Abstract
Thermal stress in hot humid conditions limits cattle production. The objectives for this study were to estimate genetic parameters for hair characteristics and core body temperature under low and high temperature humidity index (THI) conditions. Hair samples were collected and measured for length and diameter. Core body temperature was measured as vaginal temperature every 15 min over a 5-d period using an iButton temperature measuring device implanted in a blank CIDR in 336 heifers from the University of Florida multibreed herd (ranging from 100% Angus to 100% Brahman). Restricted maximum likelihood procedures were used to estimate heritabilities from multiple bivariate animal models using the WOMBAT program. Estimates of heritability for hair diameter, undercoat length, topcoat length, body temperature under low THI conditions, and body temperature under high THI conditions were 0.50, 0.67, 0.42, 0.32, and 0.26, respectively. The genetic parameters estimated in this study indicate a large, exploitable genetic variance which can be selected upon to improve tolerance in cattle. Breed effects for differing compositions of Brahman and Angus were also estimated. As Brahman breed composition increased by 25% undercoat length, topcoat length, body temperature under low THI conditions, and body temperature under high THI conditions decreased by 1.32 mm, 2.94 mm, 0.11 °C, and 0.14 °C, respectively. Under both low and high THI conditions, cattle with 25% Brahman breed composition or greater maintained a significantly lower body temperature than the 100% Angus breed group. The incorporation of Brahman germplasm is recommended for herds that often experience heat stress conditions in order to increase resilience to heat stress.
Keywords: beef cattle, Bos indicus, genetic parameters, hair length, thermotolerance
INTRODUCTION
Thermal stress in hot and humid conditions limits beef cattle production. Over 65% of the world’s cattle (beef and dairy) reside in tropical or subtropical climates known for their hot and humid conditions (Burrow, 2012). In the United States alone, thermal stress results in a loss of $369 million a year (St-Pierre et al., 2003) due to reduced animal performance such as reduced feed intake and lower pregnancy rates (Amundson et al., 2006; Sunil Kamar et al., 2011; Renaudeau et al., 2012). These economic losses are expected to increase as thermal stress increases due to climate change (Hahn, 1999; Renaudeau et al., 2012). Thermal tolerance is influenced by many factors, including breed and hair coat properties (Turner and Schleger, 1960; Jenkinson et al., 1975; Gaughan et al., 1999; Olson et al., 2003; Burrow, 2012; Porto-Neto et al., 2014; Barendse, 2017). Differences within and between breeds for thermal tolerance and hair coat properties exist and are indicative of opportunities for improvement through selection (Landaeta-Hernández et al., 2011; Hamblen et al., 2018). Thermotolerance is a quantitative trait controlled by many genes and under environmental influence (Porto-Neto et al., 2014). It is also a difficult and expensive trait to measure. On the other hand, coat properties are easier to measure and have a direct effect on the ability of an animal to tolerate hot and humid conditions. The objectives of this study were to estimate the heritability of hair characteristics and core body temperature. These estimates will give an indication of the possible improvement of these traits through selection.
MATERIALS AND METHODS
Population and Phenotype Data
The University of Florida Institutional Care and Use Committee approved the research protocol used in this study (Approval no. 201203578).
This study utilized 336 heifers from the multibreed herd of the University of Florida over 2 yr, 2017 and 2018. The UF multibreed herd has been in existence since 1988 (Elzo and Wakeman, 1998; Elzo et al., 2016, 2017). Its mating program is diallel, where sires from 6 breed groups (3 to 5 per breed group) are mated to cows belonging to each of these same 6 breed groups (35 to 50 cows per breed group). Mating is done by artificial insemination (AI; up to 2 AI per cow) followed by natural service for 60 d (single-sire mating within sire breed groups). For mating purposes, animals in the multibreed herd are assigned to 6 breed groups based on breed composition: 100% Angus = 100% to 80% Angus; 75% Angus = 79% to 60% Angus; Brangus = 62.5% Angus; 50% Angus = 59% to 40% Angus; 25% Angus = 39% to 20% Angus; and 100% Brahman: 19% to 0% Angus. Angus, Brahman, and Brangus sires are chosen from outside sources to be as representative as possible of their respective national populations. Sires from the 75% Angus, 50% Angus, and 25% Angus are chosen primarily from within the multibreed herd for availability reasons, and from outside herds when available. Heifers used in this study were progeny of sires and dams from the current national purebred populations (Angus, Brahman, Brangus) and Angus–Brahman crossbred animals from the multibreed herd.
Heifers were managed the same across both years. Heifer calves were kept with their dams on bahiagrass pastures with free access to mineral supplement (Lakeland Animal Nutrition, Lakeland, FL) at the University of Florida Beef Research Unit until weaning at 6 to 8 mo of age. Postweaning, heifers continued to be maintained on bahiagrass pastures at the Beef Research Unit and were supplemented with bermudagrass hay and cottonseed meal during the winter months.
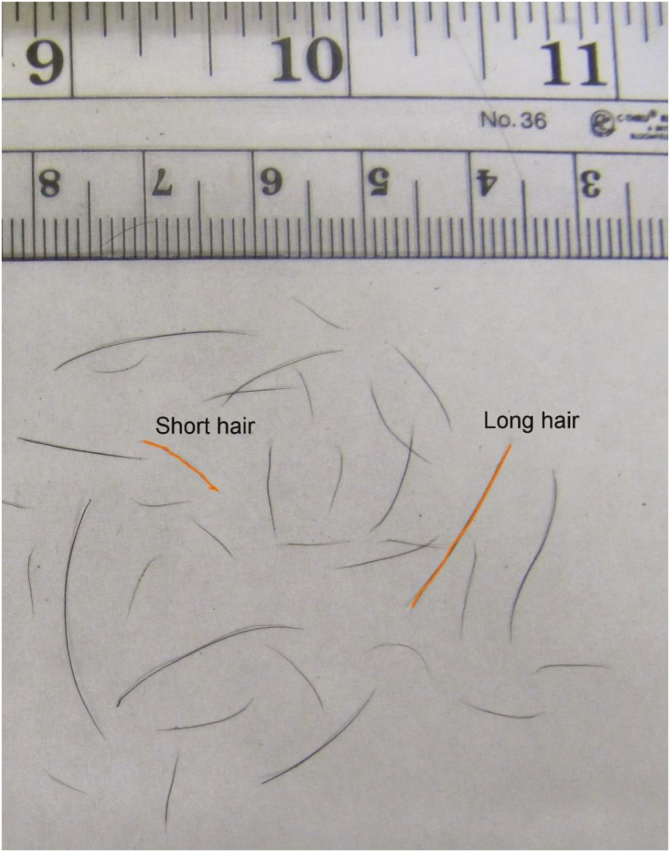
Hair samples were collected from the shoulder, 4 inches down from spine and halfway along horizontal axis of each animal, when heifers were in the chute for controlled internal drug release (CIDR) insertion. Hair samples were spread on a white paper and photographed (Fig. 1). A ruler was always included in the picture to serve as a scale by which pixels could be converted into millimeters. Hair samples were measured for length and diameter using ImageJ software (Schindelin et al., 2012). Hairs with extremely short or long lengths were not considered for measurement. Five long and 5 short hairs for each individual were selected for measurement to evaluate the length of the topcoat and undercoat, respectively. All 10 hairs were measured for diameter. The averages of the 5 short and long hairs were used in the statistical analysis.
Figure 1.
Hair samples photographed alongside a ruler, as viewed during ImageJ analysis, with a short and long hair highlighted, representing the undercoat and topcoat, respectively.
Core body temperature was measured as vaginal temperature on 89 heifers during summer of 2017 from July 17 to July 21, 205 heifers during summer 2018 from June 6 to June 10, and 42 heifers during summer 2018 from August 22 to August 26. Body temperature was recorded at 15-min intervals for 5 d using an iButton data logger (iButton type DS1922L, temperature range −40 to 85 °C, accuracy of 11-bit for ±0.0625 °C resolution, Maxim Integrated, San Jose, CA) (Dikmen et al., 2014) inserted into a blank CIDR device and then into the vagina of each animal. Each iButton was calibrated before the study started and pre-programmed to record body temperature at 15-min intervals on a 24-h cycle. Ambient environmental conditions were monitored using HOBO data loggers which continuously record temperature, humidity dew-point temperature with HOBO-U23 data logger (Onset Computer Corp., Bourne, MA), and black globe temperature by using HOBO-U22 data logger. The temperature humidity index (THI) was calculated as: THI = (1.8 × T + 32) − [(0.55 − 0.0055 × RH) × (1.8 × T − 26)], where T = air temperature (°C) and RH = relative humidity (%) (NRC, 1971). This equation has been shown to be a good indicator of heat stress (Dikmen and Hansen, 2009). Only body temperatures from the 3 continuous days when cattle were on pasture undisturbed were analyzed. Body temperatures on days 1 and 5 when the CIDRs were inserted and removed were excluded from analysis due to human interaction. Based on the thresholds defined by the livestock weather hazard guide (LWSI, 1970) and the THI level encountered during our experiment, THI conditions between 68 and 70 were considered low THI while THI between 84 and 86 were considered high THI. Body temperatures at low and high THI for each individual were calculated by averaging all the body temperature measurements collected during the time that the THI windows occurred. This was accomplished for each heifer by averaging the body temperature from all 15-min windows when the heifer was exposed to a particular THI interval (low or high).
Statistical Analyses
Statistical analyses were performed using SAS 9.4 (SAS Inst. Inc., Cary, NC). The MEANS procedure was used to produce descriptive statistics for hair characteristics and body temperature data.
The GLM procedure of SAS was used to analyze the effect of breed group on each individual trait. The model for each trait included year of birth, temperature collection group, and breed group as fixed effects. Breed-group least squares means were separated using LSMEANS with the PDIFF option.
To estimate the linear and quadratic effect of percent Brahman genetics and Brahman–Angus heterosis effects as a function of heterozygosity, the breed groups were recoded as 0, 1, 1.5, 2, 3, and 4, indicating 0%, 25%, 37.25%, 50%, 75%, and 100% Brahman genetics, respectively. Heterozygosity was calculated as (Sire Angus fraction * Dam Brahman fraction + Sire Brahman fraction * Dam Angus fraction). The model included year of birth, temperature collection group as fixed effects and the linear and quadratic breed composition as covariates. If heterosis or quadratic breed effects were not significant, they were removed from the final model.
Restricted maximum likelihood procedures were used to estimate heritability from 4 bivariate animal models using the WOMBAT software (Meyer, 2007). Undercoat and topcoat, undercoat and diameter, topcoat and diameter, and core body temperature under low and high THI conditions were analyzed as pairwise traits. In matrix notation the basic model for each of the 4 animal models was:
where y is a vector of the observations for the 2 traits; X is an incidence matrix relating observations of fixed effects; β is a vector of the fixed effects for each trait; Z is an incidence matrix relating observations to random animal effects; u is a vector of the multiple trait random effects; and e is a vector of the random residual errors for all measured traits and animals. Contemporary group was fitted as the only fixed effect in all 4 animal models. Contemporary groups were defined based on age, breed group (based on breed composition), and temperature measurement group for a total of 34 unique contemporary groups. A 4-generation pedigree file with 1,091 individuals containing the identification of the animal, sire, and dam was used to define relationships among animals in the data set. The pedigree included 334 animals with records for both hair characteristics and vaginal temperature, and 797 animals without records (parents, grandparents, etc.). Two animals missing 1 or more phenotypes were excluded from analysis. There were 143 sires and 385 dams, of which 54 sires and 225 dams had progeny with records in the data. The remaining sires and dams were grandparents and great-grandparents of the progeny with records. None of the sires or dams had phenotypic data in the data set.
RESULTS AND DISCUSSION
Hair Characteristics
Descriptive statistics for the hair measurements are provided in Table 1. Overall, there was a large variation in diameter and both the topcoat and undercoat length. The topcoat length had a slightly greater variation than undercoat length. This level of variation in both hair length phenotypes is indicative of opportunities for selective improvement.
Table 1.
Basic statistics for hair characteristics and core body temperature under low and high THI conditions
| Trait | n | Mean | SD | CV (%) |
|---|---|---|---|---|
| Diameter, mm | 332 | 0.22 | 0.11 | 50.00 |
| Undercoat, mm | 329 | 7.49 | 3.03 | 40.45 |
| Topcoat, mm | 329 | 14.57 | 6.42 | 44.06 |
| Temp at low THI, °C | 336 | 38.57 | 0.32 | 0.83 |
| Temp at high THI, °C | 336 | 39.72 | 0.42 | 1.05 |
The least squares means of the hair characteristics by breed composition are shown in Table 2. No significant differences between breed groups were found for the diameter measurements. Across both hair length measurements, the breed group with the greatest Brahman percentage had the shortest length. The percentage of Brahman breed composition was found to have a significant linear effect (P < 0.05) for both topcoat and undercoat. The group with the highest Brahman percentage had significantly shorter hair for both the undercoat and topcoat than all the other breed groups, including the 75% Brahman percentage group.
Table 2.
Least squares means and standard errors of hair lengths by breed composition
| Breed group | n | Diameter, mm | Undercoat, mm | Topcoat, mm |
|---|---|---|---|---|
| 100% Angus 0% Brahman | 54 | 0.22 ± 0.01 | 8.82 ± 0.37a | 17.17 ± 0.68a |
| 75% Angus 25% Brahman | 50 | 0.22 ± 0.01 | 8.54 ± 0.39a | 14.79 ± 0.72b |
| Brangus | 60 | 0.23 ± 0.01 | 8.67 ± 0.36a | 15.82 ± 0.66a,b |
| 50% Angus 50% Brahman | 49 | 0.22 ± 0.01 | 6.88 ± 0.38b | 13.14 ± 0.70b |
| 25% Angus 75% Brahman | 33 | 0.25 ± 0.02 | 5.97 ± 0.46b | 10.82 ± 0.84c |
| 0% Angus 100% Brahman | 89 | 0.23 ± 0.01 | 4.88 ± 0.25c | 8.57 ± 0.47d |
| I1 | 329 | 0.21 ± 0.01 | 9.92 ± 0.33 | 19.28 ± 0.63 |
| b11 | 329 | 0.02 ± 0.01 | −1.16 ± 0.59S | −2.94 ± 0.19S |
| b21 | 329 | −0.004 ± 0.003 | −0.61 ± 0.14S | −0.51 ± 0.15S |
a–dLeast squares means within column that do not have a common superscript differ, P < 0.05.
1Intercept (I), linear (b1), and quadratic (b2) effect of percent Brahman genetics.
SLinear or quadratic effect significant at P < 0.05.
There was no significant heterosis effect on diameter or topcoat length, but there was for undercoat length. As Brahman breed composition increased by 25%, undercoat and topcoat length decreased by 1.16 ± 0.59 mm and 2.94 ± 0.19 mm, respectively. These results support previous findings that indicine cattle have shorter, slicker hair coats than taurine cattle (Olson et al., 2003; Landaeta-Hernández et al., 2011; Barendse, 2017). Across all measurements and breed groups, hair length tended to decrease as indicine influence increased. However, the Brangus breed group had the most variation in hair length, even compared to the 2 purebred groups. This is similar to results reported by Porto-Neto et al. (2018) which found that crosses of indicine and taurine cattle have been shown to have a wide range of intermediate coat types as a result of the multifactorial genetic basis of hair length.
Core Body Temperature
Vaginal temperature allows for a robust and continuous monitoring of core body temperature and is not as invasive as implanted transmitters or sensitive to factors such as water and feed intake, as is the case with rumen boluses. The use of vaginal temperature loggers to monitor body temperature was validated by Vickers et al. (2010) where the relationship between vaginal and rectal temperature was found to be r = 0.81 in healthy cows not at peak lactation. Descriptive statistics for the temperature traits are provided in Table 1. Both temperature measurements had similar coefficient of variation ranging from 0.83 to 1.05, with the temperature under high THI showing greater variation. A smaller coefficient of variation for the body temperature measurements was expected due to tight thermoregulation in homeotherms, which aims at maintaining body temperature within a narrow range necessary for optimal cellular and molecular function (Nakamura and Morrison, 2008). Moreover, small differences in body temperature are likely biologically relevant as a rise of 1 °C or less in body temperature has been shown to reduce cattle performance (Kadzere et al., 2002).
The least squares means for core body temperature across the 6 breed groups under low and high THI are shown in Table 3. Under high THI conditions, the breed group with the highest Brahman breed percentage maintained the lowest core body temperature (38.85 ± 0.04). Under both low and high THI conditions, the breed group with the highest Angus percentage maintained a significantly higher core body temperature than all other breed groups, including the breed group with the second highest Angus percentage. These results indicate that even a small percentage of Brahman breed composition may allow a heifer to maintain a lower core body temperature under heat stress conditions. Least square means for core body temperature under both low and high THI conditions decreased from 100% Angus (38.79 ± 0.05 °C and 39.34 ± 0.06 °C, respectively) to 100% Brahman (38.58 ± 0.04 °C and 38.85 ± 0.04 °C, respectively) as indicated by a significant linear effect (P < 0.05). Under high THI conditions, the 100% Angus heifers had a 0.44 °C and 0.49 °C higher core body temperature compared to the 75% and 100% Brahman heifers, respectively. It is important to point out that under a high THI, the pure-bred Brahman group had the lowest body temperature and was statistically different than all the other breed groups except for the 75% Brahman breed group. This is in agreement with results presented by Dikmen et al. (2018) where a minimum of 75% Brahman genetics was required to increase the ability to regulate body temperature under severe heat stress.
Table 3.
Least squares means and standard errors of core body temperature by breed composition
| Breed group | n | Temp at low THI, °C | Temp at high THI, °C |
|---|---|---|---|
| 100% Angus 0% Brahman | 54 | 38.79 ± 0.05a | 39.34 ± 0.06a |
| 75% Angus 25% Brahman | 50 | 38.52 ± 0.06b | 39.09 ± 0.06b |
| Brangus | 60 | 38.51 ± 0.05b | 39.04 ± 0.06b |
| 50% Angus 50% Brahman | 49 | 38.54 ± 0.05b | 39.02 ± 0.06b |
| 25% Angus 75% Brahman | 33 | 38.47 ± 0.06b | 38.90 ± 0.06b,c |
| 0% Angus 100% Brahman | 89 | 38.58 ± 0.04b | 38.85 ± 0.04c |
| I1 | 334 | 38.94 ± 0.03 | 39.43 ± 0.04 |
| b11 | 334 | −0.11 ± 0.02S | −0.14 ± 0.02S |
| b21 | 334 | 0.02 ± 0.01 | 0.03 ± 0.01 |
a–cLeast squares means within column that do not have a common superscript differ, P < 0.05.
1Intercept (I), linear (b1), and quadratic (b2) effect of percent Brahman genetics.
SLinear or quadratic effect significant at P < 0.05.
There was no significant heterosis effect on body temperature under low or high THI. Body temperature decreased by 0.14 ± 0.02 °C and 0.11 ± 0.02 °C under high and low THI conditions, respectively, as Brahman breed composition increased by 25%. Differences in heat tolerance among breeds and especially between tropically adapted indicine cattle and nonadapted taurine cattle have been reported in multiple studies (Gaughan et al., 1999; Brown-Brandl et al., 2006; Chan et al., 2010; Gaughan et al., 2010; Dikmen et al., 2018). Both Gaughan et al. (1999) and Dikmen et al. (2018) also evaluated internal body temperature of cattle under heat stress. Gaughan et al. (1999) reported that purebred Brahmans had rectal temperatures 1.3 °C lower than the purebred Herefords in the study. The more extreme difference reported by Gaughan et al. (1999) may be a result of more extreme heat stress. Gaughan et al. (1999) maintained steers in a climatically controlled room with a THI of 95.6 ± 4.7 while this study utilized naturally occurring heat stress during the Florida summer with high THI conditions between 84 and 86 THI. Dikmen et al. (2018) utilized naturally occurring heat stress and reported that in a crossbred Brahman–Angus population, cattle with ½ or greater Brahman influence had significantly lower vaginal temperatures than cattle with ¼ or less Brahman influence. Dikmen et al. (2018) also observed that the differences in vaginal temperature among the breed groups was only evident during high heat stress. In the present study, although the 100% Angus breed group had significantly higher body temperature than the other breed groups, none of the other breed groups were significantly different from each other for core body temperature under low THI. The decrease in body temperature as Brahman breed composition increased was also greater under high THI conditions than low THI conditions, indicating that differences in thermoregulative abilities may only be evident under high heat stress conditions.
Heritabilities
Heritability estimates for hair characteristics and body temperatures under low and high THI conditions are provided in Table 4. Heritability was estimated to be 0.50 for diameter, 0.67 for undercoat, and 0.42 for topcoat. While heritability for hair length or diameter have not been previously estimated in cattle, heritability for coat score has been estimated to be 0.63 (Turner and Schleger, 1960). Jenkinson et al. (1975) estimated the heritability of hair follicle length to range from 0.38 to 0.69, with heritability estimates higher in the more intensely managed research herd used compared to the commercial herd used. The similarity in the heritability estimates for the 3 different hair measurements reflects the similarity of the phenotypes. The heritability for body temperature under low THI conditions (0.32) was slightly higher than that of body temperature under high (0.26) THI conditions. These heritability estimates are similar to those reported for rectal temperature in a Brahman × Angus crossbred population (0.19; Riley et al., 2012) and dairy cattle (0.17; Dikmen et al., 2012). Both studies utilized cattle located in Florida. The heritabilities estimated in this study may be slightly higher than those in Dikmen et al. (2012) and Riley et al. (2012) due to the use of vaginal temperature rather than rectal temperature and the use of heifers rather than cows. The values of heritability estimated in this study indicate a large, exploitable genetic variance which can be used in selection programs to improve heat tolerance in cattle.
Table 4.
Genetic (σ2a) and residual (σ2e) variance and heritability (h2) estimates for hair lengths and core body temperature under low and high THI conditions with standard errors (in parentheses)
| Trait | σ2a | σ2e | h2 |
|---|---|---|---|
| Diameter, mm | 0.15 (0.16) | 0.15 (0.13) | 0.50 (0.45) |
| Undercoat, mm | 23.35 (7.37) | 11.65 (5.99) | 0.67 (0.18) |
| Topcoat, mm | 3.55 (1.51) | 4.96 (1.35) | 0.42 (0.16) |
| Temp at low THI, °C | 0.39 (0.24) | 0.81 (0.15) | 0.32 (0.18) |
| Temp at high THI, °C | 0.36 (0.23) | 1.02 (0.02) | 0.26 (0.16) |
IMPLICATIONS
The genetic parameters estimated in this study indicate that hair characteristics are highly heritable and core body temperature under various THI conditions is moderately heritable. These values of heritability indicate a large, exploitable genetic variance in body hair length and core body temperature which can be selected upon to improve heat tolerance in cattle. As Brahman breed influence increased both hair length and body temperature decreased, indicating that cattle producers can improve thermotolerance by incorporating Brahman germplasm into their herds.
Footnotes
This research was supported by USDA-NIFA Grant #2017-67007-26143. During the study, Serdal Dikmen was supported by a grant from TUBITAK - BIDEB 2219.
LITERATURE CITED
- Amundson J. L., Mader T. L., Rasby R. J., and Hu Q. S.. 2006. Environmental effects on pregnancy rate in beef cattle. J. Anim. Sci. 84:3415–3420. doi: 10.2527/jas.2005-611 [DOI] [PubMed] [Google Scholar]
- Barendse W. 2017. Climate adaptation of tropical cattle. Annu. Rev. Anim. Biosci. 5:133–150. doi: 10.1146/annurev-animal-022516-022921 [DOI] [PubMed] [Google Scholar]
- Brown-Brandl T. M., Nienaber J. A., Eigenberg R. A., Mader T. L., Morrow J. L., and Dailey J. W.. 2006. Comparison of heat tolerance of feedlot heifers of different breeds. Livest. Sci. 105:19–26. doi: 10.1016/j.livsci.2006.04.012 [DOI] [Google Scholar]
- Burrow H. M. 2012. Importance of adaptation and genotype × environment interactions in tropical beef breeding systems. Animal 6:729–740. doi: 10.1017/S175173111200002X [DOI] [PubMed] [Google Scholar]
- Chan E. K., Nagaraj S. H., and Reverter A.. 2010. The evolution of tropical adaptation: comparing taurine and zebu cattle. Anim. Genet. 41:467–477. doi: 10.1111/j.1365-2052.2010.02053.x [DOI] [PubMed] [Google Scholar]
- Dikmen S., Cole J. B., Null D. J., and Hansen P. J.. 2012. Heritability of rectal temperature and genetic correlations with production and reproduction traits in dairy cattle. J. Dairy Sci. 95:3401–3405. doi: 10.3168/jds.2011-4306 [DOI] [PubMed] [Google Scholar]
- Dikmen S., and Hansen P. J.. 2009. Is the temperature-humidity index the best indicator of heat stress in lactating dairy cows in a subtropical environment? J. Dairy Sci. 92:109–116. doi: 10.3168/jds.2008-1370 [DOI] [PubMed] [Google Scholar]
- Dikmen S., Khan F. A., Huson H. J., Sonstegard T. S., Moss J. I., Dahl G. E., and Hansen P. J.. 2014. The SLICK hair locus derived from Senepol cattle confers thermotolerance to intensively managed lactating Holstein cows. J. Dairy Sci. 97:5508–5520. doi: 10.3168/jds.2014-8087 [DOI] [PubMed] [Google Scholar]
- Dikmen S., Mateescu R. G., Elzo M. A., and Hansen P. J.. 2018. Determination of the optimum contribution of Brahman genetics in an Angus-Brahman multibreed herd for regulation of body temperature during hot weather. J. Anim. Sci. 96:2175–2183. doi: 10.1093/jas/sky133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzo M. A., Mateescu R. G., Johnson D. D., Scheffler T. L., Scheffler J. J., Carr C., Rae D. O., Wasdin J. G., Driver M. D. and Driver J. D.. 2017. Genomic-polygenic and polygenic predictions for nine ultrasound and carcass traits in Angus-Brahman multibreed cattle using three sets of genotypes. Livest. Sci. 202:58–66. doi: 10.1016/j.livsci.2017.05.027 [DOI] [Google Scholar]
- Elzo M. A., Mateescu R., Thomas M. G., Johnson D. D., Martinez C. A., Rae D. O., Wasdin J. G., Driver M. D. and Driver J. D.. 2016. Growth and reproduction genomic-polygenic and olygenic parameters and prediction trends as Brahman fraction increases in an Angus-Brahman mutlibreed population. Livest. Sci. 190:104–112. doi: 10.1016/j.livsci.2016.06.011 [DOI] [Google Scholar]
- Elzo M. A., and Wakeman D. L.. 1998. Covariance components and prediction for additive and nonadditive preweaning growth genetic effects in an Angus-Brahman multibreed herd. J. Anim. Sci. 76:1290–1302. doi: 10.2527/1998.7651290x [DOI] [PubMed] [Google Scholar]
- Gaughan J. B., Mader T. L., Holt S. M., Josey M. J., and Rowan K. J.. 1999. Heat tolerance of Boran and Tuli crossbred steers. J. Anim. Sci. 77:2398–2405. doi: 10.2527/1999.7792398x [DOI] [PubMed] [Google Scholar]
- Gaughan J. B., Mader T. L., Holt S. M., Sullivan M. L., and Hahn G. L.. 2010. Assessing the heat tolerance of 17 beef cattle genotypes. Int. J. Biometeorol. 54:617–627. doi: 10.1007/s00484-009-0233-4 [DOI] [PubMed] [Google Scholar]
- Hahn G. L. 1999. Dynamic responses of cattle to thermal heat loads. J. Anim. Sci. 77(Suppl. 2):10–20. doi: 10.2527/1997.77suppl_210x [DOI] [PubMed] [Google Scholar]
- Hamblen H., Hansen P. J., Zolini A. M., Oltenacu P. A., and Mateescu R. G.. 2018. Thermoregulatory response of Brangus heifers to naturally occurring heat exposure on pasture. J. Anim. Sci. 96:3131–3137. doi: 10.1093/jas/sky224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen P. J. 2004. Physiological and cellular adaptations of zebu cattle to thermal stress. Anim. Reprod. Sci. 82–83:349–360. doi: 10.1016/j.anireprosci.2004.04.011 [DOI] [PubMed] [Google Scholar]
- Jenkinson D. M., Mason I. L., and Nay T.. 1975. Inheritance of some sweat gland and hair follicle characteristics in cattle. Aust. J. Biol. Sci. 28:417–424. doi: 10.1071/BI9750417 [DOI] [PubMed] [Google Scholar]
- Kadzere C. T., Murphy M. R., Silanikove N. and Maltz E.. 2002. Heat stress in lactating dairy cows: a review. Livest. Prod. Sci. 77:59–91. doi: 10.1016/S0301-6226(01)00330-X [DOI] [Google Scholar]
- Landaeta-Hernández A., Zambrano-Nava S., Hernández-Fonseca J. P., Godoy R., Calles M., Iragorri J. L., Añez L., Polanco M., Montero-Urdaneta M., and Olson T.. 2011. Variability of hair coat and skin traits as related to adaptation in Criollo Limonero cattle. Trop. Anim. Health Prod. 43:657–663. doi: 10.1007/s11250-010-9749-1 [DOI] [PubMed] [Google Scholar]
- LWSI 1970. Livestock weather safety index. NOAA National Weather Service, UK. [Google Scholar]
- Meyer K. 2007. WOMBAT: a tool for mixed model analyses in quantitative genetics by restricted maximum likelihood (REML). J. Zhejiang Univ. Sci. B 8:815–821. doi: 10.1631/jzus.2007.B0815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., and Morrison S. F.. 2008. A thermosensory pathway that controls body temperature. Nat. Neurosci. 11:62–71. doi: 10.1038/nn2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC 1971. A guide to environmental research on animals - National Research Council (U.S.). Committee on Physiological Effects of Environmental Factors on Animals - Google Books. Natl. Acad. Sci., Washington, DC. [Google Scholar]
- Olson T. A., Lucena C., Chase C. C. Jr., and Hammond A. C.. 2003. Evidence of a major gene influencing hair length and heat tolerance in Bos taurus cattle. J. Anim. Sci. 81:80–90. doi: 10.2527/2003.81180x [DOI] [PubMed] [Google Scholar]
- Porto-Neto L. R., Bickhart D. M., Landaeta-Hernandez A. J., Utsunomiya Y. T., Pagan M., Jimenez E., Hansen P. J., Dikmen S., Schroeder S. G., Kim E. S., et al. 2018. Convergent evolution of slick coat in cattle through truncation mutations in the prolactin receptor. Front. Genet. 9:57. doi: 10.3389/fgene.2018.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto-Neto L. R., Reverter A., Prayaga K. C., Chan E. K., Johnston D. J., Hawken R. J., Fordyce G., Garcia J. F., Sonstegard T. S., Bolormaa S., et al. 2014. The genetic architecture of climatic adaptation of tropical cattle. PLoS One 9:e113284. doi: 10.1371/journal.pone.0113284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudeau D., Collin A., Yahav S., de Basilio V., Gourdine J. L., and Collier R. J.. 2012. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 6:707–728. doi: 10.1017/S1751731111002448 [DOI] [PubMed] [Google Scholar]
- Riley D. G., Chase J. C., Coleman S. W., and Olson T. A.. 2012. Genetic assessment of rectal temperature and coat score in Brahman, Angus, and Romosinuano crossbred and straightbred cows and calves under subtropical summer conditions. Livest. Sci. 148:109–118. doi: 10.1016/j.livsci.2012.05.017 [DOI] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9:676–682. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre N. R., Cobanov B. and Schnitkey G.. 2003. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 86:52–77. doi: 10.3168/jds.S0022-0302(03)74040-512613848 [DOI] [Google Scholar]
- Sunil Kamar B. V., Ajeet K. and Meena K.. 2011. Effect of heat stress in tropical livestock and different strategies for its amelioration. J. Stress Physiol. Biochem. 7:45–54. [Google Scholar]
- Turner H. G., and Schleger A. V.. 1960. The significance of coat type in cattle. Aust. J. Agric. Econ. 11:645–663. doi: 10.1071/AR9600645 [DOI] [Google Scholar]
- Vickers L. A., Burfeind O., von Keyserlingk M. A., Veira D. M., Weary D. M., and Heuwieser W.. 2010. Technical note: comparison of rectal and vaginal temperatures in lactating dairy cows. J. Dairy Sci. 93:5246–5251. doi: 10.3168/jds.2010-3388 [DOI] [PubMed] [Google Scholar]