemplified in Supplemental Table 1. We extracted parameters g and d with their associated Student’s t and p values.
Statistical analyses
Data were analyzed in Excel (Microsoft) and in Sigmaplot 9.0. Linear regressions to evaluate the relationship between the growth rate constant, g, or other parameters and survival were implemented using the polynomial linear routine of Sigmaplot 9.0. Sample comparisons were performed by Student’s t-test, using SigmaStat 3.5 (Systat Software, Point Richmond, CA), with p set at 0.05 for significance.
RESULTS
The patients analyzed were enrolled in five clinical trials beginning February 1996 and continuing until 2010. In total, 268 patients with mCRPC were treated with experimental therapies. Full clinical information on these patients can be found in the primary trial report and summarized here in Table 1 [14 – 18, 20, 21]. Analysis of the tumor regression and growth data for the patients treated with ketoconazole plus alendronate or with thalidomide plus docetaxel was previously reported [5]. The current analysis evaluates data from the original two studies, plus data obtained in three additional trials, providing both an internal validation of the growth rate constant and a view of prostate cancer therapy over time [14 – 18]. Table 1 summarizes the trials. As seen from the table, the patient groups were, at entry, very similar to one another. Additionally, for three trials for which PSA data were available prior to study enrollment, the median tumor growth rate constants before entry were also not significantly different.
Table 1.
Clinical Characteristics
| Study | Thal | K + A | T + D | ATTP | TRIC | |
|---|---|---|---|---|---|---|
| Start date | 2/27/96 | 3/23/99 | 12/20/99 | 3/13/05 | 5/22/03 | |
| Number of patients assessed | 54 | 62 | 50 | 54 | 47 | |
| Median age (range) | 68 (50–83) | 72 (51–85) | 71 (52–83) | 66 (44–79) | 66 (47–81) | |
| Median Gleason score (range) | 8 (5–10) | 8 (4–9) | 8 (6–9) | 8 (5–10) | 8 (5–10) | |
| ECOG PS (%) 0 | 28 | 19 | 22 | 13 | 30 | |
| 1 | 66 | 70 | 78 | 80 | 64 | |
| 2 | 6 | 11 | - | 7 | 6 | |
| One or more prior chemotherapies (%) | 26 | N/A | 10 | 19 | 13 | |
| Secondary hormonal treatment (%) | N/A | 58% | 61% | 95% | 100% | |
| Soft tissue lesions (%) | 84% | 22% | 41%1 | 62% | 46% | |
| Lactate dehydrogenase, U/L | N/A | 196 (137–525) | 190 (143–777) | 198.5 (122–397) | 181 (115–916) | |
| Alkaline phosphatase, U/L | N/A | 134.5 (71–4000) | 126 (73–591) | 107 (45–720) | 77 (26–1752) | |
| Hemoglobin | N/A | 13.05 (9–15.4) | 13.2 (7.2–15.1) | 12.7 (8.3–14.3) | 12.6 (7.5–16.4) | |
| Overall survival (K-M) All patients2 | 457 | 635 | 730 | 8462 | 8012 | |
| Enrollment PSA ≤ median3,4 | 520ns | 762ns | 882* | 907ns | 1140** | |
| Enrollment PSA > median3,5 | 333ns | 592ns | 466* | 779ns | 520** | |
| Parameters describing treatment response | ||||||
| Log median growth or regression rates (days−1) and median doubling or half-times (days) | ||||||
| Log of pre-enrollment growth rate | −1.82 | N/A | N/A | −1.89 | −1.96 | |
| Pre-enrollment doubling time | 47 | N/A | N/A | 53 | 66 | |
| Log of growth rate on therapy6 | −2.08 | −2.66 | −2.57 | −3.25 | −2.07 | |
| Doubling time on therapy7 | 83 | 320 | 260 | 1244 | 124 | |
| Correlation of log growth rate with OS | −0.386 | −0.723 | −0.748 | −0.492 | −0.413 | |
| Log of regression rate on therapy8 | −1.52 | −1.66 | −1.74 | −1.52 | −1.57 | |
| Tumor half life on therapy9 | 23 | 32 | 38 | 23 | 26 | |
| Median times on study or to nadir (days) | ||||||
| Time on therapy10 | 70 | 240 | 203 | 424 | 147 | |
| Time to nadir11 | 14 | 59 | 75 | 168 | 0 | |
| Correlation of time to nadir with OS | 0.24ns | 0.624 | 0.435 | 0.476 | 0.27ns | |
| Time from nadir to off therapy | 56 | 181 | 128 | 256 | 147 | |
| Median PSA levels and medians of ratios | ||||||
| Enrollment PSA12 | 121.9 | 75.2 | 60.5 | 101.6 | 93 | |
| Nadir PSA13 | 70.2 | 22.2 | 19.2 | 9.45 | 67.6 | |
| Ratio nadir PSA/enrollment PSA | 0.69 | 0.38 | 0.52 | 0.082 | 1 | |
| Correlation of nadir ratio with OS | −0.16ns | −0.508 | −0.512 | −0.383 | −0.22ns | |
| Off therapy PSA14 | 133.4 | 48.8 | 41.5 | 65.5 | 203 | |
| Ratio off therapy PSA/nadir PSA | 1.9 | 2.19 | 2.16 | 6.93 | 3.0 | |
Measurable
Thal vs. TRIC, p = 0.002; Thal vs. ATTP, p = 0.007. All other pair-wise comparisons, p > 0.05, ns
Pair-wise comparisons of OS on study PSA ≤ median vs. on study PSA > median: ns = p > 0.05, [*] = p < 0.05, [**] = p < 0.001
Within group of “enrollment PSA ≤ median”: TRIC vs. ATTP, p = 0.025; vs. T+D, p = 0.040; vs. K+ A, p = 0.006; vs. Thal, p = 0.002. All other pair-wise comparisons, p > 0.05, ns
Within group of “enrollment PSA > median”, all pair-wise comparisons, p > 0.05, ns
Thal vs. TRIC, n.s.; K + A vs. T + D, ns; Thal or TRIC vs. T + D or K + A, p < 0.001; ATTP vs. all others, p < 0.001. Doubling time = 0.0693/10 −log growth rate
T + D vs. Thal, p < 0.005, T + D vs. ATTP, p = 0.012; all others ns (For TRIC, n = 5).
Tumor half life = 0.0693/10 −log regression rate
Thal vs. all others, p < 0.001, TRIC vs. K + A or T + D, p < 0.05; K + A vs. T + D, ns; ATTP vs. all others p < 0.001
TRIC vs. Thal, p = 0.011, TRIC vs. all others, p < 0.001; Thal vs. all others, p < 0.001; K + A vs. T + D, ns; ATTP vs. all others, p < 0.001
All pair-wise comparisons, p > 0.05, ns
TRIC vs. Thal, ns; K + A vs. T + D, ns; Thal or TRIC vs. K + A or T + D or ATTP, p = 0.008 or less; K+A vs. ATTP, p = 0.042; T + D vs. ATTP, ns
Thal vs. TRIC, n.s; K + A vs. T + D or ATTP, ns; Thal or TRIC vs. K + A or T + D or ATTP, p < 0.001
Abbreviations: Thal: Thalidomide; K + A: Ketoconazole + alendronate; T + D: Thalidomide + docetaxel; TRICOM: PSA vaccine study; ATTP: Avastin, thalidomide, taxotere, prednisone; N/A: not available; ns: not significant.
Our kinetic model could be fitted to the serial measurement data in 79% of all patients (50% with both regression (d) and growth (g) rates, 7% with only d and 22% with only g being statistically significant). The vast majority of the 21 % of the data sets that did not fit any of the three equations did so due to scatter. In only one such case of the 268 assessed cases could this be ascribed to a low PSA signal. In thirteen cases (4.8%), 10 of these being from the ATTP series, the PSA value dropped to a low level and continued at this low level for a considerable period, a behavior that cannot be fitted by our regression/growth equations. To fit such PSA profiles one can use a modification of Eq. (1) in which a lag period is modeled before tumor re-growth is initiated. At that time the growth rate constant then fits tumor growth. Insufficient cases are as yet available to explore this approach in detail. As examples of the raw data that we have analyzed, Supplemental Figure 1 depicts representative patterns of PSA kinetics for twelve patients enrolled in the five clinical trials, two representative cases being shown for each trial. In all of these cases, both the g and the d parameters of Eq. (1) were found with p values < 0.05.
Consistent with previous observations [4, 5], overall survival correlated strongly (p < 0.05) with g (as log g) for all trials (Table 1 and Figure 1). As in the prior analyses [4, 5], survival did not correlate with log d in any trial (Supplemental Figure 2). Survival did not correlate with the start of treatment PSA level (as log) (Supplemental Figure 3, panels A through D) except in the PSA-TRICOM study (panel E) where a very significant correlation (p = 0.008) was found. In most cases, survival correlated strongly (p < 0.05) also with the conventional measures of treatment efficacy (depth of nadir and time to nadir, Table 1). In all cases, the correlation of OS with log g was stronger than with the depth of the nadir or the time to reach the nadir, and in some cases, substantially stronger.
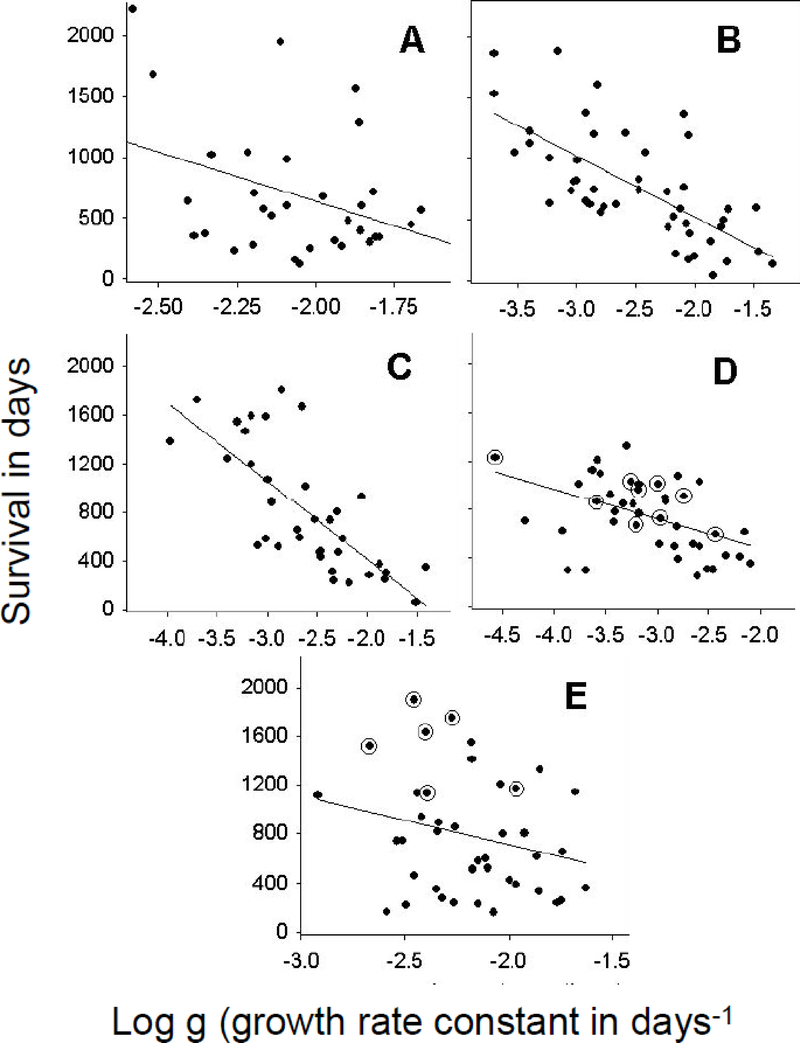
Figure 1:
Dependence of patient survival (Y axis in days) on the log of the growth rate constants. All X-axes are logarithmic scales. Growth rate constants (g, per day) were derived using Eq. (1) or Eq. (3). (A) thalidomide (R = 0.38, p = 0.027); (B) ketoconazole plus alendronate (R = 0.69, p < 0.0001); (C) thalidomide plus docetaxel (R = 0.74, p < 0.0001); (D) ATTP (R = 0.42, p = 0.005); and (E) PSA-TRICOM (R = 0.25, p = 0.13) studies, respectively. The encircled points signify patients still alive at close of follow-up.
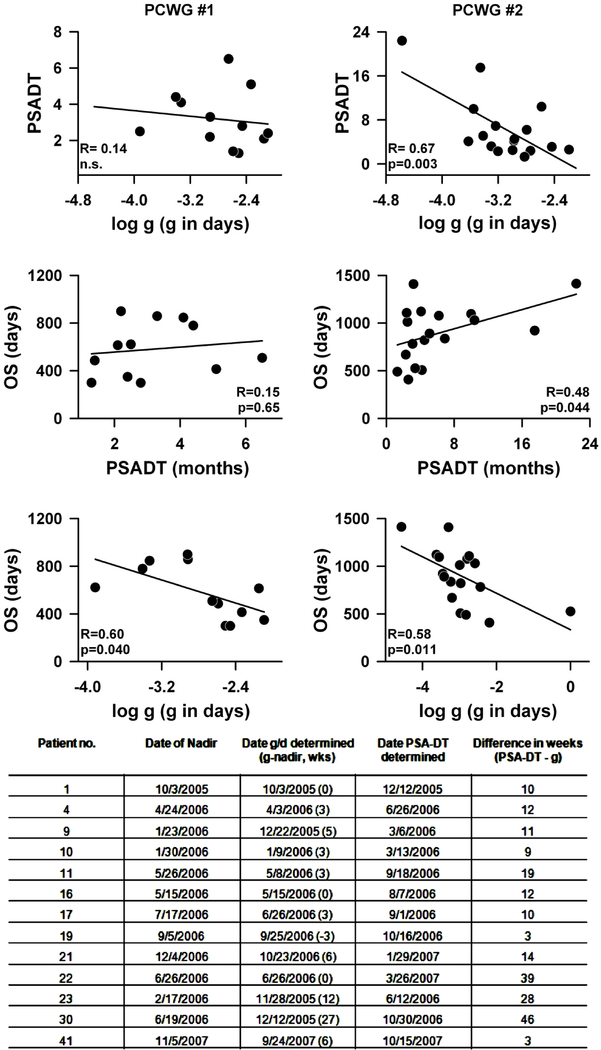
PSA kinetics have emerged as important values for predicting survival and response to therapy in prostate cancer. PSA doubling time (PSA-DT), the length of time it takes for a PSA to double based on an exponential growth pattern, and PSA velocity (PSA-V), the rate of change of PSA over time, have been shown in retrospective analyses as valuable prognostic values. Memorial Sloan- Kettering Cancer Center supports an online tool that can calculate the rate of rise of PSA, expressed as the velocity in nanograms/ml/year, or the PSA doubling time, in months or years [http://www.mskcc.org/applications/nomograms/prostate/PsaDoublingTime.aspx]. Having established a high correlation of g with overall survival we compared g with PSA-DT. Our hypothesis was that g would perform better than PSA-DT since both PSA-DT and PSAV are useful in clinical situations where there is no active intervention and growth without concomitant regression is occurring [6 – 13]; whereas g can be estimated when both regression and growth are occurring simultaneously. We thus compared g and PSA-DT in patients enrolled on the ATTP trial. The results are shown in Figure 2. Comparisons were made using two sets of data: The first data set consisted of data gathered in the early part of the study when clinical decisions were made in accordance with the guidelines of the Prostate-Specific Antigen Working Group (PCWG #1 in the figure) [22] – with progression scored when two consecutively rising PSA levels were obtained and the PSA-DT estimated over the time period from the nadir to the progression end-point. By comparison, the second data set was obtained from the latter part of the study when clinical decisions followed the guidelines of the Prostate Cancer Clinical Trials Working Group 2 (PCWG #2 in the figure) [23]. Because the PCWG #2 guidelines ignore early changes in PSA, the data sets collected following PCWG #2 guidelines included a larger number of PSA values beyond a nadir. Furthermore, because treatment continued until clinical progression was seen (radiographic or symptomatic), the PSA-DT was estimated over the time period from the point of progressive disease (defined as above to be two consecutively rising PSA levels) to the time treatment was terminated. As seen in Figure 2, we correlated estimates of the PSA-DT with the values for the growth rate constant, g, determined using Eq. (1), and then correlated PSA-DT and g separately with OS. For each of the datasets, the top panels compare PSA-DT versus log g. For PCWG #1 the regression of PSA-DT on log g is not significant, shown in the upper left panel (R = 0.14, n.s.). Also not significant (R = 0.15, n.s.) is the regression of OS against PSA-DT as shown in the middle panel on the left. However, as shown for the entire data set in Figure 1, log g exhibited a significant correlation with OS (lower left panel, R = 0.6; p = 0.04). In contrast, the values of PSA-DT estimated using the PCWG #2 data set correlate well with log g (R = 0.67, p = 0.003, top right panel). When the value of OS is regressed on these estimates of PSA-DT (middle right panel) a significant correlation is observed (R = 0.48, p = 0.044), albeit not as good as the results obtained when log g was correlated with OS (lower right panel, R = 0.58; p = 0.011). The higher correlation of PCWG#2 with OS relative to PCWG#1 is not surprising since the latter data set has PSA values obtained long after the nadir has occurred so that the PSA values begin to represent a tumor quantity that is influenced primarily by treatment refractory tumor that is growing without much concomitant tumor regression.
Figure 2:
Comparison of PSA-DT and g were made using two data sets: PCWG #1 = data gathered early in the study when the guidelines of the Prostate-Specific Antigen Working Group [22] were followed in making clinical decisions. Progression scored if two consecutively rising PSA levels were obtained. PSA-DT estimated from the nadir to the progression end-point. PCWG #2 = data obtained in the latter part of the study when the guidelines of the Prostate Cancer Clinical Trials Working Group 2 [23] were used to make clinical decisions. Treatment continued until radiographic or symptomatic progression was seen, or as long as treatment was tolerated. PSA-DT estimated from the point of progressive disease to the time treatment was terminated. For each of the datasets, the top panels compare PSA-DT versus log g, the middle panels show the regression of OS against PSA-DT while the lower panels depict the correlation of log g with OS. Values of PSA-DT estimated using the data set from the latter part of the study correlate well with log g values and OS. The table at the bottom of the figure compares thirteen randomly chosen data sets from the ATTP study. The table summarizes when in the clinical course a reliable g or PSA-DT value comparable to that obtained with the complete data set and able to predict OS could be determined. A value for g comparable to that obtained with the entire data set could be estimated a median of 12 weeks earlier than a PSA-DT (number in parentheses is how many weeks before the nadir). The PSA-DT was calculated using the Memorial Sloan Kettering Cancer Center online tool [http://www.mskcc.org/applications/nomograms/prostate/PsaDoublingTime.aspx].
Next, we compared the time required to obtain an accurate estimate of PSA-DT or g, comparable to that obtained with the complete data set. In this exercise, thirteen randomly chosen data sets from the ATTP study were analyzed. The table in Figure 2 summarizes the results. A value for the growth rate constant comparable to that obtained with the entire data set could be estimated a median of 12 weeks (25% to 75% range: 8 to 21 weeks) earlier than a PSA-DT with similar predictive accuracy. Supplemental Figure 4 shows this in detail for one of the thirteen randomly chosen data sets.
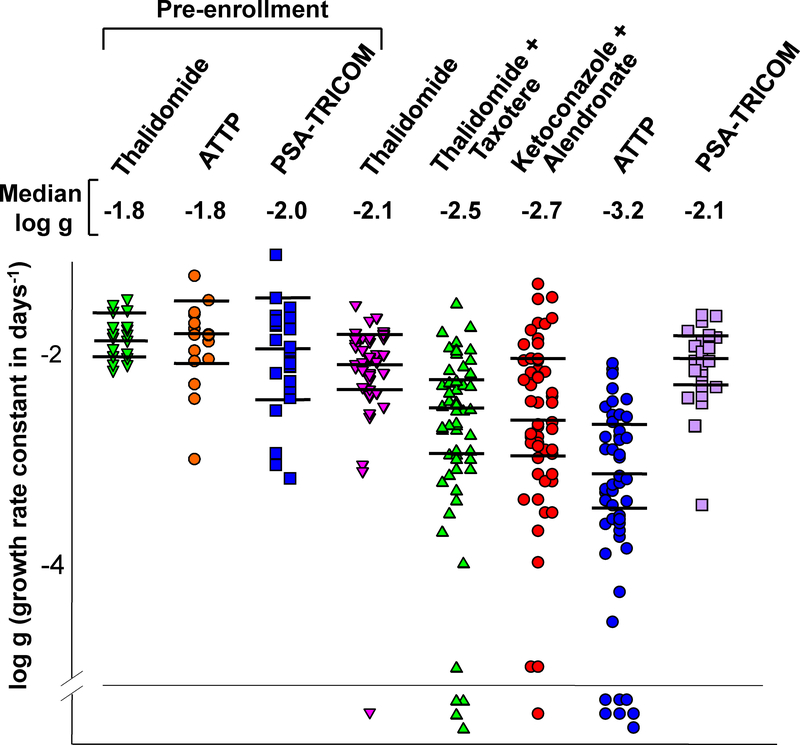
Figure 3 presents scatter plots of the log g values extracted with a p < 0.05, in patients enrolled on the various protocols. The first three scatter plots graphed on the left of the figure depict g values calculated prior to enrollment, in the three trials in which these data were available. Note that throughout more than a decade, the median pre-study values and their distribution has remained relatively constant, reflecting consistency in the biology of mCRPC in patients enrolled at the NCI. The median values, appearing below the name of each study, demonstrate that while thalidomide as a single agent had minimal to no benefit, subsequent chemotherapy regimens have had a greater effect. The g observed with the most recent clinical trial, designated ATTP (median g = 0.000676 days-1, log g, 10−3.17), is more than one log lower than that observed with single agent thalidomide (median g = 0.00832 days-1, log g = 10−2.08), studied a decade earlier. Clinically this would translate into approximately a 10-fold slower rate of rise of the patient’s PSA, while on trial. Symbols below the line in Figure 3 identify patients for whom a g value could not be calculated, their PSA curves demonstrating only regression (kinetic model could not be fitted, see earlier comment). Clinically these were scored as a complete PSA response. Supplemental Figure 5 (top panels) depicts the pre- and post-treatment g values for the individual patients for which both sets of data were available, allowing one to examine the treatment effect in individual patients. For patients on the thalidomide study (panel A) or on the PSA-TRICOM study (panel C), pre-treatment and on-study values were very similar, consistent with the results shown in the scatter plot. In contrast, for many patients receiving ATTP, therapy substantially reduced their tumor’s on-study g. Thus the data in Figure 3 show that successive chemotherapy regimens have achieved greater efficacy as evidenced by both greater reductions in g, and a greater number of patients achieving a complete PSA response. Such an effect, however, was not observed with the PSA-TRICOM vaccine where on-study g values were not statistically different (t-test, p = 0.46) from pre-enrollment g values for patients receiving vaccine. Finally, Supplemental Figure 6 presents dot plots of the distribution of the best-fit decay (regression, d) rate constants in the five studies conducted over time. Unlike the marked slowing in the growth rate constant shown in Figure 3, the decay constant has remained unchanged over time indicating the enhanced efficacy of current therapies is occurring because of a greater effect on g, rather than an effect on d.
Figure 3:
Dot plots of the distribution of the best-fit growth rate constants in studies conducted over time. The horizontal lines in each set are the median values and the 95% confidence intervals. The Y-axis is the logarithm of the derived growth rate constant. The regression rate constants for patients prior to enrollment on a study are also shown for the thalidomide, ATTP, and PSA-TRICOM trials. Median g values for each study are listed above the dot plots. Symbols below the horizontal line represent patients who achieved a complete response (CR).
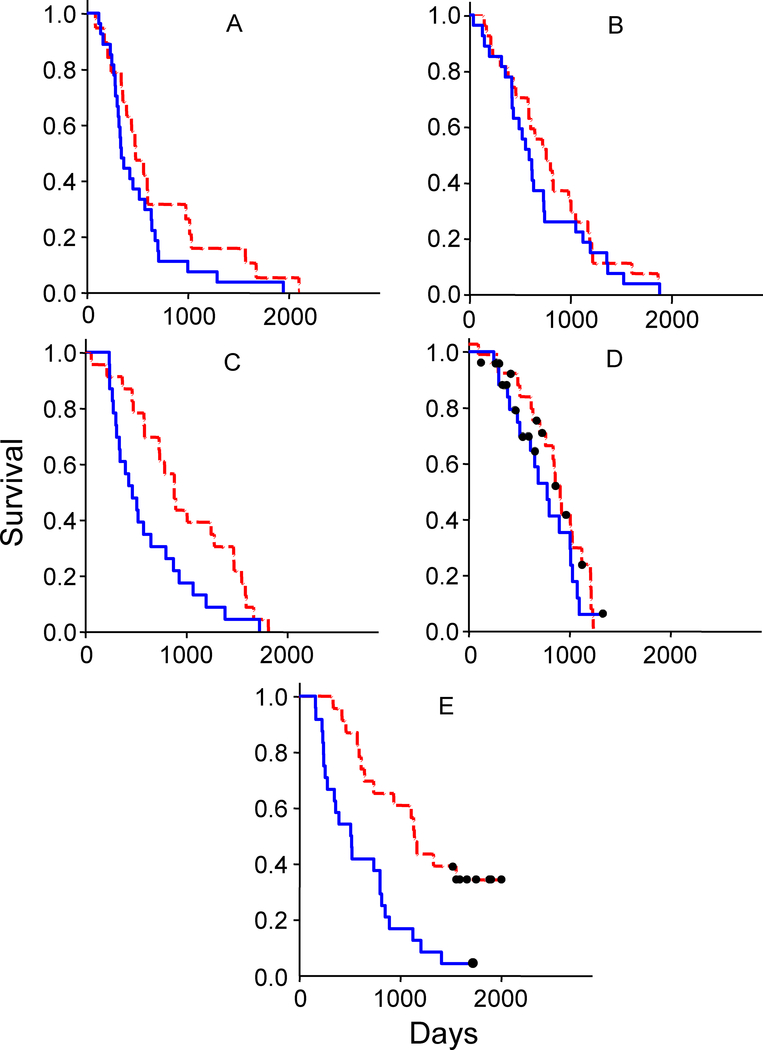
Figure 4 (panels A through E) shows Kaplan-Meier survival analyses for all five studies. For each trial survival is compared between patients whose initial PSA quantity was above or below the median PSA value. A differential survival benefit based on initial tumor quantity as indicated by PSA is most pronounced for patients treated on the PSA-TRICOM study (Compare the upper curve in panel E with all other plots). Furthermore, in the PSA-TRICOM study, on considering both the variables of initial PSA and g, it became apparent that the greatest benefit from the vaccine was obtained with those smaller tumors (quantified by initial PSA) that had smaller g values (data not shown). Given the lack of an effect on g during the vaccine’s administration (Figure 3 and Supplemental Figure 5), this suggests lower initial tumor burdens that grow slower may derive greater benefit following administration of a vaccine.
Figure 4:
Kaplan Meier survival analyses. In each study, cases were stratified by initial PSA signal and divided into below (dashed red lines) and above (solid blue lines) the median signal for that study. Panels (A) through (E) depict data from: (A) thalidomide, (B) ketoconazole plus alendronate, (C) thalidomide plus docetaxel, (D) ATTP and (E) PSA-TRICOM clinical trials, respectively. In (F), data for all five studies are combined, but here the data sets were further stratified into those below and above the median g (growth rate) for that set, and the data for those with low initial PSA, and also low g depicted. The survival curves are color-coded: thalidomide (red), ketoconazole alendronate (black), thalidomide docetaxel (blue), ATTP (light green), and PSA-TRICOM (dark green). In panels D, E and F, patients still alive are depicted by black dots.
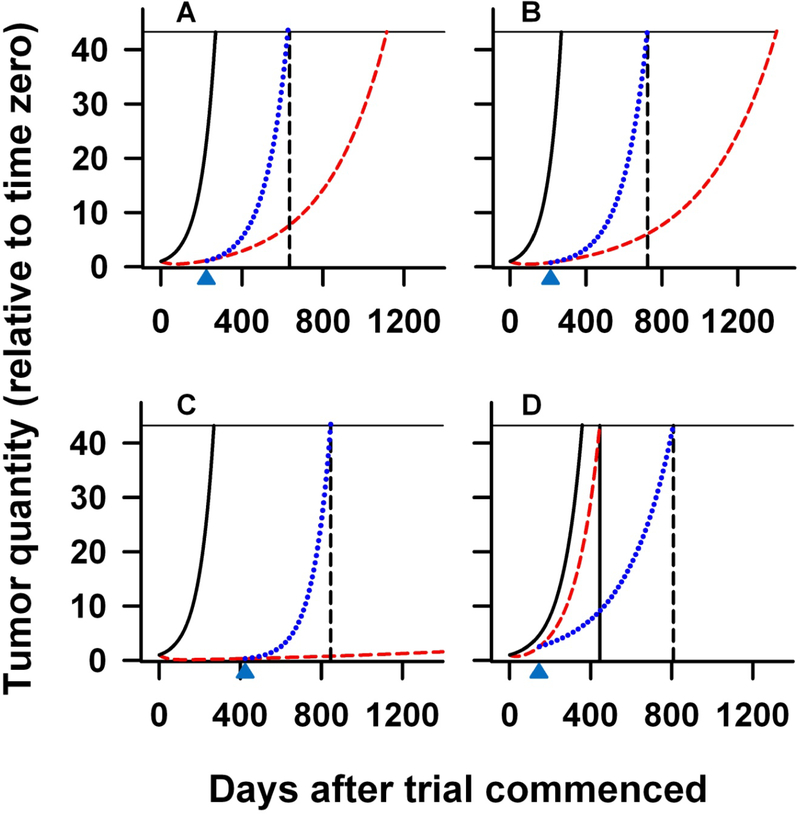
Finally, we evaluated the ability of the growth rate constant to predict survival. Eq. (1) describes how the tumor quantity, f, at a given time designated t, depends on the rate parameters for regression (d) and for growth (g) and on the time since therapy was initiated (t). We can then inquire as to what the tumor quantity, f, (or in this case its indicator, the PSA value) would be, had growth continued with these g and d values until the patient’s death. To do this, we substituted the median time until death for t in Eq. (1). This computation assumes that tumors grow at relatively constant rates until death, and do not follow Gompertzian kinetics [24, 25]. The value so computed using the data from the thalidomide trial (as a therapy that approximates a placebo) indicates a median PSA signal at death of approximately 5400, or 44 times the enrollment value. Assuming that the median amount of tumor (i.e., PSA) at time of death is similar for patients enrolled on the different clinical trials (i.e., 44-fold over enrollment value), we can model using Eq. (1), the expected OS (t in Eq. 1) had the rise in PSA value continued in all cases at the g obtained during study. This is plotted in Figure 5 with the solid black line showing the prediction to reach a tumor quantity that is 44 times the original (the solid black vertical line) if growth continued at the g and d obtained prior to enrolling on treatment (this model used all the pre-treatment values given their constancy over more than decade). This contrasts with the dashed red line that shows the prediction if growth continued at slower g values attained obtained on treatment. For the three chemotherapy trials, ketoconazole plus alendronate trial (A) thalidomide plus docetaxel (B), and ATTP one can see that the time at which the prediction intersects the horizontal solid line is a substantial over-estimate for the measured overall survival (indicated by the black vertical dashed line). That is, had the tumor growth rates continued at the values attained while on study, patients in the chemotherapy trials should have survived very much longer before reaching a PSA quantity 44 times the original. The dotted blue line in each chart represents an alternative model that shows the increased g value after treatment was discontinued (indicated as an upward-directed arrow at the bottom of each chart) needed to reach a PSA value 44-times the original at the measured OS. These rates approximate the pre-treatment values and are in A, B, and C, 4.2-fold, 3.0-fold, and 20.6-fold higher than the respective on-study rates. The findings in these three chemotherapy trials suggest that, upon discontinuing therapy, the growth rate constants increase substantially. In contrast with the TRICOM trial (D), the calculation based on the on-study rate (dashed red line) represents an under-estimate for observed OS. Here, the value of 681 days, predicted for OS had growth continued at the on-study level with the PSA-TRICOM vaccine, is somewhat less than that observed (801 days) and as expected only slightly longer than had the pre-study rate continued – demonstrating the small effect TRICOM had on g while patients were “monitored on study”. In these latter patients it appears that g, rather than increasing after therapy was discontinued, decreased.
Figure 5:
Projected PSA curves computed using derived median g and d rate constants, substituted into Eq. (1). Panels (A) through (D) depict predictions derived from (A) ketoconazole plus alendronate; (B) thalidomide plus docetaxel; (C) ATTP, and (D) PSA-TRICOM studies, respectively. In each plot, the leftmost black solid lines are the projected PSA curves based on the mean of the pre-treatment g values from the thalidomide, ATTP and PSA-TRICOM studies; while the red curve is the projected PSA curve using, in each study, the appropriate g and d values calculated using PSA values obtained while patients were on study. The dashed vertical black line in each panel denotes the median OS of that study. To have the predicted PSA curve for the three chemotherapy trials (A, B and C) intersect the horizontal line (at a relative PSA signal of 44) at the actual median OS requires fitting the curve to a model (dotted blue curve) in which the growth rate, g, reverts to a higher rate when study therapy ceases (depicted by the black arrow) and continues until death at a relative PSA signal of 44. These rates are in A, B, and C, 4.2-fold, 3.0-fold, and 20.6-fold higher than the respective on-study rate in each case, and very similar to the pre-treatment rates shown as the solid black curves. In the PSA-TRICOM study (D) the blue curve, that intersects the horizontal line at the OS, is modeled with a projected slower growth rate after treatment that is 0.50 fold the on-study rate.
DISCUSSION
In the present study we have retrospectively examined PSA measurements obtained in more than a decade of clinical trials in mCRPC at a single institution. The studies represent the evolution of therapy in mCRPC, and include a vaccine trial. Three principal conclusions can be reached. First, the measured growth rate constant (g) of the change in PSA signal correlates strongly with overall survival (OS), making g a novel clinical trial endpoint that may be useful as a predictor of OS, and that may allow comparison across clinical trials. We show that while a patient is enrolled on trial g is a more accurate predictor of OS than PSA-DT and provides an accurate assessment of treatment benefit a median of twelve weeks earlier. Second, evidence is presented that demonstrates, in most cases, increased therapeutic efficacy in successive chemotherapy trials, the derived g values declining over the decade. This example shows how the growth rate constant can be used to evaluate and compare clinical trial outcomes. Third, our kinetic modeling also suggests benefit accrues only while a patient receives therapy, with no permanent change in disease biology, with one exception. Thus, despite a marked reduction in g and tumor burden and a prolonged time on therapy, the ATTP combination did not enhance survival to the extent expected. In contrast, the greater OS of patients enrolled on the PSA-TRICOM trial despite the small effect on g during therapy suggests a late benefit, perhaps due to acquisition of a beneficial immune response that can limit tumor growth rate [26, 27]. This suggests a delayed decrease in g and is consistent with several placebo-controlled immunotherapy trials in mCRPC that have shown improved OS without improvement in time to progression [21, 28, 29]
In previous studies we have demonstrated both in mCRPC and in renal cell carcinomas that g can be determined using data obtained while patients were receiving therapy [4, 5]. These informative g values can be obtained either from analyzing direct radiologic measurements of tumor size [4] or from proxy measurements using the PSA signal ([5] and the present study). These g values can be determined using the formulae summarized in Methods. The values of g correlate with OS, the gold standard of oncology clinical trials. Since many patients enroll in additional studies or receive other therapies that can confound OS analysis, the present method has the potential to determine the efficacy of a therapy unencumbered by subsequent therapies. Note that we do not require that the PSA signal be in any exact way a measure of the total quantity of prostate tumor in the patient. Rather, we wish to suggest that the traditional use of this signal as a rough indicator of tumor quantity could be improved by a more precise kinetic analysis during chemotherapy.
The concept that a kinetic analysis of growth rate can be performed while a patient is receiving chemotherapy has multiple implications. One is that calculation of g can be performed while a patient is still apparently responding to therapy. As shown in Figures 2 and Supplemental Figure 4, g can be calculated accurately from PSA values obtained at defined intervals well before disease progression is documented. By comparison PSA-DT can only be accurately calculated after there is clinical evidence of tumor growth as demonstrated by values substantially above the nadir. From a patient’s perspective, if g values were determined across different therapeutic regimens, this could allow determination of ongoing clinical benefit despite a continued rise in PSA, since the kinetics of that rise could be accurately predicted. From a drug development perspective, g values could allow assessment of agents that slow tumor growth (i.e., are cytostatic or cytolentic) when conventional response criteria are not met [30]. Whether this kinetic approach will apply to all combinations of tumor types and anticancer therapeutic choices remains to be determined. We have shown similar results for bevacizumab (a targeted therapy) and ixabepilone (a cytotoxic therapy) in renal cell cancer. We have also now obtained validation of our predictions regarding OS in the context of large Phase III trial settings [31, 32].
The data in Table 1 indicate the g value is more closely correlated with OS than are the traditional indicators, depth of the nadir (min) and the time to nadir (tmin). Figure 2 shows that in these patients undergoing treatment the g value are also more closely correlated with OS than PSA-DT. Notably, when PSA-DT is calculated using PCWG#1 criteria, 2 consecutive rises over nadir, it does not correlate with OS. Only when more extensive data are gathered so that the PSA-DT can be calculated using PSA values to the point of clinical disease progression can one then see a correlation of PSA-DT with OS. This latter estimate of PSA-DT is also more closely correlated with g as well. This is not surprising since PSA-DT (and PSAV) have been developed and validated not in patients receiving therapy, but rather in those being followed off therapy [6 – 13]. In situations such as those reported here, where a therapy is being administered and regression and growth are occurring simultaneously, g can be discerned, along with d, using the simple equations we describe. Finally, correlations of the traditional indicators, depth of the nadir (min) and the time to nadir (tmin), with OS are not surprising, given that these parameters depend on the biologically more fundamental parameter g and therefore correlate strongly with it. Indeed, we have previously shown [5] that min and tmin and a rough estimate of PSA-DT can be directly computed if g and d are both known. The converse computation – deriving estimates of g and d from values of the nadir and the time to nadir – can also be performed but this requires the use of a nomogram previously reported in Supplementary materials [5]. Investigators can explore this methodology at http://www.tumorgrowthanalyses.com
Examining the clinical and kinetic data in Table 1 and the g values recorded in Figure 3 we conclude that chemotherapies have had increasing impact on tumor growth rates (although not on decay rate, d, as shown in Supplemental Figure 6), and demonstrates how the growth rate constant might be used in drug development to compare data obtained in similar patient populations enrolled on different trials. Interestingly, this benefit apparently accrues only while a therapy is administered, since the overall survivals do not reflect the improvements in response rates reported in the trials, nor the great differences in g values found while the patients were on study. It is possible that tumors revert to pre-enrollment growth rates after therapy is discontinued. Such a conclusion was reached in our analysis of data from a trial of renal cancers treated with bevacizumab (4), and in the present data set as extrapolated in Figure 5. In all data sets examined to date, g correlates with survival. And yet survival falls short of that predicted from the g obtained while on clinical trial. These extrapolations, if valid, suggest that on stopping non-vaccine therapies, the tumors reverted to a higher growth rate than that measured while on study. The analysis suggests that these patients might have derived a greater survival benefit, and for those on ATTP a substantial survival benefit, had treatment continued beyond the arbitrary line of “progression”, at which treatment was discontinued. Continued monitoring of PSA levels during extended therapy to determine whether or not tumors continue to grow at the reduced rate could test the predictions of this analysis. The model further suggests the post-therapy biology of the host is different following treatment with PSA-TRICOM, and could be consistent with subsequent acquisition of a beneficial immune response that prolonged survival as has been discussed by others [33 – 35].
Finally, while other metrics such as the Halabi nomogram [36] can predict survival probabilities based on clinical and laboratory parameters in patients with mCRPC, the growth rate constant defined in this study can be used to evaluate the effect of various treatments over time. This can potentially be used in any stage of prostate cancer (not just metastatic castration resistant disease) and only requires serial PSA values, not imaging or other laboratory values as required for the Halabi nomogram.
In conclusion, we report a retrospective analysis of over a decade of clinical trials in mCRPC using a novel method that determines a tumor growth rate constant using PSA values obtained while patients receive therapy. The calculated growth rate constant, g, is sufficiently sensitive to detect differences across a decade of therapies that have been increasingly more effective at reducing tumor burden. The growth rate constant data can also be used to model predicted survival, which yields estimates consistent with the hypothesis that tumors lose the on-study benefit after therapy is discontinued. A clinical trial is needed to test whether continued drug treatment with concomitant, on-going measurement of PSA fitting a stable growth rate constant might improve outcome. The model also suggests that a PSA-TRICOM vaccine substantially prolongs survival and we suspect this may have been secondary to acquisition or continued expansion of an immune response leading to slowing of tumor growth rate after vaccination was discontinued. These studies contribute to validation of the growth rate constant as a novel endpoint indicative of response to therapy in clinical trials and indicative of survival; validation with additional randomized studies is a vital next step.
Supplementary Material
Statement of Translational Relevance.
The pace with which new cancer therapies are developed is too slow. The FDA often requires that therapies demonstrate a survival advantage and this is becoming increasingly difficult. It is intuitive that faster growing tumors will lead to reduced survival. It follows that if one could accurately determine this growth rate – as a growth rate constant – one could predict when a patient will die, and draw conclusions as to therapeutic efficacy. We show that regression and growth can be mathematically dissected, and a growth rate constant determined. This growth rate constant in turn can predict survival. We show that this growth rate constant can be used to compare different clinical trial outcomes. Using this kinetic model we also suggest that the extent of benefit from our therapies has not been maximal since we stop our therapies too early. This model also suggests that there may be long-term benefit from the PSA-TRICOM vaccine. Together these studies suggest that determination of the tumor growth rate constant may serve as a new clinical trial endpoint for drug development.
Abbreviations
- PSA
prostate specific antigen
- mCRPC
metastatic castration resistant prostate cancer
- OS
overall survival
- PSA-DT
PSA doubling time
- PSA- TRICOM
An experimental vaccine composed of recombinant poxviral vectors containing transgenes for PSA and three co-stimulatory molecules (TRICOM) B7.1, ICAM-1, and LFA-3
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures 2009. Atlanta: American Cancer Society; 2009. http://www.cancer.org/docroot/STT/stt_0.asp [Google Scholar]
- 2.Petrylak D Therapeutic options in androgen-independent prostate cancer: building on docetaxel. BJU Int. 2005; 96 Suppl 2:41–6. [DOI] [PubMed] [Google Scholar]
- 3.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005; 294:238–44. [DOI] [PubMed] [Google Scholar]
- 4.Stein WD, Yang J, Bates SE, Fojo T. Bevacizumab reduces the growth rate constants of renal carcinomas: a novel algorithm suggests early discontinuation of bevacizumab resulted in a lack of survival advantage. Oncologist. 2008; 13: 1055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein WD, Figg WD, Dahut W, Stein AD, Hoshen MB, Price D, Bates SE, Fojo T. Tumor growth rates derived from data for patients in a clinical trial correlate strongly with patient survival: a novel strategy for evaluation of clinical trial data. Oncologist. 2008; 13: 1046–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Amico AV, Chen MH, Roehl KA, Catalona WJ. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004a; 351:125–35. [DOI] [PubMed] [Google Scholar]
- 7.Hanks GE, Hanlon AL, Lee WR, Slivjak A, Schultheiss TE. Pretreatment prostate-specific antigen doubling times: clinical utility of this predictor of prostate cancer behavior. Int J Radiat Oncol Biol Phys. 1996; 34:549–553 [DOI] [PubMed] [Google Scholar]
- 8.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999; 281:1591–7. [DOI] [PubMed] [Google Scholar]
- 9.Laufer Pound, Laufer M, Pound CR, Carducci MA, Eisenberger MA. Management of patients with rising prostate-specific antigen after radical prostatectomy. Urology. 2000; 55:309–15. [DOI] [PubMed] [Google Scholar]
- 10.Egawa S, Arai Y, Tobisu K, Kuwao S, Kamoto T, Kakehi Y, Baba S. Use of pretreatment prostate-specific antigen doubling time to predict outcome after radical prostatectomy. Prostate Cancer Prostatic Dis. 2000; 3:269–274. [DOI] [PubMed] [Google Scholar]
- 11.Stephenson AJ, Aprikian AG, Souhami L, Behlouli H, Jacobson AI, Begin LR, Tanguay S. Utility of PSA doubling time in follow-up of untreated patients with localized prostate cancer. Urology. 2002; 59:652–6. [DOI] [PubMed] [Google Scholar]
- 12.Ward JF, Blute ML, Slezak J, Bergstralh EJ, Zincke H. The long-term clinical impact of biochemical recurrence of prostate cancer 5 or more years after radical prostatectomy. J Urol. 2003; 170:1872–6. [DOI] [PubMed] [Google Scholar]
- 13.D’Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Prostate specific antigen doubling time as a surrogate end point for prostate cancer specific mortality following radical prostatectomy or radiation therapy. J Urol. 2004b; 172(5 Pt 2):S42–6; discussion S46–7. [DOI] [PubMed] [Google Scholar]
- 14.Figg WD, Dahut W, Duray P, Hamilton M, Tompkins A, Steinberg SM, Jones E, Premkumar A, Linehan WM, Floeter MK, Chen CC, Dixon S, Kohler DR, Krüger EA, Gubish E, Pluda JM, Reed E. A randomized phase II trial of thalidomide, an angiogenesis inhibitor, in patients with androgen-independent prostate cancer. Clin Cancer Res. 2001; 7(7): 1888–93. [PubMed] [Google Scholar]
- 15.Figg WD, Liu Y, Arlen P, Gulley J, Steinberg SM, Liewehr DJ, Cox MC, Zhai S, Cremers S, Parr A, Yang X, Chen CC, Jones E, Dahut WL. A randomized, phase II trial of ketoconazole plus alendronate versus ketoconazole alone in patients with androgen independent prostate cancer and bone metastases. J Urol. 2005; 173(3): 790–6. [DOI] [PubMed] [Google Scholar]
- 16.Figg WD, Arlen P, Gulley J, Fernandez P, Noone M, Fedenko K, Hamilton M, Parker C, Kruger EA, Pluda J, Dahut WL. A randomized phase II trial of docetaxel (taxotere) plus thalidomide in androgen-independent prostate cancer. Semin Oncol. 2001; 28(4 Suppl 15): 62–6. [DOI] [PubMed] [Google Scholar]
- 17.Dahut WL, Gulley JL, Arlen PM, Liu Y, Fedenko KM, Steinberg SM, Wright JJ, Parnes H, Chen CC, Jones E, Parker CE, Linehan WM, Figg WD. Randomized phase II trial of docetaxel plus thalidomide in androgen-independent prostate cancer. J Clin Oncol. 2004; 22(13): 2532–9. [DOI] [PubMed] [Google Scholar]
- 18.Ning YM, Gulley JL, Arlen PM, et al. Phase II trial of bevacizumab, thalidomide, docetaxel, and prednisone in patients with metastatic castration-resistant prostate cancer. J Clin Oncol 2010; 28:2070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arlen PM, Skarupa L, Pazdur M, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol 2007; 178:1515–20. [DOI] [PubMed] [Google Scholar]
- 20.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother 2010; 59:663–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 2010; 28:1099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999; 17:3461–7. Erratum in: J Clin Oncol. 2007; 25:1154. J Clin Oncol. 2000; 18:2644. [DOI] [PubMed] [Google Scholar]
- 23.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Prostate Cancer Clinical Trials Working Group. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008; 26:1148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. A Gompertz curve or Gompertz function, named after Benjamin Gompertz, is a type of mathematical model for a time series, where growth is slowest at the start and end of a time period. It is described by the equation y(t)=aebe(ct) where a is the upper asymptote, c is the growth rate, b, c are negative numbers and e is the base of the natural logarithms (e = 2.71828...).
- 25.Laird AK (1964). “Dynamics of tumor growth.” Br J Cancer 13:490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlom J, Arlen PM, Gulley JL. Cancer vaccines: moving beyond current paradigms. Clin Cancer Res 2007; 13:3776–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gulley JL, Madan RA and Arlen PM. Enhancing efficacy of therapeutic vaccinations by combination with other modalities. Vaccine 2007; 25S:B89–B86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Small E, Schellhammer P, Higano C, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 2006; 24:3089–94. [DOI] [PubMed] [Google Scholar]
- 29.Schellhammer P, Higano C, Berger E, et al. A randomized, double-blind, placebo-controlled, multi-center, phase III trial of sipuleucel-T in men with metastatic, androgen independent prostatic adenocarcinoma (AIPC). AUA Annual Meeting, 2009. [cited April 2010]. Available from: http://www.aua2009.org/program/lbsciforum.asp [Google Scholar]
- 30.Rixe O, Fojo T. Is cell death a critical end point for anticancer therapies or is cytostasis sufficient? Clin Cancer Res. 2007; 13:7280–7. [DOI] [PubMed] [Google Scholar]
- 31.Fojo AT, Stein WD, Wilkerson J, and Bates SE. Kinetic analysis of breast tumor decay and growth following ixabepilone plus capecitabine (IXA + CAP) versus capecitabine alone (CAP) to discern whether the superiority of the combination is a result of slower growth, enhanced tumor cell kill, or both. J Clin Oncol 28:15s, 2010. (suppl; abstr 1096) [Google Scholar]
- 32.Wilkerson J, Stein WD, Kim ST, Huang X, Motzer RJ, Fojo AT, Bates SE. Validation of a kinetic analysis of renal cancer regression and growth following treatment with sunitinib and interferon-alfa (IFN-α): Analysis of the pivotal randomized trial. J Clin Oncol 28:15s, 2010. (suppl; abstr 4597). [Google Scholar]
- 33.Higano C, Saad F, Somer B, et al. A phase III trial of GVAX immunotherapy for prostate cancer versus docetaxel plus prednisone in asymptomatic, castration-resistant prostate cancer (CRPC) [abstract]. ASCO Genitourinary Cancers Symposium 2009; LBA150. [Google Scholar]
- 34.Salazar LG and Disis ML. Cancer Vaccines: The role of tumor burden in tipping the scale toward vaccine efficacy. JCO 23(30): 7397–7398. [DOI] [PubMed] [Google Scholar]
- 35.Madan RA, Mohebtash M, Schlom J, Gulley JL. Therapeutic vaccines in metastatic castration-resistant prostate cancer: principles in clinical trial design. Expert Opin Biol Ther 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol 2003; 21:1232–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.