Abstract
This study was conducted to investigate the effects of Clostridium butyricum addition to diets in late gestation and lactation on the reproductive performance and gut microbiota for sows. A total of 180 healthy Landrace × Yorkshire sows at 90 d of gestation were randomly assigned to one of four groups, with 45 replicates per group, receiving a basal commercial diet (Control, 0% C. butyricum) or diet added with 0.1% C. butyricum (1 × 108 CFU/kg of feed), 0.2% C. butyricum (2 × 108 CFU/kg of feed), 0.4% C. butyricum (4 × 108 CFU/kg of feed), respectively. The experiment was conducted from 90 d of gestation to weaning at 21 d of lactation. The results showed that the interval between piglet born was linearly (P < 0.05) decreased, and the duration of farrowing was significantly (quadratic, P < 0.05) shortened as C. butyricum addition increased. There was a linear (P < 0.05) increase in litter weight at weaning and litter weight gain. The concentrations of IgG and IgM in colostrum, and IgM in milk were linearly increased (P < 0.05) as C. butyricum addition. Serum MDA concentrations of sows at parturition and 14 d in lactation, and piglets at 14 and 21 d of age were linearly (P < 0.05) decreased, respectively. The serum total antioxidant capacity concentrations of sows at parturition and 14 and 21 d in lactation, and piglets at 14 and 21 d of age were linearly (P < 0.05) increased as C. butyricum addition, respectively. There was a linear decrease in the serum endotoxin concentration of sows on 21 d in lactation (P < 0.05). The serum cortisol concentrations of piglets at 14 and 21 d of age were both significantly (quadratic, P < 0.05) decreased. The 0.2% C. butyricum increased the relative abundance of Bacteroidetes (P = 0.016) at phylum level, Prevotellaceae_NK3B31_group, Prevotella_1, Prevotellaceae_UCG-003, Prevotella_9, Alloprevotella (P < 0.05) at genus level, and decreased the relative abundance of Proteobacteria, Gemmatimonadetes, Actinobacteria (P < 0.001) at phylum level, and Clostridium_sensu_stricto_1, Streptococcus, Escheruchia-Shigella, Sphingomonas, Succinivibrio (P < 0.05) at genus level and Firmicutes/Bacteroidetes ratio (P = 0.020). In conclusion, the present research indicated that dietary addition with C. butyricum could shorten the duration of farrowing and enhance the growth performance of suckling piglets. Moreover, 0.2% C. butyricum administration to sows changed the composition of intestinal microbiota, especially increased the relative abundance of Prevotella.
Keywords: antioxidation, Clostridium butyricum, gut microbiota, reproductive performance, sows
INTRODUCTION
Perinatal sows suffer various stress conditions from the environment and physiology, such as type of housing, high temperature, metabolic disorders, and inflammation (Oliviero et al., 2008; Williams et al., 2013; Cheng et al., 2018), which always adversely affect the health, duration of farrowing, and reproduction of sows (Oliviero et al., 2010; Ruediger and Schulze, 2012). Recent studies reported that the stressors that the sows undergo during the perinatal period may be related to the intestinal microbiota (Hayakawa et al., 2016; Cheng et al., 2018). Many studies have reported that the application of probiotics can affect the composition of intestinal microbiota and improve the health and reproductive performance of sows (Alexopoulos et al., 2004; Hayakawa et al., 2016; Link et al., 2016; Tsukahara et al., 2018).
Clostridium butyricum, also known as a butyric acid-producing probiotics, is contributed to regulate the balance of intestinal microecology, promote the proliferation of beneficial bacteria, such as Bifidobacterium spp. and Lactobacillus spp., and suppress the proliferation of harmful bacteria, such as Escherichia coli (Takahashi et al., 2004; Kong et al., 2011). Many studies reported that C. butyricum has a positive effect on anti-inflammation, repairing the intestinal epithelial cells, and improving immunity (Gao, 2012a, 2012b; Yang et al., 2012; Kanai et al., 2015; Wang et al., 2015). Clostridium butyricum has been widely used as probiotics in human food and medicine, but there have been few reports about C. butyricum application on the sows.
The purpose of this experiment is to investigate the effects of C. butyricum addition to sow diets in late gestation and lactation on health, intestinal microbial composition and reproductive performance.
MATERIALS AND METHODS
This experiment was performed at a commercial pig farm in Sichuan Province in China. Animal management and sampling procedures were approved by the Animal Care and Use Committee of the Sichuan Agricultural University and were performed in accordance with the National Research Council’s Guideline for the Care and Use of Laboratory Animals.
Experimental Design and Animals
A total of 180 mixed-parity Landrace × Yorkshire sows (parity 2.42 ± 1.19; back fat, BF, 14.73 ± 1.35 mm, mean ± SD) bred with semen from a pool of Landrace boars were selected. On 90 d of gestation, the sows were randomly assigned to one of four groups according to their parity and BF, with 45 replicates per group. The dietary treatments included a basal commercial gestation and lactation diet (control, 0% C. butyricum; Table 1) or the same basal diet added with 0.1% C. butyricum (1 × 108 CFU/kg of feed), 0.2% C. butyricum (2 × 108 CFU/kg of feed), 0.4% C. butyricum (4 × 108 CFU/kg of feed) from 90 d of gestation to weaning at 21 d of lactation, respectively. The C. butyricum strain provided by Chengdu Yukang Technology Co. Ltd. was C. butyricum UCN-12, containing C. butyricum with a count of 108 CFU per gram of product. Antibiotics were excluded from the experimental diets. All the sows were fed 2.80 kg of experimental diet from 90 d of gestation to parturition. The sows were housed in individual stalls and fed twice a day (08:00 and 15:00 hours) during gestation. At 110 d of gestation, sows were moved to farrowing room. The day of parturition was defined as day 0 of lactation, and the piglets were weaned at day 21 of lactation. At farrowing, birthweight of the alive piglets and the number of alive piglets, stillborn piglets, malformed piglets, and mummies was recorded, respectively. According to the number of effective tits of sows, litters were standardized to ~12 piglets per sow within 24 h after birth by cross-fostering within treatment. Piglets were weighted after standardization of the litters and at weaning, and underwent routine processing procedures (ear notching, tail docking, castration, and supplemental iron injection) within 3 d after farrowing. The feed allowance was progressively increased stepwise from 1.0 kg at day 0 of lactation, by 1.0 kg/d, up to their maximum feed intake and then allowed free access to feed to 21 d of lactation (weaning). Feed allocation and refusals were recorded on a daily basis. Sows were fed four times (08:00, 11:00, 15:00, and 20:00 hours) per day during lactation. In addition, the piglets had free access to water throughout lactation but had no access to creep feed. Temperature in the farrowing house was maintained between 20 and 25 °C. After weaning, the sows were transferred to the gestation house. Estrous detection was done twice (09:00 and 16:00 hours) in the presence of a boar, and the time of estrus was recorded, which was used to calculate the wean-to-estrus interval.
Table 1.
Ingredients and chemical composition of basal diets (as-fed basis, %)
| Ingredients, % | Gestation | Lactation |
|---|---|---|
| Yellow corn | 33.58 | 40.08 |
| Wheat | 20 | 28 |
| Soybean meal, 43% CP | 14.5 | 18.2 |
| Fish meal, 67% CP | 0 | 2 |
| Expanded soybean | 0 | 5 |
| Wheat bran | 8 | 0 |
| Soybean hulls | 18 | 0 |
| l-Lys HCl, 98% | 0.03 | 0.28 |
| l-Thr, 98.5% | 0 | 0.1 |
| dl-Met, 99% | 0 | 0.03 |
| Limestone | 1.4 | 1.3 |
| CaHPO4 | 1.2 | 1.2 |
| Choline chloride, 50% | 0.15 | 0.15 |
| Salt | 0.4 | 0.4 |
| Vitamin–mineral premix1 | 2.74 | 3.26 |
| Total | 100 | 100 |
| Nutrient content, % | ||
| Digestible energy, Mcal/kg | 2.85 | 3.20 |
| CP | 15.03 | 18.76 |
| Crude fiber | 8.75 | 2.48 |
| Calcium | 1.09 | 1.08 |
| Total phosphorus | 0.63 | 0.69 |
| Available phosphorus | 0.38 | 0.43 |
| Total lysine | 0.73 | 1.11 |
1Vitamin–mineral premix provided the following per kilogram of basal diet: vitamin A, 8,000 IU; vitamin D3, 2,000 IU; vitamin E, 12.5 IU; vitamin K 2.5 mg; Biotin, 0.2 mg; folacin, 0.25 mg; niacin, 17.5 mg; pantothenic acid, 12.5 mg; riboflavin, 8.0 mg; thiamin, 1.0 mg; vitamin B6, 3.00 mg; vitamin B12, 15 μg; copper, 16 mg; iodine, 0.3 mg; iron, 165 mg; manganese, 30 mg; selenium, 0.3 mg; zinc, and 165 mg. The sources of the trace elements were CuSO4·5H2O, KI, FeSO4, MnSO4·H2O, Na2SeO3, and ZnSO4, respectively.
Sample Collection
The BF thickness was measured at 65 mm to the left side of the dorsal midline at the last rib level using the ultrasound (Renco Lean-Meatier; Renco Corporation, Minneapolis, MN) and recorded at 89 d of gestation and at days 1 and 28 of lactation. The total litter size was calculated as the sum of alive piglets, stillborn piglets, and mummies. At farrowing, the birth time of the first and last piglets (born alive or stillborn or mummified) was recorded respectively, and the difference was defined as duration of farrowing. The interval between piglet born was calculated as duration of farrowing divided by the total litter size. Sow fasting blood samples (10 mL) were collected from ear marginal veins at farrowing (day 0 of lactation) and 14 and 21 d of lactation before the morning meal. Blood samples of piglets were collected from the anterior vena cava at 14 and 21 d of age, respectively. All the blood samples were collected into vacuum tubes (5 mL; Jiangsu Yuli medical instrument Co., Ltd, Jiangsu, China). Samples were immediately placed on ice then centrifuged at 3,000 × g for 10 min at room temperature and the serum was stored at −20 °C.
After parturition, colostrum samples (20 mL) were collected by hand-milking before any suckling by the piglets. At day 14 of lactation, milk samples (20 mL) were collected. Briefly, piglets were separated from their dams, and then 2 mL oxytocin was injected into the ear vein of each sow. Each sample was a mixture from the anterior, middle, and posterior functional glands by hand milking. Half of colostrum and milk samples were centrifuged at 3,000 × g for 15 min at room temperature, then supernatant was collected used for measurement of Immunoglobulin G & M concentrations (ELISA Kits supplied by Nanjing Jiancheng Bioengineering Institute, Nanjing, China). All the samples were refrigerated at −20 °C prior to subsequent analysis.
Fresh feces samples of sows with no disease and diarrhea were collected into sterile tubes, and then saved in liquid nitrogen immediately until transferring them to a freezer at −80 °C at 110 d of gestation and 14 and 21 d of lactation.
Analysis for Milk Composition
Frozen colostrum and milk samples were thawed at 4 °C, and 10 mL of each sample were used to analyze milk fat, protein, and lactose contents with an ultrasonic milk analyzer (Milkyway-CP2; Hangzhou Simple Technology Company Ltd, Hangzhou, China). The results were presented as the percentage in colostrum and milk.
Analysis of Oxidant and Antioxidant Content
The contents of malondialdehyde (MDA), total antioxidant capacity (T-AOC), the activities of glutathione peroxidase (GSH-Px), catalase (CAT), and superoxide dismutase (SOD) were assessed in the serum of sows and piglets using specific assay kits (Nanjing Institute of Jiancheng Biological Engineering, Nanjing, China) as previously described by Shen et al. (2015) and Wang et al. (2016).
Hormone and Endotoxin Assays
Serum prolactin, cortisol, and endotoxin were determined with their respective commercial ELISA kit (Catalog No. H095, H094, and H255, respectively; Nanjing Institute of Jiancheng Biological Engineering, Nanjing, China). The limit for determination of prolactin, cortisol, and endotoxin were 5.0 ng/mL, 5.0 ng/mL, and 3 EU/mL, respectively. The intra- and interassay coefficients of variation were <10% and 12% all for prolactin, cortisol, and endotoxin.
Short-Chain Fatty Acid Analysis
Short-chain fatty acids (SCFAs) including acetate, propionate, and butyrate in faces samples were analyzed with a previous method (Gu et al., 2017). Briefly, 2 g of faces samples was weighed into a 10-mL centrifuge tube and added with 5 mL of deionized water. After the tube was capped, the content was vortex mixed for 30 s, left to stand for 30 min at 4 °C and then centrifuged (1,000 × g, 4 °C) for 10 min. The supernatant (1.2 mL) was removed by aspiration into another 5-mL centrifuge tube, added with 0.24 mL of 25% metaphosphate, vortex mixed for 30 s and then left to stand for 30 min at 4 °C. Next, the contents were centrifuged (1,000 × g, 4 °C) for 10 min and then 1.2 mL of the supernatant was removed by aspiration, added with 23.3 μL of 210 mmol/L cortonic acid, and vortex mixed for 30 s. Then 0.3 mL of the mixed solution was removed into another 2-mL tube, added with 0.9 mL Carbinol, and vortex mixed for 30 s for the following gas chromatography analysis. The samples were analyzed by CP-3800 gas chromatography (Varian, Inc.) equipped with a microinjector (10 μL), a flame ionization detector, and a capillary chromatographic column (CP-FFAP, 25 m × 0.32 mm × 0.3 μm). The injector temperature was 220 °C, detector temperature was 250 °C, hydrogen flux was 40 mL/min, and air flux was 450 mL/min. The temperature program was as follows: 100 °C hold 1 min, increase to 190 °C at 20 °C/min, increase to 190 °C hold 3 min. Peaks were identified by comparing their retention times with individual reference standard fatty acids.
Microbial Analyses
Total bacterial DNA of faces sample from the control group (n = 5) and 0.2% C. butyricum (n = 5) group sows at 110 d gestation was extracted using the MO BIO PowerFecal DNA Isolation Kit (Catalog No. 12830-50, MO BIO Laboratories, Inc.) according to the manufacturer’s protocols. Before sequencing, the integrity of the extracted genomic DNA was determined by electrophoresis on a 1% (w/v) agarose gel. The concentration and purity of the extracted genomic DNA were measured. According to the concentration, DNA was diluted to 1 ng/μL using sterile water. The DNA samples were sent to Novogene Bioinformatics Technology (Beijing, China) to perform amplicon pyrosequencing on the Illumina HiSeq PE250 platforms. The V4 hypervariable region of the 16S rRNA gene was amplified using 515F and 806R primer (5′-GTGCCAGCMGCCGCGGTAA-3′ and 5′-GGACTACHVGGGTWTCTAAT-3′, respectively). Raw paired-end reads obtained by Illumina HiSeq sequencing were spliced. The splicing sequences were called raw tags. Quality filtering on the raw tags was performed under specific filtering conditions to obtain the high-quality clean tags (Bokulich et al., 2013) according to the QIIME (V1.7.0, http://qiime.org/index.html;Caporaso et al., 2010) quality controlled process. And then chimeric filtering was performed to get effective tags (Figure 1A). The effective tags were mapped to operational taxonomic units (OTUs) using Uparse software (v7.0.1001 http://drive5.com/uparse/) at 97% sequence similarity. Representative sequence for each OTU was screened for further annotation. The Ribosomal Database Project classifier Version 2.2 was used to assign a taxonomic rank to each representative sequence. OTUs abundance information were normalized using a standard of sequence number corresponding to the sample with the least sequences. Subsequent analysis of alpha diversity and beta diversity were all performed basing on this output normalized data. The relative abundance of each OTU was examined at different taxonomic levels. At the phylum level, as the sum of the top 10 phyla with relative abundance exceeded 98%, we selected the top 10 phyla for statistical analysis with the control group as the reference. At the genus level, we selected genera with relative abundance of more than 0.2% in at least one sample for statistical analysis.
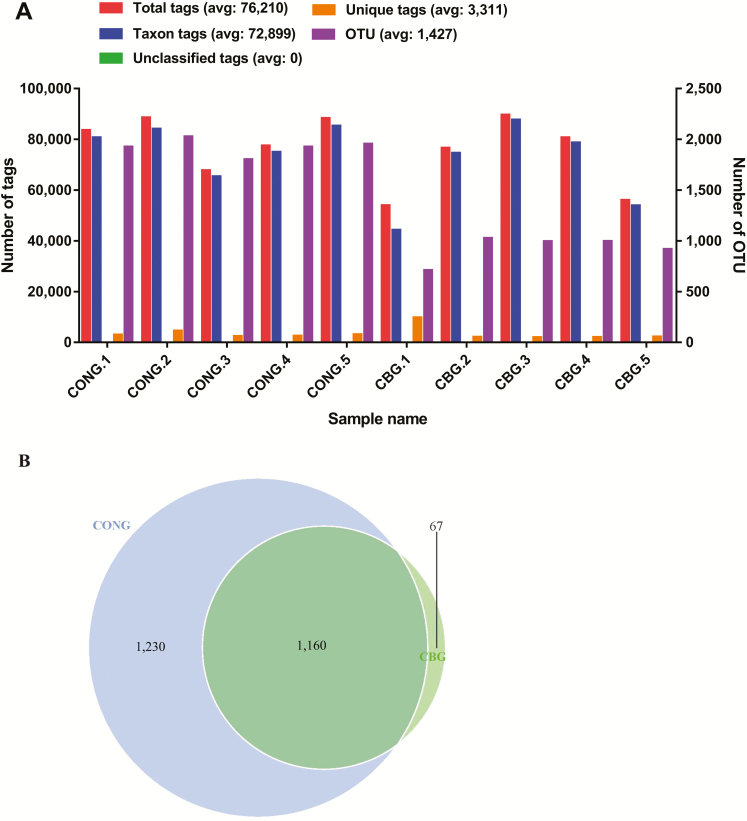
Figure 1.
Tags number, OTUs number, and Venn diagrams. Total tags are equivalent to effective tags. Taxon tags refer to the number of tags used to build OTUs. Unique tags, the sequence that cannot be clustered to OTUs will not be used for subsequent analysis. Unclassified tags refer to the number of Tags that do not receive annotation information. OTUs, operational taxonomic units. CONG, the control group at gestation; CBG, the 0.2% C. butyricum group at gestation.
Statistical Analysis
A total of 7 sows (6 in the control group and 1 in 0.4% C. butyricum group) were excluded from this experiment during pregnancy due to abortion or premature delivery. When did statistical analysis on the reproductive performance of gestation sows, 5 sows were excluded (1 in the control group, 2 in the 0.2% C. butyricum group, and 2 in the 0.4% C. butyricum group, respectively) because of the total litter size of the sows <7. When did statistical analysis on the duration of farrowing, 6 data were excluded (1 in the 0.4% C. butyricum group, 3 in the 0.4% C. butyricum group, and 2 in the 0.4% C. butyricum group, respectively) because the average interval between piglet born was more than 45 min. There were only 3 malformed piglets in total, so the malformed piglets were considered as stillborn piglets.
Twenty-seven sows (1 in the control group, 9 in the 0.1% C. butyricum group, 9 in the 0.2% C. butyricum group, and 8 in the 0.4% C. butyricum group) were excluded in the lactation due to foot injuries of sows or death of the piglets more than half.
The original data were checked by using Grubbs’ test method. If (α, n)S, Xp was considered as the outlier. The data were performed to check homogeneity of variances test and normal distribution of the residuals before using parametric analyses. Statistical analyses were performed through Mixed procedure of SAS 9.4 (SAS Institute Inc., Cary, NC) in a completely randomized design. The following statistical model was used: Yij = μ + Ti + eij, where Y is the analyzed variable, μ is the overall mean, Ti is the fixed effect of the ith treatment, and eij is the error term specific to the sow identified assigned to the ith treatment. Orthogonal polynomial contrasts were used to analyze the differences between the control group and C. butyricum groups and to determine the linear and quadratic effects of the increasing concentrations of C. butyricum included in the diet, with the replicate serving as the experimental unit.
Data of relative abundance of gut microbiota were analyzed through Glimmix procedure of SAS 9.4. Diversity within communities (Alpha diversity) calculations and taxonomic community assessments were performed by QIIME software (Version 1.7.0). Cluster analysis was preceded by principal component analysis (PCA), which was applied to reduce the dimension of the original variables using the FactoMineR package and ggplot2 package in R software (Version 2.15.3).
Differences were considered statistically significant when P < 0.05 and as a trend to significance when 0.05 ≤ P < 0.10.
RESULTS
Reproductive Performance of Sows
The interval between piglet born was linearly (P < 0.05) decreased and the duration of farrowing was significantly (quadratic, P < 0.05) shortened as C. butyricum addition increased (Table 2). As shown in Table 3, there was a linear increase in litter weight at weaning and litter weight gain (P < 0.05) as C. butyricum addition increased. The wean-to-estrus interval was significantly decreased (P = 0.045) with C. butyricum addition compared with the control (0 % C. butyricum).
Table 2.
Reproductive performance of sows1
| C. butyricum, % of diet | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Items | 0 | 0.1 | 0.2 | 0.4 | Con vs. C. butyricum | Linear | Quadratic |
| Total number born | 13.60 ± 0.44 | 14.44 ± 0.38 | 13.93 ± 0.40 | 14.62 ± 0.39 | 0.133 | 0.154 | 0.860 |
| No. born alive | 12.45 ± 0.44 | 13.31 ± 0.43 | 12.79 ± 0.41 | 13.64 ± 0.41 | 0.115 | 0.096 | 0.966 |
| Litter weight alive at parturition, kg | 18.10 ± 0.57 | 18.32 ± 0.59 | 17.55 ± 0.50 | 19.08 ± 0.61 | 0.756 | 0.270 | 0.236 |
| Average weight of piglets born alive, kg | 1.47 ± 0.03 | 1.39 ± 0.03 | 1.40 ± 0.04 | 1.41 ± 0.04 | 0.078 | 0.367 | 0.140 |
| Duration of farrowing, min | 246.6 ± 11.6 | 225.6 ± 11.8 | 204.8 ± 10.6 | 229.6 ± 12.1 | 0.053 | 0.341 | 0.023 |
| Interval between piglet born, min | 18.5 ± 1.0 | 15.7 ± 0.8 | 14.6 ± 0.7 | 15.8 ± 0.8 | 0.001 | 0.046 | 0.004 |
1Values are means ± SE. N = 38, 45, 43, and 42 for 0%, 0.1%, 0.2%, and 0.4% C. butyricum group for statistical analysis, respectively.
Table 3.
Growth performance of suckling piglets, feed intake, BF loss of sows during lactation, and weaning–estrus interval1
| C. butyricum, % of diet | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Items | 0 | 0.1 | 0.2 | 0.4 | Con vs. C. butyricum | Linear | Quadratic |
| Litter size after cross-foster, n | 12.32 ± 0.08 | 12.39 ± 0.08 | 12.36 ± 0.08 | 12.30 ± 0.08 | 0.764 | 0.730 | 0.515 |
| Litter weight after cross-foster, kg | 17.81 ± 0.38 | 17.73 ± 0.42 | 17.67 ± 0.41 | 17.64 ± 0.46 | 0.793 | 0.780 | 0.913 |
| Litter size at weaning, n | 10.22 ± 0.25 | 10.50 ± 0.22 | 10.50 ± 0.22 | 10.56 ± 0.22 | 0.249 | 0.357 | 0.546 |
| Litter weight at weaning, kg | 59.23 ± 2.27 | 60.92 ± 2.12 | 65.43 ± 2.04 | 65.11 ± 1.88 | 0.057 | 0.030 | 0.299 |
| Litter weight gain, kg | 41.43 ± 2.26 | 43.19 ± 2.11 | 47.76 ± 1.93 | 47.47 ± 1.82 | 0.046 | 0.023 | 0.278 |
| ADFI during lactation, kg/d | 5.66 ± 0.09 | 5.51 ± 0.07 | 5.66 ± 0.08 | 5.72 ± 0.09 | 0.781 | 0.317 | 0.416 |
| BF loss during lactation, mm | 3.11 ± 0.15 | 3.14 ± 0.16 | 3.00 ± 0.12 | 2.92 ± 0.14 | 0.582 | 0.253 | 0.903 |
| Wean-to-estrus interval, d | 6.00 ± 0.57 | 5.03 ± 0.48 | 4.83 ± 0.47 | 4.67 ± 0.45 | 0.045 | 0.084 | 0.290 |
1Values are means ± SE. N = 37, 36, 36, and 36 for 0%, 0.1%, 0.2%, and 0.4% C. butyricum group for statistical analysis, respectively.
Milk Composition and Immunoglobulin Concentrations
As shown in Table 4, the lactose and protein content in milk were both linearly increased (P < 0.05) as C. butyricum addition increased. The concentrations of IgG and IgM in colostrum, and the concentrations of IgM in milk were linearly increased (P < 0.05) as C. butyricum addition increased, respectively. There was a tendency to increase the concentration of IgG in milk with C. butyricum addition (P = 0.085; Table 4).
Table 4.
Effect of Clostridium butyricum addition in late gestation and lactation on composition and immunoglobulin concentrations of sow colostrum and milk1
| C. butyricum, % of diet | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Items | 0 | 0.1 | 0.2 | 0.4 | Con vs. C. butyricum | Linear | Quadratic |
| Colostrum | |||||||
| Fat, % | 6.59 ± 0.76 | 6.66 ± 0.42 | 7.00 ± 0.54 | 6.97 ± 0.46 | 0.670 | 0.596 | 0.821 |
| Lactose, % | 3.50 ± 0.84 | 3.55 ± 0.32 | 3.90 ± 0.72 | 3.80 ± 0.46 | 0.425 | 0.120 | 0.691 |
| Protein, % | 16.66 ± 0.56 | 16.63 ± 0.41 | 16.70 ± 0.64 | 17.61 ± 0.26 | 0.576 | 0.151 | 0.468 |
| IgG, mg/mL | 60.24 ± 1.89 | 63.84 ± 3.14 | 65.51 ± 2.35 | 70.19 ± 3.39 | 0.067 | 0.020 | 0.846 |
| IgM, mg/mL | 49.52 ± 2.44 | 52.14 ± 2.77 | 60.17 ± 3.39 | 62.14 ± 3.37 | 0.025 | 0.005 | 0.398 |
| Milk | |||||||
| Fat, % | 6.28 ± 0.38 | 6.51 ± 0.51 | 6.69 ± 0.50 | 7.23 ± 0.60 | 0.181 | 0.922 | 0.951 |
| Lactose, % | 4.70 ± 0.28 | 5.20 ± 0.12 | 5.43 ± 0.05 | 5.49 ± 0.24 | 0.007 | 0.013 | 0.122 |
| Protein, % | 3.50 ± 0.12 | 3.43 ± 0.11 | 3.77 ± 0.04 | 4.26 ± 0.17 | 0.031 | <0.001 | 0.208 |
| IgG, mg/mL | 39.14 ± 2.21 | 41.20 ± 1.33 | 44.52 ± 2.16 | 44.23 ± 2.06 | 0.085 | 0.071 | 0.306 |
| IgM, mg/mL | 33.79 ± 0.91 | 36.85 ± 2.24 | 42.08 ± 2.33 | 43.19 ± 1.79 | 0.006 | 0.002 | 0.201 |
1Values are means ± SE, n = 6 per treatment.
Oxidative and Antioxidative Indicators in the Serum of Sows and Piglets
Serum MDA concentrations of sows at parturition and on 14 d in lactation were linearly and quadratically (P < 0.05) decreased as C. butyricum addition increased, respectively. Serum MDA concentrations of piglets at 14 d of age were linearly (P < 0.05) decreased as C. butyricum addition increased (Table 5). The serum T-AOC concentrations of sows at parturition and 14 d in lactation were linearly and quadratically affected (P < 0.05) as C. butyricum addition increased, respectively. The serum T-AOC concentrations of sows at 21 d in lactation were linearly elevated (P < 0.05) as C. butyricum addition increased. The serum total superoxide dismutase (T-SOD) concentrations of sows at 14 d in lactation were linearly elevated (P < 0.05) as C. butyricum addition increased. However, the serum GSH-Px concentrations of sows at parturition was linearly decreased (P < 0.05) as C. butyricum addition increased (Table 6). The serum T-AOC concentrations of piglets at 14 and 21 d of age were both linearly and quadratically (P < 0.05) influenced as C. butyricum addition increased (Table 7).
Table 5.
Effect of Clostridium butyricum addition in late gestation and lactation on MDA concentrations in serum of sows and piglets1
| C. butyricum, % of diet | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Items | 0 | 0.1 | 0.2 | 0.4 | Con vs. C. butyricum | Linear | Quadratic |
| Serum MDA of sows, nmol/mL | |||||||
| At parturition | 7.55 ± 0.70 | 6.75 ± 0.59 | 3.86 ± 0.26 | 4.07 ± 0.30 | <0.001 | <0.001 | 0.022 |
| Day 14 of lactation | 7.55 ± 1.21 | 5.91 ± 0.82 | 3.67 ± 0.23 | 4.49 ± 0.30 | 0.004 | 0.008 | 0.028 |
| Day 21 of lactation | 5.05 ± 0.60 | 6.34 ± 0.87 | 5.09 ± 0.77 | 5.92 ± 0.29 | 0.350 | 0.614 | 0.890 |
| Serum MDA of piglets, nmol/mL | |||||||
| Day 14 of age | 5.14 ± 0.32 | 4.61 ± 0.30 | 3.89 ± 0.19 | 3.77 ± 0.33 | 0.005 | 0.002 | 0.162 |
| Day 21 of age | 3.71 ± 0.36 | 3.75 ± 0.22 | 2.79 ± 0.35 | 3.29 ± 0.18 | 0.202 | 0.169 | 0.168 |
1Values are means ± SE, n = 6 per treatment.
Table 6.
Effect of Clostridium butyricum addition in late gestation and lactation on serum antioxidative indicators of sows1
| C. butyricum, % of diet | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Items | 0 | 0.1 | 0.2 | 0.4 | Con vs. C. butyricum | Linear | Quadratic |
| At parturition, U/mL | |||||||
| T-AOC | 8.65 ± 1.51 | 8.14 ± 1.05 | 7.96 ± 1.62 | 14.94 ± 1.6 | 0.328 | 0.003 | 0.044 |
| T-SOD | 77.15 ± 7.41 | 60.36 ± 7.41 | 70.31 ± 3.81 | 72.13 ± 4.45 | 0.135 | 0.979 | 0.157 |
| CAT | 1.95 ± 0.31 | 1.37 ± 0.19 | 1.62 ± 0.34 | 1.88 ± 0.19 | 0.295 | 0.822 | 0.177 |
| GSH-Px | 763.7 ± 33.0 | 742.2 ± 19.8 | 724.1 ± 66.0 | 618.1 ± 58.6 | 0.230 | 0.036 | 0.593 |
| Day 14 of lactation, U/mL | |||||||
| T-AOC | 10.16 ± 1.55 | 11.12 ± 1.61 | 10.82 ± 2.14 | 23.34 ± 2.33 | 0.039 | <0.001 | 0.028 |
| T-SOD | 60.28 ± 4.32 | 69.05 ± 2.51 | 68.93 ± 3.75 | 73.15 ± 2.15 | 0.016 | 0.021 | 0.342 |
| CAT | 2.06 ± 0.29 | 2.16 ± 0.13 | 2.39 ± 0.62 | 2.74 ± 0.76 | 0.540 | 0.326 | 0.962 |
| GSH-Px | 1000.0 ± 33.2 | 899.5 ± 41.8 | 948.3 ± 58.1 | 995.1 ± 44.4 | 0.328 | 0.705 | 0.174 |
| Day 21 of lactation, U/mL | |||||||
| T-AOC | 7.08 ± 1.93 | 11.68 ± 1.27 | 10.36 ± 1.31 | 13.35 ± 1.83 | 0.020 | 0.026 | 0.513 |
| T-SOD | 39.82 ± 0.92 | 41.38 ± 2.01 | 35.11 ± 1.52 | 36.94 ± 2.04 | 0.313 | 0.091 | 0.424 |
| CAT | 1.27 ± 0.18 | 1.55 ± 0.17 | 2.27 ± 0.32 | 1.71 ± 0.25 | 0.051 | 0.161 | 0.030 |
| GSH-Px | 1003.1 ± 50.1 | 840.6 ± 57.0 | 844.9 ± 27.1 | 865.6 ± 62.0 | 0.017 | 0.147 | 0.066 |
1Values are means ± SE, n = 6 per treatment.
Table 7.
Effect of Clostridium butyricum addition in late gestation and Lactation on serum antioxidative indicators of piglets1
| C. butyricum, % of diet | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Items | 0 | 0.1 | 0.2 | 0.4 | Con vs. C. butyricum | Linear | Quadratic |
| Day 14 of age, U/mL | |||||||
| T-AOC | 5.10 ± 0.72 | 12.22 ± 2.2 | 17.20 ± 2.78 | 19.44 ± 1.88 | <0.001 | <0.001 | 0.048 |
| T-SOD | 51.15 ± 5.91 | 53.14 ± 3.87 | 44.36 ± 2.34 | 49.77 ± 3.20 | 0.666 | 0.606 | 0.442 |
| CAT | 1.63 ± 0.13 | 1.62 ± 0.15 | 1.68 ± 0.18 | 1.94 ± 0.14 | 0.519 | 0.116 | 0.505 |
| GSH-Px | 365.4 ± 31.3 | 398.6 ± 52.7 | 403.3 ± 37.9 | 412.1 ± 21.0 | 0.376 | 0.429 | 0.669 |
| Day 21 of age, U/mL | |||||||
| T-AOC | 8.15 ± 1.96 | 12.17 ± 1.53 | 23.82 ± 2.88 | 22.08 ± 3.37 | 0.001 | <0.001 | 0.034 |
| T-SOD | 52.01 ± 3.87 | 56.33 ± 5.87 | 48.28 ± 4.02 | 49.10 ± 5.64 | 0.893 | 0.471 | 0.974 |
| CAT | 1.67 ± 0.15 | 1.67 ± 0.16 | 1.71 ± 0.16 | 1.80 ± 0.20 | 0.764 | 0.554 | 0.898 |
| GSH-Px | 529.2 ± 43.1 | 527.6 ± 47.0 | 599.6 ± 52.5 | 626.1 ± 36.7 | 0.302 | 0.092 | 0.898 |
1Values are means ± SE, n = 6 per treatment.
Serum Endotoxin, Cortisol, and Prolactin
As shown in Table 8, there was a tendency to decrease the serum endotoxin concentration of sows on day 14 in lactation with C. butyricum addition (P = 0.072). There was a linear decrease in the serum endotoxin concentration of sows on day 21 in lactation (P < 0.05) as C. butyricum addition increased. Serum endotoxin concentrations of piglets at 14 and 21 d of age were linearly decreased (P < 0.05) as C. butyricum addition increased, respectively. The serum cortisol concentrations of piglets at days 14 and 21 of age were both significantly (quadratic, P < 0.05) decreased as C. butyricum addition increased.
Table 8.
Effect of Clostridium butyricum addition in late gestation and lactation on endotoxin, prolactin, and cortisol in serum of sows or piglets1
| C. butyricum, % of diet | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Items | 0 | 0.1 | 0.2 | 0.4 | Con vs. C. butyricum | Linear | Quadratic |
| At parturition, sows | |||||||
| Endotoxin, EU/mL | 407.7 ± 71.0 | 284.5 ± 58.4 | 318.3 ± 44.5 | 326.9 ± 89.3 | 0.230 | 0.585 | 0.354 |
| Prolactin, ng/mL | 234.34 ± 7.14 | 282.26 ± 36.13 | 194.55 ± 22.64 | 201.72 ± 27.98 | 0.787 | 0.137 | 0.911 |
| Cortisol, ng/mL | 115.81 ± 41.65 | 96.42 ± 25.05 | 100.88 ± 25.30 | 113.60 ± 40.96 | 0.762 | 0.955 | 0.667 |
| Day 14 of lactation, sows | |||||||
| Endotoxin, EU/mL | 588.6 ± 86.8 | 328.1 ± 112.3 | 476.4 ± 70.0 | 394.2 ± 61.0 | 0.072 | 0.298 | 0.372 |
| Prolactin, ng/mL | 251.40 ± 24.38 | 242.39 ± 29.78 | 248.81 ± 17.29 | 228.58 ± 27.67 | 0.699 | 0.553 | 0.857 |
| Cortisol, ng/mL | 118.24 ± 46.38 | 99.03 ± 25.91 | 105.14 ± 35.05 | 110.29 ± 28.43 | 0.743 | 0.948 | 0.747 |
| Day 21 of lactation, sows | |||||||
| Endotoxin, EU/mL | 643.9 ± 86.7 | 346.7 ± 76.2 | 360.5 ± 44.0 | 320.7 ± 45.3 | 0.001 | 0.009 | 0.039 |
| Prolactin, ng/mL | 221.75 ± 30.39 | 224.85 ± 25.36 | 181.01 ± 36.56 | 243.67 ± 20.75 | 0.877 | 0.351 | 0.255 |
| Cortisol, ng/mL | 47.39 ± 8.71 | 37.75 ± 4.94 | 38.57 ± 7.99 | 32.22 ± 4.68 | 0.174 | 0.168 | 0.700 |
| Day 14 of age, piglets | |||||||
| Endotoxin, EU/mL | 371.4 ± 50.2 | 281.7 ± 43.1 | 248.0 ± 46.2 | 144.1 ± 21.6 | 0.008 | 0.002 | 0.612 |
| Cortisol, ng/mL | 46.27 ± 1.45 | 37.89 ± 3.35 | 36.34 ± 1.15 | 39.47 ± 2.80 | 0.006 | 0.116 | 0.014 |
| Day 21 of age, piglets | |||||||
| Endotoxin, EU/mL | 284.3 ± 44.0 | 215.5 ± 28.6 | 163.0 ± 13.9 | 69.1 ± 12.5 | <0.001 | <0.001 | 0.689 |
| Cortisol, ng/mL | 64.22 ± 4.79 | 49.53 ± 3.56 | 41.73 ± 2.65 | 44.83 ± 3.74 | <0.001 | 0.003 | 0.006 |
1Values are means ± SE, n = 6 per treatment.
The SCFAs Concentration in Faeces Samples of Sows
As shown in Table 9, the acetate and propionate concentrations in faeces samples of sows at 110 d of gestation were linearly increased (P < 0.05) as C. butyricum addition increased, respectively. The butyrate concentrations in faces samples of sows at 110 d of gestation and at 14 d in lactation were linearly (P < 0.05) elevated as C. butyricum addition increased, respectively. The butyrate concentrations in faeces samples of sows at 21 d in lactation were linearly and quadratically (P < 0.05) influenced as C. butyricum addition increased.
Table 9.
Effect of Clostridium butyricum addition in late gestation and lactation on SCFAs concentration in faces of sows1
| C. butyricum, % of diet | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Items | 0 | 0.1 | 0.2 | 0.4 | Con vs. C. butyricum | Linear | Quadratic |
| Day 110 of gestation, μmol/g | |||||||
| Acetic acid | 36.39 ± 4.70 | 45.00 ± 3.48 | 48.04 ± 5.58 | 55.89 ± 7.34 | 0.052 | 0.024 | 0.663 |
| Propionic acid | 14.84 ± 1.22 | 22.01 ± 2.67 | 22.65 ± 2.87 | 25.64 ± 3.90 | 0.018 | 0.024 | 0.306 |
| Butyric acid | 6.78 ± 0.62 | 6.46 ± 0.64 | 9.67 ± 1.37 | 11.27 ± 0.84 | 0.041 | <0.001 | 0.956 |
| Day 14 of lactation, μmol/g | |||||||
| Acetic acid | 44.52 ± 5.64 | 41.14 ± 4.46 | 42.89 ± 5.65 | 45.27 ± 4.05 | 0.808 | 0.800 | 0.631 |
| Propionic acid | 22.39 ± 4.78 | 21.62 ± 1.72 | 18.83 ± 2.61 | 16.01 ± 0.94 | 0.106 | 0.992 | 0.775 |
| Butyric acid | 6.92 ± 0.82 | 6.52 ± 0.71 | 10.98 ± 1.23 | 11.63 ± 1.08 | 0.026 | <0.001 | 0.556 |
| Day 21 of lactation, μmol/g | |||||||
| Acetic acid | 42.03 ± 7.08 | 45.94 ± 2.91 | 48.90 ± 6.12 | 45.67 ± 5.56 | 0.471 | 0.681 | 0.464 |
| Propionic acid | 20.40 ± 3.91 | 20.77 ± 2.16 | 22.02 ± 3.42 | 18.43 ± 3.37 | 0.997 | 0.664 | 0.558 |
| Butyric acid | 6.82.1 ± 0.91 | 8.78 ± 0.83 | 12.57 ± 1.72 | 11.44 ± 0.70 | 0.006 | 0.006 | 0.041 |
1Values are means ± SE, n = 6 per treatment.
Fecal Microbiota
As shown in Figure 1, a total of 762,101 tags were obtained from all faces samples, ranging from 53,916 to 89,558. In total, 14,267 OTUs at the 97% identity level were obtained from all samples and used for downstream analyses. Venn diagram was used to evaluate the distribution of OTUs among the two groups. Based on the analyses, a total of 1,160 OTUs co-existed in the two groups. The number of OTUs in 0.2% C. butyricum group was obviously less than that in the control group (4,639 vs. 9,628).
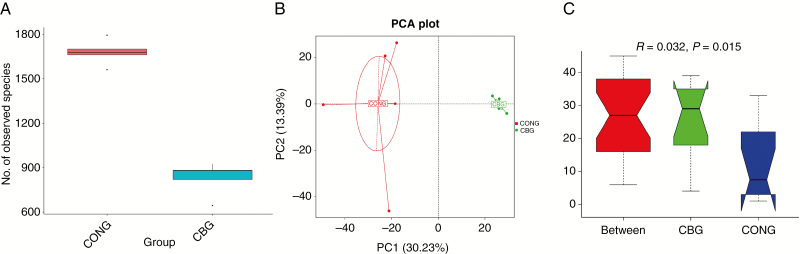
Alpha diversity and beta diversity can reflect the richness and diversity of microbial communities, the observed species index was calculated for alpha diversity (Figure 2A) and a PCA was performed for beta diversity (Figure 2B). The observed species index was obviously higher (P < 0.05) in the faces samples of sows offered 0.2% C. butyricum. The principal component analysis showed that 0.2% C. butyricum had an important effect on intestinal microbial diversity. The fecal microbiota from the control group and 0.2% C. butyricum group were divided into two different clusters that separated clearly in the PCA (Figure 2B). Moreover, the PCA plots indicated that the variation among samples in the composition of fecal microbiota in 0.2% C. butyricum group was smaller than that in the control group. Anosim analysis is used to test whether the differences between groups are significantly greater than those within groups. The results showed significant differences between groups, which indicated C. butyricum significantly affected the structure of intestinal microbial communities (Figure 2C).
Figure 2.
Alpha diversity, beta diversity, and significance test of microbial community structure. (A) The observed species index analyses, P < 0.001; (B) principal component analysis; (C) anosim analyses show the difference of microbial community structure. R-value is >0, indicating significant differences between groups. The reliability of statistical analysis is expressed by P-value.
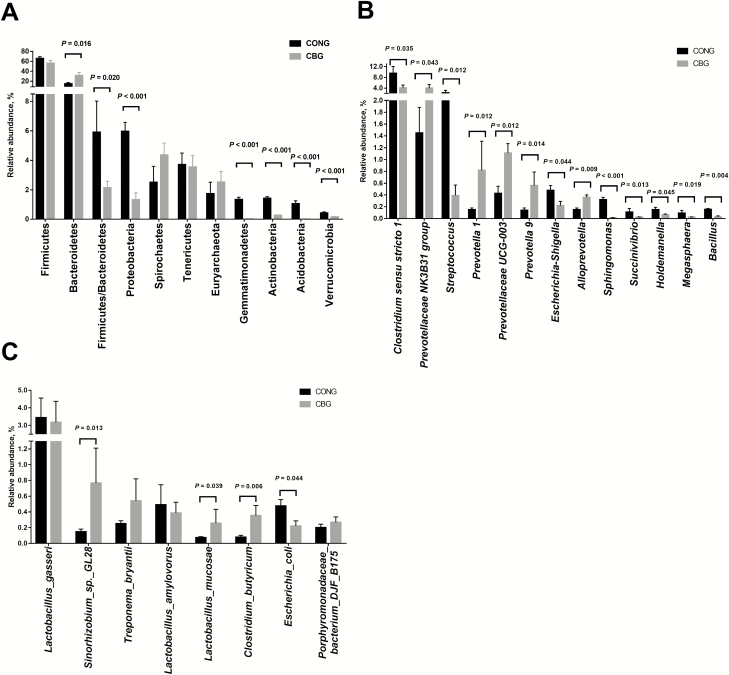
The top 10 phyla and Firmicutes/Bacteroidetes ratio were chosen for significance analyses (Figure 3A). The results suggest that the 0.2% C. butyricum increased the relative abundance of Bacteroidetes (P = 0.016), and decreased the relative abundance of Proteobacteria, Gemmatimonadetes, Actinobacteria, Acidobacteria, Verrucomicrobia (P < 0.001), and Firmicutes/Bacteroidetes ratio (P = 0.020). A total of 52 genera (the relative abundance >0.2% in at least one sample) were chosen for significance analyses, and 13 genera with significant differences were presented in Figure 3B. The 0.2% C. butyricum increased the relative abundance of Prevotellaceae_NK3B31_group (P = 0.043), Prevotella_1 (P = 0.012), Prevotellaceae_UCG-003 (P = 0.012), Prevotella_9 (P = 0.014), and Alloprevotella (P = 0.009), and decreased the relative abundance of Clostridium_sensu_stricto_1 (P = 0.035), Streptococcus (P = 0.012), Escheruchia-Shigella (P = 0.044), Sphingomonas (P < 0.001), Succinivibrio (P = 0.013), Holdemanella (P = 0.045), Megasphaera (P = 0.019), and Bacillus (P = 0.004). The top eight species were chosen for significance analyses (Figure 3C). The results suggest that the 0.2% C. butyricum increased the relative abundance of Sinorhizobium_sp._GL28 (P = 0.013), Lactobacillus_mucosae (P = 0.039), and Clostridium_butyricum (P = 0.006) and decreased the relative abundance of Escherichia_coli (P = 0.044).
Figure 3.
The relative abundances of fecal microbiota composition at the phylum level (A), genus (B), and the species level (C). A denotes the relative abundances of top 10 phyla of fecal microbiota composition. B denotes the relative abundances of thirteen genera (%, >0.2% in at least one sample) with significant difference. C denotes the top eight species (%, >0.01% in at least one sample) of fecal microbiota composition. Data were expressed as means ± SE, n = 5 for each treatment. CONG, the control group at gestation; CBG, the 0.2% Clostridium butyricum group at gestation.
DISCUSSION
In the present study, we focused on the effect of C. butyricum UCN-12 addition to sow diets in late gestation and lactation on reproductive performance. The results showed that C. butyricum UCN-12 addition shortened the duration of farrowing, increased the growth performance of suckling piglets and reduced weaning–estrus interval of sows. Moreover, 0.2% C. butyricum UCN-12 addition (2 × 108 CFU/kg of feed) showed the greatest effects when cost was taken into account.
Previous studies have shown that C. butyricum could improve antioxidant capacity and immune function in animals (Yang et al., 2012; Wang et al., 2015; Sun et al., 2016). In the current study, the C. butyricum decreased the MDA concentration and increased the T-AOC concentration in serum of sows at parturition. This may be one of the reasons C. butyricum UCN-12 addition shortened the duration of farrowing. As we know, a large number of free radicals (H2O2, O2⋅– and HO⋅) is produced due to special physiological metabolism during the late pregnancy and lactation, leading to metabolic disorders and inflammation of sows and digestion and absorption function impaired, diarrhea rate increased and immune function impaired of piglets (Saker et al., 2008; Pereira and Martel, 2014). However, if excessive free radicals cannot be removed in time, oxidation stress will occur. Oxidative stress may be one of the important factors for causing longer farrowing duration (Cui et al., 2016; Muns et al., 2016; Royer et al., 2016). The C. butyricum can produce enzymes such as hydrogen peroxide enzyme, T-SOD, which can eliminate reactive oxygen (Wang et al., 2017). In addition, the metabolite such as butyric acid and hydrogen also have antioxidant function (Ohsawa et al., 2007; Hamer et al., 2009).
The growth performance of suckling piglets was increased with C. butyricum addition in the late pregnancy and lactation. The most likely explanation was that the quality of the milk was improved with C. butyricum addition. Indeed, in our study the C. butyricum addition increased the lactose and protein content in milk. Previous studies reported that probiotics increase the fat (Bacillus) and protein content (C. butyricum compound) in milk of sows or fat content (Prevotella bryantii) in milk of dairy cows (Alexopoulos et al., 2004; Chiquette et al., 2008; Inatomi et al., 2017). However, the reason why probiotics affect milk composition needs further research. In addition, the colostrum and milk from C. butyricum group sows contained more immunoglobulin M and G, which was beneficial for the health of piglets. Many reports on different animal species found that C. butyricum or other probiotics can stimulate the immune system to enhance the immune function of animals (Yang et al., 2012; Jang et al., 2013; Zanello et al., 2013). Unfortunately, we did not measure the milk yield for other reasons in this study. However, the previous studies reported that probiotics compound containing C. butyricum increase milk production in sows (Inatomi et al., 2017; Tsukahara et al., 2018). Another explanation was that the ability of piglets to resist stress was increased with .butyricum addition. Our results showed that C. butyricum decreased the MDA concentration and increased T-AOC concentration in serum of piglets. MDA is a biological marker of oxidative stress, and elevated MDA level indicate that animals are in a state of intense oxidative stress (Del Rio et al., 2005). This was consistent with previous reports on poultry, mice, weaned piglets, and aquatic animals, which found that the C. butyricum has the function of improving the animal’s growth performance and antioxidant capacity (Sun et al., 2016; Duan, 2017a, 2017b; Miao et al., 2018).
The gut microbiota not only affected the intestinal metabolism of nutrients such as carbohydrates (Rowland, et al., 2018) and minerals (Liu et al., 2018) but also played a critical role in animal health. Our results showed that 0.2% C. butyricum addition changed the composition of intestinal microbiota. The 0.2% C. butyricum increased the relative abundance of Bacteroidetes and decreased the relative abundance of Proteobacteria, Gemmatimonadetes, Actinobacteria, Acidobacteria, Verrucomicrobia, and Firmicutes/Bacteroidetes ratio. At genus level, the 0.2% C. butyricum mainly increased the relative abundance of Prevotella and decreased the relative abundance of Clostridium_sensu_stricto_1, Streptococcus, Escheruchia-Shigella, Sphingomonas, Succinivibrio, Holdemanella, Megasphaera, and Bacillus. At species level, the 0.2% C. butyricum mainly increased the relative abundance of Clostridium_butyricum, Lactobacillus_mucosae, Sinorhizobium_sp._GL28 and decreased the relative abundance of Escherichia_coli. Bacteria from the Bacteroidetes phylum, especially Prevotella, are known to be potent at fermenting fiber into SCFAs such as acetate, propionate, and butyrate (De Filippo et al., 2010). In addition, a higher abundance of Prevotella is reportedly a dominant feature of the fecal microbiota in healthy pigs (Dou et al., 2017). In the present study, the SCFAs in the feces from C. butyricum groups were increased. The SCFAs could inhibit proliferation of harmful bacteria (such as E. coli and Salmonella) and at the same time promote the beneficial bacteria (such as Lactobacillus and Bifidobacterium) proliferation and its mechanism may be due to the reduced pH, which changes the cell permeability of harmful bacteria. The SCFAs, such as butyrate and propionate, induced the differentiation of colonic regulatory T cells, which exerted a central role in the suppression of inflammatory and allergic responses (Furusawa et al., 2013; Castillo and Hand, 2018). Inflammatory bowel disease and colon cancer are associated with a high proportion of Actinobacteria bacteria and Streptococcus bacteria, respectively (Frank et al., 2007; Brim et al., 2017). The E. coli has been considered as an agent responsible for outbreak of hemorrhagic colitis which could be inhibited by C. butyricum (Takahashi et al., 2004). Previous studies have reported that probiotics reduced cortisol concentration in the serum of piglets, while endotoxin from Escherichia_coli further increased cortisol concentration (Collier et al., 2011). Our results suggested that 0.2% C. butyricum decreased the relative abundance of Escherichia_coli and cortisol, endotoxin concentrations in serum of piglets. It is worth mention that piglets can obtain probiotics indirectly from the sow and by her environment, enriched by sow feces or feed spillage (Yan et al., 2019). Several studies have shown that the addition of probiotics in the late pregnancy and lactation period of the sow can reduce the diarrhea and mortality of piglets and improve the growth performance of suckling piglets by inhibiting the proliferation of pathogenic bacteria or by enhancing certain immune mechanisms (Kritas et al., 2015).
CONCLUSIONS
The present research indicated that dietary addition with C. butyricum in late gestation and lactation could shorten the duration of farrowing and enhance the growth performance of suckling piglets by improving the antioxidant properties. Moreover, 0.2% C. butyricum administration to sows changed the composition of intestinal microbiota, especially increased the relative abundance of Prevotella.
Conflict of interest statement. None declared.
Footnotes
The work was supported by National Key R&D Program of China (grant no. 2018YFD0501000) and Sichuan Province “135” Breeding Tackle Project (grant no. 2016NYZ0052)
LITERATURE CITED
- Alexopoulos C., Georgoulakis I. E., Tzivara A., Kritas S. K., Siochu A., and Kyriakis S. C.. 2004. Field evaluation of the efficacy of a probiotic containing Bacillus licheniformis and Bacillus subtilis spores, on the health status and performance of sows and their litters. J. Anim. Physiol. Anim. Nutr. (Berl.). 88:381–392. doi: 10.1111/j.1439-0396.2004.00492.x [DOI] [PubMed] [Google Scholar]
- Bokulich N. A., Subramanian S., Faith J. J., Gevers D., Gordon J. I., Knight R., Mills D. A., and Caporaso J. G.. 2013. Quality-filtering vastly improves diversity estimates from illumina amplicon sequencing. Nat. Methods 10:57–59. doi: 10.1038/nmeth.2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brim H., Yooseph S., Lee E., Sherif Z. A., Abbas M., Laiyemo A., Varma S., Torralba M., Dowd S., Nelson K.,. et al. 2017. A microbiomic analysis in African Americans with colonic lesions reveals Streptococcus sp. VT162 as a marker of neoplastic transformation. Genes. 8:314. doi: 10.3390/genes8110314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., Fierer N., Peña A. G., Goodrich J. K., Gordon J. I.,. et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7:335–336. doi: 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo P. A. C., and Hand T. W.. 2018. A little fiber goes a long way. Immunity 48:844–846. doi: 10.1016/j.immuni.2018.05.001 [DOI] [PubMed] [Google Scholar]
- Cheng C., Wei H., Yu H., Xu C., Jiang S., and Peng J.. 2018. Metabolic syndrome during perinatal period in sows and the link with gut microbiota and metabolites. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.01989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquette J., Allison M. J., and Rasmussen M. A.. 2008. Prevotella bryantii 25A used as a probiotic in early-lactation dairy cows: effect on ruminal fermentation characteristics, milk production, and milk composition. J. Dairy Sci. 91:3536–3543. doi: 10.3168/jds.2007-0849 [DOI] [PubMed] [Google Scholar]
- Collier C. T., Carroll J. A., Ballou M. A., Starkey J. D., and Sparks J. C.. 2011. Oral administration of Saccharomyces cerevisiae Boulardii reduces mortality associated with immune and cortisol responses to Escherichia coli endotoxin in pigs. J. Anim. Sci. 89:52–58. doi: 10.2527/jas.2010-2944 [DOI] [PubMed] [Google Scholar]
- Cui Y., Hao Y., Li J., Bao W., Li G., Gao Y., and Gu X.. 2016. Chronic heat stress induces immune response, oxidative stress response, and apoptosis of finishing pig liver: a proteomic approach. Int. J. Mol. Sci. 17:393. doi: 10.3390/ijms17050393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J. B., Massart S., Collini S., Pieraccini G., and Lionetti P.. 2010. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA. 107:14691–14696. doi: 10.1073/pnas.1005963107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio D., Stewart A. J., and Pellegrini N.. 2005. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 15:316–328. doi: 10.1016/j.numecd.2005.05.003 [DOI] [PubMed] [Google Scholar]
- Dou S., Gadonna-Widehem P., Rome V., Hamoudi D., Rhazi L., Lakhal L., Larcher T., Bahi-Jaber N., Pinon-Quintana A., Guyonvarch A.,. et al. 2017. Characterisation of early-life fecal microbiota in susceptible and healthy pigs to post-weaning diarrhoea. PLoS One 12:e0169851. doi: 10.1371/journal.pone.0169851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y., Zhang Y., Dong H., Wang Y., and Zhang J.. 2017b. Effect of the dietary probiotic Clostridium butyricum on growth, intestine antioxidant capacity and resistance to high temperature stress in kuruma shrimp Marsupenaeus japonicus. J. Therm. Biol. 66:93–100. doi: 10.1016/j.jtherbio.2017.04.004 [DOI] [PubMed] [Google Scholar]
- Duan Y., Zhang Y., Dong H., Wang Y., Zheng X., and Zhang J.. 2017a. Effect of dietary Clostridium butyricum on growth, intestine health status and resistance to ammonia stress in Pacific white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 65:25–33. doi: 10.1016/j.fsi.2017.03.048 [DOI] [PubMed] [Google Scholar]
- Frank D. N., St Amand A. L., Feldman R. A., Boedeker E. C., Harpaz N., and Pace N. R.. 2007. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA. 104:13780–13785. doi: 10.1073/pnas.0706625104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y., Obata Y., Fukuda S., Endo T. A., Nakato G., Takahashi D., Nakanishi Y., Uetake C., Kato K., Kato T.,. et al. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504:446–450. doi: 10.1038/nature12721 [DOI] [PubMed] [Google Scholar]
- Gao Q., Qi L., Wu T., and Wang J.. 2012a. Clostridium butyricum activates TLR2-mediated Myd88-independent signaling pathway in HT-29 cells. Mol. Cell. Biochem. 361:31–37. doi: 10.1007/s11010-011-1084-y [DOI] [PubMed] [Google Scholar]
- Gao Q., Qi L., Wu T., and Wang J.. 2012b. Ability of Clostridium butyricum to inhibit Escherichia coli-induced apoptosis in chicken embryo intestinal cells. Vet. Microbiol. 160:395–402. doi: 10.1016/j.vetmic.2012.06.009 [DOI] [PubMed] [Google Scholar]
- Gu Y., Song Y., Yin H., Lin S., Zhang X., Che L., Lin Y., Xu S., Feng B., Wu D., and Fang Z.. 2017. Dietary supplementation with tributyrin prevented weaned pigs from growth retardation and lethal infection via modulation of inflammatory cytokines production, ileal FGF19 expression, and intestinal acetate fermentation. J. Anim. Sci. 95:226. doi: 10.2527/jas.2016.0911 [DOI] [PubMed] [Google Scholar]
- Hamer H. M., Jonkers D. M., Bast A., Vanhoutvin S. A., Fischer M. A., Kodde A., Troost F. J., Venema K., and Brummer R. J.. 2009. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin. Nutr. 28:88–93. doi: 10.1016/j.clnu.2008.11.002 [DOI] [PubMed] [Google Scholar]
- Hayakawa T., Masuda T., Kurosawa D., and Tsukahara T.. 2016. Dietary administration of probiotics to sows and/or their neonates improves the reproductive performance, incidence of post-weaning diarrhea and histopathological parameters in the intestine of weaned piglets. Anim. Sci. J. 87:1501–1510. doi: 10.1111/asj.12565 [DOI] [PubMed] [Google Scholar]
- Inatomi T., Amatatsu M., Romero-Pérez G. A., Inoue R., and Tsukahara T.. 2017. Dietary probiotic compound improves reproductive performance of porcine epidemic diarrhea virus-infected sows reared in a Japanese commercial swine farm under vaccine control condition. Front. Immunol. 8:1877. doi: 10.3389/fimmu.2017.01877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y. D., Kang K. W., Piao L. G., Jeong T. S., Auclair E., Jonvel S., D’Inca R., and Kim Y. Y.. 2013. Effects of live yeast supplementation to gestation and lactation diets on reproductive performance, immunological parameters and milk composition in sows. Livest. Sci. 152:167–173. doi: 10.1016/j.livsci.2012.12.022 [DOI] [Google Scholar]
- Kanai T., Mikami Y., and Hayashi A.. 2015. A breakthrough in probiotics: Clostridium butyricum regulates gut homeostasis and anti-inflammatory response in inflammatory bowel disease. J. Gastroenterol. 50:928–939. doi: 10.1007/s00535-015-1084-x [DOI] [PubMed] [Google Scholar]
- Kong Q., He G. Q., Jia J. L., Zhu Q. L., and Ruan H.. 2011. Oral administration of Clostridium butyricum for modulating gastrointestinal microflora in mice. Curr. Microbiol. 62:512–517. doi: 10.1007/s00284-010-9737-8 [DOI] [PubMed] [Google Scholar]
- Kritas S. K., Marubashi T., Filioussis G., Petridou E., Christodoulopoulos G., Burriel A. R., Tzivara A., Theodoridis A., and Pískoriková M.. 2015. Reproductive performance of sows was improved by administration of a sporing bacillary probiotic (Bacillus subtilis C-3102). J. Anim. Sci. 93:405–413. doi: 10.2527/jas.2014-7651 [DOI] [PubMed] [Google Scholar]
- Link R., Reichel P., and Kyzeková P.. 2016. The influence of probiotics on reproductive parameters of sows and health of their sucklings. Folia Vet. 60:43–46. doi: 10.1515/FV-2016-0028 [DOI] [Google Scholar]
- Liu J. B., Xue P. C., Cao S. C., Liu J., Chen L., and Zhang H. F.. 2018. Effects of dietary phosphorus concentration and body weight on postileal phosphorus digestion in pigs. Anim. Feed. Sci. Tech. 242:86–94. doi: 10.1016/j.anifeedsci.2018.06.003 [DOI] [Google Scholar]
- Miao R. X., Zhu X. X., Wan C. M., Wang Z. L., Wen Y., and Li Y. Y.. 2018. Effect of Clostridium butyricum supplementation on the development of intestinal flora and the immune system of neonatal mice. Exp. Ther. Med. 15:1081–1086. doi: 10.3892/etm.2017.5461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muns R., Malmkvist J., Larsen M. L., Sørensen D., and Pedersen L. J.. 2016. High environmental temperature around farrowing induced heat stress in crated sows. J. Anim. Sci. 94:377–384. doi: 10.2527/jas.2015-9623 [DOI] [PubMed] [Google Scholar]
- Ohsawa I., Ishikawa M., Takahashi K., Watanabe M., Nishimaki K., Yamagata K., Katsura K., Katayama Y., Asoh S., and Ohta S.. 2007. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 13:688–694. doi: 10.1038/nm1577 [DOI] [PubMed] [Google Scholar]
- Oliviero C., Heinonen M., Valros A., Hälli O., and Peltoniemi O. A.. 2008. Effect of the environment on the physiology of the sow during late pregnancy, farrowing and early lactation. Anim. Reprod. Sci. 105:365–377. doi: 10.1016/j.anireprosci.2007.03.015 [DOI] [PubMed] [Google Scholar]
- Oliviero C., Heinonen M., Valros A., and Peltoniemi O.. 2010. Environmental and sow-related factors affecting the duration of farrowing. Anim. Reprod. Sci. 119:85–91. doi: 10.1016/j.anireprosci.2009.12.009 [DOI] [PubMed] [Google Scholar]
- Pereira A. C., and Martel F.. 2014. Oxidative stress in pregnancy and fertility pathologies. Cell Biol. Toxicol. 30:301–312. doi: 10.1007/s10565-014-9285-2 [DOI] [PubMed] [Google Scholar]
- Rowland I., Gibson G., Heinken A., Scott K., Swann J., Thiele I., and Tuohy K.. 2018. Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 57:1–24. doi: 10.1007/s00394-017-1445-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer E., Barbé F., Guillou D., Rousselière Y., and Chevaux E.. 2016. Development of an oxidative stress model in weaned pigs highlighting plasma biomarkers’ specificity to stress inducers. J. Anim. Sci. 94:48–53. doi:10.2527/jas.2015–9857 [Google Scholar]
- Ruediger K., and Schulze M.. 2012. Post-farrowing stress management in sows by administration of azaperone: effects on piglets performance. J. Anim. Sci. 90:2331–2336. doi: 10.2527/jas.2011-4661 [DOI] [PubMed] [Google Scholar]
- Saker M., Soulimane Mokhtari N., Merzouk S. A., Merzouk H., Belarbi B., and Narce M.. 2008. Oxidant and antioxidant status in mothers and their newborns according to birthweight. Eur. J. Obstet. Gynecol. Reprod. Biol. 141:95–99. doi: 10.1016/j.ejogrb.2008.07.013 [DOI] [PubMed] [Google Scholar]
- Shen Y., Wan H., Zhu J., Fang Z., Che L., Xu S., Lin Y., Li J., and Wu D.. 2015. Fish oil and olive oil supplementation in late pregnancy and lactation differentially affect oxidative stress and inflammation in sows and piglets. Lipids 50:647–658. doi: 10.1007/s11745-015-4024-x [DOI] [PubMed] [Google Scholar]
- Sun J., Ling Z., Wang F., Chen W., Li H., Jin J., Zhang H., Pang M., Yu J., and Liu J.. 2016. Clostridium butyricum pretreatment attenuates cerebral ischemia/reperfusion injury in mice via anti-oxidation and anti-apoptosis. Neurosci. Lett. 613:30–35. doi: 10.1016/j.neulet.2015.12.047 [DOI] [PubMed] [Google Scholar]
- Takahashi M., Taguchi H., Yamaguchi H., Osaki T., Komatsu A., and Kamiya S.. 2004. The effect of probiotic treatment with Clostridium butyricum on enterohemorrhagic Escherichia coli O157:H7 infection in mice. FEMS Immunol. Med. Microbiol. 41:219–226. doi: 10.1016/j.femsim.2004.03.010 [DOI] [PubMed] [Google Scholar]
- Tsukahara T., Inatomi T., Otomaru K., Amatatsu M., Romero-Pérez G. A., and Inoue R.. 2018. Probiotic supplementation improves reproductive performance of unvaccinated farmed sows infected with porcine epidemic diarrhea virus. Anim. Sci. J. 89:1144–1151. doi: 10.1111/asj.13040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F. Y., Liu J. M., Luo H. H., Liu A. H., and Jiang Y.. 2015. Potential protective effects of Clostridium butyricum on experimental gastric ulcers in mice. World J. Gastroenterol. 21:8340–8351. doi: 10.3748/wjg.v21.i27.8340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wu Y., Wang Y., Xu H., Mei X., Yu D., Wang Y., and Li W.. 2017. Antioxidant properties of probiotic bacteria. Nutrients. 9:521. doi: 10.3390/nu9050521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. S., Zhou P., Liu H., Li S., Zhao Y., Deng K., Cao D. D., Che L. Q., Fang Z. F., Xu S. Y.,. et al. 2016. Effects of inulin supplementation in low- or high-fat diets on reproductive performance of sows and antioxidant defence capacity in sows and offspring. Reprod. Domest. Anim. 51:492–500. doi: 10.1111/rda.12707 [DOI] [PubMed] [Google Scholar]
- Williams A. M., Safranski T. J., Spiers D. E., Eichen P. A., Coate E. A., and Lucy M. C.. 2013. Effects of a controlled heat stress during late gestation, lactation, and after weaning on thermoregulation, metabolism, and reproduction of primiparous sows. J. Anim. Sci. 91:2700–2714. doi: 10.2527/jas.2012-6055 [DOI] [PubMed] [Google Scholar]
- Yan H. L., Zhang L., Guo Z. D., Zhang H. F., and Liu J. B.. 2019. Production phase affects the bioaerosol microbial composition and functional potential in swine confinement buildings. Animals. 9:90. doi: 10.3390/ani9030090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. M., Cao G. T., Ferket P. R., Liu T. T., Zhou L., Zhang L., Xiao Y. P., and Chen A. G.. 2012. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult. Sci. 91:2121–2129. doi: 10.3382/ps.2011-02131 [DOI] [PubMed] [Google Scholar]
- Zanello G., Meurens F., Serreau D., Chevaleyre C., Melo S., Berri M., D’Inca R., Auclair E., and Salmon H.. 2013. Effects of dietary yeast strains on immunoglobulin in colostrum and milk of sows. Vet. Immunol. Immunopathol. 152:20–27. doi: 10.1016/j.vetimm.2012.09.023 [DOI] [PubMed] [Google Scholar]