Abstract
Background/Aims
Food intolerance/malabsorption, particularly histamine intolerance (HIT), may cause nonspecific functional gastrointestinal and extraintestinal symptoms. We evaluated gastrointestinal and extraintestinal symptoms in patients with HIT.
Methods
In an analysis of outpatients’ charts we identified 133 patients, who presented with recurring nonspecific functional gastrointestinal, extraintestinal symptoms, and a diamine oxidase value <10 U/mL, indicative of HIT. A standardized anonymous questionnaire with symptoms of HIT based on known symptoms and the 4 histamine receptors including gastrointestinal, cardiovascular, respiratory and skin complaints was developed, and sent by mail to the patients.
Results
In the 62 patients that completed the questionnaire, bloating was the most common and most serious symptom. Other commonly reported gastrointestinal symptoms were postprandial fullness, diarrhea, abdominal pain, and constipation. The presence of 2 from a list of 24 symptoms resulted in 276 various symptom combinations. From calculated 2.024 possible combinations of 3 symptoms the patients with HIT presented 1.975 combinations.
Conclusions
The knowledge of this wide variability of symptoms and complex symptom combinations in patients with HIT may help to clinically recognize and diagnose HIT.
Keywords: Diamine oxidase, Histamine, Irritable bowel syndrome, Gluten, Gastrointestinal diseases
INTRODUCTION
Food intolerance/malabsorption syndromes are reported in up to 20% of the population in westernized countries and, particularly histamine intolerance (HIT) may cause nonspecific GI complaints and extraintestinal symptoms [1]. In HIT a disproportionate amount of histamine in the body is thought to result from the consumption of food with high histamine content, and a reduced ability of mainly the enzyme diamine oxidase (DAO) to digest histamine [2]. The clinical diagnosis of HIT is challenging and the symptoms may occur according to the known 4 receptors of histamine [3]. Here, we describe various symptoms and complex symptom combinations in patients with HIT. This may help to clinically recognize and diagnose HIT.
METHODS
In a retrospective analysis of outpatients’ charts, we identified 133 patients (male:female, 30:103; median age, 39 years; age range, 18–81 years), who presented with recurring functional nonspecific abdominal complaints and who had a DAO value <10 U/mL (median, 5.8 U/mL; range, 1.5–9.9 U/mL). In our outpatient setting we decided to include serum DAO determination in the evaluation of patients who present with recurring functional nonspecific abdominal complaints [4]. A thorough anamnesis, concerning abdominal complaints and a timely relation to ingestion of histamine-containing food or drinks, including pharmaceutical treatments which might influence HIT, was performed. All of the patients were examined for and did not show lactose intolerance, fructose malabsorption, Helicobacter pylori infection or celiac disease.
A registered dietician was consulted to introduce and observe, using a food diary, a histamine-elimination diet for 2 weeks. If the patients were free of or had noteworthy improvement of GI symptoms, together with a DAO value <10 U/mL at first presentation, then the diagnosis of HIT was established [5]. All patients received written information about HIT and the dietician helped to develop an individually tailored long-time diet to ensure nutritional adequacy and to provide sustained relief.
In the morning, at the first presentation, blood drawings were performed after overnight fasting (>12 hours) and determination of DAO in serum was made with a radio extraction assay (Sciotec Diagnostic Technologies, Tulln, Austria) within 3 days. We used hydrogen (H2) breath tests for exclusion of lactose intolerance and fructose malabsorption (Gastrolyzer; Bedfont Scientific Inc., Kent, England). Either histologic evaluation of gastric mucosa or an enzyme-linked IgA immunosorbent assay (ELISA; Serion, Würzburg, Germany) were done and showed absence of H. pylori infection. For screening of celiac disease antibodies against tissue transglutaminase were determined with anti-tTG IgA ELISA (Euro-Diagnostica AB, Malmö, Sweden). All patients >50 years old were screened by colonoscopy and no pathology was present. None of the patients showed signs of infection.
A standardized anonymous questionnaire, regarding symptoms the patients experienced before the diagnosis of HIT and/or what complaints they have due to ingesting foods with high histamine content, was established. This questionnaire was based on known symptoms and the 4 histamine receptors according to the literature [2,3], and it was sent by mail to the patients. Every questionnaire listed 23 symptoms in 4 categories including GI, cardiovascular, respiratory and skin complaints, and for every symptom a severity score from 0 (no symptoms) to 5 (very intense) was included. Space was provided for patients to add other symptoms. Of 133 selected patients 69 (51.9%) returned the questionnaire, but 7 envelopes were returned unopened. Therefore, 62 questionnaires (46.6%) were included in this anonymous evaluation.
Qualitative data are presented as absolute and relative frequencies; quantitative parameters are summarized as mean and SD or, median and interquartile range (IQR) according to distribution, respectively. Data distribution was assessed by the Shapiro-Wilk test and the severity of symptoms is stated as the median of the reported symptom severities. Statistical analyses were performed with SPSS version 23.0 (IBM Corp., Armonk, NY, USA) and R version 3.4.2 (R Core Team, Vienna, Austria) was used to determine the frequency of combinations of symptoms. Written informed consent was obtained from each patient included in the study. The study conforms to the ethical guidelines of the Declaration of Helsinki and was approved by the Ethical Committee of the Johannes Kepler University in Linz, Austria (approval No. K-107-16).
RESULTS
Bloating was the most common symptom among these patients (92%) and was observed as the most severe symptom with a severity score of 4 (Table 1). Other common HIT-related GI symptoms included: postprandial fullness (73%), diarrhea (71%), abdominal pain (68%), and constipation (55%) as shown in Fig. 1. Eighteen patients (29%) added other symptoms in the space provided (burning sensation in mouth, on tongue, or anus, migraine, shaking-heavy legs, weakness, leg edema, listlessness, fatigue, insomnia, reduced power of concentration, heartburn, dry skin, ophthalmitis, anxiety, suffocating, sore throat, hoarseness, cardiac arrhythmia, and arterial hypertension) and assigned them a high symptom severity score of 4. Two of these patients added 2 other symptoms.
Table 1.
Symptom Severity Score in Patients with Histamine Intolerance
| Symptom | Symptom severity score |
|---|---|
| Bloating | 4 |
| Abdominal pain, intestinal colic, diarrhea, postprandial fullness, dysmenorrhea, flush, belching, palpitations, rhinorrhea | 3 |
| Constipation, pruritus | 2.5 |
| Nausea, emesis, rash, swollen reddened eye lids, headache, dizziness, nose congestion, sneezing, dyspnea | 2 |
| Hypotonia | 1.5 |
| Collapse | 1 |
Score will range 0 (no symptoms) to 5 (very intense). None of the symptoms was indicated with a symptom severity code 5.
Fig. 1.
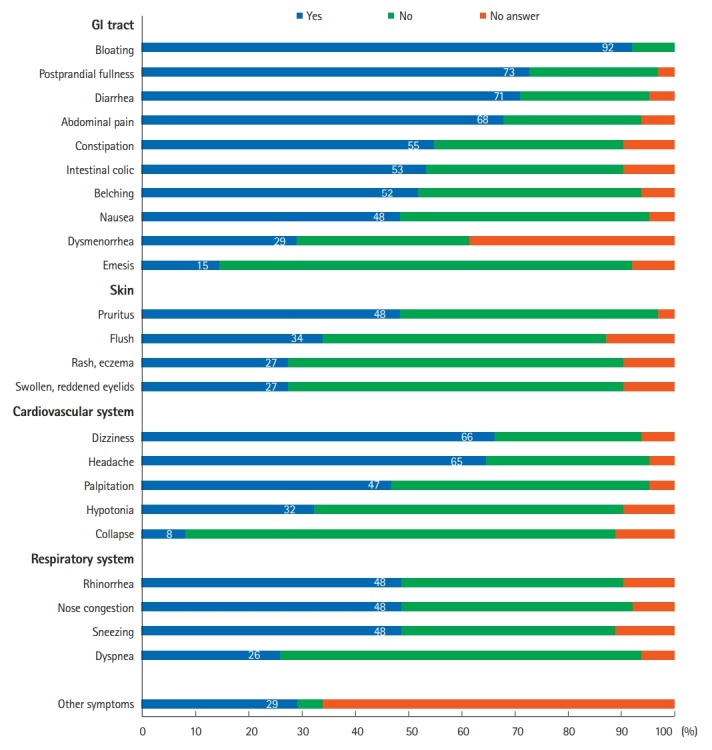
Frequency and distribution of symptoms in patients with histamine intolerance.
According to the symptom categories, all the GI symptoms were mentioned most frequently, followed by cardiovascular symptoms. The presence of 2 of the 24 symptoms in all 4 categories resulted in 276 various combinations. Of the GI symptom combinations, bloating was the most frequent in combination with other GI symptoms, and 115 various combinations were reported to occur in more than 25% of patients (Table 2).
Table 2.
Most Common Combinations of 2 Symptoms in Patients with Histamine Intolerance
| Symptom combinations | Percent of patients | Symptom combinations | Percent of patients |
|---|---|---|---|
| Bloating - postprandial fullness | 69 | Diarrhea - nausea | 42 |
| Diarrhea - nose congestion | |||
| Diarrhea - sneezing | |||
| Dizziness - constipation | |||
| Dizziness - intestinal colic | |||
| Dizziness - palpitation | |||
| Bloating - palpitation | |||
| Postprandial fullness - intestinal colic | |||
| Postprandial fullness - nose congestion | |||
| Abdominal pain - nose congestion | |||
| Bloating - diarrhea | 66 | Diarrhea - intestinal colic | 40 |
| Bloating - abdominal pain | Headache - intestinal colic | ||
| Headache - nausea | |||
| Postprandial fullness - nausea | |||
| Abdominal pain - nausea | |||
| Bloating - dizziness | 63 | Diarrhea - constipation | 39 |
| Bloating - headache | Diarrhea - palpitation | ||
| Diarrhea - pruritus | |||
| Diarrhea - belching | |||
| Dizziness - belching | |||
| Dizziness - nausea | |||
| Headache - constipation | |||
| Abdominal pain - belching | |||
| Abdominal pain - rhinorrhea | |||
| Abdominal pain - sneezing | |||
| Abdominal pain - dizziness | 55 | Dizziness - nose congestion | 37 |
| Dizziness - pruritus | |||
| Dizziness - sneezing | |||
| Headache - belching | |||
| Headache - nose congestion | |||
| Postprandial fullness - constipation | |||
| Postprandial fullness - sneezing | |||
| Postprandial fullness - pruritus | |||
| Abdominal pain - palpitation | |||
| Postprandial fullness - abdominal pain | 53 | Constipation - intestinal colic | 35 |
| Bloating - intestinal colic | Dizziness - rhinorrhea | ||
| Headache - palpitation | |||
| Headache - pruritus | |||
| Postprandial fullness - rhinorrhea | |||
| Diarrhea - dizziness | 52 | Abdominal pain - pruritus | 34 |
| Bloating-belching | Intestinal colic - nausea | ||
| Intestinal colic - nose congestion | |||
| Intestinal colic - sneezing | |||
| Rhinorrhea - sneezing | |||
| Diarrhea - headache | 50 | Intestinal colic - belching | 32 |
| Diarrhea - abdominal pain | Intestinal colic - rhinorrhea | ||
| Dizziness - headache | Bloating - flush | ||
| Bloating - constipation | Bloating - hypotonia | ||
| Postprandial fullness - diarrhea | Belching - sneezing | ||
| Postprandial fullness - headache | Postprandial fullness - palpitation | ||
| Pruritus - nose congestion | |||
| Pruritus - sneezing | |||
| Headache - rhinorrhea | |||
| Headache - sneezing | |||
| Rhinorrhea - nose congestion | |||
| Bloating - nose congestion | 48 | Constipation - palpitation | 31 |
| Bloating - rhinorrhea | Nausea - belching | ||
| Nausea - nose congestion | |||
| Nausea - sneezing | |||
| Belching - pruritus | |||
| Postprandial fullness - flush | |||
| Postprandial fullness - hypotonia | |||
| Pruritus - palpitation | |||
| Pruritus - rhinorrhea | |||
| Palpitation - nose congestion | |||
| Bloating - nausea | 47 | Diarrhea - flush | 29 |
| Bloating - pruritus | Nausea - pruritus | ||
| Bloating - sneezing | Belching - nose congestion | ||
| Postprandial fullness - dizziness | Flush - headache | ||
| Abdominal pain - headache | Nose congestion - sneezing | ||
| Abdominal pain - intestinal colic | |||
| Diarrhea - rhinorrhea | 45 | Intestinal colic - pruritus | 27 |
| Postprandial fullness - belching | Intestinal colic - palpitation | ||
| Bloating - dysmenorrhea | |||
| Bloating - other symptoms | |||
| Constipation - belching | |||
| Constipation - nose congestion | |||
| Nausea - rhinorrhea | |||
| Belching - rhinorrhea | |||
| Dizziness - hypotonia | |||
| Palpitation - sneezing | |||
| Abdominal pain - constipation | 44 | Abdominal pain - dysmenorrhea | 26 |
| Abdominal pain - hypotonia | |||
| Diarrhea - swollen, reddened eyelids | |||
| Belching - hypotonia | |||
| Belching - palpitation | |||
| Pruritus - flush | |||
| Headache - hypotonia | |||
| Palpitation - rhinorrhea |
Two patients showed only 3 or fewer symptoms, but 96.8% of patients with HIT presented with more than 3 symptoms. The median of the number of symptoms was 10 (IQR, 7), and the mean value was 11.1 symptoms (SD, ± 4.8). Less than 10 symptoms were indicated by 40.3% of patients, 14.5% of patients had 10 symptoms and 45.2% showed more than 10 symptoms (Fig. 2). From the calculated 2.024 possible combinations of 3 symptoms, patients presented with 1.975 combinations, and only 49 combinations were not displayed. From the 10.626 possible combinations of 4 symptoms, 9.899 combinations were presented and 727 combinations did not occur.
Fig. 2.
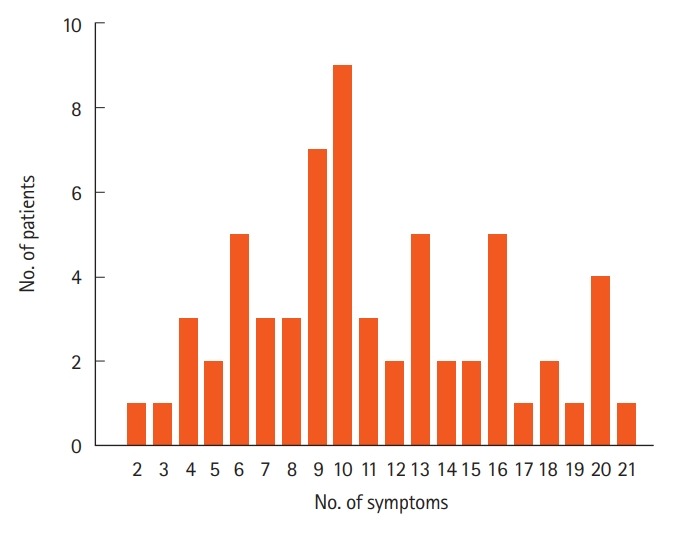
Number of symptom combinations in patients with histamine intolerance.
DISCUSSION
HIT is assumed to be due to a GI-DAO deficit and the name was created with reference to the term lactose intolerance. A disproportionate amount of histamine in the body is thought to result from the consumption of food with high histamine content, and a reduced ability of mainly the enzyme DAO to digest histamine [6]. The clinical diagnosis of HIT is challenging but patients with low serum DAO values, 2 or more GI symptoms described for HIT, and a reduction of symptoms due to a histamine-reduced diet may be diagnosed with HIT [4,5]. In the present study we describe the wide variability of GI, extraintestinal and combinations of symptoms associated with HIT. Polymorphisms identified for the genes coding for DAO and for histamine receptors may help explain these symptoms [7,8]. Complex symptom combinations may influence disease expression and the individual response to diets or treatments.
Disorders such as IBS are one of the main reasons for consultation in primary care and IBS is associated with high symptom burden causing a reduced quality of life. The diagnosis of IBS solely relies on self-reported symptoms by patients, and is in many cases self-diagnosed and self-treated [9]. Currently Rome IV criteria as classification and diagnostic criteria in IBS are used but they have limitations in clinical practice since many patients do not demonstrate the required criteria. In Rome IV the recurring symptom of abdominal pain with change in bowel habits, including diarrhea, constipation, bloating and distension, was described as main IBS symptom [10]. We here found bloating to be the most frequent and most severe symptom in HIT. Frequently, patients also reported postprandial fullness, diarrhea, abdominal pain and constipation which resemble IBS-linked symptoms. However, IBS patients were shown to believe that food, including histamine-containing food, triggers their GI symptoms [11].
Another new, symptom-based disorder is non-celiac gluten sensitivity (NCGS) and it is associated with the consumption of gluten-containing products [12]. Since there are no diagnostic criteria available for NCGS they are also referred to as “people without celiac disease avoiding gluten.” [13] It was assumed that people with NCGS are a group of patients with IBS who are self-diagnosed and perform self-treatment with a gluten-free diet [14]. However most gluten-containing foods and drinks also contain histamine and/or they are consumed with added histamine-containing seasonings. We described that GI and extraintestinal symptoms in NCGS resemble those which can be found in HIT [15].
Functional dyspepsia (FD) is a disorder with a heterogeneous group of symptoms in the epigastric region and again, with absence of any organic disease. For FD a number of explanations was employed but pathophysiology is unknown and despite all attempts the treatment of FD remains insufficient [16]. Abdominal pain and postprandial fullness are described in FD as main symptoms [17] and patients with HIT reported these also to be prominent symptoms (Table 2).
However, a symptom is an experience observed as different from normal and depends very much on the individual patients’ subjective interpretation. Symptoms alone or symptom complexes are rarely, if ever, diagnostic [9]. Although, included patients experienced recurring symptoms caused by food ingestion with high histamine content, in this study a recall bias cannot be entirely excluded. So far, no laboratory values have been found to correlate with symptoms in IBS, NCGS, or FD. Although, serum DAO values are not fully established to reflect GIDAO activity [1] and determination of DAO in serum with currently available assays has limitations [18], the diagnosis of HIT may be supported with measurements of serum DAO [4,5].
Since there are symptom correlations and parallels in IBS, NCGS, and FD with HIT, determination of serum DAO values in well-defined patients with recurring functional non-specific GI symptoms in IBS, NCGS, and FD are necessary. In conclusion, the knowledge of this wide and complex variability of symptoms in patients with HIT may assist clinically recognizing and diagnosing histamine intolerance.
Footnotes
FINANCIAL SUPPORT
The authors received no financial support for the research, authorship, and/or publication of this article.
CONFLICT OF INTEREST
W.J.S. received speaking honoraria from Sciotec. The other authors declare no competing interests.
AUTHOR CONTRIBUTION
Study design: Schnedl WJ, Enko D, Mangge H. Patient recruitment and data collection: Schnedl WJ. Laboratory data determination: Schenk M, Mangge H. Data analysis: Schnedl WJ, Lackner S, Holasek SJ. Discussion of results: Schnedl WJ, Schenk M, Holasek SJ, Mangge H. Writing the manuscript: Schnedl WJ, Lackner S, Enko D. Approval of final manuscript: all authors.
REFERENCES
- 1.Reese I, Ballmer-Weber B, Beyer K, et al. German guideline for the management of adverse reactions to ingested histamine: guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the German Society for Pediatric Allergology and Environmental Medicine (GPA), the German Association of Allergologists (AeDA), and the Swiss Society for Allergology and Immunology (SGAI) Allergo J Int. 2017;26:72–79. doi: 10.1007/s40629-017-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maintz L, Novak N. Histamine and histamine intolerance. Am J Clin Nutr. 2007;85:1185–1196. doi: 10.1093/ajcn/85.5.1185. [DOI] [PubMed] [Google Scholar]
- 3.Jones BL, Kearns GL. Histamine: new thoughts about a familiar mediator. Clin Pharmacol Ther. 2011;89:189–197. doi: 10.1038/clpt.2010.256. [DOI] [PubMed] [Google Scholar]
- 4.Mušič E, Korošec P, Šilar M, Adamič K, Košnik M, Rijavec M. Serum diamine oxidase activity as a diagnostic test for histamine intolerance. Wien Klin Wochenschr. 2013;125:239–243. doi: 10.1007/s00508-013-0354-y. [DOI] [PubMed] [Google Scholar]
- 5.Lackner S, Malcher V, Enko D, Mangge H, Holasek SJ, Schnedl WJ. Histamine-reduced diet and increase of serum diamine oxidase correlating to diet compliance in histamine intolerance. Eur J Clin Nutr. 2019;73:102–104. doi: 10.1038/s41430-018-0260-5. [DOI] [PubMed] [Google Scholar]
- 6.Kovacova-Hanuskova E, Buday T, Gavliakova S, Plevkova J. Histamine, histamine intoxication and intolerance. Allergol Immunopathol. 2015;43:498–506. doi: 10.1016/j.aller.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Maintz L, Yu CF, Rodríguez E, et al. Association of single nucleotide polymorphisms in the diamine oxidase gene with diamine oxidase serum activities. Allergy. 2011;66:893–902. doi: 10.1111/j.1398-9995.2011.02548.x. [DOI] [PubMed] [Google Scholar]
- 8.Sadek B, Stark H. Cherry-picked ligands at histamine receptor subtypes. Neuropharmacology. 2016;106:56–73. doi: 10.1016/j.neuropharm.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949–958. doi: 10.1001/jama.2015.0954. [DOI] [PubMed] [Google Scholar]
- 10.Schmulson MJ, Drossman DA. What is new in Rome IV. J Neurogastroenterol Motil. 2017;23:151–163. doi: 10.5056/jnm16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Böhn L, Störsrud S, Törnblom H, Bengtsson U, Simrén M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. 2013;108:634–641. doi: 10.1038/ajg.2013.105. [DOI] [PubMed] [Google Scholar]
- 12.Newberry C, McKnight L, Sarav M, Pickett-Blakely O. Going gluten free: the history and nutritional implications of today’s most popular diet. Curr Gastroenterol Rep. 2017;19:54. doi: 10.1007/s11894-017-0597-2. [DOI] [PubMed] [Google Scholar]
- 13.Choung RS, Unalp-Arida A, Ruhl CE, Brantner TL, Everhart JE, Murray JA. Less hidden celiac disease but increased gluten avoidance without a diagnosis in the United States: findings from the National Health and Nutrition Examination Surveys from 2009 to 2014. Mayo Clin Proc. 2017;92:30–38. doi: 10.1016/j.mayocp.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Giorgio R, Volta U, Gibson PR. Sensitivity to wheat, gluten and FODMAPs in IBS: facts or fiction? Gut. 2016;65:169–178. doi: 10.1136/gutjnl-2015-309757. [DOI] [PubMed] [Google Scholar]
- 15.Schnedl WJ, Lackner S, Enko D, Schenk M, Mangge H, Holasek SJ. Non-celiac gluten sensitivity. People without celiac disease avoiding gluten: is it due to histamine intolerance? Inflamm Res. 2018;67:279–284. doi: 10.1007/s00011-017-1117-4. [DOI] [PubMed] [Google Scholar]
- 16.Holtmann G, Shah A, Morrison M. Pathophysiology of functional gastrointestinal disorders: a holistic overview. Dig Dis. 2017;35:5–13. doi: 10.1159/000485409. [DOI] [PubMed] [Google Scholar]
- 17.Talley NJ. Functional dyspepsia: new insights into pathogenesis and therapy. Korean J Intern Med. 2016;31:444–456. doi: 10.3904/kjim.2016.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boehm T, Pils S, Gludovacz E, et al. Quantification of human diamine oxidase. Clin Biochem. 2017;50:444–451. doi: 10.1016/j.clinbiochem.2016.12.011. [DOI] [PubMed] [Google Scholar]


