Summary.
Amyloid beta (Aβ) peptides have been implicated in both Alzheimer’s disease (AD) and glaucoma and have been shown to be the key etiological factor in these dangerous health complications. On the other hand, it is well known that Aβ peptide can be generated from its precursor protein and massively released from the blood to nearby tissue upon the activation of platelets due to their involvement in innate immunity and inflammation processes. Here we review evidence about the development of AD and glaucoma neuronal damage showing their dependence on platelet count and activation. The correlation between the effect on platelet count and the effectiveness of anti-AD and anti-glaucoma therapies suggest that platelets may be an important player in these diseases.
Keywords: Beta-amyloid peptide, Alzheimer’s, Glaucoma, Platelets
Introduction
Amyloid beta (Aβ) peptides are 36–43 amino acids in length, have a specific sequence that is slightly different between mammalian species (GenScript database), and are produced in many cell types by cleavage of the longer amyloid precursor protein (APP). Due to hydrogen bonding between the peptide bonds of parallel monomers, Aβ forms dimeric or tetrameric oligomers, even at very low concentrations, while in higher concentrations it associates into β-pleated sheets, tending to join in misfolded aggregations known as amyloid plaques (Lomakin et al., 1997; Tjernberg et al., 1999). Mutations within Aβ and its precursor affect this aggregation, which is the basis of familial early-onset diseases (reviewed and studied in Hatami et al., 2017). A common factor in a number of health problems is the accumulation of Aβ in tissues, including different cancerous tissues (Hansel et al., 2003; Jin et al., 2017), the zone of traumatic brain injury (Johnson et al., 2010), skeletal muscles in special cases of myositis (Askanas et al, 1992), myocardium with diastolic dysfunction (Gianni et al., 2010), and the placenta during preeclampsia (Buhimschi et al., 2014). Aβ peptides also accumulate and are thought to be important players in two other well-known health problems, Alzheimer’s disease (AD) and glaucoma. Both of these diseases have hereditary forms, usually developing as an early onset, and sporadic forms, usually affecting elderly subjects. In this review, we concentrate on these two sporadic diseases and the evidence for a common systemic source of Aβ, which accumulates in and damages tissues during the course of these diseases, and its relation to platelets.
Alzheimer’s disease (AD)
This disease is a slowly developing progressive dementia, with extensive damage to neurons in the brain and their connections (reviewed in: Zott et al., 2018) that is accompanied by reactive astrogliosis and even astroglial atrophy (Verkhratsky and Rodríguez, 2018). It has been shown that the first signs of change in the AD brain are in the olfactory bulb, suggesting that the olfactory tracts provide a portal of entry to the brain for any pathogenic agent(s) that might be responsible for induction of the disease (Ohm and Braak, 1987; Mann et al., 1988). Next, damage to the entorhinal cortex follows, which is known to convey olfactory information and is a major input and output structure of the hippocampus. The hippocampus, in turn, is affected in the next stage of the disease, which is characterized by disorientation in space and memory problems. Damage then spreads to other cortical parts, leading to destruction of virtually all isocortical association areas and severe cognitive impairment (Braak and Braak, 1991). Microscopically, the AD-affected brain is characterized by extracellular amyloid-like senile plaques and intracellular neurofibrillary tangles. Since the time that beta-amyloid (Aβ) peptides were found to be the major constituent of amyloid cerebrovascular senile plaques described by Dr. Alois Alzheimer in the brain of dementia patients, these peptides became associated with the development of AD (Glenner and Wong, 1984). The Aβ hypothesis was formulated, suggesting that an imbalance between production and clearance of Aβ (Aβ dyshomeostasis) is an early, often initiating factor in AD (Selkoe and Hardy, 2016). However, it was discovered that Aβ plaques were sometimes present in cognitively normal individuals, while neuronal death also occurred in brain regions devoid of plaques (Sloane et al., 1997). It then became clear that only oligomers of Aβ peptides are toxic to brain cells and that there is no direct correlation between the manifestation of the disease and plaque burden (reviewed in: Sengupta et al., 2016). The most common view is that increased concentrations of Aβ oligomers trigger neuronal dysfunction and network alterations, with secondary damage produced by hyperphosphorylated tau protein aggregated in tangles (reviewed in: Jeong, 2017; Mroczko et al., 2018).
Glaucoma
This disease is a slowly developing, progressive blindness. In many cases glaucoma involves elevated intraocular pressure (IOP) that precedes damage to the optic nerve and vision loss. Disproportionate IOP develops due to a constraint on the outflow of liquid from the eye, in some cases due to vein occlusion (Săninoiu, 1992; Călugăru and Călugăru, 2001). This leads to accumulation of excessive aqueous humor, especially if anatomical problems have complicated the alternative escape (drainage) of the aqueous humor through the trabecular meshwork. Due to disrupted hemodynamics there are also clearly visible anatomical changes in the eye, termed cupping, in the zone of the entrance of blood vessels and the optic nerve to the retina, and the level of damage in this zone is the hallmark of glaucoma. The common view is that IOP at first harms the optic nerve vasculature, leading to optic nerve damage, or that it directly causes damage to the optic nerve, which consists of the axons of retinal ganglion cells (RGC), affecting also the soma of these cells, and finally leads to blindness (Mann et al., 2019). It is also known that many glaucoma cases exhibit only moderate or even normal intraocular pressure.
It is still a matter of debate which factors in glaucoma induce damage to retinal cells, but Aβ is evidently involved. It was shown that Aβ accumulates in the retina, with the highest concentration colocalizing with the layer of apoptotic retinal ganglion cells in experimental glaucoma, while application of synthetic Aβ induces significant RGC apoptosis in vivo in a dose-and time-dependent manner. Anti-Aβ treatment was effective in prevention of RGC apoptosis in glaucoma patients (Yoneda et al., 2005; Guo et al., 2007; Ning et al., 2008, Ito et al., 2012; see also reviews: Sivak, 2013; Ratnayaka et al., 2015). An increased risk of developing AD was established for persons diagnosed with glaucoma and certain other ophthalmic conditions (Lee et al., 2019). All these results suggest common pathological mechanisms in glaucoma and AD.
The association of AD and glaucoma with inflammation and infection
Glaucoma is often initiated by inflammation, and there is a 50% coincidence of glaucoma with chronic uveitis, which can be induced by an autoimmune reaction or a reaction caused by infection (Netland and Denton, 2006; also reviewed in: Bodh et al., 2011). The most common eye infections correlated with glaucoma are caused by herpes simplex virus or varicella zoster virus (Miserocchi et al., 2002). In herpetic anterior uveitis 75% of patients develop elevated IOP (Hoeksema et al., 2017). Various periodontal bacteria may also cause the development of glaucoma (Astafurov et al., 2014). The cause of inflammation leading to glaucoma can be chemical- or laser-induced trauma (Ştefan et al., 2016) or simple mechanical trauma (Markovic et al., 2017). Thus, in many cases glaucoma is a response to inflammation with or without infection.
Similarly, AD pathological changes clearly correspond to regions with inflammation (Akiyama et al., 2000). Inflammatory signaling cytokines, especially the expression and signaling of the cytokines interleukin 12 and interleukin 23, can affect the cerebral amyloid load and the development of AD (Vom Berg et al., 2012; Griffin, 2013; Wilcock and Griffin, 2013). Brain inflammation also develops after trauma (Corps et al., 2015). Interestingly, it was shown that temporary Aβ plaques appeared in the brain of an AD mouse model after mild brain trauma and then disappeared after 7 days, which was correlated with the post-traumatic concentration of soluble Aβ oligomers in the brain (Washington et al., 2014). Aβ plaques and oligomers may also be found in the brains of human patients within hours of traumatic brain injury (TBI) in non-AD patients (Roberts et al., 1991; Ikonomovic et al., 2004; also reviewed in: Johnson et al., 2010). Like in glaucoma, there are significant associations between AD and various pathogens, including Herpes simplex virus type 1 (HSV-1), cytomegalovirus, other Herpesviridae, Chlamydophila pneumoniae, spirochetes, Helicobacter pylori, and various periodontal pathogens (Harris and Harris, 2015, 2018; Maheshwari and Eslick, 2015). Recently, the gram-negative periodontal pathogen Porphyromonas gingivalis, which causes chronic periodontitis, was also identified in the brain of AD patients (Dominy et al., 2019), while previously this pathogen was found to cause brain inflammation, neurodegeneration, and Aβ production in a wild type mouse model (Ilievski et al., 2018). Genes upregulated in the AD brain match those upregulated by multiple bacteria, viruses, fungi, or protozoa in immunocompetent cells (Carter, 2017). Lyme spirochetes are also suspected of causing AD (Eimer et al., 2018). Recently, the evidence for human transmission of Aβ pathology after the treatment of children with human cadaver-derived growth hormone was reported, and it was suggested that this was an example of the prion-like spread of AD (Jaunmuktane et al., 2015), although it could be the spread of a septic agent provoking AD development. All these results suggest that Aβ pathology in AD is, in many cases, a response to inflammation and is sometimes a result of infection, which raises the question of why the buildup of Aβ in both glaucoma and AD is correlated with these processes.
Aβ as a defense peptide
It has been shown that Aβ peptide has strong antibiotic activity against both Gram-negative and Gram-positive bacteria as well as fungi and viruses (Lukiw et al., 2010; Soscia et al., 2010; Bourgade et al., 2014; White et al., 2014). Aβ peptide also protects mice against microbial infection in in vivo experiments (Kumar et al., 2016). It has been suggested that Aβ is a previously unrecognized antimicrobial agent that may normally function in the innate immune system (Soscia et al., 2010; Kumar et al., 2016; Kucheryavykh et al., 2017; Inyushin et al., 2017; Gosztyla et al., 2018). We have shown that during thrombosis in skin blood vessels Aβ peptides are generated and released in the nearby skin tissue, functioning as an antibiotic against infection (Kucheryavykh et al., 2018). The appearance of Aβ peptide in the tissues during thrombosis suggests that it has an important function in the platelet defense arsenal. It has been shown that Aβ peptide oligomers aggregated into fibrils entrap microbes (Kumar et al., 2016) or can bind herpesvirus surface glycoproteins, accelerating Aβ deposition and leading to protective viral entrapment (Eimer et al. 2018). Additionally, we suggest that an antimicrobial effect can be achieved by the pore-forming properties of Aβ peptide (Inyushin et al., 2017; Kucheryavykh et al., 2017, 2018). It was shown that soluble Aβ peptide oligomers at low concentrations (number) perforate cell membranes by forming tetrameric channels penetrable by K+ ions and do so at higher concentrations by forming Ca++-permeable hexameric pores (Kawahara et al., 1997; Lin et al., 2001; Lal et al., 2007). An excess of Ca++ permeability through these pores induces calcium dyshomeostasis and is extremely toxic (Kawahara, 2010; Sepulveda et al, 2010). We have shown that synthetic Aβ peptide perforates the external membrane of yeast (Kucheryavykh et al., 2018), and it is known that natural peptide antibiotics with channel-forming activity kill target cells by this same mechanism (Harder et al., 1997; Hancock and Chapple, 1999). Thus, Aβ may be released as a response to infection (Inyushin et al., 2017; Eimer et al. 2018), and this release is likely triggered by tissue damage and inflammation (Kucheryavykh et al., 2018).
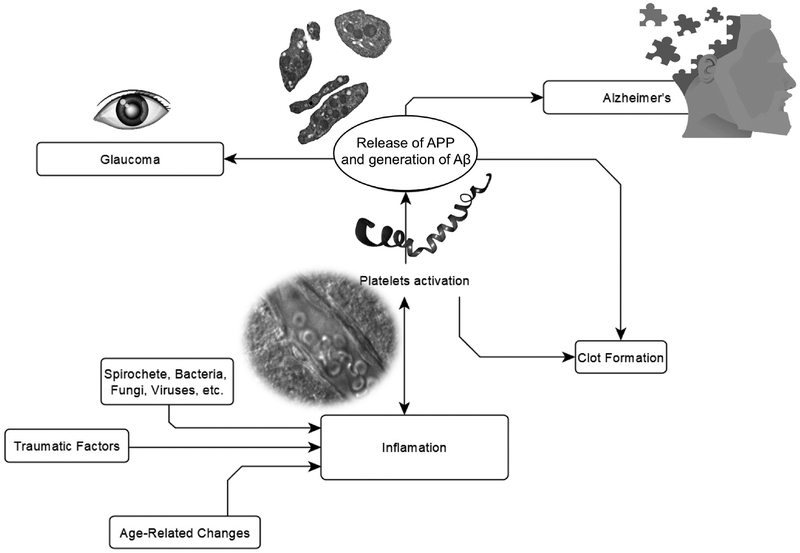
Platelets are a source of systemic APP and Aβ
Aβ peptides are released by different types of cells. In the brain the most established sources of Aβ are neurons and astrocytes, but there is another important systemic source of these peptides—platelets (reviewed in: Inyushin et al., 2017). Platelets are small anuclear cells derived from the processes of the megakaryocyte precursor cell, and their production is tightly regulated (reviewed in: Kutler, 1996, 2014). They contain diverse types of granules that include alpha granules, dense granules, and lysosomes (Sharda, Flaumenhaft, 2018). Besides coagulation factors, platelet α-granules contain amyloid precursor protein (APP), and full-length APP (containing Aβ peptide) is liberated upon platelet degranulation (Bush et al., 1990; Van Nostrand et al., 1990; Rosenberg et al., 1997; Sevush et al., 1998; Baskin et al., 2000, Padovany et al., 2001). APP represents about half of all proteins secreted from agonist-treated platelets (Van-Nostrand et al., 1990). APP is itself a Kunitz-type protease inhibitor, which effectively inhibits chymotrypsin, trypsin, and other proteolytic enzymes (Van Nostrand et al., 1990; Ledesma et al., 2000) and promotes the activation of coagulation factor XII, affecting the hemostasis and temporal stability of the thrombus (Schmaier, 2016; Zamolodchikov et al., 2016). Brain vessels endothelial cell enzymes can cleave the platelet-released APP, forming Aβ, especially if activated platelets adhere directly to the endothelial cells (Davies et al, 1998). Platelets also generate Aβ peptide itself and are the primary source (~90%) of this peptide in human blood (Chen et al., 1995). The generation of Aβ peptide in platelets involves a regulated secretory vesicle pathway (Hook et al., 2005, 2008).
While in neurons the release of Aβ and cleavage by β-secretase occurs in the soluble intracellular environment (cytoplasm), cleavage by γ-secretase occurs within the transmembrane domain of APP when it is inserted in the membrane (Selkoe, 2004; Haass and Selkoe, 2007). Hook et al. (2008) called this the constructive secretory pathway.
The regulated secretory vesicle pathway (in which APP is present in soluble, non-transmembrane form in intracellular vesicles) releases full-length, soluble APP or Aβ and other fragments. This is known to occur in platelets (Bush et al., 1990, 1993; Van Nostrand et al., 1990) and in chromaffin cells (Tezapsidis et al., 1998). Full-length soluble APP thus has β- and γ-secretease sites accessible for direct proteolytic cleavage in solution (Tezapsidis et al., 1998). The specialized cathepsin B enzyme works as a β-secretase in this pathway. It was suggested that the major portion of secreted, extracellular Aβ peptides is produced by the regulated secretory pathway (Tezapsidis et al., 1998, 2013; Hook et al., 2005, 2009, 2014). Aβ peptides secreted by platelets are similar to those found in the senile plaques of AD patients (Scheuner et al., 1996). Production of shorter Aβ peptides ending at residue 40 (Aβ40) increases if platelets are closely packed in the thrombus, while production of Aβ42 does not depend on platelet density (Casoli et al., 2007).
Platelets are an important component of the inflammation and immune response
Platelets are best known as cellular mediators of thrombosis, but they are also immune cells that initiate and accelerate many inflammatory conditions. In some contexts these conditions are protective, whereas in others they contribute to adverse inflammatory outcomes (reviewed in: Klinger and Jelkmann, 2002; Morrell et al., 2014, 2019; Manne et al., 2017; Łukasik et al., 2018). Platelets have receptors activated by viral and bacterial antigens and in response release microbicidal peptides (Yount et al., 2004; Trier et al., 2008; Yeaman, 2010; Seyoum et al., 2018). We have shown that during thrombosis after skin injury there is a massive release of Aβ peptide and that this peptide can perforate yeast cell membranes while not affecting somatic cell membranes at the same concentration (Kucheryavykh et al., 2018). However, Aβ peptide is not the only weapon in the platelet arsenal, as other antibacterial peptides were identified long ago (Yeaman et al., 1993, 1997; Krijgsveld et al., 2000; Kupferwasser et al., 2002; Tang et al., 2002). Like Aβ, one of these antibacterial peptides present in rabbit has a variable length of 72–73 amino acids and is cleaved from a longer precursor (Yount et al., 2004).
Platelets express numerous specialized Toll-like receptors (TLR) that recognize microbe-associated threats. Moreover, they (1) interact with other immune cells using cell-specific adhesion molecules, (2) attach neutrophils and monocytes at the site of lesion and also activate them as well as themselves, (3) release multiple antibacterial factors, and (4) participate in both innate and acquired immune responses (reviewed in: Morell et al., 2014; Łukasik et al., 2018). Stimulation of TLR type 2 (TLR2) amplifies P-selectin expression on the surface of platelets, enhances the pro-inflammatory response of platelets, and increases the formation of platelet-neutrophil aggregations necessary for the innate response (Blair et al., 2009). It was previously shown that the presence of long-chain bacterial polyphosphates or bacteria in the bloodstream promotes platelet activation in a FXII-dependent manner, which may contribute to sepsis-associated thrombotic processes (Zilberman-Rudenko et al., 2018). It is also known that the Aβ peptides that are released by platelets are recognized by TLR2 on the surface of lymphocytes, similarly to other pore-formers, such as nystatin and amphotericin B, inducing a cellular response and leading to the secretion of inflammation factors and tumor necrosis factors (Razonable et al., 2005; Chen et al., 2006; Liu et al., 2012). This additional activation may add positive feedback to the inflammation process.
An important component of innate immunity, FcγRIIB, is the only inhibitory receptor for the fragment crystallizable region (Fc region) of IgM antibodies, which are involved in the complex regulation of acquired defense against infection (Smith and Clatworthy, 2010). On the surface of B lymphocytes this receptor inhibits IgM production until the necessary concentration is achieved. The affinity of FcγRIIB to soluble monomeric IgM is low, and it is activated only when the concentration of IgM is sufficiently high. However, Aβ liberated by platelets can shift this regulation, because FcγRIIb is also a receptor for soluble Aβ oligomers, and it was shown that both cell-derived and synthetic Aβ oligomers bind to the immobilized FcγRIIb ectodomain (Lee et al., 2018). Thus, overproduction of Aβ may lead to overproduction of IgM, provoking pathological inflammation. The overall mechanism of how Aβ affects the regulation of tissue inflammation is unclear. But it is known that FcγRIIB mediates Aβ neurotoxicity and memory impairment in AD, soluble Aβ oligomers interact with FcγRIIb in vitro and in AD brains, and inhibition of their interaction blocks synthetic Aβ neurotoxicity (Kam et al., 2013). FcγRIIb is significantly upregulated in the hippocampus of AD brains and neuronal cells exposed to synthetic Aβ (Kam et al., 2013).These results show that Aβ effects on the brain can also be indirect.
Platelets in AD and glaucoma
Glaucoma is strongly correlated with coagulation abnormalities. Platelets are hyperactivated in patients with different types of glaucoma and tend to aggregate (Hoyng et al., 1985; Bojić and Skare-Librenjak, 1998–1999; Matsumoto et al., 2001; Kuprys et al., 2014). In primary open angle glaucoma platelet aggregation and the fibrinolytic system may become triggers of vascular damage that can lead to microcirculatory defect at the optic nerve head (Matsumoto et al., 2001). An age-dependent association between spontaneous platelet aggregation and the presence of glaucoma was also observed (p<0.05; Hoyng et al., 1985). Anti-platelet effects of anti-glaucomatous eye drops was reported (Moschos et al., 2017), suggesting that glaucoma-related Aβ originates from platelets. A study in human glaucoma patients has shown that the number of platelets (the mean plateletcrit and platelet distribution width) is increased (Li et al., 2016). Aβ co-localizes with apoptotic retinal ganglion cells (RGCs) in experimental glaucoma in rat and induces significant RGC apoptosis in vivo in a dose- and time-dependent manner (Guo et al., 2007). Similarly, Aβ appears in the RGC layer in monkey retina after chronic ocular hypertension (Ito et al., 2012). In AD patients, accumulation of Aβ in the eye is more uniform in retina and is spread also to the eye lens, unlike in “pure” glaucoma (van Wijngaarden et al., 2017). Data on Aβ accumulation suggest some similarity in the mechanisms of AD and primary glaucoma, but there is no clear statistical correlation between these diseases (Tsolaki et al., 2011).
Correspondingly, abnormal clotting was found in AD patients and was correlated with cognitive ability, and it was suggested that APP is involved by perturbing clotting parameters (Suidan et al., 2018). Persons with AD dementia have a ~200% higher risk of stroke, according to a study of a large cohort of AD patients in Finland and Taiwan (Tolppanen et al., 2013; Chi et al., 2013). Clots are formed in atherosclerotic lesions (Badimon and Vilahur, 2014), with APP and Aβ peptide present in advanced human atherosclerotic plaques, and these substances originated from platelets (De Meyer et al., 2002). We also reported that Aβ peptide can be detected by immunocytochemistry in and around blood vessels in the brain after experimental thrombosis, and that this peptide is released to the tissue from platelets (Kucheryavikh et al., 2017). During clot formation, the density of platelets in the lumen of the thrombotic vessel is significantly increased (more than 300–500 times), thus allowing a massive release of Aβ peptide (Kucheryavikh et al., 2017). Platelet inclusions in cerebral blood vessels are the first signs of disease in an AD mouse model (Kniewallner et al., 2016). Microinfarcts are closely related to AD pathology (Kövari et al., 2013; Saito and Ihara, 2014, 2016), and there is a correlation with intracranial vessel arteriosclerosis (Dolan et al., 2010; Lathe et al., 2014), in which microclots are chronically formed in brain blood vessels during arteriosclerosis (Holvoet and Collen, 1998; Badimon and Vilahur, 2014). These findings illustrate the fact that the coagulation system, and platelets in particular, are involved in AD. There are many additional pieces of evidence that AD is a thrombo-hemorrhagic disorder (Schmaier, 2016).
The activated platelets in AD retain greater amounts of APP (Davies et al., 1997) while showing increased platelet adhesion and thrombus formation (Canobbio et al., 2016). In a transgenic mouse model of AD, platelets were found to be the major contributors to cerebral amyloid angiopathy (CAA), which then forms a shield of insoluble Aβ around brain blood vessels (Gowert et al., 2014). Recently, using parabiosis between APPswe/PS1dE9 transgenic AD mice and their wild-type littermates, it was demonstrated that human Aβ originating from transgenic AD model mice entered the circulation and accumulated in the brains of wild-type mice and formed cerebral amyloid angiopathy and Aβ plaques after a 12-month period of parabiosis (Bu et al., 2018). These authors did not study the source of blood-derived Aβ but suggested that it may be platelets.
Liberation of α-granule content is mediated by specific receptors on the platelet. Some of the most important platelet membrane receptor classes that produce platelet activation and generation of Aβ during traumatic/inflammatory tissue damage were previously reviewed (Inyushin et al., 2017). These include the purinergic receptors P2X and P2Y, which are activated by ADP released from damaged tissues, and the thromboxane receptors (TxA2); both these types signal via the specific G protein Gq. It was found that RxR retinoid receptor ligands strongly inhibit Gq, thus impeding both purinergic and thromboxane receptors on the surface of platelets and drastically reducing platelet activation/agregation (Moraes et al., 2007). Interestingly, the RXR ligand bexarotene induces miraculously rapid clearance of soluble Aβ, thereby reducing plaque burden and improving cognition in a mouse model of AD (Cramer et al., 2012; Mariani et al., 2017). Moreover, bexarotene rescues glaucoma phenotypes in mice (Dheer et al., 2019). While the authors of these studies on bexarotene effects proposed alternative explanations for their results, we suggest that platelet deactivation may also be involved. Another antiplatelet drug, cilostazol, inhibits the activation-dependent membrane surface glycoprotein GPIIb/IIIa on the surface of platelets, preventing their activation (Inoue et al., 1999). Cilostazol may reduce the decline of cognitive function in AD patients and patients with mild cognitive impairment (Taguchi et al., 2013; Tai et al., 2017) as well as glaucoma (Okamoto et al., 2010). Similarly, blocking only purinergic receptors on the surface of platelets by antiplatelet drugs, such as clopidogrel, decreased plaque burden in AD model mice (Donner et al., 2016), while the effects of this drug on glaucoma are unknown. These investigators showed that Aβ stimulates the integrin receptor in platelets, leading to the release of ADP and the protein clusterin, and that this protein promoted β-sheet folding and the formation of fibrillar Aβ aggregates (Gowert et al., 2014; Donner et al., 2016). The authors did not discuss the source of Aβ but did confirm the existence of specific mechanisms involving platelets in the process of Aβ aggregation and showed the importance of platelets in AD development.
On the other hand, thrombocytopenia (a low platelet count) is extremely rare in AD patient cohorts. Out of 20,591 FDA reports on cases of AD-type dementia, only0.4% developed thrombocytopenia, appearing in the advanced stages of the disease after long use of certain anti-AD medicines (eHealthMe, 2018). It has been reported that, while AD patients have similar platelet counts as age-matched controls, their platelets are in a more activated state (Sevush et al., 1998). Twenty years ago these authors (Sevush et al., 1998) concluded that, in light of evidence that platelets are the principal source of both APP and Aβ peptide in human blood, it is possible that AD platelet activation reflects, or even contributes to, the pathogenesis of AD. Unfortunately, this idea has never been tested directly.
Recently, we have shown that thrombocytopenic animals produce lower levels of Aβ peptide during clotting (Kucheryavykh et al., 2017). Therefore, induction of mild thrombocytopenia may be a novel approach to reducing the damaging effects of overproduction of systemic Aβ.
X-ray treatment of glaucoma and AD
Exposure to X-rays directly affects blood platelet count. For glaucoma it was shown that a mild X-ray dose rapidly ameliorates retinal damage (Anderson et al., 2005; Bosco et al., 2012; Howell et al., 2012). This effect was first shown for whole-body irradiation. Destroying the bone marrow and replacing it with donor bone marrow in a genetic model of mouse glaucoma (DBA/2J mice) reduced glaucoma retinal damage after whole-body irradiation (Anderson et al., 2005). Later, the same group reported that only a single local X-ray treatment (7.5 Gy) of an individual eye in young mice provided the eye with long-term protection from glaucoma. They claim that it had no effect on the contralateral eye (Howell et al., 2012). This X-ray effect was challenged, however, by the research showing that there was no protective outcome if the X-ray irradiation was applied locally (Johnson et al, 2014). Neither of these studies investigated the effects on platelets.
Similar effects for whole-body irradiation and head-only irradiation were reported for AD (Simard et al., 2006; Mildner et al., 2011). The whole-body irradiation (10 Gy) was used first in experiments with a transgenic mouse strain (APPSwe/PS1), genetically modified to develop Aβ plaques, and it was shown that the amount of these plaques after X-ray irradiation was reduced by more than 50% (Simard et al., 2006). Later, whole-body and partial-body (lower part of the body only) irradiation was used in mouse models of AD (APPswe/PS1, APPswe, and APP23 mice) (Mildner et al., 2011). At the dosage used in these experiments, the bone marrow and its entire hematopoietic lineage became ablated, and the work of both Simard and coworkers as well as that of Mildner, used the repopulation of animals with donor bone marrow to study the effects of newly generated monocytes. After whole-body irradiation senile plaque formation ceased, and the authors concluded that monocytes/microglia were responsible, although thrombocytopenia was not assessed in these experiments. After irradiation of the lower part of the body the effect on plaque formation in brain parenchyma was insignificant (Mildner et al., 2011).
Following the success in treatment of tracheo-bronchial amyloidosis (TBA) with irradiation (20 Gy, administered as 10×2Gyover two weeks), it was proposed to use mild levels of radiation to treat AD (Bistolfi, 2008). Indeed, the other research group used direct irradiation of the cranium to show that radiation causes plaques to became wiped out in the hippocampus and cortex. In AD transgenic mice, high doses of X-rays produced a very clear (>50%) reduction in amyloid deposition, and the authors attributed this to yet undetermined immune mechanisms (Marples et al., 2012; also see WO2012034019A1 Patent Application, 2010). Later, the same group, using different regimes of X-ray irradiation of the cranium, assessed not only Aβ plaque accumulation but also other AD parameters (Marples et al., 2016; Wilson and Marples, 2016). In this work only one side of the brain was irradiated, and a lead irradiation jig was used to shield the other side of the brain and all other tissues from the treatment field. The number of Aβ plaques in the irradiated side of the brain was then compared with the number of plaques in the shielded non-irradiated side. The authors found a clear difference between treated and untreated hemispheres. Anyway, one can see that at higher multiple doses, both shielded and irradiated hemispheres showed fewer plaques, suggesting that the effect of X-ray irradiation is not entirely local (Marples et al., 2016). Similarly, a visible difference can be observed between sham-irradiated and irradiated brains, suggesting that the nonlocal effects of irradiation were much more pronounced (Marples et al., 2016). How effective was the shielding of, for example, lungs, is difficult to determine from the description of the author’s work.
It is known that X-ray irradiation of the upper chest, even at low doses (150 mGy), can produce thrombocytopenia. It was shown also that endothelial cell irradiation lowered their ability to attract migrating megakaryocytes (MKs). Moreover, the adhesion of MKs to human vascular endothelial cells (HUVECs) was markedly reduced when these cells were exposed to radiation, which was accompanied by a decreased production of platelets by MKs (Chen et al., 2017). While there is evidence of platelet production in bone marrow/spleen (Davis et al., 1997), it was found recently that the majority of platelet production from the pro-platelet processes of megakaryocyte-type extravascular progenitors is mainly concentrated in the pulmonary capillary bed of the lungs (Howell and Donahue, 1937; Zucker-Franklin and Philipp, 2000; Lefrançais et al., 2017). Thus, special shielding of the lower chest is needed to exclude the effects of a reduction of platelet count on plaque formation.
Additionally, a case report was published in which a low dose of X-rays generated by a CT scan (5× 50 mGy=250 mGy) produced miraculous, but transient, recovery of a patient with terminal AD after cranium imaging studies (Cuttler et al., 2016). CT scanning is performed by a 3D X-ray instrument, which to some extent also applies X-ray irradiation along the Z-axis to the lung during cranial imaging. It must be mentioned that the thrombocytopenia after the low doses of radiation is usually temporary and reverses in a few weeks due to compensatory effects (Ebbe et al., 1986).
The overall results on X-ray effects are interesting and can be interpreted from different points of view. While we suggest that reduction of the platelet count can explain the reported effects of ionizing radiation on both AD and glaucoma, this subject surely needs further in-depth exploration.
Blood transfusion in glaucoma and AD treatment
Blood transfusion unquestionably affects platelet count and activation. Unfortunately, there are no recent studies devoted to the effects of blood transfusion on glaucoma. We have found only old review of effects in human patients and it was found that blood transfusion can dramatically reduce glaucoma retinal damage. The suggested explanation was that it helps to restore eye hemodynamics and reduce hypoxia, especially if patients previously have some general impairment of the cardiovascular system (reviewed in: Iusupov and Medvedev, 1965).
Blood transfusion has been shown to have profound effects on AD development. Bu and coworkers (Bu et al., 2018) showed that the blood from transgenic AD mice, which usually develop Aβ plaques and neuronal brain damage by 6–7 months of age, can induce similar plaques and damage in normal wild type animal brain after constant transfusion. Constant exchange of blood was achieved by parabiosis, in which the vascular system of one animal is surgically united with the vascular system of another so that they shared a common reservoir of blood. These authors used APPswe/PS1dE9 transgenic AD mice, producing human Aβ, which can be easily distinguished from intrinsic mouse Aβ. Parabiosis between these AD mice and their wild-type littermates has shown that human Aβ originating from transgenic AD model mice enters the circulation and accumulates in the brains of wild-type mice and forms cerebral amyloid angiopathy and Aβ plaques after a 12-month period (Bu et al., 2018). While the source of blood-derived Aβ was not specifically studied, it was suggested that it might be platelets (Bu et al., 2018).
Vice versa, the transfusion of blood from healthy subjects can reduce plaque formation in AD. It was previously shown that parabiosis between young animals and aged mice mitigates age-related impairment of cognitive function, memory, and vascular function, but exclusively neuronal mechanisms were suggested (Katsimpardi et al., 2014; Villeda et al., 2014). This was followed by a preclinical study with mice (Middeldorp et al., 2016) and clinical trials in which blood from young persons was transfused into AD patients (PLASMA study NCT02256306). Because the study was not specifically focused on platelets, the preclinical animal trial did not investigate if a simple blood dilution (reducing the platelet count) by physiological solution will produce a similar effect. In the clinical trial there is still no published data on whether the effect of saline is any different from plasma transfusion and whether both are positive. If the platelet count is involved in the effect, it is possible that simple plasma dilution (platelet count lowering with saline) will have positive effects.
The overall results concerning the effects of blood transfusion are very interesting and can be interpreted from different points of view. While we suggest that reducing the platelet count can explain the reported effects on both AD and glaucoma, this subject surely needs further in-depth exploration.
Why older age is a factor in sporadic glaucoma and AD
The greatest known risk factor for AD is increasing age, and the majority of people with AD are 65 and older, similarly people have six times more risk to get glaucoma if they are over 65 years old. Correspondingly, there are clear age-related changes in platelet count, function, and reactivity, driven by changes in hematopoietic tissue, the composition of the blood, and vascular health. These age-related changes are particularly pertinent given that thrombotic disease is much more prevalent in the elderly population (Jones, 2016). As an example, acute coronary syndromes and atrial fibrillation -the most frequent indications for platelet inhibition or anticoagulation- occur mostly in older patients, as does stroke, transient ischemic attack, myocardial infarction, systemic embolism, deep vein thrombosis, and pulmonary embolism (Andreotti et al., 2015). It is known that infarcts are closely related to AD pathology (Kövari et al, 2013; Saito and Ihara, 2014, 2016), as well as the intracranial vessel arteriosclerosis in which microclots are chronically being formed in brain blood vessels (Holvoet and Collen, 1997; Dolan et al., 2010; Lathe et al., 2014; Badimon and Vilahur, 2014). Platelets are responsible for APP and Aβ peptide present in advanced human atherosclerotic plaques (De Meyer et al., 2002).Thus stroke is associated with AD among elderly individuals (Honig et al., 2003).
Similarly, a10 year follow up study within the population of Taiwan have shown that glaucoma is strongly correlated to stroke (Lee et al., 2017). The similar strong correlation was found for myocardial infarction and glaucoma in large India and Taiwan studies (Mondal et al., 2013; Chen et al., 2016).
Platelet count is stable up to about 60 years old in humans, and then falls about 8% (reviewed in Jones, 2016) in the general population, this may be as a compensation for the increased platelet reactivity which is augmented with age in an almost linear fashion. Similar changes occur in other mammalian species with aging (Balduini and Noris, 2014; Jones, 2016). Platelets are hyper-activated in AD (Jarre et al, 2014) and glaucoma (Kuprys et al., 2014), and these hyper-activated platelets more aggressively damage healthy vessels (Kniewallner et al., 2018).
Therefore, regulation of platelet count and activation, especially in old age, may be a novel approach to reducing the damaging effects of platelet-generated systemic Aβ in AD and glaucoma.
Conclusions
Aβ peptide is implicated in both AD and glaucoma and has been shown to be the important etiological factor for these dangerous health complications.
Aβ has many functions, but is well established as an antimicrobial/antibiotic peptide.
It is known that Aβ peptide is generated from its precursor protein and massively released to the blood upon the activation/aggregation of platelets, and is thereby accumulated locally from a systemic source.
Platelets are an important part of the inflammation and immune response, and both glaucoma and AD correlate with significant local inflammation.
Platelets are the main source of systemic APP and Aβ.
Platelet hyper-activation has been conclusively shown to be an aspect of both AD and glaucoma.
Induction of mild thrombocytopenia and/or the reduction of platelet activation may be an effective approach to reduce the damaging effects of systemic Aβ.
Fig. 1.
Platelets generate Aβ, and this activity is affected by inflammation and age-related changes.
Acknowledgements.
This research was supported by NIH grants SC1GM122691 to L.K. and SC2GM111149 to M.I. The funding sources had no role in study design; data collection, analysis, or interpretation; or the decision to submit this article.
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G and Wyss-Coray T (2000). Inflammation and Alzheimer’s disease. Neurobiol. Aging 21, 383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MG, Libby RT, Gould DB, Smith RS and John SW (2005). High-dose radiation with bone marrowtransfer prevents neurodegeneration in aninherited glaucoma. Proc. Natl. Acad. Sci. USA 102, 4566–4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreotti F, Rocca B, Husted S, Ajjan RA, ten Berg J, Cattaneo M, Collet JP, De Caterina R, Fox KA, Halvorsen S, Huber K, Hylek EM, Lip GY, Montalescot G, Morais J, Patrono C, Verheugt FW, Wallentin L, Weiss TW and Storey RF (2015). ESC Thrombosis Working Group, Antithrombotic therapy in the elderly: expert position paper of the European Society of Cardiology Working Group on Thrombosis. Eur. Heart J 7, 36, 3238–3249. [DOI] [PubMed] [Google Scholar]
- Askanas V, Engel WK and Alvarez RB (1992). Light and electron microscopic localization of beta-amyloid protein in muscle biopsies of patients with inclusion-body myositis. Am. J. Pathol 141, 31–36. [PMC free article] [PubMed] [Google Scholar]
- Astafurov K, Elhawy E, Ren L, Dong CQ, Igboin C, Hyman L, Griffen A, Mittag T and Danias J (2014). Oral microbiome link to neurodegeneration in glaucoma, PLoS One 9, e104416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badimon L and Vilahur G (2014). Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Int. Med 276, 618–632. [DOI] [PubMed] [Google Scholar]
- Balduini CL and Noris P (2014). Platelet count and aging,Haematologica 99, 953–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin F, Rosenberg RN, Iyer L, Hynan L and Cullum CM (2000). Platelet APP isoform ratios correlate with declining cognition in AD. Neurology 54, 1907–1909. [DOI] [PubMed] [Google Scholar]
- Bistolfi F (2008). Localized amyloidosis and Alzheimer’s disease: the rationale for weekly long-term low dose amyloid-based fractionated radiotherapy. Neuroradiol. J 21, 683–692. [DOI] [PubMed] [Google Scholar]
- Blair BS, Rex O, Vitseva L, Beaulieu K, Tanriverdi S, Chakrabarti S, Hayashi C, Genco CA, Iafrati M and Freedman JE (2009). Stimulation of Toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ. Res 104, 346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodh SA, Kumar V, Raina UK, Ghosh B and Thakar M (2011).Inflammatory glaucoma. Oman J. Ophthalmol 4, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojić L and Skare-Librenjak L (1998–1999). Circulating platelet aggregates in glaucoma. IntOphthalmol 22, 151–154. [DOI] [PubMed] [Google Scholar]
- Bosco A, Crish SD, Steele MR, Romero CO, Inman DM, Horner PJ, Calkins DJ and Vetter ML (2012). Early reduction of microglia activation by irradiation in a model of chronicglaucoma. PLoS One 7, e43602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgade K, Garneau H, Giroux G, Le Page AY, Bocti C, Dupuis G,Frost EH and Fülöp T Jr (2014). β-Amyloid peptides display protective activity against the human Alzheimer’s disease-associated herpes simplex virus-1. Biogerontology 16, 85–98. [DOI] [PubMed] [Google Scholar]
- Braak H and Braak E (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82, 239–259. [DOI] [PubMed] [Google Scholar]
- Bu XL, Xiang Y, Jin WS, Wang J, Shen LL, Huang ZL, Zhang K, Liu YH, Zeng F, Liu JH, Sun HL, Zhuang ZQ, Chen SH, Yao XQ, Giunta B, Shan YC, Tan J, Chen XW, Dong ZF, Zhou HD, Zhou XF, Song W and Wang YJ (2018). Blood-derived amyloid-β protein induces Alzheimer’s disease pathologies. Mol. Psychiatry 23, 1–9. [DOI] [PubMed] [Google Scholar]
- Buhimschi IA, Nayeri UA, Zhao G, Shook LL, Pensalfini A, Funai EF, Bernstein IM, Glabe CG and Buhimschi CS (2014). Protein misfolding, congophilia, oligomerization, and defective amyloid processing in preeclampsia. Sci. Transl. Med 6, 245ra92. [DOI] [PubMed] [Google Scholar]
- Bush AI, Martins RN, Rumble B, Moir R, Fuller S, Milward E, Currie J, Ames D, Weidemann A, Fischer P, Multhaup G, Beyreuther K and Masters CL (1990). The amyloid precursor protein of Alzheimer’s disease is released by human platelets. J. Biol. Chem 265, 15977–15983. [PubMed] [Google Scholar]
- Bush AI, Beyreuther K and Masters CL (1993). The beta A4 amyloid protein precursor in human circulation, Ann. N.Y. Acad. Sci 695, 175–182. [DOI] [PubMed] [Google Scholar]
- Călugăru M and Călugăru D (2001). Prevalence of glaucoma suspect in patients with central vein occlusion. Oftalmologia 51, 80–84. [PubMed] [Google Scholar]
- Canobbio I, Visconte C, Oliviero B, Guidetti G, Zarà M, Pula G and Torti M (2016). Increased platelet adhesion and thrombus formation in a mouse model of Alzheimer’s disease. Cell Signal 28, 1863–1871. [DOI] [PubMed] [Google Scholar]
- Carter CJ (2017). Genetic, transcriptome, proteomic, and epidemiological evidence for blood-brain barrier disruption and polymicrobial brain invasion as determinant factors in Alzheimer’s disease. J. Alzheimers Dis. Rep 1, 125–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casoli T, Di Stefano G, Giorgetti B, Grossi Y, Balietti M, Fattoretti P and Bertoni-Freddari C (2007). Release of beta-amyloid from high-density platelets: implications for Alzheimer’s disease pathology. Ann. N.Y. Acad. Sci 1096, 170–178. [DOI] [PubMed] [Google Scholar]
- Chen M, Inestrosa CC, Ross GS and Fernandez HL (1995). Platelets are the primary source of amyloid P-peptide in human blood. Biochem. Biophys. Res. Commun 213, 96–103. [DOI] [PubMed] [Google Scholar]
- Chen K, Iribarren P, Hu J, Chen J, Gong W, Cho EH, Lockett S, Dunlop NM and Wang JM (2006). Activation of Toll-like receptor 2 on microglia promotes cell uptake of Alzheimer disease-associated amyloid beta peptide. J. Biol. Chem 281, 3651–3659. [DOI] [PubMed] [Google Scholar]
- Chen YY, Hu HY, Chu D, Chen HH, Chang CK and Chou P(2016). Patients with primary open-angle glaucoma may develop ischemic heart disease more often than those without glaucoma: An 11-year population-based cohort study. PLoS One 11, e0163210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Shen M, Zeng D, Wang C, Wang S, Chen S, Tang Y, Hu M, Chen M, Su Y, Ran X, Xu Y and Wang J (2017). Effect of radiation-induced endothelial cell injury on platelet regeneration by megakaryocytes. J. Radiat. Res 58, 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi NF, Chien LN, Ku HL, Hu CJ and Chiou HY (2013). Alzheimer disease and risk of stroke: a population-based cohort study. Neurology 80, 705–711. [DOI] [PubMed] [Google Scholar]
- Corps KN, Roth TL and McGavern DB (2015). Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol 72, 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE,Casali BT, Restivo JL, Goebel WD, James MJ, Brunden KR, Wilson DA and Landreth GE (2012). ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science 335, 1503–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttler JM, Moore ER, Hosfeld VD and Nadolski DL (2016). Treatment of alzheimer disease with ct scans: A case report. Dose Response 14, 1559325816640073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies TA, Billingslea AM, Long HJ, Tibbles H, Wells JM, Eisenhauer PB, Smith SJ, Cribbs DH, Fine RE and Simons ER (1998). Brain endothelial cell enzymes cleave platelet-retained amyloid precursor protein. J. Lab. Clin. Med 132, 341–350. [DOI] [PubMed] [Google Scholar]
- Davies TA, Long HJ, Sgro K, Rathbun WH, McMenamin ME, Seetoo K, Tibbles H, Billingslea AM, Fine RE, Fishman JB, Levesque CA, Smith SJ, Wells JM and Simons ER (1997). Activated Alzheimer disease platelets retain more beta amyloid precursor protein. Neurobiol. Aging 18, 147–153. [DOI] [PubMed] [Google Scholar]
- Davis RE, Stenberg PE, Levin J and Beckstead JH (1997). Localization of megakaryocytes in normal mice and following administration of platelet antiserum, 5-fluorouracil, or radiostrontium: evidence for the site of platelet production. Exp. Hematol 25, 638–648. [PubMed] [Google Scholar]
- De Meyer GRY, De Cleen DMM, Cooper S, Knaapen MWM, Jans DM, Martinet W, Herman AG, Bult H and Kockx MM (2002). Platelet phagocytosis and processing of β-amyloid precursor protein as a mechanism of macrophage activation in atherosclerosis. Circ. Res 90, 1197–1204. [DOI] [PubMed] [Google Scholar]
- Dheer Y, Chitranshi N, Gupta V, Sharma S, Pushpitha K, Abbasi M, Mirzaei M, You Y, Graham SL and Gupta V (2019). Retinoid X receptor modulation protects against ER stress response and rescues glaucoma phenotypes in adult mice. Exp. Neurol 314, 111–125. [DOI] [PubMed] [Google Scholar]
- Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB and OBrien RJ (2010). Atherosclerosis, dementia, and alzheimer disease in the Baltimore longitudinal study of aging cohort. Ann. Neurol 68, 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, Nguyen M, Haditsch U, Raha D, Griffin C, Holsinger LJ, Arastu-Kapur S, Kaba S, Lee A, Ryder M, Potempa B, Mydel P, Hellvard A, Adamowicz K, Hasturk H, Walker GD, Reynolds EC, Faull RLM, Curtis MA, Dragunow M and Potempa J (2019). Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv 5, eaau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner L, Fälker K, Gremer L, Klinker S, Pagani G, Ljungberg LU,Lothmann K, Rizzi F, Schaller M, Gohlke H, Willbold D, Grenegard M and Elvers M (2016). Platelets contribute to amyloid-β aggregation in cerebral vessels through integrin αIIbβ3-induced outside-in signaling and clusterin release. Sci. Signal 9, ra52. [DOI] [PubMed] [Google Scholar]
- Ebbe S, Phalen E and Yee T (1986). Postirradiationthrombocytopoiesis: suppression, recovery, compensatory states, and macromegakaryocytosis. Prog. Clin. Biol. Res 215, 71–89. [PubMed] [Google Scholar]
- eHealthMe (2018). Personalized medication management, https://www.ehealthme.com/, accessed 25 December 2018
- Eimer WA, Vijaya Kumar DK, Navalpur Shanmugam NK, Rodriguez AS, Mitchell T, Washicosky KJ, György B, Breakefield XO, Tanzi RE and Moir RD (2018). Alzheimer’s disease-associated β-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron 99, 56–63. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gen Script Database, β-Amyloid peptide, https://www.genscript.com/peptide/RP10017-_Amyloid_1_42_human.html
- Gianni D, Li A, Tesco G, McKay KM, Moore J, Raygor K, Rota M, Gwathmey JK, Dec GW, Aretz T, Leri A, Semigran MJ, Anversa P, Macgillivray TE, Tanzi RE and del Monte F (2010). Protein aggregates and novel presenilin gene variants in idiopathic dilated cardiomyopathy. Circulation 121, 1216–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner GG and Wong CW (1984). Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun 120, 885–890. [DOI] [PubMed] [Google Scholar]
- Gosztyla ML, Brothers HM and Robinson SR (2018). Alzheimer’s amyloid-β is an antimicrobial peptide: A review of the evidence. J. Alzheimers Dis 62, 1495–1506. [DOI] [PubMed] [Google Scholar]
- Gowert NS, Donner L, Chatterjee M, Eisele YS, Towhid ST, Münzer P, Walker B, Ogorek I, Borst O, Grandoch M, Schaller M, Fischer JW, Gawaz M, Weggen S, Lang F, Jucker M and Elvers M (2014). Blood platelets in the progression of Alzheimer’s disease. PLoS One 9, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WST (2013). Neuroinflammatory cytokine signaling andAlzheimer’s disease. N. Engl. J. Med 368, 770–771. [DOI] [PubMed] [Google Scholar]
- Guo L, Salt TE, Luong V, Wood N, Cheung W, Maass A, Ferrari G, Russo-Marie F, Sillito AM, Cheetham ME, Moss SE, Fitzke FW and Cordeiro MF (2007). Targeting amyloid-beta in glaucoma treatment. Proc. Natl. Acad. Sci. USA 104, 13444–13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C and Selkoe DJ (2007). Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol 8, 101–112. [DOI] [PubMed] [Google Scholar]
- Hancock REW and Chapple DS (1999). Peptide antibiotics. Antimicrob. Agents Chemother 43, 1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel DE, Rahman A, Wehner S, Herzog V, Yeo CJ and Maitra A (2003). Increased expression and processing of the Alzheimer amyloid precursor protein in pancreatic cancer may influence cellular proliferation. Cancer Res 63, 7032–7037. [PubMed] [Google Scholar]
- Harder J, Bartels J, Christophers E and Schröder JM (1997). A peptide antibiotic from human skin. Nature 387 p. 861. [DOI] [PubMed] [Google Scholar]
- Harris SA and Harris EA (2015). Herpes simplex virus type 1 and other pathogens are key causative factors in sporadic Alzheimer’s disease. J. Alzheimers Dis 48, 319–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SA and Harris EA (2018). Molecular mechanisms for herpes simplex virus type 1 pathogenesis in Alzheimer’s disease. Front. Aging Neurosci 10, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatami A, Monjazeb S, Milton S and Glabe CG (2017). Familial Alzheimer’s disease mutations within the amyloid precursor protein alter the aggregation and conformation of the amyloid-β peptide. J. Biol. Chem 292, 3172–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeksema L, Jansonius NM and Los LI (2017). Risk factors for secondary glaucoma in herpetic anterior uveitis. Am. J. Ophthalmol 181, 55–60. [DOI] [PubMed] [Google Scholar]
- Holvoet P and Collen D (1997). Thrombosis and atherosclerosis. Curr. Op. Lipidol 8, 320–328. [DOI] [PubMed] [Google Scholar]
- Honig LS, Tang MX, Albert S, Costa R, Luchsinger J, Manly J, Stern Y and Mayeux R (2003). Stroke and the risk of Alzheimer disease. Arch. Neurol 60, 1707–1712. [DOI] [PubMed] [Google Scholar]
- Hook V, Toneff T, Bogyo M, Greenbaum D, Medzihradszky KF, Neveu J, Lane W, Hook G and Reisine T (2005). Inhibition of cathepsin B reduces beta-amyloid production in regulated secretory vesicles of neuronal chromaffin cells: evidence for cathepsin B as a candidate beta-secretase of Alzheimer’s disease. Biol. Chem 386, 931–940. [DOI] [PubMed] [Google Scholar]
- Hook V, Schechter I, Demuth H-U and Hook G (2008). Alternative pathways for production of beta-amyloid peptides of Alzheimer’s disease. Biol. Chem 389, 993–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook V, Kindy M, Reinheckel T, Peters C and Hook G (2009). Genetic cathepsin B deficiency reduces b-amyloid in transgenic mice expressing human wild-type amyloid precursor protein, Biochem. Biophys Res. Commun 386, 284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook G, Yu J, Sipes N, Pierschbacher MD, Hook V and Kindy MS (2014). The cysteine protease cathepsin B is a key drug target and cysteine protease inhibitors are potential therapeutics for traumatic brain injury. J. Neurotrauma 31, 515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell WH and Donahue DD (1937). The production of blood platelets in the lungs. J. Exp. Med 65, 177–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Soto I, Zhu X, Ryan M, Macalinao DG, Sousa GL, Caddle LB, MacNicoll KH, Barbay JM, Porciatti V, Anderson MG, Smith RS, Clark AF, Libby RT and John SW (2012). Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J. Clin. Invest 122, 1246–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyng PF, Greve EL, Frederikse K, Geijssen C and Oosting H (1985). Platelet aggregation and glaucoma. Doc. Ophthalmol 61, 167–173. [DOI] [PubMed] [Google Scholar]
- WO2012034019A1 Patent Application, (2010). https://patents.google.com/patent/WO2012034019A1/, accessed 18 January 2019
- Ikonomovic MD, Uryu K, Abrahamson EE, Ciallella JR, Trojanowski JQ, Lee VM, Clark RS, Marion DW, Wisniewski SR and DeKosky ST (2004). Alzheimer’s pathology in human temporal cortex surgically excised after severe brain injury. Exp. Neurol 190, 192–203. [DOI] [PubMed] [Google Scholar]
- Ilievski V, Zuchowska PK, Green SJ, Toth PT, Ragozzino ME, Le K, Aljewari HW, O’Brien-Simpson NM, Reynolds EC and Watanabe K (2018). Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration and amyloid beta production in wild type mice. PLoS One 13, e0204941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Sohma R and Morooka S (1999). Cilostazol inhibits the expression of activation-dependent membrane surface glycoprotein on the surface of platelets stimulated in vitro. Thromb. Res 93, 137–143. [DOI] [PubMed] [Google Scholar]
- Inyushin MY, Sanabria P, Rojas L, Kucheryavykh Y and Kucheryavykh L (2017). Aβ peptide originated from platelets promises new strategy in anti-alzheimer’s drug development. Biomed. Res. Int 3948360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Shimazawa M, Tsuruma K, Mayama C, Ishii K, Onoe H, Aihara M, Araie M and Hara H (2012). Induction of amyloid-β(1–42) in the retina and optic nerve head of chronic ocular hypertensive monkeys. Mol. Vis 18, 2647–2657. [PMC free article] [PubMed] [Google Scholar]
- Jarre A, Gowert NS, Donner L, Münzer P, Klier M, Borst O, Schaller M, Lang F, Korth C and Elvers M (2014). Pre-activated blood platelets and a pro-thrombotic phenotype in APP23 mice modeling Alzheimer’s disease. Cell. Signal 26, 2040–2050. [DOI] [PubMed] [Google Scholar]
- Jaunmuktane Z, Mead S, Ellis M, Wadsworth JD, Nicoll AJ, Kenny J, Launchbury F, Linehan J, Richard-Loendt A, Walker AS, Rudge P, Collinge J and Brandner S (2015). Evidence for human transmission of amyloid-β pathology and cerebral amyloid angiopathy. Nature 525, 247–250. [DOI] [PubMed] [Google Scholar]
- Jeong S (2017). Molecular and cellular basis of neurodegeneration inAlzheimer’s disease. Mol. Cells 40, 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin WS, Bu XL, Liu YH, Shen LL, Zhuang Z, Jiao SS, Zhu C, Wang QH, Zhou HD, Zhang T and Wang YJ (2017). Plasma amyloid-beta levels in patients with different types of cancer. Neurotox. Res 31, 283–288. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart W and Smith DH (2010). Traumatic brain injury and amyloid-β pathology: a link to Alzheimer’s disease? Nat. Rev. Neurosci 11, 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Cepurna WO, Choi D, Choe TE and Morrison JC (2014). Radiation pretreatment does not protect the rat optic nerve from elevated intraocular pressure-induced injury. Invest. Ophthalmol. Vis. Sci 56, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CI (2016). Platelet function and ageing, Mamm. Genome 27,358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam TI, Song S, Gwon Y, Park H, Yan JJ, Im I, Choi JW, Choi TY, Kim J, Song DK, Takai T, Kim YC, Kim KS, Choi SY, Choi S, Klein WL, Yuan J and Jung YK (2013). FcγRIIb mediates amyloid-β neurotoxicity and memory impairment in Alzheimer’s disease. J. Clin. Invest 123, 2791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS,Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ and Rubin LL (2014). Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara M (2010). Neurotoxicity of β-amyloid protein: oligomerization, channel formation, and calcium dyshomeostasis. Curr. Pharm. Des 16, 2779–2789. [DOI] [PubMed] [Google Scholar]
- Kawahara M, Arispe N, Kuroda Y and Rojas E (1997). Alzheimer’s disease amyloid β-protein forms Zn2+-sensitive, cation- selective channels across excised membrane patches from hypothalamic neurons. Biophys. J 73, 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger MH and Jelkmann W (2002). Role of blood platelets in infection and inflammation. J. Interferon Cytokine Res 22, 913–922. [DOI] [PubMed] [Google Scholar]
- Kniewallner KM, Wenzel D and Humpel C (2016). Thiazine red(+) platelet inclusions in cerebral blood vessels are first signs in an Alzheimer’s disease mouse model. Sci. Rep 6, 28447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniewallner KM, Foidl BM and Humpel C (2018). Platelets isolated from an Alzheimer mouse damage healthy cortical vessels and cause inflammation in an organotypic ex vivo brain slice model. Sci. Rep 8, 15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kövari E, Herrmann FR, Hof PR and Bouras C (2013). The relationship between cerebral amyloid angiopathy and cortical microinfarcts in brain ageing and Alzheimer’s disease. Neuropath. App. Neurobiol 39, 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krijgsveld J, Zaat SA, Meeldijk J, van Veelen PA, Fang G, Poolman B, Brandt E, Ehlert JE Kuijpers AJ, Engbers GH, Brandt E, Ehlert JE, Kuijpers AJ, Engbers GH, Feijen J and Dankert J (2000). Thrombocidins, microbicidal proteins from human blood platelets, are C-terminal deletion products of CXC chemokines. J. Biol. Chem 275, 20374–20381. [DOI] [PubMed] [Google Scholar]
- Kucheryavykh LY, Dávila-Rodríguez J, Rivera-Aponte DE, Zueva LV, Washington AV, Sanabria P and Inyushin MY (2017). Platelets are responsible for the accumulation of β-amyloid in blood clots inside and around blood vessels in mouse brain after thrombosis. Brain Res. Bull 128, 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucheryavykh LY, Kucheryavykh YV, Washington AV and Inyushin MY (2018). Amyloid beta peptide is released during thrombosis in the skin. Int. J. Mol. Sci 19, 1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar DKV, Choi HS, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, Lefkowitz A, McColl G, Goldstein LE, Tanzi RE and Moir RD (2016). Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci. Transl. Med 8, 340ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferwasser LI, Yeaman MR, Shapiro SM, Nast CC and Bayer AS (2002). In vitro susceptibility to thrombin-induced platelet microbicidal protein is associated with reduced disease progression and complication rates in experimental Staphylococcus aureus endocarditis: Microbiological, histopathologic, and echo-cardiographic analyses. Circulation 105, 746–752. [DOI] [PubMed] [Google Scholar]
- Kuprys PV, Forte S, Wanderling C, Walker L, Grybauskas A,Samples JR, Zaparackas Z, Yue B and Knepper PA (2014). Primary open-angle glaucoma patients have superactivated platelets: A sticky conundrum, Invest. Ophthalmol. Vis. Sci 55, 531. [Google Scholar]
- Kutler DJ (2014). Milestones in understanding platelet production: a historical overview. Br J. Haematol 165, 248–258. [DOI] [PubMed] [Google Scholar]
- Kutler DJ (1996). The physiology of platelets production, Stem Cells 14, 88–101 [DOI] [PubMed] [Google Scholar]
- Lal R, Lin H and Quist AP (2007). Amyloid beta ion channel: 3D structure and relevance to amyloid channel paradigm. Biochim. Biophys. Acta 1768, 1966–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R, Sapronova A and Kotelevtsev Y (2014). Atherosclerosis and Alzheimer - diseases with a common cause? Inflammation, oxysterols, vasculature. BMC Geriatr 14, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledesma MD, da Silva JS, Crassaerts K, Delacourte A, de Strooper B and Dotti CG (2000). Brain plasmin enhances APP α-cleavage and Aβ degradation and is reduced in Alzheimer’s disease brains. EMBO Rep 1, 530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Kuo LL, Tan EC and Lee OK (2017). Is normal-tension glaucoma a risk factor for stroke?-A 10-year follow-up study. PLoS One 12, e0179307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim J and Choi S (2018). Endogenous amyloid-β mediates memory forgetting in the normal brain. Biochem. Biophys. Res. Commun 506, 492–497. [DOI] [PubMed] [Google Scholar]
- Lee CS, Larson EB, Gibbons LE, Lee AY, McCurry SM, Bowen JD, McCormick WC and Crane PK (2019). Associations between recent and established ophthalmic conditions and risk of Alzheimer’s disease. Alzheimers Dement 15, 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrançais E, Ortiz-Muñoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, Thornton EE, Headley MB, David T, Coughlin SR, Krummel MF, Leavitt AD, Passegué E and Looney MR (2017). The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 544, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Cao W and Sun X (2016). Role of platelet parameters on neovascular glaucoma: A retrospective case-control study in china. PLoS One 11, e0166893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Bhatia R and Lal R (2001). Amyloid β protein forms ion channels: implications for Alzheimer’s disease pathophysiology. FASEB J 15, 2433–2444. [DOI] [PubMed] [Google Scholar]
- Liu S, Liu Y, Hao W, Wolf L, Kiliaan AJ, Penke B, Rübe CE, Walter J, Heneka MT, Hartmann T, Menger MD and Fassbender K (2012). TLR2 is a primary receptor for Alzheimer’s amyloid β peptide to trigger neuroinflammatory activation. J. Immunol 188, 1098–1107. [DOI] [PubMed] [Google Scholar]
- Lomakin A, Teplow DB, Kirschner DA and Benedeki GB (1997). Kinetic theory of fibrillogenesis of amyloid β-protein. PNAS 94, 7942–7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łukasik ZM, Makowski M and Makowska JS (2018). From blood coagulation to innate and adaptive immunity: the role of platelets in the physiology and pathology of autoimmune disorders. Rheumatol. Int 38, 959–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ, Cui JG, Yuan LY, Bhattacharjee PS, Corkern M, Clement C, Kammerman EM, Ball MJ, Zhao Y, Sullivan PM and Hill JM (2010). Acyclovir or Aβ42 peptides attenuate HSV-1-induced miRNA-146a levels in human primary brain cells. Neuroreport 21, 922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusupov AIu. and Medvedev AN (1965). Blood transfusion in the therapy of glaucoma. [Article in Russian], Vestn. Oftalmol 78, 56–60. [PubMed] [Google Scholar]
- Maheshwari P and Eslick GD (2015). Bacterial infection and Alzheimer’s disease: a meta-analysis, J. Alzheimers Dis 43, 957–966. [DOI] [PubMed] [Google Scholar]
- Mann DM, Tucker CM and Yates PO (1988). Alzheimer’s disease: an olfactory connection? Mech. Ageing Dev 42, 1–15. [DOI] [PubMed] [Google Scholar]
- Mann C, Anders F, Liu H, Brockhaus K, Liu A, Grus FH, Pfeiffer N, Thanos S and Prokosch V (2018). Morphological and quantitative changes in retinal and optic nerve vessels in experimental glaucoma model with elevated IOP for 7 weeks., Klin. Monbl. Augenheilkd (in German) (in press). [DOI] [PubMed] [Google Scholar]
- Manne BK, Xiang SC and Rondina MT (2017). Platelet secretion in inflammatory and infectious diseases. Platelets 28, 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani MM, Malm T, Lamb R, Jay TR, Neilson L, Casali B,Medarametla L and Landreth GE. (2017). Neuronally-directed effects of RXR activation in a mouse model of Alzheimer’s disease. Sci. Rep 7, 42270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic V, Vukovic D, Radosavljevic A and Marjanovic I (2017). Acquired ectropion uveae and secondary glaucoma due to trauma: report of 3 cases. Eur. J. Ophthalmol 27, e1–e4. [DOI] [PubMed] [Google Scholar]
- Marples B, McGee MC, Martinez AA, Michael DB, Wilson GD and Fontanesi J (2012). A new use for an old treatment: Radiation therapy and Alzheimer’s disease. Radiation Oncol 84, Suppl.: S107. [Google Scholar]
- Marples B, McGee M, Callan S, Bowen SE, Thibodeau BJ, Michael DB, Wilson GD, Maddens ME, Fontanesi J and Martinez AA (2016). Cranial irradiation significantly reduces beta amyloid plaques in the brain and improves cognition in a murine model of Alzheimer’s Disease (AD). Radiother Oncol 118, 43–51. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Matsuhashi H and Nakazawa M (2001). Normal tension glaucoma and primary open angle glaucoma associated with increased platelet aggregation. Tohoku J. Exp. Med 193, 293–299. [DOI] [PubMed] [Google Scholar]
- Middeldorp J, Lehallier B, Villeda SA, Miedema SS, Evans E, Czirr E, Zhang H, Luo J, Stan T Mosher KI, Masliah E and Wyss-Coray T (2016). Preclinical assessment of young blood plasma for Alzheimer disease. JAMA Neurol 1, 73, 1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A, Schlevogt B, Kierdorf K, Böttcher C, Erny D, Kummer MP, Quinn M, Brück W, Bechmann I, Heneka MT, Priller J and Prinz M (2011). Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer’s disease. J. Neurosci 31, 11159–11171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miserocchi E, Waheed NK, Dios E, Christen W, Merayo J, Roque M and Foster CS (2002). Visual outcome in herpes simplex virus and varicella zoster virus uveitis. A clinical evaluation and comparison. Ophthalmology 109, 1532–1537. [DOI] [PubMed] [Google Scholar]
- Mondal L, Baidya K, Choudhury H and Roy R (2013). Myocardial infarction increases progressive visual field defects in well treated early primary open angle glaucoma-a prospective case control study. J. Indian Med. Assoc 111, 398–399. [PubMed] [Google Scholar]
- Moraes LA, Swales KE, Wray JA, Damazo A, Gibbins JM, Warner TD and Bishop-Bailey D (2007). Nongenomic signaling of the retinoid X receptor through binding and inhibiting Gq in human platelets. Blood 109, 3741–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell CN, Aggrey AA, Chapman LM and Modjeski KL (2014). Emerging roles for platelets as immune and inflammatory cells. Blood 123, 2759–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell CN, Pariser DN, Hilt ZT and Vega Ocasio D (2019). The platelet napoleon complex-small cells, but big immune regulatory functions. Annu. Rev. Immunol 37, 125–144. [DOI] [PubMed] [Google Scholar]
- Moschos MM, Moustafa GA, Papakonstantinou VD, Tsatsos M, Laios K and Antonopoulou S (2017). Anti-platelet effects of anti-glaucomatous eye drops: an in vitro study on human platelets. Drug Des. Devel. Ther 11, 1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroczko B, Groblewska M, Litman-Zawadzka A, Kornhuber J and Lewczuk P (2018). Cellular receptors of amyloid β oligomers (aβos) in Alzheimer’s disease. Int. J. Mol. Sci 19, 1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland PA and Denton NC (2006). Uveitic glaucoma. Contemp Ophthalmol 5, 1–6. [Google Scholar]
- Ning A, Cui J, To E, Ashe KH and Matsubara J (2008). Amyloid-beta deposits lead to retinal degeneration in a mouse model of Alzheimer disease. Invest Ophthalmol. Vis. Sci 49, 5136–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohm TG and Braak H (1987). Olfactory bulb changes in Alzheimer’s disease, Acta Neuropathol 73, 365–369. [DOI] [PubMed] [Google Scholar]
- Okamoto N, Ito Y, Nagai N, Murao T, Takiguchi Y, Kurimoto T and Mimura O (2010). Preparation of ophthalmic formulations containing cilostazol as an anti-glaucoma agent and improvement in its permeability through the rabbit cornea. J. Oleo Sci 59, 423–430. [DOI] [PubMed] [Google Scholar]
- Padovani A, Pastorino L, Borroni B, Colciaghi F, Rozzini L,Monastero R, Perez J, Pettenati C, Mussi M, Parrinello G, Cottini E, Lenzi GL, Trabucchi M, Cattabeni F and Di Luca M (2001) Amyloid precursor protein in platelets: a peripheral marker for the diagnosis of sporadic AD. Neurology 57, 2243–2248. [DOI] [PubMed] [Google Scholar]
- Plasma for Alzheimer’s Symptom Amelioration (PLASMA) Study Clinical Trial, https://clinicaltrials.gov/ct2/show/NCT02256306, accessed 8 January 2019
- Ratnayaka JA, Serpell LC and Lotery AJ (2005). Dementia of the eye: the role of amyloid beta in retinal degeneration. Eye (Lond) 29, 1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razonable RR, Henault M, Watson HL and Paya CV (2005). Nystatin induces secretion of interleukin (IL)-1beta, IL-8, and tumor necrosis factor alpha by a toll-like receptor-dependent mechanism, Antimicrob. Agents Chemother 49, 3546–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts GW, Gentleman SM, Lynch A and Graham DI (1991). βA4 amyloid protein deposition in brain after head trauma. Lancet 338, 1422–1423. [DOI] [PubMed] [Google Scholar]
- Rosenberg RN, Baskin F, Fosmire JA, Risser R, Adams P, Svetlik D, Honig LS, Cullum CM and Weiner MF (1997). Altered amyloid protein processing in platelets of patients with Alzheimer disease. Arch. Neurol 54, 139–144 [DOI] [PubMed] [Google Scholar]
- Saito S and Ihara M (2014). New therapeutic approaches for Alzheimer’s disease and cerebral amyloid angiopathy. Front Aging Neurosci 6, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S and Ihara M (2016). Interaction between cerebrovascular disease and Alzheimer pathology. Curr. Op. Psych 29,168–173. [DOI] [PubMed] [Google Scholar]
- Săninoiu M (1992). The relation between retinal venous occlusions and glaucoma, Oftalmologia 36, 163–171. [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D and Younkin S (1996). Secreted amyloid P-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat. Med 2, 864–870. [DOI] [PubMed] [Google Scholar]
- Schmaier AH (2016). Alzheimer disease is in part a thrombohemorrhagic disorder. J. Thromb. Haemost 14, 991–994. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ (2004). Cell biology of protein misfolding: the examples of Alzheimer’s and Parkinson’s diseases. Nat. Cell Biol 6, 1054–1061. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ and Hardy J (2016). The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med 8, 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta U, Nilson AN and Kayed R (2016). The role of amyloid-β oligomers in toxicity, propagation, and immunotherapy. EBioMedicine 6, 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda FJ, Parodi J, Peoples RW, Opazo C and Aguayo LG (2010). Synaptotoxicity of Alzheimer beta amyloid can be explained by its membrane perforating property. PLoS One 5, e11820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevush S, Jy W, Horstman LL, Mao WW, Kolodny L and Ahn YS (1998). Platelet activation in Alzheimer disease. Arch. Neurol 55, 530–536. [DOI] [PubMed] [Google Scholar]
- Seyoum M, Enawgaw B and Melku M (2018). Human blood platelets and viruses: defense mechanism and role in the removal of viral pathogens. Thromb J 16, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharda A and Flaumenhaft R (2018). The life cycle of platelet granules. F1000 Res 7, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP and Rivest S (2006). Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron 49, 489–502. [DOI] [PubMed] [Google Scholar]
- Sivak JM (2013). The aging eye: common degenerative mechanisms between the Alzheimer’s brain and retinal disease, Invest Ophthalmol. Vis. Sci 54, 871–880. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Pietropaolo MF, Rosene DL, Moss MB, Peters A, Kemper T and Abraham CR (1997). Lack of correlation between plaque burden and cognition in the aged monkey. Acta Neuropathol 94, 471–478. [DOI] [PubMed] [Google Scholar]
- Smith KG and Clatworthy MR (2010). Fc gamma RIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat. Rev. Immunol 10, 328–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M,Hyman B, Burton MA, Goldstein LE, Duong S, Tanzi RE and Moir RD (2010). The Alzheimer’s disease-associated amyloid β-protein is an antimicrobial peptide. PLoS One 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ştefan C, Timaru CM, Iliescu DA, Schmitzer S, De Algerino S, Batras M and Hosseini-Ramhormozi J (2016). Glaucoma after chemical burns and radiation. Rom J. Ophthalmol 60, 209–215. [PMC free article] [PubMed] [Google Scholar]
- Suidan GL, Singh PK, Patel-Hett S, Chen ZL, Volfson D, Yamamoto-Imoto H, Norris EH, Bell RD and Strickland S (2018). Abnormal clotting of the intrinsic/contact pathway in Alzheimer disease patients is related to cognitive ability. Blood Adv 2, 954–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Takata Y, Ihara M, Kasahara Y, Tsuji M, Nishino M, Stern D and Okada M (2013). Cilostazol improves cognitive function in patients with mild cognitive impairment: a retrospective analysis. Psychogeriatrics 13, 164–169. [DOI] [PubMed] [Google Scholar]
- Tai SY, Chen CH, Chien CY and Yang YH (2017). Cilostazol as an add-on therapy for patients with Alzheimer’s disease in Taiwan: a case control study. BMC Neurol 17, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YQ, Yeaman MR and Selsted ME(2002). Antimicrobial peptides from human platelets. Infect. Immun 70, 6524–6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezapsidis N, Li HC, Ripellino JA, Efthimiopoulos S,Vassilacopoulou D, Sambamurti K, Toneff T, Yasothornsrikul S, Hook VY and Robakis NK (1998). Release of nontransmembrane full-length Alzheimer’s amyloid precursor protein from the lumenar surface of chromaffin granule membranes. Biochemistry 37, 1274–1282. [DOI] [PubMed] [Google Scholar]
- Tezapsidis N, Li H-G, Ripellino JA, Efthimiopoulos S, Vassilacopoulou D, Sambamurti K, Toneff T, Toledo JB, Shaw LM and Trojanowski JQ (2013). Plasma amyloid beta measurements - a desired but elusive Alzheimer’s disease biomarker. Alzheimers Res. Ther 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjernberg LO, Pramanik A, Björling S, Thyberg P, Thyberg J, Nordstedt C, Berndt KD, Terenius L and Rigler R (1999). Amyloid beta-peptide polymerization studied using fluorescence correlation spectroscopy. Chem. Biol 6, 53–62. [DOI] [PubMed] [Google Scholar]
- Tolppanen AM, Lavikainen P, Solomon A, Kivipelto M, Soininen H and Hartikainen S (2013). Incidence of stroke in people with Alzheimer disease: a national register-based approach. Neurology 80, 353–358. [DOI] [PubMed] [Google Scholar]
- Trier DA, Gank KD, Kupferwasser D, Yount NY, French WJ, Michelson AD, Kupferwasser LI, Xiong YQ, Bayer AS and Yeaman MR (2008). Platelet antistaphylococcal responses occur through P2X1 and P2Y12 receptor-induced activation and kinocidin release. Infect. Immun 76, 5706–5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolaki F, Gogaki E, Tiganita S, Skatharoudi C, Lopatatzidi C, Topouzis F and Tsolaki M (2011). Alzheimer’s disease and primary open-angle glaucoma: is there a connection? Clin. Ophthalmol 5, 887–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nostrand WE, Schmaier AH, Farrow JS and Cunningham DD (1990). Protease nexin-II (amyloid β-protein precursor): A platelet α-granule protein. Science 248, 745–748. [DOI] [PubMed] [Google Scholar]
- Van Wijngaarden P, Hadoux X, Alwan M, Keel S and Dirani M (2017). Emerging ocular biomarkers of Alzheimer disease. Clin. Exp. Ophthalm 45, 54–61. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A and Rodríguez JJ (2018). Cell-autonomous astrocytopathy in Alzheimer’s disease. Acta Physiol. (Oxf) 223, e13070. [DOI] [PubMed] [Google Scholar]
- Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, Wabl R, Udeochu J, Wheatley EG, Zou B, Simmons DA, Xie XS, Longo FM and Wyss-Coray T (2014). Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice, Nat. Med 20, 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vom Berg J, Prokop S, Miller KR, Obst J, Kälin RE, Lopategui-Cabezas I, Wegner A, Mair F, Schipke CG, Peters O, Winter Y, Becher B and Heppner FL (2012). Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease-like pathology and cognitive decline. Nat. Med 18, 1812–1819. [DOI] [PubMed] [Google Scholar]
- Washington PM, Morffy N, Parsadanian M, Zapple DN and Burns MP (2014). Experimental traumatic brain injury induces rapid aggregation and oligomerization of amyloid-beta in an Alzheimer’s disease mouse model. J. Neurotrauma 31, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MR, Kandel R, Tripathi S, Condon D, Qi L, Taubenberger J and Hartshorn KL (2014). Alzheimer’s associated β-Amyloid protein inhibits influenza a virus and modulates viral interactions with phagocytes. PLoS One 9, e101364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM and Griffin WST (2013). Down’s syndrome, neuroinflammation, and Alzheimer neuropathogenesis. J. Neuroinflammation 10, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GD and Marples BA (2016). New use for an old treatment: Radiation therapy and Alzheimer’s disease. Radiat. Res 185, 443–448. [DOI] [PubMed] [Google Scholar]
- Yeaman MR (2010). Platelets in defense against bacterial pathogens, Cell Mol. Life Sci 67, 525–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman MR, Ibrahim AS, Edwards JE Jr, Bayer AS and Ghannoum MA (1993). Thrombin-induced rabbit platelet microbicidal protein is fungicidal in vitro. Antimicrob. Agents Chemother 37, 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman MR, Tang Y-Q, Shen AJ, Bayer AS and Selsted ME (1997). Purification and in vitro activities of rabbit platelet microbicidal proteins. Infect. Immun 65, 1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda S, Hara H, Hirata A, Fukushima M, Inomata Y and Tanihara H (2005). Vitreous fluid levels of beta-amyloid((1–42)) and tau in patients with retinal diseases. Jpn J. Ophthalmol 49, 106–108. [DOI] [PubMed] [Google Scholar]
- Yount NY, Gank KD, Xiong YQ, Bayer AS, Pender T, Welch WH and Yeaman MR. (2004). Platelet microbicidal protein 1: structural themes of a multifunctional antimicrobial peptide, Antimicrob. Agents Chemother 48, 4395–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamolodchikov D, Renné T and Strickland S (2016). The Alzheimer’s disease peptide β-amyloid promotes thrombin generation through activation of coagulation factor XII. J. Thromb. Haemost, 14, 995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman-Rudenko J, Reitsma SE, Puy C, Rigg RA, Smith SA, Tucker EI, Silasi R, Merkulova A, McCrae KR, Maas C, Urbanus RT, Gailani D, Morrissey JH, Gruber A, Lupu F, Schmaier AH and McCarty OJT (2018). Factor XII Activation promotes platelet consumption in the presence of bacterial-type long-chain polyphosphate in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol 38, 1748–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zott B, Busche MA, Sperling RA and Konnerth A (2018). What happens with the circuit in Alzheimer’s disease in mice and humans? Annu. Rev. Neurosci 41, 277–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D and Philipp CS (2000). Platelet production in the pulmonary capillary bed: new ultrastructural evidence for an old concept. Am. J. Pathol 157, 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]