Abstract
The objective of this study was to investigate the impact of lipid peroxidation in a dose-dependent manner on growth, health, and oxidative stress status of nursery pigs. A total of 2,200 weaned pigs (5.95 ± 0.20 kg BW) were housed in 100 pens (22 pigs per pen) in a randomized complete block design based on initial BW and sex. Pigs were randomly assigned within blocks to 5 dietary treatments, consisting of a corn–soybean meal-based diet supplemented with 5% of either control corn oil (iodine value = 118, FFA = 0.06%, anisidine value = 3, peroxide value = 3 mEq/kg oil) or peroxidized corn oil (iodine value = 120, FFA = 0.35%, anisidine value = 30, peroxide value = 163 mEq/kg oil). These 2 diets were blended to obtain 5 levels of peroxidation with final treatments designated as 0 (diet with 5% control oil), 25%, 50%, 75%, and 100% (diet with peroxidized corn oil) peroxidation. Diets were fed ad libitum for 43 d. Blood samples were collected on d 33 from 20 pigs per treatment to determine serum oxidative stress markers and vitamin E concentrations and again on d 43 (14 d after vaccination) to determine immune response to porcine circovirus type 2 (PCV2) and Mycoplasma hyopneumoniae (Mhyo). Gain:feed ratio decreased linearly (P = 0.023) with increasing peroxidation, but pen ADG and ADFI were not affected. Number of pigs removed for medical treatment, total number medically treated, pigs culled for low end weight, and mortality increased, and full-value pigs linearly decreased (P < 0.04) with increasing peroxidation. Consequently, total pen gain (weight of viable pigs that remained in test pens at the end of the study minus weight of pigs placed) decreased linearly (P < 0.01) with increasing peroxidation. Antibody titers to Mhyo and PCV2 increased postvaccination (P < 0.001), but did not differ due to dietary treatment. Serum concentrations of malondialdehyde, 8-hydroxy-2′-deoxyguanosine, and protein carbonyl were not affected by peroxidation. Total antioxidant capacity and serum vitamin E concentrations decreased (P = 0.01) linearly with increasing peroxidation. Data show a dose-dependent negative impact of lipid peroxidation on pig productivity when determined under field population conditions, being primarily manifested by increased mortality, number of pigs medically treated, and number of culled pigs (≤13.6 kg BW). Results underscore the importance of proper assessment of lipid peroxidation as part of quality control to prevent oxidative stress and performance losses in weaned pigs.
Keywords: lipid peroxidation, nursery pigs, oxidative stress, pig viability
INTRODUCTION
Improving the efficiency of swine production is essential for the economic viability and the sustainability of the swine industry. Because energy is one of the most expensive components of swine diets, cost-competitive energy dense ingredient sources such as rendered fat, restaurant grease, and vegetable oils, including those from distiller’s dried grains with solubles (DDGS), or blends thereof are commonly used. However, quality and peroxidative status of these sources are highly variable and could be detrimental to animal health and productivity (van Kempen and McComas, 2002; Song et al., 2014; Kerr et al., 2015). Indeed, peroxidized lipids have been shown to decrease performance of swine and broilers and to induce oxidative stress in most cases (Lu et al., 2014; Shurson et al., 2015, Hung et al., 2017). This response appeared to be linear with inclusion of lipids with increasing peroxidation values (DeRouchey et al., 2004; Liang et al., 2015; Rosero et al., 2015; Hanson et al., 2016). However, the effect of peroxidized lipids on the immune system of food animals remains to be elucidated. The ultimate impact of lipid peroxidation on health, viability, and mortality of pigs housed under the rigors of commercial conditions, such as high population density and greater immune stress, is unclear. Therefore, a study was conducted in a commercial nursery retrofitted for research, to assess the effects of feeding weaned pigs with peroxidized oils on growth, efficiency of gain and ability to thrive. It was hypothesized that feeding weaned pigs diets with peroxidized corn oil would compromise growth, but also health status, by promoting oxidative stress. It was expected that weaned pigs and especially smaller pigs are most vulnerable to oxidative stress. The objective of this study was to investigate the impact of progressive increases in lipid peroxidation on performance, health (medical treatment and viability) and oxidative status of nursery pigs housed under commercial conditions.
MATERIALS AND METHODS
Animals and Dietary Treatments
All animal protocols were approved and conducted under the supervision of licensed veterinarians. Procedures used in this study were consistent with the Guide for the Care and Use of Animals in Agricultural Research and Teaching (FASS, 2010). This experiment was conducted in a commercial nursery facility that was retrofitted for research (White Hall, IL), owned and operated by The Hanor Family of Companies. Newly weaned nursery pigs (PIC TR-4 sire × Camborough sow; n = 2,200; mean BW = 5.95 ± 0.2 kg) were allotted to a randomized complete block design based on initial BW at weaning and sex and randomly assigned within blocks to 5 dietary treatments. Pigs were housed in 2 identical rooms with 22 pigs per pen using a combined total of 100 pens (20 pens per treatment). Treatment pens were randomly distributed within each room with equal representation. Pens were 5.6 m2 in dimension and contained one 6-hole feeder and 2 nipple drinkers. Pigs had ad libitum access to feed and water. Weaned pigs were judged by company veterinarians as having high health at placement, so results reflect the response of high health pigs to peroxidized lipid levels. Pigs were derived from a sow farm, that was positive for porcine reproductive and respiratory syndrome virus (1-7-4 RFLP strain), but were stable based on monthly blood sampling. The sow farm was positive for mycoplasma pneumonia and swine influenza virus but considered stable.
Dietary treatments consisted of 5 levels of lipid peroxidation to present a dose–response challenge of increasing peroxidation and treatments were administered for the duration of the nursery phase (43 d). Diets were corn–soybean meal-based formulated to meet the NRC (2012) nutrient recommendation and were supplemented with 5% of either edible, refined, bleached, and deodorized corn oil or the same oil that was peroxidized. Two diets (containing control or peroxidized oil) were manufactured by a commercial feed mill (Hanor Company, Greenfield, IL). The remaining dietary treatments were obtained by mixing those 2 diets on farm by using a computerized feeding system (Feedlogic Corporation, Wilmar, MN) capable of blending, weighing, and recording feed delivered to individual pens. Final treatments consisted of diets designated as 0% (diet with 5% control oil), 25%, 50%, 75%, and 100% (diet with 5% peroxidized corn oil) peroxidation.
Peroxidized corn oil was obtained by continuous exposure of corn oil to heat (65 °C) and forcing air through the oil at a constant rate of 20 L/min for 12 d. This was done in a 3,785 L capacity, horizontal, stainless steel fat tank, provided with 2 heaters located on each end of the tank. Air was distributed through the oil through 4 PVC pipes with multiple 1 mm diameter holes to allow for increased exposure of oil to air. Once daily, the oil was pumped from the bottom of the fat tank back into the top of the fat tank for 30 min to thoroughly mix the oil. Throughout the peroxidation process, oil samples were taken daily and analyzed for 2-thiobarbituric acid value (TBA) and peroxide value (PV) according to AOCS (method Cd 19-9, 2010) and AOAC (method 965.33, 2007), respectively. After 12 d of peroxidation, a TBA value of 0.08 absorbance units at 530 nm and 150 mEq/kg of oil for PV were reached, and the peroxidation process was stopped. Lipids (both peroxidized and control oil) were subsequently stabilized with liquid antioxidant containing tertiary butyl hydroquinone (TBHQ) (Rendox CQ, Kemin Industries, Inc., Des Moines, IA), added at 0.1% of the lipid to prevent further peroxidation.
Pigs were fed in 3 nursery phases, with Phase 1 diets offered from days 0 to 7, Phase 2 diets from d 8 to 20, followed by Phase 3 diets fed from days 21 to 43 (Table 1). All pigs were weighed and amount of residual feed in the feeders was determined on day 0, when switching diets on days 7 and 20, and at the end of the study on day 43 for growth performance calculations. ADG was expressed as biological ADG and calculated as the weight gain of pigs within each pen during the period divided pig days. Weight gain of pigs in the pen was calculated as the total weight of pigs in the pen at the end of the period plus the weight gained of any pigs that died during the period, minus the total weight of pigs in the pen at the beginning of the period. Pig days represented the number of days in the period multiplied by the number of pigs at the end of the period plus the number of days that pigs that died were present in the pen). ADFI was calculated as total feed supplied during the period minus feed remaining at the end of the period divided by total pig days. Gain:feed was calculated as total gain in the period divided by total feed intake in the period. Total pen gain was calculated as the difference of the total weight of pigs in the pen at the end of the study and the total weight of pigs in the pen at the start of the study. Thus, this measure considers only the pigs that finished the study and represents the total kilograms of pig weight produced (on a pen basis), which is practically relevant.
Table 1.
Composition of experimental diets, as-fed basis
| Item | Phase 1 | Phase 2 | Phase 3 |
|---|---|---|---|
| Ingredient, % | |||
| Corn, 8.5% CP | 8.55 | 40.29 | 41.03 |
| Soybean meal | 23.54 | 26.75 | 35.40 |
| Corn distillers’ dried grains with solubles, 6.5% fat | 6.0 | 8.0 | 16.0 |
| Oat groats, steamed | 20.0 | 0 | 0 |
| Whey permeate1 | 20.0 | 7.0 | 0 |
| Plant-based protein product2 | 7.5 | 5.5 | 0 |
| Nursery concentrate3 | 6.5 | 3.5 | 0 |
| Corn oil4 | 5.0 | 5.0 | 5.0 |
| l-lysine·HCl | 0.48 | 0.43 | 0.25 |
| dl-methionine | 0.22 | 0.17 | 0.05 |
| l-threonine | 0.10 | 0.07 | 0.00 |
| Limestone | 0.30 | 1.15 | 1.15 |
| Monocalcium phosphate, 21% P | 0.31 | 0.80 | 0.40 |
| Salt | 0.35 | 0.40 | 0.43 |
| Cu chloride | 0.03 | 0.03 | 0.00 |
| Zn oxide | 0.42 | 0.42 | 0.00 |
| Vitamin mineral premix5 | 0.15 | 0.15 | 0.15 |
| Organic acid blend6 | 0.30 | 0.10 | 0.10 |
| Sorbent7 | 0.20 | 0.20 | 0.00 |
| Choline chloride, 60% | 0.07 | 0.07 | 0.00 |
| Enzyme blend8 | 0 | 0 | 0.06 |
| Analyzed composition, % | |||
| Moisture | 8.40 | 9.63 | 10.19 |
| CP | 25.8 | 25.8 | 24.5 |
| Crude fat | 8.55 | 6.97 | 9.32 |
| Ca | 0.46 | 0.80 | 0.68 |
| P | 0.56 | 0.59 | 0.53 |
1DairyLac 80 (International Ingredients Corp., Monett, MO).
2Contained NF8 (Nutraferma, Sioux City, IA), dried fermentation biomass (Ajinomoto Heartland, Inc., Fort Lee, NJ), and NuPro (Alltech, Nicholasville, KY).
3Contained corn gluten meal, 45%; dextrose, 35%; soy hulls, 5.6%; AA blend, 4%; phytase, 0.7%; other ingredients.
4Control corn oil or peroxidized corn oil. Peroxidized corn oil was created by heating (65 °C) refined, bleached, and deodorized corn oil with constant air supply (20 L/min) for 12 d. Lipids were stabilized with liquid antioxidant (TBHQ) after peroxidation.
5Supplied per kilogram of complete diet: 105.0 mg of zinc, 100.0 mg of iron, 45.0 mg of manganese, 15.0 of copper, 0.7 mg of iodine, 0.3 of selenium, 9,923 IU of vitamin A, 1,654 IU of vitamin D3, 77.1 mg of vitamin E, 3.9 mg of vitamin K, 44.1 mg of vitamin B12, 9.9 mg of riboflavin, 33.1 mg of d-pantothenate, 55.1 mg of niacin, 3.3 mg of thiamine, 5.5 mg of pyridoxine, 0.9 mg of folic acid, and 0.3 mg of biotin.
6AviPlus (Vetagro Inc., Chicago, IL).
7Flo-Bond (Agri-Tec, Amarillo, TX).
8Supplied per kilogram of complete diet: phytase, 1,200 FTU (Quantum Blue, AB Vista, Marlborough, UK) and xylanase, 8,000 units (Porzyme, Danisco Animal Nutrition, Marlborough, UK).
The decision to remove a pig from a test pen or treat a pig was guided by protocols established by attending veterinarians and was based on poor or declining body condition, lameness, injury, or suspected respiratory distress. Pigs were removed from test pens to sick pens to allow for more targeted medical treatment and to assure humane care. They were weighed, tagged, and recorded with removal reason and date. Removed pigs remained on test were maintained on their designated dietary treatments, and outcome of pigs in sick pens was tracked by tag number. If pigs recovered and were heavier than 13.6 kg at the end of the study, they were considered viable pigs and were included in the performance calculations. Pigs weighing ≤13.6 kg at the end of the trial were considered in the non-full-value category and were culled (computed to be 4 SD from mean). Deaths and all lightweight or substandard pigs were recorded at the end of the nursery period to consider viability of pigs (full-value pigs).
To determine the impact of peroxidized lipids on the ability of pigs to respond to an immune challenge, pigs were vaccinated according to the established protocol designed by the attending veterinarian and head of veterinary services. Vaccines included a porcine reproductive and respiratory syndrome (PRRS) modified live virus mixed with a Mycoplasma hyopneumoniae (Mhyo) bacterin vaccine (0.5 mL dose; Ingelvac PRRS MLV and Ingelvac MycoFLEX, Boehringer Ingelheim Vetmedica, Inc., St Joseph, MO) at placement and a porcine circovirus type 2 (PCV2) and Mhyo combination vaccine (1 mL dose; Ingelvac FLEXcombo, Boehringer Ingelheim Vetmedica, Inc., St Joseph, MO) at ~9 wk of age. This procedure was based on sow farm health; pigs placed at this and other nursery sites are from a single sow farm flow.
Blood samples were collected from 20 pigs per treatment (10 lightweight pigs,~12.0 kg BW, and 10 heavyweight pigs,~21.8 kg BW), selected from the 20 lightest and 20 heaviest pens, prior to (on day 33) and 14 d after the second vaccine dose to determine antibody titers to vaccination. The second blood collection was 4 d after all pigs had been sent to the finisher barns (day 43 of the study), but sampled pigs remained in the nursery rooms and continued to receive their assigned diets until the second bleeding day. Samples collected on day 33 were used to assess oxidative status (total antioxidant capacity, malondialdehyde, nucleic acid damage, and protein carbonyl) and vitamin E concentrations in serum. Blood samples (~8 mL) were obtained via jugular venipuncture using 10-mL vacuum serum tubes. Immediately after collection, blood samples were placed at 4 °C and allowed to clot for ~12 h before serum separation. Serum was obtained by centrifugation at 1,000 × g for 12 min at room temperature and subsequently frozen at −20 °C for further analyses.
Chemical Analysis
Representative oil samples were submitted to commercial laboratories to analyze for moisture, insoluble impurities, unsaponifiable matter, free fatty acids, iodine value, peroxide value (initial, 4 h, and 24 h active oxygen method), anisidine value (New Jersey Feed Laboratory Inc., Trenton, NJ), oxidative stability index, hexanal, 2,4-decadienal, and tertiary butyl hydroquinone (Kemin Industries, Inc., Des Moines, IA) according to AOAC (2007) and AOCS (2010) (Table 2). Peroxidation measures of the supplemental lipid portion of the final experimental diets were calculated from the ratios that the control diet and the peroxidized lipid diet were mixed (0%, 25%, 50%, 75%, and 100%) to obtain the final diets (Table 3). Serum samples were submitted to the Veterinary Diagnostic Laboratory at Iowa State University (Ames, IA) for analyses of antibody titers and vitamin E concentrations. Antibody titers to PCV2 and Mhyo were determined by ELISA (INgezim Circo IgG, Ingenasa, Madrid, Spain for PCV2; IDEXX M. hyo. Ab Test, Idexx Laboratories Inc., Westbrook, USA for Mhyo). Titer results are presented as the sample to positive (S/P) ratio, calculated as the sample absorbance minus the negative control mean, divided by the difference between the positive and negative control means. Serum vitamin E concentrations were determined by HPLC. Briefly, serum lipids were extracted using ethanol and hexane/chloroform and dissolved in a chromatographic mobile phase (95:5; 95% being 90:10 methanol:chloroform, 5% water). Then concentrations were determined by HPLC (Dionex PDA-3000, Dionex Corp., Sunnyvale, CA) set at a wavelength of 292 nm. Separation was achieved using a reverse-phase Pecosphere C18 (4.6 × 33 mm) column (PerkinElmer, Inc., Waltham, MA).
Table 2.
Composition of experimental corn oil, as is basis1
| Item | Control | Peroxidized |
|---|---|---|
| Moisture, % | 0.07 | 0.36 |
| Insoluble impurities, % | 0.02 | 0.01 |
| Unsaponifiable matter, % | 0.82 | 0.85 |
| Free fatty acids, % | 0.06 | 0.35 |
| Iodine value | 118 | 120 |
| Peroxide value, mEq/kg fat | ||
| Initial2 | 3 | 163 |
| 4 h AOM3 | 5 | 148 |
| 20 h AOM | 7 | 442 |
| Anisidine value4 | 3 | 30 |
| Oxidative stability index5, h | 47.8 | 0.8 |
| Hexanal, ppm | <5 | 61 |
| 2,4-Decadienal, ppm | 15 | 1,080 |
| TBHQ6, ppm | 197 | 18 |
1Analysis of oxidative stability index, hexanal, 2,4-decadienal and TBHQ were conducted by Kemin Industries (Des Moines, IA), while the other analyses were conducted by New Jersey Feed Laboratory Inc. (Trenton, NJ).
2The New Jersey Feed Laboratory Inc. (method 965.33; AOAC, 2007) and Kemin Industries, Inc. both analyzed initial peroxide value and the mean of these values is reported here.
3Active oxygen method, Cd 12 to 57 (AOCS, 2010).
4Anisidine value is a relative measure used to determine aldehyde content of peroxidized oils.
5OSI, method Cd 12B-92 (AOCS, 2010).
6Tertiary butyl hydroquinone, liquid antioxidant added at 0.1% of lipid.
Table 3.
Calculated lipid peroxidation composition of supplemental lipids of the experimental diets1
| Level of peroxidation2 | |||||
|---|---|---|---|---|---|
| Item | 0 | 25 | 50 | 75 | 100 |
| Peroxide value, mEq/kg fat | |||||
| Initial | 3.3 | 43.2 | 83.2 | 123.1 | 163.0 |
| 4 h AOM3 | 4.6 | 40.5 | 76.3 | 112.2 | 148.0 |
| 20 h AOM | 6.8 | 115.6 | 224.4 | 333.2 | 442.0 |
| Anisidine value4 | 2.5 | 9.5 | 16.5 | 23.4 | 30.4 |
| Oxidative stability index, h | 47.8 | 36.0 | 24.3 | 12.5 | 0.8 |
| Hexanal, ppm | 0.0 | 15.3 | 30.5 | 45.8 | 61.0 |
| 2,4-Decadienal, ppm | 15.0 | 281.3 | 547.5 | 813.8 | 1080.0 |
1Calculated from the relative contribution of analyzed peroxidation measures of the control oil and those of the peroxidized oil presented in Table 2.
2Treatments represent a control diet with 5% refined, bleached, and deodorized corn oil (0% peroxidation) and a diet with 5% peroxidized corn oil (100% peroxidation). Intermediate diets were created by mixing the control and peroxidized diets on-farm.
3Active oxygen method.
4Anisidine value is a relative measure used to determine aldehyde content of peroxidized oils.
Serum concentrations of malondialdehyde (MDA) were measured using a commercial kit, following manufacturer’s instructions (OxiSelect TBARS Assay Kit, Cell Biolabs Inc., San Diego). Total antioxidant capacity (TAC) in serum was determined according to a colorimetric method previously described by Erel (2004). Concentrations of 8-hydroxy-2′-deoxyguanosine (8-OHdG) were measured in serum using a commercial kit, according to manufacturer’s instructions (DNA/RNA Oxidative Damage EIA Kit; Cayman Chemical Company, Ann Arbor, MI). Protein carbonyl groups were quantified in serum using a commercial kit (Protein Carbonyl Colorimetric Assay Kit, Cayman Chemical Company, Ann Arbor, MI) with modifications. Briefly, serum samples were diluted 1:3 with 0.01 M phosphate-buffered saline (pH 7.0) to obtain protein levels in the range of 1 to 10 mg/mL, then incubated with 10 mM dinitrophenylhydrazine (DNPH) in 2.5 M HCl (for serum samples) or 2.5 M HCl alone (for sample blanks) for 1 h in the dark. Trichloroacetic acid (TCA, 10% final concentration) was added to precipitate proteins in the DNPH samples and their appropriate blanks. The DNPH samples and sample blanks were washed with 10% TCA, which was followed by 3 ice-cold acetone washes. Finally, DNPH samples and their blanks were resuspended with 6 M guanidine hydrochloride (pH 6.5) and aliquoted into 96-well plates. Absorbances were measured at 360 nm in a multi-detection microplate reader (Synergy HT, BioTek Instruments, Inc., Winooski, VT). Carbonyl content (nmol/mL) was determined with the corrected absorbances (DNPH sample absorbance minus sample blank absorbance) and the extinction coefficient of DNPH. To normalize the carbonyl content to the amount of protein present in the resuspended 6 M guanidine HCl samples, protein concentrations were determined using a bicinchoninic acid assay kit (Pierce BCA Protein Assay Kit, Thermo Fisher Scientific Inc., Rockford, IL). Final carbonyl content was expressed as nmol carbonyl/mg protein.
All analyses were conducted in duplicate; intra- and interassay CV were 1.9% and 1.3% for MDA, 2.2% and 3.5% for TAC, 9.3% and 12.4% for 8-OHdG, 0.7% and 0.8% for protein carbonyl, respectively.
Statistical Analysis
Two pens were removed from the study due to issues with the feed delivery system at the beginning of the trial. Growth performance, health status (percentage of sick pigs, pigs receiving medical treatment, and culling rate), number of full-value pigs at nursery close-out and mortality data were analyzed using PROC Mixed of SAS (v. 9.4; SAS Inst. Inc., Cary, NC), testing for fixed effects of peroxidation. Pen was the experimental unit and weight block was used as random effect.
Antibody titers, markers of oxidative stress, and vitamin E concentrations were analyzed by PROC Mixed of SAS. The model included fixed effects of peroxidation, day of sampling, BW category (heavy- vs. lightweight) and relevant interactions. Pig was considered the experimental unit and pig nested within pen was used as random effect (for antibody titers only). Antibody titers were analyzed as repeated measures using day of sampling.
For all analyses, mean values were compared by Tukey–Kramer test where appropriate. Main effects were partitioned into linear, quadratic, cubic, and fourth degree polynomials to determine trends in the data associated with the quantitative peroxidation treatment structure as described in detail by Maindonald (1992). Significance of trend analysis and differences between treatments were considered significant at P < 0.05 and tendencies were declared at 0.05 ≤ P < 0.10.
RESULTS
Growth Performance and Health Status
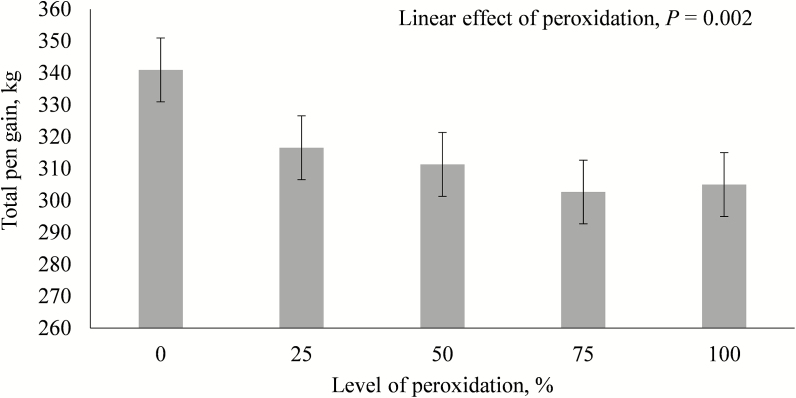
Increasing levels of peroxidation tended to quadratically impact ADG during days 1 to 20 (P = 0.06) and ADFI during days 7 to 20 (P = 0.052), 1 to 20 (P = 0.057), and 1 to 43 (P = 0.076), showing an initial decrease and then increase in these response variables (Table 4). Feed efficiency (G:F) tended to decrease linearly with increasing levels of peroxidation in the feed during days 7 to 20 (P = 0.068) and linearly decreased the first 20 d of the study (P = 0.020) and for the overall nursery period (P = 0.023). Final pen BW gain calculated as total pen weight at the end of the nursery minus pen weight at the start of the nursery decreased linearly (P = 0.002) with increasing peroxidation (Figure 1).
Table 4.
Effects of lipid peroxidation dose on growth performance, health status, and viability of nursery pigs1
| Level of peroxidation | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | 0% | 25% | 50% | 75% | 100% | SEM | Perox2 | Linear | Quad |
| BW, kg | |||||||||
| Day 1 | 5.95 | 5.95 | 5.95 | 5.94 | 5.95 | 0.17 | 0.998 | 0.794 | 0.830 |
| Day 7 | 6.93 | 6.69 | 6.72 | 6.74 | 6.74 | 0.21 | 0.606 | 0.370 | 0.273 |
| Day 20 | 12.03 | 11.98 | 11.85 | 11.97 | 12.01 | 0.28 | 0.759 | 0.878 | 0.275 |
| Day 43 | 22.61 | 22.35 | 22.60 | 22.33 | 22.56 | 0.48 | 0.889 | 0.880 | 0.635 |
| ADG, g/d | |||||||||
| Days 1 to 7 | 97 | 90 | 89 | 93 | 90 | 8 | 0.945 | 0.655 | 0.614 |
| Days 7 to 20 | 409 | 406 | 399 | 399 | 408 | 8 | 0.664 | 0.702 | 0.181 |
| Days 1 to 20 | 299 | 290 | 281 | 285 | 290 | 7 | 0.264 | 0.242 | 0.060 |
| Days 20 to 43 | 460 | 449 | 465 | 451 | 454 | 12 | 0.795 | 0.760 | 0.957 |
| Days 1 to 43 | 386 | 373 | 377 | 372 | 377 | 8 | 0.510 | 0.298 | 0.264 |
| ADFI, g/d | |||||||||
| Days 1 to 7 | 102 | 98 | 97 | 98 | 100 | 5 | 0.924 | 0.811 | 0.370 |
| Days 7 to 20 | 487 | 476 | 479 | 482 | 490 | 10 | 0.305 | 0.444 | 0.052 |
| Days 1 to 20 | 349 | 338 | 338 | 341 | 346 | 8 | 0.396 | 0.855 | 0.057 |
| Days 20 to 43 | 786 | 761 | 777 | 768 | 780 | 17 | 0.578 | 0.906 | 0.254 |
| Days 1 to 43 | 581 | 561 | 568 | 565 | 574 | 12 | 0.299 | 0.656 | 0.076 |
| G:F, g/kg | |||||||||
| Days 1 to 7 | 960 | 910 | 888 | 891 | 884 | 58 | 0.873 | 0.340 | 0.595 |
| Days 7 to 20 | 840 | 853 | 829 | 831 | 822 | 10 | 0.188 | 0.068 | 0.600 |
| Days 1 to 20 | 856 | 860 | 830 | 840 | 831 | 10 | 0.068 | 0.020 | 0.687 |
| Days 20 to 43 | 586 | 588 | 598 | 587 | 583 | 7 | 0.624 | 0.716 | 0.226 |
| Days 1 to 43 | 665 | 669 | 664 | 659 | 652 | 5 | 0.144 | 0.023 | 0.216 |
| Removed, %3 | 5.18 | 7.95 | 9.32 | 10.49 | 10.10 | 1.24 | 0.019 | 0.002 | 0.151 |
| Treated, %4 | 5.37 | 9.42 | 11.82 | 11.42 | 10.34 | 1.31 | 0.003 | 0.003 | 0.006 |
| Culled, %5 | 0.21 | 0.72 | 0.91 | 1.90 | 1.18 | 0.45 | 0.074 | 0.021 | 0.286 |
| Mortality, %6 | 0.97 | 1.36 | 2.50 | 2.73 | 2.88 | 0.73 | 0.270 | 0.033 | 0.620 |
| Full-value pigs, %7 | 98.85 | 97.82 | 96.59 | 95.22 | 95.95 | 0.94 | 0.049 | 0.005 | 0.325 |
1Values represent least squares means of 20 pens with 22 pigs per pen. Peroxidized corn oil was created by heating (65 °C) corn oil with constant air supply (20 L/min) for 12 d. Oil was stabilized with liquid antioxidant (TBHQ) after peroxidation. Treatments represent a control diet with 5% refined, bleached, and deodorized corn oil (0% peroxidation) and a diet with 5% peroxidized corn oil (100% peroxidation). Intermediate diets were created by mixing the control and peroxidized diets on-farm.
2Main effect of peroxidized corn oil. Main effects were partitioned into linear, quadratic (Quad), cubic, and fourth degree polynomials for trend analysis of peroxidation treatments.
3Pigs removed from original pens to sick pens due to sickness or small size. Removed pigs remained on test, were maintained on their designated dietary treatments, and outcome of pigs in sick pens was tracked. If pigs recovered and were heavier than 13.6 kg at the end of the study, they were considered full-value pigs.
4Pigs in original and sick pens that were treated with injectable medicine.
5Pigs weighing 13.6 kg or less at the end of the trial period.
6Pigs that died in original and sick pens.
7All pigs at the end of the trial period minus culled pigs and mortality.
Figure 1.
Impact of lipid peroxidation on total gain of pens of pigs during the 43-d nursery period. Levels of peroxidation were represented by a control diet with 5% refined, bleached, and deodorized corn oil (0% peroxidation) and a diet with 5% peroxidized corn oil (100% peroxidation). Intermediate diets were created by mixing the control and peroxidized diets on-farm. Peroxidized corn oil was created by heating (65 °C) corn oil with constant air supply (20 L/min) for 12 d. Oil was stabilized with liquid antioxidant (TBHQ) after peroxidation. Each value represents the mean of 20 pens with 22 pigs per pen (at the start of the study). Pen gain represents the total pig weight produced per pen and was calculated as total of weight of viable pigs present in each pen at the end of the study minus the sum of the weight of pigs in each pen at the start of the study.
Percentage of pigs removed, culled for extreme low weight and treated increased linearly (P < 0.03) with increasing level of peroxidation. Likewise, mortality increased linearly (P < 0.04) while percentage of full-value pigs decreased linearly (P < 0.01) with increasing dietary peroxidation level (Table 4).
Serology
Antibody titers to Mhyo and PCV2 increased after the second vaccination (P < 0.01; 0.06 vs. 0.22 for Mhyo and 0.26 vs. 0.35 for PCV2, on days 33 and 47, respectively), yet there were no differences due to dietary treatment, BW category, or their interaction (Table 5). Serum concentrations of 8-OHdG and protein carbonyl were not affected by peroxidation level, BW category or their interaction. Serum MDA was 19% higher (P < 0.01) in heavy BW pigs than in light weight pigs, but concentrations did not differ due to peroxidation level or peroxidation by BW interaction. Total antioxidant capacity in serum increased by 10% (P < 0.03) in heavy BW pigs compared to lightweight pigs and reduced linearly (P = 0.05) as peroxidation level increased. Vitamin E concentrations decreased linearly (P = 0.01) with increasing peroxidation level of the diet. Concentrations of vitamin E were 10% higher (P < 0.04) in heavyweight pigs compared with lightweight pigs (Table 5).
Table 5.
Effects of lipid peroxidation dose and BW on serum oxidative status and antibody titers of nursery pigs1
| Level of peroxidation2 | BW group3 | P-value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | 0% | 25% | 50% | 75% | 100% | SEM | Light | Heavy | SEM | Perox4 | Linear | Quad | BW | Perox*BW |
| Vitamin E, ppm | 1.24 | 1.32 | 1.23 | 0.99 | 1.15 | 0.06 | 1.12 | 1.24 | 0.04 | 0.004 | 0.011 | 1.000 | 0.033 | 0.275 |
| 8OHdG, pg/mL5 | 1,799 | 2,034 | 1,948 | 2,002 | 1,686 | 164 | 1,987 | 1,801 | 104 | 0.530 | 0.623 | 0.120 | 0.209 | 0.636 |
| TAC, µM Trolox eq/mL6 | 100.0 | 97.1 | 89.1 | 84.7 | 92.9 | 4.13 | 88.4 | 97.1 | 2.60 | 0.069 | 0.051 | 0.102 | 0.021 | 0.842 |
| MDA, µm7 | 9.08 | 10.93 | 10.20 | 9.26 | 9.25 | 0.54 | 8.90 | 10.58 | 0.38 | 0.153 | 0.487 | 0.087 | 0.003 | 0.757 |
| Protein carbonyl, pmol/mg | 1,214 | 1,237 | 1,200 | 1,201 | 1,189 | 29.2 | 1,203 | 1,213 | 18.5 | 0.810 | 0.363 | 0.780 | 0.711 | 0.411 |
| Mhyo, S/P ratio8,9,10 | 0.164 | 0.100 | 0.12 | 0.13 | 0.17 | 0.02 | 0.13 | 0.15 | 0.01 | 0.177 | 0.600 | 0.032 | 0.265 | 0.663 |
| PCV2, S/P ratio8,9,10 | 0.28 | 0.30 | 0.31 | 0.30 | 0.33 | 0.02 | 0.32 | 0.29 | 0.01 | 0.589 | 0.177 | 0.984 | 0.111 | 0.107 |
1Each value within level of peroxidation represents a mean of 20 pigs and each value within BW group represents a mean of 50 pigs.
2Peroxidized corn oil was created by heating (65 °C) corn oil with constant air supply (20 L/min) for 12 d. Oil was stabilized with liquid antioxidant (TBHQ) after peroxidation. Treatments represent a control diet with 5% refined, bleached, and deodorized corn oil (0% peroxidation) and a diet with 5% peroxidized corn oil (100% peroxidation). Intermediate diets were created by mixing the control and peroxidized diets on-farm.
3Pigs sampled on day 33 of the study were grouped into light (mean BW was 12.0 kg) and heavy (mean BW was 21.8 kg) BW groups.
4Main effect of peroxidized corn oil. Main effects were partitioned into linear, quadratic (Quad), cubic, and fourth degree polynomials for trend analysis of peroxidation treatments
58-Hydroxy-2’-deoxyguanosine.
6Total antioxidant capacity.
7Malondialdehyde.
8Antibody titers to Mycoplasma hyopneumoniae (Mhyo) and porcine circovirus type 2 (PCV2).
9Titer results are presented as the sample to positive (S/P) ratio, calculated as the sample absorbance minus the negative control mean, divided by the difference between the positive and negative control means.
10Main effect of sampling day (P < 0.001). Titers were 0.06 vs. 0.22 for Mhyo and 0.26 vs. 0.35 for PCV2 when measured on days 33 and 43, respectively.
DISCUSSION
Several compounds are produced during lipid peroxidation and various analytical tests can be employed to measure oxidation, such as peroxide value, anisidine value, thiobarbituric acid reactive substances (TBARS), and these are customarily performed in the industry (Kerr et al., 2015). In the current study, peroxidation of corn oil as measured by primary (peroxides) and secondary (aldehydes) oxidation compounds was comparable with values reported previously in the literature (Liu et al., 2014a; Rosero et al., 2015; Lindblom et al., 2018a; Overholt et al., 2018). While these and other studies (DeRouchey et al., 2004; Yuan et al., 2007; Lu et al., 2014; Hanson et al., 2016, Hung et al., 2017; Lindblom et al., 2018b; Overholt et al., 2018) have demonstrated adverse effects on pig growth performance, the impact on health status and viability of pigs fed peroxidized oils must be proven in a commercial setting with sufficient replications. This is critical information for financial evaluation, and it extends the biological implications of this stressor in young pigs.
In the present study, feed efficiency of pigs was reduced as lipid peroxidation level in the diet increased. Moreover, health status of pigs was clearly compromised by increasing peroxidation level in diets, which was shown by a greater percentage of pigs removed and medically treated. This was further reinforced by a dose-responsive decrease in viability of pigs at the end of the study. In this, and other instances where treatment compromises viability and ability to thrive, ADG and ADFI can be misleading because they represent growth of pigs that were sufficiently resilient to the stressor. When calculating total pen gain, pens with pigs that were culled or died were penalized because fewer pigs contributed to the total weight of the pen at the end of the nursery period, resulting in lower total pig gain per pen. Thus, total pig gain per pen was reduced (up to 11%) with increasing peroxidation level.
To our knowledge, this is the first study to quantitate the negative impact of peroxidation level of supplemental lipids on weaned pig viability. This study presents population responses to peroxidation dose, which included removed pigs for significant medical treatment, excessively light pigs, mortality, and total medical treatment. These are otherwise often ignored, but they are particularly pertinent to the industry, because the financial value of retaining viable pigs is a powerful driver of return over investment. These measures also help to better describe the outcomes of progressive peroxidation product intake on weaned pigs; which are presumed to be the most vulnerable phase to diet lipid peroxidation. Rosero et al. (2015) demonstrated that lipid peroxidation diminished growth, function and morphology of the small intestine, and decreased digestion of energy and lipids in nursery pigs in a dose-related manner which, not only support the results of the present study but gave incentive to conduct this study.
To investigate possible impacts of peroxidation on the immune system of pigs, we measured serum antibody titers to vaccines in the heaviest and lightest pigs, speculating that the potential negative effects of lipid peroxidation may be greater in lighter pigs. However, antibody titers were unaffected by peroxidation or BW of pigs. Toxicity of dietary lipid peroxides (as indicated by enlarged livers) has been reported in rats (Kanazawa et al., 1985) and pigs (Liu et al., 2014c; Lu et al., 2014, Lindblom et al., 2018a). Reduced immune capacity has been described by Dibner et al. (1996), who observed reduced lymphocyte proliferation in pigs and reduced IgA concentration in intestinal tissue of broilers fed peroxidized poultry fat. Liang et al. (2015) demonstrated a 48% reduction in secretory IgA and a reduction of ~40% in CD4 molecules in jejunal mucosa of broilers fed peroxidized soy oil. On the other hand, Liu et al. (2014b) did not find differences in serum IgA and IgG in pigs fed 10% peroxidized lipids. More recently, Lindblom et al. (2018a) found significant change in circulating neutrophil function between pigs fed peroxidized compared with fresh soy oil. Despite the lack of effects on antibody titers due to dietary treatments in the present study, the increase in number of pigs that received medical treatment and were removed or culled indicates that pigs were sensitive to peroxidation, and perhaps immunocompromised.
Poor health status may result from excessive oxidative stress as reported in dairy cattle (Sordillo and Aitken, 2009). Consumption of peroxidized lipids induced oxidative stress in pigs and broilers, as indicated by increased concentrations of MDA as measured by TBARS (Ringseis et al., 2007; Yuan et al., 2007; Tavárez et al., 2011; Delles et al., 2014; Liu et al., 2014b; Lu et al., 2014; Rosero et al., 2015; Liang et al., 2015; Hanson et al., 2016, Hung et al., 2017, Lindblom et al., 2018a), protein carbonyl (Delles et al., 2014; Lu et al., 2014, Lindblom et al., 2018a), and altered activities of antioxidant enzymes, such as catalase, superoxide dismutase, and glutathione peroxidase (Luci et al., 2007; Ringseis et al., 2007; Yuan et al., 2007; Boler et al., 2012; Delles et al., 2014; Liang et al., 2015, Lindblom et al., 2018a). Frequently used as a marker of oxidative stress, the TBARS assay measures the production of a conjugated-double-bond compound formed from the reaction of TBARS with MDA. In contrast to aforementioned publications, serum MDA concentrations were not affected by dietary peroxidation level, but concentrations were greater in heavy BW pigs compared with their lighter counterparts. Most recent studies did not find differences in TBARS levels in serum of pigs consuming peroxidized soy oil, but liver (Lindblom et al., 2018a) and urinary (Lindblom et al., 2018a; Overholt et al., 2018) TBARS levels were increased due to peroxidation. Lindblom et al. (2018a) suggested that measuring urinary vs. circulating levels of MDA could be a more reliable method to assess oxidative status in pigs due to the rapid metabolism and excretion of MDA. It was expected that heavier pigs would be more capable of overcoming dietary induced oxidative stress than lighter pigs, which was supported by greater TAC and vitamin E status in these pigs. Thus, the observed increase in MDA concentrations in this category of pigs was unexpected and conflicts with the other serology data provided in this study.
The molecule 8-OHdG is the product of reactive oxygen species (ROS) attack on purines and is an indicator of nucleic acid damage caused by oxidative imbalance (Sen and Chakraborty, 2011). The expected increase in this marker in pigs that were fed peroxidized lipids was not detected, which is consistent with the results obtained for serum protein carbonyl. Carbonyls are derived from the reaction of proteins with ROS and have been found to increase in pigs due to consumption of peroxidized lipids (Lu et al., 2014; Lindblom et al., 2018a). Despite the lack of differences in these oxidative stress markers in the present study, there was a reduction in serum TAC and vitamin E concentrations in pigs fed increasing dietary peroxidation levels. This suggests that TAC along with the antioxidant properties of vitamin E was able to minimize oxidative stress to levels that were similar across dietary treatments. Linear decreases in TAC were also detected by Liang et al. (2015) when feeding broilers with increasing dietary PV levels and Rosero et al. (2015) in pigs fed oils with increasing levels of peroxidation.
Vitamin E has been reported to decrease in animals fed peroxidized lipids (Luci et al., 2007; Ringseis et al., 2007; Boler et al., 2012; Hanson et al., 2016), and this is consistent with our findings. In contrast, feeding oxidized DDGS increased serum vitamin E concentrations in pigs (Hanson et al., 2015). It has been demonstrated that vitamin E concentrations decrease in complete diets ensuing addition of peroxidized oils (Luci et al., 2007; Ringseis et al., 2007; Hanson et al., 2016). In the current study, vitamin E was not analyzed in oils or in the complete diet; hence, there is a possibility that part of the observed decrease in serum vitamin E reflected a reduced vitamin E intake due to partial destruction of vitamin E in the diet. Hanson et al. (2016) reported that dietary vitamin E concentration was reduced by ~16% in diets with peroxidized oil compared with control diets, but serum vitamin E concentrations were reduced by ~70% in pigs fed peroxidized lipids.
In conclusion, we demonstrated in a large field study that increasing levels of peroxidation significantly increased mortality and decreased health status of pigs, as indicated by greater number of pigs medicated and pigs that were excessively light, resulting in reduced total pig viability by the end of the nursery. Feed efficiency and especially total pen gain were impaired with increasing levels of peroxidation in diets, demonstrating the metabolic cost of a dose–response increase in lipid peroxide intake. Response to vaccine and indicators of oxidative stress in serum (MDA, 8-OHdG, and protein carbonyl) were not affected by increasing levels of lipid peroxidation in the diets. However, serum vitamin E status and total antioxidant capacity were reduced by increasing levels of peroxidized lipids, which may have contributed to the pigs’ ability to maintain oxidative stress markers to similar levels across treatments. Thus, quantification of peroxidation level of lipid sources for swine is critically important to design quality control programs for oil and fat sources and to increase profitability of pork production, especially for weaned pigs that are expected to be the most vulnerable to poor lipid quality.
Conflict of interest statement. None declared.
Footnotes
Funded in part by the North Carolina Pork Council (Raleigh, NC). Appreciation is expressed to The Hanor Company (Franklin, KY) for providing access to their facilities and their staff for assistance in conducting the study. The assistance of Roel Becerra and Santa Maria Mendoza with preparing the peroxidized oil and Karen Murphy and Jordan Wood with chemical analyses is greatly appreciated.
LITERATURE CITED
- American Oil Chemists’ Society (AOCS). 2010. Official methods and recommended practices of the AOCS. 6th ed. Champaign, IL:AOCS. [Google Scholar]
- AOAC International. 2007. Official methods of analysis.18th ed. Rev. 2. In: Howitz W. and Latimer G. W. Jr., editors. Gaithersburg, MD:AOAC International. [Google Scholar]
- Boler D. D., Fernández-Dueñas D. M., Kutzler L. W., Zhao J., Harrell R. J., Campion D. R., McKeith F. K., Killefer J., and Dilger A. C.. 2012. Effects of oxidized corn oil and a synthetic antioxidant blend on performance, oxidative status of tissues, and fresh meat quality in finishing barrows. J. Anim. Sci. 90:5159–5169. doi: 10.2527/jas.2012-5266 [DOI] [PubMed] [Google Scholar]
- Delles R. M., Xiong Y. L., True A. D., Ao T., and Dawson K. A.. 2014. Dietary antioxidant supplementation enhances lipid and protein oxidative stability of chicken broiler meat through promotion of antioxidant enzyme activity. Poult. Sci. 93:1561–1570. doi: 10.3382/ps.2013-03682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRouchey J. M., Hancock J. D., Hines R. H., Maloney C. A., Lee D. J., Cao H., Dean D. W., and Park J. S.. 2004. Effects of rancidity and free fatty acids in choice white grease on growth performance and nutrient digestibility in weanling pigs. J. Anim. Sci. 82:2937–2944. doi: 10.2527/2004.82102937x [DOI] [PubMed] [Google Scholar]
- Dibner J. J., Atwell C. A., Kitchell M. L., Shermer W. D., and Ivey F. J.. 1996. Feeding of oxidized fats to broilers and swine: effects on enterocyte turnover, hepatocyte proliferation and the gut associated lymphoid tissue. Anim. Feed Sci. Technol. 62:1–13. doi:10.1016/S0377-8401(96)01000-0 [Google Scholar]
- Erel, O. 2004. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 37:277–285. doi:10.1016/j.clinbiochem.2003.11.015 [DOI] [PubMed] [Google Scholar]
- FASS. 2010. Guide for the care and use of agricultural animals in research and teaching. 3rd ed. Champaign, IL: FASS. [Google Scholar]
- Hanson A. R., Urriola P. E., Wang L., Johnston L. J., Chen C., and Shurson G. C.. 2016. Dietary peroxidized maize oil affects the growth performance and antioxidant status of nursery pigs. Anim. Feed Sci. Tech. 216:251–261. doi:10.1016/j.anifeedsci.2016.03.027 [Google Scholar]
- Hanson A. R., Wang L., Johnston L. J., Baidoo S. K., Torrison J. L., Chen C., and Shurson G. C.. 2015. Effect of feeding peroxidized dried distillers grains with solubles to sows and progeny on growth performance and metabolic oxidative status of nursery pigs. J. Anim. Sci. 93:135–146. doi: 10.2527/jas.2014-7371 [DOI] [PubMed] [Google Scholar]
- Hung Y. T., Hanson A. R., Shurson G. C., and Urriola P. E.. 2017. Peroxidized lipids reduce growth performance of poultry and swine: a meta-analysis. Anim. Feed Sci. Tech. 231:47–58. doi:10.1016/j.anifeedsci.2017.06.013 [Google Scholar]
- Kanazawa K., Kanazawa E., and Natake M.. 1985. Uptake of secondary autoxidation products of linoleic acid by the rat. Lipids 20:412–419. doi:10.1007/BF02534231 [DOI] [PubMed] [Google Scholar]
- van Kempen T. A., and McComas S.. 2002. Infrared spectroscopy as a tool for assessing fat quality. J. Appl. Poult. Res. 11:191–201. doi:10.1093/japr/11.2.191 [Google Scholar]
- Kerr B. J., Kellner T. A., and Shurson G. C.. 2015. Characteristics of lipids and their feeding value in swine diets. J. Anim. Sci. Biotechnol. 6:30. doi: 10.1186/s40104-015-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F., Jiang S., Mo Y., Zhou G., and Yang L.. 2015. Consumption of oxidized soybean oil increased intestinal oxidative stress and affected intestinal immune variables in yellow-feathered broilers. Asian-Australas. J. Anim. Sci. 28:1194–1201. doi: 10.5713/ajas.14.0924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom S. C., Gabler N. K., Dilger R. N., Olson Z. F., Loving C. L., and Kerr B. J.. 2018a. Influence of feeding thermally peroxidized soybean oil on oxidative status in growing pigs. J. Anim. Sci. 96:545–557. doi: 10.1093/jas/sky005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom S. C., Gabler N. K., and Kerr B. J.. 2018b. Influence of feeding thermally peroxidized soybean oil on growth performance, digestibility, and gut integrity in growing pigs. J. Anim. Sci. 96:558–569. doi: 10.1093/jas/sky004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Chen C., Kerr B. J., Weber T. E., Johnston L. J., and Shurson G. C.. 2014c. Influence of thermally oxidized vegetable oils and animal fats on growth performance, liver gene expression, and liver and serum cholesterol and triglycerides in young pigs. J. Anim. Sci. 92:2960–2970. doi: 10.2527/jas.2012-5709 [DOI] [PubMed] [Google Scholar]
- Liu P., Kerr B. J., Chen C., Weber T. E., Johnston L. J., and Shurson G. C.. 2014a. Methods to create thermally oxidized lipids and comparison of analytical procedures to characterize peroxidation. J. Anim. Sci. 92:2950–2959. doi: 10.2527/jas.2012-5708 [DOI] [PubMed] [Google Scholar]
- Liu P., Kerr B. J., Weber T. E., Chen C., Johnston L. J., and Shurson G. C.. 2014b. Influence of thermally oxidized vegetable oils and animal fats on intestinal barrier function and immune variables in young pigs. J. Anim. Sci. 92:2971–2979. doi: 10.2527/jas2012-5710 [DOI] [PubMed] [Google Scholar]
- Lu T., Harper A. F., Zhao J., Estienne M. J., and Dalloul R. A.. 2014. Supplementing antioxidants to pigs fed diets high in oxidants: I. Effects on growth performance, liver function, and oxidative status. J. Anim. Sci. 92:5455–5463. doi: 10.2527/jas.2013-7109 [DOI] [PubMed] [Google Scholar]
- Luci S., König B., Giemsa B., Huber S., Hause G., Kluge H., Stangl G. I., and Eder K.. 2007. Feeding of a deep-fried fat causes PPARalpha activation in the liver of pigs as a non-proliferating species. Br. J. Nutr. 97:872–882. doi: 10.1017/S0007114507669256 [DOI] [PubMed] [Google Scholar]
- Maindonald J. H. 1992. Statistical design, analysis and presentation issues. New Zeeland J. Agric. Res. 35:121–144. doi: 10.1080/00288233.1992.10417710 [DOI] [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. 11th rev. ed. Washington, DC:National Academies Press. [Google Scholar]
- Overholt M. F., Dilger A. C., Boler D. D., and Kerr B. J.. 2018. Influence of feeding thermally peroxidized soybean oil on growth performance, digestibility, and gut integrity in finishing pigs. J. Anim. Sci. 96:2789–2803. doi: 10.1093/jas/sky091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringseis R., Piwek N., and Eder K.. 2007. Oxidized fat induces oxidative stress but has no effect on NF-kappab-mediated proinflammatory gene transcription in porcine intestinal epithelial cells. Inflamm. Res. 56:118–125. doi: 10.1007/s00011-006-6122-y [DOI] [PubMed] [Google Scholar]
- Rosero D. S., Odle J., Moeser A. J., Boyd R. D., and van Heugten E.. 2015. Peroxidised dietary lipids impair intestinal function and morphology of the small intestine villi of nursery pigs in a dose-dependent manner. Br. J. Nutr. 114:1985–1992. doi: 10.1017/S000711451500392X [DOI] [PubMed] [Google Scholar]
- Sen S., and Chakraborty R.. 2011. The role of antioxidants in human health. In: Andreescu S. and Hepel M., editors, Oxidative stress: diagnostics, prevention, and therapy. ACS symposium series. Washington, DC:American Chemical Society. [Google Scholar]
- Shurson G. C., Kerr B. J., and Hanson A. R.. 2015. Evaluating the quality of feed fats and oils and their effects on pig growth performance. J. Anim. Sci. Biotechnol. 6:10. doi: 10.1186/s40104-015-0005-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R., Chen C., Johnston L. J., Kerr B. J., Weber T. E., and Shurson G. C.. 2014. Effects of feeding diets containing highly peroxidized distillers dried grains with solubles and increasing vitamin E levels to wean-finish pigs on growth performance, carcass characteristics, and pork fat composition. J. Anim. Sci. 92:198–210. doi: 10.2527/jas.2013-6334 [DOI] [PubMed] [Google Scholar]
- Sordillo L. M., and Aitken S. L.. 2009. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 128:104–109. doi: 10.1016/j.vetimm.2008.10.305 [DOI] [PubMed] [Google Scholar]
- Tavárez M. A., Boler D. D., Bess K. N., Zhao J., Yan F., Dilger A. C., McKeith F. K., and Killefer J.. 2011. Effect of antioxidant inclusion and oil quality on broiler performance, meat quality, and lipid oxidation. Poult. Sci. 90:922–930. doi: 10.3382/ps.2010-01180 [DOI] [PubMed] [Google Scholar]
- Yuan S., Chen D., Zhang K., and Yu B.. 2007. Effects of oxidative stress on growth performance, nutrient digestibilities and activities of antioxidative enzymes of weanling pigs. Asian-Australas. J. Anim. Sci. 20:1600–1605. doi: 10.5713/ajas.2007.1600 [DOI] [Google Scholar]