Abstract
Background:
The safety and efficacy of aspirin for the primary prevention of cardiovascular disease in patients with diabetes mellitus remains controversial.
Design:
A meta-analysis to investigate the effects of aspirin for the prevention of cardiovascular disease in diabetes mellitus.
Methods:
Ten randomized controlled trials were selected using MEDLINE, EMBASE and CENTRAL databases until 27 September 2018. Risk ratios (RRs) with 95% confidence intervals (CIs) and risk differences (RDs) reported as incident events per 1000 person-years were calculated.
Results:
In 33,679 patients, aspirin did not significantly reduce the risk of major adverse cardiovascular outcomes (RR 0.93, 95% CI 0.87–1.00, P = 0.06; RD −0.68 incident cases per 1000 person-years (95% CI −1.54, 0.17)), cardiovascular mortality (RR 0.95, 95% CI 0.83–1.09, P = 0.49; RD 0.11 incident cases per 1000 person-years (95% CI −0.80, 1.02)), myocardial infarction (RR 0.91, 95% CI 0.75–1.11, P = 0.36; RD −0.66 incident cases per 1000 person-years (95% CI −2.07, 0.75)), or stroke (RR 0.91,95% C, 0.76–1.10, P = 0.33; RD −0.55 incident cases per 1000 person-years (95% CI −1.57, 0.47)). There was a significantly higher risk of total bleeding associated with aspirin (RR 1.29, 95% CI 1.07–1.55, P = 0.01; RD 1.49 incident cases per 1000 person-years (95% CI 0.36, 2.61)).
Conclusion:
The use of aspirin for primary prevention of cardiovascular disease in patients with diabetes mellitus increases the risk of total bleeding without reducing the risk of major adverse cardiovascular outcomes.
Keywords: Cardiovascular disease, diabetes mellitus, primary prevention, aspirin
Background
Aspirin is widely used in patients with established cardiovascular disease (CYD) to prevent major adverse cardiovascular events (MACEs).1,2 Diabetes mellitus (DM) is associated with a higher risk of CVD.3,4 While the use of aspirin therapy in DM has increased over time, the role of aspirin for the primary prevention of CVD in DM remains controversial.4–6 The American College of Cardiology/American Heart Association and American Diabetes Association guidelines advocate the use of low dose aspirin in diabetes patients tailored to the individual risk of CVD and bleeding;6 whereas the 2016 European Society of Cardiology (ESC) guidelines give the use of aspirin for primary prevention in DM a class III recommendation (evidence or general agreement that given treatment is not effective and might be harmful in some cases; therefore its use is not recommended).5
While the current guidelines are largely based on the previous randomized controlled trials (RCTs), a recently published, large ASCEND trial has shed further light on this topic and has the potential to impact clinical practice.3
Aim
We performed a meta-analysis to update the evidence base regarding the efficacy and safety of aspirin for the primary prevention of CVD in patients with DM.
Methods
This trial-level meta-analysis was carried out according to the Cochrane Collaboration guidelines and PRISMA statement. We searched RCTs in MEDLINE, EMBASE and the CENTRAL databases until 27 September 2018 using broad search terms (‘aspirin’, ‘salicylic arid’, ‘salicylates’, ‘diabetes mellitus’, ‘primary prevention’, ‘myocardial infarction’, ‘stroke’, ‘transient ischemic attack’, ‘revascularization’, ‘bleeding’ and ‘mortality’). Two authors (MUK and ST) screened studies based on prespecified inclusion criteria: (a) RCTs reporting data on 500 or more diabetes patients (to provide more reliable estimates)7 receiving aspirin for the primary prevention of CVD for one year or more; and (b) reporting primary or secondary cardiovascular and bleeding outcomes of interest.
Two independent authors (MUK and ST) extracted data on the baseline variables of participants, treatment groups, events, crude estimates, sample size and followup duration of trials on a standard data collection form. Appendices of trials were reviewed for additional information. When provided, data extraction was done on an intention to treat principle. If a trial reported data on different lengths of follow-up, we defaulted to abstracting data on the longer follow-up duration. Authors (MSK and ST) assessed the quality of each trial on the Cochrane risk of bias scale (Table 1).
Table 1.
Baseline characteristics of the selected studies and participants.
| Study/year | Location | Population | Diabetes patients (n) |
Age groups |
Men (%) | Aspirin dose | Medication compliance (%) |
Follow-up (years) |
CRoBb |
|---|---|---|---|---|---|---|---|---|---|
| PHS 198911 | USA | Healthy men | 533 | 40–84 | 100 | 325 mg every other day | – | 5.0 | ***** |
| ETDRS 19928 | USA | Diabetes patients | 3711 | 18–70 | 56.5 | 650 mg daily | 91.8 | 5.0 | ***** |
| HOT 19981,2 | Europe, America, Asia | Patients with hypertension | 1501 | 50–80 | 53.0 | 75 mg daily | – | 3.8 | ***** |
| PPP 20031,3 | Italy | >50 Years with known cardiovascular risk factors | 1031 | 64.3a | 48.2 | 100 mg daily | 71.8 | 3.7 | ***** |
| WHS 20051,4 | USA | Healthy women | 1027 | ≥45 | 0.0 | 100 mg on alternate days | – | 10.1 | ****** |
| POPADAD 20089 | UK | Participants >40 years with diabetes | 1276 | ≥40 | 44.1 | 100 mg daily | 50.0 | 6.7 | ****** |
| JPAD 201710 | Japan | Diabetes patients | 2160 | 65a | 55.0 | 81 or 100 mg daily | 90.0 | 10.3 | ****** |
| JPPP 20141,5 | Japan | Patients >60 years with multiple risk factors | 4903 | 60–85 | 42.3 | 100 mg daily | 76.0 | 5.0 | ***** |
| ASCEND 20183 | UK | Participants >40 years with diabetes | 15480 | >40 | 62.5 | 100 mg daily | 70.0 | 7.4 | ****** |
| ASPREE 20181,6 | Australia, USA | Healthy elderly >65 years | 2057 | ≥65 | 43.6 | 100 mg daily | 72.9 | 4.7 | ****** |
Average age.
CRoB consists of seven domains: randomization (selection bias); allocation concealment (selection bias); blinding of patients and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete data reporting (attrition bias); selective reporting (reporting bias) and other biases. Each domain carries one star and five or more stars qualify for a good quality study.
ASPREE: Aspirin in Reducing Events in the Elderly Trial; ASCEND: A Study of Cardiovascular Events in Diabetes Trial; BMD: British Male Doctors study; CRoB: Cochrane Risk of Bias Scale; ETDRS: Early Treatment Diabetic Retinopathy Study; HOT: Hypertension Optimal Treatment trial; JPAD: Japanese Primary Prevention of Atherosclerosis with Aspirin for Diabetes; JPPP: Japanese Primary Prevention Project; PHS: Physicians’ Health Study; POPADAD: Prevention Of Progression of Arterial Disease And Diabetes; PPP: Primary Prevention Project; WHS: Women’s Health Study.
The data were adjudicated by SUK and any disagreements related to data or quality assessment of the trials were resolved by mutual consensus or referring to the original article. Estimates from each trial were selected most closely to approximate the target primary endpoint of MACE, which consisted of non-fatal myocardial infarction (MI), non-fatal stroke and cardiovascular death. The secondary endpoints were MI, stroke, cardiovascular death, angina, revascularization, transient ischemic attack, all-cause mortality, cancer, cancer-related death, total bleeding, gastrointestinal bleeding and intracranial hemorrhage (ICH).
Comprehensive neta-analysis software version 3.0 (Biostat, Englewood, NJ, USA) was used for performing meta-analysis. Risk ratios (RRs) with 95% confidence intervals (CIs) were used as summary statistics, which were derived from an analysis with adjusted models by person-years (a measure integrating study duration) to compensate for potential differences in study follow-up duration. Risk differences (RDs) were reported as incident events per 1000 person-years. Outcomes were pooled using the DerSimonian and Laird random effects model. Heterogeneity was assessed using Cochrane Q statistics and I2 with values of 75% or greater consistent with a high degree of heterogeneity. Publication bias was assessed using Egger’s regression test. All analyses were conducted at the 5% significance level.
Results
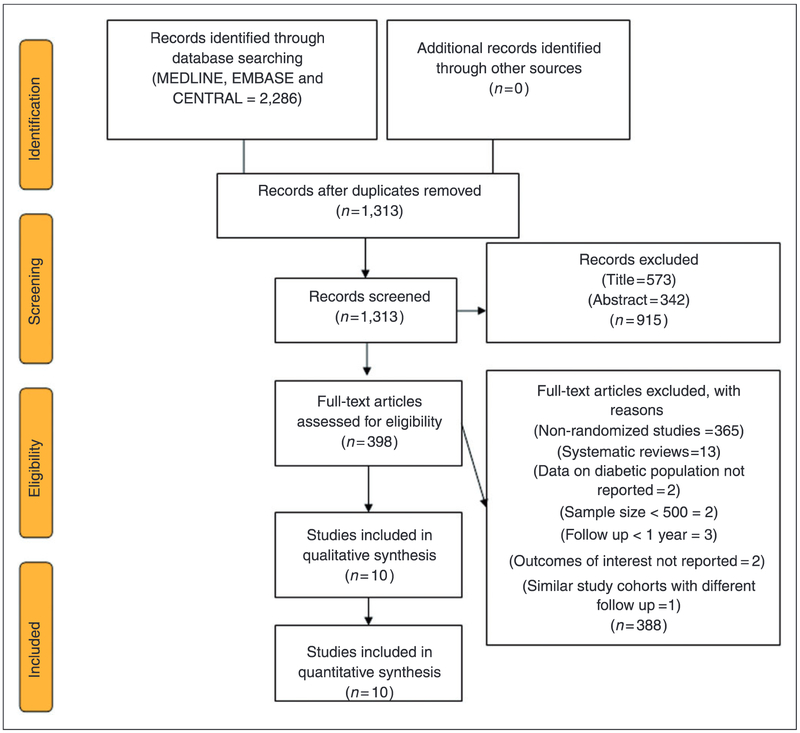
The initial electronic search yielded 2286 citations; 973 citations were removed as duplicates and 915 studies were excluded at title and abstract level screening. Furthermore, 388 full text articles were removed based on a priori selection criteria (Figure 1).
Figure 1.
PRISMA flow chart showing study selection process.
Finally, 10 RCTs (33,679 patients) were included in the analysis. Four trials3,8–10 were conducted exclusively in diabetes patients and six trials11–16 provided subgroup analysis data for diabetes patients. Most trials used an aspirin dose of 100 mg/day and the average drug compliance across the trials was 74.5%. The weighted mean follow-up duration was 6.17 ± 2.41 years. Table 1 describes the baseline characteristics of included RCTs and participants, while Table 2 demonstrates the effect of aspirin on all outcomes of interest.
Table 2.
Effect of aspirin on clinical endpoints.
| Outcome | No. of studies |
Patients (n) |
Patients with events/total no. (%) |
Rate ratio (95% CI) |
P value (I2) |
Risk difference/ 1000 patient-years (95% CI) |
|
|---|---|---|---|---|---|---|---|
| ASPIRIN | CONTROL | ||||||
| MACE | 9 | 33,146 | 1341/16,483 (8.13%) | 1452/16,663 (8.71%) | 0.93 (0.87, 1.00) | 0.06 (0%) | −0.68 (−1.54, 0.17) |
| Cardiovascular death | 6 | 25,159 | 543/12,497 (4.34%) | 580/12,662 (4.58%) | 0.95 (0.83, 1.09) | 0.49 (13%) | 0.11 (−0.80, 1.02) |
| Myocardial infarction | 8 | 26,719 | 610/13,286 (4.59%) | 666/13,433 (4.95%) | 0.91 (0.75, 1.11) | 0.36 (53%) | −0.66 (−2.07, 0.75) |
| Stroke | 7 | 27,033 | 443/13,345 (3.31%) | 513/13,688 (3.74%) | 0.91 (0.76, 1.10) | 0.33 (33%) | −0.55 (−1.57, 0.47) |
| Angina | 3 | 4467 | 115/2149 (5.35%) | 134/2318 (5.78%) | 0.90 (0.71, 1.14) | 0.38 (0%) | −0.46 (−1.87, 0.95) |
| Revascularization | 3 | 17,787 | 383/8897 (4.30%) | 436/8890 (4.90%) | 0.88 (0.77, 1.00) | 0.06 (0%) | −0.82 (−1.70, 0.06) |
| Transient ischemic attack | 4 | 19,947 | 197/9889 (1.99%) | 237/10,058 (2.35%) | 0.84 (0.69, 1.01) | 0.06 (0%) | −0.36 (−0.84, 0.12) |
| All-cause mortality | 7 | 27,216 | 1367/13,524 (10.10%) | 1409/13,692 (10.29%) | 0.0.99 (0.90, 1.09) | 89 (23%) | 0.27 (−0.98, 1.52) |
| Cancer-related death | 3 | 17,617 | 366/8805 (4.15%) | 360/8812 (4.08%) | 1.07 (0.78, 1.46) | 0.67 (48%) | 0.95 (−1.89, 3.79) |
| Total bleeding | 5 | 24,439 | 479/12,134 (3.94%) | 380/12,305 (3.08%) | 1.29 (1.07, 1.55) | 0.01 (24%) | 1.49 (0.36, 2.61) |
| Gastrointestinal bleeding | 4 | 19,947 | 198/9889 (2.00%) | 145/10,058 (1.44%) | 1.50 (0.92, 2.45) | 0.10 (63%) | 1.08 (0.09, 2.07) |
| Intracranial hemorrhage | 3 | 18,667 | 68/9246 (0.73%) | 62/9421 (0.65%) | 1.13 (0.80, 1.60) | 0.48 (0%) | 0.11 (−0.18, 0.41) |
| Cancer | 3 | 17,787 | 970/8897 (10.90%) | 973/8890 (10.94%) | 0.0.99 (0.88, 1.11) | 80 (8%) | −0.06 (−1.42, 1.30) |
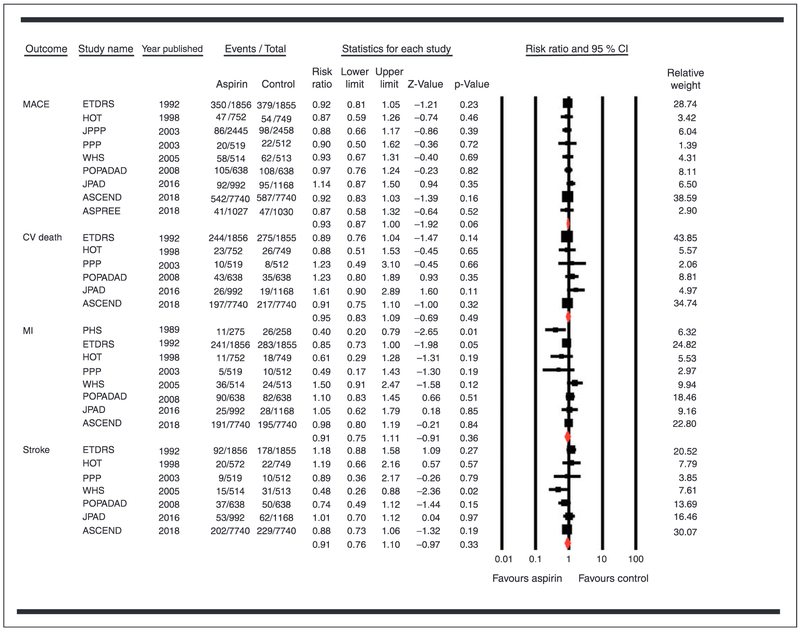
The use of aspirin was not associated with a reduction in the risk of MACEs (RR 0.93, 95% CI 0.87–1.00, P = 0.06; RD −0.68 incident cases per 1000 person-years (95% CI −1.54, 0.17)), cardiovascular mortality (RR 0.95, 95% CI 0.83–1.09, P = 0.49; RD 0.11 incident cases per 1000 person-years (95% CI −0.80, 1.02)), MI (RR 0.91, 95% CI 0.75–1.11, P = 0.36; RD −0.66 incident cases per 1000 person-years (95% CI −2.07, 0.75)), or stroke (RR 0.91, 95% CI 0.76–1.10, P = 0.33; RD −0.55 incident cases per 1000 person-years (95% CI −1.57, 0.47)) (Figure 2).
Figure 2.
Forest plot comparing aspirin versus control for major adverse cardiovascular events (MACEs), cardiovascular mortality, myocardial infarction (MI) and stroke.
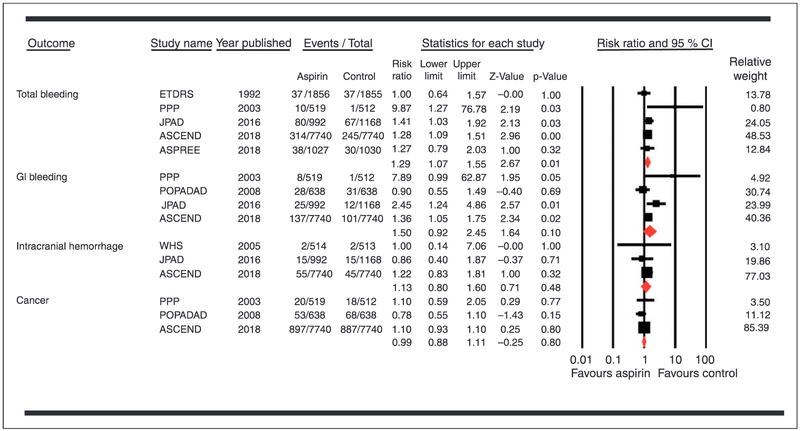
Conversely, there was a significantly higher risk of total bleeding associated with use of aspirin compared with control (RR 1.29, 95% CI 1.07–1.55, P = 0.01; RD 1.49 incident cases per 1000 person-years (95% CI 0.36, 2.61)) (Figure 3).
Figure 3.
Forest plot comparing aspirin versus control for safety outcomes.
Aspirin had no significant effect on the risk of other cardiocvascular outcomes, mortality, gastrointestinal bleeding, ICH or the incidence of cancer (Table 2). Eggers’ regression test did not show a publication bias (P value (two-tailed) = 0.23).
Conclusion
This up-to-date meta-analysis suggests that over a mean follow-up duration of 6 years, the use of aspirin in 33,679 diabetes patients was not associated with a significant reduction in MACEs. Conversely, there was a significantly higher risk of bleeding associated with the use of aspirin. These findings are novel and demand serious clinical consideration regarding the role of aspirin for the primary prevention of cardiovascular outcomes in diabetes patients.
The Anti-Thrombotic Trialists’ Collaboration reported that aspirin reduces the risk of cardiovascular outcomes by approximately 25% in patients with vascular disease and diabetes;17 whereas earlier RCTs of diabetes showed no cardiovascular benefit with use of aspirin.8–10 The recent ASCEND trial (15,480 patients) showed that the modest cardiovascular benefit achieved by aspirin was largely offset by bleeding events.3 In absolute terms, approximately 91 patients would need to be treated to prevent one serious cardiovascular event and approximately 112 would need to be treated to cause a major bleed over a mean follow-up of 7.4 years. Comparatively, our cumulative analysis suggests a number needed to harm of approximately 109 cases, while 192 cases would need to be treated to prevent one MACE. These findings indicate that the risk of using aspirin clearly outweighs any potential benefit. Furthermore, like the ASCEND trial, the proposed benefit of cancer prevention with the use of aspirin was also not observed in this analysis.3
We compared our results with previous meta-analyses. Butalia et al. (seven RCTs, 11,618 patients) showed that aspirin prevented 109 MACEs per 10,000 patients at the cost of 19 major bleeding events (the RR for the later was not statistically significant).18 Kunutsor et al. (10 RCTs, 16,690 patients) showed similar results with a 10% reduction in RR of MACEs (P = 0.03) with aspirin, but without significantly increasing the rates of bleeding (RR 2.23, 95% CI 0.79–6.34).19 However, this study included some trials with extremely small sample sizes (i.e. 68 patients), which poses the risk of small study effects.7 Kokoska et al. (six RCTs, 10,117 patients) were inconclusive regarding the safety and efficacy of aspirin in diabetes.20 Compared to these meta-analyses,18–20 the current study should be considered more valid due to robust inclusion criteria which allowed us to generate reliable estimates. Furthermore, this meta-analysis is updated with new evidence on this topic, which enabled us to assess relevant clinical outcomes in the largest pool of trials and participants at an extended follow-up duration.
However, this study does have certain shortcomings. Due to lack of access to individual patient data, heterogeneities related to certain variables, i.e. baseline cardiovascular risk, hemoglobin A1c level, duration of diabetes, BMI or weight could not be adjusted for. Despite this, it appears that each of the trials recruited patients with overall low cardiovascular risk, based on several different cardiovascular risk assessment criteria. The trials included were published from 1989 to 2018 and represent obvious variations in terms of the use of cardiovascular risk-modifying therapies, such as statins or antihypertensive agents, which can ultimately impact cardiovascular outcomes. Finally, it is possible that certain outcomes of interest were not adequately powered for across all the trials.
In summary, our current analysis suggests that aspirin should not be used for the primary prevention of cardiovascular outcomes in diabetes patients in view of the lack of cardiovascular benefits and a higher risk of bleeding. These findings are in line with ESC guidelines and demand an assessment and review of the American professional guidelines.
Acknowledgments
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Mattioli AY, Manenti A and Farinetti A. Sex differences in adherence to guidelines in aspirin prescription in a population of low-risk cardiovascular patients. Eur J Prey Cardiol 2018; 25: 606–607. [DOI] [PubMed] [Google Scholar]
- 2.Eindhoven DC, Hilt AD, Zwaan TC, et al. Age and gender differences in medical adherence after myocardial infarction: women do not receive optimal treatment – the Netherlands claims database. Eur J Prev Cardiol 2018; 25: 181–189. [DOI] [PubMed] [Google Scholar]
- 3.Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018; 379: 1529–1539. [DOI] [PubMed] [Google Scholar]
- 4.Janssen VE, Visseren FL, de Boer A, et al. Combined use of polypill components in patients with type 2 diabetes mellitus. Eur J Prev Cardiol 2018; 25: 1523–1531. [DOI] [PubMed] [Google Scholar]
- 5.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention and Rehabilitation (EACPR). Eur Heart J 2016; 37: 2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Circulation 2010; 121: 2694–2701. [DOI] [PubMed] [Google Scholar]
- 7.Schwarzer G, Carpenter JR and Rücker G. Small-Study Effects in Meta-Analysis In: Meta-Analysis with R. Cham: Springer International Publishing, 2015, pp. 107–141. [Google Scholar]
- 8.Early Treatment Diabetic Retinopathy Study Investigators. Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. ETDRS Investigators. JAMA 1992; 268: 1292–1300. [DOI] [PubMed] [Google Scholar]
- 9.Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337: a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saito Y, Okada S, Ogawa H, et al. Low-dose aspirin for primary prevention of cardiovascular events in patients with type 2 diabetes mellitus: 10-year follow-up of a randomized controlled trial. Circulation 2017; 135: 659–670. [DOI] [PubMed] [Google Scholar]
- 11.Steering Committee of the Physicians’ Health Study Research Group. Final Report on the Aspirin Component of the Ongoing Physicians’ Health Study. N Engl J Med 1989; 321: 129–135. [DOI] [PubMed] [Google Scholar]
- 12.Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet (London, England) 1998; 351: 1755–1762. [DOI] [PubMed] [Google Scholar]
- 13.Sacco M, Pellegrini F, Roncaglioni MC, et al. Primary prevention of cardiovascular events with low-dose aspirin and vitamin e in type 2 diabetic patients. Results of the Primary Prevention Project (PPP) trial. Diabetes Care 2003; 26: 3264–3272. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Cook NR, Lee I-M, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352: 1293–1304. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA 2014; 312: 2510–2520. [DOI] [PubMed] [Google Scholar]
- 16.McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med 2018; 379: 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324: 71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butalia S, Leung AA, Ghali WA, et al. Aspirin effect on the incidence of major adverse cardiovascular events in patients with diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Diabetol 2011; 10: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunutsor SK, Seidu S and Khunti K. Aspirin for primary prevention of cardiovascular and all-cause mortality events in diabetes: updated meta-analysis of randomized controlled trials. Diabet Med 2017; 34: 316–327. [DOI] [PubMed] [Google Scholar]
- 20.Kokoska LA, Wilhelm SM, Garwood CL, et al. Aspirin for primary prevention of cardiovascular disease in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract 2016; 120: 31–39. [DOI] [PubMed] [Google Scholar]