Abstract
Background:
Studies of the timing of ESRD have primarily defined “early” versus “late” initiation of dialysis using eGFR-based criteria. Our objective was to determine the theoretical time that could be spent in CKD stage 5 prior to reaching a conservative eGFR threshold of 5 mL/min/1.73 m2 compared to the actual time spent in CKD stage 5 by risk factors of interest.
Methods:
870 Chronic Renal Insufficiency Cohort participants with CKD stage 5 who started renal replacement therapy (RRT) were included for retrospective study. We used mixed models to estimate the person-specific trajectory of renal function. We then used these individual trajectories to estimate the amount of time that would be spent in CKD stage 5 (between eGFR of 15 and 5 mL/min/1.73 m2) and compared this estimate to the actual time spent in CKD stage 5 prior to ESRD (between eGFR of 15 mL/min/1.73 m2 and ESRD).
Results:
We found the median observed time between eGFR of 15 mL/min/1.73 m2 to RRT was 9.6 months, but the median predicted time between eGFR of 15 mL/min/1.73 m2 to eGFR of 5 mL/min/1.73 m2 was 17.7 months. Some of the largest differences between the predicted and actual amount of time spent in CKD stage 5 were noted among those with SBP<140 mm Hg (9.7 months longer predicted compared to actual), proteinuria <1 g/g (9.1 months), and serum albumin ≥3.5 g/dL (9.0 months).
Conclusion:
We found marked differences between the actual and predicted time spent in CKD stage 5 based on risk factors of interest. We believe that placing timing of dialysis initiation in the perspective of time is novel, and may identify subgroups of patients who may derive particular benefit from a more concerted effort to delay RRT.
Keywords: CKD, ESRD, timing of dialysis initiation
Introduction
The transition from advanced chronic kidney disease (CKD) to end-stage renal disease (ESRD) represents a vulnerable period, when multiple physiologic and psychosocial changes occur as patients prepare for either dialysis or kidney transplantation. Observational studies have suggested a lack of survival benefit to early initiation of dialysis or earlier preemptive transplantation [1–4]. A large randomized controlled trial (the Initiating Dialysis Early and Late [IDEAL] trial) also did not show a survival benefit to earlier (eGFR of 10–14 mL/min/1.73m2) versus later dialysis initiation (5–7 mL/min/1.73m2) [2]. The 2015 Kidney Disease Outcomes Quality Initiative guidelines for hemodialysis do not currently specify an eGFR threshold at which dialysis should be initiated [5]. However, in the United States, over half of all patients begin dialysis at an eGFR above 10 mL/min/1.73 m2 despite the lack of known benefit to earlier RRT initiation [6].
A number of studies have examined the timing of initiation of renal replacement therapy (RRT) based on the level of eGFR at the start of dialysis or receipt of kidney transplant [7–11]. However, few studies have characterized “early” versus “late” RRT initiation based on the amount of additional time that could be gained if nephrologists were to adopt a strategy of explicitly targeting a low eGFR threshold (e.g. eGFR of 5 mL/min/1.73 m2) in clinical practice. Quantifying “early” versus “late” RRT initiation in metrics of time as opposed to eGFR level may be more useful, given that the amount of time left before dialysis is of greatest concern for most patients with CKD stage 5. For providers, this data may provide a framework for discussions regarding the time that could potentially be available if patients were to partner with their providers to try to delay the initiation of dialysis for as long as possible.
In this study, our objective was to compare the predicted amount of time that would be spent between entry into CKD stage 5 and eGFR of 5 mL/min/1.73 m2 against the actual observed time between entry into CKD stage 5 and initiation of RRT among participants of the Chronic Renal Insufficiency Cohort (CRIC) study. We also examined the extent to which risk factors known to affect CKD progression were associated with differences between the predicted and actual time between CKD stage 5 and RRT initiation.
Material and Methods
Study population
The CRIC study is a NIH-sponsored, multi-center, observational cohort that enrolled patients from seven clinical centers located throughout the United States [13]. Participants with estimated glomerular filtration rate (eGFR) between 20–70 mL/min/1.73 m2 based on the Modification of Diet in Renal Disease (MDRD) equation were recruited for study between June of 2003 and September of 2008. The inclusion and exclusion criteria have been previously published [13,14]. CRIC participants were followed annually at in-person study visits, during which medical history, medication use, co-morbidity, and laboratory data were routinely assessed. For this study, we included the 870 CRIC participants with data available in CKD stage 5 (eGFR below 15 mL/min/1.73 m2), of whom 772 started RRT during our follow-up period.
Modeling renal function trajectory across CKD stages
We estimated renal function using the CKD-EPI creatinine-based equation [15]. We used creatinine to estimate GFR because it is the biomarker most commonly used in clinical practice and therefore results can be most easily extrapolated to patient care. We used linear mixed modeling to estimate changes in eGFR over time from CRIC enrollment until ESRD onset using all data available from time of entry into CRIC. Our mixed models included person-specific linear and quadratic time terms to accommodate non-linearities in eGFR trajectory and provide a flexible fit for each individual as previously described [16]. We adjusted these mixed models for age at the time of cohort entry and race (black vs. non-black).
Examination of time spent in each CKD stage according to factors of interest
We first categorized patients by their predicted versus actual observed time to RRT into four scenarios: 1) predicted time longer than actual observed time to RRT with RRT occurring during the follow-up period; 2) predicted time shorter than actual observed time to RRT with RRT occurring during the follow-up period 3) predicted time shorter than end of study with no observed RRT during the follow-up period, and 4) predicted time longer than end of study with no observed RRT during the follow-up period. The goal of this analysis was to provide an overall qualitative description of the distribution of patients included for analysis.
Next, we used the person-specific trajectories described above to estimate differences in the predicted versus actual amount of time spent in CKD stage 5 based on the presence or absence of individual factors of interest. For this analysis, we only included the 772 participants who were observed to develop ESRD during our follow-up period. Factors that we examined included demographic characteristics (age at entry into CKD stage 5 [categorized as ≥60 years versus <60 years], sex, and race and ethnicity [white, black, or Hispanic]), co-morbidities (presence or absence of cardiovascular disease [by self-report], uncontrolled blood pressure [SBP ≥versus <140 mm Hg], obesity [body mass index ≥ or <30 kg/m2], diabetes [yes/no], tobacco use [yes/no], and medication use [angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB), statins, and diuretics]), and laboratory findings that may factor into the decision to initiate RRT (hemoglobin A1c among diabetic patients [< or ≥7.5%], proteinuria [< or ≥1 g/g], serum albumin [≥ or <3.5 g/g], sodium [≥ or <130 meq/L], potassium [≥ or <5.5 meq/L], bicarbonate [≥ or <22 meq/L], and serum blood urea nitrogen [≥ or < 50 mg/dL]). For all non-fixed covariates, we used the values at the first visit upon entry into CKD stage 5 (eGFR of 15 mL/min/1.73 m2) to define their presence or absence as risk factors for progression.
We tested whether the differences between the predicted time to eGFR of 5 mL/min/1.73 m2 and the actual time to RRT based on each factor of interest were statistically significantly different using chi-square, Wilcoxon rank sum or Kruskal-Wallis tests. We chose an eGFR threshold of 5 mL/min/1.73 m2 to define “late” RRT initiation since much of the literature on the timing of dialysis initiation has used this as the lower eGFR threshold of interest, and this was the lower threshold in one of the treatment groups in the IDEAL trial [2,12].
Although our main focus was on unadjusted analyses as our primary models, we also used multivariate logistic regression including all demographic, co-morbidity, and laboratory data as predictors of interest to determine which factors were most strongly associated with odds of a >8-month difference between the predicted and actual time spent in CKD stage 5 among those observed to develop ESRD during our follow-up period. We selected an 8-month period as our outcome of interest because this represented the median difference between the predicted and actual time spent in CKD stage 5. We used backwards selection (with a threshold p-value of <0.10) to identify the factors most predictive of early RRT initiation and included these factors in our final multivariate logistic regression model.
We used de-identified data from the NIDDK Biorepository for analysis, which administratively censored data as of March 2013. The UCSF Institutional Review Board considers this study exempt human subjects research.
Results
Study Participants
Characteristics of participants included for analysis at the first visit when eGFR fell below 15 mL/min/1.73 m2 are shown in Table 1. Median age was 60.8 years, 52% were black, and 66% had diabetes. Mean hemoglobin was 11.4 g/dL, and median proteinuria was 1.7 g/g. Participants who were excluded were older and had higher BUN and lower proteinuria. Of the 870 participants who entered CKD stage 5, 754 (87%) had predicted time to ESRD that was longer than the actual observed time to ESRD, versus only 18 participants (2%) had predicted time to ESRD that was shorter than the actual observed time to ESRD. In addition, among those not observed to develop ESRD during the follow-up period (98 out of 870 participants), 6 participants had a predicted time to ESRD that was shorter than the end of study and 92 participants had a predicted time to ESRD that would have been longer than the end of study.
Table 1.
Baseline characteristics of CRIC participants at entry into CKD stage 5.
| Baseline Characteristics (N=870) | N (%) or Mean ± SD |
|---|---|
| Median age (years) [interquartile range] | 60.9 [52.2–67.8] |
| Female | 380 (43.7) |
| Other | 38 (4.4) |
| Obese | 496 (57.1) |
| Any cardiovascular disease | 393 (45.2) |
| Diabetes | 573 (65.9) |
| Systolic BP (mm Hg) [IQR] | 137.3 [122.7–155.3] |
| Unknown | 248 (29) |
| Current smoker | 137 (15.8) |
| Serum potassium meq/L | 4.5 ± 0.6 |
| Hemoglobin (g/dL) | 11.4 ± 1.6 |
| Serum CO2 (mmol/L) | 22.1 ± 3.5 |
| Serum Urea Nitrogen (mg/dL) | 50.8 ± 18.0 |
| Serum albumin (g/dL) | 3.7 ± 0.5 |
| Urine protein/creatinine ratio (g/g) [IQR] | 1.7 [0.7–3.8] |
Factors associated with observed time to RRT
Characteristics of participants who entered CKD stage 5 but did not initiate RRT during our study period are shown in Supplemental Table 1. Among the 98 participants who did not develop ESRD, 51 died during the study follow-up period. The observed median time to RRT was 9.6 months, but the median predicted time to an eGFR of 5 mL/min/1.73 m2 was 17.7 months. Thus, the overall median difference between predicted and actual time spent in CKD stage 5 was 8.1 months.
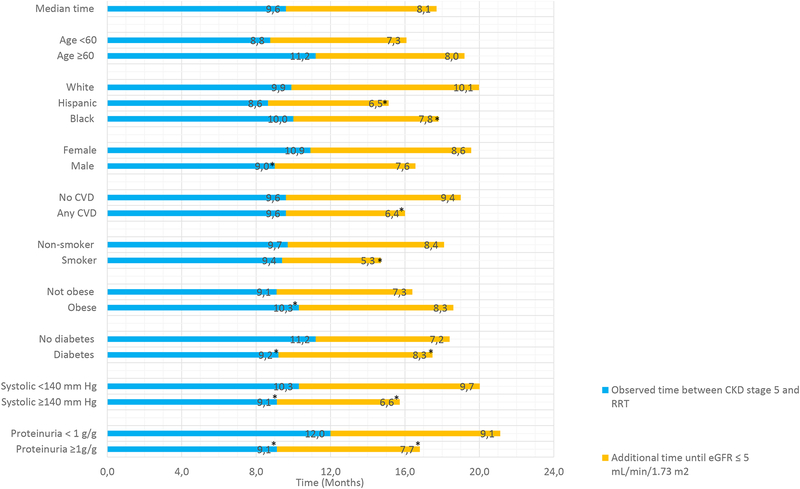
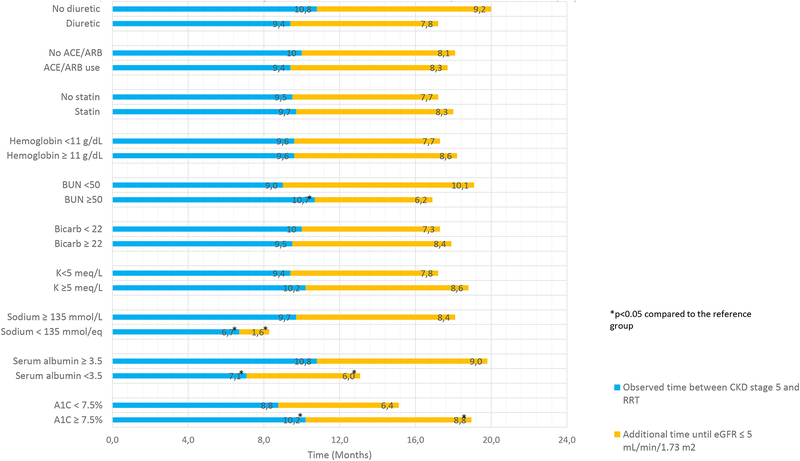
We examined the amount of observed (i.e., actual) time spent in CKD stage 5 according to each factor of interest in separate univariate analyses. We found that demographic and co-morbid factors including age, race, presence of CVD, and smoking were not statistically significantly associated with the observed time spent in CKD stage 5 (Figure 1A). In contrast, sex, obesity, lack of diabetes, controlled SBP, and proteinuria <1 g/g were associated with statistically significantly longer observed time in CKD stage 5 (Figure 1A). Low sodium, lower serum urea nitrogen, lower A1c, and lower serum albumin were associated with shorter time spent in CKD stage 5 (Figure 1B). In contrast, use of various medications including ACE/ARBs, statins, and diuretics was not associated with time spent in CKD stage 5.
Figure 1A.
Observed versus predicted time available in CKD stage 5 based on demographic and comorbid data associated with progression of CKD.
Reference group used in tests for statistically significant differences for all characteristics is the first row for each subdivision (e.g. age < 60 years, white race).
Figure 1B.
Observed versus predicted time available in CKD stage 5 based on medication and laboratory data associated with progression of CKD.
Reference group used in tests for statistically significant differences for all characteristics is the first row for each subdivision (e.g. no diuretic, no ACE/ARB).
Differences between time to RRT and predicted time to eGFR of 5 mL/min/1.73m2
Next, we determined the additional time that could have been spent in CKD stage 5 if an eGFR threshold of 5 mL/min/1.73 m2 had been used for RRT initiation according to each factor of interest in univariate analysis. The amount of additional time was longest for white participants (10.1 months), participants with no cardiovascular disease (9.4 months), low proteinuria (9.1 months), low serum urea nitrogen concentration (10.1 months), and high serum albumin concentration (9.0 months) as shown in Figure 1B. In contrast, the smallest differences between the predicted and actual time spent in CKD stage 5 were observed among participants with low serum sodium (1.6 months, Figure 1A) and among smokers (5.3 months, Figure 1B).
When we used multivariate logistic regression models to determine factors independently associated with larger differences between the predicted and actual time to initiation of RRT (greater than the median), we found that age, race/ethnicity, smoking status, cardiovascular disease, SBP, and proteinuria were statistically significantly predictors (Table 2). In our final multivariate model, age ≥60 years was associated with a 1.5 times higher odds of having a large difference between predicted and actual time to RRT (95% CI 1.09–2.06). White race, non-smokers, absence of cardiovascular disease, and controlled SBP were also associated with higher odds of larger discrepancies between the predicted and actual time available in CKD stage 5 prior to RRT (Table 2).
Table 2.
Odds of starting dialysis earlier than expected (more than 8 months prior to predicted time to eGFR of <5 mL/min/1.73 m2) in final multivariate analysis.
| Characteristic (N=772) | Odds ratio (95% CI) |
|---|---|
| Age ≥60 years (vs. <60 years) | 1.50 (1.09–2.06) |
| Hispanic | 0.82 (0.53–1.26) |
| No cardiovascular disease (versus presence of CVD) | 1.75 (1.28–2.41) |
| Non-smoker (versus smoker) | 1.59 (1.03-.2.45) |
| Systolic BP <140 mm Hg (versus ≥140 mm Hg) | 1.40 (1.02–1.93) |
| Urine protein/cre ratio <1g/g (versus ≥1 g/g) | 1.24 (0.88–1.73) |
Discussion
Most observational and randomized controlled studies have not demonstrated a benefit to early initiation of RRT, and some studies have suggested that this strategy may be associated with harm [17–19]. Yet, despite these data, the mean eGFR at the time of dialysis initiation has increased to over 10 mL/min/1.73 m2 over the last decade [6,20]. Although many studies have examined reasons for early versus late RRT initiation based on eGFR criteria, few studies have been able to quantify the magnitude of additional time that could be spent in CKD stage 5 if RRT were to be initiated at a more conservative eGFR threshold (e.g. <5 mL/min/1.73 m2). In our study, we found that even among CRIC participants, RRT could potentially be delayed by a median of 8 months if patients could be managed medically until reaching an eGFR of 5 mL/min/1.73m2. The greatest discrepancies between the actual and predicted times spent in CKD stage 5 were noted among white participants, those with controlled SBP and without substantial proteinuria, and in the absence of cardiovascular disease in both univariate and multivariate analyses. In contrast, tobacco use, low serum sodium, and higher serum urea nitrogen were associated with smaller discrepancies between the observed and predicted time in CKD stage 5. We believe our study identifies characteristics of individuals who may benefit from a more concerted effort to delay the initiation of RRT.
Currently the Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines recommend that the decision to initiate dialysis should not be based solely on any particular eGFR threshold among asymptomatic patients [5]. However, current trends in practice still show that dialysis is being initiated at higher eGFR levels despite the lack of demonstrated survival or quality of life benefit with this approach [9,20]. We believe our estimation of the potential magnitude of additional time that could be spent in CKD stage 5 if RRT were started at a more conservative threshold is informative and supports the need to reassess the threshold for uremic symptoms that may warrant initiation of RRT. These data may be helpful to providers in timing the appropriate placement of dialysis access, counseling of patients regarding their prognosis, and identifying the subsets of patients in whom it may be possible to delay RRT initiation for a substantial period of time. Given that the average annual cost of hemodialysis is around $90,000 per year, delaying dialysis by 8 months could represent average savings of $60,000 per patient in the United States.[21] While we acknowledge that widespread implementation of a policy where dialysis is initiated later could provide cost-savings, such a treatment strategy will also necessitate more personnel support to closely monitor patients during the advanced stages of CKD if dialysis initiation is delayed.”
Whereas prior randomized controlled trials have suggested a benefit to continuation of ACE inhibitors in the setting of CKD stage 4 [22], fewer studies have examined this question within a large number of patients with CKD stage 5. We found that continuation of ACE inhibitor or ARB use in CKD stage 5 was not associated with substantial differences in the observed time spent in CKD stage 5. The presence of uncontrolled proteinuria was associated with less additional predicted time in CKD stage 5, although this difference was small (1.4 months). It is possible that the relatively small estimated difference between predicted and observed time in stage 5 CKD based on proteinuria explains the lack of apparent benefit of ACE or ARB use, although we caution that our results are observational and may not imply causation.
Of interest, we did find older age to be associated with longer observed time spent in CKD stage 5, but the actual difference between those older versus younger than 60 years of age was small (on the order of three months). Although our findings are consistent with those of prior studies, which have described slower progression of CKD and later onset of RRT needs in older individuals [23,24], our data suggest that age may be less important than previously described when considered in terms of time. This discrepancy highlights the novelty of our study in its translation of risk estimates (represented by odds ratios) with associated time differences in the onset of RRT by risk factors such as age. There was also little difference in the additional time that could be available in CKD stage 5 by age based on the modeled time in CKD stage 5.
Of the laboratory factors we studied, low serum urea nitrogen, high serum albumin, and eunatremia were associated with longer amount of additional time that could theoretically be spent in CKD stage 5. However, none of these factors remained important in analysis that adjusted for other clinical factors. Surprisingly, elevated hemoglobin A1c was associated with longer observed time and larger discrepancies in the amount of additional time that could be spent in CKD stage 5. Although the reasons for this observation are unclear, we speculate that lower hemoglobin A1c among those with diabetes may reflect the severity of renal disease (i.e., “burnt out diabetes”) rather than tight blood glucose control [25]. This possibility is supported by the observation that when we examined diabetes as the factor of interest (rather than hemoglobin A1c), those with diabetes spent less time in CKD stage 5 compared with those without diabetes (but the difference in time was small).
The strengths of our study include the use of a well-characterized, nationally representative cohort of CKD patients with longitudinal follow-up and careful ascertainment of co-morbidities and outcomes of interest in one of the largest cohorts of patients with stage 5 CKD. In addition, we believe our time-centric approach to the study of timing of dialysis initiation to be novel and our data informative for providers and patients, especially for the purposes of prognostication and counseling. However, we note several limitations to our study, including its observational nature and the lack of granular data on acute kidney injury events that may have occurred in the immediate period prior to RRT initiation. We also do not have data on factors that influenced the actual decision to initiate RRT or the contribution of symptoms that may have driven such decisions. In addition, participants in CRIC may not be representative of non-study participants, and the amount of time spent in CKD that we describe may be representative of participants receiving optimal therapy under the care of nephrologists. However, we do note that a prior study using the CRIC cohort has described the median eGFR at time of dialysis initiation to be around 10 mL/min/1.73 m2, which is consistent with practice patterns in the United States as described previously.[26] Finally, we acknowledge that our findings may be reflective of practice patterns in the United States and may not reflect practice patterns in other countries.
In conclusion, we believe that initiation of RRT occurs substantially earlier than would be predicted, although we are unable to pinpoint the exact reasons for the decision to start dialysis. Aside from eGFR, there appear to other factors associated with the time spent in CKD stage 5 and the decision to initiate dialysis. We have identified characteristics of individuals who may be more likely to have slow progression of disease and spend a significant amount of time in CKD stage 5. Further studies are needed to identify the subset of patients who may safely delay initiation of RRT and to elucidate the impetus behind the complex decision to initiate RRT by both patients and providers alike.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health R01 DK115629 to EK and KLJ and K24 DK085153 to KLJ. The CRIC Study was conducted by the CRIC Study Investigators and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The data from the CRIC Study reported here were supplied by the NIDDK Central Repositories. This manuscript was not prepared in collaboration with Investigators of the CRIC Study and does not necessarily reflect the opinions or views of the CRIC Study, the NIDDK Central Repositories, or the NIDDK.
Footnotes
Disclosures
None
References
- 1.Akkina SK, Connaire JJ, Snyder JJ, Matas AJ, Kasiske BL: Earlier is not necessarily better in preemptive kidney transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2008;8:2071–2076. [DOI] [PubMed] [Google Scholar]
- 2.Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, Harris A, Johnson DW, Kesselhut J, Li JJ, Luxton G, Pilmore A, Tiller DJ, Harris DC, Pollock CA: A randomized, controlled trial of early versus late initiation of dialysis. The New England journal of medicine 2010;363:609–619. [DOI] [PubMed] [Google Scholar]
- 3.Korevaar JC, Jansen MA, Dekker FW, Boeschoten EW, Bossuyt PM, Krediet RT: Evaluation of DOQI guidelines: early start of dialysis treatment is not associated with better health-related quality of life. American journal of kidney diseases: the official journal of the National Kidney Foundation 2002;39:108–115. [DOI] [PubMed] [Google Scholar]
- 4.Pan Y, Xu XD, Guo LL, Cai LL, Jin HM: Association of early versus late initiation of dialysis with mortality: systematic review and meta-analysis. Nephron Clinical practice 2012;120:c121–131. [DOI] [PubMed] [Google Scholar]
- 5.KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. American journal of kidney diseases: the official journal of the National Kidney Foundation 2015;66:884–930. [DOI] [PubMed] [Google Scholar]
- 6.Leurs P, Machowska A, Lindholm B: Timing of dialysis initiation: when to start? Which treatment? Journal of renal nutrition: the official journal of the Council on Renal Nutrition of the National Kidney Foundation 2015;25:238–241. [DOI] [PubMed] [Google Scholar]
- 7.Bargman JM: Timing of Initiation of RRT and Modality Selection. Clinical journal of the American Society of Nephrology: CJASN 2015;10:1072–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crews DC, Scialla JJ, Boulware LE, Navaneethan SD, Nally JV Jr., Liu X, Arrigain S, Schold JD, Ephraim PL, Jolly SE, Sozio SM, Michels WM, Miskulin DC, Tangri N, Shafi T, Wu AW, Bandeen-Roche K: Comparative effectiveness of early versus conventional timing of dialysis initiation in advanced CKD. American journal of kidney diseases: the official journal of the National Kidney Foundation 2014;63:806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Hare AM, Wong SP, Yu MK, Wynar B, Perkins M, Liu CF, Lemon JM, Hebert PL: Trends in the Timing and Clinical Context of Maintenance Dialysis Initiation. Journal of the American Society of Nephrology: JASN 2015;26:1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slinin Y, Guo H, Li S, Liu J, Morgan B, Ensrud K, Gilbertson DT, Collins AJ, Ishani A: Provider and care characteristics associated with timing of dialysis initiation. Clinical journal of the American Society of Nephrology: CJASN 2014;9:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu MK, O’Hare AM, Batten A, Sulc CA, Neely EL, Liu CF, Hebert PL: Trends in Timing of Dialysis Initiation within Versus Outside the Department of Veterans Affairs. Clinical journal of the American Society of Nephrology: CJASN 2015;10:1418–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosansky SJ: Early dialysis initiation, a look from the rearview m irror to what’s ahead. Clinical journal of the American Society of Nephrology: CJASN 2014;9:222–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT, Chronic Renal Insufficiency Cohort Study I: The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. Journal of the American Society of Nephrology: JASN 2003;14:S148–153. [DOI] [PubMed] [Google Scholar]
- 14.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI, Chronic Renal Insufficiency Cohort Study G: Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clinical journal of the American Society of Nephrology: CJASN 2009;4:1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, Investigators C-E: Estimating glomerular filtration rate from serum creatinine and cystatin C. The New England journal of medicine 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ku E, Johansen KL, McCulloch CE: Time-Centered Approach to Understanding Risk Factors for the Progression of CKD. Clinical journal of the American Society of Nephrology: CJASN 2018;13:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beddhu S, Samore MH, Roberts MS, Stoddard GJ, Ramkumar N, Pappas LM, Cheung AK: Impact of timing of initiation of dialysis on mortality. Journal of the American Society of Nephrology: JASN 2003;14:2305–2312. [DOI] [PubMed] [Google Scholar]
- 18.Kazmi WH, Gilbertson DT, Obrador GT, Guo H, Pereira BJ, Collins AJ, Kausz AT: Effect of comorbidity on the increased m ortality associated with early initiation of dialysis. American journal of kidney diseases: the official journal of the National Kidney Foundation 2005;46:887–896. [DOI] [PubMed] [Google Scholar]
- 19.Susantitaphong P, Altamimi S, Ashkar M, Balk EM, Stel VS, Wright S, Jaber BL: GFR at initiation of dialysis and m ortality in CKD: a meta-analysis. American journal of kidney diseases: the official journal of the National Kidney Foundation 2012;59:829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ku E, Johansen KL, Portale AA, Grimes B, Hsu CY: State level variations in nephrology workforce and tim ing and incidence of dialysis in the United States among children and adults: a retrospective cohort study. BMC nephrology 2015;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.United States Renal Data System: Annual Data Report: Chapter 9 Healthcare Expenditures for Persons with ESRD. Ntaional Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases 2018 [Google Scholar]
- 22.Hou FF, Zhang X, Zhang GH, Xie D, Chen PY, Zhang WR, Jiang JP, Liang M, Wang GB, Liu ZR, Geng RW: Efficacy and safety of benazepril for advanced chronic renal insufficiency. The New England journal of medicine 2006;354:131–140. [DOI] [PubMed] [Google Scholar]
- 23.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL: Frailty, dialysis initiation, and mortality in end-stage renal disease. Archives of internal medicine 2012;172:1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crews DC, Scialla JJ, Liu J, Guo H, Bandeen-Roche K, Ephraim PL, Jaar BG, Sozio SM, Miskulin DC, Tangri N, Shafi T, Meyer KB, Wu AW, Powe NR, Boulware LE: Predialysis health, dialysis timing, and outcomes among older United States adults. Journal of the American Society of Nephrology: JASN 2014;25:370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovesdy CP, Park JC, Kalantar-Zadeh K: Glycemic control and burnt-out diabetes in ESRD. Seminars in dialysis 2010;23:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosansky SJ, Clark WF, Eggers P, Glassock RJ: Initiation of dialysis at higher GFRs: is the apparent rising tide of early dialysis harmful or helpful? Kidney international 2009;76:257–261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.