Abstract
The current study aims to evaluate the effects of different gestation dietary Met/Lys (methionine, Met/lysine) ratios on the production performance of sows. Specifically, it measured the effect of Met on plasma urea and AA concentrations and placental vascular density of pregnant sows. A total of 325 multiparous sows (third parity, Large × White) were randomly allocated to five dietary treatments (n = 65) with five dietary Met/Lys ratios 0.27 (nutrient requirements of swine [NRC] 2012 level), 0.32, 0.37, 0.42, and 0.47). The litter size and weight at birth were measured and recorded. Blood samples were obtained on days 0, 40, 90, and 114 of gestation, and placenta samples were collected at parturition. The effects of different dietary Met/Lys ratios on the reproductive performance were evaluated based on the prolificacy of sows as either high (≥13 total piglets born) or low (<13 total piglets born). The results showed that dietary Met/Lys ratio had no significant effect on the reproductive performance of lower prolificacy sows (P > 0.05). However, for high-prolificacy sows, litter weight of born alive significantly increased in 0.37 Met/Lys ratios group compared with control group (P < 0.05). The gestation dietary Met/Lys ratio showed significant quadratic effects on the litter birth weight and percentage of piglets born with weight <0.9 kg (P < 0.05), and the Met/Lys ratios to achieve the best reproductive performance determined to be 0.37. Furthermore, plasma urea concentrations of sows also changed with Met/Lys ratios quadratically (P < 0.05). Increasing dietary Met/Lys ratios elevated the concentration of most plasma AA. Although the dietary Met/Lys ratio had no significant effect on the placental vascular density (P > 0.05), the gestation dietary Met/Lys ratio showed significant quadratic effects on the placental vascular density (P < 0.05). In addition, the birth weight of piglets of high-prolificacy sows was positively correlated with the placental vascular density (P < 0.01). Taken as a whole, the dietary Met/Lys ratio showed a quadratic curve relation with birth weight performance and placental angiogenesis performance, to which 0.37 ratio contributed to the best performance of high-prolificacy sows.
Keywords: methionine, placental angiogenesis, pregnant sows, reproductive performance
INTRODUCTION
As the initiating AA in the synthesis of virtually all eukaryotic proteins, methionine (Met) cannot be synthesized by the animal organism (Rees et al., 2006; Liu et al., 2017). Met is considered as the second or third limiting AA in the diets of pigs (Gaines et al., 2005). Elevating dietary Met level can improve the growth performance, muscle development, and muscular antioxidant capacity of pigs (Rincker et al., 2004; Martínez et al., 2017). However, little attention has been paid to the requirements of pregnant sows for Met. As genetic improvement has significantly increased the litter size of sows, dietary Met maybe insufficient to meet the protein synthesis requirement of more fetuses and placentae. Besides serving as a substrate for protein synthesis, Met is also a major methyl donor involved in one-carbon transfer, resulting in altered methylation of DNA and RNA and acetylation of histones, which further cause the epigenetic changes in growing embryos (Kalhan and Marczewski, 2012; Kalhan, 2013). These epigenetic changes may affect fetal growth and cause long-term morbidity of the offspring. Betaine and folic acid act as the source of methyl groups for the conversion of homocysteine (Hcy) to Met (Kalhan, 2013). It has been demonstrated that gestational betaine or folic acid supplementation has positive effects on the reproductive performance of sows (Lindemann, 1993; van Wettere et al., 2012). However, few studies have been focused on the effect of the Met levels or Met/Lys ratios in gestation diet on the reproductive performance of sows.
During pregnancy, the placenta is the unique organ for nutrient exchange between the mother and fetus. Therefore, the development of placenta is closely related to the growth of the fetus (Jansson and Powell, 2007). The main factors that affect the nutrient exchange efficiency of the placenta include blood vessel density, blood flow rate, distance across the epithelial cell bilayer, and width of the folded region of the bilayer (Arroyo and Winn, 2008). Abnormal development of placental blood vessels is one of the main cause of intrauterine growth retardation (IUGR) (Meegdes et al., 1988). Further studies revealed that the vascular density was reduced in the corresponding placenta of IUGR fetus (Meegdes et al., 1988; Chen et al., 2002). Nevertheless, the effect of dietary Met on the placental angiogenesis and reproductive performance of sows remains unclear. Therefore, this study aims to evaluate the optimum Met requirement of pregnant sows and investigate the effects of dietary Met/Lys ratio on placental vascular density of sows.
MATERIALS AND METHODS
Experimental Design, Animals, and Housing
A total of 325 multiparous sows (Large × White; third parity) with an initial body backfat thickness (14.55 ± 1.73 mm; measured at weaning day) and previous litter size (12.64 ± 2.29) were randomly allocated into five groups (65 sows each group) and feed diets with five standardized ileal digestible (SID) Met/Lys dietary ratios of 0.27, 0.32, 0.37, 0.42, and 0.47. All sows were fed with the same diet from weaning to mating (day 0 of gestation), and then fed with the experimental diets in individual stalls until farrowing. All sows were moved to individual farrowing crates with stalls on day 109 of gestation. During the experimental period, sows with illness, serious lameness, death, or reproductive failure such as return to estrus, abortion, or non-pregnancy after breeding were culled. The experimental protocol was approved by the institutional animal ethics committee of Huazhong Agricultural University. All animals were managed according to the general routines of the herd.
Feeding System and Diets
The SID Met/Lys ratio of the basal diet was formulated to be 27% without the supplementation of dl-Met. The diet was maintained at almost constant levels of Lys (average SID Lys 0.71%), Thr (average SID Thr 0.48%), and Trp (average SID Trp 0.14%). The supplemented amount of dl-Met for five dietary treatments was 0, 0.4, 0.7, 1.1, and 1.4 g/kg (Table 1). Sows were fed 2.0 kg/d from days 0 to 3 of gestation, 2.8 kg/d from days 4 to 30 of gestation, 2.3 kg/d from days 31 to 90 of gestation, and 2.9 kg/d from day 91 of gestation to farrowing. All sows were fed once a day at 6:00 a.m.
Table 1.
Ingredients and nutrient compositions of the experimental diets
| Item | Met/Lys = 0.27 | Met/Lys = 0.32 | Met/Lys = 0.37 | Met/Lys = 0.42 | Met/Lys = 0.47 |
|---|---|---|---|---|---|
| Feedstuff % | |||||
| Corn | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Sorghum | 24.54 | 24.54 | 24.54 | 24.54 | 24.54 |
| Barley | 37.89 | 37.89 | 37.89 | 37.89 | 37.89 |
| Soybean hulls | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Rice bran | 7.50 | 7.50 | 7.50 | 7.50 | 7.50 |
| Soybean meal | 9.35 | 9.35 | 9.35 | 9.35 | 9.35 |
| Palm meal | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| 98% lysine | 0.28 | 0.28 | 0.28 | 0.28 | 0.28 |
| dl-methionine | 0.00 | 0.04 | 0.07 | 0.11 | 0.14 |
| l-threonine | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Limestone | 1.44 | 1.43 | 1.42 | 1.42 | 1.40 |
| Calcium phosphate (monocalcium) | 0.89 | 0.88 | 0.87 | 0.84 | 0.83 |
| Sodium chloride | 0.47 | 0.46 | 0.44 | 0.44 | 0.43 |
| Choline chloride | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 |
| Premix1 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Total % | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Composition (calculated) 2 | |||||
| NE, kcal/kg | 2,317.25 | 2,318.70 | 2,320.15 | 2,321.60 | 2,323.04 |
| CP, % | 13.92 | 13.94 | 13.96 | 13.98 | 14.00 |
| SID Lys, % | 0.71 | 0.71 | 0.71 | 0.71 | 0.71 |
| SID Met, % | 0.19 | 0.23 | 0.26 | 0.30 | 0.33 |
| SID Thr, % | 0.48 | 0.48 | 0.48 | 0.48 | 0.48 |
| SID Trp, % | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 |
| dMet/dLys | 0.27 | 0.32 | 0.37 | 0.42 | 0.47 |
| CF, % | 3.57 | 3.57 | 3.57 | 3.57 | 3.57 |
| NDF, % | 14.53 | 14.53 | 14.53 | 14.53 | 14.53 |
| ADF, % | 7.78 | 7.78 | 7.78 | 7.78 | 7.78 |
| Starch, % | 44.74 | 44.74 | 44.74 | 44.74 | 44.74 |
| Ash, % | 6.03 | 5.99 | 5.96 | 5.92 | 5.89 |
| Ca, % | 1.22 | 1.21 | 1.21 | 1.20 | 1.20 |
| P, % | 1.05 | 1.05 | 1.04 | 1.04 | 1.03 |
1The premix is produced by YangXiang Joint Stock Company and contains nutrients such as vitamins and trace elements.
2Calculated chemical concentrations using values for feed ingredients from NRC (2012).
Recording and Sampling
The litter size, number of mummy and stillborn fetuses, and the birth weight of born live were recorded immediately within 24 h after farrowing of the all sows of the experiment. Sixty sows (12 sows each group, backfat thickness at weaning day: 14.1 ± 0.80 mm, 14.58 ± 0.67 mm, 14.42 ± 0.78 mm, 14.42 ± 1.00 mm, and 14.67 ± 0.98 mm; previous litter size: 12.41±3.28, 12.33±1.78, 12.25±1.66, 12.41±1.68, and 12.75±2.42) were randomly selected for blood sampling. Blood samples were collected at 2 h after feeding via jugular venepuncture into heparinized tubes on days 0, 40, 90, and 114 of gestation. Samples were centrifuged at 2,000 × g; 15 min at 4 ℃. Plasma was transferred to 1.5 microcentrifuge tubes (National Scientific) and stored at −80 ℃ until analysis. Eight sows (backfat thickness at farrowing day: 18.38 ± 1.69, 17.5 ± 2.2, 17.57 ± 2.07, 17.38 ± 2.13, 18.13 ± 2.53) were selected randomly and observed in each group immediately after farrowing. To match individual piglets with their placentae, as each piglet was farrowed, its umbilical cord was clamped next to the piglet, and the most distal end of the umbilical cord visible at the vulva was attached by a silk suture with a numbered tag (to indicate the birth order of the piglet), and then the umbilical cord was cut and allowed to retract into the birth canal. At least five piglets and placentae with the same number on the tag were selected randomly, and then weighed and recorded. The sows were continuously monitored until all the placentae were expelled, and the placentae with the same number on the tag were collected, and then weighed and recorded (Wilson et al., 1999; Song et al., 2018). The placental samples were collected and placed on ice. A 5 × 5 cm section of the placenta was excised at the position 2–3 cm away from the umbilicus, placed into small plastic cassettes (Sakura Finetek USA Inc., Torrance, CA) and fixed in buffered formalin overnight at room temperature. The information of the selected sows for placentae collecting, the placentae from each randomly selected sows, and the corresponding particular pigs are shown in Table 2.
Table 2.
The information of sows for placentae collecting, the placentae, and the corresponding particular pigs (litter size ≥ 13)
| Item | Met/Lys ratio | P-value | ||||
|---|---|---|---|---|---|---|
| 0.27 | 0.32 | 0.37 | 0.42 | 0.47 | ||
| Number of sows, n | 6 | 5 | 5 | 7 | 4 | |
| Number of placentae, n | 39 | 35 | 31 | 48 | 24 | |
| Backfat thickness at farrowing day, mm | 18.50 ± 1.52 | 17.40 ± 0.55 | 18.40 ± 1.52 | 17.29 ± 2.29 | 19.75 ± 2.22 | 0.22 |
| Placental weight, kg | 0.33 ± 0.02 | 0.31 ± 0.07 | 0.33 ± 0.03 | 0.31 ± 0.06 | 0.31 ± 0.02 | 0.86 |
| Birth weight of corresponding pig, kg | 1.48 ± 1.6 | 1.43 ± 1.35 | 1.51 ± 1.32 | 1.47 ± 1.85 | 1.49 ± 1.04 | 0.95 |
| Placental efficiency | 4.44 ± 0.59 | 4.93 ± 1.07 | 4.6 ± 0.42 | 4.82 ± 0.87 | 4.81 ± 0.42 | 0.77 |
| Birth weight of pig in whole group | 1.43 ± 0.16 | 1.5 ± 0.15 | 1.53 ± 0.19 | 1.46 ± 0.18 | 1.51 ± 0.22 | 0.18 |
Chemical Analyses
Plasma samples were assayed for urea concentrations using a colorimetric method involving reaction with phenol and hypochlorite, as previously described (Wu and Knabe, 1994). Plasma concentrations of AA were analyzed by HPLC methods involving precolumn derivatization with o-phthaldialdehyde (Wu and Knabe, 1994). AA standards and other chemicals were obtained from Sigma Chemical.
Determination of Vascular Density of the Placenta
Placental section in buffered formalin was incubated in 70% ethanol overnight at 4 °C. The samples were then incubated in a graded series of ethanol (2 h 95% ethanol, 2 h absolute ethanol, and overnight absolute ethanol), xylene (2 × 2 h xylene, overnight xylene), and paraffin (2 × 2 h paraffin, overnight paraffin). The sections were trimmed and embedded in fresh paraffin for sectioning. The paraffin-embedded tissues were then sectioned (4 μm), placed on coated glass slides, processed through a graded series of xylene and ethanol, stained with hematoxylin and eosin, processed through a graded series of ethanol and xylene, and coverslipped using Permount. Images of three to four fields from each slide with stained tissues were projected using a projecting microscope (ECLIPSE Ti, Nikon, Japan), and the area occupied by the placental tissue was traced. In these areas, the placental vessels were traced so that the area and number of all vessels could be determined. The areas were then quantified via image analysis and evaluated for the relative area of placental vessels per unit tissue area in placental stroma. Images of five fields of placental fold from each slide with stained tissues were projected to evaluate the relative number of placental vessels per unit tissue area in the placental fold (Vonnahme et al., 2001).
Calculations and Statistical Analyses
Statistical analyses were conducted using the PROC MIXED procedure of SAS 9.2 (SAS Inst. Inc., Cary, NC), with each individual sow as an experimental unit. Dietary Met/Lys ratio was specified as fixed effects. In the mixed model, the response variables included litter size, litter weight of born alive, placental weight, placental placental efficiency, vascular density, urea concentrations, and AA concentrations. A χ2 test was conducted to test the significant differences in the rate of weak piglets among the five groups. Regression analyses were performed to evaluate the linear and quadratic effects of dietary Met/Lys ratio. Spearman correlations were used to determine the association between dietary Met/Lys ratio and placental vascular density. Data obtained by ANOVA were shown as the mean and SEM. Statistical significance was declared at P < 0.05, and tendencies were declared at 0.05 < P < 0.10.
RESULTS
Reproductive Performance of Sows
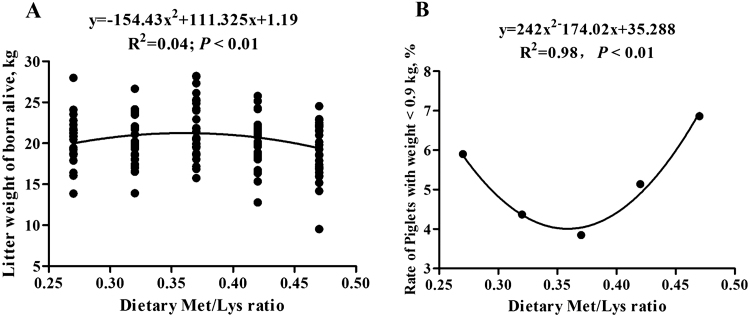
As shown in Table 3, when the litter size was <13, the dietary Met/Lys ratio showed no significant effects on total born litter size, alive litter size, number of mummy and stillborn fetuses, and average pig birth weight. Placental weight and placental efficiency were also not effected by increasing dietary Met/Lys ratio (Table 2). However, for high-prolificacy sows, the litter weight of born alive in 0.37 ratio group was greater than that of 0.27, 0.42, and 0.47 ratio groups (P < 0.05; Table 4). The litter weight of born alive changed with the dietary Met/Lys ratio quadratically (P < 0.05). According to the quadratic polynomial regression equation (y = −154.43x2 + 111.325x + 1.19, R2 = 0.04, P < 0.05; Figure 1A), the litter weight was the maximum at the dietary Met/Lys ratio of 0.36. The rate of piglets with weight <0.9 kg also changed with dietary Met/Lys ratio quadratically (P < 0.05). According to the quadratic polynomial regression equation (y = 242x2 − 174.02x + 35.288, R2 = 0.98, P < 0.05; Figure 1B), the dietary Met/Lys ratio of 0.36 resulted in the minimum rate of piglets with weight <0.9 kg. The rate of piglets with weight <0.9 kg in 0.37 ratio group was lower than that in 0.27 ratio group (3.85% vs. 5.90%). Additionally, the total litter size of 0.47 ratio group was significantly increased compared with that of 0.42 ratio group in high-prolificacy sows.
Table 3.
Effect of gestation dietary Met/Lys ratio on reproductive performance (litter size < 13)
| Item | Met/Lys ratio | SEM | P-value1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.27 | 0.32 | 0.37 | 0.42 | 0.47 | Linear | Quadratic | ANOVA | ||
| Number of sows, n | 37 | 33 | 26 | 25 | 22 | ||||
| Litter size, n | |||||||||
| Total born | 10.57 | 10.45 | 10.23 | 10.64 | 10.23 | 0.14 | 0.61 | 0.87 | 0.85 |
| Born alive | 10.35 | 10.39 | 10.04 | 10.44 | 10.09 | 0.14 | 0.64 | 0.89 | 0.87 |
| Mummy | 0.19 | 0.03 | 0.15 | 0.04 | 0.09 | 0.00 | 0.31 | 0.41 | 0.29 |
| Stillborn | 0.03 | 0.03 | 0.04 | 0.16 | 0.05 | 0.01 | 0.36 | 0.36 | 0.18 |
| Average pig birth weight, kg | 1.66 | 1.67 | 1.68 | 1.64 | 1.65 | 0.02 | 0.82 | 0.92 | 0.98 |
| Litter weight of born alive, kg | 17.12 | 17.22 | 16.65 | 17.26 | 16.12 | 0.29 | 0.39 | 0.61 | 0.76 |
| Rate of piglets with weight < 0.9 kg, %2 | 2.35 | 3.24 | 2.33 | 4.25 | 2.32 | 0.02 | 0.78 | 0.77 | 0.38 |
1Orthogonal polynomial contrast coefficients were used to determine the linear and quadratic effects of increasing dietary Met/Lys ratios.
2For analyses, the square root arcsine transformation for proportions was used. Results shown are transformed back into percentages.
Table 4.
Effect of gestation dietary Met/Lys ratio on reproductive performance (litter size ≥ 13)
| Item | Met/Lys ratio | SEM | P-value1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.27 | 0.32 | 0.37 | 0.42 | 0.47 | Linear | Quadratic | ANOVA | ||
| Number of sows, n | 26 | 30 | 33 | 33 | 38 | ||||
| Litter size, n | |||||||||
| Total born | 14.62 | 14.30 | 14.61 | 14.79 | 13.92 | 0.11 | 0.18 | 0.14 | 0.06 |
| Born alive | 14.15 | 13.73 | 14.18 | 14.15 | 13.42 | 0.11 | 0.14 | 0.14 | 0.09 |
| Mummy | 0.27 | 0.27 | 0.09 | 0.30 | 0.26 | 0.04 | 0.87 | 0.68 | 0.54 |
| Stillborn | 0.85 | 0.60 | 0.55 | 0.82 | 0.92 | 0.09 | 0.78 | 0.77 | 0.63 |
| Average pig birth weight, kg | 1.43 | 1.50 | 1.53 | 1.46 | 1.51 | 0.01 | 0.24 | 0.26 | 0.18 |
| Litter weight of born alive, kg | 20.19b | 20.46ab | 21.76a | 20.50b | 19.41b | 0.25 | 0.25 | 0.02 | 0.04 |
| Rate of piglets with weight < 0.9 kg, %2 | 5.98 | 4.37 | 3.85 | 5.14 | 6.86 | 0.04 | 0.59 | 0.02 | 0.29 |
a,bMeans within a row with different superscripts indicate significant differences (P < 0.05).
1Orthogonal polynomial contrast coefficients were used to determine the linear and quadratic effects of increasing dietary Met/Lys ratios.
2For analyses, the square root arcsine transformation for proportions was used. Results shown are transformed back into percentages.
Figure 1.
Quadratic relationship for litter weight of born alive between each groups (A). Each black point represents the litter weight of each high-prolificacy sow in different Met/Lys ratio groups; quadratic relationship for rate of piglets with weight ≤0.9 kg between each groups (B). Each black point represents the rate of piglets with weight ≤0.9kg of the whole high-prolificacy sows in different Met/Lys ratio groups.
Plasma Urea Concentrations
For high-prolificacy sows, concentrations of urea in plasma did not differ among the control and Met-supplemented groups of at days 0, 40, or 90 of gestation. However, the concentrations of urea in plasma changed with dietary Met/Lys ratio quadratically (P < 0.05). The concentrations of urea in plasma of high-prolificacy sows showed the lowest level at 0.37 Met/Lys ratio group (Table 5).
Table 5.
Effect of dietary Met/Lys ratio on the level of plasma urea concentration in the blood of sows (litter size ≥ 13)
| Gestation, d | Met/Lys ratio, mmol/L | SEM | P-value1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.27 | 0.32 | 0.37 | 0.42 | 0.47 | Linear | Quadratic | ANOVA | ||
| 0 | 5.25 | 6.09 | 5.97 | 5.86 | 5.72 | 0.16 | 0.35 | 0.60 | 0.62 |
| 40 | 5.45 | 4.85 | 4.22 | 4.92 | 4.73 | 0.15 | 0.29 | 0.11 | 0.14 |
| 90 | 4.65 | 4.70 | 4.18 | 4.18 | 4.56 | 0.10 | 0.42 | 0.25 | 0.34 |
| 114 | 5.83a | 5.22 | 4.48b | 4.57 | 5.2 | 0.19 | 0.09 | 0.03 | 0.10 |
a,bMeans within a row with different superscripts indicate significant differences (P < 0.05).
1Orthogonal polynomial contrast coefficients were used to determine the linear and quadratic effects of increasing dietary Met/Lys ratios.
Plasma Concentrations of AA
Plasma Met, isoleucine, leucine, and valine concentrations increased linearly (P < 0.01) and quadratically (P < 0.01), and concentrations of lysine, crystine, and glutamine increased linearly (P < 0.01) and quadratically (P = 0.08; P = 0.06; P = 0.11) as Met/Lys ratio increased (day 114 of gestation; Table 6). The plasma threonine and serine concentration increased linearly (P < 0.05; P = 0.05) by increasing dietary Met/Lys ratio. The increasing dietary Met/Lys ratio levels did not affect the plasma concentrations of arginine, histidine, phenylalanine, tryptophan, alanine, asparagine, aspartic acid, glutamic acid, glycine, proline, tyrosine, and carnosine (day 114 of gestation; Table 6).
Table 6.
Effect of dietary Met/Lys ratio on concentrations of amino acids in the plasma of sows at parturition (litter size ≥ 13)
| Item | Met/Lys ratio | SEM | P-value1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.27 | 0.32 | 0.37 | 0.42 | 0.47 | Linear | Quadratic | ANOVA | ||
| Number of sows | 6 | 6 | 6 | 7 | 6 | ||||
| EAA, 2 µmol/L | |||||||||
| Arginine | 15.42 | 15.96 | 18.66 | 16.86 | 17.59 | 0.76 | 0.34 | 0.5 | 0.68 |
| Histidine | 31.9 | 33.4 | 35.16 | 38.23 | 37.7 | 1.32 | 0.08 | 0.21 | 0.52 |
| Isoleucine | 45.42b | 41.21b | 52.98ab | 56.22ab | 65.71a | 2.71 | <0.01 | <0.01 | <0.05 |
| Leucine | 58.35b | 53.40b | 62.17b | 77.98b | 104.37a | 4.95 | <0.01 | <0.01 | <0.01 |
| Lysine | 50 | 60.05 | 62.83 | 85.68 | 86.47 | 6.76 | <0.01 | 0.08 | 0.22 |
| Methionine | 16.15b | 20.36ab | 22.16ab | 23.88ab | 26.41a | 1.27 | <0.01 | <0.01 | 0.1 |
| Phenylalanine | 38.59 | 38.03 | 41.14 | 41.8 | 45.5 | 1.37 | 0.06 | 0.16 | 0.44 |
| Threonine | 140.24ab | 117.56b | 175.78ab | 199.87a | 186.97a | 10.15 | <0.05 | <0.05 | <0.05 |
| Tryptophan | 61.44 | 65.53 | 67.59 | 66.76 | 61.31 | 2.49 | 0.96 | 0.58 | 0.9 |
| Valine | 82.31bc | 69.31c | 88.19bc | 105.68ab | 118.02a | 5.18 | <0.01 | <0.01 | <0.01 |
| NEAA, 3 µmol/L | |||||||||
| Alanine | 82.89 | 92.72 | 93.46 | 91.04 | 91.96 | 3.04 | 0.45 | 0.54 | 0.83 |
| Asparagine | 333.95 | 501.69 | 522.88 | 558.72 | 624.51 | 49.24 | 0.07 | 0.17 | 0.45 |
| Aspartic acid | 12.05 | 12.07 | 13.58 | 11.88 | 16.32 | 1.02 | 0.25 | 0.43 | 0.63 |
| Crystine | 6.29 | 7.45 | 9.23 | 11.21 | 12.61 | 0.98 | <0.01 | 0.06 | 0.24 |
| Glutamic acid | 185.46 | 136.37 | 160.44 | 154.19 | 168.03 | 10.83 | 0.25 | 0.43 | 0.72 |
| Glutamine | 1,322.35b | 2,131.68ab | 1,663.88ab | 2,075.2ab | 2,335.18a | 132.84 | <0.01 | 0.11 | 0.1 |
| Glycine | 170.07 | 203.66 | 185.94 | 177.31 | 189.87 | 7.67 | 0.81 | 0.86 | 0.72 |
| Proline | 112.91 | 127.99 | 156.85 | 129.57 | 173.11 | 9.85 | 0.08 | 0.22 | 0.3 |
| Serine | 49.58ab | 45.16b | 58.06a | 52.49ab | 56.65a | 1.57 | 0.05 | 0.15 | <0.05 |
| Tyrosine | 35.54 | 45.19 | 49.22 | 50.19 | 47.52 | 2.5 | 0.1 | 0.1 | 0.35 |
| Carnosine, µmol/L | 14.45 | 13.76 | 14.35 | 15.74 | 13.2 | 0.64 | 0.91 | 0.87 | 0.8 |
a–cMeans within a row with different superscripts indicate significant differences (P < 0.05).
1Orthogonal polynomial contrast coefficients were used to determine the linear and quadratic effects of increasing dietary Met/Lys ratios.
2Essential AA.
3Nonessential AA.
Placental Vascular Density
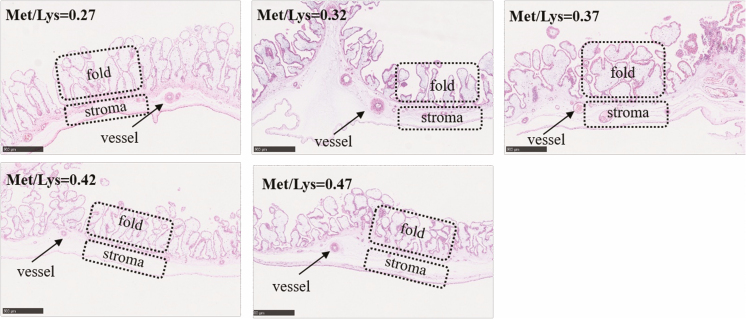
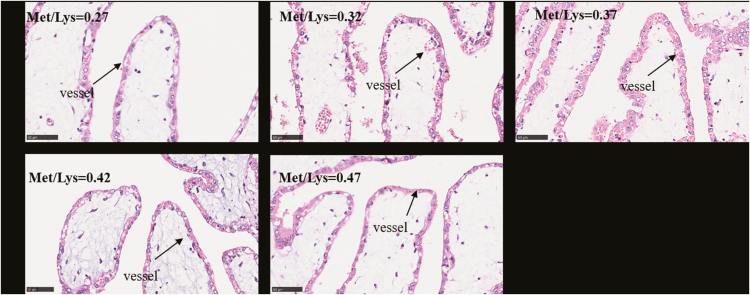
We measured the placental vascular density of sows whose litter size was no smaller than 13. The histological structures stained by Hematoxylin Eosin (H&E) to represent the vessel distributions in the placental stroma and fold were, respectively, presented in Figures 2 and 3.
Figure 2.
Hematoxylin & Eosin (H&E) staining of tissues. The images show the vessel distributions in placental tissues, every placenta was collected at the same position 2–3 cm away from the umbilicus. The arrows indicate the placental vessels in stroma (200× magnification).
Figure 3.
Hematoxylin & Eosin (H&E) staining of tissues. The images show the vessel distributions in fold of placental tissues. The arrows indicate the placental vessels in fold (1,600× magnification).
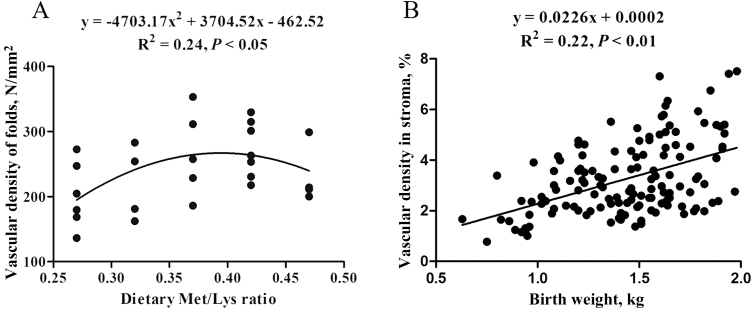
On the day of farrowing, the vascular density of placental stroma and the number of capillaries per unit area of placental folds increased to the maximum as the Met/Lys ratio rose from 0.27 to 0.37, and then started to decrease (Table 7). However, there was not a positive correlation between the vascular density of placental stroma and birth weight of piglets (P = 0.17). Nonetheless, the vascular density of placental stroma in the 0.37 ratio group was 19.77% and 42.96% higher than that in the 0.27 and 0.47 ratio groups, respectively. As shown in Figure 4B, there was a positive correlation between the vascular density of placental stroma and birth weight of piglets (R2 = 0.22; P < 0.05). For the vascular density of placental fold, the number of capillaries per unit area of placental folds changed with dietary Met/Lys ratio quadratically (P < 0.05). According to the quadratic polynomial regression equation (y = −4,703.17x2 + 3704.52x − 462.52, R2 = 0.24, P < 0.05), the maximum number of capillaries per unit area of placental folds was observed at the Met/Lys ratio of 0.39. There was no significant difference among vascular density of placental fold in each groups, although the number of capillaries per unit area of placental folds in 0.37 ratio group was 41.35% and 29.10% higher than that in 0.27 and 0.47 ratio groups, respectively.
Table 7.
Effect of dietary Met/Lys ratio on placental vascular density (litter size ≥ 13)1
| Item | Met/Lys ratio | SEM | P-value1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.27 | 0.32 | 0.37 | 0.42 | 0.47 | Linear | Quadratic | ANOVA | ||
| Number of sows, n | 6 | 6 | 4 | 7 | 4 | ||||
| Vascular density of the stroma, % | 3.25 | 3.32 | 3.86 | 3.07 | 2.70 | 0.15 | 0.29 | 0.17 | 0.27 |
| Number of sows, n | 6 | 4 | 5 | 7 | 4 | ||||
| Number of capillaries per unit area (1 mm2) of the folds | 205.82 | 241.15 | 290.92 | 275.4 | 225.34 | 10.21 | 0.08 | 0.04 | 0.13 |
1Because of that placentae with impairment and calcification were culled or eliminated, sows were eliminated when placentae samples was <3.
Figure 4.
Quadratic relationship for placental vascular density between each groups (A). Each black point represents the means of placental vascular density in fold within sow. At less five randomly selected placentae were collected, and at less five randomly selected regions in visual fields of placental fold were choosen to analyze the vascular density; relationships between birth weight and placental vascular density (B). Each black point represents the means of placental vascular density in fold within placentae corresponding to the birth weight of the piglets. At less five randomly selected regions in visual fields of placental stroma were choosen to analyze the vascular density.
DISCUSSION
NRC (2012) recommends that the Met/Lys ratio should be 0.27 for pregnant sows whose anticipated litter size is 13.5 (Usa, 2012). However, the nutrient recommendations for pigs in Denmark state that the recommended dietary Met/Lys ratio is 0.48 for pregnant sows with larger anticipated littler size than NRC (2012) (Tybirk et al., 2013). The results of the present study showed that the optimal Met/Lys ratio for high-prolificacy sows is 0.37, and elevation of the ratio from 0.27 to 0.37 could increase the litter weight and the average weight of piglets born alive as well as decrease the rate of piglets with low birth weight by 2.13% in high-prolificacy sows. However, the changes in dietary Met/Lys ratio did not show obvious effects on the reproductive performance of lower prolificacy sows. The current NRC (2012) recommendation for the Met intake of sows is based on an assumed ideal protein ratio and metabolism studies of gestation sows (Everts and Dekker, 1995; Usa, 2012). However, the Met/Lys ratio calculated according to the AA concentrations and accretion in fetal pigs was higher than 0.27 with increasing gestational age from days 60 to 114 (Wu et al., 1999). Thus, the Met/Lys ratio in the diet for pregnant sows might need to be increased along with the increase in the number of fetuses and placentae as the litter size is elevated by genetic improvement.
As the indicator for the adequacy of the AA supply, plasma urea concentrations decreased quadratically as dietary Met/Lys ratios increasing from 0.27 to 0.47 at day 114 of gestation. Similarly to the effect on birth weight of piglets, 0.37 dietary Met/Lys ratio shows the lowest level of plasma urea concentration in high-prolificacy sows and underlines the highest utilization of dietary AA. Met is not only required for protein synthesis but also acts as main methyl donor in one-carbon transfer, in which serine and glycine involved (Rees et al., 2006). The changes in plasma glycine, serine, and Hcy suggest a role for perturbations in one carbon transfer as a potential contributor to the fetal growth restriction (Kalhan and Marczewski, 2012). Met supplementation increased plasma concentration of serine isoleucine, leucine, valine, and threonine, which were proved increase remarkably of concentrations in fetal pigs during late pregnancy (Wu et al., 1999).
In mammals such as pigs, IUGR is associated with placental dysfunction, which is characterized by a number of pathologic features, such as reduced syncytiotrophoblast surface area, increased thickness of the exchange barrier formed by the trophoblast, fetal capillary endothelium and reduced placental vascular density (Arroyo and Winn, 2008; Sanchis et al., 2017). Abnormal development of placental blood vessels is one of the main causes of IUGR, as the placental vascular density showed a significant reduction in IUGR fetus compared with that in normal fetus (Chen et al., 2002). Although Steve Ford and colleagues found that placental vascular density was not affected by uterine breed or day of gestation (Biensen et al., 1999). Our results showed a positive association between the litter birth weight and vascular density of placentae in high-prolificacy sows, and elevating the Met/Lys ratio in the diet of high-prolificacy sows increased the number of capillaries per unit area of placental folds at term, as well as the litter birth weight. Increased placental weight or increased placental efficiency was considered to be the main reasons of increased piglet birth weight (Biensen et al., 1999). However, increasing dietary Met/Lys ratio did not alter placental weight or placental efficiency, and these observations are similar to those of other studies irrespective whether supplementation was arginine (Gao et al., 2012) or AA blend (include Met; Dallanora et al., 2017). Steve Ford and colleagues found that, during late gestation, the placental weight and placental surface area remained constant, while the vascular density of the Meishan placenta and the endometrium increased (Biensen et al., 1999). These results indicate that, for high-prolificacy sows, the increased vascular density is crucial for nutrient uptake from a limited surface area of contact between the placenta and endometrium to meet the rapid growth of fetus during late gestation.
It is important to note that our results showed that the effect of the elevation of gestation dietary Met/Lys ratio on promoting microangiogenesis in fold is more obvious than promoting angiogenesis in strom (vascular density in 0.37 group increased 41.35% in fold vs. 19.77% in strom). Although the most rapid developmental progress of the placenta and the vascular in placental stroma develop is observed between days 20 and 60 of gestation in pigs, the intense placental angiogenesis in fold to form capillaries could still be observed at late pregnancy with the rapid increase in the demand of the fetuses for nutrient uptake and waste removal (Knight et al., 1977; Wilson et al., 1998; Wu et al., 2005). This results suggest that it may occur mainly at the late pregnancy that the increasing of gestation dietary Met/Lys ratio improves the placental angiogenesis, especially the intense angiogenesis in fold of the placenta and then promotes the birth weight of piglets. Considering the cost of Met supplementation, we are interested in investigating the effects of increasing Met level on the placental angiogenesis and highest birth weight of piglets of sows only in late pregnancy. Although arginine was considered as a key controller of placental angiogenesis, late pregnancy supplementation with L-arginine had no effect on piglet birth weight and other productive performance (Bass et al., 2017). And dietary arginine supplementation during early gestation improves the increased the number of pigs born alive, live litter birth weight of piglets, which were associated with nitric oxide, polyamine-dependent mechanisms (Li et al., 2015). Another researches show that maternal arginine benefited the embryonic growth rate and placental vascularization during early gestation (Novak et al., 2012). In fact, Bin’s research had found that increased dietary Met level during the late pregnancy (day 90 to parturition) significantly increased the birth weights and the survival ratio of the piglets, but the study did not indicate the effects of increased dietary Met level on placental efficiency or placental angiogenesis (Bin et al., 2018). These research results indicated that the underlying mechanisms of Met affecting placental angiogenesis were during late pregnancy.
Nevertheless, a dietary excess of Met after increased Hcy and altered DNA methylation shows a pro-oxidant effect and is involved in various pathological developments (Yin et al., 2018). As our result showed, the birth weight of piglets and vascular density showed significant decrease in 0.47 group compared with 0.37 group in high-prolificacy sows. This phenomenon may be associated with Hcy, an important intermediate metabolite of Met. The exposure to Hcy was reported to impair vascular development, as indicated by the reduced expression of vascular endothelial growth factor A (Oosterbaan et al., 2012). The vasculotoxic property of Hcy may be associated with the reduction of placental vascular density, which would reduce the reproductive performance of the sows when the Met/Lys ratio exceeded 0.37 in our study, but further testing is necessary for confirmation.
In conclusion, the NRC (2012) recommended dietary Met/Lys ratio of 0.27 is insufficient for the best reproductive performance of high-prolificacy sows. For high-prolificacy sows, increasing the dietary Met/Lys ratio has a quadratic effect on sow birth weight of piglets especially the low birth weight of piglets and placental angiogenesis in fold. It was demonstrated that the dietary Met/Lys ratio of 0.37 has the best effect on performance of high-prolificacy sows. Therefore, appropriate dietary Met/Lys ratio during gestation for high-prolificacy sows is a specific nutritional strategy that should be employed to maximize reproductive performance.
ACKNOWLEDGMENT
We thank the YangXiang Joint Stock Co., Ltd. for providing the sow farm.
Conflict of interest statement. None declared.
Footnotes
This study was supported by College of Animal Science and Technology, Huazhong Agricultural University, PR China.
LITERATURE CITED
- Arroyo J. A., and Winn V. D.. 2008. Vasculogenesis and angiogenesis in the IUGR placenta. Semin. Perinatol. 32:172–177. doi: 10.1053/j.semperi.2008.02.006 [DOI] [PubMed] [Google Scholar]
- Bass B. E., Bradley C. L., Johnson Z. B., Zier-Rush C. E., Boyd R. D., Usry J. L., Maxwell C. V., and Frank J. W.. 2017. Influence of dietary-arginine supplementation of sows during late pregnancy on piglet birth weight and sow and litter performance during lactation. J. Anim. Sci. 95:248–256. doi: 10.2527/jas.2016.0986 [DOI] [PubMed] [Google Scholar]
- Biensen N. J., Wilson M. E., and Ford S. P.. 1999. The impacts of uterine environment and fetal genotype on conceptus size and placental vascularity during late gestation in pigs. J. Anim. Sci. 77:954–959. doi: 10.2527/1999.774954x [DOI] [PubMed] [Google Scholar]
- Bin P., Azad M. A. K., Liu G., Zhu D., Kim S. W., and Yin Y.. 2018. Effects of different levels of methionine on sow health and plasma metabolomics during late gestation. Food Funct. 9:4979–4988. doi: 10.1039/c8fo01477a [DOI] [PubMed] [Google Scholar]
- Chen C. P., Bajoria R., and Aplin J. D.. 2002. Decreased vascularization and cell proliferation in placentas of intrauterine growth-restricted fetuses with abnormal umbilical artery flow velocity waveforms. Am. J. Obstet. Gynecol. 187:764–769. doi: 10.1067/mob.2002.125243 [DOI] [PubMed] [Google Scholar]
- Dallanora D., Marcon J., Walter M. P., Biondo N., Bernardi M. L., Wentz I., and Bortolozzo F. P... 2017. Effect of dietary amino acid supplementation during gestation on placental efficiency and litter birth weight in gestating gilts. Livest. Sci. 197:30–35. doi: 10.1016/j.livsci.2017.01.005 [DOI] [Google Scholar]
- Everts H., and Dekker R. A.. 1995. Effect of protein supply during pregnancy on body composition of gilts and their products of conception. Livest. Prod. Sci. 43:27–36. doi: 10.1016/0301-6226(95)00004-5 [DOI] [Google Scholar]
- Gaines A. M., Yi G. F., Ratliff B. W., Srichana P., Kendall D. C., Allee G. L., Knight C. D., and Perryman K. R.. 2005. Estimation of the ideal ratio of true ileal digestible sulfur amino acids:lysine in 8- to 26-kg nursery pigs. J. Anim. Sci. 83:2527–2534. doi: 10.2527/2005.83112527x [DOI] [PubMed] [Google Scholar]
- Gao K., Jiang Z., Lin Y., Zheng C., Zhou G., Chen F., Yang L., and Wu G.. 2012. Dietary L-arginine supplementation enhances placental growth and reproductive performance in sows. Amino Acids 42:2207–2214. doi: 10.1007/s00726-011-0960-9 [DOI] [PubMed] [Google Scholar]
- Jansson T., and Powell T. L.. 2007. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin. Sci. (Lond.). 113:1–13. doi: 10.1042/CS20060339 [DOI] [PubMed] [Google Scholar]
- Kalhan S. C. 2013. One-carbon metabolism, fetal growth and long-term consequences. Nestle Nutr. Inst. Workshop Ser. 74:127–138. doi: 10.1159/000348459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalhan S. C., and Marczewski S. E.. 2012. Methionine, homocysteine, one carbon metabolism and fetal growth. Rev. Endocr. Metab. Disord. 13:109–119. doi: 10.1007/s11154-012-9215-7 [DOI] [PubMed] [Google Scholar]
- Knight J. W., Bazer F. W., Thatcher W. W., Franke D. E., and Wallace H. D.. 1977. Conceptus development in intact and unilaterally hysterectomized-ovariectomized gilts: interrelations among hormonal status, placental development, fetal fluids and fetal growth. J. Anim. Sci. 44:620–637. doi: 10.2527/jas1977.444620x [DOI] [PubMed] [Google Scholar]
- Li J., Xia H., Yao W., Wang T., Li J., Piao X., Thacker P., Wu G., and Wang F.. 2015. Effects of arginine supplementation during early gestation (days 1 to 30) on litter size and plasma metabolites in gilts and sows. J. Anim. Sci. 93:5291–5303. doi: 10.2527/jas.2014-8657 [DOI] [PubMed] [Google Scholar]
- Lindemann M. D. 1993. Supplemental folic acid: a requirement for optimizing swine reproduction. J. Anim. Sci. 71:239–246. doi: 10.2527/1993.711239x [DOI] [PubMed] [Google Scholar]
- Liu G., Yu L., Fang J., Hu C. A., Yin J., Ni H., Ren W., Duraipandiyan V., Chen S., Al-Dhabi N. A.,. et al. 2017. Methionine restriction on oxidative stress and immune response in DSS-induced colitis mice. Oncotarget 8:44511–44520. doi: 10.18632/oncotarget.17812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez Y., Li X., Liu G., Bin P., Yan W., Más D., Valdivié M., Hu C. A., Ren W., and Yin Y.. 2017. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids 49:2091–2098. doi: 10.1007/s00726-017-2494-2 [DOI] [PubMed] [Google Scholar]
- Meegdes B. H., Ingenhoes R., Peeters L. L., and Exalto N.. 1988. Early pregnancy wastage: relationship between chorionic vascularization and embryonic development. Fertil. Steril. 49:216–220. [DOI] [PubMed] [Google Scholar]
- Novak S., Paradis F., Patterson J. L., Pasternak J. A., Oxtoby K., Moore H. S., Hahn M., Dyck M. K., Dixon W. T., and Foxcroft G. R.. 2012. Temporal candidate gene expression in the sow placenta and embryo during early gestation and effect of maternal progenos supplementation on embryonic and placental development. Reprod. Fertil. Dev. 24:550–558. doi: 10.1071/RD10312 [DOI] [PubMed] [Google Scholar]
- Oosterbaan A. M., Steegers E. A., and Ursem N. T.. 2012. The effects of homocysteine and folic acid on angiogenesis and VEGF expression during chicken vascular development. Microvasc. Res. 83:98–104. doi: 10.1016/j.mvr.2011.11.001 [DOI] [PubMed] [Google Scholar]
- Rees W. D., Wilson F. A., and Maloney C. A.. 2006. Sulfur amino acid metabolism in pregnancy: the impact of methionine in the maternal diet. J. Nutr. 136(6 Suppl):1701S–1705S. doi: 10.1093/jn/136.6.1701S [DOI] [PubMed] [Google Scholar]
- Rincker M. J., Hill G. M., Link J. E., and Rowntree J. E.. 2004. Effects of dietary iron supplementation on growth performance, hematological status, and whole-body mineral concentrations of nursery pigs. J. Anim. Sci. 82:3189–3197. doi: 10.2527/2004.82113189x [DOI] [PubMed] [Google Scholar]
- Sanchis E. G., Cristofolini A. L., Fiorimanti M. R., Barbeito C. G., and Merkis C. I.. 2017. Apoptosis and cell proliferation in porcine placental vascularization. Anim. Reprod. Sci. 184:20–28. doi: 10.1016/j.anireprosci.2017.06.009 [DOI] [PubMed] [Google Scholar]
- Song T., Lu J., Deng Z., Xu T., Yang Y., Wei H., Li S., Jiang S., and Peng J... 2018. Maternal obesity aggravates the abnormality of porcine placenta by increasing N-methyladenosine. Int. J. Obes. (Lond.). 42(10): 1812. doi:10.1038/s41366-018-0113-2 [DOI] [PubMed] [Google Scholar]
- Tybirk P., Sloth N. M., and Jørgensen L. . 2013. Nutrient requirement standards[J]. SEGES-VSP. http://www.pigresearchcentre.dk/~/media/pdf/eng/Normer naeringstoffer% 20UK/Nutrient% 20standards% (Accessed 20 April 2015. [Google Scholar]
- Usa N. C. 2012. Nutrient requirements of swine. Nutrient Requirements of Swine, 2012, 44(3). [Google Scholar]
- Vonnahme K. A., Wilson M. E., and Ford S. P.. 2001. Relationship between placental vascular endothelial growth factor expression and placental/endometrial vascularity in the pig. Biol. Reprod. 64:1821–1825. doi: 10.1095/biolreprod64.6.1821 [DOI] [PubMed] [Google Scholar]
- van Wettere W. H., Herde P., and Hughes P. E.. 2012. Supplementing sow gestation diets with betaine during summer increases litter size of sows with greater numbers of parities. Anim. Reprod. Sci. 132:44–49. doi: 10.1016/j.anireprosci.2012.04.007 [DOI] [PubMed] [Google Scholar]
- Wilson M. E., Biensen N. J., and Ford S. P.. 1999. Novel insight into the control of litter size in pigs, using placental efficiency as a selection tool. J. Anim. Sci. 77:1654–1658. doi: 10.2527/1999.7771654x [DOI] [PubMed] [Google Scholar]
- Wilson M. E., Biensen N. J., Youngs C. R., and Ford S. P.. 1998. Development of Meishan and Yorkshire littermate conceptuses in either a Meishan or Yorkshire uterine environment to day 90 of gestation and to term. Biol. Reprod. 58:905–910. doi: 10.1095/biolreprod58.4.905 [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F. W., Hu J., Johnson G. A., and Spencer T. E.. 2005. Polyamine synthesis from proline in the developing porcine placenta. Biol. Reprod. 72:842–850. doi: 10.1095/biolreprod.104.036293 [DOI] [PubMed] [Google Scholar]
- Wu G., and Knabe D. A.. 1994. Free and protein-bound amino acids in sow’s colostrum and milk. J. Nutr. 124:415–424. doi: 10.1093/jn/124.3.415 [DOI] [PubMed] [Google Scholar]
- Wu G., Ott T. L., Knabe D. A., and Bazer F. W.. 1999. Amino acid composition of the fetal pig. J. Nutr. 129:1031–1038. doi: 10.1093/jn/129.5.1031 [DOI] [PubMed] [Google Scholar]
- Yin J., Ren W., Chen S., Li Y., Han H., Gao J., Liu G., Wu X., Li T., Woo Kim S.,. et al. 2018. Metabolic regulation of methionine restriction in diabetes. Mol. Nutr. Food Res. 62:e1700951. doi: 10.1002/mnfr.201700951 [DOI] [PubMed] [Google Scholar]