Abstract
The title compound, C17H18O2, crystallizes in two-dimensional sheets, in which the 2-(pentyloxy)dibenzo[b,d]furan molecules are arranged in a head-to-head and tail-to-tail fashion that enables hydrophobic interactions between fully extended 2-pentoxy chains and π-π stacking between dibenzofuran rings in adjacent rings. Nearest intermolecular π-π stacking contacts are 3.3731 (12) Å. The molecule is nearly planar with an r.m.s. deviation of 0.0803 Å from the mean plane defined by the nineteen non-hydrogen atoms.
Keywords: crystal structure, dibenzofuran, π-π stacking interactions, hydrophobic interactions, P450 inhibitor
Structure description
Natural products and their structurally diverse derivatives play a major role in drug discovery and development (Cragg et al., 1997). Derivatives of dibenzofuran, a three-ring fused system, have shown interesting biological activities as therapeutics for diseases such as cancer, thombosis, tuberculosis, etc (Yurtasş et al., 2016; Kantevari et al., 2011; Chiranjeevi et al., 2013). Our lab is studying the design and synthesis of new inhibitors of P450 enzymes, which are a superfamily of heme proteins involved in the metabolism and detoxification of endogenous and exogenous compounds (Sridhar et al., 2017). P450s are involved in the bioactivation of certain procarcinogens leading to the production of carcinogenic species. The development of potent and selective P450 enzyme inhibitors has attracted considerable attention over the years and has become an important cancer prevention target (Alexander et al., 1995; Foroozesh et al., 1997; Sridhar et al., 2017; Foroozesh et al., 2013).
Previous studies in our laboratory have shown that P450 1A1 accommodates linear polycyclic aromatic molecules while P450 1A2 prefers triangle-shaped molecules. [The 1A1 nomenclature designates enzymes belonging to family 1, subfamily A, polypeptide 1, as encoded by the CYP1A1 gene; the 1A2 notation is similarly defined.] These substrate preferences have led to the design of several triangle-shaped carbazole derivatives in an attempt to synthesize potentially selective inhibitors for P450 1A2 over P450 1A1. We are also interested in synthesizing molecules that have fused-ring systems such as dibenzofuran in our pursuit of active P450 inhibitors.
In the crystalline state, the 2-pentyloxy substituent in the title compound occurs in a fully extended, linear conformation in which it is nearly coplanar with the dibenzofuran ring system (Fig. 1). The r.m.s. deviation from the mean plane defined by the nineteen non-hydrogen atoms is 0.0803 Å, with the largest departure being seen for O2 at 0.178 (2) Å. The distinctive feature of the molecular packing is an arrangement of the molecules into two-dimensional sheets that are neither parallel nor orthogonal to any cell axis or face (Fig. 2). Within these sheets, adjacent molecules are juxtaposed in a head-to-head and tail-to-tail fashion such that pseudo twofold rotational symmetry relates them (Fig. 3). An apparent consequence of this pattern is the creation of interchain hydrophobic interactions between pentyloxy groups, which likely assist in supporting their fully extended, linear disposition. Molecules of 2-(pentyloxy)dibenzo[b,d]furan in adjacent sheets enjoy π-π stacking interactions that slightly offset the centroid of the furan ring of one molecule above that of a neighboring molecule such that the centroid-to-centroid distance is 4.070 (3) Å (Fig. 4, red line). The length of the perpendicular segment between adjacent furan rings, defined with one point as a furan centroid, is 3.3731 (12) Å (Fig. 4, blue line). These separations are comparable to the 3.72–3.76 Å distances between molecules in the structure of dibenzofuran itself (Dideberg et al., 1972).
Figure 1.
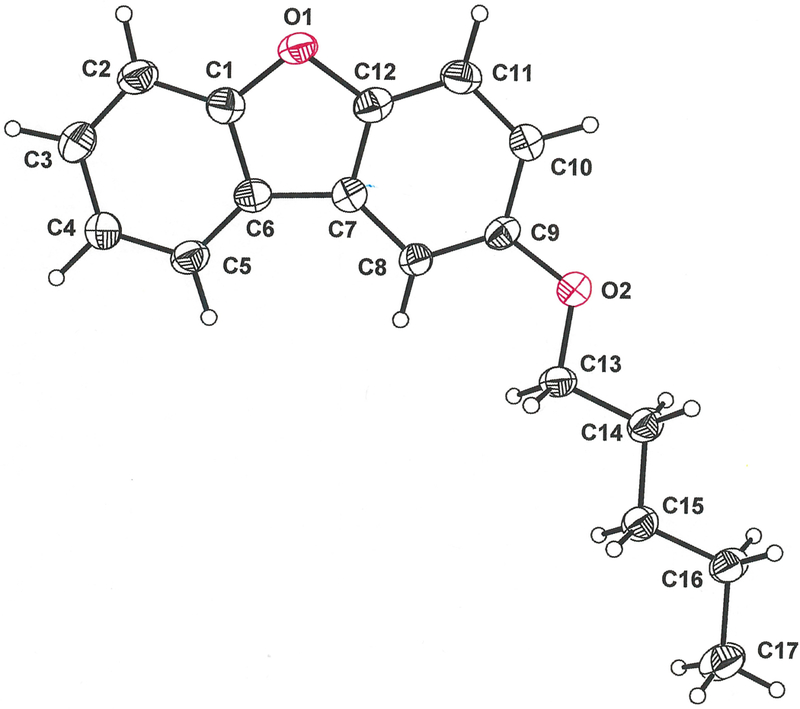
The title molecule with the atom-labeling scheme and 50% probability ellipsoids.
Figure 2.
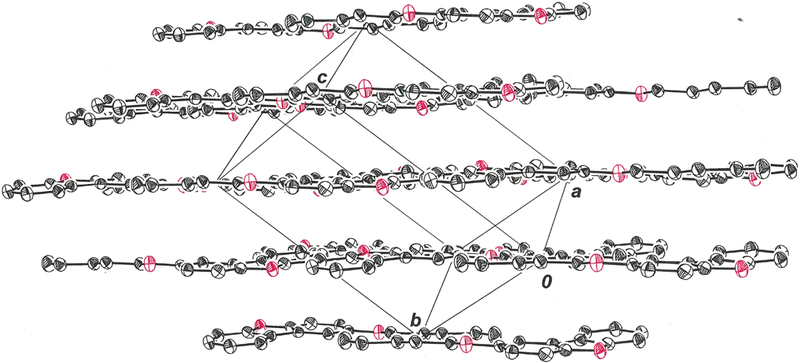
Packing of molecules of 2-(pentyloxy)dibenzo[b,d]furan within the unit cell. Displacement ellipsoids are shown at the 50% probability level.
Figure 3.
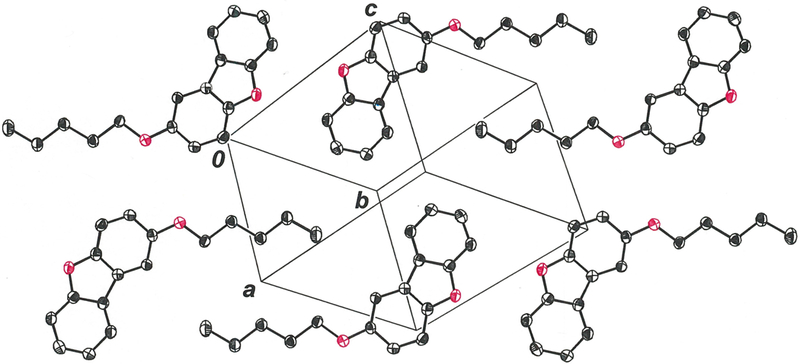
Molecules of 2-(pentyloxy)dibenzo[b,d]furan within a sheet, showing the tail-to-tail arrangement of pentyloxy substituents. Displacement ellipsoids are shown at the 50% probability level.
Figure 4.
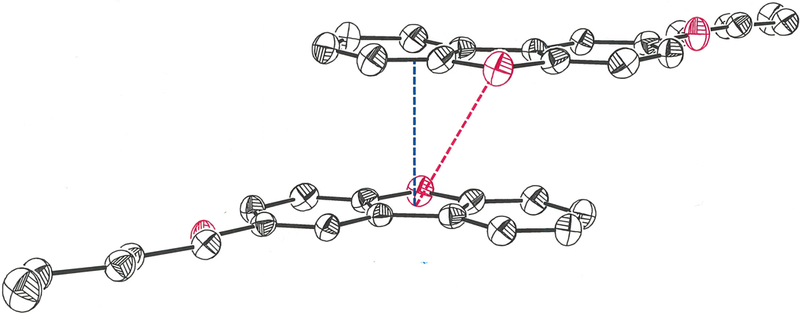
Molecules of 2-(pentyloxy)dibenzo[b,d]furan in adjacent sheets. The furan ring centroid-to-centroid distance is depicted with the red dashed line, while the separation along a perpendicular from a furan ring centroid is shown in blue. Displacement ellipsoids are shown at the 50% probability level.
Synthesis and crystallization
The starting material, 2-hydroxydibenzofuran (0.10 g, 0.54 mmol), was dissolved in 10 mL of acetone under an N2 atmosphere. To this solution, solid potassium carbonate was then added (0.3 g, 4 eq.). The reaction mixture was stirred at room temperature for 30 minutes before the dropwise addition of pentyl bromide (0.10 mL, 0.59 mmol). Mild heating to 45°C was applied for 4–5 h, during which time the reaction progress was monitored using thin layer chromatography. The reaction mixture was cooled to room temperature, vacuum filtered, and then taken to dryness under reduced pressure. The crude solid residual was purified by flash chromatography on a silica column eluted with 10:90 EtOAc:hexanes to afford the target compound as a white solid. Crystals were obtained by slow cooling of a warm solution in ethyl acetate:hexanes (2:1, v:v). Yield: 0.35 g, 85%. Rf: 0.80 (10:90 EtOAc:hexanes, UV). 1H NMR (300 MHz, (δ, ppm in CDCl3): 7.94 (d, J = 8.2 Hz, 1H), 7.59 (d, J = 8.3 Hz, 1H), 7.52–7.44 (m, 3H), 7.39–7.33 (m, 1H), 7.11–7.06 (m, 1H), 4.09 (t, J = 6.5 Hz, 2H), 1.89 (q, J = 7.2 Hz, 2H), 1.41–1.59 (m, 4H), 1.00 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, (δ, ppm in CDCl3): 156.9, 155.4, 150.8 127.0, 124.6, 124.5, 122.4, 120.5, 115.7, 112.0, 104.6, 69.0, 29.1, 28.3, 22.5, 14.1. GC–MS: 254, 183.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 1.
Table 1.
Experimental details
| Crystal data | |
|---|---|
| Chemical formula | C17H18O2 |
| Mr | 254.31 |
| Crystal system, space group | Triclinic, P |
| Temperature (K) | 150 |
| a, b, c (Å) | 7.841 (4), 8.203 (4), 11.080 (6) |
| α, β, γ (°) | 79.131 (7), 85.616 (6), 74.077 (6) |
| V (Å3) | 672.8 (6) |
| Z | 2 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.08 |
| Crystal size (mm) | 0.39 × 0.23 × 0.15 |
| Data collection | |
| Diffractometer | Bruker SMART APEX CCD |
| Absorption correction | Multi-scan (SADABS; Krause et al., 2015) |
| Tmin, Tmax | 0.790, 0.987 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 4601,1806, 1345 |
| Rint | 0.029 |
| θmax (°) | 22.7 |
| (sin θ/λ)max (Å−1) | 0.544 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.052, 0.152, 1.11 |
| No. of reflections | 1806 |
| No. of parameters | 244 |
| H-atom treatment | All H-atom parameters refined |
| Δρmax, Δρmin (e Å−3) | 0.23, −0.24 |
Computer programs: APEX3 and SAINT (Bruker, 2016), SHELXT (Sheldrick, 2015a), SHELXL2018/1 (Sheldrick, 2015b) and SHELXTL (Sheldrick, 2008).
Supplementary Material
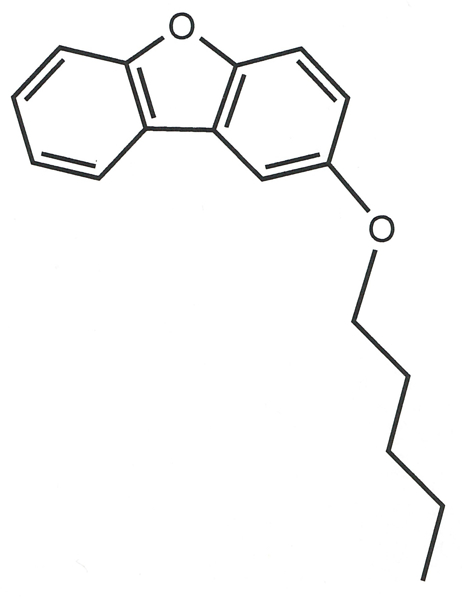
Chemical scheme
Acknowledgments
Funding information
Funding for this research was provided by: National Institutes of Health (award No. G12MD007595; award No. TL4GM118968; award No. 5RL5GM118966; award No. R25GM060926); National Science Foundation, Division of Chemistry (grant No. MRI: 1228232; award No. MRI: 0619770); Louisiana Board of Regents (grant No. LEQSF-(2002-03)-ENH-TR-67).
Structural data
Crystal structure: contains datablock I. DOI: https://doi.org//10.1107/S2414314618013068/bx4012sup1.cif
Structure factors: contains datablock I. DOI: https://doi.org//10.1107/S2414314618013068/bx4012Isup2.hkl
Crystal data
| C17H18O2 | Z = 2 |
| Mr = 254.31 | F(000) = 272 |
| Triclinic, P | = 1.255 Mg m−3 |
| a = 7.841 (4) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 8.203 (4) Å | Cell parameters from 2845 reflections |
| c = 11.080 (6) Å | θ = 2.6–22.7° |
| α = 79.131 (7)° | μ = 0.08 mm− |
| β = 85.616 (6)° | T = 150 K |
| γ = 74.077 (6)° | Block, colorless |
| V = 672.8 (6) Å3 | 0.39 × 0.23 × 0.15 mm |
Data collection
| Bruker SMART APEX CCD diffractometer | 1806 independent reflections |
| Radiation source: fine-focus sealed tube | 1345 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.029 |
| Detector resolution: 8.3333 pixels mm−1 | θmax = 22.7°, θmin = 1.9° |
| φ and ω scans | h = −8→8 |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | k = −8→8 |
| Tmin = 0.790, Tmax = 0.987 | l = −12→11 |
| 4601 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.052 | Hydrogen site location: difference Fourier map |
| wR(F2) = 0.152 | All H-atom parameters refined |
| S = 1.11 | w = 1/[σ2(Fo2) + (0.0701P)2 + 0.5606P] where P = (Fo2 + 2Fc2)/3 |
| 1806 reflections | (Δ/σ)max < 0.001 |
| 244 parameters | Δϱmax = 0.23 e Å−3 |
| 0 restraints | Δϱmin = −0.24 e Å−3 |
Special details
Experimental.
The diffraction data were obtained from 3 sets of 400 frames, each of width 0.5° in ω, collected at ϕ = 0.00, 90.00 and 180.00° and 2 sets of 800 frames, each of width 0.45° in ϕ, collected at ω = –30.00 and 210.00°. The scan time was 30 sec/frame.
Geometry.
All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | |
|---|---|---|---|---|
| O1 | 0.0899 (2) | 0.7991 (2) | 0.40165 (18) | 0.0332 (6) |
| O2 | 0.2926 (2) | 0.3931 (3) | 0.04899 (18) | 0.0349 (6) |
| C1 | −0.0704 (4) | 0.7661 (4) | 0.4407 (3) | 0.0306 (7) |
| C2 | −0.1831 (4) | 0.8459 (4) | 0.5259 (3) | 0.0345 (8) |
| H2 | −0.152 (3) | 0.931 (3) | 0.559 (2) | 0.019 (7)* |
| C3 | −0.3401 (4) | 0.8019 (4) | 0.5513 (3) | 0.0369 (8) |
| H3 | −0.433 (5) | 0.856 (4) | 0.611 (3) | 0.056 (10)* |
| C4 | −0.3820 (4) | 0.6819 (4) | 0.4941 (3) | 0.0351 (8) |
| H4 | −0.504 (4) | 0.650 (4) | 0.516 (3) | 0.033 (7)* |
| C5 | −0.2659 (4) | 0.6033 (4) | 0.4094 (3) | 0.0326 (8) |
| H5 | −0.290 (4) | 0.518 (4) | 0.369 (3) | 0.033 (8)* |
| C6 | −0.1074 (4) | 0.6445 (3) | 0.3816 (3) | 0.0285 (7) |
| C7 | 0.0414 (3) | 0.5998 (3) | 0.2969 (2) | 0.0264 (7) |
| C8 | 0.0839 (4) | 0.4924 (4) | 0.2096 (3) | 0.0271 (7) |
| H8 | 0.005 (4) | 0.427 (4) | 0.197 (2) | 0.027 (7)* |
| C9 | 0.2361 (3) | 0.4897 (3) | 0.1411 (2) | 0.0265 (7) |
| C10 | 0.3479 (4) | 0.5903 (4) | 0.1570 (3) | 0.0309 (7) |
| H10 | 0.454 (4) | 0.588 (4) | 0.106 (3) | 0.033 (8)* |
| C11 | 0.3068 (4) | 0.6953 (4) | 0.2441 (3) | 0.0332 (8) |
| H11 | 0.387 (3) | 0.760 (3) | 0.255 (2) | 0.018 (6)* |
| C12 | 0.1539 (4) | 0.6982 (4) | 0.3117 (3) | 0.0297 (7) |
| C13 | 0.2041 (4) | 0.2662 (4) | 0.0379 (3) | 0.0293 (7) |
| H13A | 0.208 (3) | 0.182 (4) | 0.115 (3) | 0.029 (7)* |
| H13B | 0.072 (4) | 0.319 (3) | 0.023 (2) | 0.025 (7)* |
| C14 | 0.2947 (4) | 0.1780 (4) | −0.0661 (3) | 0.0311 (7) |
| H14B | 0.289 (3) | 0.264 (3) | −0.139 (3) | 0.019 (7)* |
| H14A | 0.423 (4) | 0.131 (4) | −0.048 (2) | 0.030 (7)* |
| C15 | 0.2184 (4) | 0.0354 (4) | −0.0873 (3) | 0.0321 (7) |
| H15A | 0.220 (3) | −0.045 (4) | −0.009 (3) | 0.029 (7)* |
| H15B | 0.091 (4) | 0.087 (4) | −0.111 (2) | 0.031 (7)* |
| C16 | 0.3193 (4) | −0.0593 (4) | −0.1866 (3) | 0.0344 (8) |
| H16B | 0.327 (4) | 0.024 (4) | −0.264 (3) | 0.032 (8)* |
| H16A | 0.447 (4) | −0.110 (3) | −0.163 (2) | 0.030 (7)* |
| C17 | 0.2355 (5) | −0.1938 (5) | −0.2144 (4) | 0.0479 (9) |
| H17A | 0.295 (4) | −0.255 (4) | −0.279 (3) | 0.040 (9)* |
| H17C | 0.229 (5) | −0.279 (5) | −0.140 (4) | 0.068 (12)* |
| H17B | 0.106 (5) | −0.136 (5) | −0.242 (3) | 0.074 (12)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
|---|---|---|---|---|---|---|
| O1 | 0.0338 (12) |
0.0302 (12) |
0.0416 (13) |
−0.0115 (9) | 0.0012 (9) | −0.0171 (10) |
| O2 | 0.0346 (12) |
0.0360 (12) |
0.0393 (12) |
−0.0138 (9) | 0.0081 (9) | −0.0164 (10) |
| C1 | 0.0280 (16) |
0.0264 (16) |
0.0365 (17) |
−0.0044 (13) |
−0.0030 (13) |
−0.0062 (14) |
| C2 | 0.0376 (18) |
0.0279 (17) |
0.0403 (19) |
−0.0080 (14) |
−0.0004 (14) |
−0.0131 (15) |
| C3 | 0.0377 (18) |
0.0330 (18) |
0.0382 (19) |
−0.0032 (14) |
0.0024 (14) | −0.0125 (15) |
| C4 | 0.0325 (17) |
0.0360 (18) |
0.0385 (18) |
−0.0098 (14) |
0.0043 (14) | −0.0110 (15) |
| C5 | 0.0376 (18) |
0.0263 (17) |
0.0352 (18) |
−0.0086 (14) |
−0.0004 (14) |
−0.0091 (14) |
| C6 | 0.0304 (16) |
0.0252 (16) |
0.0296 (16) |
−0.0063 (12) |
−0.0038 (13) |
−0.0042 (13) |
| C7 | 0.0270 (15) |
0.0254 (16) |
0.0256 (16) |
−0.0056 (12) |
−0.0026 (12) |
−0.0025 (13) |
| C8 | 0.0249 (15) |
0.0276 (16) |
0.0306 (17) |
−0.0072 (13) |
−0.0001 (12) |
−0.0093 (13) |
| C9 | 0.0278 (15) |
0.0236 (16) |
0.0277 (16) |
−0.0027 (12) |
−0.0044 (12) |
−0.0079 (13) |
| C10 | 0.0284 (16) |
0.0338 (17) |
0.0316 (17) |
−0.0101 (13) |
0.0027 (14) | −0.0073 (14) |
| C11 | 0.0310 (17) |
0.0324 (17) |
0.0398 (19) |
−0.0120 (14) |
−0.0043 (14) |
−0.0085 (15) |
| C12 | 0.0300 (16) |
0.0265 (16) |
0.0324 (17) |
−0.0041 (12) |
−0.0063 (13) |
−0.0072 (14) |
| C13 | 0.0312 (17) |
0.0242 (16) |
0.0353 (19) |
−0.0094 (13) |
−0.0015 (13) |
−0.0085 (14) |
| C14 | 0.0309 (18) |
0.0268 (17) |
0.0354 (19) |
−0.0048 (13) |
−0.0017 (13) |
−0.0087 (15) |
| C15 | 0.0322 (18) |
0.0289 (17) |
0.0357 (19) |
−0.0066 (14) |
−0.0017 (14) |
−0.0087 (15) |
| C16 | 0.0365 (19) |
0.0305 (18) |
0.038 (2) | −0.0077 (14) |
−0.0024 (14) |
−0.0128 (16) |
| C17 | 0.057 (2) | 0.041 (2) | 0.053 (2) | −0.0146 (18) |
−0.0008 (19) |
−0.023 (2) |
Geometric parameters (Å, °)
| O1—C1 | 1.378 (3) | C10—C11 | 1.375 (4) |
| O1—C12 | 1.392 (3) | C10—H10 | 0.96 (3) |
| O2—C9 | 1.382 (3) | C11—C12 | 1.361 (4) |
| O2—C13 | 1.428 (3) | C11—H11 | 0.96 (3) |
| C1—C2 | 1.375 (4) | C13—C14 | 1.504 (4) |
| C1—C6 | 1.394 (4) | C13—H13A | 0.99 (3) |
| C2—C3 | 1.370 (4) | C13—H13B | 1.02 (3) |
| C2—H2 | 0.94 (3) | C14—C15 | 1.514 (4) |
| C3—C4 | 1.387 (4) | C14—H14B | 0.96 (3) |
| C3—H3 | 1.01 (4) | C14—H14A | 1.00 (3) |
| C4—C5 | 1.378 (4) | C15—C16 | 1.514 (4) |
| C4—H4 | 1.06 (3) | C15—H15A | 0.99 (3) |
| C5—C6 | 1.373 (4) | C15—H15B | 1.00 (3) |
| C5—H5 | 0.96 (3) | C16—C17 | 1.516 (4) |
| C6—C7 | 1.451 (4) | C16—H16B | 1.00 (3) |
| C7—C8 | 1.390 (4) | C16—H16A | 1.01 (3) |
| C7—C12 | 1.386 (4) | C17—H17A | 0.97 (3) |
| C8—C9 | 1.362 (4) | C17—H17C | 0.99 (4) |
| C8—H8 | 0.96 (3) | C17—H17B | 1.04 (4) |
| C9—C10 | 1.399 (4) | ||
| C1—O1—C12 | 105.0 (2) | C10—C11—H11 | 119.0 (15) |
| C9—O2—C13 | 118.3 (2) | C11—C12—O1 | 125.3 (2) |
| C2—C1—O1 | 124.3 (3) | C11—C12—C7 | 122.9 (3) |
| C2—C1—C6 | 123.6 (3) | O1—C12—C7 | 111.8 (2) |
| O1—C1—C6 | 112.1 (2) | O2—C13—C14 | 107.2 (2) |
| C3—C2—C1 | 116.7 (3) | O2—C13—H13A | 111.4 (15) |
| C3—C2—H2 | 124.2 (15) | C14—C13—H13A | 110.9 (16) |
| C1—C2—H2 | 119.0 (15) | O2—C13—H13B | 112.0 (14) |
| C2—C3—C4 | 121.4 (3) | C14—C13—H13B | 111.1 (14) |
| C2—C3—H3 | 122.5 (19) | H13A—C13—H13B | 104 (2) |
| C4—C3—H3 | 116.0 (19) | C13—C14—C15 | 113.5 (2) |
| C5—C4—C3 | 120.6 (3) | C13—C14—H14B | 108.3 (15) |
| C5—C4—H4 | 119.8 (15) | C15—C14—H14B | 111.2 (15) |
| C3—C4—H4 | 119.6 (15) | C13—C14—H14A | 108.4 (16) |
| C6—C5—C4 | 119.6 (3) | C15—C14—H14A | 109.8 (16) |
| C6—C5—H5 | 117.8 (17) | H14B—C14—H14A | 105 (2) |
| C4—C5—H5 | 122.6 (17) | C16—C15—C14 | 112.6 (2) |
| C5—C6—C1 | 118.1 (3) | C16—C15—H15A | 110.3 (16) |
| C5—C6—C7 | 136.4 (3) | C14—C15—H15A | 107.9 (15) |
| C1—C6—C7 | 105.4 (2) | C16—C15—H15B | 109.5 (15) |
| C8—C7—C12 | 119.6 (2) | C14—C15—H15B | 108.6 (16) |
| C8—C7—C6 | 134.6 (2) | H15A—C15—H15B | 108 (2) |
| C12—C7—C6 | 105.8 (2) | C15—C16—C17 | 112.8 (3) |
| C9—C8—C7 | 117.8 (3) | C15—C16—H16B | 109.9 (16) |
| C9—C8—H8 | 122.2 (16) | C17—-C16—H16B | 109.1 (16) |
| C7—C8—H8 | 120.0 (16) | C15—C16—H16A | 108.9 (15) |
| C8—C9—O2 | 124.2 (2) | C17—C16—H16A | 112.5 (15) |
| C8—C9—C10 | 121.8 (3) | H16B—C16—H16A | 103 (2) |
| O2—C9—C10 | 113.9 (2) | C16—C17—H17A | 115.1 (18) |
| C11—C10—C9 | 120.3 (3) | C16—C17—H17C | 110 (2) |
| C11—C10—H10 | 119.5 (17) | H17A—C17—H17C | 108 (3) |
| C9—C10—H10 | 120.1 (17) | C16—C17—H17B | 110 (2) |
| C12—C11—C10 | 117.5 (3) | H17A—C17—H17B | 106 (3) |
| C12—C11—H11 | 123.5 (15) | H17C—C17—H17B | 106 (3) |
Footnotes
Supporting information file. DOI: https://doi.org//10.1107/S2414314618013068/bx4012Isup3.cml checkCIF report
References
- Alexander DL & Jefcoate CR (1995). Proc. Amer. Assoc. Cancer Res. 36, 152, abstract 905. [Google Scholar]
- Bruker (2016). APEX3, SADABS and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA. [Google Scholar]
- Chiranjeevi B, Koyyada T, Prabusreenivasan S, Kumar V, Sujitha P, Kumar CG, Sridhar B, Shaik S & Chandrasekharam M (2013). RSCAdv. 3, 16475–16485. [Google Scholar]
- Cragg GM, Newman DJ & Snader KM (1997). J. Nat. Prod. 60, 52–60. [DOI] [PubMed] [Google Scholar]
- Dideberg O, Dupont L & André JM (1972). Acta Cryst. B28, 1002–1007. [Google Scholar]
- Foroozesh M, Jiang Q, Sridhar J, Liu J, Dotson B & McClain E (2013). J. Undergrad. Chem. Res. 12, 89–91. [PMC free article] [PubMed] [Google Scholar]
- Foroozesh M, Primrose G, Guo Z, Bell LC, Alworth WL & Guengerich FP (1997). Chem. Res. Toxicol. 10, 91–102. [DOI] [PubMed] [Google Scholar]
- Kantevari S, Yempala T, Yogeeswari P, Sriram D & Sridhar B (2011). Bioorg. Med. Chem. Lett. 21, 4316–4319. [DOI] [PubMed] [Google Scholar]
- Krause L, Herbst-Irmer R, Sheldrick GM & Stalke D (2015). J. Appl. Cryst. 48, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick GM (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed] [Google Scholar]
- Sheldrick GM (2015a). Acta Cryst. A71, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick GM (2015b). Acta Cryst. C71, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar J, Goyal N, Liu J & Foroozesh M (2017). Molecules, 22, 1143/1–1143/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurttaş L, Abu Mohsen U, Ozkan Y, Cobanoglu S, Levent S & Kaplancikli ZA (2016). J. Enzyme Inhib. Med. Chem. 31, 1177–1183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


