Abstract
Buprenorphine partial opioid agonist pharmacotherapy, a key treatment for opioid use disorders (OUDs), is underutilized in the United States. Qualitative interviews, conducted in 2012/2013 and repeated in 2015, identified systemic barriers to providing buprenorphine treatment in Ohio. A representative sample of Ohio’s Alcohol, Drug Abuse and Mental Health Services (ADAMHS) county boards (n=18) was selected based on percentage of OUD admissions, density of buprenorphine prescribers, and county board area population. Boards reported that the barriers to the use of buprenorphine in 2012/2013 included a) negative attitudes toward the use of buprenorphine among substance use disorder treatment providers, b) a lack of prescribers, and c) lack of funding. The 2015 interviews suggested that the lack of prescribers surpassed lack of funding as the main impediment to buprenorphine expansion. Negative provider attitudes were no longer problematic. Concerns about buprenorphine diversion, however, had emerged as a new barrier. This paper offers recommendations for future policy efforts to overcome these barriers and expand the use of evidence-based opioid treatments. It highlights the need for payers and policymakers to increase the number of buprenorphine prescribers to make best use of funding available to fight the opioid epidemic.
Keywords: Buprenorphine, opioid use disorders, evidence-based practices, substance use disorders
Expanding access to treatment services is an essential component of the response to the opioid overdose epidemic (Rudd et al. 2016; Compton, Boyle, and Wargo 2015) that resulted in 90 American deaths per day in 2015 (Rudd et al. 2016) and a four-fold increase in opioid overdose deaths from 1999 to 2015 (Centers for Disease Control and Prevention [CDC] 2016). Historically, treatment for opioid use disorders (OUD) in the United States has emphasized behavioral therapies and strong linkages and preferences for 12-step orientations and an “abstinence only” approach to recovery (Wilson and Cohen 2015; Cook 1988; Van Wormer and Davis 2013). Access to opioid agonist therapy (OAT) with methadone (a full opioid agonist) or buprenorphine (a partial opioid agonist) has been limited.
The National Institute on Drug Abuse, World Health Organization (WHO), and American Society of Addiction Medicine (ASAM), however, advocate for the use of OAT plus counseling to address OUD and promote sustained recovery (Volkow et al. 2014; WHO 2003; ASAM 2015). Agonist and partial agonist pharmacotherapies block opioid receptors and, when paired with cognitive behavioral therapy, have greater treatment retention rates and reductions in use of illicit opioids compared to behavioral therapy alone (Dugosh et al. 2016; Mattick et al. 2014).
Methadone has been the primary pharmacotherapy for opioid dependence, but it is only available in regulated opioid treatment programs where medication is dispensed in a controlled environment, rather than prescribed on an outpatient basis. When approved by the U.S. Food and Drug Administration in 2002, buprenorphine was projected to play an important role in treating OUDs because, as a partial opioid agonist, it is a safer medication than a full opioid agonist such as methadone (Thomas et al. 2014), and can be prescribed in an office setting. The Drug Addiction and Treatment Act of 2000 (DATA 2000) (Substance Abuse and Mental Health Services Administration [SAMHSA] 2015) permits prescribers who complete training (8 hours for physicians and 24 hours for nurse practitioners and physician assistants) to receive a waiver from federal regulations prohibiting the use of narcotics to treat narcotic addiction. Prescribers with a DATA 2000 certification waiver can treat up to 30 patients with buprenorphine in the first year. Physicians may request authorization to increase their caseload to 100 in the second year, and up to 275 active patients per year after that, under current regulations (SAMHSA 2016).
Despite buprenorphine’s promise for OUD, adoption has been limited. In 2012, 96% of states had more individuals with OUDs than buprenorphine capacity to treat those individuals (Jones et al. 2015). In 2016, 27% of specialty SUD facilities offered buprenorphine (SAMHSA 2017). In 2016, there were no DATA 2000 waivered prescribers available in 60% of rural counties within the United States (Andrilla, Coulthard, and Larson 2017). In Ohio in 2012, nearly 20% of buprenorphine DATA 2000 waivered prescribers were inactive, not providing buprenorphine treatment (Parran et al. 2017).
Prescribers’ low buprenorphine adoption rates reflect concerns about reimbursement (Netherland et al. 2009), the requirement for DATA 2000 training and associated DEA oversight (Molfenter, Sherbeck, Zehner, and Starr 2015), a fear of being inundated with buprenorphine requests (Huhn and Dunn 2017), and concerns about diversion of buprenorphine for non-prescription use (Huhn and Dunn 2017). The low adoption rate prompted an investigation of barriers that inhibited routine use of buprenorphine as an opioid agonist therapy in Ohio.
Ohio has the fourth highest age-adjusted drug overdose mortality rate in the United States (Rudd et al. 2016). In 2013, 24% of Ohio SUD treatment centers used buprenorphine for OUD treatment (SAMHSA 2014). Ohio’s regional Alcohol, Drug Addiction and Mental Health Services (ADAMHS) boards play a key role in developing regional SUD treatment policies, provide funds for addiction services, and serve as the local public health authority in addressing the opioid epidemic. Each ADAMHS board represents one to five counties. These Boards develop policy based on public health trends, emerging evidence-based practices, analyses of requests for services, admission statistics, and stakeholder input. The boards differ from traditional insurers in that they provide grants in aid rather than payment for claims submitted. The boards also provide funding through the Substance Abuse and Prevention Treatment (SAPT) block grant and local tax levies. The ADAMHS boards are in regular contact with patients to address complaints and assist with service access. They are also in contact with service providers to discuss service barriers and needs.
The study was designed to assess perceived barriers to buprenorphine adoption among Ohio’s county board payers across two sets of interviews. Initial interviews were completed in 2012/2013 and follow-up interviews in 2015. The analysis examines how perceived barriers to using buprenorphine for OUD treatment evolved over time within Ohio’s Boards. During this period (2012 to 2015), the opioid overdose death rate (age-adjusted) increased by 41% (CDC 2016), leading to a greater focus on OUDs by the media, public health officials, and SUD treatment providers.
Methods
Study participants and interviews.
The interviews were conducted as part of a cluster randomized trial focused on improving access to buprenorphine pharmacotherapy for OUDs (Molfenter et al. 2013). A representative sample of ADAMHS county Boards (n =18) was selected based on opioid admission rates, buprenorphine prescriber density, and county board area population. The sample included 27 of Ohio’s 88 counties encompassing 52% of the state’s total population and 44% (65/315) of all publicly funded SUD treatment providers with more than 100 admissions per year. These providers are SUD specialty treatment clinics and typically do not include primary care practices. No other Ohio county boards were approached to participate in this study. No county boards refused to participate in the interviews. The board’s highest-ranking clinical officer or the executive director completed the county board interviews between November 1, 2012, to February 28, 2013, for Round 1 and between February 1 to March 31, 2015, for Round 2. Ninety-two percent of the individual participants who participated in Round 1 participated in Round 2 interviews.
The interview guide included fixed and open-ended response items that queried the boards’ reasons for supporting buprenorphine, the barriers to adoption they had encountered, and the actions taken to overcome these barriers. The fixed questions asked if certain barriers were present (i.e., insufficient funds, negative attitudes about medication-assisted treatment [MAT], high cost of buprenorphine, physicians unwilling to prescribe buprenorphine, addiction treatment providers’ lack of MAT knowledge, a lack of criminal justice support, or concerns regarding diversion). Table 1 summarizes the descriptive characteristics of the participating boards and the providers they represent.
Table 1.
Site characteristics for the participating boards and their provider networks
| N | % | ||
|---|---|---|---|
| Counties Represented (n=27) | |||
| County boards (n=18) | Title | Count | % |
| General Job Description (of interview participants) | Executive Director | 8 | 44.4 |
| Vice President/Director | 9 | 50.0 | |
| Medical Director | 1 | 5.6 | |
| People Served | <1000 | 4 | 22.2 |
| 1000–1999 | 6 | 33.3 | |
| 2000–3999 | 5 | 27.8 | |
| 4000+ | 3 | 16.7 | |
| Funds Used for Buprenorphine* | Medicaid | 18 | 100.0 |
| SAPT Block Grant | 7 | 38.9 | |
| Tax Levy | 13 | 72.2 | |
| State Grant | 1 | 5.6 | |
| Federal Grant | 3 | 16.7 | |
| Board provider network (n=36)* | |||
| Services** | Detoxification | 12 | 33.3 |
| Outpatient | 30 | 83.3 | |
| Intensive Outpatient | 26 | 72.2 | |
| Residential | 17 | 47.2 | |
| Physicians on Staff | Yes | 19 | 52.8 |
| No | 17 | 47.2 | |
| Use of Methadone On-Site | Yes | 5 | 13.9 |
| No | 31 | 86.1 | |
= This data does not represent interview data
= Multiple responses were possible.
Qualitative Analysis.
A qualitative content analysis approach was used to review the data. In a multi-stage process of data reduction, exploration, and reconstitution, the researchers first sought consensus on code nomenclature and definitions to develop a codebook and ensure consistent coding. Code categories were developed during the review of the first wave of data. The same codes were used for the second wave of coding with a few new codes added due to wave two interview responses. For each round, once coding was complete, text queries of specific codes were run using ATLAS.ti (2015). Four members of the research team generated, discussed, and revised code summaries to produce a list of core themes based on frequency of mentions and saliency of comments made, with examples of text attached to each theme. The coding for each wave was done independent of the other wave. The themes extracted from each wave were compared. For the final data presentation, the team selected quotes to illustrate themes from each wave based on clarity, brevity, and typicality, or to represent similar comments from a range of participants. A thematic count supported the assessment of change in the perceived barriers to the use of buprenorphine. This study received approval from the Institutional Review Board at the University of Wisconsin–Madison.
Results
During the 2012/13 and 2015 interviews, all (n=18) ADAMHS boards viewed buprenorphine as a viable option to treat OUD. Board reasons to support the use of buprenorphine remained consistent over time – a desire to respond to the opioid epidemic and to prevent overdose deaths:
“Our county jail is saying that 60% of the people coming into the jail are going through withdrawal from opiates. We are being overrun with this,” and
“We identified this huge increase in opioid addiction coming through the door. That is what kicked off our search for what are the best practices out there and that is how we got involved with buprenorphine.”
Boards encouraged the use of buprenorphine over the period of the study because they perceived it to be effective:
“We’re using buprenorphine as a detox medication because it gets people into the clinical and educational components of treatment a lot easier.”
“Our providers are really using buprenorphine to get people retained in treatment until they have the skills to stay sober in the long term, until they have the recovery supports around them.”
Barriers to greater use of buprenorphine
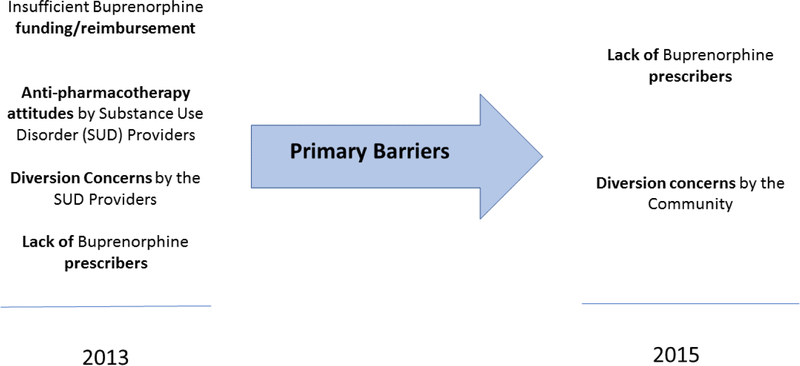
Despite the broad evidence base (Mattick et al. 2014; Thomas et al. 2014) supporting buprenorphine’s effectiveness in treating OUDs, barriers to buprenorphine use were observed in both sets of interviews. Respondents identified payment environment, anti-pharmacotherapy attitudes by SUD providers, diversion concerns, and physician prescribing capacity as barriers to buprenorphine use.
Insufficient funds to pay for buprenorphine was a primary barrier in 2012/13. Buprenorphine can cost $360/month per patient (Jones et al. 2015). Providers must use public funds to pay for the medication for uninsured patients who cannot afford the expense. In 2013, a board member explained, “We really don’t have money to fund medication therapy.” In the 2015 interviews, however, funding was no longer a major barrier. Because of increased attention to the opioid epidemic, more funds were available through local tax levies and state and federal opioid treatment grants. In addition, Medicaid resources increased in Ohio in 2014 following Medicaid expansion under the Affordable Care Act (ACA). Even though these changes reduced a significant barrier, buprenorphine adoption rates remained low.
Anti-pharmacotherapy attitudes toward the use of medications to treat addictions were deeply embedded in the SUD specialty treatment provider community at the time of the 2012/2013 interviews. The sentiment expressed in many parts of the SUD treatment community was that pharmacotherapy “is substituting one drug for another.” A board member explained, “Some clinicians were questioning why we would give a drug to an addict.” To counteract these perceptions, boards initiated educational campaigns to increase provider knowledge about buprenorphine, and some boards added contract incentives for using buprenorphine in SUD treatment. During the study period, moreover, clinicians began to witness the benefits of buprenorphine therapy firsthand. A board provider noted, “Once the client is on [buprenorphine], it becomes so much easier to treat them. They can actually pay attention.” The broader understanding and use of buprenorphine among the SUD provider community contributed to the boards being less concerned about provider anti-pharmacotherapy attitudes during the 2015 interviews. As one board member stated, “The perception towards buprenorphine is definitely much better than it was a couple years ago with our treatment providers.”
Local communities, however, became more concerned with use of buprenorphine pharmacotherapy. In 2012/13 concerns about community resistance were barely mentioned, but they were a prominent theme in 2015. A board interviewee noted, “When we first announced (in 2014) that we were going to be doing buprenorphine, the community and courts weren’t terribly happy with the idea.” The resistance seemed to be based on potential diversion of buprenorphine to individuals who did not have a prescription for the medication. As one participant described, “The public is not favorable to buprenorphine use due to the diversion rate we are seeing.”
Concerns regarding buprenorphine diversion were strong among the boards in both the 2012/13 and 2015 data collections. They did not want to be perceived as contributing to their community’s illicit drug problem. One board reported, “We know there’s a lot of diversion going on out there but that looks bad on us even though we got stricter guidelines about how we deliver [buprenorphine] to the patient.” Moreover, the boards believed that diverted medication often originated from cash-based medical practices that do not follow the buprenorphine diversion prevention policies. A board member explained, “The thing is diversion isn’t coming from our funded programs. It’s coming from the for-profit docs out in the community.” Boards “want[ed] to make sure that any referrals are going to providers that are working directly with our agencies.” In 2015, boards perceived increased diversion concerns among community stakeholders:
“Law enforcement, prosecuting attorneys, and some judges are not supportive of using Suboxone® because they see the misuse and that concerns them.”
“The public as well as local law enforcement were having issues with it (Suboxone®) being sold on the street, diversion, and those kinds of things.”
Ohio’s county boards reported that communities had minimal or incorrect information about SUDs in general, and more specifically about use of buprenorphine. As a result, in the 2015 interviews, boards reported conducting outreach through community forums and community-based opioid prevention task forces. Interviewees felt that these forums were beneficial and should continue to be offered:
“ We have sponsored a large symposium where we had about 250 people there and also have done some town hall meetings where we’ve talked about buprenorphine and are getting ready to do another round of community educational sessions.”
“Our community conversations have begun to open doors that were not opened five years ago.”
Physician capacity for buprenorphine was a concern in the 2012/13 interviews because few physicians were authorized to prescribe buprenorphine. Boards met the task of recruiting physicians with frustration:
“There are many [doctors] that don’t want to treat that population. They have full caseloads… and they just don’t want to be bothered.” Or,
“Our providers went around to identify different physicians that would be willing to be involved as a referral site for us and we got a tremendous amount of negative or uninterested response.”
As a result, existing prescribers faced additional pressure to meet the increasing need for buprenorphine therapy services (Molfenter, Sherbeck, Zehner, Quanbeck, et al. 2015). Limited physician capacity continued to be an issue in 2015 and emerged as a primary barrier to expanding buprenorphine therapy. Boards reported being unable to fully expend the budgeted funds secured to pay for buprenorphine because they could not engage physicians willing to become prescribers. Boards also increased efforts to recruit physicians through provided training about the need to address the opioid epidemic, Continuing Medical Education courses on the efficacy of buprenorphine, and presentations at hospital grand rounds, evening educational events, and at physician residency programs in the state. In spite of these efforts, physician disinterest in buprenorphine prescribing continued.
In sum, Table 2 rank orders counts of the number of thematic mentions in 2012/2013 versus 2015 to illustrate changes in the relative perceived barriers and concerns. The rankings and qualitative findings found that in 2012/13 boards perceived the primary barriers to use of buprenorphine as provider attitudes, physician prescribing capacity, and insufficient funds to pay for buprenorphine. In 2015, physician prescribing capacity was the only strong barrier that remained from 2012/2013. Provider attitudes toward buprenorphine treatment had improved, while community concerns about buprenorphine diversion emerged as a barrier in 2015.
Table 2:
Prominent Themes, with Quotes and Frequency Counts
| Top Items 2012/13 |
Frequency Count |
Top Items 2015 |
Frequency Count |
|---|---|---|---|
| Insufficient Funding Reimbursement We don’t have enough funds for MAT, because we would have to take it from existing programming. |
15 | Lack of Buprenorphine Prescribers Physician capacity is our biggest barrier. Most of the practitioners … are family practitioners meaning that their clientele is families and the elderly and so forth. The stigma just doesn’t sit well with that. |
13 |
| Anti-Pharmacotherapy Attitudes Those in recovery say if you are taking a pill, then you’re not in recovery. |
13 | ||
| Diversion Concerns (Providers) Buprenorphine is becoming one of the drugs that are being abused and sold on the street. |
9 | Diversion Concerns (Community) Some people in the community equate Suboxone distribution to pill mills where there was a lot of misuse. Still some resistance from especially the police courts with buprenorphine versus Vivitrol or something that’s a non-opiate. |
11 |
| Lack of Buprenorphine Prescribers Doctors do not want to deal with this population. |
8 |
Discussion
Limited buprenorphine access has significant implications for states that received federal funding to combat the opioid epidemic. Our findings suggest that funding alone will have limited effect in addressing the opioid epidemic. States need strategies that can engage new buprenorphine prescribers to reduce the gap between the demand for OAT and the ability to access OAT (Thomas et al. 2014). States need to dedicate resources to recruiting prescribers. Specialty treatment providers that committed more resources to prescriber recruitment were found to have greater opioid treatment pharmacotherapy capacity in a 2017 sample of 160 SUD providers (Knudsen, 2018). Other practice changes at the provider level could include a) developing effective, replicable prescriber recruiting practices that SUD treatment organizations can learn and apply, b) marketing the need for and benefits of buprenorphine prescribing to non-addiction medicine physicians and, c) expanding the number of opioid treatment programs dispensing methadone and buprenorphine.
For these outreach efforts to be most effective, public payers and treatment organizations need to address other barriers to buprenorphine prescribing. Physicians not wanting to become buprenorphine prescribers cite their highest concerns as “not enough time” to prescribe buprenorphine, the need for addiction treatment specialty back-up to address their lack of confidence in treating patients with SUDs (Hutchinson et al. 2014; Netherland et al. 2009), and lack of available psychosocial or counseling support for these patients (Hutchinson et al. 2014). Some prescribers with waivers, moreover, have low rates of prescribing; 22% to 49% of waivered physicians treat 5 or fewer buprenorphine patients. (Stein et al. 2016; Sigmon 2015). Physicians not prescribing to capacity cite insufficient time for more patients, insufficient reimbursement, and concerns about diversion as reasons for not prescribing to capacity; having information about local counseling resources and being paired with an experienced provider, conversely, were strategies likely to increase willingness to increase caseloads of patients using buprenorphine (Huhn and Dunn 2017).
Three models used to address physician concerns related to time constraints and need for more behavioral health support include a) pairing the prescriber with a nurse or a team of behavioral health professionals (LaBelle et al. 2016), b) developing a “hub and spoke” method where physicians in the community (spoke) refer patients to specialty treatment “hubs” until they are stabilized (Stoller 2015; Brooklyn and Sigmon 2017) and c) Project ECHO (Extension for Commnity Healthcare Outcomes) training where physicians can hear other physicians provide case presentations about patients on buprenorphine pharmacotherapy by webinar or video media. The Massachusetts Nurse Care Model focuses physician time on prescribing services while the nurses manage counseling and diversion prevention services (e.g., urine drug screens and medication reconciliations) (Labell et al. 2016). The Hub and Spoke and Project ECHO models have the benefit of giving the physician SUD specialist support through the Hub and the Project ECHO network.
Another emerging mechanism to increase buprenorphine prescribing capacity is through new laws allowing nurse practitioners (NPs) and physician assistants (PAs) to prescribe buprenorphine. Section 303 of the Comprehensive Addiction and Recovery Act (CARA) made these provider categories eligible for buprenorphine waivers after 24 hours of education by a qualified provider (United State Congress 2016). NPs and PAs can then prescribe buprenorphine to 30 patients at a time in the year following the training and 100 at a time in the years thereafter.
Buprenorphine diversion studies have documented several explanations for the diversion of buprenorphine (Wish et al. 2012). Lofwall and Havens (2012) discovered that daily use of diverted buprenorphine occurred in 4.5% of their sample; people who could not access buprenorphine through the health care system often used diverted buprenorphine to self-treat opioid withdrawal. Buprenorphine does not seem to be the drug of choice for many opioid users, however. In one study of patients who had used opioid analgesics to get high over the past 30 days, 97% stated they preferred to use opioids intended for pain relief (e.g., fentanyl, hydromorphone, morphine, or tramadol) over buprenorphine (Cicero, Surratt, and Inciardi 2007). In practice, the amount of diversion occurring could be reduced by applying evidence-based diversion prevention practices that include a) regular behavioral therapy sessions, b) random urine drug screens; and c) random buprenorphine medication counts (Lofwall et al. 2011).
Study limitations include generalizability, as, the sample was recruited from one US state. It is unknown if these findings would generalize to payer practices and opinions in other states. Second, the research, while containing a representative sample, did not include all Ohio ADAMHS boards. Within the boards, interviews were completed with a single person within the organization, possibly providing a limited perspective of the conditions with that board area. Furthermore, responses may reflect payers that had greater interest in expanding buprenorphine, since this was conducted as part of a randomized control trial to increase buprenorphine use. Future research should attempt to understand how barriers to opioid pharmacotherapy treatment evolve, as the opioid epidemic continues to expand and receive policy attention and state and federal funding.
In summary, prescriber capacity and concerns regarding diversion remained persistent barriers to broader use of buprenorphine pharmacotherapy across the study period. Buprenorphine administration involves prescriber recruitment, workflow adjustments, and diversion prevention strategies that must be addressed in order to build the capacity to provide this pharmacotherapy and maximize the federal dollars that are being allocated to tackle the public health crisis caused by the opioid epidemic in the United States.
Figure #1:
Progression of Buprenorphine Barriers
ACKNOWLEDGEMENTS
The research and preparation of the manuscript were supported by grants from the National Institutes on Drug Abuse (R01 DA030431) & (R01 DA41415). Author x (for anonymity purposes, will be provided post review) receives support from the National Institute on Drug Abuse (UG1 DA015815, P50 DA018165, and R33 DA035640). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Contributor Information
Todd Molfenter, Center for Health Enhancement Systems Studies, University of Wisconsin-Madison, 1513 University Ave., Madison, WI 53706.
Maureen Fitzgerald, Center for Health Enhancement Systems Studies, University of Wisconsin-Madison, 1513 University Ave., Madison, WI 53706, maureen.fitzgerald@wisc.edu.
Nora Jacobson, School of Nursing, University of Wisconsin-Madison, 701 Highland Avenue, Madison, WI 53705, najacobson@wisc.edu.
Dennis McCarty, Oregon Health and Science University-Portland State University, School of Public Health, Portland, OR, mccartyd@ohsu.edu.
Andrew Quanbeck, School of Medicine and Public Health, University of Wisconsin-Madison, arquanbe@wisc.edu.
Mark Zehner, School of Medicine and Public Health/Tobacco Research and Intervention, 1930 Monroe St., Madison, WI 53711, mark.zehner@ctri.wisc.edu.
References
ATLAS.ti, Version 7.0. Scientific Software Development GmbH.
- American Society of Addiction Medicine (ASAM). 2015. The ASAM national practice guideline for the use of medications in the treatment of addiction involving opioid use Chevy Chase, MD: American Society of Addiction Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrilla CHA, Coulthard C, and Larson EH. 2017. Barriers rural physicians face prescribing buprenorphine for opioid use disorder. Annals of Family Medicine 15 (4):359–62. doi: 10.1370/afm.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooklyn JR, and Sigmon SC. 2017. Vermont hub-and-spoke model of care for opioid use disorder: development, implementation, and impact. Journal of Addiction Medicine 11 (4):286–92. doi: 10.1097/adm.0000000000000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). 2016a. Wide-ranging Online Data for Epidemiologic Research (WONDER) U.S. Department of Health & Human Services; https://wonder.cdc.gov/ (accessed December 21, 2016). [Google Scholar]
- Centers for Disease Control and Prevention (CDC). 2016b. Injury prevention & control: opioid overdose Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; http://www.cdc.gov/drugoverdose/states/state_prevention.html (accessed June 14, 2016). [Google Scholar]
- Cicero TJ, Surratt HL, and Inciardi J. 2007. Use and misuse of buprenorphine in the management of opioid addiction. Journal of Opioid Management 3 (6):302–8. [DOI] [PubMed] [Google Scholar]
- Compton WM, Boyle M, and Wargo E. 2015. Prescription opioid abuse: Problems and responses. Preventive Medicine 80:5–9. doi: 10.1016/j.ypmed.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Cook CH 1988. The Minnesota model in the management of drug and alcohol dependency: Miracle, method or myth? Part I. The Philosophy and the Programme★. British Journal of Addiction 83 (6):625–34. [DOI] [PubMed] [Google Scholar]
- Dugosh K, Abraham A, Seymour B, McLoyd K, Chalk M, and Festinger D. 2016. A systematic review on the use of psychosocial interventions in conjunction with medications for the treatment of opioid addiction. Journal of Addiction Medicine 10 (2):93–103. doi: 10.1097/adm.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn AS, and Dunn KE. 2017. Why aren’t physicians prescribing more buprenorphine? Journal of Substance Abuse Treatment 78:1–7. doi: 10.1016/j.jsat.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson E, Catlin M, Andrilla CH, Baldwin LM, and Rosenblatt RA. 2014. Barriers to primary care physicians prescribing buprenorphine. Annals of Family Medicine 12 (2):128–33. doi: 10.1370/afm.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Campopiano M, Baldwin G, and McCance-Katz E. 2015. National and state treatment need and capacity for opioid agonist medication-assisted treatment. American Journal of Public Health 105 (8):e55–63. doi: 10.2105/ajph.2015.302664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korthuis PT, McCarty D, Weimer M, Bougatsos C, Blazina I, Zakher B, Grusing S, Devine B, and Chou R. 2017. Primary care-based models for the treatment of opioid use disorder: A scoping review. Annals of Internal Medicine 166 (4):268–78. doi: 10.7326/m16-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen H, Brown R, Jacobson N, Horst J, Kim J, Collier E, Starr S, and Molfenter T 2018. Resource needs, pharmacotherapy, and physician recruitment in specialty substance use disorder treatment organizations. Journal of Addiction Medicine [DOI] [PMC free article] [PubMed]
- LaBelle CT, Han SC, Bergeron A, and Samet JH. 2016. Office-Based Opioid Treatment with Buprenorphine (OBOT-B): Statewide implementation of the Massachusetts Collaborative Care Model in community health centers. Journal of Substance Abuse Treatment 60:6–13. doi: 10.1016/j.jsat.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofwall MR, Wunsch MJ, Nuzzo PA, and Walsh SL. 2011. Efficacy of continuing medical education to reduce the risk of buprenorphine diversion. Journal of Substance Abuse Treatment 41 (3):321–9. doi: 10.1016/j.jsat.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Lofwall MR, and Havens JR. 2012. Inability to access buprenorphine treatment as a risk factor for using diverted buprenorphine. Drug and Alcohol Dependence 126 (3):379–83. doi: 10.1016/j.drugalcdep.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, and Davoli M. 2014. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews (2):Cd002207. doi: 10.1002/14651858.CD002207.pub4. [DOI] [PubMed] [Google Scholar]
- Molfenter T, Kim JS, Quanbeck A, Patel-Porter T, Starr S, and McCarty D. 2013. Testing use of payers to facilitate evidence-based practice adoption: Protocol for a cluster-randomized trial. Implementation Science 8:50. doi: 10.1186/1748-5908-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfenter T, Sherbeck C, Zehner M, and Starr S. 2015a. Buprenorphine prescribing availability in a sample of Ohio specialty treatment organizations. Journal of Addictive Behaviors, Therapy & Rehabilitation 4:2. doi: 10.4172/2324-9005.1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfenter T, Sherbeck C, Zehner M, Quanbeck A, McCarty D, Kim JS, and Starr S. 2015b. Implementing buprenorphine in addiction treatment: Payer and provider perspectives in Ohio. Substance Abuse Treatment, Prevention, and Policy 10 (1):13. doi: 10.1186/s13011-015-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherland J, Botsko M, Egan JE, Saxon AJ, Cunningham CO, Finkelstein R, Gourevitch MN, Renner JA, Sohler N, Sullivan LE, Weiss L, and Fiellin DA. 2009. Factors affecting willingness to provide buprenorphine treatment. Journal of Substance Abuse Treatment 36 (3):244–51. doi: 10.1016/j.jsat.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parran TV, Muller JZ, Chernyak E, Adelman C, Delos Reyes CM, Rowland D, and Kolganov M. 2017. Access to and payment for office-based buprenorphine treatment in Ohio. Substance Abuse 11:1178221817699247. doi: 10.1177/1178221817699247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, and Scholl L. 2016. Increases in drug and opioid-involved overdose deaths - United States, 2010–2015. MMWR. Morbidity and Mortality Weekly Report 65 (5051):1445–52. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- Sigmon SC 2015. The untapped potential of office-based buprenorphine treatment. JAMA Psychiatry 72 (4):395–6. doi: 10.1001/jamapsychiatry.2014.2421. [DOI] [PubMed] [Google Scholar]
- Stein BD, Sorbero M, Dick AW, Pacula RL, Burns RM, and Gordon AJ. 2016. Physician capacity to treat opioid use disorder with buprenorphine-assisted treatment. JAMA 316 (11):1211–1212. doi: 10.1001/jama.2016.10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller KB 2015. A collaborative opioid prescribing (CoOP) model linking opioid treatment programs with office-based buprenorphine providers. Addiction Science & Clinical Practice 10 (1):A63. doi: 10.1186/1940-0640-10-S1-A63. [DOI] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA). National Survey of Substance Abuse Treatment Services (N-SSATS): 2016. Data on Substance Abuse Treatment Facilities. BHSIS Series S-93, HHS Publication No. (SMA) 17–5039 Rockville, MD: SAMHSA; 2017. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA). 2014b. National Survey of Substance Abuse Treatment Services (N-SSATS): 2013. Data on substance abuse treatment facilities Rockville, MD: Substance Abuse and Mental Health Services Administration; http://www.samhsa.gov/data/sites/default/files/2013_N-SSATS/2013_N-SSATS_National_Survey_of_Substance_Abuse_Treatment_Services.pdf [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA). 2015. Physician waiver qualifications: The Drug Addiction Treatment Act of 2000 (DATA 2000) https://www.samhsa.gov/medication-assisted-treatment/buprenorphine-waiver-management/apply-for-physician-waiver (accessed January 17, 2018).
- Substance Abuse and Mental Health Services Administration (SAMHSA). 2016. New rule helps finalize move to provide more medication-assisted treatment to people with opioid disorders https://www.samhsa.gov/newsroom/press-announcements/201609271100 (accessed January 17, 2018).
- Thomas CP, Fullerton CA, Kim M, Montejano L, Lyman DR, Dougherty RH, Daniels AS, Ghose SS, and Delphin-Rittmon ME. 2014. Medication-assisted treatment with buprenorphine: assessing the evidence. Psychiatric Services (Washington, D.C.) 65 (2):158–70. doi: 10.1176/appi.ps.201300256. [DOI] [PubMed] [Google Scholar]
- United States Congress. The Comprehensive Addiction and Recovery Act (CARA). 2016. Public Law 114–198 (Original Bills as Introduced in 114th Congress: S.524/H.R.953) https://www.govtrack.us/congress/bills/114/s524 (accessed July 23, 2018).
- Van Wormer K, and Davis DR. 2013. Addiction treatment: a strengths perspective 3rd ed. Boston, MA: Cengage Learning. [Google Scholar]
- Volkow ND, Frieden TR, Hyde PS, and Cha SS. 2014. Medication-assisted therapies--tackling the opioid-overdose epidemic. New England Journal of Medicine 370 (22):2063–6. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]
- Wilson B, and Cohen A. 2015. The Big Book of Alcoholics Anonymous (including 12 steps, guides & prayers) CreateSpace Independent Publishing Platform. [Google Scholar]
- Wish ED, Artigiani E, Billing A, Hauser W, Hemberg J, Shiplet M, and DuPont RL. 2012. The emerging buprenorphine epidemic in the United States. Journal of Addictive Diseases 31 (1):3–7. doi: 10.1080/10550887.2011.642757. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2003. Essential medicines: WHO model list (revised March 2005) Geneva, Switzerland: World Health Organization. [Google Scholar]