Abstract
The objective of this study was to determine whether feeding tannin-containing hays to heifers and mature beef cows influences enteric methane (CH4) emissions and nitrogen (N) excretion relative to feeding traditional legume and grass hays. Fifteen mature beef cows (Exp. 1) and 9 yearling heifers (Exp. 2) were each randomly assigned to treatment groups in an incomplete bock design with 2 periods and 6 types of hays with 3 hays fed each period (n = 5 cows and 3 heifers per treatment). Groups were fed tannin-containing [birdsfoot trefoil (BFT), sainfoin (SAN), small burnet (SML)] or non-tannin-containing [alfalfa (ALF), cicer milkvetch (CMV), meadow bromegrass (MB)] hays. Each period consisted of 14 d of adjustment followed by 5 d of sample collection. Nine cows and 9 heifers were selected for the measurement of enteric CH4 emissions (sulfur hexafluoride tracer gas technique), and excretion of feces and urine, while dry matter intake (DMI) was measured for all animals. The concentration of condensed tannins in SAN and BFT was 2.5 ± 0.50% and 0.6 ± 0.09% of dry matter (DM), respectively, while SML contained hydrolyzable tannins (4.5 ± 0.55% of DM). Cows and heifers fed tannin-containing hays excreted less urinary urea N (g/d; P < 0.001) and showed lower concentrations of blood urea N (mg/dL; P < 0.001) than animals fed ALF or CMV, indicating that tannins led to a shift in route of N excretion from urine to feces. Additionally, cows fed either BFT or CMV showed the greatest percentage of retained N (P < 0.001). Enteric CH4 yield (g/kg of DMI) from heifers (P = 0.089) was greatest for MB, while daily CH4 production (g/d) from heifers (P = 0.054) was least for SML. However, digestibility of crude protein was reduced for cows (P < 0.001) and heifers (P < 0.001) consuming SML. The results suggest that tannin-containing hays have the potential to reduce urinary urea N excretion, increase N retention, and reduce enteric CH4 emissions from beef cattle. The non-bloating tannin-free legume CMV may also reduce environmental impacts relative to ALF and MB hays by reducing N excretion in urine and increasing N retention.
Keywords: enteric methane, hay, legume, nitrogen, tannin, urea
Introduction
There were 103 million cattle in the United States in July of 2017, an increase of approximately 4% since July of 2015 (USDA, 2017). One of the challenges emerging from an increasing number of livestock is the concomitant increase in the production of greenhouse gases, including carbon dioxide, methane (CH4), and nitrous oxide (Stackhouse-Lawson et al., 2012). Approximately 80% of the greenhouse gas emissions from beef production occur during the cow–calf phase (Beauchemin et al., 2011). This includes emissions from cattle and their manure, as well as indirect emissions from the production of feed and manufactured inputs such as fertilizer and herbicides (Beauchemin et al., 2010). Thus, mitigation of greenhouse gas emissions from beef production during the cow–calf phase is crucial to reducing the national greenhouse gas inventory.
Feeds containing natural phytocompounds such as condensed and hydrolysable tannins represent a sustainable means of reducing environmental impacts of ruminants; tannin-containing feeds reduce enteric CH4 emissions and urinary N excretion (Maamouri et al., 2011; Tan et al., 2011; Aguerre et al., 2016). The effects of tannins on animal performance and environmental impacts have been studied under year-round grazing conditions using fresh forages (Woodward et al., 2004; Maamouri et al., 2011), but there is much less information on the effects of tannin-containing hays. It has been assumed that tannins are labile and highly reactive molecules that are inactivated during the hay-making process (e.g., Makkar and Singh, 1991). Nevertheless, more recent research suggests that conserved tanniferous forages (i.e., sainfoin hay) have significant bioactive properties against gastrointestinal nematodes, similar to those observed in the fresh forage, suggesting that the biological properties of tannins remain in the hay despite the changes that occur during the process of making hay (Heckendorn et al., 2006). In addition, there is tremendous variability in the chemical structure of condensed tannins, such as variation in the degree of polymerization, orientation, and proportion of functional groups within the molecule, which influence their functions and activity (Mueller-Harvey, 2006; Hatew et al., 2016). Thus, we hypothesized that the presence of tannins, coupled with the nutritional characteristics of the chosen hays, could function similarly to fresh forage and decrease CH4 emissions and N excretion from cattle relative to traditional, non-tannin-containing grass and legume hays. The use of hay rather than fresh forage is particularly relevant to cow–calf production during the winter in cold-temperate climates (Beauchemin et al., 2010) when cows are often in the late stages of gestation and have a greater protein requirement.
The objective of this study was to determine whether feeding tannin-containing hays to mature beef cows and yearling heifers influences enteric CH4 emissions and N excretion relative to feeding non-tannin-containing hays.
MATERIALS AND METHODS
Animals and Treatments
This study was conducted at the Utah State University Animal Science Farm, located in Wellsville, UT, according to procedures approved by the Utah State University Institutional Animal Care and Use Committee (Approval # 2542).
Fifteen mature Angus cows (Exp. 1) and 9 yearling Angus heifers (Exp. 2) were each randomly assigned to treatment groups in an incomplete block design with 2 periods and 6 types of hays with 3 hays fed each period (n = 5 cows and 3 heifers per treatment). The mean body weight (BW) was 676 ± 16 kg for cows and 464 ± 19 kg for heifers. Hays fed were: birdsfoot trefoil (BFT; Lotus corniculatus L., variety Langille), sainfoin (SAN; Onobrychis viciifolia Scop., variety Shoshone), small burnet (SML; Sanguisorba minor Scop., variety Delar), alfalfa (ALF; Medicago sativa L., variety DKA43-22RR), cicer milkvetch (CMV; Astragalus cicer L., variety Monarch), and meadow bromegrass (MB; Bromis riparius Rehmann, variety Cache; Jensen et al., 2004). Birdsfoot trefoil and SAN are tannin-containing legumes (Hunt et al., 2014; Wang et al., 2015), SML is a tannin-containing forb (Barry and McNabb, 1999), ALF and CMV are non-tannin-containing legumes (Broderick and Albrecht, 1997), and MB is a grass. All hays were cut in June of 2016 and at that time the legumes and SML were in the early flowering stage of development while MB was in the heading stage. Hays were harvested with a disc-bine swather with a rubber roll conditioner, double raked and baled (1.2 m × 0.9 m × 2.4 m bales). Birdsfoot trefoil took 2 d longer than the other hays to dry and none of the hays suffered any rain damage. Soils were sampled and fertilized in the spring according to the soil sample results. The selection of the forages used in this study was based on their positive characteristics of adaptation, establishment, yield, nutritive value, and persistence when grown under irrigation in the Intermountain West (MacAdam et al., 1997).
Each period consisted of 14 d of adaptation to the assigned hay followed by 5 d of sample collection. During adaptation, animals were fed their respective hays in group pens. Afterwards, they were randomly assigned to individual adjacent pens (2.54 m × 2.36 m) with concrete floors located inside a covered barn. Once in the individual pens, cattle were given 2 d to adjust to the new environment. Each animal was given ad libitum access to hay, water, and a trace mineral salt block (American Stockman®, Kansas City, KS; mineral composition: minimum 96% NaCl, 320 mg/kg Zn, 380 mg/kg Cu, 2,400 mg/kg Mn, 2,400 mg/kg Fe, 70 mg/kg I, and 40 mg/kg Co).
For Exp. 1, the cows were randomly assigned to receive either BFT, CMV, or MB hays (5 cows/hay treatment) in period 1. For period 2, the cows were re-randomized and assigned to either ALF, SAN, or SML hay treatments (5 cows/hay treatment). The 14-d adaptation period to the new hay was used to eliminate any carry-over effects and to allow the cows time to become familiar with the new treatment. During sample collection, dry matter intake (DMI) was determined for all cows. Three of the 5 cows per treatment were used for total collection of urine and feces, as well as for the determination of digestibility, DMI, nitrogen (N) excretion, and enteric CH4 production.
In Exp. 2, the heifers were assigned to ALF, SAN, or MB hay in period 1 (3 heifers/hay treatment). In period 2 of this study, the same 9 heifers were re-randomized and assigned to either BFT, CMV, or SML hay (3 heifers/hay treatment). As with the cows, the 14-d adaptation period to the new hay was used to eliminate any carry-over effects and to allow the heifers time to become familiar with the new treatment. All heifers were used for total collection of feces and urine, as well as for determination of DMI, enteric CH4 production, hay digestibility, and N excretion.
Sample Collection and Analysis
All animals were weighed when they were brought into the barn 2 d before the start of the collection period (not fasted). They were weighed again the day after the final day of collection (not fasted). An average of the 2 weights was used to calculate BW.
Orts were collected once daily at 0500 h and subsamples (100 g on an as-fed basis) were collected for each animal and composited by animal at the end of the collection period. Core samples were obtained from the bales of hay fed (500 g on an as-fed basis for each hay treatment) and were composited by treatment. Feed samples (offered and refused) were stored at −20 °C at the end of the collection period. Upon completion of the study, the samples were thawed, ground using a Wiley Mill (Thomas Scientific, Swedensboro, NJ) to pass a 2-mm screen, and split in half using a Riffle Splitter (Humbolt Manufacturing Company, Elgin, IL). One portion was freeze dried (Labconco Corporation, Kansas City, MO) and the other was oven-dried. Oven-dried samples were used to determine dry matter (DM) concentration (AOAC, 1990; Method 967.03) and organic matter concentration (AOAC, 1990; Method 942.05). Freeze-dried samples were analyzed for neutral detergent fiber (NDF) (Van Soest et al., 1991), acid detergent fiber (ADF) (AOAC, 1990; Method 973.18), total N (AOAC, 1990; Method 990.03), total condensed (Grabber et al., 2013) or hydrolysable (Hartzfeld et al., 2002) tannin concentration, and total nonstructural carbohydrates which consisted of ethanol soluble carbohydrates (Dubois et al., 1956) and starch (Hall, 2009). For the analysis of condensed tannins, standards were isolated from each species used in the present study (BFT and SAN), whereas hydrolysable tannins were analyzed using a methyl gallate standard. Hay samples were also analyzed for non-fibrous carbohydrate concentration using near-infrared reflectance spectroscopy (Utah State Analytical Laboratories, North Logan, UT). To calibrate for this, determination of crude protein (CP), amylase-treated NDF, and ash of calibration samples was made according to AOAC (2012), Methods 984.13, 2002.04, and 942.05, respectively. Non-fibrous carbohydrate concentration was calculated as suggested by NRC (2001) as 100 – [(NDF-2.0) + CP + 2.5 + ash], which assumes concentrations of 2.0 and 2.5% for neutral detergent insoluble CP (Hall, 2000) and fat, respectively (NIRS Forage and Feed Testing Consortium, Hillsboro, WI) in forage samples. For the analysis of CH4 output as a percentage of gross energy intake, the gross energy content of CH4 was assumed to be 13.3 (Armstrong and Blaxter, 1957; Lebedeva, 1964; Duchowicz et al., 2007). Previously reported values of gross energy were used for MB (McCaughey et al., 1999), CMV (Wegert, 1977), SAN (Peirett, 2005), ALF, and BFT (Ingalls et al., 1965). Values of gross energy for SML, however, are not readily available so a value for forbs from Vangilder et al. (1982) was used for that forage.
Urine was continuously collected for the duration of the 5-d collection period using indwelling silicone flushing urinary catheters. Urine from each animal was weighed every 8 h (0500, 1300, and 2100 h) for 5 consecutive days for determination of the average total amount of urine produced in a 24-h cycle. Subsamples of 30 g per animal were obtained after each weighing and immediately frozen at −20 °C. The samples were thawed and 96% sulfuric acid was used to acidify the urine at a pH <3.0. Given the low environmental temperatures at the time of the study and previous findings showing that adding acid before or 6 h after urine collection does not affect urinary concentration of N or urine urea N in heifers (Knowlton et al., 2010), we followed this procedure to avoid the hazard of acid use in the pens. Subsamples were then combined to create a composite sample for each animal in each period. These composite samples were analyzed for urea N (Dimension Xpand Plus, Siemens Healthcare Diagnostics Inc., Newark, DE) and total N (FP-528 Protein/Nitrogen Determinator, Leco Corporation, Saint Joseph, MI).
Feces were collected 3 times daily (0500, 1300, and 2100 h) for 5 consecutive days for determination of the total amount of feces produced in a 24-h cycle. At these times, all feces were removed from the pens and weighed individually for each animal. Subsamples of approximately 200 g were taken 3 times daily after weighing and mixing, and were immediately frozen at −20 °C. The fecal samples were thawed and subsampled to create a 400 g composite sample for each animal in each period. Each composite sample was freeze dried (Labconco Corporation, Kansas City, MO) and the remaining sample was dried to a constant weight in a forced air oven at 60 °C. Oven-dried samples were ground using a Wiley Mill (Thomas Scientific, Swedensboro, NJ) to pass a 2-mm screen and subsequently analyzed for organic matter concentration (AOAC, 1990; Method 942.05) and DM concentration by drying further at 100 °C for 24 h (AOAC, 1990; Method 967.03). All freeze-dried samples were analyzed for NDF, ADF, total N, and condensed or hydrolysable tannin concentration as previously described. Fecal analysis data were used in the calculations of apparent in vivo digestibility of DM, organic matter, NDF, ADF, and CP from fecal excretion and differences in these parameters between offered feed and orts. Also, N retained (g/d) by the animals was calculated by subtracting the amount of N excreted in feces and urine from N intake.
On the day after the end of the collection period, a blood sample was obtained for each animal from the medial coccygeal vein in the tail (Becton Dickinson Vacutainer System, Rutherford, NJ). The blood was allowed to clot, and serum was separated by centrifugation (2,300 × g for 25 min at 15 °C), extracted from the tubes using a disposable pipette, placed into 2-mL tubes, and frozen at −20 °C. The serum was thawed and analyzed for urea N (Dimension Xpand Plus, Siemens Healthcare Diagnostics Inc., Newark, DE).
Enteric CH4 emissions were measured for individual animals using the sulfur hexafluoride (SF6) tracer gas technique (Johnson et al., 2007). A slow-release permeation tube was put into the rumen of each animal at least 2 d prior to the first sample collection period. Each animal was fitted with a halter on the first day of the collection period. An evacuated canister was then connected to the halter via capillary tubing for the collection of exhaled air adjacent to the nostrils of the animal (Johnson et al., 2007). Canisters were changed every 24 h during the 5-d collection period. Additional samples of air were taken adjacent to the animals to measure background atmospheric concentrations of CH4 and SF6, which were used to adjust the values obtained from the animals (Williams et al., 2011). Each day, the canisters were transported to the laboratory, pressurized with N gas, and subsampled. The subsamples were analyzed for CH4 and SF6 concentration by gas chromatography (Chavez et al., 2006).
Statistical Analysis
Experiments 1 and 2 were analyzed separately for cows and heifers. Period and period × days were not significant for the responses and therefore removed from the model. The data in each experiment were analyzed using PROC MIXED (SAS Institute Inc., Cary, NC; SAS/STAT 14.1) in which treatment, days, and treatment × days were fixed factors and animal was a random factor. A first-order autoregressive error structure was also included for repeated measures on each animal. The covariance structure that best fit the data was selected according to the Schwartz’s Bayesian criterion (Littell et al., 1998). The treatment × day interaction was not significant except for daily urine and fecal outputs for the cows. Profile plots showed the treatment effect was similar in trend but differed in magnitude depending on days, thus main effects of the treatment averaged over days were tested. Differences among the means were analyzed using pairwise differences of least squares means with Tukey’s method for multiplicity adjustment. The model diagnostics included testing for normal distribution of the error residuals and homogeneity of variance. The assumptions were adequately held. Linear regressions were also carried out to explore the relationship between fecal excretion of condensed tannins and the fecal excretion of N. Probability values were considered significant at P ≤0.10.
RESULTS
Tannin Concentration and Nutritional Analysis of the Hays
All nutritional components and tannin concentrations are reported on a DM basis (Table 1). Crude protein concentrations of MB and SML were 8.1% and 11.7%, respectively, while CMV contained 19.7% and ALF contained 18.7% CP. For NDF, SML showed a concentration of 27.9% and the concentration for MB was 64.3%. Similarity, SML contained 24.3% ADF and MB contained 41.6% ADF. The concentrations of condensed tannins for BFT and SAN were 0.6% and 2.5%, respectively. Small burnet contained 4.5% hydrolysable tannins.
Table 1.
Chemical composition of the hays used in the studies on a dry matter basis
| Forage source1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tannin-containing hays | Non-tannin-containing hays | |||||||||||
| Item | BFT | SD | SAN | SD | SML | SD | ALF | SD | CMV | SD | MB | SD |
| Dry matter, % | 90.2 | 90.8 | 89.0 | 91.2 | 90.7 | 92.6 | ||||||
| Organic matter, % | 89.9 | 91.5 | 91.2 | 89.0 | 88.1 | 92.5 | ||||||
| Crude protein, % | 14.1 | 0.10 | 13.7 | 0.00 | 11.7 | 0.05 | 18.7 | 0.15 | 19.7 | 0.10 | 8.1 | 0.10 |
| Neutral detergent fiber, % | 40.3 | 0.20 | 42.3 | 0.15 | 27.9 | 0.25 | 38.0 | 0.10 | 32.3 | 0.35 | 64.3 | 0.10 |
| Acid detergent fiber, % | 31.5 | 0.25 | 35.7 | 0.35 | 24.3 | 0.30 | 30.6 | 0.05 | 28.3 | 0.15 | 41.6 | 0.10 |
| Total nonstructural carbohydrates, % | 10.2 | 0.05 | 9.2 | 0.10 | 13.6 | 0.00 | 7.1 | 0.35 | 7.3 | 0.30 | 8.5 | 0.15 |
| Ethanol soluble carbohydrates, % | 9.3 | 0.10 | 7.9 | 0.05 | 11.2 | 0.05 | 6.4 | 0.30 | 6.8 | 0.25 | 8.1 | 0.15 |
| Starch, % | 1.0 | 0.05 | 1.4 | 0.05 | 2.5 | 0.05 | 0.7 | 0.05 | 0.6 | 0.05 | 0.4 | 0.00 |
| Non-fibrous carbohydrates, % | 40.2 | 37.6 | 37.7 | 36.1 | 36.9 | 15.2 | ||||||
| Gross energy concentration, Mcal/kg | 4.62 | 4.72 | 4.53 | 4.52 | 4.42 | 4.42 | ||||||
| Condensed tannin, % | 0.6 | 0.09 | 2.5 | 0.50 | – | 0.2 | 0.00 | 0.2 | 0.01 | 0.1 | 0.00 | |
| Hydrolysable tannin, % | – | – | 4.5 | 0.55 | – | – | – |
1Treatment: birdsfoot trefoil (BFT), cicer milkvetch (CMV), meadow bromegrass (MB), alfalfa (ALF), sainfoin (SAN), and small burnet (SML).
2Previously reported values of gross energy concentration were used for ALF and BFT (Ingalls et al., 1965), CMV (Wegert, 1977), MB (McCaughey et al., 1999), and SAN (Peirett, 2005).
3Values of gross energy concentration for SML were not readily available, so a value for forbs from Vanglider et al. (1982) was used for that forage.
Condensed tannins were determined for BFT, SAN, ALF, CMV, and MB (Grabber et al., 2013). Hydrolysable tannins were determined for SML only (Hartzfield et al., 2002). Items containing a hyphen were not determined in the present study.
Intake and Digestibility
For cows, DMI did not differ among treatments (Table 2). Dry matter digestibility was greater for cows consuming CMV than for those consuming SAN (P = 0.034), SML (P = 0.023), or ALF (P = 0.080). Cows consuming BFT also had greater DM digestibility than cows consuming SAN (P = 0.062) or SML (P = 0.042). Organic matter digestibility was greater for cows fed MB compared to other treatments except for SML.
Table 2.
Daily dry matter intake, digestibility of hay constituents (on a dry matter basis), and enteric methane (CH4) emissions for cows and heifers
| Forage source1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Tannin-containing hays | Non-tannin-containing hays | |||||||
| Item | BFT | SAN | SML | ALF | CMV | MB | SEM | P-value |
| Experiment 1: cows (n = 3 cows/forage source) | ||||||||
| Dry matter intake, g/kg of body weight2 | 14.7 | 12.4 | 14.1 | 15.8 | 14.2 | 10.8 | 1.26 | 0.167 |
| Digestibility of hay constituents | ||||||||
| Dry matter, % | 66.7ab | 57.3c | 56.6c | 58.4bc | 68.1a | 62.8abc | 3.32 | 0.101 |
| Organic matter, % | 60.5bc | 55.8c | 67.4ab | 58.8c | 57.9c | 71.1a | 2.96 | 0.022 |
| Crude protein, % | 76.5a | 63.0b | 30.2c | 73.4a | 79.4a | 61.6b | 4.23 | <0.001 |
| Neutral detergent fiber, % | 48.1ab | 21.1c | 26.0c | 29.1c | 36.1bc | 56.8a | 6.39 | 0.013 |
| Acid detergent fiber, % | 39.3bc | 20.9c | 31.8bc | 29.7bc | 44.1ab | 55.8a | 6.11 | 0.015 |
| CH4 emissions | ||||||||
| CH4, g/animal/d | 308.2 | 290.5 | 208.6 | 289.3 | 265.5 | 259.4 | 44.60 | 0.505 |
| CH4, g/kg of dry matter intake | 33.6a | 36.7a | 21.3b | 27.8ab | 31.8a | 37.8a | 4.21 | 0.055 |
| CH4, g/kg of body weight | 0.49 | 0.47 | 0.30 | 0.47 | 0.42 | 0.39 | 0.060 | 0.157 |
| CH4, % of gross energy intake | 9.8a | 10.3a | 6.3b | 8.2ab | 9.6a | 11.5a | 1.22 | 0.058 |
| Experiment 2: heifers (n = 3 heifers/forage source) | ||||||||
| Dry matter intake g/kg of body weight | 21.2ab | 18.3bc | 15.3d | 22.5a | 18.2bc | 16.2cd | 1.26 | 0.005 |
| Digestibility of hay constituents | ||||||||
| Dry matter, % | 64.0ab | 57.2bc | 50.2d | 56.3cd | 67.1a | 54.1cd | 2.66 | 0.008 |
| Organic matter, % | 67.2a | 58.1bc | 63.9a | 53.0c | 59.8ab | 63.2ab | 3.82 | 0.050 |
| Crude protein, % | 76.5a | 63.0bc | 30.2d | 73.4ab | 79.4a | 61.6c | 4.23 | <0.001 |
| Neutral detergent fiber, % | 42.9a | 28.9b | 22.7b | 28.1b | 45.3a | 45.7a | 5.18 | 0.026 |
| Acid detergent fiber, % | 42.3a | 28.9b | 27.9b | 27.7b | 50.0a | 42.7a | 5.23 | 0.039 |
| CH4 emissions | ||||||||
| CH4, g/animal/d | 255.8a | 223.4a | 179.9b | 257.9a | 227.4a | 245.9a | 22.18 | 0.054 |
| CH4, g/kg of dry matter intake | 27.1b | 26.9b | 26.7b | 34.8b | 28.2b | 36.8a | 4.54 | 0.089 |
| CH4, g/kg of body weight | 0.59 | 0.49 | 0.41 | 0.52 | 0.45 | 0.53 | 0.070 | 0.398 |
| CH4 % of gross energy intake | 7.9b | 7.6b | 7.9b | 6.9b | 8.5b | 11.2a | 1.00 | 0.095 |
a–dMeans in the same row with different superscripts differ (P ≤ 0.10).
1Treatment: birdsfoot trefoil (BFT), cicer milkvetch (CMV), meadow bromegrass (MB), alfalfa (ALF), sainfoin (SAN), and small burnet (SML).
2Five cows were utilized for dry matter intake.
Crude protein digestibility was greatest for cows consuming BFT, ALF, or CMV, intermediate for cows consuming SAN or MB, and least for cows consuming SML (P < 0.001; Table 2). Cows fed MB had greater NDF digestibility compared to other treatments except for BFT (P = 0.013). Digestibility of NDF was least for cows fed SAN, SML, or ALF, although these treatments were not different from CMV. Cows showed greater digestibility of ADF for MB compared to other treatments except for CMV.
For heifers, DMI was greater for ALF than for all other hays, except for BFT, and DMI was less for SML than for all treatments except MB (P = 0.005; Table 2). The digestibility of DM was greater for heifers fed CMV than for all other hays, except for BFT (P = 0.008). Heifers fed SML had reduced DM digestibility values compared to BFT, SAN, and CMV. The digestibility of organic matter was least for heifers fed ALF although not different from SAN.
Similar to cows, heifers fed BFT or CMV showed greater digestibilities of CP compared to the other treatments except ALF, and CP digestibility was least for heifers fed SML (P < 0.001; Table 2). Heifers offered BFT, CMV, or MB showed greater NDF (P = 0.026) and ADF (P = 0.039) digestibilities than heifers offered SAN, SML, or ALF.
Enteric Methane Emissions
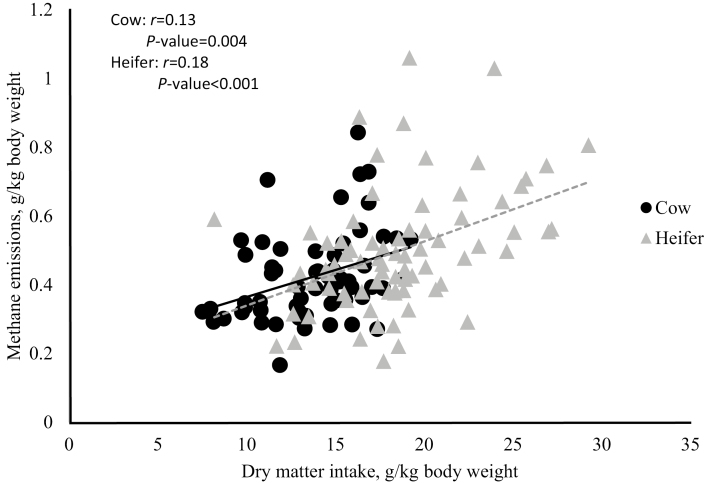
There were no differences among treatments for CH4 emitted per cow per day (Table 2). However, when CH4 was expressed as g CH4/kg DMI, the SML treatment produced less compared to other treatments (P = 0.055) except ALF and all other treatments were similar to each other. Additionally, Fig. 1 shows that there is a positive relationship for both cows (P = 0.004) and heifers (P < 0.001) between CH4 emissions (g/kg BW) and DMI (g/kg BW).
Figure 1.
Linear relationship between methane emissions (g/kg body weight) and dry matter intake (g/kg body weight) for cows (Exp. 1, n = 3 cows/treatment) and heifers (Exp. 2, n = 3 heifers/treatment).
Heifers that were offered SML emitted less CH4 (g/animal/d) than heifers offered the other hays (P = 0.054; Table 2). However, there were no differences in CH4 emissions among the hays when expressed as g CH4/kg BW. When expressed as CH4 yield (g/kg DMI), heifers fed MB produced the greatest CH4 emissions while all other forages were similar (P = 0.089). As a percentage of gross energy intake, enteric CH4 was greatest for heifers that consumed MB (P = 0.095) but there were no differences among any of the other treatments.
Excretion of Nitrogen in Urine and Feces, and Blood Urea Nitrogen
Total daily urinary output was greatest for cows consuming CMV, but similar among cows consuming the other hays (Table 3). Heifers that consumed SAN or MB produced the least urine daily while, similar to cows, heifers fed CMV had greater urinary output than the other treatments except BFT (P < 0.001). In contrast, heifers fed CMV produced the least feces per kg of BW per day, while heifers fed ALF produced the greatest amount of feces (P = 0.020), though ALF was not different from MB. For both cows (P < 0.001) and heifers (P < 0.001), the total daily excretion of N in urine (g/d) was greatest for those fed ALF, followed by CMV, and least for those fed SML. Feeding MB also resulted in less daily N loss in urine than BFT or SAN for heifers (P < 0.001) and less N loss in urine than SAN for cows (P < 0.001). Similarly, when expressed as g N/L of urinary output, animals fed SML produced the least N and those fed SAN or ALF produced the greatest amounts of N. In contrast, the amount of N excreted in feces was greatest for cows fed SML and less for cows fed MB compared to other treatments except BFT (P = 0.001). Also, heifers fed SML or ALF excreted more fecal N, heifers fed SAN excreted more than heifers fed BFT or CMV, and heifers fed MB excreted the least N in feces (P < 0.001).
Table 3.
Urine and fecal output, excretion of nitrogen (N) in urine, excretion of N and tannins in feces, N intake and retention, and blood urea N for cows and heifers
| Forage source1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Tannin-containing hays | Non-tannin-containing hays | |||||||
| Item | BFT | SAN | SML | ALF | CMV | MB | SEM | P-value |
| Experiment 1: cows (n = 3 cows/forage source) | ||||||||
| Urine output, L/d | 13.3b | 10.4b | 10.7b | 13.2b | 18.9a | 12.0b | 1.94 | 0.074 |
| Fecal output, g of dry matter/kg of body weight | 4.9 | 5.3 | 6.2 | 5.8 | 4.5 | 4.0 | 0.78 | 0.440 |
| Total N in urine, g/d | 58.4d | 90.9c | 29.4e | 180.1a | 112.4b | 56.5d | 8.92 | <0.001 |
| Total N in urine, g/L of urine | 4.7c | 8.9b | 2.3d | 13.8a | 6.5c | 4.9c | 0.87 | <0.001 |
| Total N in feces, g/d | 52.7cd | 75.3b | 152.6a | 90.7b | 70.6bc | 42.2d | 11.24 | 0.001 |
| Urine urea N, g/d | 36.0b | 45.2b | 11.3c | 101.1a | 102.2a | 39.3b | 12.56 | <0.001 |
| Blood urea N, mg/dL | 7.8c | 11.5b | 2.8d | 15.2a | 16.0a | 8.2c | 1.26 | <0.001 |
| N from urea, % of total urinary N | 61.6bc | 49.7cd | 37.1d | 55.4bc | 89.9a | 69.2b | 7.54 | 0.006 |
| N intake, g/d | 247.3b | 197.7c | 182.3c | 329.5a | 332.5a | 100.3d | 11.93 | <0.001 |
| N retention, g/d | 132.5b | 37.7d | 18.9e | 58.5c | 158.5a | 9.7e | 6.10 | <0.001 |
| Total N excreted, g/d | 111.1c | 166.2b | 182.0b | 283.1a | 182.9b | 98.6c | 14.41 | <0.001 |
| Total N excreted, % of ingested N | 45.5c | 81.6b | 90.2ab | 82.8ab | 53.4c | 91.1a | 3.48 | <0.001 |
| Excretion of condensed tannins in the feces | ||||||||
| Tannins excreted in feces2, % | 0.90c | 1.45b | 1.84a | 0.41e | 0.64d | 0.35e | 0.12 | <0.001 |
| Tannins excreted in feces, g/d | 27.9c | 47.7b | 82.2a | 14.6d | 18.1cd | 9.4e | 5.78 | <0.001 |
| Experiment 2: heifers (n = 3 heifers/forage source) | ||||||||
| Urine output, L/d | 17.7ab | 5.7d | 11.3c | 14.8bc | 20.8a | 6.4d | 1.71 | <0.001 |
| Fecal output, g of dry matter/kg of body weight | 7.8bc | 6.3cd | 7.5bcd | 9.8a | 5.9d | 9.2ab | 0.78 | 0.020 |
| Total N in urine, g/d | 66.1c | 47.8d | 17.1f | 138.8a | 103.2b | 29.4e | 7.48 | <0.001 |
| Total N in urine, g/L of urine | 3.9c | 8.3ab | 1.6d | 10.0a | 4.9bc | 5.1bc | 1.41 | 0.001 |
| Total N in feces, g/d | 80.3c | 92.6b | 102.5a | 108.7a | 74.5c | 46.1d | 4.26 | <0.001 |
| Urine urea N, g/d | 54.3b | 21.7c | 12.0d | 104.9a | 82.9a | 15.5d | 7.62 | <0.001 |
| Blood urea N, mg/dL | 14.2b | 14.2b | 2.8d | 22.9a | 19.5a | 9.5c | 1.38 | <0.001 |
| N from urea, % of total urinary N | 77.7 | 48.6 | 69.0 | 78.9 | 85.2 | 52.8 | 10.48 | 0.147 |
| N intake, g/d | 231.4b | 203.2b | 127.2c | 333.8a | 287.3a | 93.8d | 19.76 | <0.001 |
| N retention, g/d | 88.7ab | 72.7b | 10.5c | 89.6ab | 112.5a | 16.4c | 9.67 | <0.001 |
| Total N excreted, g/d | 145.6c | 138.7c | 121.3d | 248.6a | 177.8b | 75.8e | 9.93 | <0.001 |
| Total N excreted, % of ingested N | 61.6c | 65.8c | 90.4a | 74.8b | 62.0c | 81.5b | 3.45 | <0.001 |
| Excretion of tannins in the feces | ||||||||
| Tannins excreted in feces2, % | 1.23b | 1.94a | 1.73a | 0.31d | 0.49c | 0.30d | 0.13 | <0.001 |
| Tannins excreted in feces, g/d | 43.1c | 70.6a | 59.7b | 14.4d | 13.9d | 9.7e | 7.60 | <0.001 |
a–fMeans in the same row with different superscripts differ (P ≤ 0.10).
1Treatment: alfalfa (ALF), birdsfoot trefoil (BFT), cicer milkvetch (CMV), meadow bromegrass (MB), sainfoin (SAN), and small burnet (SML).
2Tannins excreted in the feces as a percentage of fecal dry matter output.
Cows consuming SML excreted the least urinary urea N (P < 0.001; Table 3). Similarly, heifers consuming SML or MB excreted the least urinary urea N (P < 0.001), whereas both cows and heifers fed ALF or CMV produced the most urinary urea N. Additionally, for heifers, BFT resulted in greater levels of urinary urea N excretion than those fed SAN. Cows (P < 0.001) and heifers (P < 0.001) had the greatest blood urea N concentrations when ALF or CMV treatments and the least when fed SML. Blood urea N concentration for animals on the SAN treatment was greater than the BFT or MB treatments for cows and greater than the MB treatment for heifers. For the percentage of urinary N from urea, SML-fed cows produced less compared to other treatments except for SAN, whereas CMV-fed cows produced the greatest amount (P = 0.006).
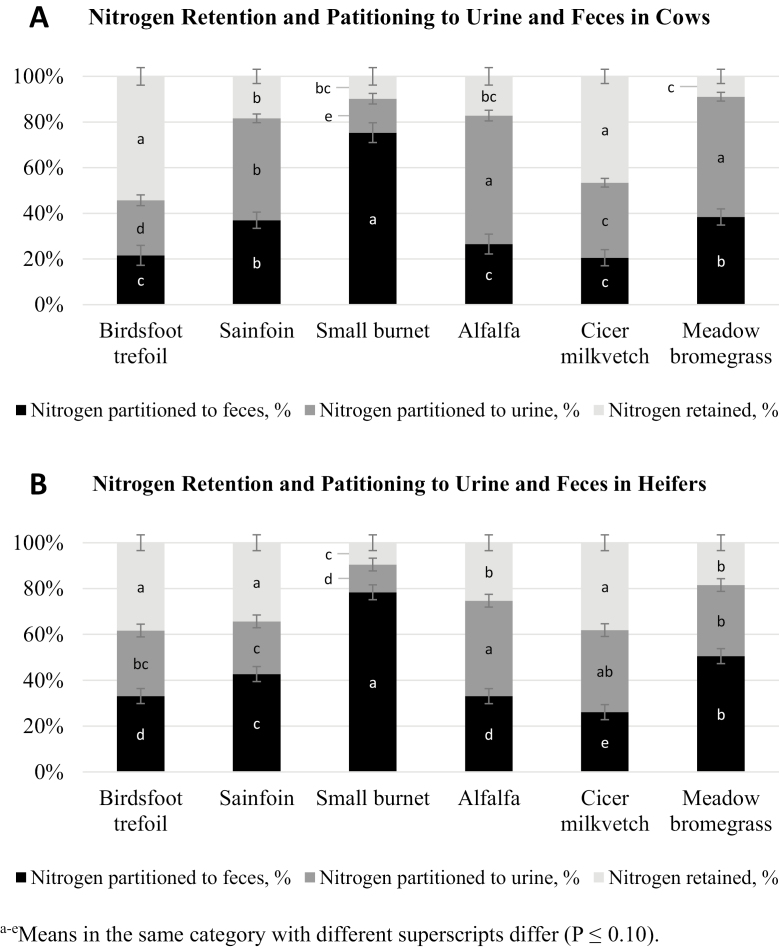
Figure 2 shows the percentage of ingested N that was retained by animals in each treatment, as well as the partitioning of ingested N to urine and feces. The percentage of N consumed that was retained was greatest for cows (Fig. 2A) consuming BFT or CMV (P < 0.001). The percentage of N that was partitioned to feces relative to the N consumed was greatest for cows consuming SML, intermediate for cows consuming SAN or MB, and least for cows consuming BFT, ALF, or CMV (P = 0.001). In contrast, cows fed ALF or MB partitioned more N to urine than cows fed tannin-containing hays or CMV (P < 0.001). Also, cows fed SML partitioned the least N to urine and cows fed BFT partitioned less than cows fed CMV which partitioned less than cows fed SAN. Daily N retention (g/d; Table 3) was least for cows consuming SML or MB hay, greater for cows fed SAN followed by ALF, then BFT, and greatest for cows fed CMV.
Figure 2.
Nitrogen retention and partitioning of nitrogen to urine and feces for A) cows (Exp. 1, n = 3 cows/treatment) and B) heifers (Exp. 2, n = 3 heifers/treatment).
For heifers, feeding SML or MB resulted in the least N retained (g/d; Table 3). Feeding BFT, SAN, or CMV resulted in the greatest percentage of N retained while feeding SML or MB led to the least percentage of N retained (Fig. 2B). Nitrogen partitioned to feces was greatest for heifers offered SML and least for heifers offered CMV (P < 0.001). Also, MB-fed heifers partitioned more N to feces than SAN-fed heifers and SAN-fed heifers partitioned more N to feces than BFT- or ALF-fed heifers. In contrast, heifers that consumed ALF partitioned more N to urine than all other treatments except CMV, and heifers that consumed SML partitioned the least N to urine (P < 0.001). Heifers fed SAN partitioned less N to urine than heifers fed CMV or MB, but did not differ from BFT. Feeding ALF resulted in the greatest total N excreted (g/d; Table 3) for both cows (P < 0.001) and heifers (P < 0.001).
Excretion of Tannins in the Feces
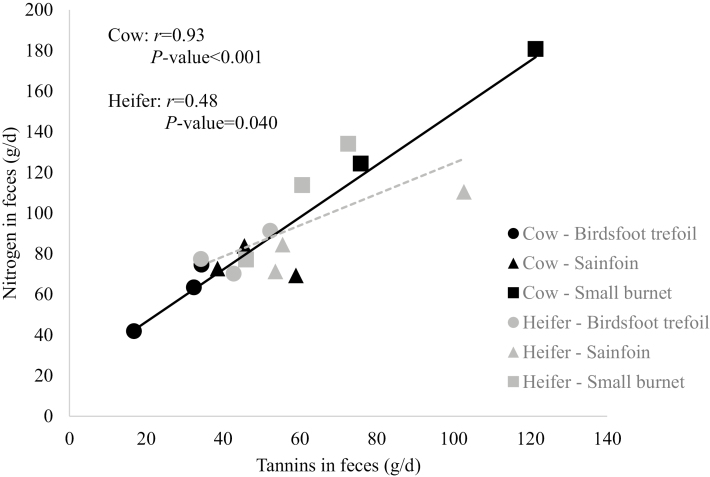
For cows (P < 0.001) and heifers (P < 0.001) ingesting SML or SAN resulted in the greatest excretion of tannin in feces (g/d; Table 3). Fecal tannin excretion was intermediate for animals fed BFT, ALF, or CMV, and least for MB-fed animals. A positive relationship was observed between the fecal excretion of condensed tannins and the fecal excretion of N (Fig. 3). As the daily amounts of fecal tannins increased, N excreted daily in feces also increased for cows (P < 0.001) and heifers (P = 0.040).
Figure 3.
Linear relationship between nitrogen and tannins in feces for cows (Exp. 1, n = 3 cows/treatment) and heifers (Exp. 2, n = 3 heifers/treatment) that consumed tannin-containing hays in the study.
Discussion
Enteric Methane Production
In a life cycle analysis of the greenhouse gas emissions of a grain-finished beef operation in western Canada, mature cows were found to contribute the greatest proportion of greenhouse gas emissions, and these emissions were dominated by enteric CH4 (Beauchemin et al., 2010). The quantity of enteric CH4 emissions from individual animals is influenced by the quantity and composition of feed consumed, the rumen microorganisms and fermentation process, and the efficiency with which an animal converts feed into meat or milk (Johnson and Johnson, 1995).
Methane emissions are negatively correlated with intake levels because passage rate increases with increments in food intake, which reduces the residence time of digesta in the rumen and thus the rate of methane production. Thus, a standardized approach to compare methane emissions across animals consuming different types of forages involves reporting this variable per unit of intake. In the present study, heifers consuming MB, the grass hay with the greatest fiber concentration, had greater CH4 emissions per kg of DMI than heifers consuming any of the other hays. This is consistent with the idea that forages with greater fiber concentration result in greater levels of CH4 production in cattle (Van Soest, 1994). Heifer CH4 emissions in g/kg of BW were greater than those of cows, and heifers weighed less than cows, but they consumed greater amounts of forage (kg DMI/kg BW) than mature cows. Also, daily CH4 produced (g/kg BW) was positively correlated with DMI for both cows and heifers which is similar to previous findings that daily CH4 production increases with increased DMI (Beauchemin and McGinn, 2006). As a percentage of gross energy intake or a proportion of DMI, however, there were no consistent differences between cows and heifers.
Feeding the SML hay to cows resulted in decreased CH4 emissions (g/kg DMI). This hay had the greatest amount of tannin and the tannin present was a hydrolysable tannin (unlike the condensed tannin present in SAN and BFT). When hydrolysable tannins were added to ALF and barley silage beef diets at a concentration of 1.5%, CH4 emissions as a proportion of intake were suppressed (Aboagye et al., 2018). In the present study, the concentration of hydrolysable tannins in the SML hay was 4.5%. For cows, CH4 as a proportion of intake was reduced on the SML hay diet, and for heifers CH4 per animal per day was reduced with this hay.
Hay intakes by cows and heifers were low, which could be attributed to the high fiber contents in MB and the presence of stems in the legumes and forb. Cattle, both young and mature have been shown to selectively consume (sort for) the shorter, more nutritious particles in their rations (e.g., leaves in our study), while selectively refusing (sort against) longer, fibrous particles (e.g., stems in our study), which may limit intake (Greter and DeVries, 2011).
Excretion of Tannins in Feces, Excretion of Nitrogen in Urine and Feces, and Blood Urea Nitrogen
There are significant concerns over the global environmental impacts of livestock production and its associated N losses to the environment (Bouwman et al., 2013). A shift in N excretion from urine to feces in livestock can help ameliorate this problem as fecal N is less volatile than urinary N. Urinary urea is quickly transformed to ammonia, and urinary N is a source of nitrate and nitrous oxide (Whitehead, 1995; Oenema et al., 2005). Nitrate is produced by the oxidation of ammonia and is a major pollutant of water (Eckard et al., 2010), whereas nitrous oxide is a byproduct of nitrification and denitrification in the soil (Bremmer, 1997) and is a significant greenhouse gas (Sakadevan and Nguyen, 2016). Thus, the greater levels of urinary N from cattle fed forages such as the ALF treatment would infer greater nitrous oxide and ammonia losses to the atmosphere.
Feeding some tannin-containing hays in the present study (e.g., SAN for heifers, and SML for both cows and heifers) shifted the partitioning of N from urine to feces, consistent with other studies that trace the fate of N in diets containing hydrolysable (Deaville et al., 2010) or condensed tannin (Ahnert et al., 2015). While the SML treatment also resulted in reduced N retention, heifers fed SAN showed elevated N retention in conjunction with the shift in N excretion to the feces. This result can be attributed to the formation of protein–tannin complexes in the rumen, which are stable in the pH range of 3.5 to 7 (Bunglavan and Dutta, 2013) and thus increase the metabolizable amino acid flow to the small intestine (Barry and McNabb, 1999).
Estimated N retention (%) for cows ranged from 9.8% for SML hay to 54.5% for BFT hay; for heifers, N retention ranged from 9.6% for SML to 38.4% for BFT. Retained N was particularly high for condensed tannin-containing legumes and CMV considering that typical estimates for beef cows are in the order of 7% (IPCC, 2006). However, N retention values ranging from 26.5% (Houseknecht et al., 1992) to 53.4% (Schwinghammer et al., 1986) have been reported in steers and up to 44.8% in ewes supplemented with Acacia cyanophylla, a tree that contains condensed tannins (Maamouri et al., 2011). Likewise, tannins in this study could have elevated the retention values for BFT and SAN given that some condensed tannins (e.g., those from the genus Lotus) enhance the concentration of growth hormone, which in turn stimulates N retention in ruminants (Aerts et al., 1999). In addition, an adequate supply of fermentable energy to the rumen (Miller et al., 2001) from elevated NDF digestibility in BFT and CMV might have contributed to an increase in N retention. Alternatively, the concentration of N in feces could have been underestimated in animals fed tannin-containing legumes if certain tannin–protein complexes went undetected as artifact lignin (Hanley et al., 1992) or as other artifacts that prevented the total detection of N in feces.
The amount of urine produced daily by an animal is related to the amount of minerals and protein consumed. According to Church (1979), high levels of protein in the diet lead to increased water consumption and thus, increased urine production. Bannink et al. (1999) also found a linear relationship between excreted N, potassium, and sodium and urine production. Both cows and heifers consuming CMV, which had high concentrations of CP but without tannins, showed high levels of daily urinary N excretions and subsequently the greatest urine output. Likewise, cows and heifers fed ALF had the greatest percentage of N partitioned to urine. This can be attributed to the high concentration of CP in this hay, a lack of condensed tannins in the forage’s tissues, or more digestible NDF that resulted in considerable N degradation in the rumen. In contrast, the BFT and SML treatments showed less partitioning of the ingested N to urine, suggesting that the tannins contained in these hays protected the protein from degradation in the rumen.
Animals consuming BFT excreted less urea N in urine but fecal N excretion did not increase, meaning that N retention was higher for BFT. High N retention in cows and heifers fed BFT may be due to condensed tannins shifting the site of protein digestion from the rumen to the small intestine (Waghorn et al., 1987). Protein degradation and efficiency of bacterial protein synthesis have been reported to be greater in BFT than in non-tannin-containing legumes like ALF (Dahlberg et al., 1988). In contrast, animals fed SML, along with animals fed MB had lower N retention values. This suggests that for SML, the protein–tannin complexes disassociated and the tannins then formed bonds with digestive enzymes (Mole and Waterman, 1987), tannin–protein complexes disassociated but reformed again before the protein was utilized by the animal (McNabb et al., 1998), or they did not disassociate in the abomasum and small intestine (Frutos et al., 2004), thereby reducing the efficiency of N utilization by the animal. For MB, this is likely due to the lack of tannin and thus the inability to form protective protein–tannin complexes in the rumen (Bunglavan and Dutta, 2013), allowing the majority of the consumed N to be excreted rather than being retained by the animals. A lower concentration of energy may have also limited the use of plant protein for the synthesis of microbial protein in the rumen.
The concentrations of condensed tannins found in BFT and SAN hays were both less than values typically observed in fresh forages of the same species (John and Lancashire, 1981). This was expected because condensed tannins are reactive and labile molecules, so drying promotes the inactivation of their biological properties in herbivores (e.g., Makkar and Singh, 1991). In addition, condensed tannins in BFT (procyanidin-rich tannin type) differ from those present in SAN (hetero- and homopolymers containing both procyanidin and prodelphinidin units) (Marais et al., 2000; Hatew et al., 2016), and thus differential responses to drying as a function of chemical structure may be possible. For instance, other studies have found that conserved forages containing condensed tannins, such as SAN, maintain their bioactive properties—similar to fresh forage—enabling them to act against gastrointestinal nematodes (Heckendorn et al., 2006). The condensed tannins in both the BFT and SAN hays appeared to affect N digestion and its mode of excretion. Reduced levels of both blood urea N and urea N in urine were found in cows and heifers consuming hays containing tannins. Tannins in SAN and SML may have been a factor in the greater fecal excretion of N in both cows and heifers; however, feeding MB hay also resulted in high fecal N excretion. Additionally, tannins were detected in the feces of animals consuming tannin-containing hays and as fecal concentration of tannins increased so did the concentration of N, likely due to the affinity of tannins for forming bonds with protein and other chemicals (Bunglavan and Dutta, 2013).
Blood urea N and urinary urea N result from the absorption of excess ammonia from the rumen (Lobley and Milano, 1997). The concentration of urea in blood is regarded as an indicator of the degradable protein supply to the rumen (Kebreab et al., 2004). Therefore, the smaller amounts of both blood urea N and urinary urea N found for tannin-containing hays compared with the non-tannin legumes, ALF and CMV, may be due to protein binding by tannins in the rumen. Animals fed MB hay also had less total N excreted in urine, urinary urea N, and blood urea N. However, this pattern can be explained by the reduced dietary intake of N (Yan et al., 2007), given the low CP content (8% of DM) observed in MB hay.
CONCLUSION
Tannin-containing hays have the potential to reduce enteric CH4 emissions from beef cattle, particularly when animals have a low level of intake, such as the cows in this study. Cows consuming the hydrolysable tannin-containing hay (SML), at lower levels of intake, showed reductions in CH4 yield (g/kg DMI) while heifers consuming this same hay at greater levels of intake did not show reduced CH4 yield. Tannin-containing hays also reduced N excretion, increased N retention, and shifted N excretion from urine to feces. For instance, feeding SML—a hydrolysable tannin-containing forb—substantially shifted the excretion of N from urine to feces. However, despite these benefits, feeding SML was not beneficial for the nutrition of cows or heifers relative to other hays given the reduced digestibilities and low levels of N retention were observed for this hay (approximately 10% of DM). In contrast, the other tannin-containing hays explored in this study, SAN and BFT, generally led to high levels of N retention and SAN also shifted some of the N excretion from urine to feces. Furthermore, the non-tannin-containing hay CMV also enhanced N utilization through attributes other than the presence of tannins in the plant’s tissues (i.e., a greater supply of synchronous sources of fermentable energy to match the high concentration of N present in this hay).
Thus, these hays can contribute to more environmentally sustainable cow–calf production while maintaining or enhancing levels of animal productivity. However, certain tannin-containing hays (e.g., SML) may have some negative impacts on N utilization, whereas other tannin-free hays (e.g., CMV) have the opposite effect and may also enhance the efficiency of beef cattle production.
Footnotes
This study was conducted with financial support from the U.S. Department of Agriculture (USDA-NIFA Award No. 2016-69004-24855). The paper is published with the approval of the Director, Utah Agricultural Experiment Station, and Utah State University, as journal paper number 9126. Thanks are extended to D. Vedres (Lethbridge Research and Development Centre, AB, Canada) for analyzing the methane samples. We acknowledge R. Stott for veterinary services, and P. Armstrong, J. Taylor, M. Endicott, and J. Hadfield for technical support.
Literature Cited
- Aboagye I. A., Oba M., Castillo A. R., Koenig K. M., Iwaasa A. D., and Beauchemin K. A.. 2018. Effects of hydrolyzable tannin with or without condensed tannin on methane emissions, nitrogen use, and performance of beef cattle fed a high-forage diet. J. Anim. Sci. 96:5276–5286. doi: 10.1093/jas/sky352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aerts R. J., Barry T. N., and McNabb W. C.. 1999. Polyphenols and agriculture: beneficial effects of proanthocyanidins in forages. Agric. Ecosyst. Environ. 75:1–12. doi: 10.1016/S0167-8809(99)00062-6 [DOI] [Google Scholar]
- Aguerre M. J., Capozzolo M. C., Lencioni P., Cabral C., and Wattiaux M. A.. 2016. Effect of quebracho-chestnut tannin extracts at 2 dietary crude protein levels on performance, rumen fermentation, and nitrogen partitioning in dairy cows. J. Dairy Sci. 99:4476–4486. doi: 10.3168/jds.2015-10745 [DOI] [PubMed] [Google Scholar]
- Ahnert S., Dickhoefer U., Schultz F., and Susenbeth A.. 2015. Influence of ruminal quebracho tannin extract infusion on apparent nutrient digestibility, nitrogen balance and urinary purine derivative excretion in heifers. Livest. Sci. 177:63–70. doi: 10.1016/j.livsci.2015.04.004 [DOI] [Google Scholar]
- AOAC 1990. Official methods of analysis. 15th ed. Assoc. Off. Anal. Chem, Arlington, VA. [Google Scholar]
- AOAC 2012. Official methods of analysis. 19th ed. Assoc. Off. Anal. Chem, Gaithersburg, MD. [Google Scholar]
- Armstrong D. G., and Blaxter K. L.. 1957. The heat increment of steam-volatile fatty acids in fasting sheep. Br. J. Nutr. 11:247–272. doi: 10.1079/BJN19570044 [DOI] [PubMed] [Google Scholar]
- Bannink A., Valk H., and Van Vuuren A. M.. 1999. Intake and excretion of sodium, potassium, and nitrogen and the effects on urine production by lactating dairy cows. J. Dairy Sci. 82:1008–1018. doi: 10.3168/jds.S0022-0302(99)75321-X [DOI] [PubMed] [Google Scholar]
- Barry T. N., and McNabb W. C.. 1999. The implications of condensed tannins on the nutritive value of temperate forages fed to ruminants. Br. J. Nutr. 81:263–272. doi:10.1017/S0007114599000501 [PubMed] [Google Scholar]
- Beauchemin K. A., Janzen H. H., Little S. M., McAllister T. A., and McGinn S. M.. 2010. Life cycle assessment of greenhouse gas emissions from beef production in western Canada: a case study. Agric. Syst. 103:371–379. doi: 10.1016/j.agsy.2010.03.008 [DOI] [PubMed] [Google Scholar]
- Beauchemin K. A., Janzen H. H., Little S. M., McAllister T. A., and McGinn S. M.. 2011. Mitigation of greenhouse gas emissions from beef production in western Canada – evaluation using farm-based life cycle assessment. Anim. Feed Sci. Technol. 166–167: 663–667. doi: 10.1016/j.anifeedsci.2011.04.047 [DOI] [Google Scholar]
- Beauchemin K. A., and McGinn S. M.. 2006. Enteric methane emissions from growing beef cattle as affected by diet and level of intake. Can. J. Anim. Sci. 86:401–408. doi: 10.4141/A06-021 [DOI] [Google Scholar]
- Bouwman L., Goldewijk K. K., Van Der Hoek K. W., Beusen A. H., Van Vuuren D. P., Willems J., Rufino M. C., and Stehfest E.. 2013. Exploring global changes in nitrogen and phosphorus cycles in agriculture induced by livestock production over the 1900-2050 period. Proc. Natl Acad. Sci. USA 110:20882–20887. doi: 10.1073/pnas.1012878108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremmer J. M. 1997. Sources of nitrous oxide in soils. Nutr. Cycle Agroecosyst. 49:7–16. doi:10.1023/A:1009798022569 [Google Scholar]
- Broderick G. A., and Albrecht K. A.. 1997. Ruminal in vitro degradation of protein in tannin-free and tannin-containing forage legume species. Crop Sci. 37:1884–1891. doi: 10.2135/cropsci1997.0011183X003700060037x [DOI] [Google Scholar]
- Bunglavan S. J., and Dutta N.. 2013. Use of tannins as organic protectants of proteins in digestion of ruminants. J. Livest. Sci. 4:67–77. [Google Scholar]
- Chavez A. V., Thompson L. C., Iwaasa A., Scott S., Olson M. E., Benchaar C., Veira D. M., and McAllister T. A.. 2006. Effect of pasture type (alfalfa vs. grass) on methane and carbon dioxide production by yearling beef heifers. Can. J. Anim. Sci. 86:409–418. doi: 10.4141/A05-081 [DOI] [Google Scholar]
- Church D. C. 1979. Digestive physiology and nutrition of ruminants. 2nd ed. O&B Books, Corvallis, OR. [Google Scholar]
- Dahlberg E. M., Stern M. D., and Ehle F. R.. 1988. Effects of forage source on ruminal microbial nitrogen metabolism and carbohydrate digestion in continuous culture. J. Anim. Sci. 66:2071–2083. doi:10.2527/jas1988.6682071x. [DOI] [PubMed] [Google Scholar]
- Deaville E. R., Givens D. I., and Mueller-Harvey I.. 2010. Chestnut and mimosa tannin silages: effects in sheep differ for apparent digestibility, nitrogen utilization and losses. Anim. Feed Sci. Technol. 157:129–138. doi: 10.1016/j.anifeedsci.2010.02.007 [DOI] [Google Scholar]
- Dubois M., Gilles K. A., Hamilton J. K., Rebers P. A., and Smith F.. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350–356. doi: 10.1021/ac60111a017 [DOI] [Google Scholar]
- Duchowicz P. R., Garro J. C. M., Andrada M. F., Castro E. A., and Fernandez F. M.. 2007. QSPR modeling of heats of combustion for carboxylic acids. QSAR Comb. Sci. 26:647–652. doi: 10.1002/qsar.200630073 [DOI] [Google Scholar]
- Eckard R. J., Grainger C., and de Klein C. A. M.. 2010. Options for the abatement of methane and nitrous oxide from ruminant production. Livest. Sci. 130:47–56. doi: 10.1016/j.livsci.2010.02.010 [DOI] [Google Scholar]
- Frutos P., Hervas G., Giraldez F. J., and Mantecon A. R.. 2004. Review. Tannins and ruminant nutrition. Span. J. Agric. Res. 2:191–202. doi: 10.5424/sjar/2004022-73 [DOI] [Google Scholar]
- Grabber J. H., Zeller W. E., and Mueller-Harvey I.. 2013. Acetone enhances the direct analysis of procyanidin- and prodelphinidin-based condensed tannins in Lotus species by the butanol-HCl-iron assay. J. Agric. Food Chem. 61:2669–2678. doi: 10.1021/jf304158m [DOI] [PubMed] [Google Scholar]
- Greter A., and DeVries T.. 2011. Effect of feeding amount on the feeding and sorting behaviour of lactating dairy cattle. Can. J. Aim. Sci. 91:47–54. doi: 10.4141/CJAS10067 [DOI] [Google Scholar]
- Hall M. B. 2000. Calculation of non-neutral detergent fiber carbohydrate content of feeds that contain non-protein nitrogen. University of Florida, Gainesville, FL; p. A-25 (Bulletin, 339). [Google Scholar]
- Hall M. B. 2009. Analysis of starch, including maltooligosaccharides, in animal feeds: a comparison of methods and a recommended method for AOAC collaborative study. J. AOAC Int. 92:42–49. [PubMed] [Google Scholar]
- Hanley T. A., Robbins C. T., Hagerman A. E., and McArthur C.. 1992. Predicting digestible protein and digestible dry matter in tannin-containing forages consumed by ruminants. Ecology 73:537–571. doi: 10.2307/1940759 [DOI] [Google Scholar]
- Hartzfeld P. W., Forkner R., Hunter M. D., and Hagerman A. E.. 2002. Determination of hydrolyzable tannins (gallotannins and ellagitannins) after reaction with potassium iodate. J. Agric. Food Chem. 50:1785–1790. doi:10.1021/jf0111155 [DOI] [PubMed] [Google Scholar]
- Hatew B., Stringano E., Mueller‐Harvey I., Hendriks W. H., Carbonero C. H., Smith L. M., and Pellikaa W. F.. 2016. Impact of variation in structure of condensed tannins from sainfoin (Onobrychis viciifolia) on in vitro ruminal methane production and fermentation characteristics. J. Anim. Physiol. Anim. Nutr. 100:348–360. doi: 10.1111/jpn.12336 [DOI] [PubMed] [Google Scholar]
- Heckendorn F., Häring D. A., Maurer V., Zinsstag J., Langhans W., and Hertzberg H.. 2006. Effect of sainfoin (Onobrychis viciifolia) silage and hay on established populations of Haemonchus contortus and Cooperia curticei in lambs. Vet. Parasitol. 142:293–300. doi: 10.1016/j.vetpar.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Houseknecht K. L., Bauman D. E., Fox D. G., and Smith D. F.. 1992. Abomasal infusion of casein enhances nitrogen retention in somatotropin-treated steers. J. Nutr. 122:1717–1725. doi: 10.1093/jn/122.8.1717. [DOI] [PubMed] [Google Scholar]
- Hunt S. R., MacAdam J. W., and Griggs T. C.. 2014. Lignification and tannin localization during the development of birdsfoot trefoil stems. Crop Sci. 54:1876–1886. doi: 10.2135/cropsci2013.09.0592 [DOI] [Google Scholar]
- Ingalls J. R., Thomas J. W., Benne E. J., and Tesar M.. 1965. Comparative response of wether lambs to several cuttings of alfalfa, birdsfoot trefoil, bromegrass, and reed canarygrass. J. Anim. Sci. 24:1159–1164. doi: 10.2527/jas1965.2441159x [DOI] [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC) 2006. Guidelines for national greenhouse gas inventories http://www.ipcc-nggip.iges.or.jp/public/2006gl/index.htm (Accessed February 2, 2019) .
- Jensen K. B., Waldron B. L., Larson S. R., and Peel M. D.. 2004. Registration of ‘Cache’ meadow bromegrass. Crop Sci. 44:2263. doi: 10.2135/cropsci2004.2263a [DOI] [Google Scholar]
- John A., and Lancashire J. A.. 1981. Aspects of the feeding and nutritive value of Lotus species. Proc. N. Z. Grassl. Assoc. 42:152–159. [Google Scholar]
- Johnson K. A., and Johnson D. E.. 1995. Methane emissions from cattle. J. Anim. Sci. 73:2483–2492. doi: 10.2527/1995.7382483x. [DOI] [PubMed] [Google Scholar]
- Johnson K. A., Westberg H. H., Michal J. J., and Cossalman M. W.. 2007. The SF6 tracer technique: methane measurement from ruminants. In: Makkar H. P. S. and Vercoe P. E., editors. Springer, Dordrecht, the Netherlands: p. 33–67. [Google Scholar]
- Kebreab E., Mills J. A. N., Crompton L. A., Bannik A., Dijkstra J., Gerrits W. J. J., and France J.. 2004. An integrated mathematical model to evaluate nutrient partition in dairy cattle between the animal and its environment. Anim. Feed Sci. Technol. 112:131–154. doi: 10.1016/j.anifeedsci.2003.10.009 [DOI] [Google Scholar]
- Knowlton K. F., McGilliard M. L., Zhao Z., Hall K. G., Mims W., and Hanihan M. D.. 2010. Effective nitrogen preservation during urine collection from Holstein heifers fed diets with high or low protein content. J. Dairy Sci. 93:323–329. doi: 10.3168/jds.2009-2600 [DOI] [PubMed] [Google Scholar]
- Lebedeva N. D. 1964. Heats of combustion of monocarboxylic acids. Russ. J. Phys. Chem. 38:1435–1437. [Google Scholar]
- Littell R. C., Henry P. R., and Ammerman C. B.. 1998. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 76:1216–1231. doi: 10.2527/1998.7641216x [DOI] [PubMed] [Google Scholar]
- Lobley G. E., and Milano G. D.. 1997. Regulation of hepatic nitrogen metabolism in ruminants. Proc. Nutr. Soc. 56:547–563. doi:10.1079/PNS19970057 [DOI] [PubMed] [Google Scholar]
- Maamouri O., Atti A., Kraiem K., and Mahouachi M.. 2011. Effects of concentrate and Acacia cyanophylla foliage on nitrogen balance and milk production for grazing ewes. Livest. Sci. 139:264–270. doi: 10.1016/j.livsci.2011.01.018 [DOI] [Google Scholar]
- MacAdam J. W., Whitesides R. E., Winger M. B., and Buffer S.. 1997. Pasture species for grazing-based dairy production under irrigation in the Intermountain West. In: Proc. XVIII Int. Grass. Congr., Winnipeg, MB and Saskatoon, SK, Canada p. 99–100. [Google Scholar]
- Makkar H. P. S., and Singh B.. 1991. Effect of drying conditions on tannin, fibre and lignin levels in mature oak (Quercus incana) leaves. J. Sci. Food Agric. 54:323–328. doi: 10.1002/jsfa.2740540302 [DOI] [Google Scholar]
- Marais J. P., Mueller-Harvey I., Brandt E. V., and Ferreira D.. 2000. Polyphenols, condensed tannins, and other natural products in Onobrychis viciifolia (sainfoin). J. Agric. Food Chem. 48:3440–3447. doi:10.1021/jf000388h [DOI] [PubMed] [Google Scholar]
- McCaughey W. P., Wittenberg K., and Corrigan D.. 1999. Impact of pasture type on methane production by lactating beef cows. Can. J. Anim. Sci. 79:221–226. doi: 10.4141/A98-107 [DOI] [Google Scholar]
- McNabb W. C., Peters S. J., Foo L. Y., Waghorn G. C., and Jackson S. J.. 1998. Effect of condensed tannins prepared from several forages on the in vitro precipitation of ribulose-1,5-bisphosphate carboxylase (rubisco) protein and its digestion by trypsin (EC 2.4.21.4) and chymotrypsin (EC 2.4.21.1). J. Sci. Food Agric. 77:201–212. doi:10.1002/(SICI)1097-0010(199806)77:2<201::AID-JSFA26>3.0.CO;2-J [Google Scholar]
- Miller L. A., Moorby J. M., Davies D. R., Humphries M. O., Scollan N. D., MacRae J. C., and Theodorou M. K.. 2001. Increased concentration of water-soluble carbohydrate in perennial ryegrass (Lolium perenne L.): milk production from late-lactation dairy cows. Grass Forage Sci. 56:383–394. doi: 10.1046/j.1365-2494.2001.00288.x [DOI] [Google Scholar]
- Mole S., and Waterman P. G.. 1987. Tannic acid and proteolytic enzymes: enzyme inhibition or substrate deprivation? Phytochemistry 26:99–102. doi:10.1016/S0031-9422(00)81490–9 [Google Scholar]
- Mueller-Harvey I. 2006. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 86:2010–2037. doi: 10.1002/jsfa.2577 [DOI] [Google Scholar]
- National Research Council (NRC) 2001. Nutrient requirements of dairy cattle. 7th rev. ed. Natl. Acad. Press, Washington, DC. [Google Scholar]
- Oenema O., Wrange N., Velthof G. L., van Groenigen J. W., Dolfing J., and Kuikman P. J.. 2005. Trends in global nitrous oxide emissions from animal production systems. Nutr. Cycle Agroecosyst. 72:51–65. doi: 10.1007/s10705-004-7354-2 [DOI] [Google Scholar]
- Peirett P. G. 2005. Prediction of the gross energy value of Mediterranean forages. J. Food Agric. Environ. 3:102–104. doi: 10.1234/4.2005.667 [DOI] [Google Scholar]
- Sakadevan K., and Nguyen M. L.. 2016. Chapter four – livestock production and its impact on nutrient pollution and greenhouse gas emissions. Adv. Agron. 141:147–184. doi: 10.1016/bs.agron.2016.10.002 [DOI] [Google Scholar]
- Schwinghammer K. A., Knapp F. W., Boling J. A., and Schillo K. K.. 1986. Physiological and nutritional response of beef steers to infestations of the horn fly (Diptera: Muscidae). J. Econ. Entomol. 79:1010–1015. doi: 10.1093/jee/79.4.1010 [DOI] [PubMed] [Google Scholar]
- Stackhouse-Lawson K. R., Rotz C. A., Oltjen J. W., and Mitloehner F. M.. 2012. Carbon footprint and ammonia emissions of California beef production systems. J. Anim. Sci. 90:4641–4655. doi: 10.2527/jas.2011-4653. [DOI] [PubMed] [Google Scholar]
- Tan H. Y., Sieo C. C., Abdullah N., Liang J. B., Huang X. D., and Ho Y. W.. 2011. Effects of condensed tannins from Leucaena on methane production, rumen fermentation and populations of methanogens and protozoa in vitro. Anim. Feed Sci. Technol. 169:185–193. doi: 10.1016/j.anifeedsci.2011.07.004 [DOI] [Google Scholar]
- United States Department of Agriculture (USDA) 2017. Cattle http://usda.mannlib.cornell.edu/usda/current/Catt/Catt-07-21-2017.pdf (Accessed October 10, 2017) .
- Vangilder L. D., Torgerson O., and Porath W. R.. 1982. Factors influencing diet selection by white-tailed deer. J. Wildl. Manage. 46:711–718. [Google Scholar]
- Van Soest P. J. 1994. Nutritional ecology of the ruminant. 2nd ed. Cornell University, Ithaca, NY. [Google Scholar]
- Van Soest P. J., Robertson J. B., and Lewis B. A.. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Waghorn G. C., Ulyatt M. J., John A., and Fisher M. T.. 1987. The effect of condensed tannins on the site of digestion of amino acids and other nutrients in sheep fed on Lotus corniculatus L. Br. J. Nutr. 57:115–126. doi:10.1079/BJN19870015 [DOI] [PubMed] [Google Scholar]
- Wang Y. T. McAllister A., and Acharya S.. 2015. Condensed tannins in sainfoin: composition, concentration, and effects on nutritive and feeding value of sainfoin forage. Crop Sci. 55:13–22. doi: 10.2135/cropsci2014.07.0489 [DOI] [Google Scholar]
- Wegert W. 1977. Production and forage quality of cicer milkvetch (Astragalus cicer L.) hay at six stages of maturation. Master’s thesis. Western State Colorado University, Gunnison. [Google Scholar]
- Whitehead D. C. 1995. Grassland Nitrogen. CAB Int., Wallingford, UK. [Google Scholar]
- Williams S. R. O., Moate P. J., Hannah M. C., Ribaux B. E., Whales W. J., and Eckard R. J.. 2011. Background matters with the SF6 tracer method for estimating enteric methane emissions from dairy cows: a critical evaluation of the SF6 procedure. Anim. Feed Sci. Technol. 170:265–276. doi: 10.1016/j.anifeedsci.2011.08.013 [DOI] [Google Scholar]
- Woodward S. L., Waghorn G. C., and Laboyrie P. G.. 2004. Condensed tannins in birdsfoot trefoil (Lotis corniculatus) reduce methane emissions from dairy cows. Proc. N. Z. Soc. Anim. Prod. 64:160–164. [Google Scholar]
- Yan T., Frost J. P., Keady T. W., Agnew R. E., and Mayne C. S.. 2007. Prediction of nitrogen excretion in feces and urine of beef cattle offered diets containing grass silage. J. Anim. Sci. 85:1982–1989. doi: 10.2527/jas.2006-408 [DOI] [PubMed] [Google Scholar]