Abstract
Background:
Human papillomavirus (HPV) and HPV-associated cancer rates are high among men who have sex with men (MSM). The US Advisory Committee on Immunization Practices (ACIP) recommends HPV vaccination for all MSM through age 26. We examined trends in HPV vaccine uptake among young US MSM between 2014–2017.
Methods:
Cochran-Armitage tests and estimated annual percentage changes (EAPC) were used to examine annual trends (2014–2017) in HPV vaccination initiation among US MSM ≤26 years as of 2011 who participated in a nationwide annual cross-sectional online survey. We identified independent correlates of HPV vaccination in 2017 using Poisson regression modeling.
Results:
There were 2,381 participants in 2014; 4,143 in 2015; 3,926 in 2016; and 3,407 in 2017. Mean age was 23.5 years, 39% lived in metropolitan areas, and 37% lived in the South. HPV vaccination significantly increased (p<0.0001) from 22.5% in 2014 to 37.6% in 2017 (EAPC=17.4%). HPV vaccination was significantly greater for MSM who were younger, had health insurance, saw a healthcare provider (HCP) in the past 12-months, resided in the Northeast, resided in metropolitan areas, had higher household income, disclosed their sexual identity to HCP, and had gonorrhea/chlamydia diagnosis in the past 12-months.
Conclusions:
HPV vaccination among MSM increased from 2014–2017, but vaccine uptake varied significantly by MSM subgroup. Despite favorable trends, the HPV vaccination coverage for this population (37.6%) is less than half of the Healthy People 2020 target (80%). Additional efforts are needed to increase coverage.
Keywords: MSM, HPV, vaccine, trend, risk
Short Summary
Between 2014–2017, HPV vaccination coverage increased from 22.5% to 37.6% among MSM participants in the American Men’s Internet Survey (AMIS).
INTRODUCTION
Human papillomavirus (HPV) infection is the most common sexually transmitted infection (STI), with an overall genital HPV prevalence of 25.1% among men in the US.1 The prevalence of penile and anal (anogenital) HPV among adult men in the US is approximately 20%.2 Prevalence of infection is much higher among men who have sex with men (MSM). Anogenital HPV prevalence is approximately 50–60% among HIV-negative MSM and 90% or higher among HIV-positive MSM.2 About 13,477 cases of HPV-attributable cancers are diagnosed in men annually.3
In 2007, the Advisory Committee on Immunization Practices (ACIP) first established a recommendation for HPV vaccination in girls and young women.4 Two years later, the FDA expanded licensure of the vaccine to males. In 2011, ACIP expanded HPV vaccination recommendations to include routine administration to males aged 11–12 years and catch-up recommendations for males aged 13–21 years. Currently, MSM and immunocompromised individuals are recommended to receive the HPV vaccine through age 26. Although the FDA expanded approved use of HPV vaccines in October 2018 to include all women and men aged 27 – 45,5 the ACIP recommendations have not changed as of March 2019.6
Several studies have examined characteristics associated with HPV vaccine uptake in MSM. Associated factors include having any health insurance, visiting health care provider (HCP) in the preceding 12 months, HCP recommendation, HIV status, and disclosure of sexual identity.7–13 Disclosure of sexual identity is an important factor in increasing HPV vaccination rates among MSM because providers are able to recommend appropriate testing, vaccination, and treatment for diseases that disproportionately affect the MSM community, including HPV, other STIs, and HIV.14 Previous studies have shown that 33%−75% of sexual minority persons may not disclose their identity to their HCP.15,16
Location is often associated with HPV vaccination coverage. A study based on the 2012–2013 National Immunization Survey-Teen (NIS-Teen) identified that adolescent boys living in urban areas had higher odds of initiation and completion of the HPV vaccine series compared to adolescent boys living in non-urban areas.17 A second study utilizing insurance claims data from 2009–2014 reported significant differences in HPV vaccine uptake based on region and location. Adolescents in Western states had the highest rates of HPV vaccination, while adolescents in Southern states had the lowest. Urban-based adolescents were more likely to receive HPV vaccinations compared to rural-based adolescents, except in the Northeast.18
Coverage of HPV vaccination among all individuals, including MSM, remains low in regards to the Healthy People 2020 target.19 Although there is information on HPV vaccination coverage in MSM, very few studies have evaluated temporal trends in vaccine coverage or geographic distribution of HPV vaccine uptake among MSM, and none have evaluated yearly trend over multiple years.12,20 This study examines trends of HPV vaccination coverage among MSM in the US using data from a nationwide online survey of MSM between 2014 and 2017.
MATERIALS AND METHODS
PARTICIPANTS
We analyzed data from the American Men’s Internet Survey (AMIS). AMIS is an annual web-based, cross-sectional survey used to collect self-reported data on demographics, HIV risk behaviors, HIV and STD testing behaviors, and use of preventive services among a convenience sample of internet-using MSM in the US. Each AMIS cycle is conducted from approximately August through December of the corresponding year. Eligibility criteria include: being 15 years of age or older, identifying as male, residing in a US state or dependent area, the ability to take the survey in English or Spanish, and having had anal or oral sex with a man at least once. Most participants are recruited via advertisements on websites and mobile apps. Some are recruited using direct e-mails to the previous year’s participants, but only data from the initial year of participation were used for the present trend analyses. The study was conducted in compliance with Federal Regulations Governing Protection of Human Subjects and was reviewed and approved by Emory University’s Institutional Review Board. Detailed methods regarding survey design and recruitment have been previously reported.21
MEASURES
The study’s primary outcome of interest was self-reported receipt of ≥ 1 dose of HPV vaccine among AMIS respondents between 2014–2017. We included men in our study who would have been no older than 26 years of age in 2011 to account for all men who would have been included in the 2011 ACIP age-related recommendation for HPV vaccination among MSM. This restricted our sample to men aged 15 to 29 years old in 2014, 15 to 30 years old in 2015, 15 to 31 years old in 2016, and 15 to 32 years old in 2017. Data was not provided on age at vaccination. Other variables included in the analyses were race/ethnicity, education level, annual household income, Census region of residence, urban/rural classification of county of residence (urbanicity), health insurance status, having seen a HCP in the past 12 months, disclosure of sexual identity to HCP, anal sex without using a condom with male partner(s) in the past 12 months, ever tested for HIV, HIV status, ever diagnosed with a viral STI, diagnosed with gonorrhea, chlamydia or syphilis in the past 12 months, and number of male sexual partners in the past 12 months. Number of sexual partners was categorized as seen in similar studies.12,13
DATA ANALYSIS
Trends in the annual prevalence of HPV vaccination coverage were examined using Cochran-Armitage tests.22 Trends were also summarized using estimated annual percentage changes (EAPC), a relative measure of percent change, which were calculated using a logistic regression model with a dichotomized variable for HPV vaccine receipt as the outcome and survey year as the exposure variable.23 Parameter estimates from the model were used to calculate EAPC and upper and lower 95% confidence intervals (95% CI). Trend analyses were conducted for the overall sample by survey year, stratified by age. Trends in region and urbanicity were then evaluated, based on a priori significant findings among adolescents. We performed bivariate analyses using Pearson χ2 to assess associations between HPV vaccination in 2017 and demographic, behavioral, and health-related characteristics. Potential factors associated with HPV vaccination were evaluated using a multivariate Poisson regression with robust standard errors to estimate adjusted prevalence ratios (aPR) for variables found to be statistically significant in bivariate analyses.24 Significance was assessed at an α = 0.05 level. All analyses were conducted using SAS v.9.4 (SAS Institute, Cary, NC).
Sensitivity Analysis
We performed a sensitivity analysis to evaluate the results of our age cohort selection process. Only men ≤ 26 years of age in each survey year were included. All other methods of analysis remained the same.
RESULTS
During the study period, 39,591 eligible individuals completed the AMIS survey, 16,607 (42%) met our HPV-related age criteria, and 15,723 (95%) were not recruited from a previous study year. Of those meeting initial eligibility criteria, HPV vaccination status was reported by 13,857 (88%) participants. The mean age of these participants was 23.5 years. Participants were mainly non-Hispanic white, college educated, living in a large central metro area, living in the South, and had annual household income ≥$40,000. Participant demographic characteristics were similar across all 4 years of data (Table 1). Overall, 4,290 (31%) participants reported receiving ≥ 1 HPV vaccine.
TABLE 1.
Sociodemographic and Health-Related Characteristics of MSM Self-Reporting ≥1 HPV Vaccination: American Men’s Internet Survey, 2014–2017
| 2014 n = 535 n (%) |
2015 n = 1,178 n (%) |
2016 n = 1,295 n (%) |
2017 n = 1,282 n (%) |
All Years n = 4,290 n (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | ||||||||||
| 15–21 | 213 | (39.8) | 618 | (52.5) | 623 | (48.1) | 683 | (53.3) | 2,137 | (49.8) |
| 22–32* | 322 | (60.2) | 560 | (47.5) | 672 | (51.9) | 599 | (46.7) | 2,153 | (50.2) |
| Race/Ethnicity | ||||||||||
| Non-Hispanic White | 351 | (65.6) | 784 | (66.6) | 874 | (67.5) | 753 | (58.7) | 2,762 | (64.4) |
| Non-Hispanic Black | 18 | (3.4) | 48 | (4.1) | 77 | (5.9) | 59 | (4.6) | 202 | (4.7) |
| Hispanic/Latino† | 105 | (19.6) | 203 | (17.2) | 204 | (15.8) | 323 | (25.2) | 835 | (19.5) |
| Other‡ | 57 | (10.7) | 124 | (10.5) | 123 | (9.5) | 124 | (9.7) | 428 | (10.0) |
| Education | ||||||||||
| High school degree or less | 97 | (18.1) | 283 | (24.0) | 279 | (21.5) | 361 | (28.2) | 1,020 | (23.8) |
| Some college or technical degree | 198 | (37.0) | 438 | (37.2) | 472 | (36.4) | 390 | (30.4) | 1,498 | (34.9) |
| College degree or postgraduate | 229 | (42.8) | 439 | (37.3) | 519 | (40.1) | 506 | (39.5) | 1,693 | (39.5) |
| Region | ||||||||||
| Northeast | 120 | (22.4) | 319 | (27.1) | 310 | (23.9) | 300 | (23.4) | 1,049 | (24.5) |
| Midwest | 108 | (20.2) | 224 | (19.0) | 290 | (22.4) | 258 | (20.1) | 880 | (20.5) |
| South | 169 | (31.6) | 346 | (29.4) | 400 | (30.9) | 433 | (33.8) | 1,348 | (31.4) |
| West | 138 | (25.8) | 289 | (24.5) | 295 | (22.8) | 289 | (22.5) | 1,011 | (23.6) |
| Location | ||||||||||
| Large central metro | 224 | (41.9) | 465 | (39.5) | 559 | (43.2) | 552 | (43.1) | 1,800 | (42.0) |
| Suburban | 108 | (20.2) | 260 | (22.1) | 287 | (22.2) | 288 | (22.5) | 943 | (22.0) |
| Small/medium metro | 164 | (30.7) | 347 | (29.5) | 343 | (26.5) | 364 | (28.4) | 1,218 | (28.4) |
| Rural | 39 | (7.3) | 105 | (8.9) | 106 | (8.2) | 76 | (5.9) | 326 | (7.6) |
| Health insurance status | ||||||||||
| Any§ | 463 | (86.5) | 1,024 | (86.9) | 1,057 | (81.6) | 1,094 | (85.3) | 3,638 | (84.8) |
| None | 0 | (0.0) | 48 | (4.1) | 55 | (4.2) | 72 | (5.6) | 175 | (4.1) |
| Annual household income | ||||||||||
| <$20,000 | 104 | (19.4) | 229 | (19.4) | 235 | (18.1) | 207 | (16.1) | 775 | (18.1) |
| $20,000–$39,999 | 120 | (22.4) | 219 | (18.6) | 223 | (17.2) | 220 | (17.2) | 782 | (18.2) |
| $40,000–$74,999 | 115 | (21.5) | 188 | (16.0) | 232 | (17.9) | 213 | (16.6) | 748 | (17.4) |
| >$75,000 | 119 | (22.2) | 276 | (23.4) | 294 | (22.7) | 343 | (26.8) | 1,032 | (24.1) |
| Seen health care provider (HCP) in last 12 months | ||||||||||
| Yes | 460 | (86.0) | 1,044 | (88.6) | 1,076 | (83.1) | 1,033 | (80.6) | 3,613 | (84.2) |
| No | 28 | (5.2) | 97 | (8.2) | 91 | (7.0) | 101 | (7.9) | 317 | (7.4) |
| Out to HCP | ||||||||||
| Yes | 391 | (77.6) | 749 | (68.5) | 824 | (69.1) | 392 | (61.5) | 2,356 | (69.1) |
| No | 113 | (22.4) | 344 | (31.5) | 354 | (30.9) | 245 | (38.5) | 1,056 | (30.9) |
| Ever tested for HIV | ||||||||||
| Yes | 438 | (81.9) | 838 | (71.1) | 932 | (72.0) | 885 | (69.0) | 3,093 | (72.1) |
| No | 91 | (17.0) | 320 | (27.2) | 356 | (27.5) | 379 | (29.6) | 1,146 | (26.7) |
| HIV Positive | ||||||||||
| Yes | 53 | (9.9) | 53 | (4.5) | 67 | (5.2) | 44 | (3.4) | 217 | (5.1) |
| No | 473 | (88.4) | 1,097 | (93.1) | 1,216 | (93.9) | 1,217 | (94.9) | 4,003 | (93.3) |
| Diagnosed with Gonorrhea or Chlamydia, past 12 months‖ | ||||||||||
| Yes | 66 | (12.3) | 145 | (12.3) | 146 | (11.3) | 164 | (12.8) | 521 | (12.1) |
| No | 463 | (86.5) | 1,014 | (86.1) | 1,127 | (87.0) | 1,088 | (84.9) | 3,692 | (86.1) |
| Diagnosed with Syphilis, past 12 months‖ | ||||||||||
| Yes | 21 | (3.9) | 28 | (2.4) | 44 | (3.4) | 42 | (3.3) | 135 | (3.1) |
| No | 508 | (95.0) | 1,131 | (96.0) | 1,229 | (94.9) | 1,210 | (94.4) | 4,078 | (95.1) |
| Diagnosed with viral STI, ever‖ | ||||||||||
| Yes | 77 | (14.4) | 109 | (9.3) | 143 | (11.0) | 112 | (8.7) | 441 | (10.3) |
| No | 446 | (83.4) | 1,036 | (87.9) | 1,120 | (86.5) | 1,132 | (88.3) | 3,734 | (87.0) |
| Anal sex without condom, past 12 months | ||||||||||
| Yes | 371 | (69.3) | 756 | (64.2) | 871 | (67.3) | 842 | (65.7) | 2,840 | (66.2) |
| No | 109 | (20.4) | 265 | (22.5) | 279 | (21.5) | 260 | (20.3) | 913 | (21.3) |
| Number of male sexual partners, past 12 months | ||||||||||
| 1 | 72 | (17.1) | 158 | (17.7) | 206 | (21.7) | 154 | (16.6) | 590 | (18.5) |
| 2–3 | 73 | (17.3) | 217 | (24.3) | 195 | (20.5) | 218 | (23.5) | 703 | (22.0) |
| 4–5 | 58 | (13.7) | 127 | (14.2) | 150 | (15.8) | 146 | (15.7) | 481 | (15.1) |
| >5 | 219 | (51.9) | 392 | (43.9) | 399 | (42.0) | 411 | (44.2) | 1,421 | (44.5) |
Note: Percentages may not add to 100% due to missing data
This age category includes men who were 26 years of age or younger in 2011 and thus could have been vaccinated under the expanded recommendations for MSM. The age category was incremented for each survey year to include men up to age 29 in 2014, 30 in 2015, 31 in 2016, and 32 in 2017.
Any race
Including Asian, Native Hawaiian, Other Pacific Islander, American Indian, Alaskan Native, and Multiracial
Including private health plan purchased through an employer, private health plan purchased through an exchange (i.e. Obamacare), Medicaid or Medicare, other Medical Assistance program, TRICARE, Veterans Administration coverage or other health care plan
Diagnosed by physician
HPV VACCINATION COVERAGE TRENDS
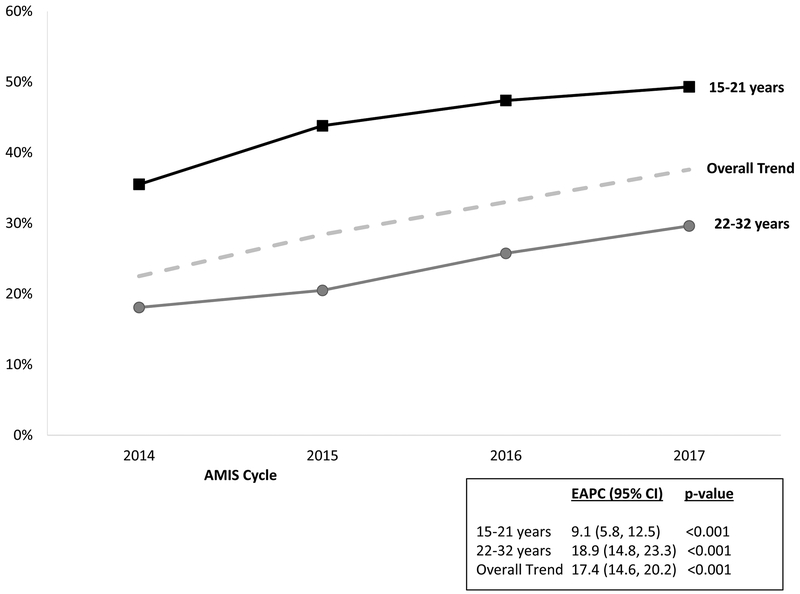
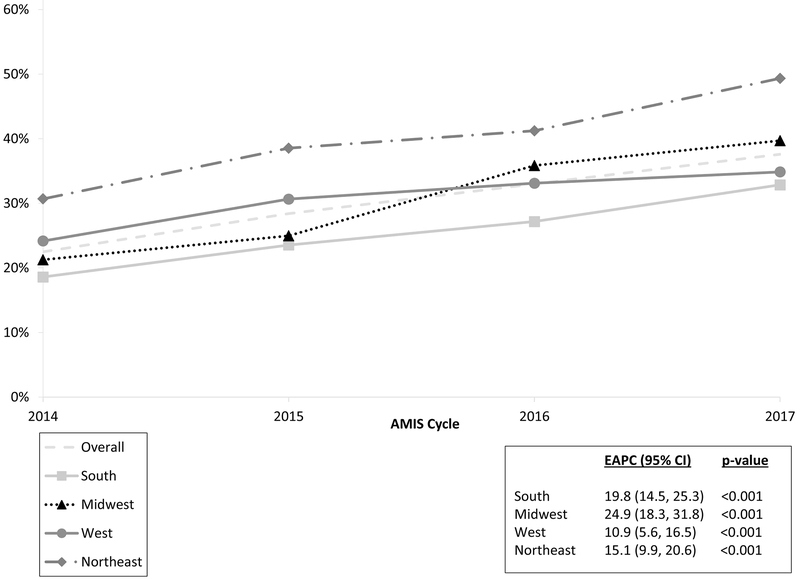
HPV vaccination coverage significantly increased (Z-statistic = −13.05, p-value < 0.0001) from 22.5% (535/2381) in 2014 to 37.6% (1282/2125) in 2017, an absolute increase of 15.1%. The relative increase, EAPC, was 17.4% per year (95% CI: 14.6% - 20.2%) (Figure 1). Vaccination among individuals aged 15–21 years increased from 35.5% to 49.3% (EAPC = 9.1%; 95% CI: 5.8% – 12.5%), and from 18.1% to 29.6% (EAPC = 18.9%; 95% CI: 14.8% - 23.3%) among those aged 22–32 years (Figure 1). HPV vaccination coverage increased between 2014 and 2017 in all categories of region and urbanicity (Figure 2). For participants who lived in rural areas, coverage significantly increased from 2014–2016 (EAPC = 14.3%; 95% CI: 4.0% – 25.5%) but remained constant from 2016–2017 (Figure 3). HPV vaccination coverage was highest in the Northeast region where the proportion vaccinated significantly increased from 30.7% to 49.3% (Figure 4; EAPC = 19.8%; 95% CI: 14.5% – 25.3%).
Figure 1:
Percent of MSM who self-reported receiving ≥ 1 HPV vaccine overall and by age from the American Men’s Internet Survey, 2014 to 2017.
Figure 2:
Percent of MSM who self-reported receiving ≥ 1 HPV vaccine by urbanicity from the American Men’s Internet Survey, 2014 to 2017.
FIGURE 3:
Percent of MSM who self-reported receiving ≥ 1 HPV vaccine by region from the American Men’s Internet Survey, 2014 to 2017.
FIGURE 4:
Percent of MSM who self-reported receiving ≥ 1 HPV vaccine in men ever eligible and in a sensitivity analysis limited to men age 26 or younger at time of survey completion, American Men’s Internet Survey, 2014 to 2017.
CORRELATES OF HPV VACCINATION
We limited analyses to 2017 data in order to reflect the most current state of potential correlates between HPV vaccination and participant characteristics. Among the 3,407 MSM participants from 2017 for which HPV vaccine information was available, bivariate analyses identified that HPV vaccination was significantly associated with age, region, urbanicity, household income, having any health insurance, having seen a HCP in the past 12 months, having disclosed sexual identity to their HCP, and having been diagnosed with gonorrhea or chlamydia in the past 12 months (Table 2). Most of these factors remained significantly and independently correlated with HPV vaccination in multivariable regression analysis (Table 3). Age was the strongest independent factor in our cohort analysis. Participants aged 15–21 years were over two times as likely to have initiated HPV vaccination compared to men aged 22–32 years (aPR = 2.1, 95% CI: 1.94, 2.33). HPV vaccination was more likely among participants who had health insurance, visited an HCP in past 12 months, disclosed their sexual identity to their HCP, diagnosed with gonorrhea and/or chlamydia in the past 12 months, and were living in the Northeast and in large central metropolitan areas.
TABLE 2.
Bivariate Correlates of MSM Self-Reporting ≥1 HPV Vaccination: American Men’s Internet Survey, United States, 2017
| No Reported HPV Vaccine | ≥1 HPV Vaccination | |||||
|---|---|---|---|---|---|---|
| Total n | n | (%) | n | (%) | P-value* | |
| Age (years) | <0.0001 | |||||
| 15–21 | 1,385 | 702 | (33.0) | 683 | (53.3) | |
| 22–32† | 2,022 | 1,423 | (67.0) | 599 | (46.7) | |
| Race/Ethnicity | 0.1393 | |||||
| Non-Hispanic White | 1,999 | 1,246 | (59.7) | 753 | (59.8) | |
| Non-Hispanic Black | 196 | 137 | (6.6) | 59 | (4.7) | |
| Hispanic/Latino‡ | 827 | 504 | (24.1) | 323 | (25.7) | |
| Other§ | 325 | 201 | (9.6) | 124 | (9.8) | |
| Education | 0.0701 | |||||
| High school degree or less | 886 | 525 | (25.2) | 361 | (28.7) | |
| Some college or technical degree | 1,052 | 662 | (31.8) | 390 | (31.0) | |
| College degree or postgraduate | 1,404 | 898 | (43.1) | 506 | (40.3) | |
| Region | <0.0001 | |||||
| Northeast | 608 | 308 | (14.5) | 300 | (23.4) | |
| Midwest | 650 | 392 | (18.4) | 258 | (20.2) | |
| South | 1,318 | 885 | (41.6) | 433 | (33.8) | |
| West | 829 | 540 | (25.4) | 289 | (22.6) | |
| Location | <0.0001 | |||||
| Large central metro | 1,371 | 819 | (38.5) | 552 | (43.1) | |
| Suburban | 710 | 422 | (19.9) | 288 | (22.5) | |
| Small/medium metro | 1,055 | 691 | (32.5) | 364 | (28.4) | |
| Rural | 269 | 193 | (9.1) | 76 | (5.9) | |
| Health insurance status | <0.0001 | |||||
| Any¶ | 2,759 | 1,665 | (86.3) | 1,094 | (93.8) | |
| None | 336 | 264 | (13.7) | 72 | (6.2) | |
| Annual household income | <0.0001 | |||||
| <$20,000 | 551 | 344 | (21.1) | 207 | (21.1) | |
| $20,000–$39,999 | 640 | 420 | (25.8) | 220 | (22.4) | |
| $40,000–$74,999 | 699 | 486 | (29.8) | 213 | (21.7) | |
| >$75,000 | 724 | 381 | (23.4) | 343 | (34.9) | |
| Seen health care provider (HCP) in last 12 months | <0.0001 | |||||
| Yes | 2,535 | 1,502 | (80.3) | 1,033 | (91.1) | |
| No | 470 | 369 | (19.7) | 101 | (8.9) | |
| Out to HCP | 0.0154 | |||||
| Yes | 932 | 540 | (55.4) | 392 | (61.5) | |
| No | 679 | 434 | (44.6) | 245 | (38.5) | |
| Ever tested for HIV | 0.2553 | |||||
| Yes | 2,318 | 1,433 | (68.1) | 885 | (70.0) | |
| No | 1,049 | 670 | (31.9) | 379 | (30.0) | |
| HIV Positive | 0.9336 | |||||
| Yes | 118 | 74 | (3.5) | 44 | (3.5) | |
| No | 3,231 | 2,014 | (96.5) | 1,217 | (96.5) | |
| Diagnosed with Gonorrhea or Chlamydia, past 12 months‖ | 0.0008 | |||||
| Yes | 356 | 192 | (9.4) | 164 | (13.1) | |
| No | 2,949 | 1,861 | (90.6) | 1,088 | (86.9) | |
| Diagnosed with Syphilis, past 12 months‖ | 0.486 | |||||
| Yes | 102 | 60 | (2.9) | 42 | (3.4) | |
| No | 3,203 | 1,993 | (97.1) | 1,210 | (96.6) | |
| Diagnosed with viral STI, ever‖ | 0.8881 | |||||
| Yes | 299 | 187 | (9.1) | 112 | (9.0) | |
| No | 2,989 | 1,857 | (90.9) | 1,132 | (91.0) | |
| Anal sex without condom, past 12 months | 0.8828 | |||||
| Yes | 2,240 | 1,398 | (76.6) | 842 | (76.4) | |
| No | 686 | 426 | (23.4) | 260 | (23.6) | |
| Number of male sexual partners, past 12 months | ||||||
| 1 | 444 | 290 | (18.7) | 154 | (16.6) | 0.3160 |
| 2–3 | 586 | 368 | (23.7) | 218 | (23.5) | |
| 4–5 | 407 | 261 | (16.8) | 146 | (15.7) | |
| >5 | 1,044 | 633 | (40.8) | 411 | (44.2) | |
P-values are based on Pearson’s χ2 test of expected frequencies. Bolded ρ-values indicate significance at the 0.05 level.
This age category includes men who were 26 years of age or younger in 2011 and thus could have been vaccinated under the expanded recommendations for MSM. The age category was incremented for each survey year to include men up to age 29 in 2014, 30 in 2015, 31 in 2016, and 32 in 2017.
Any race
Including Asian, Native Hawaiian, Other Pacific Islander, American Indian, Alaskan Native, and Multiracial
Including private health plan purchased through an employer, private health plan purchased through an exchange (i.e. Obamacare), Medicaid or Medicare, other Medical Assistance program, TRICARE, Veterans Administration coverage or other health care plan
Diagnosed by physician
TABLE 3.
Multivariate Analyses of Factors Associated with HPV Vaccination Among Study Participants: American Men’s Internet Survey, United States, 2017
| aPR* | (95% CI) | |
|---|---|---|
| 15–21 | 2.13 | (1.94–2.33) |
| 22–32† | Reference | |
| Region | ||
| Northeast | 1.35 | (1.33–1.37) |
| Midwest | 1.08 | (0.95–1.22) |
| South | Reference | |
| West | 1.1 | (1.00–1.21) |
| Location | ||
| Large central metro | 1.28 | (1.21–1.36) |
| Suburban | 1.19 | (1.13–1.25) |
| Small/medium metro | 1.04 | (0.97–1.12) |
| Rural | Reference | |
| Health insurance status | ||
| Any‡ | 1.86 | (1.57–2.21) |
| None | Reference | |
| Annual household income | ||
| <$20,000 | 0.92 | (0.87–0.96) |
| $20,000–$39,999 | 0.85 | (0.82–0.88) |
| $40,000–$74,999 | 0.81 | (0.75–0.87) |
| ≥$75,000 | Reference | |
| Seen health care provider (HCP) in last 12 months | ||
| Yes | 1.85 | (1.65–2.06) |
| No | Reference | |
| Out to HCP | ||
| Yes | 1.39 | (1.29–1.50) |
| No | Reference | |
| Diagnosed with Gonorrhea or Chlamydia, past 12 months§ | ||
| Yes | 1.20 | (1.07–1.35) |
| No | Reference |
aPR = adjusted prevalence ratio
This age category includes men who were 26 years of age or younger in 2011 and thus could have been vaccinated under the expanded recommendations for MSM. The age category was incremented for each survey year to include men up to age 29 in 2014, 30 in 2015, 31 in 2016, and 32 in 2017.
Including private health plan purchased through an employer, private health plan purchased through an exchange (i.e. Obamacare), Medicaid or Medicare, other Medical Assistance program, TRICARE, Veterans Administration coverage or other health care plan
Diagnosed by physician
Sensitivity Analysis
Limiting our analysis to men age ≤ 26 at time of survey completion resulted in 12,110 (30.6%) eligible individuals. After excluding duplicates and unknown/missing responses to the HPV vaccination question, 10,069 were included in the analysis. The overall HPV vaccination rate for all four years was 36.4%. These findings were similar to those found using our age cohort analysis (Figure 4). Overall, there was a relative increase of 18.1% in HPV vaccination coverage per year (95% CI: 15.2% - 21.1%).
DISCUSSION
This study provides evidence that HPV vaccination coverage among MSM in the US has increased significantly from 2014–2017. Among these AMIS respondents and the 4-year timeframe, the average relative yearly increase was 17% with an absolute increase of over 15%. The average relative yearly increase for respondents aged 15–21 years was 9.1%. Although there may have been increased coverage rates in later years due to individuals having more time and therefore more opportunities to receive HPV vaccination, our sensitivity analysis indicates otherwise. By evaluating men ≤ 26 years of age, we found a similar increase in coverage over the time period of our study. The benefit to our original cohort age method was that we were able to capture more men who would have been eligible for HPV vaccination. In addition, our EAPC for our original age cohort method and sensitivity analysis had very close EAPC values (17.4% vs. 18.1%), indicating that our method of age classification is an accurate way to measure HPV vaccination coverage in this study. These trends are encouraging and exceed the estimate of 17.2% from a similar study by Oliver et al.12 The 2017 NIS-Teen survey reported an increase in HPV vaccination rate among adolescents aged 13–17 years; among adolescent males, receipt of ≥ 1 dose increased from 56.0% in 2016 to 62.6% in 2017.25 In our study population, 38.0% of all MSM and 49.3% of MSM aged 15–21 years reported receiving ≥ 1 dose of the HPV vaccine in 2017, which is higher than previously reported coverage estimates of 4.8%−6.8% in 2011, 13% in 2013, and 17.2%−21.0% in 2014.7,8,11 However, these prior studies were conducted using data from the early phase of HPV vaccine implementation among boys and young men, using fewer paritcipants.12,26,27 In addition, HPV coverage rates for boys and men in the US lag behind other countries, including Australia and the UK. In Australia, 75.0% of males recommended for HPV vaccination had received at least one dose in 2016.28 In the UK, pilot studies of HPV vaccination among MSM are currently occurring; evaluation of 2016/2017 data shows that first-dose uptake among MSM is 45.5%, among participating clinics.29 Even though we observed a significant increase in HPV vaccination coverage among MSM, the overall 2017 coverage estimate is still less than the Healthy People 2020 target of 80%.19
Our results support prior findings that MSM living in large central metro areas and in the Northeast and West have higher HPV vaccine coverage rates.12,20 The South remains behind in coverage, but if the current average annual increase of 19.8% continues then coverage levels should improve over time. However, there was no increase in HPV vaccine uptake among rural MSM from 2016–2017. It will be important to continue to monitor rural areas to make sure that vaccination coverage is not stalling. In 2017, approximately 59.3% of all adolescents aged 13–17 in rural areas had received at least one HPV vaccine.25 Our results show that only 28.3% of AMIS respondents in 2017 living in rural areas reported receiving ≥1 HPV vaccine. Under-vaccination continues to be an issue in rural areas, with proposed factors ranging from being less aware of HPV or the HPV vaccine, lack of understanding of the link between HPV and cancer,30 and structural factors such as distance needed to travel to HCP and absence of alternative health resources, such as vaccine safety nets.17 Additionally, MSM in rural areas may be less comfortable disclosing their sexual identity due to anticipated stigma.31s However, our findings were independent of differences in insurance status, HCP visit and sexual identity disclosure. Therefore, lower vaccination rates may not be due to just access or stigma-related disclosures. Further research should be conducted to tease out why MSM may be experiencing a more serious gap in coverage in rural areas.
It is important that MSM feel comfortable informing their HCP of their sexual identity and that HCPs provide culturally competent care, including obtaining a sexual history, so that individuals receive relevant health care and education, including appropriate HPV vaccine recommendations. A prior study among MSM found that men who disclosed their sexual identity to their HCP were significantly more likely to receive all applicable healthcare components (i.e. HIV and other STD testing, HPV vaccine, etc.)32s In 2017, 42% of all men in our study who received ≥ 1 HPV vaccine reported that they had informed their HCP provider of their sexual identity. When we stratified on age, we found that the younger age group (15–21 years) was over 2-times more likely to have reported ≥ 1 HPV vaccine compared to the older age group (22–32 years). Under the current guidelines, men between the ages of 21 and 26 would only be offered HPV vaccination if their HCP knew that they were at risk (i.e. MSM or immunocompromised). By age group, 62% of MSM aged 15–21 and 34% of MSM aged 22–32 were out to their HCP and had been vaccinated. This is in direct contrast to the findings that 39% of MSM aged 15–21 disclosed their sexual behavior to their HCP, while 71% of MSM aged 22–32 reported disclosing their sexual behavior. This indicates a missed opportunity for older MSM; although they are more likely to report their sexual identity to their HCP, they might not be receiving appropriate vaccination recommendations.
MSM diagnosed with gonorrhea or chlamydia in the past 12 months were more likely to have received ≥ 1 HPV vaccination compared to MSM without. Among all participants reporting a diagnosis of gonorrhea or chlamydia, 54% did not report HPV vaccination. Among all participants who reported any bacterial STI in the past 12 months, 55% did not report HPV vaccination. This highlights another significant missed opportunity. More than half of men who were seen by a HCP for a bacterial STI reported not been vaccinated for HPV. Unlike other studies, we did not find an association between HIV status and HPV vaccine uptake.11,26 This could be due to low HIV prevalence, underreporting of HIV status, or lack of knowledge of HIV status in this group of MSM.
This study is subject to limitations. First, all data were self-reported; mistakes in recollection or reluctance to elaborate on confidential health information may have resulted in misclassification. However, participants completed the survey online without having to provide personal identifiers, which should have reduced social desirability bias. In addition, a previous study evaluated the self-report accuracy of HPV vaccination among young men aged 13–26 years and found an overall rate of 79.5%.33s Second, generalizability to the entire population of MSM in the U.S. is limited. Representativeness in studies of MSM is an important consideration, and while research has demonstrated comparability in the types of men recruited via social media-based recruitment and venue-time-space sampling,34s generalizability regarding education and income may be limited. Our cohort tends to be more affluent and educated due to the type of men who have access to resources to complete surveys like AMIS. Therefore, our vaccine coverage may be higher than among MSM in general. This does not impact our trend analyses because there is internal consistency in AMIS samples from year-to-year. Third, we were only able to evaluate HPV vaccine initiation, not completion. Multiple doses of HPV vaccine are recommended to provide maximum protection against oncogenic HPV genotypes, and it is worth exploring whether and how series completion rates among MSM differ from initiation rates, as well as how those rates compare to initiation and completion rates for other target populations. Any disparities observed would reveal potential areas for targeted outreach. Finally, data were not collected on when participants were vaccinated for HPV which required us to make assumptions about whether participants were first vaccinated earlier in life based on the general recommendations or later in life when vaccination is only recommended for MSM or immunocompromised people.
The prevalence of receiving ≥ 1 HPV vaccine doses among MSM has increased significantly from 2014–2017, but vaccination coverage continues to be below national targets. Important factors for HPV vaccination in MSM are having a HCP, providing that HCP with information about one’s sexual identity, and having the HCP recommend the vaccine. While previous studies have reported that MSM are often aware of and educated about the link between HPV and cancer, they are not as likely to receive a recommendation for HPV vaccination if they do not disclose their sexual identity to their provider.7,8,35s Future HPV studies should explore barriers and facilitators to HPV vaccination, including disclosure of sexual identity to HCP, particularly in rural areas.
Supplementary Material
Acknowledgments
Source of Funding: The study was funded by a grant from the MAC AIDS Fund, by the National Institutes of Health [R01MH110358] and the Emory Center for AIDS Research [NIH P30AI050409].
Footnotes
Conflicts of Interest: None declared.
REFERENCES
- 1.McQuillan GM, Kruszon-Moran D, Markowitz LE, et al. Prevalence of HPV in adults aged 18–69: United States, 2011–2014 NCHS data brief, no 280. Hyattsville, MD: National Center for Health Statistics; 2017. [Google Scholar]
- 2.Moscicki A-B, Palefsky JM. Human papillomavirus in men: an update. J Low Genit Tract Dis 2011; 15(3): 231–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.How Many Cancers Are Linked with HPV Each Year? HPV and Cancer 2018. (CDC Web site). Available at: https://www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed April 4, 2019.
- 4.Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014; 63(Rr-05): 1–30. [PubMed] [Google Scholar]
- 5.FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old (FDA Press Release). Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm622715.htm. Accessed April 4, 2019.
- 6.FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59(20): 630–632. [PubMed] [Google Scholar]
- 7.Gerend MA, Madkins K, Phillips G, et al. Predictors of Human Papillomavirus Vaccination Among Young Men Who Have Sex With Men. Sex Transm Dis 2016; 43(3): 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings T, Kasting ML, Rosenberger JG, et al. Catching Up or Missing Out? Human Papillomavirus Vaccine Acceptability Among 18- to 26-Year-old Men Who Have Sex With Men in a US National Sample. Sex Transm Dis 2015; 42(11): 601–606. [DOI] [PubMed] [Google Scholar]
- 9.Stupiansky NW, Liau A, Rosenberger J, et al. Young Men’s Disclosure of Same Sex Behaviors to Healthcare Providers and the Impact on Health: Results from a US National Sample of Young Men Who Have Sex with Men. AIDS Patient Care STDS 2017; 31(8): 342–347. [DOI] [PubMed] [Google Scholar]
- 10.Lu PJ, Yankey D, Fredua B, et al. Association of Provider Recommendation and Human Papillomavirus Vaccination Initiation among Male Adolescents Aged 13–17 Years-United States. J Pediatr 2019; 206: 33–41.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiter PL, McRee AL, Katz ML, et al. Human Papillomavirus Vaccination Among Young Adult Gay and Bisexual Men in the United States. Am J Public Health 2015; 105(1): 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliver SE, Hoots BE, Paz-Bailey G, et al. Increasing Human Papillomavirus Vaccine Coverage Among Men Who Have Sex With Men-National HIV Behavioral Surveillance, United States, 2014. J Acquir Immune Defic Syndr 2017; 75 Suppl 3: S370–s374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meites E, Markowitz LE, Paz-Bailey G, et al. HPV vaccine coverage among men who have sex with men - National HIV Behavioral Surveillance System, United States, 2011. Vaccine 2014; 32(48): 6356–6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durso LE, Meyer IH. Patterns and Predictors of Disclosure of Sexual Orientation to Healthcare Providers among Lesbians, Gay Men, and Bisexuals. Sex Res Social Policy 2013; 10(1): 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meites E, Krishna NK, Markowitz LE, et al. Health care use and opportunities for human papillomavirus vaccination among young men who have sex with men. Sex Transm Dis 2013; 40(2): 154–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossman K, Salamanca P, Macapagal K. “The doctor said I didn’t look gay”: Young adults’ experiences of disclosure and non-disclosure of LGBTQ identity to healthcare providers. J Homosex 2017; 64(10): 1390–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henry KA, Swiecki-Sikora AL, Stroup AM, et al. Area-based socioeconomic factors and Human Papillomavirus (HPV) vaccination among teen boys in the United States. BMC Public Health 2017; 18(1): 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vielot NA, Butler AM, Brookhart MA, et al. Patterns of Use of Human Papillomavirus and Other Adolescent Vaccines in the United States. J Adolesc Health 2017; 61(3): 281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Healthy People 2020: Immunization and Infectious Diseases 2018. Available at: https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed April 4, 2019.
- 20.Kahle EM, Meites E, Sineath RC, et al. Sexually Transmitted Disease Testing and Uptake of Human Papillomavirus Vaccine in a Large Online Survey of US Men Who Have Sex With Men at Risk for HIV Infection, 2012. Sex Transm Dis 2017; 44(1): 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez TH, Sineath RC, Kahle EM, et al. The Annual American Men’s Internet Survey of Behaviors of Men Who Have Sex With Men in the United States: Protocol and Key Indicators Report 2013. JMIR Public Health Surveill 2015; 1(1): e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta CR, Patel NR, Senchaudhuri P. Exact Power and Sample-Size Computations for the Cochran-Armitage Trend Test. Biometrics 1998; 54(4): 1615–1621. [Google Scholar]
- 23.Sanchez TH, Zlotorzynska M, Sineath RC, et al. National Trends in Sexual Behavior, Substance Use and HIV Testing Among United States Men Who have Sex with Men Recruited Online, 2013 Through 2017. AIDS Behav 2018; 22(8): 2413–2425. [DOI] [PubMed] [Google Scholar]
- 24.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 2005; 162(3): 199–200. [DOI] [PubMed] [Google Scholar]
- 25.Walker TY, Elam-Evans LD, Yankey D, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years - United States, 2017. MMWR Morb Mortal Wkly Rep 2018; 67(33): 909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorbach PM, Cook R, Gratzer B, et al. Human Papillomavirus Vaccination Among Young Men Who Have Sex With Men and Transgender Women in 2 US Cities, 2012–2014. Sex Transm Dis 2017; 44(7): 436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charlton BM, Reisner SL, Agenor M, et al. Sexual Orientation Disparities in Human Papillomavirus Vaccination in a Longitudinal Cohort of U.S. Males and Females . LGBT Health 2017; 4(3): 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel C, Brotherton JM, Pillsbury A, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonavalent vaccine prevent? Euro Surveill 2018; 23(41): 1700737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iyanger N, Mesher D, Checchi M, et al. Human papillomavirus (HPV) vaccination for Men who have sex with Men (MSM): 2016/17 pilot evaluation (Public Health England Report). Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/678987/HPV_msm_year1_evaluation_report.pdf. Accessed April 4, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammed KA, Subramaniam DS, Geneus CJ, et al. Rural-urban differences in human papillomavirus knowledge and awareness among US adults. Prev Med 2018; 109: 39–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.