Abstract
Introduction
Mesoporous silica nanoparticles (MSNs) are outstanding nanoplatforms for drug delivery. Herein, the most recent advances to turn MSN-based carriers into minimal side effect drug delivery agents are covered.
Areas covered
This review summarizes the scientific advances dealing with MSNs for targeted and stimuli-responsive drug delivery since 2015. Delivery aspects to diseased tissues together with approaches to obtain smart MSNs able to respond to internal or external stimuli and their applications are here described. Special emphasis is done on the combination of two or more stimuli on the same nanoplatform and on combined drug therapy.
Expert opinion
The use of MSNs in nanomedicine is a promising research field because they are outstanding platforms for treating different pathologies. This is possible thanks to their structural, chemical, physical and biological properties. However, there are certain issues that should be overcome to improve the suitability of MSNs for clinical applications. All materials must be properly characterized prior to their in vivo evaluation; furthermore, preclinical in vivo studies need to be standardized to demonstrate the MSNs clinical translation potential.
Keywords: Mesoporous Silica Nanoparticles, Targeting, Stimuli-responsive Drug Delivery, Biomedical Applications
1. Introduction
Nowadays, mesoporous silica nanoparticles (MSNs) constitute advanced inorganic nanoplatforms with an enormous interest as drug delivery systems (DDS) [1]. The main strengths of mesoporous silica nanoparticles in comparison to others are: (1) a high drug loading capability due their high surface area (> 700 m2 g-1) and pore volume (> 0.6 cm3 g-1) [2,3], (2) a tunable mesopore size (2-10 nm) and pore shape/connectivity, (3) an affordable chemical surface functionalization [4], (4) a controllable/tunable degradability under biological environments [5,6], (5) a high biocompatibility both in vitro and in vivo [7] and (6) a high level of clearance and excretion [8]. Besides their known possibilities as conventional drug delivery carriers, MSNs and related hybrid particles are suitable candidates for incorporating most of nanotechnological breakthroughs, as will be reviewed along the manuscript. In fact, mesoporous silica has gone beyond being a simple material for building nanoparticles, it has become a widespread material for connecting different units into multifunctional and degradable nanocomposites [9].
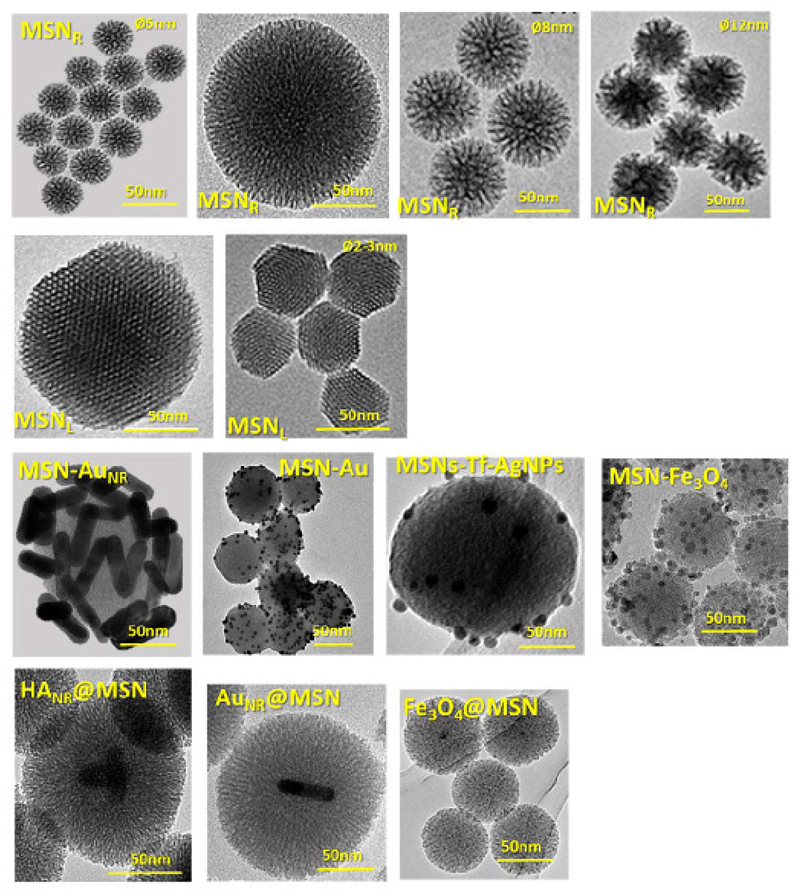
Figure 1 summarizes the main types of MSNs structures and their combination with some other inorganic nanomaterials. For instance, there are examples in which MSNs are coated with gold nanorods (MSN-AuNR), gold nanoparticles (MSN-AuNPs), silver nanoparticles (MSN-Ag) and magnetite nanocrystals (MSN-Fe3O4), whereas gold, iron oxide and upconversion nanocrystals have been also employed for the preparation of core@shell type structures.
Figure 1.
Transmission electron microscopy of several kinds of MSNs for different biomedical applications, showing (1st row) center-radial porosity (MSNR) with different particle and pore size (2-12 nm); (2nd row) longitudinal or 2D-hexagonal structure (MSNL) with different particle size (150-50 nm); (3rd row) MSNs coated with different inorganic nanoparticles such as gold nanorods (MSN-AuNR); gold nanoparticles (MSN-AuNPs), silver nanoparticles (MSN-AgNPs) and magnetite nanoparticles (MSN-Fe3O4); (4th row) core@shell structure with hydroxyapatite nanorods (HANR), gold nanorods (AuNR) and magnetite nanoparticles (Fe3O4) as core.
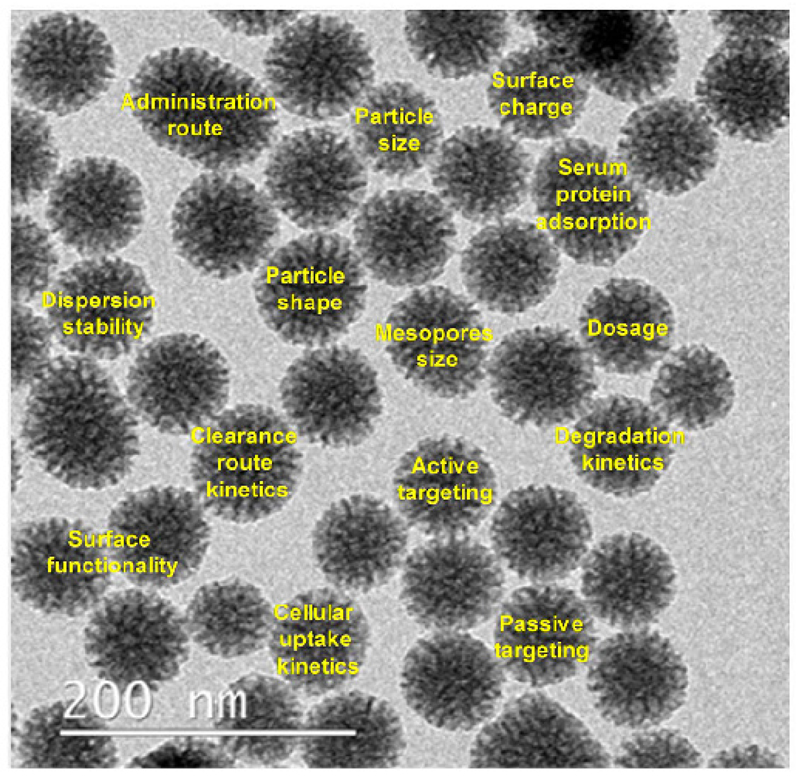
Beyond those advantages, MSNs can be fabricated in a relative large-scale synthesis showing a great variety of morphologies and functionalities, thus widening the range of possibilities in biomedical applications. In general, MSNs are easily synthetized via a modified Stöber method which, upon the appropriate modifications, permits to prepare different kinds of particles with variable sizes, mesopore sizes and connectivity [10]. In this sense, it is possible to prepare particles with either centered radial pore arrangement (MSNR) with three-dimensional structure and highly accessible mesopores [11] or 2D-hexagonal structures (honeycomb-like network) with parallel-longitudinal pores (MSNL). Moreover, during the nanoparticle design, the main factors that could influence into their biodistribution behavior should be taken into consideration (Figure 2) [12–14].
Figure 2.
Main factors influencing MSNs biodistribution and its accumulation in defense organs.
This manuscript arises from the need to update the state of the art in the use of MSNs and related nanosystems in the nanomedicine area since there has been a breakthrough in the last years in the versatility and multipurpose of these nanosystems [1]. Herein, we will only focus on the most recent advances reported for targeted and controlled release for drug delivery, although we are aware that this nanotechnology has clearly transcended into many other nanomedicine fields.
2. Selective targeting
MSNs tend to accumulate in body defense organs rich in macrophages (liver and spleen) leading to a poor accumulation in the target tissue as it happens with other nanoparticles [15]. Therefore, it is mandatory to increase the specificity towards the target tissue in order to enhance their therapeutic efficacy and hence to decrease their potential side effects [16,17].
In a conventional approach, nanocarriers are administered through the bloodstream and most of them tend to escape through aberrant neovasculature and accumulate into tumor areas. This is known as enhanced permeation and retention (EPR) effect and is the cause of the passive targeting. Once there, the active targeting based on receptor-ligand affinities is the responsible of the discrimination of diseased cells onto which the therapeutic effect would be exerted. Along this section, the most recent advances in targeting for MSNs will be tackled.
2.1. Passive targeting
Passive targeting is based on the EPR effect as mentioned above. Basically, it is based on the size of nanoparticles and their ability to extravasate through tumor vasculature, which is highly imperfect and permeable. This extravasation at the tumor would lead to a preferential accumulation into damaged tissues. In this case, since MSNs exhibit great drug loading capability, it is possible to achieve high concentration of drugs into the tumor tissue in comparison to the free drugs. However, this accumulation will only occur if MSNs can prevent fast renal clearance and uptake by the reticuloendothelial system [15]. In this sense, the non-specific adsorption of serum proteins onto the nanoparticles is highly co-related with their rapid clearance [15]. Different approaches to decrease the protein adsorption have been described based on the functionalization of the outer surfaces of MSNs, such as the functionalization with hydrophilic polymers as poly(ethylene glycol) (PEG) [18–20] or the zwitterionization of the surfaces through covalent attachment of functional groups with both positive and negative charges [21]. Today it is well-known that protein adsorption plays a pivotal role in the stealth properties [22] and affects the cell selectivity of active targeting of the MSNs [23].
In the present section the main factors that affect the biodistribution of MSNs are summarized. Properties such as size, shape, surface charge, and composition of the MSNs will influence the tissue accumulation and cellular uptake of the nanoparticles and will be described below [24,25].
2.1.1. Size and shape
In general terms, it is well-established that the optimum size of nanoparticles for biomedical application is between 10-300 nm. [1] The lower limit is set to avoid the fast-renal clearance while the upper limit is set to prevent embolisms due to aggregation into capillaries and alveoli. Numerous studies suggest that nanoparticles with a size below 100 nm exhibit optimal levels of cell internalization.
In addition, the shape of nanoparticles is also a key factor in both cell interaction and systemic biodistribution. Traditionally, spherical nanoparticles have been most employed in nanomedicine due to their relatively easy fabrication processes. However, non-spherical nanomaterials (i.e. rods, disks, cylinders and ellipsoids) show different behavior in terms of biocompatibility, biodistribution and clearance [25,26]. In this sense, rod-like MSNs have been studied to determine their destination in vivo. There was found that short-rods MSNs were retained by the liver, while long-rods MSNs were preferentially allocated in the spleen. [26]
In parallel, the effect of shape in the cellular uptake has been also studied, showing that long-rod shape displays better internalization [27]. Regarding their elimination, MSNs are mainly excreted by urine and faeces and, for a given diameter, the clearance is highly dependent on the shape. Unfortunately, there are factors beyond morphology that also affect the biodistribution and circulation time of MSNs, as there will be reviewed below.
2.1.2. Surface
Both chemical composition and surface charge play a pivotal role in the interaction with the physiological environment. These factors acquire great relevance in the case of MSNs which exhibit high surface area and pore volume. The challenges in designing MSNs are both to increase the blood circulation time and to enhance stability in physiological fluids in order to ensure their full functionality and efficacy along all their lifespan. Herein, it is also important to note that plasma proteins tend to be adsorbed onto MSNs throughout a non-specific adsorption, as it has been mentioned above. This effect, called opsonization, generates a new surface onto such particles, denoted as protein corona. This new protein surface becomes then the “visible” surface of the particle and, therefore, may affect the targeting efficiency, biodistribution, toxicity and thus the nanosystem efficacy. In the worst-case scenario, the protein corona would disrupt the uptake of nanoparticles and would trigger an immune response. Fortunately, opsonization can be avoided by creating strong hydration layers on the surface of MSNs. The most common strategy consists in decorating the nanoparticle surface with non-toxic hydrophilic polymers such as PEG in a process denoted as PEGylation. This process can be carried out by two different methodologies: covalent grafting or physical adsorption. Thus, PEGylation prevents the non-specific protein adsorption, which enhances the physiological stability and increases the blood circulation time. The stealth properties of PEGylated MSNs are strongly governed by the PEG size, surface chain density and conformation. It has been shown that molecular weights from 10,000 to 20,000 are the optimal for minimizing the non-specific adsorption of proteins [28]. However, the range of adsorption could also depend on the chemical strategy used to graft PEG to the MSNs surface. The covalently PEGylated MSNs present greater stealthiness, achieving higher blood circulation times and lower protein adhesion than the physically PEGylated MSNs.
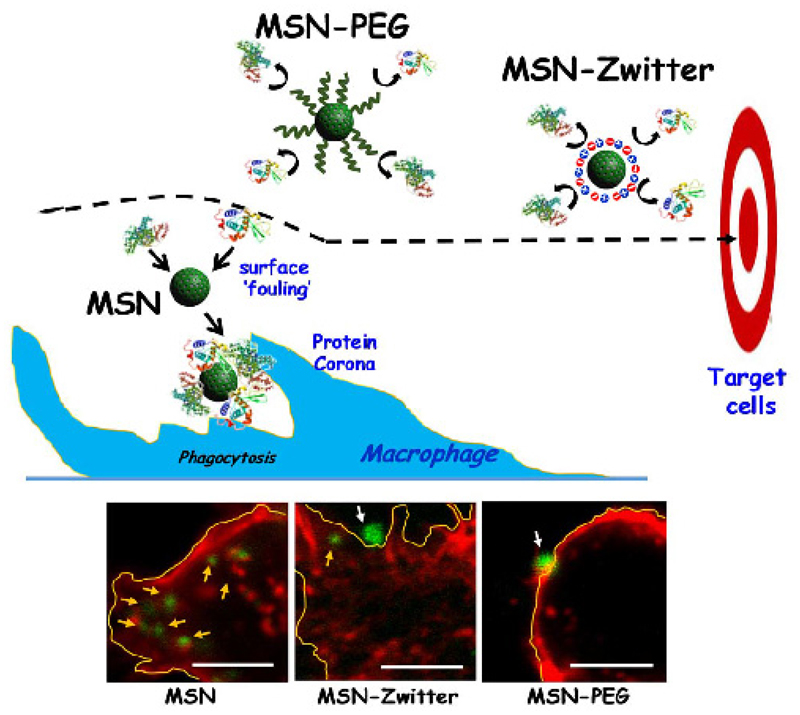
On other hand, zwitterionization has recently appeared as a powerful alternative to PEGlylation. A zwitterion surface can be defined as polyampholytes with the same number of positive and negative charges, which ensures their electrical neutrality [29]. These surfaces create a strongly fixed hydration layer forming a physical and energetic barrier that impedes non-specific adsorption of proteins [30]. Several strategies have been optimized to confer zwitterion properties to the MSNs surface. One of the most employed approaches is the covalent grafting with zwitterion polymers that present positive and negative charged groups within their backbone [31]. However, such strategy remarkably increases the hydrodynamic size of MSNs, which could represent a handicap for drug delivery applications. A possible alternative could be attaching small zwitterionic moieties such as 2-methacryloyloxyethyl phosphorylcholine. This approach was evaluated in a new multifunctional nanodevice based on polyethylenimine coated core@shell Fe3O4@MSNs. [32] The resulting nanodevice showed low protein adsorption values in Bovine Serum Albumin and Fetal Bovine Serum solutions, while allowed the co-delivery of two therapeutic agents, siRNA and daunorubicin. Additionally, our research group has also optimized a synthetic route with organosilanes throughout a very simple methodology.[33] This approach consists on the direct and simultaneously grafting of two organosilanes exhibiting functional groups with compensated positive and negative charges onto already prepared MSNs. Therefore, MSNs were functionalized via simultaneous direct post-grafting with 3-aminopropyl silanetriol and trihydroxysilylpropylmethylphosphonate, providing a zwitterion-like surface under physiological pH conditions. These zwitterion-like MSNs (MSN-Zwitter) were compared with PEGylated MSNs (MSN-PEG) using a PEG of similar length in terms of both non-specific protein adsorption and macrophage uptake. The results confirmed that both MSN-Zwitter and MSN-PEG displayed a significant reduction of serum protein adsorption and macrophages internalization with respect to unmodified MSNs. In the case of MSN-Zwitter, their reduction was up to 70-90% for protein adsorption and ca. 60% for macrophage uptake. Figure 3 displays a representative illustration showing both PEGylated and zwitterion strategies to increase the stealthy of MSNs.
Figure 3.
(Top) Schematic representation showing the more representative approaches described up to date, PEGylation and zwitterionization, to increase the resistance of non-specific protein adsorption and the stealthy to the macrophages. (Bottom) Confocal microscopy studies showing the macrophage-uptake of different MSN-type systems (bare MSNs, MSN-Zwitter and PEGylated MSN). The staining corresponds to cell-nuclei (DAPI, blue), cell membrane and cytoskeleton (phalloidin, red) and nanoparticles (fluorescein, green). Internalized nanoparticles are highlighted with yellow arrows, while those located in the outer area are marked with white arrows. Scale bar: 5 μm.
Besides PEG and zwitterions, an additional strategy for protecting the MSNs from the action of plasma proteins is the use of protocells. Briefly, protocells are lipid-coated mesoporous nanosystems which due to the presence of an outermost lipid layer are able to skip opsonization. Moreover, beyond the enhanced circulation time, those systems also show important advantages as their use overcomes the issues related to drug encapsulation, pore gating and targeting. Some differently examples of targeted protocells will be reviewed in the following sections. Furthermore, we recommend the review by Brinker and coworkers in reference [34] for the interested readers.
Besides the strategies aimed at reducing the interaction with serum proteins, another important strategy is to enhance the overall cellular uptake. This could be achieved by increasing the electrostatic interaction between the surface of the nanoparticle and the target cell membrane. Despite its non-specificity, this approach has been generally used by the scientific community for internalization in eukaryotic cells. Recently, internalization of MSNs in prokaryotic cells, such as Gram-negative bacteria, has been achieved through the external functionalization of MSNs with poly(propyleneimine) dendrimers of third generation. This nanosystem loaded with the antibiotic levofloxacin in the mesopores was highly effective in the elimination of Escherichia coli biofilms [35]. The decoration of MSNs with the polycationic dendrimers afforded high positive charge density through flexible macromolecules, providing the MSNs with bacterial membrane interaction capability and internalization thus increasing the therapeutic efficacy of the antibiotic.
2.2. Active targeting
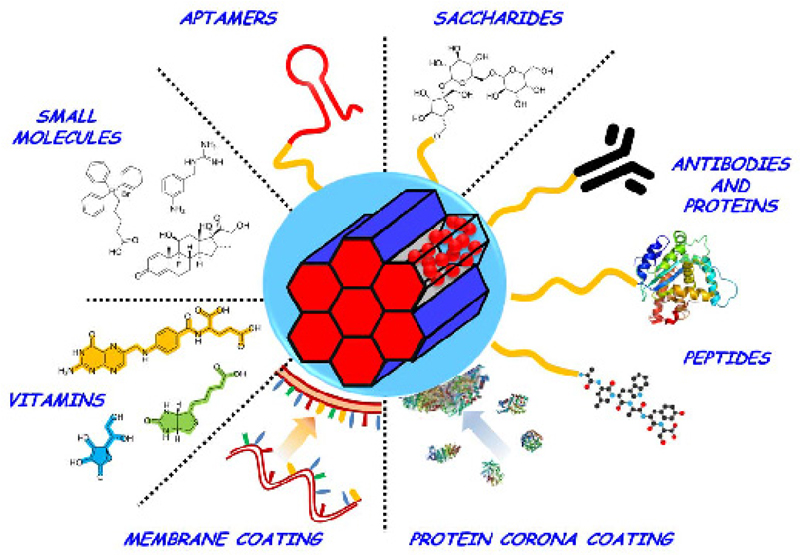
Despite significant advances made to promote the internalization of nanoparticles into tumor cells, biological recognition remains as the fundamental tool for the development of targeted therapies. This kind of targeting requires from surface modification of nanoparticles with ligands able to interact with membrane receptors overexpressed in diseased organs, tissues and cells. This strong ligand-receptor interaction will lead to a specific retention able to promote the endocytosis of nanoparticles into the target cells, complementing the EPR effect showed by most of solid tumors. In this approach, the efficiency of recognition and its latter internalization depend on several parameters, such as the abundance of the overexpressed receptors, the ligand density attached to the MSNs, and more importantly, the affinity between both counterparts. The two latter aspects are intrinsic to the nanosystems employed, so there must be accounted during their design and the strategy employed to conjugate ligands into nanoparticles. Among those strategies, amide formation and maleimide-thiol coupling are the most recurrent among the wide variety of strategies [1,36]. Conventional targeting labels include small molecules and biomacromolecules and their fragments; however, due to their interest, there have been reported examples beyond those categories, as summarized on Table 1 and Figure 4.
Table 1. Comprehensive list of active targeting ligands employed for favoring uptake of MSNs onto different cell lines.
| Substrate | Receptor | Target cell line(s) | Ref. |
|---|---|---|---|
| Antibodies | |||
| Anti-Mucin 1, TAB-004 | Mucin 1 glycoprotein | 4T1, MMT | [37] |
| Anti-TRC105 | CD105 | HUVEC, 4T1 | [38] |
| Ri7 | Transferrin (Mouse) | bEnd5, Neuro-2a | [39] |
| B220 | CD45R | CALM-AF10 | [40] |
| Anti-EGFR | HER1 | A549 | [41] |
| Trastuzumab | HER2 | SK-BR3, BT474 | [42–44] |
| Proteins | |||
| Transferrin | Transferrin receptor | BT-549, HeLa, PANC-1, HuH-7, HT1080 | [45–47] |
| ConA | Sialic acid receptor | HOS | [48] |
| Aleuria aurantia lectin | Sialyl-Lewis X antigen | DLD-1 | [50] |
| TEM1-scFv | TEM1 | Ovcar5 | [51] |
| Rec. GST-HER2-Afb | HER2 | SK-BR3 | [52] |
| Peptides | |||
| RGD family GFLGR7RGDS | αvβ3 integrins | U87-MG, MDA-MB-435, MDA-MB-231, HepG2, Neuro-2a, HeLa, SCC-7, HT-29, A375, MCF-7. |
[54] |
| NGR family | CD13 | BCEC, C6 | [55,56] |
| IL-13 | IL-13R-α2 | U251 | [57] |
| CDX | nAChR | BCEC | [58] |
| NAPamide | Melanocortin | Melanoma cell lines | [59] |
| Bld-1 | Formyl peptide receptor 1 | HT-1376, T-24 | [60] |
| cA6 | CD44 | MDA-MB-231, SK-BR3 | [61] |
| t-Lyp-1 | NRP1, NRP2 | MDA-MB 231, HUVEC | [62] |
| TAT peptides | Iα/β | HeLa; MCF-7/ADR | [63] |
| Polylysine | Unknown (electrostatic) | HeLa | [66] |
| BMP-2 derived | BMP-2 | BMSC | [68] |
| Aptamers | |||
| NCL/AS1411 | Nucleolin | MCF-7, SW480, PANC-1, PC3, MDA MB-231 | [71,72,75] |
| HB5 | HER2 | SK-BR3, MCF-7, MDA-MB-231, | [73] |
| EpCAM | EpCAM | HepG2, SW480, SW620, HT-29, HEK-293T, Y79, WERI-Rb1, Ramos B | [74–76] |
| MUC-1 | Mucin 1 glycoprotein | MDA-MB-231, C26, A549, MCF-7, CHO-K1, A2780 | [77] |
| YQ26 | CD105 | HEK-293, HUVEC, 4T1 | [78] |
| Saccharides | |||
| Hyaluronic acid | CD44, CD168, HARE | HepG2, MDA-MB-231 HEK-293, HTC-116, Ovcar8 | [80–83] |
| Small molecules | |||
| Folic Acid | FR-α | HepG2, PANC-1, U2Os, MDA-MB-231, SK-BR3, HeLa, MCF-7, MiaPaCa-2 | [84–87] |
| Biotin | BR | HOS, HeLa, MDA-MB-231, | [88–91] |
| Vitamin B12 | TCII-R | Not tested | [92] |
| Phenylboronic acid | Sialic acid receptor | HepG2 | [93] |
| TPP cations | Unknown (electrostatic) | Mitochondrion | [94,95] |
| Guanidinium cations | Unknown (electrostatic) | Mitochondrion | [96] |
| 3-ABG | NET | NB-1691 | [97,98] |
| Double targeting | |||
| RGD / TAT | αvβ3 integrins / Iα/β | HeLa | [100] |
| RGD / IL-13 | αvβ3 integrins/ IL-13R-α2 | C6, HUVEC | [101] |
| FA / Dex | FR-α / GCR | HeLa, HEK-293 | [102] |
| FA / TPP | FR-α / Mitochondrion | LNCaP | [104] |
| Biotin / TPP | BR / Mitochondrion | HOS | [105] |
Receptors: BR: biotin receptor; CD13 (aminopeptidase N, APN): cell membrane alanyl aminopeptidase; CD44 (P-glycoprotein 1, Pgp-1): Multifunctional Cell Surface Adhesion Receptor; CD45R (PTP): Protein tyrosine phosphatase; CD105 (Endoglin): type I membrane glycoprotein; CD168 (RHAMM, HMMR): Hyaluronan-mediated motility receptor; FR-α: Folic acid receptor; GCR: Glucocorticoid receptor; HARE (Stabilin-2): Human Hyaluronan Receptor for Endocytosis; HER1 (EGFR, ErbB1): human epidermal growth factor receptor 1; HER2: human epidermal growth factor receptor 2; HER2/neu (ErbB2, CD340): Tyrosine-protein kinase receptor; Iα/β: Importins α and β; IL-13R-α2: Interleukin-13 receptor α2; nAChR: Nicotine-Acetylcholine Receptor; NET: Norepinephrine transporter; NRP1 and NRP2: Neurophilins 1 and 2, coreceptors for vascular endothelial growth factor (VEGF); TEM1: antitumor endothelial marker 1; TCII-R: Transcobalamin 2 receptor. Cell Lines: Blood: Ramos: Burkitt lymphoma; Bone: BMSC: Bone Mesenchymal Stem Cells; HOS: Human osteosarcoma; Bladder: HT-1376: Bladder carcinoma; T-24: Bladder carcinoma; Brain: BCEC: brain capillary endothelial cells; NB-1691: Neuroblastoma; U87-MG: Human primary glioblastoma; U251: Human Astrocytoma; Breast: BT474: Human breast cancer cell line; BT-549: Human breast carcinoma cell line; MCF-7: Human breast cancer cell line; MCF-7/ADR: human breast cancer (doxorubicin resistant); MDA-MB-231: Human breast adenocarcinoma; SK-BR3: Human breast adenocarcinoma cell line; Cervix: HeLa: Human cervix epithelioid carcinoma; Intestine and Colon: C26: Human colorectal cancer cells; DLD-1: Colon adenocarcinoma; HT-29: Human colorectal adenocarcinoma; HCT-116: Human colon carcinoma; SW480: Colon adenocarcinoma; SW620: Colon adenocarcinoma; Epithelia: HT1080: Human fibrosarcoma; HUVEC: Human umbilical vein endothelial cell line; Eye: WERI-Rb1: Human Retinoblastoma; Kidney: HEK-293: Human embryonic kidney 293 cells; Liver: HepG2: Human hepatoblastoma-derived; HuH-7: Human hepatoma; Lung: A549: Human Lung carcinoma; Pancreas: MiaPaCa-2: Human pancreatic carcinoma; PANC-1: Human pancreatic carcinoma, epithelial-like cell line; Ovarian: A2780: human ovarian cancer cells (paclitaxel resistant); Ovcar: Human ovarian serous adenocarcinoma; Prostate: LNCap: androgen-sensitive human prostate adenocarcinoma; PC3: human prostate cancer; Skin: A375: Human amelanotic melanoma; MDA-MB-435: Amelanotic melanoma; SCC-7: Squamous cell carcinoma; Murine Lines: bEnd5: mouse brain endothelioma; C6: Rattus norvegicus brain glioma; CALM-AF10: Murine acute myeloid leukemia ; CHO-K1: Chinese hamster ovary; 4T1: Mus musculus mammary gland tumor; MMT: Murine mammary epithelial cancer cells; Neuro-2a: Mouse neuroblastoma. Abbreviations: Rec. GST-HER2-Afb: Recombinant glutathione-S-transferase-HER2-Affibody protein. Adapted and updated for the present work from reference [1].
Figure 4.
Different strategies to provide active targeting to MSNs.
2.2.1. Antibodies
Antibodies (ABs) are the most efficient and specific targeting ligands known, although they are the most sensitive, which increases the difficulty to handle them, and attach to MSNs [1]. Despite their drawbacks, their outstanding antigen-recognition capacity has seduced many research groups to use ABs as targeting components for raw MSNs and functional core@shell MSNs. To avoid the difficulty associated to their grafting, most of ABs are bound onto the surface of particles by electrostatic interactions or through covalent bonding under very mild and specific bond-forming reactions.
Another great disadvantage associated to the use of ABs is the possible induction of immune responses, which can lead to complete failure of the nanocarrier. To mitigate this issue, it is usual to find hydrophilic moieties –mainly PEG polymers– and cross-linkers connecting both subunits. This strategy provides an additional immune stealth to the system. The nanosystems reported by Vivero-Escoto and Cai can be cited as examples of the conjugation of ABs and MSNs through PEG spacers. In the first case, MSNs were connected to the TAB-004 anti-Mucin-1 antibody throughout a 2 kDa PEG and the resulting system proved to be highly sensitive to Muc-1 positive human breast cancers [37]. In the second example, biodegradable and large-pore dendritic MSNs were decorated with the TRC105 Anti-Endoglin –CD105– antibody [38], enabling the detection of particles via positron emission tomography imaging. For complementing this strategy, in which the SiO2 matrix is labelled, Fortin and coworkers designed a similar approach in which the surface of MSNs was decorated with the Ri7 anti-mouse transferrin receptor antibody and a Gd-based contrast agent for magnetic resonance imaging [39]. In this case the transferrin antigen, located at the brain, permitted to highly accumulate and detect the MSNs.
AB-coated MSNs have also proved to be highly valuable for the development of drug delivery agents. Along this line, there could be found a contribution by Buske and coworkers, who designed a system able to deliver daunorubicin to CALM-AF10 cells due to the presence of anti-B220 (CD45R) and anti-CD9 ABs [40]. Outstanding results employing ABs have also been obtained by the Vallet-Regí group with the use of a collagenase-based nanodriller with an anti-EGFR targeting [41]. In this system topotecan loaded MSNs based protocells were decorated with collagenase nanocapsules and ABs at their surface. The complete system proved to go across a thick collagen layer (which mimicked the extracellular tumor matrix) and to reach an underlying cell layer to deliver the loaded drug. ABs have also been employed for targeting silica nanocomposites. For instance, Mao and coworkers designed a system in which rod-like Bi2S3 core@shell-MSNs were chemically conjugated with trastuzumab [42]. The resulting system, able to attenuate X-rays due to the presence of a heavy cation, permitted to perform deep tissue computed tomography imaging while destroying tumors thanks to the drug loaded within the mesopores and the photothermal capacity of the core Bi2S3.
Besides common chemotherapeutics, there has also been reported the use of poly(ethyleneimine) (PEI)-coated MSNs decorated with ABs for the delivery of siRNAs. In this case, Yantasee and coworkers combined the trastuzumab targeting to HER2 with a HER2 siRNA [43], achieving a viability reduction on BT474 HER2 positive human breast cancer cells. Another interesting application of AB-targeted MSNs is their use as radiotherapy adjuvants, as reported by Tsuchimochi and coworkers. In their work, dendrimer coated MSNs decorated with anti-HER2 antibody were employed to selective internalize into SK-BR3 cells. Those cells showed increased apoptosis when X-rays were irradiated, suggesting a new feature for MSNs: sensitizing agent for radiotherapy [44].
2.2.2. Proteins
Certain proteins are involved in the accelerated metabolism of cancer cells and can be employed to target such cells. This is the case of transferrin (Tf), involved in the transportation of iron into cells, and Epidermal Growth Factor (EGF), which stimulates cell growth and differentiation. The high demand of those proteins by cancerous tissues leaded to the overexpression of specific ligands, which permitted to use them as targeting moieties [1,36]. Although Tf has been classically employed as uptake promoter, it also has been described as mesopore gating component as demonstrated by Vallet-Regí and Han groups. In the first example, a light-cleavable photolinker was employed to connect Tf to MSNs and this configuration permitted to efficiently target HT1080 cells and perform remote cleavage upon UV irradiation [45]. In the second example, Tf was bound to MSNs through an S-S bond [46], which allowed Tf disassembly under reductive conditions. This system was able to target HuH-7 cells and, once internalized, induce intracellular glutathione depletion plus doxorubicin (DOX) release, although with a limited apoptotic efficiency.
In a recent example by the Vallet-Regí group MSNs decorated with Tf act as nanoplatform for the nucleation and immobilization of AgNPs (MSNs-Tf-AgNPs). Due to the receptor mediated endocytic mechanism for the internalization of the nanosystem, the transported AgNPs dissolve in toxic Ag+ ions during the retention time within the lysosomes, following the “lysosome-enhanced Trojan horse effect”. Therefore, key proteins and transcripts involved in cell cycle regulation, cell proliferation and DNA damage are affected, as demonstrated by quantitative proteomics and validated by qPCR. This nanosystem safely delivers AgNPs to cancer cells with a therapeutic purpose [47].
In addition to Tf, other proteins have been described as targeting moieties for MSNs. This is the case of the lectin concanavalin A (ConA), able to recognize cell-surface glycans like sialic acid overexpressed in human osteosarcoma cells [48]. In one of the reported examples, an acid-labile moiety was employed to connect the carboxylate-capped MSNs with a polyacrylic acid coating layer which was further modified with the ConA. The acidic cleavage of the connecting unit permitted to disassemble the construct and release the DOX form the mesopores. Although not studied, the outstanding apoptotic efficiency of the complete system may be consequence of a possible combined effect of released ConA, which may induce an additional cell autophagy pathway in an intracellular environment [49]. Other strategy based on lectin proteins was reported by Martínez-Mañez and coworkers. In their example MSNs were bounded to Aleuria aurantia lectin throughout the Lewis X antigen trisaccharide [50]. This system proved to be disassembled in the presence of membrane’s Lewis X antigen, thus detaching the lectin gatekeeper and allowing the outflow of a fluorescent probe for colorectal DLD-1 adenocarcinoma cells. Among all the drawbacks of employing monoclonal Abs their high manufacturing costs and chemical sensitivity are two of the most important. Therefore, there is a growing interest in developing cheaper structures able to maintain the specificity of ABs, as it is the case of Affibodies (AfBs) and antibody single-chain variable fragments (scFv). Those are synthetic proteins that include the recognition regions of ABs and thus they are able to recognize antigens with high specificity. Those proteins have been also included in the development of new generation targeted devices based on MSNs. For example, Sheng and coworkers reported a scFv targeted system based on MSNs [51]. Their system proved high targeting efficiency against Ovcar5 cells, although it was not possible to determine the potential of such system because no drug was loaded into the MSNs.
One of the latest emerging disciplines in nanomedicine is the study of the protein corona that forms the immune system onto exogenous nanoparticles, as it has been highlighted before. This process leads to an assimilation of the particles by the macrophages for their subsequent elimination. Although this process is known to be avoided when hydrophilic coatings –PEG or zwitterions– are present on the particles, as mentioned above, a novel emerging possibility has come to light. In a visionary contribution, the Ryu group designed a recombinant protein with two different domains: an adhesive region plus a recognition domain. This protein was able to bind the surface of MSNs while exposing the bioactive fragment, therefore generating a targeted protein corona shield that retains targeting specificity [52]. The authors combined glutathione-S-transferase (GST), a well-known fusion-tag protein bound to a HER2 AfB and the resulting GST-HER2-AfB was able to coat MSNs through the GST region exposing the HER2 recognition tag which permitted to target the HER2-possitive SK-BR3 cells.
2.2.3. Peptides
Peptides are short amino acid sequences –less than 50– which may have similarities with their parent proteins. In some cases, the use of peptides has significant advantages like an easier synthesis and conjugation than proteins, together with a significant lower cost and reduced immune response. On the contrary, peptides do not have the outstanding antigen specificity showed by ABs; not being a limiting factor since hormones –some of them are peptides– show great specificity too. However, the interest of the peptides goes beyond the generation of targeted systems, as they also show unique properties for delivery and therapy [53]. Among recognition peptides, the RGD motif (Arg-Gly-Asp) is highly efficient to recognize αvβ3 integrins [1]. This capacity has been exploited to develop multifunctional peptides for the development of nanogates. One example is the system reported by He and coworkers, who employed a GFLGR7RGDS, cathepsin B-cleavable peptide sequence, to thread and release a β-cyclodextrin nanocap onto the mesopores [54]. Another all-purpose targeting peptidic motif is the NGR (Asn-Gly-Arg), which is able to target tumor neovasculature throughout the CD13 receptor. In this sense, a linear NGR peptide has been successfully employed for the delivery of polydopamine coated MSNs to glioma cells [55]. Apart from the linear configuration of peptides, it is usual to obtain better results with cyclic species due to their more restricted conformation. This was demonstrated by Kim and coworkers who were able to target CD13 receptor with a disulfide-bridged NGR containing peptide with a gatekeeping role [56].
Besides general low-specific peptides, there have been reported other sequences with high specificity towards receptors unique to certain cell lines. This are the cases of the Interleukin targeting peptide IL-13 (VDKLLLHLKKLFREGQFNRNFESIIICRDRTC), which has been employed to target gliomas in mice [57]; the CDX (FKESWREARGTRIERG) peptide with affinity for the nicotine-acetylcholine receptors present in brain [58]; the NAPAmide (Ac-NIe-Asp-His-d-Phe-Arg-Trp-Gly-Lys-NH2), an analog of the α-melanocyte stimulating hormone with great specificity towards malignant melanomas [59]; the Bld-1 (CSNRDARRC) peptide against cancerous bladder cell lines [60]; the cyclic A6 (CKPSSPPEECW) peptide able to achieve pore gating and target breast cell lines [61] and the tLyp-1 peptide (CGNKRTRGC) able to address neurophilin receptor present in many endothelial cells [62]. All those targeting peptides are summarized on Table 1.
As already reviewed, peptide targeting can be very a powerful tool to target nanocarriers to certain cells; however, the possibilities of peptides go beyond cell discrimination. For example the transactivator of transcription (TAT, GRKKRRQRRRPQ) peptide is known to induce particle translocation towards the nuclear membrane thanks to the importin transporters [63]. Apart from recognition by affinity, there are certain peptides capable of modifying the interaction between cells and drug delivery agents. One possibility is the functionalization of MSNs with peptides that may promote internalization [64,65]. Such is the case of polylysine, KALA (WEAKLAKALAKALAKHLAKALAKALKA) and fusogenic peptides which due to their cationic nature are able to electrostatically bind biological membranes and induce cellular uptake. Polylysine was successfully employed by Bravo et al. to create an enzyme-sensitive coating layer onto MSNs with favored uptake due to the cationic nature. This system was employed to internalize MSNs on HeLa cells and deliver the C9h therapeutic peptide (YVETLDDIFEQWAHSEDLK) known for activating the caspase-9 proapoptotic pathway [66]. For recent reviews dealing with the use of peptides as stimuli-responsive agents for controlled drug release in MSNs, please check reference [53]; and for an exhaustive review on cell-penetrating peptides, we recommend reference [67].
In addition to all the targeting features showed by peptides, there have also been reported some examples of double function peptides. This is the case of the osteogenic induction generated by dexamethasone (DEX)-loaded and peptide targeted MSNs reported by He [68]. In this model, a single peptide (KIPKASSVPTELSAISTLYL) was able to target the bone morphogenetic protein-2 (BMP-2) located in bone mesenchymal stem cells and once there, helped by the DEX release, induces the differentiation of stem cells towards osteoclasts and promote bone mineralization.
2.2.4. Aptamers
Aptamers are a special class of nucleic acids that have been evolved and selected to have high affinity for a particular template compound used for their synthesis. Among their advantages, they show (1) high affinity and specificity for their targets, (2) low complexity and relatively small size, (3) a facile synthesis and modification and (4) much lower immunogenicity than ABs. Besides, it is also remarkable that aptamers show great structural flexibility and thermal stability, which allow them to adapt to hidden epitopes and recover their active structure after thermal treatments. All those features turn aptamers into highly valuable components to develop gated and targeted nanosystems. For a recent review on the use of DNA and aptamers together with MSNs, please check reference [69]; and for an excellent up to date review on aptamer-guided nanomedicines for anticancer drug delivery, please check reference [70].
Regarding their use as targeting agents, there have been reported the use of MSNs in combination with nucleolin aptamers (NCL/AS1411) to simultaneous deliver anti-miR-155 and 5-fluorouracil (5FU) to SW480 colorectal cancer cells [71] or DOX to NCL-positive MCF-7 cells [72]. Similarly, SK-BR3 HER2 positive cells were satisfactory targeted with HB5 aptamer modified mesoporous silica–carbon nanoparticles [73]. Another very common target for aptamers is the epithelial cell adhesion molecule (EpCAM) which is involved in several cell signaling pathways, migration, proliferation and differentiation of epithelial-derived neoplasms among others. Towards this target Alibolandi and coworkers delivered 5FU to HepG2 using RNA aptamer [74] while Li and Jia’s groups delivered DM1 to SW480 [75] and DOX to SW620 colorectal cell lines [76], respectively, employing a DNA-based aptamer. Other recent examples with aptamers targeting common receptors such as mucin 1 glycoprotein [77] or CD105 [78], could be also found in the literature.
In addition to single-strands targeting aptamers, they have been also tuned to obtain pore-gating properties. To do so, two possibilities arise: thermal dehybridation of a double strand or chemical displacement. An example of the former can be found in the contribution by Lin, who employed NCL as meltable nanogate [79], while for the later approach please refer to reference [69].
2.2.5. Saccharides
Polysaccharides are widely employed as hydrophilic and biodegradable coatings for MSNs. The most extensively used polysaccharides are: chitosan, a linear polysaccharide composed of β-(1–4) linked D-glucosamine units; dextran, a branched saccharide based on α-1,6 and α-1,4-glucosidic linkages and hyaluronic acid (HA), a linear polymer consisting of D-glucuronic acid and N-acetyl-D-glucosamine linked via alternating β-(1-4) and β-(1-3) glycosidic bonds. Among them, HA has been widely employed for its interaction with the CD44 and CD168 receptors which are involved in many types of cancer. For example, Cai and coworkers designed a pH sensitive system by conjugating the HA to Hollow MSNs (HMSNs) through cleavable hydrazine bonds [80]. The system proved to internalize into HepG2 cell lines for DOX delivery. Similarly, Zeng and coworkers reported a parallel system employing a dithiol redox-cleavable linker [81], obtaining high internalization rates on HEK-293 cells. Both examples, in which HA performs a double role, show promising results in vivo since tumor growth was arrested. In addition, HA has been also used for its targeting ability. In an example by Glackin, MSNs were modified to accomplish codelivery of cisplatin and siRNA [82]. In this system, the gatekeeper role was assigned to a polyethyleneimine mesh onto which the siRNA was adsorbed. The latter grafting of HA was accomplished by EDC-NHS coupling reaction, showing great therapeutic efficiency in vivo. However, it must be considered that the HA is a polymer and therefore can present a great variety of molecular weights. In addition, its structure presents both amino and carboxylic groups in stoichiometric proportion; so, the linkage of HA towards MSNs could be an important parameter. Along this line, Arpicco and coworkers made a systematic study on both aspects [83], finding that higher molecular weight HA (200 kDa) covalently bound in one-pot onto MSNs provided better targeting capabilities due to a lower self-condensation reaction rate.
2.2.6. Small molecules
The use of small molecules for targeting purposes is gaining attention because of its simplicity and low cost. Among those reported for targeting, a classification depending of their natural –vitamins– or synthetic source can be made. The use of vitamins and related compounds has become one of the most successful targeting strategies, as typically cancerous cells show an up-regulated uptake to satisfy their accelerated metabolism. However, not all vitamins are suitable candidates for these purposes since fat-soluble vitamins which need the aid of transporters or those with high structural complexity do not perform adequately.
The most used targeting element is folic acid (FA, vitamin B9), which show a well-known reactivity that permits its incorporation in highly complex systems like nanogates. For example, Cai reported the use of FA to modify a pH-sensitive In+3-containing fluorescent chelate [84], while Qi and Wang reported two redox-sensitive models, based on FA-tagged cyclodextrins [85] and FA-modified dithiol-containing chitosan copolymer [86], respectively. Such is the relevance of FA that it is also employed as a reference targeting ligand, being an example the study that compared the drug delivery properties of two different mesostructures such as MCM-41 vs KCC-1 [87]. Another important vitamin employed as targeting label is biotin (vitamin B7 or H), whose relevance is also based on its simplicity and ease functionalization. Like FA, biotin has been employed to target MSNs-based nanosystems containing polymeric coatings [88], AuNPs caps [89], lipid bilayers [90] or surface-loaded prodrugs [91]. Another interesting vitamin for developing targeted examples is cyanocobalamine (vitamin B12), which has been elegantly coordinated to cisplatin-functionalized MSNs by Leeladee and coworkers [92] to obtain a MSN-based system with great potential for drug co-delivery.
Apart from vitamins, synthetic small molecules have been also successfully employed for the selective targeting of cancer cell populations. For example, phenyl boronic acid has been employed as a targeting element in protein capped MSNs. It is worth to note in this example the role of the connecting peptide R9PVGLIG, which is cleaved in the presence of the metalloproteinase-2 protein, releasing the protein cap and exposing a polyarginine sequence that favors the internalization [93]. Other interesting molecules are those derived from substituted triphenylphosphonium (TPP) [94,95] and guanidinium [96] cations. Those, apart from showing a facilitated non-specific uptake due to their positive charge, have also proven the ability to target mitochondria. This is of interest because any alteration may serve to disrupt the metabolism of cell and induce apoptosis. Moreover, guanidine derivatives have also additional recognition features, as demonstrated by Vallet-Regí. For example, 3 and 4-aminobenzylguanidine analogs have proved to efficiently target neuroblastoma cells due to their structural similarity with norepinephrine, whose transporter is highly overexpressed in such type of cancer [97,98].
2.2.7. Double targeting
This new strategy is recently becoming of interest for many research groups. In this approach several targeting elements are combined into a single entity, providing complementary recognition capabilities to the system. This methodology has been explored with small molecules and peptides, which do not have complex reactivity. Pioneering examples, already reviewed by us [99], were based on random functionalization of MSNs with peptide combinations: RGD-TAT [100] and RGD-IL-13 [101] for targeting HeLa and C6 plus HUVEC cancer cell lines, respectively. Those combinations mimicked the pattern of receptors present on the membranes and hence improved recognition and uptake.
However, depending on the capacities that are desired on the nanocarrier, there are other dual targeting strategies: vascular-to-cell and cell-to-organelle targeting. In this way, the pioneering example on MSNs was developed employing FA plus DEX as mitochondrial targeting [102]. In this system a random surface functionalization permitted to enter cancerous cells due to the presence of FA while once internalized DEX delivered the system into the mitochondrial area [103]. This intracellular delivery demonstrated to effectively disrupt the mitochondrial activity increasing apoptosis. As already mentioned, TPP cations are also known to produce such effect, fact that was profited by Vallet-Regí and coworkers to develop two possible approaches for cell to organelle delivery. The two systems based on an asymmetric distribution of FA and TPP onto Janus-MSNs [104] and on an engineered (internal-external) ligand disposition of TPP and biotin [105] showed enhanced uptake and thus increased delivery of drugs. Besides the use of MSNs, double targeting has been also bloomed employing other nanosystems. For an interesting review focused on the possibilities of double targeting, please check reference [106].
2.2.8. Biological membranes in targeting
As reviewed above, there are many compounds able to interact with biological membranes and cancer cells and promote a preferential uptake. However, this recognition also occurs between different types of cells such as blood, immune and mesenchymal stem cells. The particularity of these last cells is that they have tumor-tropic properties, which makes them interesting for developing bioactive coatings. In the case of stem cells, the targeting capacity was demonstrated employing upconversion (UCN) core@shell-MSNs protocells for photodynamic therapy (PDT) [107]. In the same way, red blood cells’ membranes were also employed for cloaking effect, obtaining again outstanding results enabled only by the effect of passive targeting [108].
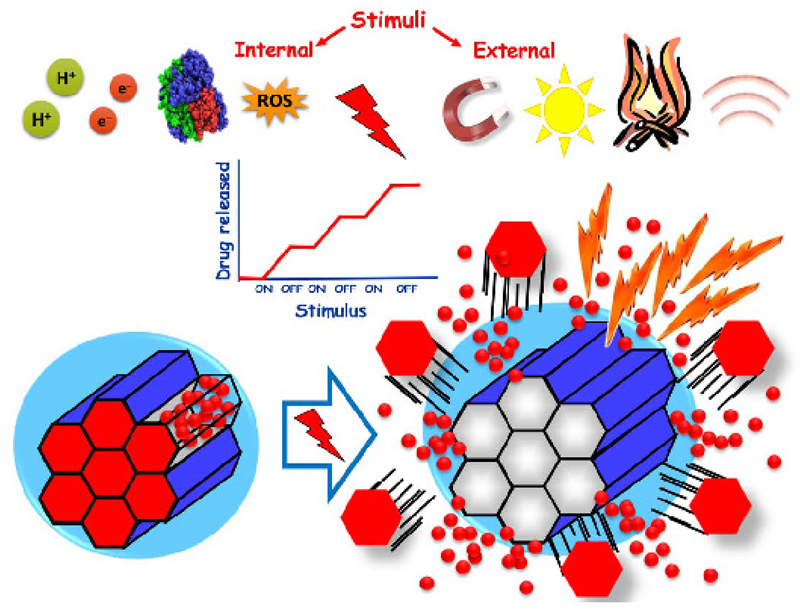
3. Stimuli-responsive MSNs
Among the three major objectives of nanomedicines - (1) trap and protect a great amount of therapeutic agents; (2) carry them to the specific site of the disease avoiding any leakage; and (3) release on-demand high local concentrations of therapeutic agents - the third objective of any nanomedicine should be releasing high local concentrations of therapeutic agents on-demand. In this sense, keeping control on the therapeutics release is a desirable feature so premature release can be avoided. This might be of great interest when the employed therapeutic might be a cytotoxic drug, so side effects on healthy tissues would be avoided.
Stimuli responsive behavior is of particular interest on MSNs, because these nanocarriers present open porosity, so it is relatively easy to introduce therapeutic agents into their network of cavities. However, it might be also relatively easy for those molecules to diffuse out [109]. Therefore, it is necessary to design strategies to cap the pore entrances to avoid premature release of the cargo. Although mesoporous matrices present pores large enough that might be capable of hosting many different types of molecules, those pores are small enough to cap them with large molecules. Those caps grafted at the pore entrances should respond to the application of certain stimuli, so the cargo could be released. Recently, Zink and co-workers have divided the mesopore capping strategies into three groups: (1) Reusable caps, that are based on a bulky capping molecule able to bind reversibly; (2) Completely reversible caps, that work on the principle of reversal of affinity of a macromolecule; and (3) Irreversible caps, that are based on a chemical bond cleavage of the capping macromolecule, which leads to the permanent separation from the host nanoparticle [110]. The last group includes a popular approach that consist on covering the external surface of the MSN with a cleavable shell that upon the application of the appropriate stimulus would detach from the nanoparticle triggering the cargo release.
In this section, we will focus on MSNs based DDS that are capable of releasing their cargos in response to the presence of several stimuli. Different endogenous (pH, redox, enzymes and small molecules) and exogenous (light, magnetic field, temperature and ultrasound) stimuli have been explored to trigger drug release (Figure 5 and Table 2). Most of single stimuli-responsive nanosystems are easily affected by different external factors and may produce side effects or low release problems. In this regard, multi-stimuli responsive DDS have been recently developed to solve these problems.
Figure 5.
Schematic representation of internal or endogenous stimuli to the pathological microenvironments (pH, redox, enzymes and small molecules) and external or exogenous (magnetic field, light, temperature and ultrasounds) stimuli that can be used to trigger on demand drug release from MSNs.
Table 2. Reviewed stimuli employed for drug delivery using MSNs.
| Stimuli | Mechanims | Ref. |
|---|---|---|
| Internal stimuli | ||
| pH | Acidic cleavage (chemical) | [48, 112, 113] |
| Host-guest interaction | [114, 115] | |
| Endosomal and lysosomal acidic pH | [116, 118] | |
| Self immolative polymers | [117] | |
| Redox | S-S cleavage disassembly | [119, 121] |
| S-S cleavage unfolding | [122] | |
| Redox induced solubility shift | [123] | |
| Enzymes | Peptide sequence cleavage | [54, 124] |
| Prodrug conversion | [125] | |
| Small molecules | Glucose concentration | [126, 127] |
| Glucose-mediated response to H2O2 | [128] | |
| Generation of additional ROS species via Fenton reaction or α-tocopheryl | [132, 133] | |
| H2O2 rich microenvironment | [134] | |
| H2O2 or NO concentration | [135] | |
| Competitive binding or displacement reactions in the presence of ATP | [140, 141] | |
| External stimuli | ||
| Light | UV-vis | [45, 146, 147, 148, 149] |
| NIR | [151] | |
| NIR to thermal conversion: photothermia | [153] | |
| conversion of NIR light to UV-vis light: upconversion | [152, 154, 155, 156, 157, 158, 159, 160, 161, 162] | |
| Temperature | Conformational changes in polymers: | |
| pNIPAM and analogs | [163, 164, 165, 166] | |
| PEG acrylates | [167] | |
| Disassembly of coiled coil motifs | [168] | |
| Magnetic fields | Increase of temperature under the action of an alternating magnetic field | |
| Superparamagnetic cores in core@MSNs: Fe3O4 and Fe3O4/Fe cores | [169, 170] | |
| IONPs in the silica network | [171, 172, 173] | |
| IONPs as mesopore caps | [174] | |
| Ultrasounds | MSNs capping with a copolymer containing a labile acetal group which is cleaved upon US application. Change of polymer conformation due to hydrophobic to hydrophilic transformation. | [180, 181, 182] |
| Multi-stimuli responsive MSNs | ||
| Dual responsive MSNs | pH or ATP concentration | [184] |
| pH or glucose concentration | [185, 186, 187] | |
| Enzyme (hyaluronidase) and redox (GSH) | [188] | |
| Sequential release of different sized cargos | Electro-stimuli and UV irradiation | [189] |
| Use of dual responsive polymers | cargo loading at 4 °C and conformation change at 37 °C: | |
| upon US irradiation | [180] | |
| upon H2O2 stimulus | [190] | |
| dual-responsive polymers as gatekeepers: | ||
| pH and thermoresponsive | [191] | |
| pH and visible light responsive | [192] | |
| Combination of chemotherapy and PTD | Drug release under acidic pH and 1O2 generation with NIR irradiation | [193] |
| 1O2 generation upon 660 nm irradiation and gatekeeper cleaving by the generated 1O2 | [194] | |
| Three gated MSNs | Redox, enzymatic hydrolysis and contact with cell membrane | [195] |
Abbreviations: ATP: Adenosine triphosphate; GSH: Glutathione; IONPs: Iron oxide nanoparticles; pNIPAM: poly(N-isopropylacrylamide); NIR: Near Infrared radiation; PEG: poly(ethyleneglycol); PDT: Photodynamic theraphy; ROS: Reactive Oxigen Species; UV-vis: Ultraviolet-Visible radiation.
3.1. Internal stimuli-responsive MSNs
The variations in metabolic and biochemical processes involved in pathologies such as cancer or inflammation diseases can be used for the design of drug delivery nanocarriers sensitive to endogenous stimuli. The physiological and biochemical differences between normal and pathological conditions (e.g., microenvironment in tumors and inflammation sites) are exploited since the responsive nanosystems do not require external mediation to trigger drug release and therefore are not invasive. In this section, we focus on the design of MSNs that respond to internal stimuli as pH levels and redox potential variations and deregulations of different enzymes or small molecules (Figure 5).
3.1.1. pH
Among the internal stimuli, pH is one of the most employed in the literature, especially associated with drug release for treatment in oncology or inflammation [1,106]. These pathologies show a significant variation of pH values over time. Tumors exhibit lower pH environments (6-7) compared with normal tissues (7.4), especially in intracellular endosomes (5) and lysosomes (< 5), and in inflamed tissues the pH can reach values of 5.5 [1,111]. In this context, the use of pH-sensitive gatekeepers as pore blockers is an interesting approach to control drug release in response to the changes of pH values, especially in cancer. In the past, the triggered release of anti-tumor drugs from mesoporous channels has mainly been studied by using polyelectrolytes, supramolecular nanovalves, pH sensitive polymers such as poly(4-vinylpyridine), poly(styrene sulfonate) or chitosan, pH-sensitive linkers (hydrazine, acetals), and acid-decomposable inorganic materials, together with others [1].
An alternative strategy is to use pH-sensitive linkers to directly graft the cytotoxic drug on the MSN surface [1]. In this context, different types of labile acid cleavage site functionalization can be used. Chen et al. developed a new pH-responsive drug delivery carrier by capping MSNs with functional peptide-coated gold nanoparticles [112], first functionalized with acid-labile α-amide-β-carboxyl groups and decorated with oligo-lysine-containing peptide. The resulting hybrid delivery system exhibited pH triggered drug release and the incorporation of RGD peptide facilitates targeting delivery to αvβ3 integrin overexpressed in cancer U87 MG cells (human glioblastoma cells). This hybrid nanocarrier was successfully internalized by these tumor cells and when it was loaded with DOX showed a dramatically decreased (ca. 60%) in tumor cell viability. Martínez-Carmona et al. developed a novel multifunctional nanocarrier based on DOX-loaded MSNs as nanoplatforms for the assembly of different building blocks to increase antitumor effectiveness and decreases toxicity in normal cells: a polyacrylic acid capping layer grafted to MSNs via an acid-cleavable acetal linker, as pH-responsive drug delivery ability, and ConA, as a targeting ligand to a cell-surface glycans sialic acids overexpressed in tumor cells [48]. This nanocarrier showed a higher internalization rate in human osteosarcoma cells (HOS), and a small DOX loading (2.5 mg mL-1) induced 95% of tumor cell death in 48 h of cell culture, 8-fold higher than that caused by the free drug. Over the past few years, poly(glycerol methacrylate)s (pGOHMAs) have emerged as an efficient alternative to classical polymers. In a study by Yan et al. the effect of anti-tumor drug camptothecin (CPT) bound to MSNs with acid-cleavable silyl ether bonds to develop a hybrid CPT-MSN nanomedicine was evaluated [113]. Only under extracellular pH conditions of tumor issues (pH 6.8) the drug was partially released from the MSNs when the acid-labile silyl ether bonds were degraded. The rate of release was controlled by changing the steric bulks of the substituents of the silicon atom, at pH 4. This DDS had a similar efficiency as CPT free at 0.5 and 1.0 μg mL-1 with an inhibition of 40% of tumor HeLa cell growth.
Regarding to host-guest interactions, in a study by Moorthy et al. MSNs were covered with tetrathio-maleimide as capping units and were functionalized with melamine groups (nitrogen-rich organic base) onto the surface by host-guest chemistry via multipoint hydrogen bonding interactions for pH-responsive in human breast MDA-MB-231 cancer cells. The nanosystem showed intracellular uptake efficiency, protecting the loaded cargo molecule (rhodamine B) inside the pores and preventing its premature release [114]. Li et al. reported a biocompatible layer-by-layer (LbL) coated MSNs, designed to release encapsulated DOX by changing the pH [115]. MSNs coated with LbL blocks were prepared by supramolecular self-assembly, based on the non-covalent bonding host–guest interactions between bis-aminated PGOHMAs and cucurbit[7]uril (CB[7]), where CB[7] serves as a molecular bridge holding two different bis-aminated polymeric layers together. These MSNs successfully released DOX by lowering the pH in MDA-MB-231 cells, decreasing the cell viability. In vivo, DOX-loaded LbL-MSNs decreased ca. 63% of tumor size on a BALB/c nude mice model induced by HeLa cancer cells.
In the other hand, Hakeem et al. studied the use of polyaspartic acid (PAsA)-anchored MSNs as a gatekeeper via amide linkage, providing a positive charge to MSNs, which contributes to effective cellular uptake by HepG2 cells [116]. In vitro release results indicated enhanced DOX release from DOX-loaded PAsA-anchored MSNs under endosomal and lysosomal acidic pH condition and translocation to the nucleus to increase cytotoxicity in HepG2 cells.
As we previous mentioned, there are many polymeric systems with acid-sensitive bonds [1]. The so-called Self Immolative Polymers (SIPs) are materials that disassemble from head-to-tail when a specific functional group is cleaved from the polymer in response to certain stimuli. In this regard, Gisbert et al. evaluated a linear SIP to cover the pore in MSNs in a single functional responsive nanocarrier, avoiding premature release of the cargo. This nanosystem showed a high loading capacity and the responsiveness of pH-sensitive SIPs to control the release. Polyurethane backbone with a t-butyloxycarbonyl protecting group on the terminal amine acted as a trigger [117]. The carbamate linkage of the trigger was the cleavage when the pH diminished, starting the sequential 1-6 elimination and decarboxylation reactions yielding CO2 and the initial monomer. MSNs capped with the acid responsive SIPs were selective internalized by human prostate adenocarcinoma LNCaP cells, indicating that SIPs could be interesting for future applications in nanomedicine.
Of special interest is the study by Pan et al. about a novel pH-responsive drug delivery platform based on a zeolite imidazole framework-8 (ZIF-8) film with a few nanometer thickness synthesized in situ on the surface of carboxylate functionalized MSNs [118]. This approach showed a pore blocking and efficient loading of small interfering RNAs. In this study the author chose a Bcl-2 siRNA, because this gene is implicated in the activation of the cellular antiapoptotic defense. The positively charged of ZIF-8 film increased the siRNA loaded into MSN and the cellular internalization and endo-lysosome escape, protecting from nuclease degradation. In addition, the ZIF-8 film was degraded in the acidic endosome and induced the intracellular release of siRNAs and DOX, increasing their efficacy in MCF-7/ADR and SKOV-3/ADR cancer cells. This technology is a promising strategy for pH-triggered, combining stimuli-responsive delivery of siRNAs and chemotherapeutic agents with an increased therapy efficacy.
3.1.2. Redox
The differences in redox potential between extracellular and intracellular environments and between normal and tumor tissues, with a dissonant production of reduce glutathione (GSH) or reactive oxygen species (ROS), allow the development and use of redox-responsive drug release systems for biomedical applications [1,111].
Among all the possibilities, surface modifications based on disulfide bonds with bulk gatekeepers are widely used. The cleavage of the disulfide bonds results in the detachment of entire polymer from MSNs. In a study developed by Sha et al. a novel method was used to successfully coat Pluronic P123 (PSMSNs) and octadecyl chain-modified (PMSNs) into MNSs [119]. Compared with the PMSNs, the PSMSNs displayed a redox-responsive drug release both in vial and in mouse 4T1 breast tumor cells, due to the cleavage of the disulfide bond by highly concentrated GSH, under which circumstance the hydrophobic chain was set free, P123 lost support and detached from the surface of the MSNs. The accumulation of P123 in the tumor in an in vivo model was enhanced by the EPR effect after coating the MSNs with P123 compared with the free P123. Redox-responsive nanocarriers for anticancer drug and gene co-delivery are promising synergistic strategy in cancer therapy, delivering high local concentration of drugs without premature release. Lin et al. developed a redox stimuli-responsive and synergistic co-delivery nanosystem for DOX and p53 gene based on MSNs and dendronized chitosan derivatives (CP) as a gatekeeper to control release [120]. The loaded DOX was control released under higher GSH situation in HeLa tumor cells, and the nanosystem showed enhanced p53 gene delivery inducing an increase in p53 protein expression in these cells. DOX and p53 co-delivered from this MSN nanocarrier induced a significant cytotoxicity in tumor cells, higher than p53 or DOX used singly, resulting in a synergistic dual delivery system promising for cancer therapeutic approach. On the other hand, polycations in the pores of MSNs allows load and controlled release of therapeutic siRNAs. Prabhakar et al. studied the combination of MSNs with PEI tethered with redox-sensitive linkers that allows a high siRNA concentration (120 mg g−1) [121]. This nanocarrier loaded with a cell-killing siRNA was efficiently internalized by MDA-MB-231 tumor cells, escaping from endosome, releasing the functional siRNA intracellularly and showing a promising long-term gene knockdown efficiency.
In another study Lee et al. designed an all-peptide gatekeeper with on-off gatekeeping capability through stimulus responsive conformational conversion and the steric bulkiness of the tryptophan unit of the zinc finger domain peptide (CXXC) [122]. Due to a reduction of the disulfide bond by GSH, the peptide conformation was converted to a random structure, which opened the mesopore releasing DOX loaded into MSNs in a controlled manner. This nanosystem was efficiently internalized and specifically induced a higher cytotoxicity in A549 human lung cancer cells.
In a study by Cheng et al. a ROS-responsive free-blockage controlled release nanosystem was explored, controlling the wetting behavior of the internal surface of nanopores on MSNs by modification with a hydrophobic phenyl sulfide [123]. Under the stimulation of ROS, the sulfide groups are oxidized triggering the release of DOX loaded into MSNs specifically in MCF-7/ADR tumor cells. This effect is possible due to the shift of nanopore environment from hydrophobic to hydrophilic, inducing a higher rate of cytotoxicity in these cells.
3.1.3. Enzymes
Cancer and other pathologies course with an overexpression and deregulations of several enzymes, which can be also used as release triggers, including esterases, matrix metalloproteinases (MMPs) and others [1]. MMPs are a family of proteins with protease activity, which are overexpressed in the tumor’s environment, in particular of liver and colon tumors. Liu et al. have described a MMPs responsive, MSN-based for DOX by using bovine serum albumin as end-cap, peptide substrate of MMPs as intermediate linker and lactobionic acid as targeting moiety [124]. The authors induced tumors by injecting HepG2 cells into nude mice. This nanocarrier was successfully accumulated at liver tumor site, delivering DOX to tumor tissue triggered by MMPs, for tumor growth decreased. In addition, Cheng et al. designed a tumor-targeted and enzyme-induced DOX delivery system, with cleavable rotaxanes anchored onto the orifices of MSNs as gatekeepers and azido-GFLGR7RGDS (multifunctional peptide with tumor-targeting, membrane-penetrating and cathepsin B-responsive functions) to stabilize it [54]. This novel nanocarrier was efficient internalized by HeLa cells thanks to integrins-mediated targeting and released DOX via enzymatic digestion of GFLG peptide due to cathepsin B overexpressed in late endosomes and lysosomes of tumor cells.
Baeza et al. studied an interesting approach consisting in the incorporation of enzymes in the nanocarrier itself [125], able to transport a non-toxic prodrug (indol-3-acetic acid, a plant growth hormone) and the enzyme (horseradish peroxidase) responsible for its conversion into cytotoxic compounds coated with a protective polymeric shell grafted to MSNs. This nanodevice was effective internalized by neuroblastoma (NB1691-luc) and leukemia (NALM6) cells and generated enough cytotoxic substances to specifically kill these cells, decreasing the side effects of current antitumor drugs. Once the nanosystems were internalized by tumor cells, intracellular enzymes degrade the protective polymeric shell and permit horseradish peroxidase to oxidize indol-3-acetic acid molecules, producing ROS that decreased human cancer cells viability.
3.1.4. Small molecules
As in the case of enzymes, the identification of some chemical species that are produced or accumulated in an unbalanced way due to a pathology can lead to their use as chemical signals to trigger the drug release by opening of the mesopores blockers or nanogates. A variety of small molecules, such as glucose, ROS or adenosine triphosphate (ATP), have been used as key molecules or trigger events.
3.1.4.1. Glucose
Glucose-sensitive MSNs may provide self-regulated insulin delivery systems for diabetes treatment in which a certain amount of insulin can be released in response to the blood glucose concentration. In a pioneering article from the group of Lin, gluconic acid-modified insulin proteins were immobilized on the exterior surface of boronic acid-functionalized MSNs, being as well caps to encapsulate cyclic adenosine monophosphate molecules inside the mesopores. The release of both gluconic acid-modified insulin and cyclic adenosine monophosphate was triggered by the introduction of saccharides such as glucose, being a double release system in which the decrease of insulin level could be overcome by delivering the cell-membrane-impermeable cyclic adenosine monophosphate into the cytosol to stimulate insulin secretion from pancreas beta cells [126].
In a similar fashion, a novel multifunctional MSNs system with integrated glucose-responsive double-drugs release and fluorescent real-time monitoring capabilities of the hypoglycemic drugs released has been recently developed. This system takes advantage of the principle of competitive binding between a hydrophobic boronic acid, glucose, and a fluorescent reporter molecule such as alizarin. This competitive binding mechanism not only ensures the response to glucose but also can cause the change of the fluorescence signal simultaneously. The signal reporter, alizarin complexone, is attached onto the surface of MSNs and then, the gluconated insulin, which acts as hypoglycemic drug and capping agent, is immobilized on MSN via a benzene-1,4-diboronic acid mediated esterification reaction. In the absence of glucose, the sandwich-type boronate ester structure formed by the diboronic acid binding to the diols of alizarin complexone and gluconated insulin simultaneously remains intact, leading to the blockage of mesopores. Furthermore, the boronate ester displays an emission peak at 570 nm under excitation of 460 nm light at this stage. When glucose is present, it binds the diboronic acid competitively and cause the dissociation of boronate ester. As a result, gluconated insulin is departed from the alizarin complexone functionalized MSNs, triggering the opening of mesopores, the disappearance of fluorescence and the diffusion of gluconated insulin and rosiglitazone maleate as the hypoglycemic drug loaded into the MSNs [127].
Another approach has been reported for insulin delivery in which glucose is not the trigger molecule, though it is necessary for the delivery event. The enzyme glucose oxidase (GOx) and insulin were encapsulated into MSNs and the insulin retention was achieved through the host-guest complex formed between 4-(imidazoyl carbamate)phenylboronic acid pinacol ester on the surface of the MSNs and α-cyclodextrin (α-CD). GOx in MSN could convert glucose to gluconic acid and generate H2O2 as byproduct and trigger event. The phenylboronic ester on the surface of MSNs could be oxidized in the presence of H2O2 that resulted in the destructive disassemble of the host-guest complex, leading to the subsequent release of the preloaded insulin. The hypoglycemic effect was in vivo investigated after transdermal administration to diabetic rats using a novel microneedles delivery device, which integrates the MSNs loaded with insulin and glucose-mediated responsive to H2O2 [128].
3.1.4.2. ROS and H2O2
Excessive amount of various ROS, such as superoxide (O2−), hydroxyl radical (•OH), hypochlorite ion (OCl−), hydrogen peroxide (H2O2) and singlet oxygen (1O2), are produced at the site of inflammation of some diseases including many types of tumors in cancer, infected tissues, injuries and neurodegenerative diseases [129–131]. Therefore, as we have mentioned for the redox stimulus, ROS pathological signals are being exploited to design oxidative triggered drug release MSNs. Typically, boronic ester and thioketal are emerging oxidation-responsive functional groups which can be readily cleaved by H2O2 in oxidative stressed microenvironments.
Sometimes the exposure of the capped MSNs nanocarrier to the disease inherent concentration of H2O2 fails to open the ROS-sensitive nanogates of the MSNs and therefore some strategies have been developed to overcome insufficient ROS concentration. A metal mediated drug delivery MSNs system responsive to hydroxyl radicals is based on the introduction of the Fenton reaction (Fe2+ + H2O2 → Fe3+ + ∙OH + OH−) into the nanosystem. The authors used thiol stabilized ZnS quantum dots (QDs) as nanocaps to regulate the release of the anticancer drug from MSNs in response to oxidative environment. Addition of catalytic amounts of divalent iron readily unseals the nanochannels at considerably low H2O2 concentrations due to the generation of highly reactive hydroxyl radicals. The exposure to •OH resulted in the oxidation of thiol groups, thus destabilizing the ZnS nanolids to open the mesopores and release the drug [132]. Another example is based on a positive feedback strategy utilized to amplify the concentration of intracellular ROS. In this case, the MSNs are loaded with the anticancer drug DOX and a ROS producing agent, α-tocopheryl succinate (α-TOS). The gatekeeper β-CD is anchored on the surface of MSNs through the ROS-cleavable thioketal linker for ROS-triggered drug release. Once the nanosystem has been taken up by tumor cells, but at the very beginning, only limited pores are open because of the existent but insufficient intracellular ROS, resulting in the simultaneous though restricted release of loaded DOX and α-TOS. Then, released α-TOS interacts with mitochondria to generate additional ROS. In other words, the intracellular ROS would be self-regenerated and amplified, which in turn facilitates the cutting of the thioketal linkage to remove the gatekeeper and led to more release of α-TOS as well as the self-accelerating release of toxic DOX from the MSNs [133].
A ROS-activated yolk-shell nanoplatform has been designed for the simultaneous delivery of protein and small-molecule anticancer drugs. The cytochrome c (Cyt c)/DOX dual therapy is achieved by immobilizing Cyt c onto the surface of yolk-shell MSNs (YMSNs) via H2O2-liable boronic ester bonds and further modifying the nanocarrier with lactic acid units to confer as well selective tumor targeting against liver cancer cells. The bioactivity of Cyt c is temporarily shielded by the boronic ester linkages and readily restored in the H2O2-rich tumor intracellular environment, therefore protecting the protein drug from biodegradation. Moreover, the immobilized Cyt c moieties effectively cap the nanochannels of YMSNs to avoid the premature delivery of DOX [134].
With the goal of treating neurological diseases caused by oxidative stress, a MSNs system able to actively cross the blood-brain barrier (BBB) and release an antioxidant drug upon ROS stimulation has been described. The MSNs are loaded with resveratrol and then coated with polylactic acid as gatekeeper followed by conjugation with a ligand peptide of low-density lipoprotein receptor to enhance the MSNs transcytosis across the BBB. The in vitro model of BBB/inflammation was established with a co-culture of rat brain microvascular endothelial cells (RBECs) and microglia cells using Transwell chambers. The RBECs on the top well can form tight junctions and create a transport barrier mimicking the BBB, while microglia on the bottom wells could be stimulated exogenously to produce abundant superoxide or nitric oxide. The conjugation of the ligand peptides markedly enhanced the migration of MSNs across the RBECs monolayer via receptor mediated transcytosis. Finally, the polylactic acid coating was degraded by the high concentration of ROS produced by microglia and resveratrol was subsequently released to reduce inflammation [135].
3.1.4.3. ATP
ATP, which is the molecular unit of currency for intracellular energy transfer, is also considered among the endogenous stimulus based on key small biomolecules. The ATP levels within the intracellular cytosol are higher than in the extracellular environment [136,137], therefore, the ATP concentration may trigger the release of drugs in the cytosol. ATP is also found to be upregulated in cancerous tissues and in processes such as chemoresitance and uncontrolled tumor growth, hence making possible to exploit differences between normal and cancer cells [138,139]. Competitive binding or displacement reactions use to be the mechanism that takes place to unblock the mesopores under the stimulus of ATP concentration.
An ATP-responsive nanocarrier for intracellular drug delivery and real-time monitoring of drug release through fluorescence resonance energy transfer has been fabricated by using graphene quantum dots (GQDs, the acceptor) as caps onto fluorescent MSNs (the donor) via an ATP aptamer. Under extracellular conditions, the fluorescence of MSNs remains in the “off” state in the low ATP level which is unable to trigger the release of drug. Once specifically recognized and internalized into the target tumor cells, in the ATP-rich cytoplasm, the ATP aptamer switches its conformation causing the shedding of the GQDs from the nanocarriers. The loaded drugs are then released and, simultaneously, the fluorescence of MSNs turns “on” along with the dissociation of GQDs, which allows monitoring drug release from the pores in real-time [140].
The same concept, intracellular and real-time monitoring drug release in response to ATP, has been achieved in a core@shell nanosystem that consists of an upconversion nanoparticles (UCNPs) core and an MSN shell (UCNPs@MSNs). The biogate for ATP-responsive drug release comprises of a dipicolylamine-zinc analogue (TDPA-Zn2+) immobilized on the exterior surface of the nanoparticle to serve as binding sites for branched polypeptides with multiple pendant carboxylate side chains which act as the capping ligands. Luminescence resonance energy transfer (LRET) occurs between the loaded drugs DOX and CPT and the UCNPs, which results in a quenched UV-vis emission of the UCNPs. The LRET is eliminated upon drug release, which appears as an enhancement on the UV-vis emission of UCNP. The drug release was triggered by ATP, which caused a competitive displacement of the polypeptide from the MSN surface due to a mechanism of competitive binding and the stronger affinity between ATP and TDPA-Zn2+ immobilized on nanoparticle surface [141].
3.2. External stimuli-responsive MSNs
The external stimuli employed to trigger the release from responsive nanoparticles include those that are remotely applied by the clinician, being under control at all times by the operator. Their main advantages include the possibility of being turned on and off as required and the ability of being applied locally into the site of the disease as desired. Many examples of MSNs externally triggered can be found in the literature (Figure 5), such as light, temperature, magnetic field or ultrasound (US).
3.2.1. Light
The use of different wavelengths light for triggering the cargo release from MSNs has become very popular in the last few years. The employed wavelengths include ultraviolet (UV), visible (vis) or near infrared (NIR), which makes this approach very versatile. In a similar way to the rest of external stimuli for triggering the release from nanoparticles, light present some benefits, such as its easy application by the operator from the outside of the body and the possibility of focusing to the targeted tissue, although the tissue penetrability is not as deep as other stimuli such as ultrasound. The most popular wavelengths employed in MSNs include UV-vis and NIR. Generally, UV-vis is normally employed for triggering the transformation of molecules or polymers because of the high energy of the photons. However, UV and visible light present low tissue penetration and potential damages to living systems. However, NIR operates in the biological transparency window, at which scatter and adsorption are minimalized leading to greater tissue penetration.
3.2.1.1. UV-visible
Among all the possibilities, UV has been the most popular type of radiation to stimulate the cargo release from MSNs, because its high power is able to break bonds [142]. UV light can also provoke the isomerization of certain molecules that might trigger the release from MSNs in what are called nanovalves [143,144]. In this sense, azobenzene derivatives have been widely employed to block the pores since they can go through a trans to cis photoisomeriazation process that might trigger the drug release from MSNs [145].
Our research group developed a proof of concept using light as stimulus to trigger the release from MSNs [45]. In this work MSNs were covered with a shell made of proteins using a photosensitive linker that was cleaved under UV radiation at 366 nm. The protein shell was functionalized on the external face with transferrin, a well-known targeting ligand that would react with overexpress receptor on the surface of cancer cells. The idea was that once the tumor cells might have had internalized MSNs, the application of UV light would trigger the drug release. This approach might be suitable for treating tumors that are accessible for light irradiation, as it is the case of melanomas.
However, UV light presents two main limitations: it might be toxic because of its high energy and it presents low penetration capability. As a possible alternative, we have developed a proof of concept using visible light to trigger the release from MSNs, because it is safer and presents higher tissue penetrability than UV light [146]. In this work we capped the pore entrances of MSNs with porphyrin-caps grafted through reactive oxygen species cleavable linkages. Although visible light has a more innocuous nature than UV light, its energy is high enough to activate some photosensitizers in the production of reactive oxygen species [147]. Our proof of concept benefits from the non-toxicity and great penetration capability of visible light and also presents a dual antitumor effect: the antitumor cargo released form the pores and the generation of reactive oxygen species. A similar dual approach on releasing cytotoxic drugs and generating singlet oxygen from MSNs was carried out using the photosensitizer chlorin e6 doped MSNs nanorods [148,149].
3.2.1.2. Near infrared
Near Infrared possesses unique advantages such as deep tissue penetration together with minimum autofluorescence and tissue scattering. In fact, NIR offers the deepest penetration of light in living tissues, with a wavelength between 650 and 900 nm. The penetration depth of NIR ranges from 1 to 2 cm, which make this stimulus more appropriated for imaging applications than controlled drug delivery [150]. The high tissue penetration depth of NIR light together with unique drug delivery capabilities of MSNs can be combined to design NIR triggered MSN based DDS [151]. The two most often approaches that have been developed include NIR-UV triggered drug release and NIR-thermal triggered drug release. The former includes the lanthanide doped upconversion nanoparticles that can transfer NIR light into UV-vis light radiation [152]; and the later includes NIR-thermal converting nanoparticles, in which precious metal nanoparticles with NIR plasma resonance need to be used [153].
3.2.1.3. Upconversion
Photosensitive nanocarriers able to act once localized in the tumor environment or the pathologically affected tissues must operate in the biological window. However, for most photosensitive nanocarriers, photoreactions are induced by UV or visible light.
Conventional administration of UV light or visible light have limited biomedical applications since brings up severe concerns of uncontrolled damages to DNA and proteins of normal tissues and also suffers from very limited depth of tissue penetration. By contrast, NIR light is a better choice for clinical therapies since it induces minimal cell damage and also has very low light absorption by water and hemoglobin, leading to the maximum tissue penetration. Upconversion (UCN) provides an effective way to convert NIR light to UV-vis light for light-triggered release. UCN is a multiphoton process in which two or more low-energy pump photons from the NIR are transferred to a higher-energy output photon with a shorter wavelength in the UV-vis region. Efficient transducer nanomaterials are based in lanthanide ions (such as Tm3+, Yb3+ and Er3+) doped nanocrystals which lead to upconversion nanoparticles (UCNPs) with potential and significant biomedical applications [154]. UCNPs combined with a mesoporous silica shell lead to core@shell nanosystems UCNPs@MSNs for NIR light triggered drug release.
For example, UCNPs@MSNs loaded with DOX and grafted with ruthenium complexes on the surface of the nanoparticles as photoactive molecular valves have demonstrated to be sensitive to ultralow-intensity NIR light. The ruthenium complexes are cleaved by 974 nm light with intensity as low as 0.35 W cm−2, which is lower than the maximum permissible exposure of skin (0.726 W cm−2), minimizing overheating problems and preventing photodamage to biological samples at such a low light intensity [155].
Recently, a polyelectrolyte bearing UV-labile pendant groups have been used as outer shell UCNPs@MSNs@polyelectrolyte, acting as gatekeeper since NIR light triggers the polymer layer disruption and drug release [156].
Multilayered conformation also makes possible the controlled release of nitric oxide when NO-releasing molecules are covalently linked to the silica as the outer shell. S-nitroso-N-acetyl-dl-penicillamine (SNAP) is a chemical progenitor of NO because it is sensitive to UV light for the release of NO. Therefore, continuous-wave NIR laser irradiation at 980 nm enables NO releasing from UCNPs@MSNs@SNAP in a light dosage-dependent manner and the effect has been investigated on platelet aggregation [157].
UCNPs@MSNs have been also reported for in vivo bioimaging in theranostics [158], Hg2+ sensing [159] due to the UCN luminescence or PDT, which uses UV-vis light to initiate a photochemical reaction between a photosensitizer and tissue oxygen to generate toxic 1O2 that can damage cancer cells. Therefore, upon NIR irradiation, the emitted UV-vis light can excite photosensitizer molecules loaded in the UCNPs@MSNs nanocarriers to generate ROS for PDT. As an example, a photosensitizer for PDT treatment, zinc phthalocyanine, has been encapsulated in UCNPs@MSNs@lipid triple layer nanoparticles. With the help of the cross-linked lipid shell, the triple layer nanoparticle can prevent the photosensitizer leaking and particle aggregation and also be modified to target the lesions [160].
Other approaches achieve synergistic effects of chemotherapy and PDT. An UCNPs@MSNs that incorporates the photosensitizer chlorin e6 in the silica network and the anticancer drug DOX loaded into the mesopores was gated by grafting a thioketal linker onto the nanoparticles surface. Upon NIR irradiation, the upconverted luminescence derived from the core stimulates Ce6 to generate ROS for PDT. In addition, the thioketal “gate” also can be cleaved by these ROS to permit drug release [161]. In another work, the blue emission derived from the UCNPs upon NIR irradiation induces the break of the gate for DOX release and also excites the photosensitizer, which had been incorporated to the silica framework, to generate ROS for PDT [162].
3.2.2. Temperature
Temperature can be employed as internal stimuli in certain pathologies, such as tumors, inflammation or infection processes, that can provoke moderate temperature increases of up to 4 or 5 °C [163]. On the other hand, temperature increases caused by other stimulus, such as the hyperthermia produced with magnetic fields, can also be employed to trigger the cargo release from MSNs. In general, water soluble polymers able to respond to temperature changes are the ideal candidates to cap the pores due to their reversible conformational changes in response to temperature and they can also contribute to improve the colloidal stability of the MSNs [164,165]. In this sense, the use of poly(N-isopropylacrylamide) (pNIPAM) to cap the mesopores from MSNs is probably the most employed strategy. pNIPAM presents a lower critical solution temperature (LCST) at 32 °C, which means that the polymer changes from hydrophilic to hydrophobic state when the temperature might be above the LCST [163]. There are also different approaches with several analogs to pNIPAM that have been employed to tune the responsive temperature as desired [166].
In the last few years, PEG acrylates have been evaluated as substitutes of pNIPAM analogs because of the better biocompatibility of PEG and the corresponding monomers. In this sense, it is possible to adjust the monomer ratio to tune the LCST temperature, offering the possibility of changing the responsive temperature as desired [167].
In a different approach, a coiled coil motif was employed to block the pores preserving the release of their cargo. The coiled coil motif consisted on two alpha-helices coiled around each other in a rope-like fashion, and when heated over physiological temperature the coiled coil structure was disassembled, triggering the release of the cargo molecules [168].
3.2.3. Magnetic fields
Magnetic fields have been widely employed in nanomedicine because they can magnetically guide the nanoparticles when using a permanent magnetic field or increase the internal temperature when employing an alternating magnetic field. The classical approaches of magnetic responsive MSNs are based on superparamagnetic microspheres with a Fe3O4@SiO2 core and a mesoporous silica shell [169]. In a similar approach, spheres with a core of magnetic Fe3O4/Fe and a mesoporous silica shell able to encapsulate ibuprofen have been explored as drug nanocarriers [170].
Our research group has developed magnetically responsive MSNs incorporating superparamagnetic iron oxide nanoparticles of ca. 5–10 nm into the silica network during the synthesis of MSNs and capping the pore entrances with the thermosensitive pNIPAM to close the pore entrances to retain the cargo inside avoiding premature release [171,172]. Then, the application of an alternating magnetic field to the responsive nanocarriers led to an increase into the local temperature, which promoted a change on the conformation of a temperature responsive polymer and leaded to the release of the cargo. In a similar approach, we added a polyamine to the thermoresponsive capping polymer so different proteins could be retained within the polymeric shell. The idea was to deliver two types of cargoes: therapeutic agents inside the mesopores and biomolecules such as certain proteins on the shell of the nanocarriers [173]. Then, the application of an alternating magnetic field provoked that the iron oxide nanoparticles increased the local temperature so the conformation of the thermoresponsive polymer changed, opening the pore entrances and triggering both cargoes, therapeutic molecules and biomolecules.
In a different approach we developed magnetically responsive MSNs grafting a single DNA strand to their surface and then loading the cargo inside the pores [174]. Separately, the complementary DNA sequence was attached to magnetic iron oxide nanoparticles of ca. 5 nm. Then, both DNA-MSNs and DNA-iron oxide nanoparticles were mixed so DNA hybridized with the subsequent capping of the pores. The employed DNA sequence presented a melting temperature of 47 °C, so the alternating magnetic field increased the temperature of the iron oxide nanoparticles encapsulated into the MSNs network and their environment. This led to the double-stranded DNA melting, which promoted the aperture of the pores capping and the subsequent and cargo release. One of the interesting aspects of this proof of concept was that the DNA coupling was reversible, so when the magnetic field might be switched off, the system might reach physiological temperature and the DNA strands would hybridize again capping the pores. Then, they could be opened and closed again, leading to a pulsatile or on-off release mechanism.
3.2.4. Ultrasound
Ultrasound is considered as one of the most promising triggers for drug delivery nanosystems, although it has already been extensively employed in the clinic. The reason for such interest relays on its capacity to non-invasively penetrate deep into the living tissues without damaging them, among others. The thermal, mechanical and chemical effects from US have been employed for developing different US responsive nanocarriers, such as liposomes, micelles and polymeric nanoparticles. This sudden interest of the drug release scientific community is due to the characteristics that ultrasound can offer to the field, besides the high tissue penetration capacity: non-invasiveness, portability, economy and spatiotemporal controllability. As a consequence, US has become one of the most promising options for controlling the drug delivery within the biomedicine field [175].
The first combination of US with mesoporous silica was carried out using bulk mesoporous materials, such as MCM-41, and capping the pores with poly(dimethylsiloxane). The application of US triggered the release of the previous loaded ibuprofen, which was employed as a model drug [176]. Afterwards, ultrasound was applied to MSNs motivated by the fast development of MSNs with nanomedicine applications in the last few years [177–179]. However, if we compare US with other external stimuli such as light or magnetic field applied to MSNs, a delay in terms of research and publications on US-responsive MSNs can be observed.
Vallet-Regí group recently started to work with this stimulus developing US-sensitive MSNs in which the pore entrances were capped with a specially designed co-polymer [180].
The designed co-polymer presented a labile acetal group that can be cleaved upon US application leading to a different molecule with changed hydrophobicity. This phase transformation from hydrophobic to hydrophilic changed the polymer conformation and, consequently, opened the pore entrances and triggered the cargo release. These designed US-responsive MSNs were not toxic in vitro. Additionally, none of the by-products from the nanocarriers before and after ultrasound irradiation affected the cell viability. Moreover, the US exposure at 1 MHz and 15 W did not damage relevant biological molecules. The US responsiveness was retained after cellular internalisation, as observed loaded with doxorubicin (anticancer drug) and evaluated with prostate cancer cells (LNCaP). Before US irradiation no cellular toxicity was observed, which meant that the pores were appropriately capped, and no premature release took place. However, when the MSNs were exposed to US, cancer cells were killed, showing the capacity of the proposed system to be used in a potential cancer therapy.
As it has been mentioned throughout this manuscript, nanocarriers can be targeted and accumulated into tumours through either passive and/or active targeting. Both approaches are based on developing nanocarriers able to circulate within the blood stream for a long time. In this sense, nanoparticles should be stable in physiological media and our previously developed US-sensitive MSNs lacked this property. The reason for that was the presence of the co-polymer on their surface that made them highly hydrophobic at physiological media which leaded to nanoparticle aggregation. Therefore, Paris et al. covered those US-sensitive MSNs with a layer of PEG to prevent aggregation and we grafted active targeting moieties to develop a PEGylated and modularly targeted US-responsive nanocarrier [181].
Although MSNs can accumulate into tumour tissue through the EPR effect or the active targeting moiety, the treatment might not be completed until the cargo could be released into the cancer cells. Therefore, it is necessary for the nanoparticles to be up taken by the cancer cells, and this might be enhanced by the presence of internalisation ligands (such as certain targeting agents) or positively charged moieties on the surface of the nanoparticles. Paris and co-workers developed a novel strategy using MSNs positively charged, as the mechanism of internalisation, which was hidden by grafting PEG chains on the nanoparticle through a thermosensitive linker. Then, the application of US produced a local increase of temperature which leaded to the cleavage of the PEG chains from the nanoparticles exposing the positive charges of the nanoparticles and, therefore, favouring the cellular uptake [182].
3.3. Multi-stimuli responsive MSNs
The precise control of the specific delivery of therapeutics at the target tissue or cells can be ensured by designing controlled drug release triggered responsive to two different stimuli that potentially could work synergistically to ensure drug release only at the target site. The MSNs versatility and functionalization capabilities makes them very attractive to incorporate at least two responsive functional moieties in the same nanosystem (multi-stimuli responsive delivery). Hence, the pore blockers may be opened either by one or another stimulus or simultaneously by both. In other situations, it is proposed to use a stimuli cascade in which one stimulus triggers the performance of the event that uncap the MSNs or leads to a sequential release of different cargos. Different combinations of endogenous, exogenous or both kind of stimuli are described in this section.
Multidrug resistance (MDR) is a great problem in successful chemotherapy. In this sense, a study by Han et al. focused on hybrid lipid-capped MSNs (LTMSNs) as redox and pH stimuli-responsive drug release to circumvent MDR [183]. Lipid molecules composing polymer D-α-tocopherol-PEG-succinate were subsequently added to self-assemble into a surrounded lipid layer via hydrophobic interaction acting as smart valves to block the pore channels of carrier. This system loaded with DOX induced higher uptake efficiency, cytotoxicity, and increased intracellular accumulation in tumor MCF-7/Adr cells.
MSN-AuNPs through ion-ligand interactions have demonstrated to release the drug molecules under both pH and ATP concentration stimuli. The MSNs externally modified with -NH2 groups were capped with L-cysteine functionalized gold nanoparticles through Cu2+ ions. A rapid release of the guest molecule from the mesoporous silica host at pH below 5 was found because the ζ-potential of the cysteine-coated AuNPs switches from negative to positive. At the same time, the disruption of the capping by formation of ion-ligand bond between Cu2+ and ATP through a competitive binding mechanism is responsible for the AuNPs removal. Therefore, under both stimuli, the AuNPs leave the MSN surface remaining associated with Cu2+, then allowing the release of the entrapped drugs and minimal release of Cu2+ [184].
pH- and glucose-stimuli responsive MSNs for the controlled release of insulin were prepared by tethering insulin molecules onto the exterior surfaces of boronic acid-functionalized MSNs via gluconic acid linker groups. The surfaces of the resulting MSNs were coated with the adhesive and pH-sensitive polymer polyacrylic acid, which was used to protect the entrapped insulin from undergoing possible enzymatic degradations in locations such as the gastrointestinal tract. Besides, the polyacrylic acid, which has excellent dispersibility and adherence to the mucosa, might also allow the permeation of such nanoparticles through the colon, and thereby improves their potential application for insulin delivery. Because of their shrinkage at low pH and swelling at high pH, the polyacrylic acid-coated MSNs exhibited pH dependent insulin release properties. In addition to pH, glucose was shown to trigger the release of insulin from the nanoparticles, which demonstrates the nanosystem dual stimuli-responsive properties for the controlled release of insulin [185].
In the same line, polyacrylic acid has been obtained through the surface-initiated atom transfer radical polymerization at the mesopore openings and after glycosylation the chains were cross-linked through the formation of boronate esters to block the pores of MSNs. The boronate esters disassociated in the presence of glucose or in acidic conditions, which lead to opening of the mesoporous channels and therefore, combination of both pH and glucose stimuli exhibited an obvious enhanced release capacity in mild acidic conditions [186].
The fact that the presence of both a low pH and sugar molecules provides cooperative effects which together control the rate of release in boronic acid-based MSNs platforms has also been demonstrated by the group of Sttodart and Zink [187].
A multi stimuli responsive DDS based on dual enzyme and GDH sensitive was studied by Wang et al. Sulfhydryl and amino-functionalized MSNs (SH/NH2-MSNs) were grafted with multifunctional HA derivatives to control drug release and reverse MDR [188]. The DOX loaded multifunctional HA derivatives modified MSNs were enzyme and redox sensitive, in respond to the intracellular stimuli of hyaluronidase and GSH successively. In tumor MCF-7/ADR cells, this DDS was efficiently internalized and endocytosed, inducing a strongest cytotoxicity. In vivo, MCF-7/ADR tumor-bearing xenograft mouse models indicated that nanosystem enhanced tumor-targeting capacity reversing cancer MDR.
For multidrug delivery, different drugs can be released either simultaneously or in sequential method. A dual-stimuli DDS which is capable of releasing two different sized drugs step by step was prepared by capping the mesopores with β-cyclodextrin (β-CD) through photocleavable bonds to control its detachment. The cavity of β-CD was blocked by ferrocene through the host-guest interaction between ferrocene and β-CD. The small cargo can be released by the escape of ferrocenium under +1.5 V electro-stimuli.
However, the bigger sized cargo was released from the MSN only after the detachment of the β-CD cap from the MSN surface upon UV irradiation. Therefore, the different sized cargoes were released successfully step by step under external electro stimuli and UV-light, respectively [189].
The drug loading and transportation of drugs with no premature release to specific locations can be also controlled with dual-responsive polymers grafted on the MSNs surface that act as gatekeepers of the mesopores. For example, a temperature and ultrasound responsive copolymer allows the cargo loading at low temperature (4 °C), taking advantage of the coil-like or open conformation that the polymer presents under these conditions. When the temperature is increased to 37 °C, the copolymer changes to an insoluble collapsed state and the nanogates are closed retaining the cargo into the pores at physiological temperature. Upon ultrasound irradiation, the hydrophobic moieties of the copolymer are cleaved, leading to an increase of the hydrophilicity of the polymer and, therefore, its conformation changes toward coil-like opening the gates and releasing the entrapped cargo [180].
In a similar fashion, a temperature and ROS-responsive copolymer tethered to the outer surface of MSNs allows cargo loading in cold water, and subsequent closing of the mesopores by raising temperature at physiological conditions. Then, upon the stimulus of H2O2, the hydrophobic phenylboronic acid groups in the copolymer backbone are promptly oxidized to hydrophilic acrylate, which increases the solubility and open the nanogates at physiological temperature, inducing the release of the cargos from the pores [190].
The combination of therapeutic strategies has been proven to be a complementary strategy for traditional chemotherapy [1], among which the combination of chemotherapy and hyperthermia therapy is widely applied. Shu et al. developed a thermo/pH-dual-stimulus controlled drug release system by anchoring a thermo/pH-responsive polymer poly((N-isopropylacrylamide)-co-methacrylic acid) onto MSNs, functionalized as the gatekeeper [191]. The pNIPAM moiety facilitates the pores of MSN opens by a thermo induced collapse of the polymeric chain. The moiety of MA is pH sensitive, inducing the opening of gatekeeper followed by the release of loaded drugs, DOX and near infrared absorbing phototherapeutic agent (indocyanine green, ICG). DOX-ICG-MSN@pNIPAM nanocarriers enhanced the HeLa tumor cytotoxicity with a synergistic chemo-photothermal effect, with a favorable photothermal conversion capability under NIR irradiation for controlled drug release. In the context of combination therapy, Wang et al. fabricated a novel visible light and pH responsive polymer-coated MSN hybrid system by electrostatic interaction between MSNs and the perylene-functionalized polymer [192]. Loaded DOX into the MSN was efficient released at acid pH and induced a significant decreased of MFC-7 cancer cells, and these results were synergistic enhanced when combined with visible light irradiation. These nanohybrids could be a great potential for cancer therapy, counteracting the hard conditions of UV light or extreme pH stimulation.
The combination of chemotherapy and PTD has been also described in a multifunctional nanoplatform with pH-responsive drug release and NIR light-triggered PDT in a multilayer core@shell@shell structure. The UCNP core was coated with a dense silica shell embedded with the photosensitizer, methylene blue trapped in the silica matrix. After that, an outer most mesoporous silica shell was the nanoreservoir to load DOX and polyethyleneimine acts as a gatekeeper to block the pore channels preventing the loaded DOX from leaking. Distortion of the polyethyleneimine layer under acidic conditions will release the loaded DOX. Besides, 1O2 species were generated with 980 nm light irradiation because the embedded photosensitizer in the sandwich shell are activated by the upconverted 660 nm red light from the UCNP core [193].
In a recent example, the trigger to open the pores is self-generated upon the application of an external stimulus. DOX loaded MSNs are capped with a Gd-DOTA complex through a 1O2 sensitive aminoacrylate linker and a (PEG)-conjugated chlorin e6 is also attached to the MSNs surface as the ROS generator. The nanosystem is effectively accumulated in tumor tissue through passive targeting and, since the Gd-DOTA complex is a T1-contrast agent, the tumor site can be readily detected using MR imaging, allowing for site-specific laser irradiation to generate 1O2. Upon 660 nm laser irradiation, the gatekeeper was cleaved by the generated 1O2 and the DOX exhibited a rapid release. The in vivo assays showed that tumor growth was significantly suppressed with this multifunctional nanosystem [194].
González-Alvarez et al. reported a three gated MSNs able to deliver their cargo triggered by different stimuli: redox environment by a disulfide bond (S1), enzymatic hydrolysis (S2), and a surfactant or being in contact with cell membrane (S3) [195]. The nanocarrier was able to deliver cargo (safranin O) only in the presence of the corresponding stimuli: a reducing agent (GSH) for S1, pancreatin for S2, and a surfactant for S3. In Caco-2 monolayers, a model for drug transport in the intestinal mucosa, cell up take results indicated that S2-MSNs were successfully internalized by these cells via endocytosis. S2-MSNs with safranin O were included in an enteric coated capsule with Eudragit FS 30 D to target colon. In rats, the coated capsule containing the MSNs delivered S2 nanoparticles in colon tissue, releasing safranin O inside the colonic cells after the enzymatic stimuli and increasing the safranin O levels as well in colonic tissue.
4. Conclusion
The unique and groundbreaking features of MSNs as drug delivery nanocarriers have attracted the attention of the nanomedicine research community. A huge amount of work has opened up with outstanding potential applications for these advanced controlled drug release systems. However, their translation to the clinic remains a challenge because there are two major issues that should be solved: the standardization of materials by appropriate synthesis and physico-chemical characterization, and the homogenization of the biological evaluation. The first premise is feasible, and it has been already properly addressed by some authors, avoiding variability and lack of reproducibility. Furthermore, the standardization of the biological evaluation, although complex, is mandatory since the variation of the experimental designs for the in vivo studies is too broad to obtain valid conclusions.
5. Expert opinion
As it has been demonstrated both in this review and the previous one from 2015, the use of MSNs is a very prolific and competitive field of research. In fact, these types of particles have proved to be outstanding platforms for the development of nanosystems capable of fighting numerous types of cancer and bacterial infections at least at the basic research level. This is possible thanks to the particular structure presented by the MSNs, which is capable of loading and retaining drugs within its porous matrix. However, the advance of knowledge and the need to transfer these materials towards advanced preclinical models have revealed new aspects and effects that must be considered for the design of medical nanosystems. In a general way, these aspects range from classical physicochemical parameters to the presence of elements of cellular/bacterial recognition, passing through the highly convenient payload retention or the in vivo determination of particle trafficking.
Currently, nanomedicine-based treatment of cancer relies on the EPR effect, a phenomenon that accumulates nanoparticles and macromolecules into most of tumor masses. However, those nanotech-drugs have a long journey before they can reach this final destination. Indeed, in this way the particles must skip the constant vigilance of the immune system and avoid self-aggregation to remain in the bloodstream long enough to reach the tumors. To do so, the particles must be able to repel the numerous adherent opsonins present in the blood serum whose purpose is to trigger the excretion of xenobiotics. The most common resource for those purposes is the use of hydrophilic polymers such as PEG that effectively protect against the deposition of proteins and prevent interaction between particles. However, the indiscriminate use of PEG in the pharmaceutical industry has begun to generate sensitivity [196], which is a great disadvantage for the development and commercialization of nanotech-drugs. To avoid this dramatic effect, the scientific community has begun to develop alternative strategies. On the one hand the use of zwitterion components permits to create low-immunogenic hydrophilic coatings suitable for achieving high blood circulation times and low hemolytic materials. Whilst on the other hand, the development of bioinspired coatings by the selection of proteins of interest could be employed for creating artificial protein-corona coatings able to avoid the action of immune system.
Apart from an adequate shape and size and the ability to overcome the action of the immune system, the lone EPR effect may not be enough to efficiently attack tumors. This is partially because a tumor is formed by a large number of cells that coexist in different states (necrosis, apoptosis, cell constrainment, etc.) and together with support cells sequestered by the tumor (macrophages or vasculature epithelial cells among others). So it is imperative that particles are able to efficiently discriminate between such cell populations. That has been accomplished by the use of active targeting modification, in order to turn particles from passively accumulated carriers into cell-homing systems. Beyond classical recognition by affinity (aptamers, proteins and ABs), the current development of targeting tags seems to be shifted to less specific but more versatile interaction. For example, short peptides and small molecules seem to be the new standard as they could be easily prepared and modified and coupled. Moreover, the recent advances on multi-targeting also offer interesting approaches like tissue-to-cell or cell-to organelle targeting, which could be convenient for more precise guidance or more efficient killing abilities. Apart from this strategy, the preference for natural or biogenic components seem to be a common denominator as their utilization has grown with the promise of obtaining an easier transfer to preclinical testing.
Besides from the improvement in the recognition of cell and bacteria populations by nanoparticles, other relevant growing field of nanomedicine is enhancing the killing capacity of the systems. To this end, two differentiated strategies have been followed, either the simultaneous co-administration of drugs or the combination of physicochemical effects together with traditional drugs. Within the first approach, the use of siRNAs for the treatment of cancer and of specific antimicrobial peptides for the treatment of infection stand out, while within the second approach the examples with silica-containing nanocomposites able to generate thermal stress (hyperthermia and photothermal effects) or oxidative (PDT) are the most representative. The use of combination therapy of drugs using either siRNAs or antimicrobial peptides has the advantage of using compounds with very little toxicity able to induce great effects; although their long-term stability is often comprised due to their fast degradability in living systems. On the other hand, the use of thermal or oxidative stress show the enormous advantage of being able to be controlled at will by the clinician, even at the cost of presenting a possible chronic toxicity if the sensitizers are not quickly cleared or excreted.
In addition to all topics aimed at improving biosafety of MSNs in living systems, there must be also considered the porosity, which is exclusive to MSNs. As already indicated, the presence of those pores in the MSNs makes them exceptional materials for the loading of chemotherapeutic drugs and antibiotics. However, in the absence of control elements, such drugs will leak from the porous matrix following concentration gradients. This results in substantial drug losses and off-target delivery that must be minimized. Fortunately, nowadays there are many systems able to regulate the permanence of drugs within the porous matrix. However, the trend observed from latest publications seems to indicate that these systems are developed under three common guidelines: a preference for biodegradable and easily-clearable materials, the ability to respond to multiple stimuli with the idea of increasing their potential applicability and finally, the possibility for further functionalization with either active targeting moieties or immune-stealth modifications.
Besides the modifications needed to turn bare MSNs into high-performing drug nanocarriers, there are also several issues to be addressed regarding the nanosystems themselves. The following are worth to be mentioned: (1) there is no homogeneity nor common criteria about the nature and size of connecting fragments; (2) there is a lack of knowledge about the possible toxic behavior of composite species like UCNs, which use fluorides and rare-earth ions, or AuNPs that do not biodegrade and finally, (3) the way a set of given materials are structured may lead to different bioavailabilities / compatibilities depending on their disposition. The design of multi-stimuli responsive MSNs should not be too complex to facilitate the translation into the in vivo study and the use of the stimulus-response nanosystem should demonstrate clear advantage in clinical settings. In any case, the following aspects must be taken into account. First, that the biosuitability of the whole will be determined by the least biocompatible component; and second, that mesoporous silica has become a connective element in many hybrid nanocomposites. This apart from auguring a new era in the development of multi-therapeutic nanoplatforms also demands from extensive knowledge of silicon metabolism and possible long-term side effects.
Moreover, there is also a big gap regarding the available in vivo experiments, as most examples are based on human cell xenografts implanted in immunosuppressed murine models; which have demonstrated to be highly limited to obtain reliable conclusions. For example, murine xenografts differ from actual diseases in both the cells populations that form the tumor and in the degree of development reached before being treated. But in addition, very few articles deal with the fate and ways of clearance of nanomaterials, which probably would be dangerously accumulated. Therefore, it would also be convenient to undertake a systematic study on how different particles (size, shape, pore symmetry and connectivity and chemical composition) are more suitable to be used as nanotech drugs. And hence, within these parameters, to know their fate in living systems (processing, accumulation and elimination in living organisms) prior to develop novel strategies and composites would be also appropriate. Accurate pre-clinical animal studies using MSNs are needed to demonstrate its worth in the field of Nanomedicine aimed at the MSNs clinical breakthrough in the next future.
Article highlights.
Mesoporous silica nanoparticles (MSNs) have evidenced to be an outstanding platform for the design of nanosystems able to treat several types of diseases.
MSNs show high versatility and multifunctionality opening the range of nanoparticles possibilities in biomedical applications.
Their stimuli-responsive and targeting properties make MSNs promising therapeutic nanocarriers minimizing side effects of drugs.
These nanosystems can be designed to tackle different situations as simultaneous co-administration of drugs or the combination of certain physicochemical effects together with conventional drugs, so therapeutic efficacy would be improved.
Funding
This paper was funded by the European Research Council, ERC-2015-AdG (VERDI), proposal No. 694160.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- [1].Baeza A, Colilla M, Vallet-Regí M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery. Expert Opin Drug Deliv. 2015;12:319–37. doi: 10.1517/17425247.2014.953051. [·· A relevant review to understand the basics of drug delivery with MSNs and the basis for this revision] [DOI] [PubMed] [Google Scholar]
- [2].Vallet-Regí M, Balas F, Arcos D. Mesoporous Materials for Drug Delivery. Angew Chemie Int Ed. 2007;46:7548–58. doi: 10.1002/anie.200604488. [DOI] [PubMed] [Google Scholar]
- [3].Vallet-Regi M, Rámila A, del Real RP, et al. A New Property of MCM-41: Drug Delivery System. Chem Mater. 2001;13:308–11. [·The first example of drug delivery with MSNs] [Google Scholar]
- [4].Vallet-Regí M, Colilla M, Izquierdo-Barba I, et al. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules. 2017;23:47. doi: 10.3390/molecules23010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Croissant JG, Fatieiev Y, Khashab NM. Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv Mater. 2017;29 doi: 10.1002/adma.201604634. 1604634. [DOI] [PubMed] [Google Scholar]
- [6].Paris JL, Colilla M, Izquierdo-Barba I, et al. Tuning mesoporous silica dissolution in physiological environments: a review. J Mater Sci. 2017;52:8761–71. [Google Scholar]
- [7].Lu J, Liong M, Li Z, et al. Biocompatibility, Biodistribution, and Drug-Delivery Efficiency of Mesoporous Silica Nanoparticles for Cancer Therapy in Animals. Small. 2010;6:1794–805. doi: 10.1002/smll.201000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lindén M. Biodistribution and Excretion of Intravenously Injected Mesoporous Silica Nanoparticles: Implications for Drug Delivery Efficiency and Safety. The Enzymes. 2018;43:155–80. doi: 10.1016/bs.enz.2018.07.007. [· A reference review dealing with biodistribution and excretion of MSNs in vivo] [DOI] [PubMed] [Google Scholar]
- [9].Castillo RR, Vallet-Regí M. Functional Mesoporous Silica Nanocomposites: Biomedical Applications and Biosafety. Int J Molec Sci. 2019;20:929. doi: 10.3390/ijms20040929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rahikkala A, Pereira SAP, Figueiredo P, et al. Mesoporous Silica Nanoparticles for Targeted and Stimuli-Responsive Delivery of Chemotherapeutics: A Review. Adv Biosyst. 2018;2 1800020. [Google Scholar]
- [11].Du X, Qiao SZ. Dendritic Silica Particles with Center-Radial Pore Channels: Promising Platforms for Catalysis and Biomedical Applications. Small. 2015;11:392–413. doi: 10.1002/smll.201401201. [DOI] [PubMed] [Google Scholar]
- [12].Goel S, Chen F, Luan S, et al. Engineering Intrinsically Zirconium-89 Radiolabeled Self-Destructing Mesoporous Silica Nanostructures for In Vivo Biodistribution and Tumor Targeting Studies. Adv Sci. 2016;3 doi: 10.1002/advs.201600122. 1600122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kramer L, Winter G, Baur B, et al. Quantitative and correlative biodistribution analysis of 89 Zr-labeled mesoporous silica nanoparticles intravenously injected into tumor-bearing mice. Nanoscale. 2017;9:9743–53. doi: 10.1039/c7nr02050c. [DOI] [PubMed] [Google Scholar]
- [14].Yu T, Hubbard D, Ray A, et al. In vivo biodistribution and pharmacokinetics of silica nanoparticles as a function of geometry, porosity and surface characteristics. J Control Release. 2012;163:46–54. doi: 10.1016/j.jconrel.2012.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang Y-N, Poon W, Tavares AJ, et al. Nanoparticle–liver interactions: Cellular uptake and hepatobiliary elimination. J Control Release. 2016;240:332–48. doi: 10.1016/j.jconrel.2016.01.020. [DOI] [PubMed] [Google Scholar]
- [16].Pochert A, Vernikouskaya I, Pascher F, et al. Cargo-influences on the biodistribution of hollow mesoporous silica nanoparticles as studied by quantitative 19 F-magnetic resonance imaging. J Colloid Interface Sci. 2017;488:1–9. doi: 10.1016/j.jcis.2016.10.085. [DOI] [PubMed] [Google Scholar]
- [17].Liu D, Auguste DT. Cancer targeted therapeutics: From molecules to drug delivery vehicles. J Control Release. 2015;219:632–43. doi: 10.1016/j.jconrel.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nissinen T, Näkki S, Laakso H, et al. Tailored Dual PEGylation of Inorganic Porous Nanocarriers for Extremely Long Blood Circulation in Vivo. ACS Appl Mater Interfaces. 2016;8:32723–31. doi: 10.1021/acsami.6b12481. [DOI] [PubMed] [Google Scholar]
- [18].He Q, Zhang Z, Gao F, et al. In vivo Biodistribution and Urinary Excretion of Mesoporous Silica Nanoparticles: Effects of Particle Size and PEGylation. Small. 2011;7:271–80. doi: 10.1002/smll.201001459. [DOI] [PubMed] [Google Scholar]
- [20].Otsuka H, Nagasaki Y, Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Deliv Rev. 2012;64:246–55. doi: 10.1016/s0169-409x(02)00226-0. [DOI] [PubMed] [Google Scholar]
- [21].Izquierdo-Barba I, Colilla M, Vallet-Regí M. Zwitterionic ceramics for biomedical applications. Acta Biomater. 2016;40:201–11. doi: 10.1016/j.actbio.2016.02.027. [DOI] [PubMed] [Google Scholar]
- [22].Schöttler S, Becker G, Winzen S, et al. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat Nanotechnol. 2016;11:372–7. doi: 10.1038/nnano.2015.330. [DOI] [PubMed] [Google Scholar]
- [23].Beck M, Mandal T, Buske C, et al. Serum Protein Adsorption Enhances Active Leukemia Stem Cell Targeting of Mesoporous Silica Nanoparticles. ACS Appl Mater Interfaces. 2017;9:18566–74. doi: 10.1021/acsami.7b04742. [DOI] [PubMed] [Google Scholar]
- [24].Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer. Nanomaterials. 2017;7:189. doi: 10.3390/nano7070189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bharti C, Gulati N, Nagaich U, et al. Mesoporous silica nanoparticles in target drug delivery system: A review. Int J Pharm Investig. 2015;5:124. doi: 10.4103/2230-973X.160844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shao D, Lu M, Zhao Y, et al. The shape effect of magnetic mesoporous silica nanoparticles on endocytosis, biocompatibility and biodistribution. Acta Biomater. 2017;49:531–40. doi: 10.1016/j.actbio.2016.11.007. [DOI] [PubMed] [Google Scholar]
- [27].Li L, Liu T, Fu C, et al. Biodistribution, excretion, and toxicity of mesoporous silica nanoparticles after oral administration depend on their shape. Nanomed Nanotechnol Biol Med. 2015;11:1915–24. doi: 10.1016/j.nano.2015.07.004. [DOI] [PubMed] [Google Scholar]
- [28].He Q, Zhang J, Shi J, et al. The effect of PEGylation of mesoporous silica nanoparticles on nonspecific binding of serum proteins and cellular responses. Biomaterials. 2010;31:1085–92. doi: 10.1016/j.biomaterials.2009.10.046. [DOI] [PubMed] [Google Scholar]
- [29].Laschewsky A, Rosenhahn A. Molecular Design of Zwitterionic Polymer Interfaces: Searching for the Difference. Langmuir. 2019;35:1056–71. doi: 10.1021/acs.langmuir.8b01789. [DOI] [PubMed] [Google Scholar]
- [30].Khung YL, Narducci D. Surface modification strategies on mesoporous silica nanoparticle ºs for anti-biofouling zwitterionic film grafting. Adv Colloid Interface Sci. 2015;226:166–86. doi: 10.1016/j.cis.2015.10.009. [DOI] [PubMed] [Google Scholar]
- [31].Chen J, Liu M, Huang L, et al. Preparation of zwitterionic polymers functionalized fluorescent mesoporous silica nanoparticles through photoinduced surface initiated RAFT polymerization in the presence of oxygen. J Taiwan Inst Chem Eng. 2018;91:570–7. [Google Scholar]
- [32].Sanchez-Salcedo S, Vallet-Regí M, Shahin SA, et al. Mesoporous core-shell silica nanoparticles with anti-fouling properties for ovarian cancer therapy. Chem Eng J. 2018;340:114–24. [Google Scholar]
- [33].Encinas N, Angulo M, Astorga C, et al. Mixed-charge pseudo-zwitterionic mesoporous silica nanoparticles with low-fouling and reduced cell uptake properties. Acta Biomater. 2019;84:317–27. doi: 10.1016/j.actbio.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Butler KS, Durfee PN, Theron C, et al. Protocells: Modular Mesoporous Silica Nanoparticle-Supported Lipid Bilayers for Drug Delivery. Small. 2016;12:2173–85. doi: 10.1002/smll.201502119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].González B, Colilla M, Díez J, et al. Mesoporous silica nanoparticles decorated with polycationic dendrimers for infection treatment. Acta Biomater. 2018;68:261–71. doi: 10.1016/j.actbio.2017.12.041. [DOI] [PubMed] [Google Scholar]
- [36].Giret S, Wong Chi Man M, Carcel C. Mesoporous-Silica-Functionalized Nanoparticles for Drug Delivery. Chem - A Eur J. 2015;21:13850–65. doi: 10.1002/chem.201500578. [DOI] [PubMed] [Google Scholar]
- [37].Dréau D, Moore LJ, Alvarez-Berrios MP, et al. Mucin-1-Antibody-Conjugated Mesoporous Silica Nanoparticles for Selective Breast Cancer Detection in a Mucin-1 Transgenic Murine Mouse Model. J Biomed Nanotechnol. 2016;12:2172–84. doi: 10.1166/jbn.2016.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Goel S, Chen F, Luan S, et al. Engineering Intrinsically Zirconium-89 Radiolabeled Self-Destructing Mesoporous Silica Nanostructures for In vivo Biodistribution and Tumor Targeting Studies. Adv Sci. 2016;3:1600122. doi: 10.1002/advs.201600122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bouchoucha M, Béliveau É, Kleitz F, et al. Antibody-conjugated mesoporous silica nanoparticles for brain microvessel endothelial cell targeting. J Mater Chem B. 2017;5:7721–35. doi: 10.1039/c7tb01385j. [DOI] [PubMed] [Google Scholar]
- [40].Mandal T, Beck M, Kirsten N, et al. Targeting murine leukemic stem cells by antibody functionalized mesoporous silica nanoparticles. Sci Rep. 2018;8:2–9. doi: 10.1038/s41598-017-18932-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Villegas MR, Baeza A, Noureddine A, et al. Multifunctional Protocells for Enhanced Penetration in 3D Extracellular Tumoral Matrices. Chem Mater. 2018;30:112–20. [Google Scholar]
- [42].Li L, Lu Y, Jiang C, et al. Actively Targeted Deep Tissue Imaging and Photothermal-Chemo Therapy of Breast Cancer by Antibody-Functionalized Drug-Loaded X-Ray-Responsive Bismuth Sulfide@Mesoporous Silica Core–Shell Nanoparticles. Adv Funct Mater. 2018;28:1–13. doi: 10.1002/adfm.201704623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ngamcherdtrakul W, Sangvanich T, Reda M, et al. Lyophilization and stability of antibody-conjugated mesoporous silica nanoparticle with cationic polymer and PEG for siRNA delivery. Int J Nanomedicine. 2018;13:4015–27. doi: 10.2147/IJN.S164393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yamaguchi H, Hayama K, Sasagawa I, et al. HER2-targeted multifunctional silica nanoparticles specifically enhance the radiosensitivity of HER2-overexpressing breast cancer cells. Int J Mol Sci. 2018;19:908. doi: 10.3390/ijms19030908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Martínez-Carmona M, Baeza A, Rodriguez-Milla MA, et al. Mesoporous silica nanoparticles grafted with a light-responsive protein shell for highly cytotoxic antitumoral therapy. J Mater Chem B. 2015;3:5746–52. doi: 10.1039/c5tb00304k. [DOI] [PubMed] [Google Scholar]
- [46].Chen X, Sun H, Hu J, et al. Transferrin gated mesoporous silica nanoparticles for redox-responsive and targeted drug delivery. Colloids Surfaces B Biointerfaces. 2017;152:77–84. doi: 10.1016/j.colsurfb.2017.01.010. [DOI] [PubMed] [Google Scholar]
- [47].Montalvo-Quiros S, Aragoneses-Cazorla G, Garcia-Alcalde L, et al. Cancer cell targeting and therapeutic delivery of silver nanoparticles by mesoporous silica nanocarriers: insights into the action mechanisms using quantitative proteomics. Nanoscale. 2019;11:4531–45. doi: 10.1039/C8NR07667G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Martínez-Carmona M, Lozano D, Colilla M, et al. Lectin-conjugated pH-responsive mesoporous silica nanoparticles for targeted bone cancer treatment. Acta Biomater. 2018;65:393–404. doi: 10.1016/j.actbio.2017.11.007. [DOI] [PubMed] [Google Scholar]
- [49].Roy B, Pattanaik AK, Das J, et al. Role of PI3K/Akt/mTOR and MEK/ERK pathway in Concanavalin A induced autophagy in HeLa cells. Chem Biol Interact. 2014;210:96–102. doi: 10.1016/j.cbi.2014.01.003. [DOI] [PubMed] [Google Scholar]
- [50].Bhat R, García I, Aznar E, et al. Lectin-gated and glycan functionalized mesoporous silica nanocontainers for targeting cancer cells overexpressing Lewis X antigen. Nanoscale. 2018;10:239–49. doi: 10.1039/c7nr06415b. [DOI] [PubMed] [Google Scholar]
- [51].Zhang Y, Guo J, Zhang XL, et al. Antibody fragment-armed mesoporous silica nanoparticles for the targeted delivery of bevacizumab in ovarian cancer cells. Int J Pharm. 2015;496:1026–33. doi: 10.1016/j.ijpharm.2015.10.080. [DOI] [PubMed] [Google Scholar]
- [52].Oh JY, Kim HS, Palanikumar L, et al. Cloaking nanoparticles with protein corona shield for targeted drug delivery. Nat Commun. 2018;9:4548. doi: 10.1038/s41467-018-06979-4. [· An example of a bifunctional protein able to cap pores and target MSNs] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hu J-J, Xiao D, Zhang X-Z. Advances in Peptide Functionalization on Mesoporous Silica Nanoparticles for Controlled Drug Release. Small. 2016;12:3344–59. doi: 10.1002/smll.201600325. [DOI] [PubMed] [Google Scholar]
- [54].Cheng Y-J, Luo G-F, Zhu J-Y, et al. Enzyme-Induced and Tumor-Targeted Drug Delivery System Based on Multifunctional Mesoporous Silica Nanoparticles. ACS Appl Mater Interfaces. 2015;7:9078–87. doi: 10.1021/acsami.5b00752. [DOI] [PubMed] [Google Scholar]
- [55].Hu J, Zhang X, Wen Z, et al. Asn-Gly-Arg-modified polydopamine-coated nanoparticles for dual-targeting therapy of brain glioma in rats. Oncotarget. 2016;7:73681–96. doi: 10.18632/oncotarget.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lee J, Oh ET, Han Y, et al. Mesoporous Silica Nanocarriers with Cyclic Peptide Gatekeeper: Specific Targeting of Aminopeptidase N and Triggered Drug Release by Stimuli-Responsive Conformational Transformation. Chem - A Eur J. 2017;23:16966–71. doi: 10.1002/chem.201704309. [DOI] [PubMed] [Google Scholar]
- [57].Shi J, Hou S, Huang J, et al. An MSN-PEG-IP drug delivery system and IL13R α2 as targeted therapy for glioma. Nanoscale. 2017;9:8970–81. doi: 10.1039/c6nr08786h. [DOI] [PubMed] [Google Scholar]
- [58].Wei X, Zhan C, Shen Q, et al. A D-peptide ligand of nicotine acetylcholine receptors for brain-targeted drug delivery. Angew Chemie - Int Ed. 2015;54:3023–7. doi: 10.1002/anie.201411226. [DOI] [PubMed] [Google Scholar]
- [59].Villaverde G, Gómez-Graña S, Guisasola E, et al. Targeted Chemo-Photothermal Therapy: A Nanomedicine Approximation to Selective Melanoma Treatment. Part Part Syst Charact. 2018;35:1800148. [Google Scholar]
- [60].Wei Y, Gao L, Wang L, et al. Polydopamine and peptide decorated doxorubicin-loaded mesoporous silica nanoparticles as a targeted drug delivery system for bladder cancer therapy. Drug Deliv. 2017;24:681–91. doi: 10.1080/10717544.2017.1309475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lee J, Oh ET, Choi MH, et al. Dual-functional cyclic peptide switch on mesoporous nanocontainers for selective CD44 targeting and on-off gatekeeping triggered by conformational transformation. New J Chem. 2018;42:12938–44. [Google Scholar]
- [62].Liu Y, Chen Q, Xu M, et al. Single peptide ligand-functionalized uniform hollow mesoporous silica nanoparticles achieving dual-targeting drug delivery to tumor cells and angiogenic blood vessel cells. Int J Nanomedicine. 2015;10:1855. doi: 10.2147/IJN.S75098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wang Y, Zhao Z, Wei F, et al. Combining autophagy-inducing peptides and brefeldin A delivered by perinuclear-localized mesoporous silica nanoparticles: A manipulation strategy for ER-phagy. Nanoscale. 2018;10:8796–805. doi: 10.1039/c8nr00872h. [DOI] [PubMed] [Google Scholar]
- [64].Roveri M, Bernasconi M, Leroux J-C, et al. Peptides for tumor-specific drug targeting: state of the art and beyond. J Mater Chem B. 2017;5:4348–64. doi: 10.1039/c7tb00318h. [DOI] [PubMed] [Google Scholar]
- [65].Zhang D, Wang J, Xu D. Cell-penetrating peptides as noninvasive transmembrane vectors for the development of novel multifunctional drug-delivery systems. J Control Release. 2016;229:130–9. doi: 10.1016/j.jconrel.2016.03.020. [DOI] [PubMed] [Google Scholar]
- [66].de la Torre C, Domínguez-Berrocal L, Murguía JR, et al. ϵ-Polylysine-Capped Mesoporous Silica Nanoparticles as Carrier of the C9h Peptide to Induce Apoptosis in Cancer Cells. Chem - A Eur J. 2018;24:1890–7. doi: 10.1002/chem.201704161. [DOI] [PubMed] [Google Scholar]
- [67].Borrelli A, Tornesello A, Tornesello M, et al. Cell Penetrating Peptides as Molecular Carriers for Anti-Cancer Agents. Molecules. 2018;23:295. doi: 10.3390/molecules23020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zhou X, Feng W, Qiu K, et al. BMP-2 Derived Peptide and Dexamethasone Incorporated Mesoporous Silica Nanoparticles for Enhanced Osteogenic Differentiation of Bone Mesenchymal Stem Cells. ACS Appl Mater Interfaces. 2015;7:15777–89. doi: 10.1021/acsami.5b02636. [DOI] [PubMed] [Google Scholar]
- [69].Castillo RR, Baeza A, Vallet-Regí M. Recent applications of the combination of mesoporous silica nanoparticles with nucleic acids: development of bioresponsive devices, carriers and sensors. Biomater Sci. 2017;5:353–77. doi: 10.1039/c6bm00872k. [DOI] [PubMed] [Google Scholar]
- [70].Alshaer W, Hillaireau H, Fattal E. Aptamer-guided nanomedicines for anticancer drug delivery. Adv Drug Deliv Rev. 2018;134:122–37. doi: 10.1016/j.addr.2018.09.011. [DOI] [PubMed] [Google Scholar]
- [71].Li Y, Duo Y, Zhai P, et al. Dual targeting delivery of miR-328 by functionalized mesoporous silica nanoparticles for colorectal cancer therapy. Nanomedicine. 2018;13:1753–72. doi: 10.2217/nnm-2017-0353. [DOI] [PubMed] [Google Scholar]
- [72].Tang Y, Hu H, Zhang MG, et al. An aptamer-targeting photoresponsive drug delivery system using “off-on” graphene oxide wrapped mesoporous silica nanoparticles. Nanoscale. 2015;7:6304–10. doi: 10.1039/c4nr07493a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wang K, Yao H, Meng Y, et al. Specific aptamer-conjugated mesoporous silica-carbon nanoparticles for HER2-targeted chemo-photothermal combined therapy. Acta Biomater. 2015;16:196–205. doi: 10.1016/j.actbio.2015.01.002. [DOI] [PubMed] [Google Scholar]
- [74].Babaei M, Abnous K, Taghdisi SM, et al. Synthesis of theranostic epithelial cell adhesion molecule targeted mesoporous silica nanoparticle with gold gatekeeper for hepatocellular carcinoma. Nanomedicine. 2017;12:1261–79. doi: 10.2217/nnm-2017-0028. [DOI] [PubMed] [Google Scholar]
- [75].Li Y, Duo Y, Bao S, et al. EpCAM aptamer-functionalized polydopamine-coated mesoporous silica nanoparticles loaded with DM1 for targeted therapy in colorectal cancer. Int J Nanomedicine. 2017;12:6239–57. doi: 10.2147/IJN.S143293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Xie X, Li F, Zhang H, et al. EpCAM aptamer-functionalized mesoporous silica nanoparticles for efficient colon cancer cell-targeted drug delivery. Eur J Pharm Sci. 2016;83:28–35. doi: 10.1016/j.ejps.2015.12.014. [DOI] [PubMed] [Google Scholar]
- [77].Pascual L, Cerqueira-Coutinho C, García-Fernández A, et al. MUC1 aptamer-capped mesoporous silica nanoparticles for controlled drug delivery and radio-imaging applications. Nanomedicine Nanotechnology. Biol Med. 2017;13:2495–505. doi: 10.1016/j.nano.2017.08.006. [DOI] [PubMed] [Google Scholar]
- [78].Tan J, Yang N, Zhong L, et al. A new theranostic system based on endoglin aptamer conjugated fluorescent silica nanoparticles. Theranostics. 2017;7:4862–76. doi: 10.7150/thno.19101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zhang Y, Hou Z, Ge Y, et al. DNA-Hybrid-Gated Photothermal Mesoporous Silica Nanoparticles for NIR-Responsive and Aptamer-Targeted Drug Delivery. ACS Appl Mater Interfaces. 2015;7:20696–706. doi: 10.1021/acsami.5b05522. [DOI] [PubMed] [Google Scholar]
- [80].Dai L, Zhang Q, Shen X, et al. A pH-responsive nanocontainer based on hydrazone-bearing hollow silica nanoparticles for targeted tumor therapy. J Mater Chem B. 2016;4:4594–604. doi: 10.1039/c6tb01050d. [DOI] [PubMed] [Google Scholar]
- [81].Huang L, Liu J, Gao F, et al. A dual-responsive, hyaluronic acid targeted drug delivery system based on hollow mesoporous silica nanoparticles for cancer therapy. J Mater Chem B. 2018;6:4618–29. doi: 10.1039/c8tb00989a. [DOI] [PubMed] [Google Scholar]
- [82].Shahin SA, Wang R, Simargi SI, et al. Hyaluronic acid conjugated nanoparticle delivery of siRNA against TWIST reduces tumor burden and enhances sensitivity to cisplatin in ovarian cancer. Nanomedicine Nanotechnology Biol Med. 2018;14:1381–94. doi: 10.1016/j.nano.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ricci V, Zonari D, Cannito S, et al. Hyaluronated mesoporous silica nanoparticles for active targeting: influence of conjugation method and hyaluronic acid molecular weight on the nanovector properties. J Colloid Interface Sci. 2018;516:484–97. doi: 10.1016/j.jcis.2018.01.072. [DOI] [PubMed] [Google Scholar]
- [84].Dai L, Zhang Q, Li J, et al. Dendrimerlike mesoporous silica nanoparticles as pH-responsive nanocontainers for targeted drug delivery and bioimaging. ACS Appl Mater Interfaces. 2015;7:7357–72. doi: 10.1021/acsami.5b00746. [DOI] [PubMed] [Google Scholar]
- [85].Chai S, Guo Y, Zhang Z, et al. Cyclodextrin-gated mesoporous silica nanoparticles as drug carriers for red light-induced drug release. Nanotechnology. 2017;28:145101. doi: 10.1088/1361-6528/aa5e74. [DOI] [PubMed] [Google Scholar]
- [86].Chen C, Yao W, Sun W, et al. A self-targeting and controllable drug delivery system constituting mesoporous silica nanoparticles fabricated with a multi-stimuli responsive chitosan-based thin film layer. Int J Biol Macromol. 2019;122:1090–9. doi: 10.1016/j.ijbiomac.2018.09.058. [DOI] [PubMed] [Google Scholar]
- [87].AbouAitah K, Swiderska-Sroda A, Farghali AA, et al. Folic acid-conjugated mesoporous silica particles as nanocarriers of natural prodrugs for cancer targeting and antioxidant action. Oncotarget. 2018;9:26466–90. doi: 10.18632/oncotarget.25470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lv G, Qiu L, Liu G, et al. pH sensitive chitosan-mesoporous silica nanoparticles for targeted delivery of a ruthenium complex with enhanced anticancer effects. DaltTrans. 2016;45:18147–55. doi: 10.1039/c6dt03783f. [DOI] [PubMed] [Google Scholar]
- [89].Castillo RR, Hernández-Escobar D, Gómez-Graña S, et al. Reversible Nanogate System for Mesoporous Silica Nanoparticles Based on Diels-Alder Adducts. Chem - A Eur J. 2018;24:6992–7001. doi: 10.1002/chem.201706100. [DOI] [PubMed] [Google Scholar]
- [90].Li Z, Zhang Y, Zhang K, et al. Biotinylated-lipid bilayer coated mesoporous silica nanoparticles for improving the bioavailability and anti-leukaemia activity of Tanshinone IIA. Artif Cells, Nanomedicine, Biotechnol. 2019;46:S578–S587. doi: 10.1080/21691401.2018.1431651. [DOI] [PubMed] [Google Scholar]
- [91].Lv G, Li K, Qiu L, et al. Enhanced Tumor Diagnostic and Therapeutic Effect of Mesoporous Silica Nanoparticle-Mediated Pre-targeted Strategy. Pharm Res. 2018;35:63. doi: 10.1007/s11095-017-2338-5. [DOI] [PubMed] [Google Scholar]
- [92].Thepphankulngarm N, Wonganan P, Sapcharoenkun C, et al. Combining Vitamin B12 and cisplatin-loaded porous silica nanoparticles via coordination: A facile approach to prepare a targeted drug delivery system. New J Chem. 2017;41:13823–9. [Google Scholar]
- [93].Liu J, Zhang B, Luo Z, et al. Enzyme responsive mesoporous silica nanoparticles for targeted tumor therapy in vitro and in vivo. Nanoscale. 2015;7:3614–26. doi: 10.1039/c5nr00072f. [DOI] [PubMed] [Google Scholar]
- [94].Qu Q, Ma X, Zhao Y. Targeted delivery of doxorubicin to mitochondria using mesoporous silica nanoparticle nanocarriers. Nanoscale. 2015;7:16677–86. doi: 10.1039/c5nr05139h. [DOI] [PubMed] [Google Scholar]
- [95].Luo GF, Chen WH, Liu Y, et al. Multifunctional enveloped mesoporous silica nanoparticles for subcellular co-delivery of drug and therapeutic peptide. Sci Rep. 2014;4 doi: 10.1038/srep06064. 6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ahn J, Lee B, Choi Y, et al. Non-peptidic guanidinium-functionalized silica nanoparticles as selective mitochondria-targeting drug nanocarriers. J Mater Chem B. 2018;6:5698–707. doi: 10.1039/c8tb01358f. [DOI] [PubMed] [Google Scholar]
- [97].Villaverde G, Baeza A, Melen GJ, et al. A new targeting agent for the selective drug delivery of nanocarriers for treating neuroblastoma. J Mater Chem B. 2015;3:4831–42. doi: 10.1039/c5tb00287g. [DOI] [PubMed] [Google Scholar]
- [98].Villaverde G, Alfranca A, Gonzalez-Murillo Á, et al. Molecular Scaffolds as Double-Targeting Agents For the Diagnosis and Treatment of Neuroblastoma. AngewChemie Int Ed. 2019;58:3067–72. doi: 10.1002/anie.201811691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Castillo RR, Colilla M, Vallet-Regí M. Advances in mesoporous silica-based nanocarriers for co-delivery and combination therapy against cancer. Expert Opin Drug Deliv. 2017;14:229–43. doi: 10.1080/17425247.2016.1211637. [DOI] [PubMed] [Google Scholar]
- [100].Pan L, Liu J, He Q, et al. MSN-Mediated Sequential Vascular-to-Cell Nuclear-Targeted Drug Delivery for Efficient Tumor Regression. Adv Mater. 2014;26:6742–8. doi: 10.1002/adma.201402752. [DOI] [PubMed] [Google Scholar]
- [101].Gao H, Xiong Y, Zhang S, et al. RGD and interleukin-13 peptide functionalized nanoparticles for enhanced glioblastoma cells and neovasculature dual targeting delivery and elevated tumor penetration. Mol Pharm. 2014;11:1042–52. doi: 10.1021/mp400751g. [DOI] [PubMed] [Google Scholar]
- [102].Xiong L, Du X, Kleitz F, et al. Cancer-Cell-Specific Nuclear-Targeted Drug Delivery by Dual-Ligand-Modified Mesoporous Silica Nanoparticles. Small. 2015;11:5919–26. doi: 10.1002/smll.201501056. [DOI] [PubMed] [Google Scholar]
- [103].Desquiret V, Gueguen N, Malthièry, et al. Mitochondrial effects of dexamethasone imply both membrane and cytosolic-initiated pathways in HepG2 cells. Int J Biochem Cell Biol. 2008;40:1629–41. doi: 10.1016/j.biocel.2007.12.010. [DOI] [PubMed] [Google Scholar]
- [104].López V, Villegas MR, Rodríguez V, et al. Janus Mesoporous Silica Nanoparticles for Dual Targeting of Tumor Cells and Mitochondria. ACS Appl Mater Interfaces. 2017;9:26697–706. doi: 10.1021/acsami.7b06906. [DOI] [PubMed] [Google Scholar]
- [105].Castillo RR, Lozano D, Vallet-Regí M. Building Block Based Construction of Membrane-Organelle Double Targeted Nanosystem for Two-Drug Delivery. Bioconjug Chem. 2018;29:3677–85. doi: 10.1021/acs.bioconjchem.8b00603. [DOI] [PubMed] [Google Scholar]
- [106].Zhu Y, Feijen J, Zhong Z. Dual-targeted nanomedicines for enhanced tumor treatment. Nano Today. 2018;18:65–85. [Google Scholar]
- [107].Gao C, Lin Z, Wu Z, et al. Stem-Cell-Membrane Camouflaging on Near-Infrared Photoactivated Upconversion Nanoarchitectures for in vivo Remote-Controlled Photodynamic Therapy. ACS Appl Mater Interfaces. 2016;8:34252–60. doi: 10.1021/acsami.6b12865. [DOI] [PubMed] [Google Scholar]
- [108].Xuan M, Shao J, Zhao J, et al. Magnetic Mesoporous Silica Nanoparticles Cloaked by Red Blood Cell Membranes: Applications in Cancer Therapy. Angew Chemie - Int Ed. 2018;57:6049–53. doi: 10.1002/anie.201712996. [DOI] [PubMed] [Google Scholar]
- [109].Manzano M, Vallet-Regí M. Mesoporous silica nanoparticles in nanomedicine applications. J Mater Sci Mater Med. 2018;29:65. doi: 10.1007/s10856-018-6069-x. [DOI] [PubMed] [Google Scholar]
- [110].Kumar N, Chen W, Cheng C-A, et al. Stimuli-Responsive Nanomachines and Caps for Drug Delivery. The Enzymes. 2018;43:31–65. doi: 10.1016/bs.enz.2018.07.003. [DOI] [PubMed] [Google Scholar]
- [111].Martínez-Carmona M, Colilla M, Vallet-Regí M. Smart Mesoporous Nanomaterials for Antitumor Therapy. Nanomaterials. 2015;5:1906–37. doi: 10.3390/nano5041906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Chen G, Xie Y, Peltier R, et al. Peptide-Decorated Gold Nanoparticles as Functional Nano-Capping Agent of Mesoporous Silica Container for Targeting Drug Delivery. ACS Appl Mater Interfaces. 2016;8:11204–9. doi: 10.1021/acsami.6b02594. [DOI] [PubMed] [Google Scholar]
- [113].Yan Y, Fu J, Wang T, et al. Controlled release of silyl ether camptothecin from thiolene click chemistry-functionalized mesoporous silica nanoparticles. Acta Biomater. 2017;51:471–8. doi: 10.1016/j.actbio.2017.01.062. [DOI] [PubMed] [Google Scholar]
- [114].Santha Moorthy M, Bharathiraja S, Manivasagan P, et al. Synthesis of surface capped mesoporous silica nanoparticles for pH-stimuli responsive drug delivery applications. Medchemcomm. 2017;8:1797–805. doi: 10.1039/c7md00270j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Li Q-L, Sun Y, Sun Y-L, et al. Mesoporous Silica Nanoparticles Coated by Layer-by-Layer Self-assembly Using Cucurbit[7]uril for in Vitro and in vivo Anticancer Drug Release. Chem Mater. 2014;26:6418–31. doi: 10.1021/cm503304p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Hakeem A, Zahid F, Zhan G, et al. Polyaspartic acid-anchored mesoporous silica nanoparticles for pH-responsive doxorubicin release. Int J Nanomedicine. 2018;13:1029–40. doi: 10.2147/IJN.S146955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Gisbert-Garzaran M, Lozano D, Vallet-Regí M, et al. Self-immolative polymers as novel pH-responsive gate keepers for drug delivery. RSC Adv. 2017;7:132–6. [Google Scholar]
- [118].Pan Q-S, Chen T-T, Nie C-P, et al. In Situ Synthesis of Ultrathin ZIF-8 Film-Coated MSNs for Codelivering Bcl 2 siRNA and Doxorubicin to Enhance Chemotherapeutic Efficacy in Drug-Resistant Cancer Cells. ACS Appl Mater Interfaces. 2018;10:33070–7. doi: 10.1021/acsami.8b13393. [· An interesting example of drug co-delivery without stimulation] [DOI] [PubMed] [Google Scholar]
- [119].Sha L, Wang D, Mao Y, et al. Hydrophobic interaction mediated coating of pluronics on mesoporous silica nanoparticle with stimuli responsiveness for cancer therapy. Nanotechnology. 2018;29 doi: 10.1088/1361-6528/aac6b1. 345101. [DOI] [PubMed] [Google Scholar]
- [120].Lin J-T, Liu Z-K, Zhu Q-L, et al. Redox-responsive nanocarriers for drug and gene co-delivery based on chitosan derivatives modified mesoporous silica nanoparticles. Colloids Surfaces B Biointerfaces. 2017;155:41–50. doi: 10.1016/j.colsurfb.2017.04.002. [DOI] [PubMed] [Google Scholar]
- [121].Prabhakar N, Zhang J, Desai D, et al. Stimuli-responsive hybrid nanocarriers developed by controllable integration of hyperbranched PEI with mesoporous silica nanoparticles for sustained intracellular siRNA delivery. Int J Nanomedicine. 2016;11:6591–608. doi: 10.2147/IJN.S120611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Lee J, Oh E-T, Yoon H, et al. A mesoporous nanocontainer gated by a stimuli-responsive peptide for selective triggering of intracellular drug release. Nanoscale. 2016;8:8070–7. doi: 10.1039/c5nr09280a. [DOI] [PubMed] [Google Scholar]
- [123].Cheng Y, Jiao X, Xu T, et al. Free-Blockage Mesoporous Anticancer Nanoparticles Based on ROS-Responsive Wetting Behavior of Nanopores. Small. 2017;13:1701942. doi: 10.1002/smll.201701942. [DOI] [PubMed] [Google Scholar]
- [124].Liu Y, Ding X, Li J, et al. Enzyme responsive drug delivery system based on mesoporous silica nanoparticles for tumor therapy in vivo. Nanotechnology. 2015;26:145102. doi: 10.1088/0957-4484/26/14/145102. [DOI] [PubMed] [Google Scholar]
- [125].Baeza A, Guisasola E, Torres-Pardo A, et al. Hybrid Enzyme-Polymeric Capsules/Mesoporous Silica Nanodevice for In Situ Cytotoxic Agent Generation. Adv Funct Mater. 2014;24:4625–33. [Google Scholar]
- [126].Zhao Y, Trewyn BG, Slowing II, et al. Mesoporous Silica Nanoparticle-Based Double Drug Delivery System for Glucose-Responsive Controlled Release of Insulin and Cyclic AMP. J Am Chem Soc. 2009;131:8398–400. doi: 10.1021/ja901831u. [DOI] [PubMed] [Google Scholar]
- [127].Zou Z, He D, Cai L, et al. Alizarin Complexone Functionalized Mesoporous Silica Nanoparticles: A Smart System Integrating Glucose-Responsive Double-Drugs Release and Real-Time Monitoring Capabilities. ACS Appl Mater Interfaces. 2016;8:8358–66. doi: 10.1021/acsami.5b12576. [DOI] [PubMed] [Google Scholar]
- [128].Xu B, Jiang G, Yu W, et al. H2O2-Responsive mesoporous silica nanoparticles integrated with microneedle patches for the glucose-monitored transdermal delivery of insulin. J Mater Chem B. 2017;5:8200–8. doi: 10.1039/c7tb02082a. [DOI] [PubMed] [Google Scholar]
- [129].Uttara B, Singh A, Zamboni P, et al. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Waris G, Ahsan H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Brieger K, Schiavone S, Miller J, et al. Reactive oxygen species: from health to disease. Swiss Med Wkly. 2012;142:w13659. doi: 10.4414/smw.2012.13659. [DOI] [PubMed] [Google Scholar]
- [132].Muhammad F, Qi W, Wang A, et al. Using oxidant susceptibility of thiol stabilized nanoparticles to develop an inflammation triggered drug release system. J Mater Chem B. 2015;3:1597–604. doi: 10.1039/c4tb01709a. [DOI] [PubMed] [Google Scholar]
- [133].Hu J-J, Lei Q, Peng M-Y, et al. A positive feedback strategy for enhanced chemotherapy based on ROS-triggered self-accelerating drug release nanosystem. Biomaterials. 2017;128:136–46. doi: 10.1016/j.biomaterials.2017.03.010. [DOI] [PubMed] [Google Scholar]
- [134].Pei Y, Li M, Hou Y, et al. An autonomous tumor-targeted nanoprodrug for reactive oxygen species-activatable dual-cytochrome c/doxorubicin antitumor therapy. Nanoscale. 2018;10:11418–29. doi: 10.1039/c8nr02358a. [DOI] [PubMed] [Google Scholar]
- [135].Shen Y, Cao B, Snyder NR, et al. ROS responsive resveratrol delivery from LDLR peptide conjugated PLA-coated mesoporous silica nanoparticles across the blood–brain barrier. J Nanobiotechnology. 2018;16:13. doi: 10.1186/s12951-018-0340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Gribble FM, Loussouarn G, Tucker SJ, et al. A Novel Method for Measurement of Submembrane ATP Concentration. J Biol Chem. 2000;275:30046–9. doi: 10.1074/jbc.M001010200. [DOI] [PubMed] [Google Scholar]
- [137].Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994;140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- [138].Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP–dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- [139].Mo R, Jiang T, DiSanto R, et al. ATP-triggered anticancer drug delivery. Nat Commun. 2014;5 doi: 10.1038/ncomms4364. 3364. [DOI] [PubMed] [Google Scholar]
- [140].Zheng F-F, Zhang P-H, Xi Y, et al. Aptamer/Graphene Quantum Dots Nanocomposite Capped Fluorescent Mesoporous Silica Nanoparticles for Intracellular Drug Delivery and Real-Time Monitoring of Drug Release. Anal Chem. 2015;87:11739–45. doi: 10.1021/acs.analchem.5b03131. [DOI] [PubMed] [Google Scholar]
- [141].Lai J, Shah BP, Zhang Y, et al. Real-Time Monitoring of ATP-Responsive Drug Release Using Mesoporous-Silica-Coated Multicolor Upconversion Nanoparticles. ACS Nano. 2015;9:5234–45. doi: 10.1021/acsnano.5b00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Mal NK, Fujiwara M, Tanaka Y. Photocontrolled reversible release of guest molecules from coumarin-modified mesoporous silica. Nature. 2003;421:350–3. doi: 10.1038/nature01362. [DOI] [PubMed] [Google Scholar]
- [143].Angelos S, Choi E, Vögtle F, et al. Photo-Driven Expulsion of Molecules from Mesostructured Silica Nanoparticles. J Phys Chem C. 2007;111:6589–92. [Google Scholar]
- [144].Lu J, Choi E, Tamanoi F, et al. Light-Activated Nanoimpeller-Controlled Drug Release in Cancer Cells. Small. 2008;4:421–26. doi: 10.1002/smll.200700903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Tarn D, Ferris DP, Barnes JC, et al. A reversible light-operated nanovalve on mesoporous silica nanoparticles. Nanoscale. 2014;6:3335. doi: 10.1039/c3nr06049g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Martínez-Carmona M, Lozano D, Baeza A, et al. A novel visible light responsive nanosystem for cancer treatment. Nanoscale. 2017;9:15967–73. doi: 10.1039/c7nr05050j. [DOI] [PubMed] [Google Scholar]
- [147].Lavado AS, Chauhan VM, Alhaj Zen A, et al. Controlled intracellular generation of reactive oxygen species in human mesenchymal stem cells using porphyrin conjugated nanoparticles. Nanoscale. 2015;7:14525–31. doi: 10.1039/c5nr00795j. [DOI] [PubMed] [Google Scholar]
- [148].Zhang W, Shen J, Su H, et al. Co-Delivery of Cisplatin Prodrug and Chlorin e6 by Mesoporous Silica Nanoparticles for Chemo-Photodynamic Combination Therapy to Combat Drug Resistance. ACS Appl Mater Interfaces. 2016;8:13332–40. doi: 10.1021/acsami.6b03881. [DOI] [PubMed] [Google Scholar]
- [149].Yang G, Sun X, Liu J, et al. Light-Responsive, Singlet-Oxygen-Triggered On-Demand Drug Release from Photosensitizer-Doped Mesoporous Silica Nanorods for Cancer Combination Therapy. Adv Funct Mater. 2016;26:4722–32. [Google Scholar]
- [150].Smith AM, Mancini MC, Nie S. Second window for in vivo imaging. Nat Nanotechnol. 2009;4:710–1. doi: 10.1038/nnano.2009.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Zhao T, Chen L, Li Q, et al. Near-infrared light triggered drug release from mesoporous silica nanoparticles. J Mater Chem B. 2018;6:7112–21. doi: 10.1039/c8tb01548a. [DOI] [PubMed] [Google Scholar]
- [152].Deng K, Li C, Huang S, et al. Recent Progress in Near Infrared Light Triggered Photodynamic Therapy. Small. 2017;13 doi: 10.1002/smll.201702299. 1702299. [DOI] [PubMed] [Google Scholar]
- [153].Zhou J, Jiang Y, Hou S, et al. Compact Plasmonic Blackbody for Cancer Theranosis in the Near-Infrared II Window. ACS Nano. 2018;12:2643–51. doi: 10.1021/acsnano.7b08725. [DOI] [PubMed] [Google Scholar]
- [154].Duan C, Liang L, Li L, et al. Recent progress in upconversion luminescence nanomaterials for biomedical applications. J Mater Chem B. 2018;6:192–209. doi: 10.1039/c7tb02527k. [DOI] [PubMed] [Google Scholar]
- [155].He S, Krippes K, Ritz S, et al. Ultralow-intensity near-infrared light induces drug delivery by upconverting nanoparticles. Chem Commun. 2015;51:431–4. doi: 10.1039/c4cc07489k. [DOI] [PubMed] [Google Scholar]
- [156].Xiang J, Ge F, Yu B, et al. Nanocomplexes of Photolabile Polyelectrolyte and Upconversion Nanoparticles for Near-Infrared Light-Triggered Payload Release. ACS Appl Mater Interfaces. 2018;10:20790–800. doi: 10.1021/acsami.8b05063. [DOI] [PubMed] [Google Scholar]
- [157].Li C, Shen J, Yang J, et al. NIR-Triggered Release of Nitric Oxide with Upconversion Nanoparticles Inhibits Platelet Aggregation in Blood Samples. Part Part Syst Charact. 2018;35 1700281. [Google Scholar]
- [158].Wang Y, Song S, Liu J, et al. ZnO-Functionalized Upconverting Nanotheranostic Agent: Multi-Modality Imaging-Guided Chemotherapy with On-Demand Drug Release Triggered by pH. Angew Chemie Int Ed. 2015;54:536–40. doi: 10.1002/anie.201409519. [DOI] [PubMed] [Google Scholar]
- [159].Ge X, Sun L, Ma B, et al. Simultaneous realization of Hg 2+ sensing, magnetic resonance imaging and upconversion luminescence in vitro and in vivo bioimaging based on hollow mesoporous silica coated UCNPs and ruthenium complex. Nanoscale. 2015;7:13877–87. doi: 10.1039/c5nr04006j. [DOI] [PubMed] [Google Scholar]
- [160].Hou B, Zheng B, Gong X, et al. A UCN@mSiO 2 @cross-linked lipid with high steric stability as a NIR remote controlled-release nanocarrier for photodynamic therapy. J Mater Chem B. 2015;3:3531–40. doi: 10.1039/c5tb00240k. [DOI] [PubMed] [Google Scholar]
- [161].Zhang T, Lin H, Cui L, et al. NIR-sensitive UCNP@mSiO 2 nanovehicles for on-demand drug release and photodynamic therapy. RSC Adv. 2016;6:26479–89. [Google Scholar]
- [162].Zhang T, Lin H, Cui L, et al. NIR-sensitive UCNP@mSiO 2 nanovehicles for on-demand drug release and photodynamic therapy. RSC Adv. 2016;6:26479–26489. [Google Scholar]
- [163].Sun R, Wang W, Wen Y, et al. Recent Advance on Mesoporous Silica Nanoparticles-Based Controlled Release System: Intelligent Switches Open up New Horizon. Nanomaterials. 2015;5:2019–53. doi: 10.3390/nano5042019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Hrubý M, Filippov SK, Štěpánek P. Smart polymers in drug delivery systems on crossroads: Which way deserves following? Eur Polym J. 2015;65:82–97. [Google Scholar]
- [165].Kotsuchibashi Y, Ebara M, Aoyagi T, et al. Recent Advances in Dual Temperature Responsive Block Copolymers and Their Potential as Biomedical Applications. Polymers (Basel) 2016;8:380. doi: 10.3390/polym8110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Karesoja M, McKee J, Karjalainen E, et al. Mesoporous silica particles grafted with poly(ethyleneoxide-block-N-vinylcaprolactam) J Polym Sci Part A Polym Chem. 2013;51:5012–20. [Google Scholar]
- [167].Ribeiro T, Coutinho E, Rodrigues AS, et al. Hybrid mesoporous silica nanocarriers with thermovalve-regulated controlled release. Nanoscale. 2017;9:13485–94. doi: 10.1039/c7nr03395h. [DOI] [PubMed] [Google Scholar]
- [168].Martelli G, Zope HR, Capell M Bròvia, et al. Coiled-coil peptide motifs as thermoresponsive valves for mesoporous silica nanoparticles. Chem Commun. 2013;49:9932. doi: 10.1039/c3cc45790g. [DOI] [PubMed] [Google Scholar]
- [169].Deng Y, Qi D, Deng C, et al. Superparamagnetic High-Magnetization Microspheres with an Fe3O4@SiO2 Core and Perpendicularly Aligned Mesoporous SiO2 Shell for Removal of Microcystins. J Am Chem Soc. 2008;130:28–9. doi: 10.1021/ja0777584. [DOI] [PubMed] [Google Scholar]
- [170].Zhao W, Gu J, Zhang L, et al. Fabrication of Uniform Magnetic Nanocomposite Spheres with a Magnetic Core/Mesoporous Silica Shell Structure. J Am Chem Soc. 2005;127:8916–7. doi: 10.1021/ja051113r. [DOI] [PubMed] [Google Scholar]
- [171].Arcos D, Fal-Miyar V, Ruiz-Hernández E, et al. Supramolecular mechanisms in the synthesis of mesoporous magnetic nanospheres for hyperthermia. J Mater Chem. 2012;22:64–72. [Google Scholar]
- [172].Guisasola E, Baeza A, Talelli M, et al. Magnetic-Responsive Release Controlled by Hot Spot Effect. Langmuir. 2015;31:12777–82. doi: 10.1021/acs.langmuir.5b03470. [DOI] [PubMed] [Google Scholar]
- [173].Baeza A, Guisasola E, Ruiz-Hernández E, et al. Magnetically Triggered Multidrug Release by Hybrid Mesoporous Silica Nanoparticles. Chem Mater. 2012;24:517–24. [Google Scholar]
- [174].Ruiz-Hernández E, Baeza A, Vallet-Regí M. Smart Drug Delivery through DNA/Magnetic Nanoparticle Gates. ACS Nano. 2011;5:1259–66. doi: 10.1021/nn1029229. [DOI] [PubMed] [Google Scholar]
- [175].Manzano M, Vallet-Regí M. Ultrasound responsive mesoporous silica nanoparticles for biomedical applications. Chem Commun. 2019;55:2731–40. doi: 10.1039/C8CC09389J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Kim H-J, Matsuda H, Zhou H, et al. Ultrasound-Triggered Smart Drug Release from a Poly(dimethylsiloxane)– Mesoporous Silica Composite. Adv Mater. 2006;18:3083–8. [Google Scholar]
- [177].Ma M, Xu H, Chen H, et al. A Drug-Perfluorocarbon Nanoemulsion with an Ultrathin Silica Coating for the Synergistic Effect of Chemotherapy and Ablation by High-Intensity Focused Ultrasound. Adv Mater. 2014;26:7378–85. doi: 10.1002/adma.201402969. [DOI] [PubMed] [Google Scholar]
- [178].Milgroom A, Intrator M, Madhavan K, et al. Mesoporous silica nanoparticles as a breast-cancer targeting ultrasound contrast agent. Colloids Surfaces B Biointerfaces. 2014;116:652–7. doi: 10.1016/j.colsurfb.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Lee S-F, Zhu X-M, Wang Y-XJ, et al. Ultrasound, pH, and Magnetically Responsive Crown-Ether-Coated Core/Shell Nanoparticles as Drug Encapsulation and Release Systems. ACS Appl Mater Interfaces. 2013;5:1566–74. doi: 10.1021/am4004705. [DOI] [PubMed] [Google Scholar]
- [180].Paris JL, Cabañas MV, Manzano M, et al. Polymer-Grafted Mesoporous Silica Nanoparticles as Ultrasound-Responsive Drug Carriers. ACS Nano. 2015;9:11023–33. doi: 10.1021/acsnano.5b04378. [DOI] [PubMed] [Google Scholar]
- [181].Paris JL, Villaverde G, Cabañas MV, et al. From proof-of-concept material to PEGylated and modularly targeted ultrasound-responsive mesoporous silica nanoparticles. J Mater Chem B. 2018;6:2785–94. doi: 10.1039/c8tb00444g. [DOI] [PubMed] [Google Scholar]
- [182].Paris JL, Manzano M, Cabañas MV, et al. Mesoporous silica nanoparticles engineered for ultrasound-induced uptake by cancer cells. Nanoscale. 2018;10:6402–8. doi: 10.1039/C8NR00693H. [DOI] [PubMed] [Google Scholar]
- [183].Han N, Zhao Q, Wan L, et al. Hybrid Lipid-Capped Mesoporous Silica for Stimuli-Responsive Drug Release and Overcoming Multidrug Resistance. ACS Appl Mater Interfaces. 2015;7:3342–51. doi: 10.1021/am5082793. [DOI] [PubMed] [Google Scholar]
- [184].Chen X, Cheng X, Soeriyadi AH, et al. Stimuli-responsive functionalized mesoporous silica nanoparticles for drug release in response to various biological stimuli. Biomater Sci. 2014;2:121–30. doi: 10.1039/c3bm60148j. [DOI] [PubMed] [Google Scholar]
- [185].Jain RN, Huang X, Das S, et al. Functionalized Mesoporous Silica Nanoparticles for Glucose- and pH-Stimulated Release of Insulin. Zeitschrift für Anorg und Allg Chemie. 2014;640:616–23. [Google Scholar]
- [186].Tan L, Yang M-Y, Wu H-X, et al. Glucose- and pH-Responsive Nanogated Ensemble Based on Polymeric Network Capped Mesoporous Silica. ACS Appl Mater Interfaces. 2015;7:6310–6. doi: 10.1021/acsami.5b00631. [DOI] [PubMed] [Google Scholar]
- [187].Yang D, Wang T, Su Z, et al. Reversing Cancer Multidrug Resistance in Xenograft Models via Orchestrating Multiple Actions of Functional Mesoporous Silica Nanoparticles. ACS Appl Mater Interfaces. 2016;8:22431–41. doi: 10.1021/acsami.6b04885. [DOI] [PubMed] [Google Scholar]
- [188].Yilmaz MD, Xue M, Ambrogio MW, et al. Sugar and pH dual-responsive mesoporous silica nanocontainers based on competitive binding mechanisms. Nanoscale. 2015;7:1067–72. doi: 10.1039/c4nr04796f. [DOI] [PubMed] [Google Scholar]
- [189].Tan L, Wu H-X, Yang M-Y, et al. The dual-stimulated release of size-selected cargos from cyclodextrin-covered mesoporous silica nanoparticles. RSC Adv. 2015;5:10393–9. [Google Scholar]
- [190].Yu F, Wu H, Tang Y, et al. Temperature-sensitive copolymer-coated fluorescent mesoporous silica nanoparticles as a reactive oxygen species activated drug delivery system. Int J Pharm. 2018;536:11–20. doi: 10.1016/j.ijpharm.2017.11.025. [DOI] [PubMed] [Google Scholar]
- [191].Shu Y, Song R, Zheng A, et al. Thermo/pH dual-stimuli-responsive drug delivery for chemo-/photothermal therapy monitored by cell imaging. Talanta. 2018;181:278–85. doi: 10.1016/j.talanta.2018.01.018. [DOI] [PubMed] [Google Scholar]
- [192].Wang G, Dong J, Yuan T, et al. Visible Light and pH Responsive Polymer-Coated Mesoporous Silica Nanohybrids for Controlled Release. Macromol Biosci. 2016;16:990–4. doi: 10.1002/mabi.201600008. [DOI] [PubMed] [Google Scholar]
- [193].Han R, Yi H, Shi J, et al. pH-Responsive drug release and NIR-triggered singlet oxygen generation based on a multifunctional core–shell–shell structure. Phys Chem Chem Phys. 2016;18:25497–503. doi: 10.1039/c6cp05308d. [DOI] [PubMed] [Google Scholar]
- [194].N VR, Han HS, Lee H, et al. ROS-responsive mesoporous silica nanoparticles for MR imaging-guided photodynamically maneuvered chemotherapy. Nanoscale. 2018;10:9616–27. doi: 10.1039/c8nr00888d. [DOI] [PubMed] [Google Scholar]
- [195].González-Alvarez M, Coll C, Gonzalez-Alvarez I, et al. Gated Mesoporous Silica Nanocarriers for a “Two-Step” Targeted System to Colonic Tissue. Mol Pharm. 2017;14:4442–53. doi: 10.1021/acs.molpharmaceut.7b00565. [DOI] [PubMed] [Google Scholar]
- [196].Yang Q, Lai SK. Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomedicine Nanobiotechnology. 2015;7:655–77. doi: 10.1002/wnan.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]