Abstract
Context:
Existing anterior cruciate ligament (ACL) injury prevention programs have failed to reverse the high rate of ACL injuries in adolescent female athletes.
Objective:
This investigation attempts to overcome factors that limit efficacy with existing injury prevention programs through the use of a novel, objective, and real-time interactive visual feedback system designed to reduce the biomechanical risk factors associated with ACL injuries.
Design:
Crossover study.
Setting:
Medical center laboratory.
Participants:
Twenty female participants (age = 19.7 ± 1.34 y; height = 1.74 ± 0.09 m; weight = 72.16 ± 12.45 kg).
Methods:
Participants performed sets of 10 bodyweight squats in each of eight training blocks (i.e., four real-time and four control blocks) and three testing blocks for a total of 110 squats. Feedback conditions were blocked and counterbalanced with half of participants randomly assigned to receive the real-time feedback block first and half receiving the control (sham) feedback first.
Results:
Heat map analysis revealed that during interaction with the real-time feedback, squat performance measured in terms of key biomechanical parameters was improved compared to when participants squatted with the sham stimulus.
Conclusion:
This study demonstrates that the interactive feedback system guided participants to significantly improve movement biomechanics during performance of a body weight squat, which is a fundamental exercise for a longer-term ACL injury risk reduction intervention. A longer training and testing period is necessary to investigate the efficacy of this feedback approach to effect long-term adaptations in the biomechanical risk profile of athletes.
1. Introduction
Anterior cruciate ligament (ACL) injuries are physically and emotionally debilitating for adolescent athletes. The trauma associated with an ACL injury contributes to significant pain1, depression2, decreased athletic identity3, and lower academic performance4. This is in addition to the potential ending of an athletic career5, greatly amplified risk of a subsequent ACL injury, likelihood for long term disability6, and risk of early osteoarthritis and chronic pain7–9. Current reports conservatively estimate $17,000-$25,000 in costs per ACL injury with an annual economic burden in the United States between $7.6 and $17.7 billion dependent on treatment type (e.g., reconstruction versus rehabilitation)10. Because of these adverse consequences, the National Public Health Agenda for Osteoarthritis has strongly recommended expansion and refinement of ACL injury prevention strategies11. Numerous prevention programs have been implemented to reduce ACL injury incidence12. Some of these have shown effectiveness in reducing ACL injury risk13–17, while others have resulted in less desirable outcomes18–20. The totality of evidence regarding the effectiveness of neuromuscular training interventions to reduce ACL injury risk unfortunately reveals that while these interventions can reduce injury risk factors in the lab their effects have not shown transfer to the actual sport environment, as injury rates have not declined despite the introduction of these programs20–24.
A common exercise, the double-leg squat, is commonly used in ACL prevention programs.14, 25. Despite this foundational movement’s integral role within ACL prevention programs, the evidence indicating only minimal efficacy in reducing ACL injury rates13–17 warrants innovative approaches that expedite biomechanical performance improvements and increase retention and transfer. One potential avenue is through visual feedback manipulation in which the squat provides the ideal exercise for such investigation. Normally during the execution of a squat, the athlete maintains an upright posture with a forward facing gaze and focal point. This position permits athletes to easily maintain focus on the feedback without disturbing normal squatting form. The movement is relatively slow and continuous, allowing for continuous presentation of feedback in a way that allows the athlete to modify the movement as it unfolds. In contrast, training tasks such as the drop vertical jump (DVJ; drop landing) are executed quickly and ballistically and simply do not afford the opportunity for online feedback to be utilized during task execution.
In the present study, we conducted a preliminary test of a visual biofeedback system designed to guide athletes to improve double leg squat biomechanics by using a strategy of “external perceptual control”26, 27 of the feedback. This type of control is achieved by moving so as to achieve a desired visual biofeedback outcome. This mode of control engages automatic and implicit motor strategies that operate more efficiently and effectively than deliberate, explicitly conscious control of movement28–37, which yields fast, robust learning, leading to high retention and transfer38–43. With the advent of motion capture and mixed reality technologies, augmented feedback has become even more accessible to researchers and clinicians and provide ample opportunity for integrating visual biofeedback into areas such as sports medicine and biomechanics. Several possible applications for such technologies with a particular focus on ACL injury prevention have been outlined44. For example, neuromuscular training can be augmented by presenting participants with an online feedback display linked to their biomechanics via 3D motion capture software45. Such real-time biofeedback has been very effective for gait retraining46–48, and many of these new technologies have already been implemented with success for non-contact ACL preventative training programs45, 49, 50. However, limitations of these previous studies are that control conditions were compared with traditional feedback50, or that biofeedback was localized to a single factor such as knee abduction/adduction45 and did not incorporate the full biomechanical profile associated with ACL injury risk.
The innovative biofeedback approach used in the current study overcomes the aforementioned limitations by simultaneously providing feedback about a wider range of biomechanical variables (e.g., knee, trunk, hip, etc.) than previous biofeedback investigations (e.g., knee only). A potential advantage of this novel approach is its ability to simultaneously convey multiple sources of information within a single comprehensive stimulus. While presenting obvious efficiencies of concurrently training several targeted biomechanical deficiencies at once, there may be additional advantages. For instance, using a typical “one variable at a time” training approach variables may be individually trained so as to improve local performance (i.e., better performance within a single variable), while having minimal beneficial effect on overall performance. This is because the individually trained variables affect performance of other variables, and the coordination of the multiple variables as a whole must be taken into consideration. When multiple variables are trained simultaneously a user does not have to reintegrate or learn to combine individual movement variables. This inclusive nature of training multiple variables through a single stimulus may avoid the problems just described and lead to a more effective training program.
The purpose of the current study was to determine the effectiveness of a real-time biofeedback stimulus that maps to a comprehensive movement profile for reducing squatting mechanics that may contribute to ACL injury risk. To contribute to previous literature that has compared real-time biofeedback to traditional interventions, we also implemented a novel sham feedback apparatus designed to limit the amount of useful feedback information available to participants during training of squat movements. The sham feedback provided a comparable visual appearance to the real feedback stimulus; however, it provided very limited information about squat biomechanics, and as such was in principle an ineffective guide for the user to use to improve squat biomechanics. The sham thus served as a control or reference condition against which to measure the efficacy of our real-time, interactive biofeedback. We hypothesized that participants would elicit significantly better squatting performance, as indexed through a novel heat map analysis, throughout acquisition and during retention (mid and post testing) when using real-time, interactive biofeedback compared to the sham feedback stimulus.
2. Method
2.1. Participants
Twenty females participated in the study (M age = 19.7, SD = 1.34 yrs; M height = 1.74, SD = 0.09 m; M weight = 72.16, SD = 12.45 kg). All participants were collegiate athletes recruited from three local universities. Nineteen participants were members of their respective basketball teams, and one participant competed in the heptathlon (a track and field event consisting of seven different sub-events). Participants had no history of neurological disorders (including any neuromuscular disabilities), musculoskeletal disabilities or disorders, or balance problems. Additionally, participants were free of any recent injuries that impaired movement, the ability to stand, or perform the body-weight squats. Two participants had past knee injuries (one ACL injury and one medial collateral ligament injury); however, the dates of occurrence were not within 5 years of their participation and performance was not unusual when compared to other participants. The study was approved by the Institutional Review Board and all participants gave written informed consent prior to participation.
2.2. Materials, Apparatus, and Stimulus Design
The real-time feedback display used in the present study was designed so that objective information about multiple kinematic and kinetic variables related to ACL injury risk51–53 could be displayed concurrently and in real time to participants. The stimulus was designed specifically to map onto a wider range of biomechanical variables (e.g., knee, trunk, hip, etc.) than previous biofeedback investigations that were isolated to a single variable (e.g., knee only). The shape of the display was a simple, two-dimensional rectangle defined by six points (see Figure 1A). The vertical and horizontal coordinates of these points were defined as a function of five kinematic and kinetic variables previously identified as ACL-injury risk factors (for justification and references see Table 1), each having a unique effect on the feedback display (see Figure 1B-E): (1) lateral trunk flexion 21, (2) knee-to-hip joint moment of force ratio (KHMr), (3) knee abduction moment of force (KAM) 21, (4) vertical ground reaction force ratio (vGRF), and (5) foot center of pressure (foot COP) location. The current values of the biomechanical variables were mapped via the geometric shape of the display (i.e., a rectangle), and as these variables changed dynamically throughout the participants’ movements, the feedback display was updated relative to these changes and displayed to the participants in real time. The feedback shape was thus not only changing in real-time but it was also reliably tied to participants’ movements.
Figure 1.
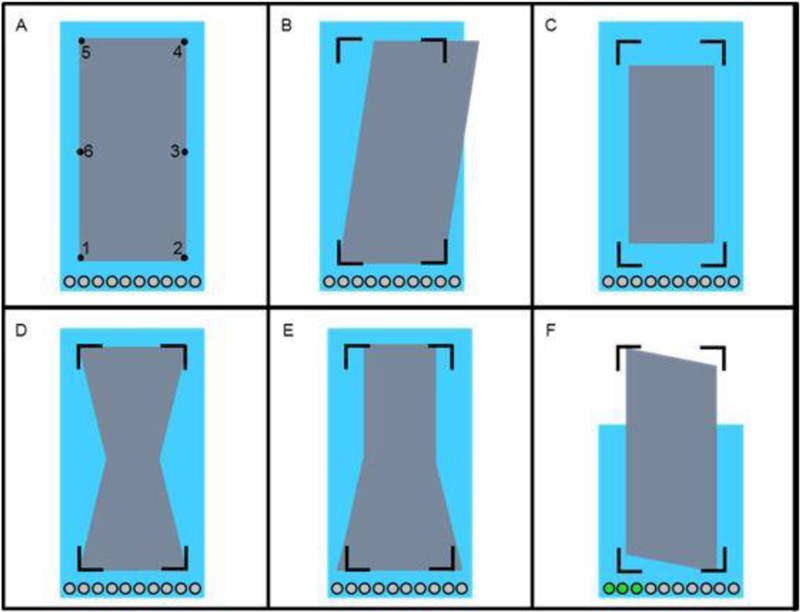
The stimulus used in the experiment. During the trials the stimulus’ shape started as a rectangle (Panel A) and was defined by points one through six. An outline of the shape’s corners (points one, two, four, and six) remained while participants were performing the squat exercise and are shown in Panels B-F. Also displayed are ten grey circles towards the bottom of each display. These were used as a visual display for counting the number of squats within a trial. As participants performed squats, the circles would change from grey to green. Depicted in Panels B-F are the effects of the trunk lean KHMr, KAM, foot COP, and vGRF variables, respectively. In Panel F, the lighter background rectangle lowered from its maximum height (displayed in Panels A-E) as a participant performed the downward movement of a squat. Accordingly, it rose during the upward movement.
Table 1:
Justification, Optimum Value, and the Effect on the Stimulus for the Included Biomechanical Variables.
| Variable | Stimulus Effect |
Justification | Optimum Value | References |
|---|---|---|---|---|
| Lateral Trunk Flexion |
Figure 1B | Lateral trunk displacement predicted ACL injuries with high sensitivity and specificity in females and flexion was also present during the time of ACL injury. | 0° | 58–60 |
| Knee-to-hip joint extensor moment of force ratio | Figure 1C | Quadriceps-dominant recruitment during dynamic movement is a risk factor for lower extremity injuries. | ≤1 | 53, 61 |
| Knee abduction moment of force |
Figure 1D | Valgus knee collapse and subsequent increased knee abduction moment occurs more frequently in ACL injury risk prone athletes. | ≤0 Nm | 62, 63 |
| Vertical ground reaction force ratio |
Figure 1E | Asymmetry in ground reaction force indicates preference for and the potential for abnormal joint loading on one limb. | 1 | 62 |
| Foot center of pressure location |
Figure 1F | COP should be located towards the back of the foot (heel) as shear forces are increased as the knee moves past the toes (during the downward phase of the squat). Video evidence also shows, at the time of injury, foot positions extending past the body’s COM | COP within the back 70% of foot length |
58, 63 |
The participants were instructed to squat so as to keep the stimulus shape as close to a perfect, symmetrical rectangle as possible. This was achieved by moving so as to produce optimum values (i.e., values associated with low ACL injury risk) of the aforementioned variables, but participants were not told that and the biomechanical variables and their optimum values were not explained to them. As the values of the variables neared or fell within optimum ranges specific to the given variable(s), a more symmetric rectangle was obtained; alternatively, the rectangle became systematically distorted by increasing amounts as the values of the variables deviated from the optimum values.
The number of squats performed by the athlete was tracked by a variable measuring knee flexion angle. A squat was considered “complete” when a participant achieved a knee flexion angle below 90° during the squat and then returned to the original standing position (see Figure 1F). This variable was visually displayed to participants (separately from the feedback rectangle just described) through the movement of a lighter-colored rectangle behind the primary feedback display shape. As a participant squatted into a lower position, the height of the background rectangle decreased. Ten circles situated below the rectangle served as a counter representing the number of “completed” squats, which would change colors from grey to green once participants had completed a full squat motion (i.e., participants were standing upright after performing the squat). If participants did not complete a full squat motion, the squat was not counted.
In addition to the real-time feedback display just described, a feedback display was also developed for the sham condition. The real-time and sham feedback displays presented identical stimulus shapes that responded, at least in part, to the same biomechanical parameters. However, the sham feedback was designed to limit the amount of useful feedback information available to participants during the squat movements. This was accomplished using a stepwise gain manipulation (see Figure 2). Movements not critical to the targeted biomechanical parameters during a squat (i.e., movements occurring close to the start/end points of squat movements) caused the sham display to respond nearly equivalently to participant movement (and thus nearly equivalently to the real feedback condition), but the influence of the biomechanical variables on the stimulus shape during the important phases of the squats were progressively eliminated from the stimulus, such that at the bottom of the squat movement the sham feedback stimulus deviated from the goal shape as a function of random noise, not from the movements of participants (i.e., it provided little to no actual feedback). This specific type of sham feedback was utilized to facilitate similar phenomenological responses to that of the experimental stimulus without promoting movements directly associated with improvements squat biomechanics.
Figure 2.

Displayed above is the technique used to derive the sham feedback. The bottom plot is a sample time series of a single squat rep performed during a sham trial. Specifically, the mid-shoulder marker is illustrated. The top plot depicts how the signal-to-noise ratio (i.e., how much of the stimulus movement was driven by participant movement or noise) corresponds to a particular movement stage of a squat.
Participants’ movements were recorded using a 10-camera motion capture system (Raptor-E, Motion Analysis Corp., Santa Rosa, CA, USA) sampling at 240 Hz. In conjunction with the motion capture system, two embedded force platforms (BP600900, AMTI, Watertown, MA, USA) sampling at 1200 Hz were used to collect ground reaction force from each foot and were synchronized with the motion system. Using a software development kit (SDK) provided by Motion Analysis Corp., the synchronized marker trajectory and force data were accessed via a custom software program—written in C++ and designed in Microsoft Visual Studio Professional 2015 (Microsoft Corp., Redmond, WA, USA) and which also incorporated OpenGL (Khronos Group, Beaverton, OR, USA) as the graphics application interface—that was designed to calculate and map the above variables to generate the visual feedback display.
The visual display was wirelessly transmitted from a desktop computer to participants using an ARIES Pro Wireless HDMI Transmitter and Receiver (Nyrius, Niagara Falls, ON, Canada). The ARIES Pro is capable of transmitting uncompressed 1080p signals up to 160 feet with a latency of < 1 ms, which allowed for maximum mobility of participants without degradation of feedback quality. Participants viewed the real-time feedback through a pair of video eyewear glasses (Wrap 1200 DX-VR; Vuzix Corp., Rochester, NY, USA) which had a 60 Hz screen refresh rate (a new frame appeared approximately every 16.67 ms). The glasses presented the feedback display in a fixed position relative to the participants’ eyes and encompassed their entire field of view. Both the ARIES Pro and glasses were powered by a portable battery pack (PowerGen Mobile Juice Pack 12000; PowerGen, Kwai Chung, Hong Kong, PRC). The wireless transmitter and battery pack were stored in a modified hydration pack designed for running (CamelBak Products, LLC, Petaluma, CA, USA). The backpack provided minimal interference to natural movement as it held the equipment securely against the body and was relatively small (length × width × height: 33 × 27 × 7.6 cm).
2.3. Procedure
In order to compare the effects of the real-time and sham feedback displays, an AB/BA or two-treatment crossover design was utilized. As the name implies, half of the participants received one condition first (e.g., ‘A’) and another condition second (e.g., ‘B’), while the other half of participants received the two identical conditions but in the opposite order. We randomly assigned participants to one of two groups: real-time biofeedback first (i.e., ‘A’) or sham feedback first (i.e., ‘B’). First, participants completed a block of 10 squats without any feedback (pre-test). Then, participants completed four blocks of 10 squats using their assigned condition (i.e., real-time biofeedback or sham) (acquisition phase 1). Before switching to the next feedback type, a block with no feedback was administered (mid retention test) followed by four blocks of 10 squats using the opposite feedback type as used in acquisition phase 1 (acquisition phase 2). Finally, participants completed a block of 10 squats without any feedback (post retention test). In total, each participant completed 110 squats—40 training squats for each feedback type (80 total) and 10 squats during each test period (30 total; see Figure 3). Participants were permitted breaks between blocks as necessary.
Figure 3.
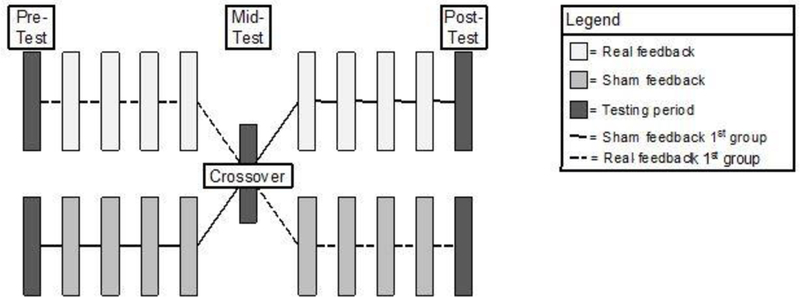
The crossover design used Each rectangle represents a block that consisted of 10 squats. The darkest rectangles represent the testing time in which no visual feedback was administered. The two rows of rectangles indicate the type of feedback used for training—either real or sham feedback. The solid and dashed black lines respectively denote the group who received the sham feedback first and the group who received the real feedback first The middle rectangle denotes the crossover point—the point that the feedback type was switched.
Each participant was outfitted with 30 retroreflective markers, with a minimum of three tracking markers on each segment, and the backpack containing the wireless transmitter and battery pack. Markers were placed on the sacrum between the L5 and S1 vertebrae, and bilaterally on the acromio-clavicular joint, anterior superior iliac spine, posterior superior iliac spine, greater trochanter, mid-thigh, medial and lateral femoral condyles, tibial tubercle, lateral and distal aspects of the shank, and medial and lateral malleoli, the heel, and central forefoot (between the second and third metatarsals). After the initial experimental preparation, all participants received identical instructions about the squat exercise and were shown an unresponsive stimulus shape that reflected the goal shape. The instructions were purposefully kept very basic as to allow for implicit discovery of how their movements related to the stimulus shape during the squat exercise; they were told only to “maintain the goal stimulus shape and size as closely as possible,” as indicated by the outlie of the shape’s corners, and, as a secondary instruction, “to squat to sufficient depth”, as indicated by the depth indicator and circle counter at the bottom of the stimulus. Participants were also asked to keep their arms crossed in front of their chest and to avoid covering any markers. A set of squats was considered complete once all ten circles’ colors changed from grey to green, indicating that ten sufficiently deep squats were performed. If participants were unable to intuitively achieve the appropriate depth, they were explicitly instructed that they must squat lower. This happened solely during the first feedback trial that participants experienced; no participants needed to be reminded again after the first trial. No other instructions were provided regarding the squats or the stimulus.
2.4. Data Reduction and Analysis
The recorded raw, three-dimensional marker positions, ground reaction forces, and center of pressure acquired from both feet were first exported from Cortex and imported into MATLAB for preprocessing. Preprocessing consisted of visual inspection of a virtual mid-shoulder marker (defined as the averaged position of the left and right shoulder markers) for each squat trial (pre-, mid-, and post-test and training trials). During the visual inspection, time series of the mid-shoulder marker’s vertical position were plotted and trimmed according to the procedure outlined in Figure 4. Only the portions of a trial where the participant was performing a squat were retained for analysis. All other marker and force data were trimmed according to the time points that were identified from the mid-shoulder marker. This procedure resulted in a time series for each squat rep across every squat set.
Figure 4.
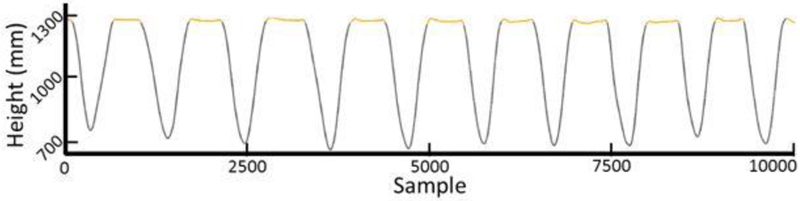
Displayed above is an example time series of the virtual mid-shoulder marker. The mid-shoulder marker was used as a template to trim and parse the time series of all other markers. The orange colored sections of the time series were removed, leaving only the portions of data during which a participant was performing a squat.
In order to quantify participants’ ability to control the stimulus shape, heat map analysis was performed on the squat data during the middle four training sets and on “reconstructed” feedback shapes obtained from the raw position and force data in the pre- and post-test sets. The heat maps provided a global assessment of squatting performance by indicating how the movement patterns of the biomechanical variables associated with ACL injury related to the target feedback shape (i.e., a rectangle). Specifically, the heat maps portrayed the percentage of time a defined space was occupied by the feedback stimulus. The heat map analysis consisted of two steps: (1) the construction of the heat maps and (2) the calculation of each heat map’s correctly occupied space. Heat maps were created using the MATLAB function inpolygon. The calculation of each heat map’s correctly occupied space consisted of first calculating the proportion of occupied space within the goal stimulus and then calculating the proportion of occupied space outside of the goal shape. The proportion of occupied space outside of the goal shape was finally subtracted from the proportion of occupied space within the goal stimulus. The possible results of this operation range from −1.00 to 1.00. A score of −1.00 indicated that the stimulus never occupied a correct location in the display while always occupying an incorrect location. A score of 1.00 indicated a stimulus shape never deviated from the goal shape and size, which meant that the relevant biomechanical parameters were achieving the desired optimal values associated with lower injury risk. These scores are transformed and presented as percentages in the following sections for ease of interpretation with higher percentages indicating better squatting performance.
In order to test for varying levels of fatigue caused by differences in the number of squats performed by each participant group, the total number of squats performed by the real- and shamfirst feedback groups were compared using an independent samples t-test. This step was necessary because participants required a few squat repetitions in order to explore and determine the appropriate squat depth, which may or may not have affected participants’ fatigue level and subsequent squatting performance.
To assess squatting performance during the acquisition phase, each trial block’s heat map scores were first averaged across each squat repetition to produce a single heat map percentage score for each trial block. Then, to assess differences in squatting performance during training (i.e., present visual feedback), two separate 2 (condition; sham and real feedback) × 4 (trial block [1, 2, 3, 4]) mixed model ANOVAs with repeated measures on the last factor (i.e., trial block) were conducted for acquisition phase 1 and acquisition phase 2.
To assess learning (i.e., absent visual feedback), a 2 (order; sham-first and real-first feedback) × 3 (test phase; pre-test, mid-test, post-test) mixed ANOVA with repeated measure on the last factor was conducted. Bonferroni adjustments were used when appropriate and an alpha level of p < .05 indicating significance was selected a priori.
3. Results
3.1. Number of Squats Performed
There were no significant differences in the number of squats performed between the real-first feedback group (M = 111.80, SD = 7.15) and the sham-first feedback group (M = 114.30, SD = 5.76), t(18) = 0.86, p = .40, d = 0.39.
3.2. Acquisition Phase Squatting Performance
There were no significant differences in overall squatting performance as measured by the heat map percentage scores for condition (F(1, 18) = 2.03, p = .17, ƞp2 = .10), trial block (F(3, 54) = 0.86, p = .47, ƞp2= .05), and a condition × trial block interaction (F(3, 54) = 0.23, p = .88, ƞp2 = .01) during acquisition phase 1. The same pattern of results held for acquisition phase 2: condition (F(1, 18) = 1.15, p = .30, ƞp2= .06), trial block (F(3, 54) = 1.90, p = .14, ƞp2 = .10), and condition × trial block interaction (F(3, 54) = .45, p = .72, ƞp2 = .02).
Since there were no significant main effects or interactions, and in order to test if squatting performance differed while engaging with the sham and real feedback regardless of condition or block, we averaged the heat map percentage scores across the eight trial blocks (acquisition phase 1 & 2) for each condition respectively and performed a paired-samples t-test to assess differences during training per feedback type (i.e., real or sham). Performance during the real-time biofeedback trials (M = 61.82%, SD = 6.42%) was better than during the sham feedback trials (M = 57.56%, SD = 8.40%), t(19) = 3.06, p = .006, d = 0.57.
3.3. Learning Phase Squatting Performance
There were no significant differences in squatting performance for order, (F(1, 18) = 0.56, p = .46, ƞp2 = 0.03), test phase (F(2, 36) = 0.19, p = .83, ƞp2 = .01), or test phase × trial block interaction (F(2, 36) = 0.43, p = .66, ƞp2 = .02) during the three test phases that assessed learning. Thus, squatting performance only improved when the real feedback shape was present during acquisition and not when the real feedback was absent for learning assessment.
4. Discussion
The purpose of this study was to determine the effectiveness of a real-time biofeedback system compared to a sham feedback system for reducing squatting mechanics that may contribute to ACL injury risk. Squatting performance, as measured through heat map analysis, was significantly better when participants interacted with the real-time biofeedback system compared to the sham. This is congruent with previous literature supporting the use of real-time biofeedback for ACL injury prevention45, 49, 50. Our study makes three distinct innovations. First, we employed an interactive, real-time stimulus that implicitly guided performance while promoting external perceptual control. Second, the developed real time biofeedback system mapped multiple biomechanical variables associated with ACL injury risk onto a single stimulus. Unlike previous systems that are isolated to one factor such as knee abduction/adduction45, our system uniquely presents athletes with a global biomechanical profile associated with ACL injury risk that includes lateral trunk flexion, knee-to-hip joint moment of force ratio, knee abduction moment of force, foot COP location, and vertical ground reaction force. Third, our inclusion of the sham feedback demonstrated that interacting with the stimulus alone was not sufficient to improve performance, but an accurate mapping from kinematics to the feedback is necessary for improved performance.
While these data provide strong initial findings for the efficacy of our real-time biofeedback system, our failure to find learning effects as measured during the mid and post retention sessions requires cautious interpretation. Motor learning is defined as relatively permanent changes in motor skill performance as a function of practice or experience54. Non-significant differences between the groups in the absence of the stimulus during both retention tests suggests that a single session with real-time biofeedback was not effective for eliciting stable motor skill changes. There are multiple possible reasons for this finding. Most importantly, and one limitation of this study, was the brief practice period—only 40 total squat repetitions were performed in a single session lasting only about 10 minutes for each participant. Implementing our interactive feedback system over multiple training sessions (i.e., over the course of weeks or months), accompanied by a progression of greater performance criteria, increased exercise difficulty, or the inclusion of more challenging exercises, may induce more stable and permanent changes in motor skill. Relatedly, a second limitation is that the practice schedule used in the current study had minimal breaks (i.e., massed practice) which is effective for acquisition, but is typically less successful for learning than when breaks are administered (i.e., distributed practice)55, 56. Third, although we used a crossover design to increase statistical power, our sample size (n =20) could nevertheless be considered small. Finally, our inability to determine how changes in squat mechanics transferred to more dynamic tasks, such as the drop vertical jump, require cautious interpretation in our real-time biofeedback’s ability to reduce ACL injury rates.
The observed performance improvements exhibited in the presence of the stimulus do show promise for its utilization in future ACL injury prevention and rehabilitation applications. The immediate gains seen in squat performance demonstrate that the stimulus was effective and support the use of real-time biofeedback integration with kinematics and kinetics44. Our design and implementation of the current stimuli, while overcoming the aforementioned technical difficulties, also demonstrate that it is feasible to map multiple variables associated with ACL injury risk in real-time. Modifying the amount of rest and feedback frequency are relatively simple changes that can be made to potentially improve learning and supports previous recommendations related to ACL injury risk reduction57.
The results of this study suggest that ACL injury prevention and rehabilitation programs could be improved by integrating a real-time, interactive biofeedback stimulus. Prevention programs could use this stimulus to improve performance of prophylactic exercises, which may lead to decreased ACL injury risk. Likewise, following an ACL injury, our approach may be particularly beneficial as a rehabilitation tool for those in the recovery stages. The integration of new factors associated with ACL injury risk suggests that it may also be possible to design similar real-time biofeedback systems to target other movement dysfunction. For example, previous investigations using real-time biofeedback for gait retraining may benefit by integrating multiple factors to supplement previously seen motor skill improvements on a single variable.
5. Conclusion
In conclusion, this study demonstrates the efficacy of novel real-time, interactive biofeedback system for improving squat performance. In a single testing session we demonstrated an immediate change in biomechanics associated with ACL injury risk when participants’ movements were guided by the interactive stimulus. Further, it appears that real-time biofeedback that is not properly mapped on to biomechanics (i.e., our sham condition) is less effective, highlighting the importance of accurate and appropriate mapping of key variables. Future research that utilizes longer testing sessions, more rest, and a more personalized dosing schedule may further the benefits of our real-time biofeedback system to elicit permanent biomechanical changes.
Acknowledgments:
The authors would like to acknowledge funding support from the National Institutes of Health/NIAMS Grants R21AR065068–01A1 and U01AR067997.
References
- 1.Tripp DA, Stanish WD, Coady C, Reardon G. The subjective pain experience of athletes following anterior cruciate ligament surgery. Psychology of Sport and Exercise 2004;5(3):339–354. [Google Scholar]
- 2.Garcia GH, Wu H-H, Park MJ, et al. Depression symptomatology and anterior cruciate ligament injury: Incidence and effect on functional outcome—a prospective cohort study. The American Journal of Sports Medicine 2016;44(3):572–579. [DOI] [PubMed] [Google Scholar]
- 3.Brewer BW, Cornelius AE, Stephan Y, Van Raalte J. Self-protective changes in athletic identity following anterior cruciate ligament reconstruction. Psychology of Sport and Exercise 2010;11(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedman KB, Glasgow MT, Glasgow SG, Bernstein J. Anterior cruciate ligament injury and reconstruction among university students. Clinical Orthopaedics and Related Research 1998;356:208–212. [DOI] [PubMed] [Google Scholar]
- 5.Giugliano DN, Solomon JL. ACL tears in female athletes. Physical Medicine and Rehabilitation Clinics of North America 2007;18(3):417–438. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz A, Kelly M, Nutton R. Arthroscopic ACL reconstruction: a 5–9 year follow-up. The knee 2002;9(3):197–200. [DOI] [PubMed] [Google Scholar]
- 7.Deacon A, Bennell K, Kiss ZS, Crossley K, Brukner P. Osteoarthritis of the knee in retired, elite Australian Rules footballers. The Medical Journal of Australia 1997;166(4):187–190. [DOI] [PubMed] [Google Scholar]
- 8.Fleming BC, Hulstyn MJ, Oksendahl HL, Fadale PD. Ligament injury, reconstruction and osteoarthritis. Current Opinion in Orthopaedics 2005;16(5):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries. The American Journal of Sports Medicine 2007;35(10):17561769. [DOI] [PubMed] [Google Scholar]
- 10.Mather RC III, Koenig L, Kocher MS, et al. Societal and economic impact of anterior cruciate ligament tears. The Journal of Bone and Joint Surgery . 2013;95(19):1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prevention CfDCa. A national public health agenda for osteoarthritis In: Services DoHaH, ed2010. [Google Scholar]
- 12.Gagnier JJ, Morgenstern H, Chess L. Interventions designed to prevent anterior cruciate ligament injuries in adolescents and adults: A systematic review and meta-analysis. The American Journal of Sports Medicine 2013;41(8):1952–1962. [DOI] [PubMed] [Google Scholar]
- 13.Gilchrist J, Mandelbaum BR, Melancon H, et al. A randomized controlled trial to prevent noncontact anterior cruciate ligament injury in female collegiate soccer players. The American Journal of Sports Medicine 2008;36(8):1476–1483. [DOI] [PubMed] [Google Scholar]
- 14.Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. The American Journal of Sports Medicine 1999;27(6):699–706. [DOI] [PubMed] [Google Scholar]
- 15.LaBella CR, Huxford MR, Grissom J, Kim K- Y, Peng J, Christoffel KK. Effect of neuromuscular warm-up on injuries in female soccer and basketball athletes in urban public high schools: cluster randomized controlled trial. Archives of Pediatrics & Adolescent Medicine 2011;165(11):1033–1040. [DOI] [PubMed] [Google Scholar]
- 16.Mandelbaum BR, Silvers HJ, Watanabe DS, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes. The American Journal of sports Medicine 2005;33(7):1003–1010. [DOI] [PubMed] [Google Scholar]
- 17.Waldén M, Atroshi I, Magnusson H, Wagner P, Hägglund M. Prevention of acute knee injuries in adolescent female football players: cluster randomised controlled trial. The BMJ 2012;344:e3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfeiffer RP, Shea KG, Roberts D, Grandstrand S, Bond L. Lack of effect of a knee ligament injury prevention program on the incidence of noncontact anterior cruciate ligament injury. The Journal of Bone and Joint Surgery 2006;88(8):1769–1774. [DOI] [PubMed] [Google Scholar]
- 19.Söderman K, Werner S, Pietilä T, Engström B, Alfredson H. Balance board training: prevention of traumatic injuries of the lower extremities in female soccer players? Knee surgery, sports traumatology, arthroscopy 2000;8(6):356–363. [DOI] [PubMed] [Google Scholar]
- 20.Sugimoto D, Myer GD, McKeon JM, Hewett TE. Evaluation of the effectiveness of neuromuscular training to reduce anterior cruciate ligament injury in female athletes: a critical review of relative risk reduction and numbers-needed-to-treat analyses. Br J Sports Med 2012:bjsports2011–090895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. Journal of athletic training 2007;42(2):311. [PMC free article] [PubMed] [Google Scholar]
- 22.Myer GD, Ford KR, Brent JL, Hewett TE. Differential neuromuscular training effects onACL injury risk factors in” high-risk” versus” low-risk” athletes. BMC musculoskeletal disorders 2007;8(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myer GD, Ford KR, Brent JL, Hewett TE. An integrated approach to change the outcome part II: targeted neuromuscular training techniques to reduce identified ACL injury risk factors. Journal of strength and conditioning research/National Strength & Conditioning Association 2012;26(8):2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myer GD, Ford KR, Brent JL, Hewett TE. An integrated approach to change the outcome part I: neuromuscular screening methods to identify high ACL injury risk athletes. Journal of strength and conditioning research/National Strength & Conditioning Association 2012;26(8):2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hewett TE, Stroupe AL, Nance TA, Noyes FR. Plyometric training in female athletes: Decreased impact forces and increased hamstring torques. The American Journal of Sports Medicine 1996;24(6):765–773. [DOI] [PubMed] [Google Scholar]
- 26.Gibson JJ. The perception of the visual world Oxford, England: Houghton Mifflin; 1950. [Google Scholar]
- 27.Powers WT. Behavior: The control of perception Chicago: Aldine; 1973. [Google Scholar]
- 28.McNevin NH, Shea CH, Wulf G. Increasing the distance of an external focus of attention enhances learning. Psychological research 2003;67(1):22–29. [DOI] [PubMed] [Google Scholar]
- 29.Mechsner F A psychological approach to human voluntary movements. Journal of Motor Behavior 2004;36(4):355–370. [DOI] [PubMed] [Google Scholar]
- 30.Shea CH, Wulf G. Enhancing motor learning through external-focus instructions and feedback. Human movement science 1999;18(4):553–571. [Google Scholar]
- 31.Wulf G Attentional focus and motor learning: a review of 15 years. International Review of Sport and Exercise Psychology 2013;6(1):77–104. [Google Scholar]
- 32.Wulf G, Dufek JS, Lozano L, Pettigrew C. Increased jump height and reduced EMG activity with an external focus. Human movement science 2010;29(3):440–448. [DOI] [PubMed] [Google Scholar]
- 33.Wulf G, McConnel N, Gärtner M, Schwarz A. Enhancing the learning of sport skills through external-focus feedback. Journal of motor behavior 2002;34(2):171–182. [DOI] [PubMed] [Google Scholar]
- 34.Wulf G, McNevin N. Simply distracting learners is not enough: More evidence for the learning benefits of an external focus of attention. European Journal of Sport Science 2003;3(5):1–13. [Google Scholar]
- 35.Wulf G, Prinz W. Directing attention to movement effects enhances learning: A review. Psychonomic bulletin & review 2001;8(4):648–660. [DOI] [PubMed] [Google Scholar]
- 36.Wulf G, Shea C, Lewthwaite R. Motor skill learning and performance: a review of influential factors. Medical education 2010;44(1):75–84. [DOI] [PubMed] [Google Scholar]
- 37.Zachry T, Wulf G, Mercer J, Bezodis N. Increased movement accuracy and reduced EMG activity as the result of adopting an external focus of attention. Brain Research Bulletin 2005;67(4):304309. [DOI] [PubMed] [Google Scholar]
- 38.Brenner E, Smeets JBJ. Quickly ‘learning’ to move optimally. Experimental Brain Research 2011;213(1):153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez L, Bootsma RJ. Non-linear gaining in precision aiming: making Fitts’ task a bit easier. Acta Psychologica 2008;129(2):217–227. [DOI] [PubMed] [Google Scholar]
- 40.Kovacs A, Buchanan J, Shea C. Perceptual influences on Fitts’ law. Experimental Brain Research 2008;190(1):99–103. [DOI] [PubMed] [Google Scholar]
- 41.Kovacs AJ, Buchanan JJ, Shea CH. Bimanual 1: 1 with 90° continuous relative phase: difficult or easy! Experimental Brain Research 2009;193(1):129–136. [DOI] [PubMed] [Google Scholar]
- 42.Shea CH, Wulf G, Whitacre CA, Park J-H. Surfing the implicit wave. The Quarterly Journal of Experimental Psychology: Section A 2001;54(3):841–862. [DOI] [PubMed] [Google Scholar]
- 43.Wang C, Kennedy DM, Boyle JB, Shea CH. A guide to performing difficult bimanual coordination tasks: just follow the yellow brick road. Experimental brain research 2013;230(1):31–40. [DOI] [PubMed] [Google Scholar]
- 44.Kiefer AW, Kushner AM, Groene J, Williams C, Riley MA, Myer GD. A commentary on real-time biofeedback to augment neuromuscular training for ACL injury prevention in adolescent athletes. Journal of sports science & medicine 2015;14(1):1. [PMC free article] [PubMed] [Google Scholar]
- 45.Ford KR, DiCesare CA, Myer GD, Hewett TE. Real-time biofeedback to target risk of anterior cruciate ligament injury: a technical report for injury prevention and rehabilitation. J Sport Rehabil 2015:2013–0138. [DOI] [PubMed] [Google Scholar]
- 46.Barrios JA, Crossley KM, Davis IS. Gait retraining to reduce the knee adduction moment through real-time visual feedback of dynamic knee alignment. Journal of Biomechanics 2010;43(11):2208–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crowell HP, Davis IS. Gait retraining to reduce lower extremity loading in runners. Clinical Biomechanics 2011;26(1):78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noehren B, Scholz J, Davis I. The effect of real-time gait retraining on hip kinematics, pain and function in subjects with patellofemoral pain syndrome. British journal of sports medicine 2010:bjsports69112. [DOI] [PubMed] [Google Scholar]
- 49.Ericksen HM, Thomas AC, Gribble PA, Armstrong C, Rice M, Pietrosimone B. Jump–landing biomechanics following a 4-week real-time feedback intervention and retention. Clinical Biomechanics 2016;32:85–91. [DOI] [PubMed] [Google Scholar]
- 50.Ericksen HM, Thomas AC, Gribble PA, Doebel SC, Pietrosimone BG. Immediate effects of real-time feedback on jump-landing kinematics. journal of orthopaedic & sports physical therapy 2015;45(2):112–118. [DOI] [PubMed] [Google Scholar]
- 51.Hewett TE, Ford KR, Myer GD, Wanstrath K, Scheper M. Gender differences in hip adduction motion and torque during a single‐leg agility maneuver. Journal of Orthopaedic Research 2006;24(3):416–421. [DOI] [PubMed] [Google Scholar]
- 52.Hewett TE, Myer GD. The mechanistic connection between the trunk, knee, and anterior cruciate ligament injury. Exercise and sport sciences reviews 2011;39(4):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Myer GD, Ford KR, Foss KDB, Liu C, Nick TG, Hewett TE. The relationship of hamstrings and quadriceps strength to anterior cruciate ligament injury in female athletes. Clinical Journal of Sport Medicine 2009;19(1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt RA, Wrisberg CA. Motor learning and performance Champaign, IL: Human Kinetics; 2005. [Google Scholar]
- 55.Donovan JJ, Radosevich DJ. A meta-analytic review of the distribution of practice effect: Now you see it, now you don’t. Journal of Applied Psychology 1999;85(5):795–805. [Google Scholar]
- 56.Lee TD, Genovese ED. Distribution of practice in motor skill acquisition: Learning and performance effects reconsidered. Research Quarterly for Exercise and Sport 1988;59(4):277287. [DOI] [PubMed] [Google Scholar]
- 57.Gokeler A, Benjaminse A, Hewett TE, et al. Feedback techniques to target functional deficits following anterior cruciate ligament reconstruction: implications for motor control and reduction of second injury risk. Sports Medicine 2013;43(11):1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. British Journal of Sports Medicine 2009;43(6):417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paterno MV. Non-operative care of the patient with an ACL-deficient knee. Current Reviews in Musculoskeletal Medicine 2017;10(3):322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zazulak BT, Hewett TE, Reeves NP, Goldberg B, Cholewicki J. Deficits in Neuromuscular Control of the Trunk Predict Knee Injury Risk:Prospective Biomechanical-Epidemiologic Study. The American Journal of Sports Medicine 2007;35(7):1123–1130. [DOI] [PubMed] [Google Scholar]
- 61.Ford KR, Myer GD, Schmitt LC, van den Bogert AJ, Hewett TE. Effect of drop height on lower extremity biomechanical measures in female athletes. Medicine and Science in Sports and Exercise 2008;40(5):S80. [Google Scholar]
- 62.Hewett TE, Myer GD, Ford KR, et al. Biomechanical Measures of Neuromuscular Control and Valgus Loading of the Knee Predict Anterior Cruciate Ligament Injury Risk in Female Athletes: A Prospective Study. The American Journal of Sports Medicine 2005;33(4):492–501. [DOI] [PubMed] [Google Scholar]
- 63.Schoenfeld BJ. Squatting Kinematics and Kinetics and Their Application to Exercise Performance. The Journal of Strength & Conditioning Research 2010;24(12):3497–3506. [DOI] [PubMed] [Google Scholar]


