Abstract
The recognition of microbe- or danger-associated molecular patterns by complement proteins initiates a cascade of events that culminate in the engagement of complement receptors on the surface of immune cells. Signaling pathways triggered by such receptors converge with those activated downstream of various pattern-recognition receptors to determine the type and magnitude of immune cell responses. The perception of complement as a regulator of immune functions has encouraged intensive investigation in the field, which has uncovered novel molecular pathways that link complement receptor-mediated signaling with homeostatic and pathologic T-cell responses. More recently, the observation that complement proteins can also act within the intracellular space to guide the fate of T cells has added new complexity to the role of complement in the regulation of adaptive immunity. In this review, we discuss fundamental mechanisms and novel concepts at the interface of complement biology and immunity and offer a critical view on how these mechanisms play a role in the maintenance of homeostasis and the development of pathological conditions in humans.
There are two sides to every question
Protagoras
Introduction
The evolution of intricate host defense systems has allowed mammals and other organisms to protect themselves against a variety of intruders, including viruses, bacteria, fungi, and foreign bodies. Prototypes of innate immune mechanisms can be found in species throughout the evolutionary tree and are mainly based on detection of pathogens and other types of insult by pattern recognition molecules (PRM), including Toll-like receptors (TLR), C-type lectin receptors, and complement1. Homologs of complement proteins, in particular, are found in very primitive invertebrates and are surprisingly conserved throughout evolution. The multiplicity of complement genes in lower species represents a mechanism for generating immune diversity, thus underscoring a vital role for complement in immunity2,3. In species located at the base of the evolutionary tree, complement is mainly responsible for microbe recognition and initiation of phagocytosis and inflammation. Notably, rather than fading away during the course of evolution, the complement system became more diversified through the emergence of additional proteins constituting the classical and lytic pathways. This evolution-driven expansion of complement’s recognition and effector mechanisms accompanied the appearance of elements representing the adaptive immune system, such as MHC-like and RAG genes2. As a result, in vertebrates, aside from participating in immunosurveillance and inflammatory mechanisms, complement gained additional roles in the regulation of T- and B-cell responses4,5.
The notion that complement modulates adaptive immunity first appeared in the 1970s, with the observation that lymphocytes produce complement proteins, and activation fragments bind to receptors located on B- and T-cell membranes6-9. These findings were further explored in the late 1990s and early 2000s after a series of high-profile reports elucidated molecular mechanisms by which complement affects B- and T-cell function10,11. The role of complement in B-cell responses has been extensively debated and will not be addressed in this review5,10,12. Here, we revisit the main findings that uncovered novel molecular pathways linking complement-mediated recognition of conserved structural motifs (microbe-associated molecular patterns [MAMPs] or endogenous stress signals, the so-called damage-associated molecular patterns [DAMPs]) with the modulation of innate and adaptive immune responses. We also offer a critical view of the complement-dependent polarization of T-cell responses and its involvement in the development of pathological conditions in humans.
Complement senses danger and triggers immune responses
Complement proteins are currently perceived as sensors and transmitters of “danger signals” represented by conserved structural motifs of exogenous or endogenous origin (MAMPs and DAMPs)13,14 The recognition of such molecular patterns (e.g., unique carbohydrate motifs on bacteria, fungi, and viruses) by soluble complement proteins elicits a proteolytic cascade culminating in the generation of protein fragments that activate complement receptors on the surface of immune and other cell types15 (Box 1). Within the complement cascade, C1q, mannose-binding lectins (MBL), ficolins, collectins, and properdin are the main sensors of MAMPs, DAMPs, and other potential stress cues, including antigen-antibody complexes and acetylated or oxidized lipid moieties14,16.
Box 1. The complement system: an overview.
The complement system comprises a network of soluble and cell membrane proteins that work in a coordinated manner toward the activation of the classical, alternative, and lectin pathways. The classical pathway is initiated by the binding of C1q to immune complexes, whereas the lectin and alternative pathways are triggered by the association of C3b, properdin, mannose-binding lectins (MBL), or ficolins with microbe-associated molecular patterns (MAMPs) or carbohydrate structures exposed on damaged cells. Activation of any of these pathways results in the formation of convertases that cleave the C3 and C5 proteins with consequent formation of the membrane attack complex (MAC, C5b-9) and generation of active fragments such as C3a, C3b, iC3b, C3dg, C4a, C4b, and C5a. While the insertion of MAC into cell membranes results in cytolysis or cell activation, other activation fragments bind their respective complement receptors present in a variety of cell types. Triggering of complement receptors culminates in biological responses that include phagocytosis, immune adherence and removal of immune complexes, cell migration, tissue regeneration, cell activation, and modulation of pattern recognition receptor (PRR)-induced responses. Notably, the alternative pathway is in a constant state of low-level activation (“tickover”), allowing for immediate response upon microbial challenge. To avoid uncontrolled activation, the complement system is tightly regulated by proteins such as carboxypeptidases, Factor H (FH), FI, complement receptor (CR)1, membrane cofactor protein (MCP), decay accelerator factor (DAF), and CD59 that accelerate the decay of the convertases or mediate the cleavage of activation fragments. System malfunction as a consequence of genetic mutations or lack of proteins inevitably leads to insufficient or excessive complement activation that triggers and/or sustains a variety of pathologic conditions, including renal, autoimmune, neurologic, hemolytic, and inflammatory diseases 137-139.
Classical and lectin pathway initiators act as danger sensors
The molecular mechanisms involved in the activation of the C1 complex (C1qr2s2) and subsequent initiation of the classical pathway have recently been elucidated by cryo-electron microscopy and small-angle X-ray scattering (SAXS)17,18. Recognition of antigen-antibody immune complexes by C1q, with consequent generation of C5a, is a key trigger for neutrophil activation and subsequent firm adhesion to the vascular endothelium, which can lead to inflammatory reactions in the joint endothelium that characterize immune complex-induced arthritis19. In addition to IgG and IgM immune complexes, C1q recognizes other structures such as lipid A, beta-sheet amyloid fibrils, pentraxins, and apoptotic cells and further binds the so-called cC1qR, gC1qR, and C1qRp receptors (Table 1)20,21. Whereas the role of C1qRp is not fully elucidated, cC1qR and gC1qR promote the C1q-mediated uptake and phagocytosis of apoptotic cells and immune complexes by macrophages and dendritic cells (DCs)22. In addition to these conventional receptors, C1q also engages the DC-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) and the leukocyte-associated Ig-like receptor (LAIR)-1 on DCs and macrophages, modulating cell differentiation and activation toward an anti-inflammatory phenotype23-25. Supporting the concept of C1q as a promoter of homeostasis, C1q-mediated uptake of apoptotic cells has been shown to limit inflammasome activation and the production of proinflammatory cytokines by phagocytes26. Consistently, C1q-deficient patients often suffer from lupus-like autoimmune disorders characterized by the presence of autoantibodies and aberrant activation of immune responses 22,27,28. In addition to downregulating inflammatory responses, C1q has also been shown to trigger mechanisms that modulate pathophysiologic processes such as tissue repair, embryo implantation, cancer development, and neurological function (Box 2)22. Interestingly, C1q has the ability to promote cell proliferation and angiogenesis which stimulates the healing of skin lesions but may also promote tumor growth29-31.
Table 1.
Complement receptors
| Complement Receptor |
Ligand | Cell Expression | Function | Reference |
|---|---|---|---|---|
| CR1 (CD35) |
C3b, C4b | Erythrocytes, leukocytes, retinal pigment epithelial cells, skin keratinocytes, and kidney podocytes | Accelerate decay of convertases, cofactor for FI, facilitate removal of immune complexes or particles coated with C3b or C4b, facilitate B cell antigen presentation | 15,163 |
| CR2 (CD21) |
iC3b, C3dg, C3d | Epithelial cells, B cells,FDCs | Co-stimulation of B-cell stimulation, induction and maintenance of immunologic memory, B-cell tolerance | 5,138,164 |
| CR3 (CD11b, CD18) MAC-1 |
iC3b | Leukocytes, FDCs | Leukocyte adherence, phagocytosis of particles coated with iC3b, modulation of IL-12 production by APCs | 138 |
| CR4 (CD11c, CD18) |
iC3b | Leukocytes, FDCs | Leukocyte adherence, phagocytosis of particles coated with iC3b | 138 |
| C3aR | C3a | Granulocytes, monocytes, macrophages, subsets of pulmonary and intestinal DCs, activated human T cells | Cell migration, tissue regeneration, activation of eosinophils and macrophages, regulation of neutrophil responses, upregulation of IL-10 production by APCs | 59,65 |
| C5aR1 (CD88) |
C5a, C5adesArg | Granulocytes, monocytes, macrophages, NK and NKT cells, subsets of DCs, endothelial cells, epithelial cells, human T cells | Cell migration, tissue regeneration, activation of immune cells, modulation of IL-12 production by APCs, modulation of PRR responses | 59,63,66 |
| C5aR2 | C5a, C5adesArg | Granulocytes, monocytes, macrophages, subsets of DCs, human T and B cells | Poorly defined, evidence points to regulation of C5aR1 activation | 59,64 |
| CRIg | C3b, iC3b | Macrophages, Kupffer cells | Phagocytosis of opsonized particles and pathogens, regulatory effect on convertase formation | 165 |
| cC1qR (calreticulin) |
Collagen domain of C1q | Ubiquitous, with the exception of erythrocytes | Phagocytic signaling through CD91 | 20,21 |
| gC1qR (p33) |
Globular domain of C1q | Ubiquitous, with the exception of erythrocytes | Potential role in phagocytosis, regulates CD8+ T-cell responses to auto-antigens | 20,28 |
| C1qRp (CD93) |
C1q | Highly expressed in platelets, monocytes and endothelial cells | Potential role in regulating phagocytosis of C3b/C4b-coated antigens | 21 |
| MCP (CD46) |
C3b | All nucleated cells | Co-factor for FI-mediated cleavage of C3b and C4b, role in reproduction, modulation of T-cell responses | 131 |
| DAF (CD55) |
C3b, C4b | Ubiquitous | Accelerate decay of convertases, costimulate T-cell activation, indirectly modulate T-cell and APC responses (via regulation of complement activity) | 166,167 |
| CD59 | C8, C9 | Ubiquitous | Prevent assembly of TCC, modulate responses of T, B, and NK cells | 168 |
| PAR1, 4 | C4a, C4adesArg | Predominantly expressed in endothelial, immune and epithelial cells | Modulate cell activation and endothelial permeability | 92 |
APC: antigen-presenting cell, CR: complement receptor, CRIg: complement receptor of the immunoglobulin family, DAF: decay accelerator factor, DC: dendritic cell, FDC: follicular dendritic cell, FI: factor I, MCP: membrane cofactor protein, NK: natural killer; PAR: protease-activated receptor; PRR: pattern recognition receptors, TCC: terminal complement complex.
Box 2. Complement in neuroimmune responses.
A great number of preclinical studies and observations from human biopsies have positioned complement activation at the heart of an inflammatory cycle in the central nervous system (CNS) that involves intricate interactions between microglia, reactive astrocytes and neuronal synaptic networks122,140. Pattern recognition molecules such as C1q and diverse complement fragments and signaling effectors, including iC3b, CR3, C3aR, C5a and C5aR1, have been shown to modulate neuroimmune responses that underlie both basal neurodevelopmental processes and chronic, age-related neurodegenerative conditions that invoke aberrant synapse elimination, cognitive deterioration, and progressive memory loss140-144. The discovery that C1q-triggered complement activation promotes the CR3-dependent microglial engulfment of iC3b-tagged synapses, thus sculpting the brain’s synaptic circuitry during development141,145,146, led to a surge of studies that revealed a prerequisite role for C3- and CR3-dependent pathways as drivers of aberrant microglial and astrocytic responses in various neurodegenerative diseases, ranging from Alzheimer’s and Huntington’s disease to Parkinson’s and multiple sclerosis (MS)139,144,147-149. A complement-microglial crosstalk aberrantly reactivates developmental programs of synaptic pruning that culminate in memory decline and cognitive impairment in models of Alzheimers’ disease (AD) and aged-related frontotemporal dementia (FTD). In addition, genetic ablation of C3 or C3aR mitigated microglial-dependent synaptic loss and cognitive impairment in a murine model of neuroinvasive West Nile Virus (WNV) infection143,144,150-152. A complement-driven loss of inhibitory synapses was also implicated in the neuronal hyperexcitability that is associated with human epilepsy153. Moreover, complement activation was recently identified as a driver of the polarization of astrocytes toward the neurotoxic A1 phenotype which propagates neuronal damage in various neurodegenerative diseases, including AD and MS154. Reactive microglia can induce A1 astrocytes via IL-1α, TNF and C1q secretion and upregulation of C3 expression in these A1 astrocytes can fuel both complement deposition on synaptic membranes and microglial activation, thus further enhancing synaptic loss via CR3+ microglia154.
Our understanding of the fundamental role of complement in the early stages of CNS neurodegeneration has been refined by studies showing that C3 activation drives early synaptic loss, preceding amyloid plaque deposition in AD, and that genetic ablation of C3 attenuates synaptic impairment in a transgenic model of age-related AD (APP/PS1 mice)143,144. C3 activation and downstream C3aR signaling were recently implicated as early drivers of tau pathology in PS19 tau transgenic mice (PS19), with C3aR inhibition leading to attenuation of neuroinflammation, synaptic loss and neurodegeneration155. Similarly, C1q instigated aberrant microglia-dependent synaptic clearance in a model of AP–associated tau pathology (Tau-P301S transgenic mice) with C1q blockage effectively reversing tau pathology and ameliorating neuroinflammation156. Collectively, these studies identify C3 and C1q as tractable targets for developing new immunomodulatory strategies in AD and other tau-related pathologies. The pervasive impact of an aberrantly activated complement-microglial axis on cognitive disorders is further reflected by GWAS and cell-based models of synaptic pruning in schizophrenia (SZ). A strong association of the C4A gene allele with SZ risk was partly attributed to enhanced neuronal C3 deposition and excessive synaptic pruning by SZ patient-derived microglia in a C4-dependent manner157.
Like C1q, other complement-associated PRMs, including the carbohydrate-binding proteins MBL, ficolins, and collectins, play a role in host defense via recognition of microorganisms and activation of the complement lectin pathway (Box 1)32-34. Notably, individuals with low levels of functional MBL in their plasma often suffer from recurrent infections during childhood that appear to be associated with decreased phagocytic activity. In addition to enhancing phagocytosis, MBL has been implicated in the modulation of antigen-presenting cell (APC) responses. Indeed, when added to cultures of monocytic THP-1 cells, purified MBL suppresses the induction of proinflammatory cytokines in response to LPS challenge. Further, myeloid DCs isolated from the blood of MBL-deficient patients show an increased production of inflammatory cytokines after stimulation with zymosan33,35,36. In line with the concept that PRMs of the lectin pathway can modulate immune responses, genetic variants of MBL, ficolins, and collectins have been correlated with the development of inflammatory diseases, such as cystic fibrosis, myocardial infarction, leprosy, rheumatoid arthritis, and Chagas disease. Collectins, in particular, have a key role in binding DAMPs expressed by the renal tissue in response to tissue hypoxia, thereby modulating complement activation, inflammation, and tissue destruction33,34.
Initiators of the alternative pathway also act as danger sensors
A potential role for properdin, the positive regulator of the alternative pathway (Box 1), as a PRM and initiator of the alternative pathway has been extensively investigated in the last decade. Whereas experimental evidence suggests that properdin is able to bind zymosan, LPS, and apoptotic cells and further activate complement, there are conflicting data regarding the requirement for initial binding of C3b prior to properdin deposition37-40. Most recently, in a mouse model of anti-glomerular basement membrane (GBM)-induced renal injury, properdin deposition was detected in the injured glomeruli of C3-deficient mice, indicating that properdin can recognize DAMPs in the absence of C3b deposition41. It is noteworthy, however, that direct binding of properdin to DAMPs, independently of C3b, has only been demonstrated under non-physiological conditions when C3 is lacking, e.g., in C3-deficient mice or in vitro assays with purified properdin. Despite the evident challenge of investigating properdin deposition under conditions closer to physiological, it is still uncertain whether the role of properdin in pattern recognition and initiation of complement activation is relevant in vivo in individuals showing healthy levels of C3.
Apart from binding C3b and stabilizing the convertases, properdin has also been suggested to bind the natural killer cell-activating receptor (NKp46), which is expressed by NK cells and developmentally related Group 1 innate lymphoid cells42. Interestingly, instead of promoting a typical activation profile, including NK cell degranulation and secretion of IFN-γ, the triggering of NKp46 by properdin results in the secretion of Xcl1, a chemokine with antimicrobial activity. Although the ability of properdin to protect mice against Neisseria meningitidis infection is impaired in the absence of NKp46, the precise biological role associated with the alternate NK activation profile requires further investigation 42.
C3 activation caused by C3 convertases, extrinsic proteases, or spontaneous hydrolysis exposes an internal thioester bond in the C3b molecule that, during its very short life, forms amide or ester bonds with amino groups and carbohydrates present on microbes or host cells lacking complement-regulatory proteins (Box 1)2,43. C3b-opsonized antigens bind to erythrocytes expressing complement receptor (CR)1 and are transported to the liver and/or spleen, where they are phagocytosed by macrophages via CR1 or the complement receptor of the immunoglobulin family (CRIg)44 (Table 1).
Whereas autologous cells express a variety of regulatory proteins that avert complement activation and therefore deposition of C3b on the cell surface, including CR1, CRIg, membrane cofactor protein (MCP), and decay-accelerator factor (DAF) (Table 1), a wealth of data indicates the presence of C3b covalently bound to the surface of APCs under physiological conditions. Such deposition on the surface of human DCs is associated with increased expression of co-stimulatory molecules and production of cytokines by these cells43. Consistent with these findings, absence of C3 has been shown to impair the differentiation and maturation of human monocyte-derived DCs. Furthermore, DCs isolated from a C3-deficient patient show an immature phenotype when compared to DCs from individuals with normal concentrations of plasma C3, suggesting that C3 is important for proper DC maturation45,46.
The C3b molecule can be further cleaved by proteases, generating iC3b and C3dg fragments that can bind CR2 (C3dg), CR3 (iC3b, C3dg), and CR4 (iC3b)2,43,47 (Table 1). Whereas the biological role of CR3 is known in greater detail than that of CR4, the structural similarity between CR3 (CD11b/CD18) and CR4 (CD11c/CD18) suggests that both receptors are responsible for the phagocytosis of iC3b-opsonized antigens and apoptotic cells43,48. Furthermore, iC3b has been shown to promote the differentiation of mouse bone marrow cells into potent myeloid-derived suppressor cells (MDSCs)49. Notably, an immune regulatory role for iC3b has also been demonstrated in a model of antigen-specific induced tolerance, and a single nucleotide polymorphism in the CD11b chain (rs1143679) has been identified as a risk factor for systemic lupus erythematosus (SLE)43,50, supporting the idea that CR3-mediated signals are associated with the suppression of inflammatory responses.
Intracellular danger sensing by complement
Growing evidence from the past 5 years indicates that complement’s danger-sensing role can also be manifested intracellularly in a tight crosstalk with other MAMP/DAMP-recognition systems51-54. One line of evidence suggests that hydrolyzed C3 [C3(H2O)], but not native C3, can be loaded intracellularly from the extracellular space51. Although a specific receptor has not yet been identified, C3(H2O) uptake appears to be a generalized phenomenon found in a variety of immune and non-immune cells51,52. While C3(H2O) can be cleaved by intracellular proteases to generate C3a upon its internalization, most of the protein (80%) returns to the extracellular space, suggesting the operation of a C3(H2O) recycling pathway51.
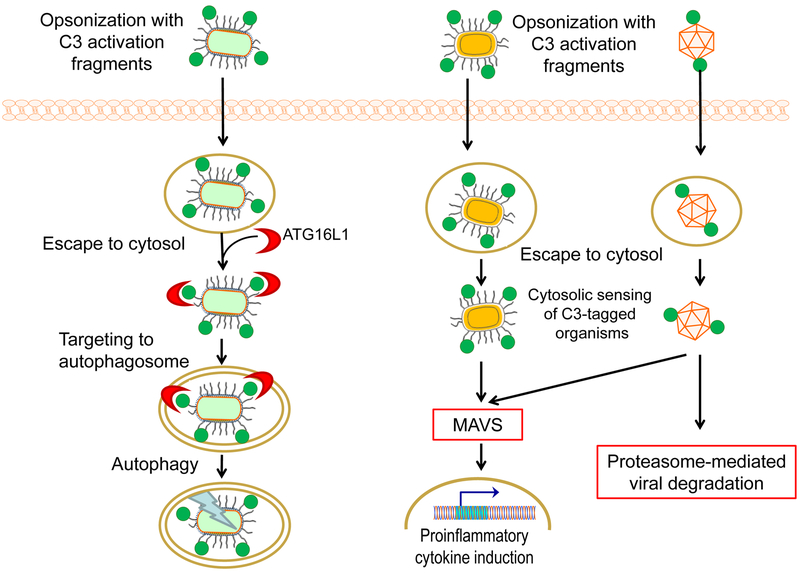
Extracellular C3-derived fragments coating non-enveloped viruses and bacteria can also be internalized via complement receptors and trigger mitochondrial anti-viral signaling (MAVS). MAVS results in the activation of the NF-κB, activating protein 1 (AP-1), and interferon regulatory factor 3 (IRF3)/IRF5/IRF7 transcription pathways, with consequent production of proinflammatory cytokines (Fig. 1). Propagation of C3-coated viruses can, moreover, be restricted via the engagement of AAA–adenosine triphosphatase (ATPase) valosin-containing protein (VCP) and proteasome-mediated degradation (Fig. 1)54. As cell activation and rapid pathogen elimination occur only in response to C3-coated virus and not virus alone, these findings suggest that an intracellular pattern-recognition receptor (PRR) senses the C3-tagged microbe and activates specific defense pathways. A similar mechanism appears to occur during infection with Francisella tularensis, in which uptake of iC3b-coated bacteria by macrophages results in membrane attack complex (MAC)-independent cell death, limiting the intracellular replication of the microbe55. Also, intracellular C3 has been associated with the regulation of autophagy via an interaction with autophagy-related protein 16-1 (ATG16L1). In a rodent model of Listeria monocytogenesis infection, C3-coated bacteria, which are sensed in the cytosol in a C3-dependent manner, trigger ATG16L1-dependent autophagy and are thus targeted to autophagolysosomes for degradation (Fig. 1)56. Interestingly, other bacteria such as Shigella flexneri and Salmonella typhimurium, which express membrane proteases capable of cleaving C3, are protected from autophagy-mediated killing56. The C3-ATG16L1 interaction has also been observed in pancreatic islet cells, regulating autophagy in insulin-secreting cells and contributing to the survival of islet cells in diabetic mice57. Further implications of intracellular complement for immune responses will be discussed below.
Figure 1. Intracellular interactions and fates of microbes opsonized with C3 in the extracellular milieu.
Left panel: Targeting of C3-opsonized bacteria to the autophagy pathway. Upon internalization, C3-coated bacteria are detected in the cytosol by the autophagy protein ATG16L1, which interacts directly with C3. This interaction initiates ATG16L1-dependent autophagy, which leads to the targeting of the bacteria to autophago-lysosomes for degradation. Right panel: Cytosolic sensing of C3-coated microbes triggers host defense. Bacteria and nonenveloped viruses that had been opsonized with C3 activation fragments in the extracellular setting can be sensed in the cytosol in a C3-dependent manner. This detection induces mitochondrial anti-viral signaling (MAVS), resulting in the activation of proinflammatory responses. Cytosolic detection of C3-opsonized viruses can, moreover, lead to proteasome-mediated viral degradation.
Overall, the information presented above indicates that multiple complement proteins recognize structural motifs present on the surface of pathogens and activate cellular pathways that contribute to the invaders’ elimination. In contrast, in the absence of infection or after an infection has resolved, complement ensures homeostasis by promoting the clearance of apoptotic cells and metabolic debris without stimulating potentially harmful inflammatory responses. Thus, the decisive element that tilts the balance between a safeguarding or injurious complement response appears to be the presence of danger motifs followed by robust complement-mediated signals.
Complement modulates innate immune responses
Microbial infection often leads to concomitant initiation of complement pathways and activation of PRRs, such as TLRs, Nod-like receptors, NLRP3 inflammasomes, and C-type lectin-like receptors on immune cells14. As mentioned above, receptors for complement activation fragments are also present on a variety of immune cells (Table 1), indicating a potential for concurrent triggering of complement receptors and PRRs on the same cell during infection. Indeed, engagement of receptors for complement activation fragments triggers the recruitment of cytoplasmic adaptor molecules allowing crosstalk with other signaling pathways58-60.
Crosstalk between complement and TLRs
The modulation of TLR4-induced responses by C5aR1-mediated signaling has been appreciated for nearly two decades61. C5aR1, a 7-transmembrane G-protein-coupled receptor (GPCR), is activated upon binding the activation fragments C5a or C5a-desArg59. The same activation fragments also bind C5aR2, a second 7-transmembrane receptor (that signals via β-arrestin) whose function is still poorly understood59. A third receptor with similar structure, C3aR, is a GPCR for the activation fragment C3a59. The cellular expression patterns of C3aR, C5aR1, and C5aR2 differ between resting and activated cells as well as between human and rodent cells. Considerable understanding of the expression of these receptors has been achieved with the investigation of green fluorescent protein (GFP) and tandem-dye Tomato fluorescent protein (tdTomato) reporter mice (Table 1)62. While numerous reports agree that myeloid cells, including neutrophils, macrophages, and subsets of DCs express C3aR, C5aR1, and C5aR2, the studies with reporter mice failed to confirm the presence of these receptors on T and B cells, suggesting that the expression of C3aR and C5aR on lymphoid cells may be an artifact resulting from unspecific antibody staining or that the signal in reporter mice was not sensitive enough to indicate the presence of these receptors in steady-state lymphoid cells62-67.
The engagement of C3aR or C5aR1 on human monocyte-derived DCs triggers the PI3K/AKT, ERK, and NF-κB signaling pathways, potentiating cell activation68. Also, the synergism between TLRs and the C3aR/C5aR1 activation pathways upregulates the expression of costimulatory molecules and the production of proinflammatory cytokines by APCs (Fig. 2)58,69-71. Thus, C3a and C5a activation fragments have been designated as “the salt and the pepper of the immune response”72. Interestingly, C5a differentially modulates TLR4-induced responses in monocytes versus macrophages, upregulating the production of inflammatory cytokines in monocytes but downregulating the response in macrophages73. Whereas both monocytes and macrophages play a key role in the initiation and resolution of inflammatory responses, monocytes act as danger sensors in the circulation and, upon activation, infiltrate tissues, where they differentiate into macrophages. It is likely that in the circulation, C5a is central to potentiating the sentinel function of monocytes. In the tissues, however, amplification of inflammation would result in organ damage; hence, C5a-mediated suppression of inflammatory responses by macrophages may represent a host-protective response. In line with this concept, LPS-induced expression of C5aR1 is upregulated in monocytes, but not in macrophages74.
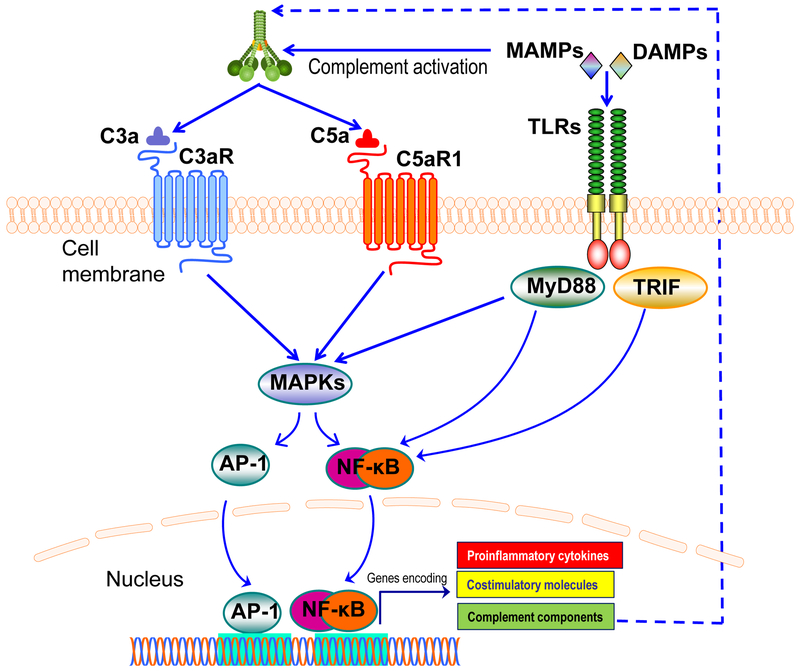
Figure 2. Synergistic interactions between complement and TLRs.
Complement and TLRs are co-activated in response to microbial infection. Certain microbe-associated molecular patterns (MAMPs), e.g., zymosan, LPS, and CpG (agonists of TLR2, TLR4, and TLR9, respectively) can activate both TLRs and complement. Complement anaphylatoxin receptor signaling stimulated by C3a or C5a synergizes with TLR-MyD88 signaling, induced either by MAMPs or endogenous danger-associated molecular patterns (DAMPs; e.g., biglycan, hyaluronan fragments, and heparan sulfate fragments). This synergy leads to enhanced activation of MAPKs and transcription factors, such as NF-κB and AP-1, resulting in upregulated expression of proinflammatory cytokines and co-stimulatory molecules. TLR activation can also upregulate the expression of complement proteins via a TRIF pathway (induced by TLR3 or TLR4 signaling), thereby generating a feed-forward loop that further amplifies inflammatory responses.
Apart from APCs, crosstalk between TLR4- and C5aR1-induced production of IFN-γ and TNF has been described in NK and NKT cells75. Furthermore, in a rodent model of polymicrobial sepsis, the activation of TLR2, TLR3, and TLR4 has been shown to promote the synthesis of factor B (FB) by macrophages and cardiac cells, with consequent activation of the alternative pathway and deposition of C3 activation fragments in vital organs such as the kidney and heart76. Similarly, in an infection model of Clostridium difficile, PAMP-induced IL-22 is required for the production of complement C3, which protects against pathogen dissemination to extra-intestinal organs, indicating a feedback mechanism between TLR- and complement-induced responses77.
Activation of CR3 in macrophages has also been implicated in modulating TLR7/8-mediated effector functions by inducing degradation of the adaptor protein MyD88 and consequent downregulation of TNF production, again pointing toward a role for CR3 in the regulation of tolerogenic responses78. Notably, such a regulatory role of CR3 (CD11b/CD18) in macrophages is not seen in DCs, in which CD11b assists the endocytosis and trafficking of TLR4 to endosomes79. In vitro studies have also suggested that additional complement-regulatory proteins such as C4b-binding protein (C4BP) and FH modulate TLR4-induced responses by DCs. Whereas C4BP prevents LPS-induced expression of indoleamine 2,3-dioxygenase (IDO), BIC-1, and proinflammatory cytokines by DCs, FH regulates the differentiation of DCs toward a tolerogenic phenotype80,81. Furthermore, MBL has been shown to bind double-stranded RNA and an extracellular domain in TLR4 and suppress TLR3- and TLR4-induced production of inflammatory cytokines36,82.
Given the broad evidence indicating modulation of TLR responses by complement, concomitant blockage of complement- and TLR-mediated pathways has been proposed as a superior therapeutic approach for diseases in which innate immunity is overactivated. However, eritoran, a synthetic lipid A analog that prevents LPS-induced activation of TLR-4, failed to reduce mortality in septic patients when administered in conjunction with standard sepsis treatment in a phase 3 study (NCT00334828). Interestingly, in an in vitro whole blood model of bacterial infection, whereas the dual blockage of TLR- and complement-mediated pathways by eritoran and the C3 inhibitor Cp40 inhibited the production of inflammatory cytokines induced by E. coli. or S. aureus, combined blockage of cell activation using an anti-CD14 antibody and Cp40 showed a greater effect in suppressing inflammation83.
Crosstalk between complement and other cell receptors
In addition to TLRs, complement-triggered pathways have also been implicated in the crosstalk with other cell receptors that modulate immune responses. A synergistic interaction between C5a- and NOD2-induced signaling has been reported in RAW 264.7 macrophages, in which engagement of NOD2 leads to an upregulation in the C5a-mediated expression of chemokines via phosphorylation of p38 MAPK84. Complement-mediated signaling also facilitates Dectin-1-mediated phagocytosis by DCs and activation of NLRP3 inflammasomes85,86. Also, deposition of sublytic levels of the terminal complex C5b-9 (Box 1) on the membranes of nucleated cells promotes the accumulation of Ca2+ in the mitochondrial matrix, loss of mitochondrial potential, and consequent activation of inflammasomes with production of IL-1β and IL-18 in vitro and in vivo85,87. Crosstalk between C5aR1- and NLRP3-induced pathways has also been observed in a rodent model of endotoxemia, in which triggering of C5aR1 upregulates the production of IL-1β88.
Additional crosstalk has been observed between C5aR1 and other GPCRs such as bradykinin receptors and C-C chemokine receptor type 5 (CCR5). In a rodent model of Trypanosoma cruzi infection, the parasite induces generation of C5a and kinins that simultaneously engage C5aR1 and the bradykinin B2 receptor (B2R). Such cooperation boosts anti-parasite immunity via production of NO and IFN-γ89. In addition, an in vitro study using macrophages infected with laboratory strains of HIV shows a requirement for C5aR1 for CCR5-mediated infection of macrophages with HIV90. Further cooperation between C5aR1- and C3aR-mediated signaling has been demonstrated in a neutrophil-dependent model of intestinal ischemia-reperfusion injury (IRI). Engagement of C3aR in neutrophils suppresses C5aR1-induced mobilization of neutrophils and intestinal inflammation, modulating the severity of IRI pathology in mice91. A new line of evidence couples complement activation with endothelial barrier responses, indicating that the activation fragment C4a acts as an agonist for the protease-activated receptors PAR1 and PAR4, thereby modulating the permeability of endothelial cells and possibly local inflammatory responses92.
These findings collectively indicate that complement shapes the type and magnitude of immune responses by cooperating with other cell defense pathways. While the physiological relevance of such crosstalk in humans requires further exploration, evidence acquired so far indicates that it both assists in the elimination of microbial intruders and contributes to the repair and maintenance of tissue homeostasis and immune tolerance.
Modulation of T-cell responses by complement
As alluded to above, complement integrates innate and adaptive immunity and can influence the quality and magnitude of T-cell activation. The stimulatory effects of complement on T-cell activation may, in part, be mediated by the action of C3a or C5a on APCs (paracrine activation). Moreover, complement also appears to exert direct effects on the functional co-stimulation and differentiation of naive CD4+ T cells (autocrine activation)4,67,93,94.
Paracrine activation of T cells
The fate of T-cell responses is regulated by signals derived from APCs: antigen presentation via MHC class-II molecules and triggering of T-cell receptors (TCRs); co-stimulation by the CD80 and CD86 molecules that engage CD28 on T cells; and a third polarizing signal coming from the cytokine milieu. IL-12 and IL-4 are the main cytokines driving the polarization of IFN-γ- and IL-4-producing Th1 and Th2 cells, respectively. Transforming growth factor (TGF)-β, IL-6, IL-1, and IL-21 are involved in the differentiation of Th17 cells, whereas IL-23 is important for Th17 cell expansion and survival. Foxp3-expressing regulatory T cells (Tregs) share a reciprocal developmental pathway with Th17 cells; thus, in the absence of the proinflammatory IL-6, TGF-β promotes the differentiation of naive CD4+ T cells into Tregs95. A plethora of data indicate that complement-induced signals on APCs influence all the three signals required to control the activation of T-cell responses4,96.
Concomitant engagement of PRRs and C3aR or C5aR1 on APCs is associated with an upregulation in the expression levels of MHC class-II and co-stimulatory molecules as well as increased production of IL-12, ensuring the polarization of CD4+ T cells toward a Th1 phenotype4,68,70,96. Indeed, thioglycolate-elicited macrophages from DAF−/− mice, which overexpress C3a and C5a, are more potent activators of Th1 responses than are macrophages from wild-type mice97. In line with these findings, DCs from C3-deficient patients show an impaired ability to stimulate alloreactive T-cell responses in vitro45,46,98. Furthermore, human T cells have been shown to produce C3 upon in vitro activation with anti-CD28, and the surfaces of activated T cells are known to be coated with iC3b fragments that mediate adherence between T cells and CR3-expressing DCs, inducing T-cell proliferation99. Given that CR3-mediated responses are tolerogenic and do not initiate a potent Th1 response, it is likely that an alternative polarization is achieved in this model. Supporting the idea that C3 is required for optimal proliferation of T cells, loading of C3-deficient DCs with apoptotic cells leads to accelerated fusion of the apoptotic cargo with lysosomes, resulting in impaired antigen presentation and decreased T-cell proliferation. These findings again support an intracellular role for C3 as a chaperone that guides the processing of an apoptotic cargo, likely modulating T-cell responses to self-antigens53.
As discussed above, like iC3b, C1q can also drive tolerogenic responses by APCs, downregulating the expression of CD86 and upregulating PD-L1 and PD-L2, with consequent differentiation of Tregs100. Moreover, whereas inhibition of FH increases the ability of DCs to induce allogenic T-cell responses, inhibition of properdin leads to an impaired allostimulatory capacity. Therefore, the balance between complement regulators (FH) and activators (properdin) appears to regulate the stimulatory capacity of DCs101.
Autocrine activation of T cells
A new concept emerged a decade ago suggesting that locally produced complement acts in an autocrine fashion to modulate CD4+ T-cell responses, independent of APCs67,93. As previously indicated, evidence shows that activated T cells produce complement components such as C3, C5, FB, and FD, with subsequent generation of C3a and C5a67,99. C3aR- and C5aR1-mediated signals on T cells have been proposed to activate the PI3K/AKT signaling pathways leading to Th1 responses, with production of IFN-γ. In contrast, ablation of C3aR- and C5aR1-mediated signals on CD4+ T cells results in the activation of an alternate signaling pathway involving the kinase PKA and consequent differentiation of Foxp3+ Tregs secreting TGF-β102,103. Furthermore, blockade of C3aR- and C5aR1-mediated signaling in natural Tregs results in increased cell-regulatory potential, abrogating autoimmune colitis in a mouse model104. Although there is a consensus that triggering C3aR and C5aR1 potentiates Th1 responses, contradictory findings exist regarding the expression of these receptors on T cells; additional research will be required to identify the circumstances under which mouse and human T cells express receptors for C3a and C5a62,63,65,66.
In contrast to C3aR and C5aR, the presence of the complement regulator MCP (CD46) on the surface of T cells is well-characterized (Table 1). Engagement of CD46 on CD4+ T cells results in either Th1 or Treg polarization, depending on local levels of IL-2 (Fig. 3). Whereas initial triggering of CD46 potentiates Th1 responses with the production of IFN-γ, accumulation of IL-2 induces a switch to IL-10 production, with consequent transition to a Th1 cell population-contraction phase that represents a self-regulatory feedback mechanism during a T-cell immune response105. It has been suggested that co-ligation of CD46 and CD35 on activated CD4+ T cells further potentiates the production of IL-10 by these cells and that the presence of monocytes modulates IL-10/IFN-γ production after CD46 stimulation106,107.
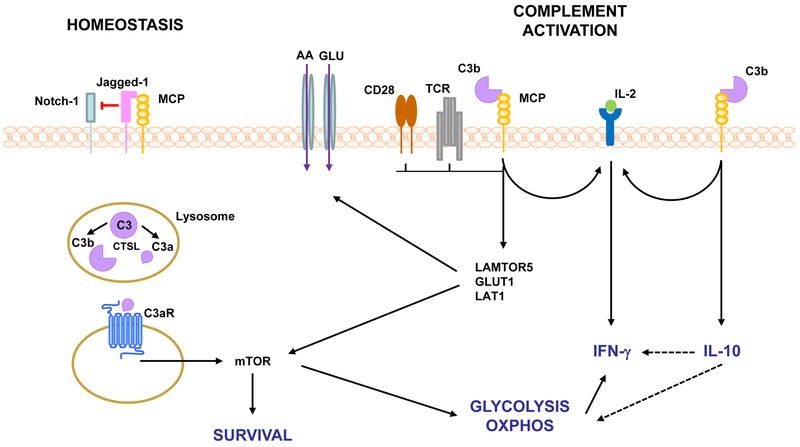
Figure 3. Complement-mediated T cell activation.
Resting CD4+ T cells have intracellular stores of C3 that can be cleaved intracellularly by CTSL. C3aR-mediated intracellular signaling induces low levels of mTOR activation that regulates T cell survival. This homeostatic state is maintained by the inhibition of Notch signaling via Jagged-1/MCP association. Engagement of TCR/CD28 during conditions favoring complement activation (infection) results in MCP activation, assembly of the IL-2 receptor and expression of LAMTOR5, GLUT1 and LAT1 leading to nutrient influx and OXPHOS and consequent induction of the Th1 cytokine IFN-γ. MCP-mediated signaling also regulates Th1 responses via collaboration with the IL-2R and induction of IL-10 with subsequent downregulation of glycolysis, OXPHOS and IFN-γ production. AA, amino acids; CTSL, cathepsin L; GLUT1, glucose transporter, LAMTOR5, late endosomal/lysosomal adaptor; LAT-1, AA transporter; mTOR, mammalian target of rapamycin; OXPHOS, oxidative phosphorylation; TCR, T cell receptor.
Whereas CD4+ T cells from CD46-deficient patients show compromised induction of Th1 responses upon stimulation with anti-CD3/CD28 or anti-CD3/CD46 in vitro, the major clinical manifestation associated with lack of CD46, atypical hemolytic uremic syndrome (aHUS), is not caused by an impaired T-cell response but by improper complement regulation60,108. Impaired B cell responses, however, and consequent common variable immunodeficiency (CVID) is observed in a few patients bearing CD46 mutations in homozygosis, an extremely rare condition108. It would be interesting, therefore, to investigate the relative contribution of CD46-mediated activation of T cells by evaluating how CD46-deficient CD4+ T cells respond in the presence of APCs and immunomodulatory factors such as C3a/C5a. In addition to C3b, the Jagged 1 protein has been identified as a ligand for CD46. Ligation of CD46 on T cells by Jagged-1 regulates the expression of Notch receptors, and crosstalk between CD46 and the Notch signaling pathway further contributes to both induction of IFN-γ and the switch to IL-10 production60. CD46-induced cytokine production by T cells is also regulated by cell metabolic pathways and dependent on the metabolism of glucose and ATP94,109 (Fig. 3, Box 3).
Box 3. Complement in immune cell metabolism.
Cell function is tightly associated with metabolic pathways and generated metabolites sustain cell survival, differentiation, proliferation, and gene expression. An association between complement and energy metabolism was initially uncovered with the observation that adipocytes, the main energy depository in the body, are a major source of FD (also known as adipsin). Expression of FD mRNA by adipocytes is dependent on the plasma concentrations of glucocorticoids, being upregulated during catabolic states, such as fasting and insulin-dependent diabetes, and downregulated in cases of genetically-determined obesity158. Another complement protein, C3a-desArg, is elevated in the plasma of obese individuals and has been shown to promote the synthesis of triglycerides by adipocytes and consequent insulin resistance, indicating an association between complement and adipose tissue metabolism159,160. To date, the understanding of a role for complement in the regulation of cell metabolism has extended from the adipose to the pancreatic and liver tissues161. Additionally, in immune cells, divergent metabolic pathways associated with distinct polarization of CD4+ cell subsets are also modulated by complement109,162. As discussed in the main text, survival and differentiation of CD4+ T cells is determined by the co-stimulation of TCR and CD46 with consequent activation of mTOR and generation of ATP via glycolysis and oxidative phosphorylation (Fig. 3)52,109. Triggering of intracellular C5aR1 during T cell activation and subsequent NLRP3-mediated secretion of IL-1β is also impacted by the production of ROS during oxygen metabolism in the mitochondria111. Similarly, in CD8+ T cells, CD46-mediated signals modulate nutrient influx and the synthesis of fatty acid, potentiating the activity of cytotoxic T cells110. As a growing body of data associates cholesterol and glucose metabolic pathways with the production of inflammatory cytokines and consequent chronic inflammatory diseases, therapeutic modulation of specific complement proteins, such as C3a and C5a, could represent an interesting approach to interfere on the cellular metabolism with the ultimate goal of restraining the production of inflammatory factors.
In addition to CD4+ T cells, complement also regulates the activation of CD8+ T cells in an autocrine fashion. In CD8+ T cells, CD46 delivers co-stimulatory signals by augmenting nutrient influx and the synthesis of fatty acid, potentiating the activity of cytotoxic T cells110. C1q also regulates the mitochondrial metabolism in effector CD8+ T cells, limiting tissue damage and autoimmune responses28.
Intracellular complement and T-cell responses
As mentioned above, a rapid and saturable recycling pathway for C3(H2O) has been described in lymphocytes that allows exchange between intracellular and extracellular stores of C351. Additional evidence indicates that human resting T cells contain intracellular stores of C3 that are cleaved into C3a and C3b by the lysosomal protease cathepsin L (CTSL)52. Such intracellular generation of C3a has been linked with homeostatic T-cell survival (Fig. 3), while externalization of C3a and binding to the C3aR on the cell surface lead to the production of proinflammatory cytokines. Interestingly, while T cells from patients with autoimmune arthritis show increased levels of intracellular C3a and production of IFN-γ, inhibition of CTSL in vitro results in the modulation of this inflammatory response52. Intracellular stores of C5a have also been observed in human T cells. It has been reported that C5a binds to the C5aR1 also present in the intracellular compartment, resulting in the assembly of the NLRP3 inflammasomes and activation of Th1 responses. Secretion of C5a further triggers the alternate receptor C5aR2 on the cell surface, which in turn restrains NLRP3-induced responses94,111.
It should be noted, however, that there are no indications that CD4+ T cells from C3-deficient patients show impaired survival112. Since the findings discussed above indicate that intracellular C3a is required for proper T-cell survival and proliferation, it has been suggested that C3a is present intracellularly in CD4+ T cells from C3-deficient patients52,112. Indeed, intracellular C3 can be observed in patients with primary and secondary C3 deficiency and C3 deficiency appears to be correlated with defects in the differentiation of memory B cells but not T cells112. While fascinating, these novel findings describing a role for intracellular C3 reveal numerous uncertainties about how the tertiary structure of C3, including its disulfide bonds, is maintained in the intracellular environment; whether the protein is glycosylated and originates from the Golgi apparatus; what decides whether the protein will be secreted; and the nature of intracellular C3a function in non-immune cells. Recent studies started to uncover some of these answers showing that in human pancreatic islet cells, intracellular C3 is transcribed from an alternative ATG codon resulting in a protein without a secretion signal, and indicating a distinct nature between “intracellular” and “extracellular” C356.
Disease states affected by complement-mediated immune responses
The vast majority of evidence linking complement-mediated T-cell responses to pathologic conditions comes from well-established disease models in rodents. Studies using complement gene-knockout strains were instrumental in revealing that imbalanced complement activation is directly associated with maladaptive T-cell responses and disease. In particular, experimental models of asthma have demonstrated that allergens drive C3a and C5a production, which in turn modulates the recruitment and activation of immune cells in the lungs and consequent release of Th2 and Th17 cytokines observed in severe asthma113-115. A C5a-mediated Th17 response is also associated with the development of chronic autoimmune arthritis in SKG mice via stimulation of IL-6 by macrophages116. Potentiation of T-cell responses by C3a and C5a further governs T cell-mediated mechanisms of organ rejection and cancer development in mice117,118. Mouse models were also key to uncovering crosstalk between C5aR1-TLR2 and CR3-TLR2 and a consequent role in the development of periodontitis119. Furthermore, imbalanced complement activation is associated with poor disease outcome in a variety of inflammatory and autoimmune disease models120-123. Despite progress in unveiling such molecular pathways in mice, certain differences between the mouse and human complement systems, such as differential expression of complement receptors, make it impossible to perfectly recapitulate human pathology using rodent models of disease94,124-126. For example, whereas the therapeutic blockage of C5aR1-induced inflammation is very efficient in reducing symptoms of arthritis in experimental models, the C5aR1 antagonist PMX-53 failed to reduce synovial inflammation in arthritis patients during a proof-of-concept clinical trial127.
Given the experimental data described above, one could expect deficiency or mutation in the CD46 (MCP) or CD55 (DAF), C3aR, or CD88 (C5aR1) genes in humans to be associated with defective T-cell responses. As discussed above, however, mutations in MCP are mainly associated with the development of aHUS, indicating that in disease states driven by persistent complement activation or dysregulation, the complement-regulatory role of MCP is more critical than the regulation of T cells108. DAF deficiency has been described in 11 patients showing severe protein-losing enteropathy. Interestingly, while CD4+ T cells from DAF−− mice show increased production of INF-γ but no gastrointestinal issues, CD4+ T cells isolated from patients show increased deposition of C3 activation fragments and production of TNF, but not INF-γ128. Notably, off-label use of eculizumab in three of these patients resulted in overall improvement in disease manifestations, including diarrhea, edema, and intestinal malabsorption129.
Whereas mutations leading to lack of function in C5aR1 have not yet been described, a frameshift mutation in the C3aR gene (C3aR1 c.355-356dup, p.Asp119Alafs*19) resulting in a premature stop codon has been described in only one patient who suffered from aHUS130. Given that aHUS often occurs as a consequence of impaired complement regulation, this finding may underscore a previously unidentified regulatory role for C3aR in humans, supporting experimental reports that C3aR-mediated signaling inhibits C5aR1-induced responses91,108,131. These observations cast doubt on whether a deficiency or lack of function of C3aR or C5aR1 is extremely rare, indicating a key role for these receptors in survival, or quite common but undiagnosed due to the absence of pathologic manifestations exclusively linked to germline mutations in these receptors. The latter suggests that in adults with a fully developed immune system, C3aR- and C5aR1-mediated immune functions may be replaced by other compensatory/overlapping immune mechanisms. Indeed, the role of complement throughout aging appears to switch from defensive (maintenance of homeostasis) to offensive (promotion of pathology)132. Taking the deficiency of C3 as an example: In childhood, C3 deficiency is associated with severe bacterial infection that can lead to death, but susceptibility to infections does not appear to be critical in adults2. Supporting this concept, complete inhibition of C3 in adult cynomolgus monkeys is not associated with increased susceptibility to infections or differential hematological profile133.
The offensive role of complement mentioned above occurs when imbalanced complement activation leads to inflammation and consequent tissue damage, dysfunction, and possible failure of a variety of organs such as the eyes, kidneys, skin, brain, and vasculature (Table 2) 2,122,132. In cancer, in particular, excessive complement activation in the tumor microenvironment has been associated with inflammation and tumor growth118. An interesting approach involving dual blockage of C5a and programmed cell death protein 1 (PD-1), initially tested in a rodent model of lung cancer, resulted in substantial enhancement of the antitumoral efficacy of the anti-PD-1 that was associated with increased numbers of CD8+ T cells in the tumor134. These findings were used as preclinical evidence to justify a phase 1 clinical trial evaluating the combined use of IPH5401 (anti-C5aR) and durvalumab (anti-PD1) in patients with advanced solid tumors (NCT03665129). Thus, while in the vast majority of diseases (Table 2), complement promotes pathogenic mechanisms by inducing inflammation, fertile ground is still available for investigating specific mechanisms linking complement and T-cell responses in human diseases.
Table 2.
Diseases associated with imbalanced complement activation.
| Disease | Mechanism | Clinical Trials |
|---|---|---|
| Age-related macular degeneration (AMD) | Genetic variants of FH, FB, C3, MCP |
NCT00935883 NCT02247479 NCT02247531 NCT02503332 NCT01527500 NCT01535950 NCT02515942 NCT02686658 |
| ANCA vasculitis | Auto-antibodies lead to complement activation and generation of C5a that drives the disease |
NCT03301467 NCT02994927 |
| Atypical hemolytic uremic syndrome (aHUS) | Genetic variants of FH, MCP, FI, C3 |
NCT00844545 NCT00844844 NCT03205995 NCT02464891 |
| Alzheimer disease (AD) | A possible mechanism described is the binding of C1q to A-beta deposits and consequent complement activation | - |
| Amyotrophic lateral sclerosis (ALS) | Clusters of complement-activated oligodendroglia and degenerating neuritis positive for C3d/C4d are detected in affected areas | |
| Cancer | Evidence of complement-induced inflammation resulting in tumor growth and metastasis | NCT03665129 |
| Cold agglutinin disease (CAD) | Presence of antibodies that activate complement |
NCT01303952 NCT03347396 NCT03347422 NCT03226678 |
| Diabetes | Evidence of increased levels of complement activation in the plasma | - |
| Epilepsy | Evidence of deposits of C1q, C3b, and C5b-9 in the vicinity of affected brain tissue | - |
| Glomerulopathies | Genetic variants of C3, FH, FI, FB; mutation in the CFHR5 gene, presence of auto-antibodies |
NCT01221181 NCT03124368 NCT02682407 NCT02384317 |
| Guillain-Barre syndrome (GBS) | Presence of antibodies that activate complement | NCT02029378 |
| Inflammatory bowel disease (IBD) | Evidence of increased complement activation in the intestinal tissue | - |
| Kidney transplant injury | Presence of anti-donor antibodies that activate complement, deposition of C4d in the transplanted organ |
NCT01919346 NCT01327573 NCT01895127 NCT01095887 NCT01756508 NCT02145182 NCT01399593 NCT02134314 |
| Multiple sclerosis (MS) | Evidence of increased complement activation in the cerebrospinal fluid of patients | - |
| Myasthenia gravis (MG) | Presence of auto-antibodies that activate complement |
NCT00727194 NCT1997229 NCT0331530 |
| Neuromyelitis optica | Presence of auto-antibodies that activate complement |
NCT00904826 NCT01892345 |
| Parkinson disease | Deposition of complement activation fragments on neuronal Lewy bodies | - |
| Paroxysmal nocturnal hemoglobinuria (PNH) | GPI anchor deficiency and defective protein anchorage, including of DAF and CD59 resulting in poor complement regulation |
NCT00122330 NCT00122317 NCT03056040 NCT03157635 NCT02534909 NCT03115996 NCT03078582 NCT03030183 NCT03225287 NCT03053102 NCT03181633 NCT03472885 NCT03439839 NCT02588833 NCT02264639 |
| Periodontitis | Local microbial-induced complement activation | NCT03694444 |
| Rheumatoid arthritis (RA) | Evidence of complement activation in the synovial membranes | |
| Schizophrenia | Risk associated with C4A variant | - |
| Skin diseases | Evidence of local deposition of complement activation fragments | NCT00005571 |
| Trauma | Evidence of increased complement activation systemically | - |
| Uveitis | Presence of auto-antibodies that activate complement | NCT01526889 |
Conclusion
Decades of research have uncovered a variety of molecular mechanisms by which complement synergizes with PRR-induced pathways in immune cells to modulate the type and magnitude of immune responses. While extensive evidence obtained in experimental models suggests that complement affects T-cell responses to cause diseases, recapitulation of such phenotypes in humans has been proven challenging, in the face of the differential expression of complement receptors in rodents and humans. Whereas common and well-characterized complement-mediated pathologies in rodents such as arthritis, asthma, and transplant rejection clearly show maladjusted T-cell responses, it is likely that human pathology may differ in terms of complement-induced injury115-117. This in no way means that T-cell responses in humans are not modulated by complement but that perhaps it is time to tune the research toward human pathology. Although recent findings show surprising correlations between C3aR and aHUS and DAF and gastrointestinal disease, various other associations between complement genes and pathology may have been neglected thus far, since they were not observed in rodents128,130. Therefore, efforts to apply therapeutic inhibition of complement to a variety of inflammatory diseases may prove beneficial for additional pathologies not currently considered to be complement-dependent (Table 2)122,129,135,136.
Key points.
Complement contains proteins that act as pattern recognition molecules.
Engagement of complement receptors in immune cells triggers signaling pathways that synergize with PRRs and other G-protein coupled receptors.
Complement modulates the activation of APCs and consequent polarization of T-cell responses.
Autocrine activation of T cells by complement has been described.
Polarization of immune responses resulting from complement overactivation is associated with pathologic conditions.
Acknowledgements
We thank Dr. Deborah McClellan for editorial assistance. JDL also thanks Dr. Ralph and Sallie Weaver for the generous endowment of his professorship. Given the broad scope of this review, we often refer to specialized review articles rather than primary literature, and we have only been able to include selected examples of the breadth of the transformative work in the field; we therefore want to thank all our colleagues who are not specifically cited for their contributions and their understanding. This work was supported by grants from the U.S. National Institutes of Health (AI068730, AI030040, DE024153, and DE024716).
Biography
John D. Lambris, Ph.D.
Dr. Ralph and Sallie Weaver Professor of Research Medicine, Department of Pathology and Laboratory Medicine, Perelman School of Medicine, University of Pennsylvania
John D. Lambris is the Dr. Ralph and Sallie Weaver Professor of Research Medicine at the University of Pennsylvania, USA. After earning his Ph.D. in biochemistry from the University of Patras, Greece, he dedicated his research to various aspects of the complement system. Research efforts in his laboratory include the elucidation of complement activation processes and crosstalk with other pathways in health and disease, and the development of complement inhibitors for therapeutic intervention.
Edimara S. Reis, Ph.D.
Research Assistant Professor, Department of Pathology and Laboratory Medicine, Perelman School of Medicine, University of Pennsylvania
Edimara S. Reis studied biological sciences at the University of Sao Paulo, Brazil, and earned a Ph.D. in Immunology from the same institution. Her interest in complement biology started during her undergraduate studies. She joined the University of Pennsylvania as a postdoctoral scientist, and her research is currently focused on the influence of complement activation in the quality and magnitude of innate and adaptive immune responses in health and disease conditions.
Dimitrios C. Mastellos, Ph.D.
Senior Researcher, Division of Biodiagnostic Sciences and Technologies, I/NRASTES, National Center for Scientific Research ‘Demokritos’
Dimitrios C. Mastellos studied biology at the University of Patras, Greece, and earned his PhD in Immunology from the Medical School of the same institution. As a postdoctoral scientist at the University of Pennsylvania, he applied animal models to interrogate the role of complement-triggered pathways in diverse pathophysiological processes. His research focuses on how complement modulates innate immune activation, host-pathogen interactions, and inflammatory signaling in health and disease.
George Hajishengallis, DDS, Ph.D.
Thomas W. Evans Centennial Professor, Department of Microbiology, School of Dental Medicine, University of Pennsylvania.
George Hajishengallis earned a D.D.S. from the University of Athens, Greece, and a Ph.D. in Immunology from the University of Alabama at Birmingham. His field of interest lies at the host-microbe interface, where his research has illuminated novel mechanisms of microbial dysbiosis, inflammation, and tissue homeostasis. His translational research has led to innovative approaches to clinical problems, such as that exemplified by complement-targeted inhibition in periodontal disease.
Footnotes
Competing interest statement
J.D. Lambris and G. Hajishengallis are inventors of patents or patent applications that describe the use of complement inhibitors for therapeutic purposes. J.D. Lambris is the founder of Amyndas Pharmaceuticals, which is developing complement inhibitors (including third-generation compstatin analogs such as AMY-101) and also the inventor of the compstatin technology licensed to Apellis Pharmaceuticals (i.e., 4(1MeW)7W/POT-4/APL-1 and PEGylated derivatives). E.S. Reis and D.C. Mastellos declare no financial interest or conflict.
References
- 1.Cooper MD & Herrin BR How did our complex immune system evolve? Nature Reviews Immunology 10, 2, doi: 10.1038/nri2686 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Ricklin D, Reis ES, Mastellos DC, Gros P & Lambris JD Complement component C3 - The "Swiss Army Knife" of innate immunity and host defense. Immunol Rev 274, 33–58, doi: 10.1111/imr.12500 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarkadis IK, Mastellos D & Lambris JD Phylogenetic aspects of the complement system. Dev Comp Immunol 25, 745–762 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Kemper C & Kohl J Novel roles for complement receptors in T cell regulation and beyond. Mol Immunol 56, 181–190, doi: 10.1016/j.molimm.2013.05.223 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Carroll MC & Isenman DE Regulation of humoral immunity by complement. Immunity 37, 199–207, doi: 10.1016/j.immuni.2012.08.002 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambris JD, Dobson NJ & Ross GD Release of endogenous C3b inactivator from lymphocytes in response to triggering membrane receptors for beta 1H globulin. J Exp Med 152, 1625–1644 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundsmo JS The leukocyte complement system. Fed Proc 41, 3094–3098 (1982). [PubMed] [Google Scholar]
- 8.Tsokos GC, Inghirami G & Lambris JD Regulation of human cytotoxic responses by complement: C3, C3b and C3d preparations enhance human allogeneic cytotoxic responses. J Immunopharmacol 8, 529–541 (1986). [DOI] [PubMed] [Google Scholar]
- 9.Eden A, Miller GW & Nussenzweig V Human lymphocytes bear membrane receptors for C3b and C3d. J Clin Invest 52, 3239–3242, doi: 10.1172/JCI107525 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC & Fearon DT C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science 271, 348–350 (1996). [DOI] [PubMed] [Google Scholar]
- 11.Montz H, Fuhrmann A, Schulze M & Gotze O Regulation of the human autologous T cell proliferation by endogenously generated C5a. Cell Immunol 127, 337–351 (1990). [DOI] [PubMed] [Google Scholar]
- 12.Rickert RC Regulation of B lymphocyte activation by complement C3 and the B cell coreceptor complex. Curr Opin Immunol 17, 237–243, doi: 10.1016/j.coi.2005.03.001 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Matzinger P The danger model: a renewed sense of self. Science 296, 301–305, doi: 10.1126/science.1071059 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Kohl J The role of complement in danger sensing and transmission. Immunol Res 34, 157–176, doi: 10.1385/IR:34:2:157 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Holers VM Complement and its receptors: new insights into human disease. Annu Rev Immunol 32, 433–459, doi: 10.1146/annurev-immunol-032713-120154 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Klop B et al. Differential complement activation pathways promote C3b deposition on native and acetylated LDL thereby inducing lipoprotein binding to the complement receptor 1. J Biol Chem 289, 35421–35430, doi: 10.1074/jbc.M114.573840 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ugurlar D et al. Structures of C1-IgG1 provide insights into how danger pattern recognition activates complement. Science 359, 794–797, doi: 10.1126/science.aao4988 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Mortensen SA et al. Structure and activation of C1, the complex initiating the classical pathway of the complement cascade. Proc Natl Acad Sci U S A 114, 986–991, doi: 10.1073/pnas.1616998114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyabe Y et al. Complement C5a Receptor is the Key Initiator of Neutrophil Adhesion Igniting Immune Complex-induced Arthritis. Sci Immunol 2, doi: 10.1126/sciimmunol.aaj2195 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eggleton P, Tenner AJ & Reid KB C1q receptors. Clin Exp Immunol 120, 406–412 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghiran I, Tyagi SR, Klickstein LB & Nicholson-Weller A Expression and function of C1q receptors and C1q binding proteins at the cell surface. Immunobiology 205, 407–420, doi: 10.1078/0171-2985-00142 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Thielens NM, Tedesco F, Bohlson SS, Gaboriaud C & Tenner AJ C1q: A fresh look upon an old molecule. Mol Immunol 89, 73–83, doi: 10.1016/j.molimm.2017.05.025 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosszu KK et al. DC-SIGN, C1q, and gC1qR form a trimolecular receptor complex on the surface of monocyte-derived immature dendritic cells. Blood 120, 1228–1236, doi: 10.1182/blood-2011-07-369728 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Son M et al. C1q and HMGB1 reciprocally regulate human macrophage polarization. Blood 128, 2218–2228, doi: 10.1182/blood-2016-05-719757 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Son M, Santiago-Schwarz F, Al-Abed Y & Diamond B C1q limits dendritic cell differentiation and activation by engaging LAIR-1. Proc Natl Acad Sci U S A 109, E3160–3167, doi: 10.1073/pnas.1212753109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serrano I, Luque A & Aran JM Exploring the Immunomodulatory Moonlighting Activities of Acute Phase Proteins for Tolerogenic Dendritic Cell Generation. Front Immunol 9, 892, doi: 10.3389/fimmu.2018.00892 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott D & Botto M The paradoxical roles of C1q and C3 in autoimmunity. Immunobiology 221, 719–725, doi: 10.1016/j.imbio.2015.05.001 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Ling GS et al. C1q restrains autoimmunity and viral infection by regulating CD8(+) T cell metabolism. Science 360, 558–563, doi: 10.1126/science.aao4555 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bossi F et al. C1q as a unique player in angiogenesis with therapeutic implication in wound healing. Proc Natl Acad Sci U S A 111, 4209–4214, doi: 10.1073/pnas.1311968111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agostinis C et al. Complement Protein C1q Binds to Hyaluronic Acid in the Malignant Pleural Mesothelioma Microenvironment and Promotes Tumor Growth. Front Immunol 8, 1559, doi: 10.3389/fimmu.2017.01559 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bulla R et al. C1q acts in the tumour microenvironment as a cancer-promoting factor independently of complement activation. Nat Commun 7, 10346, doi: 10.1038/ncomms10346 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Degn SE et al. Complement activation by ligand-driven juxtaposition of discrete pattern recognition complexes. Proc Natl Acad Sci U S A 111, 13445–13450, doi: 10.1073/pnas.1406849111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garred P et al. A journey through the lectin pathway of complement-MBL and beyond. Immunol Rev 274, 74–97, doi: 10.1111/imr.12468 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Howard M, Farrar CA & Sacks SH Structural and functional diversity of collectins and ficolins and their relationship to disease. Semin Immunopathol 40, 75–85, doi: 10.1007/s00281-017-0642-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dean MM et al. Mannose-binding lectin deficiency influences innate and antigen-presenting functions of blood myeloid dendritic cells. Immunology 132, 296–305, doi: 10.1111/j.1365-2567.2010.03365.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang M et al. Mannan-binding lectin directly interacts with Toll-like receptor 4 and suppresses lipopolysaccharide-induced inflammatory cytokine secretion from THP-1 cells. Cell Mol Immunol 8, 265–275, doi: 10.1038/cmi.2011.1 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harboe M et al. The role of properdin in zymosan- and Escherichia coli-induced complement activation. J Immunol 189, 2606–2613, doi: 10.4049/jimmunol.1200269 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harboe M et al. Properdin binding to complement activating surfaces depends on initial C3b deposition. Proc Natl Acad Sci U S A 114, E534–E539, doi: 10.1073/pnas.1612385114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kemper C, Mitchell LM, Zhang L & Hourcade DE The complement protein properdin binds apoptotic T cells and promotes complement activation and phagocytosis. Proc Natl Acad Sci U S A 105, 9023–9028, doi: 10.1073/pnas.0801015105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen JY, Cortes C & Ferreira VP Properdin: A multifaceted molecule involved in inflammation and diseases. Mol Immunol 102, 58–72, doi: 10.1016/j.molimm.2018.05.018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Flynn J et al. Properdin binds independent of complement activation in an in vivo model of anti-glomerular basement membrane disease. Kidney Int, doi: 10.1016/j.kint.2018.06.030 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Narni-Mancinelli E et al. Complement factor P is a ligand for the natural killer cell-activating receptor NKp46. Sci Immunol 2, doi: 10.1126/sciimmunol.aam9628 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erdei A et al. The versatile functions of complement C3-derived ligands. Immunol Rev 274, 127–140, doi: 10.1111/imr.12498 (2016). [DOI] [PubMed] [Google Scholar]
- 44.van Lookeren Campagne M & Verschoor A Pathogen clearance and immune adherence "revisited": Immuno-regulatory roles for CRIg. Semin Immunol 37, 4–11, doi: 10.1016/j.smim.2018.02.007 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Reis ES, Barbuto JA, Kohl J & Isaac L Impaired dendritic cell differentiation and maturation in the absence of C3. Mol Immunol 45, 1952–1962, doi: 10.1016/j.molimm.2007.10.031 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Ghannam A et al. Human C3 deficiency associated with impairments in dendritic cell differentiation, memory B cells, and regulatory T cells. J Immunol 181, 5158–5166 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Lin Z et al. Complement C3dg-mediated erythrophagocytosis: implications for paroxysmal nocturnal hemoglobinuria. Blood 126, 891–894, doi: 10.1182/blood-2015-02-625871 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vorup-Jensen T & Jensen RK Structural Immunology of Complement Receptors 3 and 4. Front Immunol 9, 2716, doi: 10.3389/fimmu.2018.02716 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsieh CC et al. The role of complement component 3 (C3) in differentiation of myeloid-derived suppressor cells. Blood 121, 1760–1768, doi: 10.1182/blood-2012-06-440214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sohn JH et al. Tolerance is dependent on complement C3 fragment iC3b binding to antigen-presenting cells. Nat Med 9, 206–212, doi: 10.1038/nm814 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elvington M, Liszewski MK, Bertram P, Kulkarni HS & Atkinson JP A C3(H20) recycling pathway is a component of the intracellular complement system. J Clin Invest 127, 970–981, doi: 10.1172/JCI89412 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liszewski MK et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity 39, 1143–1157, doi: 10.1016/j.immuni.2013.10.018 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baudino L et al. C3 opsonization regulates endocytic handling of apoptotic cells resulting in enhanced T-cell responses to cargo-derived antigens. Proc Natl Acad Sci U S A 111, 1503–1508, doi: 10.1073/pnas.1316877111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tam JC, Bidgood SR, McEwan WA & James LC Intracellular sensing of complement C3 activates cell autonomous immunity. Science 345, 1256070, doi: 10.1126/science.1256070 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brock SR & Parmely MJ Complement C3 as a Prompt for Human Macrophage Death during Infection with Francisella tularensis Strain SCHU S4. Infect Immun 85, doi: 10.1128/IAI.00424-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.King BC et al. Complement Component C3 Is Highly Expressed in Human Pancreatic Islets and Prevents beta Cell Death via ATG16L1 Interaction and Autophagy Regulation. Cell Metab 29, 202–210 e206, doi: 10.1016/j.cmet.2018.09.009 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Sorbara MT et al. Complement C3 Drives Autophagy-Dependent Restriction of Cyto-invasive Bacteria. Cell Host Microbe 23, 644–652 e645, doi: 10.1016/j.chom.2018.04.008 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Hajishengallis G & Lambris JD More than complementing Tolls: complement-Toll-like receptor synergy and crosstalk in innate immunity and inflammation. Immunol Rev 274, 233–244, doi: 10.1111/imr.12467 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klos A, Wende E, Wareham KJ & Monk PN International Union of Basic and Clinical Pharmacology. [corrected]. LXXXVII. Complement peptide C5a, C4a, and C3a receptors. Pharmacol Rev 65, 500–543 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Le Friec G et al. The CD46-Jagged1 interaction is critical for human TH1 immunity. Nat Immunol 13, 1213–1221, doi: 10.1038/ni.2454 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song WC Crosstalk between complement and toll-like receptors. Toxicol Pathol 40, 174–182, doi: 10.1177/0192623311428478 (2012). [DOI] [PubMed] [Google Scholar]
- 62.Laumonnier Y, Karsten CM & Kohl J Novel insights into the expression pattern of anaphylatoxin receptors in mice and men. Mol Immunol 89, 44–58, doi: 10.1016/j.molimm.2017.05.019 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Karsten CM et al. Monitoring and cell-specific deletion of C5aR1 using a novel floxed GFP-C5aR1 reporter knock-in mouse. J Immunol 194, 1841–1855, doi: 10.4049/jimmunol.1401401 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Karsten CM et al. Monitoring C5aR2 Expression Using a Floxed tdTomato-C5aR2 Knock-In Mouse. J Immunol 199, 3234–3248, doi: 10.4049/jimmunol.1700710 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Quell KM et al. Monitoring C3aR Expression Using a Floxed tdTomato-C3aR Reporter Knock-in Mouse. J Immunol 199, 688–706, doi: 10.4049/jimmunol.1700318 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Dunkelberger J, Zhou L, Miwa T & Song WC C5aR expression in a novel GFP reporter gene knockin mouse: implications for the mechanism of action of C5aR signaling in T cell immunity. J Immunol 188, 4032–4042, doi: 10.4049/jimmunol.1103141 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Strainic MG et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity 28, 425–435, doi: 10.1016/j.immuni.2008.02.001 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li K et al. Functional modulation of human monocytes derived DCs by anaphylatoxins C3a and C5a. Immunobiology 217, 65–73, doi: 10.1016/j.imbio.2011.07.033 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bosmann M et al. Complement activation product C5a is a selective suppressor of TLR4-induced, but not TLR3-induced, production of IL-27(p28) from macrophages. J Immunol 188, 5086–5093, doi: 10.4049/jimmunol.1102914 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hawlisch H et al. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity 22, 415–426, doi: 10.1016/j.immuni.2005.02.006 (2005). [DOI] [PubMed] [Google Scholar]
- 71.Abe T et al. Local complement-targeted intervention in periodontitis: proof-of-concept using a C5a receptor (CD88) antagonist. J Immunol 189, 5442–5448, doi: 10.4049/jimmunol.1202339 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sacks SH Complement fragments C3a and C5a: the salt and pepper of the immune response. Eur J Immunol 40, 668–670, doi: 10.1002/eji.201040355 (2010). [DOI] [PubMed] [Google Scholar]
- 73.Seow V et al. Inflammatory responses induced by lipopolysaccharide are amplified in primary human monocytes but suppressed in macrophages by complement protein C5a. J Immunol 191, 4308–4316, doi: 10.4049/jimmunol.1301355 (2013). [DOI] [PubMed] [Google Scholar]
- 74.Grant EP et al. Essential role for the C5a receptor in regulating the effector phase of synovial infiltration and joint destruction in experimental arthritis. J Exp Med 196, 1461–1471 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fusakio ME et al. C5a regulates NKT and NK cell functions in sepsis. J Immunol 187, 5805–5812, doi: 10.4049/jimmunol.1100338 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zou L et al. Complement factor B is the downstream effector of TLRs and plays an important role in a mouse model of severe sepsis. J Immunol 191, 5625–5635, doi: 10.4049/jimmunol.1301903 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hasegawa M et al. Interleukin-22 regulates the complement system to promote resistance against pathobionts after pathogen-induced intestinal damage. Immunity 41, 620–632, doi: 10.1016/j.immuni.2014.09.010 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reed JH et al. Complement receptor 3 influences toll-like receptor 7/8-dependent inflammation: implications for autoimmune diseases characterized by antibody reactivity to ribonucleoproteins. J Biol Chem 288, 9077–9083, doi: 10.1074/jbc.M112.403303 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ling GS et al. Integrin CD11b positively regulates TLR4-induced signalling pathways in dendritic cells but not in macrophages. Nat Commun 5, 3039, doi: 10.1038/ncomms4039 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olivar R et al. The Complement Inhibitor Factor H Generates an Anti-Inflammatory and Tolerogenic State in Monocyte-Derived Dendritic Cells. J Immunol 196, 4274–4290, doi: 10.4049/jimmunol.1500455 (2016). [DOI] [PubMed] [Google Scholar]
- 81.Olivar R et al. The alpha7beta0 isoform of the complement regulator C4b-binding protein induces a semimature, anti-inflammatory state in dendritic cells. J Immunol 190, 2857–2872, doi: 10.4049/jimmunol.1200503 (2013). [DOI] [PubMed] [Google Scholar]
- 82.Liu H et al. Mannan binding lectin attenuates double-stranded RNA-mediated TLR3 activation and innate immunity. FEBS Lett 588, 866–872, doi: 10.1016/j.febslet.2014.01.064 (2014). [DOI] [PubMed] [Google Scholar]
- 83.Gustavsen A et al. Combined Inhibition of Complement and CD14 Attenuates Bacteria-Induced Inflammation in Human Whole Blood More Efficiently Than Antagonizing the Toll-like Receptor 4-MD2 Complex. J Infect Dis 214, 140–150, doi: 10.1093/infdis/jiw100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang H et al. Synergistic interaction between C5a and NOD2 signaling in the regulation of chemokine expression in RAW 264.7 macrophages. Adv Biosci Biotechnol 4, 30–37, doi: 10.4236/abb.2013.48A3004 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Triantafilou K, Hughes TR, Triantafilou M & Morgan BP The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J Cell Sci 126, 2903–2913, doi: 10.1242/jcs.124388 (2013). [DOI] [PubMed] [Google Scholar]
- 86.Huang H et al. Relative contributions of dectin-1 and complement to immune responses to particulate beta-glucans. J Immunol 189, 312–317, doi: 10.4049/jimmunol.1200603 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Laudisi F et al. Cutting edge: the NLRP3 inflammasome links complement-mediated inflammation and IL-1beta release. J Immunol 191, 1006–1010, doi: 10.4049/jimmunol.1300489 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Haggadone MD, Grailer JJ, Fattahi F, Zetoune FS & Ward PA Bidirectional Crosstalk between C5a Receptors and the NLRP3 Inflammasome in Macrophages and Monocytes. Mediators Inflamm 2016, 1340156, doi: 10.1155/2016/1340156 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmitz V et al. C5a and bradykinin receptor cross-talk regulates innate and adaptive immunity in Trypanosoma cruzi infection. J Immunol 193, 3613–3623, doi: 10.4049/jimmunol.1302417 (2014). [DOI] [PubMed] [Google Scholar]
- 90.Moreno-Fernandez ME, Aliberti J, Groeneweg S, Kohl J & Chougnet CA A Novel Role for the Receptor of the Complement Cleavage Fragment C5a, C5aR1, in CCR5-Mediated Entry of HIV into Macrophages. AIDS Res Hum Retroviruses 32, 399–408, doi: 10.1089/AID.2015.0099 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu MC et al. The receptor for complement component C3a mediates protection from intestinal ischemia-reperfusion injuries by inhibiting neutrophil mobilization. Proc Natl Acad Sci U S A 110, 9439–9444, doi: 10.1073/pnas.1218815110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang H, Ricklin D & Lambris JD Complement-activation fragment C4a mediates effector functions by binding as untethered agonist to protease-activated receptors 1 and 4. Proc Natl Acad Sci U S A 114, 10948–10953, doi: 10.1073/pnas.1707364114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cravedi P et al. Immune cell-derived C3a and C5a costimulate human T cell alloimmunity. Am J Transplant 13, 2530–2539, doi: 10.1111/ajt.12405 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.West EE, Kolev M & Kemper C Complement and the Regulation of T Cell Responses. Annu Rev Immunol 36, 309–338, doi: 10.1146/annurev-immunol-042617-053245 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kapsenberg ML Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol 3, 984–993, doi: 10.1038/nri1246 (2003). [DOI] [PubMed] [Google Scholar]
- 96.Weaver DJ Jr. et al. C5a receptor-deficient dendritic cells promote induction of Treg and Th17 cells. Eur J Immunol 40, 710–721, doi: 10.1002/eji.200939333 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fang C, Miwa T & Song WC Decay-accelerating factor regulates T-cell immunity in the context of inflammation by influencing costimulatory molecule expression on antigen-presenting cells. Blood 118, 1008–1014, doi: 10.1182/blood-2011-04-348474 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sandor N, Pap D, Prechl J, Erdei A & Bajtay Z A novel, complement-mediated way to enhance the interplay between macrophages, dendritic cells and T lymphocytes. Mol Immunol 47, 438–448, doi: 10.1016/j.molimm.2009.08.025 (2009). [DOI] [PubMed] [Google Scholar]
- 99.Torok K et al. Human T cell derived, cell-bound complement iC3b is integrally involved in T cell activation. Immunol Lett 143, 131–136 (2012). [DOI] [PubMed] [Google Scholar]
- 100.Clarke EV, Weist BM, Walsh CM & Tenner AJ Complement protein C1q bound to apoptotic cells suppresses human macrophage and dendritic cell-mediated Th17 and Th1 T cell subset proliferation. J Leukoc Biol 97, 147–160, doi: 10.1189/jlb.3A0614-278R (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dixon KO, O'Flynn J, Klar-Mohamad N, Daha MR & van Kooten C Properdin and factor H production by human dendritic cells modulates their T-cell stimulatory capacity and is regulated by IFN-gamma. Eur J Immunol 47, 470–480, doi: 10.1002/eji.201646703 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van der Touw W et al. Cutting edge: Receptors for C3a and C5a modulate stability of alloantigen-reactive induced regulatory T cells. J Immunol 190, 5921–5925, doi: 10.4049/jimmunol.1300847 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Strainic MG, Shevach EM, An F, Lin F & Medof ME Absence of signaling into CD4(+) cells via C3aR and C5aR enables autoinductive TGF-beta1 signaling and induction of Foxp3(+) regulatory T cells. Nat Immunol 14, 162–171, doi: 10.1038/ni.2499 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kwan WH, van der Touw W, Paz-Artal E, Li MO & Heeger PS Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med 210, 257–268, doi: 10.1084/jem.20121525 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cardone J et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol 11, 862–871, doi: 10.1038/ni.1917 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Torok K, Dezso B, Bencsik A, Uzonyi B & Erdei A Complement receptor type 1 (CR1/CD35) expressed on activated human CD4+ T cells contributes to generation of regulatory T cells. Immunol Lett 164, 117–124, doi: 10.1016/j.imlet.2015.02.009 (2015). [DOI] [PubMed] [Google Scholar]
- 107.Charron L, Doctrinal A, Ni Choileain S & Astier AL Monocyte:T-cell interaction regulates human T-cell activation through a CD28/CD46 crosstalk. Immunol Cell Biol 93, 796–803, doi: 10.1038/icb.2015.42 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fremeaux-Bacchi V et al. Genetic and functional analyses of membrane cofactor protein (CD46) mutations in atypical hemolytic uremic syndrome. J Am Soc Nephrol 17, 2017–2025, doi: 10.1681/ASN.2005101051 (2006). [DOI] [PubMed] [Google Scholar]
- 109.Kolev M et al. Complement Regulates Nutrient Influx and Metabolic Reprogramming during Th1 Cell Responses. Immunity 42, 1033–1047, doi: 10.1016/j.immuni.2015.05.024 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arbore G et al. Complement receptor CD46 co-stimulates optimal human CD8(+) T cell effector function via fatty acid metabolism. Nat Commun 9, 4186, doi: 10.1038/s41467-018-06706-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arbore G et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4(+) T cells. Science 352, aad1210, doi: 10.1126/science.aad1210 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jimenez-Reinoso A et al. Human plasma C3 is essential for the development of memory B, but not T, lymphocytes. J Allergy Clin Immunol 141, 1151–1154 e1114, doi: 10.1016/j.jaci.2017.09.037 (2018). [DOI] [PubMed] [Google Scholar]
- 113.Lajoie S et al. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol 11, 928–935, doi: 10.1038/ni.1926 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gour N et al. C3a is required for ILC2 function in allergic airway inflammation. Mucosal Immunol 11, 1653–1662, doi: 10.1038/s41385-018-0064-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schmudde I, Laumonnier Y & Kohl J Anaphylatoxins coordinate innate and adaptive immune responses in allergic asthma. Semin Immunol 25, 2–11, doi: 10.1016/j.smim.2013.04.009 (2013). [DOI] [PubMed] [Google Scholar]
- 116.Hashimoto M et al. Complement drives Th17 cell differentiation and triggers autoimmune arthritis. J Exp Med 207, 1135–1143, doi: 10.1084/jem.20092301 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sacks SH & Zhou W The role of complement in the early immune response to transplantation. Nat Rev Immunol 12, 431–442, doi: 10.1038/nri3225 (2012). [DOI] [PubMed] [Google Scholar]
- 118.Reis ES, Mastellos DC, Ricklin D, Mantovani A & Lambris JD Complement in cancer: untangling an intricate relationship. Nat Rev Immunol 18, 5–18, doi: 10.1038/nri.2017.97 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Olsen I, Lambris JD & Hajishengallis G Porphyromonas gingivalis disturbs host-commensal homeostasis by changing complement function. J Oral Microbiol 9, 1340085, doi: 10.1080/20002297.2017.1340085 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Karsten CM et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nat Med 18, 1401–1406, doi: 10.1038/nm.2862 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pandey MK et al. Complement drives glucosylceramide accumulation and tissue inflammation in Gaucher disease. Nature 543, 108–112, doi: 10.1038/nature21368 (2017). [DOI] [PubMed] [Google Scholar]
- 122.Morgan BP The role of complement in neurological and neuropsychiatric diseases. Expert Rev Clin Immunol 11, 1109–1119, doi: 10.1586/1744666X.2015.1074039 (2015). [DOI] [PubMed] [Google Scholar]
- 123.Schonthaler HB et al. S100A8-S100A9 protein complex mediates psoriasis by regulating the expression of complement factor C3. Immunity 39, 1171–1181, doi: 10.1016/j.immuni.2013.11.011 (2013). [DOI] [PubMed] [Google Scholar]
- 124.Ueda Y, Gullipalli D & Song WC Modeling complement-driven diseases in transgenic mice: Values and limitations. Immunobiology 221, 1080–1090, doi: 10.1016/j.imbio.2016.06.007 (2016). [DOI] [PubMed] [Google Scholar]
- 125.Kotimaa J et al. Sex matters: Systemic complement activity of female C57BL/6J and BALB/cJ mice is limited by serum terminal pathway components. Mol Immunol 76, 13–21, doi: 10.1016/j.molimm.2016.06.004 (2016). [DOI] [PubMed] [Google Scholar]
- 126.Kim DD & Song WC Membrane complement regulatory proteins. Clin Immunol 118, 127–136, doi: 10.1016/j.clim.2005.10.014 (2006). [DOI] [PubMed] [Google Scholar]
- 127.Vergunst CE et al. Blocking the receptor for C5a in patients with rheumatoid arthritis does not reduce synovial inflammation. Rheumatology (Oxford) 46, 1773–1778, doi: 10.1093/rheumatology/kem222 (2007). [DOI] [PubMed] [Google Scholar]
- 128.Ozen A et al. CD55 Deficiency, Early-Onset Protein-Losing Enteropathy, and Thrombosis. N Engl J Med 377, 52–61, doi: 10.1056/NEJMoa1615887 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kurolap A et al. Eculizumab Is Safe and Effective as a Long-term Treatment for Protein-losing Enteropathy Due to CD55 Deficiency. J Pediatr Gastroenterol Nutr, doi: 10.1097/MPG.0000000000002198 (2018). [DOI] [PubMed] [Google Scholar]
- 130.Leinoe E, Nielsen OJ, Jonson L & Rossing M Whole-exome sequencing of a patient with severe and complex hemostatic abnormalities reveals a possible contributing frameshift mutation in C3AR1. Cold Spring Harb Mol Case Stud 2, a000828, doi: 10.1101/mcs.a000828 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liszewski MK & Atkinson JP Complement regulator CD46: genetic variants and disease associations. Hum Genomics 9, 7, doi: 10.1186/s40246-015-0029-z (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ricklin D, Reis ES & Lambris JD Complement in disease: a defence system turning offensive. Nat Rev Nephrol 12, 383–401, doi: 10.1038/nrneph.2016.70 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reis ES et al. Safety profile after prolonged C3 inhibition. Clin Immunol 197, 96–106, doi: 10.1016/j.clim.2018.09.004 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ajona D et al. A Combined PD-1/C5a Blockade Synergistically Protects against Lung Cancer Growth and Metastasis. Cancer Discov 7, 694–703, doi: 10.1158/2159-8290.CD-16-1184 (2017). [DOI] [PubMed] [Google Scholar]
- 135.Harris CL, Pouw RB, Kavanagh D, Sun R & Ricklin D Developments in anti-complement therapy; from disease to clinical trial. Mol Immunol 102, 89–119, doi: 10.1016/j.molimm.2018.06.008 (2018). [DOI] [PubMed] [Google Scholar]
- 136.Ricklin D, Mastellos DC, Reis ES & Lambris JD The renaissance of complement therapeutics. Nat Rev Nephrol 14, 26–47, doi: 10.1038/nrneph.2017.156 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ricklin D, Hajishengallis G, Yang K & Lambris JD Complement: a key system for immune surveillance and homeostasis. Nat Immunol 11, 785–797, doi: 10.1038/ni.1923 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Merle NS, Church SE, Fremeaux-Bacchi V & Roumenina LT Complement System Part I - Molecular Mechanisms of Activation and Regulation. Front Immunol 6, 262, doi: 10.3389/fimmu.2015.00262 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hajishengallis G, Reis ES, Mastellos DC, Ricklin D & Lambris JD Novel mechanisms and functions of complement. Nat Immunol 18, 1288–1298, doi: 10.1038/ni.3858 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stephan AH, Barres BA & Stevens B The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci 35, 369–389, doi: 10.1146/annurev-neuro-061010-113810 (2012). [DOI] [PubMed] [Google Scholar]
- 141.Stevens B et al. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178, doi: 10.1016/j.cell.2007.10.036 (2007). [DOI] [PubMed] [Google Scholar]
- 142.Hawksworth OA, Li XX, Coulthard LG, Wolvetang EJ & Woodruff TM New concepts on the therapeutic control of complement anaphylatoxin receptors. Mol Immunol 89, 36–43, doi: 10.1016/j.molimm.2017.05.015 (2017). [DOI] [PubMed] [Google Scholar]
- 143.Hong S et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716, doi: 10.1126/science.aad8373 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shi Q et al. Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci Transl Med 9, doi: 10.1126/scitranslmed.aaf6295 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bialas AR & Stevens B TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci 16, 1773–1782, doi: 10.1038/nn.3560 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 146.Schafer DP et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705, doi: 10.1016/j.neuron.2012.03.026 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Howell GR et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Invest 121, 1429–1444, doi: 10.1172/JCI44646 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Michailidou I et al. Complement C1q-C3-associated synaptic changes in multiple sclerosis hippocampus. Ann Neurol 77, 1007–1026, doi: 10.1002/ana.24398 (2015). [DOI] [PubMed] [Google Scholar]
- 149.Brennan FH, Lee JD, Ruitenberg MJ & Woodruff TM Therapeutic targeting of complement to modify disease course and improve outcomes in neurological conditions. Semin Immunol 28, 292–308, doi: 10.1016/j.smim.2016.03.015 (2016). [DOI] [PubMed] [Google Scholar]
- 150.Shi Q et al. Complement C3-Deficient Mice Fail to Display Age-Related Hippocampal Decline. J Neurosci 35, 13029–13042, doi: 10.1523/JNEUROSCI.1698-15.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lui H et al. Progranulin Deficiency Promotes Circuit-Specific Synaptic Pruning by Microglia via Complement Activation. Cell 165, 921–935, doi: 10.1016/j.cell.2016.04.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vasek MJ et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature 534, 538–543, doi: 10.1038/nature18283 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wyatt SK, Witt T, Barbaro NM, Cohen-Gadol AA & Brewster AL Enhanced classical complement pathway activation and altered phagocytosis signaling molecules in human epilepsy. Exp Neurol 295, 184–193, doi: 10.1016/j.expneurol.2017.06.009 (2017). [DOI] [PubMed] [Google Scholar]
- 154.Liddelow SA et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487, doi: 10.1038/nature21029 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Litvinchuk A et al. Complement C3aR Inactivation Attenuates Tau Pathology and Reverses an Immune Network Deregulated in Tauopathy Models and Alzheimer's Disease. Neuron 100, 1337–1353 e1335, doi: 10.1016/j.neuron.2018.10.031 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dejanovic B et al. Changes in the Synaptic Proteome in Tauopathy and Rescue of Tau-Induced Synapse Loss by C1q Antibodies. Neuron 100, 1322–1336 e1327, doi: 10.1016/j.neuron.2018.10.014 (2018). [DOI] [PubMed] [Google Scholar]
- 157.Sellgren CM et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci 22, 374–385, doi: 10.1038/s41593-018-0334-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rosen BS et al. Adipsin and complement factor D activity: an immune-related defect in obesity. Science 244, 1483–1487 (1989). [DOI] [PubMed] [Google Scholar]
- 159.Maslowska M et al. Plasma acylation stimulating protein, adipsin and lipids in non-obese and obese populations. Eur J Clin Invest 29, 679–686 (1999). [DOI] [PubMed] [Google Scholar]
- 160.Barbu A, Hamad OA, Lind L, Ekdahl KN & Nilsson B The role of complement factor C3 in lipid metabolism. Mol Immunol 67, 101–107, doi: 10.1016/j.molimm.2015.02.027 (2015). [DOI] [PubMed] [Google Scholar]
- 161.Phieler J, Garcia-Martin R, Lambris JD & Chavakis T The role of the complement system in metabolic organs and metabolic diseases. Semin Immunol 25, 47–53, doi: 10.1016/j.smim.2013.04.003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Geltink RIK, Kyle RL & Pearce EL Unraveling the Complex Interplay Between T Cell Metabolism and Function. Annu Rev Immunol 36, 461–488, doi: 10.1146/annurev-immunol-042617-053019 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Merle NS, Noe R, Halbwachs-Mecarelli L, Fremeaux-Bacchi V & Roumenina LT Complement System Part II: Role in Immunity. Front Immunol 6, 257, doi: 10.3389/fimmu.2015.00257 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sarrias MR et al. Kinetic analysis of the interactions of complement receptor 2 (CR2, CD21) with its ligands C3d, iC3b, and the EBV glycoprotein gp350/220. J Immunol 167, 1490–1499 (2001). [DOI] [PubMed] [Google Scholar]
- 165.Helmy KY et al. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell 124, 915–927, doi: 10.1016/j.cell.2005.12.039 (2006). [DOI] [PubMed] [Google Scholar]
- 166.Capasso M et al. Costimulation via CD55 on human CD4+ T cells mediated by CD97. J Immunol 177, 1070–1077 (2006). [DOI] [PubMed] [Google Scholar]
- 167.Liu J et al. The complement inhibitory protein DAF (CD55) suppresses T cell immunity in vivo. J Exp Med 201, 567–577, doi: 10.1084/jem.20040863 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kimberley FC, Sivasankar B & Paul Morgan B Alternative roles for CD59. Mol Immunol 44, 73–81, doi: 10.1016/j.molimm.2006.06.019 (2007). [DOI] [PubMed] [Google Scholar]