Abstract
There are now large-scale data on which common genetic variants confer risk for attention deficit hyperactivity disorder (ADHD). Here, we use mediation analyses to explore how cognitive and neural features might explain the association between common variant (polygenic) risk for ADHD and its core symptoms. In total, 544 participants participated (mean 21 years, 212 [39%] with ADHD], most with cognitive assessments, neuroanatomic imaging and imaging of white matter tract microstructure. We found that polygenic risk for ADHD was associated with symptoms of hyperactivity-impulsivity but not inattention. This association was mediated across multiple PRS thresholds by white matter microstructure, specifically by axial diffusivity of the right corona radiata, (maximum indirect effect β = −0.034 [CI. −0.065 to −0.01), by thickness of the left dorsomedial prefrontal [β = −0.029; CI −0.061 to −0.0047]) and area of the right lateral temporal cortex [β = 0.024; CI 0.0034 to 0.054]). Additionally, modest serial mediation was found, mapping a pathway from polygenic risk, to white matter microstructure of the anterior corona radiata, then cognition (working memory, focused attention), and finally to hyperactivity-impulsivity (working memory β = −0.014 [CI. −0.038 to −0.0026]; focused attention β = −0.011 [CI. −0.033 to −0.0017]). These mediation pathways were diagnostically specific and were not found for polygenic risk for ASD or schizophrenia. In conclusion, using a deeply phenotyped cohort, we delineate a pathway from polygenic risk for ADHD to hyperactive-impulsive symptoms through white matter microstructure, cortical anatomy and cognition.
Keywords: Polygenic risk, cognition, brain, white matter tracts, ADHD, developmental disorders
Introduction
A landmark in the genomics of attention deficit hyperactivity disorder (ADHD) was recently attained with the release of the Psychiatric Genomics Consortium genome wide association study (GWAS) of 20,183 individuals with ADHD and 35,191 controls1. This provides large-scale data on how common genetic variations are associated with the disorder. This advance also prompts the question of how common variants act to create the cardinal symptoms of the disorder, namely hyperactivity-impulsivity and inattention. The symptoms of ADHD are complex behavioral traits that likely reflect the effect of thousands of variants en masse, each conferring small amounts of risk, not just those surpassing a genome wide level of significance. Using GWAS data, an index of an individual’s genomic risk, often called the polygenic risk score (PRS) can be calculated as the sum of ADHD-associated alleles across the genome, weighted by their effect size2, 3. This polygenic risk score can be used as a starting point to examine the mechanisms that might link common genetic variant risk with symptoms.
Here, we ask which neural and cognitive features might explain the association between polygenic risk and ADHD symptoms by using mediation analyses. We first consider the microstructural properties of the white matter tracts. These tracts constitute the structural connections within the neural systems that support ADHD-related cognitive processes. ADHD is also increasingly conceptualized as the result of disrupted development of structural connectivity – a ‘developmental dysconnectome’4, 5. In support of this concept, anomalies in white matter microstructure have been associated with ADHD symptom severity in children and adults6, 7. Meta-analyses point most robustly to anomalies within the corona radiata, corpus callosum, and cerebellum, and a study of multiplex ADHD families found several of these tracts to be highly heritable6, 8, 9. We also examine cortical anatomy given repeated demonstrations of altered cortical structure in the disorder10–12. Cortical dimensions are also highly heritable and it is likely, though not yet established, that a genetic correlation will exist with ADHD13, 14.
We integrate cognition by asking if there is a causal chain that links polygenic risk with neural features, which in turn are linked to cognition and finally to symptoms. We selected candidate cognitive mediators based on two criteria: association with ADHD, and significant heritability. Several domains meet these criteria: working memory, processing speed, attentional measures and general intelligence have all been implicated in the pathogenesis of the disorder15–18. All these cognitive skills also are significantly heritable and some show genetic overlap with the disorder19–21. Recently, measures of working memory and arousal were found to mediate the association between polygenic risk for ADHD and the disorder22. Here, we seek to replicate this initial finding and additionally consider if there are pathways from polygenic risk to symptoms that will incorporate both neural features- white matter microstructure or cerebral anatomy - and cognition.
Additionally, we determine if polygenic risk for autistic spectrum disorders (ASD) is associated with symptoms of ADHD and if this association is explained by similar neurocognitive mechanisms. We consider ASD as it overlaps substantially with ADHD, at phenotypic23–25, cognitive17, 26, 27. and genetic levels28–30. We also consider PRS for schizophrenia, which while a neurodevelopmental disorder, shows less overlap with ADHD that does ASD at clinical, cognitive and genomic levels31, 32. Thus, compared to ASD, we would predict little overlap between polygenic risks for ADHD and schizophrenia in association with cognitive and clinical features.
In summary, we delineate associations between the PRS for ADHD and symptoms of the disorder, asking if they are mediated through neural and cognitive factors. We draw contrasts with polygenic risks for other disorders, predicting some overlap with the polygenic risk for ASD, but not for schizophrenia. We advance the field by examining these multiple facets – polygenic risk, the brain, cognition and symptoms – all within the same deeply phenotyped cohort, using mediation analyses to identify neural and cognitive variables that lie in the causal sequence between polygenic risk and symptoms.
Methods
In all, 544 individuals participated. Our primary analyses were conducted on the largest subpopulation (489 white, non-Hispanic individuals) to avoid issues of population stratification. However, we also report on analyses that incorporated the second largest subpopulation of 55 individuals with African American ancestry – see Supplement A1 for the determination population substructure. The general inclusion criteria were (1) age above 3 years (2) Intelligence Quotient of 70 or greater33–36. General exclusion criteria were (1) neurological disorders known to affect movement, except tic disorders; (2) psychiatric disorders other than ADHD, oppositional defiant disorder, conduct disorder, post-traumatic stress disorder, autistic spectrum disorder, mood or anxiety disorders. Symptoms of inattention and hyperactivity-disorder were ascertained using clinician-administered structured interviews – Supplement A2. The participants originated from 330 families. Overall, 190 [39%] within the white, non-Hispanic subpopulation met criteria for ADHD. Details of medications and comorbid disorders are given in Supplemental Tables B1-B3. The procedures were approved by the Institutional Review Board of the National Human Genome Research Institute. All adult participants gave written consent. Parents or guardians of children gave written consent and children gave assent.
Neuroimaging
High resolution T1 weighted anatomic sequences were collected on a 3-T GE Signa scanner. Parameters and quality control measures are detailed in the Supplement A3, and included visual inspection by at least two trained, reliable raters of the ‘raw’ data and FreeSurfer cortical reconstructions (thickness and surface area). Following quality control, 325 of 472 scans were retained. Diffusion tensor imaging (DTI) data were acquired using a single-shot, dual-spin echo, echo-planar imaging sequence (see Supplement A3). Quality control measures included the reacquisition of corrupted data in real time, visual inspection, exclusion of participants with more than two corrupted volumes, outlying fractional anisotropy, axial, radial diffusivity metrics. Overall, 383 of the original 448 DTI data sets were retained. Although there were no significant correlations between residual motion, symptoms and diffusion parameters we nonetheless regressed out residual motion along with age and sex from diffusion measures and conducted analyses on the residuals. Tract-based spatial statistics were then applied to create a group mean image, the ‘skeleton’, which represents the center of all tracts in the study cohort. The skeleton was thresholded at a FA value of >0.2 and analyses were conducted on voxels within this skeleton37.
Cognitive assessment
Working memory spans were assessed through the number of correctly recalled digits/tapping patterns in original and reverse order38. Processing speed was assessed using the visual matching task which requires participants to circle two identical numbers under time pressure; and the decision speed task requires participants to match two images under time pressure (from the Woodcock Johnson III Test of Cognitive Abilities39). General intelligence was assessed using age appropriate version of the Wechsler scales33–36. Attentional processes were measured using the Conners’ Continuous Performance Test in which participants are asked to respond when presented with any letter except “X”40. We used a principal components analysis with a varimax rotation to reduce the CPT data dimensions, – see Supplement A4, Table B4. The first emergent first factor is held to represent focused attention, the second reflects sustained attention and the final factor reflects perseverative/impulsive responding41. Missing cognitive data were imputed (missingness ranged from 3% for processing speed to 14% for working memory) using an iterative robust model-based imputation approach (R Statistical Software IRMI);42. Participants taking psychostimulants were asked to stop the medication at least 24 hours before testing. These cognitive analyses included 490 participants.
Polygenic Risk Score Calculation
Genotyping was conducted on extracted DNA (details in Supplement A5) using the Illumina HumanOmniExpressExome-8v1–2 array with genome build GRCh37/hg19. Calls were made using GenomeStudio version 2.0.3 and exported to PLINK format using GenomeStudio’s PLINK Input Report Plug-in v2.1.4. SNPs were filtered to include only those (1) present in the PGC GWAS (2) with less than 10% missing genotypes (3) Minor Allele Frequency (MAF) >= .01, genotyped in more than 99% of participants, (4) and at Hardy-Weinberg equilibrium of 10^−5. In total, 544,897 SNPs were retained for PRS analysis.
The polygenic risk scores were calculated as the sum of disorder-associated alleles across the genome, weighted by effect sizes from relevant study group within the Psychiatric Genetics Consortium: for ADHD43; for ASD44; for schizophrenia45. PRSice was used46, with base SNP clumping to remove linkage disequilibrium (LD threshold = .1, distance threshold = 250Kb). Imputation was performed using data from the 1000 Genomes Project, which includes genetic data from 26 subpopulations (The 1000 Genomes Project Consortium, 2015). We included a range of PRS at increasingly liberal significance thresholds (p < 0.01, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5) as a significant part of heritability comes from a large number of common SNPs, each of which individually has too small of an effect to be detected at the stringent genome-wide significance level, even with current sample sizes46, 47. The Psychiatric Genomics Consortium data set that was used as the basis for the PRS did not contain the recently genotyped cohort. Data from this study are being deposited in dbGAP for those individuals who consented.
Statistical Analyses
Mixed model regression tested for associations between polygenic risk scores and symptoms. Inattention and hyperactivity-impulsivity were considered separately in view of their partly distinct genetic etiologies, cognitive substrates, comorbidities and dissociable developmental trajectories48–52. Mixed model regression (implemented in R package lme453) was used as it allows the inclusion of a random term for family identity to account for nuclear or extended familial relatedness. Age and sex were entered as covariates in all analyses involving working memory and measures of attention.
Mediation analyses (using the R package Mediation54) were used to parse the significant associations between polygenic risk scores, the candidate cognitive and neuroanatomic mediators and symptoms – detailed in Supplement A6. For the cognitive mediators, a bootstrapping approach with 10,000 resamples provided a bias corrected confidence interval for estimates. We tested the five levels of polygenic risk that were significantly associated with hyperactivity-impulsivity across six cognitive domains. To correct for this multiple testing, we applied a false discovery rate and indicate the results that survived this adjustment (at q<0.05).
In the neural analyses, imaging data was first residualized to remove the effects of age, sex, motion and quality control scores. Mediation analyses of the neuroanatomic and DTI data were conducted at the voxel level to identify clusters that significantly mediated (at p< 0.05, with 1000 bootstraps per voxel) the association between PRS and symptoms. To test significance, we ran 250 permutations of the data, holding constant participant and family identity, but shuffling without repetition the residualized voxel‐wise data. For each permutation iteration we obtained a spatial map of voxel‐wise mediation (and p‐values) under the null hypothesis. We then determined the largest cluster seen at a voxel‐wise p < .05 under each iteration. After running all permutations, we established a null distribution of maximal cluster size. From this null distribution, we then determine the probability of seeing the observed cluster. These analyses returned five clusters (three in DTI and two in anatomy) that showed significant mediation consistently across PRS thresholds. A false discovery rate procedure was applied to control for multiple testing (of the five levels of PRS across the five neural mediators), and we indicate the findings that survived this adjustment (at q<0.05).
We also tested biologically plausible serial mediation pathways that included neural and cognitive variables (specifically, polygenic risk → brain regions → cognition → symptoms). These serial mediation analyses were conducted for all PRS thresholds that were significantly associated with symptoms. Such serial mediation analyses are not supported in the mixed model framework we used but are feasible for unrelated individuals, using the PROCESS Macro55:, we thus used a cohort with only one member per family.
To check the robustness of findings, we repeated analyses (1) combining the two largest subpopulations; (2) entering psychostimulant medication as a covariate, and any psychotropic medication as a covariate; (3) excluding those who had comorbid disorders; (4) confining analyses to one member of each family, thus ensuring independence of observations.
Results
Polygenic risk and symptoms
Polygenic risk for ADHD across all PRS thresholds above p<0.1, was significantly associated with symptoms of hyperactivity-impulsivity (at fdr q<0.05), but not inattention – Table 1. Neither polygenic risk for ASD nor schizophrenia was associated with ADHD symptoms. Similar results held for the combined White, non-Hispanic and African American populations, with the exception of an association between the PRS for ASD and hyperactive-impulsive symptoms at thresholds p<0.2–0.5 – Supplemental Table B5.
Table 1:
Associations between symptoms and polygenic risk scores for ADHD, ASD and schizophrenia at a range of thresholds for all White, non-Hispanic participants (N=489). A similar pattern held for the combined White, non-Hispanic and African American subpopulations-Supplemental Table B2. Standardized beta values and standard error (SE) are given.
| ADHD | ASD | Schizophrenia | |||||
|---|---|---|---|---|---|---|---|
| Symptoms | PRS threshold | β (SE) | p-value | β (SE) | p-value | β (SE) | p-value |
| Hyperactivity-impulsivity | 0.01 | 0.048 (0.046) | 0.3 | 0.043 (0.046) | 0.35 | 0.0085 (0.047) | 0.86 |
| 0.05 | 0.073 (0.045) | 0.1 | 0.045 (0.045) | 0.32 | −0.0033 (0.047) | 0.94 | |
| 0.1 | 0.1 (0.045) | 0.02 | 0.044 (0.045) | 0.33 | 0.00034 (0.046) | 0.99 | |
| 0.2 | 0.11 (0.046) | 0.02 | 0.076 (0.045) | 0.095 | 0.043 (0.047) | 0.36 | |
| 0.3 | 0.11 (0.046) | 0.02 | 0.079 (0.045) | 0.082 | 0.05 (0.046) | 0.28 | |
| 0.4 | 0.099 (0.046) | 0.03 | 0.081 (0.045) | 0.074 | 0.052 (0.046) | 0.26 | |
| 0.5 | 0.11 (0.046) | 0.02 | 0.093 (0.045) | 0.04 | 0.058 (0.046) | 0.21 | |
| Inattention | 0.01 | 0.0048 (0.045) | 0.92 | 0.0022 (0.046) | 0.96 | −0.019 (0.047) | 0.68 |
| 0.05 | 0.033 (0.045) | 0.47 | 0.0028 (0.045) | 0.95 | −0.021 (0.047) | 0.66 | |
| 0.1 | 0.05 (0.045) | 0.27 | 0.0035 (0.045) | 0.94 | 0.0014 (0.046) | 0.98 | |
| 0.2 | 0.057 (0.046) | 0.21 | 0.033 (0.045) | 0.46 | 0.037 (0.046) | 0.43 | |
| 0.3 | 0.054 (0.046) | 0.24 | 0.04 (0.045) | 0.37 | 0.047 (0.046) | 0.31 | |
| 0.4 | 0.045 (0.046) | 0.33 | 0.037 (0.045) | 0.41 | 0.056 (0.046) | 0.23 | |
| 0.5 | 0.054 (0.046) | 0.24 | 0.042 (0.045) | 0.35 | 0.055 (0.046) | 0.23 | |
Neural mediators
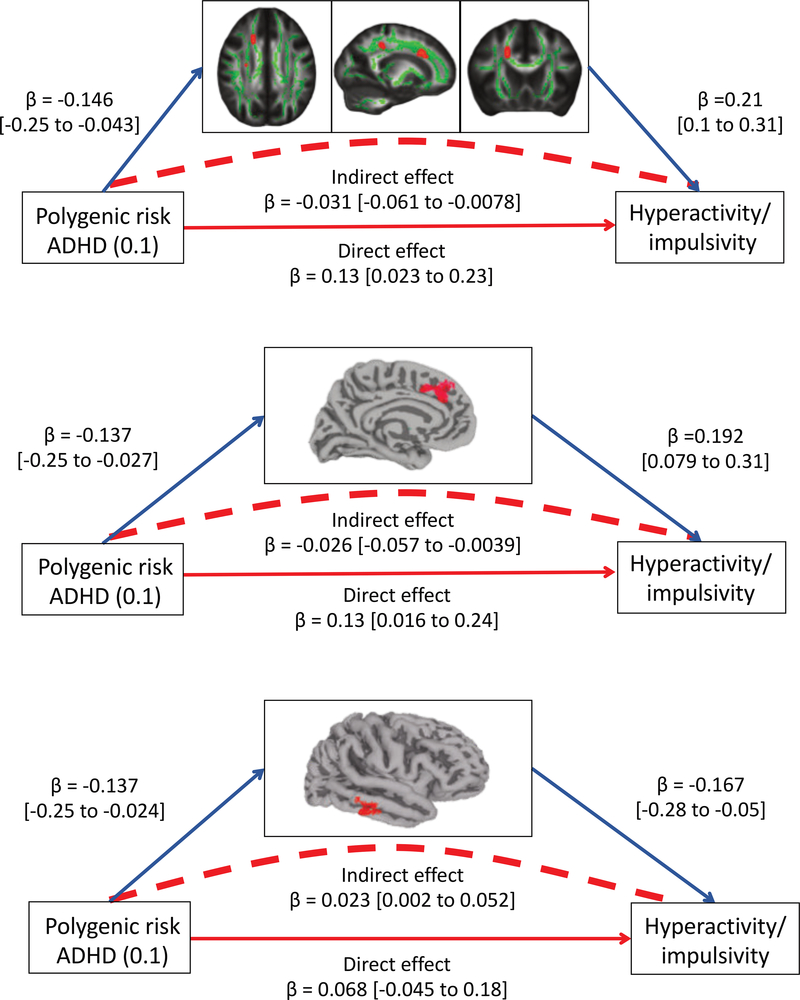
Using mediation analyses we found that some facets of white matter microstructure mediated the link between polygenic risk for ADHD and hyperactivity-impulsivity – Table 2, Figure 1. Specifically, axial diffusivity within regions of the right anterior and right superior corona radiata emerged as partial mediators across multiple PRS thresholds (at PRS<0.1, for anterior corona radiata: indirect effect β = −0.031 [CI. −0.061 to −0.0078], p value for cluster by permutation < 0.004, accounting for 29% of the genetic effect; for superior corona radiata: indirect effect β = −0.022 [CI. −0.049 to −0.0033], p = 0.02; 21% of the genetic effect).
Table 2.
White matter microstructure and cortical anatomy as mediators of the association between PRS for ADHD and symptoms of hyperactivity-impulsivity. Results are for the White non-Hispanic subpopulation (N = 352 for white matter microstructure; N= 301 for anatomic); similar results held when adding the African American subpopulation. Empirical p values for each cluster were determined by permutation.
| White matter microstructure | |||||
|---|---|---|---|---|---|
| Location | PRS threshold | MNI coordinates (x,y,z) | Cluster size (mm3) | β (CI) | p-value |
| Right anterior corona radiata | 0.1 | (18.5, 19.2, 31.5) | 104 | −0.031 (−0.061 to −0.0078) | < 0.004** |
| 0.2 | (18.5, 18.4, 32.2) | 112 | −0.033 (−0.064 to −0.0093) | < 0.004** | |
| 0.3 | (18.5, 18.4, 32.2) | 112 | −0.034 (−0.065 to −0.01) | < 0.004** | |
| 0.4 | (18.5, 18.4, 32.2) | 112 | −0.032 (−0.063 to −0.0093) | < 0.004** | |
| 0.5 | (18.5, 19.4, 32.3) | 88 | −0.025 (−0.052 to −0.0052) | 0.01** | |
| Right superior corona radiata | 0.1 | (27.5, −15.2, 25.2) | 64 | −0.022 (−0.049 to −0.0033) | 0.02** |
| 0.2 | (27.5, −12.3, 25.3) | 104 | −0.027 (−0.054 to −0.0065) | 0.004** | |
| 0.3 | (27.5, −13.6, 25.4) | 80 | −0.027 (−0.055 to −0.0063) | 0.01** | |
| 0.4 | (27.5, −13.9, 25.4) | 96 | −0.026 (−0.053 to −0.0058) | 0.008** | |
| 0.5 | (27.5, −15, 25.8) | 88 | −0.032 (−0.062 to −0.0082) | 0.01** | |
| Right anterior corona radiata and anterior thalamic radiation | 0.3 | (24.3, 20.6, 25.4) | 80 | −0.026 (−0.054 to −0.0048) | 0.01** |
| 0.4 | (24.7, 20.8, 25.2) | 80 | −0.025 (−0.054 to −0.0039) | 0.01** | |
| Cortical anatomy | ||||||
|---|---|---|---|---|---|---|
| Location | PRS threshold | MNI coordinates (x,y,z) | Cluster size (mm2) | β (CI) | p-value | |
| Left dorsomedial prefrontal cortical thickness | 0.1 | (−8, 40, 21) | 162.05 | −0.026 (−0.057 to −0.0039) | 0.04** | |
| 0.2 | (−5, 36, 33) | 367.09 | −0.029 (−0.061 to −0.0047) | 0.02** | ||
| 0.3 | (−6, 38, 26) | 175.07 | −0.024 (−0.054 to −0.002) | 0.06 | ||
| 0.4 | (−5, 33, 34) | 308.97 | −0.028 (−0.059 to −0.0048) | 0.01** | ||
| 0.5 | (−5, 27, 39) | 227.22 | −0.028 (−0.06 to −0.0056) | 0.03** | ||
| Right lateral temporal cortical surface area | 0.1 | (65, −30, −28) | 304.92 | 0.023 (0.002 to 0.052) | 0.04** | |
| 0.2 | (65, −30, −28) | 447.62 | 0.024 (0.0034 to 0.054) | 0.03** | ||
| 0.3 | (65, −29, −27) | 391.8 | 0.024 (0.003 to 0.054) | 0.02** | ||
| 0.4 | (66, −27, −27) | 207.12 | 0.022 (0.0022 to 0.052) | 0.09 | ||
FDR corrected, q < .05
Figure 1:
Figures showing mediation by white matter microstructure and cortical anatomy of the association betweenpolygenic risk (at PRS<0.1) for ADHD and hyperactive-impulsive symptoms. The indirect effect is the product of thepathway from polygenic risk to the candidate mediator, and the pathway from the mediator to hyperactivityimpulsivity.The direct effect of PRS on symptoms refers to the regression of PRS on symptoms after taking intoaccount the proposed mediator. The top panel shows mediation by white matter microstructure (axial diffusivity) ofthe regions in red. The middle panel illustrates mediation by the thickness of the left dorsomedial prefrontal cortex,and the lower panel, mediation by the surface area of the right lateral temporal cortex. Full results are given in Table 2.
Cortical dimensions also emerged as mediators – Table 2. For thickness, a region within the left dorsomedial prefrontal cortex emerged as a partial mediator (at multiple thresholds; for PRS<0.1 indirect effect β = −0.026 [CI. −0.057 to −0.0039], p value for cluster by permutation =0.04; 24% of the genetic effect). For surface area, a region within the right lateral temporal cortex fully mediated the association between polygenic risk for ADHD and hyperactivity-impulsivity (at multiple thresholds; for PRS<0.1 indirect effect β = 0.023 [CI. 0.002 to 0.052], p = 0.04; 22% of the genetic effect).
Cognitive mediators.
Three of the six cognitive variables emerged as significant mediators across multiple PRS thresholds –Table 3. Mediation was seen for working memory across PRS thresholds (for example, at PRS <0.1, indirect effect β = 0.027 [CI. 0.0051 to 0.053], p = 0.01, fdr adjusted p = 0.05; 28% of the genetic effect), IQ (indirect effect β = 0.019, [CI. 0.0036 to 0.042], p = 0.009, fdr adjusted p = 0.05; 20% of the genetic effect), and focused attention (indirect effect β = 0.016 [CI. 0.0021 to 0.037], p = 0.02, fdr adjusted p = 0.05; 17% of the genetic effect). In all cases, polygenic risk was no longer significantly associated with hyperactivity-impulsivity after each cognitive mediator was considered, implying each cognitive variable fully mediates the association between polygenic risk and symptoms. By contrast, processing speed, sustained attention and perseverative/impulsive responding did not emerge as significant mediators – Supplemental Table B7.
Table 3:
Working memory, IQ and focused attention as mediators of the relationship between PRS for ADHD and hyperactivity-impulsivity for the White non-Hispanic subpopulation (N = 438). The five levels of ADHD polygenic risk that were associated with hyperactivity-impulsivity were tested across six cognitive domains. Results for associations with impulsivity can be seen in Supplemental Table B6. Results for processing speed, sustained attention and perseverative/impulsive responding as mediators are given in Supplemental Table B7. The percent of the total effect accounted for by the mediation model is given where there was significant cognitive mediation.
| Cognitive Mediators | |||||||
|---|---|---|---|---|---|---|---|
| Working Memory | IQ | Focused Attention | |||||
| Symptoms | PRS threshold | β (CI) | p-value [%] | β (CI) | p-value [%] | β (CI) | p-value [%] |
| Hyperactivity-impulsivity | 0.1 | 0.027 (0.0051 to 0.053) | 0.01 [28%]** | 0.019 (0.0036 to 0.042) | 0.009 [20%]** | 0.016 (0.0021 to 0.037) | 0.02 [17%]** |
| 0.2 | 0.032 (0.0098 to 0.059) | 0.002 [31%]** | 0.02 (0.0041 to 0.042) | 0.008 [19%]** | 0.015 (0.0016 to 0.036) | 0.02 [14%]* | |
| 0.3 | 0.026 (0.0043 to 0.052) | 0.02 [25%]** | 0.02 (0.0038 to 0.042) | 0.009 [18%]** | 0.015 (0.0013 to 0.035) | 0.03 [13%]* | |
| 0.4 | 0.027 (0.0052 to 0.053) | 0.01 [28%]** | 0.021 (0.0039 to 0.043) | 0.007 [20%]** | 0.014 (0.0006 to 0.033) | 0.04 [13%]* | |
| 0.5 | 0.025 (0.0032 to 0.051) | 0.03 [24%]* | 0.019 (0.0031 to 0.04) | 0.01 [17%]** | 0.012 (−0.00018 to 0.031) | 0.05 [11%] | |
nominal p < 0.05
FDR corrected, q < .05
Serial mediation
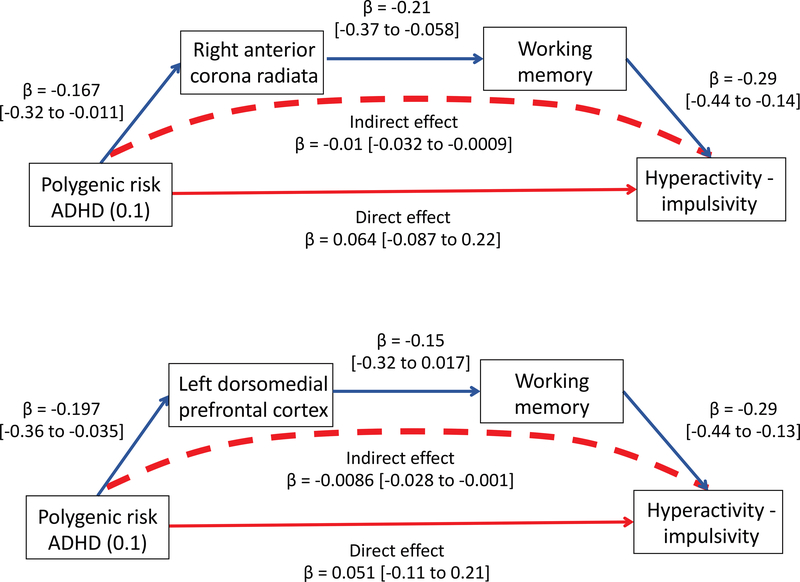
We tested for serial mediation, (asking if polygenic risk → brain regions → cognition → symptoms). Two pathways emerged – Figure 2. The first linked polygenic risk and symptoms through axial diffusivity of anterior corona radiata and then to working memory (at multiple thresholds; for PRS<0.1, indirect effect β = −0.01 [CI. −0.032 to −0.0009]; 17% of the genetic effect) and also through focused attention (β= −0.0082 [CI. −0.027 to −0.0005]; 14% of the genetic effect) – full results in Supplemental Table B8. A similar serial pathway was found acting though axial diffusivity in more superior regions of the corona radiata, linking to working memory and focused attention. Serial mediation through the thickness of the left dorsomedial prefrontal cortex was only found only with working memory (at multiple thresholds; PRS<0.1 β = −0.0086 [CI. −0.028 to −0.001]; 37% of the genetic effect) - Supplemental Table B9. There was no significant serial mediation from area of the lateral temporal cortex to cognition.
Figure 2:
Serial mediation of the association between polygenic risk for ADHD and hyperactivity-impulsivity.Polygenic risk was linked with neural features, cognition (here showing working memory) and hyperactivityimpulsivity.Top panel shows mediation by the axial diffusivity of a region in the right anterior coronaradiata, the lower by the thickness of the left dorsomedial prefrontal cortex. Serial mediation explained 17% and 37% of the total genetic effect respectively. Full results in Supplemental Tables B8 and B9.
Robustness analyses.
The pattern of results for cognitive and neural mediation mostly held when we combined the two largest subpopulations (white, non-Hispanic and African American participants) with the exception of mediation by cortical thickness) (see Supplemental Tables B10-B12). Results for cognitive and neural mediators also held when we entered psychostimulant medication as a covariate, and when we entered any psychotropic medication as a covariate. In analyses which excluded those with current comorbid disorders, mediation by cognitive factors, cortical thickness and white matter microstructure of the right superior corona radiata held; mediation by surface area did not. Finally, we repeated analyses retaining only the youngest member of each family, thus ensuring independence of observations. Again, the overall pattern of findings held for neural and cognitive variables with the exception of focused attention.
Discussion
Using a deeply phenotyped clinical cohort, we parse the neural and cognitive mechanisms that may explain the associations between polygenic risk for ADHD and its core symptoms. First, we identify mediating neural features: specifically, microstructural properties of white matter in the anterior corona radiata and the dimensions of the dorsomedial prefrontal and lateral temporal cortex. Secondly, we find mediation through some cognitive skills, including working memory, IQ and focused attention. Finally, using serial mediation, we map a possible causal chain, whereby polygenic risk impacts on white matter microstructure, that in turn is linked to cognitive features that associate with hyperactivity-impulsivity. The findings showed diagnostic specificity: neither polygenic risk for ASD nor not schizophrenia was robustly associated with ADHD symptoms in the largest, white, non-Hispanic subpopulation. The main findings held for polygenic risk scores defined across a range of thresholds and were robust to analyses which considered population structure and medication effects.
Polygenic risk scores and symptoms.
We find that polygenic risk scores for ADHD, defined using GWAS data from independent cohorts, was associated with symptoms of hyperactivity-impulsivity but not inattention. While hyperactivity-impulsivity and inattention have similar heritability estimates, their genetic correlation is around 0.6, indicating distinct as well as overlapping genetic contributors56. The two symptom domains differ in their forms of heritability: hyperactive-impulsive symptoms show more additive genetic influences (at 71%) than inattention (at 56%)57. Given that additive genetic influences are captured by common variant (SNP) heritability, this might explain why polygenic risk scores for the diagnosis of ADHD was associated with hyperactivity-impulsivity rather than inattention in our cohort.
Neural mediators.
We found that altered microstructure of white matter tracts, specifically axial diffusivity of the right anterior corona radiata, mediated the link between polygenic risk and symptoms. White matter tracts of this region connect the thalamus, anterior cingulate and lateral prefrontal cortex, comprising a network that supports a host of cognitive function including cognitive control and working memory. Thus, it is perhaps unsurprising that individual differences in white matter microstructure of the anterior corona radiata have been associated with working memory capacity58, 59 and aspects of cognitive control, such as resolving conflict between competing stimuli60. Additionally, meta-analyses have implicated anomalous white matter microstructure of this region in ADHD6. Here, we extend the literature by reporting that microstructural properties of white matter in the right corona radiata are part of the causal chain that may link polygenic risk with symptoms.
The dimensions of both the dorsomedial prefrontal cortex and right lateral temporal cortex also mediated the association between polygenic risk and symptoms. The dorsomedial prefrontal cortex plays a role in multiple cognitive functions implicated in ADHD, including cognitive control, decision making, social cognition and spatial working memory61–64. In this regard, it is noteworthy that there was serial mediation from the dimensions of the dorsomedial cortex through working memory to symptoms. The dorsomedial prefrontal and lateral temporal cortex are also functionally interconnected components of the default mode network, and are both robustly activated by making self-relevant, affective decisions65. It has been hypothesized that intrusions of the DMN into task-oriented processing results in some of the cognitive deficits seen in ADHD, including deficient sustained attention and distractibility66–68. Our current finding that common variant risk may act through the structural substrate of the DMN to produce symptoms is also consistent with recent family studies that find the DMN to be both a heritable and disorder-associated brain network8.
Cognitive mediators.
We replicate a recent report that working memory mediates the association between PRS for ADHD and symptoms22. This study also found mediation by measures of arousal which unfortunately we did not include. Why do some cognitive skills such as working memory mediate the relationship between polygenic risk for ADHD and symptoms of hyperactivity-impulsivity, whereas others such as processing speed do not? In the general population, memory and intelligence show substantial genetic correlation with one another but not with processing speed69. Working memory and IQ are also genetically correlated with ADHD, whereas a genetic overlap between ADHD and processing and attentional measures has not yet been established70, 71. Thus, our results are consistent with the presence of genetic correlations between ADHD and some cognitive skills (e.g. working memory and general intelligence) but not others (e.g. processing speed).
We also find that cognitive mediation was specific to symptoms of hyperactivity-impulsivity, not inattention. This is consistent with the concept of distinct genetic and cognitive pathways to each symptom domain. For example, twin studies find that the genetic overlap between reaction time variability and inattentive symptoms is largely distinct from the genetic overlap seen between measures of impulsive responding and symptoms of hyperactivity-impulsivity49, 72, 73. We similarly find pathways from polygenic risk to symptoms differ by domain: the cognitive skills that mediated the association between polygenic risk and hyperactivity-impulsivity did not act as mediators for inattention.
Limitations
Several limitations are noted. First, some participants were on psychotropic medications, mostly psychostimulants. While we controlled for the acute psychostimulant effects by stopping the medication at least 24 hours before testing, chronic effects are still possible. We note however, that the main results held when we excluded those on psychotropic. Some participants were related, but we controlled for the non-independence of their observations using a random term in mixed model regression analyses. We also considered the role of comorbidities and found that the pattern of results generally held when those with current comorbidities were excluded. Additionally, the results held when we included only one member from each family. The serial mediation analyses were conducted only unrelated individuals, and more robust estimates would be obtained if there were accepted mediation methods for familial data. The effect sizes for the serial mediation were modest and extension to larger cohorts is a priority. The findings on the largest subpopulation generally held when the second largest subpopulation (of African Americans) was added. Interestingly, an association with PRS for ASD and hyperactivity-impulsivity became stronger in analyses that included both subpopulations. The GWAS data from the PGC included a wide age range, and similar estimates for ADHD polygenic risk emerged from predominately childhood and adult cohorts. Nonetheless, it remains possible that the contribution of common variant risk to childhood ADHD (which includes both those who will remit and those who will persist as adults) might differ in part from its contribution to adult ADHD (which includes only those with symptom persisting from childhood Finally, while we tested many cognitive facets pertinent to ADHD, domains such as socio-emotional processing and response inhibition, which are also implicated in the disorder, were not included.
In conclusion, by leveraging multilevel data collected on a clinical cohort, we delineate pathways from polygenic risk for ADHD to hyperactive-impulsive symptoms through white matter microstructure, cortical anatomy and cognition.
Supplementary Material
Acknowledgements
The study was funded by the intramural programs of the National Human Genome Research Institute and the National Institute of Mental Health. We thank participants and their families.
Footnotes
All authors declare no conflict of interest.
References
- 1.Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E et al. Discovery Of The First Genome-Wide Significant Risk Loci For ADHD. bioRxiv 2017: 145581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Consortium IS. Common polygenic variation contributes to risk of schizophrenia that overlaps with bipolar disorder. Nature 2009; 460(7256): 748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wray NR, Goddard ME, Visscher PM. Prediction of individual genetic risk to disease from genome-wide. Genome Research 2007; 17: 1520–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Martino A, Fair DA, Kelly C, Satterthwaite TD, Castellanos FX, Thomason ME et al. Unraveling the miswired connectome: a developmental perspective. Neuron 2014; 83(6): 1335–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Human Brain Mapping 2010; 31(6): 904–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews 2012; 36(4): 1093–1106. [DOI] [PubMed] [Google Scholar]
- 7.Chuang T-C, Wu M-T, Huang S-P, Weng M-J, Yang P. Diffusion tensor imaging study of white matter fiber tracts in adolescent attention-deficit/hyperactivity disorder. Psychiatry Research: Neuroimaging 2013; 211(2): 186–187. [DOI] [PubMed] [Google Scholar]
- 8.Sudre G, Choudhuri S, Szekely E, Bonner T, Goduni E, Sharp W et al. Estimating the Heritability of Structural and Functional Brain Connectivity in Families Affected by Attention-Deficit/Hyperactivity Disorder. Jama psychiatry 2017; 74(1): 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Hu X, Ouyang L, He N, Liao Y, Liu Q et al. A systematic review and meta-analysis of tract-based spatial statistics studies regarding attention-deficit/hyperactivity disorder. Neuroscience & Biobehavioral Reviews 2016; 68: 838–847. [DOI] [PubMed] [Google Scholar]
- 10.Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biological Psychiatry 2007; 61(12): 1361–1369. [DOI] [PubMed] [Google Scholar]
- 11.Friedman LA, Rapoport JL. Brain development in ADHD. Current Opinion in Neurobiology 2015; 30: 106–111. [DOI] [PubMed] [Google Scholar]
- 12.Shaw P, Malek M, Watson B, Sharp W, Evans A, Greenstein D. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biological Psychiatry 2012; 72(3): 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Pol H, Hilleke E. Genetic influences on human brain structure: a review of brain imaging studies in twins. Human Brain Mapping 2007; 28(6): 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jansen AG, Mous SE, White T, Posthuma D, Polderman TJ. What twin studies tell us about the heritability of brain development, morphology, and function: a review. Neuropsychology review 2015; 25(1): 27–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology 2004; 18(3): 543. [DOI] [PubMed] [Google Scholar]
- 16.Huang-Pollock CL, Karalunas SL, Tam H, Moore AN. Evaluating vigilance deficits in ADHD: A meta-analysis of CPT performance. Journal of Abnormal Psychology 2012; 121(2): 360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry 2005; 44(4): 377–384. [DOI] [PubMed] [Google Scholar]
- 18.Boonstra AM, Oosterlaan J, Sergeant JA, Buitelaar JK. Executive functioning in adult ADHD: a meta-analytic review. Psychological medicine 2005; 35(8): 1097–1108. [DOI] [PubMed] [Google Scholar]
- 19.Martin J, Hamshere ML, Stergiakouli E, O’donovan MC, Thapar A. Neurocognitive abilities in the general population and composite genetic risk scores for attention‐deficit hyperactivity disorder. Journal of Child Psychology and Psychiatry 2015; 56(6): 648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bidwell L, Willcutt EG, DeFries JC, Pennington BF. Testing for neuropsychological endophenotypes in siblings discordant for attention-deficit/hyperactivity disorder. Biological Psychiatry 2007; 62(9): 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuntsi J, Eley TC, Taylor A, Hughes C, Asherson P, Caspi A et al. Co-occurrence of ADHD and low IQ has genetic origins. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics: the Official Publication of the International Society of Psychiatric Genetics 2004; 124(1): 41–47. [DOI] [PubMed] [Google Scholar]
- 22.Nigg JT, Gustafsson HC, Karalunas SL, Ryabinin P, McWeeney S, Faraone SV et al. Working Memory and Vigilance as Multivariate Endophenotypes Related to Common Genetic Risk for Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child & Adolescent Psychiatry 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Posthuma D, Polderman TJ. What have we learned from recent twin studies about the etiology of neurodevelopmental disorders? Current opinion in neurology 2013; 26(2): 111–121. [DOI] [PubMed] [Google Scholar]
- 24.Ronald A, Simonoff E, Kuntsi J, Asherson P, Plomin R. Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. Journal of Child psychology and Psychiatry 2008; 49(5): 535–542. [DOI] [PubMed] [Google Scholar]
- 25.Taylor M, Charman T, Robinson E, Plomin R, Happé F, Asherson P et al. Developmental associations between traits of autism spectrum disorder and attention deficit hyperactivity disorder: a genetically informative, longitudinal twin study. Psychological Medicine 2013; 43(8): 1735–1746. [DOI] [PubMed] [Google Scholar]
- 26.Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of child psychology and psychiatry 1996; 37(1): 51–87. [DOI] [PubMed] [Google Scholar]
- 27.Kercood S, Grskovic JA, Banda D, Begeske J. Working memory and autism: A review of literature. Research in Autism Spectrum Disorders 2014; 8(10): 1316–1332. [Google Scholar]
- 28.Martin J, Cooper M, Hamshere ML, Pocklington A, Scherer SW, Kent L et al. Biological overlap of attention-deficit/hyperactivity disorder and autism spectrum disorder: evidence from copy number variants. Journal of the American Academy of Child & Adolescent Psychiatry 2014; 53(7): 761–770. e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lionel AC, Crosbie J, Barbosa N, Goodale T, Thiruvahindrapuram B, Rickaby J et al. Rare copy number variation discovery and cross-disorder comparisons identify risk genes for ADHD. Science translational medicine 2011; 3(95): 95ra75–95ra75. [DOI] [PubMed] [Google Scholar]
- 30.Williams NM, Franke B, Mick E, Anney RJ, Freitag CM, Gill M et al. Genome-wide analysis of copy number variants in attention deficit hyperactivity disorder: the role of rare variants and duplications at 15q13. 3. American Journal of Psychiatry 2012; 169(2): 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Consortium C-DGotPG. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. The Lancet 2013; 381(9875): 1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamshere ML, Stergiakouli E, Langley K, Martin J, Holmans P, Kent L et al. Shared polygenic contribution between childhood attention-deficit hyperactivity disorder and adult schizophrenia. The British Journal of Psychiatry 2013; 203(2): 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wechsler D Wechsler abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 34.Wechsler D Wechsler Abbreviated Scale of Intelligence (2nd ed.). Psychological Corporation: San Antonio, TX, 2011. [Google Scholar]
- 35.Wechsler D, Scales PI, Index VC. Wechsler Preschool and Primary Scale of Intelligence—Fourth Edition. 2012.
- 36.Holdnack H Wechsler test of adult reading: WTAR. San Antonio, TX: The Psychological Corporation 2001. [Google Scholar]
- 37.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31(4): 1487–1505. [DOI] [PubMed] [Google Scholar]
- 38.Wechsler D Wechsler intelligence scale for children-WISC-IV. Psychological Corporation; 2003. [Google Scholar]
- 39.Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson tests of achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- 40.Conners CK. Conners’ continuous performance test. North Tonawanda, NY: Multi-Health Systems; 2000. [Google Scholar]
- 41.Egeland J, Kovalik-Gran I. Measuring Several Aspects of Attention in One Test: The Factor Structure of Conners’s Continuous Performance Test. Journal of Attention Disorders 2008; 13(4): 339–346. [DOI] [PubMed] [Google Scholar]
- 42.Templ M, Kowarik A, Filzmoser P. Iterative stepwise regression imputation using standard and robust methods. Comput Stat Data Anal 2011; 55(10): 2793–2806. [Google Scholar]
- 43.Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E et al. Discovery Of The First Genome-Wide Significant Risk Loci For ADHD. bioRxiv 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Consortium ASDWGotPG. PGC-ASD summary statistics from a meta-analysis of 5,305 ASD-diagnosed cases and 5,305 pseudocontrols of European descent (based on similarity to CEPH reference genotypes). March 2015.
- 45.Consortium SWGotPG, Ripke S, Neale BM, Corvin A, Walters JTR, Farh K-H et al. Biological Insights From 108 Schizophrenia-Associated Genetic Loci. Nature 2014; 511(7510): 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Euesden J, Lewis CM, O’Reilly PF. PRSice: Polygenic Risk Score software. Bioinformatics 2015; 31(9): 1466–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chatterjee N, Wheeler B, Sampson J, Hartge P, Chanock SJ, Park J-H. Projecting the performance of risk prediction based on polygenic analyses of genome-wide association studies. Nature genetics 2013; 45(4): 400–405e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsson H, Dilshad R, Lichtenstein P, Barker ED. Developmental trajectories of DSM-IV symptoms of attention-deficit/hyperactivity disorder: genetic effects, family risk and associated psychopathology. Journal of Child Psychology and Psychiatry 2011; 52(9): 954–963. [DOI] [PubMed] [Google Scholar]
- 49.Kuntsi J, Pinto R, Price TS, van der Meere JJ, Frazier-Wood AC, Asherson P. The separation of ADHD inattention and hyperactivity-impulsivity symptoms: pathways from genetic effects to cognitive impairments and symptoms. Journal of abnormal child psychology 2014; 42(1): 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willcutt EG, Pennington BF, Olson RK, DeFries JC. Understanding comorbidity: A twin study of reading disability and attention-deficit/hyperactivity disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 2007; 144B(6): 709–714. [DOI] [PubMed] [Google Scholar]
- 51.Wood AC, Rijsdijk F, Asherson P, Kuntsi J. Hyperactive-Impulsive Symptom Scores and Oppositional Behaviours Reflect Alternate Manifestations of a Single Liability. Behavior Genetics 2009; 39(5): 447–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiler MD, Bernstein JH, Bellinger DC, Waber DP. Processing Speed in Children With Attention Deficit/Hyperactivity Disorder, Inattentive Type. Child Neuropsychology 2000; 6(3): 218–234. [DOI] [PubMed] [Google Scholar]
- 53.Pinheiro J, Bates D, DebRoy S, Sakar D, Team RC. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 31–1311 2018. [Google Scholar]
- 54.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. mediation: R Package for Causal Mediation Analysis. Journal of Statistical Software; Vol 1, Issue 5 (2014) 2014. [Google Scholar]
- 55.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Publications; 2017. [Google Scholar]
- 56.Nikolas MA, Burt SA. Genetic and environmental influences on ADHD symptom dimensions of inattention and hyperactivity: A meta-analysis. Journal of Abnormal Psychology 2010; 119(1): 1–17. [DOI] [PubMed] [Google Scholar]
- 57.McLoughlin G, Ronald A, Kuntsi J, Asherson P, Plomin R. Genetic support for the dual nature of attention deficit hyperactivity disorder: Substantial genetic overlap between the inattentive and hyperactive–impulsive components. Journal of abnormal child psychology 2007; 35(6): 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muster R, Choudhury S, Sharp W, Sudre G, Shaw P. Mapping the neuroanatomic substrates of cognition in familial attention deficit hyperactivity disorder. Psychological Medicine In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olesen PJ, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Brain Res Cogn Brain Res 2003; 18(1): 48–57. [DOI] [PubMed] [Google Scholar]
- 60.Niogi SN, Mukherjee P, Ghajar J, McCandliss BD. Individual differences in distinct components of attention are linked to anatomical variations in distinct white matter tracts. Frontiers in neuroanatomy 2010; 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron 2006; 52(5): 921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Venkatraman V, Rosati AG, Taren AA, Huettel SA. Resolving response, decision, and strategic control: evidence for a functional topography in dorsomedial prefrontal cortex. Journal of Neuroscience 2009; 29(42): 13158–13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Horst NK, Laubach M. The role of rat dorsomedial prefrontal cortex in spatial working memory. Neuroscience 2009; 164(2): 444–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eickhoff SB, Laird AR, Fox PT, Bzdok D, Hensel L. Functional segregation of the human dorsomedial prefrontal cortex. Cerebral cortex 2014; 26(1): 304–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron 2010; 65(4): 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L et al. A lack of default network suppression is linked to increased distractibility in ADHD. Brain research 2009; 1273: 114–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wen X, Yao L, Liu Y, Ding M. Causal interactions in attention networks predict behavioral performance. The Journal of neuroscience 2012; 32(4): 1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosenberg MD, Finn ES, Scheinost D, Papademetris X, Shen X, Constable RT et al. A neuromarker of sustained attention from whole-brain functional connectivity. Nature neuroscience 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hagenaars SP, Harris SE, Davies G, Hill WD, Liewald DC, Ritchie SJ et al. Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N= 112 151) and 24 GWAS consortia. Molecular psychiatry 2016; 21(11): 1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, de Leeuw CA et al. GWAS meta-analysis (N= 279,930) identifies new genes and functional links to intelligence. bioRxiv 2017: 184853. [Google Scholar]
- 71.Martin J, Hamshere ML, Stergiakouli E, O’Donovan MC, Thapar A. Genetic risk for attention-deficit/hyperactivity disorder contributes to neurodevelopmental traits in the general population. Biological psychiatry 2014; 76(8): 664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuntsi J, Wood AC, Johnson KA, Andreou P, Arias-Vasquez A, Buitelaar JK et al. Separation of cognitive impairments in attention-deficit/hyperactivity disorder into 2 familial factors. Archives of General Psychiatry 2010; 67(11): 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wood A, Asherson P, Van Der Meere J, Kuntsi J. Separation of genetic influences on attention deficit hyperactivity disorder symptoms and reaction time performance from those on IQ. Psychological Medicine 2010; 40(6): 1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.