Abstract
Objectives
The primary objective was to investigate the left-right vocal fold symmetry in rheological and histological properties using a rabbit model. The other objective was to develop statistical models for the comparison of rheological properties between paired vocal folds.
Methods
Viscoelastic shear properties of six pairs of vocal fold lamina propria specimens were measured over a frequency range of 1 to 250 Hz by a linear, controlled-strain, simple-shear rheometer. The rheological data of the left and right vocal folds was statistically compared using the mixed-effects model approach. Six additional rabbit larynges were histologically analyzed for left–right symmetry in distribution patterns and relative densities of major extracellular matrix constituents.
Results
There were no significant differences in elastic shear modulus (p = 0.1069) and dynamic viscosity (p = 0.944) of the lamina propria between the two vocal folds of the same larynx. Left-right vocal fold symmetry in densities and distribution patterns of the key molecular constituents was also demonstrated in histological results.
Conclusion
By showing that the left and right vocal folds were rheologically and histologically symmetrical in rabbit, this study validated an underlying assumption made in many previous reports. Statistical models for the analysis of hierarchically correlated left-right vocal fold rheological data were also presented.
Keywords: Left–right vocal fold symmetry, viscoelastic shear properties, histology, animal model, mixed-effects model
Introduction
A common experimental design for vocal fold preclinical studies is to operate on one vocal fold in each animal while using the contralateral vocal fold as either normal or sham control.1–5 This experiment design assumes that tissue properties, such as histological and rheological properties, of the two healthy vocal folds belonging to an animal’s larynx are identical or very similar. To the best of our knowledge, there has been no report that comprehensively investigated this crucial assumption.
Mild or moderate left-right asymmetry of vocal fold vibration in normophonic speakers is common.6,7 Slight differences in shapes, thickness, and depths between paired human vocal folds have also been reported.8 Besides the left-right geometrical difference, a certain degree of asymmetry in biomechanical properties in human and animals has also been found by several groups: Goodyer et al. reported a better than 0.7 (with 1 being a perfect match) correlation between shear modulus of paired vocal folds in human;9 Chan and Titze observed 10% to 15% differences in elastic shear modulus G′ and dynamic viscosity η′ between the left and right vocal folds in human over the frequency range of 0.2 to 2Hz;10 Miri et al. reported an up to 25% left-right vocal fold difference in shear moduli at 0-10Hz in porcine larynges.11 However, in the latter two studies, the data was measured at a frequency range far below the normal human phonatory frequencies and was not statistically analyzed. To date, symmetry in shear properties between the paired vocal folds has not been fully studied, although the viscoelastic shear properties or rheological properties critically determine the vibratory characteristics of the vocal folds.12
To statistically compare the rheological data of the two vocal folds from the same larynx, a methodology that can account for the multilevel correlation structure among observations is required. The first level of correlation arises from multiple measurements of the same tissue sample across the test frequency range. The second level of correlation exists among measurements obtained from the same animal. If we ignore the data correlations in the statistical analysis, we misrepresent the amount of information that we actually have, and thus analysis can be flawed.13 It is obvious that the commonly used regression-fitting methods in the literature (e.g., linear least-squares regression, log-transformed linear regression) are unable to handle such hierarchical correlation structure because they assume each observation to be independent.14 Repeated measures analysis of covariance was used in several reports but only the correlation within a subject was mentioned.2,4,5,15 To our best knowledge, a statistical model that includes the hierarchical correlation structure in the analysis of vocal fold viscoelastic shear properties had not been reported.
In a recent report, our group compared viscoelastic shear properties of vocal fold tissues between human and different animal species (i.e., rabbit, porcine, and canine) at frequencies up to human phonatory frequencies.13 Mixed-effects modeling16,17 was performed on the correlated rheological data for statistical analysis.13 In the present study, viscoelastic shear properties of rabbit vocal fold lamina propria were measured at frequencies between 1 and 250 Hz using a custom-built rheometer system.12 Three mixed-effects models were developed for the statistical comparison of the left and right vocal fold rheological data.
Histological assays are performed in most animal studies to examine the microscopic structure of the extracellular matrix (ECM) in vocal fold. In addition to studying the rheological symmetry, we also investigated the left-right vocal fold symmetry in histology with regard to the distribution patterns and relative densities of major ECM molecular constituents in the present work.
The primary objective of this study was to test one of the most common assumptions in vocal fold animal research: the left-right vocal fold symmetry in rheological and histological properties. The other objective was to develop statistical models for the comparison of viscoelastic shear properties between paired vocal folds.
Materials and methods
Vocal fold tissue specimens
Larynges were harvested from twelve 5- to 8-month-old female New Zealand White rabbits weighing between 2.7 and 4 kg immediately postmortem. The rabbits were donated by other labs on campus, and none of them sacrificed specifically for this study. All the vocal folds were closely inspected by an otolaryngologist and appeared to be normal. For rheometric measurement, 12 vocal fold specimens consisting of the epithelium and the lamina propria were dissected from six larynges. The tissue samples were kept hydrated in phosphate buffered saline solution (PBS) at room temperature until measurements, which were conducted within 6 hours postmortem. The other six larynges were wrapped in gauze, soaked in PBS, and snap frozen by submerging in liquid nitrogen. The larynges were kept frozen until processed for histology.
Rheometric Instrumentation and Measurements
Measurements of viscoelastic shear properties of vocal fold specimens were conducted with a custom-built, linear, simple-shear rheometer system (Model ELF 3200, Bose Corporation, Framingham, MA) as described in previous publications.12,13 A strain sweep test was first performed, with the test frequency set at 100 Hz to identify the linear region in which viscoelastic shear properties were independent of the strain amplitude.10 Frequency sweep experiment was then performed over a frequency range of 1 to 250 Hz in the linear region of viscoelasticity.12 All of the tests were conducted in a transparent acrylic environmental chamber in which the temperature was maintained at around 37°C, with a high relative humidity.
Statistical analysis of rheological data
The mixed-effects model or mixed model approach was employed for the comparison of tissue rheological properties between paired vocal folds. In the model, the fixed effects were frequency and the laterality of the vocal fold, i.e., right or left. The model also included two levels of random effects: the animal subject and the vocal fold tissue sample. These two random effects accounted for the hierarchical correlation structure in the measurements: one level was the correlation among the rheological data of the two vocal folds from the same animal; the other level was the correlation among the data obtained from the same vocal fold. Let be the continuous outcome (log G′ or log η′) of the kth (k = 1,…, m) measurement of the jth (j = 0 for the right and 1 for the left) vocal fold in the ith (i = 1,…, n) subject. The linear mixed-effects model could be stated as:
| (1) |
where β0 is the intercept, β1k and β2 are regression coefficients for the fixed effects, and are random effects corresponding to animal subject and tissue sample, respectively, and εijk denotes the residual error. , and εijk are assumed to have zero mean with unknown variance.
The fixed effect “laterality” in the mixed model, which could only be left or right, obviously was a categorical variable. Its coefficient β2 only had one degree of freedom because the vocal fold on one side of the larynx (right side in this study) was treated as reference. However, the other fixed effect in Eq. (1), frequency, could be considered as either a continuous variable or a categorical variable. If frequency was treated as a continuous variable, its coefficient reduced to β1 from matrix β1k given that now β11 = β12 = β13 …= β1m. The model could therefore be modified to:
| (2) |
If frequency was logarithmic transformed as previously reported,13 the model was as follows:
| (3) |
In order to select the model for rheological data analysis, Akaike information criterion (AIC)18 of Eqs. (1), (2), and (3) were calculated using both G′ and η′ data sets. The statistical model with a smaller AIC number is thought to have a better quality because it can achieve a good balance between the number of model parameters and the variance explained by the model.18 Once the statistical model was determined, the null hypothesis that there were no significant differences between the left and right vocal folds in viscoelastic shear properties was tested. The level of significance was set at 0.05.
The statistical analysis was performed using the statistical software SAS 9.4 (SAS Institute, Cary, NC).
Histology and staining
Six larynges were fixed in formalin, followed by decalcification in 0.35M ethylenediaminetetraacetic acid (EDTA). The larynges were then embedded in paraffin for sectioning. Successive 5 μm-thick coronal sections of each laryngeal specimen were prepared. In addition to hematoxylin and eosin (H&E) staining, various staining methods were used to examine the key lamina propria ECM components: Picrosirius red staining19,20 was used to assess the distribution and relative content of collagen fibers in the vocal fold lamina propria. Elastin was detected by Hart’s elastin staining, which stains elastin fiber blue/black and cytoplasm yellow.21 Glycosaminoglycans (GAGs), especially the sulfated glycosaminoglycans in the tissue samples were examined by Alcian blue staining, as previously described.22 Hyaluronic acid (HA), which does not contain any sulfate groups,23 was identified with immunostaining by using biotinylated hyaluronic acid binding protein (Sigma, St. Louis, MO) as the primary antibody and horseradish peroxidase-conjugated streptavidin (Thermo Fisher Scientific, Rockford, IL) as the secondary antibody, following the procedures previously described.24
Quantitative histological analysis
A Leica DM200 upright compound microscope (Buffalo Grove, IL) and an Optronics MicroFire microscope digital CCD camera (Goleta, CA) were used to capture digital histological images of the stained slides. Images of slides with the same staining were captured using identical microscope and camera settings to ensure consistency in image acquisition conditions across samples. The relative amounts or densities of collagen, elastin, HA, and sGAG were estimated from their staining intensities and relative areas by digital image analysis using the NIH Image J software (Bethesda, MD), as previously described.22,24 Paired Student’s t test was applied to digital image analysis with alpha = 0.05.
Results
Identifying the linear region
The strain sweep test results of the vocal folds from two animals are shown in Figure 1, where the elastic shear modulus G′ were plotted as a function of shear strain at 100 Hz. For all twelve samples, G′ remained nearly constant in a small-strain, linear region of viscoelasticity, with a strain amplitude of around 2%–5%. This finding was consistent with our previous result.13
Figure 1.
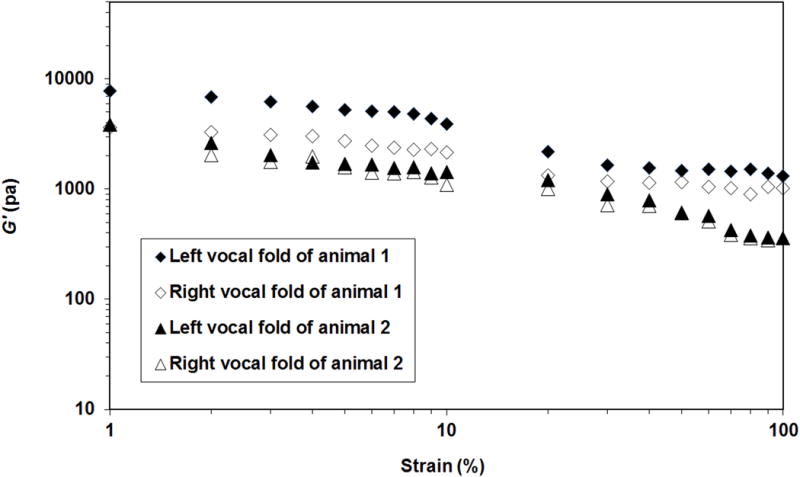
Elastic shear modulus G′ of the left and right vocal fold lamina propria from two rabbits as a function of shear strain (i.e., strain sweep; frequency=100 Hz). Based on the data of G′, the small-strain linear region of viscoelasticity can be identified with the strain amplitude at around 2-5% for rabbit vocal folds.
Viscoelastic shear properties of rabbit vocal folds
Once the linear region was identified, viscoelastic shear properties of the vocal fold samples were measured under a small strain of 2% or 3% to ensure stress-strain linearity.10 The custom rheometer system and the measurement protocol used in this study had been examined and validated in previous reports.3,12,13 Elastic shear modulus G′ and dynamic viscosity η′ of the left and right rabbit vocal folds were plotted in Figure 2 and Figure 3, respectively. Our rabbit rheological data were in good agreement with those reported in several previous studies.13,25–27
Figure 2.

Elastic shear modulus G′ of the left and right vocal fold lamina propria from six rabbits as a function of shear frequency (only upper error bars are shown for visual clarity on the plot).
Figure 3.

Dynamic viscosity η′ of the left and right vocal fold lamina propria from six rabbits as a function of shear frequency (only upper error bars are shown for visual clarity on the plot).
Statistical model selection and the analysis of rheological data
For statistical model selection, G′ and η′ of all twelve vocal fold lamina propria specimens were used to calculate the AIC values of the three mixed models. Results revealed that by treating frequency as a categorical variable, the model denoted by Eq. (1) provided the best fit to both log G′ and log η′ (Table 1). Therefore, statistical analysis of left-right symmetry in rheological data was conducted using the mixed-effects model in Eq. (1). The P values for log-transformed elastic shear modulus and dynamic viscosity were 0.1069 and 0.9433, respectively. Both P values were greater than 0.05, which indicated that the differences between the left and right vocal folds in viscoelastic shear properties were not statistically significant.
Table 1.
Akaike Information Criterion Values of Models for Logarithmic Transformed Elastic Shear Modulus and Dynamic Viscosity.
Histological results
Figure 4 shows the representative coronal sections of the laryngeal specimens. Collagen fibers, which appear red/pink in picrosirius-stained tissues, were present through the entire thickness of the lamina propria (Fig. 4B). Elastin staining was highly intense along the epithelium-lamina propria border, as previously reported.28,29 To the contrary, staining for elastin was rather sparse in the lamina propria, with its intensity slightly increased in the deeper aspect (Fig. 4C). The results of fibrous proteins (i.e., collagen, elastin) distributions were in good agreement with those obtained by immunohistochemical staining, trichrome staining, and elastin van Gieson staining.1,4,28,29 Fig. 4E shows that Alcian blue staining was quite intense throughout the vocal fold lamina propria, except in the region bordering the epithelium, where hyaluronic acid was highly concentrated (Fig. 4D). Similar Alcian blue staining pattern1,28 and the “band of hyaluronic acid”29 in rabbit vocal fold lamina propria had been reported previously. Histological examination showed that there were no appreciable differences in the morphological distributions of the key ECM constituents between the left and right vocal folds in all subjects.
Figure 4.

Representative coronal sections of rabbit larynges: (A) Hematoxylin and eosin, (B) Picrosirius red staining for collagen, (C) Hart’s elastin with arrow pointing to the subepithelial elastin band, (D) Hyaluronic acid with arrow pointing to the band of HA, and (E) Alcian blue staining with arrow pointing to the region bordering epithelium where sulfated glycosaminoglycans were largely absent. Total magnification = 25×. Laryngeal airway was narrowed in all subfigures to reduce image size.
The relative densities of the ECM constituents of interest were calculated as the fraction of the positively stained area to the total area of lamina propria. The quantitative histological results were plotted in Figure 5. Results of paired student’s t tests indicated that there were no significant left-right differences in the relative densities of collagen (p = 0.6980), elastin (p = 0.0894), hyaluronic acid (p = 0.8726), and sulfated glycosaminoglycan (p = 0.2097).
Figure 5.

Relative densities of major extracellular matrix constituents in left versus right rabbit vocal folds (n = 6).
Discussion
The left-right vocal fold symmetry in tissue properties was commonly assumed in laryngeal research.30–32 By using a rabbit model, the present study showed there were no significant differences between the left and right vocal folds in rheological and histological properties. On the other hand, results also demonstrated slight differences between the left and right vocal folds in rabbit. Similar asymmetry in anatomical structures and biomechanical properties between the two vocal folds in vocally healthy human subjects has been reported.8,10 It is known that such geometrical and biomechanical differences can contribute to asymmetrical vibrations during normal phonation in human.8,32,33 To determine whether the slight left-right vocal fold asymmetry in rabbit can also lead to asymmetrical vibrations during phonation, future investigations using imaging modalities for vocal fold vibration such as stroboscopy and videokymography are necessary.
In the current study, we developed three mixed-effects models for the rheological comparison between the left and right vocal folds. Able to take data correlation into consideration, the mixed-effects model is an appropriate approach for the statistical analysis of vocal fold rheological data generated by repeated measurement.13 Logarithmic transformed viscoelastic shear moduli were used in the models because log-transformation can greatly increase the distribution normality of G′ and η′, to subsequently ensure the validity of parametric statistical analyses.13 For our rabbit vocal fold rheological data, the AIC result indicated it was preferable to treat frequency, which is one of the fixed effects in the models, as a categorical variable. The statistical models developed in this study [Eqs. (1), (2), and (3)] can be used to compare the rheological properties of the treated vocal fold with those of the contralateral intact or sham vocal fold to conclude the efficacy of treatment in preclinical wound healing and regenerative medicine studies.
Special histology staining methods were used in this study to identify the key ECM constituents except for hyaluronic acid, which was identified by immunohistochemical staining. The histological results were consistent with published data obtained by different staining methods. It has been well accepted that the viscoelastic shear properties of the vocal fold lamina propria are predominately determined by the density and organization of its ECM molecular constituents, as well as the interactions among these macromolecules.4,13,34–36 Alteration of the composition of the lamina propria ECM can lead to changes in tissue rheological properties.1,4,34,37,38 Therefore, it is not surprising that no significant left-right vocal fold difference in normal rabbit larynges was found in histology as well. The results of rheological analysis and results of histological analysis in the present work supported each other.
Rabbit was selected for this study because it is one of the most widely used animal models in vocal fold research.5,31,39–42 Although rabbit shares similarities with human in terms of vocal fold extracellular matrix components, its vocal fold tissue layered structure and biomechanical properties are different from those of human.13,43 Therefore, further studies of human left-right vocal fold symmetry in histology and rheology are necessary. Another limitation of this study was that the sample size was relatively small. Future research in rabbit and other animal models with a larger sample size is warranted.
Conclusion
No significant differences in viscoelastic shear properties were found between the two vocal folds of the same larynx in rabbit. Histological examination also revealed there were no noticeable differences in densities and distribution patterns of major ECM constituents between the left and right vocal folds. This represented the first study that statistically investigated the left-right vocal fold symmetry in rheological and histological properties. Results of the current work validated one of the most common assumptions in vocal fold animal research.
Statistical models for the comparison of rheological data between paired vocal folds were presented in this study. These mixed-effects models can include the complex hierarchical correlation structure in data analysis and are directly applicable to vocal fold biomechanical studies.
Acknowledgments
We thank Elhum McPherson, Mindy Du, and Roger Chan for rheological data collection. We also thank Ted Mau for assistance in sample dissection. We also like to thank Brown/Goldtsein lab and Animal Resource Center in UT Southwestern Medical Center for providing animal cadavers and related records. Last but not least, we thank the staff in UTSW Histo Pathology Core especially John Shelton, Jessica Williams, Alejandro Daniel, and Anne Starling for their technical assistance in histology.
Funding: This article was supported by the National Institutes of Health, NIDCD Grants R03DC011145 and R21DC013363.
Footnotes
Conflict of Interest: None
Level of Evidence: N/A
References
- 1.Rousseau B, Hirano S, Chan RW, et al. Characterization of chronic vocal fold scarring in a rabbit model. Journal of voice: official journal of the Voice Foundation. 2004;18:116–124. doi: 10.1016/j.jvoice.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Cobell W, Duflo SM, Magrufov A, Thibeault SL. Fine needle aspiration of the vocal fold lamina propria in an animal model. The Annals of otology, rhinology, and laryngology. 2006;115:764–768. doi: 10.1177/000348940611501009. [DOI] [PubMed] [Google Scholar]
- 3.Krishna P, Regner M, Palko J, et al. The effects of decorin and HGF-primed vocal fold fibroblasts in vitro and ex vivo in a porcine model of vocal fold scarring. The Laryngoscope. 2010;120:2247–2257. doi: 10.1002/lary.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thibeault SL, Gray SD, Bless DM, Chan RW, Ford CN. Histologic and rheologic characterization of vocal fold scarring. Journal of voice: official journal of the Voice Foundation. 2002;16:96–104. doi: 10.1016/s0892-1997(02)00078-4. [DOI] [PubMed] [Google Scholar]
- 5.Thibeault SL, Klemuk SA, Chen X, Quinchia Johnson BH. In Vivo engineering of the vocal fold ECM with injectable HA hydrogels-late effects on tissue repair and biomechanics in a rabbit model. Journal of voice: official journal of the Voice Foundation. 2011;25:249–253. doi: 10.1016/j.jvoice.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonilha HS, Deliyski DD, Gerlach TT. Phase asymmetries in normophonic speakers: visual judgments and objective findings. Am J Speech Lang Pathol. 2008;17:367–376. doi: 10.1044/1058-0360(2008/07-0059). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw HS, Deliyski DD. Mucosal wave: a normophonic study across visualization techniques. J Voice. 2008;22:23–33. doi: 10.1016/j.jvoice.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Sidlof P, Svec JG, Horacek J, Vesely J, Klepacek I, Havlik R. Geometry of human vocal folds and glottal channel for mathematical and biomechanical modeling of voice production. J Biomech. 2008;41:985–995. doi: 10.1016/j.jbiomech.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Goodyer E, Hemmerich S, Muller F, Kobler JB, Hess M. The shear modulus of the human vocal fold, preliminary results from 20 larynxes. Eur Arch Otorhinolaryngol. 2007;264:45–50. doi: 10.1007/s00405-006-0133-8. [DOI] [PubMed] [Google Scholar]
- 10.Chan RW, Titze IR. Viscoelastic shear properties of human vocal fold mucosa: measurement methodology and empirical results. The Journal of the Acoustical Society of America. 1999;106:2008–2021. doi: 10.1121/1.427947. [DOI] [PubMed] [Google Scholar]
- 11.Miri AK, Mongrain R, Chen LX, Mongeau L. Quantitative assessment of the anisotropy of vocal fold tissue using shear rheometry and traction testing. J Biomech. 2012;45:2943–2946. doi: 10.1016/j.jbiomech.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan RW, Rodriguez ML. A simple-shear rheometer for linear viscoelastic characterization of vocal fold tissues at phonatory frequencies. The Journal of the Acoustical Society of America. 2008;124:1207–1219. doi: 10.1121/1.2946715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu CC, Chan RW, Sun H, Zhan X. A mixed-effects model approach for the statistical analysis of vocal fold viscoelastic shear properties. Journal of the Mechanical Behavior of Biomedical Materials. 2017;75:477–485. doi: 10.1016/j.jmbbm.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zar JH. Simple linear Regression Biostatistical Analysis. Upper Saddle River, NJ: Prentice Hall; 1998a. pp. 324–359. [Google Scholar]
- 15.Choi JS, Kim NJ, Klemuk S, et al. Preservation of viscoelastic properties of rabbit vocal folds after implantation of hyaluronic Acid-based biomaterials. Otolaryngology–head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2012;147:515–521. doi: 10.1177/0194599812446913. [DOI] [PubMed] [Google Scholar]
- 16.Harville DA. Maximum likelihood approaches to variance component estimation and to related problems. Journal of the American Statistical Association. 1977;72:320–338. [Google Scholar]
- 17.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 18.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- 19.Hahn MS, Kobler JB, Zeitels SM, Langer R. Quantitative and comparative studies of the vocal fold extracellular matrix II: collagen. The Annals of otology, rhinology, and laryngology. 2006;115:225–232. doi: 10.1177/000348940611500311. [DOI] [PubMed] [Google Scholar]
- 20.Kratky RG, Ivey J, Roach MR. Collagen quantitation by video-microdensitometry in rabbit atherosclerosis. Matrix Biology. 1996;15:141–144. doi: 10.1016/s0945-053x(96)90155-9. [DOI] [PubMed] [Google Scholar]
- 21.Watts CR, Knutsen RH, Ciliberto C, Mecham RP. Evidence for heterozygous abnormalities of the elastin gene (ELN) affecting the quantity of vocal fold elastic fibers: a pilot study. J Voice. 2011;25:e85–90. doi: 10.1016/j.jvoice.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu CC, Chan RW, Weinberger DG, Efune G, Pawlowski KS. Controlled release of hepatocyte growth factor from a bovine acellular scaffold for vocal fold reconstruction. Journal of biomedical materials research Part A. 2010;93:1335–1347. doi: 10.1002/jbm.a.32632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dicker KT, Gurski LA, Pradhan-Bhatt S, Witt RL, Farach-Carson MC, Jia X. Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater. 2014;10:1558–1570. doi: 10.1016/j.actbio.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu CC, Chan RW, Weinberger DG, Efune G, Pawlowski KS. A bovine acellular scaffold for vocal fold reconstruction in a rat model. Journal of biomedical materials research Part A. 2010;92:18–32. doi: 10.1002/jbm.a.32279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svensson B, Nagubothu SR, Cedervall J, et al. Injection of human mesenchymal stem cells improves healing of vocal folds after scar excision–a xenograft analysis. The Laryngoscope. 2011;121:2185–2190. doi: 10.1002/lary.22143. [DOI] [PubMed] [Google Scholar]
- 26.Hertegard S, Dahlqvist A, Laurent C, Borzacchiello A, Ambrosio L. Viscoelastic properties of rabbit vocal folds after augmentation. Otolaryngology–head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2003;128:401–406. doi: 10.1067/mhn.2003.96. [DOI] [PubMed] [Google Scholar]
- 27.Dahlqvist A, Garskog O, Laurent C, Hertegard S, Ambrosio L, Borzacchiello A. Viscoelasticity of rabbit vocal folds after injection augmentation. The Laryngoscope. 2004;114:138–142. doi: 10.1097/00005537-200401000-00025. [DOI] [PubMed] [Google Scholar]
- 28.Pitman MJ, Kurita T, Powell ME, et al. Vibratory function and healing outcomes after small intestinal submucosa biomaterial implantation for chronic vocal fold scar. The Laryngoscope. 2017 doi: 10.1002/lary.26883. [DOI] [PubMed] [Google Scholar]
- 29.Mau T, Du M, Xu CC. A rabbit vocal fold laser scarring model for testing lamina propria tissue-engineering therapies. The Laryngoscope. 2014;124:2321–2326. doi: 10.1002/lary.24707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Titze IR, Jiang JJ, Hsiao TY. Measurement of mucosal wave propagation and vertical phase difference in vocal fold vibration. Ann Otol Rhinol Laryngol. 1993;102:58–63. doi: 10.1177/000348949310200111. [DOI] [PubMed] [Google Scholar]
- 31.Bless DM, Welham NV. Characterization of vocal fold scar formation, prophylaxis, and treatment using animal models. Current opinion in otolaryngology & head and neck surgery. 2010;18:481–486. doi: 10.1097/MOO.0b013e3283407d87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mittal R, Erath BD, Plesniak MW. Fluid Dynamics of Human Phonation and Speech. Annual Review of Fluid Mechanics. 2013;45:437–467. [Google Scholar]
- 33.Xue Q, Mittal R, Zheng X, Bielamowicz S. A computational study of the effect of vocal-fold asymmetry on phonation. J Acoust Soc Am. 2010;128:818–827. doi: 10.1121/1.3458839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gray SD, Titze IR, Alipour F, Hammond TH. Biomechanical and histologic observations of vocal fold fibrous proteins. The Annals of otology, rhinology, and laryngology. 2000;109:77–85. doi: 10.1177/000348940010900115. [DOI] [PubMed] [Google Scholar]
- 35.Gray SD, Titze IR, Chan R, Hammond TH. Vocal fold proteoglycans and their influence on biomechanics. The Laryngoscope. 1999;109:845–854. doi: 10.1097/00005537-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Hahn MS, Jao CY, Faquin W, Grande-Allen KJ. Glycosaminoglycan composition of the vocal fold lamina propria in relation to function. The Annals of otology, rhinology, and laryngology. 2008;117:371–381. doi: 10.1177/000348940811700508. [DOI] [PubMed] [Google Scholar]
- 37.Rousseau B, Hirano S, Scheidt TD, et al. Characterization of vocal fold scarring in a canine model. The Laryngoscope. 2003;113:620–627. doi: 10.1097/00005537-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Chan RW, Gray SD, Titze IR. The importance of hyaluronic acid in vocal fold biomechanics. Otolaryngology–head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2001;124:607–614. doi: 10.1177/019459980112400602. [DOI] [PubMed] [Google Scholar]
- 39.Hansen JK, Thibeault SL, Walsh JF, Shu XZ, Prestwich GD. In vivo engineering of the vocal fold extracellular matrix with injectable hyaluronic acid hydrogels: early effects on tissue repair and biomechanics in a rabbit model. The Annals of otology, rhinology, and laryngology. 2005;114:662–670. doi: 10.1177/000348940511400902. [DOI] [PubMed] [Google Scholar]
- 40.Hertegard S, Cedervall J, Svensson B, et al. Viscoelastic and histologic properties in scarred rabbit vocal folds after mesenchymal stem cell injection. The Laryngoscope. 2006;116:1248–1254. doi: 10.1097/01.mlg.0000224548.68499.35. [DOI] [PubMed] [Google Scholar]
- 41.Cedervall J, Ahrlund-Richter L, Svensson B, et al. Injection of embryonic stem cells into scarred rabbit vocal folds enhances healing and improves viscoelasticity: short-term results. The Laryngoscope. 2007;117:2075–2081. doi: 10.1097/MLG.0b013e3181379c7c. [DOI] [PubMed] [Google Scholar]
- 42.Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich G. Effect of a synthetic extracellular matrix on vocal fold lamina propria gene expression in early wound healing. Tissue engineering. 2006;12:3201–3207. doi: 10.1089/ten.2006.12.3201. [DOI] [PubMed] [Google Scholar]
- 43.Kurita S, Nagata K, Hirano M. A comparative study of the layer structure of the vocal fold. In: Abbs DMBJH, editor. Vocal Fold Physiology: Contempo-rary Research and Clinical Issues. Vol. 1983. San Diego, CA: College-Hill Press; pp. 3–21. [Google Scholar]


