Abstract
Schizophrenia is a neurodevelopmental disorder with genetic predisposition, and stress has long been linked to its etiology. While stress affects all stages of the illness, increasing evidence suggests that stress during critical periods of development may be particularly detrimental, increasing individual’s vulnerability to psychosis. To thoroughly understand the potential causative role of stress, our group has been focusing on the prenatal methylazoxymethanol acetate (MAM) rodent model, and discovered that MAM offspring display abnormal stress reactivity and heightened anxiety prepubertally, prior to the manifestation of a hyperdopaminergic state. Furthermore, pharmacologically treating anxiety during prepuberty prevented the emergence of the dopamine dysfunction in adulthood. Interestingly, sufficiently strong stressors applied to normal rats selectively during early development can recapitulate multiple schizophrenia-related phenotypes of MAM rats, whereas the same stress paradigm during adulthood only produced short-term depression-related deficits. Altogether, the evidence is thus converging: developmental disruption (genetic or environmental) might render animals more susceptible to the deleterious effects of stress during critical time windows, during which unregulated stress can lead to the emergence of psychosis later in life. As an important region regulating the midbrain dopamine system, the ventral hippocampus is particularly vulnerable to stress, and the distinct maturational profile of its fast-spiking parvalbumin interneurons may largely underlie such vulnerability. In this review, by discussing emerging evidence spanning clinical and basic science studies, we propose developmental stress vulnerability as a novel link between early predispositions and environmental triggering events in the pathophysiology of schizophrenia. This promising line of research can potentially provide not only insights into the etiology, but also a “roadmap” for disease prevention.
Keywords: stress, psychosis, parvalbumin, perineuronal nets, critical period plasticity
Introduction
Schizophrenia is a chronic and severe psychiatric disorder that typically strikes individuals in late adolescence and early adulthood, impacting their ability to function effectively in society. For decades, research has centered on trying to better understand the cause of schizophrenia and to develop more effective treatments. One focus has been in identifying the genetic basis of schizophrenia, which has indicated that schizophrenia is polygenic in nature (Schizophrenia Working Group of the Psychiatric Genomics, 2014). However, almost all of the risk genes associated with the disorder confer only a small effect on the phenotype, and only a small percentage of genetic carriers transition to psychosis (Jagannath et al., 2017). Indeed, despite having an identical genetic background, the concordance rate of schizophrenia among identical twins is only about 50% (Cardno and Gottesman, 2000) and a substantial proportion of schizophrenia is idiopathic, without an identified family history. These evidences indicate that schizophrenia is not completely genetically determined. As a result, studies have also focused on how other risk factors could amplify the impact of genetic predisposition.
As with other major psychiatric disorders, schizophrenia is believed to arise from a combination of genetic predisposition and socio-environmental insults, such as exposure to victimization (physical abuse, sexual abuse, bullying by peers), urbanicity, social disadvantage, and others (Tsuang, 2000; van Os et al., 2010). Importantly, the impact of these socio-environmental factors is more pronounced during critical periods of development such as childhood and adolescence. Individuals with psychosis report experiencing more frequent and more severe childhood abuse than people without past or current psychiatric conditions (Mauritz et al., 2013). In addition, children and adolescents at-risk for schizophrenia, in which 20–40% will convert to the full-blown disorder (Fusar-Poli et al., 2012), experience abnormally high reactivity to stressful events (Pruessner et al., 2011) and are more likely to convert to psychosis if they show high anxiety and decreased tolerance to stress (Trotman et al., 2014; Yung et al., 2005). In fact, a multicenter study from the North American Prodrome Longitudinal Study (NAPLS) examining a large sample of adolescents at-risk found that cortisol levels, an index of stress, were significantly elevated among those who transitioned to psychosis (Walker et al., 2013).
During childhood and adolescence, a large number of dynamic alterations in physiological processes and social dynamics occurs as the person learns to adapt to their environment (Sisk and Foster, 2004; Spear, 2000). These processes, when combined with genetically influenced developmental changes, shape the neurobiological features that underlie brain maturation. Notably, these dynamic changes also make the developing brain highly vulnerable to exposure to detrimental environmental factors that can lead to the emergence of psychiatric disorders. Indeed, adolescence is a period of peak onset for many common mental disorders, including schizophrenia (Kessler et al., 2007). The neurobiological substrate for this susceptibility is currently unknown. However, it has been proposed that an elevated stress reactivity and the ongoing maturational processes of the hypothalamic-pituitary-adrenal (HPA) axis (which is part of the stress response system) during this period, as well as immaturity of cortical regions that play a role in emotion regulation, may contribute to this increased susceptibility (Gogtay et al., 2004; Romeo, 2017; Romeo and McEwen, 2006).
Given that elevated stress levels may be secondary to illness-related factors, most of the human studies are unable to discriminate true causation from association. Thus, animal studies, even with limitations, are crucial to capturing evidence to support a causative role for stress in the pathophysiology of brain development that can lead to susceptibility to psychiatric disorders, including schizophrenia. In this context, our group has been employing a neurodevelopmental disruption model that uses the mitotoxin methylazoxymethanol acetate (MAM) to garner insight into how genetic susceptibility interacts with environmental factors to lead to the pathology. Several brain regions are involved in the stress response, such as the medial prefrontal cortex, basolateral amygdala, ventral hippocampus, and ventral tegmental area. To a less neuro-inclined reader, a quick guide table summarizes the role of each of these regions in the stress response (Table 1).
Table 1 –
Main brain regions involved in the response to stress discussed in the present review.
| Brain region | Acronym | Role in stress response |
|---|---|---|
| Medial prefrontal cortex | mPFC | control of subcortical stress responses |
| Basolateral amygdala | BLA | expression of anxiety |
| Ventral hippocampus/ventral subiculum | vHipp/vSub | limbic hippocampus involved in context |
| Ventral tegmental area | VTA | dopamine neurons modulating cortical/limbic regions |
Insights from the MAM model of schizophrenia
The MAM model consists of the administration of MAM, a mitotoxin that is a DNA alkylating agent, to pregnant rats at gestational day (GD) 17 and leads to abnormal DNA methylation in the offspring (Hoareau et al., 2006), which in turn leads to neurodevelopmental disruption. As a consequence, the MAM offspring mimic many of the features found in schizophrenia (Modinos et al., 2015), including behavioral impairments (i.e., sensory gating, reversal of response strategy, social interaction) and neuroanatomical changes (thinning of limbic cortices, increased cell packing density, loss of parvalbumin neurons), in addition to a dopamine (DA) system hyperresponsivity as indicated by enhanced locomotor response to psychotomimetic drugs (i.e., phencyclidine and amphetamine) and increased number of spontaneously active DA neurons in the ventral tegmental area (VTA) (Flagstad et al., 2004; Lodge and Grace, 2007; Moore et al., 2006). Consistent with what is observed in humans, most of the schizophrenia-like changes in the MAM model appears during late adolescence/early adulthood (Gomes et al., 2016).
Dysregulation of the stress response has been proposed as a potential etiological factor in the development of schizophrenia (Gomes and Grace, 2017a). As with individuals at risk for schizophrenia (Jones et al., 2016), MAM rats also exhibit increased stress reactivity and heightened anxiety during early adolescence. We recently found that MAM rats in the juvenile period [postnatal day (PD) 22] are more responsive to stress. In response to acute footshock, juvenile MAM emitted more ultrasonic vocalizations (USVs; 22 kHz), vocalized for a longer duration and spent more time in freezing behavior compared to controls and older MAM rats (Zimmerman et al., 2013). In addition, during the peripubertal period (PD31–40), corresponding to mid-to-late adolescence in humans, MAM rats displayed anxiety-like behavior (Du and Grace, 2013), which was associated with a hyperactivity of the basolateral amygdala (Du and Grace, 2016), and showed attenuated corticosterone response to acute footshock that, unlike controls, persisted for 10 days of repeated stress exposure (Zimmerman et al., 2013). Collectively, these findings are consistent with epidemiological studies of adolescents at high-risk for schizophrenia indicating that those showing greater sensitivity to stress and increased anxiety tended to be the ones that convert to schizophrenia later in life (Devylder et al., 2013; Owens et al., 2005).
Similar to MAM rats, a blunted cortisol response to stress has also been reported in both established psychosis and individuals at-risk (Brenner et al., 2009; Pruessner et al., 2013). These findings suggest that adolescent MAM rats, as well as schizophrenia patients and individuals at-risk, may be unable to integrate appropriate responses to stress and, since a substantial cortisol/corticosterone response is necessary for homeostatic stress adaptation (McEwen and Gianaros, 2010), they also seem unable to adapt to repeated stress. It is worth mentioning that an attenuated cortisol response is thought to be a consequence of a prolonged period of hyperactivity of the HPA axis due to chronic stress (Fries et al., 2005; Pruessner et al., 2017).
Given that stress during developmental periods plays a role in schizophrenia, we proposed that alleviating anxiety and abnormal stress responsivity during these periods may prevent the emergence of the disease later in life. This idea was tested by treating MAM rats with daily administration of the anxiolytic drug diazepam during peripuberty (PD31–40) at a dose sufficient to normalize anxiety responses and amygdala activity (Du and Grace, 2013, 2016). As a result, peripubertal diazepam administration prevented the emergence of anxiety-like behavior and the higher neuronal firing rates within the BLA of MAM rats at adulthood (Du and Grace, 2016) as well as preventing the emergence of the hyperdopaminergic state observed in adult MAM rats (Du and Grace, 2013). These findings indicate that relieving stress in MAM rats around puberty circumvents the transition to the schizophrenia-like phenotype in the adult MAM rat. It is likely that other stress-relieving interventions will also be effective. Thus, these interventions applied to at-risk individuals may prevent the eventual transition to schizophrenia (Crush et al., 2018).
Deleterious effects of stress driving schizophrenia development
Based on our findings with the MAM model suggesting that stress drives the emergence of the schizophrenia phenotype and that it can be circumvented by relieving stress, we propose that maybe MAM does not “cause” schizophrenia but instead it may predispose to the emergence of pathology in the adult via increased sensitivity to environmental stressors. This is consistent with studies showing that of children at familial risk for schizophrenia, those that show heightened stress responsivity tend to convert to psychosis later in life (Owens et al., 2005). Therefore, we posit that MAM, and perhaps genetic risk factors in humans, cause the organism to show heightened susceptibility to the deleterious effects of stress during sensitive developmental periods, causing disruption to emerge later in life. Accordingly, if this model is accurate, one could predict that i) exposing normal rats to sufficiently strong stressors early in life would lead to pathophysiology in the adult that recapitulates that resulting from MAM treatment, and ii) interfering with structures that serve to limit the impact of stress exposure could mimic or exacerbate the impact of stress.
To test this, normal rats were exposed to different stressors during the same period that MAM rats were treated with diazepam (PD31–40). The stressors included either daily footshock for 10 days (PD31–40), 3 sessions of restraint stress (at PD31, 32 and 40), or the combination of 3 sessions of restraint stress plus daily footshock. All stressors impaired weight gain and induced anxiety-like responses in adults (>PD65). Exposure to daily footshock or to the combination of stressors also disrupted cognitive function. Interestingly, only the combination of stressors induced changes in DA system activity reflected by an increase in the number of spontaneously active DA neurons in the VTA and an enhanced amphetamine-induced locomotor response (Gomes and Grace, 2017b). These findings suggest that the impact on the DA system seems to depend on multiple interacting factors. Human studies have also suggested a cumulative effect of stress, indicating that experiencing two or more different types of victimization during childhood and adolescence is associated with a higher risk of developing psychotic symptoms (Arseneault et al., 2011; Kelleher et al., 2013). Together, these observations indicate that it is the repeated nature of the stress that is important, rather than a single event.
The hyperdopaminergic state observed in animals exposed to the combination of stressors during adolescence is similar to that found in MAM rats (Lodge and Grace, 2007) and is highly consistent with clinical studies. Thus, schizophrenia patients show abnormally high amphetamine-induced DA release (measured by raclopride displacement) in the striatum (Kegeles et al., 2010), consistent with the increased locomotor response to amphetamine in normal adult animals exposed to the combination of stressors during adolescence as well as in MAM rats, and an increase in fluorodopa uptake (Egerton et al., 2013) indicative of an increased number of active terminals, consistent with an increased number of spontaneously active DA neurons in the VTA (Grace, 2016). Interestingly, the increased DA neuron population activity found in animals exposed peripubertally to the combined stressor was confined to the lateral aspects of the VTA (Gomes and Grace, 2017b), which project to associative striatal regions analogous to those found to be hyper-responsive in schizophrenia patients (Weinstein et al., 2017).
What could make MAM rats or humans that are predisposed to schizophrenia have increased sensitivity to the disruptive effects of stress? Although stress is regulated by multiple brain regions, the medial prefrontal cortex (mPFC) is proposed as a primary integrator of the response to stress. In our earlier studies, we showed that the mPFC, specifically its prelimbic portion (plPFC), limited the impact of stress on activation of the BLA (Rosenkranz and Grace, 2001, 2002). Therefore, a dysfunction of the plPFC may impair the ability to respond appropriately to stress. Indeed, we found that plPFC lesions performed post-weaning (at PD25) caused the rats to show increased anxiety as adults (>PD65), and now footshock alone during PD31–40, which was inadequate to alter DA system responsivity, caused the rats to exhibit the hyperdopaminergic state in the adult (Gomes and Grace, 2017b). Also, an mPFC dysfunction in MAM animals was associated with a greater vulnerability to stress (Goto and Grace, 2006). A dysfunctional PFC has been extensively linked to the pathophysiology of schizophrenia and it can be present in the prodromal phase (Volk and Lewis, 2010). In fact, deficits in this region have been observed in the prodromal state of schizophrenia and subjects at ultra-high risk during tasks involving emotion regulation (Phillips and Seidman, 2008; van der Velde et al., 2015) as well as an abnormal connectivity between the amygdala and the PFC during emotion processing (Potvin et al., 2017). Thus, deficits in PFC function, which may be present in at-risk individuals, could limit the ability of this structure to regulate the impact of stress, increasing the vulnerability to the deleterious effects of stress and contributing to the emergence of psychiatric disorders such as schizophrenia.
In addition to the mPFC and amygdala, the hippocampus (Hipp) also plays a crucial role in modulating stress responses (McEwen et al., 2016). However, repeated stress damages the Hipp. For example, maintained stress leads to a loss of parvalbumin-containing GABAergic interneurons (PVI) in the Hipp (Czeh et al., 2005). Importantly, in schizophrenia, a PVI loss in the anterior Hipp, a region homologous to the ventral Hipp (vHipp) in rodents, is thought to lead to a hippocampal hyperactivity which in turn drives the hyperdopaminergic state associated with psychotic symptoms (Grace and Gomes, 2018). Thus, an increased VTA DA system activity induced by the combined stressors could be associated with a loss of PVI in the vHipp. Moreover, seizure-mediated activation of the BLA, an area activated during stress, decreases the number of PVI in the Hipp (Berretta et al., 2004). Hence, abnormal stress responsivity secondary to plPFC dysfunctional regulation of BLA reactivity to stress could to lead to hippocampal dysfunction, contributing to the emergence of a hyperdopaminergic state (Figure 1). Importantly, structural and functional imaging studies show hippocampal abnormalities in the prodromal state of schizophrenia (Baglivo et al., 2018; Vargas et al., 2018; Nenadic et al., 2015).
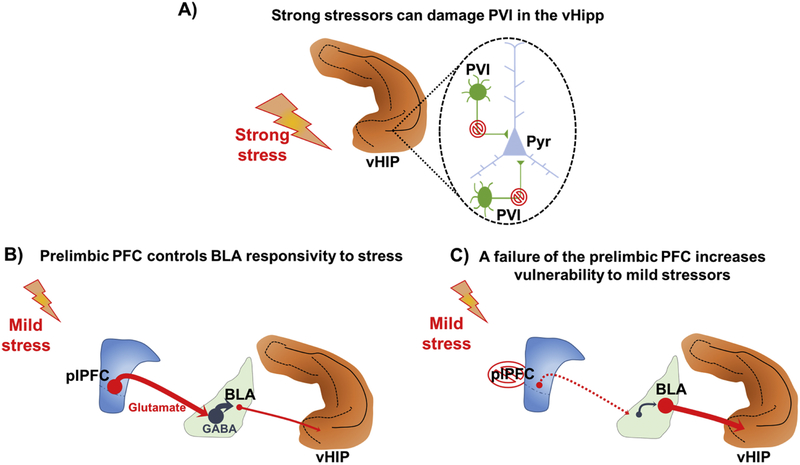
Figure 1 –
Stress as a risk factor for schizophrenia development. (A) Strong stressors during critical periods impact the ventral hippocampus (vHip) leading to a dysfunction of parvalbunin interneurons (PVI), or even to a PVI cell loss, which in turn contributes to the hyperactivity of glutamatergic pyramidal neurons that will drive a midbrain hyperdopaminergic state that underlies the emergence of psychosis. Alternately, genetic or gestational factors can increase susceptibility to mild stressor. (B) The prelimbic portion of the medial prefrontal cortex (plPFC) has been shown to control basolateral amygdala (BLA) responsivity to stress. (C) A failure of the plPFC to regulate BLA reactivity to stress could lead to a BLA glutamatergic overdrive to the vHipp which could damage PVI, contributing to the emergence of a hyperdopaminergic state.
Hippocampal PVI Dysfunction in Schizophrenia
Alterations in GABAergic circuits are proposed to be associated with numerous neurodevelopmental disorders, including schizophrenia. In fact, pathological changes in GABAergic circuits are widely reported in the PFC (Lewis et al., 2012; Volk and Lewis, 2010) and in the Hipp of schizophrenia patients (Benes et al., 2007; Konradi et al., 2011; Zhang and Reynolds, 2002). In the Hipp, GABAergic dysfunction seems to largely involve PVI, and a selective reduction in PVI density has been reported in post-mortem tissues (Konradi et al., 2011; Zhang and Reynolds, 2002). Functionally, PVIs are critical for maintaining regional excitatory-inhibitory (E/I) balance (Wöhr et al., 2015), and PVI dysregulation can potentially cause Hipp dysfunction (Pelkey et al., 2017) that is commonly associated with schizophrenia (Lieberman et al., 2018). In part, the selective vulnerability of the Hipp is attributable to its “fragile” E/I balance, and in fact the vast majority of Hipp neurons (approximately 90%) are excitatory pyramidal neurons (Freund and Buzsaki, 1996; Olbrich and Braak, 1985), which is markedly different from cortical regions where pyramidal cells and non-pyramidal cells are more balanced (Mouton, 2013). Such E/I imbalance in Hipp by default might explain its selective vulnerability to focal excitation.
Adolescent stress vulnerability is a critical period – relevance to schizophrenia
PVI dysfunction has also been extensively studied in animal models of schizophrenia, including the MAM model (Modinos et al., 2015). In MAM rats, PV reduction has been reported in the mPFC and selectively throughout subregions of the vHipp but not in their dorsal counterparts (Gill and Grace, 2014), a pattern that parallels schizophrenia patients in whom hippocampal pathologies tend to accumulate in the anterior limbic portion (Lieberman et al., 2018). Of all the Hipp regions manifesting PV reduction and/or PVI loss, the ventral subiculum (vSub) is of particular interest, as its local PVIs continue to mature until at least late adolescence (Caballero et al., 2013) and is highly susceptible to stress (O’Mara, 2005). This susceptibility may arise from their potent glutamatergic input from regions both intrinsically from hippocampal formation (e.g., CA1) and extrinsically from other brain structures (e.g., BLA, thalamus, and hypothalamus) (O’Mara, 2005). During early development, such glutamatergic input onto PVI are highly plastic as the organism adapts to the environment, potently altering regional E/I balance, and eventually triggering maturational and plasticity events termed the critical period (Hensch, 2004). However, it could remain a source of pathology if the heightened plasticity is not terminated properly, since excessive glutamatergic drive can lead to abnormal calcium accumulation in the fast-spiking PVI (Stanika et al., 2009), increased oxidative stress (Behrens et al., 2007), and increased metabolic demand (Do et al., 2009), all of which can lead to cell damage and even cell death (Berretta et al., 2001). This vulnerability continues until the closure of the critical period by the perineuronal nets (PNNs), a glycosaminoglycan matrix sheath that surrounds mainly PVI and stabilizes glutamatergic inputs to end the plastic phase, but also protect PVI from metabolic and oxidative damage (Cabungcal et al., 2013). PVIs mature at different rate across the brain, and since PNNs also form at different rates in a region-specific manner, neural system typically manifest sequential onset of diverse types of critical periods (Toyoizumi et al., 2013). Thus, we hypothesize that during early development, when PNNs are not yet fully formed, aberrant excitation onto PVIs in the vHipp, for example, could lead to cell damage, producing pathological changes related to schizophrenia (Figure 2). Furthermore, in adult animals, similar insults to the PVIs would not produce similar results, as hippocampal PVIs are largely mature and hence protected by the PNNs from damage.
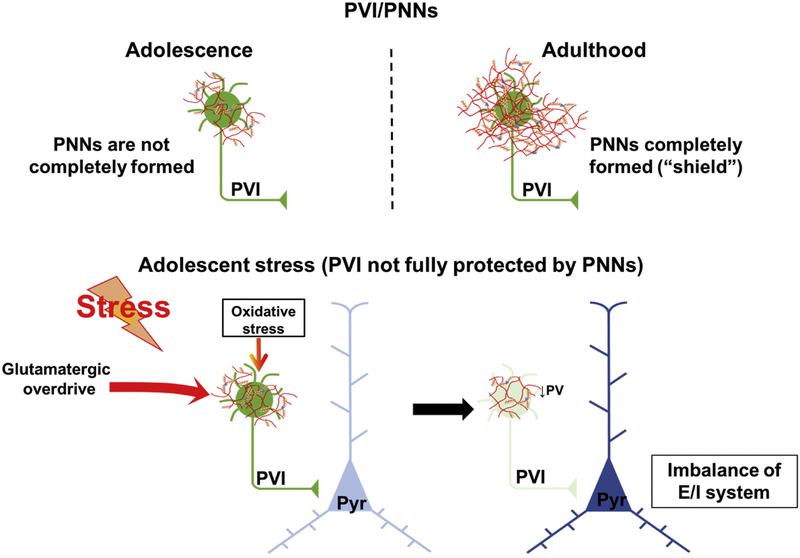
Figure 2 –
During critical periods parvalbumin interneurons (PVI) are more vulnerable to stress. This vulnerability continues until the closure of the critical period by the perineuronal nets (PNNs), a glycosaminoglycan matrix sheath that surrounds PVI to end the plastic phase, but also protect PVI from metabolic and oxidative damage. Thus, during periods when PNNs are not yet fully formed, such as adolescence, the exposure to stress can increase the oxidative stress and cause aberrant excitation onto PVIs in the vHipp, for example, leading to PVI damage/loss. This in turn results in deficits in the excitatory/inhibitory (E/I) balance producing pathological changes related to schizophrenia. In adult animals, similar insults to the PVIs would not produce the same results, as PVIs are largely protected by the PNNs from damage.
To test this, we exposed normal rats to the aforementioned combination of stressors during adolescence (PD31–40) or adulthood (PD65–74), and compared the stress-induced short- and long-term changes in the DA system activity in the VTA. Our findings indicate that the timing of stress seems to be a critical determinant of the pathology that is present in the adult. Whereas adolescent stress led to a persistent schizophrenia-like hyperdopaminergic state, adult stress produced a short-term hypodopaminergic state (Gomes et al. 2018) that is more consistent with animal models of depression (Belujon and Grace, 2014; Chang and Grace, 2014; Moreines et al., 2017). To elucidate the structural basis for the distinct stress responses observed, we examined the maturational profile of PVIs in the Hipp of animals stressed during adolescence. Confirming a previous report (Caballero et al., 2013), we found in naïve animals PVIs in the vSub continue to increase during PD31–51, suggesting a relatively immature state of the region during the stress period (PD31–40) (Zhu et al., 2018). In animals exposed to adolescent stress, a persistent decrease in the number of PVIs selectively in the vSub (but not in the dSub) was observed, suggesting potential PVI loss in the region. Furthermore, the number of mature PVIs (i.e., PVIs enwrapped by PNNs) also decreased after adolescent stress exposure, whereas adult stress does not seem to alter these markers (Zhu et al., 2018). The reduction in the number of PVIs had functional consequences, as an increase in the firing rate of the pyramidal neurons in these regions was observed (Gomes et al., 2018), which is consistent with the abnormal hippocampal activation in schizophrenia patients (Malaspina et al., 1999; Medoff et al., 2001; Schobel et al., 2013). All together, these results suggest that vulnerability to adolescent stress might indeed be a component of the critical period, and stress during this period produced distinct pathological profiles related to schizophrenia. It is noteworthy that the hippocampal PVI deficits produced by adolescent stress seemed to be region-specific, as dorsal Hipp (e.g., dorsal subiculum region) is relatively resilient to the stress effects. This is likely due to increased PNNs enwrapping in the dSub at an earlier time point.
If vulnerability to adolescent stress is indeed a critical period, or at least a critical-period-like process, one exciting possibility would be that such plastic events might be regulated by common mechanisms shared by critical periods across different brain regions (Hensch and Bilimoria, 2012). In fact, overlapping mechanisms underlying the regulation of critical period plasticity have been established from visual system and fear learning systems (Nabel and Morishita, 2013). Collectively, mechanisms that are involved in limiting critical period plasticity in adulthood are terms as plasticity “brakes” (Bavelier et al., 2010), including structural “brakes” such as formation of PNNs, molecular “brakes” such as myelin-related inhibitory signaling, and functional “brakes” such as downregulation of histone acetylation, and altered E/I balance (Nabel and Morishita, 2013). Importantly, lifting these “brakes” has been shown to reopen various forms of critical period plasticity in adulthood, resulting in an adolescent-like state (Nabel and Morishita, 2013). Therefore, to further test whether the observed adolescent vulnerability to stress is truly a critical period, we applied an established compound known to reopen the critical period, sodium valproate (VPA), in the adult and measured short and long-term stress consequences. Our results indicated that reopening the critical period of normal animals caused them to regain adolescent-like stress vulnerability, evident from the findings that stress in reopened animals induces increased VTA DA neuron population activity, vHipp hyperactivity, and reduction in numbers of PV-positive neurons (Gomes et al., 2018).
VPA has multiple mechanisms of actions, including the potentiation of GABA neurotransmission to enhance inhibitory function and enduring effects on gene transcription as a pan-HDAC inhibitor (Monti et al., 2009). HDAC inhibition is more likely to be of relevance to the observed action of re-opening of the critical period. In fact, in the primary visual cortex, enhancement of intercortical inhibition does not trigger plasticity in adults (Fagiolini and Hensch, 2000), but HDAC inhibition does (Lennartsson et al., 2015). To further test the relevance of HDAC inhibition in reopening the critical period, we utilized another potent and more selective pan-HDAC inhibitor, SAHA. SAHA has no documented action on inhibitory neurotransmission when used at the dose known to enhance cortical plasticity (Baroncelli et al., 2016), and indeed, in our study, SAHA-treated adult rats also regained adolescent-like stress vulnerability (Gomes et al., 2018). Altogether, the findings cited above indicate that adolescence is a critical period for stress vulnerability that can be reinstated (at least by HDAC inhibition) in adults. Based on these data, we posit that the interaction between stress and stress vulnerability might be an integral part of the pathophysiology of schizophrenia. Whether the onset of stress vulnerability is truly associated specifically with the critical period requires further elucidation; however, as a recent report from Smith et al. (Smith et al., 2018) addresses, schizophrenia patients are likely to express aberrant level of critical period genes, highlighting the importance of adding age- and development-specificity into future studies.
Conclusion
Converging evidence from clinical and basic science studies suggest that schizophrenia is a disorder caused by multiple interacting factors. Central to these is stress occurring during vulnerable periods. Thus, during the prepubertal period, hippocampal PVI are in a vulnerable state, and sufficient stress can lead to pathological changes including cell death of these interneurons. For stress to have this impact, it either has to be severe in nature, or experienced as more severe possibly due to inadequate stress regulation. Principle among the areas involved in stress regulation is the prelimbic prefrontal cortex, which has been shown to attenuate stress responses of the BLA (Rosenkranz and Grace, 2001, 2002). Therefore, there appears to be a balance, in which stress vulnerability and presence of stressors produce an additive effect with respect to risk for schizophrenia. Depression, which has a vulnerability period in adulthood, also can be triggered by severe stressors. Interestingly, it is known that both schizophrenia and depression run in the same families (Cross-Disorder Group of the Psychiatric Genomics, 2013). It may be that the common factor in both disorders is a genetic vulnerability to stress, such that if one can “survive” the critical period prepubertally one may be more vulnerable to depression as adults. For example, prenatal MAM exposure in rats leads to a schizophrenia phenotype, but if conversion is prevented (by relieving stress during adolescence), the adult is more susceptible to affective disorders. This is in agreement with studies showing that ultra-high risk patients (i.e., those showing attenuated psychotic signs) that do not transition to schizophrenia often show affective disorders as adults (Lin et al., 2015). Therefore, genetic susceptibility may not be directly related to schizophrenia, but instead to a vulnerability to environmental factors that can be expressed as schizophrenia or affective disorders depending on the staging of the stress exposure.
Acknowledgments
Role of funding source
This study was funded by the National Institutes of Health (NIH MH57440 to AAG).
The funding source provided salaries and supplies to construct the manuscript, and funded the work presented in the original papers cited in the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
AAG has received funds from Lundbeck, Pfizer, Otsuka, Lilly, Roche, Asubio, Abbott, Autofony, Janssen, Alkermes, Newron, and Takeda. FVG and XZ declare no conflict of interest.
References
- Arseneault L, Cannon M, Fisher HL, Polanczyk G, Moffitt TE, Caspi A, 2011. Childhood trauma and children’s emerging psychotic symptoms: A genetically sensitive longitudinal cohort study. Am J Psychiatry 168(1), 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroncelli L, Scali M, Sansevero G, Olimpico F, Manno I, Costa M, Sale A, 2016. Experience Affects Critical Period Plasticity in the Visual Cortex through an Epigenetic Regulation of Histone Post-Translational Modifications. J Neurosci 36(12), 3430–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglivo V, Cao B, Mwangi B, Bellani M, Perlini C, Lasalvia A, Dusi N, Bonetto C, Cristofalo D, Alessandrini F, Zoccatelli G, Ciceri E, Dario L, Enrico C, Francesca P, Mazzi F, Paolo S, Balestrieri M, Soares JC, Ruggeri M, Brambilla P, GET UP Group, 2018. Hippocampal Subfield Volumes in Patients With First-Episode Psychosis. Schizophr Bull 44(3), 552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK, 2010. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci 30(45), 14964–14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL, 2007. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science 318(5856), 1645–1647. [DOI] [PubMed] [Google Scholar]
- Belujon P, Grace AA, 2014. Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry 76(12), 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M, 2007. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci U S A 104(24), 10164–10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Lange N, Bhattacharyya S, Sebro R, Garces J, Benes FM, 2004. Long-term effects of amygdala GABA receptor blockade on specific subpopulations of hippocampal interneurons. Hippocampus 14(7), 876–894. [DOI] [PubMed] [Google Scholar]
- Berretta S, Munno DW, Benes FM, 2001. Amygdalar activation alters the hippocampal GABA system: “partial” modelling for postmortem changes in schizophrenia. J Comp Neurol 431(2), 129–138. [DOI] [PubMed] [Google Scholar]
- Brenner K, Liu A, Laplante DP, Lupien S, Pruessner JC, Ciampi A, Joober R, King S, 2009. Cortisol response to a psychosocial stressor in schizophrenia: blunted, delayed, or normal? Psychoneuroendocrinology 34(6), 859–868. [DOI] [PubMed] [Google Scholar]
- Caballero A, Diah KC, Tseng KY, 2013. Region-specific upregulation of parvalbumin-, but not calretinin-positive cells in the ventral hippocampus during adolescence. Hippocampus 23(12), 1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ, 2013. Early-life insults impair parvalbumin interneurons via oxidative stress: reversal by N-acetylcysteine. Biol Psychiatry 73(6), 574–582. [DOI] [PubMed] [Google Scholar]
- Cardno AG, Gottesman II, 2000. Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. Am J Med Genet 97(1), 12–17. [PubMed] [Google Scholar]
- Chang CH, Grace AA, 2014. Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol Psychiatry 76(3), 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics, C., 2013. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381(9875), 1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crush E, Arseneault L, Moffitt TE, Danese A, Caspi A, Jaffee SR, Matthews T, Fisher HL, 2018. Protective factors for psychotic experiences amongst adolescents exposed to multiple forms of victimization. J Psychiatr Res 104, 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Simon M, van der Hart MG, Schmelting B, Hesselink MB, Fuchs E, 2005. Chronic stress decreases the number of parvalbumin-immunoreactive interneurons in the hippocampus: prevention by treatment with a substance P receptor (NK1) antagonist. Neuropsychopharmacology 30(1), 67–79. [DOI] [PubMed] [Google Scholar]
- Devylder JE, Ben-David S, Schobel SA, Kimhy D, Malaspina D, Corcoran CM, 2013. Temporal association of stress sensitivity and symptoms in individuals at clinical high risk for psychosis. Psychol Med 43(2), 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M, 2009. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol 19(2), 220–230. [DOI] [PubMed] [Google Scholar]
- Du Y, Grace AA, 2013. Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology 38(10), 1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Grace AA, 2016. Amygdala Hyperactivity in MAM Model of Schizophrenia is Normalized by Peripubertal Diazepam Administration. Neuropsychopharmacology 41(10), 2455–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Chaddock CA, Winton-Brown TT, Bloomfield MA, Bhattacharyya S, Allen P, McGuire PK, Howes OD, 2013. Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol Psychiatry 74(2), 106–112. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK, 2000. Inhibitory threshold for critical-period activation in primary visual cortex. Nature 404(6774), 183–186. [DOI] [PubMed] [Google Scholar]
- Flagstad P, Mork A, Glenthoj BY, van Beek J, Michael-Titus AT, Didriksen M, 2004. Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative schizophrenia symptoms and alters amphetamine-induced dopamine release in nucleus accumbens. Neuropsychopharmacology 29(11), 2052–2064. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G, 1996. Interneurons of the hippocampus. Hippocampus 6(4), 347–470. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH, 2005. A new view on hypocortisolism. Psychoneuroendocrinology 30(10), 1010–1016. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, Barale F, Caverzasi E, McGuire P, 2012. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry 69(3), 220–229. [DOI] [PubMed] [Google Scholar]
- Gill KM, Grace AA, 2014. Corresponding decrease in neuronal markers signals progressive parvalbumin neuron loss in MAM schizophrenia model. Int J Neuropsychopharmacol 17(10), 1609–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, Winter C, Riva MA, Mortensen PB, Feldon J, Schedlowski M, Meyer U, 2013. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science 339(6123), 1095–1099. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM, 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A 101(21), 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FV, Grace AA, 2017a. Adolescent Stress as a Driving Factor for Schizophrenia Development-A Basic Science Perspective. Schizophr Bull 43(3), 486–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FV, Grace AA, 2017b. Prefrontal Cortex Dysfunction Increases Susceptibility to Schizophrenia-Like Changes Induced by Adolescent Stress Exposure. Schizophr Bull 43(3), 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FV, Rincon-Cortes M, Grace AA, 2016. Adolescence as a period of vulnerability and intervention in schizophrenia: Insights from the MAM model. Neurosci Biobehav Rev 70, 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FV, Zhu X, Grace AA, 2018. The impact of stress on the dopamine system is dependent on the state of the critical period of plasticity Program No. 449.07. 2018 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience. Online. [Google Scholar]
- Goto Y, Grace AA, 2006. Alterations in medial prefrontal cortical activity and plasticity in rats with disruption of cortical development. Biol Psychiatry 60(11), 1259–1267. [DOI] [PubMed] [Google Scholar]
- Grace AA, 2016. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci 17(8), 524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Gomes FV, 2018. The Circuitry of Dopamine System Regulation and its Disruption in Schizophrenia: Insights Into Treatment and Prevention. Schizophr Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK, 2004. Critical period regulation. Annu Rev Neurosci 27(1), 549–579. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Bilimoria PM, 2012. Re-opening Windows: Manipulating Critical Periods for Brain Development. Cerebrum 2012, 11. [PMC free article] [PubMed] [Google Scholar]
- Hoareau C, Hazane F, Le Pen G, Krebs MO, 2006. Postnatal effect of embryonic neurogenesis disturbance on reelin level in organotypic cultures of rat hippocampus. Brain Res 1097(1), 43–51. [DOI] [PubMed] [Google Scholar]
- Jagannath V, Theodoridou A, Gerstenberg M, Franscini M, Heekeren K, Correll CU, Rossler W, Grunblatt E, Walitza S, 2017. Prediction Analysis for Transition to Schizophrenia in Individuals at Clinical High Risk for Psychosis: The Relationship of DAO, DAOA, and NRG1 Variants with Negative Symptoms and Cognitive Deficits. Front Psychiatry 8, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HJ, Stergiakouli E, Tansey KE, Hubbard L, Heron J, Cannon M, Holmans P, Lewis G, Linden DE, Jones PB, Davey Smith G, O’Donovan MC, Owen MJ, Walters JT, Zammit S, 2016. Phenotypic Manifestation of Genetic Risk for Schizophrenia During Adolescence in the General Population. JAMA Psychiatry 73(3), 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, Hwang DR, Huang Y, Haber SN, Laruelle M, 2010. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry 67(3), 231–239. [DOI] [PubMed] [Google Scholar]
- Kelleher I, Keeley H, Corcoran P, Ramsay H, Wasserman C, Carli V, Sarchiapone M, Hoven C, Wasserman D, Cannon M, 2013. Childhood trauma and psychosis in a prospective cohort study: cause, effect, and directionality. Am J Psychiatry 170(7), 734–741. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Angermeyer M, Anthony JC, D.E.G. R, Demyttenaere K, Gasquet I, D.E.G. G, Gluzman S, Gureje O, Haro JM, Kawakami N, Karam A, Levinson D, Medina Mora ME, Oakley Browne MA, Posada-Villa J, Stein DJ, Adley Tsang CH, Aguilar-Gaxiola S, Alonso J, Lee S, Heeringa S, Pennell BE, Berglund P, Gruber MJ, Petukhova M, Chatterji S, Ustun TB, 2007. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry 6(3), 168–176. [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S, 2011. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res 131(1–3), 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennartsson A, Arner E, Fagiolini M, Saxena A, Andersson R, Takahashi H, Noro Y, Sng J, Sandelin A, Hensch TK, Carninci P, 2015. Remodeling of retrotransposon elements during epigenetic induction of adult visual cortical plasticity by HDAC inhibitors. Epigenetics Chromatin 8, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW, 2012. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 35(1), 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Girgis RR, Brucato G, Moore H, Provenzano F, Kegeles L, Javitt D, Kantrowitz J, Wall MM, Corcoran CM, Schobel SA, Small SA, 2018. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol Psychiatry 23(8), 1764–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Wood SJ, Nelson B, Beavan A, McGorry P, Yung AR, 2015. Outcomes of nontransitioned cases in a sample at ultra-high risk for psychosis. Am J Psychiatry 172(3), 249–258. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Weinberger DR, 1993. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology 9(1), 67–75. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA, 2007. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci 27(42), 11424–11430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Storer S, Furman V, Esser P, Printz D, Berman A, Lignelli A, Gorman J, Van Heertum R, 1999. SPECT study of visual fixation in schizophrenia and comparison subjects. Biol Psychiatry 46 (1), 89–93. [DOI] [PubMed] [Google Scholar]
- Mauritz MW, Goossens PJ, Draijer N, van Achterberg T, 2013. Prevalence of interpersonal trauma exposure and trauma-related disorders in severe mental illness. Eur J Psychotraumatol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ, 2010. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci 1186, 190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, Gray JD, 2016. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology 41(1), 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff DR, Holcomb HH, Lahti AC, Tamminga CA, 2001. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus 11 (5), 543–550. [DOI] [PubMed] [Google Scholar]
- Modinos G, Allen P, Grace AA, McGuire P, 2015. Translating the MAM model of psychosis to humans. Trends Neurosci 38(3), 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti B, Polazzi E, Contestabile A, 2009. Biochemical, molecular and epigenetic mechanisms of valproic acid neuroprotection. Curr Mol Pharmacol 2(1), 95–109. [DOI] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA, 2006. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry 60(3), 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreines JL, Owrutsky ZL, Grace AA, 2017. Involvement of Infralimbic Prefrontal Cortex but not Lateral Habenula in Dopamine Attenuation After Chronic Mild Stress. Neuropsychopharmacology 42(4), 904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton PR, 2013. Neurostereology: unbiased stereology of neural systems. John Wiley & Sons. [Google Scholar]
- Nabel EM, Morishita H, 2013. Regulating critical period plasticity: insight from the visual system to fear circuitry for therapeutic interventions. Front Psychiatry 4, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenadic I, Maitra R, Basu S, Dietzek M, Schönfeld N, Lorenz C, Gussew A, Amminger GP, McGorry P, Reichenbach JR, Sauer H, Gaser C, Smesny S, 2015. Associations of hippocampal metabolism and regional brain grey matter in neuroleptic-naïve ultra-high-risk subjects and first-episode schizophrenia. Eur Neuropsychopharmacol 25(10), 1661–1668. [DOI] [PubMed] [Google Scholar]
- O’Mara S, 2005. The subiculum: what it does, what it might do, and what neuroanatomy has yet to tell us. J Anat 207(3), 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbrich HG, Braak H, 1985. Ratio of pyramidal cells versus non-pyramidal cells in sector CA1 of the human Ammon’s horn. Anat Embryol (Berl) 173(1), 105–110. [DOI] [PubMed] [Google Scholar]
- Owens DG, Miller P, Lawrie SM, Johnstone EC, 2005. Pathogenesis of schizophrenia: a psychopathological perspective. Br J Psychiatry 186, 386–393. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Chittajallu R, Craig MT, Tricoire L, Wester JC, McBain CJ, 2017. Hippocampal GABAergic Inhibitory Interneurons. Physiol Rev 97(4), 1619–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LK, Seidman LJ, 2008. Emotion processing in persons at risk for schizophrenia. Schizophr Bull 34(5), 888–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin S, Lungu O, Tikasz A, Mendrek A, 2017. Abnormal effective fronto-limbic connectivity during emotion processing in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 72, 1–8. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Bechard-Evans L, Boekestyn L, Iyer SN, Pruessner JC, Malla AK, 2013. Attenuated cortisol response to acute psychosocial stress in individuals at ultra-high risk for psychosis. Schizophr Res 146(1–3), 79–86. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Cullen AE, Aas M, Walker EF, 2017. The neural diathesis-stress model of schizophrenia revisited: An update on recent findings considering illness stage and neurobiological and methodological complexities. Neurosci Biobehav Rev 73, 191–218. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Iyer SN, Faridi K, Joober R, Malla AK, 2011. Stress and protective factors in individuals at ultra-high risk for psychosis, first episode psychosis and healthy controls. Schizophr Res 129(1), 29–35. [DOI] [PubMed] [Google Scholar]
- Romeo RD, 2017. The impact of stress on the structure of the adolescent brain: Implications for adolescent mental health. Brain Res 1654(Pt B), 185–191. [DOI] [PubMed] [Google Scholar]
- Romeo RD, McEwen BS, 2006. Stress and the adolescent brain. Ann N Y Acad Sci 1094, 202–214. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA, 2001. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci 21(11), 4090–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA, 2002. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci 22(1), 324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics, C., 2014. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511(7510), 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, Inbar BP, Corcoran CM, Lieberman JA, Moore H, Small SA, 2013. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 78(1), 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Foster DL, 2004. The neural basis of puberty and adolescence. Nat Neurosci 7(10), 1040–1047. [DOI] [PubMed] [Google Scholar]
- Smith MR, Readhead B, Dudley JT, Morishita H, 2018. Critical period plasticity-related transcriptional aberrations in schizophrenia and bipolar disorder. Schizophr Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, 2000. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24(4), 417–463. [DOI] [PubMed] [Google Scholar]
- Stanika RI, Pivovarova NB, Brantner CA, Watts CA, Winters CA, Andrews SB, 2009. Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity. Proc Natl Acad Sci U S A 106(24), 9854–9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoizumi T, Miyamoto H, Yazaki-Sugiyama Y, Atapour N, Hensch TK, Miller KD, 2013. A theory of the transition to critical period plasticity: inhibition selectively suppresses spontaneous activity. Neuron 80(1), 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotman HD, Holtzman CW, Walker EF, Addington JM, Bearden CE, Cadenhead KS, Cannon TD, Cornblatt BA, Heinssen RK, Mathalon DH, Tsuang MT, Perkins DO, Seidman LJ, Woods SW, McGlashan TH, 2014. Stress exposure and sensitivity in the clinical high-risk syndrome: initial findings from the North American Prodrome Longitudinal Study (NAPLS). Schizophr Res 160(1–3), 104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang M, 2000. Schizophrenia: genes and environment. Biol Psychiatry 47(3), 210–220. [DOI] [PubMed] [Google Scholar]
- van der Velde J, Opmeer EM, Liemburg EJ, Bruggeman R, Nieboer R, Wunderink L, Aleman A, 2015. Lower prefrontal activation during emotion regulation in subjects at ultrahigh risk for psychosis: an fMRI-study. NPJ Schizophr 1, 15026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Kenis G, Rutten BP, 2010. The environment and schizophrenia. Nature 468(7321), 203–212. [DOI] [PubMed] [Google Scholar]
- Volk DW, Lewis DA, 2010. Prefrontal cortical circuits in schizophrenia. Curr Top Behav Neurosci 4, 485–508. [DOI] [PubMed] [Google Scholar]
- Vargas T, Dean DJ, Osborne KJ, Gupta T, Ristanovic I, Ozturk S, Turner J, van Erp TGM, Mittal VA, 2018. Hippocampal Subregions Across the Psychosis Spectrum. Schizophr Bull 44(5), 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Trotman HD, Pearce BD, Addington J, Cadenhead KS, Cornblatt BA, Heinssen R, Mathalon DH, Perkins DO, Seidman LJ, Tsuang MT, Cannon TD, McGlashan TH, Woods SW, 2013. Cortisol levels and risk for psychosis: initial findings from the North American prodrome longitudinal study. Biol Psychiatry 74(6), 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JJ, Chohan MO, Slifstein M, Kegeles LS, Moore H, Abi-Dargham A, 2017. Pathway-Specific Dopamine Abnormalities in Schizophrenia. Biol Psychiatry 81(1), 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhr M, Orduz D, Gregory P, Moreno H, Khan U, Vörckel KJ, Wolfer DP, Welzl H, Gall D, Schiffmann SN, Schwaller B, 2015. Lack of parvalbumin in mice leads to behavioral deficits relevant to all human autism core symptoms and related neural morphofunctional abnormalities. Transl Psychiatry 5, e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell’Olio M, Francey SM, Cosgrave EM, Killackey E, Stanford C, Godfrey K, Buckby J, 2005. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry 39(11–12), 964–971. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Reynolds GP, 2002. A selective decrease in the relative density of parvalbuminimmunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res 55(1–2), 1–10. [DOI] [PubMed] [Google Scholar]
- Zhu X, Gomes FV, Grace AA, 2018. Adolescent stress during critical periods alters the developmental trajectory of hippocampal parvalbumin (PV) interneurons and recapitulates circuit dysfunction implicated in schizophrenia Program No. 337.10. 2018 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience. Online. [Google Scholar]
- Zimmerman EC, Bellaire M, Ewing SG, Grace AA, 2013. Abnormal stress responsivity in a rodent developmental disruption model of schizophrenia. Neuropsychopharmacology 38(11), 2131–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]