Abstract
Objective:
Hearing impairment (HI) is common in aging adults. Aldosterone, insulin-like growth factor (IGF1), and brain-derived neurotrophic factor (BDNF), have been identified as potentially protective of hearing. The present study aims to investigate these relationships.
Methods:
The Epidemiology of Hearing Loss Study (EHLS) is a longitudinal population-based study of aging in Beaver Dam, Wisconsin which began in 1993. Baseline for the present investigation is the 1998–2000 phase. Follow-up exams occurred approximately every 5 years with the most recent occurring from 2012–2014. Hearing was measured by pure-tone audiometry. HI was defined as a pure tone average (PTA) >25 decibels hearing level in either ear. Change in PTA was the difference between follow-up examinations and baseline. Baseline serum samples were used to measure biomarkers in 2017. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated to assess the effect of biomarker levels in the lowest quintile (Q1) versus the highest (Q5) on incident HI and PTA change.
Results:
There were 1088 participants (69.3% women) at risk of HI included in analyses. The mean baseline age was 63.8 years (standard deviation=7.0). The 16-year incidence of HI was 54.9%, and was higher in men (61.1%) than women (52.1%). In age- and sex-adjusted models, aldosterone (HR=1.06, 95% CI= 0.82–1.37), IGF1 (HR=0.92, 95% CI= 0.71–1.19), and BDNF (HR=0.86, 95% CI= 0.66–1.12) levels were not associated with risk of HI. PTA change was similarly not affected by biomarker levels.
Conclusion:
Aldosterone, IGF1, and BDNF were not associated with decreased risk of age-related hearing loss in this study.
Keywords: Epidemiology, Audiology, Sensorineural hearing loss, risk factors, neuroprotective, brain-derived neurotrophic factor, insulin-like growth factor, aldosterone
Introduction:
Hearing impairment (HI) is a common health condition in older adults and risk increases with age.1–3 The risk of age-related hearing loss (presbycusis) and impairment has been shown to be at least partially modifiable.1–3 Hearing relies on cochlear and hair cell function to convert soundwaves into an electrical signal via release of neurotransmitters, and on transfer of this signal to the central nervous system via spiral ganglion cells and the auditory nerve.4–5 Preservation of this sensorineural pathway could reduce risk of presbycusis. Aldosterone, insulin-like growth factor (IGF1), and brain derived neurotrophic factor (BDNF) have been identified in previous reports as being potentially protective of hearing through mechanisms related to neuronal health. As many are affected by hearing problems in aging, a modest reduction in risk could have a large impact on the burden of disease, associated health-care and societal costs, and public health in general.
Aldosterone is a mineralocorticoid involved in regulation of ions and ion transport including sodium and potassium.6 The ion transport process has been implicated in regulation of cochlear fluid balance.7 The hearing process, especially at the hair cell level, relies on proper regulation of ion homeostasis.8 In a small cross-sectional study of 47 volunteers, higher serum aldosterone concentration was associated with lower hearing thresholds and better performance on hearing in noise tests (HINTs), implying a possible protective effect.9 In animal models, treatment with aldosterone showed improved or unchanged auditory brainstem response thresholds and was as effective as more common glucocorticoid treatments, such as prednisone.10 Also in animal models, aldosterone slowed progression of age-related hearing loss with long term administration, improved survival of spiral ganglion cells, and blocked apoptotic pathways.11–13
IGF1 is a protein involved in neurogenesis and cell survival. It is important in development and function of cells throughout the body, including cells of the inner ear, and in maintaining neuronal functioning.14–17 A cohort study of aging adults found higher levels of IGF1 were associated with decreased risk of HI after 6 years of follow-up, among participants 50 to 60 years of age at baseline, though this relationship did not exist in the cohort as a whole, when those greater than 60 years of age were included.18 In mouse models, deficiency of IGF1 was associated with worse hearing characterized by cochlear neuronal loss, delayed transmission in the central auditory pathway, and premature degeneration of the stria vascularis.19–20
BDNF is involved in neurogenesis, synaptic transmission, and cell survival (Mattson 2004).21 BDNF is involved in inner ear development in utero and may be particularly important to inner hair cell stability (Chacko 2017).22 It has improved cochlear function when applied as treatment in animal studies of ototoxic and noise-induced deafness.23–24 BDNF has also been shown to increase survival of spiral ganglion cells.25–26 As BDNF’s role in inner hair cell and neural function has been demonstrated, its potential use as a treatment for HI has been suggested.27
Much of the previous evidence of the effect of these biomarkers on hearing comes from small cross-sectional studies, from a subset of a larger cohort study, and from animal models. Replication of these findings, specifically those from human studies, in a population-based longitudinal cohort is necessary to better understand what impact these biomarkers have on hearing. If the findings of Tadros et al and Lassale et al, that serum aldosterone and IGF-1 are related to reduced risk of age-related hearing loss, can be confirmed in another population, then the applications and impact of these findings would be widespread. The current study aims to investigate the potential relationships between these biomarkers and changes in hearing in a population-based cohort of older adults.
Materials and Methods:
Study Population
Recruitment and demographic details of the Epidemiology of Hearing Loss Study (EHLS) have been previously reported.1–3, 28 Briefly, participants were residents of Beaver Dam, WI aged 43–84 during a private census from 1987 to 1988. The first EHLS exams took place from 1993 to 1995, with follow-up examinations occurring approximately every five years with the most recent exams occurring from 2014 to 2016. The 1998–2000 study phase is used as the baseline for this investigation as the laboratory samples used for biomarker assay come from this examination. Participants with an examination and with at least 1 subsequent follow-up visit are the focus of this investigation. The EHLS was approved by the Health Sciences Institutional Review Board of the University of Wisconsin, all participants provided written informed consent prior to examinations, and all study protocols were performed in accordance with the tenets of the Declaration of Helsinki.29
Hearing evaluation
Participants’ hearing was tested following a standardized protocol at each examination.1–3 Hearing tests were conducted using clinical audiometers and TDH-50 headphones (Telephonics Dynamic Headphones 50; Telephonics) or in the case of ear canal collapse as determined by otoscopic examination, ER-3A insert earphones (EARtone3A; EtymoticResearch Inc), in sound treated booths.30,31 Participants unable to be seen in the clinic site were tested in their homes or in nursing or group homes with insert earphones. All audiometers were calibrated every 6 months. Sound levels were taken monthly in the clinic booths and at the time of exam for those tested off site to ensure ANSI standards were met.32–33 Pure-tone air-conduction thresholds were measured at 0.5, 1, 2, 3, 4, 6, and 8 kHz. A pure tone average (PTA) was calculated using the thresholds at 0.5, 1, 2, and 4 kHz, the frequencies most important for speech recognition, and a high frequency PTA (HFPTA) was calculated using the thresholds at 4, 6, and 8 kHz.
Laboratory measures
Aldosterone, IGF1, and BDNF were measured in serum samples obtained during the 1998–2000 examination. Serum samples were stored at −80° Celsius until testing in 2017 by the Advanced Research and Diagnostic Laboratory at the University of Minnesota. Aldosterone was measured by chemiluminescent immunoassay (DiaSorin, Stillwater, MN) using Liaison (DiaSorin, Stillwater, MN), inter-assay coefficient of variation (CV)=5.2%. IGF1 and BDNF were measured by quantitative sandwich enzyme immunoassay using a Beckmen Coulter Biomek NXp (Beckman Coulter, Fullerton, CA), with the Human Insulin-like Growth Factor 1 and Human Brain-derived Neurotrophic Factor Quantikine ELISA kits (R&D Systems, Minneapolis, MN), CV=6.8% and 6.6%, respectively.
Other covariates
Additional information was collected on factors previously found to be associated with incident HI in this cohort.3 A detailed questionnaire was administered by trained examiners, including educational attainment and current smoking status. Diabetes mellitus was defined by a reported physician diagnosis or a measured glycosylated hemoglobin greater than 8% (or hemoglobin A1C≥6.5%), or a diagnosis of borderline diabetes with current treatment. Waist measurement was conducted following a standard protocol by trained technicians.
Statistical analysis
All analyses were conducted using SAS version 9.4 software (SAS Institute, Inc., Cary, NC).
Biomarkers
Quintiles were formed for each biomarker assay, in the overall population and sex-specifically. Sex-specific quintiles were used in sex-stratified analyses. Analyses of these biomarkers compared the lowest quintile (Q1) to the highest quintile (Q5) to investigate the potential effect of low circulating levels of each biomarker on development of HI and changes in hearing function. Additional analyses investigated the potential relationships on a continuous scale for biomarkers (per standard deviation, SD), and doubling of biomarker levels to investigate a possible dose-response relationship.
Hearing impairment
There were 1088 participants at risk of incident HI, with normal hearing in both ears, defined as PTA less than or equal to 25 decibels hearing level (dbHL) at baseline, measured biomarkers at baseline, and at least 1 subsequent follow-up examination. Incidence of HI was defined as a measured PTA greater than 25 dbHL in either ear at any follow-up examination. The relationship between biomarkers and risk of cumulative incidence of HI was analyzed using a discrete-time hazard model with a complementary log-log transformation (via Proc GENMOD specifying the cloglog link function) and a binomial distribution. The complimentary log-log function uses the maximum likelihood estimation method to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Since the complimentary log-log link has a proportional hazards assumption it is conceptually analogous to the continuous time hazard model used in Cox proportional-hazards models.34–35 Models investigating the association between biomarkers and incident HI were age- and sex-adjusted. Additional models were constructed stratified by age controlling for sex and stratified by sex controlling for age to evaluate potentially differential effects of the biomarkers. A sensitivity analysis was conducted, excluding participants tested with insert earphones at any examination phase. Finally, analyses were run investigating the relationship of the biomarkers to cumulative incidence of bilateral HI. In this analysis cases were those who had a measured PTA greater than 25 dbHL in both ears at a follow-up examination.
Change in PTA
Participants with measured PTA and biomarkers at baseline and at least 1 subsequent follow-up examination were included in analyses of change in PTA. Participants with prevalent HI at baseline were included in this analysis. Data were available for PTA change for 1908 participants, 1473 participants, and 962 participants at the 2003–2005, 2009–2010, and 2013–2015 follow-up exams, respectively. PTA change was calculated as the difference in PTA at each follow-up exam compared to baseline. A positive value indicates an increase in PTA which would mean a decline in hearing function. Linear regression modeling was used to compare the difference in PTA change between Q1 and Q5 of all three biomarkers. In this analysis positive estimates would mean those in Q1 had a larger increase in PTA than those in Q5, which could also be stated as a larger decline in hearing function.
Other analyses
Analyses were conducted to investigate that the biomarkers could be related to follow-up status. First, we compared levels of the biomarkers between those included in analysis, those with follow-up, and those without follow-up. Second, we conducted an analysis of differences in biomarker levels by competing events (loss to follow-up and death) to see if biomarker levels were predictive of such events.
Results:
Participants at risk of HI (N=1088) included in analyses, contributed an average of 11 years of follow-up (SD=4.5; range 5 to 16), had a mean age of 63.8 years (SD=7.0) at baseline and 754 (69.3%) were women. Mean levels (SD) of aldosterone, IGF1 and BDNF were 9.1 ng/dL (6.1), 74.3 ng/mL (25.4), and 21.6 ng/mL (6.2), respectively. Aldosterone and BDNF were significantly lower (p=0.02, p<0.0001, respectively) in men, while IGF1 was higher (p<0.0001) (See Table 1). Aldosterone was higher (p<0.0001) and IGF1 lower (p=0.02) in older age groups, while there was no difference (p=0.11) in BDNF by age (See Table 1).
Table 1:
Distribution of serum biomarkers in participants at risk of incident hearing impairment (N=1088)
| Aldosterone (ng/dL) | IGF1 (ng/mL) | BDNF (ng/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | |
| Overall | 9.1 | 6.1 | 2.9 – 63.6 | 74.3 | 25.4 | 20.2 – 238.8 | 21.6 | 6.2 | 2.4 – 63.0 |
| Sex† | |||||||||
| Men | 8.5 | 5.0 | 2.9 – 41.9 | 81.3 | 24.5 | 21.4 – 187.8 | 20.2 | 5.9 | 4.4 – 42.3 |
| Women | 9.4 | 6.5 | 2.9 – 63.6 | 71.2 | 25.1 | 20.2 – 238.8 | 22.2 | 6.3 | 2.4 – 63.0 |
| Age (years)‡ | |||||||||
| 53–59 | 8.1 | 5.4 | 2.9 – 60.5 | 75.1 | 25.0 | 21.4 – 187.8 | 21.6 | 6.2 | 4.7 – 63.0 |
| 60–69 | 9.2 | 5.9 | 2.9 – 63.6 | 72.7 | 24.3 | 23.0 – 219.3 | 21.8 | 6.4 | 6.8 – 60.6 |
| 70–79 | 10.8 | 7.6 | 2.9 – 58.6 | 77.2 | 28.6 | 32.7 – 238.8 | 20.9 | 6.0 | 2.4 – 34.3 |
| 80+ | 9.9 | 5.9 | 3.8 – 25.8 | 67.2 | 18.5 | 20.2 – 103.9 | 21.9 | 5.6 | 13.0 – 34.7 |
p-values for difference of biomarkers by sex (aldosterone: p=0.02, IGF1: p<0.0001, BDNF: p<0.0001)
p-values for difference of biomarkers by age group (aldosterone: p<0.0001, IGF1: p=0.02, BDNF: p=0.11)
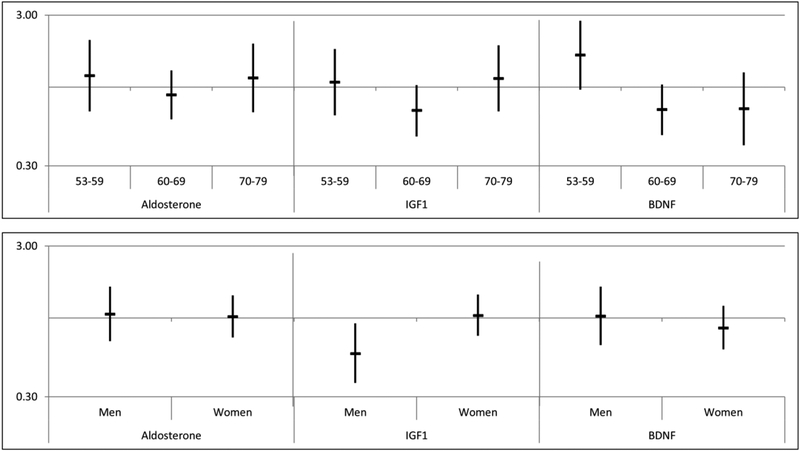
The overall 16-year cumulative incidence of HI in this follow-up period was 54.9%, 61.1% among men and 52.1% among women. The distribution of incident HI by biomarker quintiles is shown in Table 2. In age- and sex-adjusted models, aldosterone (HR=1.06, 95% CI= 0.82, 1.37), IGF1 (HR=0.92, 95% CI= 0.71, 1.19) and BDNF (HR=0.86, 95% CI= 0.66, 1.12) levels in Q1 were not associated with increased risk of incident HI in the follow-up period compared to Q5 (see Table 2). Results were similar in sex-stratified and age-stratified models (See Figure 1), though men with an IGF1 level in Q1 were less likely to develop HI compared to Q5 (HR=0.58, 95% CI=0.37, 0.92). This effect was not significant (HR=0.62, 95% CI=0.39, 1.01) when factors shown to be associated with incident HI, education, current smoking status, diabetes, and waist circumference, were included in the model.3 Excluding participants tested with insert earphones had no effect on the results. There was no significant association found between any of the biomarkers and high-frequency PTA (data not shown).
Table 2:
Distribution and age- and sex-adjusted risk (Hazard Ratios and 95% Confidence Intervals) of 16-year incident hearing impairment by biomarker quintile
| Incident Hearing impairment | |||
|---|---|---|---|
| No N (%) | Yes N (%) | HR (95%CI)† | |
| Aldosterone (ng/dL) | |||
| Q1 (<5.03) | 107 (21.8) | 135 (22.6) | 1.06 (0.82, 1.37) |
| Q2 (5.03–6.96) | 106 (21.6) | 122 (20.5) | 0.91 (0.70, 1.18) |
| Q3 (6.97–9.27) | 95 (19.4) | 109 (18.3) | 0.82 (0.63, 1.07) |
| Q4 (9.28–12.3) | 99 (20.2) | 113 (19.0) | 0.95 (0.73, 1.23) |
| Q5 (12.4+) | 83 (16.9) | 117 (19.6) | Reference |
| IGF1 (ng/mL) | |||
| Q1 (<54.05) | 96 (19.5) | 125 (20.9) | 0.92 (0.71, 1.19) |
| Q2 (54.05–66.31) | 111 (22.6) | 98 (16.4) | 0.66 (0.50, 0.86) |
| Q3 (66.32–77.76) | 107 (21.8) | 133 (22.3) | 0.87 (0.68, 1.11) |
| Q4 (77.77–93.56) | 98 (20.0) | 116 (19.4) | 0.80 (0.62, 1.04) |
| Q5 (93.57+) | 79 (16.1) | 125 (20.9) | Reference |
| BDNF (ng/mL) | |||
| Q1 (<15.80) | 77 (15.7) | 104 (17.4) | 0.86 (0.66, 1.12) |
| Q2 (15.81–19.27) | 91 (18.5) | 126 (21.1) | 1.06 (0.83, 1.36) |
| Q3 (19.28–22.43) | 99 (20.2) | 122 (20.4) | 0.94 (0.73, 1.21) |
| Q4 (22.44–25.99) | 117 (23.8) | 110 (18.4) | 0.72 (0.55, 0.93) |
| Q5 (26.00+) | 107 (21.8) | 135 (22.6) | Reference |
Adjusted for age and sex
Figure 1:
Risk of 16-year incident hearing impairment for low Aldosterone, Insulin-like growth factor (IGF1) and Brain-derived neurotrophic factor (BDNF) stratified by age (years) and sex (Hazard Ratios and 95% Confidence Intervals)†
Figure 1 caption:
†HR indicates risk for Q1 of biomarker versus Q5 controlling for sex in age-specific models and controlling for age in sex-specific models.
Analyses of incident bilateral HI also found no significant associations with Q1 of aldosterone (HR=1.01, 95% CI= 0.74, 1.37), IGF1 (HR=0.82, 95% CI=0.60, 1.13), or BDNF (HR=0.95, 95% CI=0.70, 1.30) as compared to Q5. In analyses of biomarkers modeled continuously and in dose-response models there were no significant associations between aldosterone, IGF1, or BDNF and cumulative incidence of HI in the 16-year follow-up (See Table 3) when controlling for age and sex. This was also true in sex-specific models controlling only for age (See Table 3).
Table 3:
Risk of 16-year incidence of hearing impairment per standard deviation of biomarker and doubling of biomarker: Hazard Ratio (95% Confidence Interval)†
| Overall (N=1088) | Men (N=334) | Women (N=754) | |
|---|---|---|---|
| Aldosterone | |||
| Continuous (Per SD) | 1.07 (0.99, 1.16) | 1.03 (0.89, 1.20) | 1.08 (0.98, 1.20) |
| Doubling | 1.01 (0.90, 1.13) | 0.99 (0.82, 1.19) | 1.02 (0.89, 1.17) |
| IGF1 | |||
| Continuous (Per SD) | 1.08 (0.99, 1.18) | 1.09 (0.95, 1.25) | 1.06 (0.95, 1.19) |
| Doubling | 1.11 (0.93, 1.32) | 1.26 (0.91, 1.74) | 1.03 (0.84, 1.27) |
| BDNF | |||
| Continuous (Per SD) | 0.98 (0.90, 1.07) | 0.91 (0.78, 1.06) | 1.01 (0.91, 1.12) |
| Doubling | 0.94 (0.78, 1.13) | 0.82 (0.61, 1.11) | 1.01 (0.80, 1.27) |
Controlling for age and sex in overall model and controlling for age in sex-specific models
There was no clear pattern or consistent direction of effects in the analysis of biomarkers effects on change in PTA (See Table 4). No significant difference was found between change in PTA for those in Q1 versus Q5 overall for any of the biomarkers for the entire 16-year follow-up period, or in the 10-year follow-up period (See Table 4). In the shorter 5-year follow-up period, men in Q1 of aldosterone had a significantly higher PTA change (1.49 dB larger, p=0.04) compared to men in Q5. Men in Q1 of IGF1 had a significantly smaller PTA change (1.88 dB less, p=0.01) compared to men in Q5 in this same period (See Table 4). These differences were not observed in the longer follow-up period for men or among women for any follow-up period.
Table 4:
Difference in PTA change (dbHL) between Q1 and Q5 of Aldosterone, Insulin-like growth factor (IGF1), and Brain-derived neurotrophic factor (BDNF), overall and by sex†
| PTA change 5 year follow-up | PTA change 10 year follow-up | PTA change 16 year follow-up | ||||
| Beta | p-value | Beta | p-value | Beta | p-value | |
| Overall | 0.11 | 0.80 | 0.11 | 0.87 | −1.00 | 0.32 |
| Men | 1.49 | 0.04 | −0.81 | 0.41 | −1.49 | 0.31 |
| Women | −0.58 | 0.29 | 0.65 | 0.47 | −1.58 | 0.25 |
| Overall | −0.66 | 0.14 | −0.22 | 0.75 | −0.72 | 0.49 |
| Men | −1.88 | 0.01 | −0.79 | 0.43 | −0.20 | 0.90 |
| Women | 0.09 | 0.87 | −0.04 | 0.97 | −1.26 | 0.37 |
| Overall | −0.11 | 0.79 | 1.26 | 0.06 | 0.04 | 0.97 |
| Men | −0.15 | 0.85 | 1.82 | 0.07 | 1.93 | 0.20 |
| Women | 0.13 | 0.81 | 0.91 | 0.32 | −1.38 | 0.33 |
Overall models control for age and sex, sex specific models control for age. A positive beta indicates a larger change in PTA (decline in hearing) for those in Q1 versus Q5.
Analysis to test whether biomarker levels were associated with follow-up status revealed biomarkers did not differ between those included in analysis and those without follow-up and biomarker levels were not predictive of competing events.
Discussion:
Similar to previous reports, cumulative incidence of HI was common in these analyses.3,28 In this longitudinal study of the aging senses there were no significant long term protective effects of serum aldosterone, IGF1, or BDNF against incident HI or changes in PTA overall. A protective effect of low IGF1 was found in age-adjusted stratified analyses among men though this association was likely due to residual confounding as adding factors known to be associated with incident HI attenuated the effect and it was no longer statistically significant. Similarly, the short term effects of low aldosterone and IGF1 on PTA change in men, detrimental and protective respectively, are more likely to be spurious than informative as neither effect was observed in the longer follow-up periods of 10 or 16 years.
In previous studies, higher serum aldosterone was shown to have a cross-sectional relationship to lower hearing thresholds and better performance on HINTs and was protective in animal models.9–13 Higher levels of IGF1 were shown to decrease incident HI among 50 to 60 years olds in a longitudinal cohort, and low levels proved detrimental to hearing in animal models.18–20 BDNF has been suggested as a possible treatment for deafness and was effective in treatment of ototoxic and noise-induced deafness in animal models.23–24, 27 The potentially neuroprotective effects of aldosterone, IGF1, and BDNF demonstrated in previous studies were not replicated in this study.
The lack of association between the biomarkers of interest and hearing in the present study may be due to a number of reasons. First, these biomarkers may not provide protection against hearing loss in the general human population at naturally occurring circulating levels. The findings of Tadros et al. were cross-sectional and in a limited number of participants, therefore, though there was an association, the temporality of the exposure compared to the outcome is not certain. Additionally, their subjects were a select group free of hereditary hearing loss, significant noise exposure, ear infections, drug ototoxicity, hypertension, hypercholesterolemia, heart disease, neurological disorders, and did not take certain medications.9 While this may help to ensure the reported effects were due to differences in aldosterone, it may also imply this group was not representative of the general population. Achieving an age of 58–73 years, the age of their participants, without other exposures known to increase risk of hearing loss, may indicate that these participants represent a very different group from the EHLS and the U.S. population in general. The findings of Lassale et al were for a specific age group in their population and these results similarly may not be applicable to the general population. In the present study, in a similar age group, 53–59 years, a relationship between IGF1 and hearing was not observed. This difference in findings may also be due to differences in the outcome measurement as Lassale et al used a hearing screening tool rather than the more traditional and clinically used pure tone audiometry, and perhaps some misclassification could occur as a result. They also based HIon the better ear, which would be more similar to the bilateral HI results of the present study, which still did not find an association. Finally, in regards to the animal models it is possible these findings may not apply to the more complex and uncontrolled nature of exposures and etiology of human hearing loss.
The current study has a number of strengths including a large well characterized population, long term follow-up examinations, and standardized measures of hearing function. However, the lack of association in the present study may also be due to study specific limitations. The biomarkers of interest were only measured at one time point and it may be that consistently low levels are detrimental, or changes over time may indicate risk better than a single measurement. Lassale et al were able to use an average measurement across two time-points which may have better characterized an individual’s biomarker exposure level over time. As the biomarkers were only measured at one time point in the EHLS, changes in this exposure over time could not be characterized. Additionally, we observed higher levels of aldosterone in older age groups, which is not the expected effect of age on levels, though as levels were measured at a single time point this observation may be by chance. We cannot definitively explain this and the unexpected relationship may have had an impact on our findings. Our analyses of high-frequency hearing loss did not show any associations, though study of this type of hearing loss may require a younger population with lower prevalent impairment at high frequencies. Additionally, the EHLS participants are ethnically homogenous, non-hispanic white, and findings may not be generalizable to other groups.
Conclusion:
Incident hearing impairment and decline in hearing function were common in this population-based study. Previous findings indicating protective effects of aldosterone, IGF1, and BDNF on hearing were not replicated in this 16-year follow-up period of the EHLS cohort. Based on these findings, circulating levels of aldosterone, IGF1, and BDNF in a community living U.S. adult population do not have a protective effect on hearing during the aging process.
Acknowledgments
Conflicts of Interest and Source of Funding:
The authors have no COI to declare. This work was supported by Award Number R37AG011099 from the National Institute on Aging (Dr. Cruickshanks), U10EY06594 from the National Eye Institute (Drs. R Klein and B.E.K. Klein), and an unrestricted grant from Research to Prevent Blindness. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Eye Institute, or Research to Prevent Blindness.
References:
- 1.Cruickshanks KJ, Tweed TS, Wiley TL, et al. The 5-Year Incidence and Progression of Hearing Loss: The Epidemiology of Hearing Loss Study. Arch Otolaryngol Head Neck Surg. 2003; 129(10): 1041–1046. [DOI] [PubMed] [Google Scholar]
- 2.Cruickshanks KJ, Nondahl DM, Tweed TS, et al. Education, occupation, noise exposure history and the 10-yr cumulative incidence of hearing impairment in older adults. Hear Res. 2010; 264(1–2): 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruickshanks KJ, Nondahl DM, Dalton DS, et al. Smoking, Central Adiposity, and Poor Glycemic Control Increase Risk of Hearing Impairment. J Am Geriatr Soc. 2015; 63(5): 918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao J and Ohlemiller KK. Age-Related Loss of Spiral Ganglion Neurons. Hear Res. 2010; 264(1–2): 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kidd III AR and Bao J. Recent advances in the study of age-related hearing loss: a mini-review. Gerontology. 2012; 58(6): 490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trune DR, Canlon B. Corticosteriod Therapy for Hearing and Balance Disorders. Anat Rec (Hoboken). 2012; 295(11): 1928–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Mana D, Ceranic B, Djahanbakhch O, Luxon LM. Hormones and the auditory system: A review of physiology and pathophysiology. Neuroscience. 2008; 153(4): 881–900. [DOI] [PubMed] [Google Scholar]
- 8.Trune DR. Ion homeostasis in the ear: mechanisms, maladies, and management. Curr Opin Otolaryngol Head Neck Surg. 2010; 18(5): 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tadros SF, Frisina ST, Mapes F, Frisina DR, Frisina RD. Higher serum aldosterone correlates with lower hearing thresholds: A possible protective hormone against presbycusis. Hear Res. 2005; 209(1–2): 10–18. [DOI] [PubMed] [Google Scholar]
- 10.Trune DR, Kempton JB, Kessi M. Aldosterone (Mineralocorticoid) Equivalent to Prednisolone (Glucocorticoid) in Reversing Hearing Loss in MRL/MpJ-Faslpr Autoimmune Mice. Laryngoscope. 2000; 110(11): 1902–1906. [DOI] [PubMed] [Google Scholar]
- 11.Gross ND, Kempton JB, Trune DR. Spironolactone Blocks Glucocorticoid-Mediated Hearing Preservation in Autoimmune Mice. Laryngoscope. 2002; 112(2): 298–303. [DOI] [PubMed] [Google Scholar]
- 12.Halonen J, Hinton AS, Frisina RD, Ding B, Zhu X, Walton JP. Long-term treatment with aldosterone slows the progression of age-related hearing loss. Hear Res. 2016; 336: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frisina RD, Ding B, Zhu X, Walton JP. Age-related hearing loss: prevention of threshold declines, cell loss and apoptosis in spiral ganglion neurons. Aging (Albany NY). 2016; 8(9): 2081–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashpole NM, Sanders JE, Hodges EL, Yan H, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging brain. Exp Gerontol. 2015; 68: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodríguez-de la Rosa L, Lassaletta L, Calvino M, Murillo-Cuesta S, Varela-Nieto I. The Role of Insulin-Like Growth Factor 1 in the Progression of Age-Related Hearing Loss. Front Aging Neurosci. 2017; 9: 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varela-Nieto I, Morales-Garcia JA, Vigil P, et al. Trophic effects of insulin-like growth factor-I (IGF-I) in the inner ear. Hear Res. 2004; 196(1–2): 19–25. [DOI] [PubMed] [Google Scholar]
- 17.Yamahara K, Yamamoto N, Nakagawa T, Ito J. Insulin-like growth factor 1: A novel treatment for the protection or regeneration of cochlear hair cells. Hear Res. 2015; 330(Pt A): 2–9. [DOI] [PubMed] [Google Scholar]
- 18.Lassale C, Batty GD, Steptoe A, Zaninotto P. Insulin-like Growth Factor 1 in relation to future hearing impairment: findings from the English Longitudinal Study of Ageing. Sci Rep. 2017; 7(1): 4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riquelme R, Cediel R, Contreras J, et al. A comparative study of age-related hearing loss in wild type and insulin-like growth factor I deficient mice. Front Neuroanat. 2010; 4: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cediel R, Riquelme R, Contreras J, Diaz A, Varela-Nieto I. Sensorineural hearing loss in insulin-like growth factor I-null mice: a new model of human deafness. Eur J Neurosci. 2006; 23(2): 587–590. [DOI] [PubMed] [Google Scholar]
- 21.Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004; 27(10): 589–594. [DOI] [PubMed] [Google Scholar]
- 22.Johnson Chacko L, Blumer MJF, Pechriggl E, et al. Role of BDNF and neurotrophic receptors in human inner ear development. Cell Tissue Res. 2017; 370(3): 347–363. [DOI] [PubMed] [Google Scholar]
- 23.Sly DJ, Hampson AJ, Minter RL, et al. Brain-derived neurotrophic factor modulates auditory function in the hearing cochlea. J Assoc Res Otolaryngol. 2012; 13(1): 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhai SQ, Guo W, Hu YY, et al. Protective effects of brain-derived neurotrophic factor on the noise-damaged cochlear spiral ganglion. J Laryngol Otol. 2011; 125(5): 449–454. [DOI] [PubMed] [Google Scholar]
- 25.Singer W, Panford-Walsh R, Knipper M. The function of BDNF in the adult auditory system. Neuropharmacology. 2014; 76(Pt C): 719–728. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt N, Schulze J, Warwas DP, et al. Long-term delivery of brain-derived neurotrophic factor (BDNF) from nanoporous silica nanoparticles improves the survival of spiral ganglion neurons in vitro. PLoS One. 2018; 13(3): e0194778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khalin I, Alyautdin R, Kocherga G, Bakar MA. Targeted delivery of brain-derived neurotrophic factor for the treatment of blindness and deafness. Int J Nanomedicine. 2015; 10: 3245–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schubert CR, Paulsen AJ, Nondahl DM, et al. Association Between Cystatin C and 20-Year Cumulative Incidence of Hearing Impairment in the Epidemiology of Hearing Loss Study. JAMA Otolaryngol Head Neck Surg. 2018; 144(6): 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013; 310(20): 2191–2194. [DOI] [PubMed] [Google Scholar]
- 30.Larson VD, Cooper WA, Talbott RE. Reference threshold sound-pressure levels for the TDH-50 and ER-3A earphones. J Acoust Soc AM. 1988; 84(1): 46–51. [DOI] [PubMed] [Google Scholar]
- 31.Stuart A, Strenstrom R, Tompkins C, Vandenhoff S. Test-Retest Variability in Audiometric Threshold with Supraaural and Insert Earphones among Children and Adults. Audiology. 1991; 30: 82–90. [DOI] [PubMed] [Google Scholar]
- 32.American National Standards Institute. Maximum Permissible Ambient Noise Levels for Audiometric Test Rooms. (ANSI S3.1–1999). New York, NY: ANSI; 1999. [Google Scholar]
- 33.American National Standards Institute. Specification for Audiometers. (ANSI S3.6–2010). New York, NY: ANSI; 2010. [Google Scholar]
- 34.Singer JD and Willet JB. Applied longitudinal data analysis: modeling change and event occurrence. Oxford, New York: Oxford University Press; 2003. [Google Scholar]
- 35.Alisson PD. Survival analysis using the SAS system: a practical guide. Cary, NC: SAS Institute; 1995. [Google Scholar]