Abstract
Marine cyanobacteria represent a unique source in the field of drug discovery due to the secondary metabolites they produce and the structural similarity these compounds have to endogenous mammalian receptor ligands. A series of cyanobacteria were subjected to extraction, fractionation by column chromatography and screened for affinity against CNS targets with a focus on serotonin receptors (5-HT). Out of 276 fractions screened, 21% had activity at 5-HT receptors and/or the 5-HT transporter. One sample, a cyanobacterium identified by 16S rRNA sequencing as Leptolyngbya from Las Perlas archipelago in Panama, contained a fraction with noted affinity for the 5-HT7 receptor (5-HT7R). This fraction (DUQ0002I) was screened via intracerebroventricular (ICV) injections in mice using depression and anxiety assays including the forced swim, tail suspension, elevated zero maze and light-dark preference tests. DUQ0002I decreased depression and anxiety-like behaviors in males and did not have effects in 5-HT7R knockout or female mice. Administration of DUQ0002I to the CA1 of the hippocampus induced antidepression-like, but not anxiolytic-like behaviors. Testing of further purified materials showed no behavioral effects, leading us to hypothesize that the behavioral effects are likely caused by a synergistic effect between multiple compounds in the fraction. Finally, DUQ0002I was used in a model of neuropathic pain with comorbid depression (spared nerve injury – SNI). DUQ0002I had a similar antidepressant effect in animals with SNI, suggesting a role for the 5-HT7R in the development of comorbid pain and depression. These results demonstrate the potential that cyanobacterial metabolites have in the field of neuropharmacognosy.
Keywords: Cyanobacteria, drug discovery, serotonin receptor 7, behavior, anxiety, depression, animal models
Graphical Abstract:
We found that marine cyanobacteria produce a significant number of metabolites with activity at serotonin receptors and transporters. One particular extract, with selective 5-HT7 receptor activity, demonstrated sex and anatomy-dependent behavioral effects in vivo, illustrating the potential of these organisms in lead compound drug discovery.
1. INTRODUCTION
Filamentous cyanobacteria are small, photosynthetic prokaryotes found in diverse environments around the globe. They are known to produce secondary metabolites that were first studied in freshwater environments due to the poisoning of domestic cattle drinking from freshwater with cyanobacterial blooms (Stewart, Seawright, & Shaw, 2008) and in marine environments for their involvement in “swimmer’s itch” (Osborne & Shaw, 2008). These metabolites are thought to have originally evolved as a type of chemical protection against predation (Nagle & Paul, 1999). In marine environments, these intracellular metabolites are released when cells are lysed (Organization, 1999), as when an herbivore grazes on the organism, or are released into the water as signaling molecules between cells. Regardless of their purpose, the fact that these compounds can have direct effects on animals has led to the investigation of cyanobacterial metabolites on cytotoxicity including for cancer and infection (viral, microbial, malaria) and antifeedant effects, among others (Dixit & Suseela, 2013).
What remains relatively unexplored beyond cytotoxic effects of cyanobacterial compounds is if these metabolites can modify signaling cascades in mammals, especially in the nervous system. A large number of compounds produced by cyanobacteria and other microorganisms are small linear and cyclic peptides (Ahmed, Lax, & Tidgewell, 2015; Tan, 2007; Yano et al., 2015), which structurally resemble endogenous ligands to G-protein coupled receptors (GPCRs) found in mammalian cells. To date, only a handful compounds extracted from cyanobacteria have been investigated for activity at GPCRs. These include extracts from the genus Moorea (formerly Lyngbya (N. Engene et al., 2012)) acting on the cannabinoid receptors (Gutierrez et al., 2011; Han, McPhail, Ligresti, Di Marzo, & Gerwick, 2003; Montaser, Paul, & Luesch, 2012; Sitachitta & Gerwick, 1998) and an extract from the genus Oscillatoria acting on the serotonin system identified in our previous publication (Lax et al., 2016). The goal of the present research was to further probe metabolites extracted from cyanobacteria against GPCRs found in the CNS. We sought to determine potential psychoactive effects of metabolites in affective disorders such as depression and anxiety. By doing so we hope to find compounds that could be used as chemical leads and as tools that lead to a better understanding of biological systems within the CNS, with a primary focus on serotonin (5-HT).
5-HT is one of the main monoamine neurotransmitters in the nervous system and plays a role in many physiological processes including behavior, mood, pain, learning, memory, sleep, and appetite (Evelien Gellynck et al., 2013; Monti & Jantos, 2014; Pytliak, Vargova, Mechirova, & Felsoci, 2011). Serotonin binds to serotonin receptors, which are broadly classed into seven families (5-HT1–7R) (Pytliak et al., 2011). These receptors are mainly found peripherally in the GI tract (Tuladhar, Ge, & Naylor, 2003) and smooth muscle of blood vessels (Bard et al., 1993) and in the CNS (Bonaventure et al., 2004). Many common affective disorders, including depression and anxiety, are known to be associated with 5-HT signaling. Most of the common anti-depressants (e.g. selective serotonin reuptake inhibitors (SSRIs)) target 5-HT re-uptake causing an increase in synaptic levels of 5-HT following normal release (Ferguson, 2001). This increase in serotonin can then bind to serotonin receptors (e.g. 5-HT1A, 5-HT2A, 5-HT2C, 5-HT4, 5-HT6, 5-HT7) (Pytliak et al., 2011) that are known to have effects in depression. Of these, 5-HT7R is a relatively under-studied receptor with strong potential to enhance the antidepressant effects of SSRIs when pharmacologically inhibited (Guseva, Wirth, & Ponimaskin, 2014; Tokarski, Kusek, Sowa, & Bobula, 2014) and many animal studies have shown that targeting the 5-HT7R receptor can modulate affective behaviors.
In rodents, administration of selective 5-HT7R antagonists generally decreases depression-like behaviors (Hedlund, Huitron-Resendiz, Henriksen, & Sutcliffe, 2005; Kim et al., 2014; Sarkisyan, Roberts, & Hedlund, 2010; Wesolowska, Nikiforuk, & Stachowicz, 2006). In terms of anxiety-like behavior, the role of 5-HT7R is not as clear. In mice, some studies have shown that blockade of the 5-HT7R reduces anxiety-like behavior in the open field test (Guilloux et al., 2013; Hedlund & Sutcliffe, 2007; Wesolowska et al., 2006), however, another study found that 5-HT7R agonists reduce anxiety-like behavior (Adriani et al., 2012). In human studies, the multimodal antidepressant vortioxetine (Brintellix), which acts as a 5-HT7R antagonist while also increasing serotonin concentrations through reuptake inhibition, has been shown to reduce major depressive disorder (MDD) in both short and long-term clinical trials (Pearce & Murphy, 2014). Additionally, the clinically established effects of some antipsychotic drugs, including amisulpride, aripiprazole and lurasidone most likely function through the 5-HT7R (Abbas et al., 2009; Bonaventure et al., 2007; Cates, Roberts, Huitron-Resendiz, & Hedlund, 2013). Overall, these data demonstrate that targeting the 5-HT7R can alter both affective behavior in animals and humans.
Given the possible presence of CNS active compounds in cyanobacterial metabolites and the well-established role that the 5-HT7R plays in depression and anxiety, we sought to investigate the potential of cyanobacteria to produce 5-HTR ligands and discover specific ligands for the 5-HT7R that could induce antidepressant and anxiolytic-like effects. In this study, we sought to investigate the potential of marine cyanobacteria to produce 5-HTR ligands by screening extracts taken from marine cyanobacteria near Panama and Curaçao. Crude extracts from cyanobacteria were fractionated using silica gel chromatography and screened in vitro for GPCR binding affinity by the Psychoactive Drug Screening Program (PDSP) (Besnard et al., 2012).
One particular fraction with significant binding (>50% in primary assay) and selective affinity for the 5-HT7R, DUQ0002I, was injected into the lateral ventricle of mice via intracerebroventricular (ICV) injections for initial screening in a series of depression and anxiety behavioral assays, including the forced swim, tail suspension, elevated zero maze, and light dark preference tests. We hypothesized that this fraction would alter anxiety-like and depression-like behaviors in these animals. After the initial characterization via ICV, we next explored the specificity of the in vivo results using 5-HT7R knockout (5-HT7R −/−) mice and investigated possible sex differences in the observed behavior. We explored the anatomical specificity of the behavioral effects by utilizing brain region specific targeting of DUQ0002I to the CA1 region of the hippocampus. Additionally, purified subfractions of the parent fraction DUQ0002I were tested. Finally, we utilized DUQ0002I as a therapeutic lead to determine if this fraction could alleviate the depression-like effects induced by the neuropathic pain spared nerve injury (SNI) model.
2. EXPERIMENTAL PROCEDURES
2.1. Field sampling of cyanobacteria.
Cyanobacterial samples were collected between 2011 and 2016 through snorkeling and SCUBA near Panama (Caribbean Sea and Pacific Ocean) and Curaçao. The specific sample of focus here was collected by snorkeling on January 28, 2013 while at a depth of 1 m off the coast of Mogo Mogo (GPS coordinates: 8°34’50.2” N, 79°01’10.6” W) in the Las Perlas Archipelago, Panama. A dark green/black cyanobacterial biomass was collected. The sample was fully suspended in ethanol:seawater (50:50) mixture to preserve the sample until it could be stored at −20°C prior to extraction. A voucher specimen (PLP-28Jan13–2) is deposited in the Department of Medicinal Chemistry, Graduate School of Pharmacy, Duquesne University and was given the laboratory identifier DUQ0002I.
2.2. Extraction and in vitro characterization of metabolites.
The DUQ0002I cyanobacterial biomass (75 g, dry wt) was extracted with 2:1 CH2Cl2-MeOH to afford 3.3 g of crude extract. This crude extract was fractionated over normal phase silica gel with a stepwise gradient solvent system of increasing polarity starting from 100% hexanes to 100% MeOH, to yield nine fractions (A–I). The fraction eluting with 100% methanol (fraction I (DUQ0002I), 292 mg) was chromatographed on hypersep C18 reversed phase (2000 mg/15 mL) column using methanol:water, gradient 50–100% to yield four sub-fractions, DUQ0002I-1–4 corresponding to 224.9, 5.7, 7.9 and 13.9 mg, respectively. The fraction most similar to the crude fraction, sub-fraction DUQ0002I-1 (167.5 mg), was further purified over silica gel (90.6 g) using methanol:dicloromethane, gradients 5, 8 and 10% to yield sub-fractions, DUQ0002I-1A (3.2 mg), DUQ0002I-1B (4.0 mg) and DUQ0002I-1C (7.9 mg).
NMR spectra were recorded with methanol as internal standard (δC 49.9, δH 3.34), on a Bruker 500 MHz spectrometer operating at 499.7 MHz for 1H and 125.7 MHz for 13C equipped with a 5 mm PATXI 1HD/D-13C/15N Z-GRD Probe. Column chromatography was performed using Sorbent Technologies silica gel (230–400 mesh). Solvents were evaporated on a Heidolph rotary evaporator.
2.2.1. Psychoactive Drug Screening Program binding assays.
All binding data for fractions DUQ0002A-I were performed and generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program (NIMH PDSP) utilizing a radioligand competition-binding assay. Experimental details are available online at http://pdsp.med.unc.edu/. In brief, fractions that pass primary binding criterion of greater than 50% inhibition at 10 μM are tagged for secondary radioligand binding assay to determine IC50 at the specific target of interest. To determine overall hit rates at 5-HT receptors and the serotonin transporter (SERT) for all fractions screened between 2011 and 2016, we calculated the number of fractions with >50% binding affinity at 5-HT targets (n=58) compared to the total number of our cyanobacterial fractions that have been screened by the PDSP (n=276).
2.2.2. Microscopy.
Samples of DUQ0002 used for microscopy were prepared using a piece of the sample stored in RNAlater solution. Filaments were spread out onto a slide with distilled H2O and imaged at 20× on a Nikon 1AR-HD microscope using the FITC (487 nm), TRITC (560 nm) and Cy5 (637 nm) channels.
2.2.3. 16S rRNA gene sequencing and phylogenetic analysis.
Genomic DNA was extracted from DUQ0002 that was preserved in RNAlater® solution (Thermo Fisher) after collection. Lysis buffer (10 mM Tris, 0.1 M EDTA, 0.5% (w/v) SDS, 20 μg/mL RNase, pH 8.0) was added to the cyanobacteria mass at 10× the biomass of the cells (i.e. 100 mg cells = 1000 μL lysis buffer). This was followed by 100 μL of lysozyme solution (10 mg/mL, Sigma) for 30 minutes with an incubation at 37°C. 0.01× the volume of Proteinase K (10 mg/mL, Gene Link) was then added and incubated for 1 hr at 50°C. Following this, the mixture was centrifuged at 13,000 rpm for 3 min and the remaining pellet was used with the Wizard Genomic DNA Purification Kit (Promega). Extracted genomic DNA then underwent PCR to amplify the 16S rRNA gene with primers (CYA106F and CYA1509R) previously utilized (Niclas Engene, Cameron Coates, & Gerwick, 2010; Nubel, Garcia-Pichel, & Muyzer, 1997). Successful PCR amplifications (~1370 bp) underwent PCR purification using the Min Elute PCR Purification Kit (Qiagen) and purified PCR products were used for TOPO cloning. One Shot E. coli cells along with the pCR® 4-TOPO vector with kanamycin resistance from the TOPO TA Cloning ® Kit (Invitrogen) were used and cells were plated overnight at 37°C on LB KAN plates. Successful transformants were selected and used in colony PCR with the M13F and M13R primers to confirm the presence of the 16S rRNA sequence now in the TOPO vector (~1535 bp). Purified PCR product for correct samples was sent to Beckman Coulter (now GENEWIZ) to undergo forward and reverse Sanger sequencing with the M13F and M13R primers. Our sequence for DUQ0002, along with 16S rRNA gene sequences that were obtained with sequence data available at the National Center for Biotechnology (NCBI) webpage (http://www.ncbi.nlm.nih.gov) were then used for phylogenetic analysis. Gene sequences were aligned using the Muscle algorithm and phylogeny reconstruction was done using maximum likelihood method with 500 bootstrap replicates using the MEGA (version 5.1) program (Hall, 2013). DUQ0002 16S rRNA GenBank deposit number (MH357345).
2.3. Animals.
All animal procedures were reviewed and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Animals and the Institutional Animal Care and Use Committee at Duquesne University. Experiments were performed on either male or female C57Bl/6J mice that were 8–10 weeks old. For experiments using the SNI/sham model, animals were 8–10 weeks old when SNI/sham surgeries were completed, and all behavioral testing began 10 weeks after surgery. For 5-HT7R knockout experiments, 8–10 week old 5-HT7R −/− (homozygote) mice and 5-HT7R +/+ (wildtype) sibling controls were used (Jackson Laboratories (Cat: 019453) Hedlund et al., 2003). 5-HT7R knockout mice were previously backcrossed onto a C57Bl/6J line and were re-derived in C57 female mice. All experimental mice were group housed up until cannulation surgeries were performed (this is necessary since group housing can cause the animals to lose their skull caps that are present after cannulation). Mice were maintained on a 12h light/dark cycle (lights on between 7:00AM and 7:00PM) with ad libitum access to food and water. All procedures were done during the light cycle. All behaviors performed were recorded and analyzed by a male experimenter (due to the known effects of human pheromones on rodent behavior (Sorge et al., 2014)), who was blinded to experimental treatments.
2.4. Cannulation procedures for in vivo fraction and drug delivery.
Cyanobacterial fractions, drugs, and vehicles were delivered directly into the CNS via stainless steel cannulas. For cannulation surgeries, animals were anesthetized with 3% isoflurane/0.6% oxygen. Intracerebroventricular (ICV) surgeries were done as described previously (Glascock et al., 2011) and CA1 bilateral cannulations were performed as subsequently described. Briefly, mice were placed in a stereotaxic frame and steel cannulas (one 8.00 mm for ICV and two 5.60 mm for CA1) were placed into the right lateral ventricle (ICV) or bilaterally into the CA1 region of the left and right hippocampus. The following anatomical coordinates were used: ICV – 0.5 mm anterior to bregma, 1.0 mm lateral to midline and 2.0 mm ventral to the skull; CA1 – 1.6 mm anterior to bregma, 1.0 mm lateral to midline and 1.6 mm ventral to the skull. A dental cement skullcap secured with two bone screws was used to hold cannulas in place. Mice were allowed to recover on heating pads, housed in individual cages (this is required since group housed animals often remove their cagemate’s cement skullcaps through grooming or other social behavior) and were given one week of recovery prior to the beginning of behavioral testing. Following all behavioral tests, cannula placement was verified with necropsy.
2.4.1. Fraction and compound administration for behavioral analysis.
Cyanobacterial extracts and commercial compounds for behavioral characterization were prepared as described previously (Lax et al., 2016). Briefly, samples from collection DUQ0002, fraction I (DUQ0002I), the known 5-HT7R antagonist SB258719 (SB258719 hydrochloride, Tocris) and subfractions isolated from the parent fraction (DUQ0002I-1A-C, 2–4) were dissolved in a mixture of 50% DMSO and 50% artificial cerebrospinal fluid (aCSF) containing the following (in mM): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2.0 CaCl2, 1.0 MgCl2 and 25 D-glucose, bubbled with 95% O2/5% CO2 for 20 min. prior to use. We have previously shown no behavioral effect of this vehicle preparation in mice compared to 100% aCSF vehicle (Lax et al., 2016). Microinjections were performed using a 32-gauge injector that extended 0.5 mm beyond the tip of the ICV or CA1 cannulas. The injector was attached to flexible tubing and a 1.0 μL syringe (Hamilton) that was used to deliver a total volume of 0.5 μL over a 2 min period (two syringes, injectors and sets of tubing were used for bilateral CA1 injections; 0.5 μL per CA1). The injectors were kept in place for one minute after the injections were complete to allow for complete compound infusion and all behaviors were conducted 5 minutes after compound administration. For all injections, fraction DUQ0002I and subfractions DUQ0002I-1A-C, DUQ0002-2-4 were administered at a dose of 40 μg per cannula and SB258719 was administered at a dose of 5 μg based on previously literature (Zhao et al., 2014). The fraction dose was based on PDSP screening at 4 mg/mL and adjusted for ICV injections and intra-amygdala injections that we have done previously (Kolber et al., 2010; Lax et al., 2016)._ENREF_19 50%/50% aCSF/DMSO was used as a vehicle control in all experiments.
2.5. Behavioral testing.
We determined that 5 – 8 animals would be needed per group based on previously published behavioral data (Lax et al., 2016; Lax, George, Ignatz, & Kolber, 2014) and, in part, on an a-priori power analysis using a significance level (alpha) of 0.05 (two-tailed) at 95% power. Some behavioral tests (mainly from those during initial characterization with ICV injections) have higher values since those experiments were done in duplicate to verify the veracity of the effects. For all behavioral tests, mice were habituated for 1–2 hours in the behavior room with 60 dB of white noise to block ambient background noise, temperature of 22 – 25°C, humidity of 40 – 60%. Experimenters waited an additional 30 minutes after entering the room to begin behavioral testing due to the known effects of human pheromones on rodent behavior (Sorge et al., 2014). All animals received fraction/subfractions/drug (DUQ0002I, DUQ0002I-1A-C, DUQ0002I-2–4, or SB258719) or vehicle 5 minutes prior to the beginning of testing, where they were returned to their homecages during the 5 minutes. All compound delivery was done blinded to fraction versus vehicle delivered and surgery type (e.g. SNI vs sham, where applicable). Treatment groups were randomly assigned to animals by a third-party experimenter during blinding. Animals were each tested in the same behavioral apparatus one at a time (not in parallel). For animals that were tested in more than one assay, testing was done with one week between assays and testing proceeded from the least stressful to most stressful test. This order began with elevated zero maze, followed by light-dark preference, tail suspension test and ended with forced swim test. A maximum of four tests were completed on groups of naive mice for ICV testing. Animals were individually housed for up to four weeks, including the one week of recovery post-surgery plus one week between each of the behavioral tests. In subsequent testing, only certain tests were used in different contexts but testing order always proceeded from least stressful to more stressful. No animals underwent testing in the same assay twice. All behavioral testing and analysis of behavior was done blinded to treatment and surgery type (where applicable).
2.5.1. Elevated zero maze (EOM).
The elevated zero maze was performed largely as described previously (Kulkarni, Singh, & Bishnoi, 2007). In this assay, mice were placed on an elevated circular platform (50 cm off ground, 4.5 cm wide platform, 63.5 cm diameter) with two ‘arms’ that are open, and two ‘arms’ that contain a high wall (walls are 16 cm high) enclosing the platform. Animals were recorded with an over-head videocamera (Logitech Pro 9000) and locomotion was tracked with ANY-maze software (Stoelting Co., version 4.98, Wood Dale, IL). Time spent and distance traveled in the open and closed arm were recorded for the 10 minute long test.
2.5.2. Light-dark preference (LDP) test.
The light-dark preference was performed as described previously (Bourin & Hascoet, 2003). In this assay, a box (46 cm L × 27 cm W × 31 cm H) with a ‘dark’ and ‘light’ side that take up 1/3 and 2/3 of the box space respectively is used. A door (5 cm × 6 cm) separates the two regions. First, mice are placed in the dark side with the lid on and door to the light side closed for one minute. After the one minute, the door is opened and animals were recorded with an over-head camera and tracked with ANY-maze software (Stoelting Co., version 4.98, Wood Dale, IL). Time spent and mean visit time to the light side was recorded for the 10 minute long test.
2.5.3. Tail suspension test.
The tail suspension test was performed as described previously (Can, Dao, Terrillion, et al., 2012). Briefly, mice were suspended by the distal ends of their tail in a Plexiglas enclosure (35 × 25 × 25 cm) with white sides. The animals’ movements were recorded with webcam for scoring offline in one minute long bins for a total of 6 minutes. Immobility was defined as time when the mouse did not exhibit any outward movement of its limbs or body.
2.5.4. Forced swim test.
The forced swim test was performed as described previously (Can, Dao, Arad, et al., 2012). Briefly, mice were placed in a beaker of water (~750 mL in a 1.0 L beaker, temperature 26 – 30°C), with all but their heads submerged. The animals’ movements were recorded with a Logitech Pro 9000 webcam for scoring offline in one minute long bins for a total of 6 minutes. Immobility was defined as time when the mouse did not exhibit any outward movements or when it only exhibited only movements necessary to maintain buoyancy (i.e., one paw gently moving in water).
2.5.5. Mechanical sensitivity test.
Mechanical sensitivity was measured with von Frey filaments (Touch Test) ranging from 0.02 – 2.56 g to determine the 50% withdrawal thresholds using the up/down method (Chaplan, Bach, Pogrel, Chung, & Yaksh, 1994; Dixon, 1980). Animals were placed in Plexiglas boxes on a wire mesh surface for two hours prior to testing. Paw withdrawal was defined as a full lifting of the foot off of the wire-mesh surface when a filament was applied to the lateral edge of the paw (area innervated by the intact sural branch of sciatic nerve). After baseline testing, mice underwent SNI/sham surgery (see below). One week after the surgery, mice were subjected to von Frey testing again to observe withdrawal thresholds. Additional testing was done at two, three, five, six and ten weeks after surgery.
2.6. Spared nerve injury (SNI) surgery.
For SNI, male animals were anesthetized with 10:1 ketamine/xylazine mixture injected intraperitoneally into the mice (10 μL/g) and surgery was performed as described previously (Decosterd & Woolf, 2000; Richner, Bjerrum, Nykjaer, & Vaegter, 2011). Briefly, a suture thread was tied around tibial and common peroneal branches of the sciatic nerve, both of which were ligated 2 mm distal to the suture. The sural branch of the sciatic nerve was not manipulated. Sham surgeries followed the same procedure, without manipulation of the sciatic nerve. Mice recovered on heating pads and were group housed until cannulation surgeries were performed 9 weeks after SNI/sham.
2.7. Statistical analysis.
Graph Pad Prism version 6 for Mac OS × was used for all statistical analysis. All data are presented as mean ± SEM. Statistical significance was determined between groups using un-paired t-tests or 2-ANOVAs followed by Bonferroni post hoc tests. Statistical significance was established at a 95% confidence interval (p<0.05).
2.7.1. Z-score analysis.
Z-scores were calculated for each animal in each behavior tested based on the published method (Guilloux, Seney, Edgar, & Sibille, 2011) where applicable. This method of analysis only works if each animal receives the same treatment across each behavioral experiment (this was not done during the initial screening with ICV injections and therefore this analysis was not done for those data). From each parameter measured (X = time immobile for FST and TST, open arm time and percent distance traveled in open for EOM and time in light side and mean visit to light side for LDP), an individual Z-score (Z) was calculated to determine how many standard deviations (σ) each animal was above or below the mean of the control group (μ) using the following formula: Z = (X – μ) /σ. Z-scores for individual parameters were then averaged to generate “depression” and “anxiety” emotionality scores. With this type of analysis, the control group always has an overall Z-score of zero. For the experimental groups, positive Z-scores reflect positive emotionality changes (i.e. antidepression or antianxiety), whereas negative Z-scores reflect negative emotionality changes (i.e. depression, anxiety).
3. RESULTS
3.1. Screening marine cyanobacterial fractions against serotonin receptors (5-HTRs).
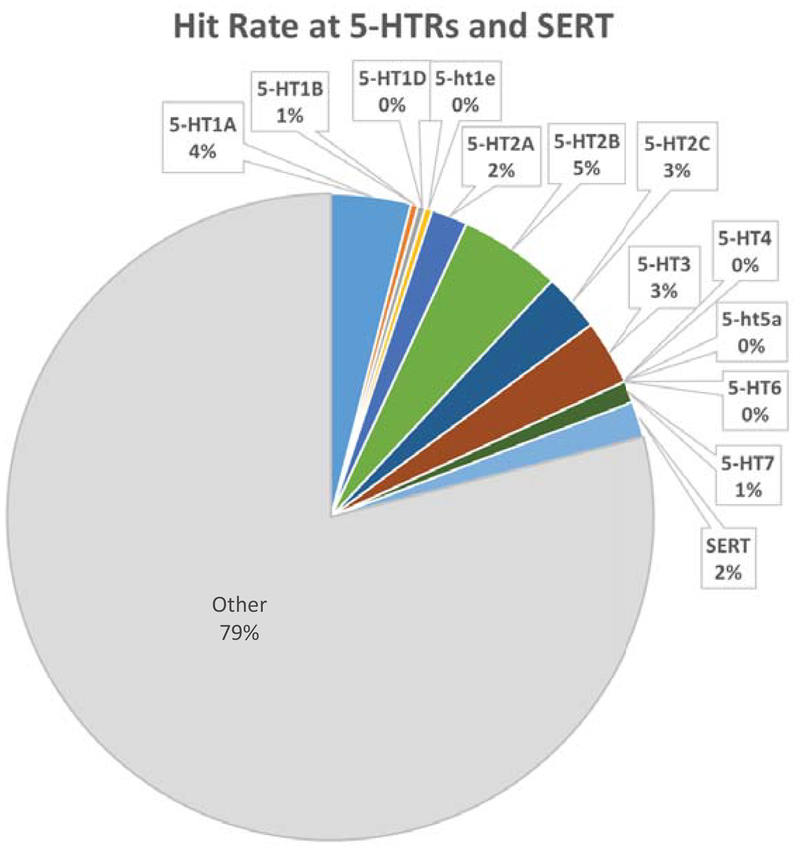
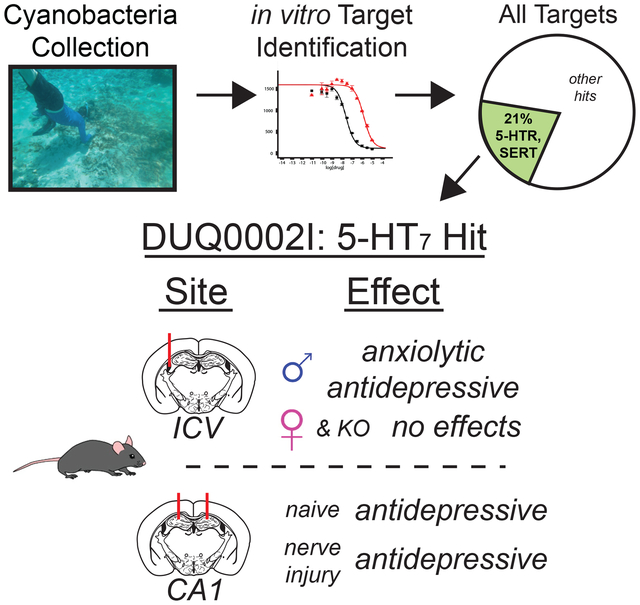
To evaluate the potential of marine cyanobacteria as a source for novel ligands to 5-HTRs and transporters, a series of cyanobacterial samples from Panama and Curaçao made from 2011 to 2016 were screened for their ability to bind to 5-HTRs and the 5-HT transporter (SERT) in a radioligand binding assays. The results of this broad screening, consisting of 276 cyanobacterial fractions, were obtained from the National Institute of Mental Health’s Psychoactive Drug Screening Program (NIMH PDSP). This initial screen indicates that 21% of the cyanobacterial fractions screened contain compounds that could modulate signaling of 5-HTRs or 5-HT transport (Figure 1). These data show the apparent evolutionary pressure that exists to produce 5-HTR metabolites. This paper focuses on a specific collection with a fraction showing specificity for the 5-HT7R.
Figure 1.
Primary binding results for initial screening of cyanobacteria collected 2011–2016. A hit is defined as greater than 50% inhibition of binding. Serotonin and serotonin transporter hits compromise 21% of hits from a total of 276 fractions screened. SERT– serotonin transporter.
3.2. 5-HT7R cyanobacterial collection, phylogeny and screening.
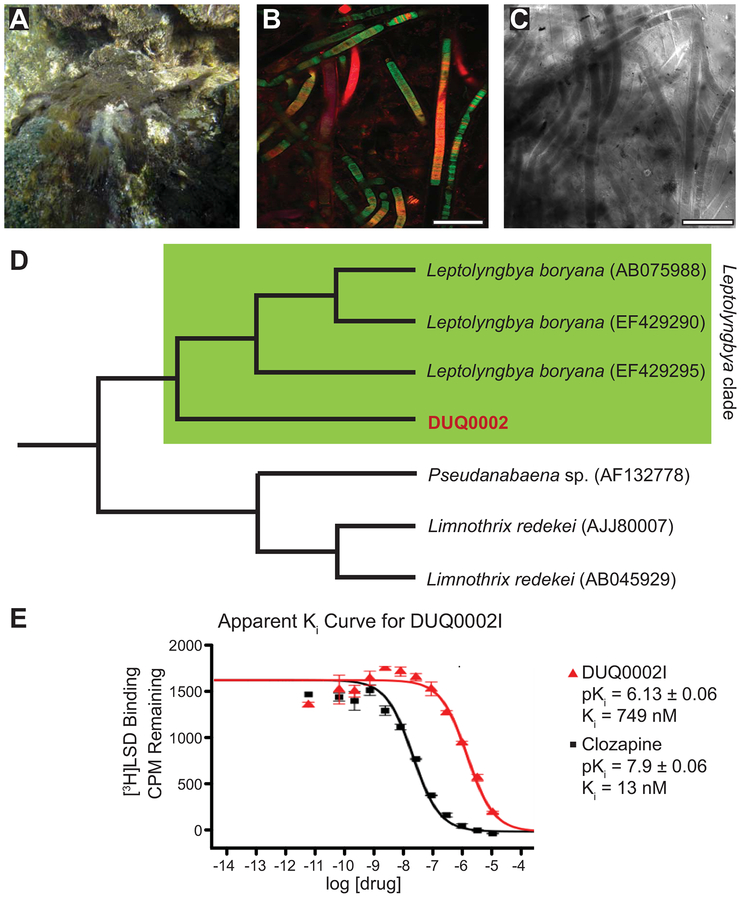
A dark green/black cyanobacterial mat was collected in ~0.6 m of water off Mogo-Mogo in the Las Perlas Archipelago, Panama (8°34’50.2”N, 79°01’10.6”W) and was given the extraction ID of DUQ0002 (Figure 2A). Confocal (Figure 2B) and transmitted light (Figure 2C) microscopy was performed in order to observe the microscopic structure of the organism. Based on the morphology of the specimen, this sample was field identified as belonging to the genera of Oscillatoria or Moorea. In order to more accurately determine the species of cyanobacteria, sequencing of the 16S rRNA gene was performed. We compared the DUQ0002 sequence with a series of previously published 16S rRNA sequences that highlighted the relatedness of marine cyanobacterial strains that produce natural products (N. Engene, Gunasekera, Gerwick, & Paul, 2013). We found that DUQ0002 groups within the Leptolyngbya clade, showing that our collection most likely belongs to this genus (Figure 2D, GenBank Number MH357345 ).
Figure 2.
Pictures of cyanobacteria DUQ0002 and phylogeny. (A) Macroscopic picture of sample DUQ0002 before collection. (B) Confocal microscopy image of DUQ0002 at 20× magnification with FITC (487 nm), TRITC (560 nm) and Cy5 (637 nm) channels. Scale bar = 100 μm. (C) Transmitted light image of DUQ0002. Scale bar = 100 μm. (D) Phylogenetic tree showing the closest related species to DUQ0002 based off of 16S rRNA sequencing. (E) Apparent Ki curve for fraction DUQ0002I showing the standard 5-HT7R ligand clozapine for comparison.
To evaluate the potential of extract DUQ0002 for lead compounds to treat pain and depression, fractions (named DUQ0002A – I) were screened using radioligand competition-binding assays. The results for the nine fractions screened are summarized in Tables 1 and 2. “Hits” for the primary assay, highlighted in yellow in Table 1, were observed for all fractions screened at one or more receptors and/or transporters.
Table 1.
PDSP primary binding assay of DUQ0002 fractions. PDSP “hits” are highlighted in yellow and represent fractions showing >50% inhibition of binding. Data represented as mean % inhibition (N = 4 separate determinations). Negative inhibition represents stimulation of binding, meaning that the fraction increased binding of the standard compound from baseline. Abbreviations: mGluR5 – metabotropic glutamate receptor 5; DOR – delta opioid receptor, KOR – kappa opioid receptor; MOR- mu opioid receptor; SERT – serotonin transporter; NET – norepinephrine transporter; ND – not determined.
| DUQ0002 | 5-HT1A | 5-HT1B | 5-HT1D | 5-HT1E | 5-HT2A | 5-HT2B | 5-HT2C | 5-HT3 | 5-HT5A | 5-HT6 | 5-HT7 | mGluR5 | DOR | KOR | MOR | SERT | NET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | −6.4 | −1.5 | −3.6 | −1.3 | −6.9 | −4.2 | −12.4 | 7.5 | −0.9 | −8.7 | −9.6 | ND | −16 | −8.6 | 7.7 | 91.7 | 9.2 |
| B | 3.7 | 9.8 | 12.1 | −7.2 | −9.2 | −7.9 | −12.7 | 15.1 | −4.8 | −10.9 | −13.4 | ND | −4.7 | −6.7 | −2.7 | 86.4 | 2.5 |
| C | 14.6 | 6.4 | 15.4 | −1.4 | −8.5 | 8.2 | −1.2 | 5.9 | 9.6 | 8.2 | 5.5 | 75.5 | 1.9 | −2.3 | 9.0 | 80.3 | 9.9 |
| D | 53.0 | 28.6 | 12.1 | 0.6 | 3.7 | 16.6 | 10.2 | 15.2 | 16.2 | 22.2 | 20.5 | 75.4 | 9.3 | 4.8 | 16.7 | 50.0 | 4.7 |
| E | 7.9 | 17.7 | 15.8 | −1.8 | −12.4 | 5.6 | −7.1 | 6.5 | 1.3 | 1.4 | −3.2 | 58.1 | 3.5 | 0.6 | 8.8 | 57.7 | 22.9 |
| F | −3.4 | 13.9 | 13.7 | 11.6 | −12.5 | 8.9 | −17.9 | 4.3 | −8.2 | −4.7 | −18.1 | 76.5 | 0.2 | 10.2 | 12.6 | 22.2 | 50.0 |
| G | 3.2 | 12.5 | 22.4 | 24.6 | −5.2 | 3.6 | −15.9 | 13.5 | −5.0 | −2.0 | −8.2 | 76.4 | 7.6 | 25.0 | 10.2 | 14.6 | 50.0 |
| H | 0.5 | −3.8 | 1.8 | 29.0 | 7.4 | 0.5 | −10.2 | 14.0 | 3.8 | 0.6 | −2.0 | 73.4 | 17.5 | 19.5 | 21.0 | −19.0 | 50.0 |
| I | 58.3 | 23.8 | 16.5 | 32.3 | 5.5 | 19.9 | 38.6 | 15.5 | 20.1 | 5.1 | 71.2 | 65.2 | 9.7 | 13.8 | 20.6 | 24.0 | 50.0 |
Table 2.
PDSP secondary binding assay of DUQ0002 fractions showing significant inhibition in primary assay. Representative IC50 values are derived from radiolabeled-ligand dose-response curves for cyanobacterial fractions (DUQ0002A-I). All values are in nM (N = 3 separate determinations). Open boxes with (−) indicate that fractions failed primary binding criterion of > 50% inhibition at 10 μM. Reference compounds: 8-OH-DPAT (5-HT1A, Ki = 0.76 nM); clozapine (5-HT7, Ki = 8 nM); fenobam (mGluR5, Ki =1.5 nM); desipramine (NET, Ki = 4.7 nM) and amitriptyline (SERT, Ki = 6.3 nM).
| DUQ0002 | 5-HT1A IC50 | 5-HT7 IC50 | mGluR5 IC50 | SERT IC50 | NET IC50 |
|---|---|---|---|---|---|
| A | - | - | - | > 10000 | - |
| B | - | - | - | > 10000 | - |
| C | - | - | 4662 | > 10000 | - |
| D | > 10000 | - | 37 | > 10000 | - |
| E | - | - | > 10000 | > 10000 | - |
| F | - | - | > 10000 | - | > 10000 |
| G | - | - | > 10000 | - | 6945 |
| H | - | - | > 10000 | - | > 10000 |
| I | 1746 | 749 | 3676 | - | > 10000 |
Fractions showing greater than 50% inhibition of binding of radioligand in the primary binding screen were subjected to secondary binding assay for IC50/Ki determination (Table 2). The apparent Ki curve for DUQ0002I and a comparison to clozapine, a serotonin receptor antagonist, is shown in Figure 2E. These apparent Ki values allow us to determine if the inhibition of binding is caused by a potent compound with a low apparent Ki or a large amount of a weakly binding compound with a high apparent Ki. Fraction DUQ0002I displayed two-fold selectivity for 5-HT7R (Ki/IC50 = 749 ng/mL) over 5-HT1AR (Ki/IC50 = 1746 ng/mL), five-fold selectivity over mGluR5 (IC50 = 3676 ng/mL), and thirteen-fold selectivity over the norepinephrine transporter (NET; Ki/IC50 = > 10,000 ng/mL). Generally, the lack of selective agonists and antagonists have been attributed to the high sequence homology amongst 5-HTR subtypes (Hoyer et al., 1994). Both 5-HT1AR and 5-HT7R target adenylyl cyclase (AC) with opposing effects; 5-HT1AR activates AC increasing the concentration of cyclic adenosine monophosphate (cAMP) whereas 5-HT7R inhibits AC, decreasing cAMP concentrations (Hannon & Hoyer, 2008).
3.3. Initial characterization of DUQ0002I in male mice via intracerebroventricular (ICV) injections.
After determining DUQ0002I affinity for the 5-HT7R, 5-HT1AR, and mGluR5 receptors, we began to characterize DUQ0002I’s potential effects using in vivo assays in male mice. Since the specific nature and sites of action in the nervous system of the active compound in DUQ0002I were unknown, we began our characterization of this fraction using ICV injections. This method of administration allows for the delivery of compounds to the lateral ventricle of the brain and disperses the compound throughout the cerebrospinal fluid of the CNS (Glascock et al., 2011). This administration route was also utilized due to the fact that it was unknown whether the active compound(s) had the ability to cross the blood-brain barrier and eliminated any possible interference of first pass metabolism of any active components.
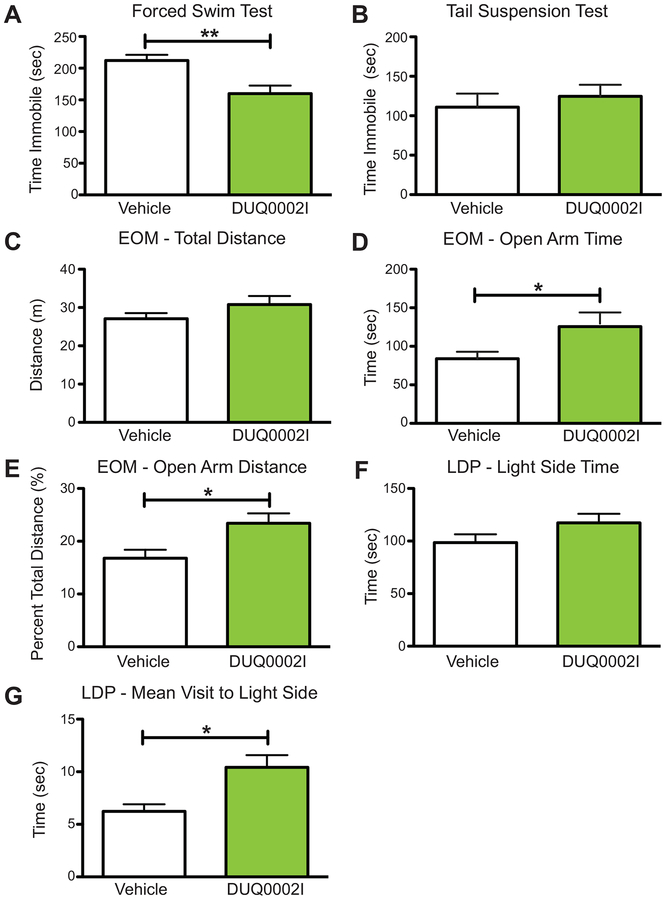
In this initial screening, we tested whether the fraction DUQ0002I could alter depression-like and anxiety-like behaviors in a group of male mice. Each animal was randomly assigned to a testing group, and all animals underwent the forced swim test, tail suspension test, elevated zero maze, and light-dark preference test. For all behavioral tests, a dose of 40 μg of DUQ0002I was used. This dose was based upon the dose that was used for the initial in vitro PDSP screening, which was done at 4 mg/mL, and then adjusted for ICV injections and intra-amygdala injections in mice that we have done previously (Kolber et al., 2010; Lax et al., 2016). Starting with the depression assays, the forced swim test was used. This standard test of depression measures the amount of time an animal spends immobile after it is placed in a beaker of water, from which it cannot escape. Longer time spent immobile in the water is correlated with greater depression-like behavior while less time spent immobile is correlated with greater antidepression-like behavior (Can, Dao, Arad, et al., 2012). Following a five-minute pretreatment with 40 μg of DUQ0002I or vehicle, animals treated ICV with DUQ0002I (n=18) spent a statistically significantly lower time immobile compared to control animals (n=18) (Figure 3A, unpaired t-test, **p=0.0019). The tail suspension test was next used to further measure changes in depression-like behavior. Similar to the forced swim test, this assay measures the amount of time an animal spends immobile after being suspended upside down by the distal end of its tail. As with the forced swim test, longer time spent immobile is indicative of depression-like behavior while less time spent immobile is associated with antidepression-like behavior (Can, Dao, Terrillion, et al., 2012). Mice pretreated with DUQ0002I (n=9) did not show any changes in total time spent immobile compared to vehicle-treated animals (n=9) (Figure 3B, unpaired t-test, p=0.5520).
Figure 3.
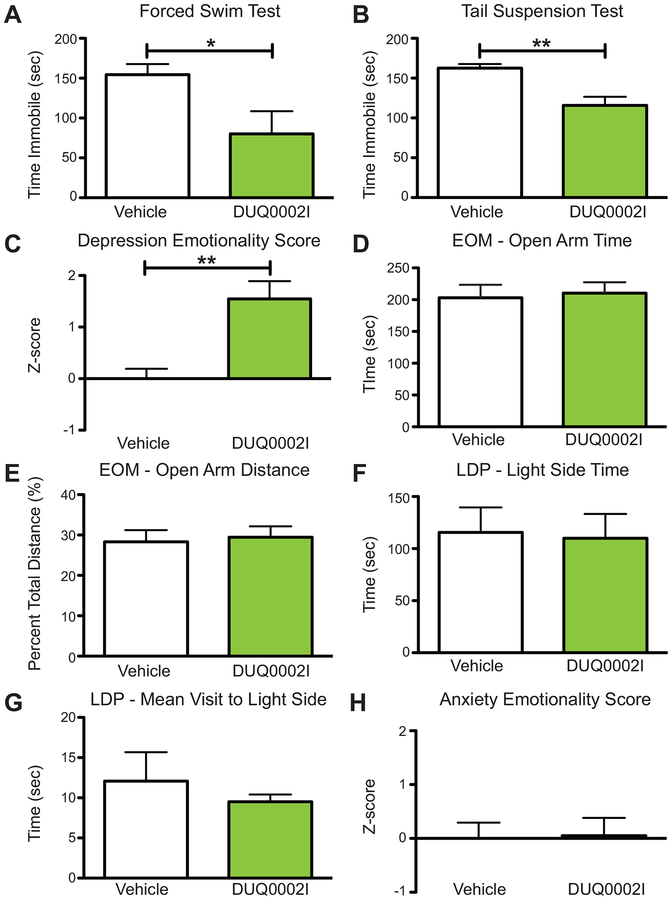
Initial characterization of the antidepression-like and anxiolytic-like effects of cyanobacterial fraction DUQ0002I in male animals via ICV administration. (A) Mice treated with DUQ0002I (n=18) decrease time immobile compared to vehicle (n=18) in the forced swim test. (B) Mice treated with DUQ0002I (n=9) show no change in time immobile compared to vehicle (n=9) in the tail suspension test. (C) Mice treated with DUQ0002I (n=18) show no changes in total distance traveled compared to vehicle (n=17) in the elevated zero maze. (D) Mice treated with DUQ0002I (n=18) show increased time spent in the open arms of the elevated zero maze (EOM) assay compared to vehicle (n=17). (E) Mice treated with DUQ0002I (n=18) also travel a greater percent of total distance traveled in the open arms of the EOM compared to vehicle (n=17). (F) Mice treated with DUQ0002I (n=19) show a trend (p=0.1203) of spending more time in the light side of the Light-Dark Preference (LDP) test during the latter half of the test compared to vehicle (n=15). (G) Mice treated with DUQ0002I (n=19) also show a higher average mean visit to the light side of the LDP test during the latter half compared to vehicle (n=15). Un-paired t-tests, *p<0.05, **p<0.01. Data shown as mean ± SEM.
In addition to studying depression-like behaviors, we also looked for changes in locomotor and anxiety-like behavior using several different assays. First, we used the elevated zero maze (EOM), a standard anxiety test which measures the amount of time and distance traveled in a circular maze with high-walled and low-walled regions, in a dimly lit room. Increased time and percent overall distance traveled in the open arms (low-walled regions) is correlated with anti-anxiety-like (anxiolytic) effects, while decreases are correlated with anxiogenic behavior (Kulkarni et al., 2007). Since this assay also measures total distance traveled, it can also be used to measure locomotor effects. Animals pretreated with DUQ0002I (n=18) showed no difference in the overall distance traveled in the EOM compared to control animals (n=17) (Figure 3C, unpaired t-test, p=0.1807), suggesting that the fraction does not cause hyperactivity. However, these same animals pretreated with DUQ0002I showed an overall statistically significantly greater amount of time spent in the open arms of the EOM (Figure 3D, unpaired t-test, *p=0.0188) and significantly greater overall percent distance (distance traveled in open/total distance traveled × 100) traveled in the open arms (Figure 3E, unpaired t-test, *p=0.0166).
Second, we used the light-dark preference (LDP) test, another test of anxiety-like behavior where animals are placed in a two-chamber box with a brightly illuminated side and a completely dark side, separated by a small door. Increases in total time spent in the light side of the box are correlated with decreased anxiety-like effects, while decreases are correlated with increases in anxiety-like effects (Bourin & Hascoet, 2003). Pretreatment with DUQ0002I (n=19) caused animals to trend towards spending more total overall time spent in the light side compared to control animals (n=15) in the latter half of the test (Figure 3F, unpaired t-test, p=0.1203). DUQ0002I also caused animals to have a greater mean visit (how long the animal spends on average when it enters the light side) to the light side compared to animals pretreated with vehicle in the latter half of the test (Figure 3G, unpaired t-test, **p=0.0025). Together, these data from both the EOM and LDP indicate an anxiolytic-like effect of DUQ0002I. Finally, we observed no changes in body temperature in animals that received DUQ0002I via ICV injection compared to vehicle.
3.4. Characterization of the specificity of DUQ0002I effects.
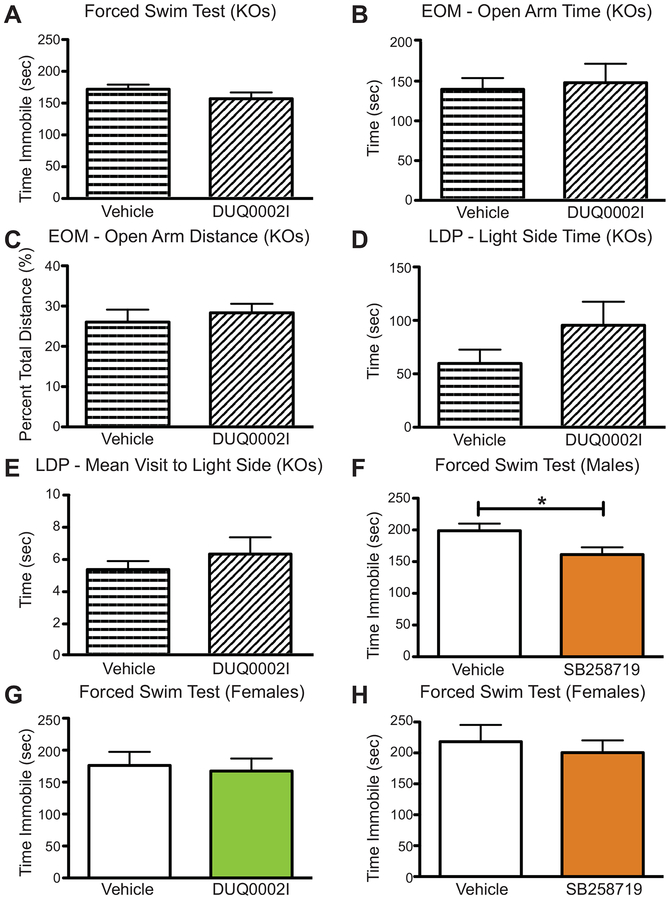
DUQ0002I showed secondary binding affinity for 5-HT1AR, 5-HT7R, and mGluR5. Given the 2-fold selectivity of 5-HT7R over 5-HT1AR and mGluR5, we tested to see if DUQ0002I had effects in 5-HT7R knockout animals. If the active compound(s) in DUQ0002I was selective for the 5-HT7R, then we would predict that there would be no behavioral changes in any of the assays we had previously tested with DUQ0002I using wildtype male mice. Here a group of both wild-type and 5-HT7R knockout male animals were tested in the forced swim test, elevated zero maze, and light-dark preference assays. In the forced swim test (Figure 4A, unpaired t-test, p=0.2490), DUQ0002I (n=6) did not cause any change on overall time immobile when compared to vehicle injected knockout mice (n=6). Similarly, DUQ0002I (n=6) did not cause animals to spend more time in the open arms (Figure 4B, unpaired t-test, p=0.7712) or travel a greater percent distance in the open arms (Figure 4C, unpaired t-test, p=0.5599) when compared to vehicle-treated knockout animals (n=6) in the elevated zero maze. Finally, in the light dark preference assay, KO animals pretreated with DUQ0002I (n=6) did not spend a greater amount of time in the light side (Figure 4D, unpaired t-test, p=0.1929) when compared to KO mice that received vehicle (n=6). These same animals also did not have a greater mean visit to the light side when compared to control KO animals (Figure 4E, unpaired t-test, p=0.4342). Together, these data in the KO animals suggest that the anti-depressive effects observed from DUQ0002I are caused by selective antagonism of the 5-HT7R.
Figure 4.
Fraction DUQ0002I shows no effects in 5-HT7R knockout animals or female animals via ICV administration. (A) Male homozygote 5-HT7R knockout (KO) mice treated with DUQ0002I (n=6) do not show any differences in time immobile compared to vehicle (n=6) in the forced swim test. (B) Male homozygote 5-HT7R KO mice treated with DUQ0002I (n=6) do not change the amount of time spent in the open arms of the EOM compared to vehicle (n=6). (C) 5-HT7R KO mice treated with DUQ0002I (n=6) also do not change percent of overall distance traveled in the open arms of the EOM compared to vehicle (n=6). (D) 5-HT7R KO mice treated with DUQ0002I (n=6) do not change the amount of time spent in the light side of the LDP test during the latter half of the test compared to vehicle (n=6). (E) 5-HT7R KO mice treated with DUQ0002I (n=6) do not change the mean visit to the light side of the LDP test during the latter half of the test compared to vehicle (n=6). (F) Male wildtype mice treated with the 5-HT7R antagonist SB258719 (n=15) show a decrease in time immobile compared to vehicle (n=17) in the forced swim test. (G) Female wildtype mice treated with DUQ0002I (n=8) do not show any differences in time immobile compared to vehicle (n=8). (H) Female wildtype mice treated with SB258719 (n=8) do not show any differences in time immobile compared to vehicle (n=7). Un-paired t-tests, *p<0.05. Data shown as mean ± SEM.
As an additional measure of 5-HT7R specificity and to help determine whether DUQ0002I is an agonist or an antagonist, we compared the effects of DUQ0002I with a known 5-HT7R antagonist, a compound that has been previously shown to have antidepression-like effects in the forced swim test (Guscott et al., 2005). In a manner similar to DUQ0002I, a five minute pretreatment of 5 μg of SB258719 caused wild-type mice (n=15) to exhibit statistically significant lower levels of immobility in the forced swim test when compared to animals that received control vehicle injections (n=15) (Figure 4F, unpaired t-test, *p=0.0275). Since antagonism of the 5-HT7R is known to cause antidepressant-like effects, the similar behavioral effects of SB258719 suggested a similar antagonistic mechanism as DUQ0002I.
Due to the known sex differences in depression and anxiety (Kokras & Dalla, 2017), we next tested DUQ0002I in female animals via ICV injection to see if it induced the same antidepression-like and anxiolytic-like effects as we had previously shown in male animals. Interestingly, administration of the same 40 μg dose of DUQ0002I in female mice (n=8) did not induce any changes in immobility time in the forced swim test when compared to control vehicle-injected mice (n=8) (Figure 4G, unpaired t-test, p=0.7675). Similarly, no differences were seen between females treated with DUQ0002I or vehicle in the tail suspension test, elevated zero maze, and light-dark preference test (data not shown). To determine if these sex differences were specific to our cyanobacteria fraction or was a broader effect of 5-HT7R antagonists, we also tested the effects of SB258719 in a separate group of wild-type female mice. As with DUQ0002I, SB258719 (n=8) failed to alter immobility time compared to control vehicle-injected animals (n=7) (Figure 4H, unpaired t-test, p=0.5986). These data suggest there may be a previously unidentified difference between males and females in the way they respond to 5-HT7R antagonists or in the distribution of 5-HT7R in their brains.
3.5. Anatomical characterization of DUQ0002I in males via hippocampal administration.
After ICV and specificity characterization of DUQ0002I, we explored which area of the brain was contributing to the observed phenotypes. Previous studies have shown that antagonists of the 5-HT7R cause antidepression and anxiolytic-like effects (Guscott et al., 2005) when specifically administered to the CA1 region of the hippocampus (Wesolowska et al., 2006). We delivered DUQ0002I via intrahippocampal injections to see if this brain region was sufficient to induce the observed effects of DUQ0002I. Only male animals were used since DUQ0002I was shown to not alter behavior in females (Figure 4G). Here, a group of animals underwent testing using the forced swim test, tail suspension test, elevated zero maze and light-dark preference test. Beginning with the forced swim test, we showed that CA1 administration of DUQ0002I (n=5) caused a statistically significant decrease in overall time immobile compared to vehicle-treated animals (n=5) (Figure 5A, unpaired t-test, *p=0.0450); in contrast to ICV delivery, we found that hippocampal-delivered DUQ0002I (n=5) also caused an antidepression-like effect in the tail suspension test compared to vehicle-treated animals (n=5) (Figure 5B, unpaired t-test, **p=0.0046).
Figure 5.
Administration of fraction DUQ0002I bilaterally to the CA1 region of the hippocampus is sufficient to induce antidepression-like effects in male animals. (A) Mice treated with DUQ0002I (n=5) decrease time immobile compared to vehicle (n=5) in the forced swim test. (B) Mice treated with DUQ0002I (n=5) decrease time immobile compared to vehicle (n=5) in the tail suspension test. (C) Mice treated with DUQ0002I (n=5) show a positive increase in emotionality when looking at the effects of the depression assays (forced swim and tail suspension) compared to vehicle (n=5). (D) Mice treated with DUQ0002I (n=5) show no difference in time spent in the open arms of the EOM compared to vehicle (n=5). (E) Mice treated with DUQ0002I (n=5) show no difference in overall percent distance traveled in the open arms of the EOM compared to vehicle (n=5). (F) Mice treated with DUQ0002I (n=5) show no difference in time spent in the light side of the LDP box during the latter half of the test compared to vehicle (n=5). (G) Mice treated with DUQ0002I (n=5) show no difference in the mean visit to the light side of the LDP box compared to vehicle (n=5). (H) Mice treated with DUQ0002I (n=5) show no changes in emotionality when looking at the effects of the combined anxiety assays (elevated zero maze and light-dark preference) compared to vehicle (n=5). Un-paired t-tests, *p<0.05, **p<0.01. Data shown as mean ± SEM.
To show overall effects of DUQ0002I in depression-like behavior, we utilized the Z-score analysis technique (Guilloux et al., 2011). This method allows for the comparison of results across different, but related, sets of data through standardization by comparison to the control group. It enables us to see the overall antidepression-like (or later anti-anxiety-like) effects of DUQ0002I by averaging the effects of the depression behavioral tests (time immobile in forced swim and tail suspension tests). Here, the parameter measured for each animal (time immobile, X) was given an individual Z-score (Z) to determine how many standard deviations (σ) each animal was above or below the mean of the control group (μ) using the following formula: Z = (X – μ) /σ. With this type of analysis, the control group always has an overall Z-score of zero. For the experimental groups, positive Z-scores reflect positive emotionality changes (i.e. antidepression or antianxiety), whereas negative Z-score reflect negative emotionality changes (i.e. depression, anxiety). Not surprisingly, a Z-score analysis of CA1 administration showed that DUQ0002I (n=5) caused a statistically significant antidepression-like effect when compared to vehicle-treated animals (n=5) (Figure 5C, unpaired t-test, **p=0.0045). These data show that CA1 administration of DUQ0002I is sufficient to induce strong antidepression-like effects.
Next, we tested to see if the anxiolytic-like effects of ICV-delivered DUQ0002I were mediated, in part, by 5-HT7R in the CA1 of the hippocampus. In the EOM test, we showed that DUQ0002I (n=5) did not change the total amount of time spent in the open arms of the maze (Figure 5D, unpaired t-test, p=0.7875) when compared to control vehicle-injected (n=5) animals via CA1 injection. The fraction also did not change the overall percent distance that the animals traveled in the open arms (Figure 5E, unpaired t-test, p=0.7806). We also tested CA1 administration of DUQ0002I in the light-dark preference test and found similar negative results. DUQ0002I (n=5) did not change the overall amount of time spent in the light side of the light-dark preference test when compared to vehicle-treated animals (n=5) (Figure 5F, unpaired t-test, p=0.8695) during the latter half of the test, nor did DUQ0002I change the overall mean visit to the light side of the box (Figure 5G, unpaired t-test, p=0.5067). Finally, as was done for the depression tests, an overall anxiety test Z-score was calculated for the CA1 administration of DUQ0002I (calculated using the time and percent distance traveled in open from the EOM and time and mean visit to the light side). In contrast to what was shown for depression behaviors, the overall emotionality score related to anxiety behaviors showed no overall difference between control and DUQ0002I treated animals (Figure 5H, unpaired t-test, p=0.9127). Combined, these data show that while DUQ0002I administration to the CA1 is sufficient to induce antidepression-like effects, it is not sufficient to induce the anxiolytic-like effects.
3.6. Bioassay-guided fractionation of DUQ0002I.
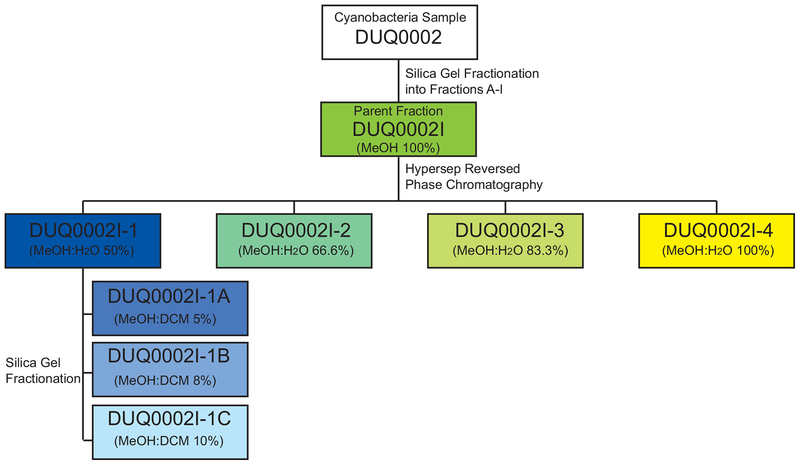
All of the alterations in depression and anxiety-like behavior seen thus far were produced using a fraction of cyanobacterial extract (DUQ0002I), which contained a mixture of various compounds. In an effort, to move beyond simply testing extracts for behavioral effects and identify specific active compounds within this fraction, DUQ0002I was purified into four subfractions (named DUQ0002I-1, −2, −3 and −4) using reverse-phase column chromatography. Sub-fractions DUQ0002I-2, DUQ0002I-3 and DUQ0002I-4 were all obtained as green pastes. In contrast, sub-fraction DUQ0002I-1 was obtained as a yellow paste and, unlike fractions DUQ0002I-2, −3 and −4, was shown by NMR to be most chemically similar to DUQ0002I. Due to its chemical similarity to the parent fraction, and the fact that these sub-fractions still contained mixtures of compounds, sub-fraction DUQ0002I-1 was further purified using silica gel chromatography to yield three more purified sub-fractions, (named DUQ0002I-1A, -B and -C). A flowchart illustrating the fractionation of DUQ0002I can be seen in Figure 6. All sub-fractions and purified sub-fractions describe herein were tested in vivo (see Figure 7) after the initial characterization of the parent fraction described below in an attempt to determine if these purified fractions contained the active compound(s).
Figure 6.
Flowchart of the fractionation of DUQ0002I.
Figure 7.
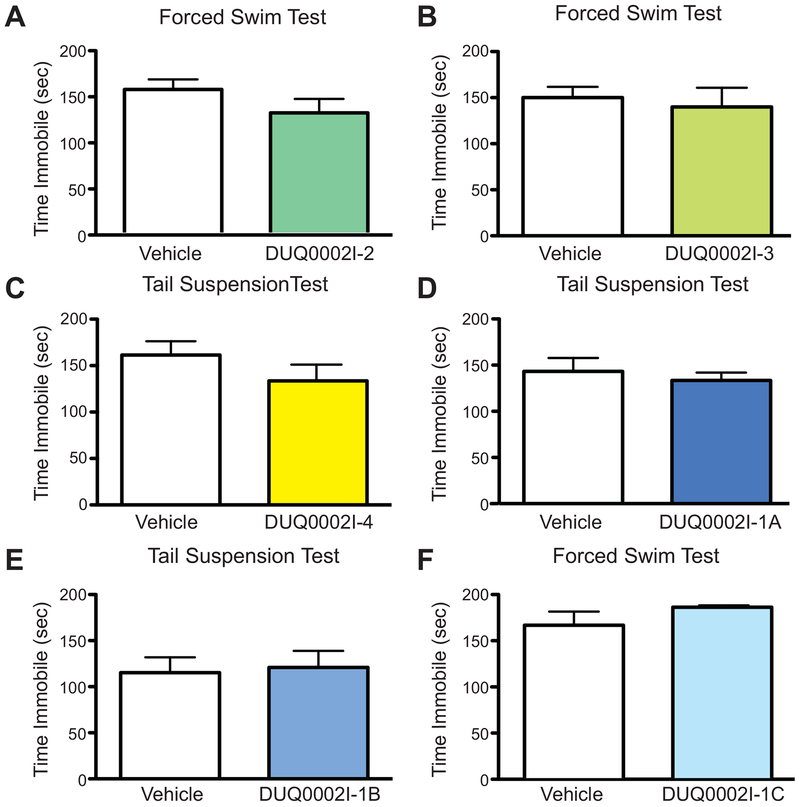
Administration of purified subfractions of DUQ0002I bilaterally to the CA1 region of the hippocampus has no effects in males. (A) Mice treated with DUQ0002I-2 (n=11) show no difference in time immobile compared to vehicle (n=12) in the forced swim test. (B) Mice treated with DUQ0002I-3 (n=6) show no difference in time immobile compared to vehicle (n=5) in the forced swim test. (C) Mice treated with DUQ0002I-4 (n=6) show no difference in time immobile compared to vehicle (n=6) in the forced swim test. (D) Mice treated with DUQ0002I-1 (n=6) show no difference in time immobile compared to vehicle (n=6) in the tail suspension test. (E) Mice treated with DUQ0002I-1B (n=6) show no difference in time immobile compared to vehicle (n=5) in the tail suspension test. (F) Mice treated with DUQ0002I-1C (n=7) show no difference in time immobile compared to vehicle (n=7) in the forced swim test. Un-paired t-tests. Data shown as mean ± SEM.
The three subfractions (DUQ0002I-2, −3 and −4) and the three purer subfractions of subfraction DUQ0002I-1 (DUQ0002I-1A, −1B and −1C) were tested via CA1 injections in separate groups of male animals that underwent either the tail suspension or forced swim tests. We predicted that since significant antidepressant-like effects were seen with the parent fraction (DUQ0002I) via CA1 injections (see Figure 5), then any subfraction(s) with the active compound(s) should have the same, or perhaps stronger behavioral effects. Since subfraction DUQ0002I-1 had chemically unique characteristics similar to the parent fraction, we predicted that one if its purified subfractions (either DUQ0002I-1A, −1B or −1C) would contain an active compound and induce the same antidepression-like effects as the parent fraction.
Interestingly, none of the subfractions or purified subfractions exhibited any statistically significant behavioral effects (DUQ0002I-2 (n=11, vehicle group, n=12) (Figure 7A, unpaired t-test, p=0.1810), DUQ0002I-3 (n=6, vehicle group n=5) (Figure 7B, unpaired t-test, p=0.6954) or DUQ0002I-4 (n=6, vehicle group n=6) (Figure 7C, unpaired t-test, p=0.2545; DUQ0002I-1A (n=6, vehicle group, n=6) (Figure 7D, unpaired t-test, p=0.5780), DUQ0002I-1B (n=6, vehicle group, n=5) (Figure 7E, unpaired t-test, p=0.8271), or DUQ0002I-1C (n=7, vehicle group, n=7) (Figures 7F, unpaired t-test, p=0.2145)). Following these negative findings, we reconfirmed the activity of the parent DUQ0002I fraction by screening it once again using NMR. No changes were observed, such that both the original NMR and this second NMR that reconfirmed activity had the same active peaks. This suggests that the parent nor subfractions had degraded. This may suggest that the activity seen in the parent fraction is caused by a synergistic effect between two or more unidentified compounds in different subfractions that do not have sub-therapeutic activity alone. For our subsequent SNI studies, we chose to test the parent fraction, while structure elucidation efforts continue to identify the individual synergistic components that could become lead compounds in drug design.
3.7. Utilizing DUQ0002I as a therapeutic lead in the spared nerve injury (SNI) model of comorbid pain and depression.
Thus far in our studies, we showed that DUQ0002I causes anxiolytic and antidepression-like effects that are specific to male animals and likely act through 5-HT7R. We also showed that the antidepression-like effects were likely caused, in part, through targeting the CA1 of the hippocampus. In all of these experiments, the animals that were tested were naïve mice; they did not have any underlying manipulation that caused them to be in a heightened state of depression. In order to understand more about how targeting the 5-HT7R may be involved in altering depression-like behavior, we decided to test DUQ0002I in animals with an underlying depression phenotype. Here, only male animals were tested since DUQ0002I was found to not have any effects in females (see Figure 4G). Since there is significant evidence that chronic pain often occurs with depression symptoms and that each condition can exacerbate the effects of the other (Bair, Robinson, Katon, & Kroenke, 2003; Von Korff, Ormel, Katon, & Lin, 1992), we decided to use the well characterized spared nerve injury (SNI) model (Decosterd & Woolf, 2000) of neuropathic pain with comorbid depression.
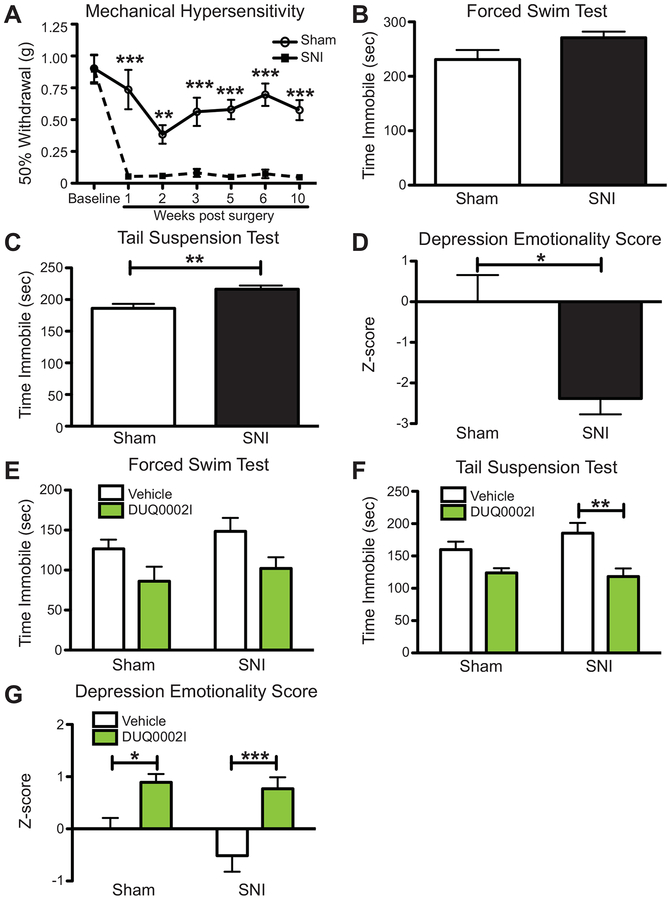
First, we wanted to determine if and when animals that received no compounds with SNI exhibited changes in mechanical sensitivity (i.e. pain-like behavior) and affective behaviors (i.e. depression-like behaviors) when compared to control sham animals. Consistent with the robust pain-like phenotype in SNI, we found that at one, three, five, six and ten weeks post-surgery, male SNI animals (n=6) had statistically significant lower withdrawal thresholds when compared to sham animals (n=6) in the ipsilateral paw (Figure 8A, 2-way ANOVA, overall effect of time ***p<0.0001, overall effect of surgery ***p<0.0001, Bonferroni post hoc tests, **p<0.01, ***p<0.001). We next determined when SNI animals developed depression-like effects in the forced swim and tail suspension tests when compared to sham animals. Using the same group of animals that underwent mechanical sensitivity testing, we found that by the five-week post-surgery time point, SNI animals showed no differences in overall time immobile in the forced swim and in tail suspension tests (data not shown). However, by the 10 week post-surgery time point, SNI animals (n=6) trended towards showing higher levels of immobility when compared to sham animals (n=6) in the forced swim test (Figure 8B, unpaired t-test, p=0.0834). SNI animals (n=6) also showed significantly higher levels of immobility in the tail suspension test when compared to control sham animals (n=6) (Figure 8C, unpaired t-test, **p=0.0037). Combining the results with a Z-score analysis (calculated using the time immobile in the forced swim and tail suspension tests) showed that overall, SNI animals showed negative depression emotionality (i.e. greater depression-like behavior) compared to sham animals (Figure 8D, unpaired t-test, *p=0.0110).
Figure 8.
Spared nerve injury (SNI) induces mechanical hypersensitivity and increases depression-like effects 10 weeks post surgery; DUQ0002I administration reverses these depression-like effects when administered to the CA1 in male animals. (A) Mice that receive SNI surgery (n=6) have lower withdrawal thresholds for up to 10 weeks post surgery compared to sham (n=6). (B) Mice that receive SNI surgery (n=6) show a trend of increasing time immobile compared to sham (n=6) in the forced swim test. (C) Mice that receive SNI surgery (n=6) increase time immobile compared to sham (n=6) in the tail suspension test. (D) Mice that receive SNI surgery (n=6) have a negative depression emotionality score when looking at the effects of the combined depression assays (tail suspension and forced swim tests) compared to sham (n=6). (E) SNI mice that receive DUQ0002I (n=12) and sham mice that receive DUQ0002I (n=10) both decrease time immobile compared to SNI animals that receive vehicle (n=10) and sham animals that receive vehicle (n=12) in the forced swim test. (F) SNI mice that receive DUQ0002I (n=12) and sham mice that receive DUQ0002I (n=10) both decrease time immobile compared to SNI animals that receive vehicle (n=10) and sham animals that receive vehicle (n=12) in the tail suspension test. (G) SNI mice that receive DUQ0002I (n=12) and sham mice that receive DUQ0002I (n=10) both show a positive increase in emotionality score when looking at the combined effects of the depression assays (forced swim and tail suspension) compared to SNI animals that receive vehicle (n=10) and sham animals that receive vehicle (n=12). A – 2-way ANOVA, main effect of time and surgery, Bonferroni post-hoc test *p<0.05, ***p<0.001; B – un-paired t-test, p=0.0834; C – un-paired t-test, **p<0.01; D – un-paired t-test, *p<0.05; E – 2-way ANOVA, overall main effect of compound; Bonferroni post hoc test n.s. and sham – unpaired t-test, +p=0.0658 and SNI – unpaired t-test, *p=0.0458; F – 2-way ANOVA, overall main effect of compound; Bonferroni post-hoc test **p<0.01 and sham – unpaired t-test, *p=0.0271 and SNI – unpaired t-test, **p=0.0032; G – 2-way ANOVA, overall main effect of compound, Bonferroni post-hoc test *p<0.05, ***p<0.001. Data shown as mean ± SEM.
Since the SNI model caused both pain-like and depression-like effects 10 weeks after surgery, we tested the effects of DUQ0002I at this time point. These data allowed us to determine if DUQ0002I would have an overall greater effect on SNI animals when compared to sham mice due to the underlying model of comorbidity. Here, a group of animals underwent either SNI or sham surgery 10 weeks prior to the beginning of behavioral testing, which included the forced swim and tail suspension tests. DUQ0002I targeted to the CA1 had no behavioral effect on SNI-induced mechanical hypersensitivity (data not shown). However, DUQ0002I had an overall effect in reducing immobility in the forced swim test in both sham (n=10) and SNI male animals (n=12) compared to vehicle-treated SNI (n=10) and sham (n=12) animals (Figure 8E, 2-way ANOVA, overall effect of compound **p=0.0063, overall effect of surgery p=0.2143, Bonferroni post hoc tests n.s.). When looking at each individual group of animals, both sham (Figure 8E, unpaired t-test, +p=0.0658) and SNI animals (Figure 8E, unpaired t-test, *p=0.0458) show near or significantly lower levels of immobility when DUQ0002I is administered to the CA1 compared to vehicle-treated animals. Similarly, DUQ0002I delivered to the CA1 reduced overall immobility in the tail suspension test for both sham and SNI animals (Figure 8F, 2-way ANOVA, overall effect of compound ***p=0.0002, overall effect of surgery p=0.4385, Bonferroni post hoc test, **p<0.01). When looking at each individual group of animals, both sham (Figure 8F, unpaired t-test, *p=0.0271) and SNI animals (Figure 8F, unpaired t-test, **p=0.0032) show similar levels of immobility when DUQ0002I is administered to the CA1 when compared to vehicle-treated animals.
To look at the overall effect that DUQ0002I had on both SNI and sham animals, a Z-score analysis for depression-like behaviors (using time immobile in the forced swim and tail suspension tests) was calculated (Figure 8G, 2-way ANOVA, overall effect of compound ***p<0.0001, overall effect of surgery p=0.1728, Bonferroni post hoc tests, *p<0.05 – sham, ***p<0.001 – SNI). This Z-score analysis shows that overall, DUQ0002I reduced depression-like behaviors in both SNI and sham animals and that these antidepression-like effects may be greater in SNI animals when compared to sham animals.
4. DISCUSSION
Overall, our data show that cyanobacteria may represent an important unexplored resource for 5-HT specific ligands. We found an extract from a marine cyanobacterium of the genus Leptolyngbya induces antidepressant and anxiolytic-like effects that are specific to the 5-HT7R and male animals using in vivo models of behavior. Additionally, we were able to show that administration of our extract to the CA1 of the hippocampus is sufficient to induce these antidepressant-like effects and that the effects are likely caused by a mixture of compounds in the fraction. Finally, using this fraction, we demonstrated that the active compounds involved had similar effects in animals with comorbid pain-like and depression-like behavior.
The genus from which our extract originated, Leptolyngbya, has global distribution in terrestrial and aquatic ecosystems (Shimura et al., 2015) and is one of the major natural product-producing genera of marine cyanobacteria (N. Engene et al., 2013). While it is unknown if the sample is still growing at the same coordinates, Leptolyngbya growing under similar environmental conditions would be predicted to produce the same types of compounds since Lyngbya, a closely related genus of cyanobacteria, has been found to grow in the same location (and produce the same compounds upon recollection) two years apart (Rossi, Roberts, Yoo, & Gerwick, 1997). The fact that our phylogenetic analysis showed that DUQ0002 belonged to Leptolyngbya, a different genus from the field identification (Oscillatoria or Moorea), highlights the importance of using 16S rRNA gene sequences to obtain more accurate identifications of field collected cyanobacteria. Recent studies using whole-genome and 16S sequencing approaches have changed the traditional phylogenetic classification of cyanobacteria (Wilmotte, 1994). Known compounds extracted from Leptolyngbya include palmyrolide A, which has potent sodium channel blocking activity without cytotoxicity (Pereira et al., 2010), crossbyanols A – D with antibiotic activity (Choi, Engene, Smith, Preskitt, & Gerwick, 2010) and honaucins A – C with the ability to inhibit proinflammtory cytokines (Choi et al., 2012). Another compound, coibamide A, a potent antiproliferative cyclic depsipeptide, was also extracted from a Leptolyngbya species from the Pacific side of Panama but from the other side of the Azuero peninsula near Coiba Island (Medina et al., 2008). DUQ0002, however, represents the first Leptolyngbya extract with GPCR-specific psychoactive effects. Overall, these data suggest that Leptolyngbya species have significant potential in the field of neuropharmacognosy.
The observed affinities for cyanobacterial secondary metabolites (Figure 1; Table 2), including those from DUQ0002I for 5-HT1AR and 5-HT7R, are very encouraging from a pharmacological viewpoint (Naumenko, Popova, Lacivita, Leopoldo, & Ponimaskin, 2014) and may represent a chemical ecology role for these serotonin receptor ligands. A possible explanation for their presence could be linked to the predator-prey relationship between Aplysia sea hares and marine cyanobacteria. In their natural environment, colonial marine cyanobacteria are preyed upon by Aplysia sea hares. This ecological relationship could link the biosynthesis of 5-HTR ligands by marine cyanobacteria to these marine mollusks due to the simple structure of their nervous system. In Aplysia, the entire CNS consists of only a small number of neurons, which contain 5-HTR and have been shown to be involved in learning and memory (Barbas, DesGroseillers, Castellucci, Carew, & Marinesco, 2003). The compounds from the cyanobacteria could be affecting the feeding behavior of the sea hares by targeting their nervous system, and provides a possible explanation for their presence. The results of the screening of all 276 fractions from Panama and Curaçao, showing 21% of all fractions have 5-HTR or 5-HT transporter activity, suggest a strong evolutionary pressure to produce 5-HT metabolites in these organisms and show the potential for cyanobacteria to be used in the drug discovery space.
The initial screening of the DUQ0002 fraction using the ICV administration route allowed us to have the broadest possible application of the fractions across the brain, brainstem and spinal cord, while overall reducing the total amount needed to see effects. It also allowed us to avoid potentially harmful systemic effects of targeting serotonin receptors (Hutcheson, Setola, Roth, & Merryman, 2011) and allowed us to test compounds that might not cross the BBB. Other broad administration routes, including intravenous, intraperitoneal or oral gavage, while more clinically relevant, would have limited our testing. Additionally, we are not overly concerned about BBB permeability or alternate administration routes of our fractions. Along with this, only one dose (40 μg) of the parent fraction (DUQ0002I) was tested. Since this initial dose worked to induce behavioral changes from the first screening tests, it was the dose that was used for the rest of the studies. Testing different doses of the parent fraction would not have provided any additional insight into the specific dose of active compound(s) needed since DUQ0002I itself is a mixture of compounds. Rather, our overall focus is on discovering and identifying compounds with efficacy in animal models at an early stage before dealing with lead optimization, modifications that could circumvent administration issues and dosing.
Our results from the initial screening of fraction DUQ0002I with ICV administration correspond well with other studies of the 5-HT7R using selective ligands in animal models of depression and anxiety. While the models used are not models of the anhedonic profile or chronic treatment, the assays that were used are well-characterized and show a strong predictive efficacy in terms of how compounds would work in humans (Q. Wang, Timberlake, Prall, & Dwivedi, 2017). In terms of depression, in rodents, administration of the selective 5-HT7R antagonist SB269970 decreases depression-like behaviors in the forced swim, tail suspension, and conflict drinking tests (Hedlund et al., 2005; Sarkisyan et al., 2010; Wesolowska et al., 2006). Other novel antagonists have shown similar antidepression-like effects (Kim et al., 2014). Moreover, male 5-HT7R knockout animals also exhibit a similar phenotype (Guscott et al., 2005). This suggests that these changes in behavior are truly dependent on the 5-HT7R. Looking at anxiety-like behavior, in mice, pharmacological blockade of the 5-HT7R reduces anxiety-like behavior in the open field test (Guilloux et al., 2013). Other studies have shown similar results with SB269970 causing anxiolytic-like effects in the Vogel drinking test and elevated plus maze in rats and the four-plate test in mice (Wesolowska et al., 2006). SB269970 has also been shown to cause a reduction in marble burying in mice, a behavior showing an anxiolytic-like effect (Hedlund & Sutcliffe, 2007). Combined, these data show that, in general, antagonists of the 5-HT7R decrease both depression and anxiety-like behaviors. Therefore, since the behavioral phenotype of DUQ0002I mimics the effects of these known antagonists, it suggests the active compound(s) in DUQ0002I is most likely acting as an antagonist of the 5-HT7R. Our data also support an overall anti-depressant and anti-anxiety role of inhibiting 5-HT7R since we were able to induce the same behavioral effect with ICV injections of a known 5-HT7R antagonist SB258719. Moreover, the fact that DUQ0002I failed to induce antidepressant and anxiolytic-like effects in 5-HT7R knockout animals suggests the selectivity of the active compounds in DUQ0002I for the 5-HT7R and indicates that the active compounds may not be acting on any other receptors, including the 5-HT1AR, NET or mGluR5. One explanation for this is that the behavioral effects of DUQ0002I were occluded by genetic ablation of the 5-HT7R, suggesting that DUQ0002I was acting as an antagonist of the 5-HT7R. However, it should be noted that in these knockout animals, already showing an antidepression-like phenotype, it may be difficult to see further antidepression-like effects, even if DUQ0002I was acting on a distinct receptor.
Our data show a potentially interesting and uncharacterized sex difference in terms of response to inhibition of the 5-HT7R in depression and anxiety-like behaviors. All of our initial antidepressant and anxiolytic-like DUQ0002I effects were found in male animals and were not replicated in female mice. Likewise, female mice did not show any behavioral changes with administration of the 5-HT7R antagonist SB258719. Despite the known sex differences in depression and anxiety (Kokras & Dalla, 2017), all of the depression studies (Hedlund et al., 2005; Kim et al., 2014; Mnie-Filali et al., 2011; Sarkisyan et al., 2010; Wesolowska et al., 2006) and anxiety studies (Guilloux et al., 2013; Hedlund & Sutcliffe, 2007; Wesolowska et al., 2006) that have looked into the behavioral effects of 5-HT7R antagonists were done using only male animals. This is also true for 5-HT7KO studies where only male mice have been tested in cognitive assays (Guscott et al., 2005; Roberts et al., 2004). The only exception is a study that found there was no hypothermic response to 5-HT in male or female mice (Hedlund et al., 2003). It is known that estrogens can interfere with the effects of antidepressants (Keers & Aitchison, 2010) and that females can exhibit higher levels of serotonin and synthesize it more slowly (Nishizawa et al., 1997). Additional work is needed to determine the mechanism behind the observed sex differences. In the pain literature, several studies have found that 5-HT7R antagonists increase pain-like behaviors in males (Brenchat et al., 2009; Liu, Reid, & Sawynok, 2013), whereas antagonists decrease pain-like behaviors in females (Amaya-Castellanos et al., 2011; Godinez-Chaparro, Barragan-Iglesias, Castaneda-Corral, Rocha-Gonzalez, & Granados-Soto, 2011). Our failure to see an effect of DUQ0002I in SNI-induced hypersensitivity may have been caused by a floor effect in the assay where male SNI-treated mice could not show additional pain behavior. Future studies with DUQ0002I will explore potential effects in the context of other models of acute and chronic pain.
The results from our anatomical studies have provided insight into where the compounds present in DUQ0002I may be acting to induce the effects observed. The initial characterization experiments with ICV injections allowed us to broadly target the 5-HT7R in the central nervous system. The lack of anatomical specificity with the ICV injections is most likely the reason behind the wide range of effects we saw in the behavioral assays. 5-HT7R expression is well-characterized in rodents and humans and is known to be expressed in a variety of regions including the cortex, hippocampus, amygdala, hypothalamus and thalamus (Bonaventure et al., 2004; E. Gellynck et al., 2013; Martin-Cora & Pazos, 2004; Varnas, Thomas, Tupala, Tiihonen, & Hall, 2004). The hippocampus is part of the limbic circuitry of the brain and serotonin in this region is involved in emotional states (Hensler, 2006) and may play a role in the action of antidepressants (Nestler et al., 2002; Vythilingam et al., 2004). Moreover, the 5-HT7R has been shown to play a role in hippocampal neuronal excitability (Tokarski, Zahorodna, Bobula, & Hess, 2003), glutamatergic synaptic transmission (Andreetta et al., 2016; Costa, Trovato, Musumeci, Catania, & Ciranna, 2012; Vasefi et al., 2013), synaptic plasticity (Costa, Sardone, Lacivita, Leopoldo, & Ciranna, 2015) and hippocampal-dependent memory (Eriksson, Golkar, Ekstrom, Svenningsson, & Ogren, 2008; Sarkisyan & Hedlund, 2009). Previous studies have shown that blocking 5-HT7R in the CA1 region of the hippocampus decreases depression-like behaviors in rodents (Wesolowska et al., 2006), and our results obtained in the forced swim and tail suspension tests show the same effect with administration of DUQ0002I. Of note, we saw antidepressant-like effects in both FST and TST with CA1 administration of DUQ0002I; with ICV injection, effects were only seen in FST. This TST difference could be a result of an insufficient dose of the active compounds in DUQ0002I reaching the area of activation with the ICV injections. We also completed a Z-score analysis (Guilloux et al., 2011) of “depression” behaviors. The Z-score analysis is a powerful that allows for the comparison of results across similar assays and has been used in studies that use multiple measures of behavior (de Sa-Calcada et al., 2015; Maluach et al., 2017; Mendez-David et al., 2017; Sargin, Oliver, & Lambe, 2016). The Z-score analysis highlights the robust anti-depressant effect that DUQ0002I had when administered to the CA1.
The strong anti-depressant effect was in contrast to our failure to observe anxiety-like behaviors with CA1 administration of DUQ0002I. We showed that there were no effects in either the elevated zero maze or light dark preference tests. The contrast between the Z-score analyses of the depression behaviors and anxiety behaviors highlights this difference very clearly. The fact that anxiolytic-like effects were not seen via CA1 administration but were seen with ICV administration also tells us that DUQ0002I has the potential to act at multiple CNS sites. Notably, the amygdala is one of the central brain regions known to be involved with anxiety and fear (Adhikari et al., 2015). The fact that 5-HT7R are expressed in the amygdala (Bonaventure et al., 2004) and that it is close to the cerebrospinal fluid system targeted with ICV injections means that it could possibly be the site of action for the anxiety-like effects of DUQ0002I.
Interestingly, in our attempt to find the active compound in DUQ0002I responsible for inducing the behavioral effects shown, we found that none of the subfractions DUQ0002I-1A, B and C and 2, 3 and 4 induced any antidepressant-like effects with CA1 administration. This suggests that a mixture of one or more compounds found in the original parent fraction itself (DUQ0002I) is responsible for the changes in behavior. While no definitive conclusions can be drawn about the number of compounds or dose of each active compound required to cause effects with our cyanobacterial fraction, this type of synergistic effect from natural products is not uncommon. Isoflavone and curcumin, two natural products, inhibit the growth of pancreatic cancer cells and alter gene expression in vitro but only in combination (Z. Wang et al., 2008). Other examples include the wide variety of plant extracts, such as terpenoids, glycosteroids, flavonoids and polyphenols, that all have weak little to no antibiotic activity on their own, but have strong synergistic antibiotic effects on a wide range of microbes (Hemaiswarya, Kruthiventi, & Doble, 2008). A similar type of synergistic effect is possibly occurring with the mixture of compounds found in DUQ0002I.
Finally, our work indicates how natural products can be used for more than simply drug discovery. Natural product research can be used to develop tools to understand biological systems. In this case, we chose to use DUQ0002I in the context of the spared nerve injury (SNI), a well-established model of neuropathic pain with comorbid depression (Norman et al., 2010; Suzuki et al., 2007), to learn more about the role of the 5-HT7R in the development of this condition. Rates of major depressive disorder (MDD) and depression symptoms are very high (13 – 82%) in chronic pain patients depending on the type of pain studied (Bair et al., 2003). In contrast, MDD only exists at a rate of 6.7% in isolation in U.S. adults (Kessler, Chiu, Demler, & Walters, 2005) and rates of chronic pain conditions, such as facial pain (4%) and fibromyalgia (2.5%) are also fairly low in isolation (NCHS, 2006). Similar to previous findings (Suzuki et al., 2007), SNI mice did not develop significant depression-like symptoms until ten-weeks post surgery, suggesting that the development of “depression” is slow to develop and is secondary to the primary hypersensitivity. The Z-score of depression-like behaviors in SNI when compared to sham animals highlight the significant behavioral changes that occur in SNI animals. With this model established, we showed that not only does DUQ0002I decrease depression-like behaviors in the forced swim and tail suspension tests in sham animals, but it also has a significant effect in SNI animals. This is similar to results that were found in another model of depression, the olfactory bulbectomy paradigm in rats, where blockade of the 5-HT7R with the selective antagonist SB269970 was found to counteract the model’s depression-inducing effects (Mnie-Filali et al., 2011). Future studies will have to investigate what other changes occur with 5-HT7R in the context of comorbidity.
Overall, our study demonstrates the power of utilizing natural products derived from marine cyanobacteria to probe the CNS. Here, we were able to show that a synergistic mixture of compounds from a species of Leptolyngbya could induce robust antidepressant and anxiolytic-effects in animal models of behavior. While the fact that our extract induced behavioral effects through a mixture of unidentified compounds does lead to some limitations, it nevertheless provided insight into some of the biology of the serotonin system. This extract shed light onto the anatomical specificity of the depression-like effects of blocking the 5-HT7R, helped us to discover a possible fundamental difference between males and females in the way the 5-HT7R serotonin system works to induce these effects, and provided evidence that the 5-HT7R may play a role in comorbid pain and depression. These discoveries illustrate the potential that these microbial secondary metabolites have for CNS drug discovery and, in the future, could possibly be used for treatment of CNS disorders related to GPCR malfunction.
ACKNOWLEDGEMENTS
We thank the Autoridad Nacional del Ambiente (ANAM), Panama for permission to make and export collections of marine cyanobacteria and the Smithsonian Tropical Research Institute (STRI) for use of boat and facilities in Panama. We would also like to thank Kh Tanvir Ahmed for sample preparations used in behavioral testing, Analise Zapadka for her work with 16S rRNA sequencing, and Anna Polaski and Heather Allen for their manuscript editing. Project was funded through (1) a grant provided by the American Pain Society and the Sharon S. Keller Fund for Chronic Pain Management Research (BJK and KJT), (2) a grant from the NIH (NCCIH R15AT008060; BJK and KJT), (3) a Fogarty International Center (FIC) International Cooperative Biodiversity Group (ICBG) grant based in Panama (2U01TW006634–06; KJT), and (4) a grant from the Duquesne University Faculty Development Fund (KJT and BJK). Ki determinations and receptor binding profiles were generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program, Contract # HHSN-271-2008-025C (NIMH PDSP). The NIMH PDSP is Directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda MD, USA. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors report no conflict of interest.
REFERENCES
- Abbas AI, Hedlund PB, Huang XP, Tran TB, Meltzer HY, & Roth BL (2009). Amisulpride is a potent 5-HT7 antagonist: relevance for antidepressant actions in vivo. Psychopharmacology (Berl), 205(1), 119–128. doi: 10.1007/s00213-009-1521-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari A, Lerner TN, Finkelstein J, Pak S, Jennings JH, Davidson TJ, … Deisseroth K (2015). Basomedial amygdala mediates top-down control of anxiety and fear. Nature, 527(7577), 179–185. doi: 10.1038/nature15698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriani W, Travaglini D, Lacivita E, Saso L, Leopoldo M, & Laviola G (2012). Modulatory effects of two novel agonists for serotonin receptor 7 on emotion, motivation and circadian rhythm profiles in mice. Neuropharmacology, 62(2), 833–842. doi: 10.1016/j.neuropharm.2011.09.012 [DOI] [PubMed] [Google Scholar]
- Ahmed KT, Lax NC, & Tidgewell KJ (2015). CNS Modulators from the Ocean In Baker BJ (Ed.), Marine Biomedicine: From Beach to Bedside (Vol. 1, pp. 247–278). Boca Raton, FL: CRC Press. [Google Scholar]
- Amaya-Castellanos E, Pineda-Farias JB, Castaneda-Corral G, Vidal-Cantu GC, Murbartian J, Rocha-Gonzalez HI, & Granados-Soto V (2011). Blockade of 5-HT7 receptors reduces tactile allodynia in the rat. Pharmacol Biochem Behav, 99(4), 591–597. doi: 10.1016/j.pbb.2011.06.005 [DOI] [PubMed] [Google Scholar]
- Andreetta F, Carboni L, Grafton G, Jeggo R, Whyment AD, van den Top M, … Barnes NM (2016). Hippocampal 5-HT7 receptors signal phosphorylation of the GluA1 subunit to facilitate AMPA receptor mediated-neurotransmission in vitro and in vivo. Br J Pharmacol, 173(9), 1438–1451. doi: 10.1111/bph.13432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, & Kroenke K (2003). Depression and pain comorbidity: A literature review. Archives of Internal Medicine, 163(20), 2433–2445. doi: 10.1001/archinte.163.20.2433 [DOI] [PubMed] [Google Scholar]
- Barbas D, DesGroseillers L, Castellucci VF, Carew TJ, & Marinesco S (2003). Multiple serotonergic mechanisms contributing to sensitization in aplysia: evidence of diverse serotonin receptor subtypes. Learn Mem, 10(5), 373–386. doi: 10.1101/lm.66103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, & Weinshank RL (1993). Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J Biol Chem, 268(31), 23422–23426. [PubMed] [Google Scholar]
- Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang XP, … Hopkins AL (2012). Automated design of ligands to polypharmacological profiles. Nature, 492(7428), 215–220. doi: 10.1038/nature11691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure P, Kelly L, Aluisio L, Shelton J, Lord B, Galici R, … Dugovic C (2007). Selective blockade of 5-hydroxytryptamine (5-HT)7 receptors enhances 5-HT transmission, antidepressant-like behavior, and rapid eye movement sleep suppression induced by citalopram in rodents. J Pharmacol Exp Ther, 321(2), 690–698. doi: 10.1124/jpet.107.119404 [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Nepomuceno D, Hein L, Sutcliffe JG, Lovenberg T, & Hedlund PB (2004). Radioligand binding analysis of knockout mice reveals 5-hydroxytryptamine(7) receptor distribution and uncovers 8-hydroxy-2-(di-n-propylamino)tetralin interaction with alpha(2) adrenergic receptors. Neuroscience, 124(4), 901–911. doi: 10.1016/j.neuroscience.2004.01.014 [DOI] [PubMed] [Google Scholar]
- Bourin M, & Hascoet M (2003). The mouse light/dark box test. Eur J Pharmacol, 463(1–3), 55–65. [DOI] [PubMed] [Google Scholar]
- Brenchat A, Romero L, Garcia M, Pujol M, Burgueno J, Torrens A, … Vela JM (2009). 5-HT7 receptor activation inhibits mechanical hypersensitivity secondary to capsaicin sensitization in mice. Pain, 141(3), 239–247. doi: 10.1016/j.pain.2008.11.009 [DOI] [PubMed] [Google Scholar]
- Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, & Gould TD (2012). The mouse forced swim test. J Vis Exp(59), e3638. doi: 10.3791/3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Dao DT, Terrillion CE, Piantadosi SC, Bhat S, & Gould TD (2012). The tail suspension test. J Vis Exp(59), e3769. doi: 10.3791/3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cates LN, Roberts AJ, Huitron-Resendiz S, & Hedlund PB (2013). Effects of lurasidone in behavioral models of depression. Role of the 5-HT(7) receptor subtype. Neuropharmacology, 70, 211–217. doi: 10.1016/j.neuropharm.2013.01.023 [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, & Yaksh TL (1994). Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods, 53(1), 55–63. [DOI] [PubMed] [Google Scholar]
- Choi H, Engene N, Smith JE, Preskitt LB, & Gerwick WH (2010). Crossbyanols A-D, toxic brominated polyphenyl ethers from the Hawai’ian bloom-forming Cyanobacterium Leptolyngbya crossbyana. J Nat Prod, 73(4), 517–522. doi: 10.1021/np900661g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Mascuch SJ, Villa FA, Byrum T, Teasdale ME, Smith JE, … Gerwick WH (2012). Honaucins A-C, potent inhibitors of inflammation and bacterial quorum sensing: synthetic derivatives and structure-activity relationships. Chem Biol, 19(5), 589–598. doi: 10.1016/j.chembiol.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa L, Sardone LM, Lacivita E, Leopoldo M, & Ciranna L (2015). Novel agonists for serotonin 5-HT7 receptors reverse metabotropic glutamate receptor-mediated long-term depression in the hippocampus of wild-type and Fmr1 KO mice, a model of Fragile × Syndrome. Front Behav Neurosci, 9, 65. doi: 10.3389/fnbeh.2015.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa L, Trovato C, Musumeci SA, Catania MV, & Ciranna L (2012). 5-HT(1A) and 5-HT(7) receptors differently modulate AMPA receptor-mediated hippocampal synaptic transmission. Hippocampus, 22(4), 790–801. doi: 10.1002/hipo.20940 [DOI] [PubMed] [Google Scholar]
- de Sa-Calcada D, Roque S, Branco C, Monteiro S, Cerqueira-Rodrigues B, Sousa N, … Correia-Neves M (2015). Exploring Female Mice Interstrain Differences Relevant for Models of Depression. Front Behav Neurosci, 9, 335. doi: 10.3389/fnbeh.2015.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I, & Woolf CJ (2000). Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain, 87(2), 149–158. doi: 10.1016/S0304-3959(00)00276-1 [DOI] [PubMed] [Google Scholar]
- Dixit R, & Suseela MR (2013). Cyanobacteria: potential candidates for drug discovery. Antonie van Leeuwenhoek, 103(5), 947–961. doi: 10.1007/s10482-013-9898-0 [DOI] [PubMed] [Google Scholar]
- Dixon WJ (1980). Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol, 20, 441–462. doi: 10.1146/annurev.pa.20.040180.002301 [DOI] [PubMed] [Google Scholar]
- Engene N, Cameron Coates R, & Gerwick WH (2010). 16S rRNA GENE HETEROGENEITY IN THE FILAMENTOUS MARINE CYANOBACTERIAL GENUS LYNGBYA1. Journal of Phycology, 46(3), 591–601. doi: 10.1111/j.1529-8817.2010.00840.x [DOI] [Google Scholar]
- Engene N, Gunasekera SP, Gerwick WH, & Paul VJ (2013). Phylogenetic inferences reveal a large extent of novel biodiversity in chemically rich tropical marine cyanobacteria. Appl Environ Microbiol, 79(6), 1882–1888. doi: 10.1128/aem.03793-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engene N, Rottacker EC, Kaštovský J, Byrum T, Choi H, Ellisman MH, … Gerwick WH (2012). Moorea producens gen. nov., sp. nov. and Moorea bouillonii comb. nov., tropical marine cyanobacteria rich in bioactive secondary metabolites. Int J Syst Evol Microbiol, 62(Pt 5), 1171–1178. doi: 10.1099/ijs.0.033761-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson TM, Golkar A, Ekstrom JC, Svenningsson P, & Ogren SO (2008). 5-HT7 receptor stimulation by 8-OH-DPAT counteracts the impairing effect of 5-HT(1A) receptor stimulation on contextual learning in mice. Eur J Pharmacol, 596(1–3), 107–110. doi: 10.1016/j.ejphar.2008.08.026 [DOI] [PubMed] [Google Scholar]
- Ferguson JM (2001). SSRI Antidepressant Medications: Adverse Effects and Tolerability. Prim Care Companion J Clin Psychiatry, 3(1), 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellynck E, Heyninck K, Andressen KW, Haegeman G, Levy FO, Vanhoenacker P, & Van Craenenbroeck K (2013). The serotonin 5-HT7 receptors: two decades of research. Exp Brain Res, 230(4), 555–568. doi: 10.1007/s00221-013-3694-y [DOI] [PubMed] [Google Scholar]
- Gellynck E, Heyninck K, Andressen KW, Haegeman G, Levy FO, Vanhoenacker P, & Van Craenenbroeck K (2013). The serotonin 5-HT7 receptors: two decades of research. Exp Brain Res, 230(4), 555–568. doi: 10.1007/s00221-013-3694-y [DOI] [PubMed] [Google Scholar]
- Glascock JJ, Osman EY, Coady TH, Rose FF, Shababi M, & Lorson CL (2011). Delivery of therapeutic agents through intracerebroventricular (ICV) and intravenous (IV) injection in mice. J Vis Exp(56). doi: 10.3791/2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinez-Chaparro B, Barragan-Iglesias P, Castaneda-Corral G, Rocha-Gonzalez HI, & Granados-Soto V (2011). Role of peripheral 5-HT(4), 5-HT(6), and 5-HT(7) receptors in development and maintenance of secondary mechanical allodynia and hyperalgesia. Pain, 152(3), 687–697. doi: 10.1016/j.pain.2010.12.020 [DOI] [PubMed] [Google Scholar]
- Guilloux JP, Mendez-David I, Pehrson A, Guiard BP, Reperant C, Orvoen S, … David DJ (2013). Antidepressant and anxiolytic potential of the multimodal antidepressant vortioxetine (Lu AA21004) assessed by behavioural and neurogenesis outcomes in mice. Neuropharmacology, 73, 147–159. doi: 10.1016/j.neuropharm.2013.05.014 [DOI] [PubMed] [Google Scholar]
- Guilloux JP, Seney M, Edgar N, & Sibille E (2011). Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: relevance to emotionality and sex. J Neurosci Methods, 197(1), 21–31. doi: 10.1016/j.jneumeth.2011.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guscott M, Bristow LJ, Hadingham K, Rosahl TW, Beer MS, Stanton JA, … McAllister G (2005). Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacology, 48(4), 492–502. doi: 10.1016/j.neuropharm.2004.11.015 [DOI] [PubMed] [Google Scholar]
- Guseva D, Wirth A, & Ponimaskin E (2014). Cellular mechanisms of the 5-HT7 receptor-mediated signaling. Front Behav Neurosci, 8, 306. doi: 10.3389/fnbeh.2014.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez M, Pereira AR, Debonsi HM, Ligresti A, Di Marzo V, & Gerwick WH (2011). Cannabinomimetic lipid from a marine cyanobacterium. J Nat Prod, 74(10), 2313–2317. doi: 10.1021/np200610t [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BG (2013). Building phylogenetic trees from molecular data with MEGA. Mol Biol Evol, 30(5), 1229–1235. doi: 10.1093/molbev/mst012 [DOI] [PubMed] [Google Scholar]
- Han B, McPhail KL, Ligresti A, Di Marzo V, & Gerwick WH (2003). Semiplenamides A-G, fatty acid amides from a Papua New Guinea collection of the marine cyanobacterium Lyngbya semiplena. J Nat Prod, 66(10), 1364–1368. doi: 10.1021/np030242n [DOI] [PubMed] [Google Scholar]
- Hannon J, & Hoyer D (2008). Molecular biology of 5-HT receptors. Behav Brain Res, 195(1), 198–213. doi: 10.1016/j.bbr.2008.03.020 [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Danielson PE, Thomas EA, Slanina K, Carson MJ, & Sutcliffe JG (2003). No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc Natl Acad Sci U S A, 100(3), 1375–1380. doi: 10.1073/pnas.0337340100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Huitron-Resendiz S, Henriksen SJ, & Sutcliffe JG (2005). 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol Psychiatry, 58(10), 831–837. doi: 10.1016/j.biopsych.2005.05.012 [DOI] [PubMed] [Google Scholar]
- Hedlund PB, & Sutcliffe JG (2007). The 5-HT7 receptor influences stereotypic behavior in a model of obsessive-compulsive disorder. Neurosci Lett, 414(3), 247–251. doi: 10.1016/j.neulet.2006.12.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemaiswarya S, Kruthiventi AK, & Doble M (2008). Synergism between natural products and antibiotics against infectious diseases. Phytomedicine, 15(8), 639–652. doi: 10.1016/j.phymed.2008.06.008 [DOI] [PubMed] [Google Scholar]
- Hensler JG (2006). Serotonergic modulation of the limbic system. Neurosci Biobehav Rev, 30(2), 203–214. doi: 10.1016/j.neubiorev.2005.06.007 [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, … Humphrey PP (1994). International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev, 46(2), 157–203. [PubMed] [Google Scholar]
- Hutcheson JD, Setola V, Roth BL, & Merryman WD (2011). Serotonin receptors and heart valve disease--it was meant 2B. Pharmacology & therapeutics, 132(2), 146–157. doi: 10.1016/j.pharmthera.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keers R, & Aitchison KJ (2010). Gender differences in antidepressant drug response. Int Rev Psychiatry, 22(5), 485–500. doi: 10.3109/09540261.2010.496448 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu W, Demler O, & Walters EE (2005). PRevalence, severity, and comorbidity of 12-month dsm-iv disorders in the national comorbidity survey replication. Archives of General Psychiatry, 62(6), 617–627. doi: 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Tae J, Lee K, Rhim H, Choo IH, Cho H, … Choo H (2014). Novel N-biphenyl-2-ylmethyl 2-methoxyphenylpiperazinylalkanamides as 5-HT7R antagonists for the treatment of depression. Bioorg Med Chem, 22(17), 4587–4596. doi: 10.1016/j.bmc.2014.07.026 [DOI] [PubMed] [Google Scholar]
- Kokras N, & Dalla C (2017). Preclinical sex differences in depression and antidepressant response: Implications for clinical research. J Neurosci Res, 95(1–2), 731–736. doi: 10.1002/jnr.23861 [DOI] [PubMed] [Google Scholar]
- Kolber BJ, Montana MC, Carrasquillo Y, Xu J, Heinemann SF, Muglia LJ, & Gereau RW (2010). Activation of metabotropic glutamate receptor 5 in the amygdala modulates pain-like behavior. J Neurosci, 30(24), 8203–8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni SK, Singh K, & Bishnoi M (2007). Elevated zero maze: a paradigm to evaluate antianxiety effects of drugs. Methods Find Exp Clin Pharmacol, 29(5), 343–348. doi: 10.1358/mf.2007.29.5.1117557 [DOI] [PubMed] [Google Scholar]
- Lax NC, Ahmed KT, Ignatz CM, Spadafora C, Kolber BJ, & Tidgewell KJ (2016). Marine cyanobacteria-derived serotonin receptor 2C active fraction induces psychoactive behavioral effects in mice. Pharm Biol, 54(11), 2723–2731. doi: 10.1080/13880209.2016.1181659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax NC, George DC, Ignatz C, & Kolber BJ (2014). The mGluR5 antagonist fenobam induces analgesic conditioned place preference in mice with spared nerve injury. PLoS One, 9(7), e103524. doi: 10.1371/journal.pone.0103524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Reid AR, & Sawynok J (2013). Antinociception by systemically-administered acetaminophen (paracetamol) involves spinal serotonin 5-HT7 and adenosine A1 receptors, as well as peripheral adenosine A1 receptors. Neurosci Lett, 536, 64–68. doi: 10.1016/j.neulet.2012.12.052 [DOI] [PubMed] [Google Scholar]
- Maluach AM, Misquitta KA, Prevot TD, Fee C, Sibille E, Banasr M, & Andreazza AC (2017). Increased Neuronal DNA/RNA Oxidation in the Frontal Cortex of Mice Subjected to Unpredictable Chronic Mild Stress. Chronic Stress (Thousand Oaks), 1. doi: 10.1177/2470547017724744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Cora FJ, & Pazos A (2004). Autoradiographic distribution of 5-HT7 receptors in the human brain using [3H]mesulergine: comparison to other mammalian species. Br J Pharmacol, 141(1), 92–104. doi: 10.1038/sj.bjp.0705576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina RA, Goeger DE, Hills P, Mooberry SL, Huang N, Romero LI, … McPhail KL (2008). Coibamide A, a potent antiproliferative cyclic depsipeptide from the Panamanian marine cyanobacterium Leptolyngbya sp. J Am Chem Soc, 130(20), 6324–6325. doi: 10.1021/ja801383f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-David I, Boursier C, Domergue V, Colle R, Falissard B, Corruble E, … David DJ (2017). Differential Peripheral Proteomic Biosignature of Fluoxetine Response in a Mouse Model of Anxiety/Depression. Front Cell Neurosci, 11, 237. doi: 10.3389/fncel.2017.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnie-Filali O, Faure C, Lambas-Senas L, El Mansari M, Belblidia H, Gondard E, … Haddjeri N (2011). Pharmacological blockade of 5-HT7 receptors as a putative fast acting antidepressant strategy. Neuropsychopharmacology, 36(6), 1275–1288. doi: 10.1038/npp.2011.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaser R, Paul VJ, & Luesch H (2012). Marine cyanobacterial fatty acid amides acting on cannabinoid receptors. Chembiochem, 13(18), 2676–2681. doi: 10.1002/cbic.201200502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti JM, & Jantos H (2014). The role of serotonin 5-HT7 receptor in regulating sleep and wakefulness. Rev Neurosci, 25(3), 429–437. doi: 10.1515/revneuro-2014-0016 [DOI] [PubMed] [Google Scholar]
- Nagle DG, & Paul VJ (1999). Production of secondary metabolites by filamentous tropical marine cyanobacteria: eclogical functions of the compounds. Journal of Phycology, 35, 1412–1421. [Google Scholar]
- Naumenko VS, Popova NK, Lacivita E, Leopoldo M, & Ponimaskin EG (2014). Interplay between serotonin 5-HT1A and 5-HT7 receptors in depressive disorders. CNS Neurosci Ther, 20(7), 582–590. doi: 10.1111/cns.12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCHS. (2006) Health, United States, 2006: With Chartbook on Trends in the Health of Americans. Hyattsville (MD). [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, & Monteggia LM (2002). Neurobiology of depression. Neuron, 34(1), 13–25. [DOI] [PubMed] [Google Scholar]
- Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, … Diksic M (1997). Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci U S A, 94(10), 5308–5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman GJ, Karelina K, Zhang N, Walton JC, Morris JS, & Devries AC (2010). Stress and IL-1beta contribute to the development of depressive-like behavior following peripheral nerve injury. Mol Psychiatry, 15(4), 404–414. doi: 10.1038/mp.2009.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nubel U, Garcia-Pichel F, & Muyzer G (1997). PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl Environ Microbiol, 63(8), 3327–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization WH (1999). Toxic Cyanobacteria in Water: A guide to their public health consequences, monitoring and management. London: E & FN Spon. [Google Scholar]
- Osborne NJ, & Shaw GR (2008). Dermatitis associated with exposure to a marine cyanobacterium during recreational water exposure. BMC Dermatol, 8, 5. doi: 10.1186/1471-5945-8-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EF, & Murphy JA (2014). Vortioxetine for the treatment of depression. Ann Pharmacother, 48(6), 758–765. doi: 10.1177/1060028014528305 [DOI] [PubMed] [Google Scholar]
- Pereira AR, Cao Z, Engene N, Soria-Mercado IE, Murray TF, & Gerwick WH (2010). Palmyrolide A, an unusually stabilized neuroactive macrolide from Palmyra Atoll cyanobacteria. Org Lett, 12(20), 4490–4493. doi: 10.1021/ol101752n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytliak M, Vargova V, Mechirova V, & Felsoci M (2011). Serotonin receptors - from molecular biology to clinical applications. Physiol Res, 60(1), 15–25. [DOI] [PubMed] [Google Scholar]
- Richner M, Bjerrum OJ, Nykjaer A, & Vaegter CB (2011). The Spared Nerve Injury (SNI) Model of Induced Mechanical Allodynia in Mice. (54), e3092. doi:doi: 10.3791/3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Krucker T, Levy CL, Slanina KA, Sutcliffe JG, & Hedlund PB (2004). Mice lacking 5-HT receptors show specific impairments in contextual learning. Eur J Neurosci, 19(7), 1913–1922. doi: 10.1111/j.1460-9568.2004.03288.x [DOI] [PubMed] [Google Scholar]
- Rossi JV, Roberts MA, Yoo H-D, & Gerwick WH (1997). Pilot scale culture of the marine cyanobacterium Lyngbya majuscula for its pharmaceutically-useful natural metabolite curacin A. Journal of applied phycology, 9(3), 195–204. [Google Scholar]
- Sargin D, Oliver DK, & Lambe EK (2016). Chronic social isolation reduces 5-HT neuronal activity via upregulated SK3 calcium-activated potassium channels. Elife, 5. doi: 10.7554/eLife.21416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisyan G, & Hedlund PB (2009). The 5-HT7 receptor is involved in allocentric spatial memory information processing. Behav Brain Res, 202(1), 26–31. doi: 10.1016/j.bbr.2009.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisyan G, Roberts AJ, & Hedlund PB (2010). The 5-HT(7) receptor as a mediator and modulator of antidepressant-like behavior. Behav Brain Res, 209(1), 99–108. doi: 10.1016/j.bbr.2010.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura Y, Hirose Y, Misawa N, Osana Y, Katoh H, Yamaguchi H, & Kawachi M (2015). Comparison of the terrestrial cyanobacterium Leptolyngbya sp. NIES-2104 and the freshwater Leptolyngbya boryana PCC 6306 genomes. DNA Res, 22(6), 403–412. doi: 10.1093/dnares/dsv022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitachitta N, & Gerwick WH (1998). Grenadadiene and grenadamide, cyclopropyl-containing fatty acid metabolites from the marine cyanobacterium Lyngbya majuscula. J Nat Prod, 61(5), 681–684. doi: 10.1021/np970576a [DOI] [PubMed] [Google Scholar]
- Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, … Mogil JS (2014). Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods, 11(6), 629–632. doi: 10.1038/nmeth.2935 [DOI] [PubMed] [Google Scholar]
- Stewart I, Seawright AA, & Shaw GR (2008). Cyanobacterial poisoning in livestock, wild mammals and birds--an overview. Adv Exp Med Biol, 619, 613–637. doi: 10.1007/978-0-387-75865-7_28 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Amata M, Sakaue G, Nishimura S, Inoue T, Shibata M, & Mashimo T (2007). Experimental neuropathy in mice is associated with delayed behavioral changes related to anxiety and depression. Anesth Analg, 104(6), 1570–1577, table of contents. doi: 10.1213/01.ane.0000261514.19946.66 [DOI] [PubMed] [Google Scholar]
- Tan LT (2007). Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry, 68(7), 954–979. doi: 10.1016/j.phytochem.2007.01.012 [DOI] [PubMed] [Google Scholar]
- Tokarski K, Kusek M, Sowa J, & Bobula B (2014). [Possible involvement of 5-HT(7) receptor in pathophysiology of affective disorders and action of antidepressant drugs]. Postepy Hig Med Dosw (Online), 68, 1104–1113. [DOI] [PubMed] [Google Scholar]
- Tokarski K, Zahorodna A, Bobula B, & Hess G (2003). 5-HT7 receptors increase the excitability of rat hippocampal CA1 pyramidal neurons. Brain Res, 993(1–2), 230–234. [DOI] [PubMed] [Google Scholar]
- Tuladhar BR, Ge L, & Naylor RJ (2003). 5-HT7 receptors mediate the inhibitory effect of 5-HT on peristalsis in the isolated guinea-pig ileum. Br J Pharmacol, 138(7), 1210–1214. doi: 10.1038/sj.bjp.0705184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnas K, Thomas DR, Tupala E, Tiihonen J, & Hall H (2004). Distribution of 5-HT7 receptors in the human brain: a preliminary autoradiographic study using [3H]SB-269970. Neurosci Lett, 367(3), 313–316. doi: 10.1016/j.neulet.2004.06.025 [DOI] [PubMed] [Google Scholar]
- Vasefi MS, Yang K, Li J, Kruk JS, Heikkila JJ, Jackson MF, … Beazely MA (2013). Acute 5-HT7 receptor activation increases NMDA-evoked currents and differentially alters NMDA receptor subunit phosphorylation and trafficking in hippocampal neurons. Mol Brain, 6, 24. doi: 10.1186/1756-6606-6-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Korff M, Ormel J, Katon W, & Lin EH (1992). Disability and depression among high utilizers of health care. A longitudinal analysis. Arch Gen Psychiatry, 49(2), 91–100. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, … Bremner JD (2004). Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol Psychiatry, 56(2), 101–112. doi: 10.1016/j.biopsych.2004.04.002 [DOI] [PubMed] [Google Scholar]
- Wang Q, Timberlake MA 2nd, Prall K, & Dwivedi Y (2017). The recent progress in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry, 77, 99–109. doi: 10.1016/j.pnpbp.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Desmoulin S, Banerjee S, Kong D, Li Y, Deraniyagala RL, … Sarkar FH (2008). Synergistic effects of multiple natural products in pancreatic cancer cells. Life Sci, 83(7–8), 293–300. doi: 10.1016/j.lfs.2008.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowska A, Nikiforuk A, & Stachowicz K (2006). Potential anxiolytic and antidepressant effects of the selective 5-HT7 receptor antagonist SB 269970 after intrahippocampal administration to rats. Eur J Pharmacol, 553(1–3), 185–190. doi: 10.1016/j.ejphar.2006.09.064 [DOI] [PubMed] [Google Scholar]
- Wilmotte A (1994). Molecular Evolution and Taxonomy of the Cyanobacteria In Bryant DA (Ed.), The Molecular Biology of Cyanobacteria (pp. 1–25). Dordrecht: Springer Netherlands. [Google Scholar]
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, … Hsiao EY (2015). Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell, 161(2), 264–276. doi: 10.1016/j.cell.2015.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Yu C, Wang C, Zhang JF, Zhou WH, Cui WG, … Xu Y (2014). Chronic resveratrol treatment exerts antihyperalgesic effect and corrects co-morbid depressive like behaviors in mice with mononeuropathy: involvement of serotonergic system. Neuropharmacology, 85, 131–141. doi: 10.1016/j.neuropharm.2014.04.021 [DOI] [PubMed] [Google Scholar]