Abstract
Purpose
Changes in blood glucose levels have been shown to influence eating in healthy individuals; however, less is known about effects of glucose on food intake in individuals who are obese (OB). The goal of this study was to determine the predictive effect of circulating glucose levels on eating in free-living OB and normal weight (NW) individuals.
Methods
Interstitial glucose levels, measured with a continuous glucose monitor (CGM) system, were obtained from 15 OB and 16 NW volunteers (age: 40 ± 14 and 37 ± 12 years; weight: 91 ± 13 and 68 ± 12 kg; hemoglobin A1c: 5.1% ± 0.7% and 5.2% ± 0.4%, respectively). While wearing the CGM, participants filled out a food log (mealtime, hunger rating, and amount of food). Glucose profiles were measured in relation to their meals [macro program (CGM peak and nadir analysis) using Microsoft® Excel].
Results
OB and NW individuals showed comparable CGM glucose levels: mean [OB = 100 ± 8 mg/dL; NW = 99 ± 13 mg/dL; P = nonsignificant (NS)] and SD (OB = 18 ± 5 mg/dL, NW = 18 ± 4 mg/dL; P = NS). Obesity was associated with slower postprandial rate of changing glucose levels (P = 0.04). Preprandial nadir glucose levels predicted hunger and food intake in both groups (P < 0.0001), although hunger was associated with greater food intake in OB individuals than in NW individuals (P = 0.008 for group interaction).
Conclusions
Premeal glucose nadir predicted hunger and food intake in a group of free-living, healthy, nondiabetic NW and OB individuals; however for a similar low glucose level stimulus, hunger-induced food intake was greater in OB than NW individuals.
Glucose nadir before a meal predicted hunger and food intake in a free-living setting. Similar hunger ratings resulted in greater food intake in people with obesity than in normal weight individuals.
Obesity is a modern health epidemic that affects more than a third of the population of the United States (1). It is a multifactorial disorder that has been linked to sedentary lifestyle and consumption of diets with high calorie content (2–4). Better understanding of food intake regulation may be important in controlling the epidemic of obesity. In the 1950s, Mayer (5) formalized the glucostatic theory, which proposed that lower glucose use in the hypothalamus of rats and mice precipitated hunger and eating. This theory was supported by both animal (6) and human studies (7, 8). Human studies have documented that rapid declines in blood glucose levels caused increased hunger and meal requests in healthy subjects (7, 8), and mild hypoglycemia increased desire for high-calorie foods (9). However, studies of how glucose patterns relate to hunger and food consumption have shown conflicting results (10–12). Moreover, these studies have been performed in controlled laboratory settings, where subjects were blinded to the time of day and restricted to an inpatient research setting (7). It is less clear whether these results accurately reflect what happens in real-life settings. Therefore, to further understand the role of blood glucose on food intake, Pittas et al (13) used a continuous glucose monitor (CGM) system in eight normal weight (NW) women in a noncontrolled free-living environment. In this study, the authors observed that hunger and desire for food were the strongest predictors of calorie intake. They also found that low interstitial glucose levels, albeit playing a smaller role, also predicted calorie intake. These studies (6–9, 13) support the glucostatic theory by demonstrating that glucose levels may be a key homeostatic signal influencing calorie intake in healthy nonobese individuals.
To date, no one has investigated the relationships between circulating glucose profiles (nadirs, peaks, and decrements), hunger, and food intake in free-living individuals with obesity. Thus, we proposed to do so by using a CGM alongside a food and hunger diary. Given that a large body of research in both animals (6) and humans (7, 13) supports the significance of glucose declines and glucose nadirs on subsequent food consumption, we hypothesized that rapid glucose declines would be associated with increased hunger and increased calorie intake, particularly of high-calorie foods. We also predicted that the influence of glucose changes on food consumption would be magnified in subjects with obesity, resulting in greater hunger and greater calorie intake. Our main goal was to investigate whether circulating glucose patterns differentially affected hunger and food intake in individuals with obesity and NW individuals.
Material and Methods
Subjects
Participants were recruited from the Yale Center for Clinical Investigation volunteer database as well as from the greater New Haven area by advertisement flyers. Forty-two participants were enrolled in the study and were divided into two groups: NW [body mass index (BMI) 18 to 25 kg/m2] and obese (OB) (BMI 30 to 40 kg/m2). Three subjects were lost to follow-up. Of the 39 subjects who completed the study, eight subjects were excluded because of poor-quality CGM data. The CGM data from the remaining 31 participants, 16 NW and 15 OB, met quality control criteria. Participants with eating disorders or any major medical or psychiatric conditions were excluded. People who were pregnant, smoking, or using illegal substances were excluded as well. Use of antihypertensive and thyroid medications was allowed in the study. Individuals taking medications known to alter glucose metabolism were excluded from the study. Study volunteers had maintained a stable weight in the 3 months before participation. The protocol of the study was approved by the Yale University Human Investigation Committee. All participants were informed and gave written consent before participation in the study.
Study design
At the initial visit, participants underwent a history and physical examination. Basic blood work was also collected. Participants with a hemoglobin A1c (HbA1c) value >5.7% or abnormal kidney (creatinine), liver (liver enzymes), and thyroid (TSH) function test results were not enrolled in the study. Eligible participants were asked to wear a CGM (G4; DEXCOM, San Diego, CA) for up to 7 days while keeping a food diary to log information about mealtime, hunger ratings at the start of the meal, and food items and amount consumed. The CGM was placed by one of the physician investigators. Approximately 1 week later, the participants returned to the research unit, and the CGM was removed and the food diary was collected.
Food diary
Each participant was given a paper-form log and instructed to document the timing of meals, their hunger level at the start of each meal, every food and drink item consumed, and the corresponding portion size.
Hunger ratings
Hunger was quantified by the participants by writing down how hungry they felt at the start of the meal (scale of 1 to 10: 1 = none and 10 = very).
Dietary intake
Reported food items and portion sizes were entered into a dietary analysis program (Nutrition Data System for Research, University of Minnesota Minneapolis, MN) to calculate calorie content. The program also calculated fat, protein, and carbohydrate content per food item and per meal.
CGM
The DEXCOM G4 (Dexcom, Inc., San Diego, CA) is a CGM system that measures glucose levels from the interstitial tissue. Participants were trained to use and calibrate the CGM and were not blinded to the CGM readings. The participants were given a glucose meter (Accu-Chek®; Roche Diabetes Care, Inc., Indianapolis, IN) to measure capillary blood glucose level. They were instructed how to use the glucose meter and asked to check their glucose levels at least every 12 hours and enter the glucose values into the CGM for calibration. Glucose values from the glucose meter were not included in the CGM data analysis.
CGM data quality control
CGM tracings were retrieved from the monitor and uploaded to the DEXCOM Studio program. To qualify for use in data analysis, the CGM tracing from each participant had to meet three quality control criteria: calibration, continuous tracing time point, and accuracy.
Calibration
The first 6 hours of each participant’s CGM tracing was removed to account for calibration time. Sensor data beyond day 7 was removed, as DEXCOM G4 guidelines recommend the sensor be changed every 7 days. The glucose level obtained from the glucometer was required to be entered into the DEXCOM monitor to calibrate the CGM tracing twice within the first 2 hours of wearing the sensor and every 12 hours thereafter. Tracings without proper calibration were discarded.
Continuous tracing time point
Each 24-hour period of CGM tracings from each participant was reviewed. One complete day of sensor readings included 288 time points of CGM measurement. Data for that day were included in the final analysis if at least 75% of the 288 time points were present. Gaps in sensor readings were dealt with by connecting the last data point before the gap to the first data point after the gap. There was an average of 10 ± 13 gaps per subject, with a duration of 20 ± 19 minutes per gap, corresponding to <3% of the total CGM use. Participants with <48 hours of time points were not included in the analysis.
Accuracy
The mean absolute relative difference (14) percentage was calculated as the difference in glucose measurement level between the DEXCOM CGM sensor and the Accu-Chek (Roche Diabetes Care, Inc.) glucometer calibration divided by the glucose level from the Accu-Chek glucometer calibration. If the average mean absolute relative difference across all calibrations was >20%, the participants’ data were discarded. Any sensor readings of “LOW” and “HIGH” were counted as a glucose reading of 40 mg/dL, the minimum glucose level detectable, and 400 mg/dL, the maximum glucose level detectable, respectively, according to the DEXCOM G4 CGM guideline.
Glucose variability analysis
Easy Glucose Variability (EasyGV) (Nathan R Hill, University of Oxford, Oxford, UK) was used to calculate various measures of glucose variability, including M-value, mean amplitude of glycemic excursions, lability index, average daily risk range, J-index, low blood glucose index, high blood glucose index, continuous overall net glycemic action, mean of daily differences, glycemic risk assessment diabetes equation, and mean average glucose (15).
CGM and food log analysis
All meals were assigned a number, starting with the first meal of the first usable CGM day and ending on the last meal of the last usable CGM day for each participant.
CGM peak and nadir analysis
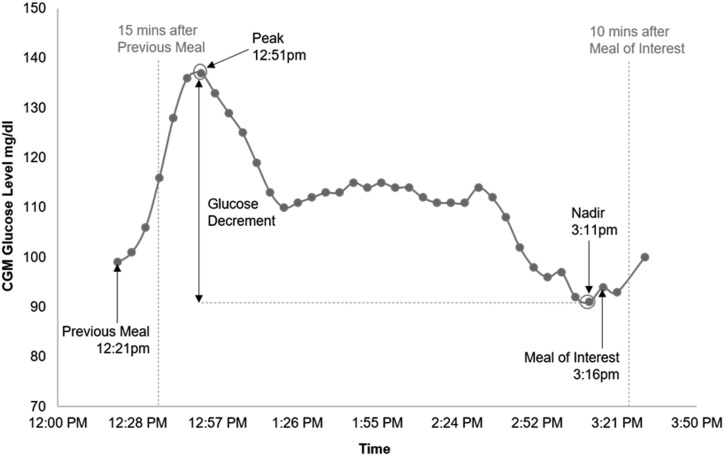
A macro was programmed using Visual Basic in Excel (Microsoft) to identify glucose peaks, nadirs, and decrements before each meal. The CGM tracing data and meal numbers were inputted into the Excel PeakNadirAuto MACRO developed by our laboratory. To correct for the lag between serum glucose values and interstitial fluid glucose values measured by the CGM sensor, the Excel-based automation determines the absolute glucose peak and nadir within a duration of 15 minutes after the previous meal and 10 minutes after the meal of interest (16). In addition, the associations between the previous meal and the following peaks and nadirs were analyzed. Figure 1 shows the CGM profile of one participant of the OB group with the continuous glucose monitor peak and nadir analysis (CPNA) data points: peak, nadir, and glucose decrement. Rate of changing glucose, a measurement of how fast it takes for a glucose level to drop from peak to nadir before the meal, was calculated by dividing (i) the difference in glucose levels between peak and nadir by (ii) the difference in time between peak and nadir.
Figure 1.
Example of the CGM tracing between two subsequent meals in a subject with obesity. The CGM peak and nadir analysis (CPNA; glucose peak, glucose nadir, and glucose decrement) is shown in relation to the meal before the meal of interest. To correct for the lag between serum glucose values and interstitial fluid glucose values measured by the CGM, glucose peak was defined as the highest glucose value at least 15 min after the previous meal, and the nadir was defined as the lowest glucose value up to 10 min after the meal of interest.
Statistical analysis
Patient characteristics were summarized using mean and SD for continuous variables and frequency and percentage for categorical variables by cohorts. Linear mixed-model analysis was used to analyze the correlation of outcome variables with predictors. Random effect of individuals was included to account for correlation of repeated assessment within the same patient. The time of day when the data were recorded was included as a covariate adjustment in the analysis. All the analyses were performed using SAS 9.4 (Cary, NC). Statistical significance was set as P < 0.05, two-sided.
Results
Subject characteristics
Thirty-one healthy nondiabetic subjects (25 women and six men) participated in the study. The participants were divided into two groups: NW volunteers [13 women and three men; age, 37 ± 12 years; BMI, 23.5 ± 1.7 kg/m2; HbA1c, 5.2% ± 0.4%] and OB volunteers (12 women and three men; age, 40 ± 14 years; BMI, 33.4 ± 2.2 kg/m2; HbA1c, 5.1% ± 0.7%). The two groups were comparable by age, sex, and ethnicity (Table 1). Both groups had stable weight and normal HbA1c levels.
Table 1.
Subject Characteristics
| OB (n = 15) | NW (n = 16) | NW vs OB P Valuea | |
|---|---|---|---|
| Age, y | 40.4 ± 14.1 | 37.3 ± 12.0 | 0.17 |
| Sex, F/M | 12/3 | 13/3 | 1.00b |
| Ethnicity | 9W/3H/2B/1A | 11W/2H/1B/2A | 0.83b |
| Weight, kg | 90.6 ± 12.6 | 67.8 ± 12.0 | <0.001 |
| BMI, kg/m2 | 33.4 ± 2.2 | 23.5 ± 1.7 | <0.001 |
| HbA1c, % | 5.1 ± 0.7 | 5.2 ± 0.4 | 0.86 |
Data are presented as mean ± SD.
Abbreviations: A, Asian; B, black; F, female; H, Hispanic; M, male; W, white.
Group comparison was analyzed by independent sample t test.
χ 2 was used to analyze group differences in sex and ethnicity.
An average of 4 ± 1 days (ranging from 2 to 6 days) of concurrent food logs and CGM recordings was available for all participants.
Food log/hunger ratings
Food intake was determined from the food logs filled out by the study participants. The average caloric consumption per meal was 412 kcal (95% CI: 332 to 492 kcal) for NW individuals and 397 kcal (95% CI: 312 to 482 kcal) for OB individuals. The average calorie consumption per day was 2252 kcal (95% CI: 1841 to 2662 kcal) for NW individuals and 2168 kcal (95% CI: 1782 to 2553 kcal) for OB individuals [P = nonsignificant (NS)] (Table 2). There were no significant group differences in caloric and macronutrient intake per meal or per day (P = NS). Also, no group differences were observed in hunger ratings at the start of each meal [NW group: 5.8 (95% CI: 5.1 to 6.5) and OB group: 5.6 (95% CI: 4.9 to 6.4); P = NS].
Table 2.
Per Meal and Per Day Energy Intake and Composition
| NW (n = 16) | OB (n = 15) | NW vs OB P Value | |
|---|---|---|---|
| Hunger | 5.8 (5.1–6.5) | 5.6 (4.9–6.4) | 0.76 |
| Per meal | |||
| Energy,a kcal/meal | 412.1 (332.1–492.1) | 397.2 (312.4–482.1) | 0.80 |
| Carbohydrate, g/meal | 45.5 (35.7–35.2) | 44.4 (34.1–54.7) | 0.88 |
| Fat, g/meal | 17.1 (13.4–20.9) | 17.8 (13.8–21.9) | 0.81 |
| Protein, g/meal | 18.8 (14.5–23.0) | 15.6 (11.1–20.1) | 0.31 |
| Per day | |||
| Energy,a kcal/d | 2251.5 (1841.1–2661.8) | 2167.8 (1782.1–2553.5) | 0.77 |
| Carbohydrate, g/d | 261.1 (212.2–310.0) | 251.8 (204.5–297.1) | 0.30 |
| Fat, g/d | 92.4 (68.3–116.5) | 97.5 (74.9–120.1) | 0.76 |
| Protein, g/d | 99.9 (81.2–118.5) | 80.9 (61.9–97.9) | 0.13 |
Data are presented as least squares mean and 95% CI from repeated measures model. Group comparison was analyzed by mixed-effect model analysis. Significance was set at P < 0.05.
Energy and macronutrient composition were calculated from self-reported food records based on Nutrition Data System for Research.
CGM
Glycemic variability
CGM data were analyzed with the EasyGV program. Mean glucose levels (NW group: 98.6 ± 13.2 mg/dL; OB group: 99.9 ± 8.3 mg/dL; P = 0.704) and SD (NW group: 18.3 ± 3.8 mg/dL; OB group: 17.5 ± 4.8 mg/dL; P = 0.599) were comparable between NW and OB individuals. The glucose variability measurements for continuous overall net glycemic action, lability index, J-index, low blood glucose index, high blood glucose index, glycemic risk assessment diabetes equation, mean amplitude of glycemic excursions, M-value, and mean average glucose were not statistically different between OB and NW individuals (all P = NS; data not shown).
CPNA
CPNA profiles before a meal
The glucose profiles of both NW and OB groups were analyzed using CPNA. Using mixed-model analysis, we observed that the rate of changing glucose was statistically significantly lower in the OB group than in the NW group [NW group: 0.52 mg/dL/min (95% CI: 0.47 to 0.57 mg/dL/min); OB group: 0.44 mg/dL/min (95% CI: 0.38 to 0.49 mg/dL/min); P = 0.04] (Table 3). This contrasts with the other CPNA parameters, which showed no group differences: glucose peak [NW group: 119.5 mg/dL (95% CI: 111.5 to 127.6 mg/dL), OB group: 121.5 mg/dL (95% CI: 113.1 to 130.0 mg/dL); P = 0.73]; nadir [NW group: 85.1 mg/dL (95% CI: 79.7 to 90.4 mg/dL), OB group: 87.1 mg/dL (95% CI: 81.4 to 92.7 mg/dL); P = 0.62]; and difference in glucose peak and nadir [NW group: 34.3 mg/dL (95% CI: 29.8 to 38.8 mg/dL), OB group: 33.9 mg/dL (95% CI: 29.0 to 38.7 mg/dL); P = 0.89].
Table 3.
Glucose Profiles From Continuous Glucose Monitoring Peak and Nadir Analysis
| OB (n = 15) | NW (n = 16) | NW vs OB P Valuea | |
|---|---|---|---|
| Glucose peak, mg/dL | 121.5 (113.1–130.0) | 119.5 (111.5–127.6) | 0.73 |
| Glucose nadir, mg/dL | 87.1 (81.4–92.7) | 85.1 (79.7–90.4) | 0.62 |
| Difference in glucose peak and nadir, mg/dL | 33.9 (29.0–38.7) | 34.3 (29.8–38.8) | 0.89 |
| Rate of changing glucose level, mg/dL/min | 0.44 (0.38–0.49) | 0.52 (0.47–0.57) | 0.04 |
Data are presented as least squares mean and 95% CI from repeated measures model. Group comparison was analyzed by mixed-effect model analysis. Significance was set at P < 0.05.
Group comparison was analyzed by mixed model analysis.
Correlations
Hunger influencing food consumption
In both NW and OB groups, hunger was significantly correlated with caloric intake (slopes: 77.9 compared with 43.7 for OB vs NW, respectively; P < 0.007) (Fig. 2a). Statistically significant differences for calorie intake/hunger score were observed between the two groups, with the OB group reporting higher caloric intake than the NW group for the same amount of hunger (P = 0.008 for group interaction).
Figure 2.
Correlation of premeal glucose nadir, hunger, and food intake. (a) A significantly linear relationship is shown between calorie intake and hunger (P < 0.0001 for each group). The slopes are significantly different (P = 0.008 for group interaction). (b) A significant linear relationship is shown between hunger and the glucose nadir before a meal (slope for OB group: −0.22; P = 0.009 and slope for NW group: −0.14; P = 0.04). However, the slopes did not differ significantly (P = 0.44). The solid black lines represent the data for each OB participant, and the dotted gray lines represent the data for each NW participant. The bold lines represent the average data of the OB (black) and NW (gray) groups.
CPNA glucose profiles influencing subsequent hunger and food consumption
Using least squared means from mixed repeated measures model analysis and controlling for daily time, lower glucose nadir was the only measurement from CPNA that was significantly associated with subsequently greater reported hunger levels (NW group: −0.14; P = 0.04 and OB group: −0.22; P = 0.009) (Fig. 2b) as well as with greater caloric consumption per meal (NW group: −20.4; P = 0.05 and OB group: −43.6; P = 0.0007) in both NW and OB groups (Table 4). No statistical group differences were observed between CPNA glucose profiles as a predictor of subsequent hunger or food consumption (Table 4). In individuals who were OB, glucose nadir negatively correlated with all three macronutrient intakes: protein (−2.12; P = 0.002), fat (−1.96; P = 0.006), and carbohydrate (−4.1; P = 0.008). Meanwhile, in NW individuals, glucose nadir showed an inverse correlation with fat intake only (−1.18; P = 0.04), but no statistically significant group differences were noted (P = NS). Of note, greater rate of changing glucose was positively associated with caloric (−796; P = 0.01), protein (−45; P = 0.008), and fat (−36.5; P = 0.04) intake in NW individuals but not in OB individuals. Glucose peak was negatively associated with protein intake in the OB group (−0.95; P = 0.03) but not in the NW group (−0.12; P = 0.73).
Table 4.
Correlation of Glucose Peaks, Nadirs, Glucose Decline, and Rate of Change Before Meal of Interest and Nutrient Consumption in Normal Weight and Obese Groups
| Predictors | Outcomes | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hunger | Caloric (kcal) | Protein (g) | Fat (g) | Carbohydrate (g) | |||||||||||
| Est | Lower/Upper | Prob | Est | Lower/Upper | Prob | Est | Lower/Upper | Prob | Est | Lower/Upper | Prob | Est | Lower/Upper | Prob | |
| Glucose peak, mg/dL | |||||||||||||||
| OB | 0.004 | (−0.10/0.11) | 0.94 | −14.3 | (−31.1/2.52) | 0.1 | −0.96 | (−1.83/−0.08) | 0.03 | −0.8 | (−1.7/0.14) | 0.09 | −0.54 | (−2.56/1.47) | 0.6 |
| NW | −0.05 | (−0.14/0.04) | 0.3 | 0.2 | (−13.5/13.9) | 0.98 | −0.19 | (−0.91/0.52) | 0.6 | −0.1 | (−0.86/0.66) | 0.79 | 0.03 | (−1.61/1.68) | 0.96 |
| Diff | 0.05 | (−0.09/0.19) | 0.46 | −14.4 | (−36.1/7.2) | 0.19 | −0.76 | (−1.90/0.37) | 0.19 | −0.69 | (−1.90/0.51) | 0.26 | −0.58 | (−3.18/2.02) | 0.66 |
| Glucose nadir, mg/dL | |||||||||||||||
| OB | −0.22 | (−0.39/−0.06) | 0.009 | −43.6 | (−68.6/−18.5) | 0.0007 | −2.12 | (−3.43/−0.81) | 0.002 | −1.96 | (−3.36/−0.56) | 0.006 | −4.1 | (−7.11/−1.07) | 0.008 |
| NW | −0.14 | (−0.27/−0.004) | 0.04 | −20.4 | (−40.5/−0.29) | 0.047 | −1 | (−2.05/0.06) | 0.06 | −1.18 | (−2.29/−0.07) | 0.04 | −1.54 | (−3.96/0.88) | 0.21 |
| Diff | −0.08 | (−0.30/0.13) | 0.44 | −23.2 | (−54.9/8.5) | 0.15 | −1.12 | (−2.79/0.54) | 0.19 | −0.78 | (−2.55/1.00) | 0.39 | −2.55 | (−6.37/1.27) | 0.19 |
| Glucose difference, mg/dL | |||||||||||||||
| OB | 0.08 | (−0.02/0.19) | 0.11 | 4.8 | (−11.5/21.2) | 0.56 | −0.02 | (−0.88/0.84) | 0.96 | 0.07 | (−0.84/0.99) | 0.87 | 1.2 | (−0.76/3.15) | 0.23 |
| NW | 0.01 | (−0.08/0.11) | 0.77 | 10 | (−4.2/24.2) | 0.17 | 0.26 | (−0.48/1.01) | 0.49 | 0.47 | (−0.33/1.27) | 0.25 | 0.79 | (−0.91/2.49) | 0.36 |
| Diff | 0.07 | (−0.07/0.21) | 0.31 | −5.2 | (−26.6/16.2) | 0.63 | −0.28 | (−1.40/0.84) | 0.62 | −0.4 | (−1.59/0.80) | 0.52 | 0.4 | (−2.16/2.97) | 0.76 |
| Rate of changing glucose, mg/dL/min | |||||||||||||||
| OB | −5.96 | (−11.8/−0.12) | 0.046 | −104 | (−1033/825.4) | 0.83 | −24.4 | (−72.8/24.06) | 0.32 | 4.73 | (−47.6/57.0) | 0.86 | 14.29 | (−97.2/125.7) | 0.8013 |
| NW | −2.09 | (−6.55/2.377) | 0.36 | −796 | (−1435/−157) | 0.01 | −45 | (−78.3/−11.7) | 0.008 | −36.5 | (−72.5/−0.545) | 0.047 | −68.9 | (−146/7.7) | 0.08 |
| Diff | −3.87 | (−11.2/3.42) | 0.3 | 692.6 | (−429/1814) | 0.23 | 20.59 | (−37.9/79.08) | 0.49 | 41.23 | (−21.9/104.4) | 0.2 | 83.2 | (−51.3/217.7) | 0.23 |
Data presented correspond to linear mixed-model analysis, whereas the probability is P value (boldface indicates significant at P < 0.05).
Abbreviations: Est, estimate; Prob, probability.
Discussion
In this study, glucose nadirs before a meal predicted subsequent hunger and food intake in a group of healthy nondiabetic individuals. The study participants, comprising OB and NW individuals, reported similar hunger and food intake, with no differences in macronutrient consumption (Table 1). Glucose profiles measured with CGM were also comparable between the OB and NW groups, including 24-hour glucose average, SD, and the EasyGV indices of glucose variability. However, when changes in glucose levels were analyzed in relation to a meal (by CPNA data analysis), statistically significant differences between OB and NW individuals were observed in the rate of changing glucose levels (from peak to nadir) (P = 0.04) but not nadir, peak, and difference in peak and nadir (P = NS). In both individuals who were OB and NW individuals, glucose nadir—but not peak difference or rate of changing of glucose—predicted hunger ratings and caloric intake. These results were statistically significant whether the two groups were analyzed together or separately (Fig. 2a; Table 4). Hunger predicted caloric intake in both groups; however, for the same reported hunger rating, individuals who were OB ate more calories per meal than NW individuals (77.9 caloric intake/hunger score, P < 0.0001 in the OB group compared with 43.7 caloric intake/hunger score, P < 0.0001 in the NW group, with a statistically significant group interaction of P = 0.008), corresponding to an excess of 32 more calories per hunger score in the OB group than in NW individuals.
OB and NW nondiabetic individuals reported similar hunger and food intake, and they were both influenced by glucose nadir. These results are in accord with previous studies (6–9, 13), showing that low blood glucose levels are homeostatic signals promoting hunger and food intake. Although glucose nadir predicted similar hunger scores and food intake in individuals who were OB and NW individuals (Fig. 2b; Table 4), for the same perceived hunger score, OB individuals ate greater amounts of calories than NW individuals (Fig. 2a). These results may indicate that although nondiabetic OB individuals have normal homeostatic physiology, the stimuli may not be appropriately processed, resulting in increased hunger and greater food intake. Indeed, in OB individuals (in comparison with NW individuals), abnormal neuronal activity patterns in eating centers of the brain in response to visual food stimuli (measured with functional MRI) have been observed during both hypoglycemia (9) and hyperglycemia (17) and in fasted and fed states (18, 19). Although we cannot exclude the possibility that our results were affected by underreporting of hunger and food intake, therapeutic strategies targeting glucose regulation of food intake may potentially be a tool to curb hunger and decrease food intake in OB individuals.
No participants in this study had a history of diabetes, and all participants had normal HbA1c levels. Glucose profiles measured by HbA1c and CGM data were similar in both groups when analyzed by glucose average and glycemic variability. CPNA of glucose levels in relation to meals (Table 3) also showed no group differences for glucose peaks, nadir, and differences between peak and nadir. The variable rate of changing glucose, however, was statistically significantly different between the two groups, with higher rates of change in glucose levels in OB individuals than in NW individuals (Table 3). These results indicate that the time needed for glucose levels to go from peak to nadir was longer in OB individuals, as both groups had the same magnitude in postprandial increments (peaks) and decrements (nadir) in glucose levels. Differences in absorption of food by the gut and/or glucose metabolism in OB individuals may explain these findings. Indeed, both delayed and accelerated gastric emptying have been shown in obesity (20), and changes in glucose absorption and decreased glucose utilization have been demonstrated in OB individuals compared with NW individuals (21). Although the rate of changing glucose did not predict next meal hunger or food intake (Table 4), it may be an indicator of the altered homeostatic environment influencing long-term energy status in OB individuals.
Differences in postprandial glucose metabolism may indirectly affect eating by influencing eating-related hormones, such as insulin and ghrelin (22). In standardized controlled research environments, higher postprandial glucose excursions and greater incremental area under the curve in response to a meal and oral glucose ingestion, respectively, have been demonstrated in OB individuals compared with NW individuals (21, 23). These changes in glucose profiles are usually accompanied by higher incremental insulin levels in OB individuals than in NW individuals (23, 24). Insulin has been shown to activate brain regions associated with food intake, including the hypothalamus (homeostasis), the frontal cortex (motivation and control), the insular cortex (sensory perception), the striatum (reward-desire), and the hippocampus (memory) (25). Postprandial increments in insulin levels may promote satiety (26); however, in obesity, brain insulin resistance may alter hunger, food preference, and food intake by an attenuated effect of insulin on feeding-related regions of the brain (25, 27). In our free-living study, no insulin levels were obtained, but higher fasting and postprandial insulin levels were expected in OB individuals (23). In a meta-analysis evaluating the effect of postprandial insulin and glucose responses on appetite and food intake, the area under the curve for postprandial insulin, but not glucose, was significantly associated with decreased hunger in all subjects, increased satiety in NW subjects, and lower energy intake in subjects who were overweight and obese (28). However, a statistically significant inverse relationship between blood glucose and energy intake in all subjects was evident only with multivariate regression analysis with individual participant data (28). Unfortunately, the studies included in this meta-analysis had fixed timing, preventing the assessment of meal initiation in relation to blood glucose levels. Nonetheless, the authors concluded that postprandial insulin, as opposed to glucose, may be a regulator of satiety for NW subjects but is blunted in subjects who are overweight or obese. Although rate of changing in glucose levels did not correlate with hunger and food intake, we cannot exclude the possibility that hormonal responses secondary to the slower rate of changing in postprandial glucose levels may promote greater food intake in OB individuals than in NW individuals.
The main limitation in this study is the use of self-reported hunger ratings and food logs. It is well known that study participants tend to underreport their food intake, an issue that has been even more evident in OB individuals (29). However, even if the participants did not accurately record their food intake and hunger ratings, we were still able to demonstrate the effects of glucose nadir on hunger and food intake in both groups. In addition, even if the underreporting was more accentuated in the OB group, it would not alter our main finding that low blood glucose levels regulate eating in both NW and OB nondiabetic individuals.
There were no major differences between the OB and NW groups based on hunger, food intake, and GCM glucose data. However, because we did not obtain other measurements of eating behavior (e.g., satiety, food craving, and food preference), we cannot completely exclude the possibility that glucose profiles may affect eating behavior in OB individuals. It is possible that the small sample of subjects per group decreased our study power to detect group differences. However, for each study participant, CGM provided a large amount of data, which substantially increased our power to detect statistically significant differences within and between groups. To better understand the relationship between glucose levels and food intake, the analysis was adjusted for the daily time when the data were recorded, as people tend to eat less at night and have less variation in glucose levels. Another limitation is the fact that the participants were not blinded for the CGM readings. However, because glucose peak and nadir were similar in the two groups, glucose readings should have affected both groups similarly. Another limitation of this study is the small number of men, which decreased our ability to investigate whether sex differences affect glucose regulation of eating.
In summary, glucose nadir (but not peak, decrement, or rate of changing in glucose) preceding a meal had a statistically significant ability to predict hunger and caloric intake in subsequent meals. This glucose-induced food intake signal was present in both NW and OB individuals without diabetes. However, in OB individuals, hunger was associated with greater food intake in response to a similar homeostatic signal (glucose nadir). These results better clarify the role of glucose levels in regulating eating. Future studies will be important to determine whether strategies to monitor and modulate glucose nadirs before a meal could control appetite and be helpful in the development of new strategies to treat obesity.
Acknowledgments
We thank the staff of the Hospital Research Unit at Yale New Haven Hospital, the clinical coordinators from the Yale Center for Clinical Investigation, and the subjects who participated in this study.
Financial Support: This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases grants [nos. K23 DK098286-02 (to R.B.-D.) and T32 DK 07058], the Diabetes Research Center (no. P30 DK045735), and the Yale Center for Clinical Investigation supported by the Clinical and Translational Science Award (no. UL1 TR001863 from the National Center for Advancing Translational Science.
Clinical Trial Information: ClinicalTrials.gov no. NCT02673203 (3 February 2016).
Disclosure Summary: J.H. received grant support from Regeneron Pharma1. R.B.-D. received grant support from Silver Palate Kitchens and GlaxoSmithKline. The remaining authors have nothing to declare.
Glossary
Abbreviations:
- BMI
body mass index
- CGM
continuous glucose monitor
- CPNA
continuous glucose monitor peak and nadir analysis
- EasyGV
Easy Glucose Variability
- HbA1c
hemoglobin A1c
- NS
nonsignificant
- NW
normal weight
- OB
obese
References and Notes
- 1. Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Apovian CM. The causes, prevalence, and treatment of obesity revisited in 2009: what have we learned so far? Am J Clin Nutr. 2010;91(1):277S–279S. [DOI] [PubMed] [Google Scholar]
- 3. Johnston CA, Moreno JP. Bridging the science-practice gap in obesity treatment. Am J Lifestyle Med. 2015;10(2):100–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Begg DP, Woods SC. The endocrinology of food intake. Nat Rev Endocrinol. 2013;9(10):584–597. [DOI] [PubMed] [Google Scholar]
- 5. Mayer J. Glucostatic mechanism of regulation of food intake. N Engl J Med. 1953;249(1):13–16. [DOI] [PubMed] [Google Scholar]
- 6. Louis-Sylvestre J, Le Magnen J. Fall in blood glucose level precedes meal onset in free-feeding rats. Neurosci Biobehav Rev. 1980;4(Suppl 1):13–15. [DOI] [PubMed] [Google Scholar]
- 7. Campfield LA, Smith FJ, Rosenbaum M, Hirsch J. Human eating: evidence for a physiological basis using a modified paradigm. Neurosci Biobehav Rev. 1996;20(1):133–137. [DOI] [PubMed] [Google Scholar]
- 8. Melanson KJ, Westerterp-Plantenga MS, Saris WH, Smith FJ, Campfield LA. Blood glucose patterns and appetite in time-blinded humans: carbohydrate versus fat. Am J Physiol. 1999;277(2):R337–R345. [DOI] [PubMed] [Google Scholar]
- 9. Page KA, Seo D, Belfort-DeAguiar R, Lacadie C, Dzuira J, Naik S, Amarnath S, Constable RT, Sherwin RS, Sinha R. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J Clin Invest. 2011;121(10):4161–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernstein LM, Grossman MI. An experimental test of the glucostatic theory of regulation of food intake. J Clin Invest. 1956;35(6):627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schultes B, Panknin AK, Hallschmid M, Jauch-Chara K, Wilms B, de Courbière F, Lehnert H, Schmid SM. Glycemic increase induced by intravenous glucose infusion fails to affect hunger, appetite, or satiety following breakfast in healthy men. Appetite. 2016;105:562–566. [DOI] [PubMed] [Google Scholar]
- 12. Rodin J, Wack J, Ferrannini E, DeFronzo RA. Effect of insulin and glucose on feeding behavior. Metabolism. 1985;34(9):826–831. [DOI] [PubMed] [Google Scholar]
- 13. Pittas AG, Hariharan R, Stark PC, Hajduk CL, Greenberg AS, Roberts SB. Interstitial glucose level is a significant predictor of energy intake in free-living women with healthy body weight. J Nutr. 2005;135(5):1070–1074. [DOI] [PubMed] [Google Scholar]
- 14. Noujaim SE, Horwitz D, Sharma M, Marhoul J. Accuracy requirements for a hypoglycemia detector: an analytical model to evaluate the effects of bias, precision, and rate of glucose change. J Diabetes Sci Technol. 2007;1(5):652–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hill NR, Oliver NS, Choudhary P, Levy JC, Hindmarsh P, Matthews DR. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther. 2011;13(9):921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sinha M, McKeon KM, Parker S, Goergen LG, Zheng H, El-Khatib FH, Russell SJ. A comparison of time delay in three continuous glucose monitors for adolescents and adults. J Diabetes Sci Technol. 2017;11(6):1132–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Belfort-DeAguiar R, Seo D, Lacadie C, Naik S, Schmidt C, Lam W, Hwang J, Constable T, Sinha R, Sherwin RS. Humans with obesity have disordered brain responses to food images during physiological hyperglycemia. Am J Physiol Endocrinol Metab. 2018;314(5):E522–E529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Le DS, Pannacciulli N, Chen K, Del Parigi A, Salbe AD, Reiman EM, Krakoff J. Less activation of the left dorsolateral prefrontal cortex in response to a meal: a feature of obesity. Am J Clin Nutr. 2006;84(4):725–731. [DOI] [PubMed] [Google Scholar]
- 19. Dimitropoulos A, Tkach J, Ho A, Kennedy J. Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite. 2012;58(1):303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Müller M, Canfora EE, Blaak EE. Gastrointestinal transit time, glucose homeostasis and metabolic health: modulation by dietary fibers. Nutrients. 2018;10(3):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meyer-Gerspach AC, Cajacob L, Riva D, Herzog R, Drewe J, Beglinger C, Wölnerhanssen BK. Mechanisms regulating insulin response to intragastric glucose in lean and non-diabetic obese subjects: a randomized, double-blind, parallel-group trial. PLoS One. 2016;11(3):e0150803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Belfort-DeAguiar R, Seo D. Food cues and obesity: overpowering hormones and energy balance regulation. Curr Obes Rep. 2018;7(2):122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Polonsky KS, Given BD, Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988;81(2):442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kreisberg RA, Boshell BR, DiPlacido J, Roddam RF. Insulin secretion in obesity. N Engl J Med. 1967;276(6):314–319. [DOI] [PubMed] [Google Scholar]
- 25. Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Häring HU. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev. 2016;96(4):1169–1209. [DOI] [PubMed] [Google Scholar]
- 26. Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci. 2014;15(6):367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tiedemann LJ, Schmid SM, Hettel J, Giesen K, Francke P, Büchel C, Brassen S. Central insulin modulates food valuation via mesolimbic pathways. Nat Commun. 2017;8:16052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Flint A, Gregersen NT, Gluud LL, Møller BK, Raben A, Tetens I, Verdich C, Astrup A. Associations between postprandial insulin and blood glucose responses, appetite sensations and energy intake in normal weight and overweight individuals: a meta-analysis of test meal studies. Br J Nutr. 2007;98(1):17–25. [DOI] [PubMed] [Google Scholar]
- 29. Livingstone MB, Black AE. Markers of the validity of reported energy intake. J Nutr. 2003;133(Suppl 3):895S–920S. [DOI] [PubMed] [Google Scholar]