Abstract
In the hindbrain and the adjacent cranial neural crest (NC) cells of jawed vertebrates (gnathostomes), nested and segmentally-restricted domains of Hox gene expression provide a combinatorial Hox-code for specifying regional properties during head development. Extant jawless vertebrates, such as the sea lamprey (Petromyzon marinus), can provide insights into the evolution and diversification of this Hox-code in vertebrates. There is evidence for gnathostome-like spatial patterns of Hox expression in lamprey; however, the expression domains of the majority of lamprey hox genes from paralogy groups (PG) 1–4 are yet to be characterized, so it is unknown whether they are coupled to hindbrain segments (rhombomeres) and NC. In this study, we systematically describe the spatiotemporal expression of all 14 sea lamprey hox genes from PG1-PG4 in the developing hindbrain and pharynx to investigate the extent to which their expression conforms to the archetypal gnathostome hindbrain and pharyngeal hox-codes. We find many similarities in Hox expression between lamprey and gnathostome species, particularly in rhombomeric domains during hindbrain segmentation and in the cranial neural crest, enabling inference of aspects of Hox expression in the ancestral vertebrate embryonic head. These data are consistent with the idea that a Hox regulatory network underlying hindbrain segmentation is a pan vertebrate trait. We also reveal differences in hindbrain domains at later stages, as well as expression in the endostyle and in pharyngeal arch (PA) 1 mesoderm. Our analysis suggests that many Hox expression domains that are observed in extant gnathostomes were present in ancestral vertebrates but have been partitioned differently across Hox clusters in gnathostome and cyclostome lineages after duplication.
Keywords: Hox expression, hindbrain segmentation, cranial neural crest, vertebrate evolution, rhombomeres, lamprey, gene regulation, axial patterning
1. Introduction:
Hox genes encode a family of highly conserved homeodomain-containing transcription factors that are found in nearly all animal genomes, playing common roles in regulating the specification of positional identities along the anterior-posterior (A-P) axis (Carroll, 1995; Graham et al., 1989). They reside in clusters, with mammals having four paralogous Hox clusters, which arose by duplication from a common ancestral complex early in vertebrate evolution (Duboule and Dolle, 1989; Parker and Krumlauf, 2017; Pascual-Anaya et al., 2013). Further duplication and gene loss events have shaped the Hox complement across vertebrate lineages (Kuraku and Meyer, 2009; Pascual-Anaya et al., 2013). Based on their sequence similarity and positions within a cluster, vertebrate Hox genes are classified into 14 paralogy groups (PG) (Krumlauf, 1994). A regulatory feature of the Hox clusters in vertebrates is that during development the timing and domains of Hox gene expression along the A-P axis are correlated with their relative gene order along the cluster, a property termed collinearity. Genes within a given Hox cluster are all transcribed in the same 5’ to 3’ orientation. Hox genes closest to the 3’ end (‘anterior’ Hox genes, such as those in PG1) show a tendency to be expressed earlier (temporal colinearity) and more anteriorly (spatial colinearity) than those closer to the 5’ end (Duboule, 2007; Duboule and Dolle, 1989; Kmita and Duboule, 2003). This results in a nested series of Hox expression domains, which create combinatorial ‘Hox codes’ that specify regional properties along the A-P axis in multiple tissues (Mallo et al., 2010).
During embryonic development the vertebrate hindbrain is transiently segmented along the A-P axis into 7 or 8 morphological units, called rhombomeres (r) (Hanneman et al., 1988; Lumsden, 2004; Lumsden and Keynes, 1989). These represent lineage-restricted cellular compartments, which respond to axial patterning signals to create distinct regional identities in each individual segment (Fraser et al., 1990; Marshall et al., 1992). Rhombomeres contain reiterated populations of neurons, which differentiate in a rhombomere-specific manner, resulting in the specialization of morphology, connectivity and function within each segment (Keynes and Lumsden, 1990; Lumsden and Keynes, 1989). The embryonic pharynx also exhibits segmentation, forming an alternating series of pharyngeal arches (PA) and pouches by out-pocketing of the endoderm. Hindbrain segmentation influences craniofacial patterning through cranial neural crest (NC) cells, which delaminate from the neural tube and migrate to the pharyngeal arches in discrete streams (Le Douarin and Kalcheim, 1999). Specific rhombomeres contribute to the different NC streams (Kontges and Lumsden, 1996; Trainor et al., 2002), with signals from surrounding tissues and between rhombomeres influencing NC migratory routes (Golding et al., 2000; Lumsden et al., 1991; Trainor and Krumlauf, 2000a; Trainor and Krumlauf, 2000b; Trainor et al., 2002). Cranial nerves connect each pharyngeal arch to branchiomotor neurons in specific rhombomeres, forming a somatotopic map of the pharyngeal arches in the hindbrain (Lumsden and Keynes, 1989; Oury et al., 2006). Thus, rhombomeres and pharyngeal segments are fundamentally coupled by the migration of cranial NC and by neuronal connectivity between the hindbrain and pharynx.
Hox genes are coupled to the gene regulatory network patterning hindbrain segments and NC. A hallmark of Hox gene expression in the hindbrain and pharynx is that anterior expression domains correspond tightly with rhombomere and pharyngeal arch boundaries, giving rise to region-specific positional Hox-codes in the hindbrain and NC (Hunt et al., 1991; Lumsden and Krumlauf, 1996). Perturbation experiments in jawed vertebrate species have revealed multiple roles for anterior Hox genes in hindbrain segmentation, segmental patterning of neurogenesis, and in patterning the skeleton of the head and neck. In the mouse, Hoxa1 is required early in hindbrain development for the formation of r5 (Chisaka et al., 1992; Dollé et al., 1993; Mark et al., 1993), while Hoxb1 influences neurogenesis in r4 (Goddard et al., 1996; Studer et al., 1996). In mice and zebrafish that lack Hoxb1, r4 neurons adopt the characteristics of those in r2, exhibiting altered migration and pathfinding of motoneurons (McClintock et al., 2002; Studer et al., 1996). In an analogous manner, Hox genes also have complex inputs into NC. Loss of Hoxa1 and Hoxb1 in the mouse neural tube results in a failure to form the r4-derived NC which migrates into PA2 (Gavalas et al., 1998; Gavalas et al., 2001). In diverse vertebrate models, loss of Hoxa2 leads to a partial transformation of PA2 skeletal derivatives into PA1-like structures (Baltzinger et al., 2005; Gendron-Maguire et al., 1993; Hunter and Prince, 2002; Rijli et al., 1993), while ectopic expression of Hoxa2 in PA1 leads to duplication of PA2 derivatives (Grammatopoulos et al., 2000; Kitazawa et al., 2015; Pasqualetti et al., 2000). Thus, Hoxa2 acts as a selector gene for specifying PA2 derivatives, while Hox paralogy group (PG) 1 genes regulate steps in the formation of NC.
Hox segmental patterning roles in the hindbrain and NC appear to be widely conserved across jawed vertebrates, based on functional studies in multiple species (Baltzinger et al., 2005; Grammatopoulos et al., 2000; Hunter and Prince, 2002; McClintock et al., 2002). Expression studies in dogfish, a cartilaginous fish, root their ancestry at least to the base of the jawed vertebrates (Oulion et al., 2011). This deep ancestry is also reflected by the sequence conservation of Hox enhancers that modulate segmental expression and by the conservation of Hox-responsive enhancer elements associated with downstream target genes (Kim et al., 2000; McEwen et al., 2009; Parker and Krumlauf, 2017; Parker et al., 2011; Parker et al., 2014b; Ravi et al., 2009). Invertebrate chordates, such as amphioxus (a cephalochordate) and ciona (a urochordate), display nested and co-linear Hox expression along the A-P neuraxis. The conservation of vertebrate-like retinoic acid response elements in the amphioxus Hox cluster suggests that ancestral chordates used in part an RA-Hox regulatory circuitry to generate nested A-P Hox expression in neural patterning (Manzanares et al., 2000; Wada et al., 2006). However, unlike vertebrates, invertebrate chordates lack rhombomeric segmentation and definitive NC. This raises two evolutionary questions: First, when in vertebrate evolution did these segmental Hox roles evolve? Second, how have these roles diverged between vertebrate lineages? Lamprey and hagfish belong to a lineage of jawless extant vertebrates (cyclostomes), which diverged early in vertebrate evolution from the lineage leading to the jawed vertebrates (gnathostomes), making them important species for addressing questions about early vertebrate evolution and diversification (Shimeld and Donoghue, 2012).
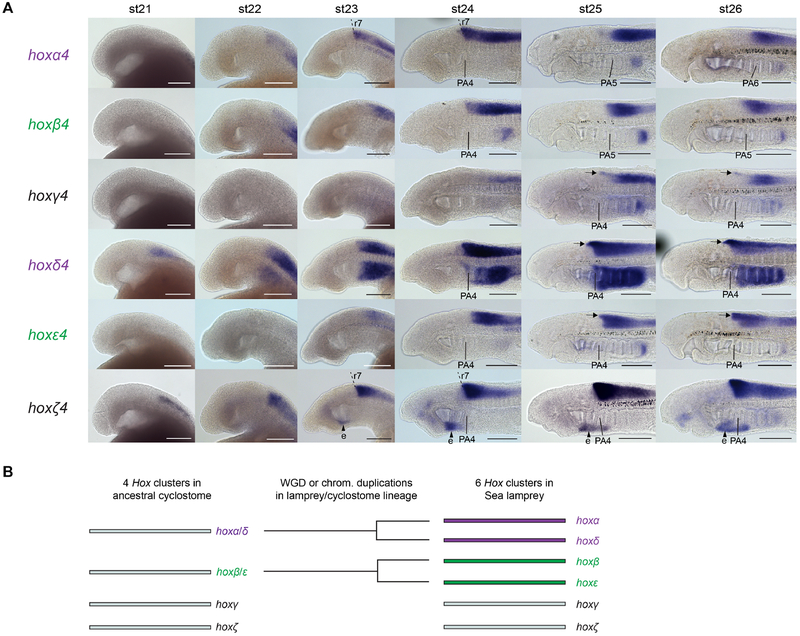
The ancestor of extant vertebrates is inferred, based on parsimony, to have had 4 Hox clusters, arising from a single ancestral chordate cluster through genomic duplication events in early vertebrates. Genomic analyses in two lamprey species – sea lamprey (Petromyzon marinus) and the closely related Arctic lamprey (Lethenteron camtschaticum) - revealed each to possess 6 hox clusters, indicative of additional duplication event/s in the lamprey/cyclostome lineage (Fig. 1A–B) (Mehta et al., 2013; Smith et al., 2018). This raises the prospect that roles for hox genes could have diversified in lamprey after these hox cluster duplications, with duplicated hox genes potentially being associated with anatomical novelties. To date, detailed expression analyses have been reported for only 4 anterior (PG1–4) hox genes in sea lamprey (Parker et al., 2014a; Parker et al., 2019), and for 5 such genes in Arctic lamprey (Takio et al., 2007). Sea lamprey was found to have transient rhombomere-restricted hox expression in the hindbrain and nested hox domains in the NC, similar to gnathostomes (Parker et al., 2014a; Parker et al., 2019; Takio et al., 2004). However, given that the sea lamprey has 14 anterior hox genes, the expression domains of the majority of lamprey hox PG1–4 genes are yet to be characterized, so it is unknown whether they are coupled to hindbrain segmentation and NC. Thus, the extent to which hox expression in the head is conserved or divergent between jawed and jawless vertebrates is still unclear, calling for a more comprehensive analysis of lamprey hox gene expression.
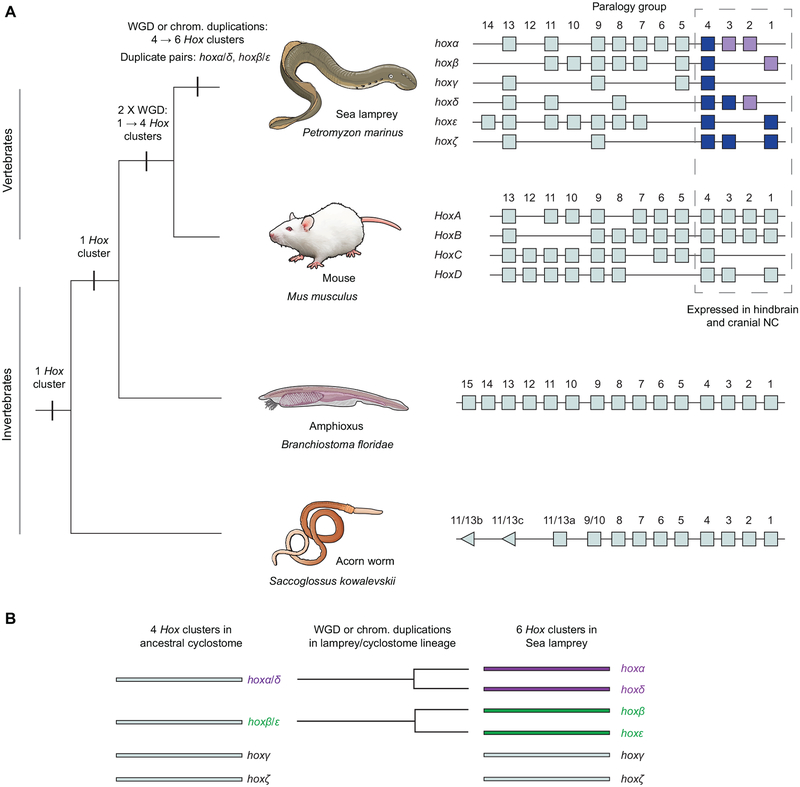
Figure 1: Hox clusters of selected deuterostomes.
(A) A phylogeny of selected deuterostomes, showing the characterised Hox clusters for given species. The duplication events that are inferred to have shaped the Hox complement of these species are indicated. These include whole genome duplication/s (WGD) in the early vertebrate lineage and WGD or chromosomal duplications in the cyclostome lineage leading to lamprey. The Hox clusters are depicted with the direction of transcription from left to right. Acorn worm hox11/13b and 11/13c show opposite direction of transcription to the rest of the hox cluster. For sea lamprey, hox genes previously characterised by in-situ hybridisation are shaded in lilac, and those characterised for the first time in this study shaded in blue. (B) A model of the duplication events that are inferred to have occurred in the lamprey/cyclostome lineage, leading to the 6 Hox clusters in Sea lamprey. Based on parsimony, it is assumed the ancestral cyclostome had 4 Hox clusters, depicted on the left. In this model, the hoxα and hoxδ clusters are paralogues that derive from a single cluster (hoxα/δ) present in the ancestral cyclostome, indicated by their purple shading. Similarly, the hoxβ and hoxε clusters derive from a single cluster (hoxβ/ε) in the ancestral cyclostome and are shaded in green.
In this study, we systematically describe the spatiotemporal expression of all 14 lamprey anterior hox genes in PG1–4 in the developing hindbrain and pharynx. We address the extent to which their expression conforms to the archetypal gnathostome hindbrain and pharyngeal hox-codes. In the context of lamprey/cyclostome-specific hox cluster duplications, we investigate whether the resulting paralogues exhibit equivalent or divergent patterns of expression. Finally, these expression patterns are used as a basis to infer shared and divergent aspects of hox cranial patterning between jawed and jawless vertebrates.
2. Materials and Methods
2.1. Lamprey embryos
Lamprey husbandry and embryo collection was performed as previously described (Nikitina et al., 2009; Parker et al., 2014a), with embryos being staged according to Tahara (Tahara, 1988), fixed in MEMFA, and dehydrated in 100% ethanol for storage at −20°C. This study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and protocols were approved by the Institutional Animal Care and Use Committee of the California Institute of Technology (lamprey, Protocol #1436–17).
2.2. Cloning of cDNA for in situ hybridization probes
In-situ probes were designed based on predicted gene sequences in the sea lamprey germline genome assembly (gPMAR100)(Smith et al., 2018), with care taken to avoid repetitive elements. Probe sequences were amplified from P. marinus genomic DNA or from st18–26 embryonic cDNA by PCR using KOD Hot Start Master Mix (Novagen). 3’ rapid amplification of cDNA ends (RACE) was performed for wnt1 using the GeneRacer Kit (Thermo Fisher Scientific). PCR products were cloned into the pCR4-TOPO vector (Thermo Fisher Scientific) and sequenced. The following PCR primers were used for amplifying probe templates, with probe lengths given:
wnt1 (729bp, partial exon and 3’UTR) F: 5’-GAACTGCACGCGGGTGGAGACTGT-3’; R: GeneRacer 3’ Nested Primer.
otx (515bp, partial exon and 3’UTR fragment) F: 5’-GTGGAAGTTTCAGCCGTTGT-3’; R: 5’-CCCGGCAAGATGTCTAACTC-3’.
hoxβ1 (674bp, 3’UTR fragment) F: 5’-ATGCTCCCTCAACTCCATCC-3’; R: 5’-TGACCTCTTCTCGCATGTAAGA-3’.
hoxε1 (338bp, partial exon 2) F: 5’-GCTGCTTCCACCAACAGG-3’; R: 5’-GAACCCCTTCGCCGAGAC-3’.
hoxζ1 (556bp, 3’UTR fragment) F: 5’-AGACATCCGGGCAATCGATT-3’; R: 5’-ATCGCTACTTCGCCAAATCG-3’.
hoxδ2 (585bp, partial exon 2) F: 5’-ACCTCTGCGCGACTCCTC-3’; R: 5’-CCAGACCTCCTCCTCCTCT-3’.
hoxδ3 (359bp, partial exon 2) F: 5’-GAGAACTCGTGCGGTGG-3’; R: 5’-TTGCCCAAACCGTGCAG-3’.
hoxζ3 (321bp, partial exon 2) F: 5’-TACCACCTCGTCGTCCAC-3’; R: 5’-GACAGCCTCGACCCCAAA-3’.
hoxα4 (301bp, partial exon 1–2) F: 5’-CTGAAGCAGCCGGTCGTG-3’; R: 5’-TGGACGAGGCTGTGTTCAAT-3’.
hoxβ4 (403bp, partial exon 1–2) F: 5’-AGCAGCAGGGACACTTGAT-3’; R: 5’-GAACGGATCTTGGTGTTGGG-3’.
Hoxγ4 (267bp, partial exon 1–2) F: 5’-ACCCGTGGATGAAGAAGGTA-3’; R: 5’-TCACCTTGGTGTTCGGTAGT-3’.
hoxδ4 (382bp, partial exon 2) F: 5’-CCAGGGACACGAGACCAAA-3’; R: 5’-GCTGGGCCTAACTCCTCAAA-3’.
hoxε4 (338bp, partial exon 2) F: 5’-CAACTATATCGGCGGGGAGT-3’; R: 5’-TGCTACTACCATTGCTGCTG-3’.
hoxζ4 (382bp, partial exon 1–2) F: 5’-GCGGTGACTTCAACCATCAA-3’; R: 5’-GCAGCTTGTGGTCCTTCTTC-3’.
krox20, hoxα2, hoxα3 probe sequences are as previously reported(Parker et al., 2014a).
2.3. In situ hybridization
Digoxygenin-labelled probes were generated by standard methods and purified using the MEGAclear Transcription Clean-up Kit (Ambion). Lamprey wholemount in situ hybridization was performed as described previously (Nikitina et al., 2009; Sauka-Spengler et al., 2007), with the following amendments to the protocol: methanol-stored embryos were first transferred into ethanol and left overnight prior to rehydration; a treatment of 0.5% acetic anhydride in 0.1M triethanolamine was added after proteinase K digestion. Hybridization was performed at 70°C for each probe. Embryos were cleared either by using a glycerol series followed by imaging in 100% glycerol, or by using a 1:2 ratio of benzyl alcohol:benzyl benzoate followed by mounting in Permount (Fisher Scientific) on microscope slides for imaging.
2.4. Sectioning
After in situ hybridization, selected embryos were transferred to 30% sucrose in phosphate-buffered saline, embedded in O.C.T compound (VWR), and cryo-sectioned to 10μm-thick sections.
2.5. Imaging
Images of BABB-cleared embryos were taken using a Zeiss Axiovert 200 microscope with an AxioCam HRc camera and AxioVision Rel 4.8.2 software. Glycerol-cleared embryo images were taken using a Leica MZ APO microscope with a Lumenera Infinity 3 camera and Infinity Analyze software. Sections were imaged using a Zeiss Axiovert 200 microscope with a Lumenera Infinity 3 camera and Micro-Manager 1.4.22 software. Images were cropped and altered for brightness and contrast using Adobe Photoshop CS5.1.
2.6. Data –Availability
Original data underlying this manuscript can be accessed from the Stowers Original Data Repository at [http://odr.stowers.org/websimr/].
3. Results
3.1. The lamprey hox complement
The sea lamprey and the Arctic lamprey each have 42 hox genes arranged in 6 clusters and 14 paralogy groups, compared to mouse with 39 hox genes across 4 clusters and 13 PG (Fig. 1) (Mehta et al., 2013; Smith et al., 2018). Within PG1–4, the Hox gene content is very similar between lamprey and mouse: both have 3 PG1, 2 PG2 and 3 PG3 genes, while lamprey has 6 PG4 genes compared to 4 in mouse (Fig. 1). Phylogenetic analyses could not resolve direct orthology between specific lamprey and gnathostome hox clusters (Mehta et al., 2013; Smith et al., 2018). Synteny analysis based on the retention of paralogous genes between lamprey hox-bearing chromosomes found significant similarity in gene content between chromosomes containing the lamprey-β and -ε clusters, and between those containing the -α and -δ clusters (Smith et al., 2018). This suggests that these pairs of chromosomes arose from duplication event/s that occurred in the cyclostome/lamprey lineage, after the split from the lineage leading to gnathostomes (Fig. 1). It has been suggested, based on parsimony, that the ancestor of all extant vertebrates had 4 Hox clusters, resulting from duplication events in an early vertebrate lineage, consistent with a recent reconstruction of vertebrate chromosomal evolution (Smith et al., 2018). Taken together, this leads to a scenario in which the common ancestor of gnathostomes and cyclostomes had 4 Hox clusters, with additional chromosome-scale (or possibly whole-genome) duplications occurring in the cyclostome/lamprey lineage, resulting in the 6 Hox clusters of extant lampreys. Of the anterior hox genes (PG1–4) in sea lamprey, only 4 have had their expression characterized by in-situ hybridization (Fig. 1 - lilac shading).
3.2. The segmental plan of the lamprey embryonic hindbrain and pharynx
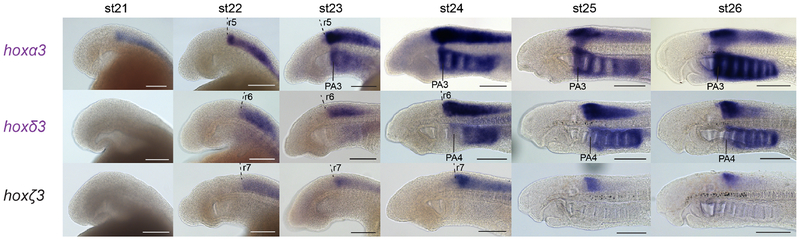
At st23.5, at least six rhombomeres can be demarcated by gene expression in the lamprey hindbrain, with wnt1 expressed in the midbrain and abutting the midbrain-hindbrain boundary, krox20(egr2) in r3/r5, hoxζ4 (a PG4 gene, described in more detail below) posterior to and abutting the r6/r7 boundary, and the anterior border of hoxα2 marking the r1/r2 boundary (Fig. 2A–D). hoxβ1 and hoxα3 exhibit discrete stripes of rhombomere-restricted expression at this stage, in r4 and r5 respectively, as previously shown (Fig. 2E–H) (Parker et al., 2014a).
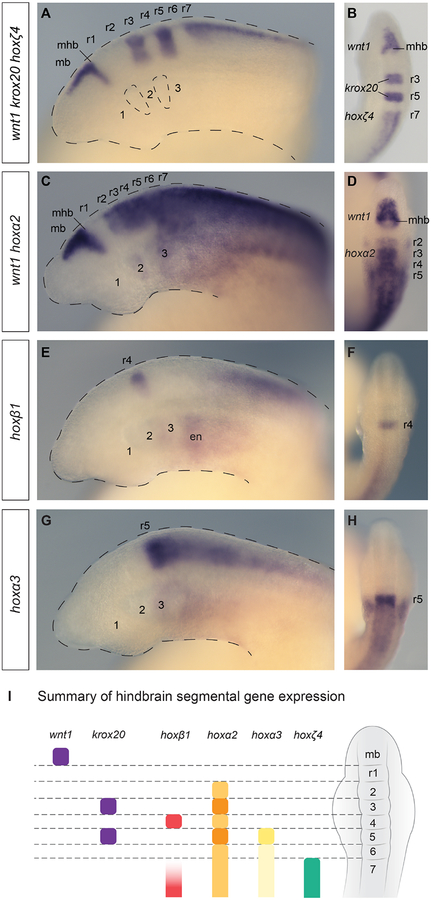
Figure 2: The lamprey hindbrain segmental plan and segmental hox expression.
Lateral (A,C,E,G) and dorsal (B,D,F,H) views of st23.5 lamprey embryos are shown. (A-B) A triple in-situ hybridisation against wnt1, krox20 and hoxζ4 (all purple) demarcates hindbrain segments in the neural tube. wnt1 marks the caudal limit of the midbrain (mb), revealing the midbrain-hindbrain boundary (mhb), while krox20 marks r3 and r5, and hoxζ4 is expressed posterior to the r6/r7 boundary. Rhombomeres (r1–r7) and pharyngeal arches (1–3) are annotated, and the head and pharyngeal pouches are outlined. (C–D) A double in-situ hybridisation against wnt1 and hoxα2, showing segmental hoxα2 expression in the hindbrain posterior to the r1/r2 boundary and wnt1 in the midbrain. hoxα2 is also expressed in the developing pharyngeal arches, posterior to PA1. (E–F) hoxβ1 is expressed in r4 and in the posterior hindbrain/spinal cord, as well as in the pharyngeal endoderm (en). (G–H), hoxα3 shows an elevated stripe of expression in r5, with lower expression levels in the neural tube posterior to r5. Expression is also seen in the pharyngeal arches, posterior to PA2. (I) A depiction of a dorsal view of a st23.5 lamprey embryo, summarising the segmental gene expression domains in the neural tube shown in (A-H), which together demarcate r1–r7.
Lamprey pharyngeal segmentation occurs between st21–26, as the pharynx is progressively segmented into a series of pharyngeal arches and pouches, ultimately comprising 8 pharyngeal arches by st26. From st23, hoxα2 is visible in the pharyngeal arches, with an anterior limit in PA2 (Fig. 2C), while hoxα3 has an anterior limit in PA3 (Fig. 2G). Together, these segmental patterns in the hindbrain and pharynx provide a topographical and temporal framework in which to analyze the expression of the anterior hox genes during lamprey head development (Fig. 2I).
3.3. hox PG1 expression
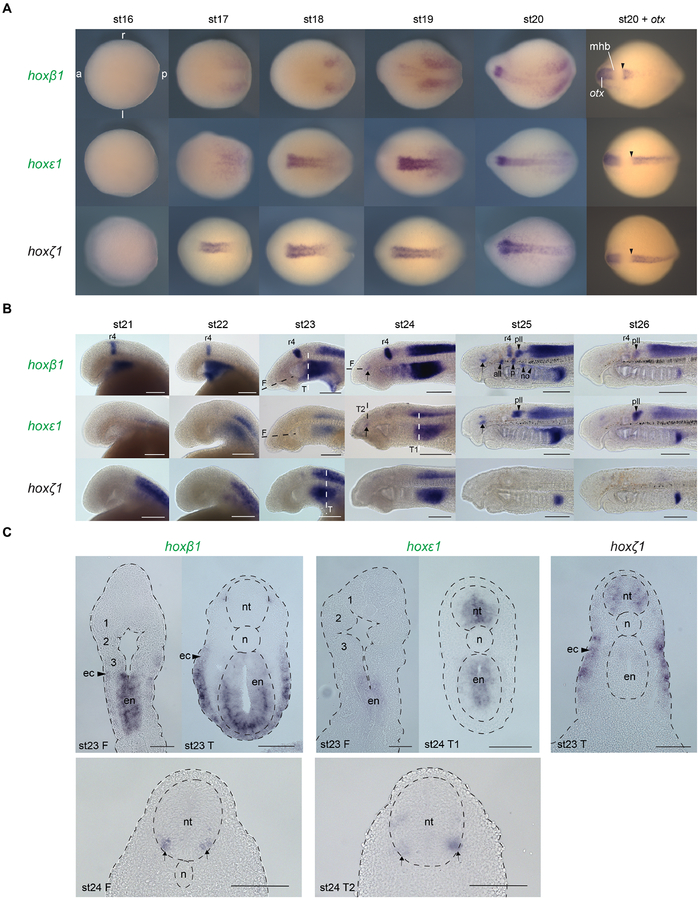
We first investigated the expression of the three lamprey PG1 genes - hoxβ1, hoxε1 and hoxζ1. In gnathostomes, PG1 genes are the earliest Hox genes to be expressed in the neuroepithelium, so we investigated their expression during early lamprey development. We detected differential timing of onset in the neuroepithelium between these genes, with hoxζ1 and hoxε1 first detectable at st17 in broad and overlapping domains. At this stage, hoxε1 expression is less detectable than that of hoxζ1, but becomes more pronounced by st18, when both genes develop clear anterior boundaries (Fig. 3A). These domains persist through st20, with hoxβ1 expression in the neural plate emerging by st19. At st20, all three PG1 genes show similar anterior borders of expression in the presumptive hindbrain (Fig. 3A – arrowheads) as compared to otx, which is expressed anterior to the midbrain-hindbrain boundary (Tomsa and Langeland, 1999). At this stage, hoxβ1 resolves into a distinct anterior stripe, which presumably corresponds to the future r4. Expression adjacent to the neural plate is also seen for hoxβ1 and hoxε1. At later stages, st21–26, hoxβ1 remains as a restricted stripe in r4, with additional expression in the posterior hindbrain and spinal cord, as previously reported (Fig. 3B) (Parker et al., 2014a). In contrast, hoxε1 and hoxζ1 expression is lost from r4, but persists more posteriorly in the neural tube, with hoxζ1 expression then disappearing from the neural tube by st25 (Fig. 3B). hoxβ1 and hoxε1 also show expression in the region of the forebrain/midbrain boundary at st24–25 (Fig. 3B - arrows). Sections reveal that these domains mark bilateral clusters of cells within the ventral neural tube (Fig. 3C), which appear to be homologous to those characterised for gnathostome Hoxa1 genes in ventral forebrain/midbrain neurons at the anterior terminus of the medial longitudinal fasciculus (McClintock et al., 2003).
Figure 3: Expression of lamprey hoxPG1 genes in the developing head.
(A) Dorsal views of st16-st20 embryos with expression of hoxPG1 genes revealed by in situ hybridisation. The anterior (a), posterior (p), left (l) and right (r) sides are annotated in the top-left image. The rightmost images show double in situ hybridisation signals for these PG1 genes with otx, which is expressed anterior to the midbrain-hindbrain boundary (mhb). These embryos are in antero-dorsal view, slightly tilted such that the anterior limits of hoxPG1 expression in the developing hindbrain can be seen more clearly (arrowheads). (B) Expression of hoxPG1 genes in st21–26 embryos, shown in lateral view with anterior to the left. Arrows mark neurons in the forebrain/midbrain. Arrowheads label cranial ganglia: all, anterior lateral line ganglion; no, nodose ganglion; p, petrosal ganglion; pll, posterior lateral line ganglion. (C) Frontal (F) and transverse (T) sections of st23–24 embryos after in situ hybridisation. The approximate planes of section are indicated in the lateral views shown in panel B. The developing pharyngeal arches are annotated (1–3) on the st23 frontal sections. Arrows indicate expression in bilateral clusters of neurons in the ventral forebrain/midbrain. In all panels, hoxβ1 and hoxε1 gene names are shaded in green to denote their paralogy relationship, as detailed in Fig. 1B. Scale bars: 200μm (B); 100μm (C). ec, ectoderm; en, endoderm; mhb, midbrain-hindbrain boundary; n, notochord; nt, neural tube; r, rhombomere.
In the pharynx, hoxβ1 is prominently expressed in the endoderm and ventral ectoderm from st21, (Fig. 3B,C). Expression is temporally dynamic in both tissues, regressing posteriorly during pharyngeal segmentation such that expression is highest posterior to the most recently formed pharyngeal pouch, with some low-level expression persisting more anteriorly. hoxε1 also displays similar endodermal expression in the pharynx, with hoxζ1 expressed in the posterior pharyngeal ectoderm (Fig. 3B,C). hoxβ1 and hoxε1 are expressed in the cranial ganglia from st25 - both genes in the posterior lateral line ganglion, hoxβ1 in the anterior lateral line, petrosal and nodose ganglia (Fig. 3B).
3.4. hox PG2 expression
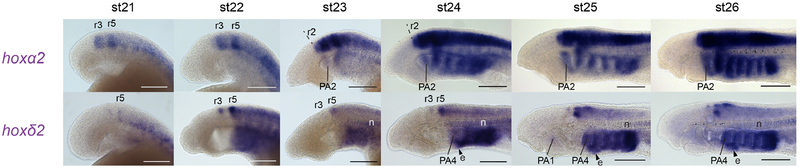
In the neural tube, hoxα2 is expressed in presumptive r3 and r5 from st21 and has lower levels of expression in r4 and posterior to r5 at that stage (Fig. 4). By st22, expression is also seen in r2 such that prominent rhombomeric stripes are visible in r2–r5. From st24 onwards, expression in the hindbrain and spinal cord persists, with an anterior limit in r2, but the rhombomere-restricted stripes of expression become less clear. hoxδ2 expression is detected in restricted domains within presumptive r5 and in dorsal r3 from st21, which persists across our developmental time-course (Fig. 4). Additional expression of lower intensity is also seen in the neural tube posterior to r5, with a dorsally-restricted domain caudal to r5 visible at st25–26.
Figure 4: Expression of lamprey hoxPG2 genes in the developing head.
Expression of hoxPG2 genes in st21–26 embryos, shown in lateral view with anterior to the left. Gene names are shaded in purple to denote their paralogy relationship, as detailed in Fig. 1B. Scale bars: 200μm. e, endostyle; n, notochord; PA, pharyngeal arch; r, rhombomere.
In the pharynx, hoxα2 is expressed in the pharyngeal arches from st23 and is maintained through later stages, with an anterior limit in PA2. At st25, this expression is prominent in the NC-derived mesenchyme, as well as in the pharyngeal arch mesoderm, as revealed by frontal sectioning (see Fig. 7B). hoxδ2 expression in the pharynx is seen from st22 and persists to later stages, with an anterior limit at st24 in the caudal half of the third pharyngeal pouch. We also observed transient, faint signal in the first pharyngeal arch from st25–26. Frontal sections at st26 show that this PA1 expression is mesodermal (see Fig. 7B), while the caudal pharyngeal expression is in the pharyngeal endoderm (pharyngeal pouch 3 to posterior) and in the mesenchyme of PA6–8. Expression was also detected for hoxδ2 in the caudal extent of the developing endostyle, posterior to PA4 at st24–26, as well as in the notochord from st23–26 (Fig. 4).
Figure 7: Rhombomeric hoxPG3–4 expression and pharyngeal expression of selected lamprey hoxPG1–4 genes.
(A) Double in situ hybridization of hox genes from PG3–4 with krox20, to resolve rhombomeric expression domains. krox20 expression is in r3 and r5. For the PG3–4 genes with clear rhombomeric boundaries, they are indicated (dashed line). hoxα3 expression in r5 was previously characterized and so is not shown (Parker et al., 2014a). (B) Frontal sections at st25–26, revealing pharyngeal hoxPG2–4 expression domains. Black arrowheads indicate anterior expression limits in the neural crest-derived pharyngeal arch mesenchyme. White arrowheads mark hoxδ2 expression in pharyngeal pouch endoderm. Pharyngeal arches are numbered (1–8). (C) A schematic frontal section of a st26 lamprey embryo indicating the different tissue layers. ec, ectoderm; en, endoderm; m, mouth; me, mesenchyme; mm, mandibular mesoderm; n, notochord; nt, neural tube.
3.5. hox PG3 expression
The PG3 genes show nested expression in the developing hindbrain, with offset anterior boundaries (Fig. 5). hoxα3 is expressed at a high level in r5 at st22, with lower expression detected in the neural tube posterior to r5. By st23, additional weak expression is detectable in r4. At these stages, hoxδ3 is expressed posterior to the r5/r6 boundary, and hoxζ3 posterior to the r6/r7 boundary, as revealed by comparison with krox20(egr2) in r3/r5 (see Fig. 7A). These patterns are temporally dynamic - from st24 onwards they break from rhombomeric registration, with each gene showing anterior expression boundaries that are non-uniform along the dorso-ventral axis. For example, at st25-st26, hoxδ3 signal is visible in the hindbrain with a sharp anterior border that aligns with the anterior side of PA4, except for a small domain in the dorsal hindbrain that protrudes rostrally from this border. Pharyngeal expression is detected for hoxα3 and hoxδ3 but not for hoxζ3 (Fig. 5). This is visible from st23, resolving into nested domains in the pharyngeal arch mesenchyme by st26: hoxα3 in PA3–8 and hoxδ3 in PA4–8 (see Fig. 7B).
Figure 5: Expression of lamprey hoxPG3 genes in the developing head.
Expression of hoxPG3 genes in st21–26 embryos, shown in lateral view with anterior to the left. hoxα3 and hoxδ3 gene names are shaded in purple to denote their paralogy relationship, as detailed in Fig. 1B. Scale bars: 200μm. PA, pharyngeal arch; r, rhombomere.
3.6. hox PG4 expression
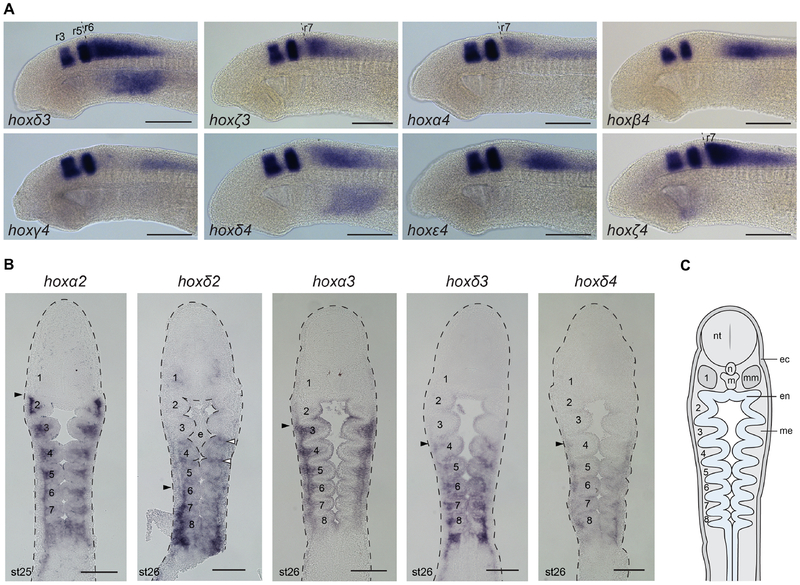
We detected expression of each of the 6 PG4 genes in the neural tube across our developmental time-course (Fig. 6A). We compared their anterior borders of expression using krox20 expression, to mark r3 and r5 (Fig. 7A), and by using the pharyngeal arches as landmarks (Fig. 6A). We sought to determine whether any lamprey PG4 genes had expression that could demarcate the r6/r7 boundary, since this is marked by PG4 genes in gnathostomes but its presence in lamprey is unclear based on morphological analysis and previous characterization of PG4 gene expression (Takio et al., 2007; Takio et al., 2004). Our analysis reveals that hoxα4 and hoxζ4 have clear anterior expression borders at the same position at st24, aligning with the anterior face of PA4 (Fig. 6A). Comparison with krox20 expression shows that this domain is caudal to r5 by approximately one rhombomere-length and is thus likely to represent the r6/r7 boundary (Fig. 7A). The other PG4 genes have anterior expression borders that are posterior to this region in the caudal hindbrain. The anterior expression limits in the neural tube are temporally dynamic for some of these PG4 genes: hoxα4 expression aligns anteriorly with PA4 at st24 but with PA6 at st26, while that of hoxβ4 aligns with PA4 at st24 and with PA5 at st26 (Fig. 6A). In other cases, such as hoxζ4, the anterior boundary is maintained across this time-course, appearing to retain a tight rhombomeric registration. hoxγ4, hoxδ4, and hoxε4 each show expression profiles that change along the dorsal-ventral axis across this time-course, with expression in the dorsal neural tube spreading more rostrally in each case, perhaps encompassing specific neuronal populations (Fig. 6A - arrows).
Figure 6: Expression of lamprey hoxPG4 genes in the developing head.
(A) Expression of hoxPG4 genes in st21–26 embryos, shown in lateral view with anterior to the left. Neural expression is seen in the posterior hindbrain and/or spinal cord for each gene. To facilitate comparison of this neural expression across time and between genes, the pharyngeal arches (PA) that are adjacent to the anterior neural expression boundaries are labelled. Arrows indicate anterior spread of dorsal neuronal expression domains at st25–26. (B) A model of the duplication events that are inferred to have occurred in the lamprey/cyclostome lineage, leading to the 6 Hox clusters in Sea lamprey. Gene names in (A) are shaded to reflect these paralogy relationships. Scale bars: 200μm. e, endostyle; PA, pharyngeal arch; r, rhombomere.
Expression is visible for each of the hoxPG4 genes within the pharynx (Fig. 6A). For hoxα4, hoxβ4, and hoxε4, this signal was only detectable in the most caudal extent of the pharynx. hoxγ4 shows faint signal in a gradient of increasing intensity from PA5 caudally, while hoxδ4 expression is visible in the mesenchyme of PA4–8 (Fig. 7B). hoxζ4 was detectable in the developing endostyle from st23 onwards, but expression in other pharyngeal domains was not seen for this gene.
Considering the model, based on patterns of conserved synteny between hox-bearing chromosomes, that the -α and -δ clusters and the -β and -ε clusters derive from duplication in the lamprey/cyclostome lineage (Fig. 6B), the lamprey PG4 genes exhibit both conservation and divergence of expression domains between paralogues from these clusters. For instance, hoxα4 and hoxδ4 are both expressed in the spinal cord and caudal hindbrain, but their precise anterior limits in the hindbrain differ. Additionally, hoxδ4 is expressed in PA4–8, while hoxα4 is only detected at the most caudal end of the pharynx. hoxβ4 and hoxε4 are also both expressed in the caudal hindbrain and spinal cord but have different anterior limits in the hindbrain (Fig. 6A).
4. Discussion:
We have characterised the expression of the 14 Hox PG1–4 genes in the developing lamprey head to address two primary questions: when did segmental Hox domains evolve in vertebrate evolution and how have they diversified between vertebrate lineages? We find many similarities in Hox expression between lamprey and gnathostome species, particularly in rhombomeric domains during hindbrain segmentation and in the cranial neural crest, enabling inference of aspects of Hox expression in the ancestral vertebrate embryonic head. We also observe differences, including variation in hindbrain domains at later stages, as well as expression in the endostyle and in PA1 mesoderm. Considering the Hox cluster duplications that preceded the cyclostome-gnathostome divergence, comparison of Hox expression and cluster organization between lamprey and gnathostomes suggests that ancestral vertebrate Hox functions have been largely retained in lamprey and gnathostomes but have been partitioned differently across duplicated clusters in each lineage. This is consistent with the observation that a Hox regulatory network underlying hindbrain segmentation is conserved to the base of vertebrates (Parker et al., 2014a).
4.1. The hox repertoire of lamprey and its relationship to gnathostome Hox clusters
The two lamprey species examined to date both have 6 Hox clusters and appear to share an identical Hox gene complement, reflecting their close phylogenetic relationship (Kuraku and Kuratani, 2006; Mehta et al., 2013; Pascual-Anaya et al., 2018; Smith et al., 2018). Despite lamprey having 6 Hox clusters compared to 4 in most tetrapods, paralogue loss has resulted in the total number of Hox genes being similar between these taxa: 43 in lamprey and 39 in mouse (Fig. 1). For PG1–4, lamprey and mouse have both retained a remarkably similar number of genes in each paralogy group. It remains unclear precisely how the 6 lamprey Hox clusters relate to the 4 Hox clusters that were presumably present in the common ancestor of gnathostomes, and to the 4 clusters in mouse, since phylogenetic analyses could not resolve 1:1 orthology between lamprey and gnathostome Hox genes/proteins (Mehta et al., 2013; Pascual-Anaya et al., 2018; Smith et al., 2018). This does not necessarily imply that lamprey and gnathostome Hox clusters arose from independent duplication events, since ancient duplication, when followed quickly by lineage separation and subsequent divergence, coupled with species-specific patterns of codon and amino acid usage, could weaken the signal of these phylogenetic events (Qiu et al., 2011). Indeed, recent reconstructions based on comparisons of gene order at the chromosomal level between vertebrate species are consistent with a model in which the ancestor of cyclostomes and gnathostomes had 4 Hox clusters (Smith et al., 2018). If this model is accurate, it has an important ramification with respect to the ancestry of the Hox segmental patterning functions seen in gnathostomes (Onimaru and Kuraku, 2018; Parker et al., 2016). Since paralogous segmental enhancers exist in gnathostomes, such as the r5 enhancers of Hoxb3 and Hoxa3, and these paralogues are posited to have arisen from duplication before the split between gnathostome and cyclostome lineages, then such segmental regulation presumably also pre-dates this split, as supported by the expression analyses presented here and in previous studies (Parker et al., 2014a; Takio et al., 2007; Takio et al., 2004).
The two additional Hox clusters found in lamprey most likely derive from duplication event/s that occurred in the lamprey/cyclostome lineage. In support of this, comparisons of gene content between lamprey Hox-bearing chromosomes suggests that the chromosomes containing the -β and -ε clusters derive from such duplication, as well as those bearing the -α and -δ clusters (Smith et al., 2018). Thus, comparisons between lamprey Hox paralogues from the -β and -ε clusters and from the -α and -δ clusters could illuminate patterns of functional divergence that may underlie their retention subsequent to duplication, as discussed below.
A recent genomic and transcriptomic analysis of another cyclostome species - the Japanese inshore hagfish, Eptatretus burgeri - identified 40 Hox genes spread across 6 predicted clusters (Pascual-Anaya et al., 2018). Comparison with the 6 sea lamprey hox clusters reveals many similarities, with each species having the same number of characterized paralogues in PG1–4: 3 PG1, 2 PG2, 3 PG3 and 6 PG4 genes. These similarities might suggest that the duplication event/s that gave rise to the 6 Hox clusters of lamprey preceded the lamprey-hagfish divergence, which is estimated to have occurred more than 400 million years ago (Kuraku and Kuratani, 2006). However, phylogenetic analyses were unable to identify clear one-to-one orthology relationships between hagfish Hox genes and those of lamprey and gnathostomes (Pascual-Anaya et al., 2018). Indeed, lamprey -β and -ε genes consistently cluster with each other in these trees but do not consistently group with genes from any hagfish cluster/s, which appears to support a more recent divergence of the lamprey -β and -ε clusters in the lamprey lineage. In summary, it is presently unclear how the 6 Hox clusters of sea lamprey relate to those of hagfish. Future chromosomal level synteny comparisons using a hagfish genome assembly may help to illuminate these relationships.
4.2. The hindbrain hox-code and rhombomeric expression domains
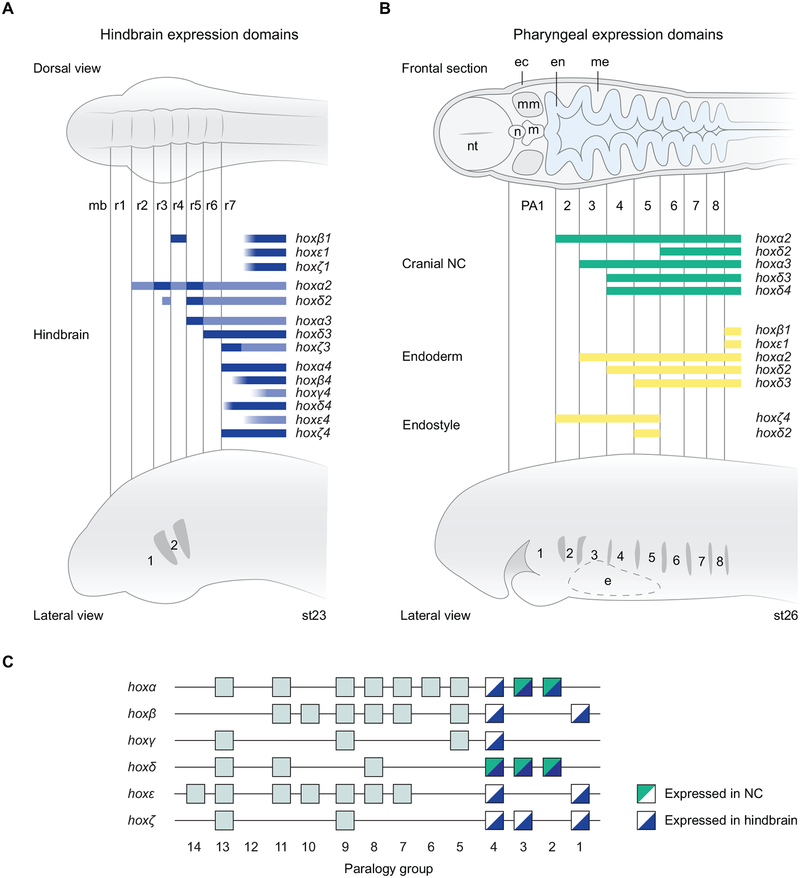
In lamprey, transient rhombomeric segmentation has been described through analyses of morphology, neuro-anatomy and segmental gene expression, being particularly apparent between st22–st24 (Horigome et al., 1999; Kuratani et al., 1998). Segmentally-restricted Hox expression, which maps to rhombomere boundaries, had previously been revealed at these stages for three anterior hox genes in lamprey: hoxβ1, hoxα2 and hoxα3 and compared directly with the expression domains of two genes involved in segmentation (kreisler(mafb) and krox20(egr2)) (Parker et al., 2014a). Collectively, these genes show similar rhombomere-restricted expression domains compared with their gnathostome counterparts, suggesting conservation of a hindbrain gene regulatory network in lamprey. Here, we have expanded upon this initial analysis by demonstrating that all 14 PG1–4 genes are dynamically expressed in the developing hindbrain at the stages examined, with 8 genes exhibiting segmentally-restricted expression at st23: hoxβ1, -α2, -δ2, -α3, -δ3, -ζ3, -α4 and -ζ4 (Fig. 8A,C). The six genes lacking segmentally-restricted expression do not have sharp anterior borders and their expression resides in the caudal hindbrain, where segmental markers are not apparent. Electron microscopy and immunolabelling approaches delineated r1–r6 in Arctic lamprey embryos but did not reveal an r6/r7 boundary (Horigome et al., 1999; Kuratani et al., 1998). However, the sharp expression boundaries we identified for hoxα4 and hoxζ4 suggest that an r6/r7 boundary exists in lamprey (Fig. 7A), at least at the level of gene expression, and that some of the Hox genes are no longer tightly coupled to this segment border (Fig. 8A). During mouse hindbrain segmentation, Hoxb4 and Hoxd4 are expressed on the posterior edge of the presumptive r6/r7 boundary and are required for its formation, while Hoxa4 and Hoxc4 are expressed more posteriorly in the neural tube (Prin et al., 2014). Similarly, in zebrafish, hoxb4a and hoxd4 have anterior expression aligning with the r6/r7 boundary, while the other hox4 paralogues are expressed more posteriorly in the hindbrain and have expression that is not clearly coupled to any rhombomere boundary (Prince et al., 1998a; Prince et al., 1998b). Thus, the PG4 genes in lamprey and gnathostomes show similar rhombomeric expression characteristics, with some being tightly coupled to the r6/r7 boundary and others being uncoupled from rhombomere boundaries.
Figure 8: Summary of lamprey hoxPG1–4 gene expression in the hindbrain and cranial NC.
(A) A summary figure depicting segmental domains of expression of lamprey hoxPG1–4 genes in the hindbrain at st23, shown relative to dorsal (top) and lateral (bottom) schematic representations of the lamprey embryonic head. Rhombomeres (r1–r7) and pharyngeal arches (1–2) are annotated. The blue shading indicates domains of gene expression, with darker shading indicating higher levels of expression as detected by in-situ hybridisation. (B) A summary of expression domains of lamprey hoxPG1–4 genes in the cranial NC (green) and endoderm (yellow) at st26, shown relative to schematic representations of the embryonic head in frontal section (top) and lateral view (bottom). Expression in the endoderm-derived endostyle is also shown (yellow). The pharyngeal arches (1–8) are labelled. hoxβ1 and hoxε1 have dynamic expression in the endoderm, which retreats caudally during development, being associated with the most recently formed pharyngeal pouch. (C) A depiction of the lamprey hox clusters, with hoxPG1–4 genes marked according to whether they are expressed in NC, hindbrain or both. This reveals that all of the hoxPG1–4 genes are expressed in the hindbrain, while only genes from hoxα and hoxδ clusters appear to be expressed in cranial NC at the stages examined. e, endostyle; ec, ectoderm; en, endoderm; m, mouth; mb, midbrain; me, mesenchyme; mm, mandibular mesoderm; n, notochord; nt, neural tube.
From this data, and by comparison with gnathostomes, we can reconstruct aspects of Hox expression that were present in the ancestral vertebrate hindbrain and which have been conserved across vertebrates: expression of a PG2 gene up to the r1/r2 boundary, a PG1 gene in r4, elevated expression of a PG3 gene in r5, and expression of a PG4 gene up to the r6/r7 boundary (Fig. 8A,C). Additionally, r1 is devoid of hox PG1–4 expression during lamprey hindbrain segmentation; this is also seen in gnathostomes, although Hox expression in specific neurons of r1 has been detected at later stages of hindbrain development in some species (McClintock et al., 2003).
A striking aspect of hox expression in the arctic lamprey hindbrain is that anterior hoxα3 domains do not appear to be segmentally restricted at later stages of hindbrain development (st25-st26), despite the maintenance of segmental krox20 and ephC expression (Murakami et al., 2004; Takio et al., 2007). A similar escape from segmental restriction is seen for hoxα3 in sea lamprey, with rhombomeric registration observed at earlier stages (Fig. 5). Our results reveal that segmental expression perdures through later stages for some lamprey hox genes, such as hoxβ1 and -ζ4, while others appear to escape segmental restriction, including hoxδ2 and -δ3. Certain PG4 genes − hoxγ4, -δ4 and -ε4 - also exhibit non-uniform anterior expression boundaries at later stages, however it is unclear whether these align with segments, particularly in the caudal hindbrain.
In gnathostome embryos, such escape from segmental registration has not been observed, as once Hox expression becomes refined to specific segments and bands of neuronal progenitors over time the domains remain aligned within rhombomere-derived territories during later embryogenesis (Gavalas et al., 2003; Prince et al., 1998b; Wingate and Lumsden, 1996). This is regulated in part through a multi-step process whereby early domains are established by cis-elements that integrate inputs from signaling pathways and the segmental pattern is actively maintained at later stages by auto-and cross-regulatory interactions (Gould et al., 1998; Manzanares et al., 2001; Studer et al., 1998). This suggests that in the lamprey hindbrain there may be key regulatory differences in how and whether Hox genes remain coupled to segmentation at later stages, resulting in a temporal relaxation in segmental constraints compared with gnathostomes. This may enable some hox genes to be co-opted to perform additional non-segmental roles at later stages of development. Nevertheless, such early segmentation has a lasting effect on the neuronal architecture of the larval lamprey hindbrain, as seen by the segmental arrangement of reticulospinal neurons and the general A-P positioning of cranial nerve motor nuclei (Gilland and Baker, 2005; Murakami et al., 2004; Osorio et al., 2005).
A recent study focusing on hagfish Hox genes revealed segmented and nested domains in the embryonic hindbrain, supporting conservation of aspects of this ancestral Hox pattern, although differences were also observed, such as the absence of detectable Hox1 expression from r4 at the stages examined (Pascual-Anaya et al., 2018). Taken together, this points to the existence of an ancient gene regulatory network for Hox-patterning in the hindbrain and pharynx that has been broadly conserved across all vertebrates, but that also exhibits lineage-specific diversification (Parker et al., 2016).
4.3. The neural crest Hox-code
The lamprey pharynx comprises 8 pharyngeal arches, which are populated by NC cells migrating in three streams from the hindbrain, broadly equivalent to the three anterior streams of gnathostomes, although a vagal NC stream from the caudal hindbrain appears to be absent in lamprey (Green et al., 2017; Horigome et al., 1999; McCauley and Bronner-Fraser, 2003; Meulemans and Bronner-Fraser, 2002). PA1 is homologous to the mandibular arch of gnathostomes; however, rather than giving rise to the jaw, it forms the velum, a cyclostome-specific piston-like valve involved in ventilating the larval lamprey pharynx (Miyashita, 2016). PA2 is homologous to the hyoid arch of gnathostomes, forming the velar support cartilage, while PA3–8 hold gills, like the posterior pharyngeal arches in aquatic gnathostomes. In gnathostomes, Hox PG2–4 genes have nested expression in pharyngeal arch NC that is broadly conserved between species, but paralogues often exhibit differences in expression levels (Parker et al., 2018). Previous studies in lamprey found conservation of select Hox domains in NC populations between lamprey and gnathostomes (Takio et al., 2007; Takio et al., 2004). Our data expands on this by showing that none of the PG1–4 genes are expressed in PA1 NC at the stages examined, similar to gnathostomes, and that there are nested domains of expression of five lamprey hox genes in PA2–5 (Fig. 8B).
We observe that only genes from hoxα and hoxδ clusters appear to have nested expression in lamprey cranial NC at the stages examined (Fig. 8C). hoxα2 is the only PG2 gene expressed in PA2 at these stages, with hoxα3 the only PG3 gene in PA3, and hoxδ4 the only PG4 gene in PA4. This suggests that there may be little functional overlap in NC patterning between hox genes from the same paralogy group in lamprey. In contrast, some paralogous Hox genes share NC expression domains in gnathostome species and exhibit a degree of functional redundancy (e.g. hoxa2b and hoxb2a in zebrafish PA2, Hoxa3 and Hoxb3 in mouse PA3) (Hunter and Prince, 2002; Manley and Capecchi, 1997). This shared activity of paralogues indicates that these Hox domains in NC were probably a feature of the ancestral, pre-duplicated Hox cluster. If so, then after the Hox cluster duplications in ancestral vertebrates, divergent vertebrate lineages have differentially retained NC expression of their paralogous Hox genes. However, it is not immediately apparent whether retention versus loss of the NC expression domains of duplicated Hox genes has an adaptive significance. Testing the functional roles of lamprey hox genes in determining the identity of skeletal elements in the head by CRISPR approaches will be an interesting avenue for future research.
4.4. Endodermal hox expression domains
In chick and dogfish, endodermal Hox expression has been shown to correlate with specific pharyngeal pouches: Hoxb1 expression progressively shifts caudally such that it is only present in the most recently formed pharyngeal pouch, Hoxa2 is associated with the 2nd pharyngeal pouch and Hoxa3 transiently with the 3rd pouch (Shone et al., 2016). Our analysis reveals dynamic hoxβ1 and -ε1 expression in the most recently formed pouch in lamprey (Fig. 3B, 8B), suggesting that the posterior limit of the pharynx is homologous between lamprey and gnathostome species. An RA-dependent role for Hox1 in defining the posterior limit of the pharynx has been shown in amphioxus (Schubert et al., 2005) and this expression is conserved in a hemichordate, Saccoglossus kowalevskii (Gillis et al., 2012), suggesting that this role for Hox1 genes in pharyngeal development traces its evolution deep into the deuterostome lineage and has been conserved in many extant chordates. Expression was also detected for other hox genes in the lamprey pharyngeal endoderm at the stages we examined: hoxα2 up to the 2nd pouch (st25), hoxδ2 up to the 3rd pouch (st26) and hoxδ3 up to the 4th pouch (st26), although this was often at low levels relative to their expression in other domains (Fig. 7, 8B). This suggests that these genes may play similar roles in patterning the pharyngeal endoderm compared to their homologues in gnathostomes.
Among extant vertebrates, lamprey species are unique in possessing an endostyle, which plays a role in filter feeding in larval lampreys and has other functions including regulating iodine uptake. The lamprey endostyle evaginates from endoderm in the ventral pharynx and is transformed into a thyroid during metamorphosis (Kluge et al., 2005). We detected expression in the endostyle for two hox genes: hoxζ4 throughout the A-P extent of the endostyle and hoxδ2 restricted to the caudal end (Fig. 8B). In urochordates, Hox1 genes have been implicated in endostyle patterning (Canestro et al., 2008; Yoshida et al., 2017), while Hox3 genes are required for normal thyroid development in mice (Manley and Capecchi, 1995; Manley and Capecchi, 1998). This suggests that there may be similar requirements for Hox genes in patterning these endoderm-derived pharyngeal organs across chordates. However, nonorthologous Hox genes appear to be utilised in each of these cases, so further investigation is required to establish whether these reflect conserved ancestral patterning networks or whether this Hox patterning has been acquired independently in different lineages.
4.5. Patterns of sub-functional divergence between paralogues
Phylogenetic and synteny analyses suggest that the lamprey hox-β and -ε clusters and the -α and -δ clusters arose from chromosome-scale duplication event/s in lamprey/cyclostomes, after the gnathostome-cyclostome divergence (Smith et al., 2018). Comparing the PG1–4 gene complement between these duplicated clusters indicates that they have retained Hox paralogues to a high degree. This is interesting given the importance of Hox genes in development of the body plan and regional specializations. This raises the question of how the lamprey lineage may have utilized these duplicated hox genes and the possibilities they may offer in regulating anatomical novelties.
Comparisons of spatiotemporal expression between the pairs of lamprey hox paralogues from these duplicated clusters are summarized in Table 1, which illuminate patterns of divergence that may underlie their retention subsequent to duplication. Divergence is seen in the anterior limits of expression between paralogues, such as for hoxα3 and hoxδ3 in hindbrain and NC. In other cases, paralogues differ more drastically in tissue specificity, for instance hoxδ4 retains expression in NC up to PA4, which is presumably ancestral since it is a feature of certain gnathostome PG4 genes, while hoxα4 has lost expression in this domain. Differences in initiation and maintenance of expression are also seen between paralogues. For example, hoxβ1 has later onset in the neural plate than hoxε1 and is maintained in r4 while hoxε1 expression is lost from this domain (Fig. 3). In mouse, differences in onset and maintenance between Hoxa1 and Hoxb1 are attributable to specific cis-regulatory elements that are associated with each gene: both have a 3’ RARE for early neural expression, while Hoxb1 is maintained in r4 by an auto-regulatory element that is lacking from Hoxa1 (Dupe et al., 1997; Marshall et al., 1994; Popperl et al., 1995). This suggests that homologous regulatory elements may also have been partitioned between lamprey hoxβ1 and hoxε1 after their duplication in the lamprey/cyclostome lineage. Similar patterns of sub-functionalisation with respect to r4 expression have been demonstrated for zebrafish hoxb1a and hoxb1b, which resulted from the teleost whole genome duplication (McClintock et al., 2001; McClintock et al., 2002). The re-occurrence of this sub-functional partitioning in multiple lineages may be stochastic, or it may reflect features in the organization of HoxPG1 cis-regulation such that there is an increased likelihood of sub-functionalisation occurring due to the modularity and functional independence of the enhancer elements that mediate initiation versus maintenance of expression. hoxβ1 and hoxε1 are both expressed in ventral fore-/mid-brain neurons, an expression domain seen for Hoxa1 orthologues in various gnathostomes as well as for Hoxc1 in zebrafish (McClintock et al., 2001; McClintock et al., 2003). Thus, lamprey hoxβ1 resembles gnathostome Hoxb1 genes in some aspects of its expression, such as its maintenance in r4, but reflects Hoxa1 genes in other respects, such as expression in fore-/midbrain neurons. The partitioning of these expression domains across HoxPG1 genes in lamprey and gnathostomes provides further evidence that many ancestral HoxPG1 functions have been retained in distantly related vertebrate lineages but have been rearranged differently across HoxPG1 genes after duplication, a phenomenon termed function shuffling (McClintock et al., 2001).
Table 1:
Conserved and divergent expression features of paralogous lamprey Hox gene pairs that arose from duplication in the lamprey/cyclostome lineage.
| Paralogous gene pair | Conserved expression features | Divergent expression features |
|---|---|---|
| Hoxβ1, hoxε1 | Pharyngeal endoderm Fore-/midbrain neurons Posterior lateral line ganglion |
Early onset in neural plate (hoxε1) Maintenance in r4 (hoxβ1) Anterior lateral line, petrosal and nodose ganglia (hoxβ1) |
| hoxα2, hoxδ2 | PA6-posterior NC | PA2–5 NC (hoxα2) Rhombomeric domains Notochord (hoxδ2) PA1 mesoderm (hoxδ2) |
| hoxα3, hoxδ3 | r6-posterior PA4-posterior NC |
r5 (hoxα3) PA3 NC (hoxα3) pharyngeal endoderm (hoxδ3) |
| hoxα4, hoxδ4 | Caudal hindbrain Spinal cord |
r6/r7 boundary (hoxα4) PA4-posterior NC (hoxδ4) Maintenance in hindbrain (hoxδ4) |
| Hoxβ4, hoxε4 | Caudal hindbrain Spinal cord |
Anterior limit in hindbrain |
Did the duplication/s in lamprey/cyclostomes add any new functions to Hox genes or simply result in shuffling an ancient set of Hox patterning functions such that they became partitioned across duplicated Hox clusters? In zebrafish, PG1 and PG5 genes exhibit such partitioning of ancestral functions across paralogues subsequent to the teleost whole genome duplication (Bruce et al., 2001; Jozefowicz et al., 2003; McClintock et al., 2001; McClintock et al., 2003; Prince and Pickett, 2002). In contrast, the lamprey PG2 genes may represent a different case, with more dramatic differences in expression between paralogues. These include expression domains for hoxδ2 that, to our knowledge, have not been seen for PG2 genes in other vertebrate species, such as in PA1 mesoderm and in the endostyle (Table1). These may reflect ancestral vertebrate Hox2 functions that have been retained in lamprey but lost in gnathostomes. However, such expression domains have not been characterized for invertebrate deuterostome Hox2 genes, so it is unclear whether they are ancestral to vertebrates. Another intriguing possibility is that these expression domains evolved in the lamprey/cyclostome lineage, after duplication of the hoxα and -δ clusters (neo-functionalisation). Coupled with these non-canonical expression domains, hoxδ2 genes in both sea lamprey and arctic lamprey show a high degree of sequence divergence relative to other vertebrate Hox2 genes (Pascual-Anaya et al., 2018). This may reflect either relaxation of selective constraint or positive selection for discrete functions after duplication in the lamprey/cyclostome lineage. It will be interesting to address the functional significance of these expression domains.
4.6. Conclusion
In conclusion, our analysis suggests that many Hox expression domains that are observed in extant gnathostomes were present in ancestral vertebrates but have been partitioned differently across Hox clusters in gnathostome and cyclostome lineages after duplication. On top of this conserved Hox patterning ground-plan, lamprey also shows differences in spatiotemporal Hox expression, which may or may not be ancestral. These include tissue domains that are either not present or not associated with Hox expression in gnathostomes, such as the endostyle and PA1 mesoderm. Understanding how these conserved and divergent Hox expression domains relate to vertebrate head evolution will require examination of Hox functional roles in lamprey using CRISPR knockout approaches (Square et al., 2015). Such approaches could test the assumption that segmental Hox expression plays equivalent roles in lamprey and gnathostomes and could address the functional significance of the differences in Hox expression that we observe. Characterization of lamprey Hox enhancer elements and comparison with those of gnathostomes will enable inference of common ancestral Hox regulatory mechanisms in vertebrates, and may elucidate how Hox functions have been differentially partitioned across Hox clusters in lamprey versus gnathostome lineages (Parker et al., 2014b). Looking deeper in chordate evolution, these studies will provide a platform for regulatory comparisons with non-vertebrate deuterostomes (Minor et al., 2018), to investigate how vertebrate segmental Hox regulation arose.
Highlights:
Sea lamprey provides insights into the evolution of the Hox-code in vertebrates
The lamprey/cyclostome lineage has experienced additional Hox cluster duplications
Many gnathostome Hox expression domains were present in ancestral vertebrates
Some patterns are partitioned differently across Hox clusters in different lineages
Many similarities in Hox expression in rhombomeric domains and cranial neural crest
Acknowledgements
We thank Stephen Green, Dorit Hockman, Tetsuto Miyashita, and Megan Martik for lamprey husbandry assistance, and the Stowers Institute Histology facility for sectioning assistance. HJP and RK were supported by the Stowers Institute (RK grant #2013–1001). MEB was supported by grants RO1NS108500 and R35 NS111564.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baltzinger M, Ori M, Pasqualetti M, Nardi I, Rijli FM, 2005. Hoxa2 knockdown in Xenopus results in hyoid to mandibular homeosis. Dev Dyn 234, 858–867. [DOI] [PubMed] [Google Scholar]
- Bruce AE, Oates AC, Prince VE, Ho RK, 2001. Additional hox clusters in the zebrafish: divergent expression patterns belie equivalent activities of duplicate hoxB5 genes. Evol. Dev 3, 127–144. [DOI] [PubMed] [Google Scholar]
- Canestro C, Bassham S, Postlethwait JH, 2008. Evolution of the thyroid: anterior-posterior regionalization of the Oikopleura endostyle revealed by Otx, Pax2/5/8, and Hox1 expression. Dev Dyn 237, 1490–1499. [DOI] [PubMed] [Google Scholar]
- Carroll SB, 1995. Homeotic genes and the evolution of arthropods and chordates. Nature 376, 479–485. [DOI] [PubMed] [Google Scholar]
- Chisaka O, Musci TS, Capecchi MR, 1992. Developmental defects of the ear, cranial nerves and hindbrain resulting from targeted disruption of the mouse homeobox gene Hox-1.6. Nature 355, 516–520. [DOI] [PubMed] [Google Scholar]
- Dollé P, Lufkin T, Krumlauf R, Mark M, Duboule D, Chambon P, 1993. Local alterations of Krox-20 and Hox gene expression in the hindbrain suggest lack of rhombomeres 4 and 5 in homozygote null Hoxa-1 (Hox-1.6) mutant embryos. Proc. Natl. Acad. Sci. USA 90, 7666–7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboule D, 2007. The rise and fall of Hox gene clusters. Development 134, 2549–2560. [DOI] [PubMed] [Google Scholar]
- Duboule D, Dolle P, 1989. The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. EMBO J. 8, 1497–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupe V, Davenne M, Brocard J, Dolle P, Mark M, Dierich A, Chambon P, Rijli FM, 1997. In vivo functional analysis of the Hoxa-1 3’ retinoic acid response element (3’RARE). Development 124, 399–410. [DOI] [PubMed] [Google Scholar]
- Fraser S, Keynes R, Lumsden A, 1990. Segmentation in the chick embryo hindbrain is defined by cell lineage restrictions. Nature 344, 431–435. [DOI] [PubMed] [Google Scholar]
- Gavalas A, Ruhrberg C, Livet J, Henderson CE, Krumlauf R, 2003. Neuronal defects in the hindbrain of Hoxa1, Hoxb1 and Hoxb2 mutants reflect regulatory interactions among these Hox genes. Development 130, 5663–5679. [DOI] [PubMed] [Google Scholar]
- Gavalas A, Studer M, Lumsden A, Rijli FM, Krumlauf R, Chambon P, 1998. Hoxa1 and Hoxb1 synergize in patterning the hindbrain, cranial nerves and second pharyngeal arch. Development 125, 1123–1136. [DOI] [PubMed] [Google Scholar]
- Gavalas A, Trainor P, Ariza-McNaughton L, Krumlauf R, 2001. Synergy between Hoxa1 and Hoxb1: the relationship between arch patterning and the generation of cranial neural crest. Development 128, 3017–3027. [DOI] [PubMed] [Google Scholar]
- Gendron-Maguire M, Mallo M, Zhang M, Gridley T, 1993. Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell 75, 1317–1331. [DOI] [PubMed] [Google Scholar]
- Gilland E, Baker R, 2005. Evolutionary patterns of cranial nerve efferent nuclei in vertebrates. Brain Behav Evol 66, 234–254. [DOI] [PubMed] [Google Scholar]
- Gillis JA, Fritzenwanker JH, Lowe CJ, 2012. A stem-deuterostome origin of the vertebrate pharyngeal transcriptional network. Proc Biol Sci 279, 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J, Rossel M, Manley N, Capecchi M, 1996. Mice with targeted disruption of Hoxb1 fail to form the motor nucleus of the VIIth nerve. Development 122, 3217–3228. [DOI] [PubMed] [Google Scholar]
- Golding JP, Trainor P, Krumlauf R, Gassmann M, 2000. Defects in pathfinding by cranial neural crest cells in mice lacking the Neuregulin receptor ErbB4. Nat Cell Biol 2, 103–109. [DOI] [PubMed] [Google Scholar]
- Gould A, Itasaki N, Krumlauf R, 1998. Initiation of rhombomeric Hoxb4 expression requires induction by somites and a retinoid pathway. Neuron 21, 39–51. [DOI] [PubMed] [Google Scholar]
- Graham A, Papalopulu N, Krumlauf R, 1989. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell 57, 367–378. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos GA, Bell E, Toole L, Lumsden A, Tucker AS, 2000. Homeotic transformation of branchial arch identity after Hoxa2 overexpression. Development 127, 5355–5365. [DOI] [PubMed] [Google Scholar]
- Green SA, Uy BR, Bronner ME, 2017. Ancient evolutionary origin of vertebrate enteric neurons from trunk-derived neural crest. Nature 544, 88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanneman E, Trevarrow B, Metcalfe WK, Kimmel CB, Westerfield M, 1988. Segmental pattern of development of the hindbrain and spinal cord of the zebrafish embryo. Development 103, 49–58. [DOI] [PubMed] [Google Scholar]
- Horigome N, Myojin M, Ueki T, Hirano S, Aizawa S, Kuratani S, 1999. Development of cephalic neural crest cells in embryos of Lampetra japonica, with special reference to the evolution of the jaw. Dev Biol 207, 287–308. [DOI] [PubMed] [Google Scholar]
- Hunt P, Gulisano M, Cook M, Sham MH, Faiella A, Wilkinson D, Boncinelli E, Krumlauf R, 1991. A distinct Hox code for the branchial region of the vertebrate head. Nature 353, 861–864. [DOI] [PubMed] [Google Scholar]
- Hunter MP, Prince VE, 2002. Zebrafish hox paralogue group 2 genes function redundantly as selector genes to pattern the second pharyngeal arch. Dev Biol 247, 367–389. [DOI] [PubMed] [Google Scholar]
- Jozefowicz C, McClintock J, Prince V, 2003. The fates of zebrafish Hox gene duplicates. J Struct Funct Genomics 3, 185–194. [PubMed] [Google Scholar]
- Keynes R, Lumsden A, 1990. Segmentation and the origins of regional diversity in the vertebrate central nervous system. Neuron 4, 1–9. [DOI] [PubMed] [Google Scholar]
- Kim CB, Amemiya C, Bailey W, Kawasaki K, Mezey J, Miller W, Minoshima S, Shimizu N, Wagner G, Ruddle F, 2000. Hox cluster genomics in the horn shark, Heterodontus francisci. Proc Natl Acad Sci U S A 97, 1655–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T, Fujisawa K, Narboux-Neme N, Arima Y, Kawamura Y, Inoue T, Wada Y, Kohro T, Aburatani H, Kodama T, Kim KS, Sato T, Uchijima Y, Maeda K, Miyagawa-Tomita S, Minoux M, Rijli FM, Levi G, Kurihara Y, Kurihara H, 2015. Distinct effects of Hoxa2 overexpression in cranial neural crest populations reveal that the mammalian hyomandibular-ceratohyal boundary maps within the styloid process. Dev Biol 402, 162–174. [DOI] [PubMed] [Google Scholar]
- Kluge B, Renault N, Rohr KB, 2005. Anatomical and molecular reinvestigation of lamprey endostyle development provides new insight into thyroid gland evolution. Dev Genes Evol 215, 32–40. [DOI] [PubMed] [Google Scholar]
- Kmita M, Duboule D, 2003. Organizing axes in time and space; 25 years of colinear tinkering. Science 301, 331–333. [DOI] [PubMed] [Google Scholar]
- Kontges G, Lumsden A, 1996. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development 122, 3229–3242. [DOI] [PubMed] [Google Scholar]
- Krumlauf R, 1994. Hox genes in vertebrate development. Cell 78, 191–201. [DOI] [PubMed] [Google Scholar]
- Kuraku S, Kuratani S, 2006. Time scale for cyclostome evolution inferred with a phylogenetic diagnosis of hagfish and lamprey cDNA sequences. Zoolog. Sci 23, 1053–1064. [DOI] [PubMed] [Google Scholar]
- Kuraku S, Meyer A, 2009. The evolution and maintenance of Hox gene clusters in vertebrates and the teleost-specific genome duplication. Int J Dev Biol 53, 765–773. [DOI] [PubMed] [Google Scholar]
- Kuratani S, Horigome N, Ueki T, Aizawa S, Hirano S, 1998. Stereotyped axonal bundle formation and neuromeric patterns in embryos of a cyclostome, Lampetra japonica. J Comp Neurol 391, 99–114. [PubMed] [Google Scholar]
- Le Douarin N, Kalcheim C, 1999. The Neural Crest, 2nd ed Cambridge University Press, Cambridge, UK; New York, NY, USA. [Google Scholar]
- Lumsden A, 2004. Segmentation and compartition in the early avian hindbrain. Mech Dev 121, 1081–1088. [DOI] [PubMed] [Google Scholar]
- Lumsden A, Keynes R, 1989. Segmental patterns of neuronal development in the chick hindbrain. Nature 337, 424–428. [DOI] [PubMed] [Google Scholar]
- Lumsden A, Krumlauf R, 1996. Patterning the vertebrate neuraxis. Science 274, 1109–1115. [DOI] [PubMed] [Google Scholar]
- Lumsden A, Sprawson N, Graham A, 1991. Segmental origin and migration of neural crest cells in the hindbrain region of the chick embryo. Development 113, 1281–1291. [DOI] [PubMed] [Google Scholar]
- Mallo M, Wellik DM, Deschamps J, 2010. Hox genes and regional patterning of the vertebrate body plan. Dev Biol 344, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley N, Capecchi M, 1995. The role of Hoxa-3 in mouse thymus and thyroid development. Development 121, 1989–2003. [DOI] [PubMed] [Google Scholar]
- Manley N, Capecchi M, 1998. Hox group 3 paralogs regulate the development and migration of the thymus, thyroid and parathyroid glands. Dev Biol 195, 1–15. [DOI] [PubMed] [Google Scholar]
- Manley NR, Capecchi MR, 1997. Hox group 3 paralogous genes act synergistically in the formation of somitic and neural crest-derived structures. Dev Biol 192, 274–288. [DOI] [PubMed] [Google Scholar]
- Manzanares M, Bel-Vialer S, Ariza-McNaughton L, Ferretti E, Marshall H, Maconochie MK, Blasi F, Krumlauf R, 2001. Independent regulation of initiation and maintenance phases of Hoxa3 expression in the vertebrate hindbrain involves auto and cross-regulatory mechanisms. Development 128, 3595–3607. [DOI] [PubMed] [Google Scholar]
- Manzanares M, Wada H, Itasaki N, Trainor PA, Krumlauf R, Holland PW, 2000. Conservation and elaboration of Hox gene regulation during evolution of the vertebrate head. Nature 408, 854–857. [DOI] [PubMed] [Google Scholar]
- Mark M, Lufkin T, Vonesch JL, Ruberte E, Olivo JC, Dolle P, Gorry P, Lumsden A, Chambon P, 1993. Two rhombomeres are altered in Hoxa-1 mutant mice. Development 119, 319–338. [DOI] [PubMed] [Google Scholar]
- Marshall H, Nonchev S, Sham MH, Muchamore I, Lumsden A, Krumlauf R, 1992. Retinoic acid alters hindbrain Hox code and induces transformation of rhombomeres 2/3 into a 4/5 identity. Nature 360, 737–741. [DOI] [PubMed] [Google Scholar]
- Marshall H, Studer M, Popperl H, Aparicio S, Kuroiwa A, Brenner S, Krumlauf R, 1994. A conserved retinoic acid response element required for early expression of the homeobox gene Hoxb-1. Nature 370, 567–571. [DOI] [PubMed] [Google Scholar]
- McCauley DW, Bronner-Fraser M, 2003. Neural crest contributions to the lamprey head. Development 130, 2317–2327. [DOI] [PubMed] [Google Scholar]
- McClintock JM, Carlson R, Mann DM, Prince VE, 2001. Consequences of Hox gene duplication in the vertebrates: an investigation of the zebrafish Hox paralogue group 1 genes. Development 128, 2471–2484. [DOI] [PubMed] [Google Scholar]
- McClintock JM, Jozefowicz C, Assimacopoulos S, Grove EA, Louvi A, Prince VE, 2003. Conserved expression of Hoxa1 in neurons at the ventral forebrain/midbrain boundary of vertebrates. Dev Genes Evol 213, 399–406. [DOI] [PubMed] [Google Scholar]
- McClintock JM, Kheirbek MA, Prince VE, 2002. Knockdown of duplicated zebrafish hoxb1 genes reveals distinct roles in hindbrain patterning and a novel mechanism of duplicate gene retention. Development 129, 2339–2354. [DOI] [PubMed] [Google Scholar]
- McEwen GK, Goode DK, Parker HJ, Woolfe A, Callaway H, Elgar G, 2009. Early evolution of conserved regulatory sequences associated with development in vertebrates. PLoS Genet. 5, e1000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta TK, Ravi V, Yamasaki S, Lee AP, Lian MM, Tay BH, Tohari S, Yanai S, Tay A, Brenner S, Venkatesh B, 2013. Evidence for at least six Hox clusters in the Japanese lamprey (Lethenteron japonicum). Proc Natl Acad Sci U S A 110, 16044–16049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulemans D, Bronner-Fraser M, 2002. Amphioxus and lamprey AP-2 genes: implications for neural crest evolution and migration patterns. Development 129, 4953–4962. [DOI] [PubMed] [Google Scholar]
- Minor PJ, Clarke DN, Andrade Lopez JM, Fritzenwanker JH, Gray J, Lowe CJ, 2018. I-SceI Meganuclease-mediated transgenesis in the acorn worm, Saccoglossus kowalevskii. Dev Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, 2016. Fishing for jaws in early vertebrate evolution: a new hypothesis of mandibular confinement. Biol Rev Camb Philos Soc 91, 611–657. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Pasqualetti M, Takio Y, Hirano S, Rijli FM, Kuratani S, 2004. Segmental development of reticulospinal and branchiomotor neurons in lamprey: insights into the evolution of the vertebrate hindbrain. Development 131, 983–995. [DOI] [PubMed] [Google Scholar]
- Nikitina N, Bronner-Fraser M, Sauka-Spengler T, 2009. The sea lamprey Petromyzon marinus: a model for evolutionary and developmental biology. Cold Spring Harb Protoc 2009, pdb emo113. [DOI] [PubMed] [Google Scholar]
- Onimaru K, Kuraku S, 2018. Inference of the ancestral vertebrate phenotype through vestiges of the whole-genome duplications. Brief Funct Genomics 17, 352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio J, Mazan S, Retaux S, 2005. Organisation of the lamprey (Lampetra fluviatilis) embryonic brain: insights from LIM-homeodomain, Pax and hedgehog genes. Dev Biol 288, 100–112. [DOI] [PubMed] [Google Scholar]
- Oulion S, Borday-Birraux V, Debiais-Thibaud M, Mazan S, Laurenti P, Casane D, 2011. Evolution of repeated structures along the body axis of jawed vertebrates, insights from the Scyliorhinus canicula Hox code. Evol. Dev 13, 247–259. [DOI] [PubMed] [Google Scholar]
- Oury F, Murakami Y, Renaud JS, Pasqualetti M, Charnay P, Ren SY, Rijli FM, 2006. Hoxa2-and rhombomere-dependent development of the mouse facial somatosensory map. Science 313, 1408–1413. [DOI] [PubMed] [Google Scholar]
- Parker HJ, Bronner ME, Krumlauf R, 2014a. A Hox regulatory network of hindbrain segmentation is conserved to the base of vertebrates. Nature 514, 490–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker HJ, Bronner ME, Krumlauf R, 2016. The vertebrate Hox gene regulatory network for hindbrain segmentation: Evolution and diversification: Coupling of a Hox gene regulatory network to hindbrain segmentation is an ancient trait originating at the base of vertebrates. BioEssays 38, 526–538. [DOI] [PubMed] [Google Scholar]
- Parker HJ, De Kumar B, Green SA, Prummel KD, Hess C, Kaufman CK, Mosimann C, Wiedemann LM, Bronner ME, Krumlauf R, 2019. A Hox-TALE regulatory circuit for neural crest patterning is conserved across vertebrates. Nat Commun 10, 1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker HJ, Krumlauf R, 2017. Segmental arithmetic: summing up the Hox gene regulatory network for hindbrain development in chordates. Wiley Interdiscip Rev Dev Biol 6. [DOI] [PubMed] [Google Scholar]
- Parker HJ, Piccinelli P, Sauka-Spengler T, Bronner M, Elgar G, 2011. Ancient Pbx-Hox signatures define hundreds of vertebrate developmental enhancers. BMC Genomics 12, 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker HJ, Pushel I, Krumlauf R, 2018. Coupling the roles of Hox genes to regulatory networks patterning cranial neural crest. Dev Biol 444 Suppl 1, S67–S78. [DOI] [PubMed] [Google Scholar]
- Parker HJ, Sauka-Spengler T, Bronner M, Elgar G, 2014b. A reporter assay in lamprey embryos reveals both functional conservation and elaboration of vertebrate enhancers. PLoS One 9, e85492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Anaya J, D’Aniello S, Kuratani S, Garcia-Fernandez J, 2013. Evolution of Hox gene clusters in deuterostomes. BMC Dev Biol 13, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Anaya J, Sato I, Sugahara F, Higuchi S, Paps J, Ren Y, Takagi W, Ruiz-Villalba A, Ota KG, Wang W, Kuratani S, 2018. Hagfish and lamprey Hox genes reveal conservation of temporal colinearity in vertebrates. Nat Ecol Evol 2, 859–866. [DOI] [PubMed] [Google Scholar]
- Pasqualetti M, Ori M, Nardi I, Rijli FM, 2000. Ectopic Hoxa2 induction after neural crest migration results in homeosis of jaw elements in Xenopus. Development 127, 5367–5378. [DOI] [PubMed] [Google Scholar]
- Popperl H, Bienz M, Studer M, Chan SK, Aparicio S, Brenner S, Mann RS, Krumlauf R, 1995. Segmental expression of Hoxb-1 is controlled by a highly conserved autoregulatory loop dependent upon exd/pbx. Cell 81, 1031–1042. [DOI] [PubMed] [Google Scholar]
- Prin F, Serpente P, Itasaki N, Gould AP, 2014. Hox proteins drive cell segregation and non-autonomous apical remodelling during hindbrain segmentation. Development 141, 1492–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince VE, Joly L, Ekker M, Ho RK, 1998a. Zebrafish hox genes: genomic organization and modified colinear expression patterns in the trunk. Development 125, 407–420. [DOI] [PubMed] [Google Scholar]
- Prince VE, Moens CB, Kimmel CB, Ho RK, 1998b. Zebrafish hox genes: expression in the hindbrain region of wild-type and mutants of the segmentation gene, valentino. Development 125, 393–406. [DOI] [PubMed] [Google Scholar]
- Prince VE, Pickett FB, 2002. Splitting pairs: the diverging fates of duplicated genes. Nat Rev Genet 3, 827–837. [DOI] [PubMed] [Google Scholar]
- Qiu H, Hildebrand F, Kuraku S, Meyer A, 2011. Unresolved orthology and peculiar coding sequence properties of lamprey genes: the KCNA gene family as test case. BMC Genomics 12, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi V, Lam K, Tay BH, Tay A, Brenner S, Venkatesh B, 2009. Elephant shark (Callorhinchus milii) provides insights into the evolution of Hox gene clusters in gnathostomes. Proc Natl Acad Sci U S A 106, 16327–16332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijli FM, Mark M, Lakkaraju S, Dierich A, Dollé P, Chambon P, 1993. A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell 75, 1333–1349. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Meulemans D, Jones M, Bronner-Fraser M, 2007. Ancient evolutionary origin of the neural crest gene regulatory network. Dev Cell 13, 405–420. [DOI] [PubMed] [Google Scholar]
- Schubert M, Yu JK, Holland ND, Escriva H, Laudet V, Holland LZ, 2005. Retinoic acid signaling acts via Hox1 to establish the posterior limit of the pharynx in the chordate amphioxus. Development 132, 61–73. [DOI] [PubMed] [Google Scholar]
- Shimeld SM, Donoghue PC, 2012. Evolutionary crossroads in developmental biology: cyclostomes (lamprey and hagfish). Development 139, 2091–2099. [DOI] [PubMed] [Google Scholar]
- Shone V, Oulion S, Casane D, Laurenti P, Graham A, 2016. Mode of reduction in the number of pharyngeal segments within the sarcopterygians. Zoological Lett 2, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JJ, Timoshevskaya N, Ye C, Holt C, Keinath MC, Parker HJ, Cook ME, Hess JE, Narum SR, Lamanna F, Kaessmann H, Timoshevskiy VA, Waterbury CKM, Saraceno C, Wiedemann LM, Robb SMC, Baker C, Eichler EE, Hockman D, Sauka-Spengler T, Yandell M, Krumlauf R, Elgar G, Amemiya CT, 2018. The sea lamprey germline genome provides insights into programmed genome rearrangement and vertebrate evolution. Nat Genet 50, 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Square T, Romasek M, Jandzik D, Cattell MV, Klymkowsky M, Medeiros DM, 2015. CRISPR/Cas9-mediated mutagenesis in the sea lamprey Petromyzon marinus: a powerful tool for understanding ancestral gene functions in vertebrates. Development 142, 4180–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer M, Gavalas A, Marshall H, Ariza-McNaughton L, Rijli FM, Chambon P, Krumlauf R, 1998. Genetic interactions between Hoxa1 and Hoxb1 reveal new roles in regulation of early hindbrain patterning. Development 125, 1025–1036. [DOI] [PubMed] [Google Scholar]
- Studer M, Lumsden A, Ariza-McNaughton L, Bradley A, Krumlauf R, 1996. Altered segmental identity and abnormal migration of motor neurons in mice lacking Hoxb-1. Nature 384, 630–634. [DOI] [PubMed] [Google Scholar]
- Tahara Y, 1988. Normal stages of development in the lamprey, Lampetra reissneri (Dybowski). Zool. Sci 5, 109–118. [Google Scholar]
- Takio Y, Kuraku S, Murakami Y, Pasqualetti M, Rijli FM, Narita Y, Kuratani S, Kusakabe R, 2007. Hox gene expression patterns in Lethenteron japonicum embryos--insights into the evolution of the vertebrate Hox code. Dev Biol 308, 606–620. [DOI] [PubMed] [Google Scholar]
- Takio Y, Pasqualetti M, Kuraku S, Hirano S, Rijli FM, Kuratani S, 2004. Evolutionary biology: lamprey Hox genes and the evolution of jaws. Nature 429, 1–2. [DOI] [PubMed] [Google Scholar]
- Tomsa JM, Langeland JA, 1999. Otx expression during lamprey embryogenesis provides insights into the evolution of the vertebrate head and jaw. Dev Biol 207, 26–37. [DOI] [PubMed] [Google Scholar]
- Trainor P, Krumlauf R, 2000a. Plasticity in mouse neural crest cells reveals a new patterning role for cranial mesoderm. Nat Cell Biol 2, 96–102. [DOI] [PubMed] [Google Scholar]
- Trainor PA, Krumlauf R, 2000b. Patterning the cranial neural crest: Hindbrain segmentation and Hox gene plasticity. Nat Rev Neurosci 1, 116–124. [DOI] [PubMed] [Google Scholar]
- Trainor PA, Sobieszczuk D, Wilkinson D, Krumlauf R, 2002. Signalling between the hindbrain and paraxial tissues dictates neural crest migration pathways. Development 129, 433–442. [DOI] [PubMed] [Google Scholar]
- Wada H, Escriva H, Zhang S, Laudet V, 2006. Conserved RARE localization in amphioxus Hox clusters and implications for Hox code evolution in the vertebrate neural crest. Dev Dyn 235, 1522–1531. [DOI] [PubMed] [Google Scholar]
- Wingate RJ, Lumsden A, 1996. Persistence of rhombomeric organisation in the postsegmental hindbrain. Development 122, 2143–2152. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Nakahata A, Treen N, Sakuma T, Yamamoto T, Sasakura Y, 2017. Hox-mediated endodermal identity patterns pharyngeal muscle formation in the chordate pharynx. Development 144, 1629–1634. [DOI] [PubMed] [Google Scholar]