Abstract
The objective of the current study was to determine the requirement of standardized ileal digestible (SID) CP for maximal litter gain in high-yielding lactating sows due to insufficient supply of either His, Leu, Val, Ile, or Phe. The content of SID Lys was formulated at 95% of the recommended level, while that of Met, Met+Cys, Thr, and Trp was formulated at 100% of the recommended level or slightly greater using crystalline AA. A total of 540 parity 1 to 5 sows (L×Y, DanBred, Herlev, Denmark) were included in the study from day 3 after farrowing until weaning at day 26. Sows were allocated to six dietary treatments increasing in SID CP content (96, 110, 119, 128, 137, and 152 g/kg). Litters were standardized to 14 piglets at day 3 ± 2 after farrowing. At day 3 ± 2 after farrowing and at day 26 ± 3, sow BW and back fat, and litter weight were recorded. On a subsample of 72 sows (parity 2 to 4), litters were also weighed at days 10 and 17 ± 3, and milk and blood were sampled at day 3 ± 2 d, and 10, 17 and at 24 ± 3 d in lactation. Sow body pools of protein and fat were determined on the 72 sows at days 3 ± 2 and 26 ± 3 d using the D2O dilution technique. All data were subjected to ANOVA, and to linear and quadratic polynomial contrasts. Variables with quadratic effects or days in milk × treatment interactions were analyzed using linear regression or one-slope linear broken line using the NLMIXED procedure of SAS. Average daily litter gain reached a breakpoint at 125 g SID CP/kg (as-fed). Multiparous sows had a greater litter gain than primiparous sows (3.33 vs. 3.02 kg/d above the breakpoint; P < 0.001) but litter size (13.1 ± 0.1) at weaning were unaffected by dietary treatment (P = 0.62). Sow BW loss was minimized at 102 g SID CP/kg. Concentrations of protein and casein in milk increased linearly with increasing SID CP (P < 0.001). Milk urea reached a minimum at 111–118 g SID CP/kg (P < 0.05) and milk fat a maximum at 116 g SID CP/kg (P < 0.05). In conclusion, 125 g SID CP/kg feed was required to maximize litter gain.
Keywords: blood metabolites, CP, crystalline AA, hyper-prolific sows, litter growth, milk composition
INTRODUCTION
Inclusion of crystalline AA in diets for lactating sows is often both environmentally and economically favorable, because it concomitantly offers the possibilities of meeting AA requirements, improving the dietary AA profile, and reducing the CP supply. In previous studies when lactating sows were fed low-CP diets supplemented with crystalline AA, apparent nitrogen utilization increased, and nitrogen losses to the environment decreased without compromising sow productivity (Huber et al., 2015; Huber et al., 2018). Furthermore, Pedersen et al. (2019) found an improvement in feed efficiency of 3% to 6% when reducing excess supply of CP by including crystalline AA.
Recently, two dose-response studies aiming at determining the dietary CP requirement for high-yielding lactating sows were carried out. Firstly, Strathe et al. (2017b) increased dietary CP by adding more soybean meal. Consequently, Lys and all other essential AA increased when dietary CP increased. It was reported that lactating sows require 135 g standardized ileal digestible (SID) CP/kg as-fed to maximize litter gain. Secondly, Højgaard et al. (2019) and Pedersen et al. (2019) expected to find a lower requirement for SID CP when increasing the inclusion of crystalline AA to improve the dietary AA profile. Therefore, they aimed at keeping SID Lys, Met, Met+Cys, Thr, and Trp at or slightly above the recommended level by inclusion of crystalline AA, while SID Leu, Ile, His, Phe, Tyr, and Val increased due to increasing dietary CP. However, no breakpoint was detected because the lowest dose of SID CP tested (116 g/kg as-fed) was too high to reveal a breakpoint. Therefore, the current study was carried out with CP supply well below the previous level.
The objective of the current study was to detect how much SID CP was required for maximal litter gain in high-yielding lactating sows due to insufficient supply of either His, Leu, Val, Ile, or Phe. SID CP was planned to be investigated in the interval of 91–151 g/kg as-fed. The content of SID Lys was formulated at 95% of the recommended level, while that of Met, Met+Cys, Thr, and Trp was formulated at 100% of the recommended level or slightly greater and added crystalline to different extent. The authors hypothesized that milk production and in turn daily litter gain was compromised if dietary CP supply was inadequate.
MATERIALS AND METHODS
The study was carried out in a commercial herd (Lemvig, Denmark), and complied with Danish laws and regulations for the humane care and use of animals in research (The Danish Ministry of Justice, 1995). Protocols were approved by The Danish Animal Experimentation Inspectorate.
Experimental Design, Animals, and Housing
The study was designed as a dose-response experiment to determine the minimum SID CP level required to maximize daily litter gain. The study included a total of 540 parity 1 to 5 crossbred sows (Danish Landrace × Danish Yorkshire, DanBred, Herlev, Denmark) mated with DanBred Duroc semen (Ornestation Mors, Redsted, Denmark). For 30 consecutive weeks, 3×6 sows stratified for parity were randomly allocated in a complete block design to 1 of 6 dietary treatments varying in SID CP concentration (Table 1). The distribution of parities (25, 26, 20, 16, and 13% of first to fifth parity, respectively) was similar in all six treatment groups and the average parity in each group was 2.7 ± 0.1.
Table 1.
Ingredients and chemical composition of the low-CP and high-CP
| Diets1 | ||
|---|---|---|
| Low-CP | High-CP | |
| Ingredient, g/kg “as-fed” | ||
| Barley | 430 | 267 |
| Wheat | 350 | 350 |
| Oat | 50.0 | 50.0 |
| Soybean meal, dehulled | 27.3 | 237 |
| Sugar beet pulp | 30.0 | 30.0 |
| Wheat bran | 34.6 | 4.6 |
| Soy oil | 16.0 | 16.0 |
| Palm oil | 7.0 | 7.0 |
| L-Lys | 9.28 | 0.88 |
| DL-Met | 1.80 | 0.35 |
| L-Thr | 2.90 | 0.08 |
| L-Trp | 1.48 | 0.00 |
| Monocalcium phosphate | 12.2 | 9.4 |
| Limestone | 12.7 | 13.2 |
| Salt | 5.0 | 5.1 |
| Choline chloride | 1.8 | 1.8 |
| Levucell SB 10 ME Titan 2 | 0.1 | 0.1 |
| Vitamin and mineral premix3 | 7.1 | 6.6 |
| Phyzyme XP4 | 0.5 | 0.5 |
| Composition, g/kg “as-fed” | ||
| DM | 861 | 868 |
| CP | 111 | 178 |
| SID5 CP | 91 | 151 |
| Fat | 46.9 | 46.5 |
| Ash | 49.7 | 54.8 |
| Calcium | 9.0 | 9.0 |
| Phosphorous | 5.6 | 5.5 |
| Energy, MJ NE/kg6 | 9.8 | 9.8 |
| Energy, Danish Feed Units/kg7 | 1.07 | 1.07 |
1Diets represent the two extreme diets corresponding to treatment 1 and 6, whereas the 4 other diets were created by mixing these two in different proportions, cf. Table 2.
2Levucell SB (Lallemand Animal Nutrition, Toulouse, France), is an active dry yeast probiotic containing the yeast Saccharomyces cerevisiae CNCM I-1079 1.0*1010 cfu/gm.
3The following amounts were provided per kg diet: 9418 IU vitamin A; 1998 IU 25-hydroxy vitamin D 3 (HyD, DSM Nutritional Products, Basel, Switzerland); 176 mg DL-alfatocoferol; 2.35 mg vitamin B1; 5.89 mg vitamin B2; 3.53 mg vitamin B6; 0.02 mg vitamin B12; 17.65 mg D-pantothenic acid; 23.54 mg niacin; 1.77 mg folic acid; 85.94 mg iron (FeSo4); 13.00 mg copper (CuSO4); 47.08 mg manganese (MnO); 0.23 mg iodine (Ca(IO3)2); 0.37 mg selenium (Na2SeO3).
4Phyzyme XP provided 500 phytase activity (FTU) per kg of diet (Danisco Animal Nutrition – DuPont, Marlborough, United Kingdom).
5Planned SID values were calculated based on SID coefficients (Pedersen and Boisen, 2002) of the feed ingredient composition.
6Calculated in Eva Pig using table values for Danish feed ingredients.
7Danish Feed Units are potential physiological energy closely related to NE (Patience, 2012).
Sows received their dietary treatments from the day after parturition (day 1) to weaning at day 26 ± 3 and litters were standardized at day 3 ± 2 to 14 piglets (average BW: 1.73 ± 0.02 kg). Sows were moved to the farrowing unit 7 d before expected farrowing and placed in individual farrowing crates. The herd had six farrowing sections and each week a block of 3×6 sows was moved into one of these sections and included in the study. Within each section, sows were randomly assigned a crate. Each crate was equipped with a covered creep area with an infrared censored heating lamp (VengSystem A/S, Roslev, Denmark) that gradually regulated the temperature from 34°C at farrowing to 22°C 15 d after farrowing. Room temperature was kept at 20°C and the air was ventilated using negative pressure by wall inlets. Artificial light was on from 0630 to 1530 hours.
Sows and piglets were managed, treated and vaccinated by the stock personnel according to the general routines of the herd. On days 3 or 4 after farrowing, all piglets were tail docked and given iron injection (0.5 mL; Solofer Vet., Pharmacosmos A/S, Holbæk, Denmark), and male piglets were castrated surgically using post-operative analgesia (0.1 mL; Melovem, Dopharma B.V., VX Raamsdonksveer, The Netherlands). Furthermore, iron was provided to the piglets as an oral supplement via the piglet water drinking nipples throughout the whole lactation period (1%; Opti-Jern, R2 Agro-Nutriscan, Hedensted, Denmark).
Diets and Feeding
Two diets; a low-CP (91 g SID CP/kg; Table 1) and a high-CP (151 g SID CP/kg), respectively, were formulated and mixed in different proportions to obtain six dietary CP levels (Table 2). Both diets were formulated based on the Danish feed evaluation system to contain 95% of the recommended level for SID Lys (Tybirk et al., 2016), while that of Met, Met+Cys, Thr, and Trp was formulated at 100% of the recommended level or slightly greater. The inclusion of crystalline Lys, Met, Thr, and Trp were greater in the low- than in the high-CP diet. In the formulation, it was taken into consideration that Met is required in larger amounts in the low-CP when Cys is insufficient in the diet (Ball et al., 2006), resulting in Met content being greater and Met+Cys being lower in the low-CP diet than in the high-CP diet. Furthermore, Trp increased slightly due to the increase in soybean meal. The low-CP diet was formulated to have lower SID contents of Leu, Ile, His, Phe, Tyr, and Val than the high-CP diet. Barley, wheat, soybean meal, and sugar beet pulp were the main ingredients. Both diets were formulated to be isoenergetic based on the Danish feed evaluation system (Danish Feed Units) which is a potential physiological energy system closely related to the NE system (Patience, 2012). Furthermore, they were formulated to ensure that the six dietary treatments would contain increasing CP content by the greater inclusion of soybean meal at the expense of barley.
Table 2.
Proportions of the low- and high-CP diets and analyzed chemical compositions of the six dietary treatments (as-fed)1,2
| Dietary treatments | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Proportions, % | ||||||
| Low-CP | 100.0 | 74.5 | 58.2 | 41.8 | 25.5 | 0.0 |
| High-CP | 0.0 | 25.5 | 41.8 | 58.2 | 74.5 | 100.0 |
| Chemical composition, g/kg | ||||||
| DM | 876 | 877 | 877 | 877 | 878 | 878 |
| CP | 117 | 133 | 143 | 153 | 163 | 178 |
| Fat | 41.3 | 41.1 | 40.9 | 40.7 | 40.5 | 40.3 |
| Ash | 44.2 | 45.6 | 46.5 | 47.4 | 48.3 | 49.8 |
| Calcium | 7.9 | 7.9 | 7.8 | 7.7 | 7.7 | 7.6 |
| Phosphorous | 5.3 | 5.3 | 5.2 | 5.2 | 5.2 | 5.2 |
| Energy, Danish Feed Units/kg3 | 1.09 | 1.09 | 1.09 | 1.09 | 1.09 | 1.09 |
| Total amino acids, g/kg | ||||||
| Lys | 8.63 | 8.84 | 8.96 | 9.07 | 9.19 | 9.36 |
| Met | 3.01 | 2.96 | 2.93 | 2.90 | 2.87 | 2.82 |
| Met + Cys | 5.20 | 5.36 | 5.46 | 5.56 | 5.66 | 5.80 |
| Thr | 5.83 | 6.01 | 6.12 | 6.23 | 6.34 | 6.51 |
| Trp | 1.89 | 2.02 | 2.10 | 2.18 | 2.26 | 2.39 |
| Ile | 3.77 | 4.62 | 5.16 | 5.70 | 6.25 | 7.10 |
| Leu | 7.27 | 8.68 | 9.56 | 10.45 | 11.35 | 12.73 |
| His | 2.49 | 2.96 | 3.26 | 3.56 | 3.86 | 4.33 |
| Phe | 5.07 | 5.99 | 6.56 | 7.14 | 7.73 | 8.63 |
| Phe + Tyr | 8.82 | 10.45 | 11.46 | 12.50 | 13.53 | 15.13 |
| Val | 4.92 | 5.74 | 6.25 | 6.78 | 7.30 | 8.12 |
| Digestible amino acids, g/kg4 | ||||||
| SID CP | 96 | 110 | 119 | 128 | 137 | 152 |
| SID Lys | 7.93 | 8.01 | 8.05 | 8.08 | 8.12 | 8.16 |
| SID Met | 2.82 | 2.75 | 2.71 | 2.66 | 2.61 | 2.54 |
| SID Met + Cys | 4.33 | 4.52 | 4.65 | 4.77 | 4.90 | 5.08 |
| SID Thr | 5.11 | 5.19 | 5.25 | 5.30 | 5.35 | 5.42 |
| SID Trp | 1.62 | 1.74 | 1.81 | 1.88 | 1.95 | 2.06 |
| SID Ile | 3.12 | 3.91 | 4.40 | 4.91 | 5.42 | 6.20 |
| SID Leu | 6.04 | 7.30 | 8.09 | 8.89 | 9.70 | 10.94 |
| SID His | 2.10 | 2.53 | 2.81 | 3.09 | 3.36 | 3.79 |
| SID Phe | 4.22 | 5.06 | 5.58 | 6.12 | 6.66 | 7.49 |
| SID Phe + Tyr | 7.22 | 8.72 | 9.66 | 10.61 | 11.57 | 13.05 |
| SID Val | 3.96 | 4.73 | 5.21 | 5.71 | 6.20 | 6.95 |
1Diets were fed to sows from the day after farrowing (day 1) until weaning (days 26 ± 3).
2Mean values of the analyzed content of the four batches of the low-CP diet (treatment 1) and the high-CP diet (treatment 6), respectively. Treatment 2 through 5 was calculated based on the inclusion level of the low-CP- and high-CP diet.
3Danish Feed Units are potential physiological energy closely related to NE (Patience, 2012).
4The content of SID CP and AA were calculated based on analyzed total values, inclusion level of treatment 1 and 6, and on SID digestibility coefficients (Pedersen and Boisen, 2002) of the feed ingredients.
From farrowing until weaning, the sows were fed three equally sized portions daily. The feedings occurred between 0430 and 0900 hours, 1200 and 1630 hours, and 1900 and 2400 hours in 6-, 8-, and 10-h intervals, respectively. At day 3, the total feed allowance was 3.3 kg/d and it was gradually increased until day 17 of lactation to a maximum of 7.5 kg/d for primiparous sows and 8.4 kg/d for multiparous sows. This feeding strategy allowed for an ADFI throughout lactation of 6.2 kg and 6.9 kg for primi- and multiparous sows, respectively. Daily, the feed allowance was adjusted on an individual basis, and sows with major feed refusals had their feed allowance reduced. Feed refusals were not recorded, but in general, sows ate their daily ration.
Sows were provided feed by air-assisted transport using a SpotMix feeding system (Schauer Agrotronic, Prambachkirchen, Austria) to avoid that feed residues from previous feedings were mixed in the pipes. This feeding system allowed usage of individual feeding curves and mixing of the right proportion of the low- and high-CP diet to target the dietary CP level planned for individual sows and the weight of each component was recorded at each meal for individual sows. Water was added just before the feed reached the trough. Sows had free access to water from drinking nipples in the trough.
Feed Sampling and Analysis
The low- and high-CP diets were manufactured (DLG, Tjele, Denmark) four times throughout the study with approximately 8-wk intervals. At each manufacturing, 10 kg samples were taken of each diet during the production process. Subsequently, each sample was split into subsamples using a 32-slot riffle sample divider (Rationel Kornservice, Esbjerg, Denmark). In total, two subsamples per diet were analyzed in duplicates at two commercial feed testing laboratories (Eurofins Steins Laboratory A/S, Vejen, Denmark; Evonik Nutrition & Care GmbH, Frankfurt, Germany) following the European Commission Directives [EC] 64/1998 and [EC] 152/2009.
Based on registrations of individual daily feed allowances of the low-CP diet and the high-CP diet for the individual sow and the chemical analysis of the four batches of the two diets, the dietary composition was calculated for individual sows and formed the basis for calculating the average chemical composition of treatments 1 through 6 (Table 2). SID coefficients (Pedersen and Boisen, 2002) for the feed ingredient composition were applied to the total analyzed chemical composition of the diets. The mean SID CP content of the six dietary treatments provided for the 540 sows were 96, 110, 119, 128, 137, and 152 g/kg as-fed, and for the 72-sows (subset of the 540 sows) it was 92, 107, 116, 125, 135, and 149 g/kg as-fed.
Overall, dietary concentrations of SID Lys, Met, Met+Cys, Thr, and Trp were almost constant across dietary treatments due to a greater inclusion of crystalline AA in the low-CP diet than in the high-CP diet. When comparing low and high SID CP diets (treatments 1 and 6, respectively), the deviations of SID Lys, Met, Met + Cys, and Thr amounted to 3, 10, 15, and 6%, respectively. The content of Trp varied by 21% from highest to lowest concentration due to the planned increase in soybean meal from treatment 1 to 6. As planned, SID Leu, Ile, His, Phe, Tyr, and Val varied considerably more (50, 45, 45, 44, 45, and 43%, respectively).
When comparing to Danish recommendations, SID Lys varied marginally between 94% and 97% of recommended level (Table 3) and was on purpose supplied slightly below (~5%), which was fulfilled. Furthermore, the supplied levels ensured that concentrations of SID Met, Met+Cys, Thr, and Trp were equally limiting or supplied in slight excess of Lys across dietary treatments. However, Met+Cys was slightly below the recommended level in the low-CP diet, and amounted to 86% of the Danish recommendations. Furthermore, Lys was slightly undersupplied to prevent underestimation of the ratios of Leu, Ile, His, Phe, Tyr, and Val relative to Lys which was planned to be more limiting than Lys in the low-CP-diet and less limiting than Lys in the high-CP diet.
Table 3.
Analyzed content of SID protein and AA in percentage of Danish recommendations (Tybirk et al., 2016)
| Standardized ileal digestible1 | Analyzed content, % of recommended level2 | |||||
|---|---|---|---|---|---|---|
| Dietary treatment | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Protein | 70 | 81 | 87 | 94 | 101 | 112 |
| Lys | 94 | 95 | 96 | 96 | 97 | 97 |
| Met | 105 | 102 | 101 | 99 | 98 | 95↓ |
| Met + Cys | 86↓ | 90↓ | 93↓ | 95↓ | 98 | 102 |
| Thr | 94 | 95 | 96 | 97 | 98 | 100 |
| Trp | 96 | 103 | 108 | 112 | 116 | 123 |
| Ile | 66↓ | 83↓ | 94↓ | 105 | 116 | 133 |
| Leu | 62↓ | 75↓ | 83↓ | 92↓ | 100 | 113 |
| His | 64↓ | 77↓ | 86↓ | 94↓ | 103 | 116 |
| Phe | 92↓ | 110 | 122 | 134 | 145 | 164 |
| Phe + Tyr | 76↓ | 92↓ | 102 | 112 | 122 | 138 |
| Val | 62↓ | 74↓ | 82↓ | 90↓ | 97 | 109 |
1Standardized ileal digestible: The content of SID CP and AA were calculated based on analyzed total values, inclusion level of treatment 1 and 6, and on SID digestibility coefficients (Pedersen and Boisen, 2002) of the feed ingredients.
2According to Danish recommendations (Tybirk et al., 2016), values marked with ↓ denote AA that were more limiting than Lys within each diet, whereas AA without any marks were supplied in excess of Lys or were equally limiting.
Data Collection and Chemical Analysis
At standardization and at weaning (referred to as 3 and 26 days in milk [DIM], respectively), BW of sows was measured using a walk-in scale (Bjerringbro Vægte ApS, Bjerringbro, Denmark), and back fat (BF) thickness of sows was measured by ultrasonography at the P2 site using a Sono-Grader II (Renco Corporation, Golden Valley, MN, United States). In the following reproductive cycle, weaning-to-estrus interval (WEI) and total born piglets were registered. Litter weight was recorded at 3 ± 2 and 26 ± 3 DIM using a weight trolley (Bjerringbro Vægte ApS, Bjerringbro, Denmark).
On a subsample of 72 parity 2 to 4 sows, additional measurements were made. Litter weight were also recorded at 10 and 17 DIM (±3 d). Blood and milk were sampled from the sows on day 3 ± 2, and at 10, 17, and 24 DIM (±3 d). On each sampling day, blood was drawn from the jugular vein 4 h (3–5) after feeding and collected into sodium heparin vacutainer tubes (9 mL Vacuette NH Sodium Heparin; Greiner Bio One International GmbH, Kremsmünster, Austria). The samples were stored on ice until centrifugation at 1,558 × g for 12 min at 4°C. Plasma was harvested and stored at −20°C until analysis. Plasma was analyzed for concentrations of plasma urea nitrogen (PUN), creatinine, NEFA, triacylglycerol (TAG), glucose and lactate by spectrophotometry according to standard procedures (ADVIA 1800 Clinical Chemistry System, Siemens Healthcare A/S, Ballerup, Denmark). Before milk sampling, piglets were removed from the sow for at least 30 min and then sows were given 2 mL i.m. injection of oxytocin (10 IU/mL; Intervet Denmark A/S, Ballerup, Denmark) to induce milk letdown. Milk (80 mL) was sampled manually from 4 to 5 teats, filtered through gauze and stored at −20°C until analysis or freeze drying. Milk was analyzed for concentrations of DM, lactose, fat, true protein, casein, and urea by infrared spectroscopy (MilkoScan 4000, Foss Electric, Hillerød, Denmark). Freeze dried milk was analyzed for concentrations of total AA by ion exchange chromatography (Biochrom 20 plus, Biochrom Ltd, Cambridge, United Kingdom) according to the European Commission Directive [EC] 152/2009.
Deuterium oxide (D2O) dilution space was measured on 3 ± 2 and 26 ± 3 DIM to derive the body water content using the D2O dilution technique as described by Theil et al. (2002). An initial blood sample was drawn from the jugular vein and then, a dose of 0.0425 g D2O solution (40%; Sigma Aldrich, Brøndby Denmark) per kg BW was injected i.m. in the neck of the sow. The exact amount of D2O solution injected was determined from the weight of the syringe before and after injection. Another blood sample was collected 5 to 7 h after injection after D2O was equilibrated with body water. The samples were centrifuged, and plasma was harvested and stored at −20°C until analysis.
Calculations and Statistical Analyses
Average daily gain of the litter was calculated as the difference between the litter weight at standardization and at weaning inclusive weights of dead piglets or piglets removed from the litter in the experimental period, divided by number of days. The daily milk yield was estimated based on litter ADG and the litter size as described by Hansen et al. (2012). The gross energy of milk was estimated, using the equation reported by Chwalibog (2006): GE (MJ/kg) = (38.9, MJ/kg × milk fat, % + 23.8, MJ/kg × milk protein, % + 16.3, MJ/kg × lactose, %)/100%. The total D2O dilution space was calculated as described by Theil et al. (2002). Body pools of protein and fat at 3 ± 2 and 26 ± 3 DIM were estimated from D2O dilution space, BF measurements, and BW according to equations by Rozeboom et al. (1994) developed for Landrace × Yorkshire gilts.
All statistical analyses were conducted using SAS Enterprise Guide 7.1 (SAS Inst. Inc., Cary, NC, United States). For the 540-sow dataset, the following model was used to analyze the response variables:
| (1) |
in which Yijk is the response variable, µ is the overall mean, α i is the fixed effect of dietary treatment (i = 1,…,6), β j is the fixed effect of parity (j = primi or multi), ν k is the random effect of block (k = 1, 2,…., 30) with sow as the experimental unit, and ε ijk is the random error component, which was assumed to be N (0, σ 2). For analysis of changes in sow BW and BF, and ADG of litter, initial measures of BW, BF and litter weight were used as covariates. When estimating the least squared means, the skewed distribution between primi- and multiparous sows were considered. The interaction between dietary treatment and parity was tested but was not significant (P > 0.05) and consequently not included in the final model.
For the 72-sow dataset, repeated measures of plasma metabolites, milk composition and ADG of litter were analyzed using the following model:
| (2) |
in which Yijkl is the response variable, µ is the overall mean and α i is the fixed effect of dietary treatment (i = 1,…, 6), β j is the effect of DIM (j = 3, 10, 17, 24), αβ ij is the interaction term between dietary treatment and DIM, γDjl is a covariate to account for the actual day in milk (D ranging from −3 to +3) for the lth sow within jth DIM, ν k is the random effect of block (k = 1, 2,…., 6), ω jl is the random effect of the lth sow (l = 1,2,….72) in the jth DIM and ε ijkl is the random error component which was assumed to be N (0, σ 2). For litter ADG, litter weight at standardization were used as a covariate. Body pools and changes in body pools of protein and fat were analyzed using equation 2 without β j. Sow BW was used as a covariate when analyzing changes in body protein and fat pools. Plasma concentrations of NEFA and lactate were log transformed to obtain variance homogeneity.
Most traits were subjected to ANOVA using the MIXED procedure of SAS. However, farrowing rate and occurrence of piglets within litters below 5 kg at weaning was considered as binomial responses and were analyzed using a logistic regression performed using PROC GLIMMIX in SAS.
Results on normally distributed data are given as least squared means and SEM. Results on log-transformed data are given as back-transformed estimates and the 95% confidence intervals. Statistical significance was declared at P < 0.05. The Tukey-Kramer test was used in multiple comparisons of means to adjust the P-values. Orthogonal polynomial contrasts were used to evaluate linear and quadratic effects of SID CP. Coefficients of the orthogonal polynomial contrasts were generated by the IML procedure of SAS using actual means of SID CP/kg for each dietary treatment. Statistical significance was declared at P < 0.05 and trends at 0.05 ≤ P ≤ 0.10.
In addition, one-slope linear and quadratic broken-line models were fitted via the NLMIXED procedure of SAS to estimate breakpoints for variables that showed quadratic significant effects or trends of SID CP and/or treatment × DIM interactions (Robbins et al., 2006; Gonçalves et al., 2016). Based on recordings of the daily feed allowances of the individual sow, chemical analysis of the low-CP and high-CP diets, and digestibility coefficients of CP for the diets, an average SID CP concentration was calculated for the individual sow and used as the explanatory variable. The linear broken-line model consists of two-line segments: a straight line with a slope (positive or negative) and a plateau.
When the plateau was located at Xi ≥ ω, the model for Xi < ω, can be written as:
| (3) |
When the plateau was located at Xi ≤ ω, the model for Xi > ω, can be written as:
| (4) |
Yij is the observed response variable associated with the ith sow, within the jth block. All sows were randomly assigned to a specific concentration of SID CP in g/kg feed, Xi. For the model, φ is the horizontal asymptote of the plateau (maximum/minimum response), β is the slope for the increasing/decreasing straight line segment having values of Xi smaller or greater than the plateau, ω is the breakpoint of SID CP in g/kg feed, bj is the random effect of block (j = 1, 2,…., 30) being , and ε ij are the random errors that are assumed to be . Differently from equations 3 and 4, the quadratic broken line has either (ω − Xi) or (Xi − ω) squared, depending on whether the plateau was located below or above the breakpoint. When there was a significant effect of parity (primi vs. multi) or DIM, the fixed effect of parity or DIM was incorporated both in β and ω. When no breakpoint was detected, a linear regression model was fitted. The output of the models was evaluated according to the following criteria and only the best fitting model, if any converged, were presented in the results: 1) Akaike information criteria and −2 log likelihood fit statistics, where smaller values indicate a better fit to data. 2) The P-value for the slope of the line segment below/above the breakpoint where P-values below 0.15 were accepted for breakpoint analysis. 3) Critical evaluation of the parameter estimates and their gradients, and careful inspection of the graphical output was also considered important (Robbins et al., 2006), and 4) confidence intervals (upper and lower) were within the studied range of SID CP.
RESULTS
Litter Characteristics
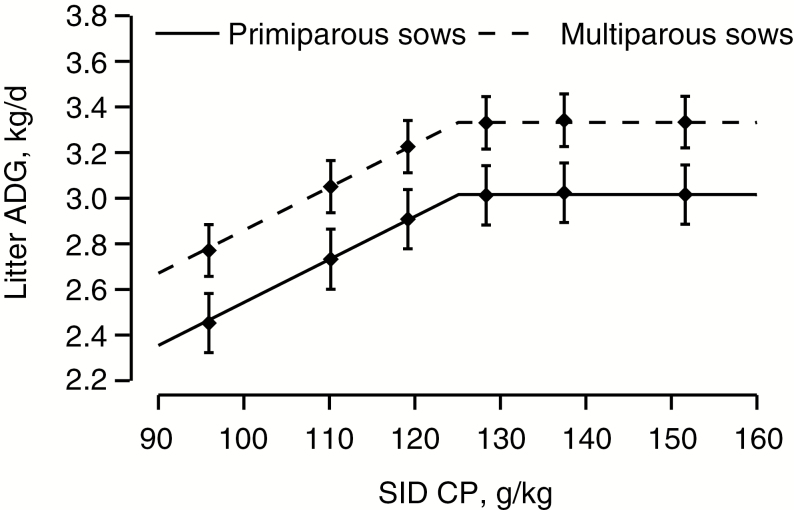
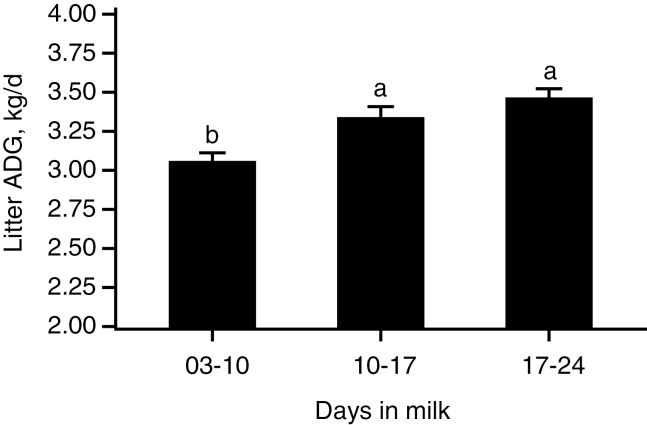
Weaning litter size (13.1 ± 0.1; Table 4) was similar between the six dietary treatments (P = 0.62), but proportion of piglets within litters below 5 kg at weaning decreased linearly as SID CP increased (P < 0.001). According to results of the linear broken-line model for litter ADG (days 3 to 26), the estimated SID CP requirement to maximize ADG was 125 g SID CP/kg (Figure 1; P < 0.001). Litter ADG differed between primi- and multiparous sows (P < 0.001) and reached a plateau at 3.02 and 3.33 kg/d, respectively (P < 0.001). Average daily litter gain increased as lactation progressed (P < 0.001; Figure 2).
Table 4.
Effect of increasing SID CP on sow and litter performance, and subsequent reproduction (n = 540)1
| Dietary treatment, SID CP g/kg2 | Parity | P-value3,4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 96 | 110 | 119 | 128 | 137 | 152 | |||||||||
| Item | 1 | 2 | 3 | 4 | 5 | 6 | SEM | Primi | Multi | SEM | Trt | Parity | Lin | Quad |
| Sows | ||||||||||||||
| Parity | 2.7 | 2.7 | 2.6 | 2.7 | 2.6 | 2.7 | 0.15 | 0.98 | – | NS | NS | |||
| Days in milk, d | 26 | 26 | 26 | 26 | 26 | 26 | 0.14 | 27.1 | 25.6 | 0.12 | 0.41 | <0.001 | NS | NS |
| ADFI, kg | 6.3b | 6.6a | 6.5a | 6.6a | 6.5a | 6.6a | 0.06 | 5.9 | 6.7 | 0.06 | <0.001 | <0.001 | *** | ** |
| SID protein intake, g/d | 604f | 725e | 780d | 846c | 898b | 999a | 8.11 | 734 | 835 | 7.43 | <0.001 | <0.001 | *** | * |
| SID Lys intake, g/d | 50.0b | 52.7a | 52.7a | 53.2a | 53.0a | 53.8a | 0.60 | 47.7 | 54.2 | 0.56 | <0.001 | <0.001 | *** | ** |
| Sow BW day 3, kg | 255 | 252 | 252 | 252 | 249 | 254 | 2.80 | 205 | 268 | 2.47 | 0.62 | <0.001 | NS | NS |
| BW change (days 3–26), kg/d | −0.45b | −0.24a | −0.28ab | −0.30ab | −0.25a | −0.29ab | 0.06 | −0.34 | −0.29 | 0.05 | <0.05 | 0.34 | NS | * |
| Sow BF day 3, mm | 15.9 | 15.2 | 15.7 | 15.8 | 16.1 | 15.6 | 0.32 | 15.2 | 15.9 | 0.28 | 0.35 | <0.05 | NS | NS |
| BF change (days 3–26), mm/d | −0.13 | −0.15 | −0.14 | −0.13 | −0.14 | −0.13 | 0.01 | −0.15 | −0.13 | 0.01 | 0.44 | <0.05 | NS | NS |
| Piglets | ||||||||||||||
| Litter size day 3 | 14.0 | 14.0 | 14.0 | 14.0 | 14.0 | 14.0 | – | – | – | – | – | |||
| Litter size day 26 | 13.0 | 13.1 | 13.1 | 13.2 | 13.2 | 13.0 | 0.10 | 13.2 | 13.0 | 0.09 | 0.62 | <0.05 | NS | NS |
| Litter weight day 3, kg | 24.3 | 24.5 | 24.2 | 23.6 | 24.2 | 24.4 | 0.49 | 21.9 | 25.0 | 0.44 | 0.81 | <0.001 | NS | NS |
| ADG of litter (day 3–26), kg/d | 2.69c | 2.97b | 3.15ab | 3.25a | 3.26a | 3.25a | 0.06 | 2.94 | 3.15 | 0.05 | <0.001 | <0.001 | *** | *** |
| Piglets within litters, below 5 kg at weaning, % | 12.4a | 6.5b | 6.9ab | 4.6b | 4.5b | 4.3b | – | 6.9 | 5.8 | – | <0.001 | 0.32 | *** | NS |
| Subsequent reproduction | ||||||||||||||
| Weaning-to-estrus interval, d | 5.0 | 4.6 | 4.9 | 5.0 | 5.5 | 5.1 | 0.46 | 6.7 | 4.4 | 0.38 | 0.85 | <0.001 | NS | NS |
| Farrowing rate, % | 96.7 | 91.0 | 95.5 | 97.7 | 94.2 | 96.8 | – | 97.2 | 95.2 | – | 0.35 | 0.33 | NS | NS |
| Total born piglets | 20.0 | 19.5 | 20.1 | 20.2 | 19.5 | 19.9 | 0.40 | 18.6 | 20.3 | 0.32 | 0.76 | <0.001 | NS | NS |
| Response | Model5 | BP6 | Equation | P-value7 | ||||||||||
| ADFI, kg | LB | 105 ± 6.1 | Yi = 6.0primi or 6.8multi − 0.03 × (105 − Xi) for Xi < 105 | 0.13 | ||||||||||
| SID protein intake, g/d | L | Yi = 533primi or 635multi + 7.1 × (Xi − 96) for Xi > 96 | <0.001 | |||||||||||
| SID Lys intake, g/d | LB | 100 ± 1.0 | Yi = 48primi or 55multi − 0.75 × (100 − Xi) for Xi < 100 | <0.001 | ||||||||||
| Sow BW change, kg/d | LB | 102 ± 4.2 | Yi = −0.27 − 0.03 × (102 − Xi) for Xi < 102 | 0.12 | ||||||||||
| ADG of litter, kg/d | LB | 125 ± 2.9 | Yi = 3.02primi or 3.33multi − 0.019 × (125 − Xi) for Xi < 125 | <0.001 | ||||||||||
a–fWithin a row, values without common superscript letters, differ (P < 0.05).
1For litter ADG, litter weight at day 3 was used as a covariate in the model. For sow BF change, sow BF thickness at day 3 was used as covariate in the model.
2Treatments 1 through 6: The calculated mean SID CP content for the dietary treatment fed to sows in the given treatment group.
3 P-values for the dietary treatment (Trt) and Parity are from the ANOVA test using the MIXED procedure of SAS. The interaction, Trt × Parity was not significant (P > 0.05).
4Orthogonal polynomial contrasts were used to evaluate linear (lin) and quadratic (quad) effects of SID CP: NS = not significant, *<0.05, **<0.01, ***<0.001.
5Best fitting model, L = linear line, LB = linear broken-line.
6BP = breakpoint for the linear broken-line.
7The P-value for the slope where P-values below 0.15 were accepted.
Figure 1.
The effect of increasing SID CP on litter ADG based on the 540-sow dataset, described by linear broken-line models: Litter ADG increased until a breakpoint at 125 g SID CP/kg and reached a plateau at 3.02 kg/d and 3.33 kg (P < 0.001) for primi- and multiparous sows, respectively. For Xi < 125 ± 2.9, ADG = 3.02primi or 3.33multi - 0.019 × (125 - Xi), and 95% confidence interval for the breakpoint [119;131]. The value, Xi, is the concentration of SID CP, g/kg, for the individual sow, i. The symbols, ♦, are the least squared means for dietary treatment 1 through 6 (96, 110, 119, 128, 137, and 152 g SID CP). The vertical lines (|) are the 95% CI for the least squared means within each treatment.
Figure 2.
Litter ADG, for the 72-sow dataset, at 3–10, 10–17, and 17–24 d in milk (P <0.001). Error bars indicate the SEM.
Sow Performance
Using a linear broken-line model, ADFI was maximized at 105 g SID CP/kg to 6.0 kg/d and 6.8 kg/d for primi- and multiparous sows, respectively. For sows fed the low CP diet with 96 g SID CP/kg, the ADFI was slightly reduced by 4.4% and 5.0% to 5.7 kg/d and 6.5 kg/d, for primi- and multiparous sows, respectively (Table 4). Likewise, the daily Lys intake was slightly reduced when sows were fed below 100 g SID CP/kg. Maximum average daily Lys intake was 48 g/d and 55 g/d for primi- and multiparous sows, respectively, whereas at 96 g SID CP/kg the Lys intake was reduced by 6.3% and 5.5% to 45 g/d and 52 g/d, respectively. However, when SID CP increased from 96 to 152 g/kg, the daily SID CP intake increased linearly 75% from 533 to 931 g/d and 63% from 635 to 1,033 g/d for primi- and multiparous sows, respectively.
The dietary treatments did not affect subsequent WEI (P = 0.85), farrowing rate (P = 0.35) or total born piglets in the next litter (P = 0.76). Loss of BF thickness were not statistically different between dietary treatments (P = 0.44). Based on a linear broken-line regression model, sows required only 102 g SID CP/kg to minimize their BW loss to 0.27 kg/d. The BW loss was increased to 0.45 kg/d at 96 g SID CP/kg, which is equivalent to a total of 6 and 10 kg of weight loss during the entire lactation. There was no difference in the body weight loss between primi- and multiparous sows, respectively (P = 0.34). For the 72-sows dataset, the change in sow body fat pool from day 3 of lactation until weaning was unaffected by dietary treatments (P = 0.77; Table 5), whereas the change in body protein changed from body protein gain of 1.15 kg at or above the breakpoint of 106 g SID CP/kg to body protein loss of 0.35 kg at 92 g SID CP/kg (corresponding to dietary treatment 1 for the 72-sow dataset).
Table 5.
The effect of increasing SID CP on sow body composition and body mobilization (n = 72)1
| Dietary treatment, SID CP g/kg2 | P-value3,4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 92 | 107 | 116 | 125 | 135 | 149 | |||||
| Item | 1 | 2 | 3 | 4 | 5 | 6 | SEM | Trt | Lin | Quad |
| Body fat day 3, kg | 60.8 | 59.3 | 62.8 | 57.4 | 59.5 | 59.8 | 1.77 | 0.20 | NS | NS |
| Body protein day 3, kg | 44.0 | 44.3 | 43.7 | 44.6 | 44.2 | 44.2 | 0.30 | 0.23 | NS | NS |
| Body fat change (days 3–26), kg | −14.0 | −12.3 | −14.2 | −14.1 | −13.3 | −15.8 | 2.10 | 0.77 | NS | NS |
| Body protein change (days 3–26), kg | −0.40b | 1.11ab | 1.12ab | 0.89ab | 1.71a | 1.02ab | 0.52 | <0.05 | * | * |
| Response | Model5 | BP6 | Equation | P-value7 | ||||||
| Body protein change (days 3–26), kg | LB | 106 ± 7.3 | Yi = 1.15 − 0.1 × (106 − Xi) for Xi < 106 | 0.14 | ||||||
abWithin a row, values without common superscript letters, differ (P < 0.05).
1For changes in pools of body fat and protein, sow BW and back fat thickness was used as a covariate in the model, respectively.
2Treatment 1 through 6: The calculated mean SID CP content for the dietary treatment fed to sows in the given treatment group.
3 P-values for the dietary treatment (Trt) are from the ANOVA test using the MIXED procedure of SAS.
4Orthogonal polynomial contrasts were used to evaluate linear (lin) and quadratic (quad) effects of SID CP: NS = not significant, *<0.05.
5Best fitting model, L = linear line, LB = linear broken-line.
6BP = breakpoint for the linear broken-line.
7The P-value for the slope where P-values below 0.15 were accepted.
Plasma Metabolites
Plasma NEFA was not affected by dietary CP concentration (P = 0.31) but was greatest in early lactation and decreased as lactation progressed (P < 0.001; Table 6). Based on a linear broken-line analysis, the plasma concentration of creatinine was at 3, 10, 17, and 24 DIM maximized at and above a breakpoint of 118 g SID CP/kg Furthermore, linear broken-line analyses showed that for 3, 10, and 17 DIM, PUN was minimized at 104, 103, and 101 g SID CP/kg, respectively, whereas PUN increased linearly at 24 DIM from 92 to 149 g SID CP/kg. Plasma glucose concentrations decreased with increasing SID CP supply until reaching a breakpoint at 123 g SID CP/kg.
Table 6.
Effect of increasing SID CP on plasma creatinine, urea, glucose, TAG, lactate, and NEFA at 3, 10, 17, and 24 DIM (n = 72)
| Dietary treatment, SID CP g/kg1 | DIM | P-value2,3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 92 | 107 | 116 | 125 | 135 | 149 | ||||||||||||
| Item | 1 | 2 | 3 | 4 | 5 | 6 | SEM | 3 | 10 | 17 | 24 | SEM | Trt | DIM | Trt×DIM | Lin | Quad |
| Creatinine, µmol/L | 145c | 144c | 157ab | 160a | 157ab | 147bc | 4.6 | 161a | 154b | 143d | 148c | 2.37 | <0.05 | <0.001 | 0.56 | NS | ** |
| Urea, mmol/L | 1.40e | 1.93de | 2.33d | 3.23c | 3.95b | 4.92a | 0.16 | 2.21d | 2.82c | 3.18b | 3.63a | 0.09 | <0.001 | <0.001 | <0.001 | *** | * |
| Glucose, mmol/L | 5.69 | 5.55 | 5.43 | 5.35 | 5.30 | 5.43 | 0.12 | 5.25c | 5.53ab | 5.65a | 5.41c | 0.08 | <0.10 | <0.001 | 0.33 | * | † |
| TAG, mmol/L | 0.22 | 0.23 | 0.20 | 0.22 | 0.20 | 0.21 | 0.01 | 0.21 | 0.20 | 0.21 | 0.23 | 0.01 | 0.71 | 0.24 | 0.31 | NS | NS |
| Lactate, mmol/L | 1.6 [1.4;1.9] | 1.7 [1.4;1.9] | 1.5 [1.3;1.7] | 1.4 [1.2;1.6] | 1.4 [1.2;1.6] | 1.3 [1.1;1.6] | – | 1.5 [1.4;1.7] | 1.5 [1.3;1.7] | 1.4 [1.3;1.6] | 1.5 [1.3;1.6] | 0.15 | 0.20 | 0.73 | * | NS | |
| NEFA, µmol/L | 106 [70;160] | 85 [55;132] | 122 [81;184] | 150 [99;226] | 108 [71;164] | 95 [62;144] | – | 248a [174;353] | 163a [116;229] | 77b [55;110] | 46c [32;64] | 0.31 | <0.001 | 0.79 | NS | NS | |
| Response | Model4 | DIM | BP5 | Equation | P-value6 | ||||||||||||
| Creatinine, µmol/L | LB | – | 118 ± 5.7 | Yi = 165DIM3 or 157DIM10 or 148DIM17 or 151DIM24 − 0.45 × (118 − Xi) for Xi < 118 | <0.05 | ||||||||||||
| Glucose, mmol/L | LB | – | 123 ± 9.3 | Yi = 5.16DIM3 or 5.42DIM10 or 5.49DIM17 or 5.30DIM24 + 0.01 × (123 − Xi) for Xi < 123 | 0.06 | ||||||||||||
| Urea nitrogen, mmol/L | LB | 3 | 104 ± 6.6 | Yi = 1.47 + 0.04 × (Xi − 104) for Xi > 104 | <0.01 | ||||||||||||
| LB | 10 | 103 ± 3.1 | Yi = 1.29 + 0.08 × (Xi − 103) for Xi > 103 | <0.0001 | |||||||||||||
| LB | 17 | 101 ± 2.6 | Yi = 1.24 + 0.09 × (Xi − 101) for Xi > 101 | <0.0001 | |||||||||||||
| L | 24 | Yi = 1.43 + 0.08 × (Xi − 92) for Xi > 92 | <0.0001 | ||||||||||||||
abcdeWithin a row, values without common superscript letters, differ (P < 0.05).
1Treatment 1 through 6: The calculated mean SID CP content for the dietary treatment fed to sows in the given treatment group.
2 P-values for the dietary treatment (Trt) and DIM are from the ANOVA test using the MIXED procedure of SAS.
3Orthogonal polynomial contrasts were used to evaluate linear (lin) and quadratic (quad) effects of SID CP: NS = not significant, †<0.10, *<0.05, **<0.01, ***<0.001.
4Best fitting model, L = linear line, LB = linear broken-line.
5BP = breakpoint for the linear broken-line.
6The P-value for the slope where P-values below 0.15 were accepted.
Milk Production
The estimated daily milk yield and milk constituents are presented in Table 7. When fitting linear broken line for milk yield, there was no breakpoint observed at 3 and 10 DIM. However, at 17 DIM the milk yield was maximized to 15.5 kg/d at and above 120 g SID CP/kg and at 24 DIM the milk yield was maximized to 15.9 kg/d at and above 125 g SID CP/kg. At 10 DIM, linear broken-line analysis showed that milk fat content was constant at 8.01% when sows were fed at and below 116 g SID CP, whereafter the milk fat content decreased to 6.75% at 149 g SID CP/kg. Milk urea content was minimized between 92 g SID CP/kg and breakpoints of 118, 115, and 111 g SID CP/kg at 10, 17, and 24 DIM, respectively, where after it increased with elevated dietary CP levels (P < 0.05). The concentration of milk protein and casein increased linearly from 92 to 149 g SID CP/kg (P < 0.001) at 3, 10, and 24 DIM, whereas at 17 DIM, the concentration of milk protein and casein increased until a breakpoint at 138 and 135 g SID CP/kg, respectively (P < 0.001). Likewise, the concentration of essential AA in milk CP at 17 DIM increased until a breakpoint of 132 g SID CP/kg (P < 0.01), whereas at 10 DIM, there was observed a linear increase (P < 0.01) and no changes were observed at 3 DIM.
Table 7.
Effect of increasing SID CP on milk yield and milk composition at 3, 10, 17, and 24 DIM (n = 72)
| Dietary treatment, SID CP g/kg1 | DIM | P-value2,3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 92 | 107 | 116 | 125 | 135 | 149 | ||||||||||||
| Item | 1 | 2 | 3 | 4 | 5 | 6 | SEM | 3 | 10 | 17 | 24 | SEM | Trt | DIM | Trt × DIM | Lin | Quad |
| Milk yield, kg/d4 | 11.4b | 12.3ab | 12.5ab | 13.1a | 12.8a | 12.9a | 0.53 | 7.0c | 13.0b | 14.9a | 15.1a | 0.53 | <0.01 | <0.001 | <0.05 | *** | * |
| DM, % | 17.7 | 17.9 | 18.4 | 18.3 | 18.0 | 18.0 | 0.35 | 19.1a | 18.2b | 17.6c | 17.3c | 0.35 | 0.22 | <0.001 | 0.37 | NS | † |
| Lactose, % | 5.20 | 5.15 | 5.11 | 5.12 | 5.15 | 5.13 | 0.05 | 4.96d | 5.07c | 5.23b | 5.30a | 0.05 | 0.30 | <0.001 | 0.25 | NS | NS |
| Fat, % | 7.29 | 7.35 | 7.63 | 7.47 | 7.01 | 6.96 | 0.35 | 8.10a | 7.63a | 6.94b | 6.47c | 0.35 | 0.13 | <0.001 | <0.10 | NS | † |
| Protein, % | 4.35e | 4.62d | 4.76cd | 4.90bc | 5.10ab | 5.27a | 0.11 | 5.34a | 4.59c | 4.62c | 4.78b | 0.11 | <0.001 | <0.001 | <0.001 | *** | NS |
| Casein, % | 3.28e | 3.57d | 3.69cd | 3.84bc | 4.04ab | 4.14a | 0.10 | 4.19a | 3.54c | 3.57c | 3.74b | 0.10 | <0.001 | <0.001 | < .001 | *** | NS |
| Urea, mg/dL | 6.29bc | 6.48abc | 6.05c | 6.52abc | 7.24ab | 7.49a | 0.28 | 4.68c | 6.46b | 7.79a | 7.78a | 0.18 | <0.01 | <0.001 | < .05 | *** | † |
| Energy, MJ/kg5 | 4.72 | 4.80 | 4.94 | 4.90 | 4.80 | 4.80 | 0.13 | 5.24a | 4.89b | 4.65c | 4.53c | 0.13 | 0.37 | <0.001 | 0.26 | NS | † |
| Concentration, g/100 g CP | |||||||||||||||||
| Total AA | 89.9 | 89.5 | 90.3 | 90.2 | 90.2 | 90.4 | 0.50 | 90.3 | 89.7 | 90.3 | – | 0.41 | 0.63 | 0.24 | 0.15 | NS | NS |
| EAA | 40.8b | 40.7b | 41.5ab | 41.7ab | 42.0a | 42.3a | 0.30 | 42.3a | 41.1b | 41.1b | – | 0.20 | <0.001 | <0.001 | <0.001 | *** | NS |
| NEAA | 49.1 | 48.8 | 48.7 | 48.6 | 48.2 | 48.2 | 0.31 | 48.0b | 48.6b | 49.2a | – | 0.24 | <0.10 | <0.001 | 0.56 | ** | NS |
| Response | Model6 | DIM | BP7 | Equation | P-value8 | ||||||||||||
| Milk yield, kg/d | LB | 17 | 120 ± 6.9 | Yi = 15.5 − 0.09 × (120 − Xi) for Xi < 120 | <0.05 | ||||||||||||
| LB | 24 | 125 ± 10 | Yi = 15.9 − 0.08 × (125 − Xi) for Xi < 125 | 0.06 | |||||||||||||
| Fat, % | LB | 10 | 116 ± 8.3 | Yi = 8.01 − 0.04 × (Xi − 116) for Xi >116 | <0.05 | ||||||||||||
| Protein, % | L | 3 | Yi = 5.2 + 0.009 × (Xi − 92) for Xi > 92 | <0.05 | |||||||||||||
| L | 10 | Yi = 4.0 + 0.02 × (Xi − 92) for Xi > 92 | <0.001 | ||||||||||||||
| LB | 17 | 138 ± 4.5 | Yi = 5.1 − 0.02 × (138 − Xi) for Xi < 138 | <0.001 | |||||||||||||
| L | 24 | Yi = 4.3 + 0.02 × (Xi − 92) for Xi > 92 | <0.001 | ||||||||||||||
| Casein, % | L | 3 | Yi = 4.1 + 0.007 × (Xi − 92) for Xi > 92 | <0.001 | |||||||||||||
| L | 10 | Yi = 2.9 + 0.02 × (Xi − 92) for Xi > 92 | <0.001 | ||||||||||||||
| LB | 17 | 135 ± 4.0 | Yi = 3.97 − 0.02 × (135 − Xi) for Xi < 135 | <0.001 | |||||||||||||
| L | 24 | Yi = 3.3 + 0.02 × (Xi − 92) for Xi > 92 | <0.001 | ||||||||||||||
| Urea, AU9 | LB | 10 | 118 ± 7.8 | Yi = 5.84 + 0.07 × (Xi − 118) for Xi > 118 | <0.05 | ||||||||||||
| LB | 17 | 115 ± 9.5 | Yi = 7.07 + 0.05 × (Xi − 115) for Xi > 115 | <0.05 | |||||||||||||
| LB | 24 | 111± 9.5 | Yi = 7.12 + 0.04 × (Xi − 111) for Xi > 111 | <0.05 | |||||||||||||
| EAA, g/100 g CP | L | 10 | Yi = 39.9 + 0.04 × (Xi − 92) for Xi > 92 | <0.01 | |||||||||||||
| LB | 17 | 132 ± 4.8 | Yi = 42.0 − 0.06 × (132 − Xi) for Xi < 132 | <0.01 | |||||||||||||
abcdWithin a row, values without common superscript letters, differ (P < 0.05).
1Treatments 1 through 6: The calculated mean SID CP content for the dietary treatment fed to sows in the given treatment group.
2 P-values for the dietary treatment (Trt) and DIM are from the ANOVA test using the MIXED procedure of SAS.
3Orthogonal polynomial contrasts were used to evaluate linear (lin) and quadratic (quad) effects of SID CP: NS = not significant, †<0.10, *<0.05, **<0.01, ***<0.001.
4Milk yield was estimated using equations from Hansen et al. (2012).
5Gross energy of milk was estimated, using the equation: GE (MJ/kg) = (38.9, MJ/kg × milk fat, % + 23.8, MJ/kg × milk protein, % + 16.3, MJ/kg × lactose, %)/100%, given by Chwalibog (2006)
6Best fitting model, L = linear line, LB = linear broken-line.
7BP = breakpoint for the linear broken-line.
8The P-value for the slope where P-values below 0.15 were accepted.
9Urea in milk was measured in arbitrary units (AU) because the instrument was not calibrated.
DISCUSSION
SID CP Requirement
This study revealed that high-yielding lactating sows require 125 g SID CP/kg to maximize litter gain, when concentrations of SID Lys, Met, Met+Cys, Thr, and Trp were kept close to the recommended level. SID Leu, Ile, His, Phe, (Phe+Tyr), and Val increased substantially from treatment 1 to 6 and one or more of these are the underlying reason as to why the litter ADG responded negatively below the breakpoint. The inclusion of crystalline AA per kg diet went from a high level in the low-CP diet (9.29 g L-Lys, 1.80 g DL-Met, 2.90 g L-Thr, and 1.48 g L-Trp) to a low level in the high-CP diet (0.88 g L-Lys, 0.35 g DL-Met, and 0.08 g L-Thr). Previously, a 540-sow study carried out in the same herd revealed that sows require 135 g SID CP/kg to maximize litter gain when investigated in the interval 104–150 g SID CP/kg using soybean meal as the primary AA source (Strathe et al., 2017b). However, in the study by Strathe and coworkers, Lys and all other essential AA were increased concomitantly with the increase in SID CP with a fairly constant inclusion of crystalline AA across dietary treatments of maximum 3.4 g/kg L-Lys, 0.8 g/kg DL-Met, 0.8 g/kg L-Thr, and 0.2 g/kg L-Val. In another recent study by Huber et al. (2018), the ideal scenario to find the absolute minimal CP requirement was carried out by adding all limiting AA crystalline, i.e., synthetic diets. In that study, there was a maximum inclusion per kg diet of 4.2 g L-Lys HCl, 1.7 g DL-Met, 2.7 g L-Thr, 0.9 g L-Trp, 1.7 g L-Ile, 1.4 g L-Leu, 12 g L-His, 3.1 g L-Phe, and 4.0 g L-Val. Huber et al. (2018) found that total dietary CP could be reduced from 162 to 127 g/kg without any negative consequences on sow productivity. This CP level was lower than the 125 g SID CP/kg (~150 g total dietary CP/kg) found to maximize litter gain in current study, because the current study reported the amount of CP required as SID CP, whereas the study carried out by Huber and coworkers reported the total CP level. Extremely low dietary CP levels can be obtained using synthetic diets, i.e., all essential AA are added crystalline (pharma- and feed-grade). However, feeding a highly synthetic diet using AA that are not available on a feed-grade basis (L-Ile, L-Leu, L-His, and L-Phe) does not have any practical or economical application. Before the current study, a similar study with the same experimental arrangement and within the same herd found that there was no effect of dietary treatment on litter ADG when SID CP were investigated in the interval of 116 to 153 g/kg (Højgaard et al., 2019). However, in that study, the milk protein content was lower in sows receiving treatment 1 containing 116 g SID CP, indicating that those sows were marginally undersupplied with CP. Followed up by energy utilization calculations, sows that received treatment 2 (126–128 g SID CP/kg) had the greatest feed efficiency when evaluated by NE corrected for body mobilization (Pedersen et al., 2019). Findings by Højgaard et al. (2019) and Pedersen et al. (2019) thus agree with the present study in that the SID CP may be reduced to 125–128 g/kg without compromising sow productivity, milk protein content, and feed efficiency, though with the conclusion that the investigated interval of SID CP was too narrow to detect any breakpoint for litter gain (Højgaard et al., 2019; Pedersen et al., 2019).
Within the investigated range of dietary CP concentration (96–152 g SID CP/kg) and the conditions of the present study, data indicate that the dietary AA profile was most optimal around the breakpoint for litter gain of 125 g SID CP/kg. Evaluating the dietary AA profile across the dietary treatments in relation to the Danish recommendations (Tybirk et al., 2016), Val and Leu (both 62% of the recommended level) was most likely limiting milk production in dietary treatment 1, followed by His (64%), Ile (66%), and Phe+Tyr (76%; Table 3). In contrast, Met+Cys (86%), Lys (94%), Thr (94%), and Trp (96%) were all supplied much closer to the recommended level (Tybirk et al., 2016). Evaluating dietary treatment 6, all AA except Met (95%) were supplied in excess of Lys (97%). Consequently, the clear breakpoint observed between treatment 3 and 4 for litter ADG were not caused by Lys, Thr, Met or Trp, but most likely caused by Val, Leu, and/or His. In the breakpoint for litter ADG at 125 g SID CP/kg, the dietary ratios of SID Val, Leu, and His relative to Lys were 69, 107, and 37%, respectively. Especially, the SID Val:Lys-ratio of 69% was substantially lower than the 85% recommended by NRC (2012) and also lower than the 76% hitherto recommended in Denmark (Tybirk et al., 2016). However, the previous recommendations were based on few studies. In agreement with the current study, previous literature have proven that SID Val:Lys-ratios at 65%–70% does not compromise sow productivity (Paulicks et al., 2003; Craig et al., 2016; Højgaard et al., 2019).
Litter gain is the best determinant of nutrient requirements of the lactating sow (Trottier et al., 2014). However, sow BW and BF loss should also be considered to ensure sow longevity and reproductive performance in the subsequent cycle (King and Dunkin, 1986; Clowes et al., 2003). Though, in the current study, the use of litter ADG as the determinant of the protein requirement is reasonable because sow BF loss was independent of dietary CP, and BW loss was minimized at a lower SID CP level (102 g/kg) than the CP required to maximize litter gain (125 g SID CP/kg). Conversely, in the study by Strathe et al. (2017b), the BW loss of the sows was minimized at a greater SID CP level (143 g/kg) than where the litter gain was maximized (135 g/kg). Therefore, it seems feasible that the reduction in BW loss of the sows in the study carried out by Strathe et al. (2017b) was driven by the increase in dietary Lys rather than CP.
Sow Body Mobilization and Metabolism
Unaccountably, the loss of BW of up to 10 kg in current study was very low compared with previous studies where BW losses of up to 20 kg throughout lactation was observed (Højgaard et al., 2019; Strathe et al., 2017b; Pedersen et al., 2019). When operating with sow BW measurements, the total weight change might be underestimated due to greater gut fill at weaning compared with day 3. However, this issue is not different from previous studies and not of concern when comparing the differences among treatment groups. In the current study, the slightly greater BW loss observed below the breakpoint of 102 g SID CP/kg was most likely a result of muscle protein mobilization, whereas above the breakpoint, the sows seemed to gain minor amounts of body protein according to estimated body composition of the sows using the D2O dilution technique. Because 1 g of retained body protein binds approximately 4.2 g body water, whereas 1 g of retained body fat only binds around 0.17 g water (Noblet and Etienne, 1987), the positive protein retention may have minimized the total loss of BW in sows receiving 102–152 g SID CP/kg even though the sows across all dietary treatments lost substantial amounts (14.4 kg) of body fat during lactation.
The concentration of NEFA gives an indication of the body fat mobilization pattern (Revell et al., 1998; Valros et al., 2003; Mosnier et al., 2010) and as for body fat estimated from the D2O dilution technique and BF measurements, there was no difference between the dietary treatments. However, the NEFA concentration was found to peak in early lactation and decline as lactation progressed. This is in agreement with observations made by Strathe et al. (2017a) and Pedersen et al. (2016) and confirms that sows are not capable of increasing the feed intake to the same extent as the milk yield increases in early lactation (Cools et al., 2014). Though sows in the current study did not have ad-libitum access to feed, and an upper limit was set on the feed allowance to prevent the sows from stop eating. Irrespective, the greater NEFA concentration in early lactation was most likely due to sows compensating inadequate supply of energy whereby mobilization of body fat reserves occurred to support a high milk production.
Creatinine is the product of protein turnover and was expected to be an indicator of body protein mobilization (Neil et al., 1996; Heo et al., 2008; Yang et al., 2009). However, when plasma creatinine was maximized at 118 g SID CP/kg it more likely reflects the total protein mass of the sows, because a larger muscle mass have a greater energy requirement and therefore needs a greater phosphorylation rate of creatine to creatinine to provide energy (Mosnier et al., 2010).
Urea nitrogen in blood and milk are both indicators of protein quantity and protein quality and can be used as rapid parameters of dietary AA requirements for lactating sows (Coma et al., 1995). In the current study, dietary SID CP increased from dietary treatments 1 to 6, and consequently, the diets were not isonitrogenous. Therefore, the observed changes in urea nitrogen in blood and milk could be due to differences in total nitrogen intake. This was clearly reflected in PUN which increased linearly from very low levels of SID CP/kg (92–104 g) and the PUN increase was more pronounced throughout lactation as the daily CP intake increased. However, reaching a minimum in urea nitrogen concentration in milk and a maximum in milk fat content around 111–118 g SID CP/kg may indicate an improvement in protein quality offset by the addition of crystalline AA thereby improving the AA profile and minimizing the energy expenditure from catabolism of excess AA. In line with this, Pedersen et al. (2019) recently showed that energy utilization peaked when sows were fed 128 g SID CP/kg. Evidently, in the current study, lower CP intake was needed to minimize sow BW loss, maximize concentration of milk fat, and minimize concentrations of urea in plasma and milk, than that needed to maximize milk yield and litter ADG.
Milk Yield, Milk Protein, and AA Content
The milk yield in the current study was estimated using ADG of the litter and the litter size (Hansen et al., 2012). Based on the 72-sow dataset, sows were supplied enough SID CP when fed between 92 and 149 g SID CP at 3 DIM, whereas at 10 DIM the milk yield decreased by 7% from 13.3 kg/d at 125 g SID CP/kg to 12.4 kg/d at 92 g SID CP/kg. Even though the slope below the breakpoint was not statistically significant it is worth mentioning that the milk yield apparently was negatively affected by the lower SID CP supply at 10 DIM and was clearly negatively affected at SID CP levels below 120–125 g SID CP/kg at 17 and 24 DIM, like the overall ADG for the 540-sow dataset. Furthermore, these findings are supported by plasma glucose which decreased with increasing SID CP supply until reaching a breakpoint at 123 g SID CP/kg. Plasma glucose is the main substrate for milk lactose synthesis but is tightly regulated in plasma to maintain homeostasis. Since the concentration of starch and fiber was similar between the dietary treatments, it was not expected to see any dietary effects. Therefore, this breakpoint was most likely related to less mammary uptake of glucose caused by a lower demand for milk synthesis when the sow was undersupplied with SID CP.
As SID CP increased from 92 to 149 g/kg, the true milk protein and casein content increased steadily, though, with a plateau at 17 DIM being reached at 135–138 g SID CP/kg. Overall, this indicates that extra dietary CP was redirected towards milk protein synthesis. However, because the ADG of the litter did not continue to increase at SID CP levels above 125 g/kg, and because milk fat decreased above the SID CP level, it seems that piglets cannot benefit from very high protein to energy ratios in milk, and this was also observed by (Pedersen et al., 2019).
Milk protein consists of two types of proteins: caseins and whey proteins. Caseins are the most abundant in sow milk (Gallagher et al., 1997) and are the sources of dietary proteins produced by the mammary gland for the specific purpose of supplying AA to the piglets (Darragh and Moughan, 1998; Mavromichalis et al., 2001). Looking at the AA content of total milk CP, divided into total-, essential-, and nonessential AA, the overall concentration of total AA in g/100 g CP were relatively constant. In agreement with Csapo et al. (1996), the concentration of essential AA decreased throughout lactation whereas the concentration of non-essential AA increased slightly. The content of essential AA in g/100 g milk CP showed the same pattern as milk true protein and casein at 10 and 17 DIM. The increase in essential AA with increasing SID CP/kg could be ascribed to changes in the ratio of casein to whey proteins in the milk.
CONCLUSION
High-yielding lactating sows require 125 g SID CP/kg to maximize litter gain when adequate Lys, Met, Thr, and Trp were ensured by crystalline addition and the underlying reason was most likely due to adequate supply of Val, Leu or His, which amounted to 69, 107, and 37% relative to Lys, respectively.
ACKNOWLEDGMENTS
Amino acid analyses of feedstuffs and milk were sponsored by Evonik Nutrition & Care Gmbh Germany branch Denmark.
Conflict of interest statement. None declared.
LITERATURE CITED
- Ball R. O., Courtney-Martin G., and Pencharz P. B.. 2006. The in vivo sparing of methionine by cysteine in sulfur amino acid requirements in animal models and adult humans. J. Nutr. 136(6 Suppl):1682S–1693S. doi: 10.1093/jn/136.6.1682S [DOI] [PubMed] [Google Scholar]
- Chwalibog A. 2006. Nutritive value and nutrient requirements [In Danish: Næringsværdi og næringsbehov]. 7th rev. ed. Samfundslitteratur, Denmark. [Google Scholar]
- Clowes E. J., Aherne F. X., Foxcroft G. R., and Baracos V. E.. 2003. Selective protein loss in lactating sows is associated with reduced litter growth and ovarian function. J. Anim. Sci. 81:753–764. doi: 10.2527/2003.813753x [DOI] [PubMed] [Google Scholar]
- Coma J., Carrion D., and Zimmerman D. R.. 1995. Use of plasma urea nitrogen as a rapid response criterion to determine the lysine requirement of pigs. J. Anim. Sci. 73:472–481. doi: 10.2527/1995.732472x [DOI] [PubMed] [Google Scholar]
- Cools A., Maes D., Decaluwé R., Buyse J., van Kempen T. A., Liesegang A., and Janssens G. P.. 2014. Ad libitum feeding during the peripartal period affects body condition, reproduction results and metabolism of sows. Anim. Reprod. Sci. 145:130–140. doi: 10.1016/j.anireprosci.2014.01.008 [DOI] [PubMed] [Google Scholar]
- Craig A., Henry W., and Magowan E.. 2016. Effect of phase feeding and valine-to-lysine ratio during lactation on sow and piglet performance. J. Anim. Sci. 94:3835–3843. doi: 10.2527/jas.2016-0648 [DOI] [PubMed] [Google Scholar]
- Csapo J., Martin T., Csapo-Kiss Z., and Hazas Z.. 1996. Protein, fats, vitamin and mineral concentrations in porcine colostrum and milk from parturition to 60 days. International Dairy Journal. 6: 881–902. doi: 10.1016/0958-6946(95)00072-0 [DOI] [Google Scholar]
- Darragh A. J., and Moughan P. J.. 1998. The composition of colostrum and milk. In: Verstegen M. W. A., Moughan P. J., and Schrama J. W., editors. The lactating sow. Wageningen (Netherlands): Wageningen Academic Publishers; p. 3–22. [Google Scholar]
- Gallagher D. P., Cotter P. F., and Mulvihill D. M.. 1997. Porcine milk proteins: a review. Int. Dairy J. 7:99–118. doi: 10.1016/S0958-6946(96)00056-8 [DOI] [Google Scholar]
- Gonçalves M. A., Bello N. M., Dritz S. S., Tokach M. D., DeRouchey J. M., Woodworth J. C., and Goodband R. D.. 2016. An update on modeling dose-response relationships: accounting for correlated data structure and heterogeneous error variance in linear and nonlinear mixed models. J. Anim. Sci. 94:1940–1950. doi: 10.2527/jas.2015-0106 [DOI] [PubMed] [Google Scholar]
- Hansen A. V., Strathe A. B., Kebreab E., France J., and Theil P. K.. 2012. Predicting milk yield and composition in lactating sows: a Bayesian approach. J. Anim. Sci. 90:2285–2298. doi: 10.2527/jas.2011-4788 [DOI] [PubMed] [Google Scholar]
- Heo S., Yang Y. X., Jin Z., Park M. S., Yang B. K., and Chae B. J.. 2008. Effects of dietary energy and lysine intake during late gestation and lactation on blood metabolites, hormones, milk composition and reproductive performance in primiparous sows. Anim. Reprod. Sci. 88:247–255. doi: 10.1016/j.anireprosci.2008.04.031 [DOI] [PubMed] [Google Scholar]
- Huber L., de Lange C. F., Krogh U., Chamberlin D., and Trottier N. L.. 2015. Impact of feeding reduced crude protein diets to lactating sows on nitrogen utilization. J. Anim. Sci. 93:5254–5264. doi: 10.2527/jas.2015-9382 [DOI] [PubMed] [Google Scholar]
- Huber L-A., Rudar M., Trottier N. L., Cant J. P., and de Lange C. F.. 2018. Whole-body nitrogen utilization and tissue protein and casein synthesis in lactating primiparous sows fed low-and high-protein diets. J. Anim. Sci. 96:2380–2391. doi: 10.1093/jas/sky047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Højgaard C. K., Bruun T. S., Strathe A. V., Zerrahn J.-E., and Hansen C. F.. 2019. High-yielding lactating sows maintained a high litter growth when fed reduced crude protein, crystalline amino acid-supplemented diets. Livest. Sci. 226:40–47. [Google Scholar]
- King R., and Dunkin A.. 1986. The effect of nutrition on the reproductive performance of first-litter sows 4. The relative effects of energy and protein intakes during lactation on the performance of sows and their piglets. Anim. Sci. 43:319–325. doi: 10.1016/0301-6226(94)90039-6 [DOI] [Google Scholar]
- Mavromichalis I., Parr T. M., Gabert V. M., and Baker D. H.. 2001. True ileal digestibility of amino acids in sow’s milk for 17-day-old pigs. J. Anim. Sci. 79:707–713. doi: 10.2527/2001.793707x [DOI] [PubMed] [Google Scholar]
- Mosnier E., Etienne M., Ramaekers P., and Père M. C.. 2010. Metabolic status during the peri partum period affects the voluntary feed intake and the metabolism of the lactating multiparous sow. Livest. Sci. 127:127–136. doi: 10.1016/j.livsci.2009.06.023 [DOI] [Google Scholar]
- Neil M., Ogle B., and Anner K.. 1996. A two-diet system and ad libitum lactation feeding of the sow 1. Sow performance. Anim. Sci. 62:337–347. doi: 10.1017/S135772980001465X [DOI] [Google Scholar]
- Noblet J., and Etienne M.. 1987. Body composition, metabolic rate and utilization of milk nutrients in suckling piglets. Reprod. Nutr. Dev. 27:829–839. doi:10.1051/rnd:19870609 [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed. Washington (DC): The National Academies Press. [Google Scholar]
- Patience J. F. 2012. Feed efficiency in swine rev. ed. Wageningen (The Netherlands): Wageningen Academic. [Google Scholar]
- Paulicks B. R., Ott H., and Roth-Maier D. A.. 2003. Performance of lactating sows in response to the dietary valine supply. J. Anim. Physiol. Anim. Nutr. (Berl). 87:389–396. [DOI] [PubMed] [Google Scholar]
- Pedersen C., and Boisen S.. 2002. Establishment of tabulated values for standardized ileal digestibility of crude protein and essential amino acids in common feedstuffs for pigs. Acta Agric. Scand. Section A Anim. Sci. 52:121–140. doi: 10.1080/090647002320229374 [DOI] [Google Scholar]
- Pedersen T. F., Bruun T. S., Feyera T., Larsen U. K., and Theil P. K.. 2016. A two-diet feeding regime for lactating sows reduced nutrient deficiency in early lactation and improved milk yield. Livest. Sci. 191:165–173. doi: 10.1016/j.livsci.2016.08.004 [DOI] [Google Scholar]
- Pedersen T. F., Chang C. Y., Trottier N. L., Bruun T. S., and Theil P. K.. 2019. Effect of dietary protein intake on energy utilization and feed efficiency of lactating sows. J Anim Sci. 97(1):779–793. doi: 10.1093/jas/sky462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell D. K., Williams I. H., Mullan B. P., Ranford J. L., and Smits R. J.. 1998. Body composition at farrowing and nutrition during lactation affect the performance of primiparous sows: I. Voluntary feed intake, weight loss, and plasma metabolites. J. Anim. Sci. 76:1729–1737. doi: 10.2527/1998.7671729x [DOI] [PubMed] [Google Scholar]
- Robbins K. R., Saxton A. M., and Southern L. L.. 2006. Estimation of nutrient requirements using broken-line regression analysis. J. Anim. Sci. 84 Suppl:E155–E165. doi: 10.2527/2006.8413_supple155x [DOI] [PubMed] [Google Scholar]
- Rozeboom D. W., Pettigrew J. E., Moser R. L., Cornelius S. G., and el Kandelgy S. M.. 1994. In vivo estimation of body composition of mature gilts using live weight, backfat thickness, and deuterium oxide. J. Anim. Sci. 72:355–366. doi: 10.2527/1994.722355x [DOI] [PubMed] [Google Scholar]
- Strathe A. V., Bruun T. S., Geertsen N., Zerrahn J-E., and Hansen C. F.. 2017b. Increased dietary protein levels during lactation improved sow and litter performance. Anim. Feed Sci. Technol. 232:169–181. doi: 10.1016/j.anifeedsci.2017.08.015 [DOI] [Google Scholar]
- Strathe A., Bruun T., and Hansen C.. 2017a. Sows with high milk production had both a high feed intake and high body mobilization. Animal. 11:1913–1921. doi: 10.1017/S1751731117000155 [DOI] [PubMed] [Google Scholar]
- The Danish Ministry of Justice. 1995. Animal testing act, consolidation act no. 726 of September 9, 1993 (as amended by act no. 1081 of December 20, 1995). The Danish Ministry of Justice, Copenhagen, Denmark. [Google Scholar]
- Theil P. K., Nielsen T. T., Kristensen N. B., Labouriau R., Danielsen V., Lauridsen C., and Jakobsen K.. 2002. Estimation of milk production in lactating sows by determination of deuterated water turnover in three piglets per litter. Acta Agric. Scand. Section A Anim. Sci. 52:221–232. doi: 10.1080/090647002762381104 [DOI] [Google Scholar]
- Trottier N., Johnston L., and de Lange C.. 2014. Applied amino acid and energy feeding of sows. In: Farmer C., editor, The gestating and lactating sow. Wageningen (the Netherlands): Wageningen Academic Publishers; p. 117–146. [Google Scholar]
- Tybirk P., Sloth N. M., Kjeldsen N., and Shooter L.. 2016. Danish nutrient requirement standards [In Danish: Normer for næringsstoffer]. 23rd rev. ed. SEGES Danish Pig Research Centre, Axelborg, Denmark. [Google Scholar]
- Valros A., Rundgren M., Špinka M., Saloniemi H., Rydhmer L., Hultén F., Uvnäs-Moberg K., Tománek M., Krejcí P., and Algers B.. 2003. Metabolic state of the sow, nursing behaviour and milk production. Livest. Prod. Sci. 79:155–167. doi: 10.1016/S0301-6226(02)00154-9 [DOI] [Google Scholar]
- Yang Y. X., Heo S., Jin Z., Yun J. H., Choi J. Y., Yoon S. Y., Park M. S., Yang B. K., and Chae B. J.. 2009. Effects of lysine intake during late gestation and lactation on blood metabolites, hormones, milk composition and reproductive performance in primiparous and multiparous sows. Anim. Reprod. Sci. 112:199–214. doi: 10.1016/j.anireprosci.2008.04.031 [DOI] [PubMed] [Google Scholar]