Abstract
Inhibiting Ca2+/calmodulin-dependent protein kinase II (CaMKII) over activation can decrease detrimental cardiac remodeling that leads to dilated cardiomyopathy, cell death, and heart failure. We previously showed that cellular retinoic acid binding protein 1 (Crabp1) knockout mice (CKO) exhibited a more severe isoproterenol (ISO)-induced heart failure and cardiac remodeling phenotype with elevated CaMKII activity in the heart, suggesting a cardiac-protective function of Crabp1 through modulating CaMKII activity. Here we examine whether the highly selective, endogenous ligand of Crabp1, all-trans retinoic acid (RA), can attenuate ISO-induced cardiac dysfunction. We also examine if this attenuation involves Crabp1 and the inhibition of CaMKII. RA pre-treatment followed by ISO challenge effectively restores ejection fraction in wild type, but not in CKO mice. This is correlated with reduced CaMKII autophosphorylation at T287 and phospholamban phosphorylation at T17, a substrate of CaMKII. RA pretreatment also reduces ISO-induced apoptosis in WT heart. Cell culture experiments confirm that RA inhibits CaMKII phosphorylation, which requires Crabp1. Molecular data reveal interaction of Crabp1 with the kinase and regulatory domains of CaMKII, and that RA selectively enhances Crabp1 interaction with the regulatory domain, suggesting a potential regulatory role for holo-Crabp1 in CaMKII activation. Together, these data demonstrate that RA bound Crabp1 plays a protective role in β-adrenergic stimulated cardiac remodeling, which is partially attributed to its dampening CaMKII activation. Targeting Crabp1 provides a potentially new therapeutic strategy for managing heart diseases.
Keywords: retinoic acid, Crabp1, CaMKII, cardiac remodeling
1. Introduction
Sympathetic stimulation of β1-adrenergic receptors activates protein kinase A (PKA) and Ca2+/calmodulin-dependent protein kinase II (CaMKII) signaling in the heart and induces positive inotropic and chronotropic responses. However, persistent activation of CaMKII triggers various cardiac remodeling including arrhythmia, dilated cardiomyopathy, inflammation, fibrosis, hypertrophy and heart failure (Feng and Anderson, 2017; Grimm and Brown, 2010; Woo and Xiao, 2012). Without stimulation, CaMKII is auto-regulated by its regulatory domain that inhibits the kinase domain to block enzymatic activity. When intracellular [Ca2+] is elevated, Ca2+/calmodulin (CaM) binds to the regulatory domain of CaMKII, relieving autoinhibition; while inducing autophosphorylation at T287 to prompt autonomous kinase activity (Erickson, 2014). The autonomous activation of CaMKII by T287 autophosphorylation lasts until it is dephosphorylated by protein phosphatase 1 or 2A (Strack et al., 1997). In contrast, autophosphorylation at T306/T307 interferes with Ca2+/CaM binding, and Ca2+/CaM binding can hinder this inhibitory autophosphorylation (Coultrap et al., 2012; Hudmon and Schulman, 2002). Therefore, it is widely proposed that regulating CaMKII activity can be a therapeutic strategy for heart failure. To this end, several small molecule and peptide inhibitors that target either the kinase domain or the regulatory domain have been used experimentally. However, these are not yet clinically applied due to concerns over non-specific effects on other kinases and channels (Pellicena and Schulman, 2014).
All trans retinoic acid (RA) has been demonstrated to alleviate hypertrophy and fibrosis after myocardial infarction and transverse aortic constriction (Choudhary et al., 2007; Paiva et al., 2005). RA has also been shown to elicit a protective effect against cardiac arrhythmia and hypertrophy induced by β-adrenergic receptor agonist, isoproterenol (ISO), and angiotensin II (Kang and Leaf, 1996; Palm-Leis et al., 2004; Wang et al., 2002). However, it remains to be determined what mediates this cardiac-protective effect of RA.
Cellular retinoic acid binding protein 1 (Crabp1) is known to bind RA with a high affinity (Fiorella et al., 1993). Although these mutant mice did not exhibit apparent abnormal, developmental phenotype as also reported in previous studies (de Bruijn et al., 1995; Gorry et al., 1994), we previously found that CKO mice developed dilated cardiomyopathy and hypertrophy later in life (Park et al., 2018). In careful examination, we found that adult CKO mice were more susceptible to ISO–induced cardiac remodeling, resulting in ventricular apoptosis, necrosis, fibrosis, and hypertrophy. Interestingly, these mice also had significantly altered CaMKII activation in the heart (Park et al., 2018). These previous data prompted this current study to examine whether, RA, the high affinity, endogenous ligand of Crabp1, has a role in protecting the heart, and if this involves Crabp1 and altered CaMKII regulation. This study also reports the molecular relationship between Crabp1 with CaMKII.
2. Materials and Methods
2.1. Materials and reagents
To assess the role of RA on CaMKII autophosphorylation induced by β-adrenergic stimulation, all-trans RA (R2625) and ISO (I6504) were purchased from Sigma Aldrich. RA was dissolved in dimethyl sulfoxide (DMSO) and diluted with corn oil (C8267, Sigma-Aldrich). The primary antibodies used for western blot are as follows: P-CaMKII (sc-32289), P-PLN at the T17 (sc-17024-R), Gapdh (sc-32233), His-probe (sc-8036), and Actin (sc-58673) were purchased from Santa Cruz Biotechnology. P-CaMKII (12716) and Gapdh (5174) were purchased from Cell Signaling. Other antibodies are P-PLN at S16 (Ab15000, Abcam), Crabp1 (C1608, Sigma-Aldrich), Flag (F1804, Sigma-Aldrich), Caspase 3 (ADI-AAP-113D, ENZO Life Sciences Inc. and AF835, R&D Systems) and CaMKÎ (LS-C100735, LifeSpan BioSciences, Inc). M2 agarose beads are bought from Sigma-Aldrich and Ni-nitrilotriacetic acid (Ni-NTA) agarose beads are from Qiagen. TUNEL staining kits were purchased from Biotium, Hayward, CA.
2.2. Plasmid constructs and cell culture
The plasmids of mouse cDNA for Camk2D and Crabp1 were described earlier (Park et al., 2018). Camk2D fragment constructs were PCR amplified using the primer sets spanning the amino acids indicated in Fig. 5B and subcloned into Eco-R1/Bam-H1 site of modified pCMX-PL1 vector containing Flag tag. HEK293T, C2C12 myoblasts, and GL261 were maintained in high glucose DMEM supplanted with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. C2C12 myoblasts were differentiated in DMEM containing 1% horse serum (HS) and 1% penicillin-streptomycin for 4 days. Expression vectors for Flag-CaMKIIδ, and Flag-Crabp1 were transfected into HEK 293T cells by calcium phosphate method.
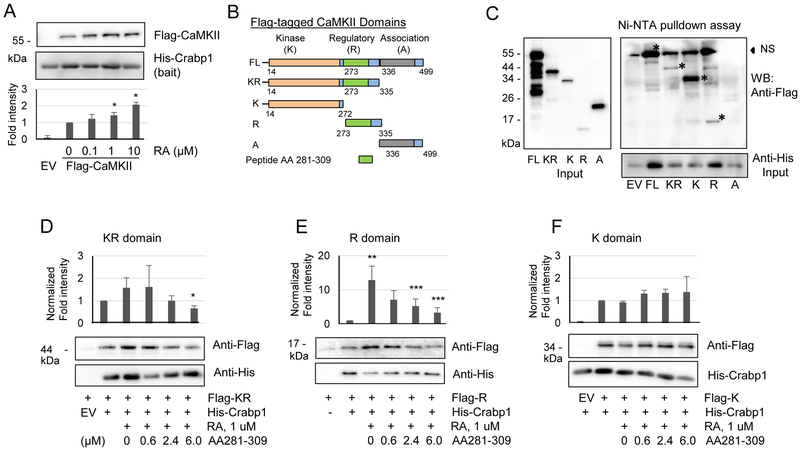
Fig. 5.
RA increases Crabp1 binding to the regulatory domain of CaMKII. (A) RA increases the direct interaction of His-Crabp1 with TnT-generated Flag-CaMKII. Statistical analyses are shown at the bottom. *P < 0.05 vs. control without RA (n=4, student t-test). (B) CaMKII domain map and plasmid constructs (FL, KR, K, R and A) are shown. (C) His-Crabp1 pulldown by Ni-NTA beads co-precipitates Flag-FL, -KR, -K and -R proteins except for Flag-A (the left and bottom panels showing protein input). * signs mark the specific, interacting bands. NS denotes nonspecific bands that slightly overlap with FL. (D-F) Competition assays with CaMKII peptide (residues 281-309) for interaction with KR, R or fragments. The peptide competes with Crabp1 binding to the KR fragment (D) and the R domain (E). (F) The peptide failed to compete with Crabp1 for interaction with K domain. Results show representatives of three independent blots. Statistical analyses are shown above the blot images. *P < 0.05 column 6 vs. column 2, **P < 0.01 column 3 vs. column 2 and **P < 0.05 columns 5 and 6 vs. column 3 (student t-test).
2.3. Crabp1 Protein Expression and Purification
His-Crabp1, was cloned into pET-15b using Nde1/BamH1 restriction sites and expressed in Rosetta™ DE3 E. coli. His-Crabp1 was grown in LB medium + ampicillin (150 μg/mL) at 37°C, 250 rpm until OD600 ~0.6-0.8. Protein expression was induced with 1 mM Isopropyl β-D-1-thiogalactopyranoside (IPTG) at 25°C, 250 rpm overnight. Cells were lysed in 1 X PBS, pH=8.0, 10mM imidazole, and 1X Halt™ Protease Inhibitor Cocktail (Thermofisher). His-Crabp1 was affinity-captured on a Ni-NTA column (Qiagen) using a 10mM-500mM imidazole gradient. Fractions containing purified His-Crabp1 were concentrated and subjected to gel filtration chromatography using a Superose 6 column (GE Healthcare Life Sciences) and exchanged into 1 X PBS, pH=8.0. Protein concentration was measured using UV absorbance at 280nm.
2.4. Experimental mice, ISO injection and tissue preparation
Experimental procedures were conducted according to NIH guidelines and approved by the University of Minnesota Institutional Animal Care and Use Committee. We used WT and CKO mice to induce ISO challenge and RA protection (Park et al., 2018). Male WT and CKO mice (16-20 weeks of age) were divided into four groups each: control, RA, ISO and RA/ISO groups. Control and RA groups were intraperitoneally injected with corn oil or RA. ISO/RA groups were pre-treated with RA (5 mg/kg/day) for 4 h, then administered with ISO (160 mg/kg) for 30 min (acute treatment). For one day ISO exposure, mice were treated with intraperitoneal injections of ISO (160 mg/kg/day). For chronic ISO exposure, mice were treated with daily intraperitoneal injections of ISO (30 mg/kg/day) for 7 days. RA and RA/ISO groups were daily pre-treated with RA (5 mg/kg/day) for 4hr prior to ISO treatment. At the end of ISO injection period, hearts were quickly excised and cross-cut in the middle of ventricles. Base part was quickly frozen in liquid nitrogen for protein analyses, and apex part embedded in Tissue-Plus O.C.T. Compound (Fisher Healthcare) without fixation was snap frozen for histological staining.
2.5. Echocardiography
Cardiac dimensions are acquired in 2-D-guided M mode images using Vevo 2100 (VisualSonics Inc. Toronto, Canada) on a parasternal short axis view of the heart. All measurements were performed under anesthesia with 1.5-2% isoflurane mixed with 0.5 L/min 100% O2. Heart rate was maintained above at least 300 bpm and body temperatures were maintained between narrow ranges (37.0 ± 1.0 °C) to avoid confounding effects of hypothermia. Measurements were averaged over 3 consecutive beats (Park et al., 2018).
2.6. TUNEL staining and Caspase 3 cleavage assay
Frozen heart sections were used. We performed terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL) staining using CF™ Dye TUNEL Assay Apoptosis Detection Kit (Biotium) (Park et al., 2018). DAPI was used for nuclear staining. Images were obtained using Leica DM5500B microscope under 20x magnification. TUNEL signals overlapped with DAPI from multiple random fields were counted and expressed as the percentage fraction of the approximate number of cells per field. Caspase 3 cleavage was assessed by detecting cleaved Caspase 3 on western blots.
2.7. Protein extraction, co-immunoprecipitation and western blot
Cytosolic lysates including membranous fractions were obtained from frozen heart tissues and cells in Triton X-100 lysis buffer (10 mM Tis-HCl, pH 7.9, 50 mM NaCl, 0.5 M sucrose, 0.1 mM EDTA, and 0.5% Triton X-100) supplemented immediately before homogenization with protease inhibitor cocktail, 1 mM PMSF, 1mM NaF, 1 mM dithiothreitol, 17.5 mM β-glycerophosphate, and 1 mM Na3VO4. Samples for CaMKII (50 μg) were separated in 8 or 10% SDS-polyacrylamide gel and for PLN (20 μg) in 13.8% and transferred to PVDF membrane (Park et al., 2018).
2.8. In vitro interaction assay
Pulldown was performed as described with slight modification (Park et al., 2018). His pulldown assay: purified His-Crabp1 was captured by Ni-NTA agarose beads for 2 h and used as bait. Flag-CaMKIIδ (FL, KR, K, R and A) were synthesized with TnT T7-coupled reticulocyte lysate system (Promega) or expressed in HEK293T cells. His-Crabp1/Ni-NTA agarose beads were incubated with Flag-CaMKIIδ in total 500 μl of binding buffer in the presence or absence of RA as indicated concentration for 2h or overnight. M2 pulldown assay: Flag-CaMKIIδ (and fragments) was incubated with M2 agarose beads and purified 2 μM His-Crabp1 in total 500 μl of binding buffer in the presence or absence of RA as indicated concentration for 2h or overnight.
2.9. Statistical analysis of the data
Comparisons among groups were evaluated with Student’s t-test or ANOVA with two-way (Student-Newman-Keuls method) when appropriate. Differences were considered significant at P < 0.05. All values are presented as means ± standard deviations (SDs).
3. Results
3.1. RA restores ISO-induced cardiac dysfunction and augmented CaMKII activation via Crabp1
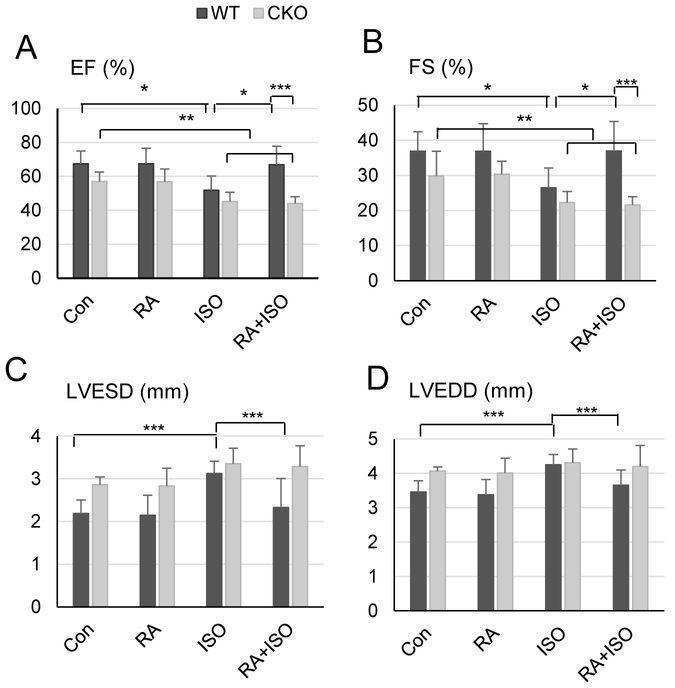
Our previous study showed reduced cardiac function of CKO mice, manifested as dilated cardiomyopathy and hypertrophy, and these mice underwent more severe cardiac remodeling after ISO treatment (Park et al., 2018). Thus we examined the effect of RA, a widely known high affinity, endogenous ligand of Crabp1, on cardiac function using 2-D-guided M mode echocardiography. As we reported earlier, we consistently found that CKO mice exhibit significantly reduced systolic performance based upon ejection fraction (EF) in unmanipulated (control) conditions (Fig. 1A). Importantly, ISO treatment (30 mg/kg/d) for 7 d significantly lowered EF in both genotypes (from 67.4 ± 7.6% to 51.8 ± 8.5% in WT, from 57.0 ± 5.6% to 45.1 ± 5.5% in CKO), which could be recovered by RA pre-treatment (5 mg/kg/d) only in WT (to 66.9 ± 10.9%), but not CKO, mice (to 44.0 ± 3.9%). Fractional shortening (FS) (Fig. 1B) was also reduced in both genotypes, but recovered only in WT mice. The improvement of cardiac function elicited by RA pre-treatment in WT is indicated by the diminution of ventricular dilation both during systole (3.12 ± 0.29 mm ISO to 2.33 ± 0.68 mm RA/ISO) and diastole (4.25 ± 0.3 mm ISO to 3.65 ± 0.44 mm RA/ISO) (Fig. 1C and D). These series of echocardiographic evaluations of WT and CKO mice show that RA exerts a cardiac protective effect, which requires Crabp1.
Fig. 1.
RA prevents cardiac dysfunction in WT, but not CKO, mice induced by β-adrenergic stimulation. M-mode echocardiography was performed on the WT and CKO mice pre-treated with RA and then ISO for 7 d at the age of 16-24 weeks (n = 5-10). Data were expressed as means ± SDs. Ejection fraction (EF, panel A) and fractional shortening (FS, panel B) represent cardiac function. Left ventricular end-systolic dimension (LVESD, panel C) and left ventricular end-diastolic dimension (LVEDD, panel D) represent internal diameters of left ventricle. *P < 0.01, **P < 0.05, ***P < 0.001 (two-way ANOVA, Student-Newman-Keuls method).
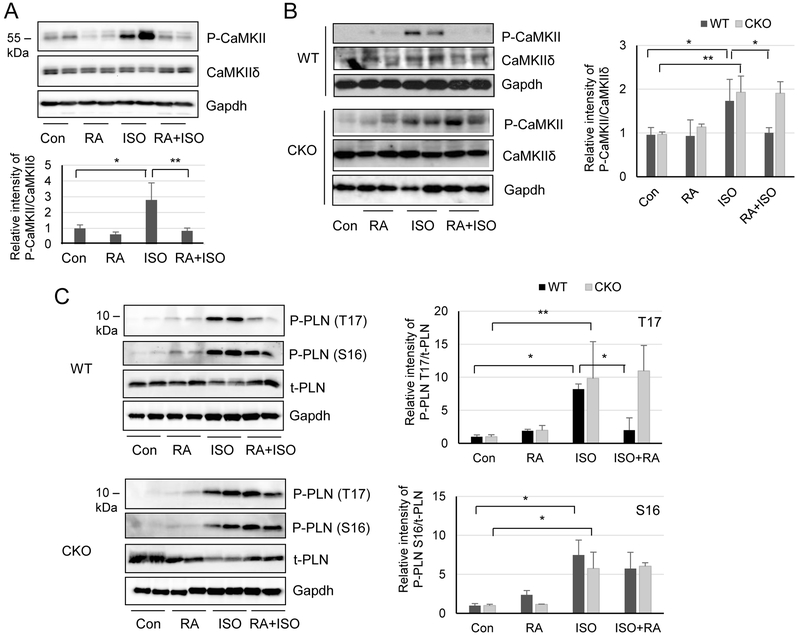
We then investigated whether RA modulated ISO-induced CaMKII autophosphorylation and activity through Crabp1. RA is known to elicit various biological activities by primarily binding to nuclear RA receptors (RARs) to regulate gene expression, which usually takes a longer period of time (Benbrook et al., 2014). We first monitored the effect of RA on acute (30 min) ISO-induced CaMKII activation. Fig. 2A shows that 4h pre-treatment with RA in mice effectively abolished acute (30 min) ISO induction of CaMKII autophosphorylation at T287 (compare ISO and RA/ISO lanes). We then further monitored the effect on CaMKII activation following a longer exposure to ISO (24 h) in both WT and CKO mice (Fig. 2B). ISO dramatically elevated the level of CaMKII autophosphorylation in both genotypes. Importantly, pretreatment with RA effectively blocks this CaMKII phosphorylation in WT (upper panels), but not CKO mice (lower panels), supporting a role for Crabp1 in this presumed non-canonical effect of RA. In the control treatment with RA alone, CaMKII seems to be slightly, but negligibly, activated. Finally, we monitored the effect on CaMKII activation, assessed by the phosphorylation of its substrate phospholamban (PLN), following an extended ISO treatment (7 days). As shown in Fig. 2C, ISO induces phospholamban (PLN) phosphorylation at both T17 and S16, the target sites of CaMKII and protein kinase A, respectively (Kranias and Hajjar, 2012) in both WT and CKO mice. But RA pre-treatment dampens phosphorylation at T17 (CaMKII target site) only in WT (upper panels, compare ISO and RA/ISO lanes), but not CKO mice (lower panels, compare ISO and RA/ISO lanes). Furthermore, RA itself had little effect on phosphorylation at S16 (PKA target site). These results, together, show that, RA can suppress β-adrenergic challenges specific to CaMKII activation and Crabp1 is involved in this suppressive activity.
Fig. 2.
RA inhibits CaMKII autophosphorylation and PLN phosphorylation at T17 in WT, but not CKO, mice. (A) Western blots detecting CaMKII phosphorylation in acute ISO treatment (30 min). WT mice were pretreated with RA for 4h (RA) and followed by ISO treatment for 30 min (RA+ISO). Controls include control vehicle (Con), RA alone (RA) and ISO alone (ISO). *P < 0.05 and **P < 0.01 (n=4, student t-test). (B) Western blots detecting CaMKII phosphorylation in 1 day ISO treatment. Experimental groups are as in A. Densitometric analysis is shown on the right panel. *P < 0.01 and **P < 0.01 (n=4-10, two-way ANOVA). (C) Western blot detecting the effect on phosphorylation of PLN at T17 (CaMKII target) and S16 (PKA target) in 7 d ISO treatment. RA dampens PLN phosphorylation at T17 (not S16) in WT, but not in CKO, mice. t-PLN indicates total PLN loading control. Densitometric analyses are shown on the right panel (upper panel for P-PLN T17 and lower panel for P-PLN S16). *P < 0.05 and **P < 0.01 (n=4, two-way ANOVA).
3.2. RA attenuates ISO-induced apoptosis in WT but not CKO heart
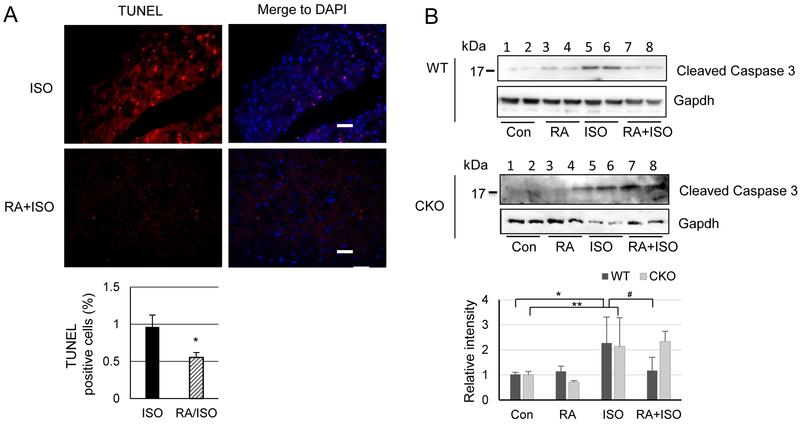
Excessive β-adrenergic stimulation has been shown to trigger ventricular cell death (Feng and Anderson, 2017). Our previous report of CKO mice has revealed that deleting Crabp1 aggravates cell death in the heart (Park et al., 2018). We then used TUNEL assay to detect whether RA could protect mice against ISO-triggered cell death in the heart. As shown in Fig. 3A, RA indeed significantly decreases ISO-induced cell death (0.96 ± 0.34% to 0.55 ± 0.15%) only in WT mice. We then compared apoptosis in WT and CKO mice (2 mice examined for each group) by assessing caspase 3 cleavage as shown in Fig. 3B. Consistent results were obtained by using two different caspase 3 antibodies. The result show that ISO treatment increases the cleavage of caspase 3 in both genotypes (lanes 5 & 6), but RA pre-treatment decreases apoptotic caspase 3 cleavage only in WT (upper panel). The decreased apoptosis in WT mice is evident because the cleaved caspase 3 signal intensity in the RA/ISO group (lanes 7 and 8) is apparently weaker than that in the ISO group (lanes 5 & 6). On the contrary, for CKO mice (lower panel), the cleaved caspase 3 signal intensity in the RA/ISO group is comparable to that in the ISO group, further supporting the protective effect of RA through Crabp1.
Fig. 3.
RA attenuates ISO-induced apoptosis in WT mice. (A) Tissue sections from WT mice (n = 3) were stained for TUNEL analysis and DAPI. Bottom graph shows statistical analyses of apoptotic cells from multiple random fields. P < 0.05, RA+ISO vs. ISO (Student’s t-test). (B) Caspase 3 cleavage assay. WT and CKO mice were pre-treated with RA prior to ISO treatment for 24 h (RA+ISO). Controls include vehicle (con), RA alone (RA) and ISO alone (ISO). Con and RA treatment have little effect on caspase cleavage (Cleaved Caspase 3). ISO treatment increases the cleavage in both genotypes, but RA pretreatment (RA+ISO) decreases apoptotic cleavage only in WT (upper panel), but not CKO (lower panel), mice. Statistical analyses are shown at the bottom. *P < 0.01 and **P < 0.05 (n=6, two-way ANOVA).
3.3. RA-Crabp1 inhibits CaMKII
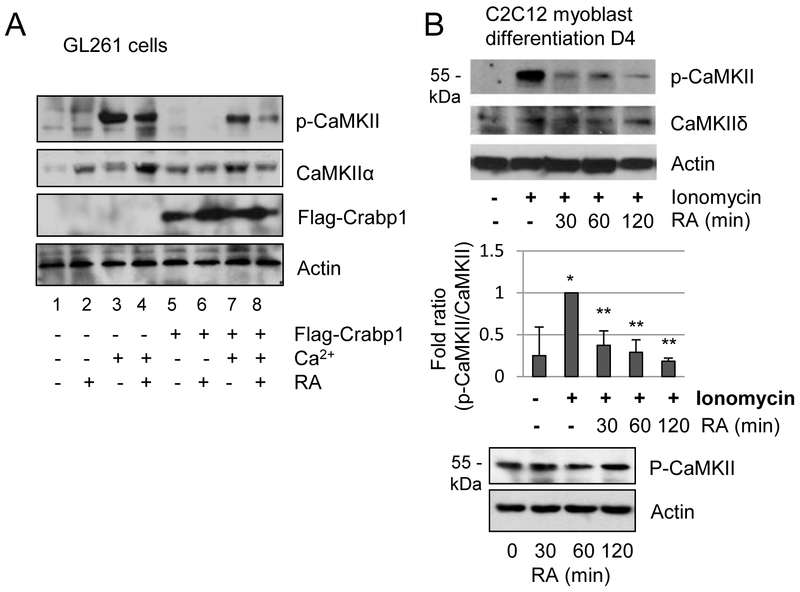
The above results show that RA can help to preserve cardiac function, protecting the heart from ISO-induced apoptotic cell death, which is correlated with dampened CaMKII activation. However, this occurs only in WT, but not CKO, mice. These results prompted further examination of the effect of RA-Crabp1 on CaMKII activity. We first conducted a gain-of-function experiment, by examining CaMKII phosphorylation in the GL261 glioma cell line (Ausman et al., 1970). Crabp1 expression is absent in GL261, but can be reconstituted by ectopic Crabp1 expression.. This is followed by pre-treatment with RA and then stimulation with Ca2+. As shown in Fig. 4A, gain of Crabp1 effectively impairs CaMKII phosphorylation after Ca2+ stimulation (compare 3 and 7 lanes), and that RA further decreases its phosphorylation (compare 7 and 8 lanes). We then used C2C12 myoblasts, in which differentiation is known to induce endogenous Crabp1 expression (Supplementary Fig. S1). In this 4-day myocyte differentiation model, Ionomycin, a Ca2+ ionophore, strongly induces CaMKII phosphorylation as expected (Fig. 4B,). Importantly, pre-treatment with RA also significantly blocks CaMKII phosphorylation (top panel). For a control, pre-treatment with RA alone in this model appears to have little effect on CaMKII activation (lower panel).
Fig. 4.
RA inhibits CaMKII autophosphorylation through Crabp1. (A) GL261 glioma cells, where Crabp1 is not expressed, were transfected with Flag-Crabp1 or empty vector and pre-treated with RA (0.1 μM) for 30 min prior to Ca2+ stimulation for 15 min. (B) Differentiated C2C12 cells were pre-treated with RA for the indicated time prior to 2 μM ionomycin stimulation for 15 min. Statistical analyses are shown in the middle. *P < 0.05 vs. control vehicle and **P < 0.05 vs. ionomycin only (n=3, student t-test). Lower panels show the control, RA alone has negligible effect on CaMKII.
3.4. RA enhances Crabp1 interaction with CaMKII at the regulatory domain
We further examined the molecular relationship of Crabp1 with CaMKII, and whether RA has an effect on this relationship. We previously found that Crabp1 directly interacts with CaMKII (Park et al., 2018). Here, the in vitro protein interaction assay using Flag-CaMKII generated from TnT reactions (Promega) and purified recombinant His-Crabp1 clearly indicates that RA indeed enhances Crabp1 interaction with CaMKII (Fig. 5A). We then dissected CaMKII into a series of fragments, including the kinase (K), regulatory (R) and association (A) domains (Fig. 5B). Fig. 5C shows in vitro interaction assay results that, Crabp1 interacts with the full length (FL), the contiguous kinase-regulatory fragment (KR), the kinase domain (K), and the regulatory domain (R) (interacting bands are marked with * signs), but not the association domain (A). A competition assay (Fig. 4D and 4E) using a small CaMKII peptide spanning amino acids 281-309 (located in the R domain) (Sigma) demonstrates that this CaMKII peptide notably competes with the KR (Left of Fig. 4D) and R (right of Fig. 4D) fragments, but not the K fragment (Fig. 4E) for interaction with Crabp1 in the presence of RA. These results reveal that Crabp1 interacts with the kinase and regulatory domains of CaMKII, and that residues 291-309 of the CaMKII R domain may play a role in this interaction with Crabp1. Furthermore, RA selectively enhances Crabp1 interaction with the regulatory domain of CaMKII.
4. Discussion
Excessive and/or persistent β-adrenergic stimulation is observed in various heart diseases such as arrhythmia and heart failure. Thus, β-adrenergic blockers are one of the standard therapeutics for heart failure. Specifically, β-adrenergic stimulation activates PKA and, indirectly, CaMKII which targets Ca2+ handling proteins. Although CaMKII inhibition and deletion has relatively little effect on the acute physiological contractile response to β-adrenergic stimulation, it is known that CaMKII over-activation is responsible for pathological processes such as arrhythmogenesis, cardiomyocyte death, fibrosis and hypertrophy (Beckendorf et al., 2018; Grimm and Brown, 2010). Therefore, targeting CaMKII (inhibition) is receiving more attention as an alternative therapeutic strategy for managing heart pathologies (Cuello and Lorenz, 2016; Kreusser et al., 2016). We have previously shown that Crabp1 deletion resulted in hyper-activation of CaMKII, especially in the condition of β-adrenergic stimulation, which suggested a protective role for Crabp1 in reducing maladaptive cardiac remodeling (Park et al., 2018). Since RA is known to bind to Crabp1 in the cytoplasm, predictably, RA can be protective, via Crabp1-binding, to dampen CaMKII hyper-activation in certain pathological conditions.
The current study demonstrates that RA indeed inhibits CaMKII activation, which requires Crabp1, in vivo. Crabp1 deletion in mice results in a phenotype of dilated cardiomyopathy that does not yet develop apparent disease symptoms; however, it causes more dilated ventricular dimensions (both at systole and diastole) and further reduces cardiac function (EF and FS) under the condition of ISO challenge. Since Crabp1 deletion abolishes the effect of RA suppression of CaMKII autophosphorylation and its activity, as assessed by PLN phosphorylation at T17 in vivo, it is tempting to speculate that Crabp1 can be an endogenous regulator of CaMKII, through which RA can exert a new non-genomic, or non RA receptor (RAR)-mediated, activity. However, this CKO model is a whole-body knockout of the Crabp1 gene, possibilities remain that Crabp1 may also affect other target molecules and/or tissues besides CaMKII and the heart, which could contribute to this dampening effect on β-adrenergic stimulation. Future studies are needed to examine these possibilities.
CaMKII is auto-activated by multiple post-translational modifications such as phosphorylation at T287, oxidation at M281/M2 (Erickson et al., 2008), O-GlcNAcylation at S280 (Erickson, 2014) and S-nitrosylation at C290 (Gutierrez et al., 2013) under physiological or pathological conditions. In the inactive state, the regulatory domain of CaMKII is superposed on the kinase domain until Ca2+/CaM binds to the regulatory domain (aa 304-312) and initiates conformational change that exposes the kinase domain. Initial activation by Ca2+/CaM induces autophosphorylation at the T287 which is a signature modification of autonomous activation even when CaM leaves and [Ca2+] drops (Beckendorf et al., 2018; Lai et al., 1987). Our data that RA inhibits CaMKII autophosphorylation only in the presence of Crabp1 in vitro and in vivo clearly demonstrate that CaMKII-dampening effect of RA requires Crabp1. It is interesting that although Crabp1 can directly bind to the kinase domain and the regulatory domain, RA seems to enhance Crabp1 binding only to the regulatory domain, suggesting that RA/Crabp1 somehow specifically targets the regulation of CaMKII. Since CaMKII regulation involves extremely complicated multiple mechanisms, which could not be unambiguously determined by a simple in vitro kinase assay, the detailed molecular mechanism underlying the effect of Crabp1-RA remains to be carefully determined in future studies.
CaMKII is known to interact with multiple proteins and molecules. Coenzyme A generated during metabolism binds to the regulatory domain without Ca2+ and activates CaMKII (McCoy et al., 2013). N-Methyl-D-aspartate (NMDA) receptor subunit N2B binds to the T-site of CaMKII to inhibit dephosphorylation at T286, an important mechanism for the induction of long-term potentiation (Mayadevi et al., 2016; Shen and Meyer, 1999). GTPase Rem2 directly interacts with the association domain and substrate domain of CaMKII and inhibits the kinase (Royer et al., 2018). Previously, a natural inhibitor protein (CaMKIINα) was found to bind to the T-site of CaMKII in brain and has been developed as a peptide inhibitor known as CaMKIINtide (Chang et al., 1998; Gomez-Monterrey et al., 2013). CaMKII peptide and its small molecule inhibitors are used only in the laboratory as an experimental tool due to their potential “off-of-target” side-effects as a therapeutic. We demonstrate here that Crabp1 is an endogenous regulator (inhibitor) of CaMKII, which can be enhanced by its natural high-affinity ligand, RA. To this end, RA has been reported to have cardioprotective effects against cardiac remodeling in adult animals (Azevedo et al., 2010; Minicucci et al., 2010; Paiva et al., 2005; Palm-Leis et al., 2004). Although nuclear RARs play important roles, particularly in cardiac development (Pan and Baker, 2007), our current study suggests that the cardioprotective effect of RA in adult hearts can also involve its non-RAR-mediated signaling through Crabp1. As such, Crabp1, as an endogenous regulator of CaMKII activity, can also serve as a new therapeutic target to modulate CaMKII activity in managing heart pathologies. Furthermore, the RA-enhanced, Crabp1-mediated regulation of CaMKII, as observed in this study, provides insight for the development of retinoid-based therapeutics for these pathologies.
5. Conclusions
Crabp1 deletion is associated with reduced cardiac function in isoproterenol, a β-adrenoceptor agonist, induced condition, which involves the modulation of CaMKII activation. RA can restore cardiac function through, at least partially, Crabp1. This Crabp1-mediated protective effect of RA is attributed to the dampening of CaMKII autophosphorylation.
Supplementary Material
Acknowledgements
This study was supported by NIH grants DK54733, DK60521, and the Dean’s Commitment and the Distinguished McKnight Professorship of University of Minnesota to LNW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ausman JI, Shapiro WR, Rall DP, 1970. Studies on the Chemotherapy of Experimental Brain Tumors: Development of an Experimental Model. Cancer Res. 30, 2394–2400. [PubMed] [Google Scholar]
- Azevedo PS, Minicucci MF, Chiuso-Minicucci F, Justulin LA Jr, Matsubara LS, Matsubara BB, Novelli E, Seiva F, Ebaid G, Campana AO, Zornoff LAM, Paiva SAR, 2010. Ventricular Remodeling Induced by Tissue Vitamin A Deficiency in Rats. Cell. Physiol. Biochem. 26, 395–402. 10.1159/000320563 [DOI] [PubMed] [Google Scholar]
- Beckendorf J, van den Hoogenhof MMG, Backs J, 2018. Physiological and unappreciated roles of CaMKII in the heart. Basic Res. Cardiol. 10.1007/s00395-018-0688-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrook DM, Chambon P, Rochette-Egly C, Asson-Batres MA, 2014. History of retinoic acid receptors. Subcell. Biochem. 70, 1–20. 10.1007/978-94-017-9050-5_1 [DOI] [PubMed] [Google Scholar]
- Chang BH, Mukherji S, Soderling TR, 1998. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc. Natl. Acad. Sci. U. S. A. 95, 10890–5. 10.1073/pnas.95.18.10890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary R, Palm-Leis A, Scott RC, Guleria RS, Rachut, EricPan J, Baker KM, Pan J, 2007. All-trans retinoic acid prevents development of cardiac remodeling in aortic banded rats by inhibiting the renin-angiotensin system. Am. J. Physiol. Circ. Physiol. 294, H633–H644. 10.1152/ajpheart.01301.2007 [DOI] [PubMed] [Google Scholar]
- Coultrap SJ, Barcomb K, Bayer KU, 2012. A significant but rather mild contribution of T286 Autophosphorylation to Ca2+/CaM-stimulated CaMKII activity. PLoS One 7, e37176 10.1371/journal.pone.0037176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello F, Lorenz K, 2016. Inhibition of cardiac CaMKII to cure heart failure: step by step towards translation? Basic Res. Cardiol. 111, 66 10.1007/s00395-016-0582-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn DRH, Oerlemans F, Hendriks W, Baats E, Ploemacher R, Wieringa B, van Kessel AG, 1995. Normal development, growth and reproduction in cellular retinoic acid binding protein-I (CRABPI) null mutant mice. Differentiation 58, 141–148. 10.1046/j.1432-0436.1995.5820141.x [DOI] [PubMed] [Google Scholar]
- Erickson JR, 2014. Mechanisms of CaMKII activation in the heart. Front. Pharmacol 10.3389/fphar.2014.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JR, Joiner MA, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham A-JL, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME, 2008. A Dynamic Pathway for Calcium-Independent Activation of CaMKII by Methionine Oxidation. Cell 133, 462–474. 10.1016/j.cell.2008.02.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng N, Anderson ME, 2017. CaMKII is a nodal signal for multiple programmed cell death pathways in heart. J. Mol. Cell. Cardiol. 103, 102–109. 10.1016/j.yjmcc.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorella PD, Giguère V, Napoli JL, 1993. Expression of cellular retinoic acid-binding protein (type II) in Escherichia coli: Characterization and comparison to cellular retinoic acid-binding protein (type I). J. Biol. Chem. 268, 21545–21552. [PubMed] [Google Scholar]
- Gomez-Monterrey I, Sala M, Rusciano MR, Monaco S, Maione AS, Iaccarino G, Tortorella P, D’Ursi AM, Scrima M, Carotenuto A, De Rosa G, Bertamino A, Vernieri E, Grieco P, Novellino E, Illario M, Campiglia P, 2013. Characterization of a selective CaMKII peptide inhibitor. Eur. J. Med. Chem. 62, 425–434. 10.1016/j.ejmech.2012.12.053 [DOI] [PubMed] [Google Scholar]
- Gorry P, Lufkin T, Dierich A, Rochette-Egly C, Décimo D, Dollé P, Mark M, Durand B, Chambon P, 1994. The cellular retinoic acid binding protein I is dispensable. Proc. Natl. Acad. Sci. U. S. A. 91, 9032–6. 10.1073/pnas.91.19.9032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm M, Brown JH, 2010. β-Adrenergic receptor signaling in the heart: Role of CaMKII. J. Mol. Cell. Cardiol. 10.1016/j.yjmcc.2009.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez DA, Fernandez-Tenorio M, Ogrodnik J, Niggli E, 2013. NO-dependent Ca MKII activation during β-adrenergic stimulation of cardiac muscle. Cardiovasc. Res. 100, 392–401. 10.1093/cvr/cvt201 [DOI] [PubMed] [Google Scholar]
- Hudmon A, Schulman H, 2002. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem. J. 364, 593–611. 10.1042/BJ20020228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JX, Leaf A, 1996. Protective effects of free polyunsaturated fatty acids on arrhythmias induced by lysophosphatidylcholine or palmitoylcarnitine in neonatal rat cardiac myocytes. Eur. J. Pharmacol. 297, 97–106. 10.1016/0014-2999(95)00701-6 [DOI] [PubMed] [Google Scholar]
- Kranias EG, Hajjar RJ, 2012. Modulation of cardiac contractility by the phopholamban/SERCA2a regulatome. Circ. Res. 110, 1646–1660. 10.1161/CIRCRESAHA.111.259754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreusser MM, Lehmann LH, Wolf N, Keranov S, Jungmann A, Gröne HJ, Müller OJ, Katus HA, Backs J, 2016. Inducible cardiomyocyte-specific deletion of CaM kinase II protects from pressure overload-induced heart failure. Basic Res. Cardiol. 111, 65 10.1007/s00395-016-0581-2 [DOI] [PubMed] [Google Scholar]
- Lai Y, Nairn AC, Gorelick F, Greengard P, 1987. Ca2+/calmodulin-dependent protein kinase II: identification of autophosphorylation sites responsible for generation of Ca2+/calmodulin-independence. Proc. Natl. Acad. Sci. 84, 5710–5714. 10.1073/pnas.84.16.5710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayadevi M, Lakshmi K, Suma Priya SD, John S, Omkumar RV, 2016. Protection of α-CaMKII from dephosphorylation by GluN2B subunit of NMDA receptor is abolished by mutation of Glu96 or His282 of α-CaMKII. PLoS One 11, e0162011 10.1371/journal.pone.0162011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy F, Darbandi R, Lee HC, Bharatham K, Moldoveanu T, Grace CR, Dodd K, Lin W, Chen S-I, Tangallapally RP, Kurokawa M, Lee RE, Shelat AA, Chen T, Green DR, Harris RA, Lin S-H, Fissore RA, Colbran RJ, Nutt LK, 2013. Metabolic activation of CaMKII by coenzyme A. Mol. Cell 52, 325–39. 10.1016/j.molcel.2013.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minicucci MF, Azevedo PS, Oliveira SA, Martinez PF, Chiuso-Minicucci F, Polegato BF, Justulin LA, Matsubara LS, Matsubara BB, Paiva SAR, Zornoff LAM, 2010. Tissue vitamin A insufficiency results in adverse ventricular remodeling after experimental myocardial infarction. Cell. Physiol. Biochem. 26, 523–530. 10.1159/000322320 [DOI] [PubMed] [Google Scholar]
- Paiva SAR, Matsubara LS, Matsubara BB, Minicucci MF, Azevedo PS, Campana AO, Zornoff LAM, 2005. Retinoic acid supplementation attenuates ventricular remodeling after myocardial infarction in rats. J. Nutr. 135, 2326–8. 10.1093/jn/135.10.2326 [DOI] [PubMed] [Google Scholar]
- Palm-Leis A, Singh US, Baker KM, Pan J, Olsovsky GD, Herbelin BS, 2004. Mitogen-activated Protein Kinases and Mitogen-activated Protein Kinase Phosphatases Mediate the Inhibitory Effects of All- trans Retinoic Acid on the Hypertrophic Growth of Cardiomyocytes. J. Biol. Chem. 279, 54905–54917. 10.1074/jbc.m407383200 [DOI] [PubMed] [Google Scholar]
- Pan J, Baker KM, 2007. Retinoic Acid and the Heart. Vitam. Horm 10.1016/S0083-6729(06)75010-5 [DOI] [PubMed] [Google Scholar]
- Park SW, Persaud SD, Ogokeh S, Meyers TA, Townsend DW, Wei LN, 2018. CRABP1 protects the heart from isoproterenol-induced acute and chronic remodeling. J. Endocrinol. 236, 151–165. 10.1530/JOE-17-0613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicena P, Schulman H, 2014. CaMKII inhibitors: From research tools to therapeutic agents. Front. Pharmacol. 10.3389/fphar.2014.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer L, Marr MT, Kenny K, Tzvetkova B, Cochrane JC, Herzog JJ, Paradis S, 2018. The Ras-like GTPase Rem2 is a potent inhibitor of calcium/calmodulin-dependent kinase II activity. J. Biol. Chem. 293, 14798–14811. 10.1074/jbc.ra118.003560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Meyer T, 1999. Dynamic control of caMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science (80-. ). 284, 162–166. 10.1126/science.284.5411.162 [DOI] [PubMed] [Google Scholar]
- Strack S, Barban MA, Wadzinski BE, Colbran RJ, 1997. Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. J. Neurochem. 68, 2119–28. 10.1046/j.1471-4159.1997.68052119.x [DOI] [PubMed] [Google Scholar]
- Wang H-J, Zhu Y-C, Yao T, 2002. Effects of all-trans retinoic acid on angiotensin II-induced myocyte hypertrophy. J. Appl. Physiol. 92, 2162–8. 10.1152/japplphysiol.01192.2001 [DOI] [PubMed] [Google Scholar]
- Woo AYH, Xiao RP, 2012. β-Adrenergic receptor subtype signaling in heart: From bench to bedside. Acta Pharmacol. Sin. 10.1038/aps.2011.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.