Abstract
The present study aimed to investigate the influence of dietary butyrate supplementation on muscle fiber-type composition and mitochondrial biogenesis of finishing pigs, and the underlying mechanisms. Thirty-two LY (Landrace × Yorkshire) growing pigs with BW of 64.9 ± 5.7 kg were randomly allotted to either control (basal diet) or butyrate diets (0.3% butyrate sodium). Compared with the control group, diet supplemented with butyrate tended to increase average daily gain (P < 0.10). Pigs fed butyrate diet had higher intramuscular fat content, marbling score and pH24 h, and lower shear force and L*24 h in longissimus thoracis (LT) muscle than that fed control diet (P < 0.05). Interestingly, supplemented with butyrate increased (P < 0.05) the mRNA level of myosin heavy chain I (MyHC-I) and the percentage of slow-fibers, and decreased (P < 0.05) the mRNA level of MyHC-IIb in LT muscle. Meanwhile, pigs in butyrate group had an increase in mitochondrial DNA (mtDNA) copy number and the mRNA levels of mtDNA-encoded genes (P < 0.05). Moreover, feeding butyrate diet increased PGC-1α (PPAR γ coactivator 1α) level, decreased miR-133a-3p level and increased its target gene level (TEAD1, TEA domain transcription factor 1), increased miR-208b and miR-499-5p levels and decreased their target genes levels (Sp3 and Sox6, specificity protein 3 and SRY-box containing gene 6; P < 0.05) in the LT muscle. Collectively, these findings suggested that butyrate promoted slow-twitch myofiber formation and mitochondrial biogenesis, and the molecular mechanism may be via upgrading specific microRNAs and PGC-1α expression, finally improving meat quality.
Keywords: butyrate, finishing pigs, mitochondrial biogenesis, myofiber-related microRNAs, myofiber type, PGC-1α
INTRODUCTION
Skeletal muscle accounts for 40–50% of total body weight and is composed of four muscle fiber types that are classified to type I (slow-oxidative), IIa (fast-oxidative), IIb (fast-glycolytic), and IIx (fast-glycolytic between type IIa and type IIb) according to the morphisms of myosin heavy chain (Pette and Staron, 2000). These different myofiber types show great differences in physicochemical and metabolic characteristics and can switch in the following way: I ↔ IIa ↔ IIx ↔ IIb (Schiaffino and Reggiani, 2011; Joo et al., 2013). In contrast with type IIb fiber, type I fiber shows higher oxidative metabolism, more mitochondria content, lower glycolytic enzyme content, and myosin ATPase activity and is resistant to fatigue. In animal production, muscle fiber-type composition can be a major determinant for meat quality assessment, and numerous studies had demonstrated that muscle fiber characteristics are responsible for many aspects of meat quality, including meat color, tenderness, postmortem meat pH, water holding capacity, and intramuscular fat (IMF) content (Leseigneurmeynier and Gandemer, 1991; Bowker et al., 2005; Choe et al., 2008; Joo et al., 2013; Kim et al., 2013). It is commonly believed that muscles with a greater proportion of slow-twitch or oxidative fibers (MyHC-I and MyHC-IIa), which have greater oxidative capacity, lead to the better meat quality than those with fast-twitch or glycolysis fibers (Ryu and Kim, 2005; Choe et al., 2008; Choe and Kim, 2014). Recently, several researches have suggested that animal breed, exercise, feeding strategies, and nutrition level can influence muscle fiber types (Lefaucheur et al., 2004; Andersen et al., 2005; Röckl et al., 2007; Yang et al., 2015; Zhang et al., 2015; Li et al., 2017; Liu et al., 2019a, 2019b). Therefore, it is possible to regulate the composition of muscle fiber types through nutritional manipulation.
Butyrate is a short-chain fatty acid produced in large amounts from anaerobic bacteria fermentation of dietary fibers in the large intestine (Hamer et al., 2008). In addition to being an energy source, butyrate may mediate the impacts of gut microbiota and nutrition on metabolism, immunity, as well as the pathogenesis of diabetes mellitus, obesity, and inflammatory bowel disease (Huang et al., 2017b). In rodent models, diet supplemented with butyrate improved mitochondrial function leading to type I oxidative fiber phenotype and physiology through induction of PGC-1α expression in both maternal and high-fat diets (Gao et al., 2009; Henagan et al., 2015; Huang et al., 2017b). Additionally, previous study has been suggested that a diet supplemented with butyrate improved many aspects of meat quality in poultry (Zhang et al., 2011). Moreover, butyrate also acts as an inhibitor of histone deacetylase (HDAC) which inhibits deacetylation of histone and various transcription factors, thus influencing mRNA and microRNAs expression (Davie, 2003; Scott et al., 2006; Gao et al., 2009). MicroRNAs are noncoding regulatory RNAs that can function in myofiber-type transformation via modulating the expression of growth-related gene, which is related to muscle fiber phenotype expression (van Rooij et al., 2009).
Although others have investigated the effects of dietary butyrate on metabolism, indicating that butyrate may shift fiber-type characteristics and metabolic properties. However, much of this work was performed in other species, and there are few reported results regarding the effects of butyrate in pigs and its effect on meat quality. Thus, our hypothesis was that butyrate could improve pork quality. Therefore, the objective was to evaluate the effects of butyrate on skeletal muscle fiber type and mitochondrial biogenesis in finishing pigs, and to investigate potential mechanism for the effects of butyrate.
MATERIALS AND METHODS
Animals and Experimental Design
The experimental protocol involved in the present study was approved by the Animal Care and Use Committee of Sichuan Agricultural University. A total of thirty-two growing barrows (large white × landrace) with an average body weight of 64.99 ± 5.71 kg were used in an 8-wk experiment. At the beginning of the experiment, pigs were randomly allotted to 2 groups with 8 duplicates pens (2 pigs per pen) and were balanced for initial body weight and ancestry across 2 treatment groups. Pigs in control group were fed a basal diet, and those in experimental group were fed the basal diet supplemented with 0.3% sodium butyrate (provided by Hangzhou King Techina Technology Co., Ltd.). The addition level of sodium butyrate was determined according to the study of Lu et al. (2012) in sows.
Diets and Feeding Management
The basal diet was formulated to meet the NRC (2012) recommendation for the nutrient requirements of 75- to 100-kg finishing pigs. Diets were fed in meal form throughout the experiment. All pigs had free access to water and feed. Details of ingredient composition and calculated nutrient level of diets are given in Table 1.
Table 1.
Ingredient composition and nutritional levels of the basal diet (as-fed basis)
| Ingredients | % | Nutrient level1 | Content |
|---|---|---|---|
| Corn, 7.8% crude protein | 84.00 | Digestible energy, MJ/kg | 14.23 |
| Soybean meal, 44% crude protein | 10.87 | Crude protein, % | 12.13 |
| Wheat bran | 1.00 | Ca, % | 0.52 |
| Soybean oil | 1.50 | Total P, % | 0.42 |
| Limestone | 0.63 | Available P, % | 0.24 |
| Dicalcium phosphate | 0.53 | Total lysine, % | 0.78 |
| Salt | 0.35 | True digestible lysine, % | 0.69 |
| l-Lysine, 50% | 0.48 | Total Met + Cys, % | 0.39 |
| dl-Methionine | 0.07 | Total Thr, % | 0.49 |
| l-Threonine | 0.15 | ||
| Tryptophan | 0.04 | ||
| Choline chloride | 0.15 | ||
| Vitamin and mineral premix2 | 0.23 |
1All data were calculated values.
2Provided the following (per kilogram of complete diet): 100 mg of Fe (as ferrous sulfate); 15 mg of Cu (as copper sulfate); 120 mg of Zn (as zinc sulfate); 40 mg of Mn (as manganese sulfate); 0.3 mg of Se (as Na2SeO3); 0.25 mg of I (as KI); 13,500 IU of vitamin A; 2250 IU of vitamin D3; 24 IU of vitamin E; 6.2 mg of riboflavin; 25 mg of nicotinic acid; 15 mg of pantothenic acid; 1.2 mg of vitamin B12; and 0.15 mg of biotin.
The experiment was performed at the Research Base of the Institute of Animal Nutrition in Sichuan Agricultural University. During the experimental periods, all pigs were housed in an environmentally controlled room with completely slatted floors. Each pen (2.5 × 1.5 m) was equipped with a 1-sided feeder and a stainless-steel nipple drinker to allow the pigs ad libitum access to feed and water. The room temperature was maintained at 20–22 °C and relative humidity was controlled at 65–75% (Yan et al., 2019a). After 56 d of experiment, 1 pig with the same ancestry was selected from each pen and then slaughtered. The initial and final body weight and feed intake of pigs in each pen were recorded to calculate growth performance including average daily gain (ADG), average daily feed intake (ADFI), and gain to feed ratio (G:F) (Liu et al., 2018; Yan et al., 2019b).
Slaughter Surveys and Sample Collection
Pigs were transported to the slaughter house, electrically stunned, exsanguinated, peeled, eviscerated, and split down the midline according to standard commercial procedures. Hot carcass weight was recorded to calculate carcass yield (= hot carcass weight/live weight × 100%). Middle backfat depth opposite the first rib, last rib, and last lumbar vertebrae was measured. About 5.0-cm-thick chops of the longissimus thoracis (LT) muscle from the 10th to 13th ribs of each left carcass were collected with 30-min postmortem and vacuum-packed in individual polyethylene bags at 4 °C for meat quality determination. Loin-eye area was determined by tracing the outline of the exposed surface muscle area at 10th rib of the left side carcass and the area was measured using a planimeter. Within 20-min postmortem, about 100 g of LT muscle sample from the right side carcass was collected anterior to the 10th rib and stored at −20 °C until lyophilization for IMF content determination. Meanwhile, an approximately 0.5-cm-thick LT muscle sample posterior to the 10th rib was collected in cryogenic vials and immediately frozen in liquid nitrogen until subsequent analysis.
Analysis of Meat Quality Traits
Meat quality traits, including color, pH, IMF, tenderness, marbling, and water-holding capacity, were analyzed following the Chinese Agriculture Industry Standard NY/T 2793-2015. Meat color (L*, a*, and b*) was collected at 24-h postmortem from 3 different locations of LT muscle (from 8th to 10th ribs) using a Minolta chromameter (CR-300, Minolta Camera, Osaka, Japan) equipped with a 2-standard observer, a pulsed xenon lamp, and a 0-viewing angle. Meat pH at 45 min and 24-h postmortem were measured using a pH meter (pH-STAR, SFK-Technology, Denmark) calibrated with pH 4.6 and 7.0 buffers equilibrated at 25 °C. The shear force of LT muscle was determined by a tenderness analyzer (TA.XT. Plus, Stable Micro Systems, Godalming, United Kingdom). Briefly, at 48-h postmortem, samples of LT muscle were equilibrated at room temperature for 0.5 h and cooked in 80 °C thermostatic water-bath until internal temperature reached 70 °C. Then, the samples within their packaging bags were cooled in running water to room temperature and stored at 4 °C for 12 h. After equilibrating at room temperature for 0.5 h, four to six 1.27-cm-diameter cores were removed parallel to the muscle fiber orientation from each chop and then were sheared perpendicular to the long axis of the cores. All data were collected using Texture Expert software version 1.22 (Stable Micro Systems, United Kingdom). Marbling score (1 = devoid and 10 = abundant) was evaluated by the National Pork Producers Council standards (1999). The IMF content of LT muscle was determined using a Soxhlet petroleum ether extraction following Zhang et al. (2015) methods, and the results were expressed as a percentage of raw meat weight.
Real-Time Quantitative PCR
Total RNA from muscle samples was extracted by TRIzol reagent (Takara, Dalian, China) following the manufacture’s protocol. After measuring quality, total RNA (1 μg) was reverse transcribed to cDNA in a final 20 μL using PrimeScript RT reagent Kit (Takara, Dalian, China) with oligo(dT) plus random primers for mRNAs and special stem-loop primer for microRNA. Real-time quantitative PCR was carried out in triplicate using the SYBR Premix Ex Taq II (Takara, Dalian, China) on ABI Prism 7900HT Sequence Detection System (Applied Biosystems). The relative expression levels of mRNAs and microRNAs were calculated using the 2−ΔΔCT method (Livak and Schmittgen, 2001) and normalized to β-actin and U6, respectively. The primers for mRNAs and microRNAs are listed in Tables 2 and 3, respectively.
Table 2.
Primers used for real-time quantitative PCR
| Gene | Primer sequence (5′ to 3′) | Accession no. |
|---|---|---|
| MyHC-I | F: GTTTGCCAACTATGCTGGGG | NM_213855.1 |
| R: TGTGCAGAGCTGACACAGTC | ||
| MyHC-IIa | F: CTCTGAGTTCAGCAGCCATGA | NM214136.1 |
| R: GATGTCTTGGCATCAAAGGGC | ||
| MyHC-IIx | F: TTGACTGGGCTGCCATCAAT | NM_001104951.2 |
| R: GCCTCAATGCGCTCCTTTTC | ||
| MyHC-IIb | F: GAGGTACATCTAGTGCCCTGC | NM_001123141.1 |
| R: GCAGCCTCCCCAAAAATAGC | ||
| Myoglobin | F: GGAAGGTGGAGGCTGATGTC | NM_214236.1 |
| R: CCGTGCTTCTTCAGGTCCTC | ||
| Tnni1 | F: AGGAACACGAGGAGCGAGAG | NM_213912.3 |
| R: CACCTTGGCGTGAAGTTCC | ||
| NRF1 | F: GCCAGTGAGATGAAGAGAAACG | AK237171.1 |
| R: CTACAGCAGGGACCAAAGTTCAC | ||
| TFAM | F: GGTCCATCACAGGTAAAGCTGAA | NM_001130211 |
| R: ATAAGATCGTTTCGCCCAACTTC | ||
| COX4 | F: CCAAGTGGGACTACGACAAGAAC | AK233334.1 |
| R: CCTGCTCGTTTATTAGCACTGG | ||
| COX1 | F: ATTATCCTGACGCATACACAGCA | AJ950517.1 |
| R: ATTATCCTGACGCATACACAGCA | ||
| Cyt c | F: TAGAAAAGGGAGGCAAACACAAG | NM_001129970.1 |
| R: TAGAAAAGGGAGGCAAACACAAG | ||
| POLG | F: CTTTGAGGTTTTCCAGCAGCAG | XM_005653521.1 |
| R: GCTCCCAGTTTTGGTTGACAG | ||
| PGC-1α | F: CCCGAAACAGTAGCAGAGACAAG | NM_213963 |
| R: CTGGGGTCAGAGGAAGAGATAAAG | ||
| Sox6 | F: AAGCCACCTCTCCATTTGC | KF933861 |
| R: CCCAGTCAGCATCTTGTTGAA | ||
| MEF2C | F: CGCTCTTCATCTTGGGTCAG | NM_001044540.1 |
| R: CGTGTGTTGTGGGTATCTCG | ||
| Sp3 | F: CAGATGGTCAGCAGGTTCAG | XM_001928783.5 |
| R: TAGCAGGAGGTGTTCCAGAG | ||
| Thrap1 | F: CATCCCTGAAGCACACAGTC | XM_005669005 |
| R: AACATCGGCACCCTTGATA | ||
| TEAD1 | F: CAGGAGCAGTGCTAACAGGG | NM_001142669.1 |
| R: GGATTTTCCAAGCCGAAGCC | ||
| β-actin | F: TCCATCGTCCACCGCAAATG | XM_003357928.4 |
| R: TTCAGGAGGCTGGCATGAGG | ||
| Cyt b | F: ATGAAACATTGGAGTAGTCCTACTATTTACC | NC_000845.1 |
| R: CTACGAGGTCTGTTCCGATATAAGG | ||
| 18S rRNA | F: GGTAGTGACGAAAAATAACAATACAGGAC | NC_010448.3 |
| R: ATACGCTATTGGAGCTGGAATTACC |
MyHC = myosin heavy chain; Tnni1 = troponin 1; PGC-1α = PPAR γ coactivator 1α; Cyt b = cytochrome b; NRF1 = nuclear respiration factor 1; TFAM = mitochondrial transcription factor A; COX = cytochrome c oxidase; Cyt c = cytochrome c; POLG = DNA polymerase gamma; Sox6 = SRY-box containing gene 6; MEF2C = myocyte enhancer factor 2C; Sp3 = specificity protein 3; Thrap1 = thyroid hormone associated protein 1; TEAD1 = TEA domain transcription factor 1.
Table 3.
Primers for reverse-transcription and real-time quantitative PCR of microRNAs
| MicroRNA | Primer sequences (5′ to 3′) |
|---|---|
| ssc-miR-23a | RT: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGA CGGAAAT |
| F: GCGATCACATTGCCAGGG | |
| R: AGTGCAGGGTCCGAGGTATT | |
| ssc-miR-208b | RT: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGA CACAAAC |
| F: GCGCGATAAGACGAACAAAAG | |
| R: AGTGCAGGGTCCGAGGTATT | |
| ssc-miR-499-5p | RT: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGA CAAACAT |
| F: GCGCGTTAAGACTTGCAGTG | |
| R: AGTGCAGGGTCCGAGGTATT | |
| ssc-miR-133a-3p | RT: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGA CCAGCTG |
| F: GCGTTGGTCCCCTTCAAC | |
| R: AGTGCAGGGTCCGAGGTATT | |
| U6 | F: CTCGCTTCGGCAGCACA |
| R: AACGCTTCACGAATTTGCGT |
Mitochondrial DNA Copy Number
Total genomic DNA was extracted from skeletal muscle samples using QIAamp DNA extraction kit (QIAGEN, GmbH Hilden, Germany). The mtDNA copy number was determined using RT-PCR as previously described with minor modifications (Zou et al., 2016). Primers specific for coding region of mtDNA (cytochrome b, cytb) were utilized for mtDNA copy number quantification, whereas primers specific for 18S nuclear gene (18S rRNA) were used for standardization. The mtDNA copy number was calculated using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
Determination of Citrate Synthase Activity
The activity of citrate synthase in the LT muscle was measured using an automatic spectrophotometric analyzer (Thermo Fisher Scientific, Inc). Briefly, about 100 mg of the LT muscle were homogenized and sonicated in 50 vol (wt/vol) of ice-chilled 0.1 M phosphate buffer (pH 7.5) containing 2 mM EDTA. After centrifugation at 2,000 × g for 15 min at 4 °C, the supernatant was collected for determination of citrate synthase at 405 nm using a commercial kit (GenMed Scientifics Inc., United States).
Metachromatic ATPase staining
ATPase staining was performed as described previously with minor modifications (Hintz et al., 1984). Briefly, LT muscle samples from pigs were frozen in isopentane near its freezing point. Serial transverse muscle sections (10 μm) were obtained from each sample with a cryostat (CM1850, Leica, Germany) at −20 °C and mounted on glass slides. The unfixed sections were preincubated at room temperature for 10 min in a buffer consisting of 20 mM CaCl2, adjusted to pH 4.25 with acetic acid. After preincubation, the following consecutive steps were carried out: 1) sections washed for two 30 s in a 100 mM Tris buffer (pH 7.8) containing 20 mM CaCl2; 2) 30 min incubation at 37 °C in 1.5 M Sigma No.221 buffer (pH 9.4) containing 20 mM CaCl2 and 2.5 mM ATP disodium salt; 3) sections rinsed in 4 changes of 1% CaCl2 (3 min each); 4) sections immersed in 2% CoCl2 for 3 min; 5) sections rinsed in 6 to 8 changes of tap water (30 s each); 6) sections immersed in 1% (NH4)2S for 30 s; 7) sections washed with several changes of tap water; 8) sections dehydrated with ethanol; and 9) sections cleared in xylene and embedded in glycerol jelly.
Western Blot Analysis
The LT muscle tissues (~10 mg) were placed in a round-bottom microcentrifuge tube, lysed in RIPA lysis buffer (50 mM Tris, pH 7.5, 5 mM EDTA, 150 mM NaCl, 0.1% wt/vol SDS, 1% vol/vol Triton X-100, 0.5% wt/vol sodium deoxycholate, 2 mM PMSF, and protease inhibitor mixture) (Beyotime, Jiangsu, China) for 10 min at 4 °C, and then homogenized with an electric homogenizer for 10 min at 4 °C. The tissue lysates were centrifuged for 20 min at 12,000 rpm at 4 °C and liquid supernatant was collected. The bicinchoninic acid (BCA) protein assay kit (Thermo, Il, United States) was used to determine the protein concentration following the manufacture’s protocol. Before loading to the gel, protein lysates were denatured at 95 to 100 °C with 5× protein loading buffer for 10 min. The proteins were separated with SDS-PAGE and followed by electrotransfer onto a polyvinylidene fluoride (PVDF) membrane (Millipore, Eshborn, Germany). After blocking with 5% skim milk, the PVDF membranes were incubated with primary antibodies at 4 °C overnight and then with HRP-labeled secondary antibodies for 2 h at room temperature. Primary antibodies specific for MyHC-I (1:1000, ABclonal), MyHC-IIa (1:1000, ABclonal), MyHC-IIb (1:1000, Proteintech), PGC-1α (1:1000, Abcam), β-actin (1:3000, Immunoway), and HRP-conjugated anti-rabbit/ mouse/goat secondary antibodies (1:4000, Beyotime) were used to detect protein expression (Liu et al., 2019c). Clarity Western ECL Substrate (Bio-Rad, Hercules, CA) was used to visualize the protein bands. The protein expression was quantified by Image Lab and normalized to β-actin expression.
Statistical Analysis
All data were analyzed by Student’s t-test using SAS (version 9.2, SAS Institute, Cary, NC). The results were presented as the means ± standard error of the mean (SEM). Differences between groups were statistically significant if P < 0.05, and there was tendency toward statistical significance if P < 0.10.
RESULTS
Growth Performance and Carcass Traits
Growth performance and carcass quality of the finishing pigs are shown in Table 4. In comparison to control group, diet supplemented with butyrate tended to increase ADG (P = 0.085). However, carcass trait was not affected by diet supplemented with butyrate (P > 0.05).
Table 4.
Effect of dietary butyrate supplementation on growth performance and carcass quality of finishing pigs
| Item1 | Control | Butyrate2 | SEM | P-value |
|---|---|---|---|---|
| Initial body weight, kg | 65.00 | 64.98 | 0.09 | 0.99 |
| Final body weight, kg | 111.18 | 113.87 | 0.54 | 0.11 |
| ADG2, g/d | 825 | 873 | 9.21 | 0.09 |
| ADFI3, kg/d | 2.60 | 2.69 | 0.04 | 0.46 |
| F:G4, kg/kg | 0.31 | 0.32 | 0.02 | 0.51 |
| Hot carcass weight, kg | 83.09 | 86.15 | 1.16 | 0.40 |
| Carcass yield, % | 72.09 | 71.46 | 0.21 | 0.12 |
| Loin eye area, cm2 | 50.92 | 48.45 | 1.41 | 0.90 |
| Backfat depth, cm | ||||
| First rib | 2.70 | 2.89 | 0.17 | 0.449 |
| Last rib | 1.83 | 1.80 | 0.13 | 0.885 |
| Last lumbar vertebra | 1.19 | 1.20 | 0.08 | 0.915 |
1Average daily gain (ADG), average daily feed intake (ADFI) and gain to feed (G:F) were analyzed with pen as the experimental unit (n = 8).
2Butyrate = basal diet with 0.3% sodium butyrate.
Meat Quality
As indicated in Table 5, the pigs subjected to butyrate treatment had higher (P < 0.05) pH24 h, and lower (P < 0.05) shear force value and L*24 h in LT muscle. As expected, noticeable increases (P < 0.05) in the marbling score and IMF content in pigs fed the butyrate diet were observed (by 60% and 11%, respectively).
Table 5.
Effect of dietary butyrate supplementation on meat quality of finishing pigs1
| Items | Control | `Butyrate2 | SEM | P |
|---|---|---|---|---|
| Drip loss, % | 2.34 | 2.13 | 0.05 | 0.13 |
| Cooking loss, % | 34.04 | 33.31 | 0.26 | 0.35 |
| Shear force, kg of force/cm2 | 5.49a | 4.26b | 0.14 | <0.05 |
| Marbling score | 1.25b | 2.00a | 0.09 | <0.05 |
| IMF content,3 % | 2.32b | 2.59a | 0.06 | <0.05 |
| pH45 min | 6.54 | 6.63 | 0.02 | 0.25 |
| pH24 h | 5.58b | 5.65a | 0.01 | <0.05 |
| L* | 59.36a | 56.73b | 0.42 | <0.05 |
| a* | 8.40 | 8.22 | 0.21 | 0.77 |
| b* | 1.20 | 1.24 | 0.06 | 0.83 |
a,bWithin a row, means with different superscript letters are significantly different (P < 0.05).
1All traits in this table were analyzed with pen as the experimental unit (n = 8).
2Butyrate = basal diet with 0.3% sodium butyrate.
3IMF = Intramuscular fat.
Muscle Fiber Types in LT Muscle
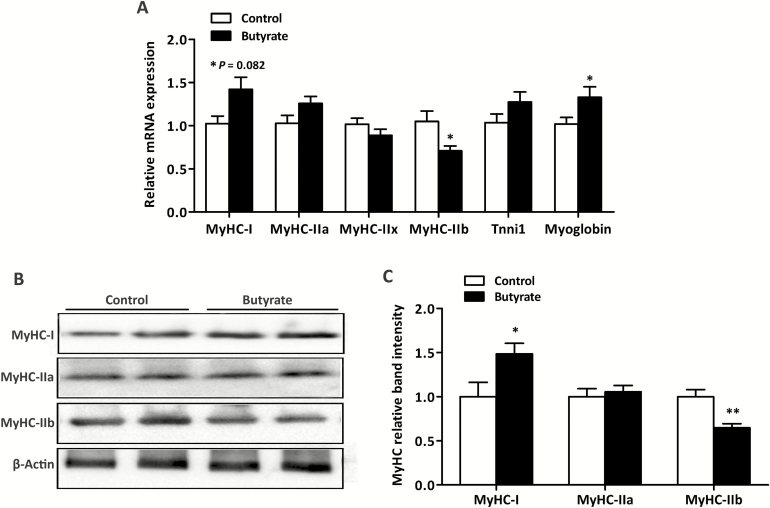
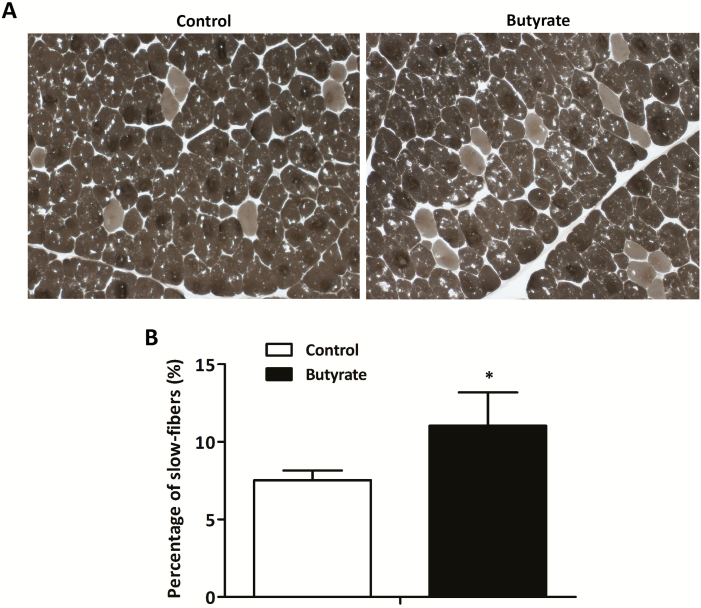
The MyHC isoforms expression and the percentage of slow-fibers in LT muscle are presented in Figures 1 and 2, respectively. In the mRNA level (Figure 1A), dietary supplemented with butyrate increased (P < 0.05) the expression of MyHC-I and myoglobin (a slow-twitch fiber marker) and decreased (P < 0.05) the expression of MyHC-IIb in LT muscle. Meanwhile, a trend for the increased expression of MyHC-IIa was observed in pigs fed butyrate diet (P = 0.082) compared with the control group. In the protein level (Figure 1B), supplemented with butyrate increased (P < 0.05) the expression of MyHC-I and decreased (P < 0.05) the expression of MyHC-IIb. In addition, pigs fed butyrate diet had higher percentage of slow fibers than that fed control diet (P < 0.05; Figure 2).
Figure 1.
Effects of dietary butyrate supplementation on expression of myosin heavy-chain (MyHC) isoforms in both mRNA (A) and protein (B) levels in LT muscle of finishing pigs. RT-PCR and western blotting were conducted to measure the mRNA and protein expression of MyHC isoforms, respectively. β-actin was utilized as a control. Control, basal diet; Butyrate, basal diet with 0.3% sodium butyrate. Data are the means ± SEM, n = 8. *P < 0.05; **P < 0.01.
Figure 2.
Metachromatic ATPase staining and percentage of slow fibers in LT muscle of finishing pigs. Slow fibers stain light gray. Original magnification, ×100. Bars: 100 μm. Control, basal diet; Butyrate, basal diet with 0.3 % sodium butyrate. Data are the means ± SEM, n = 8. *P < 0.05; **P < 0.01.
mtDNA Copy Number and mtDNA-Encoded Genes Expression in LT Muscle
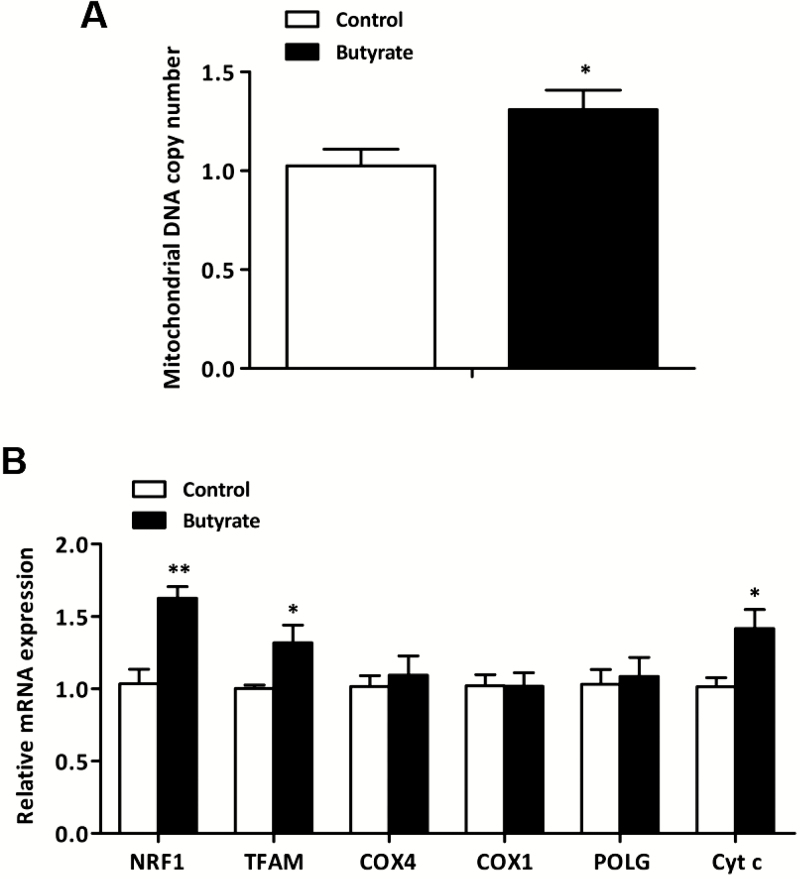
The mtDNA copy number and mtDNA-encoded genes expression are shown in Figure 3. In comparison to control group, diet supplemented with butyrate significantly increased mtDNA copy number (P < 0.05; Figure 3A) in LT muscle. In addition, supplemental butyrate led to greater mRNA levels of NRF1, TFAM, and Cytc (P < 0.05; Figure 3B).
Figure 3.
Effect of dietary butyrate supplementation on mtDNA copy number and the expression of mtDNA-encoded genes in LT muscle of finishing pigs. mtDNA copy number was calculated as the ratio of target gene (Cyt b) to internal reference gene (18S rRNA). β-actin was used as an internal reference gene for calculating the expression of mtDNA-encoded genes. Control, basal diet; Butyrate, basal diet with 0.3 % sodium butyrate. Data are the means ± SEM, n = 8. *P < 0.05; **P < 0.01.
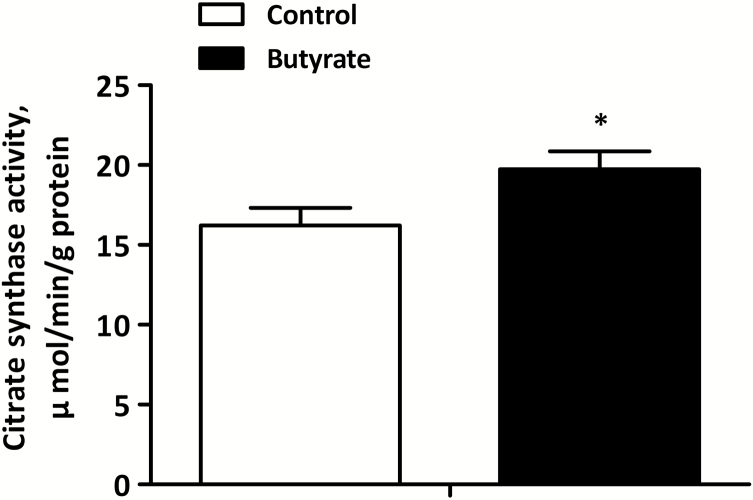
Activity of Citrate Synthase in LT muscle
The activity of citrate synthase in LT muscle is shown in Figure 4. In comparison to control group, diet supplemented with butyrate significantly improved the activity of citrate synthase (P < 0.05) in LT muscle.
Figure 4.
Effects of dietary butyrate on the activity of citrate synthase in LT muscle of finishing pigs. Control, basal diet; Butyrate, basal diet with 0.3% sodium butyrate. Data are the means ± SEM, n = 8. *P < 0.05; **P < 0.01.
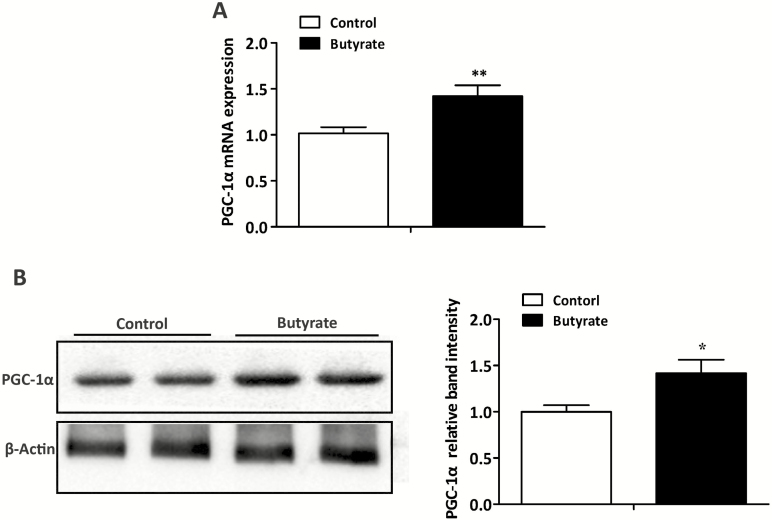
Expression of PGC-1α in LT Muscle
The expression of PGC-1α in LT muscle is shown in Figure 5. Compared with control group, diet supplemented with butyrate significantly increased the expression of PGC-1α in both mRNA (Figure 5A) and protein (Figure 5B) levels (P < 0.05).
Figure 5.
Effects of dietary butyrate supplementation on expression of PGC-1α in both mRNA (A) and protein (B) levels in LT muscle of finishing pigs. RT–PCR and western blotting were conducted to determine the mRNA and protein expression of PGC-1α, respectively. β-actin was used as a control. Control, basal diet; Butyrate, basal diet with 0.3 % sodium butyrate. Data are the means ± SEM, n = 8. *P < 0.05; **P < 0.01.
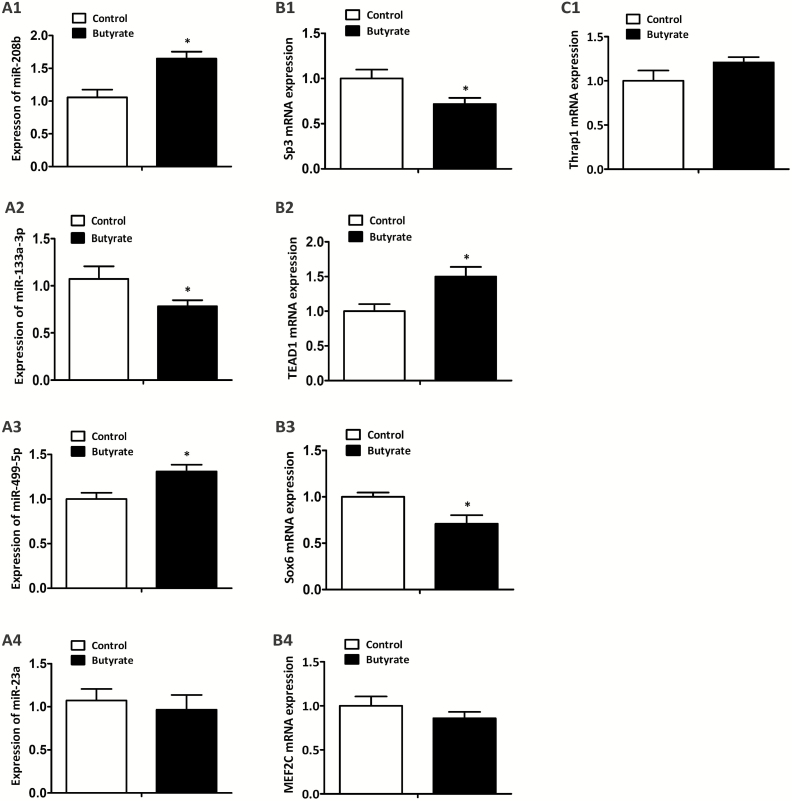
Expressions of Myofiber Type-Related MicroRNAs and Their Corresponding Target Genes in LT Muscle
The expression of myofiber type-related miRNAs (miR-208b, miR-133a-3p, miR-499-5p, and miR-23a) and their corresponding target genes are presented in Figure 6. Pigs fed with butyrate diet increased the expression of miR-208b (P < 0.05; Figure 6A1) and miR-499-5p (P < 0.05; Figure 6A3) and decreased the expression of miR-133a-3p (P < 0.05; Figure 6A2), concomitant with a decrease in the mRNA levels of Sp3 (P < 0.05; Figure 6B1) and Sox6 (P < 0.05; Figure 6B3) and an increase in the mRNA level of TEA domain transcription factor 1 (TEAD1; P < 0.05; Figure 6B2) in LT muscle than those fed with control diet, whereas no effects were observed on the mRNA level of Thrap1 (Figure 6C1), the expression of miR-23a (Figure 6A4) and its target gene MEF2C (Figure 6B4).
Figure 6.
Effects of dietary butyrate supplementation on expression of specific miRNAs and their corresponding target genes in LT muscle of finishing pigs. U6 was utilized as an internal reference gene of miR-208b (A1), miR-133a-3p (A2), miR-499-5p (A3), and miR-23a (A4). β-actin was utilized as a control of Sp3 (B1), Thrap1 (C1), TEAD1 (B2), Sox6 (B3), and MEF2C (B4). Control, basal diet; Butyrate, basal diet with 0.3% sodium butyrate. Data are the means ± SEM, n = 8. *P < 0.05.
DISCUSSION
Numerous studies have reported that dietary supplemented with butyrate had no effects on both growth and performance of weaning pigs (Biagi et al., 2007; Weber and Kerr, 2008; Fang et al., 2014). In addition, recent study have shown that supplemental 0.3% sodium butyrate for 28 d had no effects on feed intake, weight gain, and feed conversion efficiency of finishing pigs (Walia et al., 2016). Similar to previous studies, the results in our research indicated that no significant difference was observed in the growth performance and carcass traits of finishing pigs between 2 groups. However, Hanczakowska et al. (2014) reported that dietary supplemented with 3.0 g/kg sodium butyrate improved the growth performance of growing–finishing pigs including ADG and final BW. This discrepancy may be due to the difference in the form and dosage of butyrate, trial duration, and physiological stage of pigs.
It is well known that the compositions of muscle fiber types are responsible for postmortem muscle metabolism due to the differences in metabolic enzymes activities and structural proteins contents in different myofibers (Ryu and Kim, 2005; Li et al., 2017). Generally, muscles with a higher proportion of fast-twitch fiber (MyHC-IIb), which exhibits greater glycolytic capacity, influenced postmortem pH decline and leads to higher lightness and lower water-holding capacity in pigs (Choe et al., 2008; Kim et al., 2013). Meanwhile, Kim et al. (2010) reported that meat with low percentage of type IIa and IIb fibers had high L* and a*, respectively, and meat having high L* and low a*, often considered as “pale,” has high value in drip loss. Moreover, myoglobin is a biomarker of slow-twitch fiber (Kim et al., 2010), and the content of myoglobin in muscle fibers decreases in the rank order I < IIa < IIx < IIb (Lefaucheur, 2010). Therefore, from the foregoing, it is not surprising that changes in muscle fiber type will lead to changes in meat-quality traits. Previous study has shown that supplemented with butyrate promoted the meat quality of poultry (Zhang et al., 2011). Intriguingly, studies in the rodent models showed that supplementation butyrate increased the ratio of type I oxidative muscle fiber in either high-fat or maternal diets (Gao et al., 2009; Huang et al., 2017a). Additionally, the total lipid content of slow-oxidative type fiber is three times of glycolytic fiber (De Feyter et al., 2006). There was a significant positive correlation between the proportion of MyHC-I and IMF content, and negative correlation between the proportion of MyHC-IIb and IMF content (Yang et al., 2015). Accordingly, our study provided the first evidence in pigs that butyrate supplementation improved many aspects of meat quality, including greater marbling score, IMF content and pH24 h, and lower shear force. Consistent with these, an increase in the mRNA level of oxidative myofibers (MyHC-I and MyHC-IIa) and their marker (myoglobin), and a decrease in the mRNA and protein expression of glycolytic myofiber (MyHC-IIb) were observed in response to butyrate, which may partly explain the reason why butyrate improved pork quality as shown in the present study.
Differences in muscle fiber type are also reflected in mitochondrial content and oxidative capacity. In general, slow-switch muscle fibers have higher mitochondrial content and are more dependent on oxidative metabolism compared with type II muscle fibers (Kamei et al., 2004). Mitochondrial biogenesis can prevent metabolic imbalances and facilitate healthy skeletal muscle (Joseph et al., 2012). In the present study, elevated amount of mtDNA copy number and the activity of citrate synthase were detected in LT muscle with butyrate addition. As citrate synthase reflects the mitochondrial enrichment of tissue (Kelley et al., 2002), suggesting facilitated mitochondrial biogenesis in LT muscle in response to butyrate. Moreover, the initiation of mitochondrial biogenesis needs the increased transcription of both nuclear and mitochondrial DNA encoded genes (FernandezMarcos and Auwerx, 2011). In the mRNA level, dietary addition of butyrate promoted the expression of mitochondria-encoded genes including NRF1, TFAM, and Cytc, of which the first 2 genes are essential for mtDNA replication and transcription (Huang et al., 2017b). In addition, PGC-1α has been identified to induce the transcription of both nuclear- and mitochondrial-encoded genes responsible for mitochondrial biogenesis and serves as a vital coordinator and regulator of muscle fiber type and mitochondrial function (Wu et al., 1999; Lin et al., 2002; Safdar et al., 2011). As expected, an increase in PGC-1α expression was observed in LT muscle from butyrate group. Therefore, induction of PGC-1α may be a molecular mechanism by which butyrate stimulates slow-twitch fiber formation and mitochondrial biogenesis.
In addition to promoting slow-twitch muscle fiber formation by elevating PGC-1α expression, microRNAs (miRNAs) have been reported to be involved in muscle fiber transformation (van Rooij et al., 2009). MiRNAs are a group of evolutionarily highly conserved noncoding RNAs, which function in gene silencing and translational suppression by binding to the 3′ untranslated region (UTR) of their target genes (Zhang et al., 2016). Notably, miR-133a-3p, miR-23a, miR-208b, and miR-499-5p play crucial roles in myofiber specification by regulating slow-twitch or fast-twitch myofiber gene expression (van Rooij et al., 2009; Zhang et al., 2014). TEAD1 and MEF2C, as key transcriptional activators to facilitate slow-twitch fiber gene expression, are shown to be target genes of miR-133a-3p and miR-23a, respectively (Zhang et al., 2014; Shen et al., 2016). In addition to promote slow muscle phenotype, there are some transcriptional repressors to inhibit slow-twitch fiber specific gene expression including Sox6, Sp3, and Thrap1, which are targeted for inhibition by miR-208b and miR-499-5p (van Rooij et al., 2009). Interestingly, miR-133a-3p is a muscle-specific miRNA, and miR-208b/ miR-499-5p are a family of intronic miRNAs encoded by their host genes, Myh7 and Myh7b, respectively (Callis et al., 2008; van Rooij et al., 2009). As such, in our research, muscle with upregulation of slow-twitch fiber and downregulation of fast-twitch fiber, which also exhibited lower level of miR-133a-3p concurrent with greater level of target TEAD1, and higher level of miR-208b and miR-499-5p paralleled with lower level of target Sp3 and Sox6. These results suggested that improved TEAD1 mediated by decreased miR-133a-3p, together with the reduction of Sp3 and Sox6 suppressed by miR-208b and miR-499-5p, may be partly responsible for the fast-to-slow myofiber transformation.
Although we did not determine the acetylation level in the present study, it was speculated that the molecular mechanism by which butyrate regulates the expression of PGC-1α and myofiber type-related microRNAs may depend on its inhibitory activity of histone deacetylase (Davie, 2003). Inhibition of histone deacetylase stimulates transcriptional activation, which is controlled by gene promoter activity (Potthoff et al., 2007). However, activation of gene promoter requires histone acetylation, which unfolds chromatin DNA for transcription initiation and mRNA elongation (Gao et al., 2009). Interestingly, histone deacetylase inhibition is a crucial regulatory mechanism for muscle fiber-type transformation and oxidative metabolism. Transgenic mice with class II histone deacetylases knockout was shown to promote slow-twitch fiber formation through activating PGC-1α transcription (Potthoff et al., 2007). Meanwhile, treated with a class I-specific inhibitor increased mitochondrial biogenesis and oxidative capacity in vitro-cultured myotubes via increasing PGC-1α action (Ferrari et al., 2011). Additionally, microRNA levels were also changed in response to the status of histone deacetylase through deacetylation of DGCR8 (Scott et al., 2006; Wada et al., 2012).
In summary, our research revealed that dietary butyrate supplementation promoted the formation of slow-twitch myofibers and mitochondrial biogenesis in skeletal muscle, and the underlying mechanisms may be through epigenetically inducing the differential expression of myofiber type-related microRNAs and PGC-1α, ultimately contributing to the improved meat quality.
ACKNOWLEDGMENTS
We thank Xiaoqian Gao, Yaolian Hu, Hengzhi Zhang, and Weikang Wang for their assistance during the experiment, and Hangzhou King Techina Technology Co., Ltd. (Hangzhou, China) for providing sodium butyrate (30%, encapsulated).
Footnotes
This study was supported by the National Basic Research Program of China (No. 2012CB124701) and National Natural Science Foundation of China (31372323).
LITERATURE CITED
- Andersen H. J., Oksbjerg N., Young J. F., and Therkildsen M.. 2005. Feeding and meat quality - a future approach. Meat Sci. 70:543–554. doi: 10.1016/j.meatsci.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Biagi G., Piva A., Moschini M., Vezzali E., and Roth F. X.. 2007. Performance, intestinal microflora, and wall morphology of weanling pigs fed sodium butyrate. J. Anim. Sci. 85:1184–1191. doi: 10.2527/jas.2006-378. [DOI] [PubMed] [Google Scholar]
- Bowker B. C., Swartz D. R., Grant A. L., and Gerrard D. E.. 2005. Myosin heavy chain isoform composition influences the susceptibility of actin-activated S1 ATPase and myofibrillar ATPase to ph inactivation. Meat Sci. 71:342–350. doi: 10.1016/j.meatsci.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Callis T. E., Deng Z., Chen J. F., and Wang D. Z.. 2008. Muscling through the microRNA world. Exp. Biol. Med. (Maywood). 233:131–138. doi: 10.3181/0709-MR-237. [DOI] [PubMed] [Google Scholar]
- Choe J. H., Choi Y. M., Lee S. H., Shin H. G., Ryu Y. C., Hong K. C., and Kim B. C.. 2008. The relation between glycogen, lactate content and muscle fiber type composition, and their influence on postmortem glycolytic rate and pork quality. Meat Sci. 80:355–362. doi: 10.1016/j.meatsci.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Choe J. H., and Kim B. C.. 2014. Association of blood glucose, blood lactate, serum cortisol levels, muscle metabolites, muscle fiber type composition, and pork quality traits. Meat Sci. 97:137–142. doi: 10.1016/j.meatsci.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Davie J. R. 2003. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 133(7 Suppl):2485S–2493S. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- De Feyter H. M. M. L., Gert S., Hesselink M. K., Patrick S., Klaas N., and Prompers J. J.. 2006. Regional variations in intramyocellular lipid concentration correlate with muscle fiber type distribution in rat tibialis anterior muscle. Magn. Reson. Med. 56:19–25. doi: 10.1002/mrm.20924. [DOI] [PubMed] [Google Scholar]
- Fang C. L., Sun H., Wu J., Niu H. H., and Feng J.. 2014. Effects of sodium butyrate on growth performance, haematological and immunological characteristics of weanling piglets. J. Anim. Physiol. Anim. Nutr. (Berl). 98:680–685. doi: 10.1111/jpn.12122. [DOI] [PubMed] [Google Scholar]
- FernandezMarcos P. J., and Auwerx J.. 2011. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 93:884S. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari A., Galmozzi A., Mitro N., Gers E., Gilardi F., Godio C., Cermenati G., Caruso D., Mai A., and Saez E.. 2011. Inhibition of class I histone deacetylases unveils a mitochondrial signature and enhances lipid oxidation in skeletal muscle and adipose tissue. Chem. Phys. Lipids. 164:732. doi: 10.2337/db12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Yin J., Zhang J., Ward R. E., Martin R. J., Lefevre M., Cefalu W. T., and Ye J.. 2009. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer H. M., Jonkers D., Venema K., Vanhoutvin S., Troost F. J., Brummer R. J.. 2008. Review article: the role of butyrate on colonic function. Aliment. Pharm. Therap. 27:104. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- Hanczakowska E., Niwin´Ska B., Grela E. R., Weglarzy K., and Okon´ K.. 2014. Effect of dietary glutamine, glucose and/or sodium butyrate on piglet growth, intestinal environment, subsequent fattener performance, and meat quality. Czech. J. Anim. Sci. 59:460–470. doi: 10.17221/7709-CJAS. [DOI] [Google Scholar]
- Henagan T. M., Stefanska B., Fang Z., Navard A. M., Ye J., Lenard N. R., and Devarshi P. P.. 2015. Sodium butyrate epigenetically modulates high-fat diet-induced skeletal muscle mitochondrial adaptation, obesity and insulin resistance through nucleosome positioning. Br. J. Pharmacol. 172:2782–2798. doi: 10.1111/bph.13058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintz C. S., Coyle E. F., Kaiser K. K., Chi M. M., and Lowry O. H.. 1984. Comparison of muscle fiber typing by quantitative enzyme assays and by myosin ATPase staining. J. Histochem. Cytochem. 32:655–660. doi: 10.1177/32.6.6202737. [DOI] [PubMed] [Google Scholar]
- Huang Y., Gao S., Chen J., Albrecht E., Zhao R., and Yang X.. 2017a. Maternal butyrate supplementation induces insulin resistance associated with enhanced intramuscular fat deposition in the offspring. Oncotarget 8:13073–13084. doi: 10.18632/oncotarget.14375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Gao S., Jun G., Zhao R., and Yang X.. 2017b. Supplementing the maternal diet of rats with butyrate enhances mitochondrial biogenesis in the skeletal muscles of weaned offspring. Br. J. Nutr. 117:12–20. doi: 10.1017/S0007114516004402. [DOI] [PubMed] [Google Scholar]
- Joo S. T., Kim G. D., Hwang Y. H., and Ryu Y. C.. 2013. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 95:828–836. doi: 10.1016/j.meatsci.2013.04.044. [DOI] [PubMed] [Google Scholar]
- Joseph A. M., Joanisse D. R., Baillot R. G., and Hood D. A.. 2012. Mitochondrial dysregulation in the pathogenesis of diabetes: potential for mitochondrial biogenesis-mediated interventions. Exp. Diabetes Res. 2012:642038. doi: 10.1155/2012/642038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei Y., Miura S., Suzuki M., Kai Y., Mizukami J., Taniguchi T., Mochida K., Hata T., Matsuda J., Aburatani H., et al. 2004. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J. Biol. Chem. 279:41114–41123. doi: 10.1074/jbc.M400674200. [DOI] [PubMed] [Google Scholar]
- Kelley D. E., He J., Menshikova E. V., and Ritov V. B.. 2002. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- Kim G. D., Jeong J. Y., Hur S. J., Yang H. S., Jeon J. T., and Joo S. T.. 2010. The relationship between meat color (CIE L* and a*), Myoglobin content, and their influence on muscle fiber characteristics and pork quality. Korean. J. Food. Sci. An. 30:626–633. doi: 10.5851/kosfa.2010.30.4.626. [DOI] [Google Scholar]
- Kim G. D., Jeong J. Y., Jung E. Y., Yang H. S., Lim H. T., and Joo S. T.. 2013. The influence of fiber size distribution of type IIB on carcass traits and meat quality in pigs. Meat Sci. 94:267–273. doi: 10.1016/j.meatsci.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Lefaucheur L. 2010. A second look into fibre typing–relation to meat quality. Meat Sci. 84:257–270. doi: 10.1016/j.meatsci.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Lefaucheur L., Milan D., Ecolan P., and Le Callennec C.. 2004. Myosin heavy chain composition of different skeletal muscles in large white and meishan pigs. J. Anim. Sci. 82:1931–1941. doi: 10.2527/2004.8271931x. [DOI] [PubMed] [Google Scholar]
- Leseigneurmeynier A., and Gandemer G.. 1991. Lipid composition of pork muscle in relation to the metabolic type of the fibres. Meat Sci. 29:229–241. doi: 10.1016/0309-1740(91)90052-R. [DOI] [PubMed] [Google Scholar]
- Li Y. J., Li J. L., Zhang L., Gao F., and Zhou G. H.. 2017. Effects of dietary starch types on growth performance, meat quality and myofibre type of finishing pigs. Meat Sci. 131:60–67. doi: 10.1016/j.meatsci.2017.04.237. [DOI] [PubMed] [Google Scholar]
- Lin J., Wu H., Tarr P. T., Zhang C. Y., Wu Z., Boss O., Michael L. F., Puigserver P., Isotani E., Olson E. N.,. et al. 2002. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Liu J., Xue P., Cao S., Liu J., Chen L., and Zhang H.. 2018. Effects of dietary phosphorus concentration and body weight on postileal phosphorus digestion in pigs. Anim. Feed. Sci. Tech. 242:86–94. doi: 10.1016/j.anifeedsci.2018.06.003. [DOI] [Google Scholar]
- Liu J., Yan H., Cao S., Liu J., Li Z., and Zhang H.. 2019b. The response of performance in grower and finisher pigs to diets formulated to different tryptophan to lysine ratios. Livest. Sci. 222: 25–30. doi: 10.1016/j.livsci.2019.01.016. [DOI] [Google Scholar]
- Liu J., Yan H., Zhang Y., Hu Y., and Zhang H.. 2019a. Effects of dietary energy and protein content and lipid source on growth performance and carcass traits in Pekin ducks. Poultry Sci. doi: 10.3382/ps/pez217 [DOI] [PubMed] [Google Scholar]
- Liu J., Zhang Y., Li Y., Yan H., and Zhang H.. 2019c. L-tryptophan enhance intestinal integrity in diquat-challenged piglets associated with improvement of redox status and mitochondrial function. Animals 9:266. doi: 10.3390/ani9050266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., and Schmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu H., Su S., and Ajuwon K. M.. 2012. Butyrate supplementation to gestating sows and piglets induces muscle and adipose tissue oxidative genes and improves growth performance. J. Anim. Sci 90:430–432. doi: 10.2527/jas.53817. [DOI] [PubMed] [Google Scholar]
- NPPC 1999. Official color and marbling standards. Des Moines IA: National Pork Producers Council. [Google Scholar]
- NRC 2012. Nutrient requirements of swine, 11th rev. ed. Natl Acad. Press, Washington, DC. [Google Scholar]
- Pette D., and Staron R. S.. 2000. Myosin isoforms, muscle fiber types, and transitions. Microsc. Res. Tech. 50:500–509. doi:10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Potthoff M. J., Wu H., Arnold M. A., Shelton J. M., Backs J., McAnally J., Richardson J. A., Bassel-Duby R., and Olson E. N.. 2007. Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. J. Clin. Invest. 117:2459–2467. doi: 10.1172/JCI31960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röckl K. S., Hirshman M. F., Brandauer J., Fujii N., Witters L. A., and Goodyear L. J.. 2007. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes 56:2062–2069. doi: 10.2337/db07-0255. [DOI] [PubMed] [Google Scholar]
- van Rooij E., Quiat D., Johnson B. A., Sutherland L. B., Qi X., Richardson J. A., Kelm R. J. Jr, and Olson E. N.. 2009. A family of micrornas encoded by myosin genes governs myosin expression and muscle performance. Dev. Cell 17:662–673. doi: 10.1016/j.devcel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu Y. C., and Kim B. C.. 2005. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle. Meat Sci. 71:351–357. doi: 10.1016/j.meatsci.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Safdar A., Little J. P., Stokl A. J., Hettinga B. P., Akhtar M., and Tarnopolsky M. A.. 2011. Exercise increases mitochondrial PGC-1α content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J. Biol. Chem. 286:10605–10617. doi: 10.1074/jbc.M110.211466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Schiaffino S., and Reggiani C.. 2011. Fiber types in mammalian skeletal muscles. Physiol. Rev. 91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- Scott G. K., Mattie M. D., Berger C. E., Benz S. C., and Benz C. C.. 2006. Rapid alteration of microRNA levels by histone deacetylase inhibition. Cancer Res. 66:1277–1281. doi: 10.1158/0008-5472.CAN-05-3632. [DOI] [PubMed] [Google Scholar]
- Shen L., Chen L., Zhang S., Zhang Y., Wang J., and Zhu L.. 2016. MicroRNA-23a reduces slow myosin heavy chain isoforms composition through myocyte enhancer factor 2C (MEF2C) and potentially influences meat quality. Meat Sci. 116:201–206. doi: 10.1016/j.meatsci.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Wada T., Kikuchi J., and Furukawa Y.. 2012. Histone deacetylase 1 enhances microRNA processing via deacetylation of DGCR8. EMBO Rep. 13:142–149. doi: 10.1038/embor.2011.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walia K., Argüello H., Lynch H., Leonard F. C., Grant J., Yearsley D., Kelly S., Duffy G., Gardiner G. E., and Lawlor P. G.. 2016. Effect of feeding sodium butyrate in the late finishing period on salmonella carriage, seroprevalence, and growth of finishing pigs. Prev. Vet. Med. 131:79–86. doi: 10.1016/j.prevetmed.2016.07.009. [DOI] [PubMed] [Google Scholar]
- Weber T. E., and Kerr B. J.. 2008. Effect of sodium butyrate on growth performance and response to lipopolysaccharide in weanling pigs. J. Anim. Sci. 86:442–450. doi: 10.2527/jas.2007-0499. [DOI] [PubMed] [Google Scholar]
- Wu Z., Puigserver P., Andersson U., Zhang C., Adelmant G., Mootha V., Troy A., Cinti S., Lowell B., Scarpulla R. C., et al. 1999. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Yan H., Cao S., Li Y., Zhang H., and Liu J.. 2019b. Reduced meal frequency alleviates high-fat diet-induced lipid accumulation and inflammation in adipose tissue of pigs under the circumstance of fixed feed allowance. Eur J Nutr. 1–14. doi: 10.1007/s00394-019-01928-3. [DOI] [PubMed] [Google Scholar]
- Yan H., Zhang L., Guo Z., Zhang H., and Liu J.. 2019a. Production phase affects the bioaerosol microbial composition and functional potential in swine confinement buildings. Animals 9:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Chen D., Yu B., Huang Z., Mao X., Yu J., Zheng P., and He J.. 2015. Effect of dietary amylose/amylopectin ratio on growth performance, carcass traits, and meat quality in finishing pigs. Meat Sci. 108:55–60. doi: 10.1016/j.meatsci.2015.05.026. [DOI] [PubMed] [Google Scholar]
- Zhang W. H., Gao F., Zhu Q. F., Li C., Jiang Y., Dai S. F., and Zhou G. H.. 2011. Dietary sodium butyrate alleviates the oxidative stress induced by corticosterone exposure and improves meat quality in broiler chickens. Poult. Sci. 90:2592–2599. doi: 10.3382/ps.2011-01446. [DOI] [PubMed] [Google Scholar]
- Zhang C., Luo J., Yu B., Zheng P., Huang Z., Mao X., He J., Yu J., Chen J., and Chen D.. 2015. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat Sci. 102:15–21. doi: 10.1016/j.meatsci.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Zhang D., Wang X., Li Y., Zhao L., Lu M., Yao X., Xia H., Wang Y. C., Liu M. F., Jiang J., et al. 2014. Thyroid hormone regulates muscle fiber type conversion via mir-133a1. J. Cell Biol. 207:753–766. doi: 10.1083/jcb.201406068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yu B., He J., and Chen D.. 2016. From nutrient to microrna: a novel insight into cell signaling involved in skeletal muscle development and disease. Int. J. Biol. Sci. 12:1247–1261. doi: 10.7150/ijbs.16463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou T., Yu B., Yu J., Mao X., Zheng P., He J., Huang Z., Liu Y., and Chen D.. 2016. Moderately decreased maternal dietary energy intake during pregnancy reduces fetal skeletal muscle mitochondrial biogenesis in the pigs. Genes Nutr. 11:19. doi: 10.1186/s12263-016-0535-1. [DOI] [PMC free article] [PubMed] [Google Scholar]