Abstract
Cost-effective and feasible production system of meat goats requires that grazed forages are converted to profitable goat meat product. However, there are studies as how altering forage type influences ruminal fermentation parameters and animal growth performance, and interact with microbiota in meat goats. Our objective for current study was to examine whether the comparative abundance of the Bacteroidetes (B) and Firmicutes (F) bacterial phyla in meat goats fed simple and mixed forages influenced average daily gain (ADG) and rumen fermentation parameters. In the present study, a molecular approach, bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP) was applied to accomplish diversity analyses of rumen bacterial populations. Thirty-six Kiko-cross growing meat goats (body weight (BW) = 27.7 ± 2.83 kg) at approximately 7 mo of age were used in this study. Animals were randomly allocated to 3 pasture treatment groups (n = 12) as follows: 1) bermudagrass pasture (BG; Cynodon dactylon), 2) sunn hemp forage (SH; Crotalaria juncea), and 3) BG + SH forage combinations. There were 2 replicates per treatment and animals grazed these pastures for 45 d. Results indicated that treatments had similar initial BW, but final BW and ADG were higher (P < 0.01) for SH and BG + SH combinations than for BG alone. Animal ADG and rumen fermentation (acetate to propionate; A/P ratios) were highly correlated with the abundance of various bacterial populations within the rumen microbiome. There were linear decreases in percentage of Bacteroidetes (R2 = −0.84; P < 0.05) associated with decreasing ADG. In contrast, increased ADG was linearly associated with higher percentages of Firmicutes (R2 = 0.79; P < 0.05), F/B ratios (R2 = 0.88; P = 0.07), total VFA (R2 = 0.45; P < 0.05), and lower A/P ratio (R2 = −0.72; P < 0.01). This suggests that the substrates (diets) and bacterial community have the role in adapting host biological parameters in meat goats. The abundance examination of both Bacteroidetes and Firmicutes will be useful for exploring the structure of gut microbiota as an estimate of animal performance.
Keywords: ADG, acetate, Bacteroidetes, Firmicutes, meat goats
INTRODUCTION
The composition of diet fed to ruminant animals (e.g., forage vs. grain) dramatically impacts the microbial diversity and composition in the rumen (Jung and Varel, 1988; Saro et al., 2014; Grilli et al., 2016). The rumen comprises of a complex ecosystem where substrates consumed by ruminants and digested by various rumen microorganisms such as bacteria, protozoa, and fungi. In addition, grass/legume mixed pastures are often considered to have the potential to alter rumen microbiota populations and enhance animal growth performance in grazing ruminants as compared to monoculture pastures (Harris et al., 1998; Saro et al., 2014; Min et al., 2016). Alternate feeding of legumes and grasses resulted in major shifts in rumen cellulolytic bacterial populations in sheep (Jung and Varel, 1988). Therefore, undoubtedly the change in feeds results in changes in the profile of rumen microbial community of ruminant animals such as cattle, sheep, and in this case goats (Dewhurst et al., 2001; Belanche et al., 2012).
Bacteroidetes and Firmicutes are the 2 prevailing bacterial phyla in the gut of humans, mice, and pigs (Ley et al., 2005, 2006; Guo et al., 2008). In mice, an association has been revealed between the gut microbiota and energy-harvesting abilities with overweight animals, demonstrating a dissimilar ratio of the phyla Firmicutes to Bacteroidetes (F/B; Ley et al., 2005, 2006). These studies revealed that a considerable decrease in the relative abundance of Bacteroidetes and a larger proportion of Firmicutes were found in obese animals than in lean animals (Ley et al., 2005, 2006). However, volatile fatty acids (VFA) are produced in large amounts through ruminal fermentation and provide main source of energy (70% to 75%; Bergman, 1990; Mizrahi, 2011). Therefore, it seems possible that an increased supply of energy, especially of VFA, might be associated with microbial community changes and an increased average daily gain (ADG). The primary objective of the current study was to determine if the relative abundance of rumen microorganisms belonging to the 2 predominant bacterial phyla, Bacteroidetes and Firmicutes, and their ratios, impact ADG and feed efficiency in meat goats.
MATERIALS AND METHODS
This study was conducted at Caprine Research and Education Unit (CREU), Tuskegee University, Tuskegee, AL. Animals were cared for according to the “Guide for the care and use of laboratory animals” (USDA, 1985). Care and handling of all experimental animals were conducted under protocols approved by the Tuskegee University Institutional Animal Care and Use Committee (R02-2012-4-1).
Experimental Design, Animals, and Treatment Diets
Thirty-six Kiko-cross (n = 12) growing intact male goats (7 mo of age) were used in a randomized complete block design, with 12 goats in each treatment for 45 d. Goats were blocked by initial body weight (BW; 27.7 ± 2.83 kg). Before the start of the trial, goats rotationally grazed on a bermudagrass dominant pasture with alfalfa pellet supplementation at a rate of 0.2 kg per head during a 30-d adaptation period. Approximately 3 ha of pasture (0.5 ha each plot) located at the CREU, Tuskegee University, Tuskegee, AL, were used to conduct a 45-d grazing trial excluding adaptation period (30 d), between May to August 2015. All the goats were offered an estimated pasture allocation of 1.6 kg of DM per animal per day above a post-grazing pasture residual of 1,400 kg of DM/ha. Animal housing (640 × 480 cm metal shelter), plastic water troughs, and trace mineral salt blocks (Champion’s Choice, TSC Tractor Supply) were supplied on the pasture to provide shade, ad libitum access to water and minerals. Goats were offered one of the following treatments: 1) bermudagrass forage (BG; Cynodon dactylon), 2) sunn hemp forage (SH; Crotalaria juncea), and 3) BG + SH forage combinations, respectively, with 2 replicate grazing plots for forage treatment. Both BG and SH forage seeds were sown in two 0.5-ha plots for each treatment at the seeding rate of 15 kg/ha each in a randomized design (Petcher seeds, 1609 Carpenter Crossing Rd., AL). Mixtures of BG and SH forages (7 plus 8 kg mixes for BG and SH, respectively) were planted. All plots were fertilized with 50 kg of urea-N/ha, 28 d before the study commenced.
Sample Collections
Animal BW and forage samples were collected every other week to determine forage biomass and forage quality for a period of 45 d. Pasture biomass [dry matter (DM) basis] was determined by hand-clipping above ground (soil level) forage from five 0.25 m2 quadrats per paddock. Forage samples were used to estimate forage biomass production, digestibility, and nutrient content. These samples were then weighed fresh, dried in an oven at 65 °C, for 48 h, then reweighed to ascertain DM content. After biomass measurements, subsamples of 100 to 200 g were composited from the 5 field samples, ground through a 1.0 mm screen (model 4 Wiley mill; Thomas Scientific, Swedesboro, NJ) and used for nutrient components analyses. Rumen contents (total of 10 mL; 5 mL for microbial DNA analysis and 5 mL for VFA analysis) were collected at the end of trial (day 45). Rumen contents was collected (9:00 to 10:00 a.m.) with a stomach tube introduced via the mouth fitted with a small cylindrical metal strainer into 50 mL plastic bottles that were filled to capacity, covered immediately and stored at −80°C until analysis. All goats grazed experimental pastures continuously throughout the experimental period.
Laboratory Measurements
Chemical analyses were conducted on each sample in duplicate and reported on a DM basis. The DM, ash, ether extract, and minerals were analyzed according to the methods described by AOAC (1998). The TDN concentrations were calculated (Undersander et al., 1993) based on % NDF content for legume [TDN = 86.2 − (% NDF × 0.513)] and grass [105.2 − (0.667 × 64.5)]. For the combination pasture, TDN was calculated by each equation and then averaged. The composition of nitrogen (N) was measured using an organic elemental analyser (Flash 2000; CE Elantech Inc., Lakewood, NJ; AOAC, 1998). The crude protein (CP) was calculated as N × 6.25. The concentrations of neutral detergent fiber (NDF) was determined based on the procedure of AOAC (2016, method 202.04) using heat-stable α-amylase (Termamyl 120 L, type L, Novozymes A/S) and sodium sulphate, and acid detergent fiber (ADF) was determined based on AOAC (2016, method 973.18) with modifications to each procedure for use in an ANKOM Fiber Analyzer (ANKOM Technology Corp., Fairport, NY). For VFA analysis, only 4 random goats’ rumen fluids were analyzed in each treatment. The 5 mL of ruminal fluid was diluted with 1 mL of 3 M meta-phosphoric acids and VFA were determined using a GLC (model 5890 series II; Hewlet Packard Co., Palo Alto, CA) with a capillary column (30 m × 0.32 mm i.d., 1 μm phase thickness, Zebron ZB-FAAP, Phenomenex, Torrance, CA) and flame-ionization detection. The oven temperature was 170 °C held for 4 min, which was then elevated by 5 °C/min to 185 °C, and then by 3 °C/min to 220 °C, and held at this temperature for 1 min. The injector temperature was 225 °C, the detector temperature was 250 °C, and the carrier gas was helium (Eun and Beauchemin, 2007). Dietary Ca, P, Mg, K, and S concentrations were measured using a Flame atomic absorption spectrophotometer (GBC 908AA; Perkin-Elmer, Wellesley, MA) according to Solaiman et al. (2006).
DNA Extraction and bTEFAP Sequencing PCR
Microbial genomic DNA was purified from 1 mL of filtered (4 layers of cheese cloth) rumen samples according to the method described in the QIAamp DNA Mini Kit (QIAGEN Inc., Valencia, CA). The quality of DNA samples was measured using a Nanodrop spectrophotometer (Nyxor Biotech, Paris, France). For the rumen bacterial community analysis, 2 pooled samples (6 animals per sample) per treatment were created by combining equal amounts of isolated DNA samples (5 µL each) before amplifying DNA. This pooled DNA sample was investigated for bacterial diversity using a FLX bTEFAP sequencing PCR method. The bTEFAP technique was conducted at the Molecular Genomic Center, Lubbock, TX using primers covering the 530- to 1100-bp region of 16S universal Eubacterial primers, 530F (5′-GTG CCA GCMGCN GCG G) and 1100R (5′-GGG TTN CGN TCG TTG) for amplifying the 16S rRNA genes. Following PCR, all amplicon products from different samples were mixed in equal concentrations and purified using Agencourt Ampure beads (Agencourt Bioscience Corporation, MA). All DNA samples were adjusted to 100 ng/μL. A 100 ng (1 μL) aliquot of each sample DNA was used for a 50 μL PCR reaction. Samples were sequenced utilizing Roche 454 FLX titanium instruments and reagents and following manufacturer’s guidelines. In preparation for FLX bTEFAP sequencing (Roche, Nutley, NJ), the size and concentrations of the DNA fragments were measured using DNA chips and a Bio-Rad Experion Automated Electrophoresis Station. These values were confirmed using a TBS-380 Fluorometer (Turner Biosystems, Sunnyvale, CA; Hori et al., 2007). The FLX sequencing run was performed on a 70 × 75 GS Pico Titer Plate (PTP) using a Genome Sequencer FLX System (Roche, Nutley, NJ). Data quality control and analyses were directed as described by Dowd et al. (2008). HotStarTaq Plus Master Mix Kit (Qiagen, Valencia, CA) was used for PCR over the following conditions: 94 °C for 3 min followed by 32 cycles of 94 °C for 30 s; 60 °C for 40 s and 72 °C for 1 min; and a final elongation step at 72 °C for 5 min (Dowd et al., 2008).
Statistical Analyses
Data were analyzed by the GLM procedure of SAS (SAS Inst. Inc., Cary, NC) using the following statistical model:
where Yijk = observation; µ = overall mean for each parameter; Ti = effect of diet; Yj = effect of replicates; TYij = interaction between diet and replicates; and eij = random error, used to test diet, replicates, and diet × replicates interaction. Treatment means were separated by least significant differences when overall F-values were significant (P < 0.05). Main effect means for dietary treatments were reported in tables because there were no diet × replicates interactions (P > 0.10). Replications and animals were the experimental unit and were treated as a random effect. The variables included were forage biomass, VFA, ADG, and bacterial diversity analyses. The linear regression method was used to analyze the correlation among the percentages of bacterial groups, VFA and ADG. Significance levels were predetermined at P < 0.05; trends were determined at 0.05 < P < 0.10.
RESULTS AND DISCUSSION
Forage Diets, Rumen Fermentation, and Animal Growth Performance
Forage chemical composition is presented in Table 1. The level of CP, ADF, NFC, and some mineral concentrations (Ca and Mg) were greater (P < 0.05 to 0.01) for SH than for BG, and BG + SH group was intermediate. The NDF and TDN contents were greater (P < 0.01) for BG than for SH and BG + SH groups. Similarly, Min et al. (2016) reported when annual ryegrass (Lolium multiforum) was combined with legume forages including hairy vetch (Vicia villosa), berseem clover (Trifolium alexandrium), or Austrian pea (Pisum sativum), dietary CP content was increased compared to ryegrass alone. In agreement with our results, Hafley et al. (1987) and Fraser et al. (2004) reported greater CP content in legume-based forage diets than grasses. However, higher TDN contents in BG and BG + SH forages indicated that BG forage was also high-quality forage.
Table 1.
Nutrient composition (%) of bermudagrass (BG), sunn hemp (SH), and mixed forage BG + SH diets.
| Item | BG | SH | BG + SH | SEM | P-value |
|---|---|---|---|---|---|
| % DM | |||||
| Dry matter | 91.1 | 90.0 | 91.5 | 1.40 | 0.81 |
| Crude protein (CP) | 10.3c | 17.8a | 14.0b | 0.58 | 0.01 |
| Acid detergent fiber (ADF) | 37.6c | 52.0a | 44.3b | 1.17 | 0.01 |
| Neutral detergent fiber (NDF) | 64.5a | 53.1b | 56.1b | 2.21 | 0.05 |
| Nonfibrous carbohydrate (NFC) | 15.5b | 18.0a | 16.3ab | 0.45 | 0.05 |
| Total digestible nutrient1 (TDN) | 62.2 | 58.9 | 60.6 | 0.68 | – |
| Minerals | |||||
| Calcium (Ca) | 0.34b | 0.85a | 0.45ab | 0.029 | 0.01 |
| Phosphate (P) | 0.22b | 0.26ab | 0.28a | 0.018 | 0.05 |
| Magnesium (Mg) | 0.16c | 0.45a | 0.22b | 0.007 | 0.01 |
| Potassium (K) | 1.32 | 1.24 | 1.36 | 0.09 | 0.59 |
| Sulphur (S) | 0.18 | 0.16 | 0.14 | 0.016 | 0.45 |
a,bMeans within row with a different superscript differ at P < 0.05.
1The TDN concentrations were calculated (Undersander et al., 1993) based on % NDF content for legume [TDN = 86.2 − (% NDF × 0.513)] and grass [105.2 − (0.667 × 64.5)]. For the combination pasture, TDN was calculated by each equation and then averaged.
Animal growth performance and rumen VFA production are presented in Table 2. In the present study, treatments had similar initial BW, but final BW and ADG was higher (P < 0.01) for SH or BG + SH than for BG monoculture diet group. The enhanced ADG from grazing the legume SH forage or legume-grass mixtures may be attributed to improved nutritive value (Van Soest, 1982). Dry matter intake was greater when grass/legume mixed diets were fed to ruminants compared to grass alone, and the use of these mixtures generally resulted in an enhanced feed intake and particle break down (Van Soest, 1982, 1988; Dewhurst et al., 2003; Niderkorn and Baumont, 2009). Data stated enhanced ADG in goats fed legume forage SH or BG + SH mixed diets is consistent with other researchers (Wildeus et al., 2007; Min et al., 2016). Other studies reported that growing Spanish goats fed legume forage (e.g., alfalfa) diet had higher meat production (e.g., carcass yield and greater dressing percentage; Wuliji et al., 2003; Wildeus et al., 2007). The greater ADG observed in SH or BG + SH forage diets in this study was probably due to the higher CP intake (Hegarty et al., 2010; Min et al., 2016), but also a greater forage intake compared to grass-based diet (Niderkorn and Baumont, 2009). It should also be noted that grazing goats seem to select leaves from SH. The nutrient contents, especially CP and rumen digestibility would be higher in leaves than for plants cut to ground level in Table 1. Therefore, future grazing study in goats with SH forage needs to be further investigated to better calculate animal growth performance and forage intake associated with diet selection.
Table 2.
The effect of forage-based diets on animal performance, average daily gain (ADG), and molar % of volatile fatty acids (VFA) production in meat goats grazing bermudagrass (BG), sunn hemp (SH), or a mix BG + SH
| Forage treatments | |||||
|---|---|---|---|---|---|
| Item | BG | SH | BG + SH | SEM | P-value |
| Animal number | 12 | 12 | 12 | ||
| Animal performance1 | |||||
| Initial BW, kg | 26.8 | 28.9 | 27.6 | 3.49 | 0.56 |
| Final BW, kg | 31.3b | 35.9a | 32.6b | 3.49 | 0.05 |
| ADG, g/d | 98.7c | 156.3a | 118.8b | 25.25 | 0.01 |
| Rumen fermentation | |||||
| Animal number | 4 | 4 | 4 | – | – |
| Molar % | |||||
| Acetate | 73.0 | 60.0 | 69.0 | 9.50 | 0.01 |
| Propionate | 16.0 | 25.0 | 16.0 | 3.70 | 0.01 |
| Butyrate | 6.9 | 7.0 | 9.0 | 0.73 | 0.09 |
| Isobutyrate | 1.3b | 2.3a | 1.5b | 0.26 | 0.01 |
| Valerate | 1.1b | 3.1a | 1.8b | 0.34 | 0.01 |
| Isovalerate | 0.7b | 2.3a | 1.5ab | 0.26 | 0.01 |
| Total VFA, mM | 73.7b | 132.0a | 87.4b | 8.04 | 0.01 |
| A/P ratio2 | 4.6a | 2.4b | 4.4ab | 0.35 | 0.01 |
| NGR ratio3 | 5.0a | 2.7b | 4.9ab | 0.40 | 0.01 |
a,bMeans within row with a different superscript differ at P < 0.05.
1Animals grazed on SH, BG, or mix BG + SH forage for 45 d.
2A/P ratio = acetate/propionate ratio.
3NGR ratio = nonglucogenic (acetate + butyrate)/glucogenic (propionate) ratio.
The VFA, and large amount of metabolizable energy (ME) supplied by fermentation of carbohydrate in the rumen (Van Soest, 1982), can have a major impact on animal growth performance and qualities of meat and milk in ruminant animals (Hurtaud et al., 1993; Jami et al., 2014). Since most of the ME to ruminants is supplied by VFA produced by microbial fermentation in the rumen, the proportion of VFA and the efficiency with which they are used is also important. Propionate, a VFA produced from microbial carbohydrate digestion in ruminants, is a major hepatic glucogenic substrate, accounting for 65% to 80% of the net glucose (energy) supply in lactating dairy cows (Reynolds, 2003). In contrast to propionate, acetate and butyrate (nonglucogenic) do not contribute carbon atoms directly to the net synthesis of glucose (Engelking, 2015). In the present study, nonglucogenic to glucogenic ratio (NGR) was lower (P < 0.01) for SH than other treatments (Table 2). It appears that types of VFA produced might have a strong relationship with glucogenic substrate inputs and ADG as currently represented. Therefore, the feed assessment systems based on animal growth response might require an enhanced statement of rumen fermentation, focused on improving our understanding of VFA proportions produced by the diet treatments.
The VFA production is influenced by several factors such as DMI, forage chemical compositions, nutrient availability (Mizrahi, 2011; Saro et al., 2014; Liu et al., 2018), and microbial species present in the rumen (Hungate, 1966; Jung and Varel, 1988; Jami et al., 2014). In the present study, goats fed SH forage showed increased molar concentrations of acetate, propionate, valerate, butyrate, isobutyrate, and total VFA (P < 0.01 to 0.05), but A/P ratio was lower (P < 0.05) for SH than for BG treatment. Increased ruminal total VFA production and decreased A/P ratios as well as NGR ratios suggested that ruminal fermentation and feed efficiency improved with SH forage diet compared to BG or BG + SH forage diets.
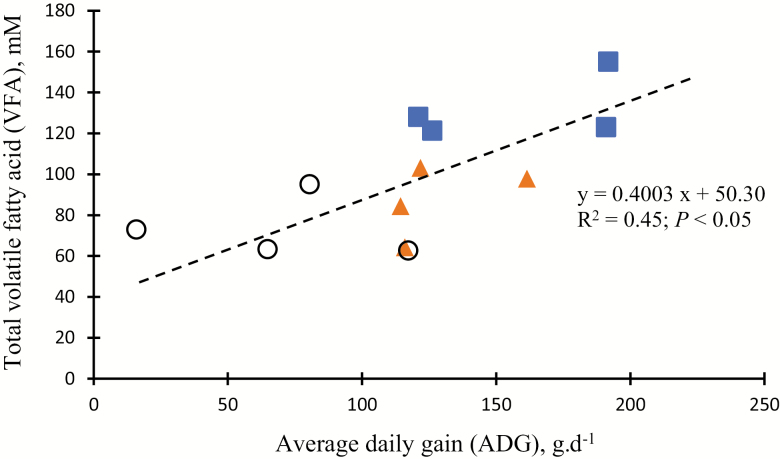
The relative concentrations of the VFA are often assumed to represent their relative rate of production. To further understand the effect of energy sources, as measured by total VFA on ADG, these values were regressed against ADG in meat goats in the present study (Fig. 1). Results indicated a positive correlation (R2 = 0.45; P < 0.05) for total VFA and ADG which is similar to other studies (Srinivas and Gupta, 1997; Packer et al., 2011). It has been shown that the higher ruminal total VFA production, the more ADG was achieved and presumably more energy was utilized for animal growth (van Nevel and Demeyer, 1996). Higher total VFA production rate was reported to be associated with better energy yield while changes in A/P ratio explained better efficiency of the energy use (Tamminga, 1979). However, this may be misleading if the individual VFA are absorbed at different rates. In the present study, total VFA levels were greater for both SH and BG + SH (132.0 and 87.4 mM, respectively) than for BG alone (73.7 mM) which partially explains why SH and BG + SH mixed forages had higher ADG compared to BG grass alone. Pasture analyses (Table 1) showed that forage samples with low NDF in SH forage had high CP. A high proportion of CP in SH could be expected to be highly rumen degradable, causing increased concentrations of rumen ammonia (Saro et al., 2014). Further studies on rumen fermentation dynamics of grazing goats could include measurement of rumen ammonia concentrations along with ruminal pH to balance fermentation of nitrogen and carbohydrates (Packer et al., 2011).
Figure 1.
Correlation between average daily gain (ADG; g/d) and the molar concentration of total volatile fatty acids (total VFA, mM) in meat goats grazed on bermudagrass (BG; ○), sunn hemp (SH; ■), or combination (BG + SH; ▲).
Predominant Microbial Community and Bacterial Phylum Changes
More than 33 bacterial species (excluding unknown bacteria; cutoff = >0.1%) were classified from the ruminal contents of the goats in this study. However, only the relative abundances of the 16 most abundant bacterial species (>1.0%) are presented in Table 3. The populations of Clostridium spp. (12.7%), Ruminococcus flavefaciens (3.8%), and Oscillospira spp. (4.2%) were relatively the most abundant species with the BG-based diet. In contrast, populations of Prevotella ruminicola (8.6%) and Quinella ovalis (6.25%) tended to be increased (P = 0.09) in SH diet (Table 3), indicating that these microbial populations may be dependent upon the increased CP levels in the SH diet compared to BG diet supporting the growth of proteolytic bacterial species (Pitta et al., 2010; Grilli et al., 2016).
Table 3.
Rumen bacterial population diversity (%) in meat goats grazing bermudagrass (BG), sunn hemp (SH), or mixed BG + SH1
| Forage treatments | ||||||
|---|---|---|---|---|---|---|
| Item | BG | SH | BG + SH | SEM | P-value | Bacterial phylum2 |
| Fibrolytic bacteria | ||||||
| Ruminococcus spp. | 6.18 | 5.94 | 6.96 | 2.06 | 0.24 | Firmicutes |
| Fibrobacter succinogenes | 2.84 | 1.85 | 2.05 | 1.51 | 0.59 | Fibrobacteres |
| Ruminoccoccus flavefaciens | 3.80a | 1.46b | 3.70a | 1.03 | 0.05 | Firmicutes |
| Proteolytic bacteria | ||||||
| Prevotella ruminicola | 7.67 | 8.58 | 7.11 | 1.94 | 0.09 | Bacteroidetes |
| Prevotella spp. | 2.93 | 2.81 | 4.52 | 1.70 | 0.08 | Bacteroidetes |
| Anaeroplasma abactoclasticum | 0.97 | 2.05 | 0.98 | 0.104 | 0.14 | Tenericutes |
| Amylolytic bacteria | ||||||
| Clostridium spp. | 12.73 | 6.60 | 7.88 | 2.99 | 0.09 | Firmicutes |
| Eubacterium spp. | 2.86 | 3.19 | 4.13 | 0.79 | 0.12 | Firmicutes |
| Selenomonas ruminantium | 0.19 | 0.35 | 1.08 | 0.31 | 0.17 | Firmicutes |
| Bacteroides spp. | 1.35 | 1.41 | 7.31 | 0.59 | 0.32 | Bacteroidetes |
| Fretibacterium fastidiosum | 8.47 | 4.10 | 7.69 | 5.49 | 0.22 | Synergistetes3 |
| Oscillospira spp. | 4.16 | 1.92 | 1.36 | 1.05 | 0.09 | Firmicutes4 |
| Quinella ovalis | 3.83 | 6.25 | 2.49 | 1.05 | 0.07 | Firmicutes5 |
| Methanogens | ||||||
| Methanobrevibactor spp. | 1.25 | 1.20 | 0.34 | 0.015 | 0.80 | Euryarchaeota |
| Others | ||||||
| Solitalea canadensis | 3.85 | 1.96 | 0.77 | 0.74 | 0.13 | Bacteroidetes6 |
| Sphingobacterium spp. | 1.89 | 3.61 | 3.30 | 0.56 | 0.14 | Bacteroidetes7 |
a,bMeans within row with a different superscript differ at P < 0.05.
1Animals grazed SH, BG, or BG + SH forage for 45 d. More than 33 bacterial species (excluding unknown bacteria; cutoff = >0.1%) were classified from the ruminal contents of the goats in this study. However, only the relative abundances of the 16 most abundant bacterial species (>1.0%) are presented in this table.
6 Weon et al. (2009 ).
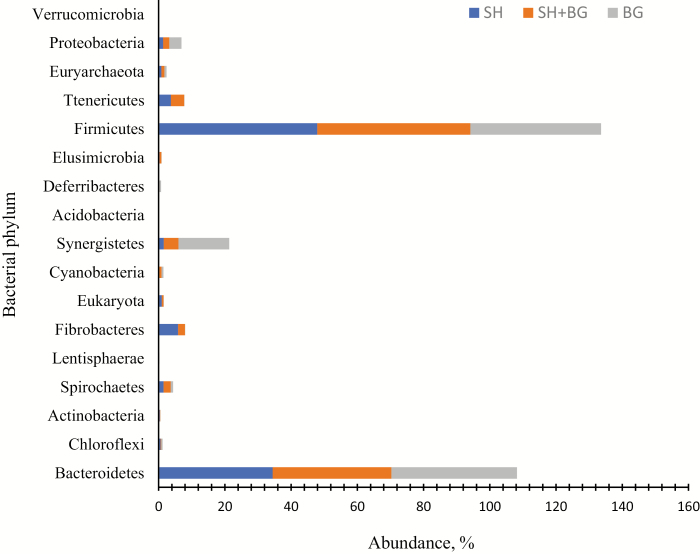
Our goal in this study was to determine whether there were any relationships between the bacterial communities inhabiting the rumen of host animals. Generally, 17 phyla were identified (Fig. 2), but only 5 were recognized in meat goats above the 1.0% threshold (Table 4). Among the major 5 bacterial phyla, the populations of Fibrobacteres (P < 0.05) and Firmicutes (P = 0.09) as well as DNA concentration (P < 0.05) were greater in animals fed SH forage diet than those consuming BG diet. Both Firmicutes (45% to 48%) and Bacteroidetes (34% to 38%) were the main bacterial phyla in the rumen of goats across the forage diets, as demonstrated in previous studies (Myer et al., 2015; Nardi et al., 2016). Even though Bacteroidetes have been found to be more plentiful in the rumen of Holstein dairy cows fed 30% roughage plus 70% concentrate diets (Jami et al., 2014), the current study showed that the rumen of meat goats exhibited a greater proportion of Firmicutes (Table 4) compared to Bacteroidetes across the forage diets. This agrees with data of Fernando et al. (2010), who reported a considerably larger number of the Firmicutes population was detected in animals fed grass hay-based (prairie hay) diet in cannulated beef steers. Likewise, de Menezes et al. (2011) fed pasture-based diet to cannulated steers and found a significant increase in Firmicutes populations compared to total mixed ration (TMR)-based diets. Results from the present experiment indicated that the 2 dominant phyla observed, in agreement with other studies of mammalian gut microbiota, were Bacteroidetes and Firmicutes, as previously described (Backhed et al., 2004; Schloss et al., 2009; Shanks et al., 2011; Min et al., 2014a, 2014b). However, the bacterial community composition was also associated with diets, gender, and time (Zhang et al., 2014; Paz et al., 2018).
Figure 2.
Predominant bacterial phyla observed in rumen samples of goats across the forage diets based on pyrosequencing of the 16S rDNA. Animals grazed sunn hemp (SH), bermudagrass (BG), or combinations (SH + BG) for 45 d. Overall, 17 phyla were detected, but only 5 were found in all meat goats as dominant phyla (cutoff: >1.0%; Table 3).
Table 4.
Predominant bacterial phylum observed in rumen samples of healthy meat goats grazing bermudagrass (BG), sunn hemp (SH), or mixed BG + SH forage diets based on pyrosequencing of the 16S rDNA1
| Forage treatments | |||||
|---|---|---|---|---|---|
| Item1 | BG | SH | BG + SH | SEM | P-value |
| Bacterial phylum, % | |||||
| Bacteroidetes (B) | 37.9 | 34.4 | 35.9 | 3.25 | 0.49 |
| Fibrobacteres | 0.08b | 5.9a | 2.1ab | 1.32 | 0.05 |
| Firmicutes (F) | 39.5 | 47.9 | 46.2 | 2.52 | 0.09 |
| Proteobacteria | 3.7 | 1.4 | 1.8 | 0.81 | 0.14 |
| Tenericutes | 0.08 | 3.7 | 4.0 | 1.86 | 0.26 |
| F/B ratio2 | 1.1 | 1.4 | 1.3 | 0.18 | 0.37 |
| DNA concentration3, ng/µL | 63.5 b | 73.9a | 73.6a | 6.23 | 0.05 |
a,bMeans within row with a different superscript differ at P < 0.05.
1Animals grazed sunn hemp (SH), bermudagrass (BG), and/or BG + SH forage for 45 d. Overall, 17 phyla were detected (Fig. 1), but only 5 were found in all meat goats as dominant phyla (cutoff: >1.0%).
2Firmicutes/Bacteroides ratio = F/B ratio.
3DNA concentration was in the extracted microbial DNA before normalization for amplicon sequencing.
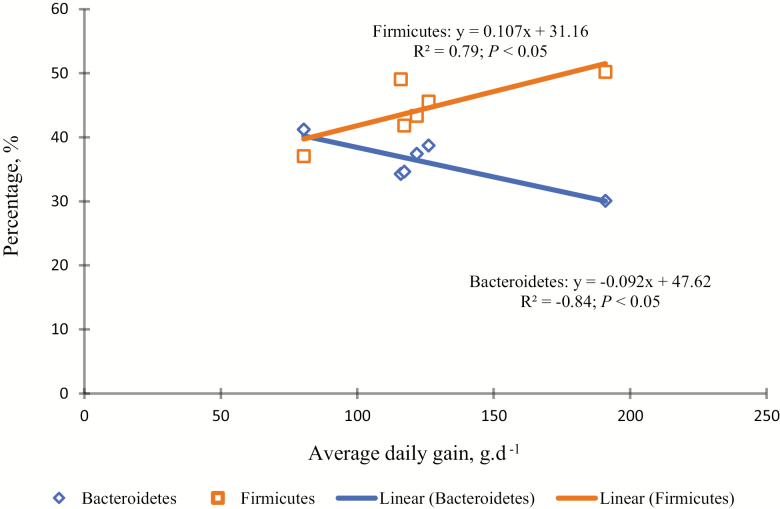
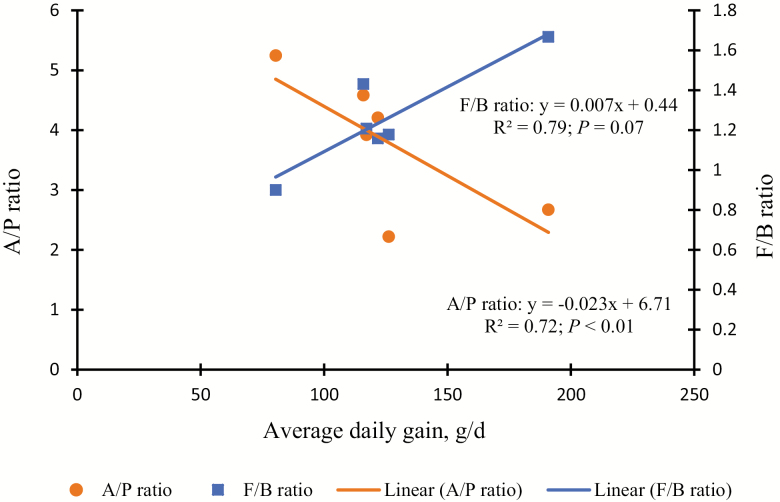
The alteration in the rumen microbiota community due to a modification in diet is of remarkable attention, as it increases the energy supply within the rumen and improves feed efficiency and ADG (Black et al., 1987; Belanche et al., 2012; Zhang et al., 2014). We assessed whether meat goat physiological parameters, such as ADG, interrelated with a modification in Firmicutes and F/B ratio. The Firmicutes population (Fig. 3; R2 = 0.79; P < 0.05) and F/B ratio (Fig. 4; R2 = −0.72; P < 0.01) were strongly correlated with ADG within forage-based diets. This is the first report that associated increased ADG with higher ruminal F/B ratio in meat goats fed forage-based diets. It has been reported that deviations in higher F/B ratio were found to be strongly correlated (Pearson R2 = 0.72, P = 2 × 10−3) with daily milk-fat yield (Jami et al., 2014). As reported previously, escalations in the abundance of Firmicutes, and shifting the F/B ratio, have been shown to influence ADG, in many of the families (e.g., Lachnospiraceae and Veillonellaceae) and genera (e.g., Acidaminococcus, Dialister, and Anaerovibrio) belong to Firmicutes and were correlated with high ADG (Myer et al., 2015; Paz et al., 2018). This suggests that the abundance of certain bacterial phylum might affect feed efficiency and ADG in meat goats rather than overall modifications in the microbiome community taxa (Paz et al., 2018).
Figure 3.
Correlation between average daily gain (ADG) and the percentages of Bacteroidetes (◊) and Firmicutes ( □ ) populations (P < 0.05) in meat goats grazed on sunn hemp (SH), bermudagrass (BG), or combination (SH + BG). For the rumen bacterial community analysis, 2 pooled DNA samples per treatment were created by combining equal amounts of isolated DNA samples (5 µL each). This pooled sample was analyzed for bacterial diversity using a bTEFAP sequencing PCR method.
Figure 4.
Correlation between ADG and Firmicutes/Bacteroidetes (F/B; ■ ) ratio, and acetate/propionate (A/P; ● ) ratios (P < 0.001 and P = 0.07, respectively) in meat goats grazed on sunn hemp (SH), bermudagrass (BG), or combination (SH + BG). For the rumen bacterial community analysis, 2 pooled DNA samples per treatment were created by combining equal amounts of isolated DNA samples (5 µL each). This pooled sample was analyzed for bacterial diversity using a bTEFAP sequencing PCR method.
An earlier study with steers fed diets containing vegetative stages of fresh wheat, showed an abundance of Firmicutes (Pitta et al., 2010), compared to reproductive stages of plant growth. High-quality forage diets or high levels of forage ratio in the diets generally enhanced the comparative richness of Firmicutes relative to Bacteroidetes (Fernando et al., 2010; Petri et al., 2013; Weimer et al., 2017) which supports the results of present study. However, these findings did not always reveal similar trends of rumen bacterial phylum of feedlot steers (Callaway et al., 2010) and dairy cattle (Jami et al., 2014; Zhang et al., 2014) compared to forage-based diets in meat goats (Min et al., 2014a, 2014b) and nonlactating cows (de Menezes et al., 2011). The Firmicutes can utilize carbohydrates such as xylan, cellulose, hemicellulose, and galactomannan as energy source (Morrison and Miron, 2000; Dassa et al., 2014). In contrast, the member of Bacteroidetes are able to utilize starch, xylan, pectin, galactomannan, and arabinogalactan (Martens et al., 2011).
The great abundance of Firmicutes within the rumen suggests that these shifts may play a role in affecting feed efficiency (Turnbaugh et al., 2006). In addition, a genera Anaerovibrio belonging to Firmicutes has been associated with succinate and propionate production, as well as lipid hydrolysis and metabolism in ruminants (Prive et al., 2013). Acidaminococcus are also associated with amino acids-fermenting bacteria and butyrate-producing bacteria (Cook et al., 1994), which was highly associated with changes in feed efficiency (Myer et al., 2015). Enhanced propionate production was related to decreased A/P ratio and confirmed that SH forage diet tended to affect the abundance of proteolytic bacterial populations such as P. ruminicola and Q. ovalis (P = 0.07 to 0.09), while lower in cellulolytic bacteria, especially R. flavefaciens (P < 0.05; Table 3). These results were consistent with the previous studies reported in dairy cows fed alfalfa forage diet compared to cornstalk or grass diets (Zhang et al., 2014; Grilli et al., 2016) and mixed forage/concentrate diets in beef steers (Fernando et al., 2010). Zhang et al. (2014) reported that alfalfa-based forage diet in dairy cows increased the proportion of genera Prevotella and Sellenomonas compared with the cornstalk-based diet.
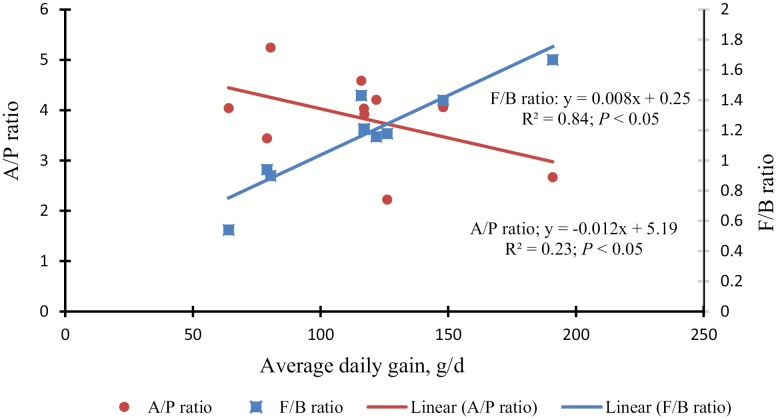
To further understand the effect of energy sources, as measured by A/P and F/B ratio on ADG, these values were regressed against ADG in meat goats in the present study (Fig. 4) and for other studies (Fig. 5; Min et al., 2014b, 2015; Wright et al., 2016). We found that there was a negative correlation (R2 = 0.72; P < 0.001) for F/B ratio and a positive correlation (R2 = 0.88; P = 0.07) for A/P ratio (Fig. 4) which is similar with other comparable studies (Fig. 5). It has been shown that as ruminal VFA production changes toward more propionate at the cost of acetate (i.e., a lower A/P ratio), more ADG was achieved and presumably more energy (Table 2) was utilized for animal growth (van Nevel and Demeyer, 1996).
Figure 5.
Correlation between average daily gain (ADG) and Firmicutes/Bacteroidetes (F/B; ■ ) ratio, and acetate/propionate (A/P; ● ) ratios (P < 0.05) in meat goats fed various forage diets (n = 10). For the rumen bacterial community analysis, 2 pooled DNA samples per treatment were created by combining equal amounts of isolated DNA samples (5 µL each). This pooled sample was analyzed for bacterial diversity using a bTEFAP sequencing PCR method. Data obtained from Min et al. (2014b, 2015), Wright et al. (2016), and current study.
In a mathematic modeling analysis, Black et al. (1987) reported that the ME efficiency was very low in ruminants fed low-quality forage diets. This resulted in high A/P ratio, because of insufficient nicotinamide‐adenine dinucleotide phosphate dehydrogenase (NADPH2) being generated from glucose metabolism to admit all the acetate to be incorporated into body lipid. Min et al. (2005) reported that the average milk production of dairy goats was 22% greater for a high-energy concentrate diet than for lower energy diets. This agrees with data of Liu et al. (2018), where VFA supplementation to young Holstein calves (10 mo. of age), especially branched chain VFA, promoted total VFA production (14%), feed conversion rate (12%), and ADG (15%) which stimulated hepatic lipid oxidation. One explanation for the higher ADG in the current study may be related to improved rumen fermentation (VFA, A/P ratio) and increased F/B bacterial community structure in SH and SH + BG diets. Lower A/P ratios and higher the phylum Firmicutes populations related to higher ADG (Waghorn and Barry, 1987; Myer et al., 2015).
The current results showed trends of increased body weight gain with an increase in Firmicutes with the 3 different forage diets. Human studies have also observed increases in body weight associated with Firmicutes species.
ACKNOWLEDGMENTS
This project was supported by the Southern Sustainable Agricultural Research and Education (S-SARE) grant (OS14-088Min).
Footnotes
The mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA.
LITERATURE CITED
- AOAC 1998. Association of official methods of analysis. 16th ed. Off. Anal. Chem. Int., Gaithersburg, MD. [Google Scholar]
- AOAC 2016. Official methods of analysis. 20th ed. Ass. Off. Anal. Chem. Int., Gaithersburg, MD. [Google Scholar]
- Backhed F., Ding H., Wang T., Hooper L. V., Koh G. Y., Nagy A. A., Semenkovich C. F., and Gordon J. Y.. 2004. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 101:15718–15723. doi:10.1073/pnas.0407076101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanche A., Doreau M., Edwards J. E., Moorby J. M., Pinloche E., and Newbold C. J.. 2012. Shifts in the rumen microbiota due to the type of carbohydrate and level of protein ingested by dairy cattle are associated with changes in rumen fermentation. J. Nutr. 142:1684–1692. doi: 10.3945/jn.112.159574 [DOI] [PubMed] [Google Scholar]
- Bergman E. N. 1990. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 70:567–590. doi: 10.1152/physrev.1990.70.2.567 [DOI] [PubMed] [Google Scholar]
- Black J. L., Gill M., Beever D. E., Thornley J. H., and Oldham J. D.. 1987. Simulation of the metabolism of absorbed energy-yielding nutrients in young sheep: efficiency of utilization of acetate. J. Nutr. 117:105–115. doi: 10.1093/jn/117.1.105 [DOI] [PubMed] [Google Scholar]
- Callaway T. R., Dowd S. E., Edrington T. S., Anderson R. C., Krueger N., Bauer N., Kononoff P. J., and Nisbet D. J.. 2010. Evaluation of bacterial diversity in the rumen and feces of cattle fed different levels of dried distillers grains plus solubles using bacterial tag-encoded FLX amplicon pyrosequencing. J. Anim. Sci. 88:3977–3983. doi: 10.2527/jas.2010-2900 [DOI] [PubMed] [Google Scholar]
- Cook G. M., Rainey F. A., Chen G., Stackebrandt E., and Russell J. B.. 1994. Emendation of the description of Acidaminococcus fermentans, a trans-aconitate- and citrate-oxidizing bacterium. Int. J. Syst. Bacteriol. 44:576–578. doi: 10.1099/00207713-44-3-576 [DOI] [PubMed] [Google Scholar]
- Dassa B., Borovok I., Ruimy-Israeli V., Lamed R., Flint H. J., Duncan S. H., Henrissat B., Coutinho P., Morrison M., Mosoni P.,. et al. 2014. Rumen cellulosomics: divergent fiber-degrading strategies revealed by comparative genome-wide analysis of six ruminococcal strains. PLoS One 9:e99221. doi: 10.1371/journal.pone.0099221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Menezes A. B., Lewis E., O’Donovan M., O’Neill B. F., Clipson N., and Doyle E. M.. 2011. Microbiome analysis of dairy cows fed pasture or total mixed ration diets. FEMS Microbiol. Ecol. 78:256–265. doi: 10.1111/j.1574-6941.2011.01151.x [DOI] [PubMed] [Google Scholar]
- Dewhurst R. J., Evans R. T., Scollan N. D., Moorby J. M., Merry R. J., and Wilkins R. J.. 2003. Comparison of grass and legume silages for milk production. 2. In vivo and in sacco evaluations of rumen function. J. Dairy Sci. 86:2612–2621. doi: 10.3168/jds.S0022-0302(03)73856-9 [DOI] [PubMed] [Google Scholar]
- Dewhurst R. J., Wadhwa D., Borgida L. P., and Fisher W. J.. 2001. Rumen acid production from dairy feeds. 1. Effects on feed intake and milk production of dairy cows offered grass or corn silages. J. Dairy Sci. 84:2721–2729. doi: 10.3168/jds.S0022-0302(01)74726-1 [DOI] [PubMed] [Google Scholar]
- Dowd S. E., Callaway T. R., Sun Y., McKeehan T., and Edrington T. S.. 2008. Evaluation of the bacterial diversity in the feces of cattle using bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP). BMC Micro. 8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelking L. R. 2015. Gluconeogenesis. In: Engelkig L. R., editor, Textbook of veterinary physiological chemistry. 3rd ed. Academic Press, MA: p. 225–230. [Google Scholar]
- Eun J. S., and Beauchemin K. A.. 2007. Assessment of the efficacy of varying experimental exogenous fibrolytic enzymes using in vitro fermentation characteristics. Anim. Feed Sci. Technol. 132:298–315. doi:10.1016/j.anifeedsci.2006.02.014 [Google Scholar]
- Fernando S. C., Purvis H. T., Najar F. Z., Sukharnikov L. O., Krehbiel C. R., Nagaraja T. G., Roe B. A., and Desilva U.. 2010. Rumen microbial population dynamics during adaptation to a high-grain diet. Appl. Environ. Microbiol. 76:7482–7490. doi: 10.1128/AEM.00388-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J., McCartney D., Najda H., and Mir Z.. 2004. Yield potential and forage quality of annual forage legumes in South Alberta and Northeast Saskatchewan. Can. J. Plant Sci. 91:811–824. doi:10.4141/cjps10177 [Google Scholar]
- Grilli D. J., Fliegerová K., Kopečný J., Lama S. P., Egea V., Sohaefer N., Pereyra C., Ruiz M. S., Sosa M. A., Arenas G. N.,. et al. 2016. Analysis of the rumen bacterial diversity of goats during shift from forage to concentrate diet. Anaerobe 42:17–26. doi: 10.1016/j.anaerobe.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Guo X., Xia X., Tang R., Zhou J., Zhao H., and Wang K.. 2008. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett. Appl. Microbiol. 47:367–373. doi: 10.1111/j.1472-765X.2008.02408.x [DOI] [PubMed] [Google Scholar]
- Hafley J., Nipper W. A., Craig W. M., Adkinson R. W., and Achacoso A. S.. 1987. Effect of growth and fertilization on crude protein degradation and in vitro protein degradation of cool season annual forages. J. Dairy Sci. 70:2550–2559. doi:10.3168/jds.S0022-0302(87)80323-5 [Google Scholar]
- Harris S. L., Auldist M. J., Clark D. A., and Jansen E. B.. 1998. Effects of white clover content in the diet on herbage intake, milk production and milk composition of New Zealand dairy cows housed indoors. J. Dairy Res. 65:389–400. doi:10.1017/S0022029998002969 [DOI] [PubMed] [Google Scholar]
- Hegarty R. S., Alcock D., Robinson D. L., Goopy J. P., and Vercoe P. E.. 2010. Nutritional and flock management options to reduce methane output and methane per unit product from sheep enterprise. Anim. Prod. Sci. 50:1026–1033. doi:10.1071/AN10104 [Google Scholar]
- Hori M., Fukano H., and Suzuki Y.. 2007. Uniform amplification of multiple DNAs by emulsion PCR. Biochem. Biophys. Res. Commun. 352:323–328. doi: 10.1016/j.bbrc.2006.11.037 [DOI] [PubMed] [Google Scholar]
- Hungate R. E. 1966. The rumen and its microbes. Academic Press, Inc., New York, NY. ISBN: 9781483263625. [Google Scholar]
- Hurtaud C., Rulquin H., and Verite R.. 1993. Effect of infused volatile fatty acids and caseinate on milk composition and coagulation in dairy cows. J. Dairy Sci. 76:3011–3020. doi: 10.3168/jds.S0022-0302(93)77640-7 [DOI] [PubMed] [Google Scholar]
- Jami E., White B. A., and Mizrahi I.. 2014. Potential role of the bovine rumen microbiome in modulating milk composition and feed efficiency. PLoS One 9:1–6. doi:10.1371/journal.pone.0085423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H. G., and Varel V. H.. 1988. Influence of forage type on ruminal bacterial populations and subsequent in vitro fiber digestion. J. Dairy Sci. 71:1526–1535. doi: 10.3168/jds.S0022-0302(88)79716-7 [DOI] [PubMed] [Google Scholar]
- Krumholz L. R., Bryant M. P., Brulla W. J., Vicini J. L., Clark J. H., and Stahl D. A.. 1993. Proposal of Quinella ovalis gen. nov., sp. nov., based on phylogenetic analysis. Int. J. Syst. Bacteriol. 43:293–296. doi: 10.1099/00207713-43-2-293 [DOI] [PubMed] [Google Scholar]
- Ley R. E., Bäckhed F., Turnbaugh P., Lozupone C. A., Knight R. D., and Gordon J. I.. 2005. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 102:11070–11075. doi: 10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R. E., Peterson D. A., and Gordon J. I.. 2006. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124:837–848. doi: 10.1016/j.cell.2006.02.017 [DOI] [PubMed] [Google Scholar]
- Liu Q., Wang C., Guo G., Huo W. J., Zhang Y. L., Pei C. X., Zhang S. I., and Wang H.. 2018. Effects of branched-chain volatile fatty acids supplementation on growth performance, ruminal fermentation, nutrient digestibility, hepatic lipid content and gene expression of dairy calves. Anim. Feed Sci. Technol. 237:27–34. doi:10.1016/j.anifeedsci.2018.01.006 [Google Scholar]
- Mackie R. I., Aminov R. I., Hu W., Klieve A. V., Ouwerkerk D., Sundset M. A., and Kamagata Y.. 2003. Ecology of uncultivated oscillospira species in the rumen of cattle, sheep, and reindeer as assessed by microscopy and molecular approaches. Appl. Environ. Microbiol. 69:6808–6815. doi: 10.1128/aem.69.11.6808-6815.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens E. C., Lowe E. C., Chiang H., Pudlo N. A., Wu M., McNulty N. P., Abbott D. W., Henrissat B., Gilbert H. J., Bolam D. N.,. et al. 2011. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 9:e1001221. doi: 10.1371/journal.pbio.1001221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B. R., Hart S. P., Sahlu T., and Satter L. D.. 2005. The effect of diets on milk production and composition, and on lactation curves in pastured dairy goats. J. Dairy Sci. 88:2604–2615. doi: 10.3168/jds.S0022-0302(05)72937-4 [DOI] [PubMed] [Google Scholar]
- Min B. R., Perkins D., Wright C., Dawod A., Min B. J., Terrill T. H., Eun J. S., Shange R., Yang S. Y., and Gurung N.. 2015. Effects of two different tannin-containing diets on ruminal fermentation profiles and microbial community changes in meat goats. Agric. Food Anal. Bact. 5:153–165. [Google Scholar]
- Min B. R., Solaiman S., Gurung N., and McElhenney W.. 2016. The effect of forage-based meat goat production systems on live performance, carcass traits and fatty acid composition of Kiko crossbred goats. J. Anim. Res. Nutri. 1:1–11. doi:10.21767/2572-5459.100006 [Google Scholar]
- Min B. R., Solaiman S., Shange R., and Eun J. S.. 2014a. Gastrointestinal bacterial and methanogenic Archaea diversity dynamics associated with condensed tannins-containing pine bark diet in goats using 16S rDNA amplicon pyrosequencing. Int. J. Micro. 2014:1–11; Article ID 141909. doi: 10.1155/2014/141909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B. R., Wright C., Ho P., Eun J. S., Gurung N., and Shang R.. 2014b. The effect of source of plant tannins on rumen fermentation characteristics and microbial diversity dynamics in goats using 16S rDNA amplicon pyrosequencing. Agric. Food Anal. Bact. 4:195–211. [Google Scholar]
- Mizrahi I. 2011. The role of the rumen microbiota in determining the feed efficiency of dairy cows. In: Rosenberg E. and Gophna U., editors, Beneficial microorganisms in multicellular life forms. Springer Berlin Heidelberg. [Google Scholar]
- Morrison M., and Miron J.. 2000. Adhesion to cellulose by Ruminococcus albus: a combination of cellulosomes and pil-proteins? FEMS Microbiol. Lett. 185:109–115. doi: 10.1111/j.1574-6968.2000.tb09047.x [DOI] [PubMed] [Google Scholar]
- Myer P. R., Smith T. P., Wells J. E., Kuehn L. A., and Freetly H. C.. 2015. Rumen microbiome from steers differing in feed efficiency. PLoS One 10:e0129174. doi: 10.1371/journal.pone.0129174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardi R. D., Marchesin G. M., Li S., Khafipour E., Plaizier K. J. C., Gianesella M., Ricci R., Andrighetto I., and Segato S.. 2016. Metagenomic analysis of rumen microbial population in dairy heifers fed a high grain diet supplemented with dicarboxylic acids or polyphenols. CMC Vet. Res. 12:1–9. doi:10.1186%2Fs12917-016-0653-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niderkorn V., and Baumont R.. 2009. Associative effects between forages on feed intake and digestion in ruminants. Animal 3:951–960. doi: 10.1017/S1751731109004261 [DOI] [PubMed] [Google Scholar]
- Packer E. L., Clayton E. H., and Cusack P. M.. 2011. Rumen fermentation and liveweight gain in beef cattle treated with monensin and grazing lush forage. Aust. Vet. J. 89:338–345. doi: 10.1111/j.1751-0813.2011.00802.x [DOI] [PubMed] [Google Scholar]
- Paz H. A., Hales K. E., Wells J. E., Kuehn L. A., Freetly H. C., Berry E. D., Flythe M. D., Spangler M. L., and Fernando S. C.. 2018. Rumen bacterial community structure impacts feed efficiency in beef cattle. J. Anim. Sci. 96:1045–1058. doi: 10.1093/jas/skx081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri R. M., Schwaiger T., Penner G. B., Beauchemin K. A., Forster R. J., McKinnon J. J., and McAllister T. A.. 2013. Characterization of the core rumen microbiome in cattle during transition from forage to concentrate as well as during and after an acidotic challenge. PLoS One 8:e83424. doi: 10.1371/journal.pone.0083424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitta D. W., Pinchak E., Dowd S. E., Osterstock J., Gontcharova V., Youn E., Dorton K., Yoon I., Min B. R., Fulford J. D.,. et al. 2010. Rumen bacterial diversity dynamics associated with changing from bermudagrass hay to grazed winter wheat diets. Microb. Ecol. 59:511–522. doi: 10.1007/s00248-009-9609-6 [DOI] [PubMed] [Google Scholar]
- Prive F., Kaderbhai N. N., Girdwood S., Worgan H. J., Pinloche E., Scollan N. D., Huws S. A., and Newbold C. J.. 2013. Identification and characterization of three novel lipases belonging to families II and V from Anaerovibrio lipolyticus 5ST. PLoS One 8:e69076. doi: 10.1371/journal.pone.0069076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C. K., Aikman P. C., Lupoli B., Humphries D. J., and Beever D. E.. 2003. Splanchnic metabolism of dairy cows during the transition from late gestation through early lactation. J. Dairy Sci. 86:1201–1217. doi: 10.3168/jds.S0022-0302(03)73704-7 [DOI] [PubMed] [Google Scholar]
- Saro C., Ranilla M. J., Tejido M. L., and Carro M. D.. 2014. Influence of forage type in the diet of sheep on rumen microbiota and fermentation characteristics. Live. Sci. 160:52–59. doi:10.1016/j.livsci.2013.12.005 [Google Scholar]
- Schloss P. D., Westcott S. L., Ryabin T., Hall J. R., Hartmann M., Hollister E. B., Lesniewski R. A., Oakley B. B., Parks D. H., Robinson C. J.,. et al. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75:7537–7541. doi: 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks O. C., Kelty C. A., Archibeque S., Jenkins M., Newton R. J., McLellan S. L., Huse S. M., and Sogin M. L.. 2011. Community structures of fecal bacteria in cattle from different animal feeding operations. Appl. Environ. Microbiol. 77:2992–3001. doi: 10.1128/AEM.02988-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaiman S. G., Shoemaker C. E., and D’Andrea G. H.. 2006. The effect of high dietary Cu on health, growth performance, and Cu status in young goats. Small Rum. Res. 66: 85–91. doi:10.1016/j.smallrumres.2005.07.0 [Google Scholar]
- Srinivas B., and Gupta B. N.. 1997. Rumen fermentation, bacterial and total volatile fatty acid (TVFA) production rates in cattle fed on urea-molasses-mineral block licks supplement. Anim. Feed Sci. Technol. 65:275–286. doi:10.1016/S0377-8401(96)01062-0 [Google Scholar]
- Tamminga S. 1979. Relation between different carbohydrate and microbial synthesis of protein. Report No. 130 Inst. Livestock Feeding and Nutri., Leystad: p. 31–50. [Google Scholar]
- Turnbaugh P. J., Ley R. E., Mahowald M. A., Magrini V., Mardis E. R., and Gordon J. I.. 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444:1027–1031. doi: 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- Undersander D., Mertens D. R., and Thiex N.. 1993. Forage analysis procedures National Forage Testing Association, Omaha, NE: http://www.foragetesting.org/files/Laboratory Procedures.pdf (Accessed 16 November 2018). [Google Scholar]
- USDA 1985. Animal Welfare. 9 CFR 1A. (Title 9, Chapter 1, Subchapter A): Washington, DC http://ecfr.gpoaccess.gov/cgi/t/text/text-idx?sid8314313bd7adf2c9f1964e2d82a88d92andc=ecfrandtpl=/ecfrbrowseTitle09/9cfrv1_02.tpl (Accessed 14 January 2017).
- Van Soest P. J. 1982. Nutritional ecology of the ruminant. O & B Books Inc., Corvallis, OR. [Google Scholar]
- Van Soest P. J. 1988. Fibre in the diet. In: Blaxter K. and Macdonald I., editors, Comparative nutrition. Libby, New York, NY: p. 215–225. [Google Scholar]
- Van Nevel C. J., and Demeyer D. I.. 1996. Control of rumen methanogenesis. Environ. Monit. Assess. 42:73–97. doi: 10.1007/BF00394043 [DOI] [PubMed] [Google Scholar]
- Vartoukian S. R., Downes J., Palmer R. M., and Wade W. G.. 2013. Fretibacterium fastidiosum gen. nov., sp. nov., isolated from the human oral cavity. Int. J. Sys. Evo. Microbiol. 63:458–63. doi:10.1099/ijs.0.041038-0. PMID 2249317 [DOI] [PubMed] [Google Scholar]
- Waghorn G. C., and Barry T. N.. 1987. Pasture as a nutrient source. In: Nicol A. M., editor, Feeding livestock on pasture. Soc. Anim. Prod. Occ. Publ. 10; p. 21–37. [Google Scholar]
- Weimer P. J., Cox M. S., Vieira de Paula T., Lin M., Hall M. B., and Suen G.. 2017. Transient changes in milk production efficiency and bacterial community composition resulting from near-total exchange of ruminal contents between high- and low-efficiency Holstein cows. J. Dairy Sci. 100:7165–7182. doi: 10.3168/jds.2017-12746 [DOI] [PubMed] [Google Scholar]
- Weon H. Y., Kim B. Y., Lee C. M., Hong S. B., Jeon Y. A., Koo B. S., and Kwon S. W.. 2009. Solitalea koreensis gen. nov., sp. nov. and the reclassification of [Flexibacter] canadensis as Solitalea canadensis comb. nov. Int. J. Sys. Evo. Microbiol. 59:1969–1975. doi:10.1099/ijs.0.007278-0. PMID 956756 [DOI] [PubMed] [Google Scholar]
- Wildeus S., Luginbuhl J. M., Turner K. E., Nutall Y. L., and Collins J. R.. 2007. Growth and carcass characteristics in goat kinds fed graded- and alfalfa-hay based diets with limited concentrate supplementation. Sheep Goats Res. 22:15–19. [Google Scholar]
- Wright C., Perkins D., Dawod A., Min B. R., Terrill T. H., Miller J. E., Vines T., and Gurung N.. 2016. The effect of phytochemical tannin containing diets on meat goat performance and drug resistant Haemonchus contortus control. Int. Vet. Health Sci. Res. 4:104–109. doi:10.19070/2332-2748-1600023 [Google Scholar]
- Wuliji T., Goetsch A. L., Sahlu T., Puchala R., and Soto-Navarro S.. 2003. Effects of different quality diets consumed continuously or after a lower quality diet on characteristics of growth of young Spanish goats. Small Rum. Res. 50:83–96. doi:10.1016/S0921-4488(03)00114-7 [Google Scholar]
- Yabuuchi E., Kaneko T., Yano I., Moss C. W., and Miyoshi N.. 1983. Sphingobacterium gen. nov., Sphingobacterium spiritivorum comb. nov., Sphingobacterium multivorum comb. nov., Sphingobacterium mizutae sp. nov., and Flavobacterium indologenes sp. nov.: glucose-nonfermenting gram-negative rods in CDC groups IIK-2 and IIb. Int. J. Syst. Bacteriol. 33:580–598. doi:10.1099/00207713-33-3-580 [Google Scholar]
- Zhang R., Zhu W., Zhu W., Liu J., and Mao S.. 2014. Effect of dietary forage sources on rumen microbiota, rumen fermentation and biogenic amines in dairy cows. J. Sci. Food Agric. 94:1886–1895. doi: 10.1002/jsfa.6508 [DOI] [PubMed] [Google Scholar]